Abstract
Vesicle fusion has long provided an easy and reliable method to form supported lipid bilayers (SLBs) from simple, zwitterionic vesicles on siliceous substrates. However, for complex compositions, such as vesicles with high cholesterol content and multiple lipid types, the energy barrier for the vesicle-to-bilayer transition is increased or the required vesicle-vesicle and vesicle-substrate interactions are insufficient for vesicle fusion. Thus, for vesicle compositions that more accurately mimic native membranes, vesicle fusion often fails to form SLBs. In this paper, we review three approaches to overcome these barriers to form complex, biomimetic SLBs via vesicle fusion: (i) optimization of experimental conditions (e.g., temperature, buffer ionic strength, osmotic stress, cation valency, and buffer pH), (ii) α-helical (AH) peptide-induced vesicle fusion, and (iii) bilayer edge-induced vesicle fusion. AH peptide-induced vesicle fusion can form complex SLBs on multiple substrate types without the use of additional equipment. Bilayer edge-induced vesicle fusion uses microfluidics to form SLBs from vesicles with complex composition, including vesicles derived from native cell membranes. Collectively, this review introduces vesicle fusion techniques that can be generalized for many biomimetic vesicle compositions and many substrate types, and thus will aid efforts to reliably create complex SLB platforms on a range of substrates.
1. Introduction
Native plasma membranes contain a complex, heterogeneous distribution of lipids and membrane proteins which interact to create important biological functions. To investigate this complex membrane environment significant progress has been made to model native membranes. The most common systems include lipid monolayers, lipid vesicles, and supported lipid bilayers (SLBs). While each system has its advantages, SLBs are particularly valuable due to their ease of formation and their lipid arrangement. SLBs constitute a single lipid bilayer on a solid substrate, typically glass, silica, or mica. The hydrophilic head groups of one lipid leaflet face the substrate where they are separated by a thin hydration layer. Their hydrophobic acyl chains interact with the acyl chains of the second lipid leaflet, whose hydrophilic head groups face the bulk solution and are available to interact with analytes (proteins, cells, nanoparticles, etc.). The SLB is stable and confined in two dimensions to the substrate surface, yet it can recapitulate the lateral lipid diffusivity of native cell membranes. Furthermore, the planar orientation of SLBs allows the use of many quantitative surface characterization techniques that are able to provide unique insights into membrane functions.
There are many techniques to create SLBs, including Langmuir-Blodgett/Schäfer deposition,[1] spin coating,[2] microcontact printing,[3] solvent-exchange deposition,[4] lipid-surfactant micelles,[5] evaporation induced assembly,[6] bubble collapse deposition,[7*] lipid dip-pen nanolithography,[8] and vesicle fusion.[9*] Langmuir-Blodgett/Schäfer (LB/LS) deposition and vesicle fusion are perhaps the most commonly used techniques to form SLBs. Briefly, LB/LS deposition is achieved by transferring a lipid monolayer contained at an air-liquid interface to a solid substrate. The substrate is passed through the lipid monolayer a second time to assemble the final SLB. SLB formation via vesicle fusion typically occurs by adsorption of lipid vesicles to a substrate, followed by vesicle rupture, fusion, and bilayer spreading. Of these techniques, vesicle fusion is the most simple, versatile, and widely accessible since it does not require sophisticated equipment to produce high quality SLBs. These advantages position vesicle fusion to play an important role in advancing SLB research platforms, particularly in regards to creating complex, multi-component SLBs that more accurately mimic native cell membranes. Thus, this article will focus on vesicle fusion techniques to form complex SLBs. Other SLB forming techniques and model lipid systems are discussed in recent review articles.[10, 11]
SLBs that contain one or two zwitterionic lipid types, and are supported on siliceous substrates (e.g., glass, silicon oxide, or mica), have long provided the foundation of model SLB systems.[12*] These simple SLBs have been exceptionally successful at mimicking the basic structure and dynamics of the plasma membrane; however, they fail to capture the highly complex lipid environment that often determines native biological functions. For example, there are about 100 lipid species in the simple red blood cell alone, and more than 600 lipid species in most plasma membranes.[13] Considering this extensive membrane diversity, SLBs with one or two lipid types can be inadequate when attempting to accurately model native cell membranes.
