Abstract
Modern evidence based guidelines in immune thrombocytopenia (ITP) are mostly based on expert opinion. Standardized clinical assessment and management plans (SCAMPs) are flexible, feedback-based practice guidelines that could be valuable for both managing patients with ITP and understanding treatment decisions and outcomes. At Boston Children’s Hospital, we have implemented a SCAMP for patients with newly diagnosed and persistent ITP. To develop the algorithm, a group of local ITP experts devised an initial guideline, which was then modified by the attending hematologists who care for ITP patients until consensus was reached. Since deviations from the algorithm are encouraged, all clinicians did not need to agree with all aspects of the algorithm. At each clinic visit, clinicians fill out data collection forms explaining practice deviations. The goals of this process are to decrease practice variation and resource utilization and learn from the outcomes and deviations that occur to continually improve our practice. SCAMPs are an innovative approach to improve quality of care in ITP.
RECENT HISTORY OF PRACTICE-BASED GUIDELINES
Immune thrombocytopenia (ITP) is an acquired bleeding disorder of children, adults, and pregnant women, which is commonly encountered by hematologists. Consensus-based “guidelines” for diagnosis and management have been published by a number of groups internationally.1–10 These guidelines are mostly based on expert opinion rather than solid clinical evidence. The 2011 guidelines from the American Society of Hematology (ASH) are supported by expert opinion, as well as prospective and retrospective data.4 However, due to the paucity of randomized trials, 44% of the recommendations in children and 64% in adults are based on grade 2B/C evidence.4 Although consensus-based guidelines from experts have been published, actual consensus on the management of both newly diagnosed and chronic ITP has not been reached and individual physician-based practices are the standard.11,12
Clinical practice guidelines (CPGs) can be implemented to reduce practice variation, resource utilization, and costs in medical conditions in which solid evidence exists.13,14 However, CPGs are fraught with limitations.15,16 CPGs do not have a mechanism for updating and modifying guidelines as new evidence is published. In addition, for most diseases, like ITP, solid evidence does not currently exist from which to base these guidelines. Furthermore, patient-centered measures, such as quality of life, are often not used in guideline development. Lastly, clinician deviations from CPGs are often not recorded or considered as part of their development.
Due to the limitations of CPGs, other practice guideline standards have been developed. Since 1988, Intermountain Healthcare, a network of hospitals and clinics, has used measures of clinical variation and outcomes data to create management standards, which they continually oversee.20 Their method involves establishing evidence-based, bestcare practice guidelines with an active variance feedback loop to modify the guidelines. They have found the practice guidelines initially undergo high rates of change but, over time, are modified less frequently but still at a stable rate. Using these methods, Intermountain has become a model for national practice standards due to the high quality and low cost of care.
Based on the model of Intermountain Healthcare, the cardiologists at Boston Children’s Hospital developed standardized clinical assessment and management plans (SCAMPs) as a quality improvement, flexible care delivery algorithm that standardizes the assessment and management of a specific patient population through data collection.17,18 Based on semiannual review of the collected data, the algorithm is designed to be periodically modified based on feedback from the previous time period.17,19 This practice guideline model encourages knowledge-based physician deviations from the guidelines as a source of innovation and potential adaptation of the algorithm.
The goals of SCAMPs are numerous (Table 1). These management algorithms reduce practice variation and healthcare resource utilization. Since data are collected prospectively and care deviations (with explanations) are encouraged, one can learn about management and treatment outcomes, as well as treatment decisions and their outcomes. In addition, with semiannual review of data, the management plan undergoes progressive modification to incorporate findings from prior management outcomes and from new developments within a specific disease field.
Table 1.
Comparison of Current Practice to SCAMPs and Clinical Practice Guidelines
| Current ITP Practice | SCAMPs* | Clinical Practice Guidelines* |
|
|---|---|---|---|
| Provide “best practice” guidelines regarding ITP diagnosis and management |
No | Yes | Yes |
| Decrease unnecessary evaluation | No | Yes | Possibly |
| Decrease practice variation | No | Yes | Yes |
| Capture data on how often guidelines are followed and why clinicians deviate from guidelines |
No | Yes | No |
| Capture data about outcomes | Only in observational clinical trials, not in standard care |
Yes | No |
| Implement new research findings into practice over time |
Yes | Yes | No |
| Incorporate findings from captured data into practice iteratively |
No | Yes | No |
No ITP clinical practice guidelines (CPGs) exist due to a paucity of evidence from comparative clinical trials. These types of trials are unlikely to occur in ITP due to the rarity of disease, financial burden of this type of investigation, and physician and patient preferences.
