Abstract
Skeletal trauma and impaired skeletal healing is commonly associated with diminished vascularity. Hypoxia inducible factor alpha (HIF-1) is a key transcription factor responsible for activating angiogenic factors during development and tissue repair. Small molecule inhibitors of the prolyl hydroxylase enzyme (PHD), the key enzyme responsible for degrading HIF-1, have been shown to activate HIF-1, and are effective in inducing angiogenesis. Here we examined the effects of several commercially available PHD inhibitors on bone marrow mesenchymal stromal cells (MSCs) in vitro and in a stabilized fracture model in vivo. Three PHD inhibitors [Desferrioxamine (DFO), L-mimosine (L-mim), and Dimethyloxalylglycine (DMOG)] effectively activated a HIF-1 target reporter, induced expression of vascular endothelial growth factor (VEGF) mRNA in vitro, and increased capillary sprouting in a functional angiogenesis assay. DFO and DMOG were applied by direct injection at the fracture site in a stabilized murine femur fracture model. PHD inhibition increased the vascularity at 14 days and increased callus size as assessed by microCT at 28 days. These results suggest that HIF activation is a viable approach to increase vascularity and bone formation following skeletal trauma.
Keywords: hypoxia inducible factor, fracture healing, vascularity, prolyl hydroxylase inhibitor
Nonhealing of fractures remains an enormous clinical problem with extremely high costs to individuals and society in suffering, direct health care costs, and indirect costs from lost work and productivity.1 A common feature of fracture nonunions is impaired blood supply related either to the initial injury or to subsequent treatment. Indeed, animal models of non-union are often created by ablation of soft tissue and vessels at the fracture site.2,3 Furthermore, in most nonunion models (as well as in clinical nonunions), hypovascularity or hypoperfusion is observed.4
Because of the recognition of importance of vascular supply to tissue repair in general and to skeletal repair more specifically, studies have focused on pharmacologic interventions to improve blood supply in these settings. Vascular endothelial growth factor (VEGF), which has been used to increase healing of nonunions and critical sized defects in rabbits,2,3,5 has not been successful increasing healing in a standard rabbit distraction model.6 Therapeutic application of VEGF may prove problematic and expensive, because of the need for recombinant protein or gene therapy approaches. As an alternative approach we explored the use of small molecules that target the hypoxia inducible factor (HIF) pathway to activate angiogenesis more globally. HIF-1 is the master regulator of response to low oxygen, and has been shown to be activated in skeletal repair.7 HIFs are transcription factors whose activity is regulated through oxygen-dependent proteolysis of the α subunit.8 HIF-1α is constitutively expressed and rapidly degraded under normoxic conditions. The first step in degradation is hydroxylation of a proline residue by one of three HIF prolyl hydroxylases (PHDs) that requires oxygen, iron, and 2-oxyglutarate as cofactors.9 Under hypoxic conditions, PHDs are inactivated, and HIF-1α accumulates and translocates to the nucleus where it dimerizes with HIF-1α to activate hypoxic responsive elements in the proximal promoter regions of HIF responsive genes. HIF target genes include factors that confer adaptive responses such as metabolic changes (glut1), and increased erythrocyte production (epo), as well as inducing angiogenesis (vegf and associated angiogenic programs).
Small molecule inhibitors of the PHDs can be used to block HIF-1α degradation and thereby activate the HIF pathway. In general, these molecules interfere with the required cofactors for PHDs as either iron chelators [e.g., Desferrioxamine (DFO)] or 2-oxyglutarate analogues [e.g., Dimethyloxalylglycine (DMOG), L-mimosine (L-mim)].10–12 We hypothesized that HIF activation could also be used to increase vascularity in a more generalizable skeletal repair model, healing of stabilized femur fractures. In this article we describe studies that evaluate several available PHD inhibitors in vitro and in vivo. Our results provide proof of principal that PHD inhibitors increase callus formation following fracture by increasing the angiogenic response.
MATERIALS AND METHODS
Animals
All animal studies were conducted under a protocol approved by the Institutional Animal Care and Use Committee. In vitro studies were carried out using bone marrow-derived mesenchymal stromal cells (MSCs) harvested from tibia and femur flushes of 6-week-old C57BL/6 mice (Jackson Labs, Bar Harbor, ME) and subjected to Ficoll column purification.7 Experimental fracture studies were carried out in 8-week-old male C57BL/6 mice.
HRE Luciferase Reporter Assay
A U2OS cell line stably expressing a luciferase reporter plasmid encoding multiple iNOS hypoxia response elements (U2OS-HRE-luc) was obtained by material transfer agreements with Drs. Ashcroft and Melillo.12,13 Confluent monolayers of the U2OS–HREluc cells were treated for 24 h with small-molecule PHD inhibitors or vehicle with triplicate wells at each concentration. Bright-Glo luciferase reagent (Promega, Madison, WI) was added and relative light units were measured in supernatants of cell lysates with a luminometer.12
Western Blotting
Western blotting was performed to evaluate HIF-1α nuclear accumulation in MSCs exposed to DFO (Sigma, St. Louis, MO), L-mim (MP Biomedical, Solon, OH), or DMOG (Frontier Scientific, Logan, UT) for 24 hs. Nuclear extracts were prepared using the NE-PER kit (Pierce, Rockford, IL). Blots were incubated with primary antibody against HIF-1α (mouse monoclonal antihuman, R&D systems, Minneapolis, MN) or TATA box binding protein as a loading control (mouse monoclonal antihuman, Abcam, Cambridge, MA). Secondary antibody (goat antimouse IgG, Cell Signaling Technology, Boston, MA) was applied and visualized using chemilumines-cence (Supersignal West Femto Dura, Pierce, Rockford, IL).
