Abstract
Objective
To determine the association of high-risk alleles in the complement factor H (CFH; Y402H, rs1061170) and age-related maculopathy susceptibility (ARMS2; A69S, rs10490924) genes with reticular macular disease (RMD), a major clinical subphenotype of age-related macular degeneration (AMD).
Methods
Using retinal images from the Columbia Macular Genetics Study, we identified 67 subject individuals with RMD. A comparison group of 64 subjects with AMD without RMD was matched by ethnicity, age, sex, and AMD clinical stage.
Results
In the RMD group, 53 of 67 subjects (79.1%) were female, the mean age was 83 years, and 47 of 67 (70.1%) had late AMD, with closely matched values in the non-RMD group. The frequencies of the CFH 402H allele were 39.6% in the RMD group (53 of 134 individuals) and 58.6% in the non-RMD group (75 of 128 individuals) (χ2=8.8; P=.003; odds ratio, 0.46 [95% confidence interval, 0.28–0.76]). The corresponding frequencies of the risk allele for ARMS2 were 44.0% (40 of 128 individuals) and 31.3% (40 of 128 individuals), respectively (χ2=4.0; P=.045; odds ratio, 1.73 [95% confidence interval, 1.04–2.90]). Homozygosity for 402Hwas particularly associated with the absence of RMD, occurring in 8 of 67 subjects (11.9%) with RMD vs 24 of 64 subjects (37.5%) without RMD(P χ.001). Retinal macular disease also was associated with hypertension among male patients.
Conclusions
The AMD-associated CFH 402H risk variant is significantly associated with the absence of RMD but enhanced risk for RMD is conferred by the ARMS2 69S AMD risk allele. These results are consistent with the hypothesis that 402H may confer a survival benefit against certain infections, some of which may cause RMD.
Clinical Relevance
Reticular macular disease may be genetically distinct from the rest of AMD.
Two major genetic loci have been associated with an increased risk of agerelated macular degeneration (AMD) through genome- wide scanning and candidate gene approaches. They are the chromosome 1q32 locus, which includes the complement factor H gene (CFH [Y402H, rs1061170]), where the common polymorphism Y402H is well established as a risk haplotype-tagging variant for AMD,1–4 and the age-related maculopathy susceptibility gene (ARMS2 [A69S, rs10490924]) on chromosome 10q26.5,6 Although the function of ARMS2 is unknown, it is significantly associated with end-stage AMD (ie, choroidal neovascularization [CNV] and geographic atrophy [GA]) and more weakly associated with early-stage AMD.5,7,8 In the case of CFH, whose role in regulating the alternative complement activation pathway is well known, a plausible scenario for disease pathogenesis is that the inflammation triggered by exposure to infectious agents or some other triggering event in genetically susceptible individuals leads to the sustained activation of complement, drusen formation, and, eventually, the development of AMD.9
One possible clinical biomarker for AMDis reticular pseudodrusen (RPD)10 or, more generally, reticular macular disease (RMD). Reticular patterns on autofluorescence and infrared reflectance images, a characteristic poxlike pattern of lesions, and RPD are joint imaging markers for RMD, which is strongly associated with advanced AMD(ie, GA and CNV).10,11 These lesions may be subretinal,12 rather than located on the subretinal pigment epithelium, as in the case of drusen. Their origin is unknown, but their compact, circumscribed, and uniform appearance on imaging modalities suggests a localized vasculopathic or inflammatory/infectious etiology.13 The importance of understanding RMD is underscored by the fact that RPD occur in only 7% to 8% of patients with AMD overall but in as many as 32% of patients with CNV11,13,14 and 21% of patients with GA on color photography,15 but more than 60% on autofluorescece imaging.16 Patients with reticular disease also have a decreased life span compared with other patients with AMD, suggesting a systemic etiology.15 The purpose of this study was to determine if there was an association of high-risk alleles in the CFH (Y402H, rs1061170) and ARMS2 (A69S, rs10490924) genes in patients with the distinct AMD phenotype RMD.
METHODS
PATIENT GROUPS
The Columbia Macular Genetics Study is approved by the institutional review board to examine the genetic basis of AMD. Informed consent was obtained from all subject individuals for participation in the study and for review of study data for publication. Imaging characteristics of RMD have been described in detail previously.11 Briefly, for color fundus or corresponding red-free photographs, RPD manifest as yellow or light interlacing networks of relatively low-contrast lesions ranging from 125 to 250 µm in width, occurring in regular patterns and welldefined domains.
