Summary
Identification of energy sources depends upon the ability to form associations between food cues and nutritional value. As such, cues previously paired with calories elicit neuronal activation in the nucleus accumbens (NAcc), which reflects the reinforcing value of food [1–4]. The identity of the physiological signals regulating this response remains elusive. Using fMRI, we examined brain response to noncaloric versions of flavors that had been consumed in previous days with either 0 or 112.5 calories from undetected maltodextrin. We report a small but perceptually meaningful increase in liking for the flavor that had been paired with calories and find that change in liking was associated with changes in insular responses to this beverage. In contrast, NAcc and hypothalamic response to the calorie-paired flavor was unrelated to liking but was strongly associated with the changes in plasma glucose levels produced by ingestion of the beverage when consumed previously with calories. Importantly, because each participant ingested the same caloric dose, the change in plasma glucose depended upon individual differences in glucose metabolism. We conclude that glucose metabolism is a critical signal regulating NAcc and hypothalamic response to food cues, and that this process operates independently from the ability of calories to condition liking.
Results
Animals readily and quickly increase their intake of flavors associated with intragastric (IG) glucose infusion and develop strong and persisting preferences for these flavors when later sampled in the absence of calories [5, 6]. The existence of this “flavor-nutrient conditioning” implies that a signal from the periphery must reach the brain to influence neural plasticity to code the acquired reinforcing properties of the flavor. Work in rodents has implicated dopaminergic signaling in the nucleus accumbens (NAcc), lateral hypothalamus, amygdala, and medial prefrontal cortex (mPFC) in flavor-nutrient conditioning [7]; however, the identity of the physiological signals regulating this response remains elusive. What is known is that the efficiency with which individual rodents use glucose as a fuel predicts how much glucose they will consume and that IG glucose infusion induces dopamine release in the striatum, an effect that is blocked by administration of the antimetabolic agent 2-deoxyglucose [8]. These data suggest that glucose metabolism may impact on dopamine release to influence flavor preference formation. In humans, increased intake is observed for flavors that have been paired with calories derived from the tasteless and odorless polysaccharide maltodextrin [9]; however, the neural correlates of this effect remain poorly understood. Using fMRI in combination with a flavor-nutrient conditioning paradigm [10–14], we sought to identify the neural circuit sensitive to calorie-predictive (CS+) versus non-calorie-predictive (CS−) flavors in humans. Moreover, we sought to determine whether CS+ brain responses are associated with calorie-driven changes in flavor liking and/or changes in circulating hormone or plasma glucose levels.
Pairing Flavors with the Carbohydrate Maltodextrin Increases Flavor Liking
Fourteen healthy subjects rated their liking, intensity, and sweetness perception of ten novel noncaloric flavored beverages before and after they repeatedly consumed one of the flavored beverages mixed with 112.5 kcal from maltodextrin (CS+) and another with no calories added (CS−) (Figure 1A). All subjects gave written informed consent to participate in our study, which was approved by the Yale University School of Medicine Human Investigation Committee. Flavors were selected to be “slightly liked” at pretest on the labeled hedonic scale [16] to reflect the methodology employed in animal models of flavor-nutrient conditioning [17]. Over four exposure days, hungry subjects ingested six times each the CS+ and CS− beverages on alternate, nonconsecutive days (Figure 1A). Importantly, as shown in Figure 1C, subjects were unable to reliably detect the presence of the maltodextrin in the flavored beverage. Therefore, any effects of conditioning must be attributed to the postoral effect of maltodextrin. A posttest followed the exposure sessions in which perceptual ratings were again collected. Finally, brain response to the CS+, CS−, and a nonexposed control flavor was assessed by fMRI (Figure 1B) using previously described delivery, collection, and analysis procedures [18]. As predicted, we observed a significant increase in liking ratings for the CS+ flavor, but not for the CS− flavor, when comparing the ratings recorded during post- versus preconditioning sessions (although a similar trend was observed for CS− liking ratings as well; see Figure 2A). In contrast, conditioning had no influence on sweetness or intensity ratings. This suggests that postoral signals can, within limits, modulate neural responses to increase flavor liking.
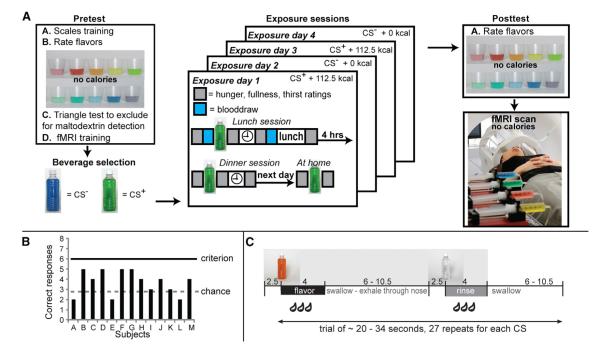
Figure 1.
