Abstract
The apicoplast and the mitochondrion of Apicomplexa cooperate in providing essential metabolites. Their co-evolution during the ancestral acquisition of a plastid and subsequent loss of photosynthesis resulted in divergent metabolic pathways compared with mammals and plants. This is most evident in their chimerical haem synthesis pathway.
Toxoplasma and Plasmodium mitochondria operate canonical TCA cycles and electron transport chains, although the roles differ between Toxoplasma tachyzoites and Plasmodium erythrocytic stages. Glutamine catabolism provides TCA intermediates in both parasites. Isoprenoid precursor synthesis is the only essential role of the apicoplast in Plasmodium erythrocytic stages. An apicoplast-located fatty acid synthesis is dispensable in these stages, which instead predominantly salvage fatty acids, while in Plasmodium liver stages and in Toxoplasma tachyzoites fatty acid synthesis is an essential role of the plastid.
Introduction
Apicomplexan parasites possess two organelles of endosymbiotic origin: a relict non-photosynthetic plastid (the apicoplast), and a mitochondrion (Figure 1), which together contribute substantially to the parasite's metabolic needs. The apicoplast and mitochondrion show tight physical [1,2] and functional collaboration. A chimerical haem pathway spans both organelles [3]. Apicoplast generated Isopentenyl pyrophosphate (IPP) is likely used in mitochondrion co-enzyme Q synthesis, and finally the Toxoplasma mitochondrion and apicoplast shared a citrate shunt [4].
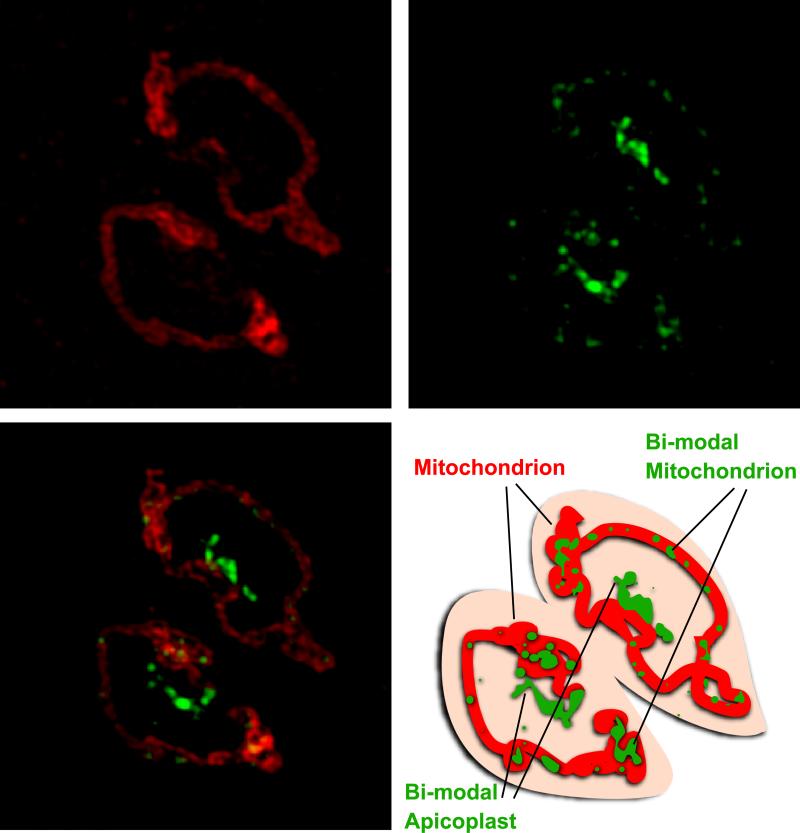
Figure 1. Fluorescence image of the mitochondrion and the apicoplast of Toxoplasma gondii.
The staining of a mitochondrial protein (TGME49_215430, [11], red) that localizes to the organelle periphery (Sheiner, unpublished data) together with a bimodally targeted mitochondrial luminal and apicoplast protein (TGME49_283830, Sheiner unpublished data, green) shows the tight proximity between the two organelles. The co-staining of the mitochondria demonstrates the difference in morphology between the luminal and peripheral compartments. TGME49_283830 (green) represents one of many examples of bimodal targeting between the two organelles. The scheme on the right depicts the outline of the two Toxoplasma tachyzoites. Bar is 1νm.
In accordance with the adaptation of each parasite to its specific host niche, the repertoire of apicoplast and mitochondrion metabolic pathways has diverged between the different phylum members [5]. Here we focus on the unique features of these pathways in Plasmodium and Toxoplasma and review our current understanding of their roles in different host environments.
