Abstract
A new, TiCl4-or SnCl4-mediated, solvent-free method was developed for the synthesis of N-Aryl benzamidines and N-phenylpicolinamidines, in moderate-to-good yield, using suitable amines and nitriles as starting materials.
1. Introduction
The amidine nucleus is found in a wide variety of biologically active molecules [1]. N-Aryl amidine exhibits activity against Mycobacterium tuberculosis, and N-alkylfuramidine shows antiprotozoan and antimicrobial activities [2]. Similarly various amidines derived from 4-amidino-2-(2-pyridyl)quinazoline [3] and 1-amino-3-(2-pyridyl)isoquinoline [3], guanidine [4], diguanidino [5], reversed diamidino 2,5-diarylfuran [5], benzimidazole [6], pyridine [7], exhibit antimycoplasmal, antimalarial, antimicrobial, antibacterial, anti-inflammatory activities. An extensive number of monoamidines have been evaluated for their utility in blocking various stages of the thrombin cascade, and numerous highly potent molecules have been reported [8].
Amidines were used as important synthon in organic synthesis in the preparation of various heterocyclic compounds, such as pyridine [7, 9], pyrimidines [9, 10], imidazoles [11], pyrazolopyrimidine [12], iminopyrimidine [13], imidazopyridine and pyrimidinopyridine [14], purine [15], benzimidazole [16], pyrimidines [17], triazaphenalene [18], triazine [19], tetraazole [19], thiadiazine [20], oxazolotriazole [21], diazirine [22], triazolopyridine [23], azetidinone [24], and pyrrole, and also used as complexing agent [25].
Several synthetic strategies have been developed for the synthesis of amidines, in which the nucleophilic addition of amine to nitrile is the most popular. Generally, nitriles were activated to the intermediate salt in the presence of EtOH/HCl [26] or NH4Cl/MeOH [27] under anhydrous condition and then reacted with amine to get amidine. While for unreactive nitriles, Lewis acid or other condensing agents were used such as anhydrous AlCl3, ZnCl2 [28], CuCl [29], Ln (III) salts [30], CaCl2 [31], Al(CH3)3 [32], SmI2 [33], Ytterbium amide [34], MeSO3H [20], and anhyd. SnCl4 [21]. Amides can be converted to imidoyl chloride using PCl5 [35, 36], which can then react with primary or secondary amine to yield amidine. In addition, amide can be O-alkylated with triethyloxonium fluoroborate at ambient temperature to yield the corresponding imidic ester fluoroborate, which then reacts with amine to yield the targeted amidine [3]. Iron pentacarbonyl was employed to the conversion of amidoximes into amidines via reductive cleavage of the N=O bond [37]. Sometimes, strong bases like LiHMDS, NaHMDS, LDA, BuLi, NaOMe [38], and NaH [20] were used as condensing agent. Similarly, Dains F. B. has shown that amidine was prepared from symmetrical diaryl and dialkyl urea and acid chloride [39, 40].
In 1998, Zhou and Zhang published the results on such a subject that amidines were successfully prepared from nitriles and nitrocompounds in the presence of TiCl4/Sm in THF. They also reported that under same reaction conditions amidine formation was not observed by treatment of nitriles with amines [41]. Thus, it was of interest to study the reactions of nitrile and amine using TiCl4 and SnCl4.
2. Results and Discussion
We would like to demonstrate in the present work that amidine could be prepared by coupling nitrile with amine in presence of TiCl4 as well as SnCl4 using neat condition in absence of samarium. At the beginning we studied the synthesis of amidine (Scheme 1, Table 1, and Entry 1) using benzonitrile and aniline as model substrates. In a typical experiment aniline (0.01 mol) and benzonitrile (0.01 mol) were heated at 100–110°C with TiCl4 or SnCl4 (0.012 mol) for 3-4 hr to complete the reaction. The obtained black reaction mixture was then neutralised with NaOH solution and extracted with dichloromethane. Product was isolated simply by evaporation of the solvent at reduced pressure. The crude product was recrystallized from hexane. The obtained product was characterised by IR, NMR, and mass spectroscopy data and compared with authentic sample. Furthermore, the reaction was carried out for several substituted aryl amines and nitriles (Table 1) under the same conditions. It is distinct that both TiCl4 and SnCl4 were found to have a potential utility for the synthesis of amidine with good-to-moderate yields under mentioned reaction conditions.
