Abstract
Among organelles, lipid droplets (LDs) uniquely constitute a hydrophobic phase in the aqueous environment of the cytosol. Their hydrophobic core of neutral lipids stores metabolic energy and membrane components, making LDs hubs for lipid metabolism. In addition, LDs are implicated in a number of other cellular functions, ranging from protein storage and degradation to viral replication. These processes are functionally linked to many physiological and pathological conditions, including obesity and related metabolic diseases. Despite their important functions and nearly ubiquitous presence in cells, many aspects of LD biology are unknown. In the past few years, the pace of LD investigation has increased, providing new insights. Here, we review the current knowledge of LD cell biology and its translation to physiology.
Keywords: organelle, energy metabolism, triacylglycerol, membranes, obesity, oil, fat
1. INTROUCTION
Lipid droplets (LDs) are dynamic cytoplasmic organelles found nearly ubiquitously in cells. They are linked to many cellular functions, including lipid storage for energy generation and membrane synthesis, viral replication, and protein degradation. LD biology is connected to myriad physiological processes, metabolic diseases, and oil production. Interest in LD biology has surged in recent years, reflecting recognition of the importance of this relatively understudied organelle and opportunities to unravel questions concerning LD biology.
Here, we focus primarily on recent discoveries in LD biology, building on other reviews (1–3), and highlight many areas of LD biology where knowledge is incomplete or controversial.
2. GENERAL PROPERTIES OF LIPID DROPLETS
2.1. Historical Aspects of Lipid Droplets
LDs were identified by light microscopy as cellular organelles in the nineteenth century. For many years, LDs often were called liposomes. In the late 1960s, a method was developed to generate vesicles in vitro, also called liposomes, and these vesicles assumed the name. Since then, LDs have been referred to as LDs, lipid bodies, fat bodies, oil bodies, spherosomes, or adiposomes. In the past decade, the field settled primarily on the name LDs.
For years, LDs were largely ignored in cell biology research, presumably because they were perceived as inert lipid globules with little functional relevance. In 1991, seminal work by Constantine Londos and coworkers (4) identified a protein, perilipin, that specifically localized to LD surfaces, and this opened the door to mechanistically studying the organelle. More recently, research on LD biology has accelerated dramatically. This reflects increased interest in the basic biology of prevalent metabolic diseases linked to LDs (e.g., obesity) and technological advances, which have shed new light on LDs and highlighted many unanswered basic questions.
2.2. Lipid Droplets Are Found in Most Cells
Nearly all cells have LDs or the capacity to form them. Several bacteria store lipids in LDs, including predominantly the actinomycetes group (e.g., Mycobacteria, Rhodococcus, Streptomyces, and Nocardia) (5).
Among eukaryotes, LDs are easily detected in budding yeast (Saccharomyces cerevisiae), facilitating genetic screens for altered LD morphology (6, 7). In higher eukaryotes, many cells have some LDs, and culturing cells with fatty acids (FAs) stimulates LD formation. Some cells, such as adipocytes or hepatocytes, exhibit many LDs at baseline owing to active lipid synthesis and storage. LD abundance varies dynamically in cells. For example, in S. cerevisiae, LDs are found most prominently during the stationary phase, and catabolism of LDs in the exponential phase is coordinated with an increased need for phospholipids during cell division (8). Also, the number of LDs increases up to severalfold in yeast with cellular stress (9, 10) and in cells of some cancers (11). The connections of LDs to these states are poorly understood.
LD number and size in different cell types or between individual cells of a population differ considerably. Many cells have small LDs (100–200-nm diameters), whereas LDs in white adipocytes have diameters up to 100 μm and fill almost the entire cytoplasm. With diameters from 100 nm to 100 μm, the corresponding surface area and volume for LDs varies by 106 and 109, respectively.
LD size can change rapidly. Oleate loading of Drosophila S2 cells increases the LD mean diameter nearly threefold within hours, corresponding to an almost 30-fold volume increase (12). LDs also grow rapidly during adipogenesis, when cells increase their capacity to synthesize lipids de novo. In contrast, LDs shrink within hours of culturing cells with limited nutrients.
2.3. Lipid Droplets Separate a Hydrophobic Phase from the Aqueous Cytosol
Among cellular organelles, LDs are uniquely composed of an organic phase of neutral lipids. This hydrophobic core is separated from the aqueous cytosol by a monolayer of surface phospholipids. The cytoplasm contains an emulsion of LDs in the cytosol. The LD phase of the emulsion provides a large interface for interactions with amphipathic molecules.
The primary neutral lipids of the LD core are sterol esters (SEs) and triacylglycerols (TGs). Their relative amount varies between cell types. For example, yeast LDs have a mix of SE and TG, possibly arranged in layers (13). LDs of adipocytes contain primarily TG, and those of macrophage foam cells contain mostly SE. Neutral lipid synthesis is catalyzed by various enzymes (14). In mammalian cells, acyl-coenzyme A (acyl-CoA):diacylglycerol acyltransferase (DGAT) enzymes, DGAT1 and DGAT2, synthesize TG, and acyl-CoA:cholesterol acyltransferase (ACAT) enzymes, ACAT1 and ACAT2, generate SEs. In yeast, the corresponding neutral lipid synthesis enzymes are Are1 and Are2, which synthesize primarily SEs, as well as Dga1 and Lro1, which synthesize TGs. Neutral lipid synthesis enzymes reside primarily in the endoplasmic reticulum (ER), with the exception of DGAT2, which also localizes to LDs (15, 16).
Various other hydrophobic lipids are found in LDs. Retinyl esters are found in LDs of hepatic stellate cells (17) and in the retina (18). LDs also contain wax esters and ether lipids, which are derived from peroxisomes and constitute 10%–20% of neutral lipids in some mammalian cells (19). Additionally, long-chain isoprenoids are found within LDs. Natural rubber consists of long-chain isoprene polymers in monolayer-bound particles that appear to be LDs of the rubber tree (20).
The LD surface comprises polar, amphipathic lipids. In mammalian LDs, phosphatidylcholine (PC) is the main surface phospholipid, followed by phosphatidylethanolamine (PE) and phosphatidylinositol (19). Compared with other membranes, LDs are deficient in phosphatidylserine and phosphatidic acid but enriched in lyso-PC and lyso-PE. More than 160 phospholipids species of varying head groups and side chains were detected in CHO cells (19). In Drosophila, PE is more abundant than PC, and this is reflected in the LD phospholipids (12). Nevertheless, PC is important in emulsifying LDs (12). LD surfaces contain other polar lipids, such as sterols, and the relative amount of these lipids depends on cell type. Sphingolipids are not present in appreciable quantities (19).
3. LIPID DROPLETS SERVE MULTIPLE FUNCTIONS IN CELLS
Foremost, LDs are intracellular lipid reservoirs, providing building blocks for membranes or substrates for energy metabolism. Packaging highly reduced, hydrophobic lipids, such as TGs, in a phase without water provides the most efficient form of energy storage. For example, an average non-obese person stores up to 2,500 kJ of metabolic energy in glycogen, but > 500,000 kJ in TGs of adipocyte LDs, enough to run ~30 marathons.
LDs also serve as organizing centers for synthesizing specific lipids. TGs, for example, are synthesized in the ER and at LDs (15). Other enzymes of lipid synthesis (e.g., ergosterol) localize to LDs, suggesting links between lipid synthesis pathways and LDs (21).
LDs have been linked to protein storage (22). For example, during embryogenesis in Drosophila, histones localize to LDs until needed for rapid nuclear division associated with embryo segmentation (23). LDs may also temporarily store unfolded membrane proteins before proteasomal degradation.
LDs are involved in hepatitis C virus (HCV) assembly. During viral replication, the HCV core protein is cleaved from the precursor viral polypeptide and binds to LDs (reviewed in Reference 24) via amphipathic helices. New HCV virions are assembled in LDs and ER membranes, where viral RNA is packaged with capsid proteins. Mature viruses are secreted as part of a lipoprotein-virus particle. LDs may provide a location for HCV core proteins until viral assembly or until cells degrade the overexpressed protein. Notably, HCV core protein localization to LDs requires DGAT1 activity, and HCV core proteins bind to DGAT1 in vitro (25). Moreover, blocking DGAT1 activity diminishes viral replication (25).
4. LIPID DROPLETS ARE ANALOGOUS TO LIPOPROTEINS
LDs share structural features with plasma lipoproteins. Both contain neutral lipid cores encased in polar lipid monolayers. Additionally, both particles are decorated with specific surface proteins, often possessing amphipathic α-helices. However, LDs (with diameters of 100 nm up to 100 microns) are generally much larger than lipoproteins [diameters range from < 20 nm (e.g., high-density lipoprotein) to ~500 nm (e.g., chylomicrons)].
Although the formation of both LDs and lipoproteins is linked to neutral lipid synthesis in the ER, their physiological functions differ: LDs primarily store lipids, and lipoproteins distribute lipids in the body. Also, only a few cell types (e.g., hepatocytes, enterocytes, and yolk sac endodermal cells) express the required proteins, such as apolipoprotein (apo) B and the microsomal TG transfer protein, for lipoprotein assembly, whereas most cells make LDs. Thus, lipoproteins may have evolved by adapting LDs to secretion.
How cells regulate the fate of newly synthesized neutral lipids—storage in LDs versus secretion on lipoproteins—is mostly unknown. Secretory cells may store lipids in LDs only after exceeding the capacity to assemble and secrete lipoproteins. Alternatively, secretion may be activated when a storage threshold is achieved. Specific LD proteins [including cell death–inducing DFF45-like effector (CIDE) proteins] may direct the LD pool of TG toward lipoprotein formation (26). Secretion of TG via lipoproteins is thought to involve their hydrolysis at LDs and resynthesis in the ER (27).
5. LIPID DROPLET FORMATION
5.1. Models of Lipid Droplet Formation
Many organelles self-replicate. However, LDs likely form de novo. In bacteria, LDs form by lipid synthesis in the cell-delimiting membrane (28). In eukaryotes, LDs may arise primarily from the ER, where the enzymes that synthesize neutral lipids reside (14). In yeast genetically engineered to lack LDs, induction of LD formation shows they invariably arise from or close to the ER (7, 29). LDs appear to remain in contact with the ER once formed, and proteins that associate with both compartments move between them (29). However, light microscopy, with limited resolution, cannot determine if such proteins reside directly on the LD surface or in ER membranes in close apposition. Many studies employing electron microscopy (EM) suggest a tight assocation of LDs and the ER. In mammalian cells, such studies show membrane cisternae, which could be connected to the ER, in close proximity to LDs (30, 31). Also, LDs in hepatocytes are tightly associated with ER membrane cisternae, marked by luminal apo B100 protein; that may be continuous with LDs (31).
Despite these findings, the molecular mechanisms of LD formation are not understood. How does a monolayer-coated LD arise from a bilayer membrane? Because neutral lipid synthesis enzymes reside in the ER, the products of these enzymes might occupy space between the membrane bilayers, forming a lens of neutral lipids. In vitro studies with ER microsomes from sunflower (Helianthus annuus) found formation of small liquid lenses of 60-nm diameter in the ER bilayer that, similar to LDs, contain freely mobile TG and recruit oleosins (32). It is unclear, however, how neutral lipids are organized into nascent LDs, whether this happens at specific locations in the ER, or whether specific proteins are involved. In mammalian cells, perilipin3/Tip47 is recruited to the ER during lipid storage (33) and was suggested to be required for LD formation (34). However, many organisms (e.g., yeast) have no perilipin 3/Tip47 ortholog, and many cells appear not to express it (e.g., Drosophila S2 cells), so this mechanism alone cannot mediate LD formation.
Several models of LD formation have been proposed (1). They include (a) ER budding, where LDs grow from the ER bilayer and remain connected or bud off (Figure 1); (b) bicelle formation in which an entire lipid lens is excised from the ER (35); (c) vesicular budding in which a bilayer vesicle forms, followed by filling of the bilayer intramembranous space with neutral lipids (36); and (d) an “eggcup” model in which a LD grows within a concave depression of the ER through transport of neutral lipids from the ER (37). The latter transfer process resembles a model for prokaryotes (28). All models posit LDs forming toward the cytosolic face of the ER membrane. However, cells, such as hepatocytes, also secrete neutral lipids into the ER lumen (38), and LDs could be derived from luminal origins. Such a model was recently proposed for yeast (29).
Figure 1.

Examples of lipid droplets (LDs). Shown are a schematic overview of LD structure, LDs in Drosophila S2 cells (middle panel), and in murine adipocytes. Images courtesy of Natalie Krahmer and Caroline Mrejen.
Which model is correct? Several obstacles prevent an easy answer. Most cells have LDs, complicating identification of nascent LDs, and systems of induced LD formation in mammals are lacking. Also, the LD size during the initial stages of formation is below the resolution of light microscopy. Thus, combinations of inducible systems with super high-resolution light microscopy and EM will likely be required to gain further insights (see the Box titled Imaging of Lipid Droplets). Alternatively, characterizing a system for in vitro LD formation from ER membranes might allow molecular dissection of different steps of formation.
