Abstract
Introduction
National cost estimates of osteoporosis and fractures in the U.S. have been based on diverse sets of provider data or selected commercial insurance claims. We sought to characterize prevalence and costs for osteoporosis using a random population-based sample of older adults.
Methods
A cross-sectional estimate of medical cost was made with 2002 data from the Medicare Current Beneficiary Survey (MCBS). MCBS combines health interviews with claims information from all payers to profile a random sample of 12,700 Medicare recipients. Three cohorts aged 65 or over were defined: 1) patients experiencing a fracture-related claim in 2002; 2) patients with a diagnosis, medication, or self-report for osteoporosis or past hip fracture; and 3) non-case controls. The total cost of patient claims was compared to that of controls using multivariate regression.
Results
Of 30.2 million elderly Medicare recipients in 2002, 1.6 million (5%) were treated for a fracture that year and an additional 7.2 million (24%) have osteoporosis without a fracture. The estimated mean impact of fractures on annual medical cost was $8600 (95%CI: $6400 to $10,800), implying a U.S. cost of $14 billion ($10 to $17 billion). Half of the non-fracture osteoporosis patients received drug treatment, averaging $500 per treated patient, or $2 billion nationwide.
Conclusions
The annual cost of osteoporosis and fractures in the U.S. elderly was estimated at $16 billion, using a national 2002 population-based sample. This amount corroborates previous estimates based on substantially different methodologies. Projected to 2008, the national cost of osteoporosis and fractures was $22 billion.
INTRODUCTION
An estimated 10 million Americans have osteoporosis, and an additional 34 million have low bone mass, indicating they are at high risk of developing the disease [1]. Over 1.5 million fractures per year are attributed to osteoporosis, including approximately 300,000 hip fractures and 700,000 vertebral fractures [2]. The morbidity and mortality associated with hip fractures are particularly high. Within one year of hip fracture, approximately one third of patients are admitted to nursing facilities [3], and the one-year case fatality rate exceeds 20% [4-6]. Osteoporosis and osteoporotic fractures are also associated with sustained disability, physical limitations, psychosocial impairment, and reduced quality of life [7-9]. With the aging of the population, the cost of osteoporosis is an increasingly significant public health concern.
Estimates of U.S. medical costs for osteoporosis range from $10 to $22 billion, even apart from the indirect costs of reductions in survival, quality of life, and productivity [3,10-13]. One of the most recent comprehensive estimates was performed by Burge using a 2001 national sample of hospitalizations and charges for hip fractures, along with updates of other provider-based data from the early 1990s. [10]
The Medicare Current Beneficiary Survey (MCBS) combines health interviews with payment information from patients, Medicare, Medicaid, and private payers to profile a representative sample of Medicare recipients [14]. It is a random, population-based sample and can be the basis of an alternate approach to using provider-based data for an estimate of the national medical cost of osteoporosis. Indeed, claims data from selected commercial insurance plans have been used to estimate osteoporosis costs [3,15-16]. MCBS is a national random sample and is unique in linking patient-level claims information on resource use and costs to self-reported medical history. Other data sources lack one or more of these items. Ideally, the sample would include all age groups, but the MCBS random sample of the elderly would represent 90% of the costs [11]. The elderly are also an important population from a health policy standpoint, as most of their expenses are covered by the Medicare program. The objectives of this research are to characterize prevalence and medical costs for osteoporosis in the elderly using the MCBS data.
METHODS
Data
The Medicare Current Beneficiary Survey is a continuous, multipurpose study of Medicare participants, conducted by the Centers for Medicaid and Medicare Services [14]. The MCBS sample is drawn from the CMS Medicare enrollment file and is statistically representative of the national Medicare population. Thus, it includes Americans over the age of 64, regardless of whether they live in the community or an institution. Though excluded from our study, it also includes younger individuals on Social Security disability and those receiving services for end stage renal disease.
MCBS provides expenditure data for all healthcare services used by Medicare beneficiaries, including those not covered by Medicare, whether paid by the patient, Medicaid, or a private insurer. The expenditure data are linked with self-reported health status and utilization from interviews with beneficiaries or qualified proxies conducted every 4 months.
MCBS has a complex sample design that follows rotating panels of beneficiaries for four years. The target sample size is 12,000, with over-sampling of smaller populations of interest, and geographic cluster sampling to facilitate interviews. For the Cost and Use file used by our study, methods are also designed to capture the costs of those who die during the year or who are otherwise enrolled for part of the year [17]. Though deaths occur for about 5% of the Medicare population annually, they account for about 15% of the costs [17]. CMS provides sampling weights on each subject so that researchers can project to the national population and calculate accurate standard errors.
