Abstract
Vestibular symptoms caused by migraine, referred to as vestibular migraine, are a frequently diagnosed but poorly understood entity. Based on recent evidence that normal subjects generate vestibular-mediated percepts of head motion and reflexive eye movements using different mechanisms, we hypothesized that percepts of head motion may be abnormal in vestibular migraine. We therefore measured motion detection thresholds in patients with vestibular migraine, migraine patients with no history of vestibular symptoms, and normal subjects using the following paradigms: roll rotation while supine (dynamically activating the semicircular canals); quasi-static roll tilt (statically activating the otolith organs); and dynamic roll tilt (dynamically activating the canals and otoliths). Thresholds were determined while patients were asymptomatic using a staircase paradigm, whereby the peak acceleration of the motion was decreased or increased based on correct or incorrect reports of movement direction. We found a dramatic reduction in motion thresholds in vestibular migraine compared to normal and migraine subjects in the dynamic roll tilt paradigm, but normal thresholds in the roll rotation and quasi-static roll tilt paradigms. These results suggest that patients with vestibular migraine may have enhanced perceptual sensitivity (e.g. increased signal-to-noise ratio) for head motions that dynamically modulate canal and otolith inputs together.
Keywords: Vestibular, migraine, vertigo, psychophysics, thresholds
1. Introduction
Vestibular migraine (VM), defined as vestibular symptoms generated by migraine mechanisms rather than other inner ear or brain abnormalities, is a very common cause of dizziness. Approximately 11% of dizzy patients [24] and 1% of the general population [25] suffer from this disorder. Despite its frequency, the pathophysiology of VM remains unclear. While neurologic symptoms are often associated with migraine as an aura that precedes the headache and are thought to result from cortical spreading depression [14], vestibular symptoms differ from other migraine auras. Vertigo is often not temporally associated with a headache and its duration can vary from seconds to days, unlike typical migraine auras that last 20–30 minutes [10]. These differences strongly suggest that vertigo in VM is usually not due to spreading depression involving vestibular regions of the cerebral cortex but rather is generated by a different, but uncertain mechanism or group of mechanisms [12].
When VM patients are evaluated between vertigo episodes, tests that focus on vestibulo-ocular and vestibulo-spinal reflexes have demonstrated a variety of abnormalities but these findings occur with a similar frequency in migraine patients with no vestibular symptoms [9]. Abnormalities observed during vertigo episodes are primarily central, but can be labyrinthine or non-specific [31]. One striking feature of VM vertigo episodes is that they are often positional in nature (e.g. provoked by changing head orientation relative to gravity) and are associated with a very high incidence of positional nystagmus [28].
To investigate the pathophysiology of VM, we used quantitative psychophysical methods because recent work in normal humans [23] and patients with pathology in visuo-motor pathways [30] suggests that the brain uses different mechanisms to generate percepts of head motion and reflexive vestibular-mediated eye movements. In particular, perception appears to be more dependent on central interactions between semicircular canal cues (which sense head rotation) and otolith cues (which sense gravity and linear acceleration) than eye movements [23].
Given the high incidence of positional vertigo and nystagmus in VM, we hypothesized that canal-otolith integration may be abnormal in these patients and that this abnormality may be reflected in perceptual measures but not by the reflexive eye movements usually used to assess patients with vestibular symptoms [13]. Furthermore, since migraine is characterized by hypersensitivity to a variety of sensory stimuli [14], we hypothesized that perceptual thresholds, defined as the stimulus magnitude where the brain can first perceive the motion signal despite the noise inherent in sensory transduction and subsequent neural processing [21], may be reduced in VM.
We investigated these hypotheses by measuring perceptual thresholds during motion paradigms that modulated activity in the semicircular canals, the otolith organs, or both the canals and otoliths simultaneously. We found a dramatic reduction in perceptual thresholds in VM compared to normal and migraine subjects when activity in the canals and otoliths was modulated in tandem, but normal thresholds when the canals or otolith organs were activated in isolation. A brief report of these results has been published [20].
