SUMMARY
SETTING
Tuberculosis (TB) infected adults attending out-patient TB clinics in Dar es Salaam, Tanzania.
OBJECTIVE
To examine the association of anemia with human immunodeficiency virus (HIV) co-infection, indicators of socio-economic status (SES) and anthropometric status in TB-infected adults.
DESIGN
Cross-sectional data collection during screening for a clinical trial.
RESULTS
Overall, 750 females and 1693 males participated in this study, of whom respectively 49% and 24% were co-infected with HIV-1. Hemoglobin levels were significantly lower in females than in males and in HIV-positive than in HIV-negative participants. HIV co-infection in this antiretroviral-naïve population was also associated with severe anemia (hemoglobin < 85 g/l) in both women (prevalence ratio [PR] = 2.07, 95%CI 1.65–2.59) and men (PR 3.45, 95%CI 2.66–4.47). Although severe anemia was negatively associated with indicators of SES, especially in males, adjustment for SES indicators only marginally changed its association with HIV co-infection. In both sexes, anemia was inversely associated with anthropometric status, independently of HIV infection and SES.
CONCLUSION
Among TB-infected adults, anemia is strongly associated with HIV co-infection and anthropometric status, independently of SES indicators. As anemia is a risk factor for morbidity and mortality in both infections, the management of anemia in TB-HIV co-infected patients warrants special attention.
Keywords: pulmonary tuberculosis, anemia, HIV, anthropometric status, socio-economic status
TUBERCULOSIS (TB) and human immunodeficiency virus (HIV) infection are major causes of morbidity and mortality worldwide, and both infections are also highly prevalent in sub-Saharan Africa. HIV infection increases the risk of developing clinical TB and accelerates TB disease progression, thus making TB one of the most common and the single most important opportunistic infection that accompanies HIV disease.1,2 Globally it is estimated that 9.4 million new cases and 1.7 million deaths from TB occurred in 2009, of which 1.1 million new cases and 0.4 million deaths were in HIV-positive people.3
Anemia is also a worldwide problem whose prevalence is highest in sub-Saharan Africa.4 Anemia is a frequent complication of both TB and HIV infection, and in both infections it is associated with increased morbidity and mortality.5-7 However, relatively little is known regarding anemia in TB-HIV co-infection. Past studies have found that hemoglobin (Hb) levels among TB-HIV co-infected individuals were considerably lower than among subjects with only one of the two infections.8-13 However, most of these studies had relatively low participant numbers and in none of them was the association of TB-HIV co-infection with anemia adjusted for possible socio-economic confounding. It therefore remains unclear whether this association is independent or a result of differences in socio-economic status (SES).
We report the results of a cross-sectional study in which we examine associations between anemia and 1) TB-HIV co-infection, 2) indicators of SES (education, income and social setting), and 3) indicators of anthropometric status and TB disease severity, in a population of 2443 TB-infected adults of both sexes.
Our expectation was that anemia would be positively associated with HIV co-infection and TB disease severity, and negatively associated with SES indicators and anthropometric status.
STUDY POPULATION AND METHODS
Data collection
We conducted a cross-sectional study among TB-infected adults who attended five out-patient TB clinics in Dar es Salaam, Tanzania, and who were evaluated for inclusion in a clinical trial.14
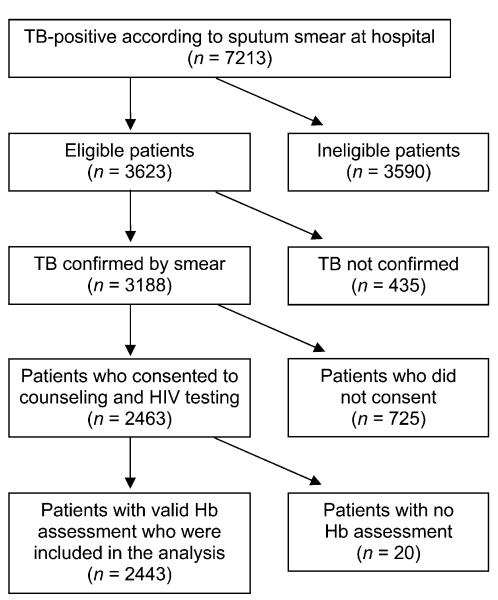
Between March 2000 and May 2005, sputum smears were obtained at the five out-patient clinics and sent to the Muhimbili National Hospital’s laboratory for microscopic analysis of acid-fast bacilli (AFB). Among the 7213 patients who tested positive (Figure), we evaluated the following eligibility criteria: age between 18 and 65 years, residence within the clinic area where the DOTS strategy was to be administered, plan to stay in the study area for 2 years, not being pregnant, not having received anti-tuberculosis treatment during the previous year and Karnofsky performance score ≥ 40%.15
Figure.
Number of participants included in and excluded from data analysis for this study. TB = tuberculosis; HIV = human immunodeficiency virus; Hb = hemoglobin.
Among the 3623 subjects who fulfilled these criteria, we obtained two additional early morning sputum samples to confirm the smear results using Ziehl-Neelsen staining of smears, and sought informed consent for HIV testing. Smear-positive TB was confirmed in 3188 individuals, 2463 of whom consented to pre-test counseling and HIV testing. HIV-1 sero-status was assessed using two sequential enzyme-linked immunosorbent assay tests (Wellcozyme, Murex Biotech, Marburg, Germany, and Enzygnost anti-HIV1+2, Behring, Milton Keynes, UK) in an alternative confirmatory testing strategy.16 Discrepant results were resolved by Western blot (Genetic Systems, Redmond, WA, USA), which was interpreted according to World Health Organization (WHO) criteria.17 Hb levels were determined by Coulter counter (Coulter Inc, Brea, CA, USA). Of the participants, 2443 also had a valid Hb assessment and were included in our analyses.
