Abstract
Aim
We determined the relationships of quantitative tests of liver function (QLFTs) to virologic responses to peginterferon (PEG) ± ribavirin (RBV) in patients with chronic hepatitis C and used serial QLFTs to define the spectrum of hepatic improvement after sustained virologic response (SVR).
Methods
Participants (N=232) were enrolled in the Hepatitis C Antiviral Long-term Treatment against Cirrhosis (HALT-C) Trial, had failed prior therapy, had bridging fibrosis or cirrhosis, and were retreated with PEG/RBV. All 232 patients had baseline QLFTs; 24 patients with SVR and 68 nonresponders had serial QLFTs. Lidocaine, [24-13C]cholate, galactose, and 99mTc-sulfur colloid were administered intravenously; [2,2,4,2-2H]cholate, [1-13C]methionine, caffeine, and antipyrine were administered orally. Clearances (Cl), breath 13CO2, monoethylglycylxylidide (MEGX), perfused hepatic mass (PHM), and liver volume were measured.
Results
Rates of SVR were 18 to 26% in patients with good function by QLFTs but <6% in patients with poor function. Hepatic metabolism, measured by caffeine kelim (P=0.02), antipyrine kelim (P=0.05), and antipyrine Cl (P=0.02), and the portal circulation, measured by cholate Cloral (P=0.0002) and cholate shunt (P=0.0003), and PHM (P=0.03), improved after SVR.
Conclusions
Hepatic dysfunction impairs the virologic response to PEG/RBV. SVR improves hepatic metabolism, the portal circulation, and perfused hepatic mass.
Keywords: Cholate clearance, Cholate Shunt, SPECT liver-spleen scan, Methionine breath test, Caffeine elimination, Antipyrine clearance, Galactose elimination capacity, MEGX, Hepatitis C, Fibrosis, Cirrhosis, Sustained virologic response, Peginterferon, Ribavirin
Introduction
More than 2.7 million Americans are infected with the hepatitis C virus, 8,000 to 10,000 die annually due to complications of chronic hepatitis C, and the number of Americans infected for 20 or more years will not peak until 2015 (1-4). As a consequence, the number of patients who will decompensate, advance to hepatocellular carcinoma, and need liver transplantation will increase (5-10).
Rates of sustained virologic response (SVR) with peginterferon/ribavirin treatment of chronic hepatitis C (11-14) are lower in patients with advanced hepatic fibrosis or cirrhosis (15-17). In the Hepatitis C Antiviral Long-term Treatment against Cirrhosis (HALT-C) Trial, patients with chronic hepatitis C with bridging fibrosis or compensated cirrhosis (Child-Turcotte-Pugh ≤6) and prior nonresponse were retreated with peginterferon/ribavirin (18). In this cohort, SVR after retreatment declined stepwise, from 23% to 9%, with increasing severity of disease, as defined by liver histology and platelet count (15). Because quantitative tests of liver function (QLFTs) measure the continuum of liver impairment, we reasoned that the relationship between SVR and disease severity might be better defined by QLFTs.
SVR reduces hepatic inflammation, fibrosis (19,20), and rates of clinical outcomes (21-25). The principal clinical manifestations of advanced chronic hepatitis C, such as varices, ascites, and encephalopathy, are linked to portal hypertension and impaired hepatic function. Beneficial effects of SVR on hepatic fibrosis and clinical outcomes are likely mediated through improvements in the portal circulation and hepatic function – improvements which could be detected by QLFTs but not by standard laboratory tests.
In this study of retreatment of patients with chronic hepatitis C with peginterferon/ribavirin, we utilized a battery of QLFTs to measure hepatic metabolism, hepatic and portal blood flow, portal-systemic shunting, and hepatic parenchymal mass. One goal was to define the relationships between severity of hepatic impairment, as measured by QLFTs, and virologic responses. In addition, we used serial QLFTs to define hepatic improvement after achievement of sustained virologic response.
Patients and Methods
This study was approved by the Data Safety and Monitoring Board, appointed for this purpose by the National Institute of Diabetes and Digestive and Kidney Disease; the US Food and Drug Administration, and the Institutional Review Boards and General Clinical Research Centers of the participating centers. The study was conducted according to the principals of the Declaration of Helsinki regarding the proper procedures for human research. Patients participating in this study had Child-Turcotte-Pugh scores ≤6 and lacked history of variceal hemorrhage, ascites, encephalopathy, spontaneous bacterial peritonitis, hepatocellular carcinoma, or biochemical deterioration. Participants signed individual informed consents for both the main HALT-C trial and the QLFT ancillary study.
The design of the main HALT-C Trial and procedures used in this QLFT study have been previously described (18,26,27). Hepatic microsomal function was measured from the elimination or metabolism of caffeine, antipyrine, and lidocaine-MEGX. Hepatic mitochondrial function was assessed using the methionine breath test. Hepatic blood flow was measured from the elimination of intravenously administered galactose and cholate. Portal inflow and portal-systemic shunt were measured from the clearance of orally administered cholate and cholate shunt. Perfused hepatic mass and liver volume were measured from SPECT liver-spleen scans. Baseline histology was staged according to Ishak – fibrosis scores from 2 to 4, and cirrhosis scores 5 or 6 (28). VR20 was defined as a negative HCV RNA at week 20 of peginterferon/ribavirin therapy, and SVR by negative HCV RNA six months or more after the end of treatment. Nonresponders had a positive HCV RNA at week 20 of treatment. Patients achieving SVR had 48 weeks of treatment and nonresponders were treated for only 24 weeks. Dose reduction or discontinuation was defined as <80 percent of target doses for both peginterferon and ribavirin in the first 20 weeks.
Study Groups
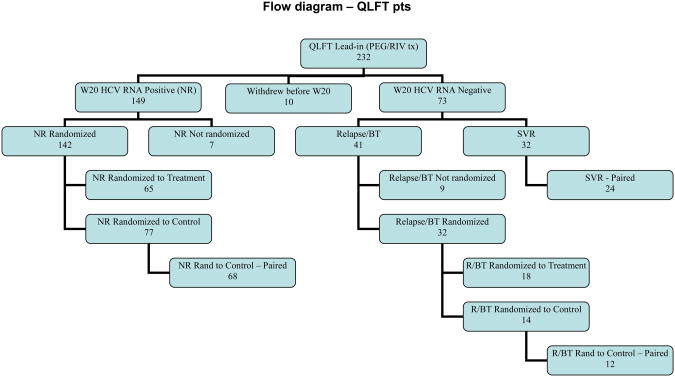
A total of 1145 patients were enrolled and retreated with peginterferon/ribavirin during the Lead-In Phase of the main HALT-C Trial (18). Two hundred thirty two of these 1145 underwent QLFTs prior to retreatment. The outcome of these 232 patients is shown in Figure 1. Relationships between hepatic function measured at baseline by QLFTs and subsequent achievement of VR20 or SVR were evaluated in these 232 patients.
