Abstract
The senescent immune system responds poorly to new stimuli; thymic involution, accumulation of memory cells against other specificities, and general refractoriness to antigen signaling all may contribute to poor resistance to infection. These same changes may pose a significant clinical barrier to organ transplantation, as transplantation tolerance requires thymic participation and integrated, tolerance-promoting responses to novel antigens. We found that after the age of 12 months, mice became resistant to the tolerance-inducing capacity of the monoclonal antibody therapy anti-CD45RB. This resistance to tolerance to cardiac allografts could be overcome by surgical castration of male mice, a procedure that led to thymic regeneration and long-term graft acceptance. The potential for clinical translation of this endocrine-immune interplay was confirmed by the ability of Lupron Depot injections, which temporarily disrupt gonadal function, to restore tolerance in aged mice. Furthermore, we demonstrated that the restoration of tolerance after surgical or chemical castration depended on thymic production of regulatory T cells (Tregs); thymectomy or Treg depletion abrogated tolerance restoration. The aging of the immune system (“immune senescence”) is a significant barrier to immune tolerance, but this barrier can be overcome by targeting sex steroid production with commonly used clinical therapeutics.
INTRODUCTION
A healthy and normally functioning immune system has many important attributes, including the ability to respond rapidly to pathogenic agents while maintaining robust self-tolerance. Many fields of medicine seek to alter this balance either to obtain a stronger immune response (for example, to defend against infection) or to curtail the response to quell autoimmunity or support transplantation tolerance. In most clinical scenarios, these two aims are at odds with one another, and fostering one is nearly always at the expense of the other. In the case of immune senescence, there is a clear decrement in the ability to respond protectively to novel antigens (1–5). However, in these same older individuals, there is also evidence of increasing failure in immune tolerance mechanisms and a greater propensity to autoimmunity (6, 7). Although several research strategies are aimed at augmenting immune responsiveness in older individuals to protect against infection, it is not yet well defined whether these same strategies may support restoration of immune tolerance.
The senescent immune response has been best characterized in terms of the response to vaccination and infectious agents. Although the peripheral immune system in aged individuals contains a high number of memory cells formed against agents encountered earlier in their lifetimes (8–10), response to novel antigens, particularly in terms of B cell–derived antibody production, can be sparse (11–13). In addition, generation of new T cells is compromised because of thymic involution (14–17). These processes combine to leave a number of “holes” within the peripheral repertoire and result in the inability of the immune system to see novel antigens.
This decreased immune reactivity may seem like an ideal scenario in which to obtain nonresponsiveness to novel antigens, which is the goal of transplantation tolerance. However, tolerance induction is not simply a process of nonresponsiveness, but an active immune program requiring a specific response (regulation) against the transplant antigens. Murine models of transplantation depend on critical, active responses from both the B lymphocyte repertoire and the thymus (18–20).
The inability of the senescent immune system to respond appropriately to novel antigens at the thymic and peripheral levels represents a daunting challenge for the attainment of peripheral tolerance. In addition, understanding this problem is central to the advancement of clinical transplant protocols because most of the clinical research protocols are performed in aged individuals in whom there has been significant thymic involution and other signs of immune senescence. Herein, we describe a clinically applicable approach to reverse immune senescence, restore thymic mass, and rejuvenate the capacity of the immune system to respond to tolerogenic stimuli. Overall, we identify immune senescence as a reversible barrier to transplantation tolerance.
RESULTS
Tolerance-inducing therapy fails in mice that are more than 1 year old
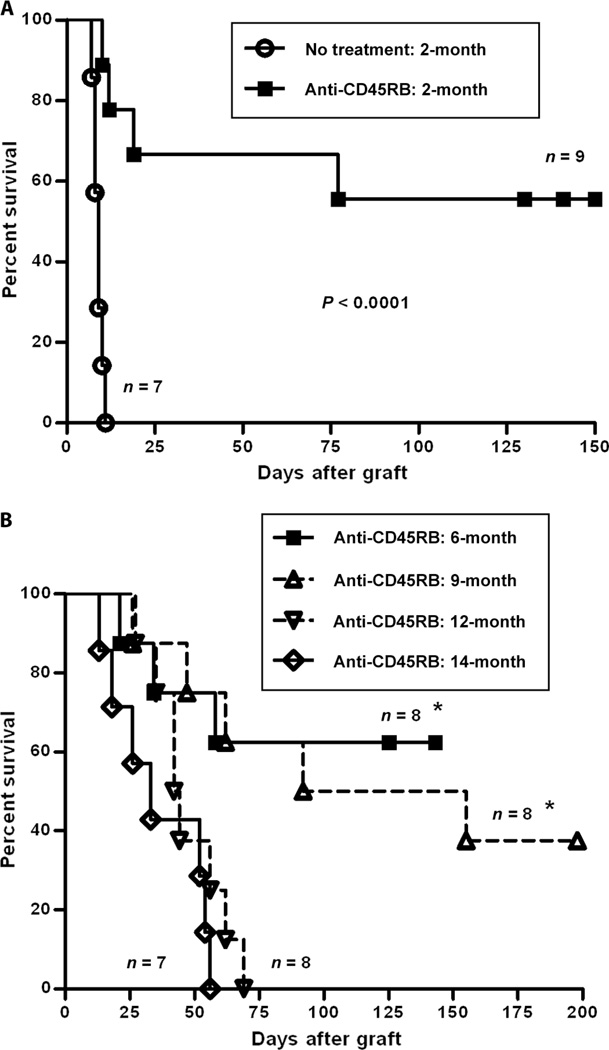
Because the induction of immune tolerance is an active process requiring antigen recognition and regulatory cell activation and differentiation, we hypothesized that this process would be diminished in aged mice. For the purposes of this investigation, we studied tolerance to cardiac allografts induced by monoclonal antibody (mAb) therapy targeting the CD45RB molecule using a standard short-term course of therapy. As previously published, we found robust tolerance induction in young mice (2 months), in which more than 50% of treated mice demonstrated long-term allograft survival. All untreated mice demonstrated rapid graft rejection by day 11 [median survival time (MST) = 9 days] (Fig. 1A). Mice at age 6 and 9months were similarly susceptible to tolerance induction. However, by 1 year of age, no treated mouse showed long-term tolerance, although there was a modest prolongation in MST of 43 days (treated) versus 10 days (untreated) (Figs. 1B and 2). By 14 months of age, the MST (33 days) was further decreased, and again, no treated animal showed long-term tolerance (Fig. 1B).
Fig. 1.
Aging eliminated the tolerance-inducing effect of anti-CD45RB on cardiac allografts. Hearts from C3H mice were transplanted into the abdominal cavity of B6 mice at different ages and treated with anti-CD45RB antibodies (100 µg, intraperitoneally, on days 0, 1, 3, 5, and 7). (A) In 2-month-old mice, all grafts were rejected rapidly without antibody treatment, but 55.6% of grafts survived beyond 150 days after anti-CD45RB therapy. (B) There were five in eight and four in eight allografts with long-term survival in 6- and 9-month-old mice, respectively; however, no allografts in 12-month-old (MST = 47.1 ± 14.1 days) and 14-month-old (MST = 36.0 ± 18.0 days) mice survived beyond 200 days. *P < 0.05, 6- and 9-month-old mice versus 12- and 14-month-old mice.
