Abstract
Chronic kidney disease (CKD) is associated with increased morbidity and mortality in coronary artery disease (CAD) patients. We compared the economic attractiveness of CAD revascularization procedures in patients with and without CKD. Our population included 6218 patients with significant CAD undergoing cardiac catheterization at Duke University between 1996 and 2001, with follow-up through 2002. We investigated the influence of CKD (creatinine clearance < 60 mL/min) upon 3-year survival and medical costs in our CAD population. Coronary artery bypass graft (CABG) surgery was an economically attractive alternative vs. percutaneous coronary intervention (PCI) or medical therapy for all patients with left main disease, three-vessel CAD patients without CKD, and two-vessel CAD patients with CKD. Medical therapy was an economically attractive strategy vs. CABG surgery or PCI for three-vessel CAD patients with CKD, two-vessel CAD patients without CKD, and all single-vessel CAD patients.
Keywords: Cost-benefit analysis, Revascularization, Coronary disease, Kidney disease
Introduction
Coronary artery disease (CAD) is associated with significant morbidity and mortality in chronic kidney disease (CKD) patients. However, CKD’s impact upon the medical costs and survival benefits associated with specific CAD treatment strategies is not known.
Clinical trials and observational studies have shown that CAD revascularization procedures (i.e., coronary artery bypass graft [CABG] surgery and percutaneous coronary intervention [PCI]) improve clinical outcomes in select patient groups. The ACC/AHA guidelines for coronary artery bypass graft surgery concluded that the primary survival benefits of CABG surgery versus Medical Therapy (MED) are in patients with left main, left main equivalent, or three-vessel CAD [1]. They also report a survival benefit for PCI versus CABG surgery in patients with single-vessel, non-left anterior descending (LAD) disease; and a survival benefit for CABG surgery versus PCI in patients with proximal LAD and in all patients with three-vessel LAD disease [2]. The ACC/AHA guidelines for PCI also recommend CABG surgery vs. PCI for multi-vessel CAD patients with diabetes, but recommend MED for the initial management of patients without severe symptoms and ischemia [3].
Recent studies have demonstrated that the presence and severity of CKD is associated with increased morbidity and mortality in CAD patients; and that this condition may alter the relative survival benefits associated with CAD revascularization procedures [4–6]. However, it is not know whether CKD significantly impacts the economic attractiveness (i.e., relative medical cost-survival benefit trade-offs) associated with CAD treatment strategies. We compared the economic attractiveness of CAD revascularization procedures in patients with and without CKD.
Materials and methods
Study population
Our clinical population included patients undergoing cardiac catheterization at Duke University Medical Center between July 1, 1996 and December 31, 2001, with follow-up through December 31, 2002. We included patients with significant coronary artery disease (≥75% stenosis in one or more major epicardial coronary segment) and excluded those with obstructive or restrictive cardiomyopathy, hemodynamic instability, valvular or congenital heart disease, or end-stage renal disease. All patients provided informed consent and study protocol approval was obtained from the Duke University Medical Center Institutional Review Board. In addition, deaths were reviewed by an independent mortality committee.
CKD-CAD strata
We divided the population into CKD-CAD strata based upon extent of chronic kidney and coronary artery disease. First, we calculated creatinine clearance using the Cockcroft-Gault equation and used this information to stratify the population into two CKD groups: patients without CKD (creatinine clearance ≥ 60 mL/min) and patients with CKD (creatinine clearance < 60 mL/min) [6, 7]. Next, we divided each CKD strata into four CAD substrata. The CAD substrata were patients with left main CAD, and those without left main disease who had one-, two-, or three-vessel CAD.
CAD treatment assignments
Patients were assigned to one of three CAD treatment groups (CABG surgery, PCI, or MED) using previously defined criteria [6]. CABG surgery and PCI group assignment designations were based upon the patient’s first revascularization procedure occurring within 30 days of their index cardiac catheterization. Patients without revascularization procedures at 30 days were assigned to the MED group. MED patients dying within 5 days of their index catheterization were excluded to avoid bias from their not having the opportunity to undergo revascularization.
Data collection
Clinical events
Baseline patient characteristics and resource use information were collected prospectively by cardiology fellows at the time of the index cardiac catheterization and recorded in the Duke Information System for Clinical Computing (DISCC) [8, 9]. Administrative information (e.g., length of stay, billing codes, admission/discharge dates) relating to the index and follow-up episodes of care at Duke University Medical Center was recorded in the Duke Hospital Information System. We also collected follow-up information on deaths and four types of non-Duke hospitalizations (i.e., myocardial infarction, CABG surgery, PCI procedure, and other hospitalization). All patients were contacted initially at 6 months after their index procedure, 1 year after their index procedure, and annually thereafter [10–12]. Follow-up was 95% complete.
Inpatient costs
All cost information (baseline and follow-up) used in this study came from Duke University Medical Center’s Transition Cost Accounting System. Transition is a bottom-up system that estimates aggregate patient costs as the sum of their component intermediate product costs [13]. Because cost information was not available for each episode of care, we developed two resource-based regression models from Duke’s cost accounting database to impute hospital costs for patients with missing financial information for hospitalizations at baseline (R2=0.93), and during the follow-up period (R2=0.88) [13].
Endpoints
We assessed 3-year survival and cumulative inpatient costs by CAD treatment strategy within each of the CKD-CAD strata. Endpoints were assessed by comparing survival and cumulative medical cost differences between pairings of more and less invasive CAD treatment strategies. In these analyses, CABG surgery was considered more invasive than PCI and PCI more invasive than MED. We prospectively excluded left main CAD patients undergoing PCI therapy and single-vessel CAD patients undergoing CABG surgery from our analyses as these therapeutic options were underrepresented in our data. Thus, while we considered all three CAD treatment strategies for multi-vessel CAD, we only compared PCI vs. MED for single-vessel disease and CABG surgery vs. MED for left main disease.
Data analysis
Baseline characteristics
Baseline characteristics are presented as percentages for discrete variables and as medians with 25th and 75th percentiles for continuous variables. Differences between dichotomous variables were tested using the chi-square statistic, and differences between continuous variables were assessed using the Kruskal–Wallis test.
