Abstract
Background & objectives:
Triple test as prenatal screening procedure does not form a part of routine health care of pregnant women in India. Hence, median values of triple test biomarkers are lacking for Indian population. This study was undertaken to establish population-specific medians for biomarkers viz. alpha-foetoprotien (AFP), human chorionic gonadotropin (hCGβ), and unconjugated estriol (uE3) for detection of Down's syndrome, Edward's syndrome and neural tube defects (NTDs) in pregnant women in north-west India.
Methods:
Serum biomarker values were derived from 5420 pregnant women between 15-20 wk of gestation who were enrolled for triple test investigations at Department of Gynecology and Obstetrics, Government Medical College and Hospital, Chandigarh, India, between January, 2007 to December, 2009. Median values were calculated for rounded weeks using database comprising pregnancies with normal outcomes only. Simple statistical analysis and log-linear regression were used for median estimation of the biomarker values.
Results:
The levels of the three biomarkers were found to be ranging from 1.38 to 187.00 IU/ml for AFP, 1.06 to 315 ng/ml for hCGβ, and 0.25 to 28.5 nmol/l for uE3. The age of women ranged from 18 to 47 yr and mean weight was 57.9 ± 9.8 kg. Data revealed that AFP, hCGβ and uE3 medians in our study population were not significantly different from those reported from other countries or when compared ethnically.
Interpretation & conclusion:
The population-specific median values for the three biomarkers (AFP, hCGβ, uE3) may be used as reference values during prenatal screening in Indian pregnant women.
Keywords: Alpha-foetoprotein (AFP), human chorionic gonadotropin (hCGβ), prenatal screening, pregnant women, unconjugated estriol (uE3)
Screening for chromosomal aneuploidies and neural tube defects (NTDs) has become an integral part of prenatal care worldwide. There have been some changes in the recommended method of prenatal screening over the last few years and advances are made to improve detection rates with better combination of maternal serum analytes. However, the issues encountered are sensitivity and specificity of multiple serum analyte combinations along with establishment of their median concentrations.
Maternal serum analytes, alpha-foetoprotein (AFP), human chorionic gonadotropin (hCGβ), and unconjugated estriol (uE3) values have been shown to be influenced by maternal weight1,2, race and ethnicity3,4,5,6,7,8, presence of certain conditions like insulin-dependent diabetes8, and smoking9,10. Such differences must be taken into consideration while calculations are made for obtaining likelihood ratios for pregnancies being affected by Down's syndrome, Edward's syndrome or neural tube defects. As the levels of the three markers change with gestational age, the results of these markers are expressed as multiples-of-median (MoM)11. These MoM values are compared with parametric population statistics and combined with a prior risk based on maternal age to calculate the likelihood of chromosomal aneuploidy and neural tube defects12.
The efficiency of any screening programme depends on the accuracy and reliability of median values and how well these represent the population being screened. In absence of any comprehensive programme for screening all pregnant women through triple test in India, the information on median values of second trimester maternal serum biomarkers is not readily available. In this study, an attempt was made to establish the representative medians for the population studied for AFP, hCGβ and uE3 in the second trimester of pregnancy.
Material & Methods
All pregnant women visiting the outpatients of Department of Obstetrics and Gynecology, Government Medical College, Chandigarh, India, between January 2007 and December 2009 were counseled about triple test and were enrolled in the study after obtaining written informed consent. The study protocol was approved by the ethical committee of the institution. Gestational age in pregnant women was determined ultrasonographically using biparietal diameter (BPD) or crown rump length (CRL). Fresh blood (3-5 ml) was collected between 15-20 wk of gestation from all pregnant women and serum was separated. Second trimester maternal serum values for AFP, hCGβ and uE3 were derived from 5420 samples from singleton, non-diabetic pregnancies tested during the study period. Serum AFP and free hCGβ concentrations were measured on DELFIA using hAFP/free hCGβ Dual kit, and uE3 by DELFIA unconjugated estriol kit purchased from PerkinElmer by time-resolved fluoroimmunoassay (PerkinElmer Life and Analytical Sciences, Finland).
The risk for pregnancy being affected by trisomy 21, trisomy 18 and NTD was calculated using Wallac LifeCycle software with Ellipse Screening Engine of PerkinElmer, using a cut-off value of 1:250 at term for Down's syndrome and 1:100 at term for Edward's syndrome. AFP MoM of more than 2.5 was used as the cut-off value for NTD risk. Patients with high-risk report were referred for a detailed foetal ultrasonography followed by a re-assessment of risk and diagnostic testing, amniocentesis, if required.
