Abstract
Caveolin-1 is an integral membrane protein primarily responsible for the formation of membrane structures known as caveolae. Caveolae are specialized lipid rafts involved in protein trafficking, cholesterol homeostasis, and a number of signaling functions. It has been demonstrated that caveolin-1 suppresses HIV-1 protein expression. We found that co-transfecting cells with HIV-1 and caveolin-1 constructs, results in a marked decrease in the level of HIV-1 transcription relative to cells transfected with HIV-1 DNA alone. Correspondingly, reduction of endogenous caveolin-1 expression by siRNA-mediated silencing resulted in an enhancement of HIV-1 replication. Further, we observed a loss of caveolin-mediated suppression of HIV-1 transcription in promoter studies with reporters containing mutations in the NF-κB binding site. Our analysis of the posttranslational modification status of the p65 subunit of NF-κB demonstrates hypoacetylation of p65 in the presence of caveolin-1. Since hypoacetylated p65 has been shown to inhibit transcription, we conclude that caveolin-1 inhibits HIV-1 transcription through a NF-κB-dependent mechanism.
Keywords: HIV-1, caveolin-1, NF-κB, acetylation, retrovirus, cholesterol
Introduction
Human Immunodeficiency Virus -1 (HIV-1) is a human retrovirus that infects CD4+ T lymphocytes and myeloid cells. Our studies and those of others have demonstrated that HIV-1 biology is critically dependent upon cholesterol (Chertova et al., 2006; Graham et al., 2003; Liao et al., 2001). HIV infection of T cells enhances expression of genes encoding proteins involved in cholesterol biosynthesis (Zheng et al., 2003; Zheng et al., 2001). Conversely, inhibiting the cholesterol biosynthetic pathways during HIV-1 infection suppresses replication of the virus (del Real et al., 2004). Cholesterol-enriched cellular membrane microdomains known as lipid rafts are the sites for HIV-1 entry and egress (Liao et al., 2001; Nguyen and Hildreth, 2000). The treatment of cells with cyclodextrins has a potent effect on the integrity of lipid rafts (Graham et al., 2003; Liao et al., 2001). Notably, HIV-1 susceptible cells treated with cholesterol-sequestering cyclodextrins are less susceptible to HIV-1 infection (Graham et al., 2003; Liao et al., 2001; Liao et al., 2003). Additionally, disruption of lipid rafts reduces virion infectivity and release from cholesterol-enriched cells (Booth et al., 2006; Gould et al., 2003; Jouvenet et al., 2006; Liao et al., 2003; Nguyen et al., 2003; Welsch et al., 2007). The dependence on cholesterol for multiple aspects of HIV-1 biology suggests that cellular mechanisms that regulate cholesterol homeostasis may modulate HIV-1 replication (del Real et al., 2004; Graham et al., 2003; Liao et al., 2001; Liao et al., 2003; Mujawar et al., 2006; Waheed et al., 2007).
In addition to cholesterol and glycosphingolipids, lipid rafts are also comprised of proteins (Hooper, 1999; Lajoie and Nabi). One such protein, caveolin-1, is a cholesterol-binding protein that is responsible for the formation of caveolae, specialized membrane structures that represent a distinct class of lipid rafts (Kurzchalia and Parton, 1999). Caveolins are a family of proteins that consist of four isoforms including: caveolin-1α, -1β, −2 and -3. These proteins are expressed in most nucleated cells, excluding T-lymphocytes (Arakawa et al., 2000; Glenney and Soppet, 1992; Matveev et al., 1999; Razani et al., 2002). Caveolae are 60-100 nm flask-like invaginations in the plasma membrane. Caveolae, like lipid rafts in general, are enriched in cholesterol, sphingolipids, and other raft-specific constituents (Dobrowsky, 2000; Fielding and Fielding, 2000; Frank et al., 2006; Harder and Simons, 1997; Hooper, 1999; Kurzchalia and Parton, 1999; Quest et al., 2004). Caveolae function as scaffolds for signaling proteins and as vesicles for intracellular cholesterol transport (Arakawa et al., 2000; Fielding and Fielding, 1997; Frank et al., 2006; Ikonen, 1997). Caveolin-1 has been reported to function as an inhibitor of signaling molecules such as endothelial nitric oxide synthase (eNOS) and is known to modulate processes such as chronic inflammation (Razani et al., 2001; Vargas et al., 2002; Yeh et al., 2004). Several studies have demonstrated that caveolin-1 regulates inflammation through the p38 MAPK and NF-κB pathways (Garrean et al., 2006; Lv et al.; Pontrelli et al., 2006; Wang et al., 2006). Another group reported that caveolin-1 null mice (cav-1 -/-) displayed a significant decrease in NF-κB activity after LPS stimulation, indicating that caveolin-1 plays a critical role in some antimicrobial defense mechanisms (Garrean et al., 2006).
Ectopic expression of caveolin-1 has been shown to suppress the replication of HIV-1 (Llano et al., 2002). However, the molecular mechanism of this inhibition of the HIV-1 life cycle by caveolin-1 remains largely undefined (Carter et al., 2010). Inhibitory effects of caveolin-1 appeared to be HIV-1 specific, as caveolin had little or no effect on other classes of viruses (Llano et al., 2002). Notably, caveolin-1 did not suppress virus species that replicated in the cytoplasm. In another study, HIV-1 infection of macrophages appeared to correlate with an induction of caveolin-1 expression in a HIV-Tat dependent manner (Lin et al., 2010). HIV-1 infection has been shown to lead to induction of a number of signal transduction pathways, some of which involve caveolin-1 (Bennasser et al., 2001; Cheng et al., 2009; Yang et al.). In the present study, we determined that the inhibitory effect of caveolin-1 on HIV-1 replication is mediated through modulation of the NF-κB-dependent inflammatory pathway. Specifically, we demonstrate that caveolin-1 inhibition of HIV-1-LTR promoter activity is mediated via suppression of NF-κB acetylation.
Results
Caveolin-1 expression suppresses HIV-1 production
Studies have shown that expression of caveolin-1 creates a restrictive cellular environment for HIV-1 replication (Lin et al., 2010; Llano et al., 2002). More recently, it has been shown that HIV-1 infection induces caveolin-1 expression in macrophages (Lin et al., 2010). These findings have intriguing implications and they may in part explain the persistence of HIV-1 infection in monocytic cells in the absence of the cytopathology observed in lymphocytes. We set out to determine the step of the HIV-1 life-cycle impacted by caveolin-1. We first verified that caveolin-1 expression decreased virus production as previously reported. To that end, 293T cells were transfected with caveolin-1 or empty vector along with HIV-1 molecular clone, pYU-2. Forty-eight hours post-transfection, virus production was measured using Western blot analysis and HIV-1 p24 ELISA. We observed a significant reduction in cell-associated virus proteins (Figure 1A) and virus present in cell culture supernatant from cells overexpressing caveolin-1 (Figure 1B). These observations confirmed that caveolin expression was associated with lower HIV replication. To further explore this relationship, 293T cells were sequentially transfected, first with control or caveolin-1 specific siRNAs and then with HIV-1 molecular clones. Cells transfected with caveolin-1 siRNA exhibited a reduction in caveolin-1 expression when compared to control cells (Figure 1C). This reduction of caveleolin-1 expression was sufficient to enhance the level of cell-associated viral proteins when compared to control cells (Figure 1C). This effect was demonstrated with two independent siRNAs. These data indicate that caveolin-1 expression restricts HIV-1 viral replication in 293T cells as previously reported.
Figure 1. Overexpression of caveolin-1 suppresses HIV-1 protein expression and virus release in 293T cells.
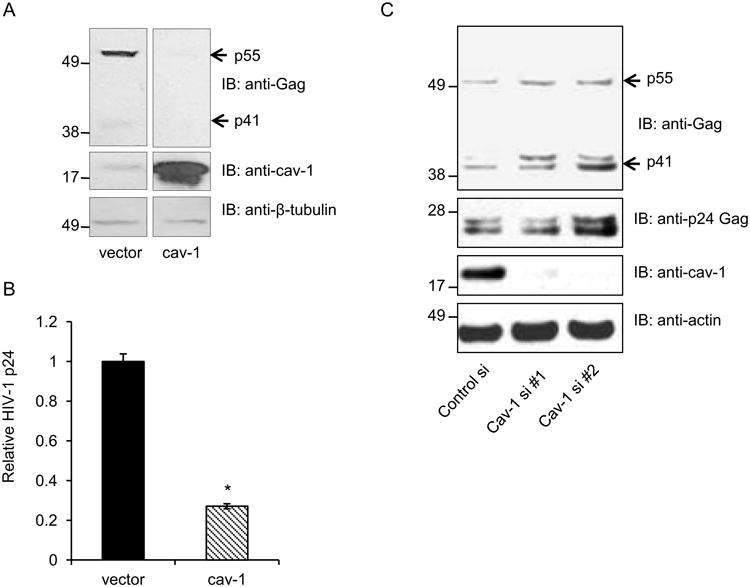
Here, 293T cells were co-transfected with HIV-1 molecular clone pYU-2 and caveolin-1expression construct or vector control. Cells and supernatants were harvested 48 hours post-transfection. A. HIV-1 Gag protein expression was analyzed by Western blot analysis with an anti-Gag mAb (upper panels). We confirmed expression of caveolin-1 by probing with a polyclonal antibody (middle panels) and used β-tubulin as a loading control (bottom panel). B. Virus particle production was assessed by performing HIV-1 p24 ELISA using supernatants from transfected cells. Graph represents 3 independent experiments done in replicates with S.E.M., p value< 0.05. C. 293T cells were first transfected with one of 2 independent caveolin-1 specific or control siRNAs, then 48 hours later transfected with pYU-2. Protein expression was analyzed after an additional 24 hours.