The apicomplexan mitochondrion
Mammalian cells have varying numbers of mitochondria that divide or fuse based on changing cellular needs, whereas Apicomplexa possess a single mitochondrion whose biogenesis coordinates with the cell-cycle [2]. Transfer of mitochondrial genes to the nucleus has occurred in all eukaryotes, allowing nuclear control over mitochondrial functions (Figure 2). The resulting loss of mitochondrial DNA-encoded genes is extreme in Apicomplexa and dinoflagellates, whose mitochondrial genomes encode only three proteins [6,7]. The organellar proteome is largely imported from the cytosol, presumably through the translocons of the outer and inner mitochondrial membranes (TOM and TIM [8]) as with other eukaryotes. Translation within the Apicomplexa mitochondrion, however, is highly divergent. Extremely fragmented ribosomal RNA genes encode products that need to be assembled into functional ribosomes [9]. No tRNAs are encoded in the mitochondrial genome [6], and no tRNA amino acyl synthetases are targeted to the organelle [10,11], rendering mitochondrial translation dependent on a flow of charged tRNAs from the cytosol, an extremely unusual process. Mitochondria are essential for both Toxoplasma and Plasmodium spp., being the synthetic site for a number of metabolites (reviewed in [5,8]). However, recent data suggests they differ in the composition and importance of their oxidative phosphorylation pathways.
Figure 2. Schematic outline of the acquisition and evolution of the mitochondrion and the apicoplast of Apicomplexa.
(1) Development of a protein import system (purple arrows show flow of proteins from their genomic place of encoding to their subcellular localization) was an important event in the evolution of a mitochondrion in the ancestor of all eukaryotes. This was accompanied by extensive gene transfer to the nuclear genome (green arrows indicate the transfer of genes to another genome or their complete loss). An algal cell (light blue) carrying a plastid (red) then began an endosymbiotic relationship with a protist host. (2) Again protein import systems were established supporting the extensive gene transfer to the nuclear genome and allowing nuclear control over a newly enslaved organelle. A stable collaboration between the two symbionts (blue arrow) drove the loss of some redundant genes (green arrows). (3) A subsequent loss of photosynthesis (green arrow) affected the distribution of tasks, such as haem synthesis, between the two organelles (blue arrow). (4) Finally the two symbionts now present in apicomplexan parasites are synchronized in their biogenesis and are tightly associated, although the biological role of this association remains unclear. (M) mitochondrion, (P) plastid, (N) nucleus, (Nm) nucleomorph.
Oxidative phosphorylation and TCA cycle
Oxidative phosphorylation is a canonical function of eukaryotic mitochondria. Tricarboxylic acid (TCA) cycle reactions are the chief source of electrons that feed the mitochondrial electron transport chain (mtETC), generating a proton gradient used for ATP synthesis by the ATP synthase complex (Figure 3).
Figure 3. TCA and mtETC in Plasmodium erythrocytic stages and in T. gondii tachyzoites.
Pyruvate from glycolysis, glutamate and gamma aminobutyric acid (GABA) from glutamine metabolism all serve as major starting points for the Plasmodium (thin black arrows, and a thin blue arrow representing a putative pathway) and Toxoplasma (thin black and red arrows) TCA cycles. Electrons from the oxidative steps in the cycle are donated to the mtETC (represented as broken arrows). Components of the mtETC are shown in purple with their names noted on the left. The asterisk notes that not all the subunits of the Apicomplexa ATP-synthase are identifiable in their genomes.
Genomic sequencing of Toxoplasma gondii and Plasmodium spp. revealed genes encoding all TCA cycle enzymes, most mtETC components and most ATP synthase complex subunits. Selective inhibition of mtETC leads to parasite demise, establishing the essential nature of these reactions. In Toxoplasma, mtETC inhibition affects ATP synthesis [12], suggesting the presence of oxidative phosphorylation. However, in Plasmodium erythrocytic stages, mtETC contribution to the ATP pool seems minor [13]. Instead, mtETC appears essential for pyrimidine biosynthesis by re-oxidation of ubiquinol, needed for the mitochondrially located dihydroorotate dehydrogenase (DHODH) [14]. While these results suggest that oxidative phosphorylation is not essential for Plasmodium erythrocytic stages, ATP synthase subunits are resistant to genetic disruption in these stages [15].
The Toxoplasma TCA cycle utilizes glucose and glutamine, as judged by stable isotope labeling and metabolomic analysis, and a GABA shunt was noted for entry of glutamine into the cycle [4] (Figure 3). The source of acetyl-CoA for priming the cycle is unclear, since the only known pyruvate dehydrogenase complex resides in the apicoplast [16-18]. Branched-chain keto acid metabolism has been proposed as an alternative source [5].