Trained research nurses collected information on demographic and socio-economic characteristics and obtained anthropometric measurements using calibrated instruments and standardized techniques. Height was measured to the nearest 0.1 cm using Seca Bodymeter 206 stadiometers (Seca, Birmingham, UK), weight to the nearest 100 g using Seca 700 balance beam scales, left mid-upper arm circumference (MUAC) at the midpoint between the acromion and olecranon to the nearest 0.1 cm using non-stretchable tailor’s tapes, and left triceps skin-fold (TSF) thickness with Holtain calipers. Arm muscle circumference (AMC) was calculated as MUAC − (π × TSF),18 and body mass index (BMI) as kg/m2.
All measurements in this cross-sectional study were obtained immediately prior to the beginning of anti-tuberculosis therapy. None of the HIV-infected subjects were receiving antiretroviral medications, as the anthropometric evaluation coincided with the time of HIV diagnosis. Each participant provided informed consent prior to enrolment. TB and, if present, HIV infection were treated following the Ministry of Health and Social Welfare regulations prevailing at that time in the absence of antiretroviral treatment.
Data analysis and ethical considerations
All data analyses were performed separately for females and males because of the strong differences between sexes, both in our outcome variables (Hb level/prevalence of severe anemia) and in our most important covariate, HIV infection. The main predictor of interest was HIV status. The main outcomes were Hb level and prevalence of severe anemia (Hb < 85 g/l for both sexes). This threshold was chosen according to the national cut-off point for referral to the district level in Tanzania.19 Prevalence of anemia (Hb < 120 g/l for females and < 130 g/l for males)20 was also considered as a possible outcome variable. However, because more than 90% of participants were anemic, severe anemia was better suited to analyze associations with other factors.
Demographic and SES indicators were age, education level, literacy, employment status, cohabiting with a partner, frequency of meat purchase in the household, money spent on food per person in the household, number of people eating in the household and the number of household assets owned,21 including the following items: sofa, television, radio, refrigerator and fan. We included these variables in the analysis of anthropometric indicators for females and/or males, either because we regard them as important possible confounders (e.g., age) or because they were associated (P < 0.2) with severe anemia or Hb level in the SES analysis for the respective sex.
Indicators of the severity of TB were the Karnofsky performance score and the number of Mycobacterium tuberculosis bacilli per sputum smear field (bacillary density according to the WHO classification22). For each participant, we chose the maximum density from all available baseline smears that had been collected prior to the initiation of anti-tuberculosis treatment.
Differences in Hb level between sexes and HIV-infected and non-infected participants were assessed using linear regression models with robust variance estimates.23
The prevalence of severe anemia was compared:
in HIV-infected and non-infected males and females, crude and adjusted for selected SES indicators;
according to SES indicators and adjusted for HIV infection; and
by categories of indicators of anthropometric status and of TB severity, adjusted for HIV infection and SES indicators.
We used Poisson regression with robust variance estimates to obtain prevalence ratios (PRs) and 95% confidence intervals (95%CIs) for binary outcomes.24 All P are from two-sided tests, and differences were considered statistically significant at P ≤ 0.05. All analyses were performed using Stata version 9.2 (Stata Corp, College Station, TX, USA).
The study protocol was approved by the Research and Publications Committee at Muhimbili University College of Health Sciences, and the Institutional Review Board of the Harvard School of Public Health. All participants provided written informed consent prior to participation.
RESULTS
Of the 2443 TB-positive study participants, 31% were women and 69% were men (Table 1). The prevalence of HIV infection was higher in women (49%) than in men (24%). The mean age was 29.8 years (standard deviation [SD] 8.4) for women and 32.2 years (SD 9.6) for men. Hb levels were significantly lower in females and in HIV co-infected participants. Accordingly, the prevalence of severe anemia was significantly higher in these groups, with TB-HIV co-infected women having the highest prevalence of severe anemia (43.4%). Differences in severe anemia and Hb for TB-HIV co-infected vs. TB-only infected participants changed only marginally when adjusting for SES indicators. Because of the strong differences between sexes regarding the prevalence of severe anemia and of HIV infection, all further analyses were performed separately for females and for males.
Table 1.
Mean Hb level and prevalence of severe anemia (Hb < 85 g/l) stratified by sex and HIV infection status
| Females |
Males |
|||
|---|---|---|---|---|
| HIV+ | HIV− | HIV+ | HIV− | |
| n | 369 | 381 | 406 | 1287 |
| Hb, g/l | 86.9 | 96.7 | 99.4 | 110.6 |
| Hb difference (95%CI) | −9.8 (−12.1 – − 7.4) | −11.2 (−13.4 – −9.1) | ||
| Adjusted Hb difference (95%CI)* | −9.9 (−12.3– −7.6) | −11.5 (−13.7– −9.4) | ||
| Prevalence of severe anemia, % | 43.4 | 21.0 | 24.6 | 7.1 |
| PR (95%CI) | 2.07 (1.65–2.59) | 3.45 (2.66–4.47) | ||
| Adjusted PR (95%CI)* | 2.09 (1.66–2.64) | 3.34 (2.54–4.40) | ||
Adjusted for age, education, frequency of meat purchase, money spent on food and number of household assets for both sexes and for literacy, employment status, living with a partner and number of people eating at home for males only.
Hb = hemoglobin; HIV = human immunodeficiency virus; + = positive; − = negative; CI = confidence interval; PR = prevalence ratio.