Survival analysis
Unadjusted Kaplan–Meier 3-year survival estimates for CAD treatment strategies were generated by CKD group. We then used the Anstrom-Tsiatis method to estimate adjusted 3-year survival by CAD treatment strategy within each CKD-CAD strata [14, 15]. We also estimated mean between-treatment-group survival differences for each CAD-CKD strata based on 1000 bootstrap datasets, estimated 90% confidence intervals (CI), and calculated the percent of samples in which the more invasive therapy was life saving versus the less invasive therapy.
Event and cost analysis
We also used the Anstrom–Tsiatis method as described above in our event and medical cost analyses. To more efficiently make use of our event and cost data, we partitioned the follow-up period into non-overlapping intervals and estimated the mean response and standard error within each interval. These results were then summed to create overall estimates of events and costs for the entire 3-year follow-up period. After calculating 3-year cumulative medical cost estimates by CAD treatment strategy and CKD-CAD strata, we then estimated mean cost differences between treatment strategies using 1000 bootstrap data sets, estimated 90% confidence intervals for the cost difference, and calculated the percent of samples in which the more invasive strategy was cost-saving.
Economic attractiveness analysis
Three situations may arise in an economic attractiveness analysis (See Fig. 1). When the more invasive CAD treatment strategy is associated with reduced survival, it is described as dominated; when it is associated with an increase in survival and a decrease in medical costs, it is dominant; and when it is associated with an increase in both survival and medical costs, its cost vs. survival benefit is assessed using a cost-effectiveness ratio (incremental discounted costs divided by the incremental discounted survival, using a 3% discount factor) [13]. By conventional standards, therapies costing >$50,000/year of life saved (YOLS) are considered cost-effective, therapies costing >$50,000/YOLS but ≤$150,000/YOLS are in the zone of economic uncertainty, and therapies costing > $150,000/YOLS are economically unattractive [13]. We estimated the median economic attractiveness (incremental medical costs divided by incremental survival) for CAD treatment strategy pairs in each CKD-CAD strata using 1000 bootstrap data sets.
Fig. 1.
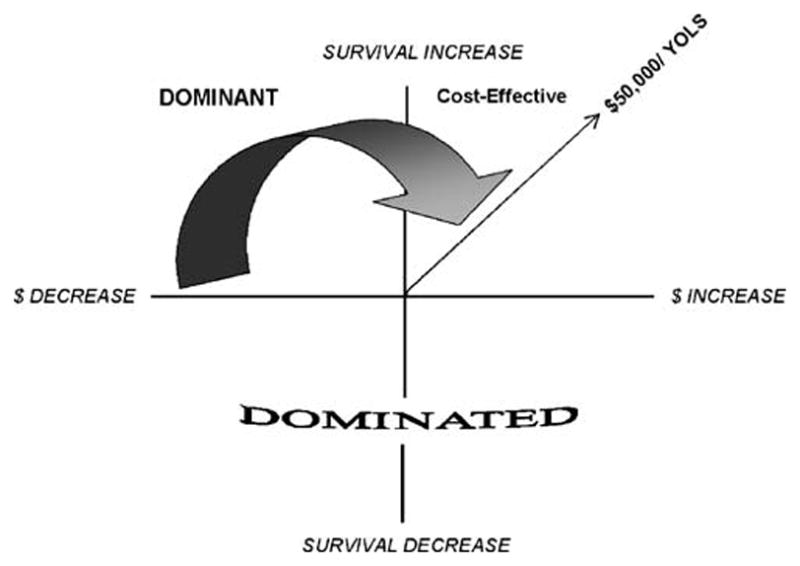
Economic attractiveness analysis categories. Year of life saved (YOLS)
Stability analysis
We evaluated the stability of our economic attractiveness estimates by calculating the percent of bootstrap samples for which the point estimates for incremental survival and incremental costs indicated that the more invasive therapy was dominant, cost-effective, or dominated when compared with the less invasive therapy. In this analysis, the dominated category shows the clinical risk (i.e., percent of times the more invasive therapy is life-taking) while the dominant and cost-effective categories show the clinical and economic benefit (i.e., percent of times the more invasive therapy is life-saving at an acceptable cost to society).
Results
Baseline characteristics
Baseline patient characteristics and resource use for the 6218 study patients (4759 without CKD and 1459 with CKD) are in Table 1 and 3-year cardiac event rates are in Table 2.
Table 1.