Figure 1.
Flow diagram of final outcome of the 232 patients enrolled in the Lead-In Phase of HALT-C and who participated in the QLFT study. The baseline QLFT studies of all 232 patients were used to define the associations of QLFTs with virologic responses. To examine the effect of SVR on hepatic function, we compared serial studies of QLFTs in 24 patients achieving SVR to 68 nonresponders and 12 relapsers randomized to long-term followup without additional treatment.
The change in hepatic function was assessed by serial studies and the impact of SVR on hepatic function was evaluated by comparison of two subgroups – patients who achieved SVR, and nonresponders undergoing long-term observation without treatment during the randomized phase of the HALT-C Trial (18). Seventy three of the 232 patients experienced VR20 (31 percent) and 32 achieved SVR (14 percent). Followup QLFT studies were performed in 24 of the 32 patients who achieved SVR. One hundred forty nine patients remained HCV RNA positive at week 20 of treatment. One hundred forty two were randomized, 65 to continued treatment and 77 to long term observation. Sixty-eight of the 77 had followup QLFT studies (Figure 1).
Forty one patients relapsed, 32 were randomized - 18 to continued treatment and 14 to long term observation (Figure 1). Twelve of the 14 patients in long-term observation underwent followup QLFT studies. The low sample size of relapsers undergoing serial QLFTs (N=12) precluded statistical comparison with the other groups.
For patients achieving SVR, the median time between baseline and followup studies was 28.8 months and the median time between end of treatment and followup studies was 19.7 months. For nonresponders, the median time between baseline and followup studies was 24.8 months and the median time between end of treatment and followup studies was 18.8 months.
Statistical Considerations
All analyses were performed with Statistical Analysis System version 9.1.3 (29,30). Patient characteristics were defined by mean, standard deviation and frequency. Differences between groups were assessed with Fisher's Exact and t-tests. Distributions of QLFT test results for the 232 Lead-In patients were defined by quartiles of results. Associations of QLFTs with VR20 and SVR, cirrhosis, and dose reduction were evaluated by Fisher's Exact and Mantel-Haenszel Chi Square tests. Univariate associations between VR20 and SVR and QLFTs were assessed using logistic regression. The independent associations of QLFTs with VR20 were evaluated in models including QLFTs, cirrhosis, and other baseline characteristics (African American race, HCV genotype, and HCV RNA level). The small number of patients achieving SVR precluded meaningful multivariate analyses of models of SVR. For the paired studies, the changes between baseline and followup test results and differences between study groups (patients achieving SVR versus nonresponders) were analyzed by paired t-tests and two-sample t-tests.
Results
Characteristics of the Study Populations
We compared selected characteristics of the 232 study patients to the remaining 913 HALT-C patients (Table 1). Study patients had lower albumin (Mean±SD: 3.76±0.4 vs 3.92±0.4, p<0.0001) and prothrombin time (INR) (1.02±0.10 vs 1.04±0.11, p=0.01), fewer were African American (10 vs. 17 percent, p=0.01), and they had higher prevalences of esophageal varices (35 vs. 22 percent, p<0.0001) and splenomegaly (37 vs. 29 percent, p=0.04). Key characteristics of the study patients were mean age 49.8 years, 75 percent male, mean body mass index (BMI) 29.5, 40 percent cirrhosis, 92% HCV genotype 1, and mean HCV RNA 6.41 ± 0.50 log10 IU/ml. Mean (± SD) laboratory values were within the normal range: bilirubin 0.7 ± 0.4 mg/dl, INR 1.02 ± 0.10, albumin 3.76 ± 0.40 g/dl, and platelet count 169,000 ± 66,000 platelets/μl.
Table 1. Characteristics of the 232 QLFT Study Subjects Compared to the Remaining 913 HALT-C Patients Treated with PEG/RBV.
| QLFT N = 232 | HALT-C N = 913 | Fisher Exact or T-test p-value | |||
|---|---|---|---|---|---|
| Mean or % | ±SD | Mean or % | ±SD | ||
| Demographics | |||||
| Age | 49.8 | 7.2 | 49.9 | 7.3 | .78 |
| BMI (kg/m2) | 29.5 | 4.9 | 29.8 | 5.6 | .40 |
| Male (%) | 75% | 71% | .25 | ||
| African American (%) | 10% | 17% | .01 | ||
| Disease Severity | |||||
| Cirrhosis (%) | 40% | 37% | .50 | ||
| Esophageal varices (%) | 35% | 22% | <0.0001 | ||
| Splenomegaly (%) | 37% | 29% | .04 | ||
| HCV Characteristics | |||||
| Genotype 1 (%) | 92% | 88% | .16 | ||
| HCV RNA (log10 IU/ml) | 6.41 | 0.50 | 6.42 | 0.54 | .78 |
| Standard Laboratory Tests | |||||
| Bilirubin (mg/dl) | 0.7 | 0.4 | 0.8 | 0.4 | .07 |
| Albumin (g/dl) | 3.76 | 0.40 | 3.92 | 0.38 | <0.0001 |
| Prothrombin time (INR) | 1.02 | 0.10 | 1.04 | 0.11 | .01 |
| Platelet Count (10−3/μL) | 169 | 66 | 169 | 64 | .99 |
| Treatment Outcomes | |||||
| SVR (%) | 14% | 16% | .42 | ||
| > 80% PEG and RBV during the first 20 Weeks (%) | 58% | 51% | .09 | ||
Abbreviations: QLFT, quantitative liver function test; HALT-C, Hepatitis C Antiviral Long-Term Treatment to Prevent Cirrhosis Trial; SD, standard deviation; PEG/RBV, peginterferon/ribavirin; BMI, body mass index; SVR, sustained virologic response.
We also compared the baseline characteristics of the 24 patients achieving SVR to those of the 68 nonresponders (Table 2). Patients achieving SVR were non-African American (P=0.03), had lower prevalence of cirrhosis (P=0.03), were less often infected with HCV genotype 1 (P=0.004), and were more likely to have received >80% of doses of PEG/RBV (P=0.01). Nonresponders had lower albumin (P=0.01) and hemoglobin (P=0.01). Patients with relapse had a prevalence of cirrhosis (58%) similar to nonresponders.