Imaging of Lipid Droplets.
Given their size (0.5 μm to tens of microns), many LDs are readily detected by light microscopy (see Figure, middle panel). Their refracting nature makes them identifiable with phase-contrast imaging. Several hydrophobic dyes can be used to image LDs. Oil red O is visible by light microscopy and typically used to stain fixed samples. Nile red and BODIPY are concentrated in LDs and fluoresce upon stimulation. They provide a robust signal in living or fixed cells. Care must be taken to avoid artifacts. BODIPY appears to be more specific for staining LDs; Nile red also stains endocytic vesicles. Additionally, BODIPY-tagged FAs can stain LDs, as the tagged FAs in many cases are taken up by cells and esterified and incorporated into neutral lipids.
Because LDs are closely associated with other organelles, it can be difficult to distinguish them from surrounding membranous organelles. This limitation was addressed by the recent development of “super high-resolution” microscopy that overcomes the classical resolution limits of light microscopy, in some cases improving resolution by a factor of 10 (i.e., from ~250 to ~25 nm) (166). In one approach, exemplified by photoactivated localization microscopy or stochastic optical reconstruction microscopy, the sequential photoactivation of single fluorescent molecules allows for the precise determination of their position by fitting a Gaussian curve to the resulting signal, leading to high-resolution reconstructed images. In a complementary approach (stimulated emission depletion microscopy), the resolution limit is increased further by depleting the excited state of fluorophores around a narrow focus of light, thus restricting molecules that emit a signal. Although these techniques are limited to live cells, they promise to develop into valuable tools for LD research.
Several approaches based on Raman spectroscopy are also used to image LDs. Most prominently, coherent anti-Stokes Raman scattering microscopy uses inelastic scattering of photons interacting with matter to detect molecule-specific vibrations and resulting blue-shifted light (167). These approaches also can quantify lipids.
EM methods are also used to analyze LDs (see Figure, right panel; LDs in murine adipocytes, courtesy of Caroline Mrejen), including transmission EM and freeze-fracture EM. Difficulties of fixing and staining lipids during sample preparation often cause LDs to appear as empty spheres in conventional EMs. Fixation and sectioning problems may lead to artifacts, but improved protocols can avoid these problems (89, 168).
5.2. Identifying Genes in Lipid Droplet Formation
To identify genes involved in LD formation, several studies utilized genome-wide screens with LD morphology as a phenotypic readout. Three screens in S. cerevisiae (6, 7, 39) identified gene deletions leading to different LD morphologies. Notably, deleting the yeast ortholog of seipin (FLD1) caused abnormal LDs. Seipin is of particular interest in LD biogenesis. It is an multimeric ER membrane protein (40) whose deficiency in humans results in lipodystrophy. Surprisingly, there was otherwise little overlap between genes identified in the yeast screens, and no single-gene deletion was found to cause the complete absence of LDs.
Genome-wide screens to find genes regulating LD morphology have been performed by RNAi in Drosophila cells (41, 42). In one screen employing oleate loading of cells, over 200 genes were identified. Interestingly, they binned into five phenotypic classes (41). In another screen, over 800 genes showed an effect on LD accumulation (42). Among overlapping hits of both screens knockdowns of proteasome genes yielded fewer LDs, of the ARF/COPI vesicular transport machinery gave large and disperse LDs, and of genes related to phospholipid synthesis led to very large LDs. The latter two classes showed defects in lipolysis, indicating functional consequences of these knockdowns. The ARF/COPI machinery functions in retrograde transport of proteins from the Golgi apparatus to the ER, but its role in lipolysis is uncertain. Adipose triglyceride lipase (ATGL), or its Drosophila homolog Brummer, fails to target to LDs in cells depleted of ARF/COPI machinery (30, 43). The link between phospholipid synthesis genes and LD size is discussed below.
Several systematic screens of genes in whole organisms also focused on lipid storage. Screens in Caenorhabditis elegans (44) and Drosophila (45) revealed a plethora of genes involved in fat storage. Although many of these genes participate in feeding behavior and energy expenditure, some may be involved directly with LDs. Other surveys identified proteins that might be important in LD formation. These include fat-inducing transcript (FIT) proteins, which are ER proteins that bind TG and have been implicated LD assembly (46, 47). FIT2 overexpression results in more LDs and knockdown in fewer LDs and TG, but there is no evidence that FIT proteins affect DGAT activity (47). How FIT proteins organize TG into LDs is unclear. Phospholipases might also be required for LD biogenesis (48).
6. LIPID DROPLETS GROW BY EXPANSION OR BY COALESCENCE
To accommodate more TG, cells form new LDs and grow existing ones (Figure 1). Adding neutral lipids to existing LDs requires local synthesis or transfer from the ER.
Neutral lipids might be synthesized locally at LDs, particularly during lipid loading. DGAT2, which catalyzes the final step of TG synthesis and is normally found in the ER, localizes to LDs during oleate loading (16, 49), as does its yeast ortholog Dga1 (29). It is unclear whether more proximal steps in TG synthesis also localize to LDs. In contrast to TG synthesis, sterol esterification likely occurs primarily in the ER because ACAT enzymes reside in the ER and do not localize to LDs (50).
The synthesis of neutral lipids in the ER necessitates their transfer to LDs, through membrane connections or via interorganelle transport by transfer proteins. Although TG transfer proteins move TG from the ER bilayer to nascent lipoproteins in the ER lumen, no such mechanism is known for cytosolic LDs.
LDs may also grow by coalescence (Figure 1) (51). Coalescence of neutral LDs minimizes the phase boundary area and free energy of the emulsion. Thus, the challenge for cells is to prevent coalescence and maintain LDs as isolated entities. LD emulsions are stabilized by surface proteins (e.g., oleosins) or surfactants (e.g., PC) (12), which prevent coalescence by lowering surface tension. In contrast, accumulation of fusogenic lipids (e.g., phosphatidic acid) might induce LD coalescence (39).
During rapid expansion, LD diameters increase more than threefold within hours—a nearly tenfold increase in surface area. Thus, cells need to synthesize and transport large amounts of phospholipids to expanding LD surfaces. Among phospholipids, PC is key for coating LDs and preventing their coalescence. PC can emulsify artificial LDs in vitro and prevent coalescence (12). During expansion, LDs become PC deficient and CTP:phosphocholine cytidylyltransferase enzymes, which catalyze the rate-limiting step in PC biosynthesis, are activated by binding to LDs (12). The de novo synthesized PC is transported to the expanding LD surface through unknown mechanisms. In this manner, de novo synthesis of PC is activated to maintain adequate PC levels at LD surfaces.
PC to coat the surfaces of LDs is also synthesized from lyso-PC and fatty acyl-CoA precursors by lyso-PC acyltransferase enzymes, which function in the Lands cycle of phospholipid remodeling of FA moieties. Lyso-PC acyltransferase 1 and 2 enzymes localize to and are active on LDs (49). They likely function in remodeling the surface phospholipids rather than providing net PC synthesis, as they cannot yield increased PC unless lyso-PC is provided.
In Drosophila cells, LD fusion happens rarely except under specific conditions, such as PC deficiency (12, 41). PC deficiency likely renders LDs prone to coalesce. A recent report found homotypic LD fusion to occur rarely in murine embryonic fibroblasts or NIH-3T3 cells under normal conditions (52), although higher rates of fusion were also reported (51). However, numerous pharmacological agents stimulated LD fusion in different cell types. These included propranolol and other drugs, albeit at supraphysiological concentrations, which may trigger fusion by inserting into and disrupting LD surface monolayers (52). Fusion was relatively slow, occurring over seconds to minutes. The volumes of fusing LDs were conserved, and surface areas were decreased, suggesting excess surface lipids are removed during fusion.
Proteins might be involved in catalyzing LD fusion. One such protein is a fat-specific protein of 27 kDa [FSP27, or cell death-inducing DFF45-like effector C (Cidec)]. In adipocytes, FSP27 is expressed as a peroxisome proliferator-activated receptor-γ (PPARγ)-regulated gene and promotes the formation of unilocular LDs (53–55). Mice lacking Fsp27/Cidec have multilocular white adipocytes, implying the protein forms large LDs (53, 55, 56). If Fsp27 is expressed ectopically in fibrobasts, LD sizes increase. The mechanism is unclear but may include promoting LD fusion or inhibiting lipolysis (53–55, 57, 58).
Because SNARE proteins (soluble N-ethylmaleimide-sensitive factor attachment receptor proteins) mediate homotypic fusion of bilayer-bounded vesicles during cellular trafficking, they are attractive candidates for LD fusion. In studies performed in NIH 3T3 cells, knockdowns of genes encoding SNAP23, syntaxin-5, and VAMP4 were reported to decrease the rate of LD fusion (59). However, it is unclear how SNARE proteins would mediate fusion of monolayer-bound vesicles, and more studies to address SNARE involvement are needed.
7. LIPID DROPLET PROTEINS
7.1. Specific Proteins Localize to the Surfaces of Lipid Droplets
LDs are characterized by specific proteins on their surfaces. Analyses of LD proteomes in different organisms yielded diverse lists of LD-associated proteins (21, 60–65). The relatively easy purification of LDs (by centrifugation) facilitates proteomic analyses, but their close association with other organelles, particularly the ER, confounds such analyses. Thus, it is often unclear which proteins are genuine LD proteins. In addition, researchers may overlook LD proteins because they have other well-known functions. For example, histones were unexpectedly found by LD proteomics and subsequently confirmed to transiently target LDs in Drosophila embryos (23). LDs may similarly transiently store other proteins that otherwise might aggregate. For example, α-synuclein, a Parkinson’s disease–associated protein prone to self-aggregation, localizes to LDs (66).
Some data on LD protein localization can be misleading. By fluorescence microscopy, many proteins reportedly localize to LDs upon oleate loading of cells. These studies assume that a characteristic ring-like appearance of proteins surrounding LDs indicates targeting to the LD-delimiting surface monolayer. In ultrastructural studies, however, membranous structures often accumulate juxtaposed to LDs, so some proteins might localize to LD-associated membranes rather than the LD surface itself (30). Indeed, many reports show no overlap between the LD signal (e.g., from neutral lipid staining) and the LD-encircling protein rings. Because light microscope resolution is ~250 nm, such proteins might actually localize to membranes at a distance from the LD surface.
7.2. Protein Signals that Mediate Lipid Droplet Targeting
How proteins target LDs is not understood, but some mechanisms are emerging (Figure 2), including (a) direct binding to the monolayer surface via amphipathic helices, (b) embedding short hydrophobic regions localized at theN terminus, (c) embedding internal protein domains that penetrate the LD with hydrophobic spans (e.g., hairpin-shaped hydrophobic helices), (d) lipid anchors, and (e) interaction with other LD-bound proteins. By contrast, proteins with hydrophilic regions on either side of bilayer-spanning helices (e.g., DGAT1) are unlikely to be on the monolayer surface of LDs, as this would be energetically unfavorable.
Figure 2.
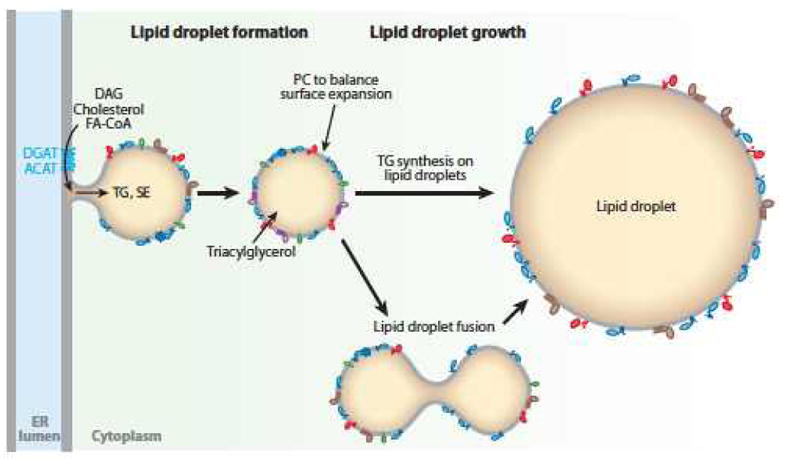
Models of lipid droplet (LD) formation and expansion. For formation, the budding model is shown, with the LD either remaining attached to the endoplasmic reticulum (ER) or detached. The neutral lipid synthesis enzymes, ACAT or DGAT, catalyze the formation of lipids that fill the core. For lipid droplet expansion, the models of local synthesis of triacylglycerol (TG) at the LD and of LD fusion (coalescence) are shown. Abbreviations: DAG, diacylglycerol; FA-CoA, fatty acyl-coenzyme A; SE, sterol ester; PC, phosphatidylcholine.