Patient characteristics, resource use, and treatments (unadjusted)
| Characteristics | Normal–mild kidney disease
|
Moderate-severe kidney disease
|
||||||
|---|---|---|---|---|---|---|---|---|
| Medical N=4,759 | PCI | CABG | P-value | Medical N=1,459 | PCI | CABG | P-value | |
| Number | 1,108 | 2,221 | 1,430 | 528 | 462 | 469 | ||
| Demographics | ||||||||
| Age, median (25th, 75th percentiles) | 60.8 (53.2, 69.0) | 57.41 (50.3, 65.5) | 62.3 (54.9, 69.5) | <0.001 | 75.3 (68.0, 80.3) | 74.9 (68.9, 79.9) | 74.2 (68.8, 78.5) | 0.123 |
| Caucasian race (%) | 63.8 | 72.3 | 78.1 | <0.001 | 63.8 | 71.8 | 73.9 | 0.001 |
| Female gender (%) | 28.1 | 26.1 | 20.4 | <0.001 | 54.1 | 58.4 | 42.0 | <0.001 |
| Chronic Kidney Disease | ||||||||
| Creatinine clearance, median (25th, 75th percentiles) | 91.4 (75.5, 115) | 99.6 (80.0, 123) | 91.2 (75.7, 113) | <0.001 | 42.7 (32.7, 52.5) | 47.7 (39.3, 54.7) | 48.0 (38.9, 54.1) | <0.001 |
| CAD risk factors | ||||||||
| Diabetes mellitus (%) | 37.4 | 24.6 | 30.7 | <0.001 | 37.3 | 27.7 | 27.5 | 0.001 |
| Hypertension (%) | 65.0 | 57.9 | 60.7 | 0.003 | 76.8 | 70.5 | 73.1 | 0.074 |
| Hyperlipidemia (%) | 53.0 | 55.3 | 57.2 | 0.107 | 50.5 | 49.7 | 53.0 | 0.570 |
| CAD history | ||||||||
| Congestive heart failure (%) | 31.5 | 11.7 | 15.1 | <0.001 | 44.7 | 22.5 | 26.0 | <0.001 |
| Myocardial infarction (%) | 53.0 | 62.2 | 50.8 | <0.001 | 50.7 | 57.5 | 52.4 | 0.083 |
| Cerebrovascular disease (%) | 13.9 | 7.0 | 9.0 | <0.001 | 21.9 | 12.9 | 18.1 | 0.001 |
| Peripheral vascular disease (%) | 14.3 | 8.1 | 11.0 | <0.001 | 23.1 | 13.4 | 18.7 | <0.001 |
| CAD severity | ||||||||
| Number diseased vessels (%) | ||||||||
| 1 | 45.1 | 55.6 | 7.9 | <0.001 | 31.4 | 47.6 | 4.6 | <0.001 |
| 2 | 26.4 | 33.5 | 26.7 | 28.6 | 36.1 | 20.4 | ||
| 3 | 28.4 | 10.8 | 65.3 | 38.3 | 15.9 | 74.8 | ||
| Left main disease (%) | 2.7 | 0.3 | 17.8 | <0.001 | 9.0 | 0.6 | 20.9 | <0.001 |
| Ejection fraction, median (25th, 75th percentiles) | 52.7 (39.3, 61.9) | 56.4 (47.8, 63.8) | 55.1 (44.2, 63.5) | <0.001 | 50.0 (32.9, 60.2) | 55.3 (43.7, 64.6) | 52.0 (40.2, 62.5) | <0.001 |
PCI Percutaneous coronary intervention, CABG coronary artery bypass graft
Table 3.
Adjusted 3-year survival by chronic kidney disease and coronary artery disease treatment group
| CAD Group | 1 VD | 2 VD | 3 VD | LMD |
|---|---|---|---|---|
| Normal–mild CKD | ||||
| PCI vs. medical (years) | ||||
| PCI | 2.898 | 2.840 | 2.713 | – |
| Medical | 2.841 | 2.782 | 2.551 | – |
| Difference | 0.057 | 0.058 | 0.162 | – |
| 90% CI | 0.004; 0.109 | −0.018; 0.138 | 0.014; 0.308 | – |
| PCI life-saving (%) | 96.8 | 88.9 | 97.0 | – |
| CABG vs. medical (years) | ||||
| CABG | – | 2.806 | 2.818 | 2.845 |
| Medical | – | 2.768 | 2.489 | 2.246 |
| Difference | – | 0.038 | 0.329 | 0.599 |
| 90% CI | – | −0.065; 0.137 | 0.212; 0.458 | 0.191; 1.120 |
| CABG life-saving (%) | – | 73.3 | 100.0 | 99.6 |
| CABG vs. PCI (years) | ||||
| CABG | – | 2.802 | 2.825 | – |
| PCI | – | 2.852 | 2.727 | – |
| Difference | – | −0.050 | 0.098 | – |
| 90% CI | – | −0.136; 0.034 | −0.030; 0.232 | – |
| CABG life-saving (%) | – | 17.1 | 89.6 | – |
| Moderate-severe CKD | ||||
| PCI vs. medical (years) | ||||
| PCI | 2.643 | 2.555 | 2.170 | – |
| Medical | 2.583 | 2.488 | 2.167 | – |
| Difference | 0.060 | 0.067 | 0.003 | – |
| 90% CI | −0.127; 0.234 | −0.108; 0.261 | −0.331; 0.350 | – |
| PCI life-saving (%) | 69.4 | 73.5 | 54.3 | – |
| CABG vs. medical (years) | ||||
| CABG | – | 2.786 | 2.540 | 2.610 |
| Medical | – | 2.426 | 2.266 | 1.881 |
| Difference | – | 0.360 | 0.274 | 0.729 |
| 90% CI | – | 0.162; 0.571 | 0.084; 0.514 | 0.302; 1.182 |
| CABG life-saving (%) | – | 99.7 | 99.0 | 99.9 |
| CABG vs. PCI (years) | ||||
| CABG | – | 2.834 | 2.569 | – |
| PCI | – | 2.583 | 2.162 | – |
| Difference | – | 0.251 | 0.407 | – |
| 90% CI | – | 0.077; 0.407 | 0.057; 0.780 | – |
| CABG life-saving (%) | – | 98.8 | 97.3 | – |
CAD Coronary artery disease, VD ventricular disease, LMD left main disease, CKD chronic kidney disease
Table 2.