Associations of severe anemia and Hb levels with SES indicators mostly showed the expected direction, with higher Hb and lower prevalence of severe anemia in socio-economically less disadvantaged participants (Table 2). In males, all SES indicators, with the exception of employment status, were significantly associated with Hb levels, whereas some of the associations of SES indicators with severe anemia did not reach statistical significance. For females, the relation of Hb and severe anemia with SES indicators was less clear-cut; only the amount of money spent on food had a significant association with both of these outcomes (P for trend < 0.01 for both outcomes).
Table 2.
Bivariate associations of severe anemia and hemoglobin levels with indicators of socio-economic status adjusted for human immunodeficiency virus infection
| Females |
Males |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence of severe anemia Hb < 85 g/l |
Hb level |
Prevalence of severe anemia Hb < 85 g/l |
Hb level |
|||||||||||||
| Covariate | n | Prevalence %* |
PR | 95%CI |
Pvalue for trend |
Hb g/l |
SD |
Pvalue for trend |
n | Prevalence %* |
PR | 95%CI |
Pvalue for trend |
Hb g/l |
SD |
Pvalue for trend |
| Age, years | 0.502 | 0.283 | 0.012 | 0.006 | ||||||||||||
| ≤20 | 60 | 20.0 | 0.73 | 0.44–1.23 | 98.4 | 17.9 | 118 | 3.4 | 0.56 | 0.20–1.51 | 110.2 | 14.5 | ||||
| 21–30† | 419 | 33.4 | 1 | — | 90.4 | 15.9 | 736 | 8.0 | 1 | — | 110.2 | 18.1 | ||||
| 31–40 | 176 | 36.9 | 1.01 | 0.80–1.27 | 91.6 | 19.1 | 542 | 15.9 | 1.49 | 1.09–2.04 | 106.2 | 20.7 | ||||
| 41–50 | 68 | 23.5 | 0.67 | 0.43–1.04 | 93.7 | 16.8 | 187 | 12.3 | 1.13 | 0.71–1.78 | 105.9 | 19.7 | ||||
| ≥51 | 20 | 25.0 | 0.93 | 0.44–1.96 | 96.5 | 16.8 | 97 | 16.5 | 1.79 | 1.07–3.02 | 102.6 | 18.7 | ||||
| Education | 0.145 | 0.369 | 0.013 | <0.001 | ||||||||||||
| None | 120 | 35.8 | 1.21 | 0.93–1.57 | 91.8 | 16.1 | 127 | 17.3 | 1.63 | 1.10–2.43 | 104.8 | 21.6 | ||||
| Grade 1-4 or adult education | 71 | 33.8 | 1.06 | 0.74–1.51 | 90.3 | 16.5 | 150 | 12.0 | 1.03 | 0.65–1.63 | 102.7 | 16.2 | ||||
| Grade 5–8† | 487 | 31.2 | 1 | — | 91.8 | 17.2 | 1182 | 10.9 | 1 | — | 108.4 | 19.2 | ||||
| Secondary and university | 66 | 28.8 | 0.93 | 0.63–1.38 | 93.6 | 18.8 | 220 | 8.6 | 0.81 | 0.52–1.25 | 111.1 | 17.7 | ||||
| Literacy | 0.430 | 0.581 | <0.001 | 0.002 | ||||||||||||
| No† | 120 | 34.2 | 1 | — | 91.3 | 16.8 | 130 | 21.5 | 1 | — | 102.8 | 21.9 | ||||
| Yes | 617 | 31.1 | 0.90 | 0.69–1.18 | 92.0 | 17.2 | 1543 | 10.4 | 0.46 | 0.33–0.65 | 108.4 | 18.8 | ||||
| Employment | 0.869 | 0.971 | 0.233 | 0.092 | ||||||||||||
| No† | 368 | 30.7 | 1 | — | 92.2 | 16.9 | 137 | 13.1 | 1 | — | 106.1 | 18.7 | ||||
| Yes | 359 | 32.9 | 1.02 | 0.83–1.25 | 91.5 | 17.3 | 1511 | 11.2 | 0.77 | 0.50–1.19 | 108.1 | 19.2 | ||||
| Living with partner | 0.302 | 0.749 | 0.062 | 0.001 | ||||||||||||
| No† | 347 | 34.9 | 1 | 91.1 | 17.7 | 922 | 11.5 | 1 | — | 107.2 | 18.8 | |||||
| Yes | 398 | 29.4 | 0.90 | 0.73–1.10 | 92.4 | 16.5 | 760 | 10.9 | 0.78 | 0.59–1.01 | 108.8 | 19.4 | ||||
| Frequency of meat purchase | 0.194 | 0.131 | 0.007 | <0.001 | ||||||||||||
| Less than once/month | 38 | 36.8 | 1.15 | 0.77–1.70 | 90.2 | 18.2 | 88 | 14.8 | 1.24 | 0.77–2.01 | 102.6 | 17.6 | ||||
| 1–4 times/month† | 409 | 33.3 | 1 | — | 91.2 | 16.1 | 822 | 12.9 | 1 | — | 106.5 | 19.4 | ||||
| >4 times/month | 294 | 29.9 | 0.90 | 0.72–1.11 | 92.8 | 18.2 | 770 | 9.1 | 0.73 | 0.55–0.97 | 110.1 | 18.7 | ||||
| Tanzanian shillings spent on food per day and person |
0.005 | 0.003 | 0.477 | <0.001 | ||||||||||||