All the pregnancies were followed up until delivery for outcome. For calculation of medians, serum biomarker values from 238 women who had an adverse pregnancy outcome (Down's syndrome, neural tube defects, congenital malformations, miscarriages, perinatal deaths) were excluded from the database. Medians were calculated for rounded weeks (15th rounded week includes gestation days from 14+4 to 15+3) using simple statistical analysis and through regression analysis. Medians for gestation weeks 15-20 of north west Indian women were compared with those reported from other countries.
Statistical analysis: Data were analyzed using Microsoft Excel (Microsoft Corporation, USA) and SPSS 16.0 (SPPS Inc., USA). Biochemical marker values were logarithm transformed and regressed against gestational age, using log-linear, quadratic, cubic and exponential equations. Median values for second trimester maternal serum markers were established as described by Vranken et al13. All serum markers were transformed to log10 data and the period of gestation was taken in decimal weeks. Least squares regression was used to establish median equations. Various equations for the three serum markers like linear, quadratic, cubic were worked out.
Results
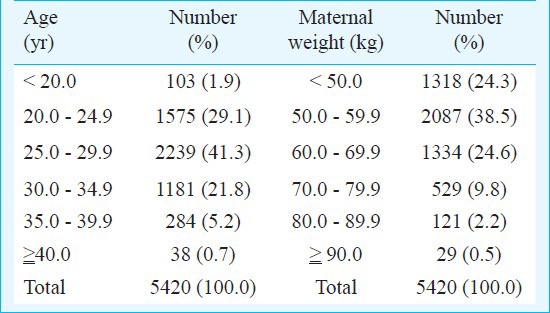
The levels of triple screen biomarkers were found to be ranging from 1.38 to 187.00 IU/ml for AFP; 1.06 to 315 ng/ml for hCGβ; and 0.25 to 28.5 nmol/l for uE3. The age of all women enrolled ranged from 18-47 yr. Among them, 94 per cent of women were less than 35 yr (Table I). The mean weight of women included in the study was 57 ± 9.8 kg.
Table I.
Distribution of pregnant women enrolled in the study with respect to age and weight
For estimation of medians for the biomarker values, simple median values were calculated. Also, biochemical marker values were logarithm transformed and regressed against gestational age in decimal weeks13. Literature cites that AFP and uE3 fit a logarithmic linear model and hCGβ fits an exponential model14. For our data, simple log-linear relationship gave a significant F test for AFP, hCGβ and uE3. The log-linear model could explain only 10 per cent of the variation in AFP and hCGβ and about 20 per cent for uE3. Multiple-of-medians (MoMs) used to report and interpret the results help to overcome this limitation and are independent of gestational age. Other models investigated (quadratic, cubic, exponential) failed to improve the value of R2. Medians were calculated using simple log-linear regression for AFP, hCGβ and uE3 and compared with those obtained using simple statistical analysis. No significant difference was observed between the median values obtained through simple statistical analysis and those obtained through simple log-linear regression, hence, medians from simple statistical analysis have been reported here and compared with those reported from other countries (Table IIa and IIb) and for other ethnicities (Table IIc and IId). Medians values for alpha foetoprotein and unconjugated estriol compared well with those reported from other countries and were not found to be significantly different.
Table IIa.
Median values of AFP for north west Indian population compared with median values from other subpopulations*
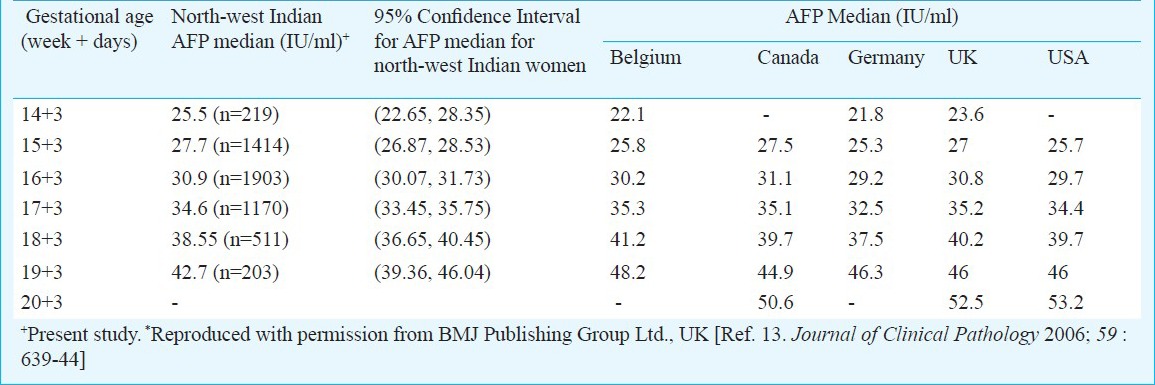
Table IIb.
