Abstract
Social groups balance flexibility and robustness in their collective response to environmental changes using feedback between behavioural processes that operate at different timescales. Here we examine how behavioural processes operating at two timescales regulate the foraging activity of colonies of the harvester ant, Pogonomyrmex barbatus, allowing them to balance their response to food availability and predation. Previous work showed that the rate at which foragers return to the nest with food influences the rate at which foragers leave the nest. To investigate how interactions inside the nest link the rates of returning and outgoing foragers, we observed outgoing foragers inside the nest in field colonies using a novel observation method. We found that the interaction rate experienced by outgoing foragers inside the nest corresponded to forager return rate, and that the interactions of outgoing foragers were spatially clustered. Activation of a forager occurred on the timescale of seconds: a forager left the nest 3–8 s after a substantial increase in interactions with returning foragers. The availability of outgoing foragers to become activated was adjusted on the timescale of minutes: when forager return was interrupted for more than 4–5 min, available foragers waiting near the nest entrance went deeper into the nest. Thus, forager activation and forager availability both increased with the rate at which foragers returned to the nest. This process was checked by negative feedback between forager activation and forager availability. Regulation of foraging activation on the timescale of seconds provides flexibility in response to fluctuations in food abundance, whereas regulation of forager availability on the timescale of minutes provides robustness in response to sustained disturbance such as predation.
Keywords: collective behaviour, complex system, flexibility, foraging, interaction rate, Pogonomyrmex barbatus, regulation, robustness, temporal dynamics, timescale
Complex biological systems are regulated by processes that operate at multiple time scales, providing flexibility in the shorter term and robustness in the longer term in response to changing conditions (Flack 2012). For example, the activation of neuron cells relies on the release of neurotransmitters at the synapse on the timescale of seconds, allowing rapid response to fluctuating environments. On a longer timescale of hours, gene regulation increases the stability and predictability of a neural response by determining which neurotransmitters are available to be released (Zupanc 2004). Negative feedback, in the form of neurotransmitter depletion by their release from the cell, prevents the system from entering a runaway process of perpetual signalling (Alon 2006).
Recent work suggests that feedback between processes that operate at multiple timescales is as important in animal behaviour as it is in other biological systems. For example, in primate societies, individuals that differ in functional role and demographic class vary in the timescale at which they decide to join or avoid fights, reducing social uncertainty about the outcome of conflict (DeDeo et al. 2011). Furthermore, the foraging behaviour of gulls in the intertidal zone is best described by models that combine immediate encounter rate with prey as well as the longer-term effect of tide cycles on prey availability (Suraci & Dill 2013). More generally, hierarchical models of foraging show that integrating information from processes occurring on short and long timescale maximizes energetic return (Lucas 1983).
Studies of social insects show that regulation of foraging occurs at different timescales, but little is known about how and why these different timescales are linked. The foraging activity of colonies of the honeybee, Apis mellifera, is regulated on the timescale of minutes through the temporal distribution of forager arrivals, leading to adjustment in foraging activity in response to short-term changes in food availability (Fernández et al. 2003). On a longer, seasonal timescale, honeybee foraging is regulated through vibration dances that vary seasonally in frequency and intensity, producing stable seasonal patterns of foraging activity (Schneider et al. 1986). The synergy between these two regulation mechanisms may help bee colonies to collectively balance flexibility and stability. In the ant Temnothorax albipennis, task allocation is determined by factors that are regulated at two different timescales. An ant’s location in the nest, which changes on a short timescale of hours, and its fat and water stores, which change on a longer timescale of days, both influence the task it performs (Robinson et al. 2009), allowing the colony to balance robustness and flexibility in task allocation.
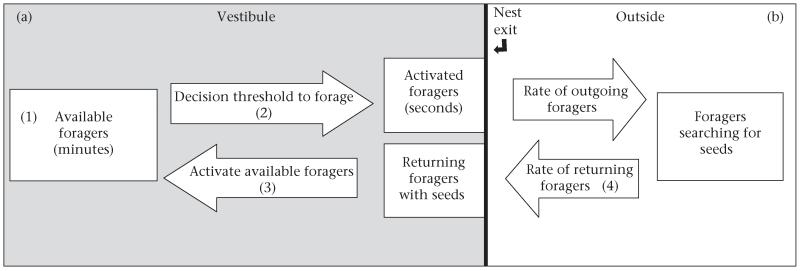
Here we examine how colonies of the red harvester ant, Pogonomyrmex barbatus, combine processes on the timescale of seconds and minutes to regulate foraging activity in response to changes in their environment. Previous work has shown that foragers search for scattered seeds, distributed by wind and flooding (Gordon 1993). A forager searches until it finds a seed, so search time corresponds to food availability (Beverly et al. 2009) (Fig. 1b) and is constrained by the risk of desiccation while searching (Feener & Lighton 1991). Foraging activity can be disturbed by predation by horned lizards (Munger 1984), which stay near foraging trails and consume foragers as they pass by. These short-term changes in foraging activity operate in the context of longer-term changes in food availability and weather, on the timescale of days (Gordon 1991; Gordon et al. 2013) and on the timescale of years, as colonies grow older and larger (Gordon 1991, 1992). Here we ask how processes operating at different timescales of seconds and minutes are linked through positive and negative feedback to allow an ant colony to balance behavioural robustness and flexibility (Fig. 1a).
Figure 1.
Diagram of the regulation of foraging behaviour in harvester ants. (a) Processes identified in the current study as occurring inside the nest vestibule. (b) Processes known from previous work to occur outside the nest. (1) Forager availability (numbers of ants) in the vestibule changes on the timescale of minutes. (2) An available forager’s decision whether to leave the nest depends on its interactions with returning foragers on the timescale of seconds. (3) Interactions of returning foragers with available foragers activate the available foragers. In addition, a returning forager can become an available forager once it drops off the food item it brought into the nest. (4) The rate at which foragers return to the nest depends on food availability.
First, we consider how the rate of forager return corresponds to the rate of interaction between returning and outgoing foragers. Previous work has shown that the rate at which outgoing foragers leave the nest depends on the rate at which successful foragers return with food (Gordon 1991; Schafer et al. 2006; Gordon et al. 2008, 2011, 2013; Prabhakar et al. 2012). This previous work is consistent with the hypothesis that foraging activity is regulated by interactions between returning and outgoing foragers inside the nest. However, these interactions have never been directly observed. Observations of laboratory -housed colonies have shown that collective behaviour of other social insects is regulated through interactions inside the nest: foragers are activated by biting in wasps (O’Donnell 2001) and by other interactions in honeybees (Fernández et al. 2003; Gruter & Farina 2009; Balbuena et al. 2012), and relocation to new nest sites is regulated by interaction rate in wasps (Sonnentag & Jeanne 2009), honeybees (Camazine et al. 1999) and rock ants (Pratt 2005). However, whether foraging regulation of harvester ants in field colonies is mediated by interactions among workers has not yet been directly observed. Here we investigate for the first time whether foraging activity depends on the interactions of foraging harvester ants inside nests in the field.
