Abstract
Brain-derived neurotrophic factor (BDNF), one of the major neurotrophic factors, plays an important role in the maintenance and survival of neurons, synaptic integrity, and synaptic plasticity. Evidence suggests that BDNF is involved in major depression, such that the level of BDNF is decreased in depressed patients and that antidepressants reverse this decrease. Stress, a major factor in depression, also modulates BDNF expression. These studies have led to the proposal of the neurotrophin hypothesis of depression. Late-life depression is associated with disturbances in structural and neural plasticity as well as impairments in cognitive behavior. Stress and aging also play a crucial role in late-life depression. Many recent studies have suggested that not only expression of BDNF is decreased in the serum/plasma of patients with late-life depression, but structural abnormalities in the brain of these patients may be associated with a polymorphism in the BDNF gene, and that there is a relationship between a BDNF polymorphism and antidepressant remission rates. This review provides a critical review of the involvement of BDNF in major depression, in general, and in late-life depression, in particular.
Keywords: BDNF, major depression, antidepressants, late-life depression, genetics
Major depressive disorder (MDD) is a major public health concern. It affects approximately 15% of the population at some point in their lives and is the leading cause of disability worldwide.1 About 9 million people are diagnosed as having MDD each year in the United States alone, and the lost productivity and treatment expenses burden the U.S. economy by more than $43 billion per year.2 Among elderly individuals (aged 65 years and older), 1% to 4% of the population exhibit MDD.3 In hospitalized elderly persons, the occurrence of MDD ranges between 10% and 35%.4,5 Major depressive disorder in elderly persons is associated with an increased number of suicide attempts and increased lethality.6 In fact, suicide in the elderly population is twice as common as in the general population;7 interestingly, MDD is one of the major causes of suicide in subjects with late-life depression because approximately 80% of people who commit suicide show depressive symptoms.8 Alexopoulos et al.9 studied the clinical characteristics that can identify elderly patients with depression at risk for suicidal ideation and found that contemporaneous severity of depression was the most important determinant of suicidal ideation over time.
Late-life depression is a heterogeneous disorder, and multiple factors play important roles in this disorder. These factors include aging, medical conditions, and cerebrovascular diseases (reviewed in Smith et al.10). Major depressive disorder is often associated with cognitive deficits; this deficit is higher in late-life than in the younger population, however.11 Butters et al.12 showed that, relative to control subjects, patients with late-life depression perform poorer in all cognitive domains and more than half of these patients exhibit significant cognitive impairment. These cognitive impairments are associated with memory impairment, poor attention, and executive dysfunctions;13,14 they often precede the onset of dementia and Alzheimer disease.15,16 Because of several factors associated with late-life depression, the neurobiological mechanisms associated with this disorder are not clearly understood. Also, not all risk factors in late-life depression are late life in origin. Several factors that are critical in the onset of MDD in younger life may also be as important in late-life depression. One example is stress and stress-related hormones, which are important in early-life, and to some extent, in late-life depression. Also, MDD in early life may predispose a person to late-life depression. Thus, there is some commonality in the pathophysiological characteristics of early- and late-life depression.17 As discussed subsequently, brain-derived neurotrophic factor (BDNF) is a critical neurotrophic factor that plays a crucial role in neural plasticity and cognition and is involved in depression. More recently, neurochemical and genetic studies indicate that BDNF may be a pathophysiological significance in late-life depression. This review focuses on the role of BDNF in depression during early and late life.
Structural Abnormalities in the Brain: Early- Versus Late-Life Depression
An emerging hypothesis suggests that the pathogenesis of MDD involves altered neural plasticity,18 resulting in the inability of the brain to make appropriate adaptive responses to environmental stimuli.19 The affective fronto-limbic circuitry, including the prefrontal cortices, the cingulate cortex, and several limbic structures (including the hippocampus), are highly involved in mediating these adaptive responses. Several lines of evidence demonstrate that these regions show structural and functional alterations in MDD. These include a reduction in cell number, density, cell body size, neuronal and glial density in frontal cortical or hippocampal brain areas, and parahippocampal cortex and cortical/laminar thickness.20–24 In addition, studies in MDD subjects show changes in synaptic circuitry in anterior limbic cortex,25 abnormal dorsolateral prefrontal cortical activity,26 impaired synaptic connectivity between the frontal lobe and other brain regions,27 neuronal atrophy, a decreased volume of the hippocampus,28–30 a decreased number of neurons and glia in cortical areas,31 and spatial cognition deficits.32 In addition, changes in the number and shape of dendritic spines,33,34 changes in the primary location of synapse formation, altered dendritic morphological characteristics of neurons in the hippocampus, and a decrease in length and number of apical dendrites35 have been reported during stress in mice and rats. Furthermore, stress hinders performance on hippocampal-dependent memory tasks and impairs induction of hippocampal long-term potentiation (LTP). These studies clearly demonstrate impaired structural and functional plasticity in MDD.
Structural abnormalities have also been reported in the brain of subjects with late-life depression. For example, structural neuroimaging findings have shown decreased volumes of subcortical and limbic structures, such as subgenual anterior cingulate, caudate, putamen, hippocampus, and amygdala.36–39 Individuals with late-life depression also show reduced activity in the prefrontal dorsolateral cortex and reduced functional connectivity between dorsolateral prefrontal cortex and anterior cingulate cortex.10,40,41,42 It has been suggested that MDD-associated alterations in neuronal and glial cell population in the frontal and subcortical circuitry changes may be related to early- and late-life depression because glial reductions are more consistent in younger depressed subjects, whereas neuronal changes are more common in elderly depressed subjects.32,43 White matter abnormalities also occur in patients with late-life depression, particularly in subcortical structures and their frontal projections, which are primarily associated with executive dysfunction (reviewed in Smith et al.10 and Alex-opoulos36). Sheline et al.44 showed greater hyper-intensities in several white matter tracts in subcortical regions in subjects with late-life depression, and volumes of some of these brain regions correlate well with executive functions. For example, whole brain white matter correlates with episodic memory, processing speed, and executive function; and whole brain gray matter correlates with processing speed.
Given the role of hippocampus in stress, memory, and cognition, several studies have examined this brain region in both early- and late-life depression. For those with MDD, it has been shown that hippocampal volume is smaller than in healthy subjects.45 Similarly, a reduction in hippocampal volume in late-life depression has also been reported by many investigators46–49 and has been further supported by meta-analyses performed in two separate studies.45,50 In a positive emission tomography study, de Asis et al.51 demonstrated deficits in bilateral activation of hippocampus of the geriatric depressed patients. These patients had memory deficits that correlated with lower hippocampal activity during both rest and activation. Ballmaier et al.52 reported that hippocampal volumes differed between depressed patients and comparison subjects but not between patients with early- and late-onset depression. When statistical mapping was performed, they found that regional surface contractions were significantly pronounced in late-onsest compared with early-onset depression in the lateral posterior of the hippocampal CA1 subfield in the left hemisphere. Hippocampal surface contractions correlated with memory measures among late-onset depressed patients. These results suggest that late-onset depressed patients are more likely to develop cognitive impairment over time than individuals with early-onset depression. Hickie et al.53 showed reduced hippocampal volumes in older people with both early- and late-onset depression. These reduced hippocampal volumes were associated with deficits in visual and verbal memory performance. Some studies, however, failed to find such changes.54,55
Not only in late-life depression, but normal aging also causes changes in hippocampal structure and, thus, altered neural plasticity (reviewed in von Bohlen und Halbach56). For example, many studies have shown reduced hippocampal volume during normal aging,57–59 which may be due to hippocampal atrophy, neuronal loss, a decrease in neuronal densities,60 or even apoptosis.61 Interestingly, these changes are related to behavioral deficits in hippocampus-dependent learning and memory.62 In addition, decreased spine densities of basal and apical dendrites of the CA1 hippocampal area in aged rats63 and a correlation in the decrease in the densities of basal dendrites in the CA1 area and impairment in spatial learning64 have been reported.
BDNF: A Key Molecule in Structural and Neural Plasticity
Neurotrophins are growth factors that are critical in regulating structural, synaptic, and morphological plasticity and in modulating the strength and number of synaptic connections and neurotransmission.65 In addition, the role of neurotrophins in the adult central nervous system is crucial because they participate in the maintenance of neuronal functions, the structural integrity of neurons, and neurogenesis in adult life,66 suggesting their biological role during the entire life span.
Neurotrophins are homodimeric proteins and are categorized into four different classes: nerve growth factor, BDNF, neurotrophin (NT)-3, and NT-4/5. Most functions of neurotrophins are mediated by the tropomycin receptor kinase (Trk) family of tyrosine kinase receptors. The interaction of neurotrophins with the Trk receptors is specific: Nerve growth factor binds with TrkA, BDNF and NT-4 both bind to TrkB, and NT-3 binds to TrkC with the highest affinity but can also bind and mediate its actions via TrkA and TrkB receptors. All neurotrophins can bind to the pan75 neurotrophin receptor (p75NTR), which plays a role in neurotrophin transport, ligand-binding specificity, and Trk functioning.67,68 In addition to the full-length TrkB receptor (TrkB.FL), several noncatalytic truncated TrkB isoforms (TrkB.T1 and TrkB.T2) have also been identified. These truncated TrkBs lack intracellular tyrosine kinase activity69 and induce signal transduction, resulting in different biological responses.70 Binding of neurotrophin to the appropriate Trk receptor leads to the dimerization and transphosphorylation of tyrosine residues in the intracellular domain of the Trk receptors and subsequent activation of signaling pathways, leading to altered transcription of critical genes. These signaling pathways include mitogen-activated protein kinase, phosphoinositide 3-kinase, and phospholipase C γ.71
The most widely distributed member of the neurotrophin family is BDNF. The BDNF gene lies on chromosome 11p13 and encodes pro-BDNF, a precursor peptide of mature BDNF. The BDNF gene contains nine 5′ noncoding exons (I-IX) linked to a common 3′ coding exon (IX), producing 22 transcripts.72 These transcripts facilitate multilevel regulation of BDNF expression and determine the tissue-specific expression.73 The BDNF is translated as 30- to 35-kDa preproproteins consisting of a preprodomain, a prodomain, and a C-terminal mature neurotrophin domain. The BDNF levels and its intracellular localization in neurons are regulated via several different mechanisms, including BDNF transcripts, messenger RNA (mRNA), protein transport, and regulated cleavage of pro-BDNF to mature BDNF. The pro-BDNF is produced in the endoplasmic reticulum, which is accumulated in the trans-Golgi network via the Golgi apparatus. Pro-BDNF can be cleaved in the endoplasmic reticulum by furin or in the regulated secretary vesicles by proconvertase enzymes. Pro-BDNF binds to sortilin, an intracellular chaperone that binds to the prodomain of BDNF to traffic it to the regulated secretory pathway, in the Golgi apparatus. This facilitates the correct folding of the mature BDNF domain. The mature BDNF domain binds to carboxypeptidase E, thereby sorting BDNF to the regulated secretary pathway.74 Pro-BDNF can also be processed by serine protease plasmin when pro-BDNF is in the extracellular milieu.75 A substitution of valine (Val) to methionine (Met) at codon 66 in the prodomain impairs this sorting of BDNF.76
The expression of the BDNF gene is tightly regulated by neuronal activity, through mechanisms dependent on calcium.77 The BDNF is present in both pre- and postsynaptic sites and can go under both retrograde and anterograde transport. In addition to BDNF, the function of a receptor for BDNF (i.e., TrkB) is also regulated in an activity-dependent manner. The TrkB is primarily localized in the synaptic sites. Further localization of TrkB occurs at the synaptic sites after neuroanal activity.74 Neuronal activity, therefore, is critical for synthesis and intracellular targeting of TrkB receptors.74 Thus, BDNF release and expression of TrkB receptors in a coordinated manner are important for optimal synaptic response.