Caveolin-mediated HIV-1 suppression is independent of modulation of cellular cholesterol
Caveolin-1 proteins bind and transport cholesterol within cells (Fielding and Fielding, 1997; Ikonen and Parton, 2000). Caveolae regulate factors that are involved cholesterol homeostasis, such as SR-BI and ABCA1 (Bist et al., 1997; Frank et al., 2006; Lin et al., 2010; Lin et al., 2009; Wong et al., 2006; Yeh et al., 2004), and some of these affect HIV-1 replication (Morrow et al., 2010; Mujawar et al., 2006; Mujawar et al., 2010). Therefore, we asked whether caveolin-mediated inhibition of HIV-1 involved disruptions in cholesterol homeostasis, as measured by changes in total cellular free cholesterol. To test this, we first transfected 293T cells with caveolin-1 or control vectors. Untransfected cells treated with cholesterol efflux inducer, LXR agonist TO-901317, or cholesterol sequestering agent β-cyclodextrin, served as positive controls for the assay. Cells were harvested two days post transfection. The cells were then fixed and stained with filipin to label total free cholesterol and subsequently analyzed by flow cytometry. As expected, treatment of cells with TO-901317 or β-cyclodextrin reduced the free cholesterol content of cells (Figure 2A). However, cells overexpressing caveolin-1 had no detectable difference in free cholesterol content when compared to controls (Figure 2B). These observations were recapitulated in both membrane fraction analyses of transfected cells, as well as fluorescent microscopy (data not shown). These results indicate that caveolin-1 does not cause significant changes in cellular cholesterol localization or content in our model system.
Figure 2. Caveolin-1 overexpression in 293T cells does not cause global changes in free cholesterol content.
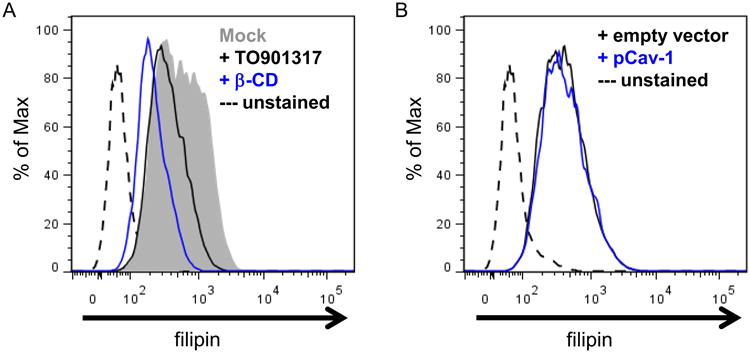
293T cells transfected with caveolin-1 or empty vector DNA were stained with fluorescent cholesterol-binding reagent filipin at 48 hours post –transfection to assess total free cholesterol in the cells. A. As a positive control for the assay, untransfected cells were treated with conditions that stimulated cholesterol efflux (5uM TO901317 for 48 hours)or a cholesterol sequestering agent (1% Beta-Cyclodextrin for 1 hour), prior to filipin staining as described in Methods. Cellular free cholesterol content was determined by flow cytometry and graphed in a histogram as indicated. Dotted lines are control cells that were not stained.
B. Caveolin-1 transfected or control cells were stained with filipin to determine the cellular cholesterol levels of transfected cells. Data for filipin-stained cell populations are plotted as histograms.
Transcription of HIV-1 is suppressed in the presence of caveolin-1
While it was known that manipulations of cholesterol content in HIV-1 susceptible cells have a major effect on virus replication (Liao et al., 2001; Liao et al., 2003), the effect of caveolin-1 on HIV-1 is still largely uncharacterized. Because HIV-1 replication involves a number of essential steps, we thought it necessary to determine at which replication steps caveolin-1 inhibited HIV-1. Since caveolin-1 is a membrane protein with roles in signaling events, we wanted to determine whether the protein affected early steps in virus replication like transcription, or late steps such as assembly and budding. It has been shown that HIV-1 Gag expression alone is sufficient to generate virus-like particles (VLP) that can be released from cells (Haynes et al., 1991; Rasmussen et al., 1990; Wang et al., 1990). We utilized a cytomegalovirus (CMV) promoter-driven Gag-GFP plasmid to examine VLP release from cells overexpressing caveolin-1. Cells and cell supernatants were collected 48-hours post-transfection in order to measure the HIV-1 VLP production by p24 ELISA. The total amount of HIV-1 Gag in the system was measured, (i.e. intracellular Gag and Gag present in cell supernatants), and those values were used to calculate the virus particle release efficiency (Figure S1). Virus particle release efficiency is the percentage of HIV-1 Gag released from the cell relative to the total amount of Gag produced in the system. No differences in release efficiency were observed between experimental conditions. This indicates that overexpression of caveolin-1 did not alter the ability of virus to exit cells. We next determined the effect of caveolin overexpression on HIV-LTR transcription. To test this we co-transfected caveolin or control plasmids with an HIV-LTR GFP-reporter construct. Flow cytometric analysis of transfected cells revealed a substantial reduction in HIV-LTR activity in cells that expressed caveolin (Figure 3A). Next we decided to test the specificity of this caveolin-dependent transcriptional suppression by using GFP reporters with either LTR or CMV promoters. The CMV promoter is constitutively active in mammalian cells, and therefore an adequate control for global changes in transcriptional activity in a cell (Fitzsimons et al., 2002). On the other hand, the HIV-LTR is highly regulated and often requires cellular activation before maximal activity can be detected (Cheng et al., 2009; Huang et al., 1994; Kinoshita et al., 1998; Malcolm et al., 2007; Marzio et al., 2002; Sadowski and Mitchell, 2005; Verhoef et al., 1999). Each viral promoter construct was co-transfected with caveolin-1 into 293T cells which were harvested 48 hours later for flow cytometric analysis. The fluorescent signals were quantified and plotted in graphs based on the relative mean fluorescence intensity (MFI). In cells transfected with CMV-Gag-GFP there were no differences between caveolin-1-transfected and control cells (Figure 3B). In contrast, caveolin-1 expression resulted in a substantial decrease in GFP signal in the LTR-GFP cells. Using HIV-1 specific antibodies in FACS analysis, we were able to observe inhibition of HIV-1 protein expression up to 70% in caveolin-1 transfected cells (data not shown).
Figure 3. Caveolin-1 suppresses HIV-1 promoter activity.
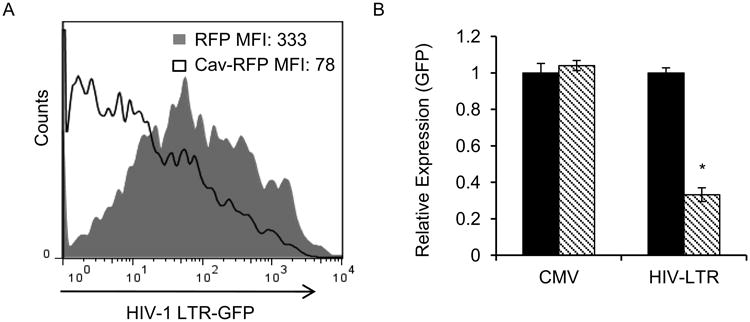
A. 293T cells were transfected with caveolin-1-RFP expression construct (filled histogram) or RFP control vector (open histogram), along with HIV-1-LTR GFP reporter. HIV-1 LTR GFP signal was measured by flow cytometry 48 hours post transfection and data was plotted in a histogram, where MFI of samples are indicated. B. 293T cells were co-transfected with caveolin-1 (stripped) or vector control (black) and either Cytomegalovirus (CMV) - or HIV-1 LTR-promoter driven Gag-GFP plasmids. Cells were harvested 48 hours post-transfection and GFP-MFI signal from the individual promoters was analyzed by flow cytometry. Graphs represent 3 independent experiments done in replicates with S.E.M., p value< 0.05.
We observed caveolin-1-mediated suppression of transcription from HIV-1 reporters in our system under basal conditions, where no stimulus like LPS or TNF-alpha was administered. We decided to test the ability of caveolin-1 to suppress Tat-activated HIV transcription. The HIV-1 Tat protein is a potent transactivator of HIV transcription and provides a robust increase in LTR activity (Huang et al., 1994; Marzio et al., 2002; Yang et al.). Caveolin-1 was found to suppress both basal and Tat-enhanced transcription in 293T cells (Figure 4A). To confirm that caveolin-1 suppresses HIV-1 promoter activity in a physiologically relevant system, we co-transfected THP-1 cells with caveolin-1 expression plasmids and an HIV-1 reporter. The cells were cultured in the absence or presence of TNF-alpha and then we measured the activity of the reporter 12 hours post transfection (Figure 4B). Further, we assessed the capacity of caveolin-1 to suppress HIV-1 transcription when p65 is overexpressed (Figure 4C). In these experiments, we were able to determine that caveolin-1 was capable of suppressing HIV-1 transcription under all conditions. Correspondingly, we observed an increase in HIV-1 transcription in THP-1 cells that were first transfected with siRNA against caveolin-1 and then infected with a VSV-G pseudotyped HIV-1 reporter virus (Figure S2). Taken together our data strongly implicate caveolin-1 as a transcriptional inhibitor of the HIV-1 promoter
Figure 4. Caveolin-1 suppresses both tat-independent and dependent HIV-1 LTR transcription.
