Abstract
The incidence of emerging infectious diseases in humans has increased within the recent past or threatens to increase in the near future. Over 30 new infectious agents have been detected worldwide in the last three decades; 60 per cent of these are of zoonotic origin. Developing countries such as India suffer disproportionately from the burden of infectious diseases given the confluence of existing environmental, socio-economic, and demographic factors. In the recent past, India has seen outbreaks of eight organisms of emerging and re-emerging diseases in various parts of the country, six of these are of zoonotic origin. Prevention and control of emerging infectious diseases will increasingly require the application of sophisticated epidemiologic and molecular biologic technologies, changes in human behaviour, a national policy on early detection of and rapid response to emerging infections and a plan of action. WHO has made several recommendations for national response mechanisms. Many of these are in various stages of implementation in India. However, for a country of size and population of India, the emerging infections remain a real and present danger. A meaningful response must approach the problem at the systems level. A comprehensive national strategy on infectious diseases cutting across all relevant sectors with emphasis on strengthened surveillance, rapid response, partnership building and research to guide public policy is needed.
Keywords: Avian influenza, chikungunya, control, emerging infections, India, Nipah virus, plague, prevention
Introduction
Emerging infectious diseases (EIDs) are diseases of infectious origin whose incidence in humans has increased within the recent past or threatens to increase in the near future. These include new, previously undefined diseases as well as old diseases with new features. These new features may include the introduction of a disease to a new location or a new population (e.g. it may present in youth where previously it was only seen in the elderly); new clinical features, including resistance to available treatments; or a rapid increase in the incidence and spread of the disease. Reappearance of a disease which was once endemic but had since been eradicated or controlled, would classify it as a re-emerging infectious disease. Emergence may also be due to a new recognition of an infectious agent in the population or the realization that an established condition has an infectious origin1.
Over 30 new infectious agents have been detected worldwide in the last three decades; 60 per cent of these are of zoonotic origin, and more than two-thirds of these have originated in the wildlife (Table)1,2,3,4. Epidemics or pandemics caused by these emerging and re-emerging infections often take a heavy toll of life and by rapidly spreading across borders are responsible for much concern and panic. Besides health, emerging infections also present a grave economic, developmental and security challenge. As a case in point SARS (severe acute respiratory syndrome), is the first severe infectious diseases to emerge in the twenty-first century. Having emerged in Guandong province of China in 2003, possibly from civet cats, SARS, a new corona virus, spread rapidly to 30 countries across Asia, Americas and Europe, with a total of 8,439 cases and 812 deaths, within 7 to 8 months5. It posed a serious threat to global health security, the health system capacities to cope where health workers themselves were at risk, and the stability and growth of economies. The economic losses to countries in Asia were estimated at UD$ 10-30 billion6.
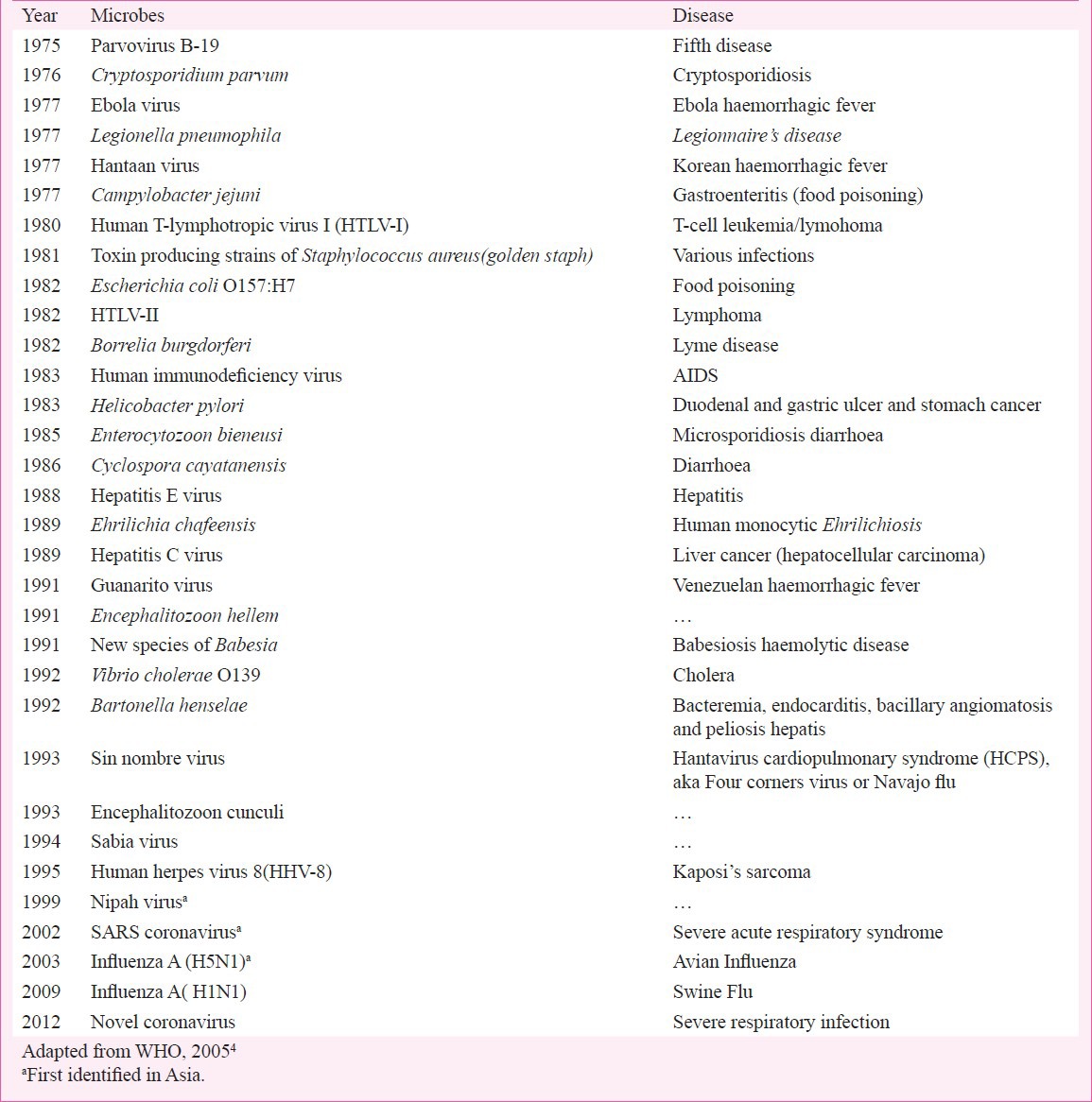
Table.