2. Methods
Perceptual thresholds were analyzed in 24 subjects. We measured thresholds in eight patients with VM and eight patients with migraine but no history of vestibular symptoms. Data from eight normal control subjects were obtained from a recent study in our laboratory using identical methods [22]. Below we describe the characteristics of the three subject groups and the motion paradigms and psychophysical tests we employed. Since we have previously published these methods [16] they are described briefly. Informed consent was obtained from all subjects as dictated by the Declaration of Helsinki and the study was approved by the institutional ethical committee (IRB).
2.1. Subjects
All eight VM subjects (7 female, 1 male) had episodic vertigo and headaches meeting International Headache Society (IHS) criteria for migraine [27]. Using the Neuhauser criteria for VM [24], six subjects had definite VM and two subjects had probable VM. The six definite VM subjects noted a clear temporal association between migraine headaches and vertigo, while the two probable VM subjects did not observe this association. When symptomatic, vertigo was provoked by changing head orientation in four patients, was exacerbated by position change in another two subjects, and was independent of head orientation in two subjects. The eight VM patients lacked otologic symptoms and had normal physical examinations while asymptomatic, including detailed vestibular and ocular motor exams. Laboratory investigations were normal in the seven VM patients who were tested, and included calorics, vestibulo-ocular reflex measured with standard earth-vertical sinusoidal rotation, audiograms, and brain MRI. The eight migraine patients (7 female, 1 male) had headaches meeting IHS criteria but no history of dizziness. Four of the migraine patients experienced aura with their headaches, which was visual in nature. The eight normal subjects (4 female, 4 male) did not have a history of migraine or dizziness, and had normal caloric and rotational testing. None of the VM, migraine, or normal subjects took prophylactic migraine medications or vestibular suppressants, and the VM and migraine subjects were studied during asymptomatic periods, at least two weeks after their most recent episode of headache or vertigo. The mean age did not differ between groups (VM = 34.9; migraine = 34.5; normal = 38; ANOVA on ranks, p = 0.65) and no subject had a history of otologic or neurologic symptoms aside from migraine (VM, migraine groups) or vertigo (VM group).
2.2. Motion stimuli
Three paradigms were used, allowing us to dynamically stimulate the canals and otoliths simultaneously (dynamic roll tilt), dynamically stimulate the canals in isolation (roll rotation) and to stimulate the otoliths in isolation (referred to as quasi-static roll tilt, following Bringoux et al. [5]). Subjects sat upright (for quasistatic and dynamic roll tilt) or supine (for roll rotation) with the head immobilized. The chair was mounted on a Moog motion platform, which was programmed to move the body en-bloc such that the head rotated about the naso-occipital axis centered between the ears. The rotational axis was earth-horizontal for the quasi-static and dynamic roll tilt paradigms and earth-vertical for roll rotation. To minimize potential non-vestibular motion cues, trials were performed in the dark (eliminating visual inputs), skin surfaces were covered and the body was padded to stabilize it within the chair (minimizing tactile cues and distributing body pressure as evenly as possible), and sounds were masked with white noise (minimizing auditory cues).
Given the need to test subjects on three motion paradigms and the relatively long duration of the psychophysical task used to define the perceptual threshold, it was necessary to limit the number of motion frequencies used in the two dynamic paradigms (dynamic roll tilt, roll rotation). Based on our prior results in normal subjects, we chose to use a relatively high frequency (1.0 Hz) where canal thresholds are normally much lower than quasi-static otolith thresholds, and a midfrequency (0.1 Hz) where canal thresholds are normally higher than quasi-static otolith thresholds [22]. Similar to normal subjects [22], we predicted in VM patients that during dynamic roll tilt central canal-otolith interactions would occur and would be observable at 0.1 Hz, but that canal inputs would dominate the perceptual threshold at 1.0 Hz.