In Plasmodium, stable isotope labeling and metabolomic analyses initially suggested that TCA metabolism involved a branched architecture bifurcating from 2-oxoglutarate [19]. However, subsequent investigations revealed that products of the seemingly reductive branch originate from uninfected erythrocytes [20] and the initial report was retracted [21]. Highly enriched parasite-infected cells show only conventional oxidative reactions, with 2-oxoglutarate as the entry point (Ke et al. in preparation). Unlike Toxoplasma, glutamine rather than glucose is the major carbon source for the TCA cycle in Plasmodium erythrocytic stages [20]. Genetic disruptions of six TCA cycle enzymes suggest that the TCA cycle is not essential for Plasmodium erythrocytic- or sexual-stage development, but is necessary for mosquito stage development (Ke et al. in preparation). Similarly, in P. berghei, where the mtETC components NADH dehydrogenase [22] and succinate dehydrogenase [23] are dispensable for erythrocytic stages, they are essential for mosquito oocyst formation.
Mitochondrial involvement in cell-death and differentiation
Recent studies link mitochondrial dynamics and autophagy in Toxoplasma [24-26]. Mitochondrial fragmentation was observed in response to both autophagy inhibition [24] and activation [25,26], creating contradictory models where autophagy either controls mitochondrial homeostasis or induces cell death. Interestingly, autophagy-mediating components associate with the apicoplast [27], and overexpression of one of them, TgATG4, results in mitochondrion and apicoplast morphological defects [27], supporting the first model. However, inhibition of autophagy led to prolonged parasite survival under monensin treatment [26] supporting the second model.
The involvement of a mitochondrial DnaK tetratricopeptide repeat protein in tachyzoite-tobradyzoite differentiation was recently proposed, joining several previous studies demonstrating a correlation between reduced mitochondrial activity and stage differentiation [28]. The mechanism remains unknown.
Haem biosynthesis, a mitochondrion/apicoplast collaboration
The genomes of Plasmodium and Toxoplasma encode the complete set of haem synthesis genes [29]. Like most non-photosynthetic organisms, the pathway starts with mitochondrial conversion of glycine into δ-aminolaevulinic acid [30]. However, the cellular localization and phylogenetic origin of the downstream enzymes tell a tale of evolutionary shuffling and rejigging. The next four steps, executed by HemB/C/D/E respectively, take place in the plastid. While HemB/C/D are of plastid origin, HemE originates from the ancestral eukaryotic host cell, an ancestry not reflected by its current place of action [31]. The subsequent steps are executed by a cytosolic HemF, and then by mitochondrial HemY and HemH. Interestingly, the mitochondrial HemY derives from the red-algal ancestor of the apicoplast [32], again a conflict between ancestry and current location. Thus, the pathway wends its way through three compartments, employing enzymes of various ancestral pathways, only to wind up back in the mitochondrial start point (Figure 4). This curious hybrid pathway likely reflects the shifts in the main sites of use for tetrapyroles following the acquisition and subsequent loss of photosynthesis [3].
Figure 4. A chimerical haem biosynthesis pathway in Apicomplexa.
The acquisition of photosynthesis and then its subsequent loss resulted in shifts as to which compartment was the main user of tetrapyrroles in the cell, and with it the location of principal responsibility for synthesis. The resulting pathway is distributed between the mitochondrion (orange), cytosol (gray) and apicoplast (red). Similarly, the enzymes involved are of different origins within the original endosymbiont [31]: either the red-algal plastid (pink) or cytoplasm (gray).
The apicoplast
A common ancestor of Apicomplexa and dinoflagellates engulfed a red alga, which underwent reduction to become a secondary plastid (Figure 2). Most dinoflagellates maintained a photosynthetic plastid, unlike the apicomplexan plastid – the apicoplast – which lost photosynthesis. The apicoplast now supports three essential metabolic functions: the synthesis of haem (above), type II fatty acids, and isoprenoid precursors.
Type II fatty acid synthesis (FASII)
Fatty acids are a core component of cellular membranes and of essential prosthetic groups [33]. De novo fatty acid synthesis occurs either via fatty acid synthesis pathway I (FASI), typically found in animals and fungi and executed by a cytosolic multi-domain polypeptide, or via FASII, which depends on several individual enzymes and is more common in prokaryotes and plastids.
Both the Toxoplasma and Plasmodium genomes encode complete sets of FASII enzymes [34], and several kinetic, structural and pharmacological studies support the roles of the corresponding proteins in FASII (reviewed in [30,35]). However, FASII was apparently lost by some Apicomplexa [36], and its importance for parasite survival differs between genera and life stages. Genetic evidence indicates that FASII is essential for the growth of Toxoplasma tachyzoites and Plasmodium liver stages but not erythrocytic or mosquito stages [37,38]. This suggests that the importance of FASII depends on the host cell or tissue environment. A recent study using lipidomics and uracyl incorporation in Plasmodium asexual stages suggests that the biogenesis of the apicoplast, and potentially other organelles, depend on salvaged precursors rather than de novo fatty acid synthesis in these stages [39].