Newly discovered microbes of public health importance
More recently, in March 2009, cases of H1N1 influenza were first reported in Mexico, followed by spread to the United States and then to rest of the world including India. By September, nearly all countries had reported H1N1 virus to the World Health Organization, with more than 17,000 deaths; of which, 12,000 were in the United States alone. Given its far reaching impact, the President of the United States in October had declared 2009 H1N1 influenza pandemic a national emergency7.
These two examples highlight the risks and underscore the need to improve preparedness at national and international levels for future pandemics. It is clear that new pathogens particularly viruses are likely to continue to emerge and spread across countries for a variety of reasons and challenge public health as never before. These will represent a serious burden, causing untold morbidity and mortality, disrupting trade and travel, and negatively affecting the economy.
This paper provides an overview of emerging infections (including re-emerging ones) globally and in India, their determinants, the national response mechanisms in place and the way forward.
Historical perspectives
Historically, over the centuries, human populations have experienced major epidemics of infectious diseases. Imported smallpox microbes carried by explorers were responsible for 10-15 million deaths in 1520-1521, effectively ending Aztec civilization. Other Amerindian and Pacific civilizations were destroyed by imported small pox and measles. Similar to smallpox, plague - a disease caused by bacteria and spread by rats had eliminated as much as a third of the European population over a five year period in the 14th century. By the middle of the 20th century, infectious diseases were no longer considered as the major cause of mortality in developed countries8,9.
Establishment of the germ theory and the identification of specific microbes as the causative agent of a wide variety of infectious diseases led to enormous progress, notably the development of vaccines and antimicrobials1. The eradication of smallpox reinforced the perception that infectious diseases could be eliminated. Improved sanitation, clean water and better living conditions along with vaccines and antimicrobial agents brought many infectious diseases under control. While the old world communicable diseases are ebbing, a slew of newer and hitherto unknown set of communicable diseases like avian influenza, SARS, etc. are on a rise10. Moreover, emergence of drug resistance such as multidrug resistant (MDR) and extensively drug resistant (XDR) tuberculosis, malaria resistant to artemisinin-combination therapy (ACT) is new global challenges requiring concerted efforts.
Human pathogens emerge and re-emerge due to interaction of multiple complex factors between the host and pathogen each driven by the need to secure the success of the species in changing environments. Adaptation by one partner to exploit new environments will often stimulate the other to modify its characteristics to take advantage of the change. The human encroachment into habitat especially in tropical regions and interface with wildlife can lead to creation of “hot spots” for emergence of new pathogens, with a potential for rapid spread among susceptible human populations, facilitated by rapid means of travel and wildlife trafficking. The mutation of a virus strain from animals, as making it infectious to humans, is a common cause of new illnesses in humans. A systematic review conducted to identify factors associated with emergence and re-emergence of human pathogens has shown changes in land use or agricultural practices and changes in human demographics and society as the major drivers associated with emergence and re-emergence of human pathogens11.
The Global burden of emerging diseases
The emerging infectious diseases account for 26 per cent of annual deaths worldwide. Nearly 30 per cent of 1.49 billion disability-adjusted life years (DALYs) are lost every year to diseases of infectious origin3,4. The burden of morbidity and mortality associated with infectious diseases falls most heavily on people in developing countries, and particularly on infants and children (about three million children die each year from malaria and diarrhoeal diseases alone)2.
A literature survey identified 1,407 species of human pathogens, with 177 (13%) species regarded as emerging or re-emerging. Distribution of emerging and re-emerging pathogens by groups shows that 37 per cent of emerging and re-emerging pathogens are viruses and prions followed by protozoa (25%). This indicates that emerging and re-emerging pathogens are disproportionately viruses11. Examples of some recent emerging and re-emerging infections are shown in Fig. 1. In addition, emergence of microorganisms resistant to antimicrobials to which these were previously sensitive to is cause of concern. Cases with such infections often fail to respond to the standard treatment, resulting in prolonged illness and increased risk of death. According to WHO, 440,000 new cases of multi-drug resistant tuberculosis (MDR-TB) emerge annually, causing at least 150,000 deaths6. A more virulent form called extensively drug-resistant tuberculosis (XDR-TB) has been reported from 64 countries. Another example of global concern relates to the specter of falciparum malaria resistant to artemisinin combination therapy (ACT) emerging at the Cambodia-Thai border which has a potential to spread across countries6.
Fig. 1.
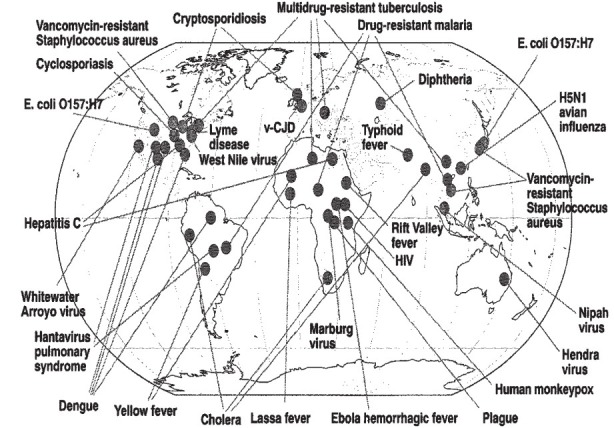
Range and recognized site(s) of origin of a variety of emerging and re-emerging infections (1976-2003). Reproduced with permission from Ref. 2.
Developing countries such as India suffer disproportionately from the burden of infectious diseases. India the second most populous country in the world is in the midst of a triple burden of diseases; the unfinished agenda of communicable diseases, non-communicable diseases linked with lifestyle changes, and emergence of new pathogens and overstretched health infrastructure13. Communicable diseases account for nearly half of India's disease burden14. Many infections are associated with poor sanitation, contaminated food, inadequate personal hygiene, or access to safe water and lack of basic health services- conditions common to large parts of India. Favourable environmental, demographic and socio-economic factors further put India at a risk of epidemics of emerging infections. Over the years increase in cases of drug resistant malaria, tuberculosis, HIV-TB co-infections and epidemics of avian influenza have demonstrated the vulnerability of India to the threat of evolving microbes.
Emerging infections in India: Trends and epidemiological features
In the recent past, India has witnessed many large outbreaks of emerging infections and most of them were of zoonotic origin. A review of the list shows that of the eight aetiological agents, five are of viral origin. Six of these infections are of zoonotic nature. The geographical origin of some of these outbreaks is depicted in Fig. 2.
Fig. 2.
