Abstract
6-aminopurine metabolism in Leishmania is unique among trypanosomatid pathogens since this genus expresses two distinct routes for adenine salvage: adenine phosphoribosyltransferase (APRT) and adenine deaminase (AAH). To evaluate the relative contributions of APRT and AAH, adenine salvage was evaluated in Δaprt, Δaah, and Δaprt/Δaah null mutants of L. donovani. The data confirm that AAH plays the dominant role in adenine metabolism in L. donovani, although either enzyme alone is sufficient for salvage. Adenosine salvage was also evaluated in a cohort of null mutants. Adenosine is also primarily converted to hypoxanthine, either intracellularly or extracellularly, but can also be phosphorylated to the nucleotide level by adenosine kinase when the predominant pathways are genetically or pharmacologically blocked. These data provide genetic verification for the relative contributions of 6-aminopurine metabolizing pathways in L. donovani and demonstrate that all of the pathways can function under appropriate conditions of genetic or pharmacologic perturbation.
Keywords: Leishmania donovani, purine salvage, adenine metabolism, adenosine metabolism, adenine phosphoribosyltransferase, adenine aminohydrolase
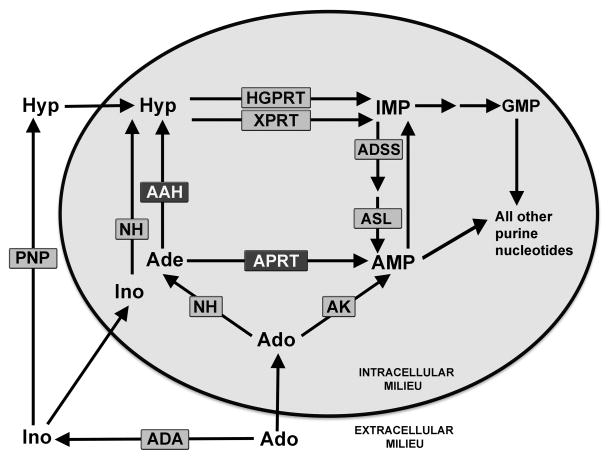
All genera of protozoan parasites are auxotrophic for purines, and, consequently, each genus has evolved a unique suite of salvage enzymes that enables purine scavenge from their hosts [1]. Among the protozoan parasites that infect humans, Leishmania, as well as Trypanosoma brucei and T. cruzi, express particularly elaborate purine acquisition pathways that encompass numerous routes for salvaging purines [1]. Leishmania also express at least four purine transporters on their plasma membrane, each of which is capable of translocating a restricted cohort of purine nucleosides or nucleobases [2,3]. It has been experimentally determined that virtually any exogenously supplied naturally occurring purine base or nucleoside can satisfy the purine requirements of L. donovani [3–5]. Once assimilated into the parasite nucleotide pool, any salvageable purine, which includes adenine and adenosine, can then be interconverted into all other purine nucleotides by the metabolic machinery of the parasite. The purine salvage pathways of Leishmania and Trypanosoma that have been deduced through both experimental studies and bioinformatic analyses are remarkably similar with one notable exception [6]. Whereas Leishmania uniquely express two distinct routes of adenine metabolism, adenine aminohydrolase (AAH) and adenine phosphoribosyltransferase (APRT) enzymes that convert adenine to hypoxanthine and AMP, respectively, both T. brucei and T. cruzi lack AAH and only metabolize the 6-aminopurine through APRT (Fig. 1). Hypoxanthine, the product of AAH catalysis, is then salvaged to the nucleotide level by hypoxanthine-guanine phosphoribosyltransferase (HGPRT) or xanthine phosphoribosyltransferase (XPRT), both of which recognize hypoxanthine as a substrate (Fig. 1) [7,8]. Adenosine metabolism and its assimilation in the parasite nucleotide pool is a bit more complex. The 6-aminopurine nucleoside has at least three immediate metabolic fates in Leishmania: 1) direct phosphorylation to AMP a reaction that is catalyzed by adenosine kinase (AK); 2) cleavage to adenine after which the nucleobase can be incorporated via the AAH/HGPRT/XPRT or APRT routes; and 3) extracellular metabolism to inosine and hypoxanthine by host enzymes or enzymes in the growth medium after which the 6-oxypurines are taken up by the parasite and fluxed through HGPRT and/or XPRT to IMP (see Fig. 1).