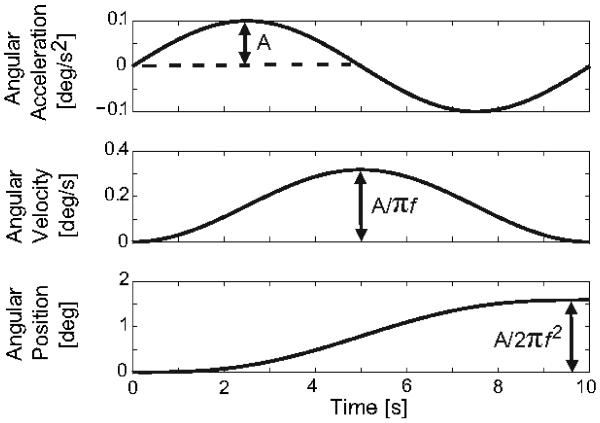
For the dynamic roll tilt (canal + otolith) and roll rotation (canal-only) paradigms, each trial consisted of a single-cycle of sinusoidal acceleration (Fig. 1). With this motion trajectory the angular velocity is unidirectional with a bell-shaped profile and the displacement is unidirectional with a sigmoidal shape. The peak velocity and total displacement of the head are proportional to the peak acceleration (Fig. 1). This motion profile was chosen because its dynamic characteristics are similar to normal voluntary head movements, it has been used successfully to test perceptual thresholds in normal subjects [4,16], and because it has no acceleration, velocity, or position discontinuities. Quasistatic roll tilt (otolith-only) thresholds were measured by positioning subjects with a constant velocity ramp of 0.125 deg/s with the velocity, peak acceleration (0.05 deg/s2), and peak jerk (0.03 deg/s3) all below the semicircular canal perceptual threshold [22]. Thresholds for the three paradigms are presented in degrees so the quasi-static and dynamic protocols can be readily compared.
Fig. 1.
Motion profiles used for the dynamic (roll tilt, roll rotation) paradigms. The peak velocity and net displacement of the head are proportional to the peak acceleration (A).
2.3. Psychophysical task
We used a one-interval direction-recognition task where a single motion stimulus was provided for each trial, the direction of motion was random, and after the motion was completed subjects had to indicate which direction (left or right) they felt they had rotated by pressing one of two buttons [16]. Perceptual thresholds were determined using a “three-down, one-up” staircase paradigm [19]. Three consecutive correct responses were required before the acceleration of the sinusoid was reduced, while the acceleration was increased after one incorrect response. Testing continued until five direction reversals occurred, and the perceptual threshold was defined as the mean of the last two reversals, corresponding to the stimulus magnitude where 79.4% of the responses were correct [19].
2.4. Data analysis
As previously described [4,16], perceptual thresholds did not differ significantly from a lognormal distribution (p > 0.2, Kolmogorov-Smirnov on log values for all subject groups and test paradigms), so all averaging and statistics were performed in logarithmic units. Mean and standard error results were transformed back to physical units for presentation.
2.5. Motion sickness and velocity storage measurements
Motion sickness susceptibility was assessed with the revised Golding questionnaire [15] and the lateral canal velocity storage integrator was assessed by calculating the dominant time constant of the horizontal VOR during sinusoidal yaw rotation about the earth-vertical axis.