Examples of proteins containing amphipathic helices as targeting signals include the HCV core protein (67), viperin (endogenous inhibitor of HCV) (68), and CTP:phosphocholine cytidylyltransferase enzymes (12, 41). How amphipathic helices distinguish the LD surface monolayer from other cellular membranes is unclear. CTP:phosphocholine cytidylyltransferase 1 binds to and is activated by PC-deficient LD surfaces (12), one of few cases where a specific lipid composition of the LD surface mediates amphipathic helix recruitment. Another example is recruitment of perilipin3/Tip47 to the ER with diacylglycerol (DAG) accumulation, which was suggested to coordinate DAG buildup with LD protein recruitment and LD formation (33).
For other organelles, specific lipids (e.g., phosphoinositides, phosphatidylserine, and phosphatidic acid) mediate protein targeting to membranes. No lipid signals are known to target proteins to LDs, but it is possible because the lipid composition of the LD surface differs from that of the ER (19, 69).
Several proteins (e.g., AAM-B, UBXD8, or ALDI) (70, 71) possess an N-terminal, hydrophobic, short domain (minimally ~28 amino acids) that is necessary and sufficient for LD targeting. These proteins localize to the ER under some conditions (e.g., in the absence of LDs), suggesting they first are inserted into the ER and subsequently transported to LDs. Their domain structure and the molecular mechanism(s) to target LDs are unknown.
Some proteins (e.g., caveolin, oleosins, and 17-hyrdoxysteroid dehydrogenases) have an internal hydrophobic domain of variable length that likely forms a hairpin structure that could be integrated into a phospholipid bilayer or LD monolayer. For plant oleosins, members of this class, the topology and some requirements have been elucidated. Oleosins are important for storing oils in seeds, which account for up to 8% of their protein. LD targeting of oleosins initiates with their synthesis at the ER (72, 73), followed by targeting to LDs. A hairpin contains a central proline knot (three prolines in 12 amino acids) that is essential for LD targeting and might induce a sharp bend between two adjacent hydrophobic protein segments (74). The hairpin domain might thermodynamically favor LD localization (i.e., the space between the ER membrane bilayer may be limiting). Alternatively, proteins recognizing such hairpins may be in involved in LD localization.
In mammalian cells, caveolins contain a similar hairpin motif. Caveolins primarily localize to caveolae at the plasma membrane but also to LDs. Initially, LD targeting of caveolin was observed in cells treated with brefeldin A, which blocks membrane trafficking, or with caveolin-1 mutants (75–77). However, targeting occurs under physiological conditions (e.g., during lipid loading) (78, 79). At the plasma membrane, caveolin topology includes a central hairpin membrane anchor and adjacent lipid-binding domains. For LDs, the topology of caveolin is unknown, but it may be similar. The hydrophobic sequences of AAM-B, ALDI, or DGAT2 may also form hairpins. For enzymes, such as DGAT2, it has yet to be demonstrated that the protein localizes directly on the LD surface (versus the adjacent ER), and, if so, whether it is active at this location.
Several different LD targeting mechanisms exist for perilipins (perilipin1, perilipin2/ADRP/adipophilin, perlipin3/Tip47, perilipin4/S3-12, and perilipin5/OXPAT), which are the first identified specific LD marker proteins (80). Perilipins are not essential for LD formation (e.g., yeast lack perilipins) (34) but are important for regulating lipid metabolism at LDs. In mammals, several perilipins are expressed ubiquitously (e.g., perilipin2/ADRP and perilipin3/Tip47), whereas others are expressed specifically in certain cell types (e.g., perilipin1 in white adipocytes and perilipin5/OXPAT in highly oxidative cell types).
Adipocytes express high levels of perilipin1, which is involved in the regulation of lipolysis. Perilipin1 contains a combination of domains interacting with LDs. Particularly important are three C-terminal hydrophobic stretches, which may penetrate LDs (81). Additional amphipathic stretches of the protein likely interact with the LD surface. Similarly, both N- and C-terminal regions of perilipin2/ADRP contribute to LD binding (82, 83). Perilipin2/ADRP localization to LDs may also involve ARF1/COPI (84).
Perilipin3/Tip47 may bind to LDs like apolipoprotein E (apo E) binds to lipoproteins. Its C-terminal four-helix bundle and α/β-domain (85) are similar to the N-terminal four-helix bundle of apo E. Apo E binds lipoproteins by opening its four-helix bundle and exposing hydrophobic, amphipathic sequences to the lipid surface (86). Perilipin3/Tip47 might use a similar mechanism. Unlike perilipin1 and perilipin2/ADRP, which are unstable when not bound to LDs, perilipin3/Tip47 is found in the cytoplasm when LDs are absent (87).
As an alternative to protein segments mediating LD interactions, lipid modifications of proteins may serve as anchors to the LD surface. Rab18, a small GTPase, localizes to LDs, where it mediates ER interactions (88, 89). By analogy to other Rabs with C-terminal lipid anchors, Rab18 might target to LDs by a prenylation anchor combined with protein-protein interactions. Unlike other Rabs, Rab18 contains one, rather than two, lipid modifications.
Proteins might also localize to LDs by interacting with LD-bound proteins. Regulated recruitment of hormone-sensitive lipase (HSL) to LDs is an example. Under basal conditions, HSL is mostly cytosolic, and access to LDs is restricted by perilipin1. Upon hormonal stimulation, perilipin1 is phosphorylated, which recruits HSL to LDs.
7.3. Cellular Pathways Involved in Targeting Proteins to Lipid Droplets
Pathways targeting proteins to LDs are less understood than the protein signals. Several mechanisms may be involved, including direct recruitment from the cytosol (Figure 2). For some membrane proteins, vesicular trafficking may be important. The ARF/COPI machinery, which mediates Golgi to ER vesicular trafficking, is required for normal LD turnover and localization of the major TG lipase, ATGL, or its Drosophila homolog, brummer, to LDs (30, 90). A fraction of ATGL colocalized with ER-exit sites (ER membrane domains dedicated to formation of secretory vesicles) and the expression of a dominant-negative Sar1 (GTPase is required for vesicles to leave the ER exit sites) inhibited ATGL targeting to LDs (30). ATGL requires a C-terminal hydrophobic stretch for LD localization (91) and may behave biochemically as a membrane-associated protein (30). Thus, ATGL may contain a hydrophobic sequence (e.g., hairpin loop) targeted to the ER and trafficked to LDs. From EM studies, cisternal structures around LDs could represent a LD-target compartment for vesicular trafficking (30). It is unclear how ATGL or similar proteins would move from this compartment to the LD surface.
Physical bridges may connect LDs and the ER (29, 31, 92). Such bridges may allow membrane-bound proteins, such as those with hairpins, to diffuse from the bilayer to the LD surface. Data from yeast support this model; fluorescently labeled LD proteins, such as Dga1, rapidly exchange with other membrane pools (29).
7.4. Removal of Lipid Droplet Proteins
Mechanisms to remove LD proteins are likely similar to those for membrane proteins. Endocytosed membrane proteins or autophagic vesicles are degraded in lysosomes. ER membrane proteins can be degraded by proteasomes (ER-associated degradation). Both mechanisms may degrade LD proteins. LDs are delivered to autophagosomes (93), and some LD proteins are modified by ubiquitin for proteasomal degradation (e.g., some perilipins) (94–100). Additionally, a nonbiased screen identified proteasome components as required for normal LD morphology (41).
Molecular links between LDs and proteasomal degradation were recently identified. Among LD proteins, ancient ubiquitous protein 1 (Aup1), located in the ER or directly on LDs, binds ubiquitin E2 ligase, Ube2g2 (101, 102). The ubiquitin E3 ligase, spartin/SPG20, localizes to LDs, and its depletion or overexpression leads to LD accumulation, perhaps from the altered turnover of LD proteins, such as perilipin2/ADRP (97, 100). These findings imply that a specific machinery degrades LD-associated proteins, but the details are unknown.
Some ER-associated degradation substrates may transiently localize to LDs (e.g., HMG-CoA reductase when proteasomal degradation was inhibited) (103). However, at least in yeast, LDs are not required for ER-associated degradation (104).
8. LIPID DROPLETS INTERACT WITH OTHER CELLULAR ORGANELLES
LDs interact with other organelles, including the ER, endosomes, mitochondria, and peroxisomes. Freeze-fracture imaging showed ER membranes in mammalian cells are often wrapped around LDs in a shape resembling an eggcup (37). Because interactions between membrane-bound organelles are implicated in lipid exchange, apposition of LDs with the ER may facilitate lipid transfer between them. Oxysterol-binding proteins localize to LDs (105), where they might be involved in lipid trafficking or neutral lipid metabolism. In steroidogenic cells, ER-LD interactions might function in the synthesis or catabolism of steroid hormones (106). In yeast, ER-LD junctions at least partly reflect intermediates of LD formation (6, 7, 29, 40), and seipin may participate in this process.
Mechanisms mediating ER association with LDs are unknown, but the small GTPase Rab18 may be involved (88, 89). Because Rab18 is recruited to LDs late, its localization likely does not reflect a role during LD formation. Rab18 localization is mutually exclusive with LD localization of perilipin 2/ADRP or a dominant-negative mutant of caveolin (88, 89), suggesting a specialized function. Indeed, Rab18 localization to LDs in cultured adipocytes is related to lipolysis stimulation (89).
Sometimes endosomal structures appear to enwrap LDs (89). These interactions may be important for delivering LDs to lysosomes by autophagy in macrophages to generate cholesterol for ABCA1-dependent efflux (107). Rab5 was detected on purified LDs and implicated in the interaction of early endosomes with LDs (108).
LDs also associate with mitochondria (109) and peroxisomes (110). These interactions may channel FAs liberated from lipolysis to sites of oxidation. Supporting this notion, exercise training increases the number of LDs and their contacts with mitochondria in skeletal muscle (111).
9. LIPID DROPLET MOVEMENT WITHIN CELLS IS SOMETIMES COORDINATED AND MICROTUBULE DEPENDENT
LDs are usually dispersed and move in small oscillations. However, in mammalian cells, perilipin1 expression leads to LD clustering, which is reversed upon perilipin1 phosphorylation (112). Similarly, when Drosophila cells (which lack perilipin1) synthesize TG for a prolonged time (e.g., during 24 h of oleate loading), newly formed LDs eventually cluster into organized superstructures (41). Increased clustering is also observed when genes involved in protein synthesis are depleted from S2 cells (41). What determines this clustering and its purpose are unknown.
LD distribution in the cell may be partially determined by active transport. LDs move directionally in axons of Aplysia by uncharacterized mechanisms (113). LDs in the Drosophila embryonal syncitium move synchronously from the periphery to a central location and back again. Although the function of this movement is unclear, its disruption leads to altered transparency of the embryo, providing a system to screen for mutants of LD transport machinery (114). Such studies revealed that LDs move bidirectionally along microtubules with dynein and kinesin motors, which pull LDs in opposite directions. Net movement depends on the relative activities of the motors. The LD protein Halo interacts with kinesin1 as part of a complex that includes the regulatory Klar protein and the perilipin-like protein Lsd2 (115). The complex appears to link LDs to microtubules and to regulate kinesin1’s activity during LD transport. Microtubules have also been implicated in LD clustering in mammalian cells, but supporting evidence is limited. Interestingly, the HCV core protein promotes clustering of LDs around microtubule organizing centers (116).
10. LIPIDS IN LIPID DROPLETS ARE MOBILIZED BY LIPASES
10.1. Lipid Droplet Catabolism by Lipolysis
In lipolysis, lipids are hydrolyzed to liberate FAs. Cells storing TG use lipolysis to generate energy and membrane lipids. In multicellular organisms, energy storage occurs predominantly in adipose tissue, and most of the knowledge concerning lipolysis comes from studies in adipocytes, where FAs are hydrolyzed sequentially by ATGL, HSL, and monoacylglycerol lipase. During lipolysis, LDs shrink as core lipids are catabolized. With decreased volume, the surface area contracts. It is unknown if excess proteins and phospholipids are resorbed into the ER or degraded.
ATGL contains an N-terminal domain with similarity to patatin, a plant acyl-hydrolase expressed highly in the potato tuber, which contains the catalytic activity. It is unknown which of the TG fatty acyl-esters are hydrolyzed by ATGL. Among the potential DAG species produced, only sn-1,2-DAG is known to act as a signaling molecule, and sn-1,3-DAG is not likely to be an optimal substrate for re-esterification by DGAT enzymes.
The ATGL N terminus also interacts with CGI-58, which has a hydrolase fold and is an ATGL activator. The activation mechanism is unknown, but it requires interaction of both proteins and CGI-58 with LDs (117). G0S2 may inhibit ATGL, although its function is unclear (118). Interaction of ATGL and perilipin1 regulates lipolysis. In mammalian adipocytes, lipolysis is initiated by hormones that trigger β-adrenergic stimulation to activate protein kinase A (PKA). In unstimulated cells, CGI-58 is bound to LD-localized perilipin1. Perilipin1 restricts basal lipolysis by sequestering CGI-58 or by sterically shielding LDs from lipolysis, or by a combination of both (119, 120). Upon activation, perilipin1 is phosphorylated by cAMP-dependent PKA at Ser492 and Ser517, releasing CGI-58 to bind and activate ATGL (121).