Fig. 1.
6-aminopurine metabolism in L. donovani. The enzymes involved in adenine and adenosine salvage into the parasite nucleotide pool are depicted. ADA, adenosine deaminase; PNP, purine nucleoside phosphorylases; AAH, adenine aminohydrolase; APRT, adenine phosphoribosyltransferase; AK, adenosine kinase; NH, nucleoside hydrolase; HGPRT, hypoxanthine-guanine phosphoribosyltransferase; XPRT, xanthine phosphoribosyltransferase; ADSS, adenylosuccinate synthetase; ASL, adenylosuccinate lyase.
The profoundly restricted growth phenotype exhibited by a conditional lethal L. donovani Δhgprt/Δxprt knockout line highlighted the importance of AAH in adenine metabolism since the only permissive growth conditions for this null mutant are a 6-aminopurine in the presence of 2′-deoxycoformycin (dCF), an inhibitor of AAH [9–11]. The Δhgprt/Δxprt promastigotes are incapable of utilizing hypoxanthine, xanthine, guanine, or their corresponding ribonucleosides as purine sources and cannot survive in adenine or adenosine as the sole purine source in the absence of dCF [9]. This inability to proliferate in adenine or adenosine alone intimates that the majority, if not all, exogenous 6-aminopurines are funneled into hypoxanthine by the metabolic machinery of the parasite and, consequently, that AAH is the predominant route for adenine salvage by L. donovani promastigotes (see Fig. 1). Furthermore, the Δhgprt/Δxprt strain also displays a profoundly incapacitated infectivity phenotype in mice implying that all purines available to the L. donovani amastigote in the mouse model of visceral leishmaniasis are either or channeled into HGPRT/XPRT substrates [9]. These findings demonstrate that the pathways of purine acquisition are likely to be very similar in both life cycle stages of the parasite and that HGPRT and XPRT play the principal roles in the salvage of extracellular purines into the parasite nucleotide pools.
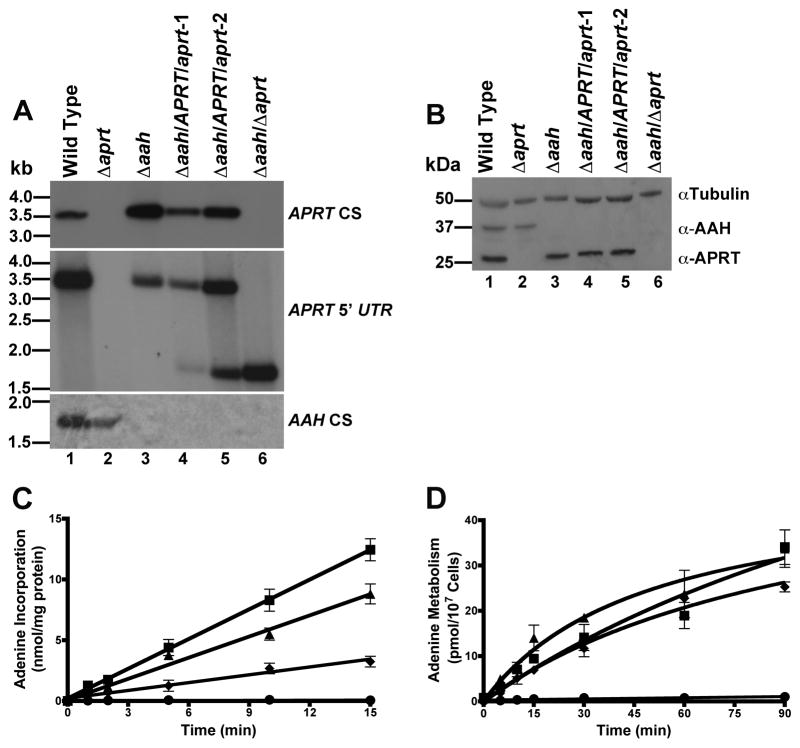
In order to dissect the relative contributions of the various adenine and adenosine salvage mechanisms in intact parasites, a series of mutants in L. donovani were created that were genetically deficient in APRT, AAH, or both APRT and AAH. The construction and phenotypic characterization of the Δaprt and Δaah cell lines have been described previously [10,12]. To generate the Δaah/Δaprt double knockout, two APRT/aprt heterozygotes, were first created within the Δaah background using independent targeted gene replacement constructs harboring two separate drug markers to generate Δaah/APRT/aprt-1 and Δaah/APRT/aprt-2 heterozygotes. The Δaah/Δaprt null parasites were then generated from the Δaah/APRT/aprt-2 heterozygote (Fig. 2A). Southern blot analysis of genomic DNA from wild type, Δaprt, Δaah, Δaah/APRT/aprt-1, Δaah/APRT/aprt-2, and Δaah/Δaprt cells confirmed the homologous gene replacement events in the genetically manipulated strains (Fig. 2A). The hybridization signals observed when the blots were probed with the APRT open reading frame, the APRT 