3-year cardiac event rates by chronic kidney disease and coronary artery disease treatment group
| Cardiac events | Normal–mild kidney disease
|
Moderate–severe kidney disease
|
||||
|---|---|---|---|---|---|---|
| Medical | PCI | CABG | Medical | PCI | CABG | |
| Acute (1–30 Days) | ||||||
| Death | 0.024 | 0.010 | 0.015 | 0.047 | 0.045 | 0.038 |
| CABG | 0.002 | 0.017 | 0.999 | 0.006 | 0.011 | 0.998 |
| PCI | 0.029 | 1.001 | 0.004 | 0.047 | 1.009 | 0.013 |
| Other | 1.045 | 0.108 | 0.202 | 1.108 | 0.149 | 0.262 |
| Total | 1.100 | 1.289 | 1.220 | 1.208 | 1.240 | 1.311 |
| 31 Days–1 Year | ||||||
| Death | 0.073 | 0.025 | 0.029 | 0.162 | 0.074 | 0.066 |
| CABG | 0.046 | 0.053 | 0.002 | 0.062 | 0.022 | 0.002 |
| PCI | 0.034 | 0.153 | 0.053 | 0.027 | 0.114 | 0.046 |
| Other | 0.603 | 0.465 | 0.431 | 0.752 | 0.642 | 0.687 |
| Total | 0.756 | 0.696 | 0.515 | 1.003 | 0.852 | 0.801 |
| 1–2 Years | ||||||
| Death | 0.057 | 0.025 | 0.025 | 0.094 | 0.042 | 0.046 |
| CABG | 0.018 | 0.016 | 0.001 | 0.009 | 0.010 | 0.003 |
| PCI | 0.032 | 0.067 | 0.029 | 0.013 | 0.059 | 0.026 |
| Other | 0.444 | 0.331 | 0.278 | 0.370 | 0.359 | 0.351 |
| Total | 0.551 | 0.439 | 0.333 | 0.486 | 0.470 | 0.426 |
| 2–3 Years | ||||||
| Death | 0.072 | 0.017 | 0.021 | 0.098 | 0.054 | 0.038 |
| CABG | 0.012 | 0.013 | 0.002 | 0.000 | 0.007 | 0.000 |
| PCI | 0.027 | 0.044 | 0.022 | 0.030 | 0.034 | 0.016 |
| Other | 0.382 | 0.306 | 0.256 | 0.323 | 0.299 | 0.433 |
| Total | 0.493 | 0.370 | 0.030 | 0.766 | 0.387 | 0.500 |
| Total | ||||||
| Death | 0.226 | 0.078 | 0.090 | 0.401 | 0.215 | 0.189 |
| CABG | 0.078 | 0.098 | 1.004 | 0.076 | 0.050 | 1.003 |
| PCI | 0.121 | 1.266 | 0.107 | 0.117 | 1.216 | 0.100 |
| Other | 2.473 | 1.201 | 1.170 | 2.565 | 1.442 | 1.746 |
| Total | 2.898 | 2.643 | 2.371 | 3.159 | 2.923 | 3.038 |
PCI Percutaneous coronary intervention, CABG coronary artery bypass graft
Patients with CKD
Across all CAD treatment strategies, patients with CKD were older and more frequently female, with greater cardiovascular disease severity than patients without CKD. In particular, they had greater incidence of congestive heart failure, with more vascular disease (cerebrovascular or peripheral vascular), multi-vessel CAD, left main disease, and 30-day mortality (Table 2). At 3-years follow-up they also had more total events, and their 3-year unadjusted survival was less than that for patients without CKD.
Across both CKD Groups
Within both CKD groups, patients assigned to MED were more frequently minorities with a greater incidence of congestive heart failure and vascular disease than patients undergoing revascularization. Patients undergoing PCI procedures had the least severe cardiovascular disease (i.e., better cardiac profile with more frequent one and two-vessel disease); whereas patients assigned to CABG surgery had more severe CAD (i.e., 3-vessel and left main disease) than those undergoing PCI. Patients in both CKD groups had similar 30-day PCI and CABG surgery rates. In both CKD groups, survival for patients receiving MED was much less than that for patients receiving CABG surgery and PCI.
Adjusted 3-year survival
Three-year adjusted survival differences among study patients were driven by differences in the extent of CKD and CAD, and by CAD treatment assignments (Table 3). Generally, patients with CKD and/or more severe CAD had greater mortality than patients without these conditions. And, while the use of more invasive treatment strategies produced modest 3-year survival gains over less invasive treatments in most CKD-CAD strata, those gains frequently did not reach statistical significance (i.e., were <95% life-saving).
PCI vs. MED
In both CKD groups, patients with less severe CAD had greater 3-year survival than patients with more severe disease. The survival benefit of PCI vs. MED was modest in most CKD-CAD strata, with the greatest difference occurring in three-vessel CAD patients without CKD.
CABG vs. MED
Survival for MED patients diminished with greater extent of CAD while that for CABG surgery patients was relatively constant. CABG surgery conferred a significant survival benefit vs. MED in patients with three-vessel or left main CAD in both CKD groups, and in two-vessel CAD patients with CKD.
CABG vs. PCI
In both CKD groups, survival for PCI patients was lower in three- vs. two-vessel CAD. And while CABG patient survival was relatively constant in all multi-vessel CAD patients without CKD, it diminished in three- as compared with two-vessel CAD patients with CKD. Among patients without CKD, PCI was associated with a modest survival benefit in two-vessel and CABG surgery was associated with a similarly modest survival benefit in 3-vessel CAD patients. However, CABG surgery had a significant survival benefit vs. PCI in multi-vessel CAD patients with CKD.
Adjusted 3-year inpatient costs
As with survival, 3-year medical cost differences were largely driven by differences in the extent of CKD and CAD and by differences in CAD treatment assignments (Table 4). Patients with CKD and more severe CAD generally had higher medical costs than patients without these conditions. Although more invasive treatment strategies were typically more expensive than less invasive strategies (i.e., < 50% cost-saving) in most CKD-CAD strata, these differences frequently were not statistically significant (i.e., < 5% cost-saving).
Table 4.