Second, we consider how forager return rate influences the probability, on the timescale of seconds, that an ant leaves the nest to forage. The more quickly a forager can find food, the lower the cost in desiccation (Lighton & Feener 1989). Thus, colony flexibility on the timescale of seconds is important for balancing the trade-off between desiccation and obtaining food. Whether a worker performs a certain task can be the result of environmental cues that lead it to reach a response threshold (Robinson 1987; Theraulaz et al. 1998; Beshers et al. 1999). We examined whether an outgoing forager requires a threshold number of interactions to become an active forager and leave the nest. Outgoing forgers may interact with both returning foragers and with other task workers inside the nest. Previous work has shown that successful, but not unsuccessful, foragers returning to the nest influence foraging activity (Schafer et al. 2006), and that the combined chemical odour of both seeds and foragers stimulates foraging, but the odour of foragers or of seeds alone does not (Greene et al. 2013). Therefore, we asked whether interactions with returning foragers with seeds were more likely to stimulate outgoing foragers to leave the nest than interactions with other ants. Because a previous study of laboratory colonies showed that interactions are not homogenously distributed in space (Pinter-Wollman et al. 2011), we also asked whether, in colonies in the field, there are interaction ‘hotspots’ close to the nest entrance, similar to those observed in the laboratory.
Third, we investigate the process on the timescale of minutes that determines how many foragers are available to interact with returning foragers. In colonies of honeybees (Anderson & Ratnieks 1999) and wasps (Jeanne 1986), workers queue near the nest entrance to receive food or building material from returning individuals, creating a pool of available workers. In harvester ants, foraging may stop for minutes when a predator, the horned lizard, interrupts foraging activity (Munger 1984). Furthermore, foraging activity recovers quickly after it is experimentally stopped for less than 5 min (Gordon et al. 2008, 2011, 2013; Prabhakar et al. 2012), but not when foraging activity is stopped for more than 10 min (Gordon 2002). Here we test the hypothesis that the number of available foragers inside the nest is related to the rate of returning foragers and determines the recovery of a colony’s foraging activity after perturbations of varying durations. Specifically we test whether a long interruption in forager return causes the outgoing foragers to go deeper into the nest, reducing the pool of available foragers.
METHODS
Materials and Experimental Procedures
Study site
To investigate how interaction rate is used in the regulation of harvester ant foraging, we conducted two experiments to manipulate the rate at which foragers returned to the nest. Experiments were performed on three colonies in August 2010 and on four other colonies in August 2011, at the site of a long-term study of a population of P. barbatus near Rodeo, New Mexico, U.S.A. (Gordon & Kulig 1996). We developed a method to observe the ants inside the nest without any direct intervention. We created a transparent ceiling for the area just inside the nest entrance where ants gathered as they entered and left the nest. We refer to this area as the ‘vestibule’.
Creating the observation vestibule
The soil around the nest entrance was lightly scraped, using a spoon, to expose the area just inside the nest entrance. Ants could leave the vestibule in two directions: (1) through the nest exit to go outside the nest or (2) down a tunnel, leading to a deeper chamber (Fig. 2). To create a ceiling, an opaque surface (either a petri dish, 8.5 cm diameter, filled with plaster in 2010, or a wooden block, 20 × 25 × 2.5 cm, in 2011) was placed on top of the excavated region. The ants were allowed to acclimate to the artificial ceiling for 2–3 days. Before beginning an experiment, we shaded the area around the nest entrance, including the vestibule, using a beach umbrella, and replaced the opaque artificial ceiling with a transparent glass sheet of the same area. Ants did not appear disturbed by this replacement and showed no alarm after 2–3 min. We waited at least 5 min before starting any experiment. At the end of each experiment, the transparent ceiling was replaced with the opaque ceiling. During the experiments, we used one video camera, Canon Vixia HF20, to film ant behaviour inside the vestibule and a second camera, Insignia NS DV720PBL2, to record the activity of foragers on the most active foraging trail.
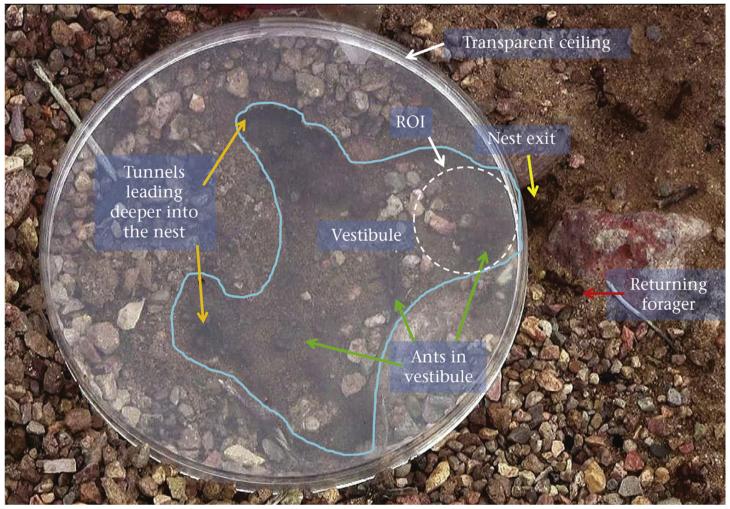
Figure 2.
Spatial arrangement of the nest vestibule. The image was taken from a frame of the video for experiment 1, colony 672. The round object is the transparent ceiling; the blue line is the boundary of the vestibule; orange arrows indicate the tunnels leading from the vestibule deeper into the nest; the yellow arrow indicates the nest exit through which foragers entered and left the foraging trail; green arrows point to ants that are standing in the vestibule; the red arrow indicates a returning forager carrying a seed; the dashed white circle near the nest exit is the region of interest (ROI) used in the spatial analysis.
Measuring ant interactions on the timescale of seconds
To count ant interactions inside the nest, we used a Matlab script that records the time and position of user-identified events. Using focal observations, we recorded all interactions of outgoing foragers from the videos of the vestibule. An interaction was recorded when the focal ant’s head came within one antenna length of another ant in the vestibule. We coded each interaction as either (1) with a returning forager carrying a seed in its mandibles or (2) with any other ant in the vestibule (including an inactive worker, another outgoing forager, or an ant performing a task other than foraging, such as midden work or nest maintenance) (Fig. 2).
Measuring foraging rates and forager availability on the timescale of minutes
We counted the number of returning and outgoing foragers on the foraging trail and the number of ants in the vestibule from the videos every 30 s in both experiments. Time series of numbers of returning and outgoing foragers on the trail, crossing an invisible line approximately 1 m from the nest entrance, as described in Prabhakar et al. (2012), were produced using the image analysis system AnTracks (http://www.antracks.org). To record the number of ants in the vestibule, we played the video on a computer, overlaid a transparency on the monitor, and drew the boundary of the vestibule (light blue line in Fig. 2). We paused the film every 30 s and counted the number of ants in the vestibule. To compare the time series obtained from the trail and the vestibule, we calculated the number of foragers travelling on the trail in each direction per 30 s.