Real-Time Polymerase Chain Reaction (PCR)
Total RNA was extracted with Trizol from MSCs exposed to agents or vehicle for 24 h. Three micrograms of RNA was reverse transcribed into cDNA using Superscript first-strand synthesis system (Invitrogen, Carlsbad, CA). Real-time PCR was performed at 57°C for 30 cycles in the Opticon Continuous Fluorescent Detector by using IQTM SYBR green supermix (Bio-Rad, Hercules, CA). Triplicates were performed for each of three samples, and results were normalized to β-actin. We used the following primers: VEGF-A: F5′-CCACGTCAGA-GAGCAACATCA-3′ and R5′-TCATTCTCTCTATGTGCTGGC-TTT-3′. β-Actin, 5′-CCCAGAGCAAGAGAGG-3′ and 5′-GTCC-AGACGCAGGATG-3′.
Fetal Mouse Metatarsal Angiogenesis Assay
Fetal mouse metatarsals were used in an angiogenesis assay as described previously.14 Briefly, E17.5 embryos were removed from timed-pregnant mice, and metatarsals were dissected, and explanted into tissue culture plates in α-MEM supplemented with 10% heat-inactivated FBS and 1% penicillin/streptomycin for 12 days with replacement of medium every 3 days. They were then exposed to for 24 h to saline, VEGF 10 ng/mL, or PHD inhibitor (DFO 10 or 50 µM, L-M 300 or 500 µM, or DMOG 500 or 700 µM). Explants were then fixed in zinc formalin for 15 min at room temperature and subsequently stained for the endothelial marker cluster of differentiation 31 (CD31) using a monoclonal rat antimouse CD31 antibody (BD Biosciences Pharmingen, San Jose, CA). Cultures were performed in triplicate and each complete experiment was repeated at least three times. Metatarsals that did not adhere to the culture dish or that became dislodged during processing were not used for subsequent analysis. Images were obtained from a Zeiss dissecting microscope at 2× magnification and imported to Image J. The images were then converted to RGB stacks and thresholding was used to measure the pixel area of capillary CD31 staining by a blinded observer (n = 3–6 per group).
Mouse Femur Fracture Model
A stabilized femur fracture model was used as previously described.15 Eight-week-old male mice were anesthetized and an intramedullary pin (25-gauge needle) inserted into the femoral shaft retrograde and a midshaft femur fracture created by manual three point bending. Faxitron imaging was used to confirm correct pin and fracture placement and animals with incorrect pin placement or incorrect fracture (incomplete, comminuted, or metaphyseal location) were euthanized and not used in subsequent analysis. Local injection at the fracture site with 20 µL of DFO (200 µM), DMOG (500 µM) or saline was performed every other day for five doses. Vascularity and callus was assessed at 14 days and bone healing at 28 days postfracture.
MicroCT Imaging
Computed tomography was used to quantify angiogenesis and bone healing using the µCT-40 system (Scanco Medical, Bassersdorf, Switzerland) and related analysis software. MicroCT angiography was performed as previously described.15 The MicroFil™ perfused, harvested, decalcified bones were scanned and the fracture site identified. Scanning was performed at a 12-µm isotropic voxel size and the volume of interest (VOI) was defined as 200 slices on either side of the fracture site. Spatial segmentation of the contrast within the bone was performed and three-dimensional angiograms were reconstructed (n = 3–7 per group). For evaluation of fracture healing at 28 days, the bones were scanned at 12-µm voxel size. A VOI was selected inclusive of 250 slices on either side of the fracture site. A segmentation threshold of 204 was used and direct calculation of bone morphometric parameters was performed (n = 8–11 per group).
Histology
To evaluate bone and cartilage healing, quantitative histomorphometry was performed.16 Femurs were fixed, decalcified, and embedded in paraffin. Transverse sections were made at 500-µm intervals and six sections were examined per specimen centered on the fracture site. Hematoxylin and eosin staining was performed and color match analysis was used to identify bone, cartilage, and void (BioQuant, Nashville, TN). Total callus area (inclusive of original cortex) and area and proportion of cartilage and bone were recorded (n = 3 per group).
Biomechanical Testing
Femurs were collected at 28 days postfracture and fresh frozen. The femora were potted in Wood’s metal in a custom potting apparatus with a gauge length of 6.5 mm. They were loaded in torsion at a rate of 3°/s until failure using an ELF 3200 testing system (Bose, Eden Prairie, MN) as previously described.15 Using the recorded test data, stiffness, yield point, maximum torque, work to failure, rotation at failure, and postyield rotation were determined. Specimens that failed at the potting site rather than the fracture site were excluded from analysis. (DMOG, Saline n = 3, DFO n = 4).
Statistical Analysis
All data is presented as mean±SE. Statistical analysis was performed using SPSS Statistics software (SPSS, Chicago, IL). Nonparametric testing was felt to be most appropriate so the Kruskal-Wallis one-way analysis of variance was used. Each treatment group was then compared to the same control group by Dunnett’s post hoc method. Significance was assigned as p = 0.05.