Study Design and Behavioral Protocol
(A) Schematic depiction of the overall study design. For full details, please see “Experimental Design” in the Supplemental Experimental Procedures. Pretest: subjects participated in a pretest, four exposure days, a posttest, and an fMRI scanning session. In the pretest, subjects were trained on how to use the scales to rate overall intensity, sweetness intensity, liking, and wanting for each of ten noncaloric flavored beverages developed in-house. After training, subjects rated each of the flavor stimuli three times. Average ratings were calculated, and two beverages were selected to be close to neutral in liking and equally well liked. One of the beverages was designated as destined to be the 112.5 calorie beverage, and the other was designated as the 0 calorie beverage. Caloric content was achieved by adding maltodextrin, which is relatively tasteless and odorless. To confirm that subjects could not detect the presence of the maltodextrin, we performed a triangle test with a separate flavored beverage (see B). Finally, subjects were trained on the fMRI procedures. Exposure sessions: subjects participated in four exposure days (two per stimulus). In each exposure day, subjects drank one of the beverages three times. Thus, each stimulus (0 calorie-paired flavored beverage and 112.5 calorie-paired flavored beverage) was exposed six times (three drinks × two days). Blood was sampled once for each beverage. Posttest: subjects rated all ten flavored beverages from the pretest (including the noncaloric version of the two exposed flavors) and filled out questionnaires. Subjects participated in one fMRI scan session within three weeks of beginning the experiment. The figure on the right depicts the setup in our mock scanner where they receive training in the procedure prior to scanning. During scanning, subjects sampled the noncaloric versions of the 0 calorie-paired flavor, the 112.5 calorie-paired flavor, as well as one of the nonexposed flavors as a control stimulus and a tasteless and odorless solution.
(C) Cartoon depicting the timing of stimulus delivery. There are two event types: “flavor” and “tasteless.” During flavor events, one of the three flavored beverages (control, 0 calorie-paired flavor, and 112.5 calorie-paired flavor) is delivered over 4 s. Note that no maltodextrin is added to the 112.5 calorie-paired flavor during scanning. Therefore, all flavors are noncaloric during scanning. Following delivery of the flavors, subjects are instructed to swallow and exhale through their nose so that the volatiles escape the oral cavity to reach the olfactory epithelium and induce retronasal olfaction, which is a key component of flavor perception. A variable period of rest (6–10.5 s) then follows. This “jitter” is important for aiding deconvolution of the hemodynamic response. Following the jitter, deionized water is delivered using the same procedure as the flavors, to rinse the mouth. During the tasteless event, the tasteless and odorless control solution is delivered over 4 s.
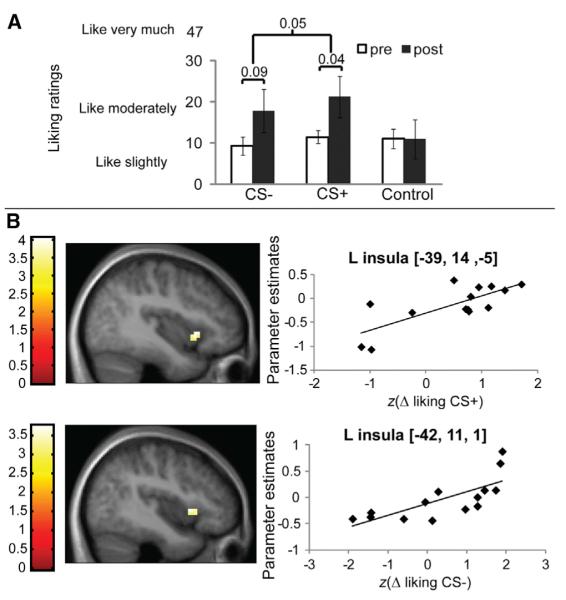
Figure 2.
Hedonic Conditioning
(A) The conditioning procedure produced a weak influence on overall liking ratings across the flavored beverages [two-way repeated-measures (RM) ANOVA, caloric load × time effect F(1,13) = 3.3; p = 0.05]. Liking ratings increased significantly in post- compared to preconditioning sessions for the 112.5 kcal-paired flavor (post hoc paired two-sample t test, p < 0.04, Bonferroni corrected), but not for the 0 kcal-paired flavor (p = 0.09). No effects were associated with a control flavor for which no conditioning sessions were performed.