3. Results
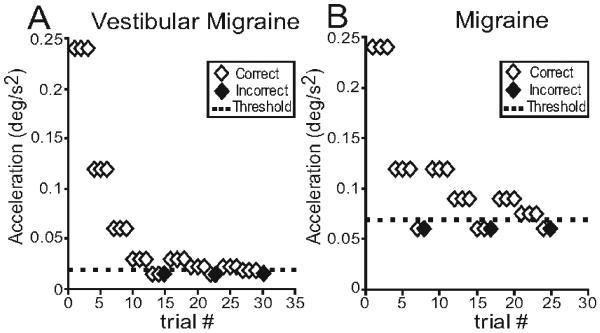
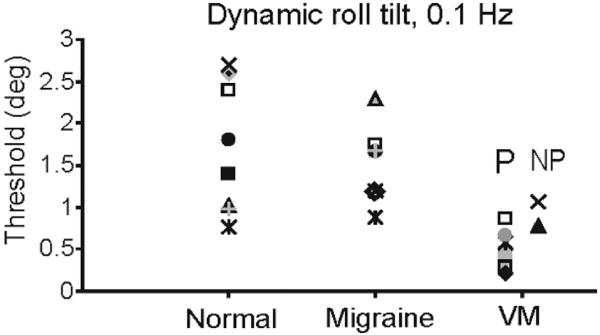
Figure 2 shows representative examples of the staircase paradigm in a VM and migraine subject for the dynamic roll tilt paradigm, which modulates canal and otolith cues in tandem. The figure illustrates results at 0.1 Hz and demonstrates that the VM subject was able to accurately detect the direction of head motion at lower peak accelerations than the migraine patient. Overall, when tested with the dynamic roll tilt paradigm at 0.1 Hz, perceptual thresholds in VM subjects (0.6 ± 0.1 deg = mean were ± one SEM) significantly lower than those measured in normal (1.7 ± 0–0.3 deg) or migraine (1.6 ± 0.2 deg) subjects (t-test, p < 0.001 for VM versus normal and VM versus migraine). Thresholds in the normal and migraine groups did not differ for this paradigm (t-test, p = 0.69). Figure 3 shows the threshold measurement for each subject during dynamic roll tilt at 0.1 Hz. Thresholds in VM patients are clearly clustered at lower values than those of normal controls or migraine subjects, and thresholds appeared to be lower in VM when vestibular symptoms had a positional component (labeled P in Fig. 3) compared to VM subjects without positional features (labeled NP).
Fig. 2.
Examples of the staircase paradigm used to determine perceptual thresholds. Data are for 0.1 Hz roll tilts and peak acceleration of the sinusoid is plotted against trial number. After three correct responses the acceleration was reduced (3-down) and after each incorrect response it was increased (1-up) until 5 reversals had occurred.
Fig. 3.
Thresholds (in deg) for each normal, migraine, and VM subject, 0.1 Hz dynamic roll tilt. VM patients are sub-divided into two groups, those with positional vertigo or positional exacerbation of vertigo (P) and those with vertigo that had no positional component (NP).
In contrast, thresholds in the three groups did not differ for 1.0 Hz dynamic roll tilt, roll rotation at either 0.1 or 1.0 Hz, or for quasi-static roll tilt (p > 0.1 for these comparisons using t-tests and ANOVA).
Thresholds measured during both dynamic roll tilt and roll rotation in all subject groups did show highpass characteristics like those expected from canal activation, with much lower thresholds at 1.0 Hz than at 0.1 Hz for both motion paradigms (p < 0.001, Holm-Sidak test). Dynamic roll tilt thresholds at 1.0 Hz were not distinguishable from roll rotation thresholds at 1.0 Hz, supporting the contention that canal cues dominate thresholds during higher frequency roll tilts [22].
No significant difference in VOR time constant was observed between the normal and VM groups (t-test: p = 0.55), nor were there differences in motion sickness susceptibility scores between the three subject groups (ANOVA: p = 0.44). Neither the VOR time constant nor the motion sickness susceptibility score correlated with dynamic roll tilt thresholds at 0.1 Hz in the VM or normal groups (p > 0.05 for Pearson r test for both parameters and subject groups).
4. Discussion
We found a dramatic reduction in perceptual motion thresholds in VM patients, compared to normal and migraine subjects, when they were tilted dynamically in the roll plane at a mid-frequency but not when they were tilted quasi-statically or rotated in roll about an earth-vertical axis. Below we consider possible explanations for this observation and conclude that in VM the motion cue derived from the central synthesis of canal and otolith inputs is most likely enhanced relative to the noise inherent in sensory transduction and subsequent signal processing. We also consider possible neural substrates for these findings and their potential contribution to the vestibular symptoms experienced in VM.
4.1. What mechanism underlies reduced dynamic tilt thresholds in VM?
Although reduced thresholds in VM were observed at only one of the two frequencies tested, the difference between VM and the migraine and normal control groups at 0.1 Hz was highly significant (p < 0.001), and hence extremely unlikely to be artifactual. Because of the time-consuming nature of the psychophysical task and the need to acquire data with three different motion paradigms, it was not feasible to test all of the patients at other mid-frequencies near 0.1 Hz. We have tested three VM patients on the dynamic roll tilt paradigm at 0.2 Hz, however, and found that their thresholds (0.57 ± 0.2) were substantially lower than normal controls (1.24 ± 0.16) at this frequency as well. This finding supports our conclusion that VM subjects have abnormally low perceptual thresholds for mid-frequency dynamic roll tilts.