Experiment 1: seed additions
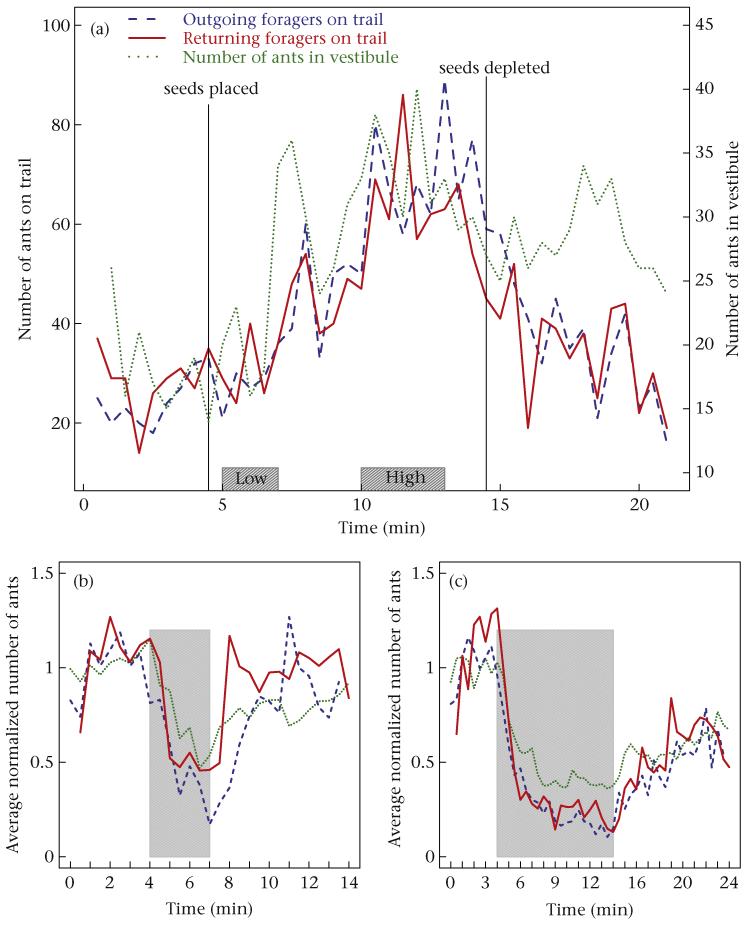
To examine the effects of forager return rate on the interactions inside the nest, we conducted ‘seed addition’ experiments with three colonies (ID: 672, 977, 486) on 21 August 2010. Five minutes after we began filming, we added a pile (~1 cm3) of millet seeds on the trail where it was being filmed, 1.5–2 m from the nest entrance, and continued filming for 10 min after seeds were depleted. Based on the number of ants on the foraging trail (Fig. 3a) and the behaviour of the returning foragers, we selected two periods that differed in foraging activity and were long enough to track the behaviour of many outgoing foragers while foraging rate was relatively stable: We refer to the first 2–3 min after seeds were provided, before foragers began to retrieve the seeds, as the ‘low return rate’ period. Forager return rate increased over time as the ants collected the seeds, and peaked immediately before the seeds were depleted (Fig. 3a). We refer to the 1–2 min during which foragers returned at the highest rate as the ‘high return rate’ period (Fig. 3a, Table 1). During both the low and high forager return rate periods, we recorded the number of foragers on the trail, the number of ants in the vestibule and the interactions of all outgoing foragers for which the entire trajectory in the vestibule could be viewed without obstruction (Table 1).
Figure 3.
(a) Number of returning and outgoing foragers on the trail and number of ants inside the vestibule for colony 486 in experiment 1; boxes indicate the low and high forager return rate periods; solid vertical lines indicate the times at which seeds were placed on the trail and depleted by the ants. (b, c) Average normalized number of returning and outgoing foragers on the trail and number of ants inside the vestibule for 3 min removals (N = 10) and 10 min removals (N = 12), respectively, in experiment 2; grey shading indicates the time during which returning foragers were prevented from returning to the nest.
Table 1.
Return rate of foragers, interaction rate of outgoing foragers and number of ants in the vestibule during the low and high forager return rate periods in experiment 1
| Colony | Forager return rate (ants/s) (Nr)* |
Mean±SD interaction rate (interactions/s) (Nf)† |
Mean±SD number of ants in vestibule (Nc‡; D§) |
|||
|---|---|---|---|---|---|---|
| Low | High | Low | High | Low | High | |
| 486 | 1.14(179) | 2.29 (176) | 1.43±0.74 (80) | 2.68±0.99 (100) | 26.16±8.4 (6; 2.63) | 35.75±4.35 (4; 1.28) |
| 672 | 0.45 (74) | 0.67 (109) | 1.11±0.69(100) | 1.55±0.75 (80) | 21.16±3.25 (6; 2.7) | 21.86±3.02 (7; 2.77) |
| 977 | 0.18 (36) | 0.82 (71) | 0.69±0.29 (34) | 1.33±0.61 (21) | 11 ±4.04 (8; 3.31) | 22.6±3.71 (5; 1.45) |
Nr: number of returning foragers.
Nf: number of focal foragers tracked.
Nc: number of counts performed.
D: duration of forager return rate period analysed (minutes).
Experiment 2: removal of retuning foragers
To examine how the duration of an interruption of foraging activity influences forager availability, we removed returning foragers for a short interval of 3 min or a long interval of 10 min. Returning foragers were collected from the foraging trail and placed inside plastic boxes and thus prevented from returning to the nest (e.g. Gordon et al. 2008, 2011; Prabhakar et al. 2012). Returning foragers were removed for 3 min (minutes 4–7 of a 14 min trial) or 10 min (minutes 4–14 in a 24 min trial)(Fig. 3b, c). Foragers collected during the experiment were released at the nest after the experiment was concluded. Experiments were conducted on four colonies over a 9-day period, 17–26 August 2011. Each removal regime (3 min or 10 min) was repeated three times on different days for each colony. We conducted four removal trials per day (two of each removal regime), and no more than one trial was conducted on a particular colony on a given day. We excluded two trials because of technical malfunction, resulting in 22 trials (3 min trials: N = 10; 10 min trials: N = 12).
To allow for comparison among trials, we normalized the time series data of the numbers of returning foragers, outgoing foragers and the numbers of ants in the vestibule by dividing the numbers of ants in each time series by the average number of ants during the 4 min before the removals began. We used these normalized time series to calculate an average time series for each measure (number of returning foragers, outgoing foragers and numbers of ants in the vestibule) in all 10 min and all 3 min trials (Fig. 3b, c).
Data Analysis
Relationship between forager return rate and interaction rate of outgoing foragers
To examine the effect of forager return rate on the interaction rate of outgoing foragers and on the number of ants in the vestibule, we calculated forager return rate, interaction rate and the average number of ants in the vestibule for each of the two foraging rate periods (low and high) of experiment 1. Forager return rate was defined as the total number of returning foragers on the trail divided by the duration of the period. The interaction rate of a focal ant was defined as the number of its interactions divided by the time it spent in the vestibule. The number of ants in the vestibule during each foraging period was defined as the average number of ants counted in the vestibule every 30 s during that foraging period (Table 1). To account for ant density in the vestibule we compared interaction rates divided by the number of ants in the vestibule in the low and high foraging periods, for each colony, using a t test.