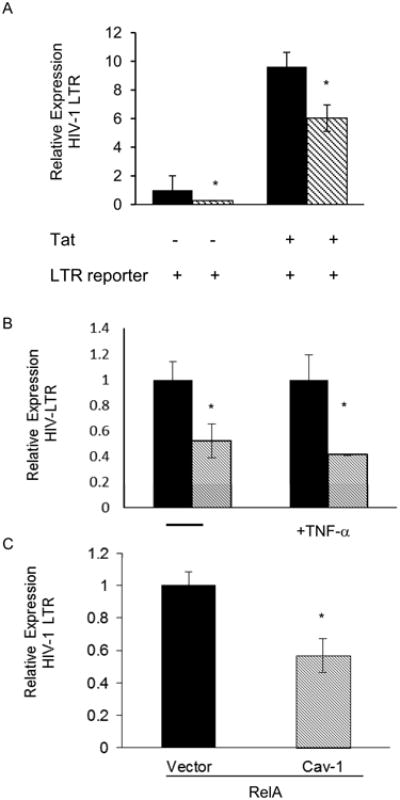
Cells were transfected with caveolin-1DNA and transcriptional activity from HIV-1 reporters was measured by flow cytometry. A. 293T cells were co-transfected with caveolin-1 (stripped) or empty vector control (black) along with HIV-1 LTR reporter and −/+HIV-1 Tat. HIV-1-LTR-GFP fluorescence was measured 24 hours post-transfection by flow cytometry. Displayed is the average of 3 independent experiments. B. THP-1 monocytic cells were transfected with vector or caveolin along with HIV-1 LTR reporter and treated with 50 ng/ml of TNF-α for the last 2hours of cell culture before harvest. The GFP signal from the HIV-1-LTR reporter was measured by flow cytometry. Caveolin-transfected samples were normalized to control vector transfected counterparts. TNF-α treated samples were normalized to 1.C. THP-1 cells were co-transfected with caveolin-1 expression construct or empty vector control along with RelA expression construct and HIV-1 LTR GFP reporter. All graphs represent at least 2 independent experiments done in replicates with S.E.M., p value< 0.05
Caveolin-1 suppression of HIV-1 replication requires NF-κB
It is well documented that HIV-1 infection induces a number signaling pathways, including the inflammatory pathway (Herbein and Varin, 2010; Ross et al., 2006). Inflammation is a host defense mechanism against infection and is largely sufficient to create an inhospitable environment for many pathogens. However, this is not the case for HIV-1 infections, which induce inflammation and in fact benefit greatly from the activation of the numerous host transcription factors that are known to mediate the inflammatory response (DeLuca et al., 1999; Montano et al., 1996; Roulston et al., 1993; Verhoef et al., 1999; Westendorp et al., 1995). Caveolin-1, on the other hand, has been shown to suppress inflammatory responses (Chidlow and Sessa; Garrean et al., 2006; Lv et al.; Wang et al., 2006). Since our data indicates that caveolin-1 specifically inhibits HIV-1-LTR transcription, we hypothesize that NF-κB could mediate caveolin's effect on HIV-1 replication. NF-κB binding sites are present in the HIV-1 LTR, and in cells other than T cells, NF-κB drives the majority of HIV transcription (DeLuca et al., 1999; Montano et al., 1996; Roulston et al., 1993; Westendorp et al., 1995). To determine the contribution NF-κB to inhibition of HIV-1 by caveolin, NF-κB reporters were co-transfected along with caveolin expression constructs into 293T cells. Fourteen hours post-transfection, the cells were treated with 10 ng/mL of TNF-α for 10 hours. After stimulation with TNF-α, cells were harvested and analyzed by microscopy and flow cytometry. Microscopy showed that the overall level of the NF-κB-GFP signal was reduced in caveolin-1 transfected cells (Figure 5A, top). We noted a difference in the intensity of RFP signal from caveolin-1-RFP transfected cells when compared to RFP vector transfected cells, possibly due to intrinsic differences in protein stability. Flow cytometry data indicated that NF-κB activity was suppressed in cells transfected with caveolin-1 (Figure 5A, lower). To determine the NF-κB -dependence of caveolin-mediated suppression of HIV-1 transcription, we next transfected cells with either wild-type HIV-LTR (wtLTR) reporters or reporter containing point mutations in the NF-κB binding site (nkLTR). We observed caveolin-1-dependent suppression of transcription only in cells transfected with a wild-type LTR. The mutant HIV-1 reporter (nkLTR) was insensitive to caveolin-1-mediated suppression (Figure 5B). To further examine the requirement for NF-κB in caveolin-mediated suppression, we next employed specific inhibitors of NF-κB activation in cells transfected with caveolin-1 and HIV-1 molecular clone pNLENG1. Transfected cells were cultured in the absence or presence of 50 μg/ml of specific IκB kinase (IKK) inhibitor peptide for 18 hours. In cells that received the IκB kinase inhibitor peptide, caveolin-1-mediated suppression was ablated (Figure 5C). These observations indicate that caveolin suppresses HIV-1 expression not by simply inhibiting NF-κB activity but by a mechanism that requires NF-κB nuclear translocation. Our results also suggest that NF-κB binding to the HIV-1 promoter is a required step for caveolin-1-mediated suppression of HIV-1 transcription.
Figure 5. Caveolin-1-dependentsuppression of HIV-1 transcription is mediated by NF-κB.
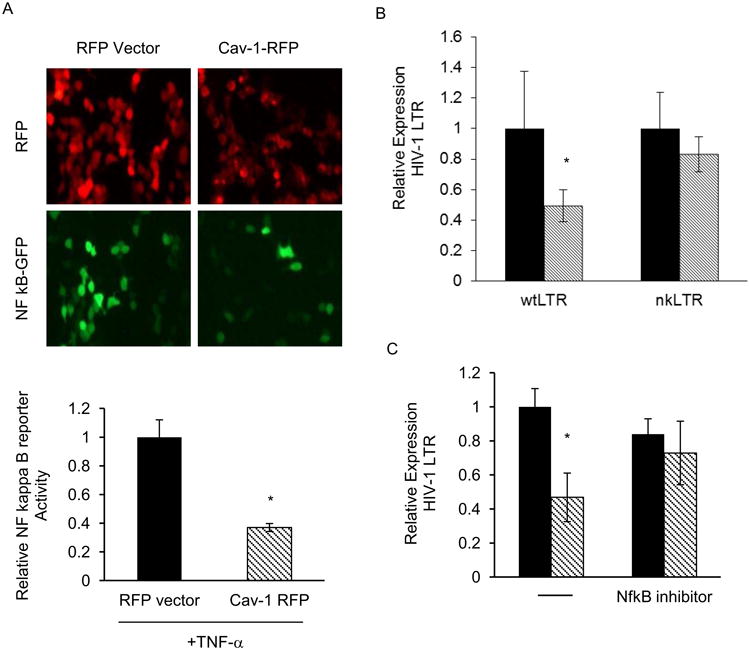
A. 293T cells were transfected with caveolin-1-RFP or control RFP expression vector with a NF-κB-GFP reporter construct then treated with 50 ng/mL of TNF-α for last 14 hours of the experiment. Cells were visualized by fluorescence microscopy (top) 24 hours post-transfection and collected and analyzed for NF-κB activity (bottom) by flow cytometry. Relative expression (MFI-GFP) is graphed for RFP-caveolin-1 expression construct (stripped bars) or RFP control vector (black bars) transfected samples. B. Cells were transfected as in (A) with HIV-1-LTR GFP reporters (wild-type, wtLTR or mutated NF-κB binding site, nkLTR) along with RFP-caveolin-1 expression construct (stripped bars) or RFP control vector (black bars) and 24 hours post-transfection reporter activity was analyzed by flow cytometry. C. 293T cells were transfected with RFP-caveolin-1 (stripped bars) or RFP control vector (black bars) expression construct with HIV-1-LTR GFP reporter, then treated with IκB kinase inhibitor peptide, SN50 (50 μg/mL) for 18 hours. HIV-1-LTR reporter activity was analyzed by flow cytometry. All data shown represents 3 independent experiments. Graph represents three independent experiments done in replicates with S.E.M. p value < 0.05.
Caveolin-1 decreases the acetylation of NF-κB p65
We have demonstrated that caveolin-1-mediated suppression of HIV-1 requires NF-κB translocation, but we have not determined the consequence of that relocation. Our data clearly show that NF-κB-dependent transcription is reduced when caveolin-1 is expressed and that nuclear NF-κB is involved. Interestingly, Chen at al. have described post-translational modifications at K310 that could alter NF-κB transcriptional activity. In their work unacetylated p65 subunit was unable to drive transcription. Nonetheless, up until this point it was unclear how a normally positive modulator of HIV-1 transcription was made to suppress viral replication by caveolin-1. Post-translational modification of p65 is required for optimum transcriptional activity (Chen et al., 2002; Chen et al., 2005). Acetylation is one such modification that has been described as a molecular switch for transcriptional activation, providing an additional layer of NF-κB regulation (Kiernan et al., 2003). Therefore we suspected that caveolin-1 overexpression affected the post-translational modification of the p65, making it less transcriptionally active. To test this possibility in 293T cells, we first co-expressed caveolin-1 with p65-myc, and then probed for acetylation specifically at K310. As predicted, we observed a significant decrease in K310 acetylation (Figure S3). These observations indicated that caveolin-1 suppresses p65 K310 acetylation.