4.1.1. Potential confounding factors
Although patients with migraine and particularly VM have been described to have increased susceptibility to motion sickness [18], we found no correlation between perceptual thresholds and motion sickness susceptibility as quantified with the revised Golding score. This is consistent with the observation that VM patients did not simply sense motion at lower amplitudes in a non-specific manner, as might be expected in subjects with low tolerance to motion sickness, but rather accurately detected motion direction at lower thresholds. Evoked potential studies (reviewed in [29]) have demonstrated that during certain forms of stimulation, responses habituate in normal subjects but not in migraineurs. While this could result in the appearance of increased sensitivity in migraine subjects, we explicitly looked for habituation in normal subjects [22] and found no evidence that this occurred. It is therefore improbable that the differences in thresholds reflect differences in habituation. Finally, the possible effects of gender on the threshold results must be considered, since the VM and migraine groups were predominantly female (reflecting the greater prevalence of migraine in women) but the normal group was equally divided between males and females. Prior work has shown that gender does not affect perceptual motion thresholds [4], however, and in our study dynamic roll tilt thresholds at 0.1 Hz were significantly smaller in the seven female VM patients than in the four normal female subjects (p = 0.02) or the seven female migraine subjects (p = 0.001), demonstrating that this difference is not due to gender.
4.1.2. Is the threshold reduction vestibular in origin?
We attempted to isolate the vestibular contribution to motion perception by masking extra-labyrinthine sensory cues. More specifically, visual information was eliminated, auditory cues were masked with white noise, and somatosensory cues were reduced by draping the body and stabilizing it in the chair. In addition, like several prior studies [4,16], we used a direction-recognition task to minimize the influence of vibration and other non-directional cues on perceptual thresholds. Since patients with bilateral vestibular hypofunction tested with this paradigm had perceptual thresholds that were approximately four times higher than normal [22], vestibular inputs appear to provide the dominant information used by the brain to sense motion direction in our test protocol.
We cannot exclude the possibility, however, that somatosensory information contributed to tilt thresholds. Migraine patients could theoretically utilize somatosensory cues more readily than normal controls since migraineurs can develop hypersensitivity to tactile stimulation during and shortly after headaches [7]. Our patients were tested a minimum of two weeks following a migraine or vertigo spell, however, and there is no evidence that tactile hypersensitivity extends far longer than the duration of a migrainous episode. More importantly, thresholds in the migraine group did not differ from normal controls, indicating that even if tactile hypersensitivity could persist in migaineurs it does not affect tilt thresholds measured with this protocol. It is therefore very unlikely that tactile or other extra-vestibular inputs contributed to the threshold reduction in VM.
4.1.3. What vestibular mechanism underlies the threshold reduction?
During dynamic roll tilt, both canal and otolith inputs are dynamically modulated in tandem. With the roll rotation paradigm we demonstrated that dynamic modulation of canal cues is not responsible for the threshold difference observed during dynamic roll tilt. The two remaining possibilities are that the central integration of canal and otolith cues is different in VM, or that there is a frequency-dependent enhancement of otolith information in VM that is not present in normal or migraine subjects. The latter remains a theoretical possibility because we tested otolith thresholds during (quasi) static roll tilt but did not look for possible increases in otolith-derived signals during dynamic stimulation of the otolith organs (e.g. using translational stimuli). This explanation is unlikely, however, since normal subjects show no evidence of this behavior [22] and because the one study that has investigated otolith function in VM suggested that otolith sensitivity is reduced, not increased, in VM compared to normal controls [3]. In contrast, there is substantial evidence that normal subjects synthesize canal and otolith inputs during roll tilts at mid-frequencies near 0.1 Hz in a non-linear manner (e.g. [23]). Reduced mid-frequency perceptual thresholds in VM are therefore more likely to reflect enhancement of the normal interaction between canal and otolith cues than a novel frequency-dependence of otolith inputs that has no known physiologic basis.