BDNF is involved in a plethora of biological functions in the brain. More importantly, it is involved in synaptic transmission and maintenance of neuronal plasticity, including regulation of synaptic activity,78,79 neurite outgrowth, phenotypic maturation, morphological plasticity, and synthesis of proteins for differentiated functioning of neurons and for synaptic functioning. BDNF is also involved in nerve regeneration, neuronal survival, neurite outgrowth, structural integrity, and neurotransmitter synthesis.80 The role of BDNF has extensively been studied in learning and memory and in cognitive functions. For example, BDNF is necessary and sufficient to induce persistence of long-term memory storage and synaptic consolidation of LTP.78,81 Behaviorally, BDNF expression increases in the rat hippocampus following behavioral tasks, such as the Morris water maze,82 the radial arm maze,83 passive avoidance,84 and contextual fear conditioning.85 TrkB also plays an important role in such learning and memory because mice over-expressing full-length TrkB show enhanced learning and memory.86 Thus, a pathological alteration of the BDNF/TrkB may lead to defects in neural maintenance and regeneration and, therefore, structural abnormalities in the brain. This type of alteration may also reduce neural plasticity and, therefore, impair the individual's ability to adapt to crisis situations. Because of the role played by BDNF/TrkB in regulating structural, synaptic, and morphological plasticity, as well as cognition, there has been great interest in their role in the pathogenic mechanisms, particularly MDD. This review focuses on the role of BDNF in stress, aging, and MDD, in general, and during late-life depression, in particular. The role of BDNF in the mechanism of action of antidepressants is also briefly discussed.
Stress and BDNF
An overactive hypothalamus-pituitary-adrenal (HPA) axis has been well established in stress, which leads to chronic elevation of adrenal glucocorticoid production, with impaired negative feedback regulation of the HPA axis.87 Stress has been shown to be one of the risk factors in late-life depression. O'Brien et al.88 showed an association between advanced age and increased HPA axis dysregulation in late-life depression. Lee et al.89 investigated the relationship between the dexamethasone suppression test, cognitive function, depressive symptoms, and hippocampal atrophy in healthy controls, Alzheimer disease patients, and patients with MDD in late life. They found that elevated cortisol was associated with poorer cognitive function across a range of domains, suggesting that HPA axis dysregulation is a risk factor for poorer cognitive performance in older persons. Also pertinent is that older humans with significant prolonged cortisol elevations show a reduced hippocampal volume and deficits in hippocampus-dependent memory tasks and that the degree of hippocampal atrophy strongly correlates with both the degree of cortisol elevation over time and current basal cortisol levels, suggesting that basal cortisol elevation may cause hippocampal damage and impair hippocampus-dependent learning and memory in humans.90 As discussed earlier, late-life depressed subjects show abnormalities in hippocampal structure, and this could be attributed to depression-related hypercortisolemia. On this basis, Kumar et al. have proposed a model suggesting that prior depressive episodes, aging, stress, and a concomitant release of cortisol and a reduced level of BDNF may cause focal atrophy, which may decrease the threshold for late-life mood disorders, leading to late-life depression.91
Although there is no direct evidence showing a relationship to hyperactive HPA axis in late-life depression and BDNF, many preclinical studies suggest a strong relationship of BDNF stress pathophysiological characteristics. The first study showing the role of BDNF in stress was from Smith et al.92 who showed that immobilization stress in rats lowers the expression of BDNF in the hippocampus, most notably in the dentate gyrus. Subsequently, this was confirmed by other investigators.93,94 Other stressors, such as social defeat, also decreased BDNF in mouse hippocampus, but interestingly, this decrease extended to cortical and subcortical regions.95 Because stress is associated with elevated levels of glucocorticoids, several studies have examined the effect of exogenous glucocorticoids on BDNF expression. For example, corticosterone (CORT) treatment in rats reduces BDNF expression in the hippocampus.92,96 In a recent study, we extensively examined the effects of CORT on BDNF expression in the rat brain and found that CORT decreased expression of BDNF in the hippocampus and in the frontal cortex.97 Interestingly, adrenalectomy increased the level of BDNF in the hippocampus,98,99 whereas supplementation of the synthetic glucocorticoid dexamethasone to adrenalectomized rats restored the level of BDNF to normal.98 These studies suggest that CORT plays a critical role in regulating the synthesis of BDNF.
We further examined the molecular basis of decreased BDNF synthesis in response to CORT treatment. The rat BDNF gene contains four distinct promoters that are linked to four main transcript forms.100 Although the functions of each BDNF transcript are not clearly known, BDNF transcripts are differentially expressed across brain regions and are differentially regulated.101,102 When we examined whether a decrease in BDNF expression by CORT is associated with alterations in the expression of a specific BDNF transcript, we found that CORT decreased the expression of selective transcripts II and IV, but not transcript I or III, in the rat frontal cortex and hippocampus.97 Other studies also suggest that immobilization stress decreased exon IV in the hippocampus103 and hypothalamus,104 leading to a decrease in total BDNF expression in these brain areas. Although the functional significance of these changes is yet to be known, it has been shown that exon II has brain-enriched expression patterns, whereas exon IV is widely expressed. Interestingly, exon IV is expressed in the cell body, and is involved in maturation of interneurons through a transsynaptic route.
Because antidepressants can regulate the levels of glucocorticoids105,106 and they are as effective in early-life depression as in a subpopulation of late-life depressed patients, it is interesting to examine whether glucocorticoid-mediated down regulation of BDNF is reversed by antidepressants and, if so, by what possible mechanism. We showed that desipramine (a norepinephrine blocker) and phenelzine (a monoamine oxidase inhibitor) increased mRNA levels of BDNF gene expression in both the frontal cortex and hippocampus, whereas fluoxetine (a serotonin reuptake blocker) increased the mRNA level of BDNF only in the hippocampus.97 Interestingly, desipramine specifically increased the expression of BDNF transcripts I and III in both the frontal cortex and hippocampus; fluoxetine increased only exon II in the hippocampus; and phenelzine increased exons I and IV in the hippocampus but only exon I in the frontal cortex. Furthermore, all the antidepressants normalized the levels of CORT. When examined, we found that desipramine reversed the CORT-induced decrease in BDNF expression in both the frontal cortex and hippocampus. Fluoxetine only partially reversed such a decrease in the hippocampus, but no effect was found in the frontal cortex. Phenelzine, on the other hand, reversed the CORT-induced decrease in BDNF, partially in the frontal cortex and completely in the hippocampus. Interesting results were noted when individual BDNF transcripts were examined after antidepressant treatment to CORT-implanted rats. We found that all the antidepressants increased mRNA levels of those BDNF transcripts that were affected when the respective antidepressant was given to healthy rats without CORT treatment. For example, desipramine increased exons I and III in the frontal cortex and hippocampus, fluoxetine increased exon II in the hippocampus, and phenelzine increased exon I in the frontal cortex and exons I and IV in the hippocampus. Surprisingly, except for an increase in exon II by fluoxetine in the frontal cortex and in exon IV by phenelzine in the hippocampus, the CORT-mediated decrease in exons II and IV persisted even after antidepressant treatment. Additionaly, despite these different effects of CORT and antidepressants on BDNF transcripts, overall, all the antidepressants increased the level of BDNF mRNA in the brain of CORT-treated rats. Although it is difficult to assess the extent of involvement of a particular exon in the regulation of overall BDNF expression, there is complete reversal by desipramine in both the frontal cortex and hippocampus because the increase in exon III was robust in these brain areas. On the other hand, in the hippocampus, fluoxetine reversed the CORT-mediated decrease of only exon II, but not exon IV; therefore, the reversal was partial. No effect of fluoxetine on total BDNF expression was observed in the frontal cortex, however, because fluoxetine did not increase either exon II or exon IV in the frontal cortex. On the other hand, phenelzine was partially effective in the frontal cortex because of its effects on exon II, but complete reversal was noted in the hippocampus because phenelzine increased the levels of both CORT-decreased exons II and IV. Thus, it appears that antidepressants reverse total BDNF expression in CORT-treated rats; the mechanisms for the down regulation of BDNF transcripts by CORT and those that affect their up regulation by antidepressants are different, however.
Recently, in an attempt to identify potential biomarkers for the onset of antidepressant action in patients with MDD, Rojas et al.107 examined glucocorticoid receptors and serum BDNF levels during antidepressant treatment. Thirty-four depressed outpatients were treated with venlafaxine, and individuals exhibiting a 50% reduction in their baseline Hamilton Depression Rating Scale score by the sixth week of treatment were considered responders. These responders showed an early improvement in parallel with an increase in BDNF levels during the first two weeks of treatment. Nonresponders showed increased glucocorticoid receptor levels by the third week and reduced serum BDNF levels by the sixth week of treatment. The authors concluded that levels of BDNF in serum and glucocorticoid receptor levels in lymphocytes may represent biomarkers that could be used to predict responses to venlafaxine treatment. Whether such a response can be predicted in late-life depression needs to be further investigated.