5′-flanking region, or the AAH coding region corresponded to the sizes of the restriction fragments predicted from the sequences of the APRT and AAH ORFs and their adjacent 5′ and 3′ UTRs [10,13]. The homozygous gene replacements in the parasite lines harboring Δaprt, Δaah, and Δaah/Δaprt lesions were corroborated by Western blot analysis of lysates prepared from wild type, heterozygous, and homozygous knockout parasites using polyclonal antisera specific for APRT [14] and AAH [10] (Fig. 2B). Equivalent loading of cell lysates on the western blot was confirmed using antisera to α-tubulin (Fig. 2B).
Fig. 2.
Molecular and biochemical characterization of wild type, Δaprt, Δaah, and Δaah/Δaprt parasites. The construction of the Δaprt and Δaah parasites has been described previously [10,12]. The Δaah/Δaprt line was created in the Δaah background by double targeted gene replacement using APRT targeting constructs that were assembled via a 4-way ligation method [19]. The primers used to amplify the APRT 5′-UTR were 5′-GAGGCCACCTAGGCCGAGATGTCACGAAACCTGCCAG-3′ and 5′-GAGGCCACGCAGGCCCGTGTGTGTCGATGCTGCTGGTATG-3′, while 5′-GAGGCCTCTGTGGCCCGCTGCGCAGTAGTGCGCTTGCTG-3′ and 5′-GAGGCCTGACTGGCCGTCGTAGGGGAAGAAGGCGATG-3′ (SfiI sites underlined) were used to amplify the 3′-UTR. To confirm the genetic lesions, Southern and western analyses were performed. (A) Total genomic DNA from wild type L. donovani (lane 1), Δaprt (lane 2), Δaah (lane 3), Δaah/APRT/aprt-1 (lane 4) Δaah/APRT/aprt-2 (lane 5), and Δaah/Δaprt (lane 6) was digested with BamHI, SalI and EcoRI, fractionated on a 1% agarose gel, and blotted onto a nylon membrane. The blot was hybridized under high stringency conditions using either the APRT coding sequence, the APRT 5′ UTR, or the AAH open reading frame as probes. (B) Lysates of exponentially growing wild type L. donovani (lane 1), Δaprt (lane 2), Δaah (lane 3), Δaah/APRT/aprt-1 (lane 4) Δaah/APRT/aprt-2 (lane 5) and Δaah/Δaprt (lane 6) were analyzed by immunoblotting using anti-APRT and anti-AAH monospecific polyclonal antisera [10,14]. The amount of protein loaded in each lane was normalized using commercially available mouse antiα-tubulin monoclonal antibody. (C) Cell-free extracts were prepared by sonication of 2 X 109 wild type or mutant parasites that had been washed in PBS and resuspended in 50 mM Tris–HCl, pH 7.4, 5 mM MgCl2, 1 mM phosphoribosylpyrophosphate, and protease inhibitor cocktail. The abilities of 50 μg of either wild type (■), Δaah (◆), Δaprt (▲) or Δaah/Δaprt (●) cell lysate to phosphoribosylated 20 μM [8-14C]adenine (50 mCi/mmol) to the nucleotide level were then assessed over a 15 min time course using the previously reported DE-81 filter binding assay [12,13,20]. (D) The capacities of live wild type (■), Δaah (◆), Δaprt (▲) or Δaah/Δaprt (●) cells to metabolize 20 μM [8-14C]adenine into phosphorylated products over a 90 min time course were measured at ambient temperature using the DE-81 filter disk method [12,13,20]. Each measurement was obtained with 107 intact parasites that had been resuspended in modified DME-L [12] lacking FBS and hemin at a concentration of 108 cells/ml. Each aliquot was washed once in 1.0 ml of PBS and the cells lysed in 50 μl of 1% Triton X-100. The extracts were applied to DE-81 filter disks and the disks washed as described [12,13,20]. Disks were air-dried, and the radioactivity incorporated was quantified by scintillation counting.