| ≤250† | 190 | 37.4 | 1 | — | 89.6 | 16.3 | 323 | 12.4 | 1 | — | 104.2 | 18.5 | ||||
| 251–499 | 197 | 30.5 | 0.82 | 0.62–1.08 | 92.5 | 16.2 | 304 | 10.9 | 0.79 | 0.52–1.20 | 108.7 | 18.8 | ||||
| 500–799 | 155 | 31.6 | 0.80 | 0.60–1.05 | 92.4 | 15.8 | 375 | 9.3 | 0.72 | 0.48–1.09 | 110.0 | 19.8 | ||||
| ≥800 | 74 | 23.0 | 0.55 | 0.35–0.86 | 94.0 | 17.7 | 407 | 11.3 | 0.87 | 0.59–1.28 | 109.4 | 20.1 | ||||
| Number of people eating at home | 0.312 | 0.541 | 0.595 | 0.007 | ||||||||||||
| 1 † | 67 | 31.3 | 1 | — | 90.6 | 18.0 | 385 | 12.2 | 1 | — | 108.2 | 20.3 | ||||
| 2 or 3 | 187 | 30.5 | 1.06 | 0.70–1.59 | 92.6 | 15.8 | 455 | 9.2 | 0.71 | 0.49–1.04 | 109.8 | 18.9 | ||||
| 4 or 5 | 235 | 32.3 | 1.17 | 0.79–1.75 | 92.0 | 16.8 | 328 | 12.2 | 0.90 | 0.61–1.32 | 108.1 | 18.7 | ||||
| 6 to 8 | 152 | 32.9 | 1.18 | 0.77–1.79 | 90.7 | 16.5 | 323 | 11.8 | 0.97 | 0.66–1.43 | 105.8 | 18.9 | ||||
| ≥9 | 103 | 33.0 | 1.19 | 0.76–1.85 | 92.5 | 20.0 | 190 | 11.1 | 1.02 | 0.64–1.63 | 106.4 | 17.9 | ||||
| Number of household assets owned‡ | 0.359 | 0.082 | 0.008 | <0.001 | ||||||||||||
| 0† | 284 | 32.7 | 1 | — | 91.2 | 16.6 | 533 | 13.9 | 1 | — | 106.2 | 19.4 | ||||
| 1 | 172 | 31.4 | 0.93 | 0.71–1.21 | 91.2 | 16.3 | 430 | 10.0 | 0.64 | 0.45–0.90 | 106.9 | 18.1 | ||||
| 2 or 3 | 217 | 33.6 | 1.02 | 0.80–1.30 | 91.9 | 18.9 | 552 | 9.6 | 0.57 | 0.41–0.79 | 109.1 | 19.2 | ||||
| 4 or 5 | 70 | 22.9 | 0.70 | 0.44–1.09 | 95.9 | 13.8 | 157 | 12.1 | 0.71 | 0.45–1.12 | 112.7 | 19.5 | ||||
Unadjusted prevalence of severe anemia in substratum.
Reference category.
From a list that included sofa, television, radio, refrigerator and fan.
Hb = unadjusted mean hemoglobin level for substratum; PR = prevalence ratio adjusted for HIV infection; P for trend = adjusted P value for each variable when introduced into the model as a continuous predictor; CI = confidence interval; SD = standard deviation.
In males, there was also a clear trend regarding the association of age with anemia, with older participants having higher prevalences of severe anemia and lower Hb levels than younger participants (P for trend < 0.02 for both outcomes). In females, however, a clear age trend was not discernable.
Hb was positively associated with nearly all measured anthropometric indicators in both sexes (Table 3). The only exception was height, which had a weaker but still positive association with Hb in males; it, however, showed an opposite trend towards lower Hb levels and higher prevalences of severe anemia in taller female study participants.
Table 3.
Bivariate associations of severe anemia and Hb level with anthropometric indicators, AFB density and Karnofsky score adjusted for HIV infection and indicators of socio-economic status*
| Females |
Males |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence of severe anemia, Hb < 85 g/l |
Hb level |
Prevalence of severe anemia, Hb < 85 g/l |
Hb level |
|||||||||||||
| Covariate | n | Prevalence %† |
PR | 95%CI |
Pvalue for trend |
Hb g/l |
SD |
Pvalue for trend |
n | Prevalence %† |
PR | 95%CI |
Pvalue for trend |
Hb g/l |
SD |
Pvalue for trend |
| Height, cm | 0.004 | 0.219 | 0.182 | 0.020 | ||||||||||||
| ≤1 58.0‡ | 449 | 27.2 | 1 | — | 92.7 | 15.9 | 140 | 13.6 | 1 | — | 104.7 | 20.4 | ||||
| 158.1–164.0 | 184 | 40.2 | 1.62 | 1.29–2.04 | 90.4 | 18.5 | 400 | 11.5 | 0.99 | 0.61–1.61 | 107.1 | 19.3 | ||||
| 164.1–169.5 | 72 | 36.1 | 1.39 | 1.01–1.90 | 91.1 | 19.6 | 484 | 11.8 | 0.99 | 0.62–1.58 | 107.5 | 19.3 | ||||
| >169.5 | 11 | 18.2 | 0.69 | 0.25–1.95 | 95.5 | 16.4 | 589 | 9.3 | 0.78 | 0.48–1.26 | 110.1 | 18.3 | ||||