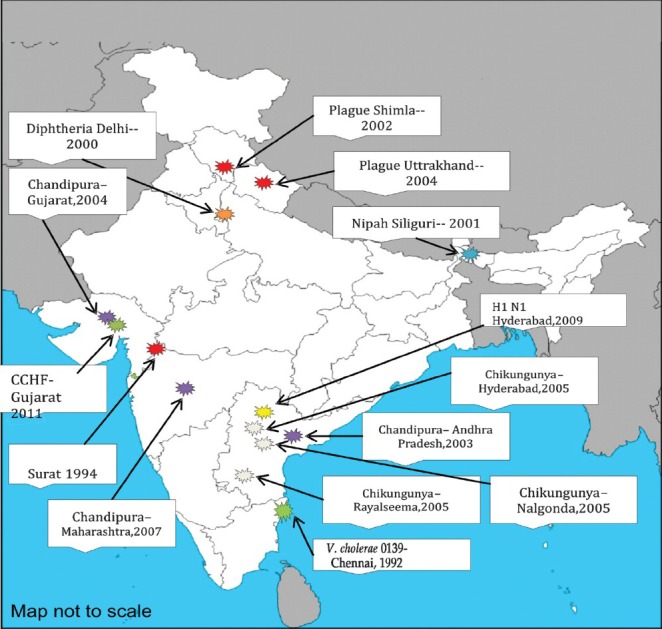
Recognized site(s) of emerging and re-emerging infections in India, 1990-2011. CCHF, Crimean - Congo haemorrhagic fever.
A large scale cholera outbreak occurred in India in 1992 starting in southern peninsular India and spreading both inland and along coast line of Bay of Bengal. Vibrio cholerae O139, a new serogroup was associated with this epidemic cholera15. Reports of cholera outbreak due to this new serogroup have come from various parts of the country16,17,18,19,20,21. Patients infected with O139 strains were much older than those infected with O1 strains. Over the previous one decade, O1 and O139 serogroups have coexisted in much of the cholera endemic areas in India and elsewhere22.
The 1994 plague outbreak in Surat in Gujarat State created an unprecedented level of panic leading to population exodus and internal migration, contributed in part by local and international media reports, and to considerable negative social, political, and economic impact. Plague infection continues to exist in sylvatic foci in many parts of India which is transmitted to humans occasionally. This has been demonstrated with focal outbreaks of plague in India in 1994, 2002 and 200423,24,25. Epidemiological investigations have attributed these recurrences to spillover from an epizootic cycle of plague in wild rodents to commensal rodents driven by climate variation26,27. Recent analysis of data from Kazakhstan shows that warmer springs and wetter summers increase the prevalence of plague in its main host, the great gerbil27. The National Centre for Disease Control (NCDC) has identified four sylvatic foci in India; the tri-junction of Karnataka, Andhra Pradesh and Tamil Nadu, later Beed belt in Maharashtra, Rohru in Himachal Pradesh and Uttarakhand25. The plague outbreak in Surat, led not only to nationwide panic but to a near international isolation of India. As a result, the country incurred direct economic losses to the tune of US $ 1.7 billion6.
The incidence of diphtheria, a vaccine preventable disease during 1980 was about 39,231, it reduced to 2817 cases in 1997. However, in the past two decades, there has been a sudden increase in diphtheria cases with more than 8000 cases reported in 200428. The primary immunization coverage for diphtheria has remained between 56 to 72 per cent in the past two decades according to WHO UNICEF estimates29. The three rounds of National Family Health Surveys (NFHS) also show that DPT 3 coverage during 1992-2006 was only 52-55 per cent30,31,32. Persistence or resurgence of diphtheria in the country seems mainly due to low coverage of primary immunization as well as boosters (Fig. 3). There have been reports of diphtheria outbreaks from various States including Delhi, Andhra Pradesh, Assam, Maharashtra, Chandigarh, Gujarat33,34,35,36,37. An epidemiological age shift has been noted in these outbreaks, with the disease now affecting older children (5-19 yr) and adults. Majority of the cases were reported from children who were unimmunized or partially immunized against diphtheria.
Fig. 3.
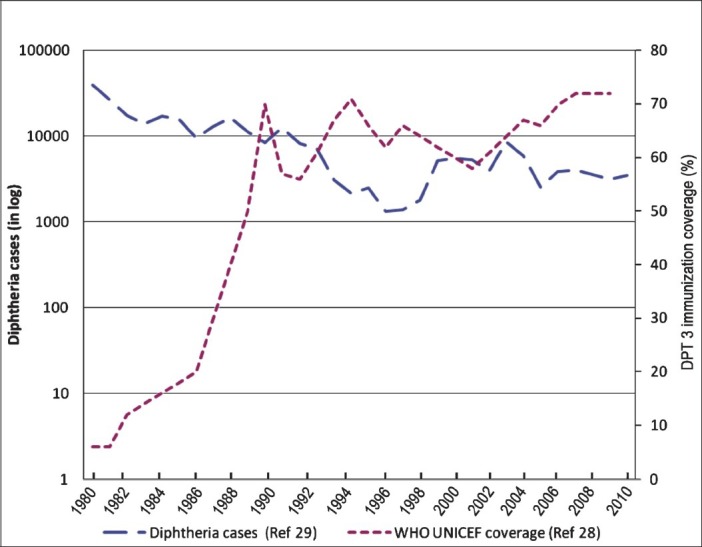
Cases of diphtheria and immunization coverage in India 1980-2010.
The Nipah virus was first recognized in 1999 during an outbreak among pig farmers in Malaysia. Since then, there have been 12 additional outbreaks, all in South Asia38. Fruit bats of the Pteropodidae family are the natural hosts for Nipah virus. Evidence shows that geographical distribution of Henipavirus (Nipah and Hendra) overlaps with that of Pteropus (Fig. 4). Over the years, the epidemiology of Nipah appears to have changed. Evidence of person to person transmission and a high case fatality rate (60-70%) were some of the alarming developments seen in Nipah outbreaks in India (2001) and Bangladesh (2001, 2006)39,40. Nipah virus has also ben categorized as a food borne disease from eating dates contaminated with urine or saliva of infected bats41.
Fig. 4.