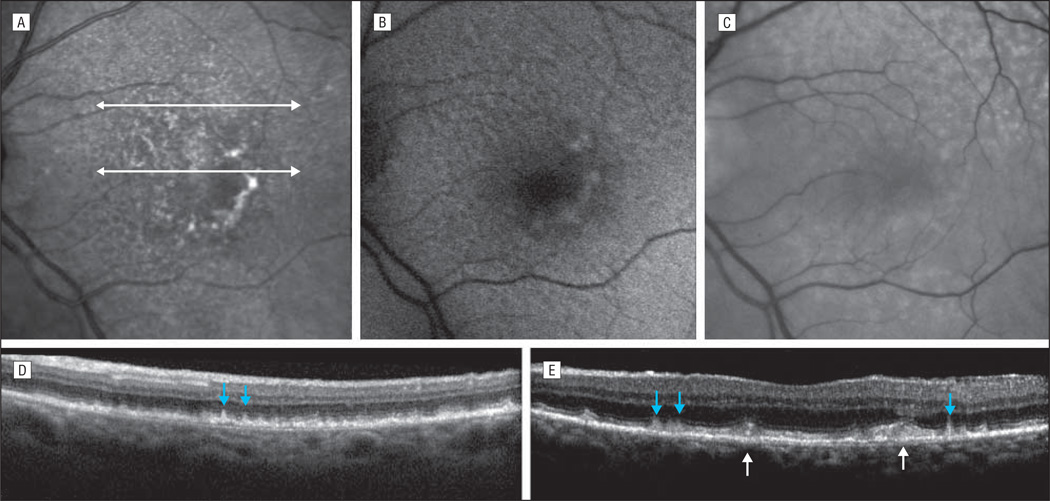
In this study, autofluorescence and infrared images were obtained using a confocal scanning laser ophthalmoscope (SLO) (HRA2/Spectralis; Heidelberg Engineering, Inc, Vista, California).1 For autofluorescence images, this instrument used blue laser light at 488 nm for illumination and a barrier filter at 500 nm. The infrared reflectance images were obtained at 810 nm. Reticular autofluorescence was defined as a grouping of ill-defined, relatively low-contrast hypoautofluorescent lesions against a background of mildly elevated autofluorescence, occurring in regular patterns and well-defined domains. Reticular infrared was defined as groups of hyporeflectant lesions against a background of mild hyperreflectance with analogous characteristics. Reticular macular disease was defined as RPD in color or red-free photography and/or a reticular pattern on SLO imaging (ie, autofluorescence or infrared). The characteristic presentations of RMD on infrared, autofluorescence, and red-free images are illustrated in the Figure, A-C. Spectral domain optical coherence tomography scans reveal the apparent deposits of RPD in the subretinal space and ordinary large soft drusen below the retinal pigment epithelium (Figure, D and E).
Figure.
Macula of a 79-year-old woman from the Columbia Macular Genetics Study with early-stage age-related macular degeneration (ie, large soft drusen) accompanied by reticular macular disease (RMD). A, Near infrared image reveals the characteristic hyporeflectant reticular pattern of RMD in the peripheral macula. The central soft drusen are hyperreflectant. White arrows point to the location of the registered spectral domain optical coherence tomography (SD-OCT) sections. B, The autofluorescence image reveals the characteristic hypoautofluorescent reticular pattern of RMD in the peripheral macula. Some of the central soft drusen are hyperautofluorescent. The quality of the image is slightly decreased by cataract. C, The red-free image demonstrates the characteristic reticular pattern of reticular pseudodrusen (RPD). D and E, The SD-OCT scans reveal the apparent deposits of RPD in the subretinal space (blue arrows) and ordinary large soft drusen below the retinal pigment epithelium (white arrows).
The correct diagnosis of RMD in our subjects—for example, distinguishing RPD from basal laminar drusen (BLD), which are also detected in a honeycomb configuration—was accomplished by multimodal imaging (ie, SLO, autofluorescence, and infrared). We applied the documented autofluorescence characterization of BLD according to Meyerle et al,17 which showed that BLD are tiny dots (30–50 µm) of hypoautofluorescence that correspond exactly on image registration to the hyperfluorescent lesions in the characteristic “starry sky” angiogram of BLD. Hence, without angiography, BLD are easily distinguishable from the much larger lesions of RMD on autofluorescence imaging.