ITP AS A MODEL DISEASE FOR SCAMPS*
Diagnosis and Laboratory Evaluation of ITP
ITP is a diagnosis of exclusion. Other than complete blood counts and review of the peripheral blood smear, there are no agreed upon diagnostic tools in an individual with a history and physical examination consistent with ITP. Nevertheless, physicians often send an array of lab tests in an individualized patient-directed fashion or based on physician preferences and past patient experiences.
The current ASH guidelines are clear that bone marrow aspirates and biopsies are no longer indicated at diagnosis in patients with typical features of ITP.4 However, current guidelines do not discuss whether bone marrow procedures should be performed in patients with chronic ITP or prior to second- or third-line therapies. Furthermore, there are no studies to guide laboratory evaluation and monitoring of chronic ITP patients. For example, does screening for other autoimmunity, such as thyroid or anti-nuclear antibody testing, help to more timely diagnosis and treatment of patients with secondary ITP?21 Which patients should be screened for an underlying immunodeficiency?22,23 SCAMPs could focus diagnostic testing in the majority of ITP patients and gather information about which patients to send more broad testing. This type of approach would reduce unnecessary testing, and we are likely to learn the answers about which patients need further testing and immunologic screening.
Management of Newly Diagnosed ITP
Some variation in ITP treatment is to be expected based on the epidemiology and clinical features of the disease. Thus, the approach to treatment of acute ITP is different in children versus adults, in bleeding versus non-bleeding patients, and in resource-limited settings versus non-limited settings. Although the 2011 ASH guidelines for the initial management of ITP are different for adults and children, there is no clear age cut-off by which a clinician should switch from the pediatric to the adult guidelines. Prospective data have shown that severe bleeding in children with newly diagnosed ITP is rare regardless of medical treatment, but retrospective data have indicated that ITP-related bleeding becomes more common as patients age.24,25 SCAMPs could incorporate age and, through various guideline iterations, the knowledge of proper age cut-offs will be improved to guide specific treatment approaches.
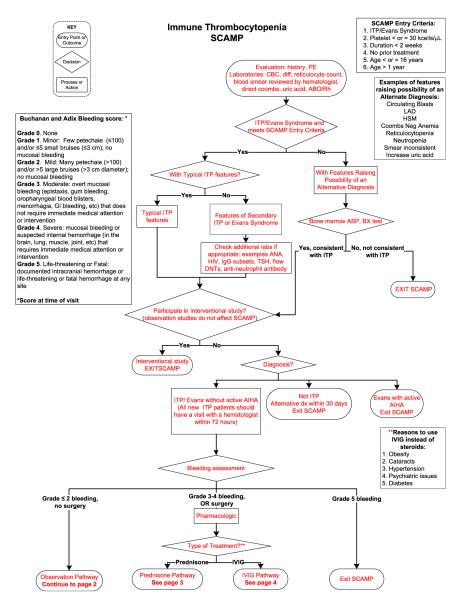
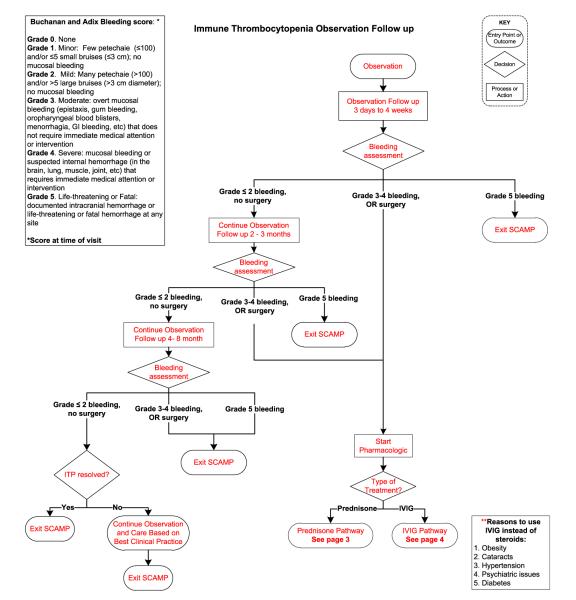
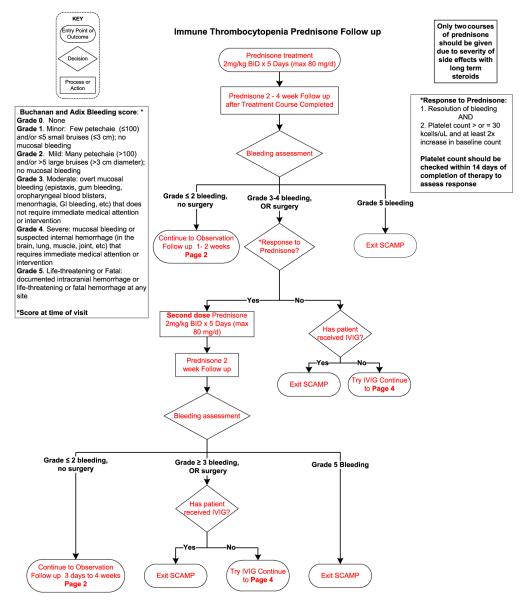
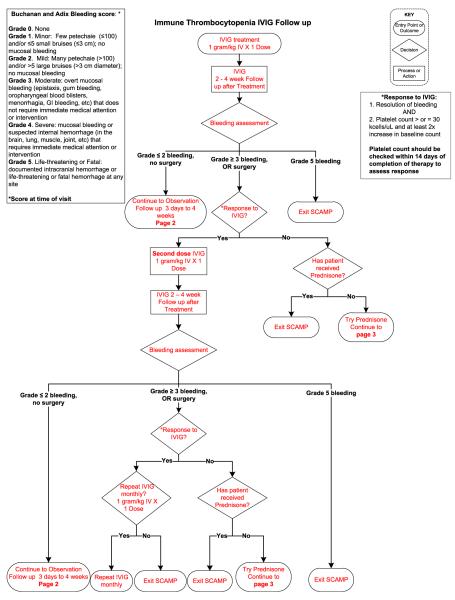