Foraging regulation on the timescale of seconds
Forager activation
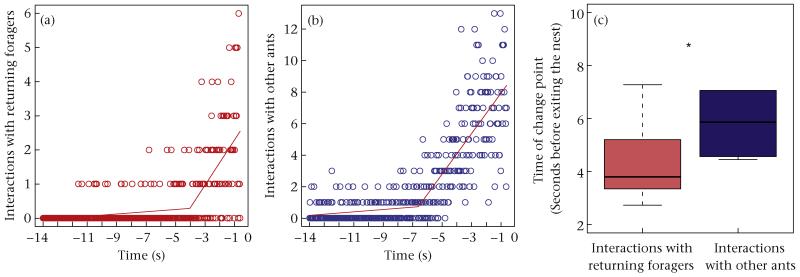
We determined the effect of interactions on the time at which an outgoing forager left the nest by examining how interaction rate changed as ants approached their departure from the nest, in each of the six foraging rate periods of experiment 1 (two foraging periods each, high and low, for one trial in each of the three colonies). The time at which each focal ant departed from the nest was set to zero. We then found the total number of interactions experienced by all focal outgoing ants during each video frame preceding departure from the nest such that if five outgoing foragers experienced interactions with other ants 1 s before they left the nest, the value at time −1 s was 5 (see Results, Fig. 5a). We used a piecewise linear regression, also known as a ‘broken-stick fit’, to detect whether and when the slope of the increase in interactions changed abruptly. The time of such an abrupt change is called the ‘change point’ of the piecewise linear regression (Toms & Lesperance 2003). Change points were obtained using the R package SiZer (The R Foundation for Statistical Computing, Vienna, Austria). We compared the effects of interactions with returning foragers, or with other ants, on the time that an outgoing forager left the nest. To do so, we used paired-sample Wilcoxon signed-ranks tests that compared the six change points in the rate of interactions with returning foragers with the six change points in the rate of interaction with all other ants.
Figure 5.
Total number of interactions with (a) returning foragers and (b) other ants at each video frame (1/30 s) before an ant left the nest, in colony 486 for the period of high forager return rate. Lines are the piecewise linear regressions. Time zero indicates when an ant left the nest and all preceding times are when it was in the vestibule. (c) Difference between the change point for interactions with returning foragers and with other ants; all colonies and foraging rate periods pooled. Boxes indicate the lower and upper quartiles; horizontal lines within boxes indicate the median, and whiskers extend to the 1.5 interquartile range from the box.
We examined whether there was an association between the location of interactions with returning foragers and the location of interactions with other ants. To characterize the spatial pattern in the location of outgoing forager interactions, we produced a utilization distribution map using a two-dimensional kernel density estimation. To test whether outgoing foragers, as they approached the nest exit (region of interest, ROI, Fig. 2), interacted more with returning foragers than with other ants, we used a Monte Carlo simulation (details in Supplementary Material).
Effect of forager return rate on forager activation
We considered how forager return rate influenced the interaction rate of outgoing foragers as they left the nest. First, we examined differences between the periods of low and high forager return rate in the change points showing the times at which interactions increased. We used paired-sample Wilcoxon signed-ranks to compare the three values of change points for the three colonies in the low foraging period with the three values of change points for the three colonies in the high foraging period of experiment 1. We compared the change points in low and high foraging periods of three categories of interactions of outgoing foragers: with returning foragers only, with ants other than returning foragers and with all ants (both returning foragers and other ants). Second, we asked whether the foraging periods differed in interaction rate before the change point and then whether they differed after the change point. For each foraging period, we determined the interaction rate before and after the change point for each focal outgoing forager. For example, the change point during the low forager return rate period for colony 486 was 7.3 s, and we calculated two interaction rates for each outgoing forager: (1) before the change point: from the moment it entered the vestibule until 7.3 s before it left the nest; and (2) after the change point: during the 7.3 s before it left the nest. We used t tests to compare interaction rates in the low and high forager return rate periods before and after the change point for each colony in experiment 1.
Foraging regulation on the timescale of minutes
The number of returning and outgoing foragers and the number of ants in the vestibule changed on the timescale of minutes in response to the seed addition (experiment 1; Fig. 3a) and removal manipulations (experiment 2; Fig. 3b, c).
To examine how the rates of outgoing and returning foragers were each related to the number of ants in the vestibule, we used time series regressions (Cowpertwait & Metcalfe 2009) on the averaged time series of all 3 min and 10 min removal trials of experiment 2 (Fig. 3b, c). The averaged time series of the number of ants in the vestibule was the dependent variable and the independent variable was the averaged time series of either returning or outgoing foragers.
We examined how a decrease in forager return rate was related to changes in the numbers of ants in the vestibule. We used t tests to compare the slopes corresponding to the decrease in forager return rate or the decrease in the rate of outgoing foragers to the decrease in numbers of ants in the vestibule. We did this for periods of decreasing return rate in both experiments, using the slopes of linear fits against time after seeds were depleted (experiment 1) and during the removal of returning foragers (experiment 2). Linear and not exponential fits were used because the Akaike Information Criterion (AIC) values were lower for the linear fits, indicating that a linear model was more appropriate for the data. For experiment 1, we performed the linear fit from the time at which ant numbers were highest until the end of the experiment (Fig. 3a). For experiment 2, the linear fit was from the time at which we began removing foragers until the end of the removals. We used the slopes of these lines to compare the rate at which the number of returning foragers, outgoing foragers and ants in the vestibule decreased over time. To allow for the comparison among slopes, we normalized the number of outgoing and returning foragers and the number of ants in the vestibule before performing the linear fit by dividing the numbers of ants in each of the three time series by the first data point used for the linear fit.
To examine when the decline in number of ants in the vestibule eventually stopped, while foragers were prevented from returning to the nest, we used a piecewise linear regression (Toms & Lesperance 2003). A change point in the number of ants in the vestibule was obtained for each trial in experiment 2, and the mean change points of the 3 min and the 10 min removal trials were compared using a t test.
We examined how an increase in the rate of outgoing foragers was related to changes in the numbers of ants in the vestibule. We compared the slopes corresponding to the increase in the rate of outgoing foragers to the increase in numbers of ants in the vestibule using a t test. We did this for the period of increase in forager return rate in the removal experiments (experiment 2) using the slopes of linear fits against time, beginning at the time that removals stopped, so that foragers were no longer prevented from returning to the nest, until the end of the trial. Linear and not exponential fits were used because the AIC values were lower for the linear fits, indicating that a linear model was more appropriate for the data.
All analyses were conducted using R version 2.12.1 and all t tests and Wilcoxon tests were two tailed.
RESULTS
Interaction Rate Increases with Foraging Rate
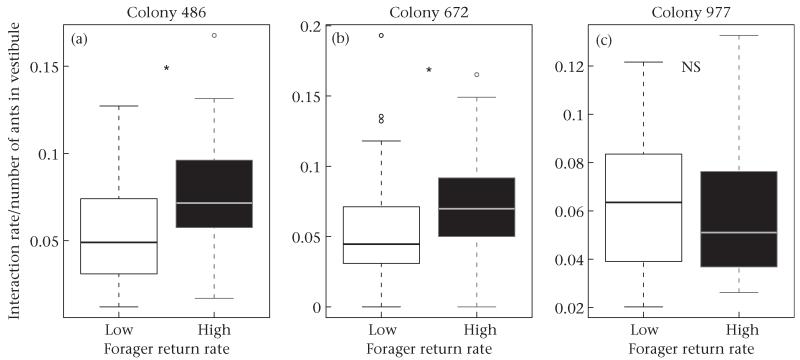
The higher the rate at which foragers returned to the nest with food, the higher the rate of interaction experienced by outgoing foragers inside the nest (Fig. 4, Fig. S2). As the rate of returning foragers increased, so did the number of ants in the vestibule (Fig. 3). Despite this relationship, the increase in interaction rate was not solely due to the increase in the number of ants in the vestibule. Interaction rate divided by the average number of ants in the vestibule was higher in the high forager return rate period than in the low forager return rate period for two of the three colonies (t test: colony 468: t178 = −4.88, P < 0.0001; colony 672: t178 = −3.71, P < 0.001; colony 977: t53 = 0.48, P = 0.63; Fig. 4).