We classified 629 subjects enrolled in the Columbia Macular Genetics Study as having early- or late-stage AMD per the international grading system.18 They underwent image analysis to determine their reticular status. From the initial set, 67 subjects were identified from photographs and/or SLO scans as having RMD by means of grading criteria described in detail previously.11 A patient was defined as having RMD if either eye had RMD. A comparison group consising of 64 ethnicity-, age-, sex-, and clinical stage–matched subjects without RMD was selected from the Columbia Macular Genetics Study. All study subjects were of European American ancestry (ie, white), as determined by a questionnaire detailing their maternal and paternal ethnic origin.
In the group of 67 reticular cases, 14 subjects (20.9%) were men, 53 (79.1%) were women, the mean age was 83 years, 20 (29.9%) had early AMD, and 47 (70.1%) had late AMD (GA and/or CNV). In the nonreticular AMD control group of 64 subjects, 17 (26.6%) were men, 47 (73.4%) were women, the mean age was 80 years, 20 subjects (31.3%) had early AMD, and 44 (68.8%) had late AMD. We could find only 2 subjects with primary GA and without RMD documented by color photography and SLO imaging; therefore, the advanced cases in the subjects with RMD (26 CNV and 21 GA) were not matched to the advanced cases in the subjects without RMD (42 CNV and 2 GA). The mean age in the initial set of 629 subjects was 77 years; also, a preponderance of women was observed in this set (59%), but not as many as in the reticular subgroup. Reticular macular disease appears to be more prevalent with increasing age, in line with the advanced stages of AMD with which it is associated. Possible reasons for the excess of women with RMD are discussed later. The medical data for subjects in the Columbia Macular Genetics Study included smoking history and the presence or absence of angina, myocardial infarction, diabetes, and hypertension.
GENOTYPING
Genomic DNA from all subjects was isolated from peripheral blood leukocytes with DNA blood kits (QIAamp DNA Blood Maxi kits; Qiagen, Valencia, California).2 The DNA samples were screened for haplotype-tagging single-nucleotide polymorphisms in CFH (Y402H, rs1061170) and ARMS2 (A69S, rs10490924). Genotyping was performed by polymerase chain reaction–restriction fragment length polymorphism and by assays (TaqMan; Applied Biosystems, Foster City, California)3 using 5 ng of DNA. The thermal cycling conditions in the 384- well thermocycler (ABI 9700; Applied Biosystems)4 consisted of an initial hold at 95°C for 10 minutes, followed by 50 cycles of a 15-second 95°C denaturation step and a 1-minute 60°C annealing and extension step. Plates were read in a sequence-detection system5 (ABI Prism 7900 HT; Applied Biosystems).
Allele and genotype frequency of the 2 haplotype-tagging single-nucleotide polymorphisms from these loci, tagging the major AMD-associated haplotypes, were characterized in the 2 groups of subjects with AMD—67 subjects with RMD and 64 subjects with AMD but without RMD matched by ethnicity, age, sex, and AMDstage—and were compared with the same data acquired previously on a Columbia AMD group of 368 subjects with latestage AMD (ie, CNV and/or GA) and an ethnicity- and age-matched Columbia group of 368 unaffected individuals.1,19 Allele frequencies for each single-nucleotide polymorphism were compared between each pair of groups. The Yates χ 2 and P values were calculated for double-sided Fisher exact tests, along with the odds ratio (OR) and 95% confidence interval (CI).
RESULTS
The frequencies of the CFH 402H risk allele were 53 of 134 (39.6% in the RMD group and 75 of 128 [58.6%] in the non-RMD group [χ 2=8.8; P=.003; OR, 0.46 {95% CI, 0.28–0.76}]), demonstrating that the 402H AMD risk allele is significantly associated with the absence of RMD among patients with AMD. The corresponding frequencies of the risk allele for ARMS2 were 59 of 134 (44.0%) and 40 of 128 (31.3%) (χ 2=4.0; P=.045; OR, 1.73 [95% CI, 1.04–2.90]), demonstrating an enhanced risk for RMD conferred by the ARMS2 69S risk allele among patients with AMD (Table). The borderline significance of this result may be owing to the relatively small numbers in our groups.
Table.