(B) A significant correlation is observed between the magnitude of hedonic conditioning to the CS+ flavor shown in (A) with the differential response in the anterior insula to the CS+ flavor versus response to the unexposed control flavor. The peak is at −39, 14, 2−5; z = 3.13, k = 16; p = 0.04, similar analyses were performed for CS−, yielding significant correlations at −42, 11, 1; z = 3.0; p = 0.03 false discovery rate (FDR) corrected across the voxels of the taste cortex region of interest. This and all subsequent images are thresholded at p < 0.005 and a cluster criterion of 5. The color bar represents the t value. The graph depicts the contrast estimate (y axis) extracted from the peak voxel plotted against the change in liking ratings posttest minus pretest.
Hedonic Conditioning Is Represented in Insular Cortex
No significant differential blood oxygen level-dependent (BOLD) responses were observed for the comparison of the noncaloric versions of the CS+ versus the CS− flavors. However, a significant correlation was observed between response in the anterior insular cortex to the CS+ flavor versus the control flavor and the magnitude of hedonic conditioning for the CS+ beverage (liking ratings recorded during post- minus preconditioning sessions) Figure 2B. This raises the possibility that postoral signals act upon insular circuits to change flavor liking, a proposal consistent with the known role of this region of insular cortex in oral sensory, homeostatic representation [19], and conditioned taste-aversion learning [20]. However, we also note that these effects might have been accounted for by exposure alone, independently of conditioning effects per se. Indeed, although liking did not change significantly for the CS− beverage, individual differences in change in liking did correlate with insular response. Future investigations will be needed to dissociate flavor conditioning from flavor exposure effects on insular sensory coding.
Glucose Metabolism Regulates NAcc and Hypothalamic Responses to the CS+ versus CS− Flavors
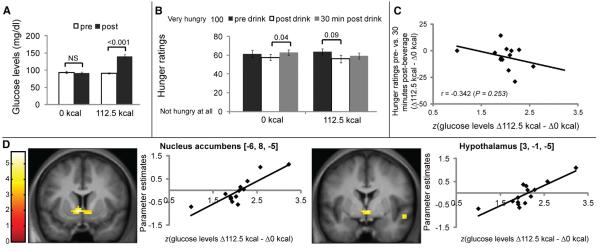
To determine whether glucose metabolism influences flavor-nutrient conditioning, we collected a blood sample just prior to, and 30 min following, beverage consumption on the conditioning days (Figure 1A). Glucose, insulin, triglycerides, hemoglobin, hematocrit, and ghrelin were measured. As expected, glucose, ghrelin, and insulin changed more during the consumption of the caloric compared to the noncaloric drink (see Table S1 available online). In particular, ingestion of the 112.5 kcal flavored beverage, but not of the 0 calorie beverage, produced marked increases in plasma glucose levels (Figure 3A). Changes in glucose levels are reflective of each individual’s metabolic response to a saccharide challenge (i.e., intestinal hydrolysis/absorption, cellular transport, and oxidation), because all subjects ingested the same amount of maltodextrin (112.5 kcal) while displaying individual-specific circulating levels of glucose at test. We also observed differences in perceived hunger associated with the ingestion of the 112.5 kcal flavored beverage compared to the 0 calorie beverage (Figure 3B), although changes in hunger levels were not associated with changes in plasma glucose levels (Figure 3C). We next computed each subject’s metabolic response given by the increases in plasma glucose levels produced by the 112.5 kcal flavored beverage minus the equivalent increases produced by the 0 calorie beverage, and the result was regressed to the brain responses obtained from the contrast (CS+ minus CS−). Strikingly, this analysis revealed a single robust (whole-brain corrected) cluster of activation that included, bilaterally, a well-defined region of the NAcc (Figure 3D). This cluster extended caudally to bilaterally involve the hypothalamus, a finding consistent with previous studies describing the glucosensing roles of the human hypothalamus [21]. A similar but nonsignificant effect was observed in the anterior medial orbitofrontal cortex. No other responses were associated with changes in plasma glucose levels even when liberal statistical thresholds were employed. We also repeated the above analysis for other metabolic markers obtained from blood samples collected during the conditioning sessions, including circulating levels of ghrelin, insulin, triglycerides, hematocrit, and hemoglobin. We found that none of the metabolic markers other than glucose was associated with flavor-driven responses in NAcc or any other region of interest. These findings indicate that, similar to rodents [22, 23], the human NAcc and hypothalamus play a critical role in flavor-nutrient conditioning. Our findings also reveal for the first time that the formation of associations between flavors and carbohydrate is tightly regulated by glucose metabolism.