ATGL’s interaction with CGI-58 also targets the lipase to LDs, where it is activated (122, 123). Similarly, interactions of ATGL and perilipin5/OXPAT (but not other perilipins) recruit ATGL to LDs, but in this case, the interaction limits lipolysis (122, 123). Because perilipin5 is expressed mostly in oxidative tissues, perilipin5’s interaction with ATGL might be used in those tissues to regulate ATGL differently than it does in others. Perilipin5/OXPAT interacts with CGI-58, possibly forming high-molecular-weight assemblies (122). These data suggest a complex interplay among ATGL, perilipins, and CGI-58 to mediate LD targeting and lipolysis (122).
HSL catalyzes the second step of lipolysis and is also regulated by hormones. PKA activation leads to HSL phosphorylation at multiple sites, of which Ser650 (human HSL) is particularly important (124). Phosphorylation increases HSL lipolytic activity twofold. This mechanism is combined with regulated recruitment of HSL to LDs (125). Specifically, PKA-phosphorylated perilipin1 (at Ser81, Ser222, and Ser276, particularly) recruits HSL from the cytosol to LDs (125). With HSL phosphorylation, recruitment to LDs increases HSL activity 100-fold (126).
In the final lipolysis step, monoacylglycerol is hydrolyzed by monoacylglycerol lipase and possibly by HSL (127). No evidence indicates the monoacylglycerol lipase–catalyzed reaction occurs at LDs or is regulated by hormones.
Lipolysis in nonadipocyte cells is not as well understood, but similar steps must exist. Unfortunately, sequence homology cannot predict functions in proteins with hydrolase motifs. Adiponurtrin/PNPLA3, another member of the patatin-like family, is expressed in liver, and a human adiponurtrin/PNPLA3 variant (I148M) is associated with nonalcoholic fatty liver disease (128). However, adiponurtrin/PNPLA3 knockout mice do not have LD and TG accumulation in liver (129, 130), so the biochemical and physiological roles of this protein are unknown.
In addition to TG, other lipids are hydrolyzed at LD surfaces by phospholipases and other enzymes. For example, anandamide and other N-acylethanolamines traffic to and are catabolized at LDs (131).
10.2. The Fate of Hydrolyzed Lipids
FAs liberated from TGs in LDs have one of several fates. They can be re-esterified to TG, used for β-oxidation to generate energy, used as building blocks for membrane lipid synthesis, or used as cofactors for cell signaling, or exported.
In adipocytes, many FAs generated by lipolysis are re-esterified by DGAT or MGAT enzymes to TG, although the percentage drops with fasting (132). It is unclear whether re-esterification occurs in the ER or at LD surfaces. Because DGAT enzymes use activated FAs bound to CoA, re-esterification includes generating fatty acyl-CoA esters by long-chain acyl-CoA synthetases (ACSLs) in a reaction that requires the input of cellular energy. Among ACSL proteins, ACSL3 and -4 are recruited to LDs specifically during lipolysis, and ACSL1 constitutively localizes there (62, 65). Specific ACSLs, therefore, may act at LD surfaces as part of the re-esterification cycle. The function of TG turnover in this futile cycle is unknown but may fine-tune lipolysis and preserve stores of essential FAs.
FAs liberated from LD hydrolysis can be utilized directly for β-oxidation. Contact sites between LDs and mitochondria and peroxisomes may directly transfer FAs (discussed above). FAs may also be used to synthesize membrane lipids. Like re-esterification, this reaction requires FA activation by attaching a CoA moiety, possibly at LDs (65, 133). FAs liberated by ATGL also serve as endogenous activators of PPARα, as shown by lack of PPARα signaling in hearts of ATGL-deficient mice (134).
10.3. Lipid Droplet Catabolism by Autophagy
Lipids in LDs can also be degraded by lipases within lysosomes after delivery by macroautophagy. Like lipolysis, macroautophagy is induced during nutrient deprivation and responds to hormonal signals. In macroautophagy of LDs (or macrolipophagy), an LD, whole or in part, is engulfed by a membrane bilayer with the activated PE-modified LC3-II protein. The resulting autophagosome containing an LD is delivered to the lysosome where acid lipases liberate FAs from TG. This last step recapitulates the fate of lipoprotein-derived TG delivered to lysosomes via endocytosis. The relative contributions of LD autophagy to LD catabolism are unclear and may vary among tissues. LD autophagy was first reported in hepatocytes, where expression levels of ATGLs and HSLs are relatively low (93). Hepatic-specific inactivation of autophagy leads to hepatic steatosis, consistent with a function of macroautophagy in hepatic lipid metabolism. The molecular events that trigger LD autophagy are unknown.
11. LIPID DROPLETS FIGURE PROMINENTLY IN PHYSIOLOGY AND DISEASE
We cannot review in detail the metabolic and physiological processes that involve lipid storage and LDs. Instead, we focus on describing LDs in various cell types and present examples of how LDs are linked to physiology and disease. Other reviews address medical aspects of LD biology (135).
11.1. Lipid Droplets and Lipid Storage in Tissues
11.1.1 Adipose tissue
Some insects and most vertebrates have highly specialized white adipocytes and adipose tissue. In most invertebrates, TG is distributed throughout the body, often in connective tissue that fills gaps between other tissues, or in enterocytes of C. elegans. In flies, fat is located predominantly in a centrally located fat body. In vertebrates, adipose tissue is distributed to distinct regions and regulated by factors, such as hormones. With the evolutionary advent of homeothermy (in birds and mammals), adipose tissue gained a function as a subcutaneous insulator (136).
Adipocytes are the most highly specialized cell type for storing lipids in LDs, and in white adipocytes, a single large LD frequently occupies most of the cytoplasm. Human fat cells can store vast quantities of energy in LDs, primarily as TG. Adipocyte LDs also store cholesterol esters and fat-soluble vitamins. As expected, the adipocyte gene expression profile reflects the high flux of lipid synthesis, storage, and turnover. Adipocyte fat content is coupled to leptin expression, the main adipocyte-derived hormone that regulates long-term energy homeostasis (e.g., by regulating food intake) (137).
Brown adipocytes in mammals catabolize lipids in mitochondria that are uncoupled from oxidative phosphorylation to generate heat. LDs are prominent but smaller and more numerous than those in white adipocytes. The study of brown adipocytes is likely to shed light on interactions among LDs, mitochondria, and FA oxidation.
11.1.2. Liver
Next to adipose tissue, liver has the greatest capacity to store lipids in LDs. At basal conditions, the fraction of the cytoplasm occupied by LDs in hepatocytes is smaller than in adipoctyes, but they can hold massive amounts of TG, as illustrated by foie gras. In humans, abnormal LD accumulation is called hepatic steatosis.
LDs in hepatocytes are typically multilocular, and their formation and consumption are dynamic in normal physiology. For example, during overnight fasting in mice, large amounts of FAs are mobilized from TG stores in white adipose tissue and taken up by the liver to form LDs, generating a fatty liver. Hepatocytes express DGATs and ACATs, and the lipid composition of LDs partly reflects substrate availability. The liver also has large numbers of stellate cells that store retinol (vitamin A) as retinyl esters in LDs (17).
11.1.3. Small intestine
LDs are dynamic, prominent organelles in intestinal enterocytes (138). They reflect the enormous capacity of small intestine to absorb fat from a meal. Enterocytes have a large surface area and significant ability to synthesize and store TGs. The intestine absorbs over 95% of the TG in a typical meal, leading to rapid formation of LDs in enterocytes until TGs are exported from the cell as part of chylomicrons. This process typically takes minutes to hours after a meal. Many aspects of this process remain unclear.
11.1.4. Yolk sac
During embryonic development in many animals, the yolk sac endoderm functions like the liver and intestine to hold lipids from the mother that are destined for the developing embryo (139). As in the intestinal or liver cells, similar processes of fat uptake, storage in LDs, and export on apoB-containing lipoproteins occur in the yolk sac (139).
11.1.5. Skeletal muscle
Oxidative (type I, slow-twitch) muscles have a high rate of oxidative metabolism and are rich in mitochondria and LDs. In obese individuals, intramyocellular TG accumulation in type I muscles is associated with insulin resistance. However, in highly trained athletes, TG stores in LDs in oxidative muscle are increased without negative consequences. This “athlete’s paradox” has been linked to DGAT1 function (140, 141). LDs in oxidative muscle are often close to mitochondria (111, 142). Maintaining coupling of lipid storage with consumption of lipids for fuel appears to be important for efficient energy utilization.
11.1.6. Adrenal cortex
LDs are prominent in the adrenal cortex. They store large amounts of SEs, presumably as cholesterol for steroid hormone synthesis. In fact, the yellow color of adrenal glands is due to lipid storage, and mice lacking ACAT1, and thus cholesterol esters and LDs, have pale-colored adrenal glands. Surprisingly, adrenocortical hormone synthesis in ACAT1 knockout mice is normal. Like adrenocortical cells, other steroidogenic cells, such as Leydig cells of the testes, often have prominent LDs. Additionally, LDs are found in oocytes.
11.1.7. Macrophages
Macrophages store lipids in LDs (e.g., after phagocytosis of modified lipoproteins in the arterial wall) until they are exported. Macrophages express ACAT and DGAT enzymes, and the content of their LDs reflects their exposure to substrates. Macrophage foam cells store large amounts of cholesterol from lipoproteins, such as low-density lipoproteins. Macrophages utilize LDs to store arachidonic acid, the precursor for bioactive lipids (e.g., prostaglandins and leukotrienes) in inflammation.
11.2. Excessive Lipid Droplet Storage and Disease
Many metabolic diseases are characterized by excessive lipid storage in LDs. In these diseases, lipids may exceed the cellular capacity to store them and to buffer against their toxic effects. Unesterified lipids, such as cholesterol or FAs and their derivatives, can trigger inflammatory responses that result in tissue damage, fibrosis, scarring, and potentially organ failure.
11.2.1. Obesity and diabetes as diseases of excessive lipid droplets
Obesity is a state of excess lipid storage and LDs in the adipose tissue, often accompanied by lipid deposition and excessive LDs in nonadipose tissue, which cause lipotoxicity or tissue dysfunction. Whether obesity results in complications, such as type 2 diabetes mellitus, may relate to the capacity of LDs in adipocytes or macrophages to store lipids. In transgenic mice, increasing adipocyte capacity to store TG results in obesity without diabetes (143, 144), thereby uncoupling the two. Similarly, increasing DGAT1 expression selectively in macrophages avoids many metabolic consequences of obesity (145). The LD capacity of macrophages to store neutral lipids therefore might profoundly influence pathophysiology. Taken together, LD-targeted strategies for therapies for pathologies associated with obesity can be aimed at blocking lipid absorption and LD formation in the small intestine, preventing influx into the system; catabolizing, rather than storing, excess lipids through oxidation; and increasing LD capacity in adipocytes or macrophages to store toxic lipids and prevent associated inflammation and tissue damage.
Obesity is often accompanied by hepatic steatosis or LD accumulation in the liver, affecting millions of people. Many individuals progress to hepatic inflammation (called nonalcoholic steatohepatitis), and some go on to develop fibrosis and liver failure. Possibly, the capacity of hepatocytes to store lipids in LDs is exceeded, promoting inflammation. Hepatic steatosis is also strongly associated with hepatic insulin resistance, although the causative mechanism is unclear. Proposed contributing mechanisms include alterations in insulin signaling pathways mediated by bioactive lipids, ER stress, and activation of inflammation signaling (146). A common theme, however, relates to limited hepatocyte capacity to secrete or store lipids, thereby leading to lipid excess and liver dysfunction.
11.2.2. Atherosclerosis and macrophage lipid droplets
Like obesity, atherosclerosis is associated with excessive deposition of lipids in tissues, in this instance cholesterol, derived from apo B-containing lipoproteins in arterial walls. Excess cholesterol is taken up and esterified by macrophages. Cholesterol esters in turn are stored in LDs until they can be mobilized and effluxed to high-density lipoproteins, which are transported to the liver for clearance of cholesterol in bile. Macrophages full of cholesterol ester–containing LDs are called foam cells. LDs protect macrophage foam cells from toxicity because of excess free cholesterol. Deletion of ACAT1, the primary enzyme responsible for esterifying cholesterol in macrophages, in mice does not prevent atherosclerosis and even exacerbates it (147). Additionally, lack of cholesterol esterification in macrophages results in deposition of large amounts of free cholesterol in the skin and brains of ACAT1 knockout mice that were crossed into a hyperlipidemic genetic background (147). These studies highlight the lipid-buffering function of LDs and how storage of potentially toxic lipids prevents lipotoxicity.
11.2.3. The heart and lipid droplets
Although FAs are a fuel for heart muscle, few large LDs are normally present in cardiac myocytes. With lipid overload, however, LDs accumulate and heart dysfunction may occur. In murine models, overexpression of ACSL1 in the heart results in cardiomyopathy and severe heart dysfunction (148). Interestingly, this can be rescued by targeting DGAT1 overexpression to the heart, which esterifies the excess fatty acyl-CoAs to store them as TG in LDs (149). In contrast, ATGL deletion causes lipid accumulation in the heart and severe cardiomyopathy, which results in death of mice (150). Intriguingly, this phenotype can be rescued by treatment with a PPARα agonist (134), which likely increases fat oxidation in the tissue.