Adjusted 3-year inpatient costs by chronic kidney disease and coronary artery disease treatment group
| CAD Group | 1 VD | 2 VD | 3 VD | LMD |
|---|---|---|---|---|
| Normal-mild CKD | ||||
| PCI vs. medical ($) | ||||
| PCI | 22,173 | 30,887 | 36,930 | – |
| Medical | 19,721 | 23,996 | 31,337 | – |
| Difference | 7,452 | 6,891 | 5,593 | – |
| 90% CI | 3,688; 10,767 | 2,294; 11,386 | −703; 11,267 | – |
| Percent PCI cost-saving | 0.3 | 1.2 | 7.1 | – |
| CABG vs. medical ($) | ||||
| CABG | – | 35,509 | 36,949 | 41,559 |
| Medical | – | 25,509 | 31,586 | 26,068 |
| Difference | – | 9,999 | 5,363 | 15,491 |
| 90% CI | – | 4,972; 15,115 | 266; 10,391 | 6,285; 23,902 |
| Percent CABG cost-saving | – | 0.1 | 4.4 | 0.7 |
| CABG vs. PCI ($) | ||||
| CABG | – | 33,673 | 36,204 | – |
| PCI | – | 30,594 | 37,765 | – |
| Difference | – | 3,079 | −1,561 | – |
| 90% CI | – | 410; 5,676 | −6,338; 3,071 | – |
| Percent CABG cost-saving | – | 2.9 | 66.3 | – |
| Moderate–severe CKD | ||||
| PCI vs. medical ($) | ||||
| PCI | 30,570 | 35,399 | 34,699 | – |
| Medical | 25,648 | 38,775 | 35,486 | – |
| Difference | 4,922 | −3,375 | −787 | – |
| 90% CI | −428; 10,128 | −14,824; 5,852 | −9,073; 6,689 | – |
| Percent PCI cost-saving | 6.6 | 69.0 | 55.0 | – |
| CABG vs. medical ($) | ||||
| CABG | – | 45,328 | 59,127 | 47,331 |
| Medical | – | 40,846 | 35,863 | 46,781 |
| Difference | – | 4,482 | 23,264 | 549 |
| 90% CI | – | −9,524; 15,871 | 13,834; 32,539 | −35,172; 25,362 |
| Percent CABG cost-saving | – | 26.7 | 0.0 | 44.4 |
| CABG vs. PCI ($) | ||||
| CABG | – | 42,850 | 53,800 | – |
| PCI | – | 34,475 | 33,429 | – |
| Difference | – | 8,375 | 20,370 | – |
| 90% CI | – | 1,570; 16,074 | 11,501; 28,680 | – |
| Percent CABG cost-saving | – | 1.8 | 0.0 | – |
CAD Coronary artery disease, VD ventricular, disease LMD left main disease, CKD chronic kidney disease
PCI vs. MED
In both CKD groups, patients with multi-vessel CAD had greater medical costs than patients with single-vessel CAD. And while cost differences reached (or neared) statistical significance for patients without CKD, this was not the case in CKD patients, where overall cost differences between therapies were much smaller.
CABG vs. MED
In comparing these patients, cost differences between therapies reached statistical significance for all patients without CKD. However, medical costs were much higher for CKD patients and differences only reached statistical significance among three-vessel CAD patients.
CABG vs. PCI
Among patients without CKD, cost differences between therapies were small and were only statistically significant in two-vessel CAD patients. However, among patients with CKD, medical costs for CABG surgery patients were greater and reached statistical significance for all multi-vessel CAD patients.
3-year economic attractiveness
PCI vs. MED
PCI was not an economically attractive alternative to MED for most single-vessel CAD patients in both CKD groups (Table 5). Among multi-vessel CAD patients, it was cost-effective in three-vessel CAD patients without CKD and dominant in two-vessel CAD patients with CKD.
Table 5.
Adjusted 3-year economic attractiveness by chronic kidney disease and coronary artery disease treatment group
| CAD Group | 1 VD | 2 VD | 3 VD | LMD |
|---|---|---|---|---|
| Normal–mild CKD | ||||
| PCI vs. medical | $149,457/YOLS | $140,129/YOLS | $38,582/YOLS | |
| CABG vs. medical | $332,506/YOLS | $20,299/YOLS | $28,588/YOLS | |
| CABG vs. PCI | Dominated | Dominant | ||
| Moderate–severe CKD | ||||
| PCI vs. medical | $112,472/YOLS | Dominant | $89,364/YOLS | |
| CABG vs. medical | $15,661/YOLS | $91,583/YOLS | $3709/YOLS | |
| CABG vs. PCI | $36,593/YOLS | $54,902/YOLS | ||
CAD Coronary artery disease, VD ventricular disease, LMD left main disease, CKD chronic kidney disease
CABG vs. MED
CABG surgery was an economically attractive strategy versus MED for patients in many CKD-CAD strata. These included all patients with left main CAD, three-vessel CAD patients without CKD, and two-vessel CAD patients with CKD.
CABG vs. PCI
In both CKD groups, CABG surgery was an economically attractive alternative vs. PCI among patients with three-vessel CAD. And, while it was cost-effective vs. PCI in two-vessel CAD patients with CKD, PCI dominated CABG surgery in patients without CKD.
Stability analysis
PCI vs. MED
PCI was economically attractive (dominant or cost-effective at <$50,000/YOLS) vs. MED in more than 50% of bootstrap samples for three-vessel CAD patients without CKD and for two-vessel CAD patients with CKD. However, the PCI vs. MED economic attractiveness point estimate was unstable (>20% dominated) in all CKD strata.
CABG vs. MED
When compared with MED, CABG surgery was an economically attractive alternative among all left main CAD patients, three-vessel CAD patient without CKD, and two-vessel CAD patients with CKD.
CABG vs. PCI
The presence of CKD directly impacted the relative economic attractiveness of CABG surgery vs. PCI. Among patients without CKD, CABG surgery was an economically unattractive alternative (dominated or >$150,000/YOLS) vs. PCI in patients with two-vessel CAD, while it was economically attractive in three-vessel CAD patients. In contrast, CABG surgery was an economically attractive alternative vs. PCI among two-vessel CAD patients with CKD, whereas, it was an economically unattractive alternative for CKD patients with three-vessel disease (Table 6).
Table 6.