Figure 4.
Difference in interaction rate divided by the number of ants in the vestibule between low (white bars) and high (black bars) forager return rate periods for the three colonies in experiment 1. Boxes indicate the lower and upper quartiles; horizontal lines within boxes indicate the median, whiskers extend to the 1.5 interquartile range from the box, and points indicate outliers.
Foraging Regulation on the Timescale of Seconds
Forager activation
The rate of interactions experienced by an outgoing forager increased during the time it spent in the vestibule. Slopes of the linear regression for all time periods and all colonies were significantly positive (see Supplementary Table S1). Approximately 3–8 s before an outgoing forager reached the nest exit, the number of its interactions began to increase substantially (Table 2).
Table 2.
Time (s) at which interactions began to increase substantially (change point) before outgoing foragers exited the nest
| Colony | All interactions |
Interactions with other ants |
Interactions with returning foragers |
|||
|---|---|---|---|---|---|---|
| Low | High | Low | High | Low | High | |
| 486 | 7.34 | 5.47 | 7.06 | 5.99 | 5.20 | 3.41 |
| 672 | 3.91 | 5.69 | 4.45 | 5.73 | 3.34 | 4.18 |
| 977 | 8.76 | 3.48 | 11.30 | 4.56 | 7.28 | 2.72 |
The time at which an outgoing forager left the nest was related to its interaction rate with returning foragers. An outgoing forager’s interaction rate with returning foragers increased later, closer to the time at which it left the nest, than did its interaction rate with other ants. The change point for interactions with returning foragers was closer to the time at which the outgoing forager left the nest than it was to the change point for interactions with other ants (paired-sample Wilcoxon signed-ranks test: V = 21, N = 6, P = 0.031; Fig. 5, Table 2).
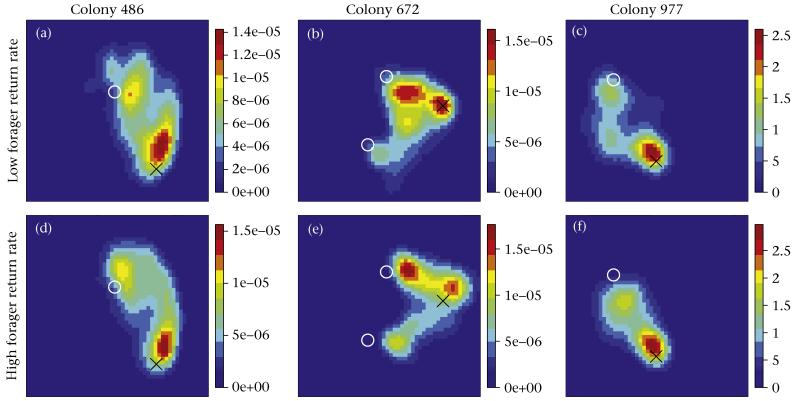
Most interactions took place near the nest exit or near the exit from the vestibule leading down into the nest. The location of interactions was similar in both the low and high forager return rate periods (Fig. 6). During the period when an outgoing forager’s interactions were more frequent, after the change point, interactions occurred close to the nest exit. Interactions before the change point occurred close to the exit leading deeper into the nest (Fig. S3).
Figure 6.
Spatial distribution of ant interactions during (a–c) low and (d–f) high forager return rate periods. Regions in red indicate many interactions and those in blue indicate few interactions (see scale bars). Black Xs denote the nest exit and white Os indicate tunnels that led further into the nest.
Whether an outgoing forager interacted with returning foragers or with other ants did not depend on its location. Interactions of outgoing foragers were equally likely with returning foragers or other ants, anywhere in the vestibule. There was no significant difference between the ratio of the number of interactions with returning foragers to the number of interactions with all other ants near the nest exit and elsewhere in the vestibule (Monte Carlo: P = vestibule > ROI, iterations = 100 000; low: colony 977 = 0.13, colony 486 = 0.25, colony 672 = 0.14; high: colony 977 = 0.36, colony 486 = 0.32, colony 672 = 0.31; Fig. S1).
Effect of forager return rate on forager activation
The time at which an outgoing forager’s interaction rate began to increase substantially did not depend on forager return rate. Change points did not differ between the low and high return rate periods, either when all interactions were considered (Wilcoxon two-sample test: W = 7, N = 3, P = 0.4) or when interactions with returing foragers (W = 7, N = 3, P = 0.4) and with all other ants (W = 6, N = 3, P = 0.7) were considered separately (Table 2).
Although the change point, the time at which an outgoing forager’s interaction rate substantially increased, did not depend on forager return rate, the magnitude of the increase in the outgoing forager’s interaction rate was strongly influenced by forager return rate. The interaction rate of outgoing foragers after the change point increased with forager return rate (t test: low versus high: colony 486: t178 = 9.59, P < 0.0001; colony 672: t178 = 2.66, P = 0.008; colony 977: t53 = 6.23, P < 0.0001) as did interaction rate divided by the number of ants in the vestibule (t test: low versus high: colony 486: t178 = −4.77, P < 0.0001; colony 672: t178 = −2.27, P = 0.02; colony 977: t53 = 0.001, P = 0.99; Fig. 7). However, before the change point, an outgoing forager’s interaction rate did not depend on forager return rate in two of the three colonies (t test: low versus high: colony 486: t178 = −0.01, P = 0.99; colony 672: t178 = −1.31, P = 0.19; colony 977: t53 = −2.33, P = 0.025) and neither did interaction rate divided by the number of ants in the vestibule (t test low versus high: colony 486: t178 = 1.27, P = 0.2; colony 672: t178 1.44, P = 0.15; colony 977: t53 = 3.14, P = 0.003; Fig. 7).
Figure 7.
Difference between the low (white) and high (black) forager’ return rate periods in outgoing foragers’ interaction rate before and after the change point. Boxes indicate the lower and upper quartiles; horizontal lines within boxes indicate the median, whiskers extend to the 1.5 interquartile range from the box, and points indicate outliers.
Foraging Regulation on the Timescale of Minutes
On the timescale of minutes, the number of ants in the vestibule was related to the number of returning foragers on the trail. Changes in the numbers of ants in the vestibule corresponded to changes in the numbers of returning foragers (time series regression: 3 min removals: R2 = 0.38, P < 0.001; 10 min removals: R2 = 0.77, P < 0.0001) and to changes in the numbers of foragers exiting the nest (time series regression: 3 min removals: R2 = 0.39, P < 0.001; 10 min removals: R2 = 0.88, P < 0.0001; Fig. 3b, c). = This indicates that when returning foragers were removed, not all ants stayed in the vestibule; some went further down into the nest.