| Body mass index, kg/m2 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||||||
| <16.0‡ | 80 | 42.5 | 1 | — | 87.6 | 18.5 | 127 | 15.7 | 1 | — | 102.8 | 17.9 | ||||
| 16.0–16.9 | 93 | 38.7 | 0.94 | 0.66–1.33 | 90.2 | 17.2 | 173 | 13.9 | 0.88 | 0.53–1.48 | 102.7 | 17.8 | ||||
| 17.0–18.4 | 138 | 39.1 | 0.98 | 0.71–1.36 | 89.2 | 17.1 | 426 | 13.6 | 0.87 | 0.55–1.39 | 105.1 | 19.1 | ||||
| 18.5–19.9 | 144 | 28.5 | 0.71 | 0.50–1.01 | 91.8 | 16.8 | 419 | 9.5 | 0.63 | 0.38–1.02 | 109.2 | 18.1 | ||||
| 20.0–21.9 | 132 | 24.2 | 0.61 | 0.42–0.90 | 94.5 | 14.6 | 335 | 6.9 | 0.44 | 0.25–0.77 | 112.7 | 19.0 | ||||
| >21.9 | 123 | 19.5 | 0.47 | 0.31–0.72 | 97.2 | 16.9 | 124 | 8.1 | 0.51 | 0.25–1.04 | 115.5 | 20.2 | ||||
| Triceps skinfold thickness, mm | <0.001 | <0.001 | 0.402 | 0.004 | ||||||||||||
| <5.0‡ | 56 | 50.0 | 1 | — | 85.4 | 15.9 | 490 | 13.1 | 1 | — | 105.4 | 18.5 | ||||
| 5.0–6.3 | 103 | 49.5 | 1.09 | 0.79–1.50 | 86.0 | 17.2 | 451 | 9.5 | 0.81 | 0.58–1.15 | 109.1 | 18.9 | ||||
| 6.4–9.8 | 204 | 30.9 | 0.72 | 0.52–1.00 | 91.9 | 17.3 | 361 | 11.9 | 1.04 | 0.74–1.47 | 109.8 | 20.0 | ||||
| >9.8 | 325 | 23.4 | 0.56 | 0.41–0.78 | 94.8 | 16.2 | 235 | 9.8 | 0.74 | 0.47–1.16 | 109.7 | 19.1 | ||||
| Mid-upper-arm circumference, cm | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||||||
| ≤21.0‡ | 240 | 45.0 | 1 | — | 86.4 | 17.2 | 365 | 20.0 | 1 | — | 99.3 | 18.7 | ||||
| 21.1–23.0 | 194 | 32.0 | 0.75 | 0.59–0.95 | 92.3 | 16.7 | 502 | 14.1 | 0.72 | 0.54–0.97 | 105.7 | 18.8 | ||||
| 23.1–24.9 | 103 | 25.2 | 0.59 | 0.41–0.84 | 93.7 | 16.3 | 354 | 4.2 | 0.27 | 0.16–0.46 | 112.4 | 16.2 | ||||
| >24.9 | 202 | 18.8 | 0.44 | 0.32–0.61 | 97.1 | 15.6 | 444 | 6.3 | 0.34 | 0.22–0.51 | 114.2 | 18.9 | ||||
| Arm muscle circumference, cm | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||||||
| <19.0‡ | 276 | 42.0 | 1 | — | 87.3 | 17.7 | 254 | 21.3 | 1 | — | 99.6 | 19.1 | ||||
| 19.0–20.6 | 198 | 29.3 | 0.73 | 0.57–0.93 | 93.2 | 15.4 | 379 | 13.5 | 0.57 | 0.41–0.80 | 106.1 | 18.9 | ||||
| 20.7–22.1 | 127 | 22.0 | 0.53 | 0.37–0.75 | 95.1 | 16.5 | 424 | 8.5 | 0.42 | 0.28–0.62 | 108.6 | 17.2 | ||||
| ≥22.2 | 87 | 18.4 | 0.46 | 0.29–0.73 | 98.6 | 15.5 | 478 | 6.7 | 0.35 | 0.24–0.53 | 114.1 | 19.0 | ||||
| AFB density in HIV-negatives | 0.538 | 0.140 | 0.014 | 0.003 | ||||||||||||
| <1 /field‡ | 110 | 22.7 | 1 | — | 98.3 | 17.4 | 278 | 5.0 | 1 | — | 112.8 | 18.5 | ||||
| 1–10/field | 102 | 22.5 | 0.95 | 0.57–1.57 | 96.7 | 15.2 | 365 | 5.8 | 1.02 | 0.52–2.00 | 111.8 | 17.3 | ||||
| >10/field | 168 | 19.0 | 0.86 | 0.54–1.39 | 95.4 | 16.1 | 644 | 8.9 | 1.82 | 1.04–3.22 | 109.0 | 18.6 | ||||
| AFB density in HIV-positives | 0.096 | 0.057 | 0.312 | 0.676 | ||||||||||||
| <1 /field‡ | 124 | 38.7 | 1 | — | 88.5 | 16.1 | 112 | 23.2 | 1 | — | 99.9 | 19.8 | ||||
| 1–10/field | 87 | 42.5 | 1.13 | 0.81–1.58 | 87.9 | 17.0 | 116 | 24.1 | 1.19 | 0.74–1.92 | 98.6 | 18.9 | ||||
| >10/field | 154 | 48.1 | 1.27 | 0.96–1.70 | 85.0 | 16.5 | 174 | 25.9 | 1.25 | 0.82–1.90 | 99.4 | 19.3 | ||||
| Karnofsky score <70% | <0.001 | <0.001 | 0.038 | <0.001 | ||||||||||||
| No† | 603 | 27.5 | 1 | — | 93.7 | 16.6 | 1476 | 10.2 | 1 | — | 108.9 | 19.1 | ||||
| Yes | 138 | 50.7 | 1.75 | 1.42-2.16 | 83.6 | 16.5 | 190 | 19.5 | 1.39 | 1.02–1.90 | 100.7 | 17.9 | ||||
Adjusted for HIV status, age, education, frequency of meat purchase, money spent on food and number of household assets for both sexes and for literacy, employment status, living with a partner and number of people eating at home, for males only
Unadjusted prevalence of severe anemia in substratum.