It should be emphasized that while roll tilt thresholds were reduced in VM at 0.1 Hz (and 0.2 Hz in a smaller sample) and were normal at 1.0 Hz, central canal-otolith interactions presumably occur across the frequency range that activates the canal and otolith organs in tandem. The effects of this interaction are observable at mid-frequencies in normal subjects [22] and patients with VM, but not at higher frequencies (where robust canal cues dominate the perceptual threshold) or with quasi-static roll tilt (where canal cues are so weak that otolith inputs appear to define the threshold).
4.2. Potential neural mechanisms for reduced tilt thresholds in VM
Since perceptual thresholds were reduced in VM, it appears that head motion cues in the vestibular regions of the thalamus or cerebral cortex are enhanced (relative to the noise) in this disorder. This could reflect primary changes in thalamic or cerebral cortical processing or these effects could be partially or completely mediated by ascending inputs from structures in the posterior fossa (see [12] for a review). Sensitization of neurons in the trigeminal nucleus occurs in migraine [8], for example, and this could enhance responses in the vestibular thalamus if a subset of thalamic neurons received dual trigeminal and vestibular innervation (similar to the mechanism that has been proposed to underlie photosensitivity in migraine [26]). Conversely, the trigeminal nucleus projects directly to the vestibular nuclei so sensitization in the thalamus or cerebral cortex could be driven via trigeminal-vestibular-thalamic pathways.
The vestibular nuclei also receive input from the cerebellum, and like visual motion perception [17], it has been recently demonstrated that perception of head motion is influenced by the midline cerebellar structures [6]. Of particular interest, given our findings that suggest abnormal canal-otolith integration in VM, is the potential role of the caudal cerebellar vermis (nodules IX and X, the nodulus and uvula). This region of the brain is required for normal canal-otolith integration [32], and all Purkinje cells in the nodulus and uvula carry head motion signals that are derived from the synthesis of canal and otolith signals [1]. In contrast, only a subset of neurons in the vestibular nuclei and the vestibular regions of the thalamus and cerebral cortex carry these integrated motion cues [2]. Altered signal processing in the caudal cerebellar vermis is therefore an attractive hypothesis to explain the changes in dynamic tilt thresholds in VM that occur without concomitant changes in canal or otolith-mediated thresholds.
If the primary neural changes that underlie reduced perceptual tilt thresholds in VM are infratentorial, then vestibular-mediated eye movements could also be abnormal in this disorder. While oculomotor responses appear similar in VM and migraine subjects [13], a small increase in the yaw VOR time constant in VM patients has been recently reported [18], although this was not clearly evident in our smaller patient population. Since the velocity storage network in the brainstem, which is under inhibitory control by the cerebellar nodulus and uvula, determines the VOR time constant and has been has been linked to motion sickness susceptibility [11], aberrant control of velocity storage by the cerebellum could contribute to both the vertigo and motion intolerance that is characteristic of VM. This potential mechanism could be evaluated further by analyzing the dynamic and spatial characteristics of eye movements produced by combined canal and otolith stimulation in VM and control subjects.
4.3. Vertigo in VM
It is unclear if the low tilt thresholds reflect central dysfunction that is a prerequisite for the development of VM or if they result from abnormal vestibular processing associated with prior vertigo episodes. In between episodes, lowered thresholds may contribute to the increased sensitivity to motion sickness that is characteristic of migraine and particularly VM [18]. If the perceptual abnormality were further enhanced, patients may overestimate the amplitude of head movements and this may generate abnormal illusions of motion, particularly when the head is reoriented relative to gravity. Abnormal motion signals derived from the synthesis of canal and otolith cues could therefore explain the high frequency of positional vertigo in VM and the even higher incidence of positional nystagmus during vertigo episodes. This is consistent with our observation that VM patients with positional vertigo have lower thresholds than VM subjects without positional symptoms.