The loss of lipoylation of plastid pyruvate dehydrogenase observed with both pharmacological [16] and genetic [40] disruption of Toxoplasma FASII had suggested that FASII supplies only specialized apicoplast lipids. However, a recent study combining metabolomic and genetic analyses indicated that most (60-80%) myristic and palmitic acids in Toxoplasma originate from FASII activity [41], making the apicoplast a significant source of cellular fatty acids. The remaining 20-40% are presumably derived from other sources, perhaps including the homolog of the multifunctional FASI enzyme found in the Toxoplasma genome, a potential remnant of its pre-photosynthetic ancestor. There is also clear evidence for lipid salvage from the host [39,42], and it appears that the contributions of de novo synthesis and salvage vary depending on circumstances. This flexibility perhaps facilitates the transition of parasites through different types of host cell during their complex life cycle.
Isoprenoid precursor biosynthesis
Isoprenoids are derivates of isopentenyl pyrophosphate (IPP) or of its isomer dimethylallyl pyrophosphate (DMAPP). Apicomplexans possess the 1-deoxy-D-xylulose-5-phosphate (DOXP) pathway for IPP synthesis [29,43], which is found mainly in eubacteria and plastids, and lack the alternative mevalonate pathway found in the cytosols of plant, animal and fungal cells.
Plasmodium spp. are sensitive to fosmidomycin [43], an inhibitor with two potential targets in the DOXP pathway [44]. Yeh and DeRisi showed that IPP can negate the effect of fosmidomycin, reinforcing the drug's specificity [45]. Moreover, plastid-less P. falciparum blood stages can be propagated in the presence of exogenous IPP, implicating the DOXP pathway as the only essential apicoplast function in Plasmodium erythrocytic stages [45]. Nair and coworkers used genetic approaches to confirm that the DOXP pathway is essential in Toxoplasma, although fosmidomycin showed little or no effect on tachyzoite growth [46]. Expressing a bacterial fosmidomycin transporter rendered Toxoplasma fully susceptible to fosmidomycin, suggesting that drug accessibility dictates sensitivity in this case [46]. In an independent study, Baumeister and coworkers reached a similar conclusion but suggest the barrier to drug entry is the host-cell rather than the parasite membranes [47].
The end uses of parasite-synthesized IPP are becoming clearer. Potential products include membrane anchors for dolichols in the ER glycosylation machinery and for ubiquinone in the mtETC. IPPs are also precursors of the prenyl tails of a range of C-terminally prenylated proteins such as Rabs [48], which are common in both Toxoplasma and Plasmodium.
Concluding remarks
The endosymbiotic organelles of Apicomplexa are crucial for parasite survival in different host settings during their complex life cycle. Studies combining metabolomics and genetic approaches have exposed interesting differences between Plasmodium and Toxoplasma in the roles of certain pathways. While genetic studies suggest that the TCA cycle is dispensable for Plasmodium erythrocytic stages, pharmacological evidence supports an essential role in Toxoplasma tachyzoite growth [4].
Similarly, the apicoplast FASII pathway is essential in Toxoplasma tachyzoites but dispensable in Plasmodium erythrocytic stages, where IPP precursor synthesis is the only essential function.
These differences may reflect the specialist versus generalist strategies adopted by Plasmodium and Toxoplasma. Malaria parasites appear to rely less on organelle metabolism in erythrocyte stages. Conversely, Toxoplasma tachyzoites, which can parasitize a large range of host cells, salvage less from their host and are more dependent on self production. Another explanation might be related to the different properties of erythrocytes and nucleated cells. This is supported by the importance of the FASII pathway in Plasmodium liver stages and the dependence of mosquito stages on an active TCA cycle – both findings are similar to those in Toxoplasma tachyzoites.
In contrast to our growing understanding of the apicoplast and mitochondrion metabolic roles, their biogenesis is currently understudied. Insights into apicoplast protein import [49-52] and division [53,54] are beginning to accumulate, pioneering this important aspect of organellar biology. Unbiased strategies are being developed aimed at enlarging the repertoire of known apicoplast proteins [55] and isolating apicoplast enriched fractions [39]. A lipidomics study performed with isolated Plasmodium asexual stage apicoplasts revealed that the majority of lipids incorporated in the apicoplast membranes are likely of host rather than algal origin [39]. The relative contribution of de novo synthesis and salvage pathways to the biogenesis of the apicoplast in Toxoplasma is yet to be established.
Apicomplexan mitochondrial biogenesis is an even more neglected area of research. Its tight association with the apicoplast has impaired the attempts to address this question. The establishment of biogenesis mutants for both organelles ([49-55] and Sheiner, unpublished) paves the way to develop strategies based on breaking their association and isolating each organelle for its separate analysis.