11.3. Lipodystrophies and Too Few Lipid Droplets
Absence or deficiency of white adipose tissue, or lipodystrophy (reviewed in Reference 151), results in an LD deficiency. Genetic causes for lipodystrophy include mutations in genes that encode for an acylglycerol-phosphate acyltransferase (AGPAT2), seipin (BCSL2), caveolin (CAV1) for generalized lipodystrophy, and lamin A/C (LMNA), peroxisome proliferator-activated receptor-γ (PPARG), Akt2/protein kinase B (AKT2), and endoprotease Face-1 (ZMPSTE24) for partial lipodystrophy. The pathogenesis of lipodystrophies is not understood for all causes but can relate to impairments in the development of white adipose tissue; reduced ability to synthesize glycerolipids and TGs; or an apparent deficiency in LD formation, as in lipodystrophy related to the absence of seipin (BSCL2). When LDs of white adipose tissue are insufficient to store TGs, the liver assumes this role, and massive hepatic steatosis often results. Associated leptin deficiency results in many metabolic abnormalities, including lipid deposition in nonadipose tissue and insulin resistance. As a consequence, leptin replacement has emerged as a therapy for some lipodystrohies.
11.4. Lipid Droplets and Cancer
Metabolism in cancer cells, including lipid metabolism (11), has received increased interest for therapeutic interventions. Most cancer cells upregulate FA synthesis, presumably to provide FAs for membrane proliferation. Some tumor cells and associated inflammatory cells have prominent LDs (11). The mechanism for LD accumulation in cancer cells is unclear but could relate to increased FA synthesis or impairments in FA oxidation.
11.5. Lipid Droplets and Lactation
LDs are prominent in mammary epithelial cells of lactating mammary glands, where they essentially serve as a secreted organelle, transferring lipid nutrients and energy from mother to child. In this process, LDs are secreted from the apical side of epithelial cells (reviewed in Reference 152), and at least three proteins are involved. Butyrophilin, a transmembrane protein, localizes to the apical plasma membrane. Xanthine oxidoreductase, an enzyme oxidizing purines and other molecules, has a role in the envelopment of LDs by the plasma membrane (153). Perilipin2/ADRP may act as a LD-specific anchor. The disruptions of butyrophilin and xanthine oxidoreductase genes resulted in impaired lactation (153, 154). Interestingly, the lactation defect in perilipin2/ADRP gene disruption was not severe, most likely because a truncated protein was still produced from the allele (155). Disruption of DGAT1 also impairs lactation in homozygous females (156), which relates to impaired development of the mammary gland and lack of LDs for milk production (157).
12. LIPID DROPLETS ARE TARGETS OF INDUSTRIAL OIL PRODUCTION
Like other organisms, plants store TG. In particular, seeds store oil, and different species store varying amounts of oil in LDs (often referred to as oil bodies) in different parts of the seed. Most plants, including soybean (Glycine max), sunflower (Helianthus annuus), safflower (Carthamus tinctorius), and Brasicaceae, store oil in the embryo itself. In other plants, such as castor bean (Ricinus communis), oil accumulates mostly in the triploid endosperm surrounding the embryonic tissue. In most of these species, the TG provides metabolic energy for the seedling upon germination. Some species, such as oat grains, fuse large numbers of LDs in the endosperm, and this oil attracts fruit predators that disperse seeds. The FA composition of TG in seeds differs widely with various chain length and levels of desaturation and is genetically controlled. The synthesis of TG is mediated by enzymes analogous to those in mammalian cells, including GPATs, AGPATs, lipin (PA hydrolase), and DGATs. In addition to DGAT1 and DGAT2, an additional DGAT enzyme was recently purified from the cytosol of peanuts (Arachis hypogaea) (158). A polymorphism of DGAT in maize (Zea mays) increases oil content substantially (159). As an additional complextiy in plants, TG generation involves cross talk between four different compartments: the cytosol for glycerol production, plastids for the production of FAs, mitochondria for the production of very-long-chain FAs in some plants, and the ER for LD (oil body) formation.
LD production in plants is part of a highly regulated differentiation program, and many TG synthetic enzymes are under combinatorial control of several transcription factors, including activators and repressors (160). Most likely from this highly integrated process, attempts to increase oil production in seeds by overexpressing TG synthesis enzymes have been disappointing. However, overexpression of Arabidposis thaliana DGAT in tobacco plants increased TG content of leaves 20-fold, providing a potential strategy for biofuel production (161).
When seeds germinate, they mobilize TG in oil bodies, using a lipase (Sdp1) similar to ATGL (162). Additional proteins, such as steroleosins, regulate lipolysis and β-oxidation during germination (163). In EMs, plant LDs appear to contact glyoxisomes, where FAs may be transported for β-oxidation (164).
Similar to animal LDs, plant oil bodies are decorated with specific proteins. Most notably, oleosins prevent the coalescence of oil bodies and shield them from lipolysis (165), with the latter function similar to that of mammalian perilipins.
Industries have targeted biodiesel production from crops, such as palm or oilseed rape. However, increasing production of these plants in which only a fraction is used for oil presents challenges because it puts them in competition for arable land with food crops. Thus, TG production in algae, or other microorganisms, for biofuel has received increasing attention. To generate biofuels, algae must be grown and harvested, and the lipids extracted and processed. Considerable effort has gone into identifying suitable species for cultivation in open ponds or photobioreactors and into optimizing other production steps. To be economcially and environmentally viable, biofuel production from algae must have a positive life-cycle analysis, meaning a positive balance of energy yield compared with the energy required for biofuel generation. This is challenging, and thus efforts to optimize growth systems and conditions are underway. Nevertheless, engineering of metabolism in algae toward oil production (e.g., by overexpressing DGAT or acetyl-CoA carboxylase enzymes) is promising.
Figure 3.
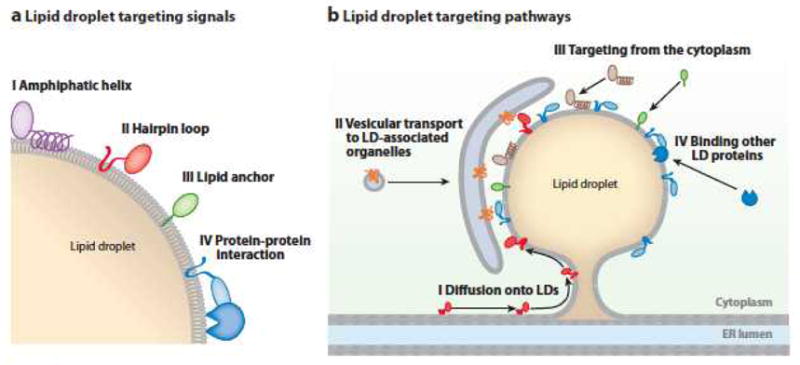
Models of proteins that target lipid droplets (LDs) and the pathways for their targeting. (a) Some examples of the types of proteins that target the phospholipid monolayer of a LD surface are shown. (b) Several mechanisms by which proteins may target the surfaces of LDs or adjacent membranes are shown. Abbreviation: ER, endoplasmic reticulum.
Acknowledgments
We acknowledge the many scientists who contributed to the knowledge in the field, some of which we were unable to cite owing to space constraints. In particular, we salute the pioneering contributions of cell biologists Constantine Londos and Richard Anderson, who helped to launch the field of LD biology. We thank current and former members of the Walther and Farese laboratories for their many contributions to the ideas contained in the review, Sylvia Richmond for assistance with manuscript preparation, and Gary Howard for editorial assistance.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Tobias C. Walther, Email: tobias.walther@yale.edu.
Robert V. Farese, Jr., Email: bfarese@gladstone.ucsf.edu.
LITERATURE CITED
- 1.Farese RV, Jr, Walther TC. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell. 2009;139:855–60. doi: 10.1016/j.cell.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujimoto T, Parton RG. Not just fat: the structure and function of the lipid droplet. Cold Spring Harb Perspect Biol. 2011;3:a004838. doi: 10.1101/cshperspect.a004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thiele C, Spandl J. Cell biology of lipid droplets. Curr Opin Cell Biol. 2008;20:378–85. doi: 10.1016/j.ceb.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem. 1991;266:11341–46. [PubMed] [Google Scholar]
- 5.Alvarez HM, Steinbuchel A. Triacylglycerols in prokaryotic microorganisms. Appl Microbiol Biotechnol. 2002;60:367–76. doi: 10.1007/s00253-002-1135-0. [DOI] [PubMed] [Google Scholar]
- 6.Fei W, Shui G, Gaeta B, Du X, Kuerschner L, et al. Fld1p, a functional homologue of human seipin, regulates the size of lipid droplets in yeast. J Cell Biol. 2008;180:473–82. doi: 10.1083/jcb.200711136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szymanski K, Binns D, Bartz R, Grishin N, Li W, et al. The lipodystrophy protein seipin is found at endoplasmic reticulum lipid droplet junctions and is important for droplet morphology. Proc Natl Acad Sci USA. 2007;104:20890–95. doi: 10.1073/pnas.0704154104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurat C, Wolinski H, Petschnigg J, Kaluarachchi S, Andrews B, et al. Cdk1/Cdc28-dependent activation of the major triacylglycerol lipase Tgl4 in yeast links lipolysis to cell-cycle progression. Mol Cell. 2009;33:53–63. doi: 10.1016/j.molcel.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto K, Takahara K, Oyadomari S, Okada T, Sato T, et al. Induction of liver steatosis and lipid droplet formation in ATF6α-knockout mice burdened with pharmacological endoplasmic reticulum stress. Mol Biol Cell. 2010;21:2975–86. doi: 10.1091/mbc.E09-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fei W, Wang H, Fu X, Bielby C, Yang H. Conditions of endoplasmic reticulum stress stimulate lipid droplet formation in Saccharomyces cerevisiae. Biochem J. 2009;424:61–67. doi: 10.1042/BJ20090785. [DOI] [PubMed] [Google Scholar]
- 11.Bozza PT, Viola JP. Lipid droplets in inflammation and cancer. Prostaglandins Leukot Essent Fatty Acids. 2010;82:243–50. doi: 10.1016/j.plefa.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Krahmer N, Guo Y, Hilger M, Lingrell S, Wilfling F, et al. Localized activation of CTP: phosphocholine cytidyltransferase (CCT) is required for phosphotidylcholine synthesis during lipid droplet expansion. Cell Metab. 2012 doi: 10.1016/j.cmet.2011.07.013. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czabany T, Wagner A, Zweytick D, Lohner K, Leitner E, et al. Structural and biochemical properties of lipid particles from the yeast Saccharomyces cerevisiae. J Biol Chem. 2008;283:17065–75. doi: 10.1074/jbc.M800401200. [DOI] [PubMed] [Google Scholar]
- 14.Buhman KK, Chen HC, Farese RV., Jr The enzymes of neutral lipid synthesis. J Biol Chem. 2001;276:40369–72. doi: 10.1074/jbc.R100050200. [DOI] [PubMed] [Google Scholar]
- 15.Kuerschner L, Moessinger C, Thiele C. Imaging of lipid biosynthesis: How a neutral lipid enters lipid droplets. Traffic. 2008;9:338–52. doi: 10.1111/j.1600-0854.2007.00689.x. [DOI] [PubMed] [Google Scholar]
- 16.Stone SJ, Levin MC, Zhou P, Han J, Walther TC, Farese RV., Jr The endoplasmic reticulum enzyme DGAT2 is found in mitochondria-associated membranes and has a mitochondrial targeting signal that promotes its association with mitochondria. J Biol Chem. 2009;284:5352–61. doi: 10.1074/jbc.M805768200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blaner WS, O’Byrne SM, Wongsiriroj N, Kluwe J, D’Ambrosio DM, et al. Hepatic stellate cell lipid droplets: a specialized lipid droplet for retinoid storage. Biochim Biophys Acta. 2009;1791:467–73. doi: 10.1016/j.bbalip.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orban T, Palczewska G, Palczewski K. Retinyl ester storage particles (retinosomes) from the retinal pigmented epithelium resemble lipid droplets in other tissues. J Biol Chem. 2011;286:17248–58. doi: 10.1074/jbc.M110.195198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartz R, Li WH, Venables B, Zehmer JK, Roth MR, et al. Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. J Lipid Res. 2007;48:837–47. doi: 10.1194/jlr.M600413-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Cornish K, Wood DF, Windle JJ. Rubber particles from four different species, examined by transmission electron microscopy and electron-paramagnetic-resonance spin labeling, are found to consist of a homogeneous rubber core enclosed by a contiguous, monolayer biomembrane. Planta. 1999;210:85–96. doi: 10.1007/s004250050657. [DOI] [PubMed] [Google Scholar]