In conclusion, given the multiple potential interactions between migraine and the vestibular system, it is likely that vertigo in VM is multi-factorial and could include labyrinthine as well as neurologic components. Despite this complexity, we have described a specific abnormality related to percepts of head motion that appears to be derived from the interaction of canal and otolith cues. Since this finding may differentiate VM patients from migraine and normal subjects, it could provide a useful diagnostic test for a disorder that currently lacks a pathognomonic finding.
Acknowledgements
We thank Drs. J. Furman, D. Zee, R. Burstein, and D. Chen. This work was supported by the Eleanor and Miles Shore 50th Anniversary Fellowship for Scholars in Medicine at Harvard Medical School [A.J.P]; the Swiss Foundation for Grants in Biology and Medicine in cooperation with the Swiss National Science Foundation [K.N]; and the National Institutes of Health [grant number DC04158].
References
- [1].Angelaki DE, et al. Computation of egomotion in the macaque cerebellar vermis. Cerebellum. 2010;9:174–182. doi: 10.1007/s12311-009-0147-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Angelaki DE, Yakusheva TA. How vestibular neurons solve the tilt/translation ambiguity. Comparison of brainstem, cerebellum, and thalamus. Ann NY Acad Sci. 2009;1164:19–28. doi: 10.1111/j.1749-6632.2009.03939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Baier B, et al. Vestibular-evoked myogenic potentials in vestibular migraine. J Neurol. 2009;256:1447–1454. doi: 10.1007/s00415-009-5132-4. [DOI] [PubMed] [Google Scholar]
- [4].Benson AJ, et al. Thresholds for the perception of whole body angular movement about a vertical axis. Aviat Space Environ Med. 1989;60:205–213. [PubMed] [Google Scholar]
- [5].Bringoux L, et al. Perception of slow pitch and roll body tilts in bilateral labyrinthine-defective subjects. Neuropsychologia. 2002;40:367–372. doi: 10.1016/s0028-3932(01)00103-8. [DOI] [PubMed] [Google Scholar]
- [6].Bronstein AM, et al. Reduced self-motion perception in patients with midline cerebellar lesions. NeuroReport. 2008;19:691–693. doi: 10.1097/WNR.0b013e3282fbf9f6. [DOI] [PubMed] [Google Scholar]
- [7].Burstein R, et al. The development of cutaneous allodynia during a migraine attack: Clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain. 2000;123:1703–1709. doi: 10.1093/brain/123.8.1703. [DOI] [PubMed] [Google Scholar]
- [8].Burstein R, et al. Chemical stimulation of the intracranial dura enhances responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol. 1989;79:964–982. doi: 10.1152/jn.1998.79.2.964. [DOI] [PubMed] [Google Scholar]
- [9].Casini AP, et al. Otoneurologic dysfunctions in migraine patients with and without vertigo. Otol Neurotol. 2009;30:961–967. doi: 10.1097/MAO.0b013e3181b4e780. [DOI] [PubMed] [Google Scholar]
- [10].Cutrer FM, Baloh RW. Migraine-associated dizziness. Headache. 1992;32:300–304. doi: 10.1111/j.1526-4610.1992.hed3206300.x. [DOI] [PubMed] [Google Scholar]
- [11].Dai M, et al. The relation of motion sickness to the spatial-temporal properties of velocity storage. Exp Brain Res. 2003;151:173–189. doi: 10.1007/s00221-003-1479-4. [DOI] [PubMed] [Google Scholar]
- [12].Furman JM, et al. Migrainous vertigo: development of a pathogenetic model structured diagnostic interview. Curr Opin Neurology. 2003;16:5–13. doi: 10.1097/01.wco.0000053582.70044.e2. [DOI] [PubMed] [Google Scholar]
- [13].Furman JM, et al. Vestibular function in migraine-related dizziness: a pilot study. J Vest Res. 2005;5:327–332. [PubMed] [Google Scholar]