Highlights.
Toxoplasma and Plasmodium posses two organelles of endosymbiotic origin: the apicoplast, and the mitochondrion.
The mitochondrion hosts a complete TCA cycle and an electron transport chain lacking complex I. Glutamine catabolism contributes to the TCA cycle.
Haem biosynthesis is an extremely unusual chimerical pathway shared between the two organelles and the cytoplasm.
Isoprenoid precursor synthesis and fatty acid synthesis are essential roles of the apicoplast. Different life stages show differential dependencies on these pathways.
Acknowledgment
We thank Boris Striepen and Muthugapatti Kandasamy for access and assistance in utilizing a Zeiss ELYRA S1 (SR-SIM) for super resolution microscopy. LS is supported by an NIH pathway to independence award (K99-AI103032). ABV is supported by NIH grants (R01-AI028398, R01-AI098413 and R56-AI100569). GMcF is supported by the Australian Research Council and a Program Grant from the National Health and Medical Research Council.
Abbreviations
- mtETC
mitochondrial electron transport chain
- TCA
tricarboxylic acid
- FAS
fatty acid synthesis
- DOXP
1-deoxy-D-xylulose-5-phosphate
- IPP
isopentenyl pyrophosphate
- DHODH
dihydroorotate dehydrogenase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kobayashi T, Sato S, Takamiya S, Komaki-Yasuda K, Yano K, Hirata A, Onitsuka I, Hata M, Mi-ichi F, Tanaka T, et al. Mitochondria and apicoplast of Plasmodium falciparum: behaviour on subcellular fractionation and the implication. Mitochondrion. 2007;7:125–132. doi: 10.1016/j.mito.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 2.Nishi M, Hu K, Murray JM, Roos DS. Organellar dynamics during the cell cycle of Toxoplasma gondii. J Cell Sci. 2008;121:1559–1568. doi: 10.1242/jcs.021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Dooren GG, Kennedy AT, McFadden GI. The use and abuse of heme in apicomplexan parasites. Antioxid Redox Signal. 2012;17:634–656. doi: 10.1089/ars.2012.4539. [DOI] [PubMed] [Google Scholar]
- 4**.Macrae JI, Sheiner L, Nahid A, Tonkin C, Striepen B, McConville MJ. Mitochondrial Metabolism of Glucose and Glutamine Is Required for Intracellular Growth of Toxoplasma gondii. Cell Host Microbe. 2012;12:682–692. doi: 10.1016/j.chom.2012.09.013. [This study demonstrate directly that the TCA cycle is fully active in Toxoplasma tachyzoites and discovers a GABA shunt as a part of their metabolic network.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seeber F, Limenitakis J, Soldati-Favre D. Apicomplexan mitochondrial metabolism: a story of gains, losses and retentions. Trends Parasitol. 2008;24:468–478. doi: 10.1016/j.pt.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Vaidya AB, Akella R, Suplick K. Sequences similar to genes for two mitochondrial proteins and portions of ribosomal RNA in tandemly arrayed 6-kilobase-pair DNA of a malarial parasite. Mol Biochem Parasitol. 1989;35:97–107. doi: 10.1016/0166-6851(89)90112-6. [DOI] [PubMed] [Google Scholar]
- 7.Vaidya AB, Mather MW. Mitochondrial evolution and functions in malaria parasites. Annu Rev Microbiol. 2009;63:249–267. doi: 10.1146/annurev.micro.091208.073424. [DOI] [PubMed] [Google Scholar]
- 8.van Dooren GG, Stimmler LM, McFadden GI. Metabolic maps and functions of the Plasmodium mitochondrion. FEMS Microbiol Rev. 2006;30:596–630. doi: 10.1111/j.1574-6976.2006.00027.x. [DOI] [PubMed] [Google Scholar]
- 9.Feagin JE, Harrell MI, Lee JC, Coe KJ, Sands BH, Cannone JJ, Tami G, Schnare MN, Gutell RR. The fragmented mitochondrial ribosomal RNAs of Plasmodium falciparum. PLoS One. 2012;7:e38320. doi: 10.1371/journal.pone.0038320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson KE, Pham JS, Kwek M, De Silva NS, Allen SM, Goodman CD, McFadden GI, de Pouplana LR, Ralph SA. Dual targeting of aminoacyltRNA synthetases to the apicoplast and cytosol in Plasmodium falciparum. Int J Parasitol. 2011;42:177–186. doi: 10.1016/j.ijpara.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Pino P, Aeby E, Foth BJ, Sheiner L, Soldati T, Schneider A, Soldati-Favre D. Mitochondrial translation in absence of local tRNA aminoacylation and methionyl tRNA Met formylation in Apicomplexa. Mol Microbiol. 2010;76:706–718. doi: 10.1111/j.1365-2958.2010.07128.x. [DOI] [PubMed] [Google Scholar]