Approach
We developed a cross-sectional, prevalence-based snapshot of osteoporosis medical costs for the sample of elderly Medicare-enrolled individuals in 2002, the latest year for which the MCBS Cost and Use file was available at the time the study was done. Because the interview portion of the survey asks whether the beneficiary has ever been told they have osteoporosis, we can additionally capture lifetime self-reported prevalence of both osteoporosis and hip fracture, even if they have no osteoporosis or fracture claim event in that year.
Study Patients
MCBS subjects were identified as study patients if they were over age 64 and met any of the following inclusion criteria in 2002:
At least one event with a primary or secondary diagnosis code of osteoporosis (ICD-9-CM 733.0x);
Diagnosis or treatment for fractures often attributed to osteoporosis (hip, thoracic and lumbar vertebra, distal forearm, pathologic fractures) or for other fractures that may be result of both bone fragility and/or trauma (pelvis, femur, tibia/fibula, patella, ankle, radius/ulna, wrist, or ribs) as indicated by primary or secondary ICD-9-CM diagnosis code, CPT, or ICD-9-CM procedure (codes available in an electronic technical appendix);
Filled prescription for a drug which had an FDA-approved indication for the treatment or prevention of osteoporosis. These included bisphosphonates (alendronate, risedronate), the selective estrogen receptor modulator (SERM) raloxifene, calcitonin, and teriparatide;
The patient had ever been told by a doctor they had osteoporosis and/or a broken hip (per response to interviewer questions)
Following two previous claims studies of osteoporosis burden [3,18], we excluded patients with malignant neoplasms (ICD-9-CM diagnoses 140.xx to 208.xx, 228.09, 238.0, 238.6, 239.2). Also excluded were those who had a primary or secondary diagnosis code suggestive of a bone disease or fracture from causes other than osteoporosis. These included:
Paget’s disease (ICD-9-CM diagnosis 731.0x) and
Transport accidents (ICD-9-CM diagnoses E800-E848) accidental falls from high heights (E881-E883, E884.1). (Falls from steps or low levels were not grounds for exclusion.)
A control sample of all sample beneficiaries not in the osteoporosis study population was also selected from within the 2002 Cost and Use file. The same exclusion criteria were applied.
Once patients were identified, all MCBS event records in the 2002 Cost and Use File were extracted from January 1, 2002 through December 31, 2002.
Outcomes
Patient characteristics measured include demographics, institutionalization, functional limitations, and self-reported chronic conditions and diseases. Event and payment information is collected for the following resources:
Hospitalization
Outpatient hospital
Physician services
Prescriptions (not available for those in long-term nursing or hospice)
Labs and radiology
Supplier services
Skilled nursing facility (SNF)
Long term nursing care
Hospice care
Home health care
Event data may come from electronic capture of Medicare medical claims, respondent interview, or transcription of Explanation of Benefit forms sent to patient by non-Medicare payers, including Medicaid and commercial insurance. (Services and payments for beneficiaries covered by Medicare risk HMO contractors were imputed from matching fee for service beneficiaries.) The manual process is subject to several reconciliation and quality control efforts. For example, prescription data originate with self-report, followed by efforts of CMS interviewers and staff to complete the information by examining pill bottles and making calls to pharmacies [17].
Resource use events are classified as related to osteoporosis disease if they include any of the following:
Diagnosis or procedure for fractures (i.e., same codes as used for patient inclusion);
Osteoporosis as a primary diagnosis (ICD-9-CM 733.0x);
Bone mineral density (BMD) scans; or
Drugs with an FDA-approved indication for treatment or prevention of osteoporosis plus estrogen, estrogen-progestin, Vitamin D, and calcium.
In a preliminary analysis, we tested adding in the claims where the provider noted osteoporosis as a complicating factor in treating the primary diagnosis (i.e., by coding it any of the secondary diagnosis positions 2-10). We found that they added less than 15% to disease-coded expenses, and so we excluded them to have a lower bound on our cost estimates.
Analysis
The osteoporosis study population is divided into two cohorts: those who were and were not treated for fractures in 2002. For the sake of brevity, they are referred to as the fracture and osteoporosis cohorts, respectively. They are compared to each other and the cohort of non-patients, called controls.
Patient characteristics and outcomes are analyzed by cohort with descriptive tables. Costs are tabulated by fracture type and payment source.
Disease-related cost is assessed with two methods: 1) totaling claims attributed to disease via primary diagnosis code or a fracture-related procedure, and 2) comparing total costs of patients to non-patient controls with a linear multivariate model to adjust for possible confounders.
The second method is especially important with this data source, as long-term care facility events, which can be expensive, are not assigned diagnosis codes. Also, as an estimate of the marginal impact of osteoporosis on total cost, a multivariate estimate can include the effects of fracture or disease on the costs of other illnesses.