Median values of uE3 for north-west Indian population compared with median values from other subpopulations*
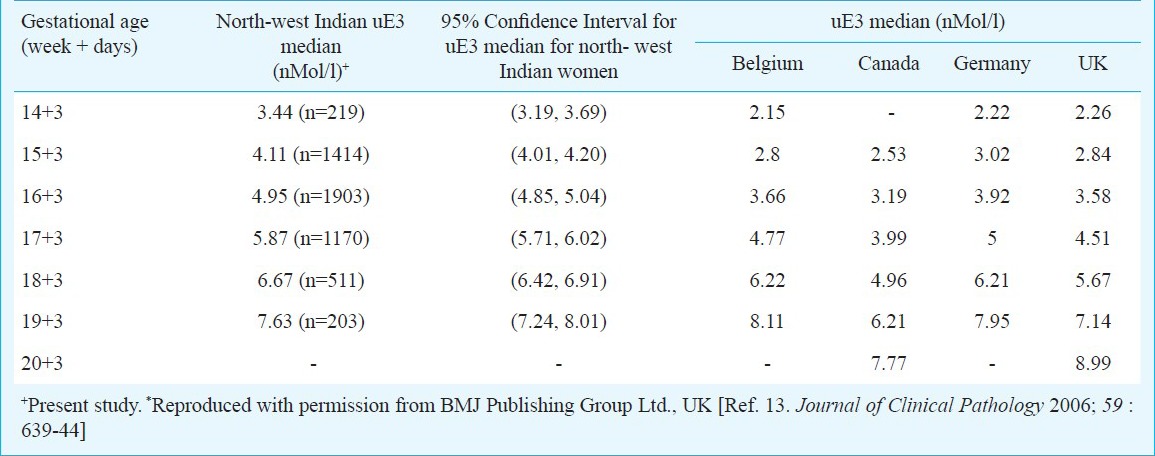
Table IIc.
Median values of AFP for north-west Indian population compared with median values from other ethnicities*
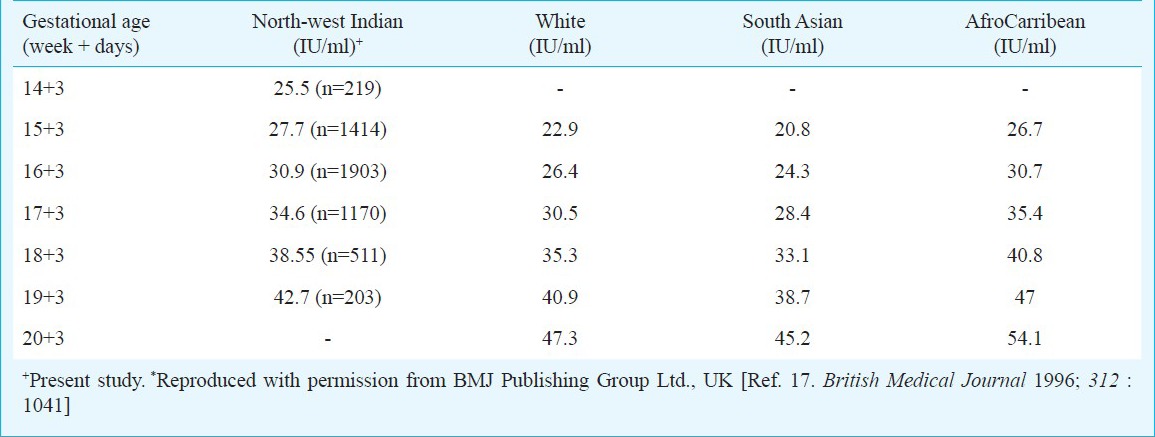
Table IId.