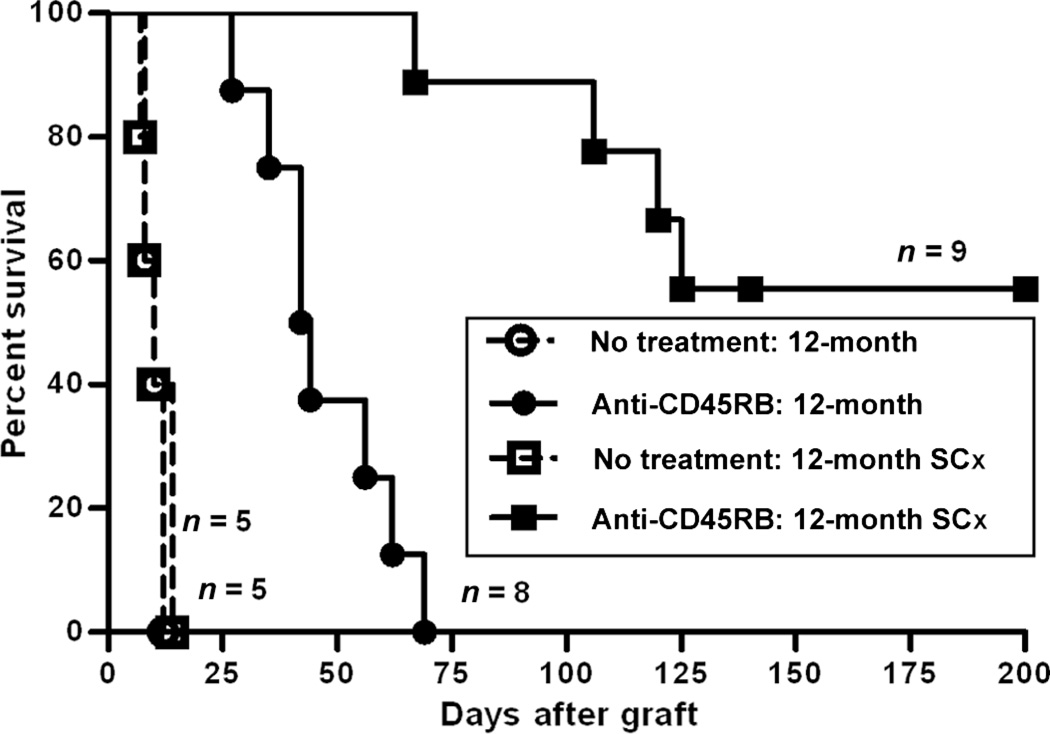
Fig. 2.
Surgical castration (SCx) restored the survival of cardiac allografts in aged mice treated with anti-CD45RB. Hearts from C3H donors were transplanted into the abdominal cavity of 12-month-old mice, some of which had undergone surgical castration 1 month before cardiac transplantation. Recipients were then treated with or without anti-CD45RB antibody. Although graft survival was significantly prolonged in the anti-CD45RB–treated, noncastrated 12-month-old mice compared with untreated mice (P < 0.001), no grafts permanently survived in the former. However, surgical castration significantly improved long-term graft survival in old mice treated with anti-CD45RB compared with normal 12-month-old mice treated with anti-CD45RB (P < 0.0001).
Castration reverses the tolerance resistance of aged mice
Because anti-CD45RB treatment requires active contribution of central tolerance mechanisms mediated by an intact thymus (18), we hypothesized that tolerance could be restored by restoration of thymic function. Diminished thymic function is a hallmark of immune senescence, and recent lines of investigation suggest that thymic involution may be driven in part by sex steroid production as the process initiates in the peripubertal period (21–24). Gonadectomy has been reported to increase thymic weight and cellularity and improve adaptive immunity (25). However, whether this rejuvenation of thymic mass also results in restoration of tolerance-promoting capacity has not been investigated. Therefore, we determined whether gonadectomy would restore tolerance susceptibility in aged mice. As shown in Fig. 2, we investigated the ability of anti-CD45RB treatment to induce tolerance in aged male mice after surgical castration. Surprisingly, we found that gonadectomy restored tolerance sensitivity to similar levels as those seen in 2-month-old mice. Castration by itself did not result in an immunosuppressed state because control, castrated animals rejected their cardiac allografts with a rapid tempo (MST = 10 days).
Lupron-mediated gonadal suppression restores tolerance induction
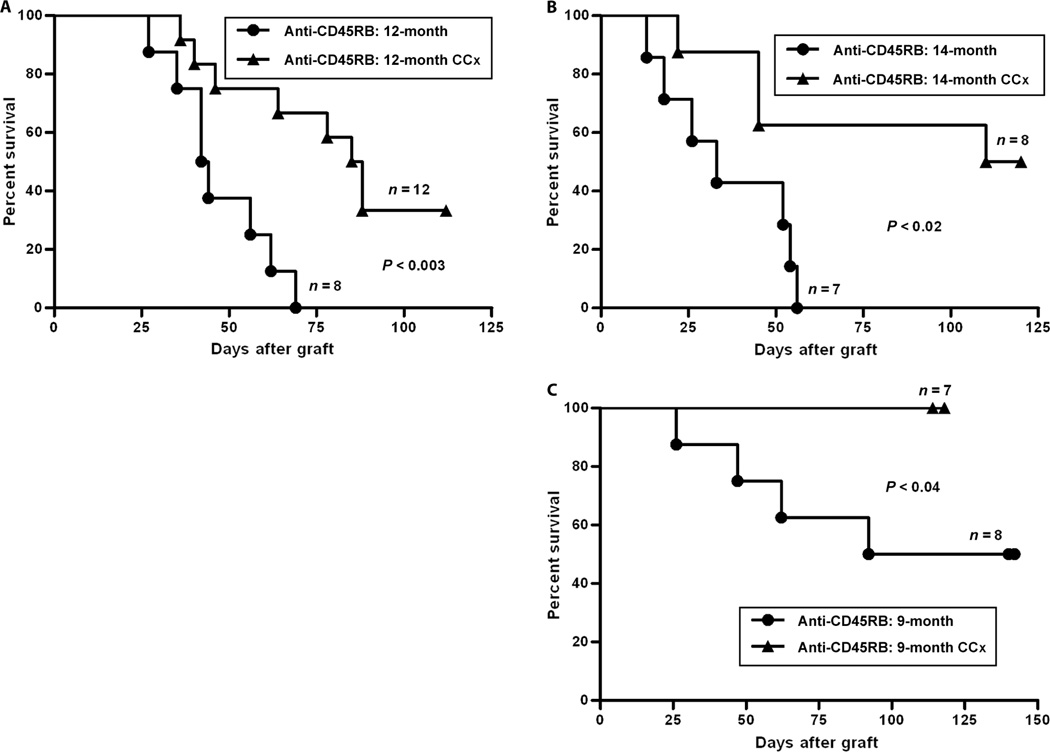
Considering the effect of gonadectomy on restoring the susceptibility to tolerance in aged mice, we further investigated whether gonadal suppression by medical therapy, which would represent a clinically appropriate intervention to promote tolerance, would be similarly effective. In addition, because gonadectomy induces a state of hypergonadotropic hypogonadism, whereas medical therapy with Lupron induces hypogonadotropism, this second intervention would also determine whether circulating gonadotropins, which increase after castration and decrease after Lupron Depot, contribute to the restoration of tolerance. To perform chemically mediated gonadal suppression (chemical castration), we injected mice with Lupron Depot 1 month before transplant and again on the day of transplant. In mice at age 12 and 14 months, in whom tolerance is not successful, gonadal suppression with Lupron Depot restored tolerance induction to levels similar to those seen both in younger mice and in surgically castrated transplant recipients (Fig. 3, A and B). Considering the success of medical therapy in restoring tolerance, we further considered whether it could augment tolerance induction in younger mice. In all age groups under 9 months, no more than about 50% of treated mice demonstrated long-term graft acceptance during the standard tolerance induction protocol with antibodies to CD45RB. We treated 9-month-old mice with Lupron Depot and found that this treatment allowed tolerance induction to be successful in 100% of treated mice (Fig. 3C). Thus, the production of circulating sex steroids in male mice at ages 9 months and greater interferes with tolerance induction, and this interference can be overcome by a widely used medical therapy.