Covariates tested included age, gender, Elixhauser comorbidity index [19], individual comorbidity indicators, number of chronic conditions, full-year institutionalization, urban residence, and race. All variables were initially tried in a fully saturated model and considered for dropping if non-significant (p > 0.10) or if their omission had little effect on the coefficients of remaining variables. One comorbidity measure was chosen and age and gender were kept regardless of significance. Adjustment for heteroskedasticity was done with weighted least squares. Alternate log-linear models and a generalized linear model with log-link (Poisson distribution) were tested for comparison.
All calculations were performed with SAS Version 9 (Cary, North Carolina) with procedures that accounted for the stratified multistage sample design of MCBS.
RESULTS
Prevalence
After applying the exclusion criteria to the 2002 MCBS sample (n = 12,697), the study sample size of elderly Medicare beneficiaries was 9182. Five percent (5%) were treated for a fracture in 2002 and an additional 24% had osteoporosis by our criteria (diagnosis, medication, or self-report for osteoporosis; or self-reported past hip fracture), but no fracture in 2002. Projected nationally to 30.2 million elderly Medicare recipients (without malignant neoplasms), 1.6 million had a fracture in 2002 and 7.2 million had osteoporosis without a fracture. See Table 1.
Table 1.
| Fracture | Osteoporosisa | Controls (non-cases) | Total | |
|---|---|---|---|---|
| Sample Size (weightedc), n | 497 | 2196 | 6489 | 9182 |
| Percent of Population | 5.4% | 23.9% | 70.7% | 100.0% |
| Population Estimate (millions) | 1.6 | 7.2 | 21.3 | 30.2 |
Diagnosis, medication, or self-report for osteoporosis, or self-reported past hip fracture, but no fracture in 2002
Excludes those with malignant neoplasms
MCBS national population weights for respondents normalized to MCBS sample size of 12,697. Raw sample sizes for fracture, osteoporosis, controls, and total, are 577, 2158, 6152, and 8887, respectively.
Figure 1 gives the prevalence rates by gender and age and illustrates the population sizes. The data reflect the higher prevalence with female gender and increased age. In particular, 15% of females and 9% of males over age 84 were treated for fracture in 2002, while 35% of females and 7% of males had osteoporosis with no fracture.
Figure 1.
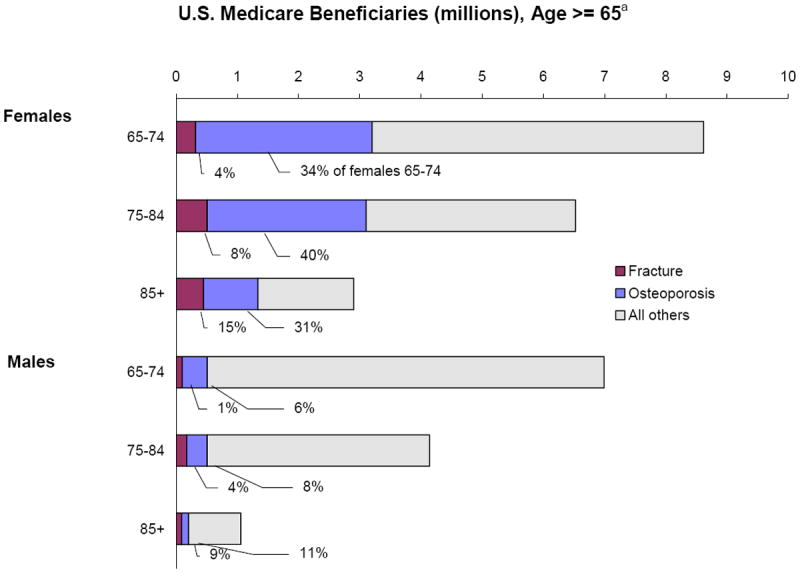
Prevalence of Fractures and Osteoporosis by Gender and Age, 2002
- Percent and Number in Elderly Medicare Population
- National Estimates, Medicare Current Beneficiary Survey
a Excludes those with malignant neoplasms
Table 2 gives detail on who entered the osteoporosis cohort by showing the percent in mutually exclusive groupings of specific inclusion criteria. A significant portion, 11%, report a past hip fracture, and an additional 41% had a claim with an osteoporosis diagnosis in 2002 (12% with diagnosis only, 29% who also self-report or receive a treatment). Patients who self-reported osteoporosis, but who had no 2002 diagnosis, treatment, or past hip fracture, make up about a quarter of the cohort.
Table 2.
- National Estimates, Medicare Current Beneficiary Survey, 2002
| Criteria Met | Percent of Cohort |
|---|---|
| ICD-9-CM diagnosis of osteoporosis only | 12% |
| ICD-9-CM diagnosis and self-report or treatment | 29% |
| Self-report osteoporosis only | 26% |
| Osteoporosis treatmenta only | 6% |
| Treatment and self-report | 16% |
| At least past hip fractureafractureb | 11% |
| All osteoporosis cohort | 100% |
FDA-indicated: alendronate, risedronate, raloxifene, calcitonin, or teriparatide
Past hip fracture and possibly any of other listed criteria
Patient Characteristics
Table 3 shows patient demographic and health characteristics by cohort. Patients represented in the fracture cohort and osteoporosis (without fracture) cohort were older, more likely to be women, Caucasian, and poorer than those in the control cohort. Fourteen percent of fracture patients were institutionalized the full year, and an additional 16% were in an institution part of the year, compared to 6% and 3%, respectively, in the control cohort.