Figure 3.
Biological Utility Conditioning
(A) Subject-normalized plasma glucose levels increased significantly 30 min following ingestion of the 112.5 kcal, but not the 0 kcal, flavored beverage [two-way RM ANOVA, caloric load × time effect F(1,13) = 157.7; p < 0.001]. Post hoc paired two-sample t test, p < 0.001; NS, statistically nonsignificant; paired two-sample t test, p = 0.39. 112.5 kcal, 112.5 kcal beverage; 0 kcal, 0 kcal beverage.
(B) No overall decreases in hunger levels were observed after ingestion of the flavored beverages (two-way RM ANOVA, caloric load × time effect; p = 0.37); however, hunger levels increased significantly 30 min following ingestion of the 0 kcal, but not the 112.5 kcal, beverage (post hoc paired two-sample t test, p = 0.04).
(C) Consistently, changes in hunger levels were not correlated with changes in glucose levels. Data are shown as standardized (z score) changes in glucose. D112.5 kcal, changes in glucose levels (post- versus preconditioning session) for the 112.5 kcal beverage. Similar definition applies for Δ0 kcal.
(D) Coronal sections showing positive correlations between parameter estimates (PEs) associated with the statistical parametric map (SPM) contrast (112.5 kcal-paired [CS+] flavor minus 0 kcal-paired [CS−] flavor) and changes in plasma glucose levels (Δglucose, in mg/dl) following ingestion of the 112.5 versus the 0 kcal beverage during the conditioning sessions in the NAcc (MNI coordinates on the left are −6, 8, −5 [z = 3.9] and right = 9, 5, −8 [z = 3.5] [graph not shown for right NAcc]) and hypothalamus (MNI coordinates = 3, −1, −5; z = 3.7). Scatter plots displaying the relationship between changes in glucose levels versus PEs from these voxels are shown to the right of each coronal section. These peaks fall within a single cluster that was found to be significant at p < 0.05 FDR corrected at the cluster level across the whole brain (number of voxels in cluster k = 107). Color scale bars correspond to SPM t values. Changes in glucose are shown as standardized (z score). When liking was included as the first regressor and Δglucose as the second, minimal changes were observed in the results. In NAcc, the peak voxels were located at −3, 5, −5 (z = 3.81) and 9, 5, −5 (z = 3.35). In hypothalamus, the peak voxels were located at 3, −1, −5 (z = 3.7) and 3, −1, −5 (z = 3.9). As in the original analysis, these peaks fall within a single cluster that was found to be significant at p < 0.05 FDR corrected at the cluster level across the whole brain.
See also Table S1.
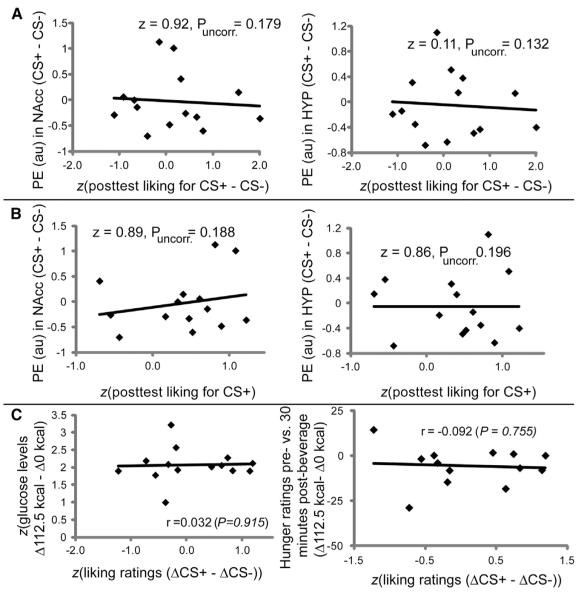
Hedonic Conditioning and Liking Are Unrelated to Plasma Glucose or NAcc/Hypothalamic Response
Next we sought to exhaustively test for associations between NAcc and hypothalamic responses and hedonic conditioning. No significant associations were observed between differential response to the CS+ versus the CS− flavors and either hedonic conditioning or postconditioning CS+ liking ratings (Figure 4). Furthermore, we regressed change in liking for each stimulus (CS+ and CS−) against response to that stimulus minus the control drink (i.e., change in liking for CS− regressed against CS− minus control and change in liking for CS+ regressed against CS+ minus control) and again observed no associations with response in the hypothalamus or NAcc. This was true even when the t map threshold was lowered to p < 0.05. We also note that changes in liking ratings were not associated with changes in either glucose or hunger levels (Figure 4C). Finally, when ratings for wanting, stimulus intensity, and sweetness were regressed to the brain responses obtained from the contrast (CS+ minus CS−), no significant foci of activation were detected in NAcc or hypothalamus. We therefore conclude that glucose metabolism regulates the formation of associations between flavors and their physiological value in the NAcc and hypothalamus independently of hedonic conditioning and perceptions of liking. Although it is certain that collecting several postingestion plasma samples (instead of only one) would have provided a more complete description of the subjects’ glucose profiles (while nevertheless increasing stress), the lack of associations between blood glucose changes and psychophysical or other measurements strongly supports the claim that the glucose measurements were sufficient to capture a robust and specific sensitivity of hypothalamic/accumbal circuits to metabolic events associated with flavor cues.