Fig. 3.
Chemical castration (CCx) restored the survival of cardiac allografts in older mice treated with anti-CD45RB. One month before transplantation, mice at 8, 11, or 13 months were injected with a 1-month Lupron Depot; an additional dose was given on the day of transplantation. (A) In mice at age of 12 months, the anti-CD45RB–treated control mice rapidly rejected their grafts. Injection of Lupron Depot enhanced the efficacy of anti-CD45RB, leading to long-term graft acceptance in more than 30% of animals (P < 0.003). (B) In mice at age of 14 months, Lupron Depot improved the long-term graft survival in 50% of anti-CD45RB–treated mice compared with no graft acceptance in control mice (P < 0.02). (C) In 9-month-old mice, 50% of control mice demonstrated long-term tolerance and 100% of Lupron Depot–treated mice demonstrated long-term tolerance (P < 0.04).
Chemical or surgical castration restores thymic architecture and increases thymic weight and number of developing regulatory T cells
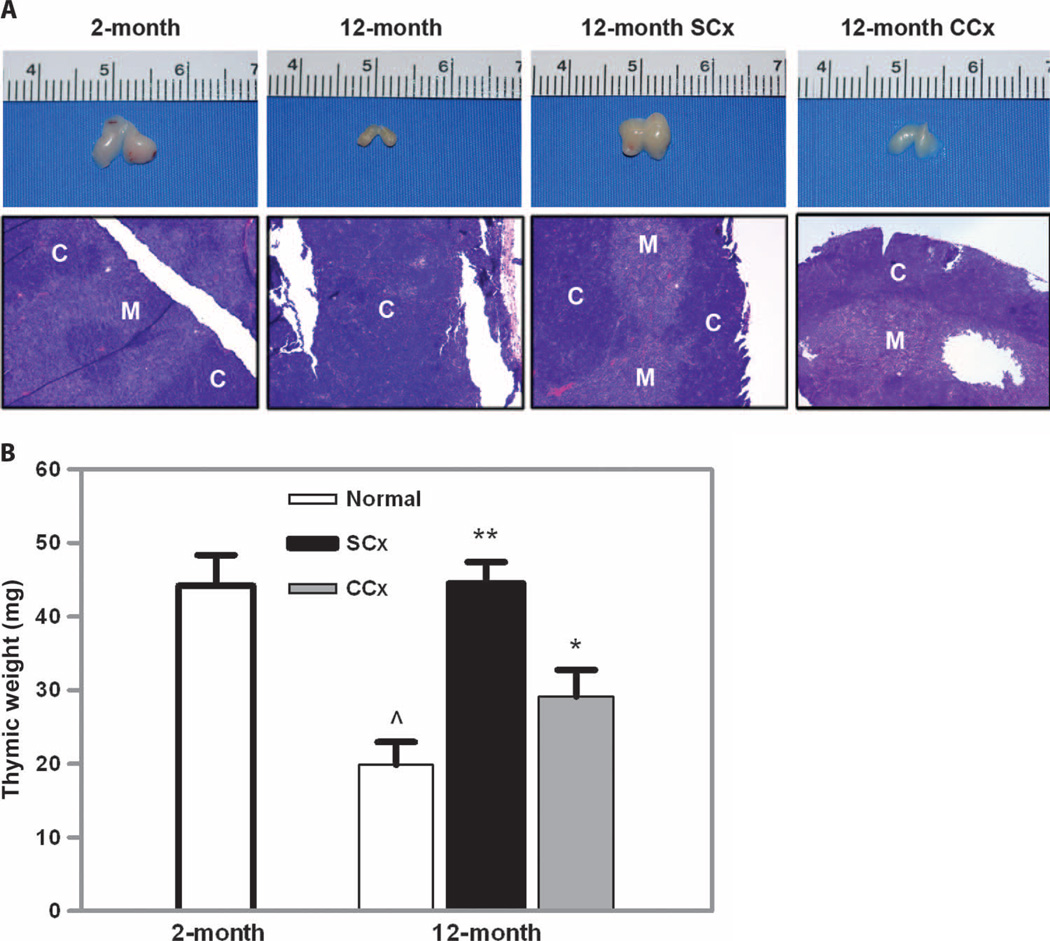
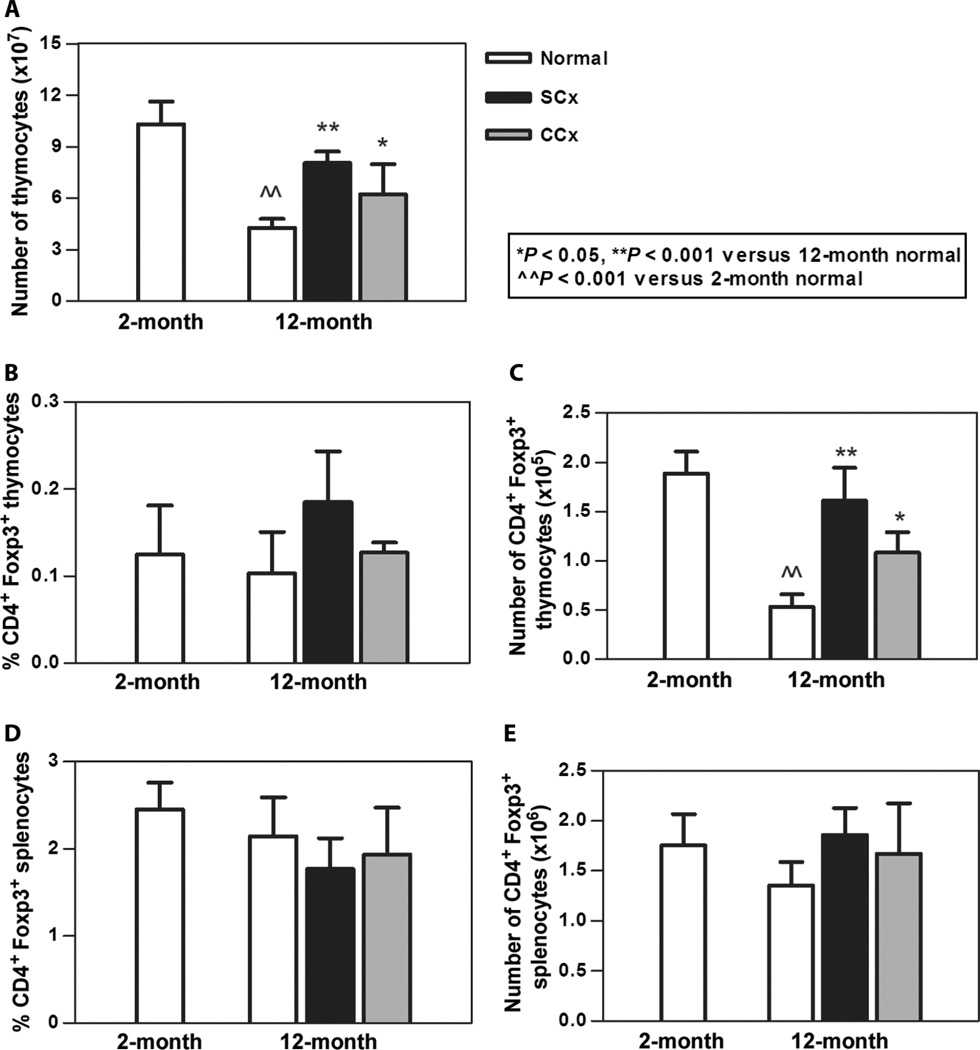
We considered that a likely effect of these therapies aimed at disrupting sex steroid production would be restoration of thymic architecture and function. Therefore, we undertook an extensive analysis of thymic weight, architecture, cellularity, and composition. As shown in Fig. 4A, visual inspection of the thymus in surgically castrated animals demonstrated an increase in size compared to age-matched controls. The thymic size was similar to that seen in 2-month-old animals as assessed by measurement of thymic weight (Fig. 4B). Chemical castration also produced an increase in thymic mass, although not to the same degree as the surgical procedure. Microscopic evaluation showed normal architecture in a specimen from a 2-month-old mouse, the loss of cortical-medullary differentiation in aged mice, and the restoration of the normal architecture in the specimen from the surgically castrated animal (Fig. 4A). This increase in thymic mass was accompanied by an increase in thymocyte number in both the surgically and the chemically castrated treatment groups (Fig. 5A). Moreover, the absolute number of regulatory T cells (Tregs), as determined by CD4+Foxp3+ cells, was increased over age-matched control animals and indistinguishable between the two treatment groups (Fig. 5, B and C). We also examined the periphery to see whether the increase in Tregs extended to secondary lymphoid organs. We observed no increase in percentage or in absolute number of Foxp3+ Tregs in the spleen (Fig. 5, D and E). Thus, both procedures aimed at suppression of gonadal function led to recovery of thymic function and a marked reconstitution of the newly developing Treg pool.