Table 3.
- National Estimates, Medicare Current Beneficiary Survey, 2002
| COHORT
|
|||
|---|---|---|---|
| Fracture | Osteoporosis | Control | |
|
| |||
| na=497 | n=2196 | n=6489 | |
|
| |||
| Age % | |||
| 85+ | 40 | 19 | 17 |
| 75-84 | 41 | 43 | 36 |
| 65-74 | 19 | 38 | 47 |
|
| |||
| Gender % | |||
| Female | 78 | 88 | 49 |
| Male | 22 | 12 | 51 |
|
| |||
| Race % | |||
| Black | 6 | 6 | 11 |
| Hispanic | 2 | 2 | 3 |
| Other | 2 | 3 | 3 |
| White | 90 | 90 | 84 |
|
| |||
| Institutionalized For Long-Term Care % | |||
| Full Year | 14 | 4 | 6 |
| Part Year | 16 | 2 | 3 |
| No Facility Days | 70 | 94 | 91 |
|
| |||
| Urban % | 68 | 73 | 72 |
|
| |||
| Have Supplemental Coverage % | 35 | 39 | 30 |
|
| |||
| Income (Mean) | $21490 | $25800 | $28490 |
|
| |||
| General Health % | |||
| Excellent | 8 | 12 | 16 |
| Very good | 18 | 25 | 29 |
| Good | 29 | 34 | 31 |
| Fair | 32 | 21 | 18 |
| Poor | 13 | 7 | 6 |
| Unknown | 1 | 1 | 1 |
|
| |||
| Functional Limitation % | |||
| Two or more | 68 | 42 | 30 |
| One | 11 | 16 | 13 |
| Zero | 21 | 42 | 56 |
|
| |||
| Mobility Limitation % | 80 | 59 | 47 |
|
| |||
| Upper Extremity Limitation % | 62 | 48 | 39 |
|
| |||
| Living Arrangement % | |||
| Alone | 35 | 39 | 28 |
| With spouse | 25 | 41 | 50 |
| With children | 14 | 11 | 9 |
| With non-family | 5 | 5 | 5 |
| Institutionalized | 21 | 5 | 7 |
|
| |||
| Comorbidities % | |||
| Arthritis | 60 | 74 | 54 |
| Hypertension | 57 | 63 | 59 |
| Diabetes | 19 | 17 | 21 |
| Emphysema/asthma/COPD | 15 | 16 | 13 |
| Stroke/brain hemorrhage | 17 | 13 | 13 |
| Myocardial infarction | 15 | 13 | 15 |
| Skin cancer | 12 | 18 | 15 |
| Other (non-skin) cancer | 10 | 13 | 11 |
| Angina pectoris/CHD | 11 | 14 | 13 |
| Alzheimer’s/dementia | 12 | 5 | 5 |
| Parkinson’s disease | 5 | 2 | 2 |
| Mental retardation | 2 | 1 | 1 |
|
| |||
| Comorbidity index (Elixhauser)- mean | 3.9 | 2.3 | 1.7 |
|
| |||
| Number of chronic conditions - mean | 2.5 | 2.3 | 1.7 |
MCBS national population weights for respondents normalized to MCBS sample size of 12,697.
Fracture and osteoporosis patients were more likely to have fair or poor self-reported health status and to have two or more functional limitations. They were also more likely to live alone. Additionally, they had a greater burden of morbidity.
Costs
Health care costs by cohort and fracture type for both disease-coded events (medication, diagnosis, or procedure for fracture or osteoporosis on claim) and all health care events are shown in Table 4. Disease-coded expense for fracture patients is $4420, while their mean per-patient total expenses in 2002 were $27,730, $19,210 more than the expenses of controls. The disease-coded expense understates the cost of the disease, as a limitation of the data is that long-term nursing and hospice care are not attributed and coded to a diagnosis. As shown in Table 3, 30% of the fracture patients have at least a part-year stay in a long-term care facility, compared to 9% of controls. Usage and cost of other services is also high for fracture patients. In particular, from additional analyses (not shown) of resource utilization, 53% have a hospitalization and 31% have a stay in a skilled nursing facility (SNF).
Table 4.