Intriguingly, Kiernan et al. observed that acetylation at residues 122/123 of p65 has the ability to lower the DNA-binding capacity of NF-κB. Hence, suppression of p65 acetylation could result in increased binding of transcriptionally inert p65 to κB, resulting in reduced expression of NF-κB-responsive genes. In lieu of having available reagents that can be used to probe for acetylation of K122/123, we assessed global acetylation levels of p65 in the presence of caveolin-1. Here, 293T cells were first co-transfected with p65-myc and caveolin-1 expression constructs or vector control. Thirty-six-hours after transfection, we prepared cell lysates which were subjected to immunoprecipitation of transfected p65 using antibodies directed against the myc-epitope in overexpressed p65 or acetylated p65 with pan-specific anti-acetyl-lysine antibodies. Next, we detected the total amount of p65 precipitated in each sample with a pan-specific p65 antibody We observed a marked reduction in the amount of p65 in the acetyl-lysine immunoprecipitates of samples overexpressing caveolin-1 when compared to vector control (Figure 6A, lane 8 and 9). However we detected similar amounts of p65 in the samples immunoprecipitated using anti-myc antibody (Figure 6A, lanes 5 and 6). These observations support the hypothesis that caveolin-1 expression can suppress global acetylation of p65.
Figure 6. Caveolin-1 overexpression decreases total acetylation of p65.
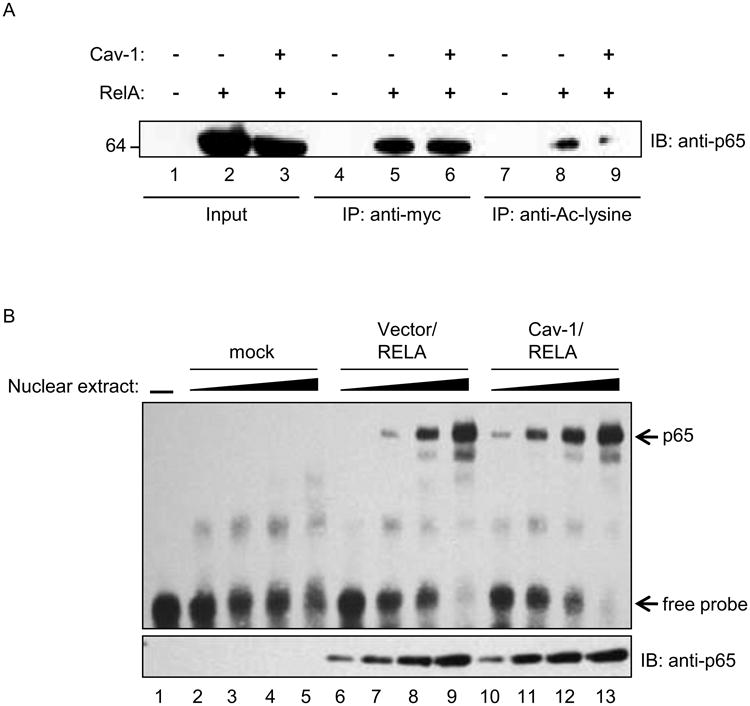
293T cells were transfected with caveolin-1 and myc-tagged RelA expression constructs as indicated. A. Total cells lysates were immunoprecipitated using either anti-myc or anti-acetyl-lysine antibodies and analyzed by and Western blot analysis. Blots were probed with monoclonal anti-p65 antibodies to determine efficacy of pulldown. B. Electrophoretic mobility shift assays (EMSA) performed with increasing volumes of nuclear extracts (0.5-4uL) from caveolin-1 and RelA transfected 293T cells. DNA binding was determined using biotinylated-HIV-1-LTR oligonucleotides and chemiluminescent detection. Replica nuclear extract samples were analyzed for p65 expression by Western blot (lower panel).
To assess if global hypoacetylation of p65 mediated by caveolin-1 results in differential DNA binding, we performed electrophoretic mobility shift assays (EMSA). For these studies, nuclear extracts were prepared from 293T cells co-transfected with caveolin-1 and p65-myc expression constructs. We observed a 3.5-fold enhancement in the level of p65 bound to kB-containing probes derived from the HIV-1 LTR when comparing nuclear extracts from caveolin-1 transfected cells to extracts from control cells (Figure 6B, compare lanes 7 and 11). The shifted species was not detected when using an HIV-1 LTR probe with mutated κB sites (data not shown). This finding was also independently verified by a plate-based transcription factor binding assay (Figure S4). Increased p65 DNA binding was transfection and concentration dependent. These findings indicate that caveolin-1 expression resulted in modifications of NF-κB that not only lowered its transcriptional activity, but also increased the DNA-binding capacity to κB sites. This occurs via combined hypoacetylation of NF-κB residues K310 and K122/123, which regulate transcriptional activity and DNA-binding, respectively (Chen et al., 2002; Kiernan et al., 2003). These observations likely explain the profound effect of caveolin-1 on HIV-1 replication in cells that require NF-κB to drive viral transcription. Our model for caveolin-mediated suppression of HIV-1 showed that caveolin alters the equilibrium between acetylated and unacetylated forms of p65 (Figure 7). This shift increases the pool of p65 that is transcriptionally inert but capable of DNA binding, thus providing a mechanism for suppressing NF-κB-dependent transcription.
Figure 7. Model of caveolin-1 mediated suppression of HIV-1 transcription via hypoacetylation of NF-κB p65 subunit.
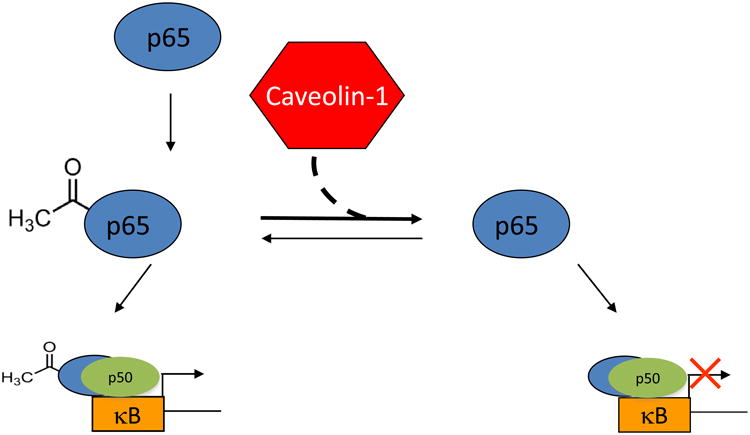
Expression of caveolin results in hypoacetylation of p65 resulting in increased binding to DNA while making the protein transcriptionally inert.
Discussion
It has been several years since the first report of caveolin-1-dependent inhibition of HIV-1 (Llano et al., 2002). During that time there have been few attempts to identify the mechanism for caveolin-1 suppression of HIV-1. A recent study demonstrated that caveolin-1 interacts with HIV-1 envelope proteins (gp41) and potentially facilitates entry (Hovanessian et al., 2004). A number of reports have indicated that caveolin-1 plays some role in modulating viral pathogenesis in vivo (Benferhat et al., 2009a; Benferhat et al., 2009b; Benferhat et al., 2008; Fermin and Garry, 2005; Hovanessian et al., 2004; Huang et al., 2007; Rey-Cuille et al., 2006; Wang et al., 2010). Based on some of our previous studies on the role of cholesterol in HIV-1 biology, we initially suspected that the mechanism of HIV-1 inhibition by caveolin would be linked to its role as a cholesterol transporter. However, data from the current study indicated that caveolin's role in cholesterol homeostasis was not essential to the mechanism by which it inhibits HIV-1 replication. Based on the known role of caveolin in modulation of inflammatory responses, we queried whether altered signal transduction could explain suppression of HIV-1 by the protein. NF-κB is the prototypical initiator of inflammatory processes in cells and is also one of the major transcription factors induced during the course of HIV-1 infection. We hypothesized that caveolin-1 inhibited HIV-1 replication by suppressing NF-κB-induced transcription.
Our results showed that instead of reducing NF-κB association with DNA, caveolin expression increased DNA binding of nuclear NF-κB. Further, mutation of the NF-κB binding site in the HIV-LTR ablated the suppressive effect of caveolin on HIV transcription. These results indicated that caveolin-mediated suppression of HIV-1 required nuclear association of NF-κB and binding to the LTR. While we were completing our studies, Wang et al published a study confirming that caveolin suppressed HIV-1 transcription through an NF-κB-dependent mechanism (Wang et al., 2011). In that study, the investigators abrogated caveolin-1 mediated suppression of HIV-1 by blocking nuclear translocation of NF-κB with the inhibitor SN50 confirming that NF-κB was necessary for caveolin-1 mediated suppression of HIV-1 replication. We extended our observations and those of the study by Wang et al. by demonstrating that binding of hypoacetylated NF-κB to the HIV-1 promoter is the specific mechanism by which caveolin-1 inhibits HIV-1.
It is possible that cells, such as macrophages, have developed mechanisms to protect themselves from the cytopathic effects of HIV-1 infection. Modulation of caveolin-1 levels during the course of infection may be a host cell response designed to combat HIV-1 replication at the level of viral transcription (Lin et al., 2010). The detailed steps that are involved in the mechanism are still somewhat unclear, but with the evidence presented in this study it is clear that caveolin-1-mediated suppression of HIV-1 requires DNA binding of NF-κB p65. This finding is similar to a phenomenon previously described when unacetylated p65 is present in the nucleus. In the absence of acetylation, NF-κB p65 suppresses transcriptional activity, while showing enhanced DNA binding capacity. Currently it is unclear how caveolin could affect acetylation of p65. One potential mechanism would be caveolin-dependent reduction of expression or activity of molecules such as SIRT-1 or p300, which are responsible for acetylation status of NF-κB. Investigating the effect of caveolin-1 on these molecules will be the subject of further studies. Understanding how caveolin modulates factors that regulate NF-κB p65 function may provide important new insights into differences in HIV-1 replication in T cells and macrophages.