Fig. 4.
Surgical and chemical castration enhanced thymic mass and restored thymic architecture. (A) Thymic mass decreased with age. Mass increased after either surgical or chemical castration. (B) This difference was quantified and confirmed a significant increase in thymic weight (*P < 0.01; **P < 0.001 versus 12-month normal; ^P < 0.01 versus 2-month normal; n=3 to 5 animals per group). This improvement in thymic mass was associated with a restoration of the normal thymic architecture as seen in (A) by the recovery of corticomedullary differentiation that had been disrupted in the noncastrated 12-month-old animal (H&E staining, ×100). C, cortex; M, medulla.
Fig. 5.
Chemical and surgical castration increased thymic cellularity and Treg numbers in aged mice. (A) Aged mice demonstrated an expected decrease in thymocyte number, whereas surgical and chemical castration resulted in an increased number of thymocytes. (B) Percentage of CD4+Foxp3+ thymocytes was not significantly increased. (C) Overall, there was an increase in the absolute number of Tregs. (D and E) Neither percentage (D) nor absolute number (E) of CD4+Foxp3+ splenocytes was increased. n = 3 to 5 animals per group for all groups.
Thymectomy or Treg depletion ablates the tolerance-restorative effect of gonadectomy and hormonal suppression
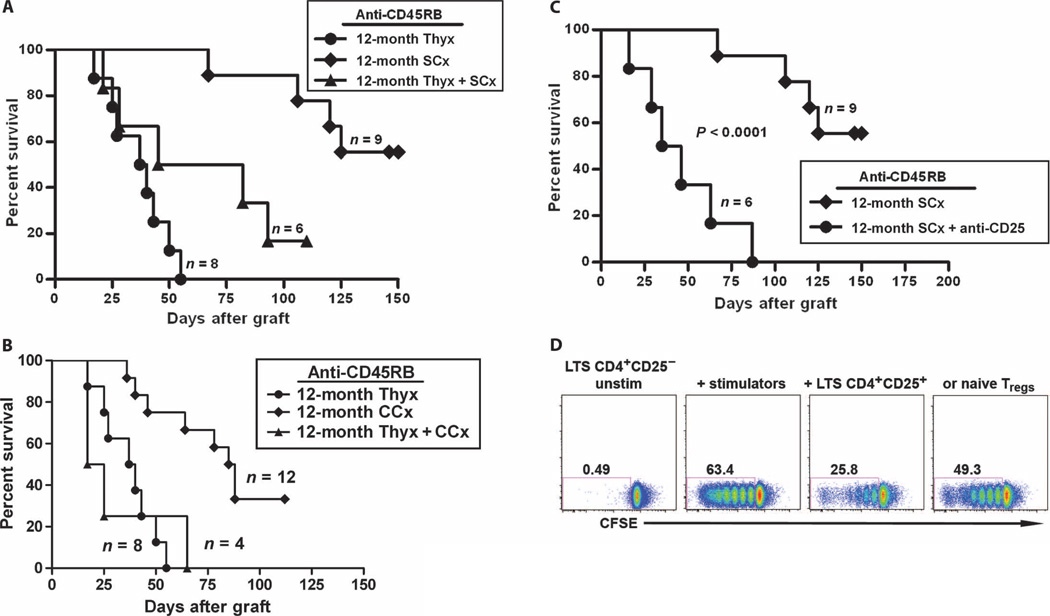
Because these tolerance-restoring interventions demonstrated an effect on thymic activity, and thymic function is required for anti-CD45RB–mediated tolerance, we hypothesized that the thymus was the principal target by which immune tolerance was restored after endocrine modulation. To confirm this hypothesis formally, we undertook both surgical (Fig. 6A) and chemical (Fig. 6B) castration in thymectomized, aged mice. After thymectomy, long-term tolerance induction was markedly reduced in mice after surgical castration and completely eliminated in mice undergoing treatment with Lupron Depot for gonadal suppression; the effect of thymectomy was not significantly different between the surgical and chemical castration groups (P > 0.05).
Fig. 6.