Reference.
AFB = acid-fast bacilli; HIV = human immunodeficiency virus; Hb = unadjusted mean hemoglobin; PR = prevalence ratio adjusted for HIV infection and socio-economic status; CI = confidence interval; P for trend = adjusted P value for each variable when introduced into the model as a continuous predictor; SD = standard deviation.
As it is known that HIV co-infected individuals have on average lower AFB counts than TB-only infected subjects,25 the association of severe anemia and Hb levels with AFB density was assessed separately for HIV-negative and -positive participants. AFB density was adversely associated with Hb levels in HIV-negative participants of both sexes and in HIV-infected females; however, only the association in HIV-negative males was significant (P for trend = 0.003). Severe anemia prevalence had a positive association with AFB density in HIV-negative males and in HIV-positive participants of both sexes, which again was only significant in HIV-negative males (P for trend = 0.014). Contrary to this, the prevalence of severe anemia decreased (non-significantly) in HIV-negative females with higher AFB counts, and Hb levels in HIV-infected males showed no association with AFB density.
Severe disability as indicated by a Karnofsky score of <70% was significantly associated with lower Hb levels and a higher prevalence of severe anemia in both sexes (females PR = 1.75, P < 0.001; males PR = 1.39, P = 0.038).
DISCUSSION
Our data show that HIV co-infection in TB patients from Dar es Salaam is strongly and significantly associated with Hb levels and with severe anemia: co-infected participants of both sexes had around 10 g/l lower mean Hb values than HIV-negative participants, and the prevalence of severe anemia was doubled in HIV co-infected females and more than tripled in co-infected males.
Like most chronic infections, both HIV and TB can cause anemia. In both infections, decreased production of red blood cells seems to play an important role as a cause of anemia. Malabsorption syndrome and nutritional deficiencies, which are relatively common in our study population, may aggravate these problems.26,27
We identified more TB-infected males than females, and HIV co-infection was more prevalent in women than in men. In principal, both findings are in agreement with those from a study in South African hospital patients with TB infection6 and an earlier report by Range et al. from Tanzania.28 Both studies identified fewer female than male TB-infected participants and found higher HIV prevalence in females, although discrepancies between sexes in our study are larger than those found by Range et al.28 Reasons for the larger difference in HIV prevalence found between sexes are hard to ascertain; however, at least some of this difference could have been caused by different health-seeking behaviors among females and males, and by the different applicability of our various exclusion criteria to the two sexes, factors that might also have influenced the results of the above cited studies.
The Hb levels found in our study population are far below mean values for healthy participants from Dar es Salaam reported in the early 1970s (respectively 126 and 140 g/l for females and males29) and those reported in a recent study carried out in Mbeya, Tanzania (respectively 136 and 155 g/l for females and males30). Although in a severely ill population this is to be expected, the size of these differences, and the fact that anemia is a strong predictor of mortality both in TB6,31 and in HIV-infected subjects,5,28 warrants special attention to this problem in TB-HIV co-infected subjects.
As with severe anemia, HIV co-infected women were also most likely to be anemic (97%); however, the prevalence of anemia was high (>85%) in all four substrata (HIV-infected/non-infected, males/females), and mean Hb levels were correspondingly low (Table 4). A comparison of our numbers with those from two other East African sites shows that the Hb levels found in Dar es Salaam were more than 10% lower in each of the four substrata than those found in Kampala, Uganda,9 but that they were comparable to those from Zomba, Malawi.11 However, the prevalence of anemia in our study population was much higher than in both other sites, although the same threshold values were used.
Table 4.
Hb levels and prevalence of anemia (Hb < 120 g/l for females and < 130 g/l for males) in adults with active tuberculosis infection from three East African sites
| Females |
Males |
|||
|---|---|---|---|---|
| HIV+ | HIV− | HIV+ | HIV− | |
| Dar es Salaam, Tanzania | ||||
| Mean Hb, g/l | 87 | 97 | 99 | 111 |
| Anemia prevalence, % | 97.0 | 92.9 | 92.9 | 85.7 |
| Kampala, Uganda9 | ||||
| Mean Hb, g/l | 98 | 110 | 113 | 122 |
| Anemia prevalence, % | 85.5 | 67.9 | 62.6 | 42.6 |
| Zomba, Malawi11 | ||||
| Mean Hb, g/l | 92 | 99 | 96 | 111 |
| Anemia prevalence, % | 86.0 | 85.3 | 91.0 | 67.3 |
Hb = hemoglobin; HIV = human immunodeficiency virus; + = positive; − = negative.
In addition to HIV co-infection, various SES indicators were also negatively associated with Hb and positively with severe anemia, with stronger associations in males. The lack of significant associations for most of these indicators in females can partly be explained by the lower number of female participants. However, most associations of severe anemia and SES indicators in females also had less extreme PRs (i.e., PRs closer to unity) than in males, which hints at a real difference between sexes.