Table 2. Baseline Characteristics of Sustained Responders and Nonresponders who underwent Serial QLFT Studies.
| SVR N = 24 | NR N = 68 | Fisher Exact or T-test p-value | |||
|---|---|---|---|---|---|
| Mean or % | ±SD | Mean or % | ±SD | ||
| Demographics | |||||
| Age | 49.4 | 6.0 | 50.1 | 7.0 | 0.65 |
| BMI (kg/m2) | 28.4 | 4.7 | 30.7 | 5.3 | 0.07 |
| Male (%) | 83% | 68% | 0.19 | ||
| African American (%) | 0% | 17.7% | 0.03 | ||
| Disease Severity | |||||
| Cirrhotic (%) | 20.8% | 48.5% | 0.03 | ||
| Esophageal varices (%) | N/A | 35% | N/A | ||
| Splenomegaly (%) | 29% | 50% | 0.15 | ||
| HCV Characteristics | |||||
| Genotype 1 (%) | 75% | 97% | 0.004 | ||
| HCV RNA (log10 IU/ml) | 6.47 | 0.50 | 6.31 | 0.51 | 0.19 |
| Standard Laboratory Tests | |||||
| Hemoglobin (g/dL) | 15.9 | 1.2 | 15.0 | 1.6 | 0.01 |
| WBC (10−3/μL) | 5.9 | 1.9 | 5.4 | 1.9 | 0.27 |
| Platelet Count(10−3/μL) | 177 | 59 | 160 | 71 | 0.31 |
| Bilirubin (mg/dl) | 0.74 | 0.37 | 0.73 | 0.32 | 0.92 |
| Albumin (g/dl) | 3.90 | 0.35 | 3.67 | 0.36 | 0.01 |
| Prothrombin time (INR) | 1.01 | 0.09 | 1.04 | 0.12 | 0.21 |
| Creatinine (mg/dL) | 0.81 | 0.17 | 0.78 | 0.15 | 0.35 |
| Treatment Course | |||||
| > 80% PEG and RBV during the first 20 Weeks (%) | 83% | 54% | 0.01 | ||
Abbreviations: QLFT, quantitative liver function test; PEG/RBV, peginterferon/ribavirin; SVR, sustained virologic responder; NR, nonresponder; SD, standard deviation; BMI, body mass index; WBC, white blood cell count.
Spectrum of Hepatic Impairment at Baseline
The spectrum of baseline hepatic functional impairment was categorized by quartiles ranging from best to worst function for each QLFT. Boundaries for the quartiles are given in Table 3. We have previously reported that the prevalences of both cirrhosis and varices increase significantly from best to worst quartiles of QLFTs (26).
Table 3. Boundaries for Quartiles of Quantitative Tests of Liver Function.
| Best Function | 25th Percentile | 50th Percentile | 75th Percentile | Worst Function | |
|---|---|---|---|---|---|
| Tests of Metabolism | |||||
| Caffeine kelim (h−1) | 0.28 | 0.09 | 0.06 | 0.04 | 0.01 |
| Antipyrine kelim (h−1) | 0.09 | 0.04 | 0.03 | 0.03 | 0.01 |
| Antipyrine Cl (ml/min) | 79 | 39 | 29 | 21 | 12 |
| MEGX15min (ng/ml) | 70 | 25 | 16 | 8 | 0 |
| MEGX30min (ng/ml) | 98 | 30 | 20 | 13 | 1 |
| MBT | 308 | 84 | 65 | 45 | 6 |
| Tests of Total Hepatic Blood Flow | |||||
| Cholate kelim (min−1) | 0.27 | 0.11 | 0.09 | 0.08 | 0.02 |
| Cholate Cliv (ml/min) | 903 | 459 | 367 | 305 | 155 |
| GEC (mg/kg·min) | 10.1 | 5.6 | 4.7 | 4.0 | 2.1 |
| Tests of Portal Circulation | |||||
| Cholate Cloral (ml/min) | 3036 | 1463 | 1113 | 771 | 255 |
| Cholate Shunt (%) | 10 | 27 | 36 | 48 | 91 |
| Tests of Hepatic Parenchyma | |||||
| Perfused Hepatic Mass | 114 | 105 | 100 | 94 | 70 |
| Liver Volume (ml) | 2690 | 1867 | 1593 | 1343 | 769 |
Abbreviations: Cl, clearance; oral, orally administered; iv, intravenously administered; k, rate constant of elimination; MEGX, monoethylglycine xylidide; GEC, galactose elimination capacity.
QLFT Quartiles and Rates of VR20 and SVR
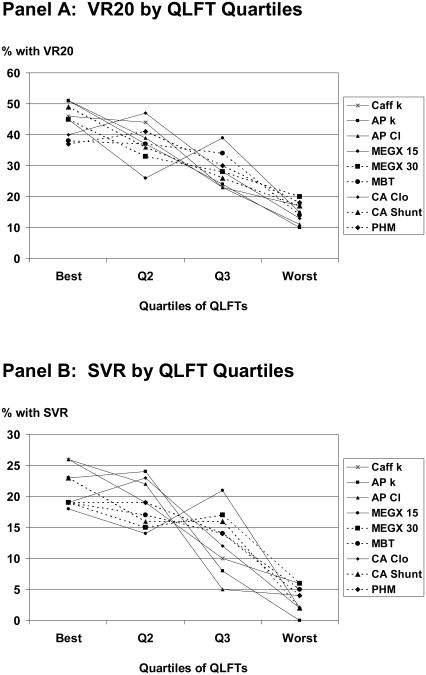
One hundred ten patients (47.4%) had greater than two log10 drop in HCV RNA by week 12 and 73 patients (32%) achieved VR20. Rates of VR20 declined as function worsened (Figure 2, Panel A). VR20 ranged from 37 to 51 percent in the quartiles of patients with the best function but was only 10 to 20 percent in the quartiles with the worst function. Rates of SVR also declined as function worsened (Figure 2, Panel B). SVR rates ranged from 18 to 26 percent in quartiles of patients with the best function, but were ≤6 percent in quartiles with worst function.
Figure 2.