Successful SLB formation via vesicle fusion largely depends on the lipid components of the vesicles being used. In attempts to increase SLB complexity, such as by incorporating cholesterol, charged lipids, or phase separating lipid compositions, complete SLB formation may no longer occur.[14**, 15] This restricts the utility of SLBs created from vesicle fusion by limiting their compositional complexity to simple binary or tertiary lipid compositions containing little or no cholesterol. Cholesterol is often neglected or underrepresented in biomimetic SLBs because it can prevent vesicle fusion and subsequent SLB formation by increasing vesicle rigidity.[14**] However, cholesterol is an essential component of plasma membranes, ranging from 15–50% of total lipid composition.[13] In native plasma membranes, cholesterol provides an important structural role by condensing acyl chains of unsaturated lipids in fluid lipid phases, and fluidizing saturated lipids that would otherwise form solid-like gel phases. Regions of cholesterol- and sphingolipid-enriched domains, termed lipid rafts,[16*] are also believed to exist in plasma membranes, where they contribute to compartmentalizing cellular processes. Lipid rafts are associated with the function of many membrane proteins and are directly linked to important pathologies including those of the central nervous system,[17, 18] viral infections,[19, 20] cardiovascular disease,[21] and certain types of cancer.[22]
Despite the difficulties in forming complex SLB systems, several vesicle fusion approaches have the capability to create SLBs with multiple lipid types and high cholesterol content on a range of substrates. In this review, we summarize three approaches to induce complex SLB formation: (i) optimizing experimental conditions, including temperature and buffer selection, (ii) the use of α-helical (AH) peptides acting as a vesicle fusion catalyst, and (iii) the use of a moving bilayer edge in a microfluidic channel. These approaches were selected due to their potential to advance the use of biomimetic SLB platforms by easily achieving SLB formation on a range of substrates and under conditions that would otherwise be unfavorable.
2. Background
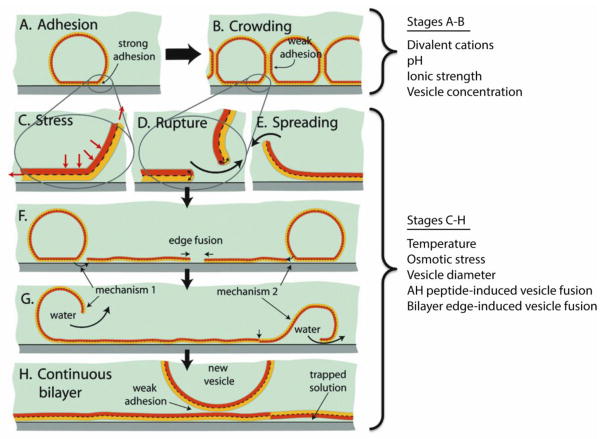
The mechanism of vesicle fusion will be briefly discussed to understand the barriers to SLB formation and how they can be overcome. Although not completely understood, the mechanism of vesicle fusion is believed to be a two-step process that relies on membrane tension, vesicle-vesicle and vesicle-substrate interactions, as shown in Figure 1.[23, 24] Small unilamellar vesicles are first adsorbed to a substrate surface. Vesicle crowding ensues and after a critical concentration (θc) of surface-adhered vesicles is reached, vesicles will rupture and fuse with each other, forming SLB patches on the substrate. The energetically unfavorable edge of SLB patches can spread on the surface and induce rupture of adsorbed vesicles to form a complete SLB (Fig. 1). Although one-step SLB formation by direct vesicle rupture is possible for certain combinations of vesicles, buffers, and substrates,[25] the surface-induced stress alone is often insufficient for vesicle rupture.
Figure 1.
Stages of SLB formation: (A) adhesion, (B) crowding, (C–E) rupture and spreading of bilayer patches that can expose either leaflet by mechanism 1 or 2, (F, G) coalescence of high energy edges and release of water/excess lipid, and (H) completed SLB. Additional vesicle adsorption to the SLB is typically weak and does not lead to their rupture or spreading. Experimental conditions and techniques that generally have the most pronounce effect on respective stages of SLB formation are listed to the right of the figure. Substrate type and chemical surface modifications are omitted from classification since these conditions generally affect the entire SLB formation process. Adapted with permission from reference [12*]. Copyright (2009) American Chemical Society.