Based on the restricted growth and noninfectious phenotype of the Δhgprt/Δxprt double knockout, it was reasonable to infer that L. donovani preferentially incorporates adenine into nucleotides via AAH-mediated deamination to hypoxanthine rather than via direct phosphoribosylation by APRT. In order to test this hypothesis, the abilities of wild type, Δaprt, Δaah, and Δaah/Δaprt cell lysates to convert [14C]adenine to the nucleotide level were compared (Fig. 2C). Because APRT is a bisubstrate reaction, phosphoribosylpyrophosphate, the ribose-phosphate donor, was added to the reaction mixtures at a concentration of 1 mM. The rates by which wild type, Δaprt, and Δaah extracts metabolized adenine into nucleotides were linear over a 15 min time course, whereas no incorporation was observed with Δaah/Δaprt cell lysates. The rates by which the 6-oxypurine [14C]hypoxanthine was incorporated into phosphorylated nucleotides served as controls for sample preparation and quality since the 6-oxypurine nucleobase is phosphoribosylated to the nucleotide level via HGPRT and XPRT and were equivalent among all four cell lines (data not shown) [5,15]. The finding that extracts prepared from the Δaah/Δaprt double null mutant fail to convert adenine to nucleotides indicate that AAH and APRT are the sole mechanisms by which adenine can be assimilated into the parasite nucleotide pool. Furthermore, because these incorporation assays were conducted at 20 μM [14C]adenine, a concentration that exceeds the Km value of APRT by an order of magnitude [16] but is comparable to the Km value determined for AAH [10], these findings strengthen the notion that AAH is the preferred route of adenine uptake in L. donovani.
The rates by which intact wild type, Δaprt, Δaah, and Δaah/Δaprt promastigotes assimilated [14C]adenine into the nucleotide pool were also assessed. No adenine conversion to nucleotides was observed for intact Δaah/Δaprt promastigotes over a 90 min interval, confirming that AAH and APRT are the exclusive routes by which adenine is metabolized in intact parasites, while wild type, Δaprt, and Δaah promastigotes incorporated adenine into phosphorylated metabolites at roughly equivalent rates (Fig. 2D). The discrepancy in the relative efficiencies by which Δaprt and Δaah cell lysates and intact parasites metabolize adenine can be ascribed to the fact that the rates and extents of adenine assimilation into anionic phosphorylated metabolites by live parasites is contingent upon the interplay of a multiplicity of cellular processes, including transport, nucleotide interconversion, and nucleic acid synthesis.