Figure 4.
Changes in Flavor Liking Fail to Regulate Calorie-Predictive Flavor Responses in Human NAcc and Hypothalamus
(A) No significant associations in NAcc or hypothalamus were found between the parameter estimates (PEs) associated with the SPM contrast (CS+ minus CS−) and standardized (z score) postconditioning CS+ liking ratings minus post-conditioning CS− liking ratings. For (A) and (B), the z and p values are uncorrected for multiple comparisons. au, arbitrary units.
(B) No significant associations in NAcc or hypothalamus were found between responses revealed by the SPM contrast (CS+ minus CS−) and standardized (z score) postconditioning liking ratings for the CS+.
(C) No significant associations were observed between standardized (z score) changes in liking ratings and standardized (z score) changes in glucose or hunger levels. For statistically nonsignificant correlations, p > 0.5. ΔCS+ is change in ratings (post- versus preconditioning) for the CS+ flavor. Similar definition applies for ΔCS−.
Discussion
Our results provide the first direct evidence that responses to flavors in the NAcc and hypothalamus, neural circuits known to be critical for the anticipation of food availability and the initiation of consummatory acts [1, 3, 24], reflect the learned association between a flavor and its ability to result in a change in blood glucose. We additionally show that this learning is independent of the ability of postoral signals to increase liking.
These results extend findings from work in rodents highlighting metabolic regulation of NAcc cue-driven activation. First, Sclafani and colleagues have shown that dopaminergic signaling in the NAcc and hypothalamus is critical for the process by which postingestive factors increase preference for flavors with which they are associated [7, 22, 25]. More recently, systemic glucose administration was shown to be sufficient to influence appetitive behaviors and dopamine release [8], and sugar-predictive cues were found to evoke greater NAcc dopamine release than saccharin-predictive cues [26]. Our findings are also consistent with previous studies indicating that lateral hypothalamic taste-responsive neurons are modulated by metabolic responses to sugars [27]. More generally, our findings establish not only that metabolic regulation of NAcc and hypothalamic activation is extendable to humans but, more crucially, that metabolic responses to glucose ingestion control NAcc and hypothalamic responses to learned cues signaling calories.
The lack of significant effects produced by changes in hedonic ratings on accumbal activation provides further support to the notion that hedonic “liking” and motivation “wanting” signals for food reward differentially affect accumbal circuits [28]. Especially relevant for flavor-nutrient conditioning (see above), accumbal microinjections of dopamine-stimulating drugs are known to enhance the motivation, but not the hedonic impact, of learned prediction signals [29]. Consistent with the above, the accumbal effects observed in our study did not depend on the detection of maltodextrin taste: the removal of maltodextrin-detecting subjects, rather than compromising generalizability, revealed that dedicated human brain circuits encode the associations between flavors and metabolic processes independently of sugar oral detection. Our findings therefore suggest that metabolic signals generated during and/or after food digestion specifically modulate the motivational component of flavor-nutrient learning. Furthermore, these results also indicate that flavors, upon associations with ensuing metabolic effects, elicit anticipatory responses even during active orosensation, i.e., during the “consummatory” phase of eating. Such superposition may have wide implications for how the brain controls the motor acts associated with active ingestive behavior.