Materials and Methods
Cells and constructs
293T human embryonic kidney cells were cultured in DMEM (GIBCO/Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum, 10mM L-glutamine, and 5mM Hepes and 100U/ml penicillin and streptomycin (complete DMEM). THP-1 monocytes were cultured in RPMI (GIBCO/Invitrogen, Carlsbad, CA, Carlsbad, CA) supplemented with 10% fetal bovine serum, 10mM L-glutamine, and 5mM HEPES (complete RPMI). pYU-2 (obtained from NIH AIDS Research and Reference Reagent Program) is a CCR5- tropic HIV-1 molecular clone derived from a human brain viral isolate. Caveolin-1 cDNA was excised from pOTB7 by EcoRI/XhoI digestion and subcloned into pcDNA3.1+ vector, (pCav1). Caveolin-1-myc-RFP and RFP-myc vectors were kind gifts from Dr. Robert Nabi (University British Columbia) were made by subcloning caveolin-1 gene into pRFP-N1 vector (Clontech, Mountain View, CA). NF-κB reporter, Cignal NFκB Reporter (GFP) Kit (CCS-013, SABioscience). Caveolin-1 silencing experiments were performed using Silencer select pre-designed caveolin-1 siRNA, 5′-GCCGUCUAUUCCAUCUAtt3′ (Ambion Inc., Foster City, CA). Wild-type and mutant HIV-1-LTR constructs were cloned into pZsgreen expression vector (cat. No. 632446, Clontech, Mountain View, CA) to generate LTR reporters as previously described (Taylor et al., 2011). The following primers were used to generate HIV-1-LTR and HIV-1-NF-κB mutant LTR reporters: wtLTR-Zsgreen- 5′-TGACATCGAGCTTGCTACAAGGGACTTTCCGCTGGGGACTTTCC-3′ and nkLTR-Zsgreen 5′-TGACATCGAGCTTGCTACAAGCCACTTTCCGCTGGGGACTTTCC-3′.
Virus Stock
Virus preparation and viral infection. Virus stocks were prepared by transfecting 293T cells with HIV-1 plasmid DNA using Lipofectamine 2000 reagent (Invitrogen). VSV-G envelope-pseudotyped virus was produced as previously reported (Zhou et al., 2005). Culture supernatants were collected 48 h after transfection, centrifuged at 1,000 × g to remove cell debris, filtered through a 0.45-um-pore-size filter, and concentrated by ultracentrifugation at 100,000 × g through a cushion of 20% sucrose in PBS. The pelleted virus was resuspended in RPMI with 10% FBS, aliquoted, and stored at −80°C. The viral titer was measured by anti-p24Gag ELISA.
Transfection
Transfections were carried out by utilizing the Lipofectamine 2000 (Invitrogen, Carlsbad, CA) protocol for plasmid DNA transfection and Oligofectamine (Invitrogen, Carlsbad, CA, Carlsbad, CA) for siRNA transfection per manufacturer's instructions. Preliminary transfections utilized pCav1 and pYU-2. siRNA transfections were done in a sequential manner to maximize the effect of caveolin-1 knockdown on HIV-1 replication. Cells were transfected with siRNA as previously described, and incubated for 24 hours. Cells were then transfected with HIV-1 provirus and incubated an additional 48 hours before they were harvested. To monitor transfection efficiency, specific siRNAs were co-transfected along with a fluorescent-tagged control siRNA. To determine transfection efficiency of expression plasmids, cells were co-transfected with pMAX vector (Amaxa Biosystems, Braunschweig, Germany) which encodes GFP along with the expression plasmids. The fluorescent signal was measured by analysis on a FACSCalibur (Becton Dickson, Fair View Lakes, NJ) flow cytometer. pNLENG1-EGP is a full length infectious molecular clone of HIV-1 containing enhanced green fluorescent protein(EGFP) between the env and nef genes. This was kindly provided by D. N. Levy (NYU).
ELISA
Virus released from infected cells was quantified using a standard Enzyme-Linked Immunosorbent Assay (ELISA) to measure viral p24 antigen in supernatants (sensitivity 50-200 pg/ml) (Liao et al., 2003). Absorbance was measured on a Fluorstar Optima plate reader (BMG Labtech).
Western blot
Protein analysis was accomplished using the NuPAGE gel electrophoresis system (Invitrogen, Carlsbad, CA). Cells were lysed on ice for 30 minutes in RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, and protease inhibitors (Roche)). Lysates were clarified at 16,000 × g at 4°C. Protein lysates were resolved on 10% Bis-Tris gels, and then transferred onto nitrocellulose using a semi-dry transfer apparatus (Bio-Rad). Membranes were blocked for 1 hour in 5% non-fat milk in PBS, followed by 3 washes in PBST (PBS, 0.5% Tween-20) before probing with primary antibodies. Membranes were then incubated with secondary antibodies conjugated to peroxidase. Chemiluminiscent substrate (ECL, GE Healthcare Life Sciences) was used for detection.
Immunoprecipitation
Cells were first pelleted at 1000 × g for 5 minutes and resuspended in NP-40 lysis buffer (50 mM Tris-HCl, pH 7.4,150 mM NaCl, 1% NP-40, 2mM EDTA, and protease inhibitors (Roche) then incubated on ice for 30 minutes. Lysates were clarified by centrifugation at 16,000 × g for 20 minutes at 4°C. Immunoprecipitation were performed using 500 μg of total protein and 10 μg/mL of either anti-myc mAb (9E10 clone) or anti-acetyl-lysine polyclonal antibodies. Samples were incubated on ice O/N and immune complexes were recovered by adding protein A/G sepharose for 1 hour at 4°C. Beads were washed twice with lysis buffer and resuspended in loading buffer and prepared for SDS-PAGE.
Antibodies
Antibodies used in this study were polyclonal rabbit anti-caveolin-1 (BD Transduction Laboratories, cat No. 610059), HIV-1 p24 KC57-FITC and KC57-p24-RD1 (Beckman Coulter, cat Nos. 6604665 and 6604667). anti-p65 mAb (Santa Cruz), anti-phospho-p65 (S276; Cell Signaling), anti-IκBα(Cell Signaling), anti-phospho- IκBα(14D4; Cell Signal), anti-acetyl-lysine antibody (#9441; Cell Signal). Anti-acetyl-lysine (21623; Abcam), anti-topoisomerase (clone H-300; Santa Cruz), and anti-RNA polymerase II (Millipore).
Flow Cytometry
Cells transfected with cDNA for fluorescent-tagged proteins were pelleted and washed with PBS to remove excess culture medium. Cells were then fixed with 50ul of 2% paraformaldehyde in PBS for 15 minutes at room temperature. The cells were then washed using 1ml of PBS and resuspended in 1ml of FACS buffer (1XPBS, 5% FCS, and 0.1% sodium azide). The cell suspension was analyzed by flow cytometry (FACSCalibur, Becton Dickson) for specific signals associated with expression of tagged proteins. Controls consisted of untransfected cells or cells transfected with empty vector. Protein expression was also assessed by staining cells with directly-conjugated monoclonal antibodies or untagged primary antibodies and directly-conjugated secondary antibodies, followed by flow cytometry analysis.
Cholesterol Analysis
Cholesterol content of fractions from sucrose gradients was measured using the Amplex Red Cholesterol Oxidase kit (Invitrogen, Carlsbad, CA). Fractions collected at the completion of lipid raft isolation were subjected to the cholesterol oxidase assay as per manufacturer's protocol. The protein concentration of cell lysates determined was by BCA assay (Pierce/THERMO). For filipin staining, transfected cells were grown on 35mm glass-bottom dishes (MatTek Corporation, Ashland, MA) under normal growth conditions and then fixed in 2% paraformaldehyde in PBS for 15 min. The cells were then incubated on ice with filipin at 50ug/ml in PBS for 30 min. Analysis of filipin staining was performed using a Nikon TE2000 wide-field microscope (Nikon Instruments, Melville, NY). All images were acquired with a plan fluor 60× 1.3 NA oil immersion objective equipped with an ultraviolet filter. The digital image acquisition was performed with Nikon Elements Advanced Research Software (Nikon Instruments, Melville, NY).