The metabolic incorporation experiments on Δaah/Δaprt lysates and parasites confirmed that AAH and APRT are the only routes of adenine metabolism in L. donovani (Fig. 2C–D). These short-term experiments, however, do not directly assess the involvement of AAH and APRT in enabling 6-aminopurines to support parasite growth. To evaluate the nutritional roles of AAH and APRT in facilitating L. donovani growth, the abilities of wild type, Δaprt, Δaah, and Δaah/Δaprt promastigotes to grow on adenine, as well as adenosine, were appraised. Included in this nutritional analysis were previously isolated gene deletion mutants with known 6-aminopurine growth deficits. These mutants include L. donovani harboring genetic lesions in adenylosuccinate synthetase (ADSS), AAH and ADSS (Δaah/Δadss), HGPRT and XPRT (Δhgprt/Δxprt), and the triple null mutant Δaah/Δhgprt/Δxprt (Table I) [9,10,17]. Nutritional assessments were performed in purine-deficient Dulbecco’s modified Eagle medium-Leishmania (DME-L) growth medium supplemented with 5% dialyzed fetal bovine serum to which either 100 μM adenine or adenosine was added. None of these cell lines, of course, could grow in purine-deficient medium (data not shown). As expected, wild type, Δaprt, and Δaah promastigotes displayed robust growth in adenine, while Δaah/Δaprt promastigotes failed to proliferate (Table I). As shown previously, neither Δadss nor Δhgprt/Δxprt promastigotes could grow in adenine [9,17], although when a Δaah mutation was inserted into each of these genetic backgrounds, the cells grew robustly [10,17]. Furthermore, pharmacologic simulation of the AAH deficiency with dCF enabled Δadss [17] and Δhgprt/Δxprt (Table 1) promastigotes to proliferate with adenine as the sole purine nutrient but prevented growth of Δaprt promastigotes by blocking adenine conversion to hypoxanthine via AAH. Collectively, these data establish that AAH is the primary route for adenine salvage into the parasite nucleotide pool, but APRT is functional in promastigotes if AAH is genetically or pharmacologically ablated. That AAH is the primary mechanism by which adenine is metabolized by Leishmania can account for the highly compromised infectivity phenotype of the Δhgprt/Δxprt parasites in mice [9,17], since host supplied adenine (or adenosine) would be siphoned into hypoxanthine, which cannot be salvaged by Δhgprt/Δxprt cells (see Fig. 1).
Table 1.
The capacity of wild type L. donovani and purine salvage mutants to grow in adenine, adenosine or purine supplemented with dCF or EHNA
| Cell Line | Purine Source
|
|||||
|---|---|---|---|---|---|---|
| Ade | Ade + dCF | Ade + EHNA | Ado | Ado + dCF | Ado + EHNA | |
| Wild Type | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| Δaah | ++ | + | ++ | ++++ | +++ | ++++ |
| Δaprt | ++++ | − | +++ | ++++ | ++ | ++++ |
| Δaah/Δaprt | − | − | − | ++++ | ++ | ++++ |
| Δadss [17] | − | ++++ | − | − | +++ | − |
| Δaah/Δadss | ++++ | ++++ | ++++ | − | ++++ | ++++ |
| Δhgprt/Δxprt | − | ++++ | − | − | ++++ | − |
| Δaah/Δhgprt/Δxprt | ++++ | +++ | ++++ | − | ++++ | ++++ |
Exponentially growing parasites were incubated in purine-free medium for 8 h, pelleted and resuspended at a density of 5 X 104 cells/mL in modified Dulbecco’s modified Eagle-Leishmania medium [12] supplemented with 5% dialyzed fetal bovine serum and either 100 μM adenine (Ade) or adenosine (Ado) in the absence or presence of either 20 μM 2′-deoxycoformycin (dCF) or 20 μM erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA). Parasites were counted with a hemacytometer after 7–10 days and scored according to their relative level of growth in each different growth condition as compared to wild type cells. (++++) represents ~100% wild type growth levels, (+++) indicates ~75% of wild type density, (++) denotes 50%, (+) corresponds to 25%, and (−) indicates no growth. All experiments were performed in triplicate with essentially identical results.