Lower levels of subjective social support are associated with late-life depression severity104 and with poorer treatment outcomes108–112 and an impairment of social support is a risk factor for developing medical and psychiatric illnesses.110,113 Taylor et al.114 examined the relationship between the BDNF Val66-Met polymorphism and social support measures in a group of older subjects (aged 60 years or older) composed of 243 with depression and 115 without depression, of whom 233 were Val66 allele homozygotes and 125 were Met66 allele carriers. After controlling for diagnosis and education level, they found that the Met66 allele was associated with lower levels of subjective social support and a trend for fewer social interactions. That study shows that a genetic polymorphism in BDNF may influence social support perception. As previously mentioned, social defeat in mice decreases BDNF not only in the hippocampus but also in cortical and subcortical structures.95
Aging and BDNF
In a human postmortem brain study, Webster et al.115 showed that BDNF mRNA is not altered in the aged hippocampus; they found that expression of full-length TrkB is lower in several subfields of the hippocampus, however, most notably in the subiculum. In the human prefrontal cortex, both BDNF116 and truncated TrkB (TrkBTK)117 mRNA levels peak in expression during young adulthood, coincident with the structural and functional maturation of the frontal cortex. Levels of both the full-length form of TrkB mRNA and the truncated form of TrkB decrease over the life span, however. In the temporal cortex, BDNF and truncated TrkB mRNA levels are highest in neonates and decreased with age. In contrast, TrkBTK mRNA levels remained constant across the life span in the temporal cortex.115
Neural plasticity is severely affected in aging, suggested by studies showing changes in hippocampal morphology and impairment in LTP.75,118,119 These studies suggest that a decline in TrkB expression during aging may be critical in lower BDNF functioning and may be causing impaired LTP. Animal studies also suggest that expression of BDNF is not altered during aging in rats.120–123 In contrary, Hayashi et al.124 showed that BDNF immunoreactivity declines in cell bodies and dendrites of the neurons in the hippocampus of aged macaque monkeys. Also, the gerbil hippocampus shows an age-dependent decrease in BDNF, which is correlated with memory loss and a decrease of memory.125 Interestingly, peripheral BDNF changes during aging. For example, Lommatzsch et al.126 demonstrated that plasma BDNF decreases with an increase in age and that there is a negative correlation between plasma BDNF and age.127,128 On the other hand, antidepressant treatment to aged rats increases the expression of BDNF in the hippocampus.129
Antidepressants and BDNF
The effects of antidepressants on the expression of the BDNF gene have been extensively investigated. In general, when given to healthy rodents, several classes of antidepressants, including monoamine oxidase inhibitors, selective serotonin reuptake inhibitors, tricyclic agents, noradrenaline reuptake inhibitors, and noradrenergic and specific serotonergic antidepressants, increase the expression of BDNF in the brain.130–134 Long-term treatment with antidepressants not only increases expression of BDNF in healthy rodents, but also reverses down regulation of BDNF caused by stress.130,135 These effects are dependent on various factors, however, including length of administration, class of antidepressant, route of administration, age of animal, and doses of the drugs. In general, an increase in BDNF expression occurs after long-term treatment,130,131,136–138 although short-term treatment with antidepressants has been shown to increase BDNF expression in the cortex.139 The effects of antidepressants, such as desipramine or fluoxetine, have also been studied in BDNF-deficient mice. These studies show that the behavioral effects of antidepressants are abolished in BDNF-deficient mice,140 suggesting that BDNF plays an important role in the behavioral effects of antidepressants.
Regulation of BDNF Exons by Antidepressants
To examine how BDNF is regulated in response to antidepressants, we administered different classes of antidepressants to healthy rats and examined whether antidepressants regulate the expression of BDNF via specific BDNF transcript(s).97 We observed interesting results, such that treatment of healthy rats with desipramine or phenelzine increased mRNA levels of total BDNF in both the frontal cortex and hippocampus, whereas fluoxetine increased the mRNA level of BDNF only in the hippocampus.97 More interestingly, when we examined the effects of antidepressants on the expression of individual exons containing BDNF transcripts, we found that desipramine specifically increased exons I and III in both the frontal cortex and hippocampus; fluoxetine increased only exon II in the hippocampus; and phenelzine effectively increased exons I and IV in the hippocampus but only exon I in the frontal cortex. In another study, Dias et al.141 examined the effects of long-term antidepressants on BDNF transcript levels in the rat hippocampus, amygdala, and cortex. They observed that desipramine increased exon III in different cortical areas, whereas fluoxetine had no significant effects on BDNF exons in any of the brain areas studied. Another recent study by Altieri et al.137 also showed no effect of long-term fluoxetine treatment on BDNF transcripts in the hippocampus. Our observation of increased exon III by desipramine is similar to the findings of Dias et al.141 but we also noted an increase in the expression of exon I. In addition, contrary to reports by Altieri et al.137 and Dais et al.141 we found a selective increase in exon II by fluoxetine in both the frontal cortex and hippocampus. Although some of these discrepancies can be attributed to route of administration or doses of drugs, these findings suggest that there is no unified mechanism for the regulation of BDNF exon(s) by antidepressants and that various classes of antidepressants may affect BDNF exon expression differently.
Antidepressant-Like Effect of BDNF
The role of BDNF in depression also stems from preclinical studies demonstrating that antidepressants regulate the expression of BDNF and BDNF shows antidepressant-like effects in animal models. In a learned helplessness model of depression, infusion of BDNF reduces escape latencies and failure rates in rodents,142,143 suggesting the effectiveness of BDNF in reducing inescapable random shock–induced depressive behavior. Similarly, intra-midbrain infusion of BDNF in rodents produces an antidepressant-like effect in the forced swim test and learned helpless models of depression.142 Infusion of BDNF in the dorsal raphe nucleus also resulted in an antidepressant-like effect in the learned helpless model of depression.144 Interestingly, the effects of BDNF on these behavioral paradigms were much longer lasting compared with classic antidepressants.143,145
BDNF Abnormalities in Early-Life Depression and Response to Antidepressants
Several studies in humans provide evidence that BDNF plays an important role in early-life depression. In an earlier study, Chen et al.146 showed that the expression of BDNF is increased in the postmortem brain of depressed subjects treated with antidepressants compared with those who were untreated. We also reported decreased expression of BDNF in prefrontal cortex and hippocampus of suicide subjects who had major depression.147 Kozicz et al.148 showed decreased BDNF also in midbrain of depressed suicide subjects. These studies have recently been replicated by Thompson et al.149 who found similar results in hippocampus of depressed subjects. Interestingly, Guilloux et al.150 for the first time reported reduced BDNF functions in amygdala of female depressed subjects.
Besides postmortem brain studies, many researchers have examined the level of BDNF in serum or platelets of depressed subjects with and without antidepressant treatment. Although the significance of measurement of BDNF in blood cells is unclear at the present time, however, there are studies showing a complete passage of intact BDNF across the blood–brain barrier by a high-capacity and saturable transport system, as well as its efflux from brain to blood.151 In addition, platelets are the major source of BDNF in serum152 and it has been suggested that platelets and neurons are developed from a common embryonic precursor in the neural crest.153 Therefore, there is a possibility that serum BDNF may represent the central nervous system state.154 This is further supported by the fact that BDNF is neuronal in origin and is expressed highly in brain. Interestingly, platelet BDNF shows similar changes postnately similar to the brain,155 suggesting that there are parallel changes in the blood and brain levels of BDNF. Karege et al.155 were the first to compare BDNF levels in the serum of depressed subjects with those in healthy controls. In 15 male and 15 female depressed patients, they found that the BDNF level was significantly lower compared with that in healthy controls. This decrease was negatively correlated with the severity of depression. Recently, the same group of investigators suggested that the decrease in serum BDNF in depressed patients is related to release mechanisms of BDNF because no change was found in the level of BDNF in blood, but serum and platelet BDNF levels were decreased in depressed patients.156 Since then, several studies have examined BDNF level in these peripheral tissues before and after antidepressant treatment. For example, Gonul et al.157 Piccinni et al.158 and Dell'Osso et al.159 reported decreases in serum BDNF level in depressed patients. BDNF levels were related to both recurrence and severity of depression.159 On the other hand, Matrisciano et al.160 examined serum BDNF levels in healthy subjects and depressed patients at baseline and after 5 weeks and 6 months of sertraline, escitalopram, or venlafaxine treatment. They found that the BDNF level was lower in depressed patients and that sertraline increased the BDNF level after 5 weeks and 6 months, whereas escitalopram increased the BDNF level only after 6 months. Venlafaxine did not change the level of BDNF. There was a negative correlation between increase in BDNF level and decrease in Hamilton Depression Rating Scale score. On the other hand, Gonul et al.157 reported that depressed patients show an increased BDNF level in serum after treatment with a variety of antidepressants for 8 weeks, including venlafaxine, sertraline, fluoxetine, paroxetine, and citalopram. Similarly, increases in the serum BDNF level by amitriptyline after 36 days, paroxetine after 4 or 8 weeks, or venlafaxine after 12 weeks of treatment to depressed patients were reported.161–163 Not only antidepressants but vagus nerve stimulation, repetitive transcranial magnetic stimulation,164 or electroconvulsive therapy,165 administered to depressed patients, also cause an increase in the serum BDNF level.
Late-Life Depression and BDNF
In elderly women with a remitted depressive episode of unipolar depression, Laske et al.166 showed significantly decreased BDNF serum levels compared with healthy female controls. In another study, Lee et al.167 did not find a correlation between serum BDNF level and depression in the elderly population; it was, however, significantly correlated with deterioration of cognitive functions. Shi et al.168 measured plasma BDNF and tissue-type plasminogen activator (tPA) in those with late-onset geriatric depression before and after 6 weeks of antidepressant treatment compared with control subjects. The tPA is involved in cleavage of pro-BDNF to mature BDNF and, thus, regulates BDNF action.75,169 The tPA has been implicated in neuronal and cognitive functions, because it mediates stress-induced decline of neuronal and cognitive functions in the mouse hippocampus.170 Shi et al.168 found that baseline plasma BDNF and tPA levels were significantly lower in late-onset geriatric depressed patients compared with controls. Also, there was a heightening tendency of plasma BDNF level after antidepressant treatment. Recently, Chu et al.171 compared the differences in plasma BDNF levels among institutionalized ethnic Chinese elderly participants with major depression, those with subclinical depression, and a nondepressed control group. They found a significantly negative association between age and plasma BDNF in the regression model and noted that plasma BDNF was significantly lower in the major depressive group than in the nondepressive group. The BDNF plasma concentrations in the subclinically depressive group and control group were also significantly different, suggesting that plasma BDNF levels were reduced in ethnic Chinese elderly patients with MDD and in those with subclinical depression. Overall, these studies demonstrate a negative correlation between serum BDNF and late-life depression.
BDNF Polymorphism and Early- and Late-Life Depression
In humans, a common single-nucleotide polymorphism (SNP) at nucleotide 196 within the 5′ pro-BDNF sequence encodes a variant BDNF at codon 66 (Val66-Met). This Met66 variant affects activity-dependent BDNF secretion.76,172 This is critical for dendritic trafficking and synaptic localization of BDNF because a Val66Met polymorphism reduces activity-dependent secretion of BDNF, without any change in the level of total BDNF.173 More interestingly, mice carrying the BDNF Met/Met or Val/Met allele show a reduced hippocampal volume and BDNF Met/Met knock-in mice have reduced dendritic arbor complexity.174 These studies are relevant to MDD in relation to observed structural abnormalities, including reduced hippocampal volume, which increases the risk for MDD.175,176 Recently, Frodl et al.177 examined the effect of the BDNF Val66Met polymorphism on hippocampal and amygdala volumes in patients with depression and in healthy control subjects, and found that MDD patients had significantly reduced hippocampal volumes. They also found smaller hippocampal volumes for depressed patients and for healthy controls carrying the Met-BDNF allele when compared with subjects homozygous for the Val-BDNF allele. No significant difference in amygdala volume was found between depressed patients and healthy controls, and no significant main effects for the BDNF Val66Met polymorphism were observed. They concluded that the Met-BDNF allele carriers might be at risk of developing smaller hippocampal volumes and might be susceptible to MDD. Interestingly, magnetic resonance imaging studies in healthy subjects showed that Val/Val homozygotes had a larger hippocampal volume than Val/Met heterozygotes.178,179 People with the Met allele also have poor hippocampal-dependent memory function and hippocampal hyperactivation during learning,76,180 which could be associated with hippocampal hypersensitivity to stress. The direct role of the BDNF Val66Met polymorphism in cognitive impairment comes from a study by Miyajima et al.181 who investigated six haplotype-tagging SNPs using a cohort of elderly individuals. They found that the presence of the Met allele reduced cognitive performance, suggesting that the Met allele is associated with reduced cognitive functioning. The magnetic resonance imaging data showed that the left and right sides of the hippocampus were 5.0% and 3.9% smaller, respectively, in those possessing the Met allele. On the other hand, Kleim et al.182 demonstrated that training-dependent increases in the amplitude of motor-evoked potentials and motor map reorganization are reduced in healthy subjects with a Val66Met polymorphism in the BDNF gene, compared with subjects without the polymorphism. These results suggest that BDNF is involved in mediating the experience-dependent plasticity of the human motor cortex. Furthermore, the Val66Met polymorphism in the BDNF gene modulates human cortical plasticity and the response to transcranial magnetic stimulation.183 Because the majority of the polymorphic relationship of BDNF gene and hippocampal volume studies are focused on early-life depression, how BDNF polymorphism leads to cognitive decline in late-life depression or whether it leads to depression in elderly is not clear and more longitudinal studies are need to clarify this issue.