- 21.Athenstaedt K, Zweytick D, Jandrositz A, Kohlwein S, Daum G. Identification and characterization of major lipid particle proteins of the yeast Saccharomyces cerevisiae. J Bacteriol. 1999;181:6441–48. doi: 10.1128/jb.181.20.6441-6448.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welte MA. Proteins under new management: Lipid droplets deliver. Trends Cell Biol. 2007;17:363–69. doi: 10.1016/j.tcb.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Cermelli S, Guo Y, Gross SP, Welte MA. The lipid-droplet proteome reveals that droplets are a protein-storage depot. Curr Biol. 2006;16:1783–95. doi: 10.1016/j.cub.2006.07.062. [DOI] [PubMed] [Google Scholar]
- 24.Herker E, Ott M. Unique ties between hepatitis C virus replication and intracellular lipids. Trends Endocrinol Metab. 2011;22:241–48. doi: 10.1016/j.tem.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herker E, Harris C, Hernandez C, Carpentier A, Kaehlcke K, et al. Efficient hepatitis C virus particle formation requires diacylglycerol acyltransferase-1. Nat Med. 2010;16:1295–98. doi: 10.1038/nm.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye J, Li JZ, Liu Y, Li X, Yang T, et al. Cideb, an ER- and lipid droplet-associated protein, mediates VLDL lipidation and maturation by interacting with apolipoprotein B. Cell Metab. 2009;9:177–90. doi: 10.1016/j.cmet.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Dolinsky VW, Gilham D, Alam M, Vance DE, Lehner R. Triacylglycerol hydrolase: role in intracellular lipid metabolism. Cell Mol Life Sci. 2004;61:1633–51. doi: 10.1007/s00018-004-3426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wältermann M, Hinz A, Robenek H, Troyer D, Reichelt R, et al. Mechanism of lipid-body formation in prokaryotes: How bacteria fatten up. Mol Microbiol. 2005;55:750–63. doi: 10.1111/j.1365-2958.2004.04441.x. [DOI] [PubMed] [Google Scholar]
- 29.Jacquier N, Choudhary V, Mari M, Toulmay A, Reggiori F, Schneiter R. Lipid droplets are functionally connected to the endoplasmic reticulum in Saccharomyces cerevisiae. J Cell Sci. 2011;124:2424–37. doi: 10.1242/jcs.076836. [DOI] [PubMed] [Google Scholar]
- 30.Soni K, Mardones G, Sougrat R, Smirnova E, Jackson C, Bonifacino J. Coatomer-dependent protein delivery to lipid droplets. J Cell Sci. 2009;122:1834–41. doi: 10.1242/jcs.045849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohsaki Y, Cheng J, Suzuki M, Fujita A, Fujimoto T. Lipid droplets are arrested in the ER membrane by tight binding of lipidated apolipoprotein B-100. J Cell Sci. 2008;121:2415–22. doi: 10.1242/jcs.025452. [DOI] [PubMed] [Google Scholar]
- 32.Lacey DJ, Beaudoin F, Dempsey CE, Shewry PR, Napier JA. The accumulation of triacylglycerols within the endoplasmic reticulum of developing seeds of Helianthus annuus. Plant J. 1999;17:397–405. [Google Scholar]
- 33.Skinner JR, Shew TM, Schwartz DM, Tzekov A, Lepus CM, et al. Diacylglycerol enrichment of endoplasmic reticulum or lipid droplets recruits perilipin 3/TIP47 during lipid storage and mobilization. J Biol Chem. 2009;284:30941–48. doi: 10.1074/jbc.M109.013995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bulankina AV, Deggerich A, Wenzel D, Mutenda K, Wittmann JG, et al. TIP47 functions in the biogenesis of lipid droplets. J Cell Biol. 2009;185:641–55. doi: 10.1083/jcb.200812042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ploegh HL. A lipid-based model for the creation of an escape hatch from the endoplasmic reticulum. Nature. 2007;448:435–38. doi: 10.1038/nature06004. [DOI] [PubMed] [Google Scholar]
- 36.Walther TC, Farese RV., Jr The life of lipid droplets. Biochim Biophys Acta. 2009;1791:459–66. doi: 10.1016/j.bbalip.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robenek H, Hofnagel O, Buers I, Robenek MJ, Troyer D, Severs NJ. Adipophilin-enriched domains in the ER membrane are sites of lipid droplet biogenesis. J Cell Sci. 2006;119:4215–24. doi: 10.1242/jcs.03191. [DOI] [PubMed] [Google Scholar]
- 38.Hamilton RL, Wong JS, Cham CM, Nielsen LB, Young SG. Chylomicron-sized lipid particles are formed in the setting of apolipoprotein B deficiency. J Lipid Res. 1998;39:1543–57. [PubMed] [Google Scholar]
- 39.Fei W, Shui G, Zhang Y, Krahmer N, Ferguson C, et al. A role for phosphatidic acid in the formation “supersized” lipid droplets. PLoS Genet. 2011;7:e1002201. doi: 10.1371/journal.pgen.1002201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Binns D, Lee S, Hilton CL, Jiang QX, Goodman JM. Seipin is a discrete homooligomer. Biochemistry. 2010;49:10747–55. doi: 10.1021/bi1013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo Y, Walther TC, Rao M, Stuurman N, Goshima G, et al. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature. 2008;453:657–61. doi: 10.1038/nature06928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beller M, Riedel D, Jänsch L, Dieterich G, Wehland J, et al. Characterization of the Drosophila lipid droplet subproteome. Mol Cell Proteomics. 2006;5:1082–94. doi: 10.1074/mcp.M600011-MCP200. [DOI] [PubMed] [Google Scholar]
- 43.Beller M, Sztalryd C, Southall N, Bell M, Jäckle H, et al. COPI complex is a regulator of lipid homeostasis. PLoS Biol. 2008;6:e292. doi: 10.1371/journal.pbio.0060292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ashrafi K, Chang FY, Watts JL, Fraser AG, Kamath RS, et al. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature. 2003;421:268–72. doi: 10.1038/nature01279. [DOI] [PubMed] [Google Scholar]
- 45.Pospisilik JA, Schramek D, Schnidar H, Cronin SJ, Nehme NT, et al. Drosophila genome-wide obesity screen reveals hedgehog as a determinant of brown versus white adipose cell fate. Cell. 2010;140:148–60. doi: 10.1016/j.cell.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 46.Gross DA, Snapp EL, Silver DL. Structural insights into triglyceride storage mediated by fat storage-inducing transmembrane (FIT) protein 2. PLoS ONE. 2010;5:e10796. doi: 10.1371/journal.pone.0010796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kadereit B, Kumar P, Wang WJ, Mirnada D, Snapp EL, et al. Evolutionarily conserved gene family important for fat storage. Proc Natl Acad Sci USA. 2008;105:94–99. doi: 10.1073/pnas.0708579105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gubern A, Barcelo-Torns M, Casas J, Barneda D, Masgrau R, et al. Lipid droplet biogenesis induced by stress involves triacylglycerol synthesis that depends on group VIA phospholipase A2. J Biol Chem. 2009;284:5697–708. doi: 10.1074/jbc.M806173200. [DOI] [PubMed] [Google Scholar]
- 49.Moessinger C, Kuerschner L, Spandl J, Shevchenko A, Thiele C. Human lysophosphatidylcholine acyltransferases 1 and 2 are located in lipid droplets where they catalyze the formation of phosphatidylcholine. J Biol Chem. 2011;286:21330–39. doi: 10.1074/jbc.M110.202424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khelef N, Buton X, Beatini N, Wang H, Meiner V, et al. Immunolocalization of acyl-coenzyme A:cholesterol O-acyltransferase in macrophages. J Biol Chem. 1998;273:11218–24. doi: 10.1074/jbc.273.18.11218. [DOI] [PubMed] [Google Scholar]
- 51.Bostrom P, Rutberg M, Ericsson J, Holmdahl P, Andersson L, et al. Cytosolic lipid droplets increase in size by microtubule-dependent complex formation. Arterioscler Thromb Vasc Biol. 2005;25:1945–51. doi: 10.1161/01.ATV.0000179676.41064.d4. [DOI] [PubMed] [Google Scholar]
- 52.Murphy S, Martin S, Parton RG. Quantitative analysis of lipid droplet fusion: inefficient steady state fusion but rapid stimulation by chemical fusogens. PloS One. 2010;5:e15030. doi: 10.1371/journal.pone.0015030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Puri V, Konda S, Ranjit S, Aouadi M, Chawla A, et al. Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage. J Biol Chem. 2007;282:34213–18. doi: 10.1074/jbc.M707404200. [DOI] [PubMed] [Google Scholar]
- 54.Keller P, Petrie JT, De Rose P, Gerin I, Wright WS, et al. Fat-specific protein 27 regulates storage of triacylglycerol. J Biol Chem. 2008;283:14355–65. doi: 10.1074/jbc.M708323200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nishino N, Tamori Y, Tateya S, Kawaguchi T, Shibakusa T, et al. FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets. J Clin Investig. 2008;118:2808–21. doi: 10.1172/JCI34090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Puri V, Virbasius JV, Guilherme A, Czech MP. RNAi screens reveal novel metabolic regulators: RIP140, MAP4k4 and the lipid droplet associated fat specific protein (FSP) 27. Acta Physiol. 2008;192:103–15. doi: 10.1111/j.1748-1716.2007.01786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jambunathan S, Yin J, Khan W, Tamori Y, Puri V. FSP27 promotes lipid droplet clustering and then fusion to regulate triglyceride accumulation. PLOS One. 2011;6:e28614. doi: 10.1371/journal.pone.0028614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gong J, Sun Z, Wu L, Xu W, Schieber N, et al. Fsp27 promotes lipid droplet growth by lipid exchange and transfer at lipid droplet contact sites. J Cell Biol. 2011;195:953–63. doi: 10.1083/jcb.201104142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boström P, Andersson L, Rutberg M, Perman J, Lidberg U, et al. SNARE proteins mediate fusion between cytosolic lipid droplets and are implicated in insulin sensitivity. Nat Cell Biol. 2007;9:1286–93. doi: 10.1038/ncb1648. [DOI] [PubMed] [Google Scholar]
- 60.Zweytick D, Athenstaedt K, Daum G. Intracellular lipid particles of eukaryotic cells. Biochim Biophys Acta. 2000;1469:101–20. doi: 10.1016/s0005-2736(00)00294-7. [DOI] [PubMed] [Google Scholar]
- 61.Liu P, Ying Y, Zhao Y, Mundy DI, Zhu M, Anderson RG. Chinese hamster ovary K2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic. J Biol Chem. 2004;279:3787–92. doi: 10.1074/jbc.M311945200. [DOI] [PubMed] [Google Scholar]
- 62.Fujimoto Y, Itabe H, Sakai J, Makita M, Noda J, et al. Identification of major proteins in the lipid droplet-enriched fraction isolated from the human hepatocyte cell line HuH7. Biochim Biophys Acta. 2004;1644:47–59. doi: 10.1016/j.bbamcr.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 63.Umlauf E, Csaszar E, Moertelmaier M, Schuetz GJ, Parton RG, Prohaska R. Association of stomatin with lipid bodies. J Biol Chem. 2004;279:23699–709. doi: 10.1074/jbc.M310546200. [DOI] [PubMed] [Google Scholar]
- 64.Wu CC, Howell KE, Neville MC, Yates JR, III, McManaman JL. Proteomics reveal a link between the endoplasmic reticulum and lipid secretory mechanisms in mammary epithelial cells. Electrophoresis. 2000;21:3470–82. doi: 10.1002/1522-2683(20001001)21:16<3470::AID-ELPS3470>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 65.Brasaemle DL, Dolios G, Shapiro L, Wang R. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J Biol Chem. 2004;279:46835–42. doi: 10.1074/jbc.M409340200. [DOI] [PubMed] [Google Scholar]
- 66.Cole NB, Murphy DD, Grider T, Rueter S, Brasaemle D, Nussbaum RL. Lipid droplet binding and oligomerization properties of the Parkinson’s disease protein α-synuclein. J Biol Chem. 2002;277:6344–52. doi: 10.1074/jbc.M108414200. [DOI] [PubMed] [Google Scholar]
- 67.Barba G, Harper F, Harada T, Kohara M, Goulinet S, et al. Hepatitis C virus core protein shows a cytoplasmic localization and associates to cellular lipid storage droplets. Proc Natl Acad Sci USA. 1997;94:1200–5. doi: 10.1073/pnas.94.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hinson ER, Cresswell P. The antiviral protein, viperin, localizes to lipid droplets via its N-terminal amphipathic alpha-helix. Proc Natl Acad Sci USA. 2009;106:20452–57. doi: 10.1073/pnas.0911679106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tauchi-Sato K, Ozeki S, Houjou T, Tagushi R, Fujimoto T. The surface of lipid droplets is a phospholipid monolayer with a unique fatty acid composition. J Biol Chem. 2002;277:44507–12. doi: 10.1074/jbc.M207712200. [DOI] [PubMed] [Google Scholar]