The ability of wild type and mutant L. donovani to utilize adenosine as a purine source was also evaluated. To differentiate among the three adenosine scavenge mechanisms that are potentially available to the parasite (see Fig. 1), the abilities of wild type, Δaprt, Δaah, and Δaah/Δaprt cells to proliferate in adenosine as the sole purine source were evaluated in the absence and presence of dCF or erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA). EHNA is a potent inhibitor of the mammalian adenosine deaminase (ADA) but not the leishmanial AAH, whereas dCF inhibits both ADA and AAH [11,18]. Wild type, Δaprt, Δaah, and Δaah/Δaprt promastigotes, as expected, were all capable of growing indefinitely in adenosine as the sole purine source in the medium. The addition of EHNA did not impact the ability of any of the four strains to utilize adenosine, or for that matter adenine, as a purine nutrient (Table 1). Similarly, dCF did not negatively affect parasite growth with adenosine but, as noted above, blocked adenine salvage by the Δaprt strain. Because, the Δaah/Δaprt strain cannot utilize adenine as a purine source, the ability of the double knockout to utilize adenosine in the presence of dCF or EHNA intimates that the parasite AK is a functional metabolic route for adenosine incorporation when extracellular adenosine metabolism is pharmacologically blocked.
To evaluate the routes of 6-aminopurine metabolism further, we also tested in parallel whether the addition of EHNA or dCF to the culture medium impacted the abilities of L. donovani strains with defined genetic lesions known to impact 6-aminopurine salvage to grow in adenosine or adenine (Table 1). As previously reported, Δhgprt/Δxprt and Δadss L. donovani cannot utilize adenine or adenosine as a purine nutrient indicating that the 6-aminopurines are funneled through hypoxanthine, a metabolically useless purine for the Δhgprt/Δxprt null mutant, and then to IMP, which cannot serve as a source of adenylate nucleotides in Δadss parasites (Fig. 1). These data suggest that robust metabolism of 6-aminopurines to 6-oxypurines renders AK and APRT functionally superfluous in wild type L. donovani. Insertion of a Δaah mutation into the Δhgprt/Δxprt or Δadss cell lines restores their capacities to grow in adenine but does not allow adenosine to serve as a purine source for Δaah/Δhgprt/Δxprt or Δaah/Δadss promastigotes. These findings support the inference that adenosine in the culture medium is efficiently converted to 6-oxypurines, a process that is initiated by ADA in the culture medium. This contention is reinforced by the observation that dCF restores the abilities of Δadss, Δaah/Δadss, and Δhgprt/Δxprt promastigotes to utilize adenosine by blocking both AAH in the parasite and ADA in the medium. Conversely, EHNA, which, like dCF, also blocks extracellular ADA but does not inhibit parasite AAH, enables Δaah/Δadss, and Δaah/Δhgprt/Δxprt to grow in adenosine, but does not permit growth of either Δadss or Δhgprt/Δxprt parasites to make use of adenosine as a purine source since these lines can still convert adenosine to hypoxanthine via a nucleoside hydrolase activity and AAH.