- [14].Goadsby PJ. Pathophysiology of migraine. Neurol Clin. 2009;27:335–360. doi: 10.1016/j.ncl.2008.11.012. [DOI] [PubMed] [Google Scholar]
- [15].Golding JF. Motion sickness susceptibility questionnaire revised and its relationship to other forms of sickness. Brain Res Bulletin. 1998;47:507–516. doi: 10.1016/s0361-9230(98)00091-4. [DOI] [PubMed] [Google Scholar]
- [16].Grabherr L, et al. Vestibular thresholds for yaw rotation about an earth-vertical axis as a function of frequency. Exp Brain Res. 2008;186:677–681. doi: 10.1007/s00221-008-1350-8. [DOI] [PubMed] [Google Scholar]
- [17].Handel B, et al. Visual motion perception deficits due to cerebellar lesions are paralleled by specific changes in cerebrocortical activity. J Neurosci. 2009;29:15126–15133. doi: 10.1523/JNEUROSCI.3972-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jeong S-H, et al. Vestibular dysfunction in migraine: effects of associated vertigo and motion sickness. J Neurol. 2009;257:905–912. doi: 10.1007/s00415-009-5435-5. [DOI] [PubMed] [Google Scholar]
- [19].Leek MR. Adaptive procedures in psychophysical research. Percept Psychophys. 2001;63:1279–1292. doi: 10.3758/bf03194543. [DOI] [PubMed] [Google Scholar]
- [20].Lewis RF, et al. Abnormal motion perception in vestibular migraine. Laryngoscope. 2011 doi: 10.1002/lary.21723. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Macmillan NA, Creelman CD. Detection Theory: A User’s Guide. 2nd ed. Lawrence Eribaum Associates; New Jersey: 2005. [Google Scholar]
- [22].Merfeld DM, et al. Perceptual direction-detection thresholds for whole body roll tilts about an earth-horizontal axis. Soc Neurosci Abst. 2009 357.20. [Google Scholar]
- [23].Merfeld DM, et al. Vestibular perception and action employ qualitatively different mechanisms. II. VOR and perceptual responses during combined tilt and translation. J Neurophysiol. 2005;94:199–205. doi: 10.1152/jn.00905.2004. [DOI] [PubMed] [Google Scholar]
- [24].Neuhauser HK, et al. The interrelations of migraine, vertigo, and migrainous vertigo. Neurology. 2001;56:436–441. doi: 10.1212/wnl.56.4.436. [DOI] [PubMed] [Google Scholar]
- [25].Neuhauser HK, et al. Migrainous vertigo: Prevelance and impact on quality of life. Neurology. 2006;67:1028–1033. doi: 10.1212/01.wnl.0000237539.09942.06. [DOI] [PubMed] [Google Scholar]
- [26].Noseda R, et al. A neural mechanism for exacerbation of headache by light. Nature Neurosci. 2010;13:239–245. doi: 10.1038/nn.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Olesen J. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalagia. 1988;8(Supp 7):9–96. [PubMed] [Google Scholar]
- [28].Polensek SH, Tusa RJ. Nystagmus during attacks of vestibular migraine: an aid in diagnosis. Audiol Neurootol. 2009;15:241–246. doi: 10.1159/000255440. [DOI] [PubMed] [Google Scholar]
- [29].Schoenen J, et al. Evoked potentials and transcranial magnetic stimulation in migraine: published data and viewpoint on their pathophysiologic significance. Clin Neurophysiol. 2003;114:955–972. doi: 10.1016/s1388-2457(03)00024-5. [DOI] [PubMed] [Google Scholar]
- [30].Seemungal BM, et al. Symptomatic recovery in Miller Fisher Syndrome parallels vestibular-perceptual and not vestibular-ocular reflex function. Front Neurol. 2011;2:2. doi: 10.3389/fneur.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].vonBrevern M, et al. Acute migrainous vertigo: clinical and oculographic findings. Brain. 2004;128:365–374. doi: 10.1093/brain/awh351. [DOI] [PubMed] [Google Scholar]
- [32].Wearne S, et al. Control of spatial orientation of the angular vestibuloocular reflex by the nodulus and uvula. J Neurophysiol. 1998;79:2690–2675. doi: 10.1152/jn.1998.79.5.2690. [DOI] [PubMed] [Google Scholar]