Nuclear Binding Assays
Transfected cells were lysed in nuclear/cytoplasmic extraction buffer from NE/PER kit (Pierce) per manufacturer's instructions. The protein content of nuclear and cytoplasmic lysates was measured by BCA assay (Pierce). After lysis aliquots were placed at −80°C until use. An equal amount of protein equivalents were placed into wells containing oligonucleotides with κB binding sites provided with NF-κB p65 Transcription Factor Assay Kit (Pierce/THERMO). Lysates were incubated in the wells for 1 hour before washing the plates with provided wash buffer. Next primary antibodies (anti-p65, dilution 1:1000), were added to each well and incubated at room temperature for 1 hour, followed by an additional wash step. Finally secondary antibody was added (horseradish peroxidase-conjugated, 1:10000), and incubated for 1 hour at room temperature. After a final wash, luminal detection solution was added to the well for 20 minutes. Chemiluminiscent signal was detected on fluorescent plate reader. Electrophoretic mobility shift assay
Nuclear extracts were obtained as described above. Extracts were thawed and added to binding reactions. Nuclear extract volumes were used between 0.5- 4.0ul with 20fmol of either wild type HIV-1 LTR-biotinylated probe (Biotin-5′-TACAAGGGACTTTCCGCTGGGGACTTTCCAGGGA-3′) or mutant probe (Biotin-5′-TACAAGCCACTTTCCGCTGGCCACTTTCCAGGGA-3′) containing point mutations in the NF-κB binding sites in final volume of 20ul. Reaction mix incubated on ice for 30 minutes. Prerun 5% native polyacrylamide in 0.5× TBE at 120V for 30 minutes in a Mini-PROTEAN tetra cell (Bio-Rad). Add 5ul of 5× loading buffer to each reaction and load onto gel. Run gel at 100V until dye front runs 2/3 down the gel at 4°C. Transfer gel on to a nylon membrane in 0.5× TBE for 30 minutes at 400 mA for 30 minutes (at constant 25 volts). Crosslink membrane with a UV crosslinker. Probes detected as described by LightShift Chemiluminescent EMSA kit protocol (Pierce/THERMO).
Statistical analysis
All experiments were reproduced two to four times, and representative experiments are shown. The average of repeat experiments was plotted and the standard deviation of mean was calculated from experimental values. The student t test was used to determine statistical significance of the differences.
Supplementary Material
Cells were transfected with HIV-1 molecular clone PY-U2 and caveolin-1 expression construct and 48 hours post-transfection, cells and viral particles in culture supernatant were collected to determine virus release efficiency. Release efficiency was assessed by measuring the cell-associated and extracellular HIV-1 Gag by ELISA. The release efficiency was calculated as the ratio of supernatant Gag to total Gag.
A. THP-1 monocytes were transfected with caveolin-1 siRNA and 48 hours later infected with VSV-G pseudotyped HIV-1-GFP. A. The fluorescent GFP signal from the single round infection was measured by flow cytometry and relative expression is plotted for indicated samples. Graph represents 3 independent experiments S.E.M, p value <0.05*. B. Western blot analysis of uninfected THP-1 cells transfected with caveolin-1-specific or control siRNAs. Cells were treated with TNF-α for indicated periods.
A. 293T cells were transfected with caveolin-1 and NF-κB expression constructs then harvested 24 hours post-transfection. Cell lysates were probed for acetylation of p65 lysine 310 in the presence of caveolin-1. Levels of acetylated p65 were calculated by densitometry after subtracting background of the blot; data shown are the average of three independent experiments.
Nuclear extracts were harvested from 293T cells transfected with caveolin-1 expression construct 24 hours post-transfection. Nuclear binding assay was performed using NF-κB p65 Transcription Factor Assay Kit (Pierce/THERMO) per manufacturer's instructions. All data shown represents 3 independent experiments. p value <0.05.
Acknowledgments
We thank Dr. Robert Nabi for providing caveolin-1-RFP constructs to this study. Dr. Bindong Liu and the FACS/BSL3 Core service and RCMI grant Award Number G12RR003032 from the National Center for Research Resources for use of facilities. The work described in this study was supported by National Institutes of Health grants F31 AI082950 (G.E.S), T32 AI007281 (G.E.S), and Vanderbilt Institute for Clinical and Translational Research Scholar Award 5 KL2 RR024977-03 (H.E.T.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arakawa R, Abe-Dohmae S, Asai M, Ito JI, Yokoyama S. Involvement ofcaveolin-1 in cholesterol enrichment of high density lipoprotein during its assembly by apolipoprotein and THP-1 cells. J Lipid Res. 2000;41:1952–1962. [PubMed] [Google Scholar]
- Benferhat R, Krust B, Rey-Cuille MA, Hovanessian AG. The caveolin-1 binding domain of HIV-1 glycoprotein gp41 (CBD1) contains several overlapping neutralizing epitopes. Vaccine. 2009a;27:3620–3630. doi: 10.1016/j.vaccine.2009.03.057. [DOI] [PubMed] [Google Scholar]
- Benferhat R, Martinon F, Krust B, Le Grand R, Hovanessian AG. The CBD1 peptide corresponding to the caveolin-1 binding domain of HIV-1 glycoprotein gp41 elicits neutralizing antibodies in cynomolgus macaques when administered with the tetanus T helper epitope. Mol Immunol. 2009b;46:705–712. doi: 10.1016/j.molimm.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Benferhat R, Sanchez-Martinez S, Nieva JL, Briand JP, Hovanessian AG. The immunogenic CBD1 peptide corresponding to the caveolin-1 binding domain in HIV^1envelope gp41 has the capacity to penetrate the cell membrane and bind caveolin-1. Mol Immunol. 2008;45:1963–1975. doi: 10.1016/j.molimm.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Bennasser Y, Contreras X, Moreau M, Le Clerc C, Badou A, Bahraoui E. HIV-1 Tat protein induces IL-10 production by human monocytes: implications of the PKC and calcium pathway. J Soc Biol. 2001;195:319–326. [PubMed] [Google Scholar]
- Bist A, Fielding PE, Fielding CJ. Two sterol regulatory element-like sequences mediate up-regulation of caveolin gene transcription in response to low density lipoprotein free cholesterol. Proc Natl Acad Sci U S A. 1997;94:10693–10698. doi: 10.1073/pnas.94.20.10693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth AM, Fang Y, Fallon JK, Yang JM, Hildreth JE, Gould SJ. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J Cell Biol. 2006;172:923–935. doi: 10.1083/jcb.200508014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter GC, Bernstone L, Baskaran D, James W. HIV-1 infects macrophages by exploiting an endocytic route dependent on dynamin, Rac1 and Pak1. Virology. 2010 doi: 10.1016/j.virol.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Chen LF, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. EMBO J. 2002;21:6539–6548. doi: 10.1093/emboj/cdf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LF, Williams SA, Mu Y, Nakano H, Duerr JM, Buckbinder L, Greene WC. NF-kappaB RelA phosphorylation regulates RelA acetylation. Mol Cell Biol. 2005;25:7966–7975. doi: 10.1128/MCB.25.18.7966-7975.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SM, Li JC, Lin SS, Lee DC, Liu L, Chen Z, Lau AS. HIV-1 transactivator protein induction of suppressor of cytokine signaling-2 contributes to dysregulation of IFN{gamma} signaling. Blood. 2009;113:5192–5201. doi: 10.1182/blood-2008-10-183525. [DOI] [PubMed] [Google Scholar]
- Chertova E, Chertov O, Coren LV, Roser JD, Trubey CM, Bess JW, Jr, Sowder RC, 2nd, Barsov E, Hood BL, Fisher RJ, Nagashima K, Conrads TP, Veenstra TD, Lifson JD, Ott DE. Proteomic and biochemical analysis of purified human immunodeficiency virus type 1 produced from infected monocyte-derived macrophages. Journal of virology. 2006;80:9039–9052. doi: 10.1128/JVI.01013-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidlow JH, Jr, Sessa WC. Caveolae, caveolins, and cavins: complex control of cellular signalling and inflammation. Cardiovasc Res. 86:219–225. doi: 10.1093/cvr/cvq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Real G, Jimenez-Baranda S, Mira E, Lacalle RA, Lucas P, Gomez-Mouton C, Alegret M, Pena JM, Rodriguez-Zapata M, Alvarez-Mon M, Martinez AC, Manes S. Statins inhibit HIV-1 infection by down-regulating Rho activity. J Exp Med. 2004;200:541–547. doi: 10.1084/jem.20040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca C, Petropoulos L, Zmeureanu D, Hiscott J. Nuclear IkappaBbeta maintains persistent NF-kappaB activation in HIV-1-infected myeloid cells. The Journal of biological chemistry. 1999;274:13010–13016. doi: 10.1074/jbc.274.19.13010. [DOI] [PubMed] [Google Scholar]
- Dobrowsky RT. Sphingolipid signalling domains floating on rafts or buried in caves? Cell Signal. 2000;12:81–90. doi: 10.1016/s0898-6568(99)00072-8. [DOI] [PubMed] [Google Scholar]
- Fermin C, Garry R. Alterations of lymphocyte membranes during HIV-1 infection via multiple and simultaneous entry strategies. Microsc Res Tech. 2005;68:149–167. doi: 10.1002/jemt.20228. [DOI] [PubMed] [Google Scholar]