In patients who require treatment, current pediatric guidelines recommend either a short course of corticosteroids or a single dose of intravenous immunoglobulin (IVIG).4 Current practice guide-lines do not help the clinician to choose between these two treatments and the outcomes of this treatment choice are not collected. In addition, the timing of follow-up of newly diagnosed patients is not standardized. SCAMPs may teach us whether specific management choices have better outcomes and at least, why physicians pick certain treatment types (Figure 1).
Figure 1.
ITP SCAMP algorithm for newly diagnosed and persistent ITP. Data collection forms not shown.
Since bleeding, rather than platelet count, often determines treatment in ITP, it is important to have consistent and validated measures of bleeding. Currently, a variety of scoring systems are used to standardize bleeding in ITP patients.26,27 These also tend to be designed by expert opinion rather than validated clinical measures. SCAMPs could be used to both modify and validate existing bleeding systems into the one that is best for use in clinical practice.
Practice Decisions in Chronic and Refractory ITP
Although the management of chronic and refractory ITP has evolved over the past 50 years, therapy continues to be based more on clinical experience than research evidence. Historically, splenectomy was a common treatment choice, particularly in adult patients. However, other options with different side effect profiles have become common second- or third-line treatments, including rituximab and the thrombopoietinmimetic agents. Furthermore, in children, in whom splenectomy is much less commonly performed, a host of treatment options are chosen, often based on physician practice and local hospital experience.11 In addition to rituximab and the thrombopoietin-mimetic agents, these agents include oral immunosuppressant agents, such as 6-mercaptopurine, mycophenolate, cyclosporine, and sirolimus.28–30
Since so many agents are used in this rare disease, it is difficult for clinicians to know which agents are most effective and if there is different efficacy within certain populations of ITP patients. A randomized study of even just two of these treatment approaches would be a massive undertaking. The 2011 study of eltrombopag versus standard care involved 85 sites in 14 countries.31 It is unlikely, outside of the drug development process, that similar studies will be performed to determine which agent works best in chronic or refractory ITP. However, a multicenter approach is required to study chronic ITP. A multicenter SCAMP is one approach to learning how best to manage chronic ITP while actively managing these patients.