Allele Frequencies of 2 htSNPs From the 2 AMD-Associated Loci
| MAF, Frequency | Comparisons, OR | |||||||
|---|---|---|---|---|---|---|---|---|
| htSNP | RMD (n=67) |
Non-RMD AMD (n=64) |
AMD (n=368) |
Controls (n=368) |
RMD and Non-RMD AMD |
RMD and All AMD |
RMD and Controls |
AMD and Controls |
| CFH Y402H, rs1061170 | 0.40 | 0.59 | 0.54 | 0.32 | 0.46 | 0.56 | 1.34 | 2.43 |
| P value | NA | NA | NA | NA | .003 | .002 | .13 | <.001 |
| LOC/ARMS2 A69S, rs10490924 | 0.44 | 0.31 | 0.43 | 0.22 | 1.73 | 1.03 | 2.74 | 2.66 |
| P value | NA | NA | NA | NA | .045 | .94 | <.001 | < 001 |
Abbreviations: AMD, age-related macular degeneration; CFH, complement factor H gene; htSNP, haplotype-tagging single-nucleotide polymorphism; LOC/ARMS2, age-related maculopathy susceptibility gene; MAF, minor allele frequency; NA, not applicable; OR, odds ratio calculated by 2 × 2 table; RMD, reticular macular disease.
Homozygosity for 402H was particularly associated with the absence of RMD. Eight of 67 subjects with RMD (11.9%) vs 24 of 64 subjects without RMD (37.5%) were homozygous for 402H (P χ.001). Homozygosity for ARMS2 showed a trend for risk of RMD. Twelve of 67 subjects with RMD (17.9%) vs 7 of 64 subjects without RMD (10.9%) were homozygous for the ARMS2 risk allele (P=.26).
The frequency of the 402H allele in the nonreticular AMD group (75 of 128 [58.6%]) was highly elevated and not significantly different from that in the large (368 latestage cases) Columbia AMD group (54.0%). The frequency of the 402H allele in the reticular AMD group (53 of 134 [39.6%]) was significantly lower than in the Columbia AMD group (53.8%, P=.002) but not significantly different from that in the large (ie, 368 cases) Columbia AMD-unaffected group (32.4%; P=.11). The frequency of the ARMS2 69S risk allele in the nonreticular AMD group (40 of 128 [31.3%]) was significantly lower than in the Columbia AMD group (43.3%; P=.008), but the frequency of the ARMS2 risk allele in the reticular AMD group (59 of 134 [44.0%]) was approximately the same.
As mentioned in the “Methods” section of the text, primary GA without evidence of RMD in any imaging modality was uncommon among our subjects: findings in only 2 such subjects could be documented. The subdivision into categories of advanced AMD was, therefore, necessarily unbalanced between the reticular and nonreticular groups; 2 of 44 (5%) had GA in the nonreticular advanced group and 21 of 47 (45%) had GA in the reticular advanced group. Thus, of all 23 subjects with GA, 2 of 23 (9%) had non-RMD and 21 of 23 (91%) had RMD. Also, the allele frequencies of the subjects with primary GA and RMD were less enriched with Y402H and more enriched with ARMS2 than those of the other subjects with RMD (8 of 21 in the GA group [38.1%] with Y402H vs 19 among 46 other subjects with RMD[41.3%]; 20 of 42 in the GA group [47.6%] with the ARMS2 risk allele vs 39 among 92 other subjects with RMD[42.4%]).
In our series, 55 of 67 subjects with RMD had bilateral disease. Furthermore, all subjects in whom reticular lesions could be found in only 1 eye had CNV in the fellow eye. Significantly more hypertension was observed among men with than without RMD(9 of 14 [64%] vs 4 of 17 [24%], data missing for 2 subjects; P=.03) and trends to more angina among all subjects with than without RMD (10 of 66 [15%] vs 4 of 63 [67%], data missing for 2 patients) and more angina among women with RMD than without RMD (7 of 52 [13%] vs 3 of 47 [6%], data missing for 1 patient). No significant differences were found with respect to smoking history, myocardial infarction, or diabetes.
COMMENT
The common CFH Y402H and ARMS2 A69S variants are well-established risk factors for AMD. Reticular macular disease, a subphenotype of AMD, has independently been shown to be associated with advanced AMD and with the risk of progression to both forms of advanced AMD.10,11,13–15 The increased female to male ratio and increased late-stage disease in subjects with RMD herein were consistent with findings in prior studies.11,15 If RMD has an inflammatory origin (eg, autoimmune disease), then the extraordinarily high proportion of women was consistent with the well-known increased susceptibility of women to autoimmune disease.20 Studies also have postulated other factors, such as the use of female replacement hormone therapy or birth control, to partly explain the higher incidence of AMD in women.21 These factors also might contribute to the higher incidence of RMD in women.