- Fielding CJ, Fielding PE. Intracellular cholesterol transport. J Lipid Res. 1997;38:1503–1521. [PubMed] [Google Scholar]
- Fielding CJ, Fielding PE. Cholesterol and caveolae: structural and functional relationships. Biochim Biophys Acta. 2000;1529:210–222. doi: 10.1016/s1388-1981(00)00150-5. [DOI] [PubMed] [Google Scholar]
- Fitzsimons HL, Bland RJ, During MJ. Promoters and regulatory elements that improve adeno-associated virus transgene expression in the brain. Methods. 2002;28:227–236. doi: 10.1016/s1046-2023(02)00227-x. [DOI] [PubMed] [Google Scholar]
- Frank PG, Cheung MW, Pavlides S, Llaverias G, Park DS, Lisanti MP. Caveolin-1 and regulation of cellular cholesterol homeostasis. Am J Physiol Heart Circ Physiol. 2006;291:H677–686. doi: 10.1152/ajpheart.01092.2005. [DOI] [PubMed] [Google Scholar]
- Garrean S, Gao XP, Brovkovych V, Shimizu J, Zhao YY, Vogel SM, Malik AB. Caveolin-1 regulates NF-kappaB activation and lung inflammatory response to sepsis induced by lipopolysaccharide. J Immunol. 2006;177:4853–4860. doi: 10.4049/jimmunol.177.7.4853. [DOI] [PubMed] [Google Scholar]
- Glenney JR, Jr, Soppet D. Sequence and expression of caveolin, a protein component of caveolae plasma membrane domains phosphorylated on tyrosine in Rous sarcoma virus-transformed fibroblasts. Proc Natl Acad Sci U S A. 1992;89:10517–10521. doi: 10.1073/pnas.89.21.10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould SJ, Booth AM, Hildreth JE. The Trojan exosome hypothesis. Proc Natl Acad Sci U S A. 2003;100:10592–10597. doi: 10.1073/pnas.1831413100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DR, Chertova E, Hilburn JM, Arthur LO, Hildreth JE. Cholesterol depletion of human immunodeficiency virus type 1 and simian immunodeficiency virus with beta-cyclodextrin inactivates and permeabilizes the virions: evidence for virion-associated lipid rafts. Journal of virology. 2003;77:8237–8248. doi: 10.1128/JVI.77.15.8237-8248.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder T, Simons K. Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains. Curr Opin Cell Biol. 1997;9:534–542. doi: 10.1016/s0955-0674(97)80030-0. [DOI] [PubMed] [Google Scholar]
- Haynes JR, Cao SX, Rovinski B, Sia C, James O, Dekaban GA, Klein MH. Production of immunogenic HIV-1 viruslike particles in stably engineered monkey cell lines. AIDS Res Hum Retroviruses. 1991;7:17–27. doi: 10.1089/aid.1991.7.17. [DOI] [PubMed] [Google Scholar]
- Herbein G, Varin A. The macrophage in HIV-1 infection: from activation to deactivation? Retrovirology. 2010;7:33. doi: 10.1186/1742-4690-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper NM. Detergent-insoluble glycosphingolipid/cholesterol-rich membrane domains, lipid rafts and caveolae (review) Mol Membr Biol. 1999;16:145–156. doi: 10.1080/096876899294607. [DOI] [PubMed] [Google Scholar]
- Hovanessian AG, Briand JP, Said EA, Svab J, Ferris S, Dali H, Muller S, Desgranges C, Krust B. The caveolin-1 binding domain of HIV-1 glycoprotein gp41 is an efficient B cell epitope vaccine candidate against virus infection. Immunity. 2004;21:617–627. doi: 10.1016/j.immuni.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Huang JH, Lu L, Lu H, Chen X, Jiang S, Chen YH. Identification of the HIV-1 gp41 core-binding motif in the scaffolding domain of caveolin-1. J Biol Chem. 2007;282:6143–6152. doi: 10.1074/jbc.M607701200. [DOI] [PubMed] [Google Scholar]
- Huang LM, Joshi A, Willey R, Orenstein J, Jeang KT. Human immunodeficiency viruses regulated by alternative trans-activators: genetic evidence for a novel non-transcriptional function of Tat in virion infectivity. EMBO J. 1994;13:2886–2896. doi: 10.1002/j.1460-2075.1994.tb06583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen E. Molecular mechanisms of intracellular cholesterol transport. Curr Opin Lipidol. 1997;8:60–64. doi: 10.1097/00041433-199704000-00002. [DOI] [PubMed] [Google Scholar]
- Ikonen E, Parton RG. Caveolins and cellular cholesterol balance. Traffic. 2000;1:212–217. doi: 10.1034/j.1600-0854.2000.010303.x. [DOI] [PubMed] [Google Scholar]
- Jouvenet N, Neil SJ, Bess C, Johnson MC, Virgen CA, Simon SM, Bieniasz PD. Plasma membrane is the site of productive HIV-1 particle assembly. PLoS Biol. 2006;4:e435. doi: 10.1371/journal.pbio.0040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan R, Bres V, Ng RW, Coudart MP, El Messaoudi S, Sardet C, Jin DY, Emiliani S, Benkirane M. Post-activation turn-off of NF-kappa B-dependent transcription is regulated by acetylation of p65. The Journal of biological chemistry. 2003;278:2758–2766. doi: 10.1074/jbc.M209572200. [DOI] [PubMed] [Google Scholar]
- Kinoshita S, Chen BK, Kaneshima H, Nolan GP. Host control of HIV-1 parasitism in T cells by the nuclear factor of activated T cells. Cell. 1998;95:595–604. doi: 10.1016/s0092-8674(00)81630-x. [DOI] [PubMed] [Google Scholar]
- Kurzchalia TV, Parton RG. Membrane microdomains and caveolae. Curr Opin Cell Biol. 1999;11:424–431. doi: 10.1016/s0955-0674(99)80061-1. [DOI] [PubMed] [Google Scholar]
- Lajoie P, Nabi IR. Lipid rafts, caveolae, and their endocytosis. Int Rev Cell Mol Biol. 282:135–163. doi: 10.1016/S1937-6448(10)82003-9. [DOI] [PubMed] [Google Scholar]
- Liao Z, Cimakasky LM, Hampton R, Nguyen DH, Hildreth JE. Lipid rafts and HIV pathogenesis: host membrane cholesterol is required for infection by HIV type 1. AIDS Res Hum Retroviruses. 2001;17:1009–1019. doi: 10.1089/088922201300343690. [DOI] [PubMed] [Google Scholar]
- Liao Z, Graham DR, Hildreth JE. Lipid rafts and HIV pathogenesis: virion-associated cholesterol is required for fusion and infection of susceptible cells. AIDS Res Hum Retroviruses. 2003;19:675–687. doi: 10.1089/088922203322280900. [DOI] [PubMed] [Google Scholar]
- Lin S, Wang XM, Nadeau PE, Mergia A. HIV infection upregulates caveolin 1 expression to restrict virus production. Journal of virology. 84:9487–9496. doi: 10.1128/JVI.00763-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Wang XM, Nadeau PE, Mergia A. HIV infection upregulates caveolin 1 expression to restrict virus production. Journal of virology. 2010;84:9487–9496. doi: 10.1128/JVI.00763-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Lin CH, Kuo CY, Yang VC. ABCA1 modulates the oligomerization and Golgi exit of caveolin-1 during HDL-mediated cholesterol efflux in aortic endothelial cells. Biochem Biophys Res Commun. 2009;382:189–195. doi: 10.1016/j.bbrc.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Llano M, Kelly T, Vanegas M, Peretz M, Peterson TE, Simari RD, Poeschla EM. Blockade of human immunodeficiency virus type 1 expression by caveolin-1. Journal of virology. 2002;76:9152–9164. doi: 10.1128/JVI.76.18.9152-9164.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv XJ, Li YY, Zhang YJ, Mao M, Qian GS. Over-expression of caveolin-1 aggravate LPS-induced inflammatory response in AT-1 cells via up-regulation of cPLA2/p38 MAPK. Inflamm Res. 59:531–541. doi: 10.1007/s00011-010-0157-9. [DOI] [PubMed] [Google Scholar]
- Malcolm T, Chen J, Chang C, Sadowski I. Induction of chromosomally integrated HIV-1 LTR requires RBF-2 (USF/TFII-I) and Ras/MAPK signaling. Virus Genes. 2007;35:215–223. doi: 10.1007/s11262-007-0109-9. [DOI] [PubMed] [Google Scholar]
- Marzio G, Vink M, Verhoef K, de Ronde A, Berkhout B. Efficient human immunodeficiency virus replication requires a fine-tuned level of transcription. J Virol. 2002;76:3084–3088. doi: 10.1128/JVI.76.6.3084-3088.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matveev S, van der Westhuyzen DR, Smart EJ. Co-expression of scavenger receptor-BI and caveolin-1 is associated with enhanced selective cholesteryl ester uptake in THP-1 macrophages. J Lipid Res. 1999;40:1647–1654. [PubMed] [Google Scholar]
- Montano MA, Kripke K, Norina CD, Achacoso P, Herzenberg LA, Roy AL, Nolan GP. NF-kappa B homodimer binding within the HIV-1 initiator region and interactions with TFII-I. Proc Natl Acad Sci U S A. 1996;93:12376–12381. doi: 10.1073/pnas.93.22.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow MP, Grant A, Mujawar Z, Dubrovsky L, Pushkarsky T, Kiselyeva Y, Jennelle L, Mukhamedova N, Remaley AT, Kashanchi F, Sviridov D, Bukrinsky M. Stimulation of the liver X receptor pathway inhibits HIV-1 replication via induction ofATP-binding cassette transporter A1. Mol Pharmacol. 2010;78:215–225. doi: 10.1124/mol.110.065029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujawar Z, Rose H, Morrow MP, Pushkarsky T, Dubrovsky L, Mukhamedova N, Fu Y, Dart A, Orenstein JM, Bobryshev YV, Bukrinsky M, Sviridov D. Human immunodeficiency virus impairs reverse cholesterol transport from macrophages. PLoS Biol. 2006;4:e365. doi: 10.1371/journal.pbio.0040365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujawar Z, Tamehiro N, Grant A, Sviridov D, Bukrinsky M, Fitzgerald ML. Mutation of the ATP cassette binding transporter A1 (ABCA1) C-terminus disrupts HIV-1 Nef binding but does not block the Nef enhancement of ABCA1 protein degradation. Biochemistry. 2010;49:8338–8349. doi: 10.1021/bi100466q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DG, Booth A, Gould SJ, Hildreth JE. Evidence that HIV budding in primary macrophages occurs through the exosome release pathway. The Journal of biological chemistry. 2003;278:52347–52354. doi: 10.1074/jbc.M309009200. [DOI] [PubMed] [Google Scholar]