Our finding also has important implications for a large literature aiming to identify neural circuits associated with risk of weight-gain susceptibility. Responses to food cues in the NAcc and hypothalamus are associated with body weight [30], sensitivity to reward [31], external food sensitivity [32], dietary restraint [33], hunger [34], overfeeding [35], failure to succeed in weight-loss preventions [36], and increases in body weight over a one-year period [37]. Although it is clear that response to food cues in this circuit reflects the reinforcement value of food cues [38–40], the physiological signal regulating this response was unknown. The current results strongly suggest that glucose metabolism generates such a critical signal. This is consistent with the fact that BOLD responses in the NAcc and hypothalamus are sensitive to manipulations of circulating plasma glucose [41]. Critically, because changes in plasma glucose levels following a standard challenge are determined by individual responses to glucose absorption and/or utilization, our findings imply that the reinforcement potency of novel drinks or foods that drive their consumption, and hence contribute to weight-gain susceptibility, may be dependent upon individual differences in glucose metabolism. Future studies directly testing this hypothesis will be critical because, if borne out, they would support the notion that individuals with diabetes and prediabetes are more susceptible to the reinforcing effects of energy-dense foods and hence more reactive to cues predicting their availability [42].
Finally, we stress that our results by no means imply that circulating blood glucose levels constitute the obligatory reinforcing signal during flavor-nutrient conditioning. Rather, these levels function as indexes of a series of complex physiological events. The expression “glucose metabolism” indicates the entire sequence of biochemical processes linking the consumption of maltodextrin (essentially a polymer consisting of D-glucose units) to a given level of glucose in plasma. Such sequence involves the breakdown of maltodextrin into absorbable glucose moieties, the active and passive preabsorptive and absorptive processes—modulated by gastric emptying—allowing glucose into enterocytes, metabolic events occurring within the enterocyte proper (including glucose oxidation), the release of gut incretin hormones such as GLP-1 that in turn regulate blood insulin and glucose levels, and the subsequent release of glucose into the circulation. Because we did not rule out the possibility that critical signaling events occur within the gastrointestinal tract, our findings are not inconsistent with the reported inability of intravenous glucose infusions to condition flavor preferences [17]. In addition, although the gut hormone ghrelin was not directly related to accumbal/hypothalamic flavor responses, it is known to regulate reward circuitry [43] and may therefore have indirectly influenced the results. We note in particular that the small intestine is a site where high glucose metabolism activity is observed in vivo, a process that in turn regulates glucose absorption and transport [44]. The above is nevertheless also consistent with the finding that vagal signals are not required for the formation of maltodextrin-based flavor-nutrient conditioning [45]. The possibility that intestinal glucose oxidation provides reinforcing signals to the central nervous system during nutrient ingestion is a fascinating topic for future research.
Experimental Procedures
Please see Supplemental Experimental Procedures for a full description of the paradigm design, methodology, and analysis.
Supplementary Material
Acknowledgments
This study was supported by NIH grants R01-DK085579 to D.M.S. and I.E.d.A. and R01-DC009997 to I.E.d.A. We thank Paul Geha for assistance with fMRI data analyses, the John B. Pierce Laboratory workshop for stimulus delivery apparatus, Cheryl Leone for blood sampling and storage, and Alexandra D’Agostino and Julie Boyle for assistance with running subjects and stimulus preparation.
Footnotes
Supplemental Information Supplemental Information includes one table and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2013.04.001.
References
- 1.Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacology (Berl.) 2007;191:439–459. doi: 10.1007/s00213-007-0741-z. [DOI] [PubMed] [Google Scholar]
- 2.Day JJ, Carelli RM. The nucleus accumbens and Pavlovian reward learning. Neuroscientist. 2007;13:148–159. doi: 10.1177/1073858406295854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog. Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- 4.Berridge KC. Food reward: Brain substrates of wanting and liking. Neurosci. Biobehav. Rev. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- 5.Holman EW. Immediate and delayed reinforcers for flavor preferences in rats. Learn. Motiv. 1975;6:91–100. [Google Scholar]