Alternately, if RMD is associated with severe systemic disease, then perhaps men are more severely affected systemically and have a greater mortality rate before reaching the age when AMD is diagnosed. Evidence was observed of an increased frequency of vascular disease among our subjects with RMD, with significantly more hypertension among men with RMD than among men without RMD. Also, trends to more angina were observed among all subjects with RMD and among women with RMD than among those without RMD. In general, if RMD is related to systemic disease, the presentation should tend to be bilateral, consistent with our findings of bilaterality in 55 of 67 subjects. Furthermore, of those 12 subjects in whom reticular lesions could be found in only 1 eye, all had CNV in the fellow eye, consistent with the possibility that RMD could be obscured in such cases. In fact, our prior study of the multimodal imaging of RMD confirmed the fading of reticular findings in the eyes of patients with CNV.11
Previous analysis of a smaller group of subjects (N=32) with reticular disease from the Wisconsin study suggested that the 402H risk allele was enriched in patients with RMD relative to the general population.15 Therefore, it was unexpected to find that the 402H risk allele was significantly associated with the absence of RMD among our group of subjects with AMD. Possible explanations for this apparent discordance might include the following: our imaging modalities for detection of RMD included SLO imaging, which we believe is obligatory for proper RMD diagnosis11 and was not available in the Wisconsin study; no explicit control group existed in the Wisconsin study other than the general population, and no control group was stratified by AMD stage, as in the present study; and the RMD subject numbers in the Wisconsin study are less than half of those in ours. For these reasons, the studies are not directly comparable.
If, in fact, the 402H variant is not associated with RMD, as suggested by the data herein, this result also appears to contradict the hypothesis of RMD as an inflammatory disorder driving advanced AMD, because AMD and inflammation are presumably related through the CFH risk allele. One might have expected that, if reticular lesions were inflammatory in origin, dysregulation of the alternative complement pathway would further exacerbate the consequences of reticular disease, leading to advanced AMD. The purely genetic interpretations of all available data include the following: the CFH gene is not at all associated with RMD one way or the other, and ARMS2, established as a risk factor for RMD herein, conveys most of the risk; a different haplotype of CFH is associated with RMD; and another as-yet undiscovered gene drives RMD. Because specific analytical methods were not used to examine possible population substructure differences between our comparison groups, we cannot eliminate the possibility that our results reflect subtle ethnic differences between these groups (ie, population substructure). However, because the allele frequencies of the 2 variants analyzed in this study (and all other>200 variants studied in these cohorts) correlated very well with all other studies (and the single-nucleotide polymorphism database, available at http://www.ncbi.nlm.nih gov/snp) reporting allele frequencies in cohorts of white ethnic origin, this is unlikely.
We may have to wait for a better understanding of the origin of RMD and its relationship to the alternative complement pathway to derive the correct explanation. However, an intriguing biological hypothesis could be suggested from the results of this study: reticular disease may be infectious in origin, with the alternative pathway being the main line of host defense against the offending pathogen(s). In that case, the alternative complement pathway response, being made more vigorous by the dysregulation of complement inhibition conferred by the 402H variant, could provide the host organism and macula with a more effective defense against the initial attack, with this defense being more important than any subsequently increased level of inflammation. This would account for the paucity of RMD in subjects with the 402H variant. Also, this explanation is in accordance with the population genetic implications of the multiple protective and risk alleles in the CFH region, in particular the hypothesis that 402H has been maintained in evolution as a common polymorphism in populations of European and African origin, because it possibly confers some survival benefit against certain infections or other triggers specific to Europe and Africa.9 Indeed, the decreased life span of patients with RMD, which suggests a systemic disorder, now also could be interpreted as decreased resistance to specific infections because these patients tend to have the “normal” CFH allele. These hypotheses become more plausible when one recalls that an infectious etiology for AMD long has been suspected, with candidate pathogens including Chlamydia pneumoniae, Helicobacter pylori, and cytomegalovirus. 22–25 Perhaps the reason that these studies have been found inconclusive, with other studies finding no relationship, is that a missing link such as RMD has been overlooked until now. We are actively pursuing additional studies of markers of infection and inflammation that might clarify these relationships; real evidence of these hypotheses awaits further work.