Panel A: Rates of VR20 declined as caffeine kelim (P=0.0001), antipyrine kelim (P=0.0002), antipyrine Cl (P=0.0002), cholate Cloral (P=0.0003), cholate shunt (P=0.0001), MEGX15min (P=0.01), MEGX30min (P=0.005), methionine breath test (P=0.02), and perfused hepatic mass (P=0.01) worsened. Panel B: Rates of SVR declined as caffeine kelim (P=0.002), antipyrine kelim (P=0.002), antipyrine Cl (P=0.002), cholate Cloral (P=0.003), cholate shunt (P=0.002), methionine breath test (P=0.04), and perfused hepatic mass (P=0.01), MEGX15min (P=0.09), and MEGX30min (P=0.07) worsened. Cholate kelim, cholate Cliv, and galactose elimination capacity, which primarily assess total hepatic blood flow, failed to correlate with either VR20 or SVR (not shown). Abbreviations: Q, quartile; QLFT, quantitative test of liver function; Caff k, rate constant of elimination of caffeine; AP k, rate constant of elimination of antipyrine; AP Cl, clearance of antipyrine; MEGX 15, concentration of monoethylglycylxylidide 15 minutes after administration of lidocaine; MEGX 30, concentration of monoethylglycylxylidide 30 minutes after administration of lidocaine; CA Clo, clearance of orally administered [2,2,4,4-2H] cholate; CA Shunt, cholate shunt; GEC, galactose elimination capacity; MBT, methionine breath test; PHM, perfused hepatic mass
Multivariate analyses of Relationships of QLFTs to VR20
Because cirrhosis (P=0.02) and platelet count (P=0.009) correlated with VR20, we examined models including QLFTs with cirrhosis or platelet count to predict VR20. After adjustment for cirrhosis, QLFTs with an independent relationship to VR20 were caffeine kelim (P=0.03), antipyrine kelim (P=0.004), antipyrine Cl (P=0.005), cholate Cloral (P=0.04), cholate shunt (P=0.02), and perfused hepatic mass (P=0.007). Similar results were obtained after adjustment for platelet count.
QLFTs with an independent relationship to VR20 after adjustment for cirrhosis, HCV genotype, HCV RNA level, and African American race, were caffeine kelim (P=0.09), antipyrine kelim (P=0.03), antipyrine Cl (P=0.03), MEGX15min (P=0.01), MEGX30min (P=0.003), cholate Cloral (P=0.09), cholate shunt (P=0.03), and perfused hepatic mass (P=0.002).
Impact of Virologic Response on Hepatic Function
Hepatic Metabolic Function
In patients achieving SVR, caffeine kelim increased by 38 percent (P=0.02), antipyrine kelim increased by 25 percent (P=0.05), and antipyrine Cl increased by 31 percent (P=0.02). MEGX15min increased by 30 percent and MEGX30min increased by 9 percent but these changes were not statistically significant (Table 4). Nonresponders had lower baseline values and did not demonstrate any significant changes for these tests between baseline and followup studies. The improvements in caffeine kelim, antipyrine kelim, and antipyrine clearance in patients achieving SVR were significant when compared to the changes in these tests in nonresponders (P=0.01, 0.05, and 0.02, respectively).
Table 4. Test Results in Patients Achieving SVR versus Nonresponders.
| SVR N |
SVR QLFT at Baseline X±SD | Δ QLFT in SVR M24-Base | P value For Δ QLFT in SVR | NR N |
NR QLFT at Baseline X±SD | Δ QLFT in NR M24-Base | P value For Δ QLFT in NR | P value ΔQLFT SVR vs NR | |
|---|---|---|---|---|---|---|---|---|---|
| Standard Laboratory Tests | |||||||||
| Bilirubin (mg/dL) | 24 | 0.74 ±0.37 | 0.07 ±0.27 | 0.21 | 68 | 0.73 plusmn;0.32 | 0.35 ±0.54 | <0.0001 | 0.002 |
| Albumin (g/dL) | 24 | 3.90 ±0.35 | 0.03 ±0.37 | 0.70 | 68 | 3.67 ±0.36 | −0.12± 0.40 | 0.02 | 0.11 |
| INR | 24 | 1.01 ±0.09 | 0.05 ±0.09 | 0.01a | 68 | 1.04 ±0.12 | 0.07 ±0.09 | <0.0001 | 0.43 |
| Platelets (10−3/μL) | 24 | 177 ±59 | 22 ±40 | 0.01 | 68 | 160 ±71 | −13 ±33 | 0.003 | 0.0001 |
| Tests of Metabolism | |||||||||
| Caffeine kelim (h−1) | 22 | 0.08 ±0.04 | 0.03 ±0.05 | 0.02 | 59 | 0.05 ±0.04 | 0 ±0.03 | 0.57 | 0.01 |
| Antipyrine kelim (h−1) | 17 | 0.04 ±0.02 | 0.01 ±0.02 | 0.05 | 40 | 0.03 ±0.01 | 0 ±0.01 | 0.87 | 0.05 |
| Antipyrine Cl (ml/min) | 17 | 39.6 ±13.6 | 12.1 ±19.6 | 0.02 | 39 | 28.9 ±13.7 | −0.6 ±8.2 | 0.63 | 0.02 |
| MEGX15min (ng/ml) | 24 | 19.4 ±12.9 | 5.9 ±18.7 | 0.14 | 64 | 16.2 ±11.7 | −0.6 plusmn;13.4 | 0.71 | 0.13 |
| MEGX30min (ng/ml) | 24 | 23.0 ±8.8 | 2.1 ±14.1 | 0.48 | 63 | 20.8 ±11.8 | 0.1 ±14.0 | 0.97 | 0.55 |
| Tests of Total Hepatic Blood Flow | |||||||||
| Cholate kelim (min−1) | 24 | 0.09 ±0.02 | 0.01 ±0.03 | 0.25 | 67 | 0.09 +0.03 | −0.01 ±0.03 | 0.03 | 0.04 |
| Cholate Cliv (ml/min) | 24 | 427 ±115 | −33 ±107 | 0.14 | 67 | 394 +125 | −60 ±121 | 0.0001 | 0.33 |
| GEC (mg/kg·min) | 23 | 4.88 ±1.10 | 0.05 ±0.84 | 0.80 | 66 | 4.75 +1.22 | −0.16 ±0.88 | 0.15 | 0.34 |
| Tests of Portal Circulation | |||||||||
| Cholate Cloral (ml/min) | 24 | 1371 ±329 | 437 ±494 | 0.0002 | 67 | 1107 ±551 | −102 ±377 | 0.03 | <0.0001 |
| Cholate Shunt (%) | 24 | 32 ±8 | −8 ±10 | 0.0003 | 67 | 41 ±15 | 0 ±16 | 0.85 | 0.003 |
| Tests of Hepatic Parenchyma | |||||||||
| Perfused Hepatic Mass | 20 | 102.3 ±4.1 | 1.6 ±3.1 | 0.03 | 65 | 96.3 ±9.0 | −2.7 ±15.6 | 0.17 | 0.04 |
| Liver Volume (ml) | 20 | 1635 ±358 | −42 ±178 | 0.30 | 65 | 1691 ±361 | −55 ±318 | 0.17 | 0.82 |
Although the change in INR was significant, the change was in the direction of worsening with SVR.