- 70.Zehmer JK, Bartz R, Liu P, Anderson RG. Identification of a novel N-terminal hydrophobic sequence that targets proteins to lipid droplets. J Cell Sci. 2008;121:1852–60. doi: 10.1242/jcs.012013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Turro S, Ingelmo-Torres M, Estanyol JM, Tebar F, Fernandez MA, et al. Identification and characterization of associated with lipid droplet protein 1: A novel membrane-associated protein that resides on hepatic lipid droplets. Traffic. 2006;7:1254–69. doi: 10.1111/j.1600-0854.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 72.Napier JA, Stobart AK, Shewry PR. The structure and biogenesis of plant oil bodies: the role of the ER membrane and the oleosin class of proteins. Plant Mol Biol. 1996;31:945–56. doi: 10.1007/BF00040714. [DOI] [PubMed] [Google Scholar]
- 73.Qu R, Wang S-M, Lin Y-H, Vance VB, Huang AHC. Characteristics and biosynthesis of membrane proteins of lipid bodies in the scutella of maize (Zea mays L.) Biochem J. 1986;235:57–65. doi: 10.1042/bj2350057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abell BM, Holbrook LA, Abenes M, Murphy DJ, Hills MJ, Moloney MM. Role of the proline knot motif in oleosin endoplasmic reticulum topology and oil body targeting. Plant Cell. 1997;9:1481–93. doi: 10.1105/tpc.9.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pol A, Luetterforst R, Lindsay M, Heinoo S, Ikonen E, Parton RG. A caveolin dominant negative mutant associates with lipid bodies and induces intracellular cholesterol imbalance. J Cell Biol. 2001;152:1057–70. doi: 10.1083/jcb.152.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fujimoto T, Kogo H, Ishiguro K, Tauchi K, Nomura R. Caveolin-2 is targeted to lipid droplets, a new “membrane domain” in the cell. J Cell Biol. 2001;152:1079–85. doi: 10.1083/jcb.152.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ostermeyer A, Paci J, Zeng Y, Lublin D, Munro S, Brown D. Accumulation of caveolin in the endoplasmic reticulum redirects the protein to lipid storage droplets. J Cell Biol. 2001;152:1071–78. doi: 10.1083/jcb.152.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pol A, Martin S, Fernandez MA, Ingelmo-Torres M, Ferguson C, et al. Cholesterol and fatty acids regulate dynamic caveolin trafficking through the Golgi complex and between the cell surface and lipid bodies. Mol Biol Cell. 2005;16:2091–105. doi: 10.1091/mbc.E04-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Le Lay S, Hajduch E, Lindsay MR, Le Liepvre X, Thiele C, et al. Cholesterol-induced caveolin targeting to lipid droplets in adipocytes: a role for caveolar endocytosis. Traffic. 2006;7:549–61. doi: 10.1111/j.1600-0854.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 80.Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem. 1991;266:11341–46. [PubMed] [Google Scholar]
- 81.Subramanian V, Garcia A, Sekowski A, Brasaemle DL. Hydrophobic sequences target and anchor perilipin A to lipid droplets. J Lipid Res. 2004;45:1983–91. doi: 10.1194/jlr.M400291-JLR200. [DOI] [PubMed] [Google Scholar]
- 82.McManaman JL, Zabaronick W, Schaack J, Orlicky DJ. Lipid droplet targeting domains of adipophilin. J Lipid Res. 2003;44:668–73. doi: 10.1194/jlr.C200021-JLR200. [DOI] [PubMed] [Google Scholar]
- 83.Targett-Adams P, Chambers D, Gledhill S, Hope RG, Coy JF, et al. Live cell analysis and targeting of the lipid droplet-binding adipocyte differentiation-related protein. J Biol Chem. 2003;278:15998–6007. doi: 10.1074/jbc.M211289200. [DOI] [PubMed] [Google Scholar]
- 84.Nakamura N, Fujimoto T. Adipose differentiation-related protein has two independent domains for targeting to lipid droplets. Biochem Biophys Res Commun. 2003;306:333–38. doi: 10.1016/s0006-291x(03)00979-3. [DOI] [PubMed] [Google Scholar]
- 85.Hickenbottom SJ, Kimmel AR, Londos C, Hurley JH. Structure of a lipid droplet protein: the PAT family member TIP47. Structure. 2004;12:1199–207. doi: 10.1016/j.str.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 86.Hatters DM, Peters-Libeu CA, Weisgraber KH. Apolipoprotein E structure: insights into function. Trends Biochem Sci. 2006;31:445–54. doi: 10.1016/j.tibs.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 87.Wolins NE, Quaynor BK, Skinner JR, Schoenfish MJ, Tzekov A, Bickel PE. S3-12, adipophilin, and TIP47 package lipid in adipocytes. J Biol Chem. 2005;280:19146–55. doi: 10.1074/jbc.M500978200. [DOI] [PubMed] [Google Scholar]
- 88.Ozeki S, Cheng J, Tauchi-Sato K, Hatano N, Taniguchi H, Fujimoto T. Rab18 localizes to lipid droplets and induces their close apposition to the endoplasmic reticulum-derived membrane. J Cell Sci. 2005;118:2601–11. doi: 10.1242/jcs.02401. [DOI] [PubMed] [Google Scholar]
- 89.Martin S, Driessen K, Nixon SJ, Zerial M, Parton RG. Regulated localization of Rab18 to lipid droplets: effects of lipolytic stimulation and inhibition of lipid droplet catabolism. J Biol Chem. 2005;280:42325–35. doi: 10.1074/jbc.M506651200. [DOI] [PubMed] [Google Scholar]
- 90.Beller M, Bulankina AV, Hsiao HH, Urlaub H, Jäckle H, Kühnlein RP. PERILIPIN-dependent control of lipid droplet structure and fat storage in Drosophila. Cell Metab. 2010;12:521–32. doi: 10.1016/j.cmet.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 91.Schweiger M, Schoiswohl G, Lass A, Radner FP, Haemmerle G, et al. The C-terminal region of human adipose triglyceride lipase affects enzyme activity and lipid droplet binding. J Biol Chem. 2008;283:17211–20. doi: 10.1074/jbc.M710566200. [DOI] [PubMed] [Google Scholar]
- 92.Wanner G, Formanet H, Theimer RR. The ontogeny of lipid bodies (spherosomes) in plant cells. Planta. 1981;151:109–23. doi: 10.1007/BF00387812. [DOI] [PubMed] [Google Scholar]
- 93.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, et al. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–35. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu G, Sztalryd C, Lu X, Tansey JT, Gan J, et al. Post-translational regulation of adipose differentiation-related protein by the ubiquitin/proteasome pathway. J Biol Chem. 2005;280:42841–47. doi: 10.1074/jbc.M506569200. [DOI] [PubMed] [Google Scholar]
- 95.Masuda Y, Itabe H, Odaki M, Hama K, Fujimoto Y, et al. ADRP/adipophilin is degraded through the proteasome-dependent pathway during regression of lipid-storing cells. J Lipid Res. 2006;47:87–98. doi: 10.1194/jlr.M500170-JLR200. [DOI] [PubMed] [Google Scholar]
- 96.Xu G, Sztalryd C, Londos C. Degradation of perilipin is mediated through ubiquitination-proteasome pathway. Biochim Biophys Acta. 2006;1761:83–90. doi: 10.1016/j.bbalip.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 97.Hooper C, Puttamadappa SS, Loring Z, Shekhtman A, Bakowska JC. Spartin activates atrophin-1-interacting protein 4 (AIP4) E3 ubiquitin ligase and promotes ubiquitination of adipophilin on lipid droplets. BMC Biol. 2010;8:72. doi: 10.1186/1741-7007-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nian Z, Sun Z, Yu L, Toh SY, Sang J, Li P. Fat-specific protein 27 undergoes ubiquitin-dependent degradation regulated by triacylglycerol synthesis and lipid droplet formation. J Biol Chem. 2010;285:9604–15. doi: 10.1074/jbc.M109.043786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Edwards TL, Clowes VE, Tsang HT, Connell JW, Sanderson CM, et al. Endogenous spartin (SPG20) is recruited to endosomes and lipid droplets and interacts with the ubiquitin E3 ligases AIP4 and AIP5. Biochem J. 2009;423:31–39. doi: 10.1042/BJ20082398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Eastman SW, Yassaee M, Bieniasz PD. A role for ubiquitin ligases and Spartin/SPG20 in lipid droplet turnover. J Cell Biol. 2009;184:881–94. doi: 10.1083/jcb.200808041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Spandl J, Lohmann D, Kuerschner L, Moessinger C, Thiele C. Ancient ubiquitous protein 1 (AUP1) localizes to lipid droplets and binds the E2 ubiquitin conjugase G2 (Ube2g2) via its G2 binding region. J Biol Chem. 2011;286:5599–606. doi: 10.1074/jbc.M110.190785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Klemm EJ, Spooner E, Ploegh HL. The dual role of ancient ubiquitous protein 1 (AUP1) in lipid droplet accumulation and ER protein quality control. J Biol Chem. 2011;286:37602–14. doi: 10.1074/jbc.M111.284794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hartman IZ, Liu P, Zehmer JK, Luby-Phelps K, Jo Y, et al. Sterol-induced dislocation of 3-hydroxy-3-methylglutaryl coenzyme A reductase from endoplasmic reticulum membranes into the cytosol through a subcellular compartment resembling lipid droplets. J Biol Chem. 2010;285:19288–98. doi: 10.1074/jbc.M110.134213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Olzmann JA, Kopito RR. Lipid droplet formation is dispensable for endoplasmic reticulum-associated degradation. J Biol Chem. 2011;286:27872–74. doi: 10.1074/jbc.C111.266452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hynynen R, Suchanek M, Spandl J, Bäck N, Thiele C, Olkkonen VM. OSBP-related protein 2 is a sterol receptor on lipid droplets that regulates the metabolism of neutral lipids. J Lipid Res. 2009;50:1305–15. doi: 10.1194/jlr.M800661-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yokoi Y, Horiguchi Y, Araki M, Motojima K. Regulated expression by PPARα and unique localization of 17β-hydroxysteroid dehydrogenase type 11 protein in mouse intestine and liver. FEBS J. 2007;274:4837–47. doi: 10.1111/j.1742-4658.2007.06005.x. [DOI] [PubMed] [Google Scholar]
- 107.Ouimet M, Franklin V, Mak E, Liao X, Tabas I, Marcel YL. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab. 2011;13:655–67. doi: 10.1016/j.cmet.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu P, Bartz R, Zehmer JK, Ying YS, Zhu M, et al. Rab-regulated interaction of early endosomes with lipid droplets. Biochim Biophys Acta. 2007;1773:784–93. doi: 10.1016/j.bbamcr.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Blanchette-Mackie EJ, Scow RO. Movement of lipolytic products to mitochondria in brown adipose tissue of young rats: an electron microscope study. J Lipid Res. 1983;24:229–44. [PubMed] [Google Scholar]
- 110.Binns D, Januszewski T, Chen Y, Hill J, Markin VS, et al. An intimate collaboration between peroxisomes and lipid bodies. J Cell Biol. 2006;173:719–31. doi: 10.1083/jcb.200511125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tarnopolsky MA, Rennie CD, Robertshaw HA, Fedak-Tarnopolsky SN, Devries MC, Hamadeh MJ. Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1271–78. doi: 10.1152/ajpregu.00472.2006. [DOI] [PubMed] [Google Scholar]
- 112.Marcinkiewicz A, Gauthier D, Garcia A, Brasaemle DL. The phosphorylation of serine 492 of perilipin a directs lipid droplet fragmentation and dispersion. J Biol Chem. 2006;281:11901–9. doi: 10.1074/jbc.M600171200. [DOI] [PubMed] [Google Scholar]
- 113.Savage MJ, Goldberg DJ, Schacher S. Absolute specificity for retrograde fast axonal transport displayed by lipid droplets originating in the axon of an identified Aplysia neuron in vitro. Brain Res. 1987;406:215–23. doi: 10.1016/0006-8993(87)90785-2. [DOI] [PubMed] [Google Scholar]
- 114.Welte MA, Gross SP, Postner M, Block SM, Wieschaus EF. Developmental regulation of vesicle transport in Drosophila embryos: forces and kinetics. Cell. 1998;92:547–57. doi: 10.1016/s0092-8674(00)80947-2. [DOI] [PubMed] [Google Scholar]
- 115.Shubeita GT, Tran SL, Xu J, Vershinin M, Cermelli S, et al. Consequences of motor copy number on the intracellular transport of kinesin-1-driven lipid droplets. Cell. 2008;135:1098–107. doi: 10.1016/j.cell.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Boulant S, Douglas MW, Moody L, Budkowska A, Targett-Adams P, McLauchlan J. Hepatitis C virus core protein induces lipid droplet redistribution in a microtubule- and dynein-dependent manner. Traffic. 2008;9:1268–82. doi: 10.1111/j.1600-0854.2008.00767.x. [DOI] [PubMed] [Google Scholar]