Quality of Life and ITP
Multiple measures of quality of life have been validated in ITP but only recently have prospective studies incorporated these outcomes into study findings.32–34 Given that quality-of-life measures often drive the choice of treatment in patients with ITP, these findings should be incorporated into SCAMPs. How physician management decisions effect quality of life in patients with ITP is an important aspect of data collection for feedback and future modifications of practice algorithms.
DISCUSSION
SCAMPs rely on management decision, treatment, and outcomes data. In addition, periodic modifications of these practice guidelines also require that clinical centers value and are committed to patient care and high quality. Based on the number of existing consensus based guidelines for ITP, it is clear this community values decision making and outcomes. Through feedback-based practice, the decisions in management can be recorded and treatment algorithms can be improved towards patient-centered care rather than individual physician-directed care (Table 2).
Table 2.
Areas Within ITP in Which Knowledge and Practice Could Be Improved Through the Use of SCAMPs
|
Amenable to a single center or multicenter SCAMP.
Requires a multicenter SCAMP.
Although the goal of practice algorithms is to provide high quality care, these types of guidelines can work in parallel with ITP research. If practice algorithms are used together in multiple centers, cohorts of phenotypically homogenous ITP patients, such as those with consistently high bleeding scores, those refractory to both IVIG and corticosteroids, or those of a particular age, can be identified for biology studies and prospective studies. By the identification of ITP patient subpopulations with the guidance of practice guidelines, clinical research opportunities could be offered to more eligible patients and patients could be more easily identified within existing clinical practice.
It is possible that the same practice guidelines cannot exist internationally, across regions, or across both local and large academic centers. Prior studies have clearly demonstrated wide variability in the approach to ITP in different countries, in terms of medication treatment versus observation, bleeding symptoms required for treatment, acceptable platelet counts, and treatment in the hospitalized setting versus outpatient.12,35 Some of these decisions may be driven by cost, availability of specific treatments, the heterogeneity within ITP, and variations in culture. However, as with any rare disease, clinical studies and improvement in care of ITP, particularly chronic ITP, will require a multicenter approach.
Few randomized trials have established the treatment approach to ITP patients. In an era of limited resources, these types of studies have become more expensive. It is unlikely that randomized clinical trials of splenectomy versus rituximab or thrombopoietinmimetic agents versus rituximab will ever be completed for many reasons, among which include patient and clinician preferences and biases. Without this data, how do clinicians know what to do? Clinicians and researchers must be innovative in the approach to treatment of ITP patients. SCAMPs are an approach to improve quality of care and an opportunity to learn from our patients and the decisions we make in their treatment.
*SCAMPs will be used as an example of a feedback-based, modifiable practice guideline throughout this text.
Acknowledgments
Publication of this article was supported by the International Cooperative ITP Study Group (ICIS).
Footnotes
Conflicts of interest: R.F. Grace has no conflict of interests.