Scheme 1.
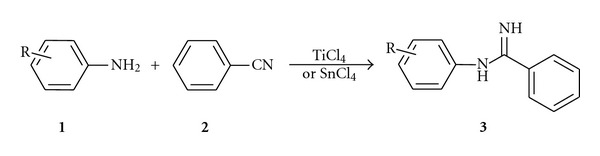
Table 1.
SnCl4/TiCl4 catalysed coupling of substituted anilines with benzonitrile.
| Entry no. | Amines | Amidinesa (isolated yield %) | M.P. °C (Lit.) | |
|---|---|---|---|---|
| by TiCl4 | by SnCl4 | |||
| 3a | Aniline | 75 | 71 | 114–116 (116) |
| 3b | 2-Cl-Aniline | 65 | 67 | 108–110 |
| 3c | 4-F-Aniline | 64 | 63 | 86–88 |
aAll products were characterised by IR, NMR, and mass spectral data and in comparison with authentic samples.
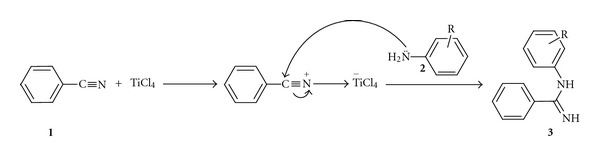
The mechanism we propose for the reaction is similar to the one reported for amidine using AlCl3 [42] and is outlined in Scheme 2: at first the complex between nitrile and TiCl4 was formed followed by nucleophilic addition of amine (2) on the more electrophilic carbon of nitriles to yield the amidine (3).
Scheme 2.
Mechanism.
With these results in hand, we tested the scope and limitations of this process; we examined the coupling reaction of various substituted benzonitriles with heteroaromatic amine, that is, 2-aminopyridine, (Scheme 3 and Table 2) by performing the reaction with the well-established reaction conditions.
Scheme 3.
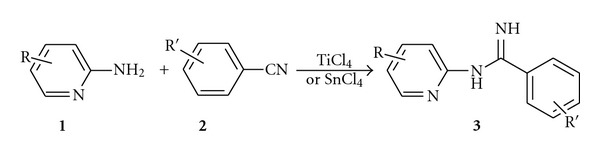
Table 2.
SnCl4/TiCl4 catalysed coupling of 2-aminopyridine with substituted benzonitriles.
| Entry no. | Amines | Nitriles | Amidinesa (isolated yield %) | M.P.°C (Lit.) | ||
|---|---|---|---|---|---|---|
| R | X | R′ | by TiCl4 | by SnCl4 | ||
| 3d | H | N | H | 72 | 61 | 96 (97-98) |
| 3e | H | N | 3-Cl | 63 | 68 | 114–116 |
| 3f | H | N | 4-Cl | 69 | 63 | 162–164 |
| 3g | H | N | 4-Br | 73 | 66 | 152–154 |
| 3h | 4-Br | N | H | 67 | 63 | 102–104 |
aAll products were characterised by IR, NMR, and mass spectral data and in comparison with authentic samples.
Similarly, to test the scope and limitations of this process; we examined the coupling reaction of various substituted anilines with heteroaromatic nitrile, that is, 2-cyanopyridine, (Scheme 4 and Table 3) by performing the reaction with the well-established reaction conditions.
Scheme 4.
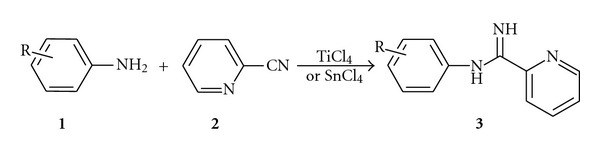
Table 3.
SnCl4/TiCl4 catalysed coupling of substituted anilines with 2-cyanopyridine.
| Entry no. | Amines | Amidinesa (isolated yield %) | M.P. °C (Lit.) | |
|---|---|---|---|---|
| R | by TiCl4 | by SnCl4 | ||
| 3i | H | 79 | 68 | 78–80 HCl Salt |
| 3j | 2-CH3 | 59 | 64 | 68–70 (68-69) |
| 3k | 4-CH3 | 64 | 61 | 54 (52-53) |
| 3l | 4-F | 78 | 69 | 72 (75-76) |
| 3m | 4-Cl | 66 | 60 | 80–82 (80–82) |
| 3n | 4-Br | 81 | 65 | 84–86 (85-86) |
| 3o | 3,4-Cl | 76 | 69 | 112 (112-113) |
aAll products were characterised by IR, NMR, and mass spectral data and in comparison with authentic samples.
3. Conclusion
In the summary, we have developed a solvent-free method of amidine formation from nitrile and amine using TiCl4 or SnCl4 in absence of expensive metal-like samarium. The reaction proceeded at 100–110°C and was completed within 3-4 hrs. In conclusion the reaction was extremely simple to carry out, and the obtained yield of amidine was good to moderate. On the basis of yield, we may conclude that TiCl4 is preferable catalyst over SnCl4.
4. Experimental
Melting points were determined by open capillary tube method and are uncorrected. Progress of the reaction was monitored by TLC (visualization was effected by exposure to UV light). Commercial reagents were used without purification for synthesis. Mass spectra were recorded on Thermo Finnigan (model- LCQ Advantage MAX) mass spectrometer. The IR spectra were recorded with KBr pellets on Perkin-Elmer Spectrum One Spectrometer. 1H NMR spectra were recorded in CDCl3 on a Bruker 300 DRX Avance instrument at 300 MHz.
4.1. Preparation of Amidines, 3a–o
Benzonitrile (1.03 g, 0.01 mol) was taken in a dry round bottom flask and to this was added a 2-aminopyridine (0.94 g, 0.01 mol). The flask was heated, after fitting a dry condenser along with a guard tube, in an oil bath at a temperature range of 80–90°C with stirring. After 30 min TiCl4 (1.3 mL, 0.012 mol) or SnCl4 (1.4 mL, 0.012 mol) was added to the flask. After addition, temperature was increased to 100–110°C, and contents of the flask were heated for 3-4 hrs. The mixture was cooled to room temperature, and the solid, thus, formed was dissolved in hot water and made alkaline with 10% NaOH. This solution was extracted with a CH2Cl2 (3 × 100 mL). Then organic layer was decolourized with activated charcoal and dried over anhydrous Na2SO4. After evaporating the solvent under reduced pressure, crude amidine was obtained. This crude product was recrystallized from hexane to get pure amidine.
N-Phenylbenzamidine (3a, C13H12N2) —
1H NMR (CDCl3, δ ppm): 4.84 (br s, 2H, NH, C=NH), 6.96–6.99 (d, J=8.1 Hz, 2H, ArH), 7.03–7.08 (t, J=7.5 Hz 1H, ArH), 7.32–7.37 (t, J=7.8 Hz, 2H, ArH), 7.42–7.49 (m, 3H, ArH), 7.85 (s, 2H, ArH); IR (KBr) ν (cm−1): 3340, 2853, 1618, 1574, 1459, 1377, 1153, 722; MS (ESI, 70 eV) m/z (%): 198 (13), 197 (100) [M + H]+.
N-(2-Chlorophenyl)benzamidine (3b, C13H11ClN2) —
1H NMR (CDCl3, δ ppm): 4.78 (br s, 2H, NH, C=NH), 6.97–7.02 (m, 2H, ArH), 7.19–7.22 (t, J=7.2 Hz,1H, ArH), 7.39–7.51 (m, 4H, ArH), 7.86 (br s, 2H, ArH); IR (KBr) ν (cm−1): 3345, 2853, 1618, 1574, 1459, 1377, 1153, 722; MS (ESI, 70 eV) m/z (%): 233 (37), 231(100) [M + H]+.
N-(4-Fluorophenyl)benzamidine (3c, C13H11FN2) —
1H NMR (CDCl3, δ ppm): 4.86 (br s, 2H, 2NH), 6.90–6.94 (m, 2H, ArH), 7.01–7.07 (t, J=8.3 Hz, 2H, ArH), 7.41–7.46 (t, J=7.3 Hz, 3H, ArH), 7.83–7.86 (d, J=6.3 Hz, 2H, ArH); IR (KBr) ν (cm−1): 3348, 2854, 1625, 1590, 1459, 1377, 1152, 777, 722; MS (ESI, 70 eV) m/z (%): 216 (17), 215 (100) [M + H]+, 198 (13).
N-(Pyridin-2-yl)benzamidine (3d, C12H11N3) —
1H NMR (CDCl3, δ ppm): 2.03 (bs, 2H, 2NH), 6.90–6.95 (m, 1H, ArH), 7.25–7.29 (d, J=9 Hz, 1H, ArH), 7.40–7.48 (m, 3H, ArH), 7.61–7.67 (m, 1H, ArH), 7.89–7.92 (m, 2H, ArH), 8.31–8.34 (m, 1H, ArH); IR (KBr) ν (cm−1): 3351, 2854, 1625, 1590, 1459, 1377, 1152, 777, 722; MS (ESI, 70 eV) m/z (%): 198 (100) [M + H]+.
3-Chloro-N-(pyridin-2-yl)benzamidine (3e, C12H10ClN3) —
1H NMR (CDCl3, δ ppm): 2.03 (bs, 2H, 2NH), 6.92–6.96 (m, 1H, ArH), 7.25–7.45 (m, 3H, ArH), 7.62–7.68 (m, 1H, ArH), 7.72–7.73 (d, J=1.5 Hz, 1H, ArH), 7.92–7.93 (t, J=1.8 Hz, 1H, ArH), 8.32–8.34 (m, 1H, ArH); IR (KBr) ν (cm−1): 3341, 2854, 1625, 1590, 1459, 1377, 1152, 777, 722; MS (ESI, 70 eV) m/z (%): 231 (100) [M + H]+.
4-Chloro-N-(pyridin-2-yl)benzamidine (3f, C12H10ClN3) —
1H NMR (CDCl3, δ ppm): 1.81 (br s, 2H, 2NH), 6.93–6.97 (m, 1H, ArH), 7.25–7.27 (t, J=8.1 Hz,1H, ArH), 7.41–7.43 (d, J=6.3 Hz, 2H, ArH), 7.63–7.69 (m, 1H, ArH), 7.85–7.89 (d, J=6.9 Hz, 2H, ArH), 8.33–8.35 (m, 1H, ArH); IR (KBr) ν (cm−1): 3344, 2853, 1618, 1594, 1462, 1377, 1125, 831, 782, 722, 538; MS (ESI, 70 eV) m/z (%): 231 (100) [M + H]+.
4-Bromo-N-(pyridin-2-yl)benzamidine (3g, C12H10BrN3) —
1H NMR (CDCl3, δ ppm): 1.78 (br s, 2H, 2NH, C=NH), 6.92–6.97 (m, 1H, ArH), 7.24–7.27 (t, J=3.9 Hz,1H, ArH), 7.55–7.60 (m, 2H, ArH), 7.63–7.69 (m, 1H, ArH), 7.72–7.81 (m, 2H, ArH), 8.32–8.35 (m, 1H, ArH); IR (KBr) ν (cm−1): 3349, 2854, 1640, 1577, 1530, 1459, 1377, 1321, 1300, 1260, 1151, 1119, 1096, 1006, 818, 769, 722, 541; MS (ESI, 70 eV) m/z (%): 278 (100), 276 (100) [M + H]+.
N-(5-Bromopyridin-2-yl)benzamidine (3h, C12H10BrN3) —
1H NMR (CDCl3, δ ppm): 1.68 (br s, 2H, NH), 7.16–7.19 (d, 1H, ArH), 7.42–7.49 (m, 3H, ArH), 7.71–7.75 (m, 1H, ArH), 7.88–7.91 (t, J=6 Hz, 2H, ArH), 8.37–8.38 (d, J=2.4 Hz, 1H, ArH); IR (KBr) ν (cm−1): 3350, 2853, 1618, 1574, 1459, 1377, 1153, 722; MS (ESI, 70 eV) m/z (%): 278 (94), 276 (100) [M + H]+, 260 (90), 259 (90).
N-Phenylpicolinamidine (3i, C12H11N3) —
1H NMR (CDCl3, δ ppm): 5.91 (br s, 2H, NH, C=NH), 6.88–7.26 (m, 4H, ArH), 7.31–7.37 (m, 2H, ArH), 7.78–7.81 (m, 1H, ArH), 8.41–8.45 (m, 1H, ArH), 8.52–8.59 (m, 1H, ArH); IR (KBr) ν (cm−1): 3346, 2853, 1618, 1574, 1459, 1377, 1153, 722; MS (ESI, 70 eV) m/z (%): 198 (100) [M + H]+, 181 (45).
N-o-tolylpicolinamidine (3j, C13H13N3) —
1H NMR (CDCl3, δ ppm): 2.18 (s, 3H, ArCH3), 5.72 (br s, 2H, NH, C=NH), 6.89–7.02 (m, 2H, ArH), 7.16–7.24 (q, J=7.8 Hz, 1H, ArH), 7.30–7.33 (dd, J=8.4 Hz,1H, ArH), 7.36–7.41 (m, 1H, ArH), 7.77–7.84 (m, 1H, ArH), 8.36–8.48 (m, 1H, ArH), 8.55–8.57 (m, 1H, ArH); IR (KBr) ν (cm−1): 3345, 1694, 1613, 1463, 1377, 1118, 721; MS (ESI, 70 eV) m/z (%): 212 (100) [M + H]+, 195 (37).
N-p-tolylpicolinamidine (3k, C13H13N3) —
1H NMR (CDCl3, δ ppm): 2.18 (s, 3H, ArCH3), 5.58 (br s, 2H, NH, C=NH), 6.90–7.01 (m, 3H, ArH), 7.18–7.26 (m, 2H, ArH), 7.36–7.40 (m, 1H, ArH), 7.56 (m, 1H, ArH), 7.77–7.84 (m, 1H, ArH); IR (KBr) ν (cm−1): 3339, 2854, 1694, 1613, 1463, 1377, 1118, 721; MS (ESI, 70 eV) m/z (%): 212 (100) [M + H]+, 195 (37).
N-(4-Fluorophenyl)picolinamidine (3l, C12H10FN3) —
1H NMR (CDCl3, δ ppm): 1.76 (br s, 1H, NH), 5.81 (br s, 1H, NH), 6.88–7.02 (m, 4H, ArH), 7.32–7.35 (m, 1H, ArH), 7.73–7.78 (m, 1H, ArH), 8.32–8.34 (d, J=6 Hz, 1H, ArH), 8.49–8.50 (d, J=6 Hz, 1H, ArH); IR (KBr) ν (cm−1): 3378, 2854, 1625, 1590, 1459,1377, 1152, 777, 722; MS (ESI, 70 eV) m/z (%): 216 (100) [M + H]+, 199 (25).
N-(4-Chlorophenyl)picolinamidine (3m, C12H10ClN3) —
1H NMR (CDCl3, δ ppm): 5.86 (br s, 2H, NH, C=NH), 6.84–6.88 (m, 2H, ArH), 7.25–7.42 (m, 3H, ArH), 7.73–7.82 (t, J=5.7 Hz, 1H, ArH), 8.29–8.40 (m, 1H, ArH), 8.51–8.59 (m, 1H, ArH); IR (KBr) ν (cm−1): 3345, 2853, 1618, 1574, 1459, 1377, 1153, 722; MS (ESI, 70 eV) m/z (%): 232 (100) [M + H]+, 215 (33).
N-(4-Bromophenyl)picolinamidine (3n, C12H10BrN3) —
1H NMR (CDCl3, δ ppm): 5.85 (br s, 2H, NH, C=NH), 6.88–6.91 (d, J=8.7 Hz, 2H, ArH), 7.38–7.50 (m, 3H, ArH), 7.79–7.84 (m, 1H, ArH), 8.36–8.39 (d, J=8.1 Hz, 1H, ArH), 8.56–8.58 (m, 1H, ArH); IR (KBr) ν (cm−1): 3376, 2854, 1640, 1577, 1530, 1459, 1377, 1321, 1300, 1260, 1151, 1119, 1096, 1006, 818, 769, 722, 541; MS (ESI, 70 eV) m/z (%): 278 (100), 276 (100) [M + H]+, 260 (20), 259 (20).
N-(3,4-Dichlorophenyl)picolinamidine (3o, C12H9Cl2N3) —
1H NMR (CDCl3, δ ppm): 5.97 (br s, 2H, 2NH), 6.84–6.88 (m, 1H, ArH), 7.12–7.17 (t, 1H, ArH), 7.39–7.43 (t, J=3.9 Hz,2H, ArH), 7.79–7.87 (t, J=9.6 Hz, 1H, ArH), 8.33–8.36 (d, J=7.8 Hz,1H, ArH), 8.56–8.58 (d, J=4.5 Hz, 1H, ArH); IR (KBr) ν (cm−1): 3345, 1694, 1613, 1463, 1377, 1118, 721; MS (ESI, 70 eV) m/z (%): 267 (100), 266(65) [M + H]+.
References
- 1.Patai S, Rappoport Z. The Chemistry of Amidines and Imidates. Vol. 27. New York, NY, USA: John Wiley & Sons; 1991. [Google Scholar]
- 2.Rahmathullah SM, Tidwell RR, Jones SK, Hall JE, Boykin DW. Carbamate prodrugs of N-alkylfuramidines. European Journal of Medicinal Chemistry. 2008;43(1):174–177. doi: 10.1016/j.ejmech.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 3.de Zwart MAH, van der Goot H, Timmerman H. Synthesis and copper-dependent antimycoplasmal activity of 1-amino-3-(2-pyridyl)isoquinoline derivatives. 2. Amidines. Journal of Medicinal Chemistry. 1989;32(2):487–493. doi: 10.1021/jm00122a033. [DOI] [PubMed] [Google Scholar]
- 4.Calas M, Ouattara M, Piquet G, et al. Potent antimalarial activity of 2-aminopyridinium salts, amidines, and guanidines. Journal of Medicinal Chemistry. 2007;50(25):6307–6315. doi: 10.1021/jm0704752. [DOI] [PubMed] [Google Scholar]
- 5.Stephens CE, Tanious F, Kim S, et al. Diguanidino and “reversed” diamidino 2,5-diarylfurans as antimicrobial agents. Journal of Medicinal Chemistry. 2001;44(11):1741–1748. doi: 10.1021/jm000413a. [DOI] [PubMed] [Google Scholar]
- 6.Göker H, Özden S, Yildiz S, Boykin DW. Synthesis and potent antibacterial activity against MRSA of some novel 1,2-disubstituted-1H-benzimidazole-N-alkylated-5-carboxamidines. European Journal of Medicinal Chemistry. 2005;40(10):1062–1069. doi: 10.1016/j.ejmech.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Bhosale SV, Patil UD, Kalyankar MB, Nalage SV, Patil VS, Desale KR. Facile synthesis of 2,6-diaryl-4-secondary aminonicotinonitriles and highly substituted unsymmetrical 2,2′-bipyridines. Journal of Heterocyclic Chemistry. 2010;47(3):691–696. [Google Scholar]
- 8.Frédérick R, Pochet L, Charlier C, Masereel B. Modulators of the coagulation cascade: focus and recent advances in inhibitors of tissue factor, factor VIIa and their complex. Current Medicinal Chemistry. 2005;12(4):397–417. doi: 10.2174/0929867053363108. [DOI] [PubMed] [Google Scholar]
- 9.Pratap R, Kumar B, Ram VJ. An efficient substituent dependent synthesis of congested pyridines and pyrimidines. Tetrahedron. 2007;63(41):10309–10319. [Google Scholar]
- 10.Agarwal N, Srivastava P, Raghuwanshi SK, et al. Chloropyrimidines as a new class of antimicrobial agents. Bioorganic and Medicinal Chemistry. 2002;10(4):869–874. doi: 10.1016/s0968-0896(01)00374-1. [DOI] [PubMed] [Google Scholar]
- 11.Cheng JF, Chen M, Liu B, Hou Z, Arrhenius T, Nadzan AM. Design and synthesis of heterocyclic malonyl-CoA decarboxylase inhibitors. Bioorganic and Medicinal Chemistry Letters. 2006;16(3):695–700. doi: 10.1016/j.bmcl.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 12.Adams ND, Schmidt SJ, Knight SD, Dhanak D. A novel synthesis of substituted 4H-pyrazolo[3,4-d]pyrimidin-4-ones. Tetrahedron Letters. 2007;48(23):3983–3986. [Google Scholar]
- 13.McCauley JA, Theberge CR, Liverton NJ. Chemoselective reactions of amidines: selective formation of iminopyrimidine regioisomers. Organic Letters. 2000;2(21):3389–3391. doi: 10.1021/ol006499j. [DOI] [PubMed] [Google Scholar]
- 14.Cartwright MW, Sandford G, Bousbaa J, et al. Imidazopyridine and pyrimidinopyridine systems from perfluorinated pyridine derivatives. Tetrahedron. 2007;63(30):7027–7035. [Google Scholar]
- 15.Szczepankiewicz BG, Rohde JJ, Kurukulasuriya R. Synthesis of purines and other fused imidazoles from acyclic amidines and guanidines. Organic Letters. 2005;7(9):1833–1835. doi: 10.1021/ol0504751. [DOI] [PubMed] [Google Scholar]
- 16.Grenda VJ, Jones RE, Gal G, Sletzinger M. Novel preparation of benzimidazoles from N-arylamidines. New synthesis of thiabendazole. Journal of Organic Chemistry. 1965;30(1):259–261. [Google Scholar]
- 17.Pratap R, Farahanullah, Raghunandan R, Maulik PR, Ram VJ. Substituent directed regioselective synthesis of 2-oxonicotonic acids and methyl nicotinates. Tetrahedron Letters. 2007;48(28):4939–4942. [Google Scholar]
- 18.Pratap R, Roy AD, Kushwaha SP, Goel A, Roy R, Ram VJ. Guanidine and amidine mediated synthesis of bridgehead triazaphenalenes, pyrimidines and pyridines through domino reactions. Tetrahedron Letters. 2007;48(33):5845–5849. [Google Scholar]
- 19.Shie JJ, Fang JM. Microwave-assisted one-pot tandem reactions for direct conversion of primary alcohols and aldehydes to triazines and tetrazoles in aqueous media. Journal of Organic Chemistry. 2007;72(8):3141–3144. doi: 10.1021/jo0625352. [DOI] [PubMed] [Google Scholar]
- 20.Zienkiewicz J, Kaszynski P, Young VG. Fused-ring thiadiazines: preparation and crystallographic characterization of 3-phenyl derivative of benzo-, pyridio[2,3-e]-, pyrazino[2,3-e]-, and tetrafluorobenzo-[1,2,4]thiadiazines. Journal of Organic Chemistry. 2004;69(7):2551–2561. doi: 10.1021/jo035835h. [DOI] [PubMed] [Google Scholar]
- 21.Sambaiah T, Reddy KK. Synthesis of 2-Aryl[1,2,4]triazolo[5,1-b]benzoxazoles by oxidative cyclization of N-(benzoxazol-2-yl)benzamidines. Synthesis. 1990;(5):422–424. [Google Scholar]
- 22.Graham WH. The halogenation of amidines. I. Synthesis of 3-halo- and other negatively substituted diazirines. Journal of the American Chemical Society. 1965;87(19):4396–4397. [Google Scholar]
- 23.Potts KT, Burton HR, Bhattacharyya J. 1,2,4-Triazoles. XIII. Derivatives of the s-triazolo[1,5-a]pyridine ring system. Journal of Organic Chemistry. 1966;31(1):260–265. [Google Scholar]
- 24.Bose AK, Kugajevsky I. Studies on lactams-VII. A new synthesis of β-amino-β-lactams. Tetrahedron. 1967;23(2):957–963. [Google Scholar]
- 25.Mahajan RK, Dhawan P. Adsorptive stripping voltammetric determination of nickel(II) using N-2-pyridyl-benzamidine as a complexing reagent. Indian Journal of Chemistry A. 2002;41(5):981–984. [Google Scholar]
- 26.Roger R, Neilson DG. The chemistry of imidates. Chemical Reviews. 1961;61(2):179–211. [Google Scholar]
- 27.Dabak K. Synthesis and protection of some amidines. Turkish Journal of Chemistry. 2002;26(4):547–550. [Google Scholar]
- 28.Bower JD, Ramage GR. Heterocyclic systems related to pyrrocoline. Part II. The preparation of polyazaindenes by dehydrogenative cyclisations. Journal of the Chemical Society (Resumed) 1957:4506–4510. [Google Scholar]
- 29.Rousselet G, Capdevielle P, Maumy M. Copper(I)-induced addition of amines to unactivated nitriles: the first general one-step synthesis of alkyl amidines. Tetrahedron Letters. 1993;34(40):6395–6398. [Google Scholar]
- 30.Forsberg JH, Spaziano VT, Balasubramanian TM, et al. Use of lanthanide(III) ions as catalysts for the reactions of amines with nitriles. Journal of Organic Chemistry. 1987;52(6):1017–1021. [Google Scholar]
- 31.Meder M, Galka CH, Gade LH. Bis(2-pyridylimino)isoindole (BPI) ligands with novel linker units: synthesis and characterization of their palladium and platinum complexes. Monatshefte fur Chemie. 2005;136(10):1693–1706. [Google Scholar]
- 32.Asproni B, Pau A, Bitti M, et al. Synthesis and pharmacological evaluation of 1-[(1,2-diphenyl-1H-4-imidazolyl)methyl]-4-phenylpiperazines with clozapine-like mixed activities at dopamine D2, serotonin, and GABAA receptors. Journal of Medicinal Chemistry. 2002;45(21):4655–4668. doi: 10.1021/jm020848t. [DOI] [PubMed] [Google Scholar]
- 33.Xu F, Sun J, Shen Q. Samarium diiodide promoted synthesis of N,N′-disubstituted amidines. Tetrahedron Letters. 2002;43(10):1867–1869. [Google Scholar]
- 34.Wang J, Xu F, Cai T, Shen Q. Addition of amines to nitriles catalyzed by ytterbium amides: an efficient one-step synthesis of monosubstituted N-arylamidines. Organic Letters. 2008;10(3):445–448. doi: 10.1021/ol702739c. [DOI] [PubMed] [Google Scholar]
- 35.Partridge MW, Smith A. Cyclic amidines. Part XXIV. Cyclisation of N-allyl-N′- arylacetamidines to imidazolines, dihydroquinazolines, and dihydrobenzodiazepines. Journal of the Chemical Society, Perkin Transactions 1. 1973:453–456. [Google Scholar]
- 36.Hill AJ, Johnston JV. Amidines derived from ethylenediamine. I. Diamidines. Journal of the American Chemical Society. 1954;76(3):920–922. [Google Scholar]
- 37.Dondoni A, Barbaro G. Synthetic reactions using transition metal complexes. Conversion of amide oximes into amidines by pentacarbonyliron and evidence for imine intermediates in the deoximation of ketoximes. Journal of the Chemical Society, Chemical Communications. 1975;(18):761–762. [Google Scholar]
- 38.Khanna IK, Yu Y, Huff RM, et al. Selective cyclooxygenase-2 inhibitors: heteroaryl modified 1,2-diarylimidazoles are potent, orally active antiinflammatory agents. Journal of Medicinal Chemistry. 2000;43(16):3168–3185. doi: 10.1021/jm0000719. [DOI] [PubMed] [Google Scholar]
- 39.Dains FB. On the action of certain acid reagents on the substituted ureas. Journal of the American Chemical Society. 1900;22(4):181–198. [Google Scholar]
- 40.Dains FB, Roberts RC, Brewster RQ. On the action of certain acid reagents on the substituted ureas and thiazole. Journal of the American Chemical Society. 1916;38(1):131–140. [Google Scholar]
- 41.Zhou L, Zhang Y. Low-valent titanium induced reductive coupling of nitriles with nitro compounds. Synthetic Communications. 1998;28(17):3249–3262. [Google Scholar]
- 42.Oxley P, Partridge MW, Short WF. Amidines. Part VII. Preparation of amidines from cyanides, aluminium chloride, and ammonia or amines. Journal of the Chemical Society (Resumed) 1947:1110–1116. [Google Scholar]