P values that are in bold typeface indicate significant change in the test from baseline or in the comparison of changes between patients achieving SVR and nonresponders. The gray shading identifies tests that improved significantly with SVR and were significant in the comparison of changes in the test between SVR and NR.
Abbreviations: Cl, clearance; oral, orally administered; iv, intravenously administered; k, rate constant of elimination; MEGX, monoethylglycine xylidide; GEC, galactose elimination capacity.
Hepatic Blood Flow
Cholate kelim, cholate Cliv, and galactose elimination capacity, did not change with SVR (Table 4). In nonresponders there was significant decline in cholate kelim (P=0.03) and cholate Cliv (P=0.0001). In comparison to patients achieving SVR, only the decline in cholate kelim in nonresponders remained significant (P=0.04).
Portal blood flow and shunt
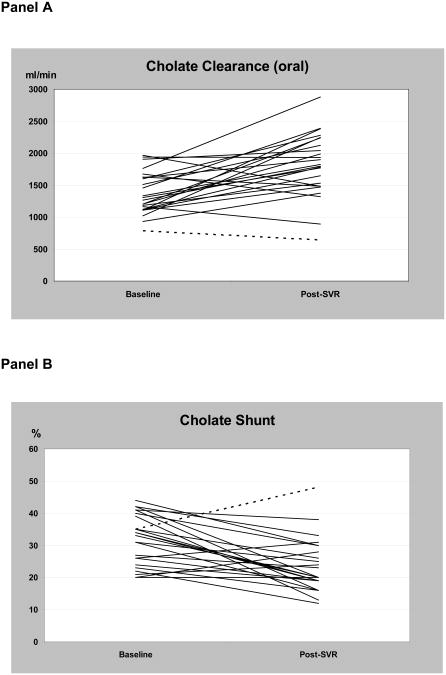
Cholate Cloral and cholate shunt improved after SVR (Figure 3) (Table 4). Cholate Cloral increased from 1371±329 ml/min to 1808±497 ml/min, an increase of 32% (P<0.0002). Cholate shunt decreased from 32±8 to 24±8 percent, a reduction in shunt of 25 percent (P<0.0003). Nonresponders had lower cholate Cloral and higher cholate shunt at baseline compared to patients who achieved SVR. In nonresponders, mean cholate Cloral decreased (P=0.03) but cholate shunt did not change. The improvements in patients achieving SVR were highly significant when compared to the changes in these tests in nonresponders (SVR vs NR: cholate Cloral P<0.0001 and cholate shunt P=0.003).
Figure 3.
SVR was associated with a 32% increase in cholate Cloral (Panel A), a measure of portal blood flow, and a 26% decrease in cholate shunt (Panel B), a measure of portal-systemic shunting. The dotted line represents one patient with increase in cholate shunt despite SVR – this patient also had the lowest cholate Cloral both at baseline and in followup.
Relapsers had lower cholate Cloral (1086±605 ml/min) and higher cholate shunt (44±20%) at baseline compared to patients who achieved SVR. In followup studies mean cholate Cloral increased (1280±689 ml/min, P=0.10) and cholate shunt decreased (32±11%, P=0.03).
Perfused Hepatic Mass and Liver Volume
Perfused hepatic mass increased and liver volume did not change after SVR (Table 4). Perfused hepatic mass was 102±4 at baseline and 104±3 after SVR (P=0.03) and liver volume was 1635±358 mL at baseline and 1592±320 mL after SVR (P=NS). Nonresponders had lower perfused hepatic mass but similar liver volumes at baseline compared to patients who achieved SVR. There was no significant change in either perfused hepatic mass or liver volume in nonresponders. The increase in perfused hepatic mass in patients achieving SVR was significant when compared to the lack of change in perfused hepatic mass of nonresponders (P=0.04).
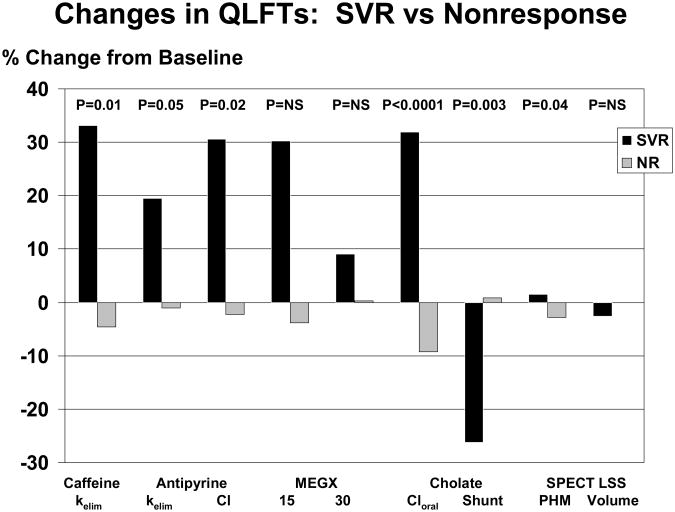
Overall, SVR was associated with increased hepatic metabolic activity, enhanced clearance from the portal circulation, reduced portal-systemic shunt, and increased perfused hepatic mass without change in liver volume (Figure 4).
Figure 4.
The percentage change between baseline and the followup studies for QLFTs are shown. The black bars depict the changes after sustained virologic response (SVR) and grey bars show the changes in patients with nonresponse (NR). Compared to patients with nonresponse, patients experiencing SVR had significant improvements in caffeine and antipyrine elimination rates (kelim), antipyrine clearance (Cl), clearance of orally administered cholate (Cloral), cholate shunt, and perfused hepatic mass (PHM).
Standard Laboratory Tests
At baseline means (±SD) of standard laboratory tests were in the normal range in all groups. Platelet count was the only standard laboratory test that improved (increased by 12%) with SVR (P=0.01). Although means of platelet counts remained within the normal range, the increase in the platelet counts of patients who achieved SVR was highly significant when compared to the decrease in the platelet counts of nonresponders (P=0.0001).