- 12.Lin SS, Gross U, Bohne W. Type II NADH dehydrogenase inhibitor 1-hydroxy-2-dodecyl-4(1H)quinolone leads to collapse of mitochondrial inner-membrane potential and ATP depletion in Toxoplasma gondii. Eukaryot Cell. 2009;8:877–887. doi: 10.1128/EC.00381-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fry M, Webb E, Pudney M. Effect of mitochondrial inhibitors on adenosinetriphosphate levels in Plasmodium falciparum. Comp Biochem Physiol B. 1990;96:775–782. doi: 10.1016/0305-0491(90)90230-q. [DOI] [PubMed] [Google Scholar]
- 14.Painter HJ, Morrisey JM, Mather MW, Vaidya AB. Specific role of mitochondrial electron transport in blood-stage Plasmodium falciparum. Nature. 2007;446:88–91. doi: 10.1038/nature05572. [DOI] [PubMed] [Google Scholar]
- 15.Balabaskaran Nina P, Morrisey JM, Ganesan SM, Ke H, Pershing AM, Mather MW, Vaidya AB. ATP synthase complex of Plasmodium falciparum:dimeric assembly in mitochondrial membranes and resistance to genetic disruption. J Biol Chem. 2011;286:41312–41322. doi: 10.1074/jbc.M111.290973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crawford MJ, Thomsen-Zieger N, Ray M, Schachtner J, Roos DS, Seeber F. Toxoplasma gondii scavenges host-derived lipoic acid despite its de novo synthesis in the apicoplast. EMBO J. 2006;25:3214–3222. doi: 10.1038/sj.emboj.7601189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleige T, Fischer K, Ferguson DJ, Gross U, Bohne W. Carbohydrate metabolism in the Toxoplasma gondii apicoplast: localization of three glycolytic isoenzymes, the single pyruvate dehydrogenase complex, and a plastid phosphate translocator. Eukaryot Cell. 2007;6:984–996. doi: 10.1128/EC.00061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foth BJ, Stimmler LM, Handman E, Crabb BS, Hodder AN, McFadden GI. The malaria parasite Plasmodium falciparum has only one pyruvate dehydrogenase complex, which is located in the apicoplast. Mol Microbiol. 2005;55:39–53. doi: 10.1111/j.1365-2958.2004.04407.x. [DOI] [PubMed] [Google Scholar]
- 19.Olszewski KL, Mather MW, Morrisey JM, Garcia BA, Vaidya AB, Rabinowitz JD, Llinas M. Branched tricarboxylic acid metabolism in Plasmodium falciparum. Nature. 2010;466:774–778. doi: 10.1038/nature09301. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Macrae JI, Dixon MW, Dearnley MK, Chua HH, Chambers JM, Kenny S, Bottova I, Tilley L, McConville MJ. Mitochondrial metabolism of sexual and asexual blood stages of the malaria parasite Plasmodium falciparum. BMC Biol. 2013;11:67. doi: 10.1186/1741-7007-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olszewski KL, Mather MW, Morrisey JM, Garcia BA, Vaidya AB, Rabinowitz JD, Llinas M. Retraction: Branched tricarboxylic acid metabolism in Plasmodium falciparum. Nature. 2013;497:652. doi: 10.1038/nature12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boysen KE, Matuschewski K. Arrested oocyst maturation in Plasmodium parasites lacking type II NADH:ubiquinone dehydrogenase. J Biol Chem. 2011;286:32661–32671. doi: 10.1074/jbc.M111.269399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hino A, Hirai M, Tanaka TQ, Watanabe Y, Matsuoka H, Kita K. Critical roles of the mitochondrial complex II in oocyst formation of rodent malaria parasite Plasmodium berghei. J Biochem. 2012;152:259–268. doi: 10.1093/jb/mvs058. [DOI] [PubMed] [Google Scholar]
- 24*.Besteiro S, Brooks CF, Striepen B, Dubremetz JF. Autophagy protein Atg3 is essential for maintaining mitochondrial integrity and for normal intracellular development of Toxoplasma gondii tachyzoites. PLoS Pathog. 2011;7:e1002416. doi: 10.1371/journal.ppat.1002416. [This is the first demonstration of autophagy in Toxoplasma.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghosh D, Walton JL, Roepe PD, Sinai AP. Autophagy is a cell death mechanism in Toxoplasma gondii. Cell Microbiol. 2012;14:589–607. doi: 10.1111/j.1462-5822.2011.01745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavine MD, Arrizabalaga G. Analysis of monensin sensitivity in Toxoplasma gondii reveals autophagy as a mechanism for drug induced death. PLoS One. 2012;7:e42107. doi: 10.1371/journal.pone.0042107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong-Hap MA, Mouammine A, Daher W, Berry L, Lebrun M, Dubremetz JF, Besteiro S. Regulation of ATG8 membrane association by ATG4 in the parasitic protist Toxoplasma gondii. Autophagy. 2013;9 doi: 10.4161/auto.25189. [DOI] [PubMed] [Google Scholar]