Taken as a whole, our findings indicate that both AAH and APRT are functional routes of adenine metabolism in L. donovani, although the robust activity of AAH can render adenine salvage by APRT useless via deamination to hypoxanthine, which requires HGPRT or XPRT and ADSS activities to then serve as a source of parasite adenylate nucleotides (Fig. 1). Adenosine metabolism is more complex in that there are multiple routes by which the nucleoside can be assimilated into the parasite nucleotide pool. All have been confirmed to be operational if the adenosine pool can be sustained by appropriate pharmacological obstruction, and genetic manipulation can perturb the adenosine flux. In pharmacologically unencumbered wild type L. donovani promastigotes, the preferred route appears to involve adenosine conversion to hypoxanthine, either intracellularly or extracellularly, since mutants blocked in hypoxanthine salvage cannot grow in the nucleoside. In contrast, AK can support adenosine salvage when ADA and AAH are both pharmacologically impeded with dCF, and APRT can enable adenosine salvage when ADA is blocked with EHNA and AAH is genetically ablated.
Highlights.
Purine salvage is an indispensable nutritional process for L. donovani
Exogenous adenine and adenosine can fulfill the purine requirements of Leishmania.
Adenine is primarily metabolized to hypoxanthine by adenine deaminase in L. donovani
Adenosine is also mostly salvaged through hypoxanthine by L. donovani
Secondary pathways for adenine and adenosine salvage are available to the parasite
Acknowledgments
This work was supported by grant AI023682 provided by the National Institute of Allergy and Infectious Diseases.
Abbreviations
- AAH
adenine aminohydrolase
- APRT
adenine phosphoribosyltransferase
- HGPRT
hypoxanthine-guanine phosphoribosyltransferase
- XPRT
xanthine phosphoribosyltransferase
- AK
adenosine kinase
- dCF
2′-deoxycoformycin
- ADSS
adenylosuccinate synthetase
- EHNA
erythro-9-(2-hydroxy-3-nonyl)adenine
- ADA
adenosine deaminase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berens RL, Krug Edward C, Marr Joseph J. Purine and Pyrimidine Metabolism. In: Marr JJ, Muller M, editors. Biochemistry and Molecular Biology of Parasites. Academic Press Ltd; London, England; San Diego, CA: 1995. pp. 89–117. [Google Scholar]
- 2.Boitz JM, Ullman B, Jardim A, Carter NS. Purine salvage in Leishmania: complex or simple by design? Trends Parasitol. 2012;28:345–52. doi: 10.1016/j.pt.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter NS, Yates PA, Gessford SK, Galagan SR, Landfear SM, Ullman B. Adaptive responses to purine starvation in Leishmania donovani. Mol Microbiol. 2010;78:92–107. doi: 10.1111/j.1365-2958.2010.07327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krenitsky TA, Koszalka GW, Tuttle JV, Adamczyk DL, Elion GB, Marr JJ. Purine salvage enzymes in man and Leishmania donovani. Adv Exp Med Biol. 1979;122B:51–6. doi: 10.1007/978-1-4684-8559-2_10. [DOI] [PubMed] [Google Scholar]
- 5.Marr JJ, Berens RL, Nelson DJ. Purine metabolism in Leishmania donovani and Leishmania braziliensis. Biochim Biophys Acta. 1978;544:360–71. doi: 10.1016/0304-4165(78)90104-6. [DOI] [PubMed] [Google Scholar]
- 6.Logan-Klumpler FJ, De Silva N, Boehme U, Rogers MB, Velarde G, McQuillan JA, Carver T, Aslett M, Olsen C, Subramanian S, Phan I, Farris C, Mitra S, Ramasamy G, Wang H, Tivey A, Jackson A, Houston R, Parkhill J, Holden M, Harb OS, Brunk BP, Myler PJ, Roos D, Carrington M, Smith DF, Hertz-Fowler C, Berriman M. GeneDB--an annotation database for pathogens. Nucleic Acids Res. 2012;40:D98–108. doi: 10.1093/nar/gkr1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen TE, Hwang HY, Jardim A, Olafson R, Ullman B. Cloning and expression of the hypoxanthine-guanine phosphoribosyltransferase from Leishmania donovani. Mol Biochem Parasitol. 1995;73:133–43. doi: 10.1016/0166-6851(94)00105-v. [DOI] [PubMed] [Google Scholar]