RESULTS
PHD Inhibitors Induce HIF Activation
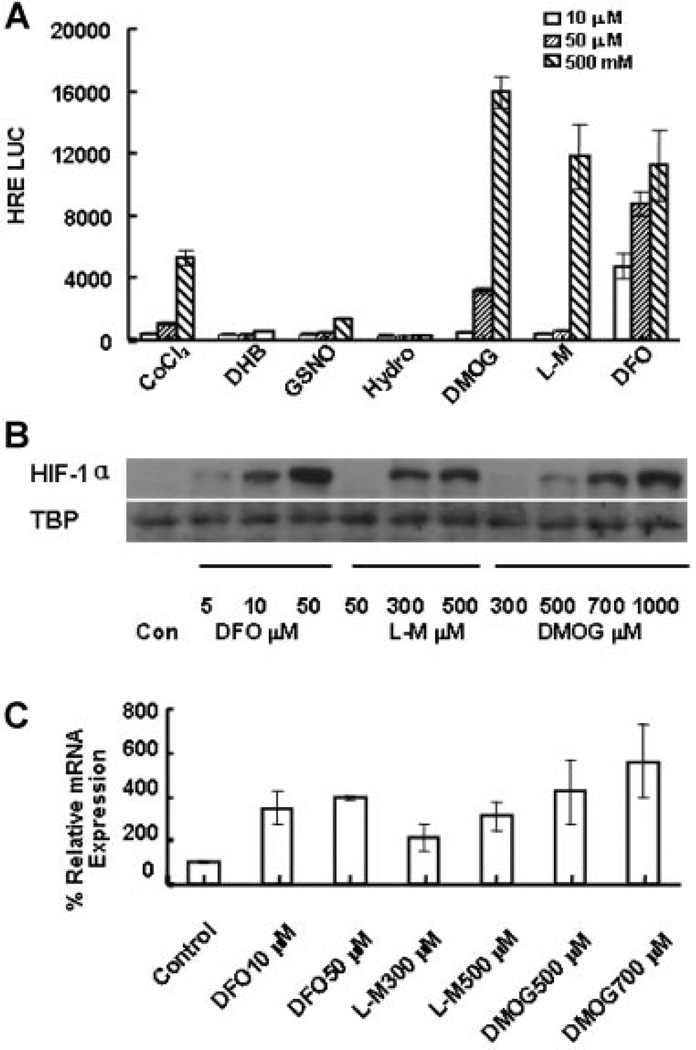
PHD inhibitors were screened for their ability to activate a HIF-responsive construct driving a luciferase reporter in U2OS cells. DMOG, L-mim, and DFO dose-dependently increased reporter activity up to 20- to 50-fold compared to controls (Fig. 1A). These changes in HIF-1 reporter activity were associated with corresponding HIF-1α protein nuclear accumulation by Western blotting (Fig. 1B) and increased VEGF mRNA levels by real-time rt-PCR in mouse MSCs (Fig. 1C).
Figure 1.
Activation of HIF by PHD inhibitors. (A) HRE luciferase assay. Seven known commercially available PHD inhibitors were tested by applying 10, 50, or 500 µM to U2OS HRE-luc reporter cells in culture. (COCl2, Cobalt Chloride; DHB, 3,4-dihydroxybenzoate; GSNO, S-nitrosoglutathione; Hydro, Hydralazine). After 24-h exposure, cells were lysed and luciferase was added. Results of the luminometer readings are shown as arbitrary relative light units. DMOG, L-mim, and DFO strongly induced the HRE reporter. (B) Western blotting for HIF-1α. MSCs were exposed to a range of doses of DFO, L-Mim, or DMOG for 24 h. Nuclear protein was extracted for Western blotting. Primary antibody to HIF-1α was used. TATA Box Binding Protein was used for loading control. Increased HIF-1α nuclear accumulation in a dose-dependent manner is observed for all three compounds. (C) VEGF expression. MSCs were treated with DFO, L-mim, or DMOG for 24 h. RNA was collected and real-time PCR was performed to evaluate VEGF expression relative to β-actin. PHD inhibitor treatment induced three- to sevenfold increased VEGF expression.
PHD Inhibitors Induce Endothelial Sprouting in Metatarsal Explants
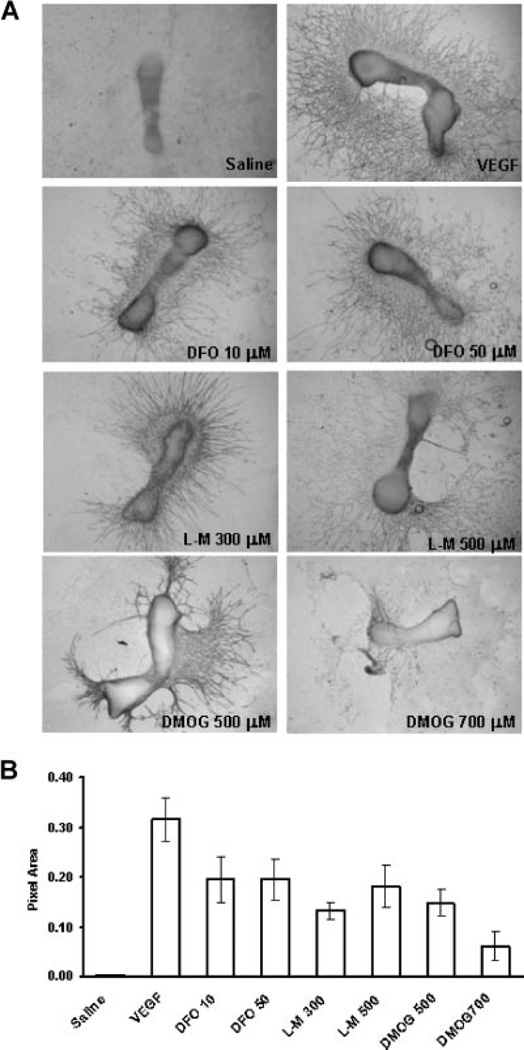
The angiogenic effects of PHDs were next examined in an established mouse metatarsal explant model (Fig. 2A). As a positive control for this assay, VEGF (10 nM) strongly induced endothelial sprouting from the metatarsals compared to vehicle treated controls. DFO, L-mim, and DMOG also enhanced endothelial sprouting. At the 700 µM dose of DMOG, less sprouting was observed and the metatarsals frequently detached from the plate, possibly due to generalized toxicity or effects of PHD inhibition on collagen processing.17 Image analysis of the area of positive CD31 staining showed an increase in capillary sprouting area measured for each of the three drugs (Fig. 2B). These results confirmed that the observed activation of the HIF and VEGF pathways were associated with activation of angiogenic programs.