- 28.Ueno A, Dautu G, Haga K, Munyaka B, Carmen G, Kobayashi Y, Igarashi M. Toxoplasma gondii: a bradyzoite-specific DnaK-tetratricopeptide repeat (DnaK-TPR) protein interacts with p23 co-chaperone protein. Exp Parasitol. 2011;127:795–803. doi: 10.1016/j.exppara.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 29.Ralph SA, van Dooren GG, Waller RF, Crawford MJ, Fraunholz MJ, Foth BJ, Tonkin CJ, Roos DS, McFadden GI. Tropical infectious diseases: metabolic maps and functions of the Plasmodium falciparum apicoplast. Nat Rev Microbiol. 2004;2:203–216. doi: 10.1038/nrmicro843. [DOI] [PubMed] [Google Scholar]
- 30.Surolia N, Padmanaban G. de novo biosynthesis of heme offers a new chemotherapeutic target in the human malarial parasite. Biochem Biophys Res Commun. 1992;187:744–750. doi: 10.1016/0006-291x(92)91258-r. [DOI] [PubMed] [Google Scholar]
- 31*.Koreny L, Obornik M, Lukes J. Make it, take it, or leave it: heme metabolism of parasites. PLoS Pathog. 2013;9:e1003088. doi: 10.1371/journal.ppat.1003088. [A concise summary of the evolution of the haem biosynthesis pathway in Apicomplexa and their photosynthetic relatives.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koreny L, Sobotka R, Janouskovec J, Keeling PJ, Obornik M. Tetrapyrrole synthesis of photosynthetic chromerids is likely homologous to the unusual pathway of apicomplexan parasites. Plant Cell. 2011;23:3454–3462. doi: 10.1105/tpc.111.089102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frenal K, Polonais V, Marq JB, Stratmann R, Limenitakis J, Soldati-Favre D. Functional dissection of the apicomplexan glideosome molecular architecture. Cell Host Microbe. 2010;8:343–357. doi: 10.1016/j.chom.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Seeber F. Soldati-Favre D: Metabolic pathways in the apicoplast of apicomplexa. Int Rev Cell Mol Biol. 2010;281:161–228. doi: 10.1016/S1937-6448(10)81005-6. [DOI] [PubMed] [Google Scholar]
- 35.Mazumdar J, Striepen B. Make it or take it: fatty acid metabolism of apicomplexan parasites. Eukaryot Cell. 2007;6:1727–1735. doi: 10.1128/EC.00255-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleige T, Limenitakis J, Soldati-Favre D. Apicoplast: keep it or leave it. Microbes Infect. 2010;12:253–262. doi: 10.1016/j.micinf.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 37.Vaughan AM, O'Neill MT, Tarun AS, Camargo N, Phuong TM, Aly AS, Cowman AF, Kappe SH. Type II fatty acid synthesis is essential only for malaria parasite late liver stage development. Cell Microbiol. 2009;11:506–520. doi: 10.1111/j.1462-5822.2008.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu M, Kumar TR, Nkrumah LJ, Coppi A, Retzlaff S, Li CD, Kelly BJ, Moura PA, Lakshmanan V, Freundlich JS, et al. The fatty acid biosynthesis enzyme FabI plays a key role in the development of liver-stage malarial parasites. Cell Host Microbe. 2008;4:567–578. doi: 10.1016/j.chom.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39**.Botte CY, Yamaryo-Botte Y, Rupasinghe TW, Mullin KA, Macrae JI, Spurck TP, Kalanon M, Shears MJ, Coppel RL, Crellin PK, et al. Atypical lipid composition in the purified relict plastid (apicoplast) of malaria parasites. Proc Natl Acad Sci U S A. 2013;110:7506–7511. doi: 10.1073/pnas.1301251110. [This study establishes apicoplats purification for the first time and describes its surprising lipid composition that is mainly of host origin.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mazumdar J, E HW, Masek K, C AH, Striepen B. Apicoplast fatty acid synthesis is essential for organelle biogenesis and parasite survival in Toxoplasma gondii. Proc Natl Acad Sci U S A. 2006;103:13192–13197. doi: 10.1073/pnas.0603391103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41*.Ramakrishnan S, Docampo MD, Macrae JI, Pujol FM, Brooks CF, van Dooren GG, Hiltunen JK, Kastaniotis AJ, McConville MJ, Striepen B. Apicoplast and endoplasmic reticulum cooperate in fatty acid biosynthesis in apicomplexan parasite Toxoplasma gondii. J Biol Chem. 2012;287:4957–4971. doi: 10.1074/jbc.M111.310144. [A thorough analysis, using both genetics and metabolomics, of the sources of cellular lipids in Toxoplasma tachyzoites.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.oppens I. Contribution of host lipids to Toxoplasma pathogenesis. Cell Microbiol. 2006;8:1–9. doi: 10.1111/j.1462-5822.2005.00647.x. [DOI] [PubMed] [Google Scholar]