Discussion
Our study is unique in that it represents the most comprehensive assessment of the relationships of hepatic function to virologic clearance in response to peginterferon/ribavirin therapy in a large, extensively characterized cohort of patients with chronic hepatitis C. We quantified hepatic metabolism, the portal circulation, perfused hepatic mass, and liver volume using a battery of QLFTs. We found that QLFTs performed at baseline prior to treatment were independent predictors of virologic response to peginterferon/ribavirin. In addition, using serial QLFTs we demonstrated that patients who achieved SVR experienced significant improvement in hepatic metabolism, portal blood flow, and portal-systemic shunt – improvements that were not detected by standard clinical or laboratory assessment.
QLFTs and Virologic Responses
Patients with chronic hepatitis C and cirrhosis respond poorly to antiviral therapy (11-17). In a previous analysis of the HALT-C cohort we categorized the severity of liver disease based upon a combination of liver histology and platelet count (15). We reported that SVR declined from 23% in patients with noncirrhotic fibrosis and >125,000 platelets/μL to a low of 9% in patients with cirrhosis and <125,000 platelets/μL. Multivariate analyses indicated that cirrhosis was a key independent pre-treatment variable predicting virologic response (15).
QLFTs assess the spectrum of liver impairment (27,31-35). QLFTs are performed by administering various test compounds and measuring their clearance from the circulation or metabolism using samples of blood, saliva, or breath, or radiologic imaging. The rate of decline in concentration of the originally administered compound or the appearance of its metabolite is proportional to hepatic metabolic function, blood flow, or shunting. We have reported that our battery of QLFTs, used in this subgroup of HALT-C patients, correlates with cirrhosis, stage of fibrosis, varices, and size of varices (26,27).
As noted above, virologic response to antiviral therapy worsens with clinical disease severity (15). In the current study, we found that virologic response declined as QLFTs assessing hepatic metabolism, portal blood flow, portal-systemic shunt, and perfused hepatic mass worsened. In the case of SVR, patients with the worst hepatic impairment on baseline QLFTs had rates of SVR of only 0 to 6 percent. In multivariate analysis QLFTs remained significant predictors of virologic response after controlling for other known predictors, including histologically-defined cirrhosis and platelet count. Additional studies would be needed to determine whether QLFTs could be used, a priori, to identify nonresponders and potentially exclude them from treatment.
Improvement in Hepatic Metabolism, Portal Blood Flow, and Portal-Systemic Shunt after SVR
The goal of therapy for chronic hepatitis C is to halt disease progression. Chronic hepatitis C progresses slowly, typically over decades of a person's life, and many years of followup are required to demonstrate a benefit of SVR on clinical complications or patient survival. Because long-term followup is often impractical, standard laboratory tests, clinical scores (MELD, CTP), and liver histology are typically used as surrogates for measuring benefits of treatment. Although SVR reduces both hepatic inflammation and hepatic fibrosis (19,20), serial assessment using liver biopsies is invasive, associated with significant risk, and prone to sampling error.
In our patients, bilirubin, INR, and albumin were essentially normal at baseline and did not improve with SVR – emphasizing the lack of sensitivity of these tests. Platelet count increased with SVR, but mean platelet count was in the normal range both at baseline and in followup after SVR – emphasizing the limited utility of platelet count as a marker for hepatic dysfunction or portal hypertension.
Impaired hepatic function and portal hypertension account for the major manifestations and clinical complications of liver disease. Because our battery of QLFTs measured both hepatic metabolism and the portal circulation we reasoned that these QLFTs could be useful surrogates to identify clinically relevant, beneficial effects of SVR. Indeed, we found that SVR was associated with improvements in hepatic metabolism, portal blood flow, and portal-systemic shunt. These physiologic improvements after SVR would, at least theoretically, reduce risk for clinical decompensation or complications. Absence of clinical complications in the long-term follow-up of patients with advanced fibrosis or cirrhosis after SVR supports this interpretation (23).
SVR improved the clearance or metabolism of caffeine, antipyrine, and lidocaine-MEGX by 9 to 38% without affecting liver volume. Caffeine is metabolized by an array of hepatic microsomal cytochrome P450 (CYP) enzymes (1A1, 1A2, 2A6, 2E1, 3A) (32), antipyrine by CYP 1A2, 2B6, 2C8, 3C9, and 2C18 (33), and lidocaine-MEGX primarily by CYP 3A4 (33,34). Ocker and colleagues used a different battery of QLFTs (aminopyrine breath test, galactose elimination capacity, sorbitol clearance, and indocyanine green clearance) to study 50 patients with chronic hepatitis C at baseline and 3 months after initiation of interferon-based therapy (35). They observed improvement in hepatic metabolism in the patients who were HCV RNA negative. We interpret these results to indicate that HCV, or inflammation and fibrosis related to HCV, interferes with the hepatic metabolism of a wide range of drugs, medications, and xenobiotics – and that these effects are reversible with effective therapy.
SVR improves portal blood flow and perfused hepatic mass, as measured by cholate Cloral and SPECT liver-spleen scan, and reduces portal-systemic shunting, as measured by cholate shunt. Reduction of hepatic inflammation and fibrosis after SVR may lower hepatic resistance to portal inflow, reduce portal pressure, and diminish portal-systemic shunt. This interpretation is further supported by our observation of a 12% increase in platelet count, and the study by Rincon and colleagues - which demonstrated a 26 percent reduction in hepatic venous pressure gradient in a subset of patients who achieved SVR (36). We observed 32 percent increase in cholate Cloral and 25 percent decrease in cholate shunt. Globally, these results suggest that SVR reverses portal hypertension, improves portal inflow, and diminishes portal-systemic shunting.
Which QLFTs carry the most promise and could potentially be applied in clinical practice? The analyses in this paper and our prior publication (26) suggest that oral cholate clearance, cholate shunt, and perfused hepatic mass by SPECT liver-spleen scan may be superior to tests of metabolism. Clearly breath tests are simplest to administer, but in our studies the methionine breath test was inferior to cholate tests or SPECT analysis. Performance of the cholate test is complex – but, we have now defined the minimal model for cholate clearance and shunt (27), reducing patient discomfort and time commitment, and limiting laboratory analytical time. SPECT requires use of radioactivity and time commitment of patient and personnel in the nuclear medicine department – but SPECT is readily available in most hospitals.
In conclusion, QLFTs, especially those that assess the portal circulation and perfused hepatic mass, are helpful in predicting likelihood of response to retreatment with peginterferon/ribavirin in patients with chronic hepatitis C. In addition, these same QLFTs detect improvements related to virologic response that are not shown by standard laboratory tests or clinical evaluation. Although our study was limited to previous nonresponders to interferon-based therapy who also had advanced fibrosis, broader application of QLFTs in the selection of patients for treatment and assessment of the impact of therapy may be warranted.