When forager return rate decreased, the numbers of ants in the vestibule remained high for a few minutes, both when seeds were depleted, and thus, when the foragers were no longer returning with seeds (experiment 1), and when returning foragers were prevented from reaching the nest (experiment 2). As seeds were depleted, the number of ants in the vestibule decreased more slowly than did either the number of returning foragers or the number of outgoing foragers on the trail (Fig. 3a, Table S2). As returning foragers were removed, the number of ants in the vestibule decreased more slowly than did the number of outgoing or returning foragers on the trail (Fig. 3b, c). For the 22 removal experiments, the slopes of the linear regressions of the decrease in the number of ants in the vestibule over time were significantly lower than those of the number of outgoing and returning foragers (paired t test: ants in vestibule versus outgoing foragers: t10 = −3.06, P = 0.006; ants in vestibule versus returning foragers: t10 = −2.8, P 0.01).
When foragers were prevented from returning to the nest, the numbers of ants in the vestibule decreased but eventually stabilized at a number greater than zero (Fig. 3c). The change in the number of ants in the vestibule depended on how long foragers were prevented from returning. During the 3 min removal experiments, the number of ants in the vestibule continued to decline for a mean ± SD of 2.93 ± 1 min (N = 10), approximately equivalent to the duration of removals. However, when foragers were prevented from returning for 10 min, the number of ants inside the vestibule continued to decline for a mean ± SD of 4.66 ± 1.72 min (N = 12), and then stayed at the new low level until foragers were allowed to return. The number of ants in the vestibule stopped decreasing during removals significantly earlier for the 3 min removals than for the 10 min removals (t test: t20 = −2.93, P = 0.009). However, we detected no difference in the minimum number of ants in the vestibule between the 10 min and 3 min removal experiments (t test: t20 = 0.67, P = 0.51).
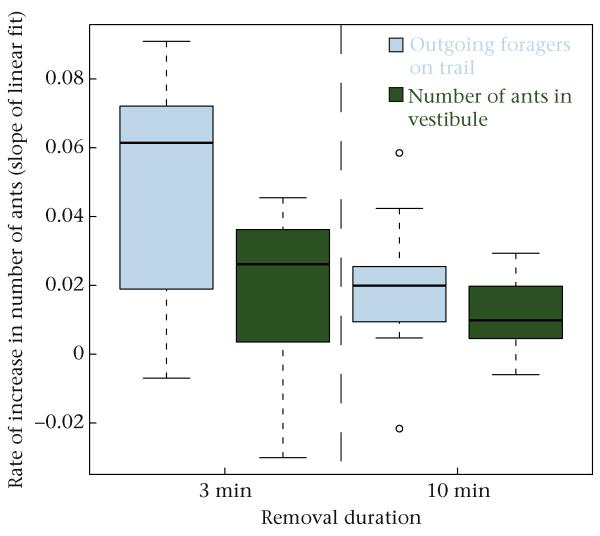
When foragers did not return to the nest for 3 min, outgoing foragers appeared to be recruited from the vestibule, but when foragers did not return for 10 min, outgoing foragers appeared to be recruited from deeper inside the nest. When foragers were allowed to return after the 3 min removals, the number of outgoing foragers on the trail increased more quickly than did the number of ants in the vestibule (paired t test: outgoing foragers versus ants in vestibule: t4 = 2.43, P = 0.038; Fig. 8). This difference suggests that outgoing foragers come from nearby, in the vestibule, after a brief interruption. However, when foragers were allowed to return after the 10 min removals, the number of outgoing foragers on the trail and the number of ants in the vestibule both increased at the same rate (paired t test: outgoing foragers versus ants in vestibule: t5 = 1.34, P = 0.21; Fig. 8), suggesting that both outgoing foragers and ants in the vestibule arrived from deeper inside the nest after a long interruption.
Figure 8.
Differences in rate of increase of the number of ants in the vestibule and outgoing foragers when foragers were allowed to return to the nest after 3 min or 10 min removals. Boxes indicate the lower and upper quartiles; horizontal lines within boxes indicate the median, whiskers extend to the 1.5 interquartile range from the box, and points indicate outliers.
Foraging resumed more quickly when foragers began to return after the 3 min removals ended than after the 10 min removals ended. The rate of increase in outgoing forager numbers was greater after the 3 min removal experiments than after the 10 min removal experiments (t test: 3 min versus 10 min: t20 = 2.4, P = 0.029; Fig. 8). However, the number of ants in the vestibule increased at the same rate after the 3 min and 10 min removals had ended (t test: 3 min versus 10 min: t20 = 0.878, P = 0.397; Fig. 8).
DISCUSSION
A harvester ant colony regulates its foraging activity by linking processes that operate at different timescales. Interactions between returning and outgoing foragers, on the timescale of seconds, stimulate outgoing foragers to leave the nest, allowing the colony to respond to current conditions of weather and food abundance. Changes in the availability of outgoing foragers, on the timescale of minutes, influence how quickly the colony can resume foraging after a disturbance such as an episode of predation. These two processes are linked through the rate at which foragers return to the nest.
A colony balances the flexibility and robustness of its foraging activity by combining negative and positive feedback (Fig. 1). Positive feedback provides flexibility, but unchecked it can lead to a runaway process (Brandman & Meyer 2008), so negative feedback adds stability (Cannon 1929; Alon 2006). For harvester ants, forager return rate and the availability of ants in the vestibule stimulate each other, thus generating positive feedback: as more foragers return to the nest, more ants are available to become outgoing foragers, which then return to the nest with food, becoming available to leave the nest again. Similarly, there is positive feedback between forager return rate and forager activation: the more foragers that return, the more interactions that outgoing foragers experience, and the faster they become outgoing foragers, which return with food to activate more foragers. The negative feedback that stabilizes this is the link between forager availability and forager activation: as more foragers become activated, fewer are available to be stimulated by returning foragers. Because the pool of foragers is finite, the negative feedback between the shorter- and longer-term processes of forager activation and forager availability prevent the numbers of foragers from increasing indefinitely in response to the positive feedback due to forager return (Fig. 1). Thus, the system has both flexibility, in response to short-term changes in food supply, and resilience to sustained disturbances, provided by linked positive and negative feedback.
Direct observations inside the nest show that the rate of forager return corresponds to the rate of interaction that available foragers experience inside the nest (Fig. 4). While this was implied by previous work (Gordon 2002; Greene & Gordon 2007; Greene & Gordon 2003; Gordon et al. 2008, 2011), to our knowledge we provide the first direct evidence of a relationship between forager return rate and interaction rate inside nests of ant colonies in natural conditions. Other studies using observation hives in laboratory settings have demonstrated a relation between forager return rate and interaction with foragers inside the nest in wasps (O’Donnell 2001) and honeybees (Fernández et al. 2003; Gruter & Farina 2009; Balbuena et al. 2012) but not in nests found in the field.
The relation between interaction rate and forager return rate depends on ant density in the vestibule (Fig. 3); it appears that the more ants that are available to interact, the higher the interaction rate. However, interaction rate divided by the number of ants in the vestibule also increased with forager return rate (Fig. 4), suggesting that other factors, in addition to ant density, contribute to the positive relation of interaction rate and forager return rate. For example, ants may modify the way they move when forager return rate is high, to increase interaction rate (Gordon et al. 1993; Pinter-Wollman et al. 2011).