Tolerance-rejuvenating effect of castration on the survival of cardiac allografts in anti-CD45RB–treated aged mice was thymus- and Treg-dependent. Twelve-month-old mice underwent surgical or chemical castration with or without thymectomy. (A and B) Thymectomy was performed 1 month before cardiac allotransplantation. Thymectomy abrogated the effect of both surgical (A, P < 0.05 for surgical castration versus thymectomy/surgical castration) and chemical (B, P < 0.005 for chemical castration versus thymectomy/chemical castration) castration on restoring tolerance induced by anti-CD45RB. (C) Castration-mediated tolerance was Treg-dependent. Recipients are treated with 250 µg of anti-CD25 intraperitoneally on days −6 and −1. CD4+CD25+ T cell depletion was verified on day 10. Depletion of Tregs resulted in a significant decrease in heart allograft survival (MST > 128.8 ± 28.2 days versus 46.0 ± 25.6 days, anti-CD25–treated, P < 0.0001). (D) Suppressive effect of Tregs from the castrated, aged long-term survivor (LTS) on native T cell response to alloantigen. To assess the function of Tregs in the castrated recipients of cardiac allograft, we used an in vitro mixed lymphocyte reaction assay. CFSE-labeled CD4+CD25− T effector cells from LTS B6 mice alone (left), or stimulated with C3H splenocytes (middle left), in the presence of CD4+CD25+ T cells from either LTS (middle right) or naïve B6 (right). Tregs from the LTS mice (3:2 responders/Tregs). Data represent one experiment performed in duplicate.
Enhanced thymic function results in production of increased numbers of Tregs, which we hypothesized were the key mediators of immune regulation in this model. To formally test this hypothesis, we eliminated Tregs by anti-CD25 treatment.Anti-CD25 treatment led to failure of tolerance induction and rapid rejection of allografts (Fig. 6C).
Finally, to verify further that Tregs from allograft tolerant recipients were suppressive, we purified Tregs from aged, castrated, long-term survivors and used them in an in vitro mixed lymphocyte reaction. In the absence of Tregs, sensitized CD4+CD25− T cells were highly responsive to C3H stimulators (Fig. 6D). Coculture with purified tolerant, aged Tregs resulted in efficient suppression of proliferation in comparison to naïve Tregs.
DISCUSSION
The effect of aging on the immune system is a complex process that drastically modifies immune responsiveness. Many studies have characterized an age-dependent decline in immune reactivity that may account for increased susceptibility to cancer and infection (6, 9, 26–28). The decreased immune responsiveness seen in aged mice and people has been proposed to be due to an inappropriate enhancement of immune regulation. Investigations have suggested that the complement of classical Foxp3+ regulatory cells and their function is unchanged to slightly increased in the senescent immune system (29, 30); it has also been suggested that their numbers remain relatively stable in the aged, involuted thymus. Thus, it might be expected that the aging immune system could effectively maintain peripheral immune tolerance. However, clinical data suggest an increasing propensity to autoimmunity associated with aging, possibly resulting from resistance of effector T cells to immune regulation that develops with aging (7).
There may also be an important central contribution to the disrupted tolerance in aged individuals. In particular, one of the characteristics of the effector arm of the aged immune system is its relative inability to respond effectively to novel antigens. Immune regulation in the transplant setting is also an orchestrated response to a novel antigen, and thus, assessment of transplantation tolerance may better highlight defects in this arm of immunity in addition to effector immunity.
To assess transplantation in the aged immune system, we studied the capacity of antibodies to CD45RB to establish transplantation tolerance across the lifetime of our mouse model. Antibodies to CD45RB exert important effects both centrally and in the periphery, as we and others have previously characterized (18, 19, 31–35). An anti-human CD45RB antibody has been developed and demonstrated to have preliminary efficacy in a monkey kidney transplant model (36). We found an age-dependent decline in the susceptibility of the immune system to transplantation tolerance induced by the anti-CD45RB antibody; mice more than 1 year of age were very resistant to transplant tolerance. We identified thymic function as a key determinant of success in this system. Despite the presence of Foxp3+ cells in the aged thymus, these cells are unable to be instructed to provide graft protection to the same degree as their younger counterparts. These findings have important ramifications for clinical research and practice. Most subjects in research protocols that assess transplant tolerance are adults, and many are more than 50 years. The thymic epithelium and thymic output are substantially decreased by the time a human subject reaches 50 years of age. Thus, thymic involution may represent a previously unconsidered barrier to transplantation tolerance.
Although some of this effect may be related to intrinsic hematopoietic decline and decreased thymopoiesis, thymic involution is also positively regulated by decreases in neuroendocrine hormones that accompany aging (23, 37–39). The effect of androgen ablation on thymic cellularity and function has been extensively described previously (24). After surgical castration, thymic cellularity is rapidly restored and this has been associated with increased levels of major histocompatibility complex (MHC) class II and other aspects of rejuvenation of the epithelium. These changes enhance the ability of the thymus to support bone marrow function and to recovery of thymocyte proliferation. In human patients receiving chemical castration for prostate cancer, increased T cell receptor excision circle (TREC) expression, a marker of recent thymic emigration, has been noted. Despite this extensive characterization, it has not previously been determined whether thymic restoration changes susceptibility to tolerance induction.
To establish a protocol that could be readily applied in the clinical setting, we explored gonadal suppression with Lupron Depot. We found that medical therapy was comparable to gonadectomy in restoring tolerance induction. This tolerance restoration was associated with increased thymic mass and Treg production. Thymectomy abrogated the restorative effect of chemical or surgical castration and confirmed the requisite role of the thymus in the establishment of transplantation tolerance. Thus, we propose that there is a required threshold of thymic function needed for tolerance induction, and this threshold may be passed during aging in our mouse model between 9 and 12 months and in humans at an as yet undefined time. Moreover, because no increase was observed in splenic Tregs, the thymectomy data also suggest that the thymus is a primary site of action and that the export of new Tregs may contribute only marginally, but vitally, to the peripheral pool.
Our strategy for tolerance induction, which involved only 1 week of anti-CD45RB antibody therapy and 1 to 2 months of gonadal suppression, was able to produce long-standing immune tolerance in a subset of mice when observed up to 120 days, without evidence of rejection. Other protocols investigating restoration of thymic function rightly suggest that permanent reversal of immune senescence may also require attention to the age-related decline in bone marrow output (40). Whether there is an age at which this feature would again result in failure of immune tolerance remains an important consideration under investigation. Overall, the combination of approaches to restore the endogenous tolerance-inducing capacity of the thymus with introduction of appropriate antigens and novel immunomodulating therapies represents a clinically translatable approach to a large number of human diseases (41).
Our methods to disrupt the neuroendocrine axis included two different mechanisms that would produce either hypergonadotropic hypogonadism (castration) or hypogonadotropic hypogonadism (Lupron). Because both were equally effective at rejuvenating the tolerance axis, we conclude that the effect was not mediated by gonadotropins but by effects on sex steroid levels. The role of sex steroids in immune tolerance is complex and not completely understood. Considering the well-established clinical data of the increased propensity for women to develop autoimmunity, it has been strongly suggested that estrogen plays an important role in mediating this effect (42). Studies on peripheral T cells have not supported this hypothesis; instead, they have suggested that estrogen serves to enhance the number and function of Tregs (43, 44). Clinically, this has been correlated with increased Treg numbers as a barrier to breast cancer treatment (45–47). In contrast, lower testosterone levels may decrease the frequency of Tregs in the peripheral circulation (48). Indeed, treatment of humans with leuprolide, which decreases testosterone levels, has been reported to decrease interleukin-6 production (49) and increase the number of circulating CD3+ cells (50, 51), consistent with our observations on the contribution of male sex hormones to tolerance induction.