Acknowledgments
This study was supported by the National Institute of Diabetes & Digestive & Kidney Diseases (contract numbers are listed below). Additional support was provided by the National Institute of Allergy and Infectious Diseases, the National Cancer Institute, the National Center for Minority Health and Health Disparities and by General Clinical Research Center grants from the National Center for Research Resources, National Institutes of Health (grant numbers are listed below). Additional funding to conduct this study was supplied by Metabolic Solutions, Inc. and by Hoffmann-La Roche, Inc., through a Cooperative Research and Development Agreement with the National Institutes of Health.
The authors wish to acknowledge the contributions of our co-investigators, study coordinators and staff at each of the participating institutions as follows:
University of Colorado School of Medicine, Denver, CO: (Contract N01-DK-9-2327, Grant M01RR-00051) Marcelo Kugelmas, MD, Carol McKinley, RN, Brenda Easley, RN, Shannon Lauriski, BS, Stephanie Shea, BA, Michelle Jaramillo.
University of California - Irvine, Irvine, CA: (Contract N01-DK-9-2320, Grant M01RR-00827) Muhammad Sheikh, MD, Norah Milne, MD, Choon Park, RN, William Rietkerk, Richard Kesler-West, M. Mazen Jamal, MD, MPH
Virginia Commonwealth University Health System, Richmond, VA: (Contract N01-DK-9-2322, Grant M01RR-00065) Charlotte Hofmann, RN, Paula Smith, RN
New England Research Institutes, Watertown, MA: (Contract N01-DK-9-2328) Michael C. Doherty, MA, Kristin K Snow, ScD, Marina Mihova, MHA
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Digestive Diseases and Nutrition, Bethesda, MD: James E. Everhart, MD, Jay H. Hoofnagle, MD, Leonard Seeff, MD.
Data and Safety Monitoring Board Members: (Chair) Gary L. Davis, MD, Guadalupe Garcia-Tsao, MD, Michael Kutner, PhD, Stanley M. Lemon, MD, Robert P. Perillo, MD.
List of Abbreviations
- SVR
sustained virologic response 6 months after the end of treatment
- PEG
peginterferon
- RBV
ribavirin
- QLFTs
quantitative liver function tests
- HALT-C
Hepatitis C Antiviral Long-term Treatment against Cirrhosis
- Cl
clearance
- PHM
perfused hepatic mass
- VR20
viral clearance at week 20 of treatment
- HCV
hepatitis C virus
- GCRC
General Clinical Research Center
- SD
standard deviation
- INR
prothrombin time international normalized ratio
- BMI
body mass index
- IND
Investigational New Drug
- MEGX
monoethylglycine xylidide
- MBT
methionine breath test
- SPECT-LSS
single photon emission computed tomographic liver spleen scan
- kelim
elimination rate constant
- GEC
galactose elimination capacity
- CTP
Child-Turcotte-Pugh
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
Footnotes
Financial disclosures: Financial relationships of the authors with Hoffmann-La Roche, Inc., are as follows: G.T. Everson, M. L. Shiffman, T. R. Morgan, and R. K. Sterling are consultants, on the speaker's bureau, and receive research support. J.C. Hoefs is on the speaker's bureau.
Financial relationships of the authors with Metabolic Solutions are: G.T. Everson, M.L. Shiffman, R. K. Sterling, and T.R. Morgan received research support; and D.A. Wagner is employed, has equity, and has intellectual property rights.
G. T. Everson and UCHSC have filed US Patent Application No. 60/647,689, “Methods for Diagnosis and Intervention of Hepatic Disorders”, 26 January 2005, and International Application Number PCT/US2006/003132 as published under the Patent Cooperation Treaty, World Intellectual Property Organization, International Patent Classification A61K 49/00 (2006.01), International Publication Number WO 2006/081521 A2, 3 August 2006 (03.08.2006).
Authors with no financial relationships to disclose are: J.L. DeSanto, T.M. Curto, and E.C. Wright.
References
- 1.Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. New Engl J Med. 1999;341:556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong GL, Simard EP, Wasley A, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus (HCV) infection in the United States, 1999-2002 (abstract) Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 3.Alter MJ. Epidemiology of hepatitis C. Hepatology. 1997;26(Suppl 1):62S–65S. doi: 10.1002/hep.510260711. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong GL, Alter MJ, McQuillan GM, Margolis HS. The past incidence of hepatitis C virus infection: implications for the future burden of chronic liver disease in the United States. Hepatology. 2000;31:777–782. doi: 10.1002/hep.510310332. [DOI] [PubMed] [Google Scholar]
- 5.Wong JB, McQuillan GM, McHutchison JG, Poynard T. Estimating future hepatitis C morbidity, mortalty, and costs in the United States. Am J Public Health. 2000;90:1562–1569. doi: 10.2105/ajph.90.10.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35–S46. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- 7.Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Seminars Liv Dis. 2000;20:17–35. doi: 10.1055/s-2000-9505. [DOI] [PubMed] [Google Scholar]
- 8.Afdahl NH. The natural history of hepatitis C. Semin Liv Dis. 2004;24:3–8. doi: 10.1055/s-2004-832922. [DOI] [PubMed] [Google Scholar]
- 9.Seeff LB, Hollinger FB, Alter HJ, Wright EC, Cain CM, Buskell ZJ, et al. Long-term mortality and morbidity of transfusion-associated non-A, non-B hepatitis: a National Heart, Lung and Blood Institute collaborative study. Hepatology. 2001;33:455–463. doi: 10.1053/jhep.2001.21905. [DOI] [PubMed] [Google Scholar]
- 10.El Serag. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–S34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 11.National Institutes of Health. Consensus Development Conference statement: management of hepatitis C:2002-June 10-12,2002. Hepatology. 2002;36:S3–220. doi: 10.1053/jhep.2002.37117. [DOI] [PubMed] [Google Scholar]