An outgoing forager’s rate of interaction, on the timescale of seconds, is associated with its decision to leave the nest. A forager leaves the nest about 3–8 s after its interaction rate has substantially increased (Table 2, Fig. 5). It is during this 3–8 s interval that forager return rate has the strongest effect on interaction rate (Fig. 7). Our results suggest that there is a threshold interaction rate that an ant must experience before it leaves the nest, just as response thresholds determine worker activation in other social insect species (Robinson 1987; Theraulaz et al. 1998; Beshers et al. 1999). In this study, we observed only those ants that eventually left the nest to forage, so further work is needed to determine whether ants that experienced fewer interactions were less likely to leave the nest.
An outgoing forager’s location in the nest appears to influence its interaction rate. There is an interaction hotspot near the exit from the nest, and another, as observed in laboratory colonies (Pinter-Wollman et al. 2011), near the opening of the tunnel leading further into the nest. The location of these interaction hotspots did not depend on forager return rate (Fig. 6). Because nest structure influences how ants move around (Burd et al. 2010) and thus where and how often they interact (Adler & Gordon 1992; Pinter-Wollman et al. 2011), the architecture of the nest probably influences the regulation of foraging.
Foragers tend to leave the nest after a substantial increase in their rate of interaction with returning foragers. These interactions with returning foragers seem to have a greater impact on a forager’s decision to leave than do interactions with other ants (Fig. 5), as suggested by other studies (Schafer et al. 2006; Greene et al. 2013). Foragers left the vestibule immediately after an increase in interaction rate with returning foragers but not after an increase in interaction rate with other ants in the vestibule (Fig. 5). Surprisingly, even though interactions with returning foragers increased close to the nest exit (Fig. S3), there were as many interactions with other ants, relative to interactions with returning foragers, near the nest exit as there were anywhere else in the vestibule (Fig. S1). Interactions among task groups are important in task allocation (Gordon & Mehdiabadi 1999), but our results indicate that interactions between outgoing foragers and any other task group have a smaller effect than interactions with returning foragers on the rate at which foragers are activated to leave the nest once foraging has begun.
Our results show that foraging is regulated, on the timescale of minutes, through a second, previously undescribed, process that determines the availability of outgoing foragers in the vestibule to meet returning foragers. How long ants linger in the vestibule depends on the rate at which foragers return to the nest (Fig. 3). Similarly, workers of honeybees (Anderson & Ratnieks 1999) and wasps (Jeanne 1986) queue near their nest entrance to receive food or building material from returning workers; how long they queue depends on the time it takes a returning worker to off-load its food or building material to another worker (Anderson & Ratnieks 1999). Waiting times of outgoing harvester ant foragers in the vestibule may depend on the time it takes each outgoing forager to reach the interaction threshold that stimulates it to leave the nest. When no foragers were leaving the nest, the numbers of ants in the vestibule eventually decreased (Fig. 3b, c), showing that ants tend to descend deeper into the nest possibly to seek higher humidity and limit the risks of desiccation (Lighton & Feener 1989). Thus, by regulating the number of ants available in the vestibule, a colony balances its response to various environmental conditions such as humidity, food availability and predation.
The number of ants available in the vestibule affects how quickly a colony can increase the rate at which outgoing foragers leave the nest. We found that foraging resumed more quickly when foragers were allowed to return after 3 min removals than after 10 min removals (Fig. 8). It is possible that foragers did not travel as far down into the nest during the shorter interruption and thus were quicker to reach the nest exit and interact with returning foragers.
Many processes contribute to variation among colonies in the regulation of foraging (Gordon 1991; Pinter-Wollman 2012). Colonies of P. barbatus vary in foraging activity (Gordon et al. 2011) as do other species of harvester ants (Cole et al. 2010; Pinter-Wollman et al. 2012). Other work suggests that colonies vary in how much each interaction with a returning forager affects the likelihood that an outgoing forager will leave the nest (Prabhakar et al. 2012; Gordon et al. 2013). We found that the behaviour of colony 977 differed slightly from that of the other two colonies, 486 and 672, in experiment 1. Its forager return rate was low and its interaction rate per ant did not increase with forager return rate. Low forager return rate may have led the ants to go deeper into the nest in this colony, or its nest structure may have made the vestibule less accessible to foragers (Pinter-Wollman et al. 2012).
Here we show that simple local interactions effectively mediate processes that provide flexibility on the timescale of seconds and robustness on the timescale of minutes. By coordinating positive and negative feedback that link processes occurring at multiple timescales, ant colonies, like other complex biological systems, can regulate their behavioural response to changing environments.
Supplementary Material
Acknowledgments
This work was funded by a National Science Foundation (NSF) Postdoctoral Fellowship in Biological Informatics to N.P.W., a National Institutes of Health (NIH) grant (5-R01GM086884) to S.H. and an NSF grant IOS-0718631 to D.M.G. We thank Lis Nelis and Roy Wollman for helpful discussion, and Mike Greene and Mark Longo for comments on the manuscript.
Footnotes
Supplementary Material
Supplementary material for this article is available, in the online version, at http://dx.doi.org/10.1016/j.anbehav.2013.05.012.