Although sex steroids may have a direct effect on immune function in the periphery by augmenting Treg and other peripheral T cell functions, it is also likely that the increase in sex steroids seen at puberty is a key trigger for thymic involution. Here, we further support the concept that this decline in central immune function may set the stage for disruptions in peripheral tolerance. In particular, the transplantation data suggest that sex steroids, through their action on the thymus, promote a state of immune rigidity in which tolerance (or reactivity) to novel antigens is limited. Estrogens have been particularly implicated in thymic involution. A recent report by Perisić and colleagues demonstrates that ovariectomy also leads to enhanced numbers of thymocytes, increased thymocyte function, and normalization of thymic subsets in a rat model (52). These findings coordinate well with the cellular events we describe and suggest that tolerance restoration would also be successful in this model. It has been suggested that the role of estrogens in thymic involution is not absolute because thymic recovery after ovariectomy may be transitory (53). Because other tissues, including fat, may produce estrogens, treatment with an aromatase inhibitor, which globally suppresses the conversion of testosterone to estrogen, may have a similar or enhanced immune-rejuvenating function.
These data support a model in which thymic involution associated with aging leads to the inability to incorporate novel antigens into the repertoire of specificities protected by recirculating regulatory cells. This resistance to tolerance induction can be overcome by elimination of sex steroids, a procedure that results in recovery of thymic mass and functional restoration of pro-tolerance novel specificities. This recovery can be achieved with the clinically applicable agent Lupron and suggests that trials of transplantation tolerance in aged individuals may not support successful immunomodulatory strategies because the thymus is unable to participate. Lupron is already being actively considered in clinical trials to modulate immunologic function in aged individuals. Three clinical trials including NCT00275262 (Study to Evaluate the Ability of Lupron Depot to Enhance Immune Function Following Bone Marrow Transplantation), NCT01338987 (Pilot Study of Lupron to Improve Immune Function After Allogeneic Bone Marrow Transplantation), and NCT00254397 (Melanoma Vaccine with Peptides and Leuprolide) are all actively evaluating the ability of Lupron to modulate immune system function. All of these trials are evaluating the capacity of endocrine modulation to enhance immunity. At present, it has not been considered whether hormonal modulation can improve transplant tolerance induction. Verifying thymic function and applying new methods, such as that described here, to restore thymic function may hasten the attainment of immune tolerance in individuals across all ages.
MATERIALS AND METHODS
Mice
Male C57BL/6 and C3H/HeJ mice were purchased from the Jackson Laboratory and housed in specific pathogen-free barrier facility. C57BL/6 mice were used at the ages of 2, 6, 9, 12, and 14 months. All procedures were conducted in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at Massachusetts General Hospital.
Cardiac transplantation
C3H/HeJ donor hearts were heterotopically transplanted into the abdominal cavity of C57BL/6 recipients. Transplantation was performed according to the Ono-Lindsey model as adapted for mice (54).
Antibody treatment
Mice were treated with intraperitoneal injections of 100 µg of rat anti-mouse CD45RB antibody (clone: MB23G2, American Type Culture Collection) on days 0, 1, 3, 5, and 7 after transplant.
For Treg depletion, 250 µg of rat anti-mouse CD25 mAb (PC61) was administrated intraperitoneally on days 6 and 1 before transplantation. These antibodies were purchased from Bio Express Inc.
Surgical castration
One month before transplantation, mice were anesthetized with 12.5% (g/liter) 2,2,2-tribromoethanol (Sigma-Aldrich), and a vertical incision was performed along the line between penis and anus. Bilateral testes along with epididymal adipose were removed with high-temperature cautery (World Precision Instruments Inc.). Peritoneal openings and skin incision were closed with absorbable suture.
Chemical castration
One month before transplantation, mice were injected with a 1-month Lupron Depot (leuprolide acetate for depot suspension), a luteinizing hormone–releasing hormone (LHRH) agonist (Takeda Pharmaceutical Co. Ltd.), at a dose of 0.6 mg per mouse, intramuscularly in the hind leg. An additional injection of one dose was repeated on the day of transplantation.
Thymectomy
Concomitant with castration, some 12-month-old mice were subjected to thymectomy as previously described (55). In brief, anesthetized mice were placed supine and a small horizontal incision was made along the upper verge of the sternum. After separation of muscle around trachea, a blunt, curved Pasteur pipette was introduced into the mediastinum and the thymus was removed by negative pressure aspiration.
Flow cytometric analysis
Thymus or spleen was harvested and single-cell suspensions were prepared. Cell suspension was rendered erythrocyte-free, and absolute number of thymocytes or splenocytes was determined. Isolated cells were washed with cold fluorescence-activated cell sorting (FACS) buffer [phosphate-buffered saline (PBS)/2% bovine serum albumin (BSA)/0.1% azide] and stained with the following antibodies: CD4 phycoerythrin (PE)–Cy7, CD8 Pacific Blue, CD25 peridinin chlorophyll protein (PerCP)–Cy5.5, and Foxp3 allophycocyanin (APC). Foxp3 staining was performed after cell fixation and permeabilization with the buffer kit provided by the manufacturer. All antibodies were purchased from eBioscience. All staining procedures were carried on ice to keep cells viable. Samples were analyzed on an LSR2 flow cytometer (Becton Dickinson) with FlowJo software (Tree Star).
Mixed lymphocyte reaction
Irradiated C3H splenocytes (0.6 × 106) were cultured with 0.3 × 106 Miltenyi bead-purified carboxyfluorescein diacetate succinimidyl ester (CFSE)–labeled CD4+CD25− T cells, 0.2 × 106 CD4+CD25+ T cells, and soluble anti-CD3 antibody (0.07 µg/ml) (2c11, Bio Express). Cells were prepared by passage of tissue through a cell strainer (70–µm pore size; Falcon; BD Biosciences). Cells were resuspended at a density of 107 cells/ml in Iscove’s modified Dulbecco’s medium (IMDM). An equal volume of 5 mM CFSE (Invitrogen) in IMDM was added, and cells were incubated at 37°C for 5 min. The reaction was quenched through the addition of an equal volume of heat-inactivated fetal calf serum (FCS) (Life Technologies). Labeled cells were washed twice and resuspended in culture medium for in vitro culture.
Histology
The intact thymus was fixed overnight in 4% formaldehyde before being processed and embedded into paraffin. Sections of 5 µm were cut and stained with hematoxylin-eosin (H&E).
Statistical analysis
Statistical analysis was performed with the Prism 5 Program (GraphPad). For survival studies, Kaplan-Meier survival curves were generated and statistical analysis was performed with the log-rank test. For thymocyte subset data analyses, statistical significance was determined by t test. A value of P <0.05 was considered significant.