- Disease-Codeda and Total, 2002
- National Estimates, Medicare Current Beneficiary Survey
| Disease-codeda | Total Expenses | |||||
|---|---|---|---|---|---|---|
| Cohort and Fracture Type b | n c | Percent of Cohort | Mean Expense | Std Error | Mean Expense | Std Error |
| Fracture | 497 | 100% | $4,420 | $435 | $27,730 | $1,360 |
| Hip/femur | 166 | 33% | 9,600 | 990 | 39,620 | 2,660 |
| Spine | 73 | 15% | 2,400 | 590 | 24,660 | 590 |
| Lower Extremity | 71 | 14% | 2,580 | 760 | 15,890 | 2,000 |
| Shoulder/humerus | 35 | 7% | 2,560 | 980 | 22,940 | 4,110 |
| Other | 152 | 30% | 980 | 240 | 22,840 | 1,910 |
| Osteoporosis | 2,196 | 100% | 280 | 12 | 9,850 | 395 |
| Control | 6,489 | 100% | 0 | 0 | 8,480 | 295 |
(Numbers rounded)
Claims with procedures, primary diagnosis codes, or medications for fractures or osteoporosis. Does not include long-term and hospice care expenses, as no procedure or diagnosis codes were available for these events
Patients with multiple fractures were assigned to one type in the order hip/femur, spine, lower extremity, shoulder/humerus, other.)
MCBS national population weights for respondents normalized to MCBS sample size of 12,697.
About a third of the fracture patients were treated for a hip fracture, and their total cost for the year averaged $39,620; disease-coded cost was $9600. Spinal fracture patients (15%) averaged $24,660 in total cost and those with lower extremity fractures, $15,890. (Patients with multiple fractures were assigned to one type in the order hip/femur, spine, lower extremity, shoulder/humerus, other.)
The osteoporosis cohort averaged $280 in disease-coded expense, while their total expenses averaged $9850, $1370 more than controls. Resource use data indicate that osteoporosis-related drug therapy accounted for $250 of this expense; 49% of the cohort was treated at a mean of $500 per treated patient. The usage in fracture patients was just about half as high, 27%. (Both figures exclude full-year long-term care patients, for whom prescription data were unavailable.)
Multivariate Analysis of Marginal Cost
A linear regression model adjusting for age, comorbidity index, gender, urban residence, and institutionalization estimated the average marginal impact of fracture on annual total cost as $8600 (95% CI: $6400 to $10,800). The estimated effects of all the model variables on cost are shown in Table 5.
Table 5.
- adjusted Adjusted for Demographics and Comorbidities, Age, Gender, Urban Residence, Institutionalization*y
- National Estimates, Medicare Current Beneficiary Survey
| Parameter Variable | Coefficient Estimate | 95% Confidence Interval | |
|---|---|---|---|
| Intercept | -$2,100 | -$7,800 | $3,700 |
| Fracture vs Controls | 8,600 | 6,400 | 10,800 |
| Osteoporosis vs Controls | -400 | -1,300 | 500 |
| Comorbidity Index | 4,400 | 3,900 | 4,900 |
| Age (continuous, years) | 40 | 0 | 100 |
| Female | -1,300 | -2,600 | 0 |
| Urban residence (cities and towns > 2500 pop) | 2,250 | 3,300 | 1,200 |
| Institutionalized full year of 2002 | 22,900 | 19,900 | 25,900 |
Linear regression, weighed least squares, stratified multistage sample design, (Numbers rounded)
Patients institutionalized for a full-year of long-term care (14% of fracture patients and 6% of control patients), are estimated to average $22,900 more in cost than those who were not. Full-year institutionalization is used as a predictor variable, because in our one year snapshot, it is not an effect of fracture: to be coded as full-year it began prior to 2002 and thus usually before the fracture claim. However, some patients are in the fracture cohort because they have a 2002 treatment for an earlier fracture that contributed to their institutionalization in 2002. As a sensitivity analysis, we tested omitting full-year institutionalization from the model; this results in a marginal cost estimate for fracture of $9500, about 8% higher than when it is included in the model. Part-year institutionalization is more likely to be an effect of a fracture, so it was not used as a predictor.
Females are estimated to average $1300 less in expense than males. Comorbidities were modeled with an index for which most chronic conditions are each counted as one, severe conditions such as cancer or AIDS as two or three [19]. For every one-unit increase in the index, costs go up by $4400. Variables for individual comorbidities, such as arthritis, diabetes, and COPD were not found to offer additional significant explanatory power.
Given the comorbidity index and the institutionalization variable, and that all subjects were at least 65 years old, the age variable was of marginal significance (p = 0.09). Living in a rural area was associated with $2200 less expense than in cities or towns.
After adjustment, the marginal one-year cost of having osteoporosis (but without a fracture that year), was not statistically significant (95%CI: -$1300 to $500).
Alternate log-linear models and generalized linear model with log-link (Poisson distribution) obtained similar results. The weighted linear least squares model was retained for ease of interpretation.