References
- Adler FR, Gordon DM. Information collection and spread by networks of patrolling ants. American Naturalist. 1992;140:373–400. doi: 10.1086/285418. [DOI] [PubMed] [Google Scholar]
- Alon U. An Introduction to Systems Biology: Design Principles of Biological Circuits. Chapman & Hall; London: 2006. [Google Scholar]
- Anderson C, Ratnieks FLW. Task partitioning in insect societies. I. Effect of colony size on queueing delay and colony ergonomic efficiency. American Naturalist. 1999;154:521–535. doi: 10.1086/303255. [DOI] [PubMed] [Google Scholar]
- Balbuena MS, Molinas J, Farina WM. Honeybee recruitment to scented food sources: correlations between in-hive social interactions and foraging decisions. Behavioral Ecology and Sociobiology. 2012;66:445–452. [Google Scholar]
- Beshers SN, Robinson GE, Mittenthal J. Response thresholds and division of labor in insect colonies. In: Detrain C, Deneubourg J-L, Pasteels JM, editors. Information Processing in Social Insects. Birkhauser Verlag; Basel: 1999. pp. 115–139. [Google Scholar]
- Beverly BD, McLendon H, Nacu S, Holmes S, Gordon DM. How site fidelity leads to individual differences in the foraging activity of harvester ants. Behavioral Ecology. 2009;20:633–638. [Google Scholar]
- Brandman O, Meyer T. Feedback loops shape cellular signals in space and time. Science. 2008;322:390–395. doi: 10.1126/science.1160617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd M, Shiwakoti N, Sarvi M, Rose G. Nest architecture and traffic flow: large potential effects from small structural features. Ecological Entomology. 2010;35:464–468. [Google Scholar]
- Camazine S, Visscher PK, Finley J, Vetter RS. House-hunting by honey bee swarms: collective decisions and individual behaviors. Insectes Sociaux. 1999;46:348–360. [Google Scholar]
- Cannon WB. Organization for physiological homeostasis. Physiological Reviews. 1929;9:399–431. [Google Scholar]
- Cole BJ, Smith AA, Huber ZJ, Wiernasz DC. The structure of foraging activity in colonies of the harvester ant, Pogonomyrmex occidentalis. Behavioral Ecology. 2010;21:337–342. [Google Scholar]
- Cowpertwait PSP, Metcalfe AV. Introductory Time Series with R. Springer; New York: 2009. [Google Scholar]
- DeDeo S, Krakauer D, Flack J. Evidence of strategic periodicities in collective conflict dynamics. Journal of the Royal Society Interface. 2011;8:1260–1273. doi: 10.1098/rsif.2010.0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feener DH, Lighton JRB. Is foraging in the desert ant, Messor pergandei (Hymenoptera, Formicidae), limited by water. Ecological Entomology. 1991;16:183–191. [Google Scholar]
- Fernández PC, Gil M, Farina WM. Reward rate and forager activation in honeybees: recruiting mechanisms and temporal distribution of arrivals. Behavioral Ecology and Sociobiology. 2003;54:80–87. [Google Scholar]
- Flack JC. Multiple time-scales and the developmental dynamics of social systems. Philosophical Transactions of the Royal Society B. 2012;367:1802–1810. doi: 10.1098/rstb.2011.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon DM. Behavioral flexibility and the foraging ecology of seed-eating ants. American Naturalist. 1991;138:379–411. [Google Scholar]
- Gordon DM. How colony growth affects forager intrusion between neighboring harvester ant colonies. Behavioral Ecology and Sociobiology. 1992;31:417–427. [Google Scholar]
- Gordon DM. The spatial scale of seed collection by harvester ants. Oecologia. 1993;95:479–487. doi: 10.1007/BF00317431. [DOI] [PubMed] [Google Scholar]
- Gordon DM. The regulation of foraging activity in red harvester ant colonies. American Naturalist. 2002;159:509–518. doi: 10.1086/339461. [DOI] [PubMed] [Google Scholar]
- Gordon DM, Dektar KN, Pinter-Wollman N. Harvester ant colony variation in foraging activity and response to humidity. PLoS One. 2013;8:e63363. doi: 10.1371/journal.pone.0063363. http://dx.doi.org/10.1371/journal.pone.0063363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon DM, Kulig AW. Founding, foraging, and fighting: colony size and the spatial distribution of harvester ant nests. Ecology. 1996;77:2393–2409. [Google Scholar]
- Gordon DM, Mehdiabadi NJ. Encounter rate and task allocation in harvester ants. Behavioral Ecology and Sociobiology. 1999;45:370–377. [Google Scholar]
- Gordon DM, Paul RE, Thorpe K. What is the function of encounter patterns in ant colonies? Animal Behaviour. 1993;45:1083–1100. [Google Scholar]
- Gordon DM, Holmes S, Nacu S. The short-term regulation of foraging in harvester ants. Behavioral Ecology. 2008;19:217–222. doi: 10.1093/beheco/arq218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon DM, Guetz A, Greene MJ, Holmes S. Colony variation in the collective regulation of foraging by harvester ants. Behavioral Ecology. 2011;22:429–435. doi: 10.1093/beheco/arq218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene MJ, Gordon DM. Cuticular hydrocarbons inform task decisions. Nature. 2003;423:32. doi: 10.1038/423032a. [DOI] [PubMed] [Google Scholar]
- Greene MJ, Gordon DM. Interaction rate informs harvester ant task decisions. Behavioral Ecology. 2007;18:451–455. [Google Scholar]
- Greene MJ, Pinter-Wollman N, Gordon DM. Interactions with combined chemical cues inform harvester ant foragers’ decisions to leave the nest in search of food. PLoS One. 2013;8:e52219. doi: 10.1371/journal.pone.0052219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruter C, Farina WM. Past experiences affect interaction patterns among foragers and hive-mates in honeybees. Ethology. 2009;115:790–797. [Google Scholar]
- Jeanne RL. The organization of work in Polybia occidentalis: costs and benefits of specialization in a social wasp. Behavioral Ecology and Sociobiology. 1986;19:333–341. [Google Scholar]
- Lighton JRB, Feener DH. Water-loss rate and cuticular permeability in foragers of the desert ant Pogonomyrmex rugosus. Physiological Zoology. 1989;62:1232–1256. [Google Scholar]
- Lucas JR. The role of foraging time constraints and variable prey encounter in optimal diet choice. American Naturalist. 1983;122:191–209. [Google Scholar]
- Munger JC. Long-term yield from harvester ant colonies: implications for horned lizard foraging strategy. Ecology. 1984;65:1077–1086. [Google Scholar]
- O’Donnell S. Worker biting interactions and task performance in a swarm-founding eusocial wasp (Polybia occidentalis, Hymenoptera: Vespidae) Behavioral Ecology. 2001;12:353–359. [Google Scholar]
- Pinter-Wollman N. Personality in social insects: how does worker personality determine colony personality? Current Zoology. 2012;58:579–587. [Google Scholar]
- Pinter-Wollman N, Wollman R, Guetz A, Holmes S, Gordon DM. The effect of individual variation on the structure and function of interaction networks in harvester ants. Journal of the Royal Society Interface. 2011;8:1562–1573. doi: 10.1098/rsif.2011.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter-Wollman N, Gordon DM, Holmes S. Nest site and weather affect the personality of harvester ant colonies. Behavioral Ecology. 2012;23:1022–1029. doi: 10.1093/beheco/ars066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar B, Dektar KN, Gordon DM. The regulation of ant colony foraging activity without spatial information. PLoS Computational Biology. 2012;8:e1002670. doi: 10.1371/journal.pcbi.1002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt SC. Quorum sensing by encounter rates in the ant Temnothorax albipennis. Behavioral Ecology. 2005;16:488–496. [Google Scholar]
- Robinson EJH, Feinerman O, Franks NR. Flexible task allocation and the organization of work in ants. Proceedings of the Royal Society B. 2009;276:4373–4380. doi: 10.1098/rspb.2009.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GE. Modulation of alarm pheromone perception in the honey bee: evidence for division of labor based on hormonally regulated response thresholds. Journal of Comparative Physiology A. 1987;160:613–619. [Google Scholar]
- Schafer RJ, Holmes S, Gordon DM. Forager activation and food availability in harvester ants. Animal Behaviour. 2006;71:815–822. doi: 10.1016/j.anbehav.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider SS, Stamps JA, Gary NE. The vibration dance of the honeybee 1. Communication regulating foraging on 2 timescales. Animal Behaviour. 1986;34:377–385. [Google Scholar]
- Sonnentag PJ, Jeanne RL. Initiation of absconding-swarm emigration in the social wasp Polybia occidentalis. Journal of Insect Science. 2009;9:11. doi: 10.1673/031.009.1101. www.insectscience.org/9.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suraci JP, Dill LM. Short timescale rate maximization by gulls and implications for predation on size-structured prey. Behavioral Ecology. 2013;24:280–292. [Google Scholar]
- Theraulaz G, Bonabeau E, Deneubourg JL. Response threshold reinforcement and division of labour in insect societies. Proceedings of the Royal Society B. 1998;265:327–332. [Google Scholar]
- Toms JD, Lesperance ML. Piecewise regression: a tool for identifying ecological thresholds. Ecology. 2003;84:2034–2041. [Google Scholar]
- Zupanc GKH. Behavioral Neurobiology. Oxford University Press; New York: 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.