- 43.Jomaa H, Wiesner J, Sanderbrand S, Altincicek B, Weidemeyer C, Hintz M, Turbachova I, Eberl M, Zeidler J, Lichtenthaler HK, et al. Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science. 1999;285:1573–1576. doi: 10.1126/science.285.5433.1573. [DOI] [PubMed] [Google Scholar]
- 44.Zhang B, Watts KM, Hodge D, Kemp LM, Hunstad DA, Hicks LM, Odom AR. A second target of the antimalarial and antibacterial agent fosmidomycin revealed by cellular metabolic profiling. Biochemistry. 2011;50:3570–3577. doi: 10.1021/bi200113y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45**.Yeh E, DeRisi JL. Chemical rescue of malaria parasites lacking an apicoplast defines organelle function in blood-stage Plasmodium falciparum. PLoS Biol. 2011;9:e1001138. doi: 10.1371/journal.pbio.1001138. [Through a clever complementation approach this study pinpoints the only essential role of the apicoplast in blood stages Plasmodium.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nair SC, Brooks CF, Goodman CD, Strurm A, McFadden GI, Sundriyal S, Anglin JL, Song Y, Moreno SN, Striepen B. Apicoplast isoprenoid precursor synthesis and the molecular basis of fosmidomycin resistance in Toxoplasma gondii. J Exp Med. 2011;208:1547–1559. doi: 10.1084/jem.20110039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baumeister S, Wiesner J, Reichenberg A, Hintz M, Bietz S, Harb OS, Roos DS, Kordes M, Friesen J, Matuschewski K, et al. Fosmidomycin uptake into Plasmodium and Babesia-infected erythrocytes is facilitated by parasite-induced new permeability pathways. PLoS One. 2011;6:e19334. doi: 10.1371/journal.pone.0019334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Howe R, Kelly M, Jimah J, Hodge D, Odom AR. Isoprenoid biosynthesis inhibition disrupts Rab5 localization and food vacuolar integrity in Plasmodium falciparum. Eukaryot Cell. 2013;12:215–223. doi: 10.1128/EC.00073-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agrawal S, van Dooren GG, Beatty WL, Striepen B. Genetic evidence that an endosymbiont-derived endoplasmic reticulum-associated protein degradation (ERAD) system functions in import of apicoplast proteins. J Biol Chem. 2009;284:33683–33691. doi: 10.1074/jbc.M109.044024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glaser S, van Dooren GG, Agrawal S, Brooks CF, McFadden GI, Striepen B, Higgins MK. Tic22 is an essential chaperone required for protein import into the apicoplast. J Biol Chem. 2012;287:39505–39512. doi: 10.1074/jbc.M112.405100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalanon M, Tonkin CJ, McFadden GI. Characterization of two putative protein translocation components in the apicoplast of Plasmodium falciparum. Eukaryot Cell. 2009;8:1146–1154. doi: 10.1128/EC.00061-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Dooren GG, Tomova C, Agrawal S, Humbel BM, Striepen B. Toxoplasma gondii Tic20 is essential for apicoplast protein import. Proc Natl Acad Sci U S A. 2008;105:13574–13579. doi: 10.1073/pnas.0803862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Dooren GG, Reiff SB, Tomova C, Meissner M, Humbel BM, Striepen B. A novel dynamin-related protein has been recruited for apicoplast fission in Toxoplasma gondii. Curr Biol. 2009;19:267–276. doi: 10.1016/j.cub.2008.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jacot D, Daher W, Soldati-Favre D. Toxoplasma gondii myosin F, an essential motor for centrosomes positioning and apicoplast inheritance. EMBO J. 2013 doi: 10.1038/emboj.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55*.Sheiner L, Demerly JL, Poulsen N, Beatty WL, Lucas O, Behnke MS, White MW, Striepen B. A systematic screen to discover and analyze apicoplast proteins identifies a conserved and essential protein import factor. PLoS Pathog. 2011;7:e1002392. doi: 10.1371/journal.ppat.1002392. [This study describes a bioinformatics approach to identify new organellar proteins and an improved system to generate Toxoplasma conditional mutants that have been shown highly efficient by many studies published since.] [DOI] [PMC free article] [PubMed] [Google Scholar]