REFERENCES
- 1.Shirahata A, Ishii E, Eguchi H, et al. Consensus guideline for diagnosis and treatment of childhood idiopathic thrombocytopenic purpura. Int J Hematol. 2006;83(1):29–38. doi: 10.1532/IJH97.05123. [DOI] [PubMed] [Google Scholar]
- 2.Del Vecchio GC, De Santis A, Giordano P, et al. Management of acute childhood idiopathic thrombocytopenic purpura according to AIEOP consensus guidelines: assessment of Italian experience. Acta Haematol. 2008;119(1):1–7. doi: 10.1159/000112837. [DOI] [PubMed] [Google Scholar]
- 3.Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115(2):168–86. doi: 10.1182/blood-2009-06-225565. [DOI] [PubMed] [Google Scholar]
- 4.Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117(16):4190–207. doi: 10.1182/blood-2010-08-302984. [DOI] [PubMed] [Google Scholar]
- 5.Guideline for diagnosis and treatment of immune thrombocytopenia. Arch Argent Pediatr. 2010;108(2):173–8. doi: 10.1590/S0325-00752010000200021. [DOI] [PubMed] [Google Scholar]
- 6.George JN, Woolf SH, Raskob GE, et al. Idiopathic thrombocytopenic purpura: a practice guideline developed by explicit methods for the American Society of Hematology. Blood. 1996;88(1):3–40. [PubMed] [Google Scholar]
- 7.De Mattia D, Del Vecchio GC, Russo G, et al. Management of chronic childhood immune thrombocytopenic purpura: AIEOP consensus guidelines. Acta Haematol. 2010;123(2):96–109. doi: 10.1159/000268855. [DOI] [PubMed] [Google Scholar]
- 8.Stasi R, Provan D. Management of immune thrombocytopenic purpura in adults. Mayo Clin Proc. 2004;79(4):504–22. doi: 10.4065/79.4.504. [DOI] [PubMed] [Google Scholar]
- 9.Guidelines for the investigation and management of idiopathic thrombocytopenic purpura in adults, children and in pregnancy. Br J Haematol. 2003;120(4):574–596. doi: 10.1046/j.1365-2141.2003.04131.x. [DOI] [PubMed] [Google Scholar]
- 10.Blanchette V, Carcao M. Approach to the investigation and management of immune thrombocytopenic purpura in children. Semin Hematol. 2000;37(3):299–314. doi: 10.1016/s0037-1963(00)90108-2. [DOI] [PubMed] [Google Scholar]
- 11.Neunert CE, Bright BC, Buchanan GR. Severe chronic refractory immune thrombocytopenic purpura during childhood: a survey of physician management. Pediatr Blood Cancer. 2008;51(4):513–6. doi: 10.1002/pbc.21621. [DOI] [PubMed] [Google Scholar]
- 12.Imbach P, Zimmerman S. Local and cultural aspects of childhood idiopathic thrombocytopenic purpura: a summary of statements from the 12 countries worldwide. J Pediatr Hematol Oncol. 2003;25(Suppl 1):S68–73. doi: 10.1097/00043426-200312001-00016. [DOI] [PubMed] [Google Scholar]
- 13.Rosoff AJ. The role of clinical practice guidelines in health care reform. Health Matrix Clevel. 1995;5(2):369–396. [PubMed] [Google Scholar]
- 14.Huttin C. The use of clinical guidelines to improve medical practice: main issues in the United States. Int J Qual Health Care. 1997;9(3):207–14. doi: 10.1093/intqhc/9.3.207. [DOI] [PubMed] [Google Scholar]
- 15.Flores G, Lee M, Bauchner H, et al. Pediatricians’ attitudes, beliefs, and practices regarding clinical practice guidelines: a national survey. Pediatrics. 2000;105:496–501. doi: 10.1542/peds.105.3.496. [DOI] [PubMed] [Google Scholar]
- 16.Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 17.Farias M, Ziniel S, Rathod RH, et al. Provider attitudes toward standardized clinical assessment and management plans (SCAMPs) Congenit Heart Dis. 2011;6(6):558–65. doi: 10.1111/j.1747-0803.2011.00579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman KG, Rathod RH, Farias M, et al. Resource utilization after introduction of a standardized clinical assessment and management plan. Congenit Heart Dis. 2010;5(4):374–81. doi: 10.1111/j.1747-0803.2010.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farias M, Friedman KG, Powell AJ, et al. Dynamic evolution of practice guidelines: analysis of deviations from assessment and management plans. Pediatrics. 2012;130(1):93–8. doi: 10.1542/peds.2011-3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James BC, Savitz LA. How Intermountain trimmed health care costs through robust quality improvement efforts. Health Aff (Millwood) 2011;30(6):1185–91. doi: 10.1377/hlthaff.2011.0358. [DOI] [PubMed] [Google Scholar]