- 117.Gruber A, Cornaciu I, Lass A, Schweiger M, Poeschl M, et al. The N-terminal region of comparative gene identification-58 (CGI-58) is important for lipid droplet binding and activation of adipose triglyceride lipase. J Biol Chem. 2010;285:12289–98. doi: 10.1074/jbc.M109.064469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang X, Lu X, Lombès M, Rha GB, Chi Y-I, et al. The G0/G1 switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab. 2010;11:194–205. doi: 10.1016/j.cmet.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brasaemle DL, Rubin B, Harten IA, Gruia-Gray J, Kimmel AR, Londos C. Perilipin A increases triacylglycerol storage by decreasing the rate of triacylglycerol hydrolysis. J Biol Chem. 2000;275:38486–93. doi: 10.1074/jbc.M007322200. [DOI] [PubMed] [Google Scholar]
- 120.Souza SC, de Vargas LM, Yamamoto MT, Lien P, Franciosa MD, et al. Overexpression of perilipin A and B blocks the ability of tumor necrosis factor α to increase lipolysis in 3T3-L1 adipocytes. J Biol Chem. 1998;273:24665–69. doi: 10.1074/jbc.273.38.24665. [DOI] [PubMed] [Google Scholar]
- 121.Subramanian V, Rothenberg A, Gomez C, Cohen AW, Garcia A, et al. Perilipin A mediates the reversible binding of CGI-58 to lipid droplets in 3T3-L1 adipocytes. J Biol Chem. 2004;279:42062–71. doi: 10.1074/jbc.M407462200. [DOI] [PubMed] [Google Scholar]
- 122.Granneman JG, Moore HP, Mottillo EP, Zhu Z, Zhou L. Interactions of perilipin-5 (Plin5) with adipose triglyceride lipase. J Biol Chem. 2011;286:5126–35. doi: 10.1074/jbc.M110.180711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang H, Bell M, Sreenevasan U, Hu H, Liu J, et al. Unique regulation of adipose triglyceride lipase (ATGL) by perilipin 5, a lipid droplet-associated protein. J Biol Chem. 2011;286:15707–15. doi: 10.1074/jbc.M110.207779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Krintel C, Osmark P, Larsen MR, Resjo S, Logan DT, Holm C. Ser649 and Ser650 are the major determinants of protein kinase A-mediated activation of human hormone-sensitive lipase against lipid substrates. PLoS ONE. 2008;3:e3756. doi: 10.1371/journal.pone.0003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang H, Hu L, Dalen K, Dorward H, Marcinkiewicz A, et al. Activation of hormone-sensitive lipase requires two steps, protein phosphorylation and binding to the PAT-1 domain of lipid droplet coat proteins. J Biol Chem. 2009;284:32116–25. doi: 10.1074/jbc.M109.006726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schweiger M, Schreiber R, Haemmerle G, Lass A, Fledelius C, et al. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem. 2006;281:40236–41. doi: 10.1074/jbc.M608048200. [DOI] [PubMed] [Google Scholar]
- 127.Taschler U, Radner FP, Heier C, Schreiber R, Schweiger M, et al. Monoglyceride lipase deficiency in mice impairs lipolysis and attenuates diet-induced insulin resistance. J Biol Chem. 2011;286:17467–77. doi: 10.1074/jbc.M110.215434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.He S, McPhaul C, Li JZ, Garuti R, Kinch L, et al. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J Biol Chem. 2010;285:6706–15. doi: 10.1074/jbc.M109.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen W, Chang B, Li L, Chan L. Patatin-like phospholipase domain-containing 3/adiponutrin deficiency in mice is not associated with fatty liver disease. Hepatology. 2010;52:1134–42. doi: 10.1002/hep.23812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Basantani MK, Sitnick MT, Cai L, Brenner DS, Gardner NP, et al. Pnpla3/adiponutrin deficiency in mice does not contribute to fatty liver disease or metabolic syndrome. J Lipid Res. 2011;52:318–29. doi: 10.1194/jlr.M011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kaczocha M, Glaser ST, Chae J, Brown DA, Deutsch DG. Lipid droplets are novel sites of N-acylethanolamine inactivation by fatty acid amide hydrolase-2. J Biol Chem. 2010;285:2796–806. doi: 10.1074/jbc.M109.058461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Leibel RL, Hirsch J, Berry EM, Gruen RK. Alterations in adipocyte free fatty acid re-esterification associated with obesity and weight reduction in man. Am J Clin Nutr. 1985;42:198–206. doi: 10.1093/ajcn/42.2.198. [DOI] [PubMed] [Google Scholar]
- 133.Fujimoto Y, Itabe H, Kinoshita T, Homma KJ, Onoduka J, et al. Involvement of ACSL in local synthesis of neutral lipids in cytoplasmic lipid droplets in human hepatocyte HuH7. J Lipid Res. 2007;48:1280–92. doi: 10.1194/jlr.M700050-JLR200. [DOI] [PubMed] [Google Scholar]
- 134.Wölkart G, Schrammel A, Dörffel K, Haemmerle G, Zechner R, Mayer B. Cardiac dysfunction in adipose triglyceride lipase deficiency: Treatment with a PPARα agonist. Br J Pharmacol. 2011;165:380–89. doi: 10.1111/j.1476-5381.2011.01490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Greenberg AS, Coleman RA, Kraemer FB, McManaman JL, Obin MS, et al. The role of lipid droplets in metabolic disease in rodents and humans. J Clin Investig. 2011;121:2102–10. doi: 10.1172/JCI46069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–56. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 137.Friedman JM. Leptin at 14 y of age: an ongoing story. Am J Clin Nutr. 2009;89:S973–79. doi: 10.3945/ajcn.2008.26788B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhu J, Lee B, Buhman KK, Cheng JX. A dynamic, cytoplasmic triacylglycerol pool in enterocytes revealed by ex vivo and in vivo coherent anti-Stokes Raman scattering imaging. J Lipid Res. 2009;50:1080–89. doi: 10.1194/jlr.M800555-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Farese RV, Jr, Cases S, Ruland SL, Kayden HJ, Wong JS, et al. A novel function for apolipoprotein B: Lipoprotein synthesis in the yolk sac is critical for maternal-fetal lipid transport in mice. J Lipid Res. 1996;37:347–60. [PubMed] [Google Scholar]
- 140.Liu L, Zhang Y, Chen N, Shi X, Tsang B, Yu YH. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J Clin Investig. 2007;117:1679–89. doi: 10.1172/JCI30565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Investig. 2007;117:1690–98. doi: 10.1172/JCI30566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shaw CS, Jones DA, Wagenmakers AJM. Network distribution of mitochondria and lipid droplets in human muscle fibres. Histochem Cell Biol. 2008;129:65–72. doi: 10.1007/s00418-007-0349-8. [DOI] [PubMed] [Google Scholar]
- 143.Chen HC, Stone SJ, Zhou P, Buhman KK, Farese RV., Jr Dissociation of obesity and impaired glucose disposal in mice overexpressing acyl coenzyme A:diacylglycerol acyltransferase 1 in white adipose tissue. Diabetes. 2002;51:3189–95. doi: 10.2337/diabetes.51.11.3189. [DOI] [PubMed] [Google Scholar]
- 144.Franckhauser S, Muñoz S, Pujol A, Casellas A, Riu E, et al. Increased fatty acid re-esterification by PEPCK overexpression in adipose tissue leads to obesity without insulin resistance. Diabetes. 2002;51:624–30. doi: 10.2337/diabetes.51.3.624. [DOI] [PubMed] [Google Scholar]
- 145.Koliwad SK, Streeper RS, Monetti M, Cornelissen I, Chan L, et al. DGAT1-dependent triacylglycerol storage by macrophages protects mice from diet-induced insulin resistance and inflammation. J Clin Investig. 2010;120:756–67. doi: 10.1172/JCI36066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nagle CA, Klett EL, Coleman RA. Hepatic triacylglycerol accumulation and insulin resistance. J Lipid Res. 2009;50(Suppl):S74–79. doi: 10.1194/jlr.R800053-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Accad M, Smith SJ, Newland DL, Sanan DA, King LE, Jr, et al. Massive xanthomatosis and altered composition of atherosclerotic lesions in hyperlipidemic mice lacking acyl CoA:cholesterol acyltransferase 1. J Clin Invest. 2000;105:711–19. doi: 10.1172/JCI9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chiu H-C, Kovacs A, Ford DA, Hsu F-F, Garcia R, et al. A novel mouse model of lipotoxic cardiomyopathy. J Clin Investig. 2001;107:813–22. doi: 10.1172/JCI10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu L, Shi X, Bharadwaj KG, Ikeda S, Yamashita H, et al. DGAT1 expression increases heart triglyceride content but ameliorates lipotoxicity. J Biol Chem. 2009;284:36312–23. doi: 10.1074/jbc.M109.049817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–37. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 151.Garg A, Agarwal AK. Lipodystrophies: disorders of adipose tissue biology. Biochim Biophys Acta. 2009;1791:507–13. doi: 10.1016/j.bbalip.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.McManaman JL, Russell TD, Schaack J, Orlicky DJ, Robenek H. Molecular determinants of milk lipid secretion. J Mammary Gland Biol Neoplasia. 2007;12:259–68. doi: 10.1007/s10911-007-9053-5. [DOI] [PubMed] [Google Scholar]
- 153.Vorbach C, Scriven A, Capecchi MR. The housekeeping gene xanthine oxidoreductase is necessary for milk fat droplet enveloping and secretion: gene sharing in the lactating mammary gland. Genes Dev. 2002;16:3223–35. doi: 10.1101/gad.1032702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ogg SL, Weldon AK, Dobbie L, Smith AJ, Mather IH. Expression of butyrophilin (Btn1a1) in lactating mammary gland is essential for the regulated secretion of milk-lipid droplets. Proc Natl Acad Sci USA. 2004;101:10084–89. doi: 10.1073/pnas.0402930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Russell TD, Palmer CA, Orlicky DJ, Bales ES, Chang BH, et al. Mammary glands of adipophilin-null mice produce an amino-terminally truncated form of adipophilin that mediates milk lipid droplet formation and secretion. J Lipid Res. 2008;49:206–16. doi: 10.1194/jlr.M700396-JLR200. [DOI] [PubMed] [Google Scholar]
- 156.Smith SJ, Cases S, Jensen DR, Chen HC, Sande E, et al. Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking DGAT. Nat Genet. 2000;25:87–90. doi: 10.1038/75651. [DOI] [PubMed] [Google Scholar]
- 157.Cases S, Zhou P, Schillingford J, Wiseman B, Fish J, et al. Development of the mammary gland requires DGAT1 expression in stromal and epithelial tissues. Development. 2004;131:3047–55. doi: 10.1242/dev.01158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Saha S, Enugutti B, Rajakumari S, Rajasekharan R. Cytosolic triacylglycerol biosynthetic pathway in oilseeds. Molecular cloning and expression of peanut cytosolic diacylglycerol acyltransferase. Plant Physiol. 2006;141:1533–43. doi: 10.1104/pp.106.082198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zheng P, Allen WB, Roesler K, Williams ME, Zhang S, et al. A phenylalanine in DGAT is a key determinant of oil content and composition in maize. Nat Genet. 2008;40:367–72. doi: 10.1038/ng.85. [DOI] [PubMed] [Google Scholar]
- 160.Baud S, Lepiniec L. Physiological and developmental regulation of seed oil production. Prog Lipid Res. 2010;49:235–49. doi: 10.1016/j.plipres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 161.Andrianov V, Borisjuk N, Pogrebnyak N, Brinker A, Dixon J, et al. Tobacco as a production platform for biofuel: Overexpression of Arabidopsis DGAT and LEC2 genes increases accumulation and shifts the composition of lipids in green biomass. Plant Biotechnol J. 2010;8:277–87. doi: 10.1111/j.1467-7652.2009.00458.x. [DOI] [PubMed] [Google Scholar]
- 162.Eastmond PJ. SUGAR-DEPENDENT1 encodes a patatin domain triacylglycerol lipase that initiates storage oil breakdown in germinating Arabidopsis seeds. Plant Cell. 2006;18:665–75. doi: 10.1105/tpc.105.040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Graham IA. Seed storage oil mobilization. Annu Rev Plant Biol. 2008;59:115–42. doi: 10.1146/annurev.arplant.59.032607.092938. [DOI] [PubMed] [Google Scholar]
- 164.Hayashi Y, Hayashi M, Hayashi H, Hara-Nishimura I, Nishimura M. Direct interaction between glyoxysomes and lipid bodies in cotyledons of the Arabidopsis thaliana ped1 mutant. Protoplasma. 2001;218:83–94. doi: 10.1007/BF01288364. [DOI] [PubMed] [Google Scholar]
- 165.Schmidt MA, Herman EM. Suppression of soybean oleosin produces micro-oil bodies that aggregate into oil body/ER complexes. Mol Plant. 2008;1:910–24. doi: 10.1093/mp/ssn049. [DOI] [PubMed] [Google Scholar]
- 166.Toomre D, Bewersdorf J. A new wave of cellular imaging. Annu Rev Cell Dev Biol. 2010;26:285–314. doi: 10.1146/annurev-cellbio-100109-104048. [DOI] [PubMed] [Google Scholar]