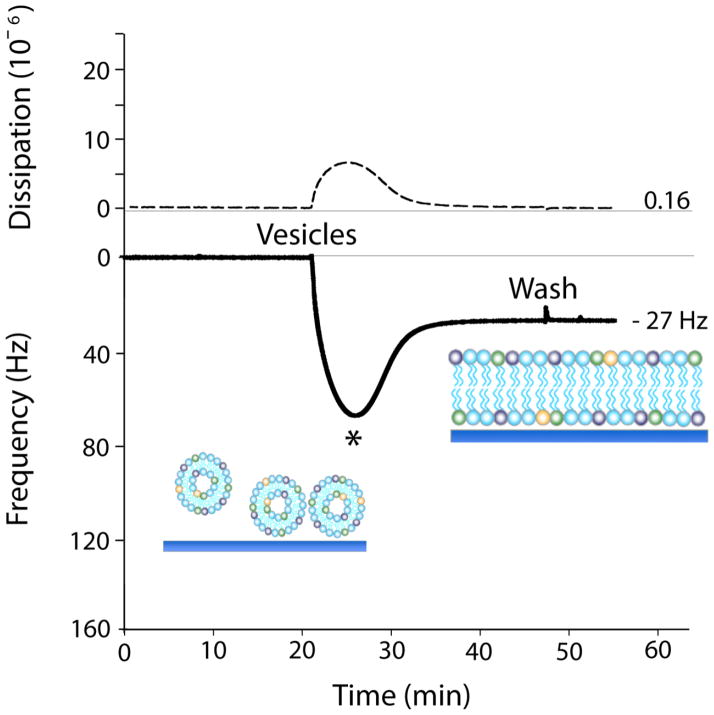
The transition from vesicles to a SLB is most commonly observed with quartz crystal microbalance with dissipation monitoring (QCM-D).[26] In this review, QCM-D results will be used to discuss the effectiveness of vesicle fusion techniques. QCM-D measures, in real-time, mass adsorbed and desorbed from a substrate by monitoring the resonance frequency change (Δf) of an oscillating quartz crystal. In the limit of thin, elastic layers, the relationship between a quartz crystal’s resonance frequency change and the mass of the adlayer is linear, and described by the Sauerbrey equation.[27] Viscoelasticity of the adlayer can also be detected by monitoring the damping of the crystal’s oscillation (ΔD), which can give insight into the conformational changes occurring during vesicle fusion and peptide-lipid interactions. Figure 2 shows frequency and dissipation plotted as a function of time during SLB formation via vesicle fusion from zwitterionic phosphatidylcholine (PC) vesicles. First, vesicles are sparsely adsorbed onto the silica surface, resulting in a large frequency drop due to the increase in associated mass from the buffer trapped within and between the intact vesicles (Fig. 1A,B). Concurrently, the adsorbed vesicles contribute to an increase in dissipation due to their viscoelastic properties. Once the vesicle surface coverage reaches a critical concentration (θc, indicated by the * in Fig. 2), the vesicles spontaneously rupture and fuse to form a continuous SLB.[28–31] The frequency increase is due to SLB displacement of adsorbed vesicles and the buffer released from within the vesicle interior (Fig. 1G). Thus, for vesicles composed of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), a complete bilayer is characterized by a dip in Δf and peak ΔD with time, resulting in a final Δf of ~ −26 Hz and a ΔD of ~ 0.2 × 10−6 (Fig. 2).[23]
Figure 2.
QCM-D frequency (solid line) and dissipation (dashed line) response plotted as a function of time for SLB formation from pure POPC vesicles via vesicle fusion. (*) indicates the critical concentration of vesicle surface coverage (θc). Adapted with permission from reference [14**].