In any case, the fact that the frequency of the 402H allele among our patients with RMD was not much greater than the frequency among our AMD-free controls suggests that patients with AMD can be roughly categorized into 2 large groups, reticular and nonreticular, with the former characterized by a near-normal distribution of CFH alleles and the latter enriched with 402H. For those patients with the “normal” CFH allele and RMD, the high risk for advanced AMD conferred by RMD is therefore unlikely to be due to enhanced complement activation by the alternative pathway but rather may be due to some other factor, such as the elevated risk from the ARMS2 locus.
Regarding the ARMS2 risk allele and RMD, because both lead to advanced AMD, with the ARMS2 allele further facilitating RMD as suggested herein, they may share mechanistic features. Further speculation must await more data on the function of the ARMS2 gene and its specific functional role in AMD.
Another striking finding was the fact that primary GA without evidence of RMD on any imaging modality appeared to be uncommon (2 of 23 subjects with GA in this study [9%]). In other words, the frequency of RMD among our subjects with primary GA was high (21 of 23 [91%]), much higher than previously reported based on color photography only (21%).16 In addition, the allele frequencies of the subjects with primary GA and RMD were less enriched with Y402H and more enriched with ARMS2 than those of the other subjects with RMD. The numbers of subjects are small, and we are continuing to collect imaging and genetic data regarding patients with GA to explore the possible significance of these findings.
CONCLUSIONS
We have found evidence of an association of the CFH risk allele 402H with the absence of RMD among patients with AMD and an increased risk for RMD conferred by the ARMS2 risk allele 69S. In addition, the possibility that RMD is associated with systemic disease is bolstered by the consistent trend for more prevalent vascular disease among our subjects with RMD, particularly hypertension and angina. Regardless of the precise etiology of RMD, we have shown evidence that advanced AMD, genetically and phenotypically, may be divided roughly into 2 major diseases, reticular and nonreticular. Reticular macular disease is defined phenotypically by its stereotypical imaging findings, is characterized by a near-normal distribution of the CFH 402H allele, and is highly enriched with the ARMS2 69S allele. Nonreticular AMD, by definition, clinically manifests with soft drusen or any other AMD lesions except those of RMD and is enriched with 402H and 69S. It is therefore probable that the pathways to advanced disease in these 2 groups are somewhat different. Further confirmation of these results in larger groups is warranted. In light of the findings of this article, a search for a systemic etiology of RMD and a reexamination of the hypothesis of an infectious etiology for AMD is suggested.
Acknowledgments
Funding/Support: This study was supported by the New York Community Trust; grants R01 EY015520 (Dr Smith), R24 EY017404 (Dr Allikmets), and R01 EY13435 (Dr Allikmets) from the National Eye Institute; and an unrestricted grant to the Department of Ophthalmology, Harkness Eye Institute, Columbia University, from Research to Prevent Blindness, Inc.
Footnotes
Author Contributions: Dr Smith had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial Disclosure: None reported.
Additional Contributions: Jennifer L. Dalberth, BA, Department of Ophthalmology, Harkness Eye Institute, Columbia University, provided editorial assistance
References
- 1.Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102(20):7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;5720;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308(5720):419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 4.Edwards AO, Ritter R, III, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;5720;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 5.Rivera A, Fisher SA, Fritsche LG, et al. Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk. Hum Mol Genet. 2005;14(21):3227–3236. doi: 10.1093/hmg/ddi353. [DOI] [PubMed] [Google Scholar]
- 6.Jakobsdottir J, Conley YP, Weeks DE, Mah TS, Ferrell RE, Gorin MB. Susceptibility genes for age-related maculopathy on chromosome 10q26. AmJ Hum Genet. 2005;77(3):389–407. doi: 10.1086/444437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leveziel N, Puche N, Richard F, et al. Genotypic influences on severity of exudative age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010;51(5):2620–2625. doi: 10.1167/iovs.09-4423. [DOI] [PubMed] [Google Scholar]