3-year economic attractiveness stability analysis
| CAD Group | 1 VD | 2 VD | 3 VD | LMD |
|---|---|---|---|---|
| Normal–mild CKD | ||||
| PCI vs. medical (%) | ||||
| Dominant | 0.20 | 1.00 | 6.00 | – |
| Cost-effectiveness | – | |||
| ≤$50,000/YOLS | 3.40 | 7.60 | 55.50 | – |
| ≤$150,000/YOLS | 46.60 | 43.80 | 28.00 | – |
| >$150,000/YOLS | 46.50 | 36.30 | 7.20 | – |
| Dominated | 3.30 | 11.30 | 3.30 | – |
| CABG vs. medical (%) | ||||
| Dominant | – | 0.10 | 3.00 | 0.70 |
| Cost-effectiveness | – | |||
| ≤$50,000/YOLS | – | 2.40 | 94.20 | 77.40 |
| ≤$150,000/YOLS | – | 21.40 | 2.80 | 19.80 |
| >$150,000/YOLS | – | 48.20 | 0.00 | 1.60 |
| Dominated | – | 27.90 | 0.00 | 0.50 |
| CABG vs. PCI (%) | ||||
| Dominant | – | 0.40 | 58.00 | – |
| Cost-effectiveness | – | – | ||
| ≤$50,000/YOLS | – | 3.00 | 25.90 | – |
| ≤$150,000/YOLS | – | 4.80 | 3.60 | – |
| >$150,000/YOLS | – | 8.20 | 2.20 | – |
| Dominated | – | 83.60 | 10.30 | – |
| Moderate–severe CKD | ||||
| PCI vs. medical (%) | ||||
| Dominant | 4.70 | 50.50 | 30.40 | – |
| Cost-effectiveness | – | |||
| ≤$50,000/YOLS | 26.40 | 14.80 | 17.10 | – |
| ≤$150,000/YOLS | 24.50 | 4.80 | 4.10 | – |
| >$150,000/YOLS | 13.50 | 2.70 | 2.80 | – |
| Dominated | 30.90 | 27.20 | 45.60 | – |
| CABG vs. medical (%) | ||||
| Dominant | – | 25.10 | 0.00 | 44.70 |
| Cost-effectiveness | – | |||
| ≤$50,000/YOLS | – | 63.50 | 12.80 | 47.50 |
| ≤$150,000/YOLS | – | 10.60 | 65.60 | 7.00 |
| >$150,000/YOLS | – | 0.50 | 20.60 | 0.70 |
| Dominated | – | 0.30 | 1.00 | 0.10 |
| CABG vs. PCI (%) | ||||
| Dominant | – | 1.70 | 0.0 | – |
| Cost-effective | – | – | ||
| ≤$50,000/YOLS | – | 81.90 | 11.00 | – |
| ≤$150,000/YOLS | – | 1.70 | 26.90 | – |
| >$150,000/YOLS | – | 13.50 | 59.50 | – |
| Dominated | – | 1.20 | 2.60 | – |
CAD Coronary artery disease, VD ventricular disease, LMD left main disease, CKD chronic kidney disease
Discussion
We assessed medical cost vs. survival benefit tradeoffs associated with CAD revascularization procedures in patients with and without CKD. Despite higher medical costs and greater mortality for CKD patients, we found that the relative economic attractiveness of CAD revascularization procedures was largely similar among left main patients where CABG was favorable and one-vessel CAD patients where MED was favorable. However, differences were found among multi-vessel CAD patients; CABG was favorable for three-vessel CAD patients without CKD and for two-vessel CAD patients with CKD, but MED was favorable for three-vessel CAD patients with CKD and for two-vessel CAD patients without CKD.
PCI vs. MED
In a systematic review of PCI vs. MED trials, Bucher and colleagues found that the use of PCI was associated with no significant difference in the risks of death or myocardial infarction [16]. We found that PCI was life-saving vs. MED in >50% of all patients with and without CKD. However these differences were only statistically significant among patients in two of the non-CKD strata. Interestingly, while PCI produces the greatest survival advantage vs. MED in three-vessel CAD patients without CKD, it was associated with no survival advantage in three-vessel CAD patients with CKD.
Unfortunately we have no economic information from clinical trials comparing PCI with MED. However, one writer’s assessment that PCI largely serves as an expensive alternative to MED was born out in our results [17]. PCI was more expensive than MED for all patients without CKD and for single-vessel CAD patients with CKD. However, PCI was less expensive than MED in >50% of CKD patients with multi-vessel CAD. Overall, PCI would be considered an economically attractive alternative to MED only among three-vessel CAD patients without CKD. While it was dominant vs. MED among two-vessel CAD patients with CKD, this estimate was unstable and PCI was dominated by MED in 27% of bootstrap samples.
CABG vs. MED
Yusuf and colleagues used information from seven trials to compare CABG with MED and concluded that the reduction in mortality achieved by surgical revascularization was proportional to the number of diseased vessels and the degree of myocardial ischemia, particularly if LAD disease was present [18]. We found that CABG surgery was lifesaving vs. MED across both CKD groups. These differences reached statistical significance for all three-vessel and left main CAD patients and for two-vessel CAD patients with CKD. The survival benefit associated with CABG surgery vs. MED in two-vessel CKD patients (0.36 years) was comparable to that in three-vessel CAD patients with and without CKD, and appears to be driven by reduced survival among two-vessel MED patients with CKD.
CABG surgery costs are typically greater than those for other CAD therapies and remain high even in patients with less severe CAD [17, 19–21]. Thus, CABG surgery is most economically attractive vs. PCI and MED in those patients for whom it confers the greatest survival advantage and for whom the costs of alternative treatments are greatest (i.e., patients with most severe CAD). We found that CABG surgery was more expensive than MED for all patients, and that these differences reached statistical significance in all strata with CKD patients. However, the survival benefits associated with surgical revascularization were frequently of such magnitude that it was economically attractive (i.e., cost-effective or dominant). Among patients without CKD, CABG surgery was an economically attractive alternative vs. MED in three-vessel or left main CAD. Among CKD patients, surgery was economically attractive vs. MED in two-vessel or left main CAD, and was marginally attractive in patients with three-vessel CAD.
CABG vs. PCI
A recent analysis of nine CABG surgery vs. PCI trials found no differences in mortality rates [22]. However, these trials included lower risk CAD patients who would not benefit from surgical revascularization. The BARI study reported a trend toward greater survival for CABG surgery vs. PCI among three-vessel CAD and significant survival benefit among multi-vessel disease patients with diabetes [23]. We found that CABG surgery was life-saving vs. PCI for all multi-vessel CAD patients with CKD and for three-vessel CAD patients without CKD. However these differences only reached statistical significance among CKD patients.
The BARI economic analysis reported that after 5-years, cumulative PCI costs were 95% of those for CABG ($2664 difference). This report also suggested that CABG surgery was more expensive than PCI with no survival benefit for two-vessel CAD patients but cost-effective in three-vessel CAD patients [21]. In our analysis, CABG surgery was more expensive than PCI among all CKD-CAD strata except for those with three-vessel disease without CKD. While our results for patients without CKD agree with the BARI economic analysis, we also found that CABG surgery was economically attractive in all multi-vessel CAD patients with CKD, a population with increased morbidity and mortality.