Nearly all anthropometric indicators were strongly and significantly associated with Hb and severe anemia in both sexes, independently of SES indicators and HIV co-infection. These associations are likely to reflect the effect of overall malnutrition, which includes iron deficiency and other micronutrient deficiencies that contribute to the etiology of anemia. In a previous study in the same population, we found that HIV co-infection is also independently associated with anthropometric indicators.32 For most of these indicators one could thus hypothesize a bi-directional relationship with Hb level, TB and HIV infection respectively, meaning that impaired health (as indicated by lower Hb levels or caused by HIV infection and/or active TB) would be both cause and consequence of declining anthropometric indicators.33
A major strength of the study is the relatively large sample size and the availability of data on SES indicators, which enables us to adjust the associations of anemia and Hb with HIV co-infection and anthropometric status for possible confounders. However, the study also has some limitations.
First, the cross-sectional design prevents us from establishing the temporal sequence of HIV infection, development of anemia and of clinical TB and the causal relationship between these three factors, SES and anthropometric status. However, it is clear that both HIV and TB infection predispose to further infection by compromising the immune system, and also have a direct impact on nutritional status and Hb levels.34-36 It is also known that low SES, nutritional deficiencies and low Hb are risk factors for HIV and TB infection. We would therefore hypothesize that most of these factors have bi- or multidirectional relationships with each other, thus forming a vicious cycle: low SES leads to nutritional deficiencies which have a negative impact on anthropometric outcomes and Hb levels and also on socio-economic activities. All this predisposes to infection with various agents, including HIV and M. tuberculosis.
Second, establishing a TB diagnosis by positive smears is likely to have excluded some HIV-co-infected subjects, who often present with lower AFB densities.25 The exclusion of subjects with TB treatment during the previous year might also have resulted in an under-representation of HIV-infected patients, who are likely to present with TB relapses. It is therefore possible that our study even underestimates the true association of TB-HIV co-infection with anemia.
Third, our study did not collect information on malaria, schistosomiasis and other possible causes of anemia. These unassessed factors could therefore have confounded some of our findings. Similarly, we are lacking information regarding the predominant type(s) of anemia in our study population which might have provided useful insights regarding the underlying causes for anemia.
CONCLUSION
Our data show that in our TB-infected study population, anemia is significantly associated with HIV co-infection and with anthropometric status independent of SES. Other studies have shown that anemia is also an important risk factor for disease progression and mortality both in HIV and in TB infection. Combined, and if confirmed in other settings, these findings would imply that the management of anemia in TB-HIV co-infected patients requires special attention.
Acknowledgements
The authors thank the study participants and the study coordinator, supervisors, nurses, and laboratory technicians who made this research possible. This study was supported by the United States National Institute of Allergy and Infectious Diseases (UO1 AI45441-01).
References
- 1.Harries AD, Hargreaves NJ, Kemp J, et al. Deaths from tuberculosis in sub-Saharan African countries with a high prevalence of HIV-1. Lancet. 2001;357:1519–1523. doi: 10.1016/S0140-6736(00)04639-0. [DOI] [PubMed] [Google Scholar]
- 2.Corbett EL, Watt CJ, Walker N, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . WHO report 2010. WHO; Geneva, Switzerland: 2010. Global tuberculosis control. WHO/HTM/TB/2010.7. [Google Scholar]
- 4.World Health Organization . Worldwide prevalence of anaemia 1993–2005: WHO global database on anaemia. WHO; Geneva, Switzerland: 2008. [Google Scholar]
- 5.Belperio PS, Rhew DC. Prevalence and outcomes of anemia in individuals with human immunodeficiency virus: a systematic review of the literature. Am J Med. 2004;116(Suppl 7A):27S–43S. doi: 10.1016/j.amjmed.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Sacks LV, Pendle S. Factors related to in-hospital deaths in patients with tuberculosis. Arch Intern Med. 1998;158:1916–1922. doi: 10.1001/archinte.158.17.1916. [DOI] [PubMed] [Google Scholar]
- 7.Morris CD, Bird AR, Nell H. The haematological and bio-chemical changes in severe pulmonary tuberculosis. Q J Med. 1989;73:1151–1159. [PubMed] [Google Scholar]
- 8.Lawson L, Yassin MA, Thacher TD, et al. Clinical presentation of adults with pulmonary tuberculosis with and without HIV infection in Nigeria. Scand J Infect Dis. 2008;40:30–35. doi: 10.1080/00365540701509899. [DOI] [PubMed] [Google Scholar]
- 9.Shah S, Whalen C, Kotler DP, et al. Severity of human immunodeficiency virus infection is associated with decreased phase angle, fat mass and body cell mass in adults with pulmonary tuberculosis infection in Uganda. J Nutr. 2001;131:2843–2847. doi: 10.1093/jn/131.11.2843. [DOI] [PubMed] [Google Scholar]
- 10.Swaminathan S, Padmapriyadarsini C, Sukumar B, et al. Nutritional status of persons with HIV infection, persons with HIV infection and tuberculosis, and HIV-negative individuals from southern India. Clin Infect Dis. 2008;46:946–949. doi: 10.1086/528860. [DOI] [PubMed] [Google Scholar]