Earlier, Tsai et al.184 studied the BDNF gene Val66Met polymorphism in MDD and healthy control subjects. They also examined the association of this polymorphism and 4-week fluoxetine therapeutic response in patients with MDD. They found no significant differences for the genotype or allele frequency of the BDNF polymorphism comparing the MDD and control groups. Furthermore, no significant differences were noted comparing the three genotype groups for depressive-cluster symptoms. A trend to improved 4-week fluoxetine antidepressant response was demonstrated, however, for heterozygous patients compared with homozygous analogs. Similarly, Choi et al.185 reported that the genotype, allele, and allele-carrier distributions for the Val66Met polymorphism did not differ significantly between patients with MDD and healthy controls; they showed, however, that the Val66Met polymorphism of BDNF was associated with citalopram efficacy, with Met-allele carriers responding better to citalopram treatment.
Recently, Licinio et al.186 studied novel genetic polymorphisms in the BDNF gene and assessed their frequencies and associations with MDD or antidepressant response. They identified 83 novel SNPs (30 in untranslated regions, 4 in coding sequences, 37 in introns, and 12 in upstream regions); three of four rare novel-coding SNPs were nonsynonymous. Association analyses of patients with MDD and controls showed that six SNPs were associated with MDD (rs12273539, rs11030103, rs6265, rs28722151, rs41282918, and rs11030101), and two haplotypes in different blocks (one including Val66 and 1 near exon VIIIh) were significantly associated with MDD. The 5′ untranslated region SNP, rs61888800, was associated with antidepressant response, however.
There have been several studies demonstrating a direct link between late-life depression and BDNF polymorphism. Hwang et al.187 reported that the BDNF Val66Met genotype distribution was significantly different between geriatric depressed patients and healthy subjects and that there was a significant excess of the Met allele in these patients compared with the control group. Duncan et al.188 found that the Val/Val genotype was associated with higher scores on the cognitive-affective factor of the Beck Depression Inventory-II scale, and somatic-vegetative factor scores, suggesting an association between the Val/Val genotype and higher levels of depression symptoms. In 245 elderly depressed white subjects and 94 elderly comparison white subjects, Taylor et al.189 examined allelic differences in the BDNF Val66Met polymorphism in late-life depression. Subjects were dichotomized as either homozygous for the Val66 allele or Met66 allele carriers. They found that depressed subjects were more likely to be Met66 allele carriers than were comparison subjects and that there was no significant relationship between genotype and age of onset, number of episodes, or family history of depression. These results suggest that Met66 allele carriers have almost double the odds of having geriatric depression than do Val66 allele homozygotes. They argued that not finding an association with age of onset or number of depressive episodes suggests that presence of the Met66 allele would not predispose a person to an earlier age of developing depression but would increase the risk in context of other genetic risk factors for depression or impairment of hippocampus function. On the other hand, Kanellopoulos et al.190 reported that elderly depressed BDNF Val/Val homozygotes had significantly higher right hippocampal volumes compared with nondepressed Val/Val subjects. There was no difference, however, between the depressed and healthy nondepressed Met carriers. In addition, depressed Met carriers had an earlier age of onset of depressive illness than Val/Val homozygotes, suggesting that neurotrophic factor production protects against pathophysiological processes triggered by depression in older adults with a later age of onset of depression.
Because white matter abnormalities are often associated with late-life depression, Alexopoulos et al.191 tested the hypothesis of whether the BDNF (Val/Met) polymorphism influences the remission rate in these patients and whether the relationship between BDNF allelic status to remission is influenced by the presence of white matter abnormalities. They found that BDNF(Met) carriers were more likely to achieve remission than BDNF(Val/Val) homozygotes after 12 weeks of escitalopram treatment. They also found that microstructural abnormalities in the corpus callosum, left superior corona radiata, and right inferior longitudinal fasciculum were associated with a lower remission rate; no significant interactions between BDNF(Val66Met) status and microstructural abnormalities in predicting remission were noted, however. These studies suggest that depressed older BDNF(Met) carriers have a higher remission rate than BDNF(Val/Val) homozygotes, which is not related to microstructural white matter abnormalities. On the other hand, Taylor et al.192 when examining whether the BDNF Val66Met polymorphism was associated with greater volumes of hyperintense lesions in depressed and nondepressed elderly persons, found that the Met66 allele is associated with a risk factor for geriatric depression, including greater magnetic resonance imaging hyperintense lesion severity. More recently, they examined the relationship between a BDNF polymorphism and antidepressant remission rates in an elderly sample with MDD, while testing for mediation effects of social support and hyper-intensities. At the 3-month evaluation, the BDNF Val66Met genotype was not associated with remission. When not controlling for multiple comparisons, Met66 allele carriers were more likely to be remitted at 6 months, with an odds ratio of 1.82. This effect persisted after controlling for lesion volume and social support, neither of which mediated this relationship. They concluded that the Met66 allele may be associated with increased odds of remission in older subjects, but also with increased time to remission.
In a detailed study, Lin et al.193 assessed both the primary effects of single loci and multilocus interactions and tested the hypothesis that the BDNF and NTRK2(TrkB) genes may contribute to the cause of geriatric depression independently and/or through complex interactions. They genotyped the BDNF gene Val66Met (rs6265) polymorphism and four SNPs (rs1187323, rs1187329, rs1778929, and rs1545285) in the NTRK2 gene in 155 elderly inpatients diagnosed with major depression and 195 age- and sex-similar control subjects. They found that the genotype distributions of all five SNPs tested were significantly different between depressed patients and control subjects. BDNF rs6265, NTRK2 rs1187323, and NTRK2 rs1778929 were also statistically different in the genotypic tests. In addition, the two-marker haplo-type derived from the rs1187323 and rs1187329 polymorphisms demonstrated a significant difference between geriatric depression and control groups according to haplotype distribution. BDNF and NTRK2 interactions were also found in the significant two-, three-, four-, and five-locus gene–gene interaction models, suggesting that the BDNF and NTRK2 genes may contribute to the risk of geriatric depression, independently and in an interactive manner. These studies show a clear link between a BDNF polymorphism and early- and late-life depression and response to antidepressant treatment.
Conclusion and Future Studies
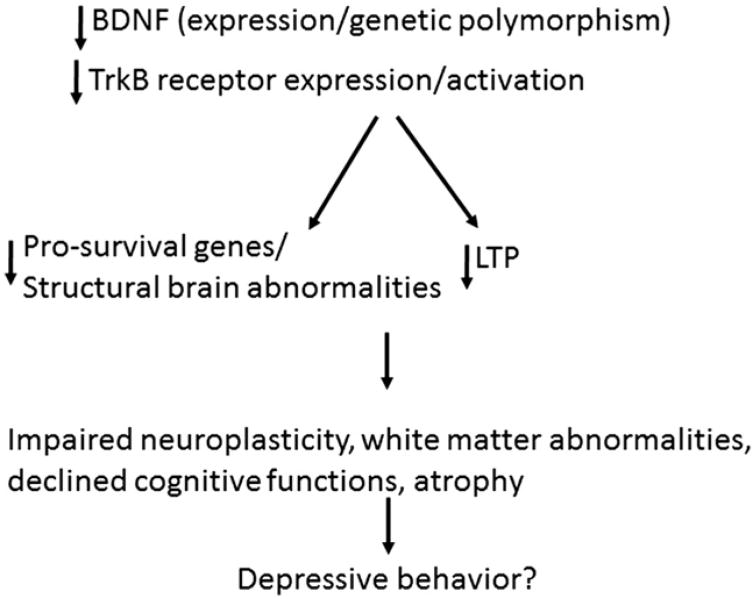
Several preclinical and clinical observations indicate that MDD may be associated with the inability of neural systems to exhibit adaptive plasticity. Given the role of BDNF and its cognate receptors in neural and structural plasticity, and that depression and antidepressants exert opposite actions on BDNF and TrkB expression and functions, it is apparent that BDNF may be crucial in the pathophysiology of MDD and in the mechanism of action of antidepressants. From the studies described in this review, it is clear that both early-onset and late-life depression are associated with altered BDNF expression. In addition, genetic polymorphic studies clearly point out a link between hippocampal volume in these patients as well as cognitive decline, which could possibly lead to depression (Fig. 1). On the other hand, BDNF studies regarding late-onset depression is not well studied and it would be interesting to see whether similar or different pattern of changes in BDNF expression, function, or genetic polymorphism occur in these patients. As far as late-life depression is concerned, it often arises in the context of other chronic medical conditions; social and psychosocial adversity; and aging, which causes changes in the brain and cognition. As discussed earlier, the expression of BDNF and TrkB decline during aging. Thus, future studies need to consider these factors.
Figure 1. Postulated hypothesis showing role of BDNF in depressive behavior.
Exactly how a decrease in BDNF expression leads to major depression is not clear. Genetic BDNF knock-in and knock-out models could possibly answer this question. Recent studies suggest that a reduction in BDNF level in BDNF heterozygous knockout mice does not produce depression-like symptoms,194 although overexpression of TrkB reduces anxiety and depressive behavior in mice.195 Thus, more in-depth studies of the relationship between TrkB and BDNF in major depression are required to answer this question.
There are many avenues in BDNF research in major depression that need further attention. For example, what role does dendritic localization of BDNF/TrkB play in altered plasticity? Recently, it has been shown that BDNF and TrkB can regulate translational machinery in dendrites.196 Moreover, BDNF induces the expression of Lim kinase 1, a protein kinase whose mRNA translation is inhibited by brain-specific microRNA-134. MicroRNA-134 is localized in dendrites and its overexpression leads to a decrease in spine size through repression of Lim kinase 1 mRNA translation.197 Thus, studying BDNF/TrkB and other interacting proteins in dendrites will further reveal their novel mechanistic roles in the development of early onset, late life, and late onset depression.
Acknowledgments
This research was supported by grants from the National Institute of Mental Health (MH081099, MH082802, and MH091509) and the American Foundation for Suicide Prevention to Dr. Y. Dwivedi.
Footnotes
The author reports no conflict of interest, financially or otherwise.