Median values of hCGβ for north-west Indian population compared with median values from other ethnicities*
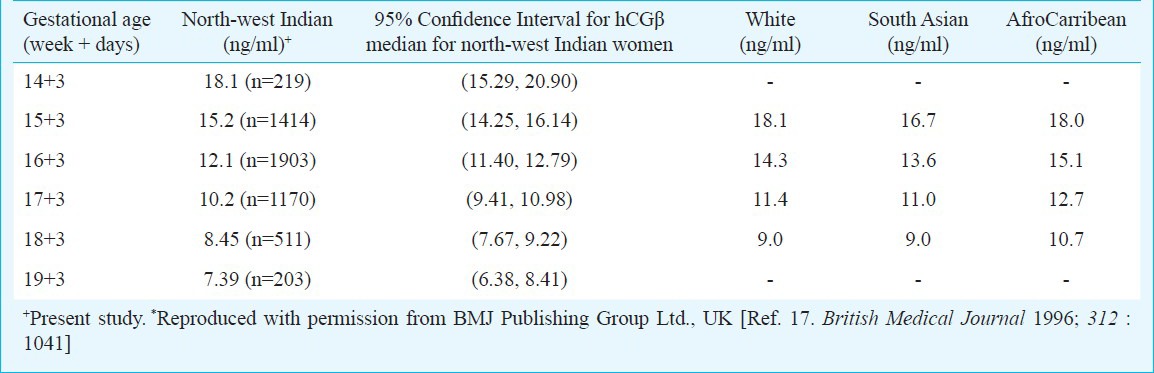
A comparison of hCGβ values could not be made for other sub-populations i.e. Belgium, Canada, Germany, UK, USA; since the available median concentrations from these countries were for intact hCG while our study measured free hCGβ for screening of chromosomal aneuploidies. However, evaluating median values for hCGβ of our study with those available in literature for other ethnicities (White, South Asian and Afrocarribean) did not show a significant difference.
Medians for different gestational ages were also compared with the overall median (ignoring gestational age) using Wilcoxon-signed rank test. The comparison revealed that there is a significant (P<0.05) difference in medians of each gestation compared to the overall median of AFP, hCGβ and uE3. A test for heterogeneity of medians was also carried out for different gestational age groups related to AFP, hCGβ and uE3 using Kruskal Wallis non parametric test which showed a significant difference between all the gestations for AFP, hCGβ and uE3 concentrations (AFP, P=0.0001; hCGβ, P=0.0001; uE3, P=0.001). In order to compare medians of two gestations at a time, Mann-Whitney-U-statistics was applied and all the comparisons were found to differ significantly (P<0.05).
Discussion
Screening for Down syndrome began in the second half of the 20th century with maternal age as the sole criterion for categorizing women as high risk for carrying Down syndrome affected foetus. Several studies have documented existence of differences in biomarker values with region13 and ethnicity4,5,6,7,8. Strengthening maternal serum screening for Down's syndrome makes it imperative to incorporate a set of median values which adequately characterize the population being screened. Various methods have been documented in literature to study the median values for second trimester maternal serum markers. Regression analysis is generally preferred as it takes into account the variation due to extreme values. However, the R2 values as reported in literature for regression analysis, though highly significant, are usually low13. For our data, the median values obtained through simple statistical analysis and regression analysis were not significantly different, and hence, simple median values were computed and compared with those reported from other countries.
No significant difference was observed when our medians derived from north-west Indian pregnant women were compared with those reported from other geographical regions. The median values of AFP and uE3 did not show any significant difference when compared with those reported from other subpopulations, though uE3 values were observed to be consistently higher from 14 to 18 wk. AFP and intact hCGβ concentrations have been observed to be higher in Black pregnant women as compared to Whites4,5,6,15 while uE3 values are reported to be not appreciably different2,7,8. In our study, AFP values were found to be higher in comparison to Whites, reported from other studies and lower to those reported for Afro-Carribeans and hence, in agreement to other studies in the literature. The difference between AFP median values relative to White decreased with gestational age, and was highest in the 15th week of pregnancy16. The median values for free β-human chorionic gonadotropin were also observed to be lower as compared to other ethnicities. On the other hand, our AFP median values were higher to those reported by Holloway and Bulusu17 for South Asian Population. This could be because most of these studies have been conducted in migrant population of Asian origin and hence, more studies in indigenous population are warranted for conclusive results.
Refinements to risk calculation through corrections for maternal weight and subsequent corrections for ethnicity have been recommended4,15,16. The correction for maternal weight is carried out in the MoM values to obtain corrected MoM values. This correction also includes correction for other conditions that affect the biomarker values, for example, insulin dependent diabetes, smoking and for ethnicity.
In conclusion, the mean values for the three biomarkers (AFP, hCGβ, uE3) in north-west Indian pregnant women may be used as reference values for Indian women. Further studies need to be done in other regions and subpopulations of the country.
Acknowledgment
The study was financially supported by Chandigarh Administration, Chandigarh.