Figure 2.
PHD inhibitors induce functional angiogenesis. (A) CD31 staining. Metatarsals were dissected from 17.5-day mouse embryos. They were cultured in MEM. PHD inhibitors at the concentrations indicated, saline, or VEGF (10 ng/mL) were applied for 24 h. Subsequent capillary sprouting is assessed by CD31 staining. Robust sprouting is observed for the treatment and positive control groups. (B) Image analysis. Pixel area of capillary sprouts identified by CD31 staining was measured using Image J. The graph represents combined results from three separate experiments.
PHD Inhibitors Increase Angiogenesis and Callus Formation following Femur Fracture
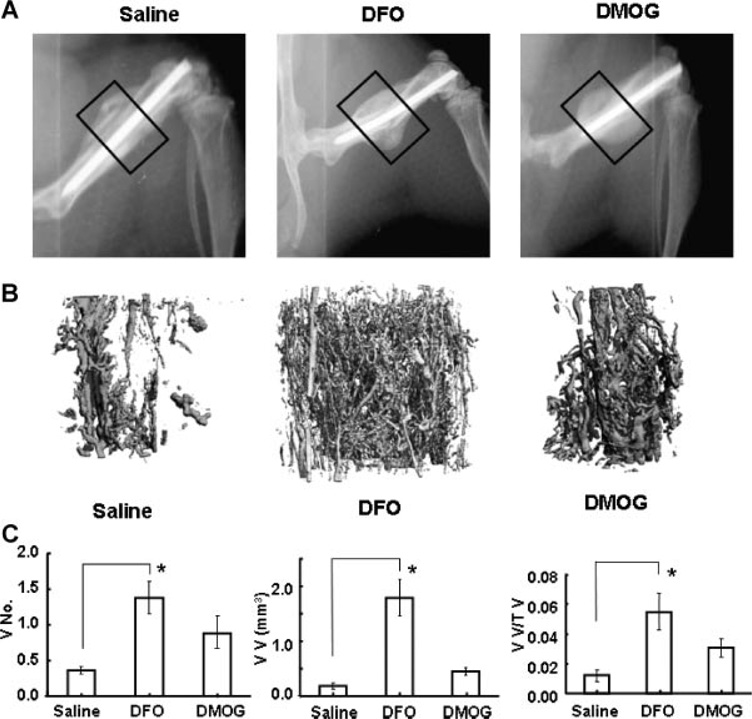
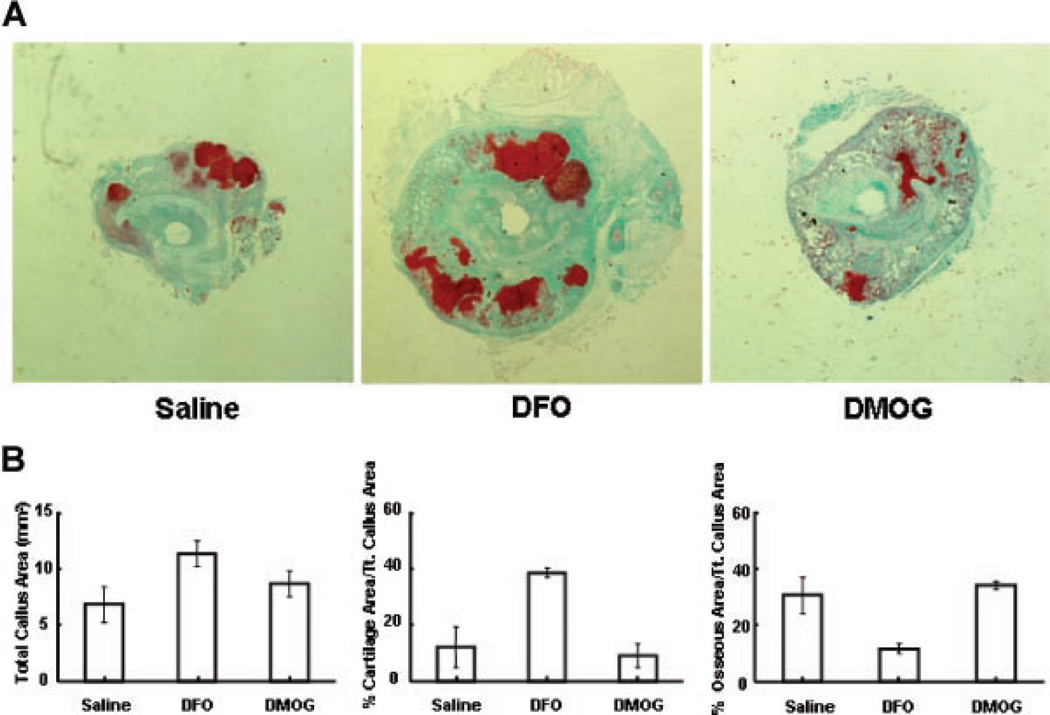
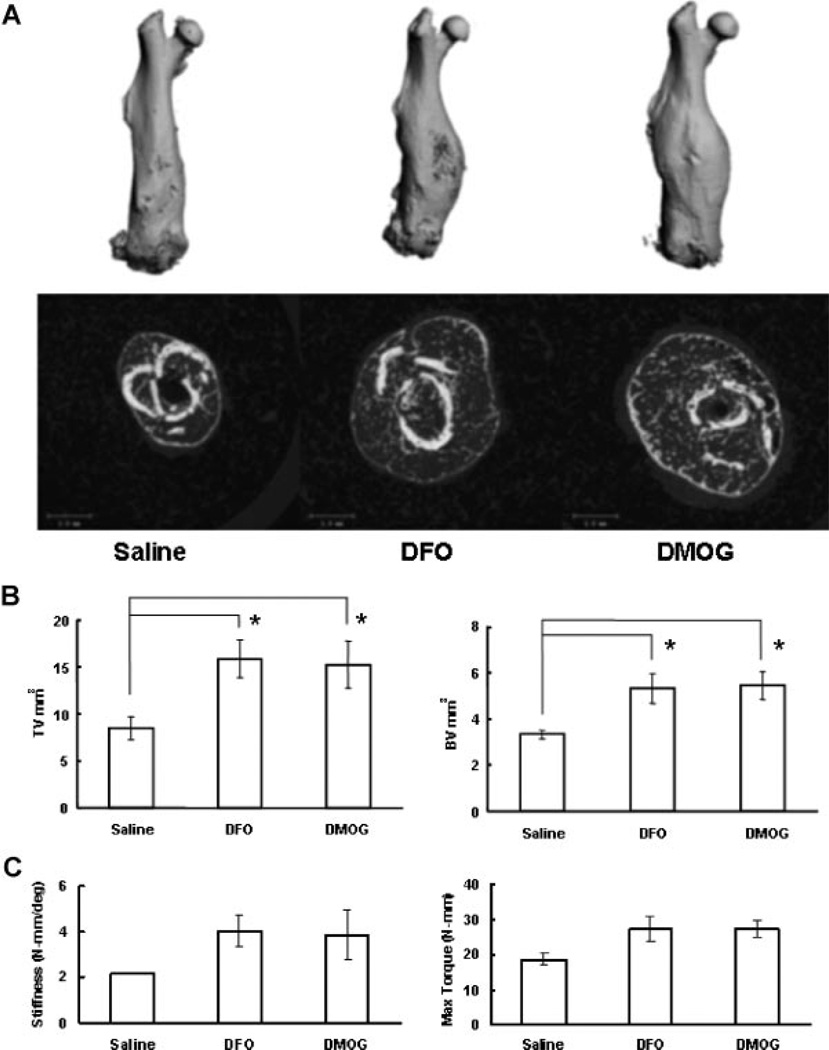
Based on the in vitro studies, we selected DFO and DMOG for in vivo testing in a stabilized femur fracture model. Each drug was administered directly into the fracture site every other day for five doses. Representative faxitron images show callus formation at 2 weeks following fracture (Fig. 3A). Three-dimensional reconstructions of the microCT angiography showed increased vascularity in the treatment groups (Fig. 3B). Quantitative analysis revealed a marked and statistically significant increase in vessel number, vessel volume, and vessel volume per total volume at the fracture site in the DFO group compared to controls. The DMOG group showed a trend toward similar increases but did not reach statistical significance (Fig. 3C). At 14 days following fracture, histomorphometry revealed a trend toward increased callus volume in the treatment groups. Qualitatively, there was increased cartilage in the DFO group compared to saline (Fig. 4A and B). MicroCT scanning at 28 days, showed a marked and statistically significant increase in total volume and bone volume of the fracture callus in both the DFO and DMOG groups compared to the saline group (Fig. 5A and B). No significant difference was seen between the groups with respect to