- 21.Demir C, Esen R, Atmaca M, et al. Prevalence of autoantibodies related to some autoimmune disordersin patients with chronic idiopathic thrombocytopenic purpura. Clin Appl Thromb Hemost. 2011;17(6):E114–8. doi: 10.1177/1076029610387588. [DOI] [PubMed] [Google Scholar]
- 22.Notarangelo LD. Primary immunodeficiencies (PIDs) presenting with cytopenias. Hematology Am Soc Hematol Educ Program. 2009:139–43. doi: 10.1182/asheducation-2009.1.139. [DOI] [PubMed] [Google Scholar]
- 23.Heeney MM, Zimmerman SA, Ware RE. Childhood autoimmune cytopenia secondary to unsuspected common variable immunodeficiency. J Pediatr. 2003;143(5):662–5. doi: 10.1067/S0022-3476(03)00445-1. [DOI] [PubMed] [Google Scholar]
- 24.Neunert CE, Buchanan GR, Imbach P, et al. Severe hemorrhage in children with newly diagnosed immune thrombocytopenic purpura. Blood. 2008;112(10):4003–8. doi: 10.1182/blood-2008-03-138487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen YC, Djulbegovic B, Shamai-Lubovitz O, et al. The bleeding risk and natural history of idiopathic thrombocytopenic purpura in patients with persistent low platelet counts. Arch Intern Med. 2000;160(11):1630–8. doi: 10.1001/archinte.160.11.1630. [DOI] [PubMed] [Google Scholar]
- 26.Fogarty PF, Tarantino MD, Brainsky A, et al. Selective validation of the WHO Bleeding Scale in patients with chronic immune thrombocytopenia. Curr Med Res Opin. 2012;28(1):79–87. doi: 10.1185/03007995.2011.644849. [DOI] [PubMed] [Google Scholar]
- 27.Buchanan GR, Adix L. Grading of hemorrhage in children with idiopathic thrombocytopenic purpura. J Pediatr. 2002;141(5):683–8. doi: 10.1067/mpd.2002.128547. [DOI] [PubMed] [Google Scholar]
- 28.Sobota A, Neufeld EJ, Lapsia S, et al. Response to mercaptopurine for refractory autoimmune cytopenias in children. Pediatr Blood Cancer. 2009;52(1):80–4. doi: 10.1002/pbc.21729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colovic M, Suvajdzic N, Colovic N, et al. Mycophenolate mophetil therapy for chronic immune thrombocytopenic purpura resistant to steroids, immunosuppressants, and/or splenectomy in adults. Platelets. 2011;22(2):153–6. doi: 10.3109/09537104.2010.520372. [DOI] [PubMed] [Google Scholar]
- 30.Breakey VR, Blanchette VS. Childhood immune thrombocytopenia: a changing therapeutic landscape. Semin Thromb Hemost. 2011;37(7):745–55. doi: 10.1055/s-0031-1297165. [DOI] [PubMed] [Google Scholar]
- 31.Kuter DJ, Rummel M, Boccia R, et al. Romiplostim or standard of care in patients with immune thrombocytopenia. N Engl J Med. 2010;363(20):1889–99. doi: 10.1056/NEJMoa1002625. [DOI] [PubMed] [Google Scholar]
- 32.Klaassen RJ, Blanchette V, Burke TA, et al. Quality of life in childhood immune thrombocytopenia: international validation of the kids’ ITP tools. Pediatr Blood Cancer. 2013;60(1):95–100. doi: 10.1002/pbc.24257. [DOI] [PubMed] [Google Scholar]
- 33.Klaassen RJ, Mathias SD, Buchanan G, et al. Pilot study of the effect of romiplostim on child health-related quality of life (HRQoL) and parental burden in immune thrombocytopenia (ITP) Pediatr Blood Cancer. 2012;58(3):395–8. doi: 10.1002/pbc.23312. [DOI] [PubMed] [Google Scholar]
- 34.Mathias SD, Bussel JB, George JN, et al. A disease-specific measure of health-related quality of life for use in adults with immune thrombocytopenic purpura: its development and validation. Health Qual Life Outcomes. 2007;5:11. doi: 10.1186/1477-7525-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matzdorff A, Neufeld EJ, Roganovic J. To treat or not to treat—from guidelines to individualized patient management. Semin Hematol. 2013;50(Suppl 1):S12–7. doi: 10.1053/j.seminhematol.2013.03.004. [DOI] [PubMed] [Google Scholar]