References
- 1.World Health Organization Health Systems. Improving Performance. Geneva: Tertiary; 2000. [Google Scholar]
- 2.Simon GE. Social and economic burden of mood disorders. Biol Psychiatry. 2003;54:208–215. doi: 10.1016/s0006-3223(03)00420-7. [DOI] [PubMed] [Google Scholar]
- 3.Blazer DG. Depression in late life: review and commentary. J Gerontol A Biol Sci Med Sci. 2003;58:249–265. doi: 10.1093/gerona/58.3.m249. [DOI] [PubMed] [Google Scholar]
- 4.Blazer D. Depression in the elderly. N Engl J Med. 1989;320:164–166. doi: 10.1056/NEJM198901193200306. [DOI] [PubMed] [Google Scholar]
- 5.Small GW. Recognition and treatment of depression in the elderly. J Clin Psych. 1991;56:11–22. [PubMed] [Google Scholar]
- 6.Katz IR, Streim J, Parmelee P. Prevention of depression, recurrences, and complications in late life. Prev Med. 1994;23:743–750. doi: 10.1006/pmed.1994.1128. [DOI] [PubMed] [Google Scholar]
- 7.Miniño AM, Arias E, Kochanek KD, et al. Deaths: final data for 2000. Natl Vital Stat Rep. 2002;50:1–119. [PubMed] [Google Scholar]
- 8.Conwell Y, Duberstein PR, Cox C, et al. Relationships of age and axis I diagnoses in victims of completed suicide: a psychological autopsy study. Am J Psychiatry. 1996;153:1001–1008. doi: 10.1176/ajp.153.8.1001. [DOI] [PubMed] [Google Scholar]
- 9.Alexopoulos GS, Bruce ML, Hull J, et al. Clinical determinants of suicidal ideation and behavior in geriatric depression. Arch Gen Psychiatry. 1999;56:1048–1053. doi: 10.1001/archpsyc.56.11.1048. [DOI] [PubMed] [Google Scholar]
- 10.Smith GS, Gunning-Dixon FM, Lotrich FE, et al. Translational research in late-life mood disorders: implications for future intervention and prevention research. Neuropsychopharmacology. 2007;32:1857–1875. doi: 10.1038/sj.npp.1301333. [DOI] [PubMed] [Google Scholar]
- 11.Thomas AJ, Gallagher P, Robinson LJ, et al. A comparison of neurocognitive impairment in younger and older adults with major depression. Psychol Med. 2009;39:725–733. doi: 10.1017/S0033291708004042. [DOI] [PubMed] [Google Scholar]
- 12.Butters MA, Whyte EM, Nebes RD, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61:587–595. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- 13.Alexopoulos GS, Meyers BS, Young RC, et al. Executive dysfunction and long-term outcomes of geriatric depression. Arch Gen Psychiatry. 2000;57:285–289. doi: 10.1001/archpsyc.57.3.285. [DOI] [PubMed] [Google Scholar]
- 14.Lockwood KA, Alexopoulos GS, Kakuma T, et al. Subtypes of cognitive impairment in depressed older adults. Am J Geriatr Psychiatry. 2000;8:201–208. [PubMed] [Google Scholar]
- 15.Saczynski JS, Beiser A, Seshadri S, et al. Depressive symptoms and risk of dementia: the Framingham Heart Study. Neurology. 2010;75:35–41. doi: 10.1212/WNL.0b013e3181e62138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dal Forno G, Palermo MT, Donohue JE, et al. Depressive symptoms, sex, and risk for Alzheimer's disease. Ann Neurol. 2005;57:381–387. doi: 10.1002/ana.20405. [DOI] [PubMed] [Google Scholar]
- 17.Tiemeier H. Biological risk factors for late life depression. Eur J Epidemiol. 2003;18:745–750. doi: 10.1023/a:1025388203548. [DOI] [PubMed] [Google Scholar]
- 18.Garcia R. Stress, synaptic plasticity, and psychopathology. Rev Neurosci. 2002;13:195–208. doi: 10.1515/revneuro.2002.13.3.195. [DOI] [PubMed] [Google Scholar]
- 19.Duman RS, Malberg J, Nakagawa S, D'Sa C. Neuronal plasticity and survival in mood disorders. Biol Psychiatry. 2000;48:732–739. doi: 10.1016/s0006-3223(00)00935-5. [DOI] [PubMed] [Google Scholar]
- 20.Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosoklija G, Toomayan G, Ellis SP, et al. Structural abnormalities of subicular dendrites in subjects with schizophrenia and mood disorders: preliminary findings. Arch Gen Psychiatry. 2000;57:349–356. doi: 10.1001/archpsyc.57.4.349. [DOI] [PubMed] [Google Scholar]
- 22.Cotter D, Mackay D, Chana G, et al. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002;12:386–394. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- 23.Miguel-Hidalgo J, Rajkowska G. Morphological brain changes in depression. Can antidepressants reverse them? CNS Drugs. 2002;16:361–372. doi: 10.2165/00023210-200216060-00001. [DOI] [PubMed] [Google Scholar]
- 24.Rajkowska G. Cell pathology in mood disorders. Semin Clin Neuropsychiatry. 2002;7:281–292. doi: 10.1053/scnp.2002.35228. [DOI] [PubMed] [Google Scholar]
- 25.Aganova EA, Uranova NA. Morphometric analysis of synaptic contacts in the anterior limbic cortex in the endogenous psychoses. Neurosci Behav Physiol. 1992;22:59–65. doi: 10.1007/BF01186670. [DOI] [PubMed] [Google Scholar]
- 26.Drevets WC, Ongür D, Price JL. Reduced glucose metabolism in the subgenual prefrontal cortex in unipolar depression. Mol Psychiatry. 1998;3:190–191. doi: 10.1038/sj.mp.4000380. [DOI] [PubMed] [Google Scholar]
- 27.Honer WG. Assessing the machinery of mind: synapses in neuropsychiatric disorders. J Psychiatry Neurosci. 1999;24:116–121. [PMC free article] [PubMed] [Google Scholar]
- 28.Sheline YI. 3D MRI studies of neuroanatomic changes in unipolar depression: the role of stress and medical comorbidity. Biol Psychiatry. 2000;48:791–800. doi: 10.1016/s0006-3223(00)00994-x. [DOI] [PubMed] [Google Scholar]
- 29.Sala M, Perez J, Soloff P, et al. Stress and hippocampal abnormalities in psychiatric disorders. Eur Neuropsychopharmacol. 2004;14:393–405. doi: 10.1016/j.euroneuro.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Frodl T, Schaub A, Banac S, et al. Reduced hippocampal volume correlates with executive dysfunctioning in depression. J Psychiatry Neurosci. 2006;31:316–323. [PMC free article] [PubMed] [Google Scholar]
- 31.Rajkowska G, Miguel-Hidalgo JJ. Gliogenesis and glial pathology in depression. CNS Neurol Disord Drug Targets. 2007;6:219–233. doi: 10.2174/187152707780619326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sackeim HA. Functional brain circuits in depression and remission. Arch Gen Psychiatry. 2001;58:649–650. doi: 10.1001/archpsyc.58.7.649. [DOI] [PubMed] [Google Scholar]
- 33.Leuner B, Shors TJ. Stress, anxiety, and dendritic spines: What are the connections? Neuroscience. 2012 Apr 20; doi: 10.1016/j.neuroscience.2012.04.021. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 34.Liston C, Gan WB. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc Natl Acad Sci USA. 2011;108:16074–16079. doi: 10.1073/pnas.1110444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McEwen BS. Effects of adverse experiences for brain structure and function. Biol Psychiatry. 2000;48:721–731. doi: 10.1016/s0006-3223(00)00964-1. [DOI] [PubMed] [Google Scholar]
- 36.Alexopoulos GS. Frontostriatal and limbic dysfunction in late-life depression. Am J Geriatr Psychiatry. 2002;10:687–695. [PubMed] [Google Scholar]
- 37.Sexton CE, Mackay CE, Ebmeier KP. A systematic review and meta-analysis of magnetic resonance imaging studies in late-life depression. Am J Geriatr Psychiatry. 2013;21:184–195. doi: 10.1016/j.jagp.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 38.Burke J, McQuoid DR, Payne ME, et al. Amygdala volume in late-life depression: relationship with age of onset. Am J Geriatr Psychiatry. 2011;19:771–776. doi: 10.1097/JGP.0b013e318211069a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamburo RJ, Siegle GJ, Stetten GD, et al. Amygdalae morphometry in late-life depression. Int J Geriatr Psychiatry. 2009;24:837–846. doi: 10.1002/gps.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aizenstein HJ, Butters MA, Wu M, et al. Altered functioning of the executive control circuit in late-life depression: episodic and persistent phenomena. Am J Geriatr Psychiatry. 2009;17:30–42. doi: 10.1097/JGP.0b013e31817b60af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crocco EA, Castro K, Loewenstein DA. How late-life depression affects cognition: neural mechanisms. Curr Psychiatry Rep. 2010;12:34–38. doi: 10.1007/s11920-009-0081-2. [DOI] [PubMed] [Google Scholar]
- 42.Khundakar AA, Thomas AJ. Morphometric changes in early- and late-life major depressive disorder: evidence from postmortem studies. Int Psychogeriatr. 2009;21:844–854. doi: 10.1017/S104161020999007X. [DOI] [PubMed] [Google Scholar]
- 43.Khundakar A, Morris C, Oakley A, et al. Morphometric analysis of neuronal and glial cell pathology in the caudate nucleus in late-life depression. Am J Geriatr Psychiatry. 2011;19:132–141. doi: 10.1097/JGP.0b013e3181df4642. [DOI] [PubMed] [Google Scholar]
- 44.Sheline YI, Price JL, Vaishnavi SN, et al. Regional white matter hyperintensity burden in automated segmentation distinguishes late-life depressed subjects from comparison subjects matched for vascular risk factors. Am J Psychiatry. 2008;165:524–532. doi: 10.1176/appi.ajp.2007.07010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 46.Hoptman MJ, Gunning-Dixon FM, Murphy CF, et al. Structural neuroimaging research methods in geriatric depression. Am J Geriatr Psychiatry. 2006;14:812–822. doi: 10.1097/01.JGP.0000238588.34205.bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steffens DC, Byrum CE, McQuoid DR, et al. Hippocampal volume in geriatric depression. Biol Psychiatry. 2000;48:301–309. doi: 10.1016/s0006-3223(00)00829-5. [DOI] [PubMed] [Google Scholar]
- 48.Lloyd AJ, Ferrier IN, Barber R, et al. Hippocampal volume change in depression: late- and early-onset illness compared. Br J Psychiatry. 2004;184:488–495. doi: 10.1192/bjp.184.6.488. [DOI] [PubMed] [Google Scholar]
- 49.O'Brien JT, Lloyd A, McKeith I, et al. A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am J Psychiatry. 2004;161:2081–2090. doi: 10.1176/appi.ajp.161.11.2081. [DOI] [PubMed] [Google Scholar]
- 50.Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, et al. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009;30:3719–3735. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Asis JM, Stern E, Alexopoulos GS, et al. Hippocampal and anterior cingulate activation deficits in patients with geriatric depression. Am J Psychiatry. 2001;158:1321–1323. doi: 10.1176/appi.ajp.158.8.1321. [DOI] [PubMed] [Google Scholar]
- 52.Ballmaier M, Narr KL, Toga AW, et al. Hippocampal morphology and distinguishing late-onset from early-onset elderly depression. Am J Psychiatry. 2008;165:229–237. doi: 10.1176/appi.ajp.2007.07030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hickie I, Naismith S, Ward PB, et al. Reduced hippocampal volumes and memory loss in patients with early- and late-onset depression. Br J Psychiatry. 2005;186:197–202. doi: 10.1192/bjp.186.3.197. [DOI] [PubMed] [Google Scholar]