- 11.van Lettow M, Kumwenda JJ, Harries AD, et al. Malnutrition and the severity of lung disease in adults with pulmonary tuberculosis in Malawi. Int J Tuberc Lung Dis. 2004;8:211–217. [PubMed] [Google Scholar]
- 12.Whalen CC, Nsubuga P, Okwera A, et al. Impact of pulmonary tuberculosis on survival of HIV-infected adults: a prospective epidemiologic study in Uganda. AIDS. 2000;14:1219–1228. doi: 10.1097/00002030-200006160-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subbaraman R, Devaleenal B, Selvamuthu P, et al. Factors associated with anaemia in HIV-infected individuals in southern India. Int J STD AIDS. 2009;20:489–492. doi: 10.1258/ijsa.2008.008370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villamor E, Mugusi F, Urassa W, et al. A trial of the effect of micronutrient supplementation on treatment outcome, T-cell counts, morbidity, and mortality in adults with pulmonary tuberculosis. J Infect Dis. 2008;197:1499–1505. doi: 10.1086/587846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karnofsky DA, Abelmann WH, Craver LF, Burchenal JH. The use of the nitrogen mustards in the palliative treatment of carcinoma. With particular reference to bronchogenic carcinoma. Cancer. 1948;1:634–656. [Google Scholar]
- 16.Urassa W, Godoy K, Killewo J, et al. The accuracy of an alternative confirmatory strategy for detection of antibodies to HIV-1: experience from a regional laboratory in Kagera, Tanzania. J Clin Virol. 1999;14:25–29. doi: 10.1016/s1386-6532(99)00043-8. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization Acquired immunodeficiency syndrome (AIDS). Proposed WHO criteria for interpreting results from Western blot assays for HIV-1, HIV-2 and HTLV-I/HTLV-II. Wkly Epidemiol Rec. 1990;65:281–283. [PubMed] [Google Scholar]
- 18.World Health Organization . Technical Report. WHO; Geneva, Switzerland: 1995. Physical status: the use and interpretation of anthropometry: report of a WHO Expert Committee. Series No 854. [PubMed] [Google Scholar]
- 19.Massawe SN, Urassa EN, Nystrom L, Lindmark G. Effectiveness of primary level antenatal care in decreasing anemia at term in Tanzania. Acta Obstet Gynecol Scand. 1999;78:573–579. [PubMed] [Google Scholar]
- 20.World Health Organization . A guide for programme managers. WHO; Geneva, Switzerland: 2001. Iron deficiency anaemia: assessment, prevention, and control. WHO/NHD/01.3. [Google Scholar]
- 21.Montgomery MR, Gragnolati M, Burke KA, Paredes E. Measuring living standards with proxy variables. Demography. 2000;37:155–174. [PubMed] [Google Scholar]
- 22.World Health Organization . Laboratory services in tuberculosis control. WHO; Geneva, Switzerland: 1998. WHO/TB/98.258. [Google Scholar]
- 23.White H. A Heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–830. [Google Scholar]
- 24.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 25.Mugusi F, Villamor E, Urassa W, Saathoff E, Bosch RJ, Fawzi WW. HIV co-infection, CD4 cell counts and clinical correlates of bacillary density in pulmonary tuberculosis. Int J Tuberc Lung Dis. 2006;10:663–669. [PubMed] [Google Scholar]
- 26.Lee SW, Kang YA, Yoon YS, et al. The prevalence and evolution of anemia associated with tuberculosis. J Korean Med Sci. 2006;21:1028–1032. doi: 10.3346/jkms.2006.21.6.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volberding PA, Levine AM, Dieterich D, Mildvan D, Mitsuyasu R, Saag M. Anemia in HIV infection: clinical impact and evidence-based management strategies. Clin Infect Dis. 2004;38:1454–1463. doi: 10.1086/383031. [DOI] [PubMed] [Google Scholar]
- 28.Range N, Ipuge YA, O’Brien RJ, et al. Trend in HIV prevalence among tuberculosis patients in Tanzania, 1991–1998. Int J Tuberc Lung Dis. 2001;5:405–412. [PubMed] [Google Scholar]
- 29.Nhonoli AM, Msuya PM, Kamuzora HL. Some normal haematological and other clinical values in Tanzanian adults. East Afr Med J. 1972;49:921–933. [PubMed] [Google Scholar]
- 30.Saathoff E, Schneider P, Kleinfeldt V, et al. Laboratory reference values for healthy adults from southern Tanzania. Trop Med Int Health. 2008;13:612–625. doi: 10.1111/j.1365-3156.2008.02047.x. [DOI] [PubMed] [Google Scholar]
- 31.Kourbatova EV, Borodulin BE, Borodulina EA, del Rio C, Blumberg HM, Leonard MK., Jr. Risk factors for mortality among adult patients with newly diagnosed tuberculosis in Samara, Russia. Int J Tuberc Lung Dis. 2006;10:1224–1230. [PubMed] [Google Scholar]
- 32.Villamor E, Saathoff E, Mugusi F, Bosch RJ, Urassa W, Fawzi WW. Wasting and body composition of adults with pulmonary tuberculosis in relation to HIV-1 coinfection, socio-economic status, and severity of tuberculosis. Eur J Clin Nutr. 2006;60:163–171. doi: 10.1038/sj.ejcn.1602281. [DOI] [PubMed] [Google Scholar]
- 33.van Lettow M, Fawzi WW, Semba RD. Triple trouble: the role of malnutrition in tuberculosis and human immunodeficiency virus co-infection. Nutr Rev. 2003;61:81–90. doi: 10.1301/nr.2003.marr.81-90. [DOI] [PubMed] [Google Scholar]
- 34.Killewo J. Poverty, TB and HIV infection: a vicious cycle. J Health Popul Nutr. 2002;20:281–284. [PubMed] [Google Scholar]
- 35.Cahn P, Perez H, Ben G, Ochoa C. Tuberculosis and HIV: a partnership against the most vulnerable. J Int Assoc Physicians AIDS Care (Chic Ill) 2003;2:106–123. doi: 10.1177/154510970300200303. [DOI] [PubMed] [Google Scholar]
- 36.Schaible UE, Kaufmann SH. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med. 2007;4:e115. doi: 10.1371/journal.pmed.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]