- Nguyen DH, Hildreth JE. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. Journal of virology. 2000;74:3264–3272. doi: 10.1128/jvi.74.7.3264-3272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontrelli P, Ursi M, Ranieri E, Capobianco C, Schena FP, Gesualdo L, Grandaliano G. CD40L proinflammatory and profibrotic effects on proximal tubular epithelial cells: role of NF-kappaB and lyn. J Am Soc Nephrol. 2006;17:627–636. doi: 10.1681/ASN.2005020202. [DOI] [PubMed] [Google Scholar]
- Quest AF, Leyton L, Parraga M. Caveolins, caveolae, and lipid rafts in cellular transport, signaling, and disease. Biochem Cell Biol. 2004;82:129–144. doi: 10.1139/o03-071. [DOI] [PubMed] [Google Scholar]
- Rasmussen L, Battles JK, Ennis WH, Nagashima K, Gonda MA. Characterization of virus-like particles produced by a recombinant baculovirus containing the gag gene of the bovine immunodeficiency-like virus. Virology. 1990;178:435–451. doi: 10.1016/0042-6822(90)90341-n. [DOI] [PubMed] [Google Scholar]
- Razani B, Combs TP, Wang XB, Frank PG, Park DS, Russell RG, Li M, Tang B, Jelicks LA, Scherer PE, Lisanti MP. Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. The Journal of biological chemistry. 2002;277:8635–8647. doi: 10.1074/jbc.M110970200. [DOI] [PubMed] [Google Scholar]
- Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H, Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. The Journal of biological chemistry. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- Rey-Cuille MA, Svab J, Benferhat R, Krust B, Briand JP, Muller S, Hovanessian AG. HIV-1 neutralizing antibodies elicited by the candidate CBD1 epitope vaccine react with the conserved caveolin-1 binding motif of viral glycoprotein gp41. J Pharm Pharmacol. 2006;58:759–767. doi: 10.1211/jpp.58.6.0006. [DOI] [PubMed] [Google Scholar]
- Ross MJ, Fan C, Ross MD, Chu TH, Shi Y, Kaufman L, Zhang W, Klotman ME, Klotman PE. HIV-1 infection initiates an inflammatory cascade in human renal tubular epithelial cells. J Acquir Immune Defic Syndr. 2006;42:1–11. doi: 10.1097/01.qai.0000218353.60099.4f. [DOI] [PubMed] [Google Scholar]
- Roulston A, Beauparlant P, Rice N, Hiscott J. Chronic human immunodeficiency virus type 1 infection stimulates distinct NF-kappa B/rel DNA binding activities in myelomonoblastic cells. Journal of virology. 1993;67:5235–5246. doi: 10.1128/jvi.67.9.5235-5246.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski I, Mitchell DA. TFII-I and USF (RBF-2) regulate Ras/MAPK-responsive HIV-1 transcription in T cells. Eur J Cancer. 2005;41:2528–2536. doi: 10.1016/j.ejca.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Taylor HE, Linde ME, Khatua AK, Popik W, Hildreth JE. Sterol regulatory element-binding protein 2 couples HIV-1 transcription to cholesterol homeostasis and T cell activation. J Virol. 2011;85:7699–7709. doi: 10.1128/JVI.00337-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas L, Nore BF, Berglof A, Heinonen JE, Mattsson PT, Smith CI, Mohamed AJ. Functional interaction ofcaveolin-1 with Bruton's tyrosine kinase and Bmx. The Journal of biological chemistry. 2002;277:9351–9357. doi: 10.1074/jbc.M108537200. [DOI] [PubMed] [Google Scholar]
- Verhoef K, Sanders RW, Fontaine V, Kitajima S, Berkhout B. Evolution of the human immunodeficiency virus type 1 long terminal repeat promoter by conversion of an NF-kappaB enhancer element into a GABP binding site. J Virol. 1999;73:1331–1340. doi: 10.1128/jvi.73.2.1331-1340.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed AA, Ablan SD, Roser JD, Sowder RC, Schaffner CP, Chertova E, Freed EO. HIV-1 escape from the entry-inhibiting effects of a cholesterol-binding compound via cleavage of gp41 by the viral protease. Proc Natl Acad Sci U S A. 2007;104:8467–8471. doi: 10.1073/pnas.0701443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LH, Coy DH, Taylor JE, Jiang NY, Moreau JP, Huang SC, Frucht H, Haffar BM, Jensen RT. des-Met carboxyl-terminally modified analogues of bombesin function as potent bombesin receptor antagonists, partial agonists, or agonists. J Biol Chem. 1990;265:15695–15703. [PubMed] [Google Scholar]
- Wang XM, Kim HP, Song R, Choi AM. Caveolin-1 confers antiinflammatory effects in murine macrophages via the MKK3/p38 MAPK pathway. Am J Respir Cell Mol Biol. 2006;34:434–442. doi: 10.1165/rcmb.2005-0376OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XM, Nadeau PE, Lin S, Abbott JR, Mergia A. Caveolin 1 inhibits HIV replication by transcriptional repression mediated through NF-{kappa}B. J Virol. 2011 doi: 10.1128/JVI.00254-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XM, Nadeau PE, Lo YT, Mergia A. Caveolin-1 modulates HIV-1 envelope-induced bystander apoptosis through gp41. Journal of virology. 2010;84:6515–6526. doi: 10.1128/JVI.02722-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch S, Keppler OT, Habermann A, Allespach I, Krijnse-Locker J, Krausslich HG. HIV-1 buds predominantly at the plasma membrane of primary human macrophages. PLoS Pathog. 2007;3:e36. doi: 10.1371/journal.ppat.0030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorp MO, Shatrov VA, Schulze-Osthoff K, Frank R, Kraft M, Los M, Krammer PH, Droge W, Lehmann V. HIV-1 Tat potentiates TNF-induced NF-kappa B activation and cytotoxicity by altering the cellular redox state. EMBO J. 1995;14:546–554. doi: 10.1002/j.1460-2075.1995.tb07030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J, Quinn CM, Brown AJ. SREBP-2 positively regulates transcription of the cholesterol efflux gene, ABCA1, by generating oxysterol ligandsfor LXR. Biochem J. 2006;400:485–491. doi: 10.1042/BJ20060914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wu J, Lu Y. Mechanism of HIV-1-TAT induction of interleukin-1beta from human monocytes: Involvement of the phospholipase C/protein kinase C signaling cascade. J Med Virol. 82:735–746. doi: 10.1002/jmv.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh M, Cole AL, Choi J, Liu Y, Tulchinsky D, Qiao JH, Fishbein MC, Dooley AN, Hovnanian T, Mouilleseaux K, Vora DK, Yang WP, Gargalovic P, Kirchgessner T, Shyy JY, Berliner JA. Role for sterol regulatory element-binding protein in activation of endothelial cells by phospholipid oxidation products. Circ Res. 2004;95:780–788. doi: 10.1161/01.RES.0000146030.53089.18. [DOI] [PubMed] [Google Scholar]
- Zheng YH, Plemenitas A, Fielding CJ, Peterlin BM. Nef increases the synthesis of and transports cholesterol to lipid rafts and HIV-1 progeny virions. Proc Natl Acad Sci U S A. 2003;100:8460–8465. doi: 10.1073/pnas.1437453100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng YH, Plemenitas A, Linnemann T, Fackler OT, Peterlin BM. Nef increases infectivity of HIV via lipid rafts. Curr Biol. 2001;11:875–879. doi: 10.1016/s0960-9822(01)00237-8. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Zhang H, Siliciano JD, Siliciano RF. Kinetics of human immunodeficiency virus type 1 decay following entry into resting CD4+ Tcells. Journal of virology. 2005;79:2199–2210. doi: 10.1128/JVI.79.4.2199-2210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cells were transfected with HIV-1 molecular clone PY-U2 and caveolin-1 expression construct and 48 hours post-transfection, cells and viral particles in culture supernatant were collected to determine virus release efficiency. Release efficiency was assessed by measuring the cell-associated and extracellular HIV-1 Gag by ELISA. The release efficiency was calculated as the ratio of supernatant Gag to total Gag.
A. THP-1 monocytes were transfected with caveolin-1 siRNA and 48 hours later infected with VSV-G pseudotyped HIV-1-GFP. A. The fluorescent GFP signal from the single round infection was measured by flow cytometry and relative expression is plotted for indicated samples. Graph represents 3 independent experiments S.E.M, p value <0.05*. B. Western blot analysis of uninfected THP-1 cells transfected with caveolin-1-specific or control siRNAs. Cells were treated with TNF-α for indicated periods.
A. 293T cells were transfected with caveolin-1 and NF-κB expression constructs then harvested 24 hours post-transfection. Cell lysates were probed for acetylation of p65 lysine 310 in the presence of caveolin-1. Levels of acetylated p65 were calculated by densitometry after subtracting background of the blot; data shown are the average of three independent experiments.
Nuclear extracts were harvested from 293T cells transfected with caveolin-1 expression construct 24 hours post-transfection. Nuclear binding assay was performed using NF-κB p65 Transcription Factor Assay Kit (Pierce/THERMO) per manufacturer's instructions. All data shown represents 3 independent experiments. p value <0.05.