- 54.Ashtari M, Greenwald BS, Kramer-Ginsberg E, et al. Hippocampal/amygdala volumes in geriatric depression. Psychol Med. 1999;29:629–638. doi: 10.1017/s0033291799008405. [DOI] [PubMed] [Google Scholar]
- 55.Pantel J, Schröder J, Essig M, et al. Quantitative magnetic resonance imaging in geriatric depression and primary degenerative dementia. J Affect Disord. 1997;42:69–83. doi: 10.1016/s0165-0327(96)00105-x. [DOI] [PubMed] [Google Scholar]
- 56.von Bohlen und Halbach O. Involvement of BDNF in age-dependent alterations in the hippocampus. Front Aging Neurosci. 2010;2:pii–36. doi: 10.3389/fnagi.2010.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Convit A, de Leon MJ, Tarshish C, et al. Hippocampal volume losses in minimally impaired elderly. Lancet. 1995;345:266. doi: 10.1016/s0140-6736(95)90265-1. [DOI] [PubMed] [Google Scholar]
- 58.Malykhin NV, Bouchard TP, Camicioli R, et al. Aging hippocampus and amygdala. Neuroreport. 2008;19:543–547. doi: 10.1097/WNR.0b013e3282f8b18c. [DOI] [PubMed] [Google Scholar]
- 59.Zhang H, Trollor JN, Wen W, et al. Grey matter atrophy of basal forebrain and hippocampus in mild cognitive impairment. J Neurol Neurosurg Psychiatry. 2011;82:487–493. doi: 10.1136/jnnp.2010.217133. [DOI] [PubMed] [Google Scholar]
- 60.Driscoll I, Hamilton DA, Petropoulos H, et al. The aging hippocampus: cognitive, biochemical and structural findings. Cereb Cortex. 2003;13:1344–1351. doi: 10.1093/cercor/bhg081. [DOI] [PubMed] [Google Scholar]
- 61.Lucassen PJ, Müller MB, Holsboer F, et al. Hippocampal apoptosis in major depression is a minor event and absent from subareas at risk for glucocorticoid overexposure. Am J Pathol. 2001;158:453–468. doi: 10.1016/S0002-9440(10)63988-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog Neurobiol. 2003;69:143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- 63.Nunzi MG, Milan F, Guidolin D, et al. Dendritic spine loss in hippocampus of aged rats. Effect of brain phosphatidylserine administration. Neurobiol Aging. 1987;8:501–510. doi: 10.1016/0197-4580(87)90124-2. [DOI] [PubMed] [Google Scholar]
- 64.von Bohlen und Halbach O, Zacher C, Gass P, et al. Age-related alterations in hippocampal spines and deficiencies in spatial memory in mice. J Neurosci Res. 2006;83:525–531. doi: 10.1002/jnr.20759. [DOI] [PubMed] [Google Scholar]
- 65.Thoenen H. Neurotrophins and activity-dependent plasticity. Prog Brain Res. 2000;128:183–191. doi: 10.1016/S0079-6123(00)28016-3. [DOI] [PubMed] [Google Scholar]
- 66.Cooper JD, Skepper JN, Berzaghi MD, et al. Delayed death of septal cholinergic neurons after excitotoxic ablation of hippocampal neurons during early postnatal development in the rat. Exp Neurol. 1996;139:143–155. doi: 10.1006/exnr.1996.0089. [DOI] [PubMed] [Google Scholar]
- 67.Barbacid M. Neurotrophic factors and their receptors. Curr Opin Cell Biol. 1995;7:148–155. doi: 10.1016/0955-0674(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 68.Schweigreiter R. The dual nature of neurotrophins. Bioessays. 2006;28:583–594. doi: 10.1002/bies.20419. [DOI] [PubMed] [Google Scholar]
- 69.Klein R, Conway D, Parada LF, et al. The trkB tyrosine protein kinase gene codes for a second neurogenic receptor that lacks the catalytic kinase domain. Cell. 1990;61:647–656. doi: 10.1016/0092-8674(90)90476-u. [DOI] [PubMed] [Google Scholar]
- 70.Yacoubian TA, Lo DC. Truncated and full-length TrkB receptors regulate distinct modes of dendritic growth. Nat Neurosci. 2000;3:342–349. doi: 10.1038/73911. [DOI] [PubMed] [Google Scholar]
- 71.Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 72.Aid T, Kazantseva A, Piirsoo M, et al. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Timmusk T, Palm K, Metsis M, et al. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. 1993;10:475–489. doi: 10.1016/0896-6273(93)90335-o. [DOI] [PubMed] [Google Scholar]
- 74.Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- 75.Pang PT, Lu B. Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: role of secreted proteins tPA and BDNF. Ageing Res Rev. 2004;3:407–430. doi: 10.1016/j.arr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 76.Egan MF, Kojima M, Callicott JH, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:144–145. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 77.Mellstrom B, Torres B, Link WA, et al. The BDNF gene: exemplifying complexity in Ca2+-dependent gene expression. Crit Rev Neurobiol. 2004;16:43–49. doi: 10.1615/critrevneurobiol.v16.i12.40. [DOI] [PubMed] [Google Scholar]
- 78.Soulé J, Messaoudi E, Bramham CR. Brain-derived neurotrophic factor and control of synaptic consolidation in the adult brain. Biochem Soc Trans. 2006;34:600–604. doi: 10.1042/BST0340600. [DOI] [PubMed] [Google Scholar]
- 79.Cowansage KK, LeDoux JE, Monfils MH. Brain-derived neurotrophic factor: a dynamic gatekeeper of neural plasticity. Curr Mol Pharmacol. 2010;3:12–29. doi: 10.2174/1874467211003010012. [DOI] [PubMed] [Google Scholar]
- 80.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bekinschtein P, Cammarota M, Izquierdo I, et al. BDNF and memory formation and storage. Neuroscientist. 2008;14:147–156. doi: 10.1177/1073858407305850. [DOI] [PubMed] [Google Scholar]
- 82.Kesslak JP, So V, Choi J, et al. Learning upregulates brain-derived neurotrophic factor messenger ribonucleic acid: a mechanism to facilitate encoding and circuit maintenance? Behav Neurosci. 1998;112:1012–1019. doi: 10.1037//0735-7044.112.4.1012. [DOI] [PubMed] [Google Scholar]
- 83.Mizuno M, Yamada K, Olariu A, et al. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurosci. 2000;20:7116–7121. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ma YL, Wang HL, Wu HC, et al. Brain-derived neurotrophic factor antisense oligonucleotide impairs memory retention and inhibits long-term potentiation in rats. Neuroscience. 1998;82:957–967. doi: 10.1016/s0306-4522(97)00325-4. [DOI] [PubMed] [Google Scholar]
- 85.Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000;3:533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- 86.Koponen E, Lakso M, Castrén E. Overexpression of the full-length neurotrophin receptor trkB regulates the expression of plasticity-related genes in mouse brain. Brain Res Mol Brain Res. 2004;130:81–94. doi: 10.1016/j.molbrainres.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 87.Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- 88.O'Brien JT, Ames D, Schweitzer I, et al. Clinical and magnetic resonance imaging correlates of hypothalamic-pituitary-adrenal axis function in depression and Alzheimer's disease. Br J Psychiatry. 1996;168:679–687. doi: 10.1192/bjp.168.6.679. [DOI] [PubMed] [Google Scholar]
- 89.Lee BK, Glass TA, McAtee MJ, et al. Associations of salivary cortisol with cognitive function in the Baltimore memory study. Arch Gen Psychiatry. 2007;64:810–818. doi: 10.1001/archpsyc.64.7.810. [DOI] [PubMed] [Google Scholar]
- 90.Lupien SJ, de Leon M, de Santi S, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- 91.Narayan M, Bremner JD, Kumar A. Neuroanatomic substrates of late-life mental disorders. J Geriatr Psychiatry Neurol. 1999;12:95–106. doi: 10.1177/089198879901200303. [DOI] [PubMed] [Google Scholar]
- 92.Smith MA, Makino S, Kvetnansky R, et al. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fuchikami M, Morinobu S, Kurata A, et al. Single immobilization stress differentially alters the expression profile of transcripts of the brain-derived neurotrophic factor (BDNF) gene and histone acetylation at its promoters in the rat hippocampus. Int J Neuropsychopharmacol. 2009;12:73–82. doi: 10.1017/S1461145708008997. [DOI] [PubMed] [Google Scholar]
- 94.Rasmusson AM, Shi L, Duman R. Downregulation of BDNF mRNA in the hippocampal dentate gyrus after re-exposure to cues previously associated with footshock. Neuropsychopharmacology. 2002;27:133–142. doi: 10.1016/S0893-133X(02)00286-5. [DOI] [PubMed] [Google Scholar]
- 95.Pizarro JM, Lumley LA, Medina W, et al. Acute social defeat reduces neurotrophin expression in brain cortical and subcortical areas in mice. Brain Res. 2004;1025:10–20. doi: 10.1016/j.brainres.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 96.Schaaf MJM, Hoetelmans RWM, de Kloet ER, et al. Corticosterone regulates expression of BDNF and trkB but not NT-3 and trkC mRNA in the rat hippocampus. J Neurosci Res. 1997;48:334–341. [PubMed] [Google Scholar]
- 97.Dwivedi Y, Rizavi HS, Pandey GN. Antidepressants reverse corticosterone-mediated decrease in BDNF expression: dissociation in regulation of specific exons by antidepressants and corticosterone. Neuroscience. 2006;139:1017–1029. doi: 10.1016/j.neuroscience.2005.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barbany G, Persson H. Regulation of neurotrophin mRNA expression in the rat brain by glucocorticoids. Eur J Neurosci. 1992;4:396–403. doi: 10.1111/j.1460-9568.1992.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 99.Chao HM, Sakai RR, Ma LY, et al. Adrenal steroid regulation of neurotrophic factor expression in the rat hippocampus. Endocrinology. 1998;139:3112–3118. doi: 10.1210/endo.139.7.6114. [DOI] [PubMed] [Google Scholar]
- 100.Nakayama M, Gahara Y, Kitamura T, et al. Distinctive four promoters collectively direct expression of brain-derived neurotrophic factor gene. Mol Brain Res. 1994;21:206–218. doi: 10.1016/0169-328x(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 101.Luberg K, Wong J, Weickert CS, et al. Human TrkB gene: novel alternative transcripts, protein isoforms and expression pattern in the prefrontal cerebral cortex during postnatal development. J Neurochem. 2010;113:952–964. doi: 10.1111/j.1471-4159.2010.06662.x. [DOI] [PubMed] [Google Scholar]
- 102.Wong J, Webster MJ, Cassano H, et al. Changes in alternative brain-derived neurotrophic factor transcript expression in the developing human prefrontal cortex. Eur J Neurosci. 2009;29:1311–1322. doi: 10.1111/j.1460-9568.2009.06669.x. [DOI] [PubMed] [Google Scholar]
- 103.Marmigere F, Givalois L, Rage F, et al. Rapid induction of BDNF expression in the hippocampus during immobilization stress challenge in adult rats. Hippocampus. 2003;13:646–655. doi: 10.1002/hipo.10109. [DOI] [PubMed] [Google Scholar]
- 104.Rage F, Givalois L, Marmigere F, et al. Immobilization stress rapidly modulates BDNF mRNA expression in the hypothalamus of adult male rats. Neurosci. 2002;112:309–318. doi: 10.1016/s0306-4522(02)00072-6. [DOI] [PubMed] [Google Scholar]