- 8.Sohrab MA, Barile G, Xu L, et al. Association of homozygous high-risk alleles in complement factor H and ARMS2 with the staging of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50:3451. e-abstract. [Google Scholar]
- 9.Hageman GS, Hancox LS, Taiber AJ, et al. AMD Clinical Study Group. Extended haplotypes in the complement factor H (CFH) and CFH-related (CFHR) family of genes protect against age-related macular degeneration: characterization, ethnic distribution and evolutionary implications. Ann Med. 2006;38(8):592–604. [PMC free article] [PubMed] [Google Scholar]
- 10.Arnold JJ, Sarks SH, Killingsworth MC, Sarks JP. Reticular pseudodrusen: a risk factor in age-related maculopathy. Retina. 1995;15(3):183–191. [PubMed] [Google Scholar]
- 11.Smith RT, Sohrab MA, Busuioc M, Barile G. Reticular macular disease. Am J Ophthalmol. 2009;148(5):733–743. doi: 10.1016/j.ajo.2009.06.028. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zweifel SA, Spaide RF, Curcio CA, Malek G, Imamura Y. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology. 2010;117(2):303.e.1–312.e.1. doi: 10.1016/j.ophtha.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Smith RT, Chan JK, Busuoic M, Sivagnanavel V, Bird AC, Chong NV. Autofluorescence characteristics of early, atrophic, and high-risk fellow eyes in agerelated macular degeneration. Invest Ophthalmol Vis Sci. 2006;47(12):5495–5504. doi: 10.1167/iovs.05-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen SY, Dubois L, Tadayoni R, Delahaye-Mazza C, Debibie C, Quentel G. Prevalence of reticular pseudodrusen in age-related macular degeneration with newly diagnosed choroidal neovascularisation. Br J Ophthalmol. 2007;91(3):354–359. doi: 10.1136/bjo.2006.101022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein R, Meuer SM, Knudtson MD, Iyengar SK, Klein BE. The epidemiology of retinal reticular drusen. Am J Ophthalmol. 2008;145(2):317–326. doi: 10.1016/j.ajo.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitz-Valckenberg S, Alten F, Steinberg JS, et al. Reticular drusen associated with geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. doi: 10.1167/iovs.11-7235. [published online April 15, 2011] [DOI] [PubMed] [Google Scholar]
- 17.Meyerle CB, Smith RT, Barbazetto IA, Yannuzzi LA. Autofluorescence of basal laminar drusen. Retina. 2007;27(8):1101–1106. doi: 10.1097/IAE.0b013e3181451617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bird AC, Bressler NM, Bressler SB, et al. The International ARM Epidemiological Study Group. An international classification and grading system for age-related maculopathy and age-related macular degeneration. Surv Ophthalmol. 1995;39(5):367–374. doi: 10.1016/s0039-6257(05)80092-x. [DOI] [PubMed] [Google Scholar]
- 19.Gold B, Merriam JE, Zernant J, et al. AMD Genetics Clinical Study Group. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006;38(4):458–462. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walsh SJ, Rau LM. Autoimmune diseases: a leading cause of death among young and middle-aged women in the United States. Am J Public Health. 2000;90(9):1463–1466. doi: 10.2105/ajph.90.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith W, Mitchell P, Wang JJ. Gender, oestrogen, hormone replacement and age-related macular degeneration: results from the Blue Mountains Eye Study. Aust N Z J Ophthalmol. 1997;25(suppl 1):S13–S15. doi: 10.1111/j.1442-9071.1997.tb01745.x. [DOI] [PubMed] [Google Scholar]
- 22.Guymer R, Robman L. Chlamydia pneumoniae and age-related macular degeneration: a role in pathogenesis or merely a chance association? Clin Experiment Ophthalmol**. 2007;35(1):89–93. doi: 10.1111/j.1442-9071.2006.01392.x. [DOI] [PubMed] [Google Scholar]
- 23.Kalayoglu MV, Bula D, Arroyo J, Gragoudas ES, D’Amico D, Miller JW. Identification of Chlamydia pneumoniae within human choroidal neovascular membranes secondary to age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2005;243(11):1080–1090. doi: 10.1007/s00417-005-1169-y. [DOI] [PubMed] [Google Scholar]
- 24.Miller DM, Espinosa-Heidmann DG, Legra J, et al. The association of prior cytomegalovirus infection with neovascular age-related macular degeneration. Am J Ophthalmol. 2004;138(3):323–328. doi: 10.1016/j.ajo.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 25.Nilsson HO, Pietroiusti A, Gabrielli M, Zocco MA, Gasbarrini G, Gasbarrini A. Helicobacter pylori and extragastric diseases: other Helicobacters. Helicobacter. 2005;10(suppl 1):54–65. doi: 10.1111/j.1523-5378.2005.00334.x. [DOI] [PubMed] [Google Scholar]