National Cost
To estimate a national cost for the U.S. elderly population, we applied the lower and upper confidence limits of the fracture cost estimate to the 1.6 million people estimated to have a fracture in 2002. The resulting mean national estimate was $14 billion, with 95% confidence intervals estimated at $10 to $17 billion for 2002. Including the drug and physician visit costs of osteoporosis patients who did not have a fracture would add an additional $2 billion to the national estimate, resulting in a total estimated cost for osteoporosis and fractures among Medicare-eligible individuals of $16 billion.
Almost 70% of the fracture and osteoporosis expenses were borne by Medicare, 4% by Medicaid, 17% by private insurers, and 10% by the patients (data not shown).
Discussion
We used the national Medicare Current Beneficiary Survey (MCBS) to estimate the prevalence and costs of fractures and osteoporosis in the U.S. Fractures occurred in 5% (1.6 million) of the elderly population in 2002, while 24% (7.2 million) had osteoporosis without a fracture in 2002. These estimates compare well with commonly cited figures of 1.5 million persons with fractures [2] and 10 million with osteoporosis [1]. Another Medicare study using a much larger sample of claims from over 900,000 recipients, found a six year period prevalence of osteoporosis or fractures of 36%, using fracture criteria similar to ours (30% for more narrowly defined fracture criteria) [20]. Based on bone mineral density scans at the femur neck taken by the National Health and Nutrition Examination 2005-2006, 5.3 million men and women (age 50 years or older) had osteoporosis and 34.5 million more had osteopenia [21].
The MCBS is especially well-suited for estimating medical costs, as it includes payments of patients and all payers, to all providers, including pharmacies, long-term care facilities, and hospices that are difficult to obtain from other sources. We estimated the national medical cost of osteoporosis and fractures to be $16 billion in 2002. A cost estimate for 2008, accounting for the 8% growth in Medicare elderly enrollees and for 27% medical inflation, would be $22 billion [22-23].
Using a random population-based patient sample with national weights like MCBS complements previous efforts to estimate national costs from provider-side data and commercial insurer data. Burge provides a recent comprehensive estimate of $19 billion for incident and prevalent fractures in 2005 [10]. They used a wide range of data and published research, including the 2001 National Inpatient Sample for hip-fracture rates and hospital charges, a cost to charge ratio, a single-county population study of other fracture rates, and updates of provider-based data and cost estimates from the early 1990s. The relation of charges to payments is highly variable by procedure and payer [24], and the lack of recent payment information is a weakness of their approach.
Parts of Burge’s approach were inflation updates of methods developed by Hoerger and colleagues and Ray and colleagues [11,13], who combined separate federally-collected national survey samples of providers of inpatient, outpatient, physician, nursing home, and other services (1992 for Ray, and 1994 for Hoerger). Ray used an expert panel to attribute the probability that a fracture at a given age is due to osteoporosis and estimated national costs of osteoporosis at $13.8 billion (1995 dollars). Hoerger included all encounters having a primary diagnosis of a limb fracture or osteoporosis, and estimated the national cost for females at $12.9 billion (1997 dollars). Ray and Hoerger relied on cost to charge ratios or imputed costs and could not include costs of outpatient medication.
Integrated claims databases from selected commercial insurance plans provide perhaps better payment data, but on narrower populations, for estimates by Sasser [16] and Orsini [3]. Orsini’s claims data source included retirees and spouses over age 64 with employer-paid coverage. Disease-related claims were $4000/year for those with a limb fracture and osteoporosis diagnosis in the study period, (7% of the cohort), $450/year for those with only an osteoporosis diagnosis (93% of the cohort), similar to our disease-coded claim totals. These figures translate to a nationwide cost estimate of $6 billion for the fractures plus $3.8 billion for osteoporosis only [3].
Rousculp used Medicaid claims from three states and a multivariate model to estimate that the marginal impact of a fracture on subsequent 12-months medical costs to be $9677 and $4007 (2002 dollars) on disease-coded expenses [17]. These figures compare well to our corresponding estimates of $8600 and $4400.
The scope of our cost estimate is limited in that we are not estimating the costs to the non-elderly population, and are not estimating indirect costs to patient and society. We also are not doing a longitudinal study that could examine individual increases in cost after a fracture or osteoporosis diagnosis [25].
With respect to our objective of estimating annual medical costs for the Medicare population, we are also limited by:
The difficulty of defining disease-related costs in claims data. For example, data is not available to determine definitively whether a fracture is attributable to weak bone mass or severe trauma.
The lack of ability to attribute long-term care to a diagnosis and the lack of drug records during periods of long-term care.
The inability to tie drug data to a diagnosis. SERMS and bisphosphonates are used for bone disease caused by both cancer and osteoporosis (though some bone cancers were excluded from the study cohorts). We also cannot identify pain or other medications used for treatment of fractures.
The age of the data (2002)
Data on many non-Medicare payments is manually collected from patient explanation of benefit forms, rather than from electronic transfer. CMS asserts the process is subject to several reconciliation and quality control efforts [17].