- 105.Fitzsimons CP, van Hooijdonk LW, Morrow JA, et al. Anti-glucocorticoids, neurogenesis and depression. Mini Rev Med Chem. 2009;9:249–264. doi: 10.2174/138955709787316001. [DOI] [PubMed] [Google Scholar]
- 106.Nikisch G. Involvement and role of antidepressant drugs of the hypothalamic-pituitary-adrenal axis and glucocorticoid receptor function. Neuro Endocrinol Lett. 2009;30:11–16. [PubMed] [Google Scholar]
- 107.Rojas PS, Fritsch R, Rojas RA, et al. Serum brain-derived neurotrophic factor and glucocorticoid receptor levels in lymphocytes as markers of antidepressant response in major depressive patients: a pilot study. Psychiatry Res. 2011;189:239–245. doi: 10.1016/j.psychres.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 108.George LK, Blazer DG, Hughes DC, et al. Social support and the outcome of major depression. Br J Psychiatry. 1989;154:478–485. doi: 10.1192/bjp.154.4.478. [DOI] [PubMed] [Google Scholar]
- 109.Blazer DG. Impact of late-life depression on the social network. Am J Psychiatry. 1983;140:162–166. doi: 10.1176/ajp.140.2.162. [DOI] [PubMed] [Google Scholar]
- 110.Lin N, Dean A. Social support and depression. A panel study Soc Psychiatry. 1984;19:83–91. doi: 10.1007/BF00583819. [DOI] [PubMed] [Google Scholar]
- 111.Wade TD, Kendler KS. The relationship between social support and major depression: cross-sectional, longitudinal, and genetic perspectives. J Nerv Ment Dis. 2000;188:251–258. doi: 10.1097/00005053-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 112.Bruce ML. Psychosocial risk factors for depressive disorders in late life. Biol Psychiatry. 2002;52:175–184. doi: 10.1016/s0006-3223(02)01410-5. [DOI] [PubMed] [Google Scholar]
- 113.Vanderhorst RK, McLaren S. Social relationships as predictors of depression and suicidal ideation in older adults. Aging Ment Health. 2005;9:517–525. doi: 10.1080/13607860500193062. [DOI] [PubMed] [Google Scholar]
- 114.Taylor WD, Züchner S, McQuoid DR, et al. Social support in older individuals: the role of the BDNF Val66Met polymorphism. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1205–1212. doi: 10.1002/ajmg.b.30754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Webster MJ, Herman MM, Kleinman JE, et al. BDNF and trkB mRNA expression in the hippocampus and temporal cortex during the human lifespan. Gene Expr Patterns. 2006;6:941–951. doi: 10.1016/j.modgep.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 116.Webster MJ, Weickert CS, Herman MM, et al. BDNF mRNA expression during postnatal development, maturation and aging of the human prefrontal cortex. Dev Brain Res. 2002;139:139–150. doi: 10.1016/s0165-3806(02)00540-0. [DOI] [PubMed] [Google Scholar]
- 117.Romanczyk TB, Weickert CS, Webster MJ, et al. Alterations in trkB mRNA in the human prefrontal cortex throughout the lifespan. Eur J Neurosci. 2002;15:269–280. doi: 10.1046/j.0953-816x.2001.01858.x. [DOI] [PubMed] [Google Scholar]
- 118.Rex CS, Kramár EA, Colgin LL, et al. Long-term potentiation is impaired in middle-aged rats: regional specificity and reversal by adenosine receptor antagonists. J Neurosci. 2005;25:5956–5966. doi: 10.1523/JNEUROSCI.0880-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- 120.Lapchak PA, Araujo DM, Beck KD, et al. BDNF and trkB mRNA expression in the hippocampal formation of aging rats. Neurobiol Aging. 1993;14:121–126. doi: 10.1016/0197-4580(93)90087-r. [DOI] [PubMed] [Google Scholar]
- 121.Croll SD, Ip NY, Lindsay RM, et al. Expression of BDNF and trkB as a function of age and cognitive performance. Brain Res. 1998;812:200–208. doi: 10.1016/s0006-8993(98)00993-7. [DOI] [PubMed] [Google Scholar]
- 122.Silhol M, Bonnichon V, Rage F, et al. Age-related changes in brain-derived neurotrophic factor and tyrosine kinase receptor isoforms in the hippocampus and hypothalamus in male rats. Neuroscience. 2005;132:613–624. doi: 10.1016/j.neuroscience.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 123.Hattiangady B, Rao MS, Shetty GA, et al. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp Neurol. 2005;195:353–371. doi: 10.1016/j.expneurol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 124.Hayashi M, Mistunaga F, Ohira K, et al. Changes in BDNF-immunoreactive structures in the hippocampal formation of the aged macaque monkey. Brain Res. 2001;918:191–196. doi: 10.1016/s0006-8993(01)03002-5. [DOI] [PubMed] [Google Scholar]
- 125.Hwang IK, Yoo KY, Jung BK, et al. Correlations between neuronal loss, decrease of memory, and decrease expression of brain-derived neurotrophic factor in the gerbil hippocampus during normal aging. Exp Neurol. 2006;201:75–83. doi: 10.1016/j.expneurol.2006.02.129. [DOI] [PubMed] [Google Scholar]
- 126.Lommatzsch M, Zingler D, Schuhbaeck K, et al. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 2005;26:115–123. doi: 10.1016/j.neurobiolaging.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 127.Ziegenhorn AA, Schulte-Herbrüggen O, Danker-Hopfe H, et al. Serum neurotrophins—a study on the time course and influencing factors in a large old age sample. Neurobiol Aging. 2007;28:1436–1445. doi: 10.1016/j.neurobiolaging.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 128.Bus BA, Tendolkar I, Franke B, et al. Serum brain-derived neurotrophic factor: Determinants and relationship with depressive symptoms in a community population of middle-aged and elderly people. World J Biol Psychiatry. 2012;13:39–47. doi: 10.3109/15622975.2010.545187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Garza AA, Ha TG, Garcia C, et al. Exercise, antidepressant treatment, and BDNF mRNA expression in the aging brain. Pharmacol Biochem Behav. 2004;77:209–220. doi: 10.1016/j.pbb.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 130.Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Coppell AL, Pei Q, Zetterström TS. Bi-phasic change in BDNF gene expression following antidepressant drug treatment. Neuropharmacology. 2003;44:903–910. doi: 10.1016/s0028-3908(03)00077-7. [DOI] [PubMed] [Google Scholar]
- 132.Van Hoomissen JD, Chambliss HO, Holmes PV, et al. Effects of chronic exercise and imipramine on mRNA for BDNF after olfactory bulbectomy in rat. Brain Res. 2003;974:228–235. doi: 10.1016/s0006-8993(03)02584-8. [DOI] [PubMed] [Google Scholar]
- 133.Xu H, Steven Richardson J, Li XM. Dose-related effects of chronic antidepressants on neuroprotective proteins BDNF, Bcl-2 and Cu/Zn-SOD in rat hippocampus. Neuropsychopharmacology. 2003;28:53–62. doi: 10.1038/sj.npp.1300009. [DOI] [PubMed] [Google Scholar]
- 134.Rogóz Z, Skuza G, Legutko B. Repeated treatment with mirtazepine induces brain-derived neurotrophic factor gene expression in rats. J Physiol Pharmacol. 2005;56:661–671. [PubMed] [Google Scholar]
- 135.Alfonso J, Frick LR, Silberman DM, et al. Regulation of hippocampal gene expression is conserved in two species subjected to different stressors and antidepressant treatments. Biol Psychiatry. 2006;59:244–251. doi: 10.1016/j.biopsych.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 136.Fujimaki K, Morinobu S, Duman RS. Administration of a cAMP phosphodiesterase 4 inhibitor enhances antidepressant-induction of BDNF mRNA in rat hippocampus. Neuropsychopharmacology. 2000;22:42–51. doi: 10.1016/S0893-133X(99)00084-6. [DOI] [PubMed] [Google Scholar]
- 137.Altieri M, Marini F, Arban R, et al. Expression analysis of brain-derived neurotrophic factor (BDNF) mRNA isoforms after chronic and acute antidepressant treatment. Brain Res. 2004;1000:148–155. doi: 10.1016/j.brainres.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 138.De Foubert G, Carney SL, Robinson CS, et al. Fluoxetine-induced change in rat brain expression of brain-derived neurotrophic factor varies depending on length of treatment. Neuroscience. 2004;128:597–604. doi: 10.1016/j.neuroscience.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 139.Miró X, Pérez-Torres S, Artigas F, et al. Regulation of cAMP phosphodiesterase mRNAs expression in rat brain by acute and chronic fluoxetine treatment. An in situ hybridization study Neuropharmacology. 2002;43:1148–1157. doi: 10.1016/s0028-3908(02)00220-4. [DOI] [PubMed] [Google Scholar]
- 140.Monteggia LM, Barrot M, Powell CM, et al. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci USA. 2004;101:10827–10832. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dias BG, Banerjee SB, Duman RS, et al. Differential regulation of brain-derived neurotrophin factor transcripts by antidepressant treatments in the adult rat brain. Neuropharmacology. 2003;45:553–563. doi: 10.1016/s0028-3908(03)00198-9. [DOI] [PubMed] [Google Scholar]
- 142.Siuciak JA, Lewis DR, Wiegand SJ, et al. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF) Pharmacol Biochem Behav. 1997;J56:131–137. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- 143.Shirayama Y, Chen AC, Nakagawa S, et al. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Altar CA. Neurotrophins and depression. Trends Pharmacol Sci. 1999;20:59–61. doi: 10.1016/s0165-6147(99)01309-7. [DOI] [PubMed] [Google Scholar]
- 145.Hoshaw BA, Malberg JE, Lucki I. Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain Res. 2005;1037:204–208. doi: 10.1016/j.brainres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 146.Chen B, Dowlatshahi D, MacQueen GM, et al. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 2001;50:260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- 147.Dwivedi Y, Rizavi HS, Conley RR, et al. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60:804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- 148.Kozicz T, Tilburg-Ouwens D, Faludi G, et al. Gender-related urocortin 1 and brain-derived neurotrophic factor expression in the adult human midbrain of suicide victims with major depression. Neuroscience. 2008;152:1015–1023. doi: 10.1016/j.neuroscience.2007.12.050. [DOI] [PubMed] [Google Scholar]
- 149.Thompson Ray M, Weickert CS, Wyatt E, et al. Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. J Psychiatry Neurosci. 2011;36:195–203. doi: 10.1503/jpn.100048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Guilloux JP, Douillard-Guilloux G, Kota R, et al. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry. 2012;17:1130–1142. doi: 10.1038/mp.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pan W, Banks WA, Fasold MB, et al. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropsychopharmacol. 1998;37:1553–1561. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 152.Yamamoto H, Gurney ME. Human platelets contain brain-derived neurotrophic factor. J Neurosci. 1990;10:3469–3478. doi: 10.1523/JNEUROSCI.10-11-03469.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pearse AG. The common peptides and the cytochemistry of their cells of origin. Basic Appl Histochem. 1980;24:63–73. [PubMed] [Google Scholar]