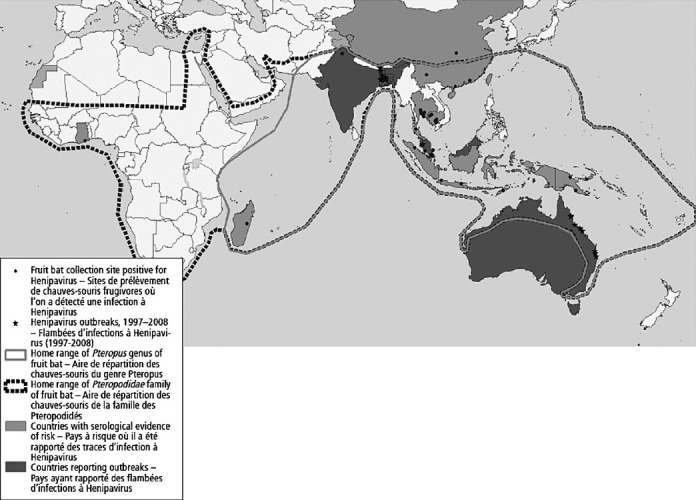
Geographical distribution of Henipavirus outbreaks and fruit bats of the Pteropodidae family, 1997-2008.
Source: Adapted from Ref. 38.
A new virus belonging to family Rhabdoviridae was isolated in 1965 in the Chandipura (Nagpur) region of India in two adult patients with febrile illness during an outbreak of febrile illness caused by chikungunya and dengue viruses42. It was named as Chandipura (CHP) virus. This virus was not considered to have an epidemic potential until an outbreak of acute encephalitis in children in Andhra Pradesh, India was attributed to CHP virus in 2003 with a case fatality rate of 55 per cent43. CHP virus is transmitted to humans by sandflies. Subsequently focal outbreaks have been reported from Gujarat (2004), and Maharashtra (2007)44,45. The outbreaks have predominantly focused in rural areas and sandflies seem to be the main vector and maintainence host of the virus. Case distribution was spotty without clustering and paediatric age group of 9 months to 14 years was involved. Neurological sequelae was rare in recovered children46.
Chikungunya fever, caused by the chikungunya virus, was first reported in Tanzania in 1953. Previous outbreaks in India (1963 and 1973) were caused by the Asian genotypes. Non-human primates act as a main reservoir of infection. After a quiescence of three decades, a resurgence of infection from southern and central parts of the country was reported in 200647,48. This has been attributed to the East African genotype earlier not prevalent in this part of the world49. Currently, 22 States and Union Territories of India have reported cases of chikungunya Fig. 5. Chikungunya is associated with joint pains lasting up to six months. Although deaths are not known to occur but the morbidity and disability caused due to chikungunya are enormous and 45.26 DALYs were lost per million population due to 2006 epidemic in India50.
Fig. 5.
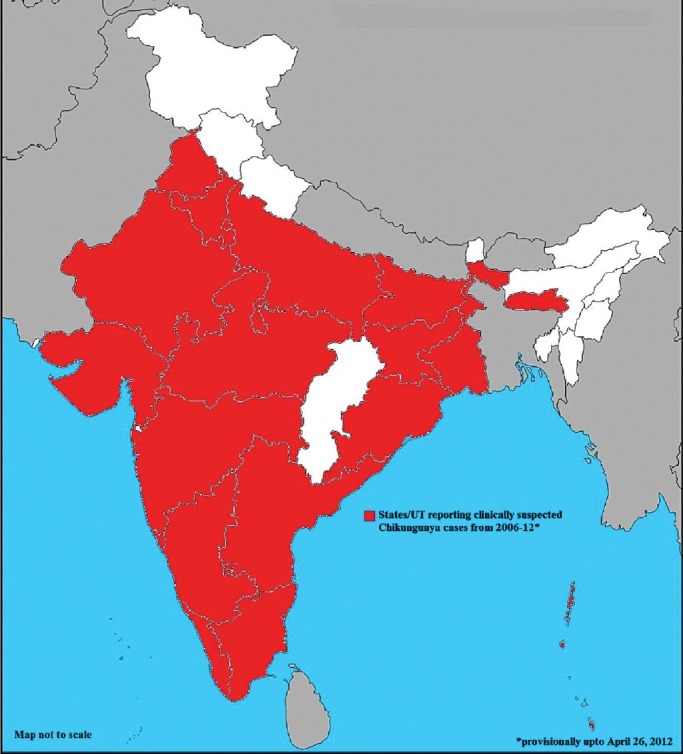
States reporting Chikungunya cases in India.
Source: Adapted from NVBDCP, Ref. 48
Avian influenza is an infection caused by Influenza A (H5N1) viruses, usually infecting poultry animals and pigs. It was first reported in 1997 in Hong Kong51,52. In 2003, changes in the strains of virus resulted in emergence of ‘novel’ Z strain and, infection to human beings by this virus, contrary to earlier belief that avian influenza virus cannot infect human beings due to differences in receptors51. Vietnam reported first human case due to H5N1 in 2003. Till date 587 persons have been infected by Influenza A (H5N1) with 346 deaths from 15 countries53. Cases of bird flu were reported in Navapur tehsil of Nandurbar district of Maharashtra. So far India has not reported any human cases of Influenza A (H5N1)54.
The pandemic HINI influenza virus emerged in humans in early April 2009 in Mexico and California. The H1N1 strain then quickly spread worldwide through human-to-human transmission. Up till August 2010, worldwide more than 214 countries had reported laboratory confirmed cases of pandemic influenza H1N1 2009, including over 18449 deaths55. On August 10, 2010, the WHO Director-General Dr Margaret Chan announced that the influenza H1N1 virus has moved into the post-pandemic period. In India, till August 8, 2010, a total of 15,4259 persons were tested for H1N1 influenza and 23.4 per cent were found to be positive including 1833 deaths. Transmission was intense in western States of Maharashtra and Gujarat56.
Crimean-Congo haemorrhagic fever (CCHF) was first described as a clinical entity in 1944-1945 in Crimea during World War II. CCHF virus circulates in an enzootic tick-vertebrate-tick cycle. The virus causes disease among smaller wildlife species, e.g. hares and hedgehogs that act as hosts for the immature stages of the tick vectors57. A CCHF outbreak was reported in Gujarat in 2011. This outbreaks was characterized by a zoonotic origin and a person-to-person spread in hospital setting. High index of clinical suspicion, early laboratory diagnosis and institution of containment measures curtailed further spread of the disease58.
Epidemiologically, major at-risk group are farmers living in endemic areas and animal handlers. The geographic range of CCHF virus is the most extensive among the tickborne viruses that affect human health, and the second most widespread of all medically important arboviruses, after dengue viruses. Changes in climatic conditions have been suggested to be one of the factors that has facilitated the survival of a large number of Hyalomma spp. ticks and of the hosts of both their immature and adult stages and consequently the increased incidence of CCHF58,59,60.