bone volume per total volume or bone mineral density of the fracture callus (data not shown), indicating that the fractures healed with a larger volume of bone of similar radio-graphic quality. Biomechanical testing of the fractured bones after 28 days healing showed a trend toward increased in stiffness and maximum torque in the treatment groups compared to controls (Fig. 5C).
Figure 3.
Local application of PHD inhibitors increases fracture vascularity. (A) Faxitron X-rays. Mice underwent femur fracture with intramedullary stabilization. Saline, DFO, or DMOG was injected locally every other day for five doses. Representative faxitron images at 14 days are shown. The boxed areas represent the region of interest for microCT angiography. (B, C) MicroCT angiography. At 14 days the mice were sacrificed and perfused with silicone lead contrast agent. The bones were decalcified and microCT scanning of the bones was performed at the fracture site. (B) Representative µCT reconstructions are shown. (C) Quantitative analysis of the µCT data showed increased vessel number (VN), vessel volume (VV), and vessel volume per tissue volume (VV/TV) in the treated groups. (Results compared by Kruskal-Wallis followed by post hoc Dunnett’s, *p < 0.05.)
Figure 4.
Effect of local application of PHD inhibitors on early callus formation. (A) Representative histology sections. Mice underwent stabilized femur fracture and were sacrificed at 2 weeks. Representative transverse sections at the fracture site stained with Safranin-O/Fast green are shown (4× magnification). (B) Quantitative fracture histomorphometry. Image analysis software was used to identify callus area. Cartilage and bone was quantified by color matching. Transverse sections were analyzed at 6 levels 500 microns apart centered at the fracture site. Total callus area (mm2), per cent cartilage, and per cent bone for each of the three groups at 14 days is shown. (Results compared by Kruskal-Wallis followed by post hoc Dunnett’s, not statistically significant.)