- 6.Sclafani A. Oral and postoral determinants of food reward. Physiol. Behav. 2004;81:773–779. doi: 10.1016/j.physbeh.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 7.Touzani K, Bodnar RJ, Sclafani A. Neuropharmacology of learned flavor preferences. Pharmacol. Biochem. Behav. 2010;97:55–62. doi: 10.1016/j.pbb.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren X, Ferreira JG, Zhou L, Shammah-Lagnado SJ, Yeckel CW, de Araujo IE. Nutrient selection in the absence of taste receptor signaling. J. Neurosci. 2010;30:8012–8023. doi: 10.1523/JNEUROSCI.5749-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeomans MR, Leitch M, Gould NJ, Mobini S. Differential hedonic, sensory and behavioral changes associated with flavor-nutrient and flavor-flavor learning. Physiol. Behav. 2008;93:798–806. doi: 10.1016/j.physbeh.2007.11.041. [DOI] [PubMed] [Google Scholar]
- 10.Holman GL. Intragastric reinforcement effect. J. Comp. Physiol. Psychol. 1969;69:432–441. doi: 10.1037/h0028233. [DOI] [PubMed] [Google Scholar]
- 11.Booth DA. Food-conditioned eating preferences and aversions with interoceptive elements: conditioned appetites and satieties. Ann. N Y Acad. Sci. 1985;443:22–41. doi: 10.1111/j.1749-6632.1985.tb27061.x. [DOI] [PubMed] [Google Scholar]
- 12.Sclafani A. Post-ingestive positive controls of ingestive behavior. Appetite. 2001;36:79–83. doi: 10.1006/appe.2000.0370. [DOI] [PubMed] [Google Scholar]
- 13.Yeomans MR, Gould NJ, Leitch M, Mobini S. Effects of energy density and portion size on development of acquired flavour liking and learned satiety. Appetite. 2009;52:469–478. doi: 10.1016/j.appet.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Brunstrom JM, Mitchell GL. Flavor-nutrient learning in restrained and unrestrained eaters. Physiol. Behav. 2007;90:133–141. doi: 10.1016/j.physbeh.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Lawless HT, Heymann H. Sensory Evaluation of Food: Principles and Practices. Kluwer Academic; Norwell, MA, USA: 1999. [Google Scholar]
- 16.Lim J, Wood A, Green BG. Derivation and evaluation of a labeled hedonic scale. Chem. Senses. 2009;34:739–751. doi: 10.1093/chemse/bjp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ackroff K, Yiin YM, Sclafani A. Post-oral infusion sites that support glucose-conditioned flavor preferences in rats. Physiol. Behav. 2010;99:402–411. doi: 10.1016/j.physbeh.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veldhuizen MG, Douglas D, Aschenbrenner K, Gitelman DR, Small DM. The anterior insular cortex represents breaches of taste identity expectation. J. Neurosci. 2011;31:14735–14744. doi: 10.1523/JNEUROSCI.1502-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Araujo IE, Geha P, Small DM. Orosensory and homeostatic functions of the insular taste cortex. Chemosens. Percept. 2012;5:64–79. doi: 10.1007/s12078-012-9117-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braun JJ, Slick TB, Lorden JF. Involvement of gustatory neocortex in the learning of taste aversions. Physiol. Behav. 1972;9:637–641. doi: 10.1016/0031-9384(72)90023-6. [DOI] [PubMed] [Google Scholar]
- 21.Smeets PA, de Graaf C, Stafleu A, van Osch MJ, van der Grond J. Functional MRI of human hypothalamic responses following glucose ingestion. Neuroimage. 2005;24:363–368. doi: 10.1016/j.neuroimage.2004.07.073. [DOI] [PubMed] [Google Scholar]
- 22.Touzani K, Bodnar R, Sclafani A. Activation of dopamine D1-like receptors in nucleus accumbens is critical for the acquisition, but not the expression, of nutrient-conditioned flavor preferences in rats. Eur. J. Neurosci. 2008;27:1525–1533. doi: 10.1111/j.1460-9568.2008.06127.x. [DOI] [PubMed] [Google Scholar]
- 23.Touzani K, Sclafani A. Conditioned flavor preference and aversion: role of the lateral hypothalamus. Behav. Neurosci. 2001;115:84–93. doi: 10.1037/0735-7044.115.1.84. [DOI] [PubMed] [Google Scholar]
- 24.Peciña S, Smith KS, Berridge KC. Hedonic hot spots in the brain. Neuroscientist. 2006;12:500–511. doi: 10.1177/1073858406293154. [DOI] [PubMed] [Google Scholar]