References
- 1.Wald NJ, Kennard A, Hackshaw A, McGuire A. Antenatal screening for Down's syndrome. (1-112).Health Technol Assess. 1998;2:i–iv. [PubMed] [Google Scholar]
- 2.Burton BK, Nieb B. Effect of maternal race and weight on hCGβ and uE3 levels in the mid-trimester. Am J Hum Genet. 1991;49(Suppl):212. [Google Scholar]
- 3.Wald NJ, Kennard A, Hackshaw A, McGuire A. Antenatal screening for Down's syndrome. J Med Screen. 1997;4:181–246. doi: 10.1177/096914139700400402. [DOI] [PubMed] [Google Scholar]
- 4.Crandall BF, Lebherz TB, Schroth PC, Matsumoto M. Alpha-fetoprotein concentrations in maternal serum: relation to race and body weight. Clin Chem. 1983;29:531–3. [PubMed] [Google Scholar]
- 5.Johnson AM. Racial differences in maternal serum alphafetoprotein screening. In: Mizejewski GJ, Porter IH, editors. Alpha-fetoprotein and congenital disorders. New York: Academic Press; 1985. pp. 183–96. [Google Scholar]
- 6.Baumgarten A. Racial difference and biological significance of maternal serum alpha-fetoprotein. Lancet. 1986;ii:573. doi: 10.1016/s0140-6736(86)90137-6. [DOI] [PubMed] [Google Scholar]
- 7.Simpson JL, Elias S, Morgan CD, Shulman L, Umstot E, Anderson RN. Second trimester maternal serum human chorionic gonadotropin and unconjugated oestriol levels in blacks and whites. Lancet. 1990;335:459–60. doi: 10.1016/0140-6736(90)91485-s. [DOI] [PubMed] [Google Scholar]
- 8.Canick JA, Panizza DS, Palomaki GE. Prenatal screening for Down syndrome using AFP, uE3 and hCGβ: effect of maternal race, insulin-dependent diabetes and twin pregnancy. Am J Hum Genet. 1990;47:A270. [Google Scholar]
- 9.Crossley JA, Aitken DA, Waugh SM, Kelly T, Connor JM. Maternal smoking: age distribution, levels of alpha-fetoprotein and human chorionic gonadotrophin, and effect on detection of Down syndrome pregnancies in second-trimester screening. Prenat Diagn. 2002;22:247–55. doi: 10.1002/pd.313. [DOI] [PubMed] [Google Scholar]
- 10.Spencer K. The influence of smoking on maternal serum AFP and free beta hCGβ levels and the impact on screening for Down syndrome. Prenat Diagn. 1998;18:225–34. doi: 10.1002/(sici)1097-0223(199803)18:3<225::aid-pd239>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 11.Wald NJ, Cuckle H, Brock JH, Peto R, Polani PE, Woodford FP. Maternal serum alpha-fetoprotein measurements in antenatal screening for anencephaly and spina bifida in early pregnancy. Report of U.K. collaborative study on alpha-fetoprotein in relation to neural tube defects. Lancet. 1977;1:1323–32. [PubMed] [Google Scholar]
- 12.Reynolds TM, Penney MD. The mathematical basis of multivariate risk screening: with special reference to screening for Down's syndrome associated pregnancy. Ann Clin Biochem. 1990;27:452–8. doi: 10.1177/000456329002700506. [DOI] [PubMed] [Google Scholar]
- 13.Vranken G, Reynolds T, Nueten JV. Medians for second-trimester maternal serum markers: geographical differences and variations caused by median multiples-of-median equations. J Clin Pathol. 2006;59:639–44. doi: 10.1136/jcp.2005.034272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palomaki GE, Bradley LA, McDowell GA Down Syndrome Working Group; ACMG Laboratory Quality Assurance Committee. Technical Standards and guidelines: Prenatal screening for Down Syndrome. Genet Med. 2005;7:344–54. doi: 10.1097/01.gim.0000167808.96439.f3. [DOI] [PubMed] [Google Scholar]
- 15.Watt HC, Wald NJ, Smith D, Kennard A, Densem J. Effect of allowing for ethnic group in prenatal screening for Down's syndrome. Prenat Diagn. 1996;16:691–8. doi: 10.1002/(SICI)1097-0223(199608)16:8<691::AID-PD946>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 16.Hsu JJ, Hsieh TT, Soong YK, Kuo B. The influence of maternal weight correction formulas in Asian Down syndrome screening using alpha-fetoprotein and free beta-human chorionic gonadotropin. J Matern Fetal Investig. 1998;8:66–70. [PubMed] [Google Scholar]
- 17.Holloway PJ, Bulusu S. For appreciable ethnic differences in false positive rates, use different cut-off points. BMJ. 1996;312:1041. doi: 10.1136/bmj.312.7037.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]