Figure 5.
Local application of PHD inhibitors increases fracture callus. (A) MicroCT images. Mice were sacrificed at 4 weeks following stabilized femur fracture and analyzed by microCT. Representative full-length reconstructions and cross sections at the fracture site are shown for the saline and treatment groups. Increased callus was noted in both treatment groups. (B) MicroCT data. Quantitative analysis of total volume (TV) and bone volume (BV) are shown graphically. Both total volume and bone volume are increased in the treatment groups. (Results compared by Kruskal-Wallis followed by post hoc Dunnett’s,*p < 0.05.) (C) Bio-mechanical testing. Biomechanical testing was performed on the specimens analyzed by microCT above. Torsional testing was performed at 3°/s to failure. Stiffness and maximum torque were calculated and are shown graphically. (Results compared by Kruskal-Wallis followed by post hoc Dunnett’s, not statistically significant.)
DISCUSSION
In this study, we demonstrate the effectiveness of small molecule inhibitors of PHD to enhance vascularity (Fig. 3) and increase callus formation (Fig. 5) in a stabilized fracture model. Our in vitro results suggest that these effects are due to their ability to increase HIF and VEGF activation (Fig. 1) and to drive vascularization following fracture. Although increased final callus size was noted in the treatment groups, it is difficult to establish improvement of fracture healing in a model in which healing is routinely observed. Our findings are in contrast to Komatsu et al.,18 who found that partial HIF inactivation (global HIF-1α hemizygous) led to increased fracture callus size and a decreased rate of apoptosis in a similar mouse femur fracture model. Although HIF can both positively and negatively impact apoptosis,19 in the present study, the overwhelming effect on vascularity may have abrogated potential negative impact of apoptosis on callus production by either recruitment of additional cells for repair, or restoring supplies of oxygen and nutrients. We have previously shown that HIF activation by PHD inhibition increases vascularity and bone healing in distraction osteogenesis.7 However, distraction osteogenesis is a specialized mode of skeletal regeneration involving predominately intramembranous bone formation,7,20 whereas the stabilized femur fracture model utilized in this study heals mainly by endochondral formation.15 There are significant differences in these two skeletal repair models in terms of local environment, cellular response, and molecular programs activated. One commonality is the stimulation of vascular response via VEGF and associated angiogenic factors.7,21–23 The finding that PHD inhibition also increased vascularity in endochondral ossification suggests utility for a broader range of clinical skeletal repair.
Because of the importance of neoangiogenesis in nonhealing skeletal defects and other wounds, attention has focused on VEGF, an established mediator of the angiogenic cascade. Local VEGF application has been successfully exploited in animal fracture, nonunion, and critical defect models to increase vascularity and bone healing,2,3,24,25 but success has not been universal,6 and use of recombinant proteins is expensive. We believe that our strategy to target PHD inhibition has advantages over VEGF therapy. First, the HIF pathway is upstream of VEGF and so also activates other associated angiogenic programs (e.g., angiopoeitins, tie 1 and tie 2).26,27 Second, HIF is also associated with activation of other response programs that include metabolic adaptation28 and cell recruitment (e.g., stromal derived factor-1).29 Also, the use of a small molecule approach is simpler than strategies requiring recombinant growth factors or gene therapy, avoiding the need for complex delivery systems such as vectors with the associated increased expense and safety concerns.
Previously, we established a link between HIF activation, VEGF upregulation, angiogenesis and increased bone formation.7,14 In the current study, we have reinforced the finding of increased VEGF activation and functional angiogenesis with the in vitro studies. Subsequently, with PHD inhibition, increased vascularity was observed at the healing fracture site in vivo. Although an effect on perfusion (rather than vascularity) at the fracture healing site cannot be completely excluded, the use of perfusion fixation at a uniform pressure prior to angiography should decrease that possibility. The cells responsible for activating the angiogenic programs have not been identified, but our in vitro studies and previous work suggest that MSCs residing in the area of the fracture have the ability to upregulate angiogenic programs. The mechanisms by which angiogenic events may impinge on bone formation programs are also not clear. Some interactions between the VEGF and BMP pathways have been observed by other investigators, but the interactions are complex and depend upon the local environment.24 Additionally, angiogenesis may stimulate bone formation by the recruitment of additional bone modeling units, which increasing evidence suggests are usually associated with an end vessel.14,30,31
A surprising finding was that of qualitatively increased cartilage formation in the fracture callus at 14 days in the DFO group (Fig. 4). A link between HIF activation and initiation of chondrogenesis in a rabbit endochondral bone formation model has been established.32 Additionally, we and others have shown that HIF activation favors chondrogenic differentiation in vitro.33,34 This finding highlights that although the treatment groups have qualitatively similar effects throughout the study, there are differences in the observed magnitudes of the effects when compared to control. This suggests that there may be dose dependency of the desired effect of HIF activation and that DMOG may be much less potent in vivo. Alternately, the differences could be related to the different mechanisms of action by which they inhibit prolyl hydroxylase, which may lead to different unintended treatment effects. For instance, DFO is an iron chelator and so has the potential to impact other pathways with iron-dependent enzymes. One notable example is tartrate resistant acid phosphatase (TRAP),35,36 which could impact bone resorption and contribute to the retention of a large callus. Further, both DFO and DMOG have been shown to induce prostaglandin E synthesis.37–39 Previous studies with DFO have attributed this effect to iron chelation, anti-inflammatory properties, and to free radical reduction. A more recent study with DMOG has proposed direct interaction between the HIF pathway and prostaglandin E2 synthetase.38 The net effects can be quite complex, as prostaglandins have a variety of pro and anti-inflammatory roles, can influence vasoconstriction, and have direct effects on bone and cartilage formation. These possibilities are under further study.