Acute encephalitis syndrome (AES) characterized by fever and seizures each year takes a heavy toll in a few States of India, especially in children below the age of 10 years. During the past five years, the incidence of AES in the country has been on the rise (Fig. 6). Of the five States reporting the disease during 2011, most cases and deaths were in Uttar Pradesh, followed by Bihar, Assam and West Bengal. As a seasonal disease, AES often occurs in outbreaks during summer or following the rains. In a recent outbreak in Muzzaffarpur district of Bihar, which began during late May 2012 had by Mid July accounted for 389 cases and 160 deaths, with a case fatality rate of 41.13 per cent62. Characteristically, in most outbreaks, the aetiological agents responsible for this life threatening disease remain undetermined, with Japanese encephalitis virus detected in about 15 per cent. Unfortunately, many gaps presently exist in our understanding of the disease epidemiology including the mode of transmission, which hamper efforts at effective control.
Fig. 6.
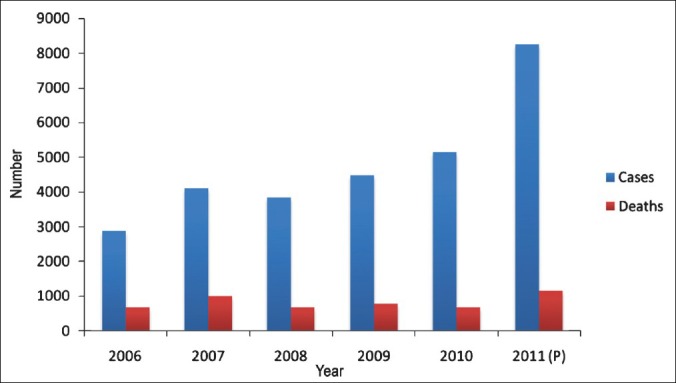
Cases and deaths due to acute encephalitis syndrome/Japanese encephalitis (AES/JE).
Source: Adapted from NVBDCP, Ref. 61
Combating emerging infections: strategies and response capacities in India
EIDs will continue to challenge public health infrastructure, test credibility of health services, and threaten to devastate health and economic development unless a strategic vision and an effective plan of action are developed to combat these. This will increasingly require the application of sophisticated epidemiologic and molecular biologic technologies, changes in human behaviour, and a national perspective. In view of this, the WHO63 has made several recommendations for national strategies including the need to strengthen epidemic preparedness and rapid response, public health infrastructure, risk communication, research and its utilization, and advocacy for political commitment and partnership building. Several initiatives are underway in the country as outlined below:
Strengthening surveillance and rapid response mechanisms
To mount an effective public health response, the surveillance forms an important cornerstone for control of emerging and re-emerging infections. After the plague outbreak in 1994, the Government constituted a technical committee to suggest measures to prevent recurrence of such outbreaks. The Central Council of Health and Family Welfare (CCHFW) is the apex political and policy formulating body with the Union Minister of Health and Family Welfare (as chairperson) and health ministers from all the States / Union Territories of the country as members. In 1995, the Council recommended the establishment of State and district level epidemiological units and revitalization of procedures of identification and reporting of outbreaks through the primary health care system. It also affirmed the need to strengthen public health system to effectively implement and evaluate national health programmes and to prevent outbreaks that can have national and international consequences64. In 1996, the National Apical Advisory Committee for National Disease Surveillance and Response System (NAAC) was created65.
These initiatives and policy level decisions led to the establishment of the National Surveillance Programme on Communicable Diseases (NSPCD) in 1997. In 1999, the Government of India constituted a technical advisory group on diseases of international public health importance. Following a detailed appraisal of the NSPCD, the Integrated Disease Surveillance Project (IDSP) was established in 2004 in 101 districts66. Since then, it has expanded to cover all States and districts in the country, meaning that each district now has a surveillance unit and a rapid response team (RRT) to quickly manage the disease outbreak in any part of the country. To augment surveillance activities and response mechanisms a wide network of epidemiologists, microbiologists and entomologists has been made available in all district and State headquarters under IDSP. IT connectivity has been established with all the States, districts and medical colleges through 776 sites for rapid data transfer, video conferencing and distance learning activities. Over the years, there has been an increase in the number of outbreaks reported by and investigated through IDSP with 1675 outbreaks in 2011 alone66.
Complying with International Health Regulations (IHR)
In 2005, the 194 member countries which are considered as States Parties passed the International Health Regulations known as IHR (2005). As a legal instrument, the aim is to ensure public health through the prevention of disease spread across borders, with limited interference to international traffic and trade. In order to do so, IHR (2005) requires all countries to assess their surveillance and response capacities, and to ensure that the core capacities are built by 201267.
At the time of the SARS outbreak, countries were only required to notify WHO of yellow fever, cholera and plague outbreaks under the IHR. After SARS, it was clear that the rules needed to be updated considering the increase in international travel and trade, and emergence and re-emergence of new international disease threats. A revised version was developed and in May 2005 it was approved by the World Health Assembly. The purpose and scope of the new regulations are not limited to any specific diseases or manner of transmission, but rather address illness or medical condition, irrespective of origin or source, that presents or could present significant harm to humans67.
As one of the signatories, India has been implementing various provisions of the IHR to enhance national and thereby global public health security by preventing and responding to acute public health risks that have the potential to cross borders and may constitute a potential threat to other countries. NCDC is the focal point for IHR in India and efforts are being made to strengthen core capacities needed under IHR (2005). In recent years, the epidemic disease act 1897 has been invoked by various States of India to tackle the challenges of communicable diseases like pandemic HINI influenza68.
Building capacity in epidemiology
Complementing Government's strategy to augment epidemiological capacities at the national, State and local level various short- and long term field epidemiology training programmes (FETP) have been started. The NCDC has been conducting short term (3 month) FETPs since 1963-1964. Since 1996, a three month regional FETP for WHO-SEAR region is being organized in NCDC each year for senior and middle level health professionals from regional countries69. A second, two year FETP was started in NCDC as a degree-granting programme, offering a Masters of Public Health in Field Epidemiology (MPH-FE). The programme takes in medical graduates with an MBBS degree70. The National Institute of Epidemiology in Chennai has been offering a two years FETP which is an in-service training programme in applied epidemiology. It trains public health leaders while providing epidemiologic services to health authorities in India71. Similarly, a new mentorship driven two year inservice training programme modeled on Epidemic Intelligence Service (EIS) run by CDC, Atlanta, is announced by the Government of India. This training programme is aimed at creating a cadre of highly skilled field epidemiologists at State and district level72.