REFERENCES AND NOTES
- 1.Hallgren HM, Bergh N, Rodysill KJ, O’Leary JJ. Lymphocyte proliferative response to PHA and anti-CD3/Ti monoclonal antibodies, T cell surface marker expression, and serum IL-2 receptor levels as biomarkers of age and health. Mech. Ageing Dev. 1988;43:175–185. doi: 10.1016/0047-6374(88)90045-0. [DOI] [PubMed] [Google Scholar]
- 2.De Greef GE, Van Tol MJ, Kallenberg CG, Van Staalduinen GJ, Remarque EJ, Tjandra YI, Hijmans W. Influence of ageing on antibody formation in vivo after immunisation with the primary T-cell dependent antigen Helix pomatia haemocyanin. Mech. Ageing Dev. 1992;66:15–28. doi: 10.1016/0047-6374(92)90070-t. [DOI] [PubMed] [Google Scholar]
- 3.Shi Y, Yamazaki T, Okubo Y, Uehara Y, Sugane K, Agematsu K. Regulation of aged humoral immune defense against pneumococcal bacteria by IgM memory B cell. J. Immunol. 2005;175:3262–3267. doi: 10.4049/jimmunol.175.5.3262. [DOI] [PubMed] [Google Scholar]
- 4.Alberti S, Cevenini E, Ostan R, Capri M, Salvioli S, Bucci L, Ginaldi L, De Martinis M, Franceschi C, Monti D. Age-dependent modifications of type 1 and type 2 cytokines within virgin and memory CD4+ T cells in humans. Mech. Ageing Dev. 2006;127:560–566. doi: 10.1016/j.mad.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Deans GD, Stiver HG, McElhaney JE. Influenza vaccines provide diminished protection but are cost-saving in older adults. J. Intern. Med. 2010;267:220–227. doi: 10.1111/j.1365-2796.2009.02201.x. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal S, Busse PJ. Innate and adaptive immunosenescence. Ann. Allergy Asthma Immunol. 2010;104:183–190. doi: 10.1016/j.anai.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Fülöp T, Jr, Larbi A, Dupuis G, Pawelec G. Ageing, autoimmunity and arthritis: Perturbations of TCR signal transduction pathways with ageing—a biochemical paradigm for the ageing immune system. Arthritis Res. Ther. 2003;5:290–302. doi: 10.1186/ar1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herndler-Brandstetter D, Veel E, Laschober GT, Pfister G, Brunner S, Walcher S, Parson W, Lepperdinger G, Grubeck-Loebenstein B. Non-regulatory CD8+CD45RO+CD25+ T-lymphocytes may compensate for the loss of antigen-inexperienced CD8+CD45RA+ T-cells in old age. Biol. Chem. 2008;389:561–568. doi: 10.1515/bc.2008.052. [DOI] [PubMed] [Google Scholar]
- 9.Lang A, Brien JD, Messaoudi I, Nikolich-Zugich J. Age-related dysregulation of CD8+ T cell memory specific for a persistent virus is independent of viral replication. J. Immunol. 2008;180:4848–4857. doi: 10.4049/jimmunol.180.7.4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta S, Gupta AA. Death of memory T-cell subsets in humans: Changes during aging. Expert Rev. Clin. Immunol. 2007;3:637–645. doi: 10.1586/1744666X.3.4.637. [DOI] [PubMed] [Google Scholar]
- 11.Huang YP, Pechere JC, Michel M, Gauthey L, Loreto M, Curran JA, Michel JP. In vivo T cell activation, in vitro defective IL-2 secretion, and response to influenza vaccination in elderly women. J. Immunol. 1992;148:715–722. [PubMed] [Google Scholar]
- 12.Deng Y, Jing Y, Campbell AE, Gravenstein S. Age-related impaired type 1 T cell responses to influenza: Reduced activation ex vivo, decreased expansion in CTL culture in vitro, and blunted response to influenza vaccination in vivo in the elderly. J. Immunol. 2004;172:3437–3446. doi: 10.4049/jimmunol.172.6.3437. [DOI] [PubMed] [Google Scholar]
- 13.Belshe RB, Newman FK, Cannon J, Duane C, Treanor J, Van Hoecke C, Howe BJ, Dubin G. Serum antibody responses after intradermal vaccination against influenza. N. Engl. J. Med. 2004;351:2286–2294. doi: 10.1056/NEJMoa043555. [DOI] [PubMed] [Google Scholar]
- 14.Pido-Lopez J, Imami N, Aspinall R. Both age and gender affect thymic output: More recent thymic migrants in females than males as they age. Clin. Exp. Immunol. 2001;125:409–413. doi: 10.1046/j.1365-2249.2001.01640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bodey B, Bodey B, Jr, Siegel SE, Kaiser HE. Involution of the mammalian thymus, one of the leading regulators of aging. In Vivo. 1997;11:421–440. [PubMed] [Google Scholar]
- 16.van den Dool C, de Boer RJ. The effects of age, thymectomy, and HIV Infection on α and β TCR excision circles in naive T cells. J. Immunol. 2006;177:4391–4401. doi: 10.4049/jimmunol.177.7.4391. [DOI] [PubMed] [Google Scholar]
- 17.Aspinall R, Andrew D. Thymic involution in aging. J. Clin. Immunol. 2000;20:250–256. doi: 10.1023/a:1006611518223. [DOI] [PubMed] [Google Scholar]
- 18.Deng S, Moore DJ, Huang X, Mohiuddin M, Lee IV MK, Velidedeoglu E, Lian MM, Chiaccio M, Sonawane S, Orlin A, Wang J, Chen H, Caton A, Zhong R, Markmann JF. Antibody-induced transplantation tolerance that is dependent on thymus-derived regulatory T cells. J. Immunol. 2006;176:2799–2807. doi: 10.4049/jimmunol.176.5.2799. [DOI] [PubMed] [Google Scholar]
- 19.Deng S, Moore DJ, Huang X, Lian MM, Mohiuddin M, Velededeoglu E, Lee IV MK, Sonawane S, Kim J, Wang J, Chen H, Corfe SA, Paige C, Shlomchik M, Caton A, Markmann JF. Cutting edge: Transplant tolerance induced by anti-CD45RB requires B lymphocytes. J. Immunol. 2007;178:6028–6032. doi: 10.4049/jimmunol.178.10.6028. [DOI] [PubMed] [Google Scholar]
- 20.Uryuhara K, Ambiru S, Dehoux JP, Oike F, Talpe S, Gianello P. Thymectomy impairs but does not uniformly abrogate long-term acceptance of semi-identical liver allograft in inbred miniature swine temporarily treated with FK506. Transplantation. 2004;77:1172–1180. doi: 10.1097/01.tp.0000121762.47432.15. [DOI] [PubMed] [Google Scholar]
- 21.Lynch HE, Goldberg GL, Chidgey A, Van den Brink MR, Boyd R, Sempowski GD. Thymic involution and immune reconstitution. Trends Immunol. 2009;30:366–373. doi: 10.1016/j.it.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hince M, Sakkal S, Vlahos K, Dudakov J, Boyd R, Chidgey A. The role of sex steroids and gonadectomy in the control of thymic involution. Cell. Immunol. 2008;252:122–138. doi: 10.1016/j.cellimm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Gray DH, Seach N, Ueno T, Milton MK, Liston A, Lew AM, Goodnow CC, Boyd RL. Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood. 2006;108:3777–3785. doi: 10.1182/blood-2006-02-004531. [DOI] [PubMed] [Google Scholar]