- 154.Lang UE, Hellweg R, Gallinat J. BDNF serum concentrations in healthy volunteers are associated with depression-related personality traits. Neuropsychopharmacology. 2004;29:795–798. doi: 10.1038/sj.npp.1300382. [DOI] [PubMed] [Google Scholar]
- 155.Karege F, Perret G, Bondolfi G, et al. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109:143–148. doi: 10.1016/s0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 156.Karege F, Bondolfi G, Gervasoni N, et al. Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol Psychiatry. 2005;57:1068–1072. doi: 10.1016/j.biopsych.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 157.Gonul AS, Akdeniz F, Taneli F, et al. Effect of treatment on serum brain-derived neurotrophic factor levels in depressed patients. Eur Arch Psychiatry Clin Neurosci. 2005;255:381–386. doi: 10.1007/s00406-005-0578-6. [DOI] [PubMed] [Google Scholar]
- 158.Piccinni A, Marazziti D, Catena M, et al. Plasma and serum brain-derived neurotrophic factor (BDNF) in depressed patients during 1 year of antidepressant treatments. J Affect Disord. 2008;105:279–283. doi: 10.1016/j.jad.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 159.Dell'Osso L, Del Debbio A, Veltri A, et al. Associations between brain-derived neurotrophic factor plasma levels and severity of the illness, recurrence and symptoms in depressed patients. Neuropsychobiology. 2010;62:207–212. doi: 10.1159/000319946. [DOI] [PubMed] [Google Scholar]
- 160.Matrisciano F, Bonaccorso S, Ricciardi A, et al. Changes in BDNF serum levels in patients with depression disorder (MDD) after 6 months treatment with sertraline, escitalopram, or venlafaxine. J Psychiatr Res. 2009;43:247–254. doi: 10.1016/j.jpsychires.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Aydemir O, Deveci A, Taneli F. The effect of chronic antidepressant treatment on serum brain-derived neurotrophic factor levels in depressed patients: a preliminary study. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:261–265. doi: 10.1016/j.pnpbp.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 162.Yoshimura R, Mitoma M, Sugita A, et al. Effects of paroxetine or milnacipran on serum brain-derived neurotrophic factor in depressed patients. Prog Neuropsychopharmacol Biol Psychiatry. 2007;3:1034–1037. doi: 10.1016/j.pnpbp.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 163.Hellweg R, Ziegenhorn A, Heuser I, et al. Serum concentrations of nerve growth factor and brain-derived neurotrophic factor in depressed patients before and after antidepressant treatment. Pharmacopsychiatry. 2008;41:66–71. doi: 10.1055/s-2007-1004594. [DOI] [PubMed] [Google Scholar]
- 164.Lang UE, Bajbouj M, Gallinat J, et al. Brain-derived neurotrophic factor serum concentrations in depressive patients during vagus nerve stimulation and repetitive transcranial magnetic stimulation. Psychopharmacology. 2006;187:156–159. doi: 10.1007/s00213-006-0399-y. [DOI] [PubMed] [Google Scholar]
- 165.Bocchio-Chiavetto L, Zanardini R, Bortolomasi M, et al. Elec-troconvulsive therapy (ECT) increases serum brain derived neurotrophic factor (BDNF) in drug resistant depressed patients. Eur Neuropsychopharmacol. 2006;16:620–624. doi: 10.1016/j.euroneuro.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 166.Laske C, Banschbach S, Stransky E, et al. Exercise-induced normalization of decreased BDNF serum concentration in elderly women with remitted major depression. Int J Neuropsychopharmacol. 2010;13:595–602. doi: 10.1017/S1461145709991234. [DOI] [PubMed] [Google Scholar]
- 167.Lee JG, Shin BS, You YS, et al. Decreased serum brain-derived neurotrophic factor levels in elderly korean with dementia. Psychiatry Investig. 2009;6:299–305. doi: 10.4306/pi.2009.6.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shi Y, You J, Yuan Y, et al. Plasma BDNF and tPA are associated with late-onset geriatric depression. Psychiatry Clin Neurosci. 2010;64:249–254. doi: 10.1111/j.1440-1819.2010.02074.x. [DOI] [PubMed] [Google Scholar]
- 169.Pang PT, Teng HK, Zaitsev E, et al. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- 170.Pawlak R, Rao BS, Melchor JP, et al. Tissue plasminogen activator and plasminogen mediate stress-induced decline of neuronal and cognitive functions in the mouse hippocampus. Proc Natl Acad Sci USA. 2005;102:18201–18206. doi: 10.1073/pnas.0509232102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chu CL, Liang CK, Chou MY, et al. Decreased plasma brain-derived neurotrophic factor levels in institutionalized elderly with depressive disorder. J Am Med Dir Assoc. 2012;13:434–437. doi: 10.1016/j.jamda.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 172.Chen ZY, Ieraci A, Teng H, et al. Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J Neurosci. 2005;25:6156–6166. doi: 10.1523/JNEUROSCI.1017-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chen ZY, Patel PD, Sant G, et al. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci. 2004;24:4401–4411. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chen ZY, Jing D, Bath KG, et al. Genetic variant BDNF (Val66-Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sheline YI, Wang PW, Gado MH, et al. Hippocampal atrophy in recurrent depression. Proc Natl Acad Sci USA. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- 177.Frodl T, Schüle C, Schmitt G, et al. Association of the brain-derived neurotrophic factor Val66Met polymorphism with reduced hippocampal volumes in depression. Arch Gen Psychiatry. 2007;64:410–416. doi: 10.1001/archpsyc.64.4.410. [DOI] [PubMed] [Google Scholar]
- 178.Pezawas L, Verchinski BA, Mattay VS, et al. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24:10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bueller JA, Aftab M, Sen S, et al. BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biol Psychiatry. 2006;59:812–815. doi: 10.1016/j.biopsych.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 180.Hariri AR, Goldberg TE, Mattay VS, et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23:6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Miyajima F, Ollier W, Mayes A, et al. Brain-derived neurotrophic factor polymorphism Val66Met influences cognitive abilities in the elderly. Genes Brain Behav. 2008;7:411–417. doi: 10.1111/j.1601-183X.2007.00363.x. [DOI] [PubMed] [Google Scholar]
- 182.Kleim JA, Chan S, Pringle E, et al. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat Neurosci. 2006;9:735–737. doi: 10.1038/nn1699. [DOI] [PubMed] [Google Scholar]
- 183.Cheeran B, Talelli P, Mori F, et al. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol. 2008;586:5717–5725. doi: 10.1113/jphysiol.2008.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tsai SJ, Cheng CY, Yu YW, et al. Association study of a brain-derived neurotrophic-factor genetic polymorphism and major depressive disorders, symptomatology, and antidepressant response. Am J Med Genet B Neuropsychiatr Genet. 2003;123B:19–22. doi: 10.1002/ajmg.b.20026. [DOI] [PubMed] [Google Scholar]
- 185.Choi MJ, Kang RH, Lim SW, et al. Brain-derived neurotrophic factor gene polymorphism (Val66Met) and citalopram response in major depressive disorder. Brain Res. 2006;1118:176–182. doi: 10.1016/j.brainres.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 186.Licinio J, Dong C, Wong ML. Novel sequence variations in the brain-derived neurotrophic factor gene and association with major depression and antidepressant treatment response. Arch Gen Psychiatry. 2009;66:488–497. doi: 10.1001/archgenpsychiatry.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hwang JP, Tsai SJ, Hong CJ, et al. The Val66Met polymorphism of the brain-derived neurotrophic-factor gene is associated with geriatric depression. Neurobiol Aging. 2006;27:1834–1837. doi: 10.1016/j.neurobiolaging.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 188.Duncan LE, Hutchison KE, Carey G, et al. Variation in brain-derived neurotrophic factor (BDNF) gene is associated with symptoms of depression. J Affect Disord. 2009;115:215–219. doi: 10.1016/j.jad.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Taylor WD, Züchner S, McQuoid DR, et al. Allelic differences in the brain-derived neurotrophic factor Val66Met polymorphism in late-life depression. Am J Geriatr Psychiatry. 2007;15:850–857. doi: 10.1097/JGP.0b013e318050c9d5. [DOI] [PubMed] [Google Scholar]
- 190.Kanellopoulos D, Gunning FM, Morimoto SS, et al. Hippocampal volumes and the brain-derived neurotrophic factor val66met polymorphism in geriatric major depression. Am J Geriatr Psychiatry. 2011;19:13–22. doi: 10.1097/jgp.0b013e3181f61d62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Alexopoulos GS, Glatt CE, Hoptman MJ, et al. BDNF val66met polymorphism, white matter abnormalities and remission of geriatric depression. J Affect Disord. 2010;125:262–268. doi: 10.1016/j.jad.2010.02.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Taylor WD, McQuoid DR, Ashley-Koch A, et al. BDNF Val66Met genotype and 6-month remission rates in late-life depression. Pharmacogenomics J. 2011;11:146–154. doi: 10.1038/tpj.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lin E, Hong CJ, Hwang JP, et al. Gene–gene interactions of the brain-derived neurotrophic-factor and neurotrophic tyrosine kinase receptor 2 genes in geriatric depression. Rejuvenation Res. 2009;12:387–393. doi: 10.1089/rej.2009.0871. [DOI] [PubMed] [Google Scholar]
- 194.MacQueen GM, Ramakrishnan K, Croll SD, et al. Performance of heterozygous brain-derived neurotrophic factor knockout mice on behavioral analogues of anxiety, nociception, and depression. Behav Neurosci. 2001;115:1145–1153. doi: 10.1037//0735-7044.115.5.1145. [DOI] [PubMed] [Google Scholar]
- 195.Koponen E, Voikar V, Riekki R, et al. Transgenic mice over-expressing the full-length neurotrophin receptor trkB exhibit increased activation of trkB/PLC-gamma pathway, reduced anxiety, and facilitated learning. Mol Cell Neurosci. 2004;26:166–181. doi: 10.1016/j.mcn.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 196.Takei N, Inamura N, Kawamura M, et al. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J Neurosci. 2004;24:9760–9769. doi: 10.1523/JNEUROSCI.1427-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Schratt GM, Tuebing F, Nigh EA, et al. A brain-specific micro-RNA regulates dendritic spine development. Nature. 2006;439:283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]