Finally, our findings suggest proof of principal that small molecule inhibitors of the PHDs can be developed to improve vascularity in the setting of long bone fractures. Similar results demonstrating the effectiveness of the PHD inhibitors to increase bone vascularity and bone healing have been recently obtained in distraction osteogenesis. The current studies raise the possibility that these compounds may be more broadly applied in bone injury and tissue regeneration for which vascularity is critical.
ACKNOWLEDGMENTS
This work was supported by NIH/NIAMS (AR 054771) (SG), UAB Orthopaedic Surgery and the John Kirklin Foundation. We wish to thank Angela Lin for assistance with torsional biomechanical testing.
Footnotes
AUTHOR CONTRIBUTIONS
X.S. performed in vitro experiments and fracture experiments. C.W. performed CT analyses and assisted with experimental design. G.R. and A.E. performed biomechanical testing and interpretation and assisted with statistical analysis. Y.W. performed metatarsal angiogenesis experiments. M.M. assisted in development of the fracture model and in manuscript preparation. C.D. and R.G. participated in experimental design, development of the fracture model and microCT angiography techniques, and data analysis. L. D. and T.C. participated in experimental design and data interpretation and manuscript revision. S.G. participated in experimental design, fracture experiments, and manuscript preparation.
REFERENCES
- 1.U.S. Bone and Joint Decade. The burden of musculoskeletal diseases in the United States. Rosemont, IL: AAOS; 2008. p. 268. [Google Scholar]
- 2.Eckardt H, Ding M, Lind M, et al. Recombinant human vascular endothelial growth factor enhances bone healing in an experimental nonunion model. J Bone Joint Surg Br. 2005;87:1434–1438. doi: 10.1302/0301-620X.87B10.16226. [DOI] [PubMed] [Google Scholar]
- 3.Street J, Bao M, deGuzman L, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA. 2002;99:9656–9661. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murnaghan M, Li G, Marsh DR. Nonsteroidal antiinflammatory drug-induced fracture nonunion: an inhibition of angiogenesis? J Bone Joint Surg Am. 2006;88(Suppl 3):140–147. doi: 10.2106/JBJS.F.00454. [DOI] [PubMed] [Google Scholar]
- 5.Li R, Stewart DJ, von Schroeder HP, et al. Effect of cell-based VEGF gene therapy on healing of a segmental bone defect. J Orthop Res. 2008;27:8–14. doi: 10.1002/jor.20658. [DOI] [PubMed] [Google Scholar]
- 6.Eckardt H, Bundgaard KG, Christensen KS, et al. Effects of locally applied vascular endothelial growth factor (VEGF) and VEGF-inhibitor to the rabbit tibia during distraction osteogenesis. J Orthop Res. 2003;21:335–340. doi: 10.1016/S0736-0266(02)00159-6. [DOI] [PubMed] [Google Scholar]
- 7.Wan C, Gilbert SR, Wang Y, et al. Activation of the hypoxia-inducible factor-1alpha pathway accelerates bone regeneration. Proc Natl Acad Sci USA. 2008;105:686–691. doi: 10.1073/pnas.0708474105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-depend-ent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ivan M, Kondo K, Yang H, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 10.Wang GL, Semenza GL. Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: implications for models of hypoxia signal transduction. Blood. 1993;82:3610–3615. [PubMed] [Google Scholar]
- 11.Warnecke C, Griethe W, Weidemann A, et al. Activation of the hypoxia-inducible factor-pathway and stimulation of angiogenesis by application of prolyl hydroxylase inhibitors. Faseb J. 2003;17:1186–1188. doi: 10.1096/fj.02-1062fje. [DOI] [PubMed] [Google Scholar]
- 12.Chau NM, Rogers P, Aherne W, et al. Identification of novel small molecule inhibitors of hypoxia-inducible factor-1 that differentially block hypoxia-inducible factor-1 activity and hypoxia-inducible factor-1alpha induction in response to hypoxic stress and growth factors. Cancer Res. 2005;65:4918–4928. doi: 10.1158/0008-5472.CAN-04-4453. [DOI] [PubMed] [Google Scholar]
- 13.Rapisarda A, Uranchimeg B, Scudiero DA, et al. Identification of small molecule inhibitors of hypoxia-inducible factor 1 transcriptional activation pathway. Cancer Res. 2002;62:4316–4324. [PubMed] [Google Scholar]
- 14.Wang Y, Wan C, Deng L, et al. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest. 2007;117:1616–1626. doi: 10.1172/JCI31581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duvall CL, Taylor WR, Weiss D, et al. Impaired angiogenesis, early callus formation, and late stage remodeling in fracture healing of osteopontin deficient mice. J Bone Miner Res. 2007;22:286–297. doi: 10.1359/jbmr.061103. [DOI] [PubMed] [Google Scholar]
- 16.Gerstenfeld LC, Alkhiary YM, Krall EA, et al. Three-dimensional reconstruction of fracture callus morphogenesis. J Histochem Cytochem. 2006;54:1215–1228. doi: 10.1369/jhc.6A6959.2006. [DOI] [PubMed] [Google Scholar]
- 17.Bickel M, Baringhaus KH, Gerl M, et al. Selective inhibition of hepatic collagen accumulation in experimental liver fibrosis in rats by a new prolyl 4-hydroxylase inhibitor. Hepatology. 1998;28:404–411. doi: 10.1002/hep.510280217. [DOI] [PubMed] [Google Scholar]