While QCM-D serves as a reliable method to confirm bilayer formation on the meso- and macroscale, it is limited in the detection of minor bilayer defects and in characterizing details in the physical properties of SLBs. Past QCM-D studies have found that for different vesicle compositions, final Δf and ΔD values can differ substantially, e.g., up to 30% for Δf.[14**, 15] The interpretation of such differences is difficult, given that Δf represents an averaged, surface-associated mass, and does not differentiate between mass contributions from the lipids, the associated buffer, or the hydration layer. Furthermore, ΔD provides qualitative insights into the structure of surface-associated mass and cannot quantitatively identify structural properties such as bilayer thickness, defects, or lipid packing density. Thus, QCM-D should be used in combination with other techniques, such as neutron reflectivity, ellipsometry, surface plasmon resonance, fluorescence recovery after photo-bleaching, and atomic force microscopy to confirm details of SLB formation and to quantify SLB properties.
3. Experimental optimization techniques to achieve vesicle fusion
Although SLB formation via vesicle fusion is a reliable and easy technique for many vesicle compositions, it is still a complex, dynamic, and highly sensitive process. As researches attempt to increase complexity of SLB platforms, additional steps or alternative techniques become necessary to induce SLB formation. Favorable SLB formation by conventional vesicle fusion largely depends on the substrate type and lipid composition of the vesicles, where lipid charge, polarity, head group size, acyl chain length, and degree of unsaturation ultimately all contribute to the ease of vesicle fusion and subsequent SLB formation. However, for researchers attempting to use SLBs to model native membranes the compositional ratios and lipid types of a vesicle are largely fixed. Yet, the experimental conditions in which vesicle fusion takes place can often be optimized to achieve SLB formation for a wide range of vesicle compositions. Conditions that are commonly manipulated include vesicle size,[30] vesicle concentration,[12*] temperature,[32] pH,[33*] flow conditions (batch or flow system),[34**] exposure to buffers of different ionic strengths,[35] the type of substrate material,[34**] and chemical surface modifications.[23] It is commonly observed that experimental conditions that work for one vesicle composition, may fail to work as well for that of another vesicle composition. However, there are a few variables that are generally more important than others since they can be successfully applied to a wide range of vesicle compositions to promote vesicle fusion. These include temperature, buffer pH, buffer ionic strength, ion type, and osmotic stress.
3.1 Effect of temperature
The critical surface coverage of vesicles required for SLB formation has been shown to be temperature dependent, thus indicating that vesicle rupture is a thermally activated process.[32] For example, Reimhult et al. have shown that a decrease in temperature requires a higher critical coverage (θc) of surface adhered vesicles (i.e., an increase in vesicle-vesicle interactions) to induce fusion, and increases the time to transition from intact vesicles to SLBs.[9*] Furthermore, temperature also affects the lipid phase, which can play a critical role for vesicle fusion. A phase change into a more fluid vesicle can promote SLB formation, especially when the temperature change involves passing through a chain melting temperature (Tm) of one of the lipid components.[12*] The chain melting temperature defines the transition of a lipid from one phase to another i.e., from the gel to the liquid-disordered (ld) phase. Vesicles with lipids in the gel phase contain higher order compared to the ld phase and are believed to resist vesicle fusion due to decreased lipid mobility and vesicle-vesicle interactions. Thus, using vesicles in the liquid phase by raising the temperature approximately 15 °C above the highest lipid Tm is a common and successful strategy for achieving reliable SLB formation.[9*, 32]
In addition to lipid structure, the presence of cholesterol and sphingolipids can also result in vesicle phase separations as they have the tendency to self-associate within the ld phase to form highly ordered, tightly packed islands, known as liquid ordered (lo) domains or lipid rafts.[36] For instance, POPC/cholesterol (55:45) undergoes a phase transition in the temperature range between 15–35 °C.[37] At 15 °C the lipid bilayer is completely in the lo phase while between 25–35 °C the lo and ld phases co-exist. With increasing temperature the ld phase increases, resulting in shorter times to reach θc, peak Δf and ΔD amplitudes decrease, and final Δf and ΔD values approach those of pure POPC vesicles.[15] Thus, a temperature increase has been successful in achieving SLB formation from vesicles containing as much as 50 mol% percent cholesterol.[38*] Without heating, vesicles may fail to consistently form SLBs when containing cholesterol compositions above 33 mol%.[39] Therefore, for vesicles containing cholesterol, raising the temperature to increase the ld phase may assist in achieving reliable SLB formation.
Using elevated temperature to prepare SLBs via vesicle fusion is one of the most frequently used techniques. However, depending on the vