Abstract
Phospholipids play an essential role in cell membrane structure and function. The length and number of double bonds of fatty acids in membrane phospholipids are main determinants of fluidity, transport systems, activity of membrane-bound enzymes, and susceptibility to lipid peroxidation. The fatty acid profile of serum lipids, especially the phospholipids, reflects the fatty acid composition of cell membranes. Moreover, long-chain n-3 polyunsatured fatty acids decrease very-low-density lipoprotein assembly and secretion reducing triacylglycerol production. N-6 and n-3 polyunsatured fatty acids are the precursors of signalling molecules, termed “eicosanoids,” which play an important role in the regulation of inflammation. Eicosanoids derived from n-6 polyunsatured fatty acids have proinflammatory actions, while eicosanoids derived from n-3 polyunsatured fatty acids have anti-inflammatory ones. Previous studies showed that inflammation contributes to both the onset and progression of atherosclerosis: actually, atherosclerosis is predominantly a chronic low-grade inflammatory disease of the vessel wall. Several studies suggested the relationship between long-chain n-3 polyunsaturated fatty acids and inflammation, showing that fatty acids may decrease endothelial activation and affect eicosanoid metabolism.
1. Introduction
Cardiovascular disease is the leading cause of mortality in many economically developed nations accounting for about 30% of all deaths [1] and its incidence is still increasing. Ongoing research aims to investigate and prevent the early development of cardiovascular risk factors such as atherosclerosis, hypertension, dyslipidemia, chronic inflammation, and insulin resistance.
The beneficial effects of n-3 polyunsaturated fatty acids (n-3 PUFAs) were proved in several observational and experimental studies. The lipid lowering action of n3-PUFAs was detected at the beginning, so these nutrients were used for the treatment of dyslipidemic disorders. Their anti-inflammatory, antithrombotic, antiatherosclerotic, and antiarrhythmogenic effects were observed later. Low-grade chronic inflammation is now recognized as a prominent process in the development of atherosclerosis and coronary heart disease. The induction of inflammation may well provide a link between hyperlipidemia and atherogenesis [2, 3].
Atherosclerosis is now considered a “systemic disease” featured by low-grade arterial inflammatory lesions that can develop through the disease progression [4].
In physiological conditions, endothelial cells synthesize and release adequate amounts of nitric oxide (NO) and prostaglandins (such as PGE2 and PGE3) and maintain a downstream balance between pro- and anti-inflammatory molecules. However, in the presence of atherosclerosis this balance disrupts leading towards an increase production of proinflammatory cytokines as interleukins 1, 2, and 6 (IL-1, 2, and 6) and tumor necrosis factor α (TNF-α), with further progression of the disease [5]. These pro-inflammatory cytokines can induce oxidative stress by enhancing the production of reactive oxygen species (ROS) by monocytes, macrophages, and leukocytes. PUFAs and their eicosanoid derivatives may play a significant role modulating the inflammatory response (Figure 1) [6].
Figure 1.
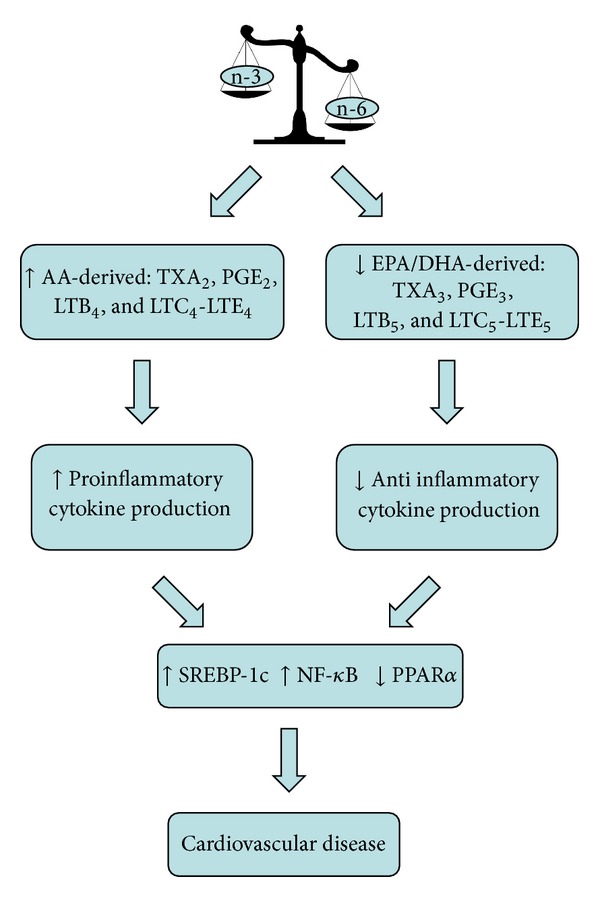
Effects of unbalanced n-6:n-3 dietary fatty acid intake on inflammatory state. EPA: eicosapentaenoic acid; DHA: docasahexaenoic acid; AA: arachidonic acid; PPAR-α: peroxisome proliferation-activated receptors-α; TX A2: thromboxane A2; TX A3: thromboxane A3; NF-κB: nuclear factor kappa light-chain enhancer of activated B cells; PGE2: prostaglandin E2; PGE3: prostaglandin E3; LTB: leukotriene; SREBP-1c: sterol regulatory binding protein 1c.
2. Metabolism of PUFAs
Unsaturated fatty acids are referred to as PUFAs when two or more double bounds are present. There are two PUFAs families, omega-3 (n-3) and omega-6 (n-6) fatty acids. They differ in location of the last double bond relative to the terminal methyl end of the molecule. The human body can produce almost all fatty acids, except linoleic acid (LA, C18:2 n-6, precursor to the n-6 series of fatty acids) and α-linoleic acid (ALA, C18:3n-3, precursor to the n-3 series of fatty acids). These two PUFAs are named “essential fatty acids” because the body cannot synthesize them [7].
Endogenous conversion (elongation and desaturation) of the initial C18 PUFA precursors results in the synthesis of longer-chain counterparts such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in the n-3 family and dihomo-γ-linoleic acid (DGLA) and arachidonic acid (AA) in the n-6 family [1].
In humans the biochemical pathways converting ALA to EPA and EPA to DHA are limited: 0.2–8% of ALA is converted to EPA (generally more in women) and 0–4% of ALA to DHA [8–12]. Results from pilot studies suggest that ALA conversion is also limited to function as a surrogate for fish consumption [13]. Thus, tissue and circulating EPA and DHA levels are primarily related to their dietary intake. Fish is the major food source of long-chain n-3 PUFAs, including EPA, DHA, and docosapentaenoic acid (DPA), while ALA is a plant n-3 fatty acid mainly found in seeds, nuts, and their oils. Thus, plant sources of n-3 fatty acids cannot currently be considered as a replacement for seafood-derived n-3 PUFAs [14]. This suggests that n-3 fatty acids derived from different sources might have their own specific effects on cardiovascular risk markers. Linoleic acid is thought to decrease the conversion of ALA into EPA and DHA by competing for the n-6-desaturase enzyme [15]. Previous studies showed that genetic variations in this enzyme may be related to cardiovascular disease [16]. Furthermore, because the two fatty acid pathways are mutually exclusive (i.e., n-3 fatty acid cannot become n-6 fatty acid and vice versa), a balanced intake of ALA and LA as precursors or of their longer-chain products EPA, DHA, and AA is required [1].
3. PUFAs and Cardiovascular Disease
There is a vast amount of epidemiologic evidence of a cardioprotective effect of fish oil-derived EPA and DHA and it was confirmed in randomized controlled trials [17–20]. Several intervention studies, such as the “Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico” (GISSI)-Prevenzione trial and the Cardiovascular Health Study, have shown that an increased intake of eicosapentaenoic acid (EPA, C20:5n-3) and docosahexaenoic acid (DHA, C22:6n-3) lowers the risk of coronary heart disease (CHD) [21, 22]. The first GISSI-Prevenzione trial, a randomized open label study in 11324 Italian patients with recent myocardial infarction, demonstrated that patients had a 15% lower combined risk of mortality, nonfatal myocardial infarction, and stroke upon supplementation for 3.5 years with 850 mg·day-1 of LC n-3 PUFA. The relative risk of cardiovascular mortality was also decreased by 30% and that of sudden death by 45%. The GISSI-Prevenzione trial demonstrated that a significant protective effect could be obtained with doses much lower than those previously considered necessary for significant beneficial effects [21].
The second GISSI-HF study, a randomized double-blind placebo-controlled trial in 6975 Italian patients with chronic heart failure, revealed a moderate decrease in both all-cause mortality admissions to hospital for cardiovascular disease upon supplementation for an average of 3.9 years with 1 g of LC n-3 PUFA daily. Again, the beneficial effects were seen in a population already treated with recommended therapies [23].
The “Japan Eicosapentaenoic acid (EPA) Lipid Intervention Study,” (JELIS) trial, was performed on 18645 Japanese men and women with hypercholesterolaemia treated with statins. Supplementation with 1.8 g EPA daily decreased major coronary events by 19% over 4.6 years. Non-fatal coronary events, rather than CHD death, were decreased [24]. This may be evident in Japanese population where a consistent reduction of CHD is observed probably because of a high background seafood intake [25, 26]. In summary, a modest intake of LC n-3 PUFA significantly decreases the risk of fatal CHD. However, higher doses and longer duration of intervention, as reported for the JELIS study, have the potential to protect from non-fatal CHD events [26]. Presently, there is evidence to suggest that the two LC n3-PUFAs—EPA (eicosapentaenoic acid, 20:5N3) and DHA (docosahexaenoic acid, 22:6N3)—exert pleiotropism (akin to those effects which have been reported for certain cardiovascular drugs) by modulating a range of diverse target mechanisms involved in cardiovascular disease development. [27–30]. Since the appearance of purified forms of DHA in the market in the 1990s, researchers have started to investigate the differential effects of EPA and DHA on cardiovascular health. However, the number of human studies is still limited in this field and the independent effects of EPA and DHA on various cardiovascular outcomes are yet to be firmly established [31].
Furthermore, the incorporation of EPA and DHA into the cell membrane influences its organisation, fluidity, and permeability, as well as the activity of transmembrane proteins, including receptors, enzymes, and ion channels. Both EPA and DHA modulate K, Na, and Ca channel activities in myocardial cells, regulating myocyte electrical excitability and contractility [32–34]. These effects are concentration dependent and appear to be mediated by the action of EPA and DHA on membrane fluidity [34], although other mechanisms, such as a direct binding of n-3 LCP to the channel, may be involved [35]. Furthermore, animal studies support the increasing evidence that DHA rather than EPA is preferentially incorporated into the myocardial cell membrane [36]. Collectively, these findings help to explain the antiarrhythmic and heart rate (HR)-lowering effects observed by DHA but not by EPA in humans [37]. In addition, incorporation of DHA into the membrane of cardiomyocytes influences the beta adrenergic system to a greater extent than EPA [38], that is, potentially another important mechanism involved in the hypotensive and anti-arrhythmic action of DHA. Furthermore, DHA incorporation into the membrane of endothelial cells stimulates ATP release from the endothelium, increasing vasodilation by stimulating nitric oxide (NO) release [39]. The induction of NO release, together with the decrease in noradrenaline levels, may also account for the BP-lowering effect of DHA [39]. A recent meta-analysis including thirty randomized controlled trials showed that prolonged fish oil intake may reduce HR, especially in populations with a high baseline HR [40]. In dyslipidemic males and postmenopausal women, this decrease appears to be mediated by DHA rather than EPA [37, 41, 42]. In contrast, no significant effect of either EPA or DHA on HR was observed in healthy males for similar dosage and treatment duration [43]. HR variability (HRV) is a strong predictor of CVD, including sudden cardiac death, arrhythmic CHD, and atrial fibrillation. Fish oils have shown anti-arrhythmic properties in animal studies [36], and several clinical and epidemiological studies have reported an association between HRV and n-3 LCP blood cell levels and/or fish oil intake [44–46].
The relationship between ALA and cardiovascular disease is less clear. Short-term trials (6–12 week) in healthy individuals showed no or low effect of a daily intake of 1.2–3.6 g of ALA on blood lipids, LDL-oxidation, lipoprotein (a), and apolipoproteins A-I and B [8, 47]. Long-term treatment with high-dose ALA (40 g/daily) showed a beneficial effect by reduction of body weight and blood LDL/total cholesterol ratio [47, 48]. Previous evidence favored recommendation for modest dietary consumption of ALA (2-3 g daily) in the primary and secondary prevention of CHD [19]. The recently published case-control study by Campos et al. [49] shows a strong inverse associations between ALA status-intake and nonfatal MI. Recent data support the assumption that ALA may protect against atherosclerosis [50, 51]. However, data from two recent epidemiologic studies suggest that high tissue ALA is related to an increased rather than decreased risk of fatal cardiovascular events [52] and sudden death [53]. Also, n-3 PUFAs may interfere with CVD with other physiological effects: the reduction of triglyceride (TG) synthesis is well recognized [54]. Moreover, the reduction of hepatic VLDL synthesis may contribute to this protective effect reducing the availability of fatty acids for triglyceride synthesis due to the decreased “de novo” lipogenesis (DNL), increasing the beta-oxidation of fatty acids, reducing the delivery of nonesterified fatty acids to the liver, reducing the activity of TG synthesis by hepatocytes, and finally increasing the hepatic synthesis of phospholipids [54–59].
In both experimental models and human studies, reduction of DNL appears to be prominent [54–60]. A different effect was reported in some studies between EPA and DHA: both EPA and DHA produced a significant reduction of TGs and LDL-cholesterol, raising effects of fish oil [61, 62]. EPA showed antiplatelet and anti-inflammatory properties [63, 64].
Recently, Buckley and coworkers [65] showed that a four-week supplementation with DHA significantly reduced TG levels in normolipidemic human subjects by 22%, while EPA decreased TG levels by 15% without reaching statistical significance. In another four-week interventional study, both EPA and DHA reduced postprandial TG without affecting fasting TG levels in healthy human subjects [66]. However, EPA and DHA seemed to reduce triglyceridaemia to the same extent when given for a long-enough period [60, 61, 67–72].
Studies in humans show that an average intake of n-3 PUFAs 3-4 g/daily decreases serum triacylglycerol concentration by 25–30% in a dose-dependent manner. The same intake does not affect total cholesterol but increases LDL cholesterol by 5–10% and HDL cholesterol by 1–3% [73].
The increase in LDL cholesterol is mainly due to a rise of the larger and the potentially less atherogenic LDL particles [74].
Hypertension (AH) and dyslipidemia (DLP) are the most important and frequent risk factors for CVD in the general population, with a prevalence of 50–80% according to different authors [75–79]. The prevalence of DLP is 40% among untreated hypertensive patients. On the other hand almost half of the patients with increased total cholesterol (TC) have systo-diastolic hypertension [80–83].
Modifications of erythrocyte-membrane fatty acids (FA) composition are an early indicator of the development of AH and lipid disorders [79]. Thus, modifications of erythrocyte-membrane FA are fairly subtle indicators of lipid metabolism pathology which manifest themselves much earlier than changes in plasma lipoproteins. Modifications of fatty acids composition of the cell membrane lipid matrix play an important role in the AH pathogenesis. Changes of the fatty acids (FA) composition with decrease of essential PUFAs may result in increase of membrane microviscosity, activation of pro-inflammatory eicosanoids synthesis and increased sensitivity of the smooth muscle cells in artery walls to the influence of vasoconstrictors [84]. That is the reason of the great interest in the study of the spectrum of erythrocyte lipids in hypertensive dyslipidemic patients.
Deficiency of n-3 PUFA is a distinctive feature of the modification of erythrocyte-membrane FA in hypertensive dyslipidemic patients. In particular Novgorodetseva and coworkers observed a significant reduction of 22:5n-3 and 22:6n-3 [79]. Deficiency of endogenous n-3 PUFAs may lead to changes in physicochemical properties of cell membranes, activation of the synthesis of proinflammatory and vasoconstrictive eicosanoids, and finally induction of a systemic inflammatory syndrome [84]. All these conditions may favor the induction and progression of atherosclerosis [85]. Thus, structural and functional changes in erythrocyte cell membranes develop in hypertension as a part of the common systemic disorder of lipid metabolism. The FA disorganization of the cell membrane may cause the development of hyperlipidemia and the progression of hypertensive disease [74]. Thus, an important role of FA in the pathogenesis of cardiovascular disease seems clear.
A large part of the cardioprotective actions of LC n-3-PUFAs is likely to be mediated by the vascular endothelium [28, 86–88]. This thin monolayer of cells plays a central role in cardiovascular homeostasis and function via the production of a range of potent autocrine and paracrine biochemical mediators that control the tone of vascular smooth muscle cells [31, 89, 90]. Vascular endothelium is also the site for the inception, progression, and clinical manifestations of atherosclerosis [91–93].
Multifactorial endothelial dysfunction induced by DLP, toxins, cigarette-smoking, and so on may be the early event in the development of atherosclerosis. The endothelium becomes “proadhesive” and induces an increased adhesion of circulating monocytes that subsequently infiltrate the arterial intima. At this level endothelial cells or macrophages release reactive oxygen species that oxidize circulating LDL into oxidized-LDL and create the “lipid streak” [94]. In the evolution from the lipid streak to the atherosclerotic plaque, numerous cytokines that cause infiltration of smooth muscle layer by leukocytes and fibroblasts and promote platelet adhesion are involved in turn [2] (Figures 2 and 3).
Figure 2.
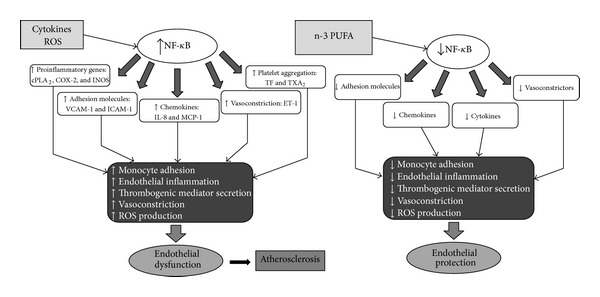
Relationship between inflammation, atherosclerosis, and n-3-PUFA. NF-κB: nuclear factor kappa light-chain enhancer of activated B cells; ROS: reactive oxygen species; cPLA2: cytosolic phospholipase A2; COX-2: cyclooxygenase-2; i-NOS: inducible nitric oxide synthase; VCAM-1: vascular cell adhesion protein 1; ICAM-1: intercellular adhesion molecule-1; IL-8: interlukin-8; MCP-1: monocyte chemoattractant protein-1; TF: tissue factor; ET-1: endothelin-1; TX A2: thromboxane A2.
Figure 3.
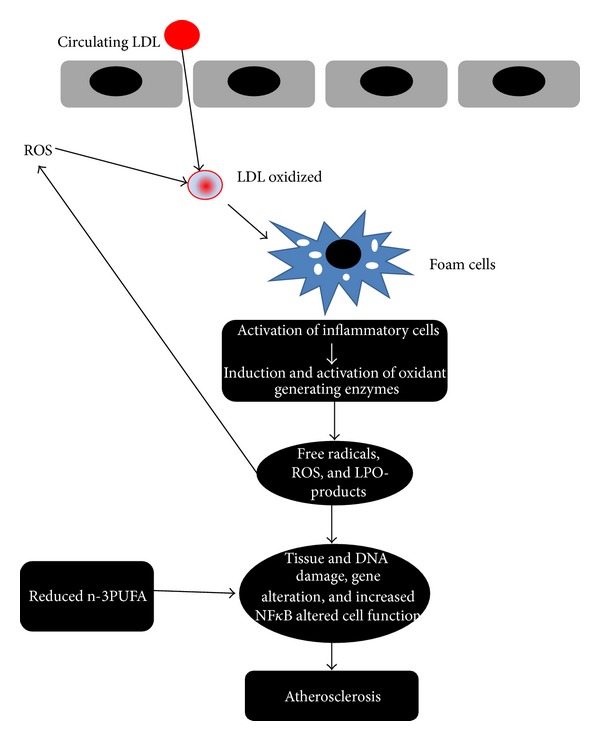
Factors and cells involved in the development of atherosclerosis. ROS: reactive oxygen species; LPO-products: lipo-peroxidation products; NF-κB: nuclear factor kappa light-chain enhancer of activated B cells.
During the migration over the vessel wall, monocytes are exposed to various proinflammatory stimuli that contribute to their differentiation into macrophages. After the absorption of lipoproteins, these macrophages become “foam cells.” Other macrophages induce a local inflammatory compartment within the vassel wall, that is, continuously powered by cytokines, activated T-helper cells and scavanger receptor activators. The inhibition of this self-maintaining process may reduce atherogenesis as demonstrated in mutant mice deficient for macrophage colony stimulating factor (M-CSF) or macrophage migration inhibitory factor (MIF) [95, 96]. In more advanced lesions, cell debris, apoptotic cells, and free cholesterol particles accumulate in the lipid-rich necrotic core of the plaque, are sometimes separated from the blood only by a thin fibrous cap. The number of inflammatory cells within the lesion is strongly associated with the risk of rupture of the so-called “vulnerable plaque.” At last, the process may propagate intraluminal thrombosis, thereby distal ischemia of heart or brain tissue [97, 98].
Through their positive antithrombotic, lipid-lowering, proendothelial functional activities, n-3 PUFAs play an important role on the mechanisms of atherosclerosis. In fact supplementation of N-3 PUFAs improves flow-mediated arterial dilatation by an increased endothelial synthesis of nitric oxide [86, 99–111]. Several, although not all, clinical trials have also found that consumption of n-3 PUFAs lowers circulating markers of endothelial dysfunction, such as E-selectin, vascular cell adhesion molecule-1, and intracellular adhesion molecule-1 [74, 112, 113]. Thus, normalization of endothelial function could partly mediate the protective effects of n-3 PUFA against CVD. A recent randomized clinical trial established the role of PUFAs on plaque stability [114]. Patients with carotid artery atherosclerosis were randomized to take placebo, fish oil (n-3 PUFAs), or seed oil (n-6 PUFAs). Subjects treated with n-3 PUFAs showed higher EPA and DHA concentration, reduction of monocytes and macrophages, and a thicker fibrous cap compared to controls and group treated with n-6 PUFAs, all changes that can enhance the stability of atherosclerotic plaques. By contrast, increased assumption of n-6 PUFAs did not affect carotid plaque fatty acid composition or stability. Plaques stability may explain the reductions in nonfatal and fatal cardiovascular events, associated with increased n-3 PUFAs intake [114]. The triglyceride-lowering, antiatherogenic, antithrombotic, and anti-inflammatory effects of n-3 PUFAs were clearly demonstrated in human studies. Conversely, their anti-arrhytmogenic effect was showed only in vitro and in animal experiments, as confirmation in humans has been limited by the absence of reliable physiological measures or biomarkers to quantify antiarrhythmic potential [115]. The main effects exerted by PUFAs in cardiovascular diseases are summarized in Table 1.
Table 1.
PUFAs and cardiovascular disease.
| PUFA effects | |
|---|---|
| (1) Anti-inflammatory | (a) ↓NF-κB activation |
| (b) EPA and DHA compete with AA for COX & 5-lipo-oxygenase enzymatic sites | |
| ⇓ | |
| Reduce the production of IL-1, IL-6, and TNF-α | |
| (c) ↑Anti inflammatory eicosanoids | |
|
| |
| (2) Cardiac energetic | (a) ↑ATP generation |
| (b) ↓O2 consumption | |
| (c) ↓Sarcoplasmic reticulum calcium concentration | |
| ⇓ | |
| Maintain normal mitochondrial function | |
|
| |
| (3) Antiarrhythmic | (a) ↑Ca2+/Mg2+ ATPase activity |
| (b) Inhibit fast voltage-dependent Na+ channels (I Na) | |
| (c) Inhibit L-type Ca2+ channels (I Ca,L) | |
| ⇓ | |
| Membrane stabilization | |
| (d) Reduced automaticity | |
| (e) Increased relative refractory period | |
|
| |
| (4) Hemodynamics | (a) Improved endothelium-independent and dependent vasodilatation |
| (b) ↓ET-1 | |
| (c) ↑NO | |
| ⇓ | |
| Improved endothelium dysfunction | |
|
| |
| (5) Ventricular remodeling and fibrosis | (a) ↑PPARγ → ↑adiponectin |
| ⇓ | |
| Attenuates ventricular remodeling | |
|
| |
| (6) Vascular | (a) ↓Platelet aggregation via ↓TXA2 |
| (b) ↓VCAM-1, ELAM-1, ICAM-1 | |
| (c) ↓monocyte endothelial adherence via ↓PAF | |
EPA: eicosapentaenoic acid; DHA: docosahexaenoic acid; AA: arachidonic acid; ET-1: endothelin-1; NO: nitric oxide; PPAR-γ: peroxisome proliferation-activated receptors-γ; TX A2: thromboxane A2; VCAM-1: vascular cell adhesion protein 1; ELAM-1: endothelial leukocyte adhesion molecule-1; ICAM-1: intercellular adhesion molecule-1; PAF: platelet activating factor; COX: cyclooxygenase.
4. PUFAs and Inflammation
Inflammation is a physiological response to tissue trauma or infection, but leukocytes, which are the effector cells of the inflammatory process, have powerful capabilities of tissue remodelling. Thus, the passage of leukocytes from the bloodstream into inflamed tissue is tightly regulated to ensure their precise localization. Recruitment of circulating neutrophils into the tissue stroma occurs during the early phases of inflammation. In this process, peptide agonists of the chemokine family are assumed to provide a chemotactic stimulus capable of supporting the migration of neutrophils across vascular endothelial cells, through the vessel wall and out into the tissue stroma. Although an initial chemokine stimulus is essential for the recruitment of flowing neutrophils by endothelial cells, stimulated by the inflammatory cytokine (TNF-α), transit across the endothelial monolayer is regulated by additional stimuli [116].
A new step in the neutrophil recruitment process that relies upon a lipid-mediated signal to regulate the migration of neutrophils across endothelial cells was recently described. This signal is supplied by the metabolism of arachidonic acid into the eicosanoid prostaglandin-D2 (PGD2) by cyclooxygenase (COX) enzymes. This step in the neutrophil recruitment process was revealed when the EPA was used as an alternative substrate for COX enzymes, leading to the generation of prostaglandin-D3 (PGD3) [117–120]. This alternative eicosanoid inhibited the migration of neutrophils across endothelial cells competing for the PGD2 receptor [121, 122]. PGD2 signalling is subordinate to the chemokine-mediated activation of neutrophils, but without the sequential delivery of this signal, neutrophils fail to penetrate the endothelial cell monolayer. The ability of dietary EPA to inhibit this process reveals an unsuspected level of regulation in the migration of inflammatory leukocytes. This may contribute to clarify the interactions between PUFAs and the inflammatory system and direct research on novel therapeutic agents that target the inflammatory system with greater affinity and/or specificity than dietary supplements of n-3-PUFAs [116].
Acute inflammation and its healing are essential processes for tissue protection. The inflammation healing mechanisms are of main interest, and in recent years, new endogenous anti-inflammatory and proresolving lipid mediators generated from PUFAs (lipoxins, resolvins, protectin, and maresin) were uncovered [123]. Lipid mediator metabolomics of self-resolving inflammatory exudates recently highlighted a new family of potent anti-inflammatory and proresolving mediators. Serhan and coworkers identified families of novel bioactive mediators derived from arachidonic acid (AA-derived lipoxins), eicosapentaenoic acid (EPA-derived E-series resolvins), and docosahexaenoic acid (DHA-derived D-series resolvins, protectin, and maresin) [124–135]. These lipid mediators promote resolution through enhanced clearance of apoptotic PMNs, chemokines, cytokines, and microbial products by macrophages [136, 137].
N-3 PUFAs may act through several mechanisms, for instance, by preventing conversion of the n-6 PUFA arachidonic acid to pro-inflammatory eicosanoids and by their conversion to potent anti-inflammatory mediators such as resolvins [124–126, 138].
A possible biological mechanism underlying the beneficial effects of LC n-3 PUFAs on inflammation and endothelial function is that they may compete with n-6 fatty acids for prostaglandin and leukotriene synthesis at the cyclooxygenase and lipoxygenase levels. LC n-3 PUFAs from fish or fish oil modulate prostaglandin metabolism by increasing the active vasodilator and inhibitor of platelet aggregation prostaglandin E3, the weak platelet aggregator and vasoconstrictor thromboxane A3, and the weak inducer of inflammation leukotriene B5. Otherwise, the production of thromboxane A2, a potent platelet aggregator and vasoconstrictor, and leukotriene B4, an inducer of inflammation and a powerful inducer of leukocyte chemotaxis and adherence is reduced [139]. Another suggested mechanism is that LC n-3 PUFAs may bind reactive oxygen species because of their multiple double bonds and lead to a decreased production of hydrogen peroxide. Hydrogen peroxide is a critical activator of the nuclear factor-κB system of transcription factors that controls the coordinated expression of adhesion molecules and of leukocyte-specific chemoattractants upon cytokine stimulation [140]. He and coworkers [141] found independent inverse associations of LC n-3 PUFAs and nonfried fish with IL-6, CRP and MMP3. The relevance of these biomarkers of inflammatory and endothelial activation in the atherogenic process is well recognized. Previous investigations suggest that both CRP and IL-6, two systemic inflammatory markers, are independent predictors of CVD and may play an important role in atherogenesis [142]. In addition, MMP3 is suggested as an independent prognostic factor in stable coronary artery disease [143]. Several lines of evidence support the main role of MMPs in plaque stability [144].
Soluble intercellular adhesion molecule-1 (sICAM-1) is thought to be a key factor in the adherence of monocytes to the endothelium and subsequent transmigration into the intima (Figure 2). The role of sICAM-1 in the pathogenesis of inflammation and atherosclerosis has been confirmed in experimental models [145]. Previous investigations also indicate that sICAM-1 is an independent predictor of CVD apart of other traditional risk factors [146]. He et al. [141] observed that fried fish but not non-fried fish consumption was significantly inversely related to sICAM-1. This finding is not expected since the frying process may reduce the content of LC n-3 PUFAs and produce trans-fatty acids. One possible explanation is that fried fish consumption may be a marker of relatively unhealthy lifestyle; those who had high fried fish consumption were more likely to suffer from dyslipidemia and were likely to be under treatment with medications (e.g., statins), which may lower sICAM-1 [147]. However, the inverse association between fried fish intake and sICAM-1 level remained significant when patients taking cholesterol-lowering medications were excluded. Anyway, further investigation is warranted [148].
LC n-3 PUFAs are believed to affect inflammatory processes mainly through two pathways: endothelial activation and changes in eicosanoid production, or a combination of the two [28]. Two hypotheses may define these molecular mechanisms. The first, predicated on the observation that n-3 PUFAs regulate the transcription of endothelial cell inflammatory genes by downregulating the activity of the nuclear factor-kB, predicts changes in the levels of adhesion receptor and chemokine expression after supplementation with EPA [149–151]. The second hypothesis proposes that upon endothelial cell activation, EPA may compete with the n-6-PUFA arachidonic acid (AA; 20:4n-6) for cyclooxygenase enzymes (COX1 and COX2) after both fatty acids are released from membrane phospholipids by endogenous phospholipases [152]. The main effects exerted by PUFAs in inflammatory processes are summarized in Table 1.
5. Conclusions
A large amount of investigations suggest, the cardioprotective effects of LC n-3 PUFAs EPA and DHA intake in human subjects, because of their lipid lowering, hypotensive, anti-arrhythmic, and anti-thrombotic properties. Moreover, studies performed in the last twenty years showed heterogeneous effects of different n-3 PUFAs on various cardiovascular outcomes, which may be of paramount relevance in primary and secondary prevention of cardiovascular disease.
Recent in vitro investigations as well as clinical studies also demonstrated that LC-n3-PUFAs significantly interact with inflammation-related mechanisms, such as endothelial activation, modification of eicosanoid metabolism, and resolution of the inflammatory process.
Low-grade chronic inflammation is present in several diseases and is characterized by abnormal circulating levels of pro- and anti-inflammatory cytokines. N-3 PUFAs may modulate inflammation, that is, suggested by the reduction of plasma inflammatory cytokines (TNF-α, Il-6) and inflammatory markers as high sensitive C reactive protein, observed after the intake of EPA and DHA. Low-grade chronic inflammation plays a key role in the induction and progression of atherosclerosis and consequently of cardiovascular disease. Taking into consideration the pleiotropic nature of their actions, we suggest that dietary intake of LC n-3 PUFAs may lead to improvements in cardio-metabolic health parameters of their antioxidant, anti-inflammatory, and antiarrhytmic actions.
Abbreviations
- n-3 PUFAs:
n-3 polyunsaturated fatty acids
- NO:
Nitric oxide
- PGE2; PGE3:
Prostaglandins
- IL-1:
Interleukins 1
- IL-2:
Interleukins 2
- IL-6:
Interleukins 6
- TNF-α:
Tumor necrosis factor α
- ROS:
Reactive oxygen species
- LA:
Linoleic acid
- ALA:
α-Linoleic acid
- EPA:
Eicosapentaenoic acid
- DHA:
Docosahexaenoic acid
- DGLA:
Dihomo-γ-linoleic acid
- AA:
Arachidonic acid
- DPA:
Docosapentaenoic acid
- CHD:
Coronary Heart Disease
- HRV:
Heart Rate Variability
- Tgs:
Triglyceride
- AH:
Arterial Hypertension
- DLP:
Dyslipidemia,
- TC:
Total cholesterol
- FA:
Fatty acids
- M-CSF:
Macrophage colony stimulating factor
- MIF:
Migration Inhibiting Factor
- PGD2:
Prostaglandin D2
- COX1-COX2:
Cycloxygenase 1-2
- TXA:
Thromboxane A
- sICAM-1:
Soluble intercellular adhesion molecule-1.
References
- 1.Abeywardena MY, Patten GS. Role of ω3 longchain polyunsaturated fatty acids in reducing cardio-metabolic risk factors. Endocrine, Metabolic and Immune Disorders. 2011;11(3):232–246. doi: 10.2174/187153011796429817. [DOI] [PubMed] [Google Scholar]
- 2.Dei Cas L, Nodari S, Manerba A. Polyunsaturated fatty acids (n-3 PUFAs) Giornale di Gerontologia. 2007;55(1):45–57. [Google Scholar]
- 3.Ross R. Atherosclerosis and inflammatory disease. New England Journal Medicine. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 4.Montecucco F, Mach F. Seminars in Immunopathology. New York, NY, USA: Springer; 2009. Atherosclerosis is an inflammatory disease. [DOI] [PubMed] [Google Scholar]
- 5.Das UN. A defect in the activity of Δ6 and Δ5 desaturases may be a factor in the initiation and progression of atherosclerosis. Prostaglandins Leukotrienes and Essential Fatty Acids. 2007;76(5):251–268. doi: 10.1016/j.plefa.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Das UN. Biological significance of essential fatty acids. Journal of Association of Physicians of India. 2006;54:309–319. [PubMed] [Google Scholar]
- 7.Patterson E, Wall R, Fitzgerald GF, et al. Health implications of high dietary Omega-6 polyunsaturated fatty acids. Journal of Nutrition and Metabolism. 2012;5:1–16. doi: 10.1155/2012/539426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burdge G. α-Linolenic acid metabolism in men and women: nutritional and biological implications. Current Opinion in Clinical Nutrition and Metabolic Care. 2004;7(2):137–144. doi: 10.1097/00075197-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Burdge GC, Finnegan YE, Minihane AM, Williams CM, Wootton SA. Effect of altered dietary n-3 fatty acid intake upon plasma lipid fatty acid composition, conversion of [13C]α-linolenic acid to longer-chain fatty acids and partitioning towards β-oxidation in older men. British Journal of Nutrition. 2003;90(2):311–321. doi: 10.1079/bjn2003901. [DOI] [PubMed] [Google Scholar]
- 10.Burdge GC, Jones AE, Wootton SA. Eicosapentanoic and docosapentanoic acids are the principal products of alpha-linoleic acid metabolism in young men. British Journal of Nutrition. 2002;88:355–363. doi: 10.1079/BJN2002662. [DOI] [PubMed] [Google Scholar]
- 11.Emken EA, Adlof RO, Gulley RM. Dietary linoleic acid influences desaturation and acylation of deuterium-labeled linoleic and linolenic acids in young adult males. Biochimica et Biophysica Acta. 1994;1213(3):277–288. doi: 10.1016/0005-2760(94)00054-9. [DOI] [PubMed] [Google Scholar]
- 12.Pawlosky RJ, Hibbeln JR, Novotny JA, Salem N. Physiological compartmental analysis of α-linolenic acid metabolism in adult humans. Journal of Lipid Research. 2001;42(8):1257–1265. [PubMed] [Google Scholar]
- 13.Goyens PLL, Spilker ME, Zock PL, Katan MB, Mensink RP. Compartmental modeling to quantify α-linolenic acid conversion after longer term intake of multiple tracer boluses. Journal of Lipid Research. 2005;46(7):1474–1483. doi: 10.1194/jlr.M400514-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.U.S Department of Agriculture. Dietary Guidelines for Americans. 7th edition. Washington, DC, USA: Government Printing Office; 2010. [Google Scholar]
- 15.Harper CR, Edwards MJ, DeFilipis AP, et al. Flaxseed oil increases the plasma concentrations of cardioprotective (n-3) fatty acids in humans. American Society For Nutrition. 2007;137(2816):83–87. doi: 10.1093/jn/136.1.83. [DOI] [PubMed] [Google Scholar]
- 16.Truong H, DiBello JR, Ruiz-Narvaez E, et al. Does genetic variation in the Delta6-desaturase promoter modify the associatio between alpha-linolenic acid and the prevalence of metabolic syndrome? The American Journal of Clinical Nutrition. 2009;89:920–925. doi: 10.3945/ajcn.2008.27107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen SN, Harris WS. New evidence for the cardiovascular benefits of long chain omega-3 fatty acids. Current Atherosclerosis Reports. 2007;9:434–440. doi: 10.1007/s11883-007-0058-8. [DOI] [PubMed] [Google Scholar]
- 18.Harris WS, Kris-Etherton PM, Harris KA. Intakes of long-chain omega-3 fatty acid associated with reduced risk for death from coronary heart disease in healthy adults. Current Atherosclerosis Reports. 2008;10(6):503–509. doi: 10.1007/s11883-008-0078-z. [DOI] [PubMed] [Google Scholar]
- 19.Mozaffarian D. Does alpha-linolenic acid intake reduce the risk of coronary heart disease? A review of the evidence. Alternative Therapies in Health and Medicine. 2005;11(3):24–31. [PubMed] [Google Scholar]
- 20.Harris WS, Miller M, Tighe AP, Davidson MH, Schaefer EJ. Omega-3 fatty acids and coronary heart disease risk: clinical and mechanistic perspectives. Atherosclerosis. 2008;197(1):12–24. doi: 10.1016/j.atherosclerosis.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 21.GISSI Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nel'Infarto miocardico. The Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 22.Lemaitre RN, King IB, Mozaffarian D, Kuller LH, Tracy RP, Siscovick DS. n-3 polyunsaturated fatty acids, fatal ischemic heart disease, and nonfatal myocardial infarction in older adults: the Cardiovascular Health Study. American Journal of Clinical Nutrition. 2003;77(2):319–325. doi: 10.1093/ajcn/77.2.319. [DOI] [PubMed] [Google Scholar]
- 23.GISSI-HF investigators. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. The Lancet. 2008;372(9645):1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 24.Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. The Lancet. 2007;369(9567):1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 25.Mozaffarian D. JELIS, fish oil, and cardiac events. The Lancet. 2007;369(9567):1062–1063. doi: 10.1016/S0140-6736(07)60504-2. [DOI] [PubMed] [Google Scholar]
- 26.Mozaffarian D. Fish and n-3 fatty acids for the prevention of fatal coronary heart disease and sudden cardiac death. American Journal of Clinical Nutrition. 2008;87(6):1991S–1996S. doi: 10.1093/ajcn/87.6.1991S. [DOI] [PubMed] [Google Scholar]
- 27.Di Minno MND, Tremoli E, Tufano A, Russolillo A, Lupoli R, Di Minno G. Exploring newer cardioprotective strategies: ω-3 fatty acids in perspective. Thrombosis and Haemostasis. 2010;104(4):664–680. doi: 10.1160/TH10-01-0008. [DOI] [PubMed] [Google Scholar]
- 28.Abeywardena MY, Head RJ. Longchain n-3 polyunsaturated fatty acids and blood vessel function. Cardiovascular Research. 2001;52(3):361–371. doi: 10.1016/s0008-6363(01)00406-0. [DOI] [PubMed] [Google Scholar]
- 29.Makris GC, Geroulakos G, Makris MC, Mikhailidis DP, Falagas ME. The pleiotropic effects of statins and omega-3 fatty acids against sepsis: a new perspective. Expert Opinion on Investigational Drugs. 2010;19(7):809–814. doi: 10.1517/13543784.2010.490830. [DOI] [PubMed] [Google Scholar]
- 30.Dimitrow PP, Jawien M. Pleiotropic, cardioprotective effects of omega-3 polyunsaturated fatty acids. Mini-Reviews in Medicinal Chemistry. 2009;9(9):1030–1039. doi: 10.2174/138955709788922638. [DOI] [PubMed] [Google Scholar]
- 31.Cottin SC, Sanders TA, Hall WL. The differential effects of EPA and DHA on cardiovascular risk factors. Diabetes and Nutritional Sciences Division, School of Medicine, King’s College London, London, UK. [DOI] [PubMed]
- 32.Xiao YF, Gomez AM, Morgan JP, Lederer WJ, Leaf A. Suppression of voltage-gated L-type Ca2+ currents by polyunsaturated fatty acids in adult and neonatal rat ventricular myocytes. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(8):4182–4187. doi: 10.1073/pnas.94.8.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li GR, Sun HY, Zhang XH, et al. Omega-3 polyunsaturated fatty acids inhibit transient outward and ultra-rapid delayed rectifier K+ currents and Na+ current in human atrial myocytes. Cardiovascular Research. 2009;81(2):286–293. doi: 10.1093/cvr/cvn322. [DOI] [PubMed] [Google Scholar]
- 34.Xiao YF, Sigg DC, Leaf A. The antiarrhythmic effect of n-3 polyunsaturated fatty acids: modulation of cardiac ion channels as a potential mechanism. Journal of Membrane Biology. 2005;206(2):141–154. doi: 10.1007/s00232-005-0786-z. [DOI] [PubMed] [Google Scholar]
- 35.Kang JX, Leaf A. Evidence that free polyunsaturated fatty acids modify Na+ channels by directly binding to the channel proteins. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(8):3542–3546. doi: 10.1073/pnas.93.8.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLennan PL. Myocardial membrane fatty acids and the antiarrhythmic actions of dietary fish oil in animal models. Lipids. 2001;36(supplement):S111–S114. doi: 10.1007/s11745-001-0692-x. [DOI] [PubMed] [Google Scholar]
- 37.Mori TA, Bao DQ, Burke V, Puddey IB, Beilin LJ. Docosahexaenoic acid but not eicosapentaenoic acid lowers ambulatory blood pressure and heart rate in humans. Hypertension. 1999;34(2):253–260. doi: 10.1161/01.hyp.34.2.253. [DOI] [PubMed] [Google Scholar]
- 38.Grynberg A, Fournier A, Sergiel JP, Athias P. Effect of docosahexaenoic acid and eicosapentaenoic acid in the phospholipids of rat heart muscle cells on adrenoceptor responsiveness and mechanism. Journal of Molecular and Cellular Cardiology. 1995;27(11):2507–2520. doi: 10.1006/jmcc.1995.0238. [DOI] [PubMed] [Google Scholar]
- 39.Hashimoto M, Shinozuka K, Gamoh S, et al. The hypotensive effect of docosahexaenoic acid is associated with the enhanced release of ATP from the caudal artery of aged rats. Journal of Nutrition. 1999;129(1):70–76. doi: 10.1093/jn/129.1.70. [DOI] [PubMed] [Google Scholar]
- 40.Mozaffarian D, Geelen A, Brouwer IA, Geleijnse JM, Zock PL, Katan MB. Effect of fish oil on heart rate in humans: a meta-analysis of randomized controlled trials. Circulation. 2005;112(13):1945–1952. doi: 10.1161/CIRCULATIONAHA.105.556886. [DOI] [PubMed] [Google Scholar]
- 41.Stark KD, Holub BJ. Differential eicosapentaenoic acid elevations and altered cardiovascular disease risk factor responses after supplementation with docosahexaenoic acid in postmenopausal women receiving and not receiving hormone replacement therapy. American Journal of Clinical Nutrition. 2004;79(5):765–773. doi: 10.1093/ajcn/79.5.765. [DOI] [PubMed] [Google Scholar]
- 42.Grimsgaard S, Bønaa KH, Hansen JB, Myhre ESP. Effects of highly purified eicosapentaenoic acid and docosahexaenoic acid on hemodynamics in humans. American Journal of Clinical Nutrition. 1998;68(1):52–59. doi: 10.1093/ajcn/68.1.52. [DOI] [PubMed] [Google Scholar]
- 43.Woodman RJ, Mori TA, Burke V, Puddey IB, Watts GF, Beilin LJ. Effects of purified eicosapentaenoic and docosahexaenoic acids on glycemic control, blood pressure, and serum lipids in type 2 diabetic patients with treated hypertension. American Journal of Clinical Nutrition. 2002;76(5):1007–1015. doi: 10.1093/ajcn/76.5.1007. [DOI] [PubMed] [Google Scholar]
- 44.Christensen JH, Skou HA, Fog L, et al. Marine n-3 fatty acids, wine intake, and heart rate variability in patients referred for coronary angiography. Circulation. 2001;103(5):651–657. doi: 10.1161/01.cir.103.5.651. [DOI] [PubMed] [Google Scholar]
- 45.Holguin F, Téllez-Rojo MM, Lazo M, et al. Cardiac autonomic changes associated with fish oil vs soy oil supplementation in the elderly. Chest. 2005;127(4):1102–1107. doi: 10.1378/chest.127.4.1102. [DOI] [PubMed] [Google Scholar]
- 46.Christensen JH, Christensen MS, Dyerberg J, Schmidt EB. Heart rate variability and fatty acid content of blood cell membranes: a dose-response study with n-3 fatty acids. American Journal of Clinical Nutrition. 1999;70(3):331–337. doi: 10.1093/ajcn/70.3.331. [DOI] [PubMed] [Google Scholar]
- 47.Geleijnse JM, De Goede J, Brouwer IA. Alpha-linolenic acid: is it essential to cardiovascular health? Current Atherosclerosis Reports. 2010;12(6):359–367. doi: 10.1007/s11883-010-0137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dodin S, Cunnane SC, Mâsse B, et al. Flaxseed on cardio-vascular disease markers in healthy menopausal women: a randomized, double-blind, placebo-controlled trial. Nutrition. 2008;24:23–30. doi: 10.1016/j.nut.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Campos H, Baylin A, Willett WC. Alpha-linolenic acid and risk of nonfatal acute myocardial infarction. Circulation. 2008;118:339–345. doi: 10.1161/CIRCULATIONAHA.107.762419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sala-Vila A, Cofán M, Pérez-Heras A, et al. Fatty acids in serum phospholipids and carotid intima-media thickness in Spanish subjects with primary dyslipidemia. The American Journal of Clinical Nutrition. 2010;92:186–193. doi: 10.3945/ajcn.2009.28807. [DOI] [PubMed] [Google Scholar]
- 51.Park Y, Lim J, Kwon Y, Lee J. Correlation of erythrocyte fatty acid composition and dietary intakes with markers of atherosclerosis in patients with myocardial infarction. Nutrition Research. 2009;29(6):391–396. doi: 10.1016/j.nutres.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 52.Warensjö E, Sundström J, Vessby B, Cederholm T, Risérus U. Markers of dietary fat quality and fatty acid desaturation as predictors of total and cardiovascular mortality: a population-based prospective study. American Journal of Clinical Nutrition. 2008;88(1):203–209. doi: 10.1093/ajcn/88.1.203. [DOI] [PubMed] [Google Scholar]
- 53.Lemaitre RN, King IB, Sotoodehnia N, et al. Red blood cell membrane α-linolenic acid and the risk of sudden cardiac arrest. Metabolism. 2009;58(4):534–540. doi: 10.1016/j.metabol.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harris WS, Bulchandani D. Why do omega-3 fatty acids lower serum triglycerides? Current Opinion in Lipidology. 2006;17(4):387–393. doi: 10.1097/01.mol.0000236363.63840.16. [DOI] [PubMed] [Google Scholar]
- 55.Faeh D, Minehira K, Schwarz JM, et al. Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes. 2005;54(1):907–913. doi: 10.2337/diabetes.54.7.1907. [DOI] [PubMed] [Google Scholar]
- 56.Clarke SD. Polyunsaturated fatty acid regulation of gene transcription: a molecular mechanism to improve the metabolic syndrome. Journal of Nutrition. 2001;131(4):1129–1132. doi: 10.1093/jn/131.4.1129. [DOI] [PubMed] [Google Scholar]
- 57.Rivellese AA, Maffettone A, Iovine C, et al. Long-term effects of fish oil on insulin resistance and plasma lipoproteins in NIDDM patients with hypertriglyceridemia. Diabetes Care. 1996;19(11):1207–1213. doi: 10.2337/diacare.19.11.1207. [DOI] [PubMed] [Google Scholar]
- 58.Saraswathi V, Morrow JD, Hasty AH. Dietary fish oil exerts hypolipidemic effects in lean and insulin sensitizing effects in obese LDLR-/- mice. Journal of Nutrition. 2009;139(12):2380–2386. doi: 10.3945/jn.109.111567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jump DB. Fatty acid regulation of hepatic lipid metabolism. Current Opinion in Clinical Nutrition and Metabolic Care. 2011;14(2):115–120. doi: 10.1097/MCO.0b013e328342991c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wada S, Yamazaki T, Kawano Y, Miura S, Ezaki O. Fish oil fed prior to ethanol administration prevents acute ethanol-induced fatty liver in mice. Journal of Hepatology. 2008;49(3):441–450. doi: 10.1016/j.jhep.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 61.Kobatake Y, Kuroda K, Jinnouchi H. Differential effects of dietary eicosapentaenoic and docosahexaenoic fatty acids on lowering of triglyceride and cholesterol levels in the serum of rats on hypercholesterolemic diet. Journal of Nutritional Science and Vitaminology. 1984;30(4):357–372. doi: 10.3177/jnsv.30.357. [DOI] [PubMed] [Google Scholar]
- 62.Rambjør GS, Wålen AI, Windsor SL, Harris WS. Eicosapentaenoic acid is primarily responsible for hypotriglyceridemic effect of fish oil in humans. Lipids. 1996;31(3, supplement):S45–S49. doi: 10.1007/BF02637050. [DOI] [PubMed] [Google Scholar]
- 63.Terano T, Kojima T, Seya A, et al. The effect of highly purified eicosapentaenoic acid in patients with psoriasis. Advance Prostaglandin, Thromboxane, and Leukotriene Research. 1989;19:610–613. [PubMed] [Google Scholar]
- 64.Hirai A, Terano T, Makuta H, et al. Effect of oral administration of highly purified eicosapentaenoic acid and docosahexaenoic acid on platelet function and serum lipids in hyperlipidemic patients. Advance Prostaglandin, Thromboxane, and Leukotriene Research. 1989;19:627–630. [PubMed] [Google Scholar]
- 65.Buckley R, Shewring B, Turner R, Yaqoob P, Minihane AM. Circulating triacylglycerol and apoE levels in response to EPA and docosahexaenoic acid supplementation in adult human subjets. British Journal of Nutrition. 2004;92(3):477–483. doi: 10.1079/bjn20041235. [DOI] [PubMed] [Google Scholar]
- 66.Park Y, Harris WS. Omega-3 fatty acid supplementation accelerates chylomicron triglyceride clearance. Journal of Lipid Research. 2003;44(3):455–463. doi: 10.1194/jlr.M200282-JLR200. [DOI] [PubMed] [Google Scholar]
- 67.Grimsgaard S, Bønaa KH, Hansen JB, Nordøy A. Highly purified eicosapentaenoic acid and docosahexaenoic acid in humans have similar triacylglycerol-lowering effects but divergent effects on serum fatty acids. American Journal of Clinical Nutrition. 1997;66(3):649–659. doi: 10.1093/ajcn/66.3.649. [DOI] [PubMed] [Google Scholar]
- 68.Egert S, Kannenberg F, Somoza V, Erbersdobler HF, Wahrburg U. Dietary α-linolenic acid, EPA, and DHA have differential effects on LDL fatty acid composition but similar effects on serum lipid profiles in normolipidemic humans. Journal of Nutrition. 2009;139(5):861–868. doi: 10.3945/jn.108.103861. [DOI] [PubMed] [Google Scholar]
- 69.Mori TA, Burke V, Puddey IB, et al. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hypedipidemic men. American Journal of Clinical Nutrition. 2000;71(5):1085–1094. doi: 10.1093/ajcn/71.5.1085. [DOI] [PubMed] [Google Scholar]
- 70.Olano-Martin E, Anil E, Caslake MJ, et al. Contribution of apolipoprotein E genotype and docosahexaenoic acid to the LDL-cholesterol response to fish oil. Atherosclerosis. 2010;209(1):104–110. doi: 10.1016/j.atherosclerosis.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 71.Woodman RJ, Mori TA, Burke V, Puddey IB, Watts GF, Beilin LJ. Effects of purified eicosapentaenoic and docosahexaenoic acids on glycemic control, blood pressure, and serum lipids in type 2 diabetic patients with treated hypertension. American Journal of Clinical Nutrition. 2002;76(5):1007–1015. doi: 10.1093/ajcn/76.5.1007. [DOI] [PubMed] [Google Scholar]
- 72.Nestel P, Shige H, Pomeroy S, Cehun M, Abbey M, Raederstorff D. The n-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid increase systemic arterial compliance in humans. American Journal of Clinical Nutrition. 2002;76(2):326–330. doi: 10.1093/ajcn/76.2.326. [DOI] [PubMed] [Google Scholar]
- 73.Harris WS. n-3 fatty acids and serum lipoproteins: human studies. The American Journal of Clinical Nutrition. 1997;65:1645S–1654S. doi: 10.1093/ajcn/65.5.1645S. [DOI] [PubMed] [Google Scholar]
- 74.De Roos B, Mavrommatis Y, Brouwer IA. Long-chain n-3 polyunsaturated fatty acids: new insights into mechanisms relating to inflammation and coronary heart disease. British Journal of Pharmacology. 2009;158(2):413–428. doi: 10.1111/j.1476-5381.2009.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goff DC, Bertoni AG, Kramer H, et al. Dyslipidemia prevalence, treatment, and control in the Multi-Ethnic Study of Atherosclerosis (MESA): gender, ethnicity, and coronary artery calcium. Circulation. 2006;113(5):647–656. doi: 10.1161/CIRCULATIONAHA.105.552737. [DOI] [PubMed] [Google Scholar]
- 76.Anderson KM, Castelli WP, Levy D. Cholesterol and mortality. 30 Years of follow-up from the Framingham Study. Journal of the American Medical Association. 1987;257(16):2176–2180. doi: 10.1001/jama.257.16.2176. [DOI] [PubMed] [Google Scholar]
- 77.Liakishev AA. Correction of lipid disorders in patients with arterial hypertension. Russian Medical Journals. 2002;10(19):878–882. [Google Scholar]
- 78.Kobalava JD, Tolkachev VV. Hypercholesterolemia and arterial hypertension. Heart. 2006;4(28):172–176. [Google Scholar]
- 79.Novgorodtseva TP, Kantur TA, Karaman YK, Antonyuk MV, Zhukova NV. Modification of fatty acids composition in erythrocytes lipids in arterial hypertension associated with dyslipidemia. Lipids in Health and Disease. 2011;10, article 18 doi: 10.1186/1476-511X-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goff DC, Bertoni AG, Kramer H, et al. Dyslipidemia prevalence, treatment, and control in the Multi-Ethnic Study of Atherosclerosis (MESA): gender, ethnicity, and coronary artery calcium. Circulation. 2006;113(5):647–656. doi: 10.1161/CIRCULATIONAHA.105.552737. [DOI] [PubMed] [Google Scholar]
- 81.Arnett DK, Jacobs DR, Luepker RV, Blackburn H, Armstrong C, Claas SA. Twenty-year trends in serum cholesterol, hypercholesterolemia, and cholesterol medication use: the Minnesota Heart Survey, 1980–1982 to 2000–2002. Circulation. 2005;112(25):3884–3891. doi: 10.1161/CIRCULATIONAHA.105.549857. [DOI] [PubMed] [Google Scholar]
- 82.Graham I, Cooney MT, Bradley D, Dudina A, Reiner Z. Dyslipidemias in the prevention of cardiovascular disease: risks and causality. Current Cardiology Reports. 2012;14(6):709–720. doi: 10.1007/s11886-012-0313-7. [DOI] [PubMed] [Google Scholar]
- 83.Martsevich SY. Treatment of lipid disorders in patients with coronary heart disease. Treating Physician. 2005;5:42–45. [Google Scholar]
- 84.Endakova EA, Novgorodtseva TP. Svetashev VI Modification of Blood Fatty Acids Composition in Case of Cardiovascular Diseases. Vol. 296. Vladivostok, Russia: Dalnauka; 2002. [Google Scholar]
- 85.Titov VN. Biological Bases Pathogenesis, Diagnostics, Preventive and Treatment of Atherosclerosis. Vol. 750. M:Altus; 2002. Atherosclerosis as a pathology polien fatty acids. [Google Scholar]
- 86.Rizza S, Tesauro M, Cardillo C, et al. Fish oil supplementation improves endothelial function in normoglycemic offspring of patients with type 2 diabetes. Atherosclerosis. 2009;206(2):569–574. doi: 10.1016/j.atherosclerosis.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mori TA. Omega-3 fatty acids and hypertension in humans. Clinical and Experimental Pharmacology and Physiology. 2006;33(9):842–846. doi: 10.1111/j.1440-1681.2006.04451.x. [DOI] [PubMed] [Google Scholar]
- 88.Wang S, Ma AQ, Song SW, Quan QH, Zhao XF, Zheng XH. Fish oil supplementation improves large arterial elasticity in overweight hypertensive patients. European Journal of Clinical Nutrition. 2008;62(12):1426–1431. doi: 10.1038/sj.ejcn.1602886. [DOI] [PubMed] [Google Scholar]
- 89.Ait-Yahia D, Madani S, Savelli JL, Prost J, Bouchenak M, Belleville J. Dietary fish protein lowers blood pressure and alters tissue polyunsaturated fatty acid composition in spontaneously hypertensive rats. Nutrition. 2003;19(4):342–346. doi: 10.1016/s0899-9007(02)00858-4. [DOI] [PubMed] [Google Scholar]
- 90.Erkkilä AT, Schwab US, De Mello VDF, et al. Effects of fatty and lean fish intake on blood pressure in subjects with coronary heart disease using multiple medications. European Journal of Nutrition. 2008;47(6):319–328. doi: 10.1007/s00394-008-0728-5. [DOI] [PubMed] [Google Scholar]
- 91.Taddei S, Ghiadoni L, Virdis A, Versari D, Salvetti A. Mechanisms of endothelial dysfunction: clinical significance and preventive non-pharmacological therapeutic strategies. Current Pharmaceutical Design. 2003;9(29):2385–2402. doi: 10.2174/1381612033453866. [DOI] [PubMed] [Google Scholar]
- 92.Stebbins CL, Stice JP, Hart CM, Mbai FN, Knowlton AA. Effects of dietary decosahexaenoic acid (DHA) on eNOS in human coronary artery endothelial cells. Journal of Cardiovascular Pharmacology and Therapeutics. 2008;13(4):261–268. doi: 10.1177/1074248408322470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.De Caterina R, Massaro M, Scoditti E, Annunziata Carluccio M. Pharmacological modulation of vascular inflammation in atherothrombosis. Annals of the New York Academy of Sciences. 2010;1207:23–31. doi: 10.1111/j.1749-6632.2010.05784.x. [DOI] [PubMed] [Google Scholar]
- 94.Ross R. Phathogenesis of atherosclerosis a prospective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 95.Qiao JH, Tripathi J, Mishra NK, et al. Role of macrophage colony-stimulating factor in atherosclerosis: studies of osteopetrotic mice. American Journal of Pathology. 1997;150(5):1687–1699. [PMC free article] [PubMed] [Google Scholar]
- 96.Bernhagen J, Krohn R, Lue H, et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nature Medicine. 2007;13(5):587–596. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- 97.Libby P. Vascular biology of atherosclerosis: overview and state of the art. American Journal of Cardiology. 2003;91(3):3A–6A. doi: 10.1016/s0002-9149(02)03143-0. [DOI] [PubMed] [Google Scholar]
- 98.van der Valk FM, van Wijk DF, Stroes ESG. Novel anti-inflammatory strategies in atherosclerosis. Current Opinion in Lipidology. 2012;23:532–539. doi: 10.1097/MOL.0b013e3283587543. [DOI] [PubMed] [Google Scholar]
- 99.Stirban A, Nandrean S, Götting C, et al. Effects of n-3 fatty acids on macro- and microvascular function in subjects with type 2 diabetes mellitus. American Journal of Clinical Nutrition. 2010;91(3):808–813. doi: 10.3945/ajcn.2009.28374. [DOI] [PubMed] [Google Scholar]
- 100.Dangardt F, Osika W, Chen Y, et al. Omega-3 fatty acid supplementation improves vascular function and reduces inflammation in obese adolescents. Atherosclerosis. 2010;212(2):580–585. doi: 10.1016/j.atherosclerosis.2010.06.046. [DOI] [PubMed] [Google Scholar]
- 101.Goodfellow J, Bellamy MF, Ramsey MW, Jones CJH, Lewis MJ. Dietary supplementation with marine omega-3 fatty acids improve systemic large artery endothelial function in subjects with hypercholesterolemia. Journal of the American College of Cardiology. 2000;35(2):265–270. doi: 10.1016/s0735-1097(99)00548-3. [DOI] [PubMed] [Google Scholar]
- 102.Engler MM, Engler MB, Malloy M, et al. Docosahexaenoic acid restores endothelial function in children with hyperlipidemia: results from the early study. International Journal of Clinical Pharmacology and Therapeutics. 2004;42(12):672–679. doi: 10.5414/cpp42672. [DOI] [PubMed] [Google Scholar]
- 103.Haberka M, Mizia-Stec K, Mizia M, et al. N-3 polyunsaturated fatty acids early supplementation improves ultrasound indices of endothelial function, but not through NO inhibitors in patients with acute myocardial infarction. N-3 PUFA supplementation in acute myocardial infarction. Clinical Nutrition. 2011;30(1):79–85. doi: 10.1016/j.clnu.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 104.Schiano V, Laurenzano E, Brevetti G, et al. Omega-3 polyunsaturated fatty acid in peripheral arterial disease: effect on lipid pattern, disease severity, inflammation profile, and endothelial function. Clinical Nutrition. 2008;27(2):241–247. doi: 10.1016/j.clnu.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 105.Tagawa H, Shimokawa H, Tagawa T, Kuroiwa-Matsumoto M, Hirooka Y, Takeshita A. Long-term treatment with eicosapentaenoic acid augments both nitric oxide-mediated and non-nitric oxide-mediated endothelium-dependent forearm vasodilatation in patients with coronary artery disease. Journal of Cardiovascular Pharmacology. 1999;33(4):633–640. doi: 10.1097/00005344-199904000-00017. [DOI] [PubMed] [Google Scholar]
- 106.Doshi SN, Naka KK, Payne N, et al. Flow-mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clinical Science. 2001;101(6):629–635. [PubMed] [Google Scholar]
- 107.Harris WS, Rambjor GS, Windsor SL, et al. N-3 fatty acids and urinary excretion of nitric oxide metabolites in humans. The Amrican Journal of Clinical Nutrition. 1997;65:459–464. doi: 10.1093/ajcn/65.2.459. [DOI] [PubMed] [Google Scholar]
- 108.Okuda Y, Kawashima K, Sawada T, et al. Eicosapentaenoic acid enhances nitric oxide production by cultured human endothelial cells. Biochemical and Biophysical Research Communications. 1997;232(2):487–491. doi: 10.1006/bbrc.1997.6328. [DOI] [PubMed] [Google Scholar]
- 109.Omura M, Kobayashi S, Mizukami Y, et al. Eicosapentaenoic acid (EPA) induces Ca2+-independent activation and translocation of endothelial nitric oxide synthase and endothelium-dependent vasorelaxation. FEBS Letters. 2001;487(3):361–366. doi: 10.1016/s0014-5793(00)02351-6. [DOI] [PubMed] [Google Scholar]
- 110.Singh TU, Kathirvel K, Choudhury S, Garg SK, Mishra SK. Eicosapentaenoic acid-induced endothelium-dependent and -independent relaxation of sheep pulmonary artery. European Journal of Pharmacology. 2010;636(1–3):108–113. doi: 10.1016/j.ejphar.2010.02.041. [DOI] [PubMed] [Google Scholar]
- 111.López D, Möller M, Denicola A, et al. Long-chain n-3 polyunsaturated fatty acid from fish oil modulates aortic nitric oxide and tocopherol status in the rat. British Journal of Nutrition. 2008;100(4):767–775. doi: 10.1017/S0007114508939854. [DOI] [PubMed] [Google Scholar]
- 112.Robinson JG, Stone NJ. Antiatherosclerotic and antithrombotic effects of omega-3 fatty acids. American Journal of Cardiology. 2006;98(4):39–49. doi: 10.1016/j.amjcard.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 113.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106(21):2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 114.Thies F, Garry JMC, Yaqoob P, et al. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. The Lancet. 2003;361(9356):477–485. doi: 10.1016/S0140-6736(03)12468-3. [DOI] [PubMed] [Google Scholar]
- 115.Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. Journal of the American College of Cardiology. 2011;58(20):2047–2068. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 116.Tull SP, Yates CM, Maskrey BH, et al. Omega-3 fatty acids and inflammation: novel interactions reveal a new step in neutrophil recruitment. PLoS Biology. 2009;7(8) doi: 10.1371/journal.pbio.1000177.e1000177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hamada T, Tsuchihashi S, Avanesyan A, et al. Cyclooxygenase-2 deficiency enhances Th2 immune responses and impairs neutrophil recruitment in hepatic ischemia/reperfusion injury. Journal of Immunology. 2008;180(3):1843–1853. doi: 10.4049/jimmunol.180.3.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gonçalves De Moraes VL, Vargaftig BB, Lefort J, Meager A, Chignard M. Effect of cyclo-oxygenase inhibitors and modulators of cyclic AMP formation on lipopolysaccharide-induced neutrophil infiltration in mouse lung. British Journal of Pharmacology. 1996;117(8):1792–1796. doi: 10.1111/j.1476-5381.1996.tb15356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Beesley JE, Pearson JD, Hutchings A. Granulocyte migration through endothelium in culture. Journal of Cell Science. 1979;38:237–248. doi: 10.1242/jcs.38.1.237. [DOI] [PubMed] [Google Scholar]
- 120.Pearson JD, Carleton JS, Beesley JE. Granylocyte adhesion to endothelium in culture. Journal of Cell Science. 1979;38:225–235. doi: 10.1242/jcs.38.1.225. [DOI] [PubMed] [Google Scholar]
- 121.Fujitani Y, Kanaoka Y, Aritake K, Uodome N, Okazaki-Hatake K, Urade Y. Pronounced eosinophilic lung inflammation and Th2 cytokine release in human lipocalin-type prostaglandin D synthase transgenic mice. Journal of Immunology. 2002;168(1):443–449. doi: 10.4049/jimmunol.168.1.443. [DOI] [PubMed] [Google Scholar]
- 122.Rajakariar R, Hilliard M, Lawrence T, et al. Hematopoietic prostaglandin D2 synthase controls the onset and resolution of acute inflammation through PGD2 and 15-deoxyΔ12-14 PGJ2. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(52):20979–20984. doi: 10.1073/pnas.0707394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Isobe Y, Kato T, Arita M. Emerging roles of eosinophils and eosinophil derived lipid mediators in the resolution of inflammation. Frontiers in Immunology. 2012;3, article 270 doi: 10.3389/fimmu.2012.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Serhan CN, Clish CB, Brannon J, et al. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated fromomega-3fatty acids via cyclooxygenase2-nonsteroidal antiinflammatory drugs and tran-scellular processing. The Journal of Experimental Medicine. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Arita M, Bianchini F, Aliberti J, et al. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. Journal of Experimental Medicine. 2005;201(5):713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tjonahen E, Oh SF, Siegelman J, et al. Resolvin E2: identification and anti-inflammatory actions. Pivotal roleof human 5-lipoxygenasein resolvin Eseries biosynthesis. Chemistry and Biology. 2006;13(11):1193–1202. doi: 10.1016/j.chembiol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 127.Serhan CN, Hong S, Gronert K, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. Journal of Experimental Medicine. 2002;196(8):1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sun YP, Oh SF, Uddin J, et al. Resolvin D1 and its aspirin-triggered 17R epimer: stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. Journal of Biological Chemistry. 2007;282(13):9323–9334. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- 129.Spite M, Norling LV, Summers L, et al. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461(7268):1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chiang N, Fredman G, Bäckhed F, et al. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells: autacoids in anti-inflammation. Journal of Biological Chemistry. 2003;278(17):14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 132.Marcheselli VL, Hong S, Lukiw WJ, et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. The Journal of Biological Chemistry. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 133.Serhan CN, Gotlinger K, Hong S, et al. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. The Journal of Immunology. 2006;176:1848–1859. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- 134.Serhan CN, Yang R, Martinod K, et al. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. Journal of Experimental Medicine. 2009;206(1):15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Serhan CN, Dalli J, Karamnov S, et al. Macrophage proresolving mediator maresin1 stimulates tissuere generation and controls pain. The FASEB Journal. 2012;26:1755–1765. doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ariel A, Fredman G, Sun YP, et al. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nature Immunology. 2006;7(11):1209–1216. doi: 10.1038/ni1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447(7146):869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. Journal of Clinical Investigation. 2011;121(2):569–581. doi: 10.1172/JCI42545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Leaf A, Weber PC. Cardiovascular effects of n-3 fatty acids. New England Journal of Medicine. 1988;318(9):549–557. doi: 10.1056/NEJM198803033180905. [DOI] [PubMed] [Google Scholar]
- 140.De Caterina R, Zampolli A. n-3 fatty acids: antiatherosclerotic effects. Lipids. 2001;36(supplement):S69–S78. doi: 10.1007/s11745-001-0685-9. [DOI] [PubMed] [Google Scholar]
- 141.He K, Liu K, Daviglus ML, et al. Associations of Dietary Long-Chain n-3 Polyunsaturated Fatty Acids and Fish With Biomarkers of Inflammation and Endothelial Activation (from the Multi-Ethnic Study of Atherosclerosis [MESA]) American Journal of Cardiology. 2009;103(9):1238–1243. doi: 10.1016/j.amjcard.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. New England Journal of Medicine. 2000;342(12):836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 143.Wu TC, Leu HB, Lin WT, Lin CP, Lin SJ, Chen JW. Plasma matrix metalloproteinase-3 level is an independent prognostic factor in stable coronary artery disease. European Journal of Clinical Investigation. 2005;35(9):537–545. doi: 10.1111/j.1365-2362.2005.01548.x. [DOI] [PubMed] [Google Scholar]
- 144.Nanni S, Melandri G, Hanemaaijer R, et al. Matrix metalloproteinases in premature coronary atherosclerosis: influence of inhibitors, inflammation, and genetic polymorphisms. Translational Research. 2007;149(3):137–144. doi: 10.1016/j.trsl.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 145.Bourdillon MC, Poston RN, Covacho C, Chignier E, Bricca G, McGregor JL. ICAM-1 deficiency reduces atherosclerotic lesions in double-knockout mice (ApoE(-/-)/ICAM-1(-/-)) fed a fat or a chow diet. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20(12):2630–2635. doi: 10.1161/01.atv.20.12.2630. [DOI] [PubMed] [Google Scholar]
- 146.Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. The Lancet. 1998;351(9096):88–92. doi: 10.1016/S0140-6736(97)09032-6. [DOI] [PubMed] [Google Scholar]
- 147.Ray KK, Morrow DA, Shui A, Rifai N, Cannon CP. Relation between soluble intercellular adhesion molecule-1, statin therapy, and long-term risk of clinical cardiovascular events in patients with previous acute coronary syndrome (from PROVE IT-TIMI 22) American Journal of Cardiology. 2006;98(7):861–865. doi: 10.1016/j.amjcard.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 148.Weber C, Erl W, Pietsch A, Danesch U, Weber PC. Docosahexaenoic acid selectively attenuates induction of vascular cell adhesion molecule-1 and subsequent monocytic cell adhesion to human endothelial cells stimulated by tumor necrosis factor-α . Arteriosclerosis, Thrombosis, and Vascular Biology. 1995;15(5):622–628. doi: 10.1161/01.atv.15.5.622. [DOI] [PubMed] [Google Scholar]
- 149.Collie-Duguid ESR, Wahle KWJ. Inhibitory effect of fish oil n-3 polyunsaturated fatty acids on the expression of endothelial cell adhesion molecules. Biochemical and Biophysical Research Communications. 1996;220(3):969–974. doi: 10.1006/bbrc.1996.0516. [DOI] [PubMed] [Google Scholar]
- 150.Shaw DI, Hall WL, Jeffs NR, Williams CM. Comparative effects of fatty acids on endothelial inflammatory gene expression. European Journal of Nutrition. 2007;46(6):321–328. doi: 10.1007/s00394-007-0669-4. [DOI] [PubMed] [Google Scholar]
- 151.Calder PC. N-3 polyunsaturated fatty acids, inflammation and immunity: pouring oil on troubled waters or another fishy tale? Nutrition Research. 2001;21(1-2):309–341. [Google Scholar]
- 152.Ruan KH, Cervantes V, So SP. Engineering of a novel hybrid enzyme: an anti-inflammatory drug target with triple catalytic activities directly converting arachidonic acid into the inflammatory prostaglandin E2. Protein Engineering, Design and Selection. 2009;22(12):733–740. doi: 10.1093/protein/gzp058. [DOI] [PMC free article] [PubMed] [Google Scholar]