Strengthening of laboratory and networks
The National Institute of Communicable Diseases (NICD) has been upgraded to National Centre for Disease Control (NCDC) as a centre of excellence with responsibility for enhanced capabilities for rapid response and laboratory based surveillance of communicable diseases73. Under the IDSP, 50 district public health laboratories are being strengthened all over the country. Alongside, a network of referral laboratories utilizing the services of existing functional laboratories in the nine States is being established. These include existing laboratories in microbiology departments of medical colleges and other large institutions for the aetiological diagnosis of outbreaks. This network would allow access to quality public health laboratory services for selected linked districts66.
Research and development
Cross-cutting research that informs key policy decisions such as rational use of drugs and pesticides; climatic change; environmental impact assessment is the cornerstone of disease prevention and control. The Government of India created a new Department of Health Research in Ministry of Health and Family Welfare in 2007. It is mandated to provide technical support in dealing with epidemics and natural calamities and development of tools for prevention. The department has specific programmes focusing on emerging and re-emerging diseases such as; identification of agent; development of diagnostic tests; formulation of case management modules and preventive strategies; establishment of laboratories to handle new, exotic and dangerous organisms73,74.
The Indian Council of Medical Research (ICMR) is the apex body in India for the formulation, coordination and promotion of biomedical research funded by Department of Health Research, Ministry of Health and Family Welfare. ICMR has a network of institutes devoted to specific infectious diseases and a chain of regional centres. In late 1990s the ICMR stepped up its funding in communicable diseases, accelerating research inputs for emerging infections75.
Information sharing and partnerships
The recent pandemic HINI influenza and avian influenza brought the international scientific community together showing the importance of effective partnerships in combating emerging infections. Under the international health regulations, national focal points are required to work closely with relevant ministries in timely identification of extraordinary public health events. As the national focal point for International Health Regulations (IHR) in India, the NCDC is in the process of identifying and partnering with other relevant ministries in identification of public health emergencies of internatonal concern (PHEIC).
Conclusions and the way forward
For a country of size and population of India, the emerging infections remain a real challenge. A meaningful response must approach the problem at the systems level. A comprehensive national strategy on infectious diseases addressing the challenges of emerging and re-emerging infections cutting across all relevant sectors, both governmental and non-governmental, should be in place. Identification of national centres of excellence and their capacity building is of critical importance. These centres of excellence should be encouraged to develop networking and partnerships between public health organizations to improve their individual scientific capacity, share best practices and expand collective knowledge base. Concerted efforts are also needed to develop advanced countermeasures such as surveillance tools, diagnostic tests, vaccines and therapeutics through basic, translational and applied research. Sensitive rapid response mechanisms at various levels of health service are the cornerstone to detect public health threats and respond quickly enough to protect valuable human lives. National commitment and comprehensive efforts are necessary at all levels of health services in order to meet the threat of emerging and re-emerging infections.
References
- 1.Bhatia R, Narain JP, Plianbangchang S. Emerging infectious diseases in East and South-East Asia. In: Detels R, Sullivan SG, Tan CC, editors. Public health in East and South-east Asia. Berkeley, USA: University of California Press; 2012. pp. 43–78. [Google Scholar]
- 2.Fauci AS. Infectious diseases: considerations for the 21st century. Clin Infect Dis. 2001;32:675–85. doi: 10.1086/319235. [DOI] [PubMed] [Google Scholar]
- 3.Taylor LH, Latham SM, Woolhouse ME. Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci. 2001;356:983–9. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Combating emerging infectious diseases in the South-East Asia Region. New Delhi: World Health Organization, WHO SEARO; 2005. World Health Organization, Regional Office for South East Asia Region. [Google Scholar]
- 5.Geneva: World Health Organization; 2003. [accessed on December 11, 2006]. Summary table of SARS cases by country, 1 November 2002 - 7 August. Available from: http://www.who.int/csr/sars/country/2003_08_15/en/index.html . [Google Scholar]
- 6.World Health Organization. Geneva, Switzerland: World Health Organization; 2007. World Health Report 2007. [Google Scholar]
- 7.World Health Organization. Situation updates - Pandemic (H1N1) 2009. [accessed on September 6, 2012]. Available from: http://www.who.int/csr/disease/swineflu/updates/en .
- 8.Hopkins DR. Princes and peasants smallpox in history. In: Hopkins DR, editor. Chicago IL: University Chicago Press; 1983. pp. 204–33. [Google Scholar]
- 9.Morens DM. Measles in Fiji, 1875: thoughts on the history of emerging infectious diseases. Pac Health Dialog. 1998;5:119–28. [Google Scholar]
- 10.Dikid T, Pala S, Ratho RK, Singh MP, Singh AJ. Chickenpox outbreak in North India: people vs administrative response. South Asian J Socio Pol Stud. 2010;10:36–41. [Google Scholar]
- 11.Woolhouse ME, Gowtage-Sequeria S. Host range and emerging and reemerging pathogens. Emerg Infect Dis. 2005;11:1842–7. doi: 10.3201/eid1112.050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geneva, Switzerland: World Health Organization; 2004. World Health Organization. The World Health Report 2004 - chaning history. [Google Scholar]
- 13.Quigley MA. Shifting burden of disease-epidemiological transition in India. Int J Epidemiol. 2006;35:1530–1. doi: 10.1093/ije/dyl244. [DOI] [PubMed] [Google Scholar]
- 14.NCMH background papers - burden of disease in India. New Delhi: National Commission on Macroeconomics and Health & Family Welfare, Ministry of Health, Government of India; 2005. Disease burden in India: estimations and causal analysis. [Google Scholar]