CKD and CAD treatment
A recent study from our group compared CAD treatment-related 5-year survival differences among patients with varying degrees of CKD [6]. Our results compliment those from this earlier study by: confirming the importance of creatinine clearance as the point where CABG surgery may be more attractive than PCI as a revascularization strategy; and by identifying the CAD strata (two-vessel CAD patients) in which optimal CAD treatment strategy differed between patients with and without CKD. We also placed the survival differences identified in these studies in an economic context.
Limitations
Our study’s horizon was limited to 3-years follow-up. Therefore questions may arise over whether our more invasive therapies might become more economically attractive after the study’s follow-up period ends. The BARI economic study reported that most of the survival benefits of CABG surgery versus PCI were evident at 3-years follow-up [21]. Thus, while we feel that a longer follow-up period would have been informative, we do not believe that a 3-year study horizon limits the ability to generalize our results. Ending enrollment in 2001 also means that our study did not include drug-eluting stents. However, drug-eluting stents have not been shown to increase patient survival [24, 25]. Instead the primary benefit of these devices is in reducing repeat revascularizations. Given the limited economic data available on drug-eluting stents, we believe that these devices will most likely cause an overall increase in the total costs of the PCI treatment strategy, making it a less attractive alternative to both MED and CABG surgery.
This is a single site study that used observational data so our findings may reflect local practice. However our survival analyses agree with results from systematic reviews and other observational studies regarding the patient groups that generally benefit from more invasive therapies [11, 12, 16, 18, 22, 26, 27]. Another potential confounder is our inability to account for duration of CKD or for changes in CKD status during the follow-up period. Nonetheless our follow-up was 95% complete and any CKD-related changes in patients events (i.e., death, myocardial infarction, CABG surgery, PCI, or other hospitalizations) would have been factored into our clinical and economic results.
Three rationales have been cited for patients to undergo CAD revascularizations: (1) to alleviate symptoms of ischemia; (2) to reduce mortality risks; and (3) to treat/prevent comorbidities (e.g., arrhythmia, heart failure, or myocardial infarction) [22]. Our results include mortality reductions and indirectly include the treatment/prevention of comorbidities in our follow-up event and medical cost data. However, we did not address potential differences in quality of life (e.g., angina pain) that could be attributed to different CAD treatments. Even in instances where we found no significant differences in survival between more and less invasive therapies, there is a possibility that certain of these patients could benefit from CABG or PCI through improvements in pain management and quality of life.
Lastly, there may be some disagreement regarding our definition of chronic kidney disease. The National Kidney Foundation defines CKD by creatinine clearance and/or proteinurea level; therefore a patient could be classified as CKD even with a creatinine clearance greater than 60 mL/min. Proteinurea was not available in our dataset, and thus could not be included in our analyses [6].
Conclusions
CKD is associated with greater cardiovascular disease burden, reduced survival, and higher medical costs in CAD patients. CABG surgery was an economically attractive alternative vs. PCI or MED for all patients with left main disease, three-vessel CAD patients without CKD, and two-vessel CAD patients with CKD. MED was an economically attractive strategy vs. CABG surgery or PCI for three-vessel CAD patients with CKD, two-vessel CAD patients without CKD, and all single-vessel CAD patients.
Acknowledgments
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant RO1-DK058188. The authors acknowledge the editorial and technical support of Maqui Ortiz.
Contributor Information
Eric L. Eisenstein, Email: eric.eisenstein@duke.edu, Duke University Medical Center, Durham, NC, USA. Duke Clinical Research Institute, P.O. Box 17969, Durham, NC 27715, USA. 2400 Pratt Street, Room 0311, Durham, NC 27705, USA
Jie L. Sun, Duke University Medical Center, Durham, NC, USA. Duke Clinical Research Institute, P.O. Box 17969, Durham, NC 27715, USA
Kevin J. Anstrom, Duke University Medical Center, Durham, NC, USA. Duke Clinical Research Institute, P.O. Box 17969, Durham, NC 27715, USA
Elizabeth R. DeLong, Duke University Medical Center, Durham, NC, USA. Duke Clinical Research Institute, P.O. Box 17969, Durham, NC 27715, USA
Lynda A. Szczech, Duke University Medical Center, Durham, NC, USA. Duke Clinical Research Institute, P.O. Box 17969, Durham, NC 27715, USA
Daniel B. Mark, Duke University Medical Center, Durham, NC, USA. Duke Clinical Research Institute, P.O. Box 17969, Durham, NC 27715, USA
References
- 1.Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA guidelines for coronary artery bypass graft surgery: executive summary and recommendations: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to revise the 1991 guidelines for coronary artery bypass graft surgery) Circulation. 1999;100(13):1464–1480. doi: 10.1161/01.cir.100.13.1464. [DOI] [PubMed] [Google Scholar]
- 2.Hannan EL, Racz MJ, McCallister BD, et al. A comparison of three-year survival after coronary artery bypass graft surgery and percutaneous transluminal coronary angioplasty. Journal of the American College of Cardiology. 1999;33(1):63–72. doi: 10.1016/s0735-1097(98)00540-3. [DOI] [PubMed] [Google Scholar]
- 3.Smith SC, Jr, Dove JT, Jacobs AK, et al. ACC/AHA guidelines for percutaneous coronary intervention (revision of the 1993 PTCA guidelines)-executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee to revise the 1993 guidelines for percutaneous transluminal coronary angioplasty) endorsed by the Society for Cardiac Angiography and Interventions. Circulation. 2001;103(24):3019–3041. doi: 10.1161/01.cir.103.24.3019. [DOI] [PubMed] [Google Scholar]