- 25.Sclafani A, Touzani K, Bodnar RJ. Dopamine and learned food preferences. Physiol. Behav. 2011;104:64–68. doi: 10.1016/j.physbeh.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCutcheon JE, Beeler JA, Roitman MF. Sucrose-predictive cues evoke greater phasic dopamine release than saccharin-predictive cues. Synapse. 2012;66:346–351. doi: 10.1002/syn.21519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Araujo IE, Gutierrez R, Oliveira-Maia AJ, Pereira A, Jr., Nicolelis MA, Simon SA. Neural ensemble coding of satiety states. Neuron. 2006;51:483–494. doi: 10.1016/j.neuron.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Berridge KC. ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiol. Behav. 2009;97:537–550. doi: 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith KS, Berridge KC, Aldridge JW. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc. Natl. Acad. Sci. USA. 2011;108:E255–E264. doi: 10.1073/pnas.1101920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoeckel LE, Weller RE, Cook EW, 3rd, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41:636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 31.Beaver JD, Lawrence AD, van Ditzhuijzen J, Davis MH, Woods A, Calder AJ. Individual differences in reward drive predict neural responses to images of food. J. Neurosci. 2006;26:5160–5166. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Passamonti L, Rowe JB, Schwarzbauer C, Ewbank MP, von dem Hagen E, Calder AJ. Personality predicts the brain’s response to viewing appetizing foods: the neural basis of a risk factor for overeating. J. Neurosci. 2009;29:43–51. doi: 10.1523/JNEUROSCI.4966-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demos KE, Kelley WM, Heatherton TF. Dietary restraint violations influence reward responses in nucleus accumbens and amygdala. J. Cogn. Neurosci. 2011;23:1952–1963. doi: 10.1162/jocn.2010.21568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldstone AP, Prechtl de Hernandez CG, Beaver JD, Muhammed K, Croese C, Bell G, Durighel G, Hughes E, Waldman AD, Frost G, Bell JD. Fasting biases brain reward systems towards high-calorie foods. Eur. J. Neurosci. 2009;30:1625–1635. doi: 10.1111/j.1460-9568.2009.06949.x. [DOI] [PubMed] [Google Scholar]
- 35.Cornier MA, Von Kaenel SS, Bessesen DH, Tregellas JR. Effects of overfeeding on the neuronal response to visual food cues. Am. J. Clin. Nutr. 2007;86:965–971. doi: 10.1093/ajcn/86.4.965. [DOI] [PubMed] [Google Scholar]
- 36.Murdaugh DL, Cox JE, Cook EW, 3rd, Weller RE. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. Neuroimage. 2012;59:2709–2721. doi: 10.1016/j.neuroimage.2011.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geha PY, Aschenbrenner K, Felsted J, O’Malley SS, Small DM. Altered hypothalamic response to food in smokers. Am. J. Clin. Nutr. 2013;97:15–22. doi: 10.3945/ajcn.112.043307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Killgore WD, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Cortical and limbic activation during viewing of high- versus low-calorie foods. Neuroimage. 2003;19:1381–1394. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- 39.O’Doherty JP, Buchanan TW, Seymour B, Dolan RJ. Predictive neural coding of reward preference involves dissociable responses in human ventral midbrain and ventral striatum. Neuron. 2006;49:157–166. doi: 10.1016/j.neuron.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 40.Schur EA, Kleinhans NM, Goldberg J, Buchwald D, Schwartz MW, Maravilla K. Activation in brain energy regulation and reward centers by food cues varies with choice of visual stimulus. Int. J. Obes. (Lond.) 2009;33:653–661. doi: 10.1038/ijo.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Page KA, Seo D, Belfort-DeAguiar R, Lacadie C, Dzuira J, Naik S, Amarnath S, Constable RT, Sherwin RS, Sinha R. Circulating glucose levels modulate neural control of desire for high-calorie foods in humans. J. Clin. Invest. 2011;121:4161–4169. doi: 10.1172/JCI57873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chechlacz M, Rotshtein P, Klamer S, Porubská K, Higgs S, Booth D, Fritsche A, Preissl H, Abele H, Birbaumer N, Nouwen A. Diabetes dietary management alters responses to food pictures in brain regions associated with motivation and emotion: a functional magnetic resonance imaging study. Diabetologia. 2009;52:524–533. doi: 10.1007/s00125-008-1253-z. [DOI] [PubMed] [Google Scholar]
- 43.Abizaid A. Ghrelin and dopamine: new insights on the peripheral regulation of appetite. J. Neuroendocrinol. 2009;21:787–793. doi: 10.1111/j.1365-2826.2009.01896.x. [DOI] [PubMed] [Google Scholar]
- 44.Kellett GL, Jamal A, Robertson JP, Wollen N. The acute regulation of glucose absorption, transport and metabolism in rat small intestine by insulin in vivo. Biochem. J. 1984;219:1027–1035. doi: 10.1042/bj2191027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sclafani A, Lucas F. Abdominal vagotomy does not block carbohydrate-conditioned flavor preferences in rats. Physiol. Behav. 1996;60:447–453. doi: 10.1016/s0031-9384(96)80018-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.