Each approach to estimating cost of illness has different limitations and it is valuable to have estimates available from a variety of methodologies. As one of the more prevalent and costly afflictions in the U.S., confidence in estimates of the cost of osteoporosis is important for national resource allocation discussions. For example, Hoerger and colleagues highlighted that among postmenopausal diseases in women, cardiovascular disease health care cost $60 billion, osteoporosis $13 billion, and breast cancer $5 billion (1997 dollars) [11]. Our estimate of $22 billion for the medical cost of osteoporosis in 2008 corroborates this and other estimates derived using quite different data sets and methodologies and thus reinforces the importance of osteoporosis.
Acknowledgments
The research was sponsored by Novartis Pharmaceuticals Corporation.
The authors gratefully acknowledge Kristijan Kahler, Novartis, and Clark Paramore, United BioSource Corporation for developing the study concept, and George Chalissery, hMetrix, for statistical programming and guidance on MCBS. We also thank Kenneth Saag, University of Alabama at Birmingham, and Wing Chan, Novartis, for input on the study design and an early version of this manuscript.
Potential Conflict of Interest/Disclosure:
The study was sponsored by Novartis Pharmaceuticals, Inc.
| Blume, Steven W: | |
| Employee: United BioSource Corporation, consulting firm to pharmaceutical, biotechnology, and medical device industries. | |
| Curtis JR: | |
| Consultant/Advisory Board: | Procter & Gamble |
| Research: | Novartis, Merck, Procter & Gamble, Eli Lilly |
| Speaker: | Novartis, Procter & Gamble, Eli Lilly |
Appendix
a Fracture Inclusion Criteria
| ICD9 Diagnosis Code Include | CPT Codes | ICD9 9 or V codes | ICD9 Proc Codes | |
|---|---|---|---|---|
| Hip/femur neck | 820 | 27120-27138, 27193-27228 | 9053 V5413 V5423 | 79.0y, 79.1y, 79.2y, 79.3y, 79.6y where y is in (1,2,3,5,6,7,9) to omit procedures on phalanges of foot or hand |
| Pelvis | 808 | |||
| Other femur | 821 | 27230-27248, 27500-27519 | 9054 V5414 V5415 V5416 V5424 V5425 V5426 | |
| Patella | 822 | 27520-27524 | ||
| Tibia/fibula | 823 | 27530-27540, 27750-27766, 27780-27832 | ||
| Ankle | 824 | 27840-27860, 28400-28485 | ||
| Multiple or ill-defined of lower limb | 827, 828 | - | ||
| Radius/ulna | 813 | 24620-24685, 25500-25620, 25650-25652 | 9052 V5410 V5411 V5412 V5420 V5421 V5422 | |
| Wrist | 814 | 25622-25645, 26600-26686 | ||
| Multiple or ill- defined of upper limb | 818, 819 | - | ||
| Spine | 805, 806 | 22305-22328, 22505-22522 | 9051, V5417, V5427 | |
| Shoulder/humerus | 809, 810, 811, 812 | 23570-23585, 23600-23616, 23620-23630, 24500-24582 | V5419, V5429 | |
| Ribs | 807.0-807.3 | 21800-21825 | ||
| Pathological fractures coded for osteoporosis | 733.1, 733.1x | - | - |
Note to editors – Primarily for use by reviewers. Electronic availability on publication may be desirable.
Contributor Information
Steven W Blume, United BioSource Corporation, Center for Health Economics & Science Policy, Bethesda, Maryland, United States.
JR Curtis, Email: jcurtis@uab.edu, University of Alabama at Birmingham, Center for Education and Research on Therapeutics of Musculoskeletal Disorders, Birmingham, Alabama, United States.