- 4.Szczech LA, Reddan DN, Owen WF, et al. Differential survival after coronary revascularization procedures among patients with renal insufficiency. Kidney International. 2001;60(1):292–299. doi: 10.1046/j.1523-1755.2001.00799.x. [DOI] [PubMed] [Google Scholar]
- 5.Szczech LA, Best PJ, Crowley E, et al. Outcomes of patients with chronic renal insufficiency in the bypass angioplasty revascularization investigation. Circulation. 2002;105(19):2253–2258. doi: 10.1161/01.cir.0000016051.33225.33. [DOI] [PubMed] [Google Scholar]
- 6.Reddan DN, Szczech LA, Tuttle RH, et al. Chronic kidney disease, mortality, and treatment strategies among patients with clinically significant coronary artery disease. Journal of the American Society of Nephrology. 2003;14(9):2373–2380. doi: 10.1097/01.asn.0000083900.92829.f5. [DOI] [PubMed] [Google Scholar]
- 7.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. American Journal of Kidney Diseases. 2002;39(2 Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 8.Rosati RA, McNeer JF, Starmer CF, Mittler BS, Morris JJ, Jr, Wallace AG. A new information system for medical practice. Archives of Internal Medicine. 1975;135(8):1017–1024. [PubMed] [Google Scholar]
- 9.Fortin DF, Califf RM, Pryor DB, Mark DB. The way of the future redux. American Journal of Cardiology. 1995;76(16):1177–1182. doi: 10.1016/s0002-9149(99)80331-2. [DOI] [PubMed] [Google Scholar]
- 10.Harris PJ, Harrell FE, Jr, Lee KL, Behar VS, Rosati RA. Survival in medically treated coronary artery disease. Circulation. 1979;60(6):1259–1269. doi: 10.1161/01.cir.60.6.1259. [DOI] [PubMed] [Google Scholar]
- 11.Califf RM, Harrell FE, Jr, Lee KL, et al. The evolution of medical and surgical therapy for coronary artery disease. A 15-year perspective. JAMA. 1989;261(14):2077–2086. [PubMed] [Google Scholar]
- 12.Mark DB, Nelson CL, Califf RM, et al. Continuing evolution of therapy for coronary artery disease. Initial results from the era of coronary angioplasty. Circulation. 1994;89(5):2015–2025. doi: 10.1161/01.cir.89.5.2015. [DOI] [PubMed] [Google Scholar]
- 13.Eisenstein EL, Mark DB. Cost effectiveness of new diagnostic tools and therapies for acute coronary syndromes. In: Topol EJ, editor. Acute coronary syndromes. Marcel Dekker; New York: 2001. pp. 811–844. [Google Scholar]
- 14.Anstrom KJ, Tsiatis AA. Utilizing propensity scores to estimate causal treatment effects with censored time-lagged data. Biometrics. 2001;57(4):1207–1218. doi: 10.1111/j.0006-341x.2001.01207.x. [DOI] [PubMed] [Google Scholar]
- 15.Eisenstein EL, Shaw LK, Anstrom KJ, et al. Assessing the clinical and economic burden of coronary artery disease: 1986–1998. Medical Care. 2001;39(8):824–835. doi: 10.1097/00005650-200108000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Bucher HC, Hengstler P, Schindler C, Guyatt GH. Percutaneous transluminal coronary angioplasty versus medical treatment for non-acute coronary heart disease: meta-analysis of randomised controlled trials. BMJ. 2000;321(7253):73–77. doi: 10.1136/bmj.321.7253.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mark DB, Hlatky MA. Medical economics and the assessment of value in cardiovascular medicine: Part II. Circulation. 2002;106(5):626–630. doi: 10.1161/01.cir.0000021408.40925.63. [DOI] [PubMed] [Google Scholar]
- 18.Yusuf S, Zucker D, Peduzzi P, et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet. 1994;344(8922):563–570. doi: 10.1016/s0140-6736(94)91963-1. [DOI] [PubMed] [Google Scholar]
- 19.Smith LR, Milano CA, Molter BS, Elbeery JR, Sabiston DC, Jr, Smith PK. Preoperative determinants of postoperative costs associated with coronary artery bypass graft surgery. Circulation. 1994;90(5 Pt 2):II124–II128. [PubMed] [Google Scholar]
- 20.Weintraub WS, Mauldin PD, Becker E, Kosinski AS, King SB., III A comparison of the costs of quality of life after coronary angioplasty or coronary surgery for multivessel coronary artery disease. Results from the Emory Angioplasty Versus Surgery Trial (EAST) Circulation. 1995;92(10):2831–2840. doi: 10.1161/01.cir.92.10.2831. [DOI] [PubMed] [Google Scholar]
- 21.Hlatky MA, Rogers WJ, Johnstone I, et al. Medical care costs quality of life after randomization to coronary angioplasty or coronary bypass surgery. Bypass Angioplasty Revascularization Investigation (BARI) Investigators. New England Journal of Medicine. 1997;336(2):92–99. doi: 10.1056/NEJM199701093360203. [DOI] [PubMed] [Google Scholar]
- 22.Rihal CS, Raco DL, Gersh BJ, Yusuf S. Indications for coronary artery bypass surgery and percutaneous coronary intervention in chronic stable angina: review of the evidence and methodological considerations. Circulation. 2003;108(20):2439–2445. doi: 10.1161/01.CIR.0000094405.21583.7C. [DOI] [PubMed] [Google Scholar]
- 23.The BARI Investigators. Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. The Bypass Angioplasty Revascularization Investigation (BARI) Investigators. New England Journal of Medicine. 1996;335(4):217–225. doi: 10.1056/NEJM199607253350401. [DOI] [PubMed] [Google Scholar]
- 24.Kong DF, Eisenstein EL, Sketch MH, Jr, et al. Economic impact of drug-eluting stents on hospital systems: a disease-state model. American Heart Journal. 2004;147(3):449–456. doi: 10.1016/j.ahj.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Morice MC, Serruys PW, Sousa JE, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. New England Journal of Medicine. 2002;346(23):1773–1780. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- 26.Brener SJ, Lytle BW, Casserly IP, Schneider JP, Topol EJ, Lauer MS. Propensity analysis of long-term survival after surgical or percutaneous revascularization in patients with multi-vessel coronary artery disease and high-risk features. Circulation. 2004;109(19):2290–2295. doi: 10.1161/01.CIR.0000126826.58526.14. [DOI] [PubMed] [Google Scholar]
- 27.Jones RH, Kesler K, Phillips HRIII, et al. Long-term survival benefits of coronary artery bypass grafting and percutaneous transluminal angioplasty in patients with coronary artery disease. Journal of Thoracic and Cardiovascular Surgery. 1996;111(5):1013–1025. doi: 10.1016/s0022-5223(96)70378-1. [DOI] [PubMed] [Google Scholar]