- 18.Komatsu DE, Bosch-Marce M, Semenza GL, et al. Enhanced bone regeneration associated with decreased apoptosis in mice with partial HIF-1alpha deficiency. J Bone Miner Res. 2007;22:366–374. doi: 10.1359/JBMR.061207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greijer AE, van der Wall E. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J Clin Pathol. 2004;57:1009–1014. doi: 10.1136/jcp.2003.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ilizarov GA. The tension-stress effect on the genesis and growth of tissues. Part I. The influence of stability of fixation and soft-tissue preservation. Clin Orthop Relat Res. 1989;238:249–281. [PubMed] [Google Scholar]
- 21.Pacicca DM, Patel N, Lee C, et al. Expression of angiogenic factors during distraction osteogenesis. Bone. 2003;33:889–898. doi: 10.1016/j.bone.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Gerstenfeld LC, Cullinane DM, Barnes GL, et al. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873–884. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 23.Ferguson C, Alpern E, Miclau T, et al. Does adult fracture repair recapitulate embryonic skeletal formation? Mech Dev. 1999;87:57–66. doi: 10.1016/s0925-4773(99)00142-2. [DOI] [PubMed] [Google Scholar]
- 24.Peng H, Usas A, Olshanski A, et al. VEGF improves, whereas sFlt1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis. J Bone Miner Res. 2005;20:2017–2027. doi: 10.1359/JBMR.050708. [DOI] [PubMed] [Google Scholar]
- 25.Geiger F, Bertram H, Berger I, et al. Vascular endothelial growth factor gene-activated matrix (VEGF165-GAM) enhances osteogenesis and angiogenesis in large segmental bone defects. J Bone Miner Res. 2005;20:2028–2035. doi: 10.1359/JBMR.050701. [DOI] [PubMed] [Google Scholar]
- 26.Kelly BD, Hackett SF, Hirota K, et al. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a con-stitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93:1074–1081. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- 27.Yamakawa M, Liu LX, Date T, et al. Hypoxia-inducible factor-1 mediates activation of cultured vascular endothelial cells by inducing multiple angiogenic factors. Circ Res. 2003;93:664–673. doi: 10.1161/01.RES.0000093984.48643.D7. [DOI] [PubMed] [Google Scholar]
- 28.Rajpurohit R, Koch CJ, Tao Z, et al. Adaptation of chondrocytes to low oxygen tension: relationship between hypoxia and cellular metabolism. J Cell Physiol. 1996;168:424–432. doi: 10.1002/(SICI)1097-4652(199608)168:2<424::AID-JCP21>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 29.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 30.Dominici M, Pritchard C, Garlits JE, et al. Hematopoietic cells and osteoblasts are derived from a common marrow progenitor after bone marrow transplantation. Proc Natl Acad Sci USA. 2004;101:11761–11766. doi: 10.1073/pnas.0404626101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khosla S, Eghbali-Fatourechi GZ. Circulating cells with osteogenic potential. Ann N Y Acad Sci. 2006;1068:489–497. doi: 10.1196/annals.1346.022. [DOI] [PubMed] [Google Scholar]
- 32.Emans PJ, Spaapen F, Surtel DA, et al. A novel in vivo model to study endochondral bone formation; HIF-1alpha activation and BMP expression. Bone. 2007;40:409–418. doi: 10.1016/j.bone.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Robins JC, Akeno N, Mukherjee A, et al. Hypoxia induces chondrocyte-specific gene expression in mesenchymal cells in association with transcriptional activation of Sox9. Bone. 2005;37:313–322. doi: 10.1016/j.bone.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 34.Kanichai M, Ferguson D, Prendergast PJ, et al. Hypoxia promotes chondrogenesis in rat mesenchymal stem cells: a role for AKT and hypoxia-inducible factor (HIF)-1alpha. J Cell Physiol. 2008;216:708–715. doi: 10.1002/jcp.21446. [DOI] [PubMed] [Google Scholar]
- 35.Alcantara O, Reddy SV, Roodman GD, et al. Transcriptional regulation of the tartrate-resistant acid phosphatase (TRAP) gene by iron. Biochem J. 1994;298(Pt 2):421–425. doi: 10.1042/bj2980421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleckenstein E, Dirks W, Dehmel U, et al. Cloning and characterization of the human tartrate-resistant acid phosphatase (TRAP) gene. Leukemia. 1996;10:637–643. [PubMed] [Google Scholar]
- 37.Camacho M, Lopez-Belmonte J, Vila L. Rate of vasoconstrictor prostanoids released by endothelial cells depends on cyclooxygenase-2 expression and prostaglandin I synthase activity. Circ Res. 1998;83:353–365. doi: 10.1161/01.res.83.4.353. [DOI] [PubMed] [Google Scholar]
- 38.Grimmer C, Pfander D, Swoboda B, et al. Hypoxia-inducible factor 1alpha is involved in the prostaglandin metabolism of osteoarthritic cartilage through up-regulation of microsomal prostaglandin E synthase 1 in articular chondrocytes. Arthritis Rheum. 2007;56:4084–4094. doi: 10.1002/art.23136. [DOI] [PubMed] [Google Scholar]
- 39.Tanji K, Imaizumi T, Matsumiya T, et al. Desferrioxa-mine, an iron chelator, upregulates cyclooxygenase-2 expression and prostaglandin production in a human macrophage cell line. Biochim Biophys Acta. 2001;1530:227–235. doi: 10.1016/s1388-1981(01)00089-0. [DOI] [PubMed] [Google Scholar]