- 24.Sutherland JS, Goldberg GL, Hammett MV, Uldrich AP, Berzins SP, Heng TS, Blazar BR, Millar JL, Malin MA, Chidgey AP, Boyd RL. Activation of thymic regeneration in mice and humans following androgen blockade. J. Immunol. 2005;175:2741–2753. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
- 25.Heng TS, Goldberg GL, Gray DH, Sutherland JS, Chidgey AP, Boyd RL. Effects of castration on thymocyte development in two different models of thymic involution. J. Immunol. 2005;175:2982–2993. doi: 10.4049/jimmunol.175.5.2982. [DOI] [PubMed] [Google Scholar]
- 26.Panda A, Arjona A, Sapey E, Bai F, Fikrig E, Montgomery RR, Lord JM, Shaw AC. Human innate immunosenescence: Causes and consequences for immunity in old age. Trends Immunol. 2009;30:325–333. doi: 10.1016/j.it.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimoyama Y, Asano N, Kojima M, Morishima S, Yamamoto K, Oyama T, Kinoshita T, Nakamura S. Age-related EBV-associated B-cell lymphoproliferative disorders: Diagnostic approach to a newly recognized clinicopathological entity. Pathol. Int. 2009;59:835–843. doi: 10.1111/j.1440-1827.2009.02466.x. [DOI] [PubMed] [Google Scholar]
- 28.Burns EA, Leventhal EA. Aging, immunity, and cancer. Cancer Control. 2000;7:513–522. doi: 10.1177/107327480000700603. [DOI] [PubMed] [Google Scholar]
- 29.Gregg R, Smith CM, Clark FJ, Dunnion D, Khan N, Chakraverty R, Nayak L, Moss PA. The number of human peripheral blood CD4+ CD25high regulatory T cells increases with age. Clin. Exp. Immunol. 2005;140:540–546. doi: 10.1111/j.1365-2249.2005.02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lages CS, Suffia I, Velilla PA, Huang B, Warshaw G, Hildeman DA, Belkaid Y, Chougnet C. Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J. Immunol. 2008;181:1835–1848. doi: 10.4049/jimmunol.181.3.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao G, Moore DJ, Lee KM, Kim JI, Duff PE, O’Connor MR, Hirohashi T, Lei J, Yang M, Markmann JF, Deng S. An unexpected counter-regulatory role of IL-10 in B-lymphocyte-mediated transplantation tolerance. Am. J. Transplant. 2010;10:796–801. doi: 10.1111/j.1600-6143.2010.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basadonna GP, Auersvald L, Khuong CQ, Zheng XX, Kashio N, Zekzer D, Minozzo M, Qian H, Visser L, Diepstra A, Lazarovits AI, Poppema S, Strom TB, Rothstein DM. Antibody-mediated targeting of CD45 isoforms: A novel immunotherapeutic strategy. Proc. Natl. Acad. Sci. U.S.A. 1998;95:3821–3826. doi: 10.1073/pnas.95.7.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rothstein DM, Livak MF, Kishimoto K, Ariyan C, Qian HY, Fecteau S, Sho M, Deng S, Zheng XX, Sayegh MH, Basadonna GP. Targeting signal 1 through CD45RB synergizes with CD40 ligand blockade and promotes long term engraftment and tolerance in stringent transplant models. J. Immunol. 2001;166:322–329. doi: 10.4049/jimmunol.166.1.322. [DOI] [PubMed] [Google Scholar]
- 34.Moore DJ, Huang X, Lee IV MK, Lian MM, Chiaccio M, Chen H, Koeberlein B, Zhong R, Markmann JF, Deng S. Resistance to anti-CD45RB-induced tolerance in NOD mice: Mechanisms involved. Transpl. Int. 2004;17:261–269. doi: 10.1007/s00147-004-0698-3. [DOI] [PubMed] [Google Scholar]
- 35.Huang X, Moore DJ, Mohiuddin M, Lian MM, Kim JI, Sonawane S, Wang J, Gu Y, Yeh H, Markmann JF, Deng S. Inhibition of ICAM-1/LFA-1 interactions prevents B-cell-dependent anti-CD45RB-induced transplantation tolerance. Transplantation. 2008;85:675–680. doi: 10.1097/TP.0b013e3181663422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen G, Luke PP, Yang H, Visser L, Sun H, Garcia B, Qian H, Xiang Y, Huang X, Liu W, Senaldi G, Schneider A, Poppema S, Wang H, Jevnikar AM, Zhong R. Anti-CD45RB monoclonal antibody prolongs renal allograft survival in cynomolgus monkeys. Am. J. Transplant. 2007;7:27–37. doi: 10.1111/j.1600-6143.2006.01598.x. [DOI] [PubMed] [Google Scholar]
- 37.Olsen NJ, Olson G, Viselli SM, Gu X, Kovacs WJ. Androgen receptors in thymic epithelium modulate thymus size and thymocyte development. Endocrinology. 2001;142:1278–1283. doi: 10.1210/endo.142.3.8032. [DOI] [PubMed] [Google Scholar]
- 38.García-Suárez O, Pérez-Pérez M, Germanà A, Esteban I, Germanà G. Involvement of growth factors in thymic involution. Microsc. Res. Tech. 2003;62:514–523. doi: 10.1002/jemt.10413. [DOI] [PubMed] [Google Scholar]
- 39.Capri M, Monti D, Salvioli S, Lescai F, Pierini M, Altilia S, Sevini F, Valensin S, Ostan R, Bucci L, Franceschi C. Complexity of anti-immunosenescence strategies in humans. Artif. Organs. 2006;30:730–742. doi: 10.1111/j.1525-1594.2006.00295.x. [DOI] [PubMed] [Google Scholar]
- 40.Dudakov JA, Khong DM, Boyd RL, Chidgey AP. Feeding the fire: The role of defective bone marrow function in exacerbating thymic involution. Trends Immunol. 2010;31:191–198. doi: 10.1016/j.it.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Chidgey AP, Layton D, Trounson A, Boyd RL. Tolerance strategies for stem-cell-based therapies. Nature. 2008;453:330–337. doi: 10.1038/nature07041. [DOI] [PubMed] [Google Scholar]
- 42.Cutolo M, Sulli A, Capellino S, Villaggio B, Montagna P, Seriolo B, Straub RH. Sex hormones influence on the immune system: Basic and clinical aspects in autoimmunity. Lupus. 2004;13:635–638. doi: 10.1191/0961203304lu1094oa. [DOI] [PubMed] [Google Scholar]
- 43.Tai P, Wang J, Jin H, Song X, Yan J, Kang Y, Zhao L, An X, Du X, Chen X, Wang S, Xia G, Wang B. Induction of regulatory T cells by physiological level estrogen. J. Cell. Physiol. 2008;214:456–464. doi: 10.1002/jcp.21221. [DOI] [PubMed] [Google Scholar]
- 44.Polanczyk MJ, Hopke C, Vandenbark AA, Offner H. Treg suppressive activity involves estrogen-dependent expression of programmed death-1 (PD-1) Int. Immunol. 2007;19:337–343. doi: 10.1093/intimm/dxl151. [DOI] [PubMed] [Google Scholar]
- 45.Generali D, Bates G, Berruti A, Brizzi MP, Campo L, Bonardi S, Bersiga A, Allevi G, Milani M, Aguggini S, Dogliotti L, Banham AH, Harris AL, Bottini A, Fox SB. Immunomodulation of FOXP3+ regulatory T cells by the aromatase inhibitor letrozole in breast cancer patients. Clin. Cancer Res. 2009;15:1046–1051. doi: 10.1158/1078-0432.CCR-08-1507. [DOI] [PubMed] [Google Scholar]
- 46.Ghebeh H, Barhoush E, Tulbah A, Elkum N, Al-Tweigeri T, Dermime S. FOXP3+ Tregs and B7-H1+/PD-1+ T lymphocytes co-infiltrate the tumor tissues of high-risk breast cancer patients: Implication for immunotherapy. BMC Cancer. 2008;8:57. doi: 10.1186/1471-2407-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]