- 15.Ramamurthy T, Yamasaki S, Takeda Y, Nair GB. Vibrio cholerae O139 Bengal: odyssey of a fortuitous variant. Microbes Infect. 2003;5:329–44. doi: 10.1016/s1286-4579(03)00035-2. [DOI] [PubMed] [Google Scholar]
- 16.Mitra R, Basu A, Dutta D, Nair GB, Takeda Y. Resurgence of Vibrio cholerae 0139, Bengal with altered antibiogram in Calcutta, India. Lancet. 1996;348:1181. doi: 10.1016/s0140-6736(05)65326-3. [DOI] [PubMed] [Google Scholar]
- 17.Jalgaonkar SV, Ingole KV, Ambhore NA, Fule RP. Re-emergence of Vibrio cholerae serogroup 0139 during June-August 1997 in Yavatmal (Maharashtra) Indian J Med Res. 1998;108:1–2. [PubMed] [Google Scholar]
- 18.Lahiri KK, Ayyagari A. Vibrio cholerae 0139 in Lucknow. Indian J Med Microbiol. 1998;16:133. [Google Scholar]
- 19.Kaur H, Lal M. Reappearance of Vibrio cholerae O139 during March-August, 1998 in Ludhiana (Punjab), India. Indian J Med Res. 1999;109:3–4. [PubMed] [Google Scholar]
- 20.Raut S, Jalgaonkar SV, Tankhiwale NS, Agrawal VA. Re-emergence of Vibrio cholerae 0139 serogroup during 1998 in Nagpur (Maharashtra), India. Indian J Med Res. 1999;109:1–2. [PubMed] [Google Scholar]
- 21.Agrawal G, Jalgaonkar SV, Jagtap PM, Kamlakar UP, Deogade NG. Emergence and re-emergence of Vibrio cholerae 0139: an epidemiological study during 1993-2002 at Nagpur, Central India. Indian J Med Sci. 2003;57:155–7. [PubMed] [Google Scholar]
- 22.Ramamurthy T, Garg S, Sharma R, Bhattacharya SK, Nair GB, Shimada T, et al. Emergence of a novel strain of Vibrio cholerae with epidemic potential in Southern and Eastern India. Lancet. 1993;341:703–4. doi: 10.1016/0140-6736(93)90480-5. [DOI] [PubMed] [Google Scholar]
- 23.Clem A, Galwankar S. Plague: a decade since the 1994 outbreaks in India. J Assoc Physicians India. 2005;53:457–64. [PubMed] [Google Scholar]
- 24.Joshi K, Thakur JS, Kumar R, Singh AJ, Ray P, Jain S, et al. Epidemiological features of pneumonic plague outbreak in Himachal Pradesh, India. Trans R Soc Trop Med Hyg. 2009;103:455–60. doi: 10.1016/j.trstmh.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 25.Mittal V, Rana UV, Jain SK, Kumar K, Pal IS, Arya RC, et al. Quick control of bubonic plague outbreak in Uttar Kashi, India. J Commun Dis. 2004;36:233–9. [PubMed] [Google Scholar]
- 26.Parmenter RR, Yadav EP, Parmenter CA, Ettestad P, Gage KL. Incidence of plague associated with increased winter-spring precipitation in Mexico. Am J Trop Med Hyg. 1999;61:814–21. doi: 10.4269/ajtmh.1999.61.814. [DOI] [PubMed] [Google Scholar]
- 27.Stenseth NC, Samia NI, Viljugrein H, Kausrud KL, Begon M, Davis S, et al. Plague dynamics are driven by climate variation. Proc Natl Acad Sci USA. 2006;103:13110–5. doi: 10.1073/pnas.0602447103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization. Immunization, surveillance, assessment and monitoring. [accessed on July 28, 2012]. Available from: http://www.who.int/immunization_monitoring/data/data_subject/en .
- 29.World Health Organization. Global health observatory data repository. [accessed on July 29, 2012]. Available from: http://apps.who.int/ghodata/?vid=80201# .
- 30.International Institute for Population Sciences (IIPS) Bombay: IIPS; 1995. National Family Health Survey (MCH and Family Planning), India 1992-93. [Google Scholar]
- 31.International Institute for Population Sciences (IIPS) and ORC Macro. Mumbai: IIPS; 2000. National Family Health Survey (NFHS-2), 1998-99: India. [Google Scholar]
- 32.International Institute for Population Sciences (IIPS) and Macro International. National Family Health Survey (NFHS-3), 2005-06: India. Mumbai: IIPS; 2007. [Google Scholar]
- 33.Murhekar MV, Bitragunta S. Persistence of diphtheria in India. Indian J Community Med. 2012;36:164–5. doi: 10.4103/0970-0218.84141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lodha R, Dash NR, Kapil A, Kabra SK. Diphtheria in urban slums in north India. Lancet. 2000;355:204. doi: 10.1016/S0140-6736(99)04847-3. [DOI] [PubMed] [Google Scholar]
- 35.Khan N, Shastri J, Aigal U, Doctor B. Resurgence of diphtheria in the vaccination era. Indian J Med Microbiol. 2007;25:434. doi: 10.4103/0255-0857.37367. [DOI] [PubMed] [Google Scholar]
- 36.Ray SK, Das Gupta S, Saha I. A report of diphtheria surveillance from a rural medical college hospital. J Indian Med Assoc. 1998;96:236–8. [PubMed] [Google Scholar]
- 37.Nath B, Mahanta TG. Investigation of an outbreak of diphtheria in Borborooah block of Dibrugarh district Assam. Indian J Community Med. 2010;35:436–8. doi: 10.4103/0970-0218.69282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nipah virus. Wkly Epidemiol Rec. 2011;86:451–5. No authors listed. [PubMed] [Google Scholar]
- 39.Harit AK, Ichhpujani RL, Gupta S, Gill KS, Lal S, Ganguly NK, et al. Nipah/Hendra virus outbreak in Siliguri, West Bengal, India in 2001. Indian J Med Res. 2006;123:553–60. [PubMed] [Google Scholar]
- 40.Hsu VP, Hossain MJ, Parashar UD, Ali MM, Ksiazek TG, Kuzmin I, et al. Nipah virus encephalitis reemergence, Bangladesh. Emerg Infect Dis. 2004;10:2082–7. doi: 10.3201/eid1012.040701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luby SP, Rahman M, Hossain MJ, Blum LS, Husain MM, Gurley E, et al. Foodborne transmission of Nipah virus, Bangladesh. Emerg Infect Dis. 2006;12:1888–94. doi: 10.3201/eid1212.060732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhatt PN, Rodrigues FM. Chandipura virus: a new arbovirus isolated in India from patients with febrile illness. Indian J Med Res. 1967;55:1295–305. [PubMed] [Google Scholar]
- 43.Narasimha Rao S, Wairagkar NS, Murali Mohan V, Khetan M, Somarathi S. Brainstem encephalitis associated with Chandipura in Andhra Pradesh outbreak. J Trop Pediatr. 2008;54:25–30. doi: 10.1093/tropej/fmm078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chadha MS, Arankalle VA, Jadi RS, Joshi MV, Thakare JP, Mahadev PV, et al. An outbreak of Chandipura virus encephalitis in the eastern districts of Gujarat state, India. Am J Trop Med Hyg. 2005;73:566–70. [PubMed] [Google Scholar]
- 45.Gurav YK, Tandale BV, Jadi RS, Gunjikar RS, Tikute SS, Jamgaonkar AV, et al. Chandipura virus encephalitis outbreak among children in Nagpur division, Maharashtra, 2007. Indian J Med Res. 2010;132:395–9. [PubMed] [Google Scholar]
- 46.Indian Council of Medical Research. Chandipura encephalitis. [accessed on February 23, 2012]. Available from: http://icmr.nic.in/pinstitute/niv/CHANDIPURA%20ENC.pdf .