- 8.Jardim A, Bergeson SE, Shih S, Carter N, Lucas RW, Merlin G, Myler PJ, Stuart K, Ullman B. Xanthine phosphoribosyltransferase from Leishmania donovani. Molecular cloning, biochemical characterization, and genetic analysis. J Biol Chem. 1999;274:34403–10. doi: 10.1074/jbc.274.48.34403. [DOI] [PubMed] [Google Scholar]
- 9.Boitz JM, Ullman B. A conditional mutant deficient in hypoxanthine-guanine phosphoribosyltransferase and xanthine phosphoribosyltransferase validates the purine salvage pathway of Leishmania donovani. J Biol Chem. 2006;281:16084–9. doi: 10.1074/jbc.M600188200. [DOI] [PubMed] [Google Scholar]
- 10.Boitz JM, Strasser R, Hartman CU, Jardim A, Ullman B. Adenine aminohydrolase from Leishmania donovani: unique enzyme in parasite purine metabolism. J Biol Chem. 2012;287:7626–39. doi: 10.1074/jbc.M111.307884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kidder GW, Nolan LL. Adenine aminohydrolase: occurrence and possible significance in trypanosomid flagellates. Proc Natl Acad Sci U S A. 1979;76:3670–2. doi: 10.1073/pnas.76.8.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boitz JM, Ullman B. Leishmania donovani singly deficient in HGPRT, APRT or XPRT are viable in vitro and within mammalian macrophages. Mol Biochem Parasitol. 2006;148:24–30. doi: 10.1016/j.molbiopara.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Hwang HY, Ullman B. Genetic analysis of purine metabolism in Leishmania donovani. J Biol Chem. 1997;272:19488–96. doi: 10.1074/jbc.272.31.19488. [DOI] [PubMed] [Google Scholar]
- 14.Zarella-Boitz JM, Rager N, Jardim A, Ullman B. Subcellular localization of adenine and xanthine phosphoribosyltransferases in Leishmania donovani. Mol Biochem Parasitol. 2004;134:43–51. doi: 10.1016/j.molbiopara.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 15.LaFon SW, Nelson DJ, Berens RL, Marr JJ. Purine and pyrimidine salvage pathways in Leishmania donovani. Biochem Pharmacol. 1982;31:231–8. doi: 10.1016/0006-2952(82)90216-7. [DOI] [PubMed] [Google Scholar]
- 16.Allen T, Henschel EV, Coons T, Cross L, Conley J, Ullman B. Purification and characterization of the adenine phosphoribosyltransferase and hypoxanthine-guanine phosphoribosyltransferase activities from Leishmania donovani. Mol Biochem Parasitol. 1989;33:273–81. doi: 10.1016/0166-6851(89)90089-3. [DOI] [PubMed] [Google Scholar]
- 17.Boitz JM, Strasser R, Yates PA, Jardim A, Ullman B. Adenylosuccinate Synthetase and Adenylosuccinate Lyase Deficiencies Trigger Growth and Infectivity Deficits in Leishmania donovani. J Biol Chem. 2013;288:8977–90. doi: 10.1074/jbc.M112.431486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uberti J, Lightbody JJ, Johnson RM. The effect of nucleosides and deoxycoformycin on adenosine and deoxyadenosine inhibition of human lymphocyte activation. J Immunol. 1979;123:189–93. [PubMed] [Google Scholar]
- 19.Fulwiler AL, Soysa DR, Ullman B, Yates PA. A rapid, efficient and economical method for generating leishmanial gene targeting constructs. Mol Biochem Parasitol. 2011;175:209–12. doi: 10.1016/j.molbiopara.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iovannisci DM, Goebel D, Allen K, Kaur K, Ullman B. Genetic analysis of adenine metabolism in Leishmania donovani promastigotes. Evidence for diploidy at the adenine phosphoribosyltransferase locus. J Biol Chem. 1984;259:14617–23. [PubMed] [Google Scholar]