References
- 1.National Osteoporosis Foundation (NOF) America’s bone health: the state of osteoporosis and low bone mass in our nation. National Osteoporosis Foundation; Washington, DC: 2002. [Google Scholar]
- 2.Keen RW. Burden of osteoporosis and fractures. Curr Osteoporos Rep. 2003;1(2):66–70. doi: 10.1007/s11914-003-0011-x. [DOI] [PubMed] [Google Scholar]
- 3.Orsini LS, Rousculp MD, Long SR, Wang S. Health care utilization and expenditures in the United States: a study of osteoporosis-related fractures. Osteoporos Int. 2005;16(4):359–371. doi: 10.1007/s00198-004-1694-2. [DOI] [PubMed] [Google Scholar]
- 4.Fisher ES, Baron JA, Malenka DJ, et al. Hip fracture incidence and mortality in New England. Epidemiol. 1991;2(2):116–122. doi: 10.1097/00001648-199103000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Jacobsen SJ, Goldberg J, Miles TP, Brody JA, Stiers W, Rimm AA. Race and sex differences in mortality following fracture of the hip. Am J Public Health. 1992;82(8):1147–1150. doi: 10.2105/ajph.82.8.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magaziner J, Simonsick EM, Kashner TM, Hebel JR, Kenzora JE. Survival experience of aged hip fracture patients. Am J Public Health. 1989;79(3):274–278. doi: 10.2105/ajph.79.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ettinger B, Black DM, Nevitt MC, et al. Contribution of vertebral deformities to chronic back pain and disability. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1992;7(4):449–456. doi: 10.1002/jbmr.5650070413. [DOI] [PubMed] [Google Scholar]
- 8.Ross PD, Ettinger B, Davis JW, Melton LJ, 3rd, Wasnich RD. Evaluation of adverse health outcomes associated with vertebral fractures. Osteoporos Int. 1991;1(3):134–140. doi: 10.1007/BF01625442. [DOI] [PubMed] [Google Scholar]
- 9.Spector TD, McCloskey EV, Doyle DV, Kanis JA. Prevalence of vertebral fracture in women and the relationship with bone density and symptoms: the Chingford Study. J Bone Miner Res. 1993;8(7):817–822. doi: 10.1002/jbmr.5650080707. [DOI] [PubMed] [Google Scholar]
- 10.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465–75. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 11.Hoerger TJ, Downs KE, Lakshmanan MC, et al. Healthcare use among U.S. women aged 45 and older: total costs and costs for selected postmenopausal health risks. J Womens Health Gend Based Med. 1999;8(8):1077–1089. doi: 10.1089/jwh.1.1999.8.1077. [DOI] [PubMed] [Google Scholar]
- 12.Max W, Sinnot P, Kao C, Sung HY, Rice DP. The burden of osteoporosis in California, 1998. Osteoporos Int. 2002;13(6):493–500. doi: 10.1007/s001980200060. [DOI] [PubMed] [Google Scholar]
- 13.Ray NF, Chan JK, Thamer M, Melton LJ., 3rd Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12(1):24–35. doi: 10.1359/jbmr.1997.12.1.24. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Medicare and Medicaid Services (CMS) [December 30, 2009];Medicare Current Beneficiary Survey. 2002 http://www.cms.hhs.gov/MCBS. [PubMed]
- 15.Desai SS, Duncan BS, Sloan AS. The cost of treating osteoporosis in a managed health care organization. J Manag Care Pharm. 2003;9(2):142–149. doi: 10.18553/jmcp.2003.9.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sasser AC, Rousculp MD, Birnbaum HG, Oster EF, Lufkin E, Mallet D. Economic burden of osteoporosis, breast cancer, and cardiovascular disease among postmenopausal women in an employed population. Womens Health Issues. 2005;15(3):97–108. doi: 10.1016/j.whi.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Medicare and Medicaid Services (CMS) Health & Health Care of the Medicare Population: 2002. Appendix A. [December 30, 2009];Technical Documentation for the Medicare Current Beneficiary Survey. 2002 http://www.cms.hhs.gov/mcbs/downloads/HHC2002appendixA.pdf.
- 18.Rousculp MD, Long SR, Wang S, Schoenfeld MJ, Meadows ES. Economic burden of osteoporosis-related fractures in Medicaid. Value Health. 2007;10(2):144–52. doi: 10.1111/j.1524-4733.2006.00161.x. [DOI] [PubMed] [Google Scholar]
- 19.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Cheng H, Gary LC, Curtis JR, et al. Estimated prevalence and patterns of presumed osteoporosis among older Americans based on Medicare data. Osteoporos Int. 2009;20(9):1507–15. doi: 10.1007/s00198-009-0835-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Looker AC, Melton LJ, Harris TB, Borrud LG, Shepherd JA. Prevalence and Trends in Low Femur Bone Density Among Older US Adults: NHANES 2005-2006 Compared with NHANES III dagger. J Bone Miner Res. 2009 doi: 10.1359/jbmr.090706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bureau of Labor Statistics. Consumer Price Index - All Urban Consumers. Medical Care. [Dec 17, 2009];Seasonally Adjusted. [Google Scholar]
- 23.Centers for Medicare and Medicaid Services (CMS) [December 30, 2009];Medicare Enrollment Reports. National Trends 1966-2008. http://www.cms.hhs.gov/MedicareEnRpts/Downloads/HISMI08.pdf. [PubMed]
- 24.Reinhardt UE. The pricing of U.S. hospital services: chaos behind a veil of secrecy. Health Aff. 2006;25(1):57–69. doi: 10.1377/hlthaff.25.1.57. [DOI] [PubMed] [Google Scholar]
- 25.Kilgore ML, Morrisey MA, Becker DJ, et al. Health Care Expenditures Associated With Skeletal Fractures Among Medicare Beneficiaries, 1999-2005. J Bone Miner Res. 2009;24(12):2050–2055. doi: 10.1359/jbmr.090523. [DOI] [PubMed] [Google Scholar]