- 47.National Vector Borne Disease Control Programme, Government of India. Chikungunya update. [accessed on January 10, 2012]. Available from: http://nvbdcp.gov.in/Doc/Facts%20about%20Chikungunya17806.pdf .
- 48.National Vector Borne Disease Control Programme, Government of India. Clinically suspected Chikungunya fever cases since. 2007. [accessed on October 26, 2012]. Available from: http://nvbdcp.gov.in/chikcd.html .
- 49.Kalantri SP, Joshi R, Riley LW. Chikungunya epidemic: an Indian perspective. Natl Med J India. 2006;19:315–22. [PubMed] [Google Scholar]
- 50.Krishnamoorthy K, Harichandrakumar KT, Krishna Kumari A, Das LK. Burden of chikungunya in India: estimates of disability adjusted life years (DALY) lost in 2006 epidemic. J Vector Borne Dis. 2009;46:26–35. [PubMed] [Google Scholar]
- 51.Peiris JS, Yu WC, Leung CW, Cheung CY, Ng WF, Nicholls JM, et al. Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet. 2004;363:617–9. doi: 10.1016/S0140-6736(04)15595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mounts AW, Kwong H, Izurieta HS, Ho Y, Au T, Lee M, et al. Case-control study of risk factors for avian influenza A (H5N1) disease, Hong Kong, 1997. J Infect Dis. 1999;180:505–8. doi: 10.1086/314903. [DOI] [PubMed] [Google Scholar]
- 53.Geneva: World Health Organization; 2003. [accessed on February 27, 2012]. Global alert and Response (GAR) Available from: http://www.who.int/csr/don/archive/year/2003/en/index.html . [Google Scholar]
- 54.Avian influenza. MOHFW press release. Situation updates- Avian Influenza (H5N1); [accessed on February 27, 2012]. Ministry of Health and Family Welfare (MOHFW), Government of India. Available from: http://mohfw.nic.in/Communi-cation%20to%20States/Press%20Release%20on%20Avian%20Flu%20dt%20190206.pdf . [Google Scholar]
- 55.World Health Organization. Situation updates- Avian Influenza (H5N1) Pandemic (H1N1) 2009 Update 112. [accessed on January 10, 2012]. Available from: http://www.who.int/csr/don/2010_08_06/en/index.html .
- 56.Ministry of Health and Family Welfare (MOHFW), Government of India. Pandemic influenza-A (H1N1). Situational updates. [accessed on January 10, 2012]. Available from: http://mohfw-h1n1.nic.in/August2010.html .
- 57.Hoogstraal H. The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J Med Entomol. 1979;15:307–417. doi: 10.1093/jmedent/15.4.307. [DOI] [PubMed] [Google Scholar]
- 58.National Centre Disease Control. Jain SK, Chhabra M, Kumar K, Thomas TG, Dikid T, Aravindan A, et al. Crimean-Congo hemorrhagic fever virus. CD Alert. 2011;14:1–8. [Google Scholar]
- 59.Watts DM, Ksiazek TG, Linthicum KJ, Hoogstraal H. Crimean-Congo hemorrhagic fever. In: Monath TP, editor. The arboviruses epidemiology and ecology. II. Boca Raton, Florida, USA: CRC Press; 1989. pp. 177–260. [Google Scholar]
- 60.Ergönül O. Crimean-Congo haemorrhagic fever. Lancet Infect Dis. 2006;6:203–14. doi: 10.1016/S1473-3099(06)70435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.National Vector Borne Disease Control Programme, Government of India. AES/JE cases and deaths in the country. [accessed on October 26, 2012]. Available from: http://nvbdcp.gov.in/je-cd.html .
- 62.National Centre for Disease Control (NCDC) Delhi: NDMC; 2012. Report on acute encephalitis syndrome in Muzaffarpur district, Bihar. [Google Scholar]
- 63.Noumea, New Caledonia: World Health Organization; [accessed on June 3, 2012]. World Health Organization, Geneva. Asia-pacific strategy for emerging diseases. Available from: http://www.wpro.who.int/internet/resources.ashx/EDT/asia_pacific.pdf . [Google Scholar]
- 64.Proceedings of the Central Council of Health and Family Welfare, Ministry of Health and Family Welfare, Government of India. [accessed on April 26, 2012]. Available from: http://hrhm-mis.nic.in/ui/who/central%20council_Final/proceeding-council.htm .
- 65.World Health Organization (WHO) Geneva: WHO; 2003. Global Consultation on strengthening national capacities for surveillance and control of communicable disease 2003. [Google Scholar]
- 66.Integrated disease surveillance project. [accessed on April 26, 2012]. Available from: http://www.idsp.nic.in .
- 67.World Health Organization (WHO) Geneva; Switzerland: WHO; 2005. International Health Regulations 2005. [Google Scholar]
- 68.Unstarred question number 4838, 15th Lok Sabha. [accessed on April 26, 2012]. Available from: http://loksabha.nic.in .
- 69.Dhariwal AK, Jain SK. Proceedings of South East Asia Regional Conference on Epidemiology, March 8-10, 2010. New Delhi: World Health Organizations, Regional Office for South-East Asia; 2010. Field epidemiology training programme in India: ten years of experience; pp. 285–8. [Google Scholar]
- 70.Sharma K, Zodpey S. Public health education in India: need and demand paradox. Indian J Community Med. 2011;36:178–81. doi: 10.4103/0970-0218.86516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murhekar MV, Manickam P, Bhatnagar T, Kaur P, Ramachandran V, Ramakrishnan R, et al. Proceedings of South East Asia Regional Conference on Epidemiology. New Delhi: World Health Organizations, Regional Office for South-East Asia; 2010. Mar 8-10, Ten years of field epidemiology training at the National Institute of Epidemiology, Chennai, India, 2001-2010; pp. 289–92. [Google Scholar]
- 72.New Delhi, India: MOHFW; 2012. Government of India, Ministry of Health and Family Welfare (MOHFW). Compendium of operational guidelines for epidemic intelligence service (EIS) like training programme in India. [Google Scholar]
- 73.New Delhi, India: MOHFW; 2010. Government of India, Ministry of Health and Family Welfare (MOHFW). Annual report to people on health 2010. [Google Scholar]
- 74.Department of Health Research, Ministry of Health and Family welfare, Government of India. [accessed on July 28, 2012]. Available from: http://www.dhr.gov.in/schemes.htm .
- 75.Kant L. Combating emerging infectious diseases in India: orchestrating a symphony. J Biosci. 2008;33:425–7. doi: 10.1007/s12038-008-0061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