- 12.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomized trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 13.Fried MW, Shiffman ML, Reddy R, Smith C, Marinos G, Goncales FL, Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 14.Hadziyannis SJ, Sette H, Jr, Morgan TR, Balan V, Diago M, Marcellin P, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–355. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- 15.Everson GT, Hoefs JC, Seeff LB, Bonkovsky HL, Naishadham D, Shiffman ML, et al. Impact of Disease Severity on Outcome of Antiviral Therapy for Chronic Hepatitis C: Lessons from the HALT-C Trial. Hepatology. 2006;44:1675–1684. doi: 10.1002/hep.21440. [DOI] [PubMed] [Google Scholar]
- 16.Everson GT, Trotter JF, Forman L, Kugelmas M, Halprin A, Fey B, et al. Treatment of Advanced Hepatitis C with a Low Accelerating Dosage Regimen of Antiviral Therapy. Hepatology. 2005;42:255–262. doi: 10.1002/hep.20793. [DOI] [PubMed] [Google Scholar]
- 17.Everson GT. Treatment of Hepatitis C in the Patient with Decompensated Cirrhosis. Clinical Gastroenterology and Hepatology. 2005;3:S106–S112. doi: 10.1016/s1542-3565(05)00699-3. [DOI] [PubMed] [Google Scholar]
- 18.Lee WM, Dienstag JL, Lindsay KL, Lok AS, Bonkovsky HL, Shiffman ML, et al. Evolution of the HALT-C Trial: pegylated interferon as maintenance therapy for chronic hepatitis C in previous interferon nonresponders. Controlled Clinical Trials. 2004;25:472–492. doi: 10.1016/j.cct.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z, et al. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. The PEG-FIBROSIS Project Group. Gastroenterology. 2002;122:1303–13. doi: 10.1053/gast.2002.33023. [DOI] [PubMed] [Google Scholar]
- 20.Camma C, Di Bona D, Schepis F, Heathcote EJ, Zeuzem S, Pockros PJ, et al. Effect of peginterferon alfa-2a on liver histology in chronic hepatitis C: a meta-analysis of individual patient data. Hepatology. 2004;39:333–42. doi: 10.1002/hep.20073. [DOI] [PubMed] [Google Scholar]
- 21.Huang JF, Yu ML, Lee CM, Dai CY, Hou NJ, Hsieh MY, et al. Sustained virological response to interferon reduces cirrhosis in chronic hepatitis C: a 1,386-patient study from Taiwan. Aliment Pharmacol Ther. 2007;25:1029–37. doi: 10.1111/j.1365-2036.2007.03297.x. [DOI] [PubMed] [Google Scholar]
- 22.Bruno S, Stroffolini T, Colombo M, Bollani S, Benvegnu L, Mazzella G, et al. Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology. 2007;45:579–87. doi: 10.1002/hep.21492. [DOI] [PubMed] [Google Scholar]
- 23.Veldt BJ, Heathcote EJ, Wedemeyer H, Reichen J, Hofmann WP, Zeuzem S, et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. 2007;147:677–84. doi: 10.7326/0003-4819-147-10-200711200-00003. [DOI] [PubMed] [Google Scholar]
- 24.Arase Y, Ikeda K, Suzuki F, Suzuki Y, Kobayashi M, Akuta N, et al. Interferon-induced prolonged biochemical response reduces hepatocarcinogenesis in hepatitis C virus infection. J Med Virol. 2007;79:1485–90. doi: 10.1002/jmv.20925. [DOI] [PubMed] [Google Scholar]
- 25.Arase Y, Ikeda K, Suzuki F, Suzuki Y, Kobayashi M, Akuta N, et al. Prolonged-interferon therapy reduces hepatocarcinogenesis in aged-patients with chronic hepatitis C. J Med Virol. 2007;79:1095–102. doi: 10.1002/jmv.20866. [DOI] [PubMed] [Google Scholar]
- 26.Everson GT, Shiffman ML, Morgan TR, Hoefs JC, Sterling RK, Wagner DA, et al. The Spectrum of Hepatic Functional Impairment in Patients with Fibrosis and Compensated Cirrhosis due to Chronic Hepatitis C: Results from the HALT-C Trial. Aliment Pharmacol Ther. 2008;27:798–809. doi: 10.1111/j.1365-2036.2008.03639.x. [DOI] [PubMed] [Google Scholar]
- 27.Everson GT, Martucci MA, Shiffman ML, Sterling RK, Morgan TR, Hoefs JC, et al. Portal systemic shunting in patients with fibrosis or cirrhosis due to chronic hepatitis C: the minimal model for measuring cholate clearances and shunt. Aliment Pharmacol Ther. 2007;26:401–410. doi: 10.1111/j.1365-2036.2007.03389.x. [DOI] [PubMed] [Google Scholar]
- 28.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–9. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 29.SAS Institute, Inc. SAS/STAT® 9.1 User's Guide. Cary, NC: SAS Institute, Inc: 2004. [Google Scholar]
- 30.Rosner B. Fundamentals of Biostatistics. 3re. Belmont, CA: Duxbury Press; 1990. [Google Scholar]
- 31.Shrestha R, McKinley C, Showalter R, Wilner K, Marsano L, Vivian B, et al. Quantitative Liver Function Tests (QLFTs) define the functional severity of liver disease in early stage cirrhosis. Liver Transplantation and Surgery. 1997;3:166–173. doi: 10.1002/lt.500030210. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka E, Kurata N, Yasuhara H. How useful is the ‘cocktail approach’ for evaluating human drug metabolizing capacity using cytochrome P450 phenotyping probes in vivo? Journal of Clinical Pharamacy and Therapeutics. 2003;28:157–165. doi: 10.1046/j.1365-2710.2003.00486.x. [DOI] [PubMed] [Google Scholar]
- 33.Wojcicki J, Kozlowski K, Drozdzik M, Wojcicki M. Comparison of MEGX (monoethylglycinexylidide) and antipyrine tests in patients with liver cirrhosis. European Journal of Drug Metabolism and Pharmacokinetics. 2002;27:243–247. doi: 10.1007/BF03192334. [DOI] [PubMed] [Google Scholar]
- 34.Reichel C, Nacke A, Sudhop T, Wienkoop G, Luers C, Hahn C, et al. The Low-Dose Monoethylglycinexylidide Test: Assessment of Liver Function with Fewer Side Effects. Hepatology. 1997;25:1323–1327. doi: 10.1002/hep.510250603. [DOI] [PubMed] [Google Scholar]
- 35.Ocker M, Ganslmayer M, Zopf S, Gahr S, Janson C, Hahn EG, et al. Improvement of quantitative testing of liver function in patients with chronic hepatitis C after installment of antiviral therapy. World J Gastroenterol. 2005;11:5521–5524. doi: 10.3748/wjg.v11.i35.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rincon D, Ripoll C, Lo Iacono O, Salcedo M, Catalina MV, Alvarez E, et al. Antiviral therapy decreases hepatic venous pressure gradient in patients with chronic hepatitis C and advanced fibrosis. Am J Gastroenterol. 2006;101:2269–74. doi: 10.1111/j.1572-0241.2006.00743.x. [DOI] [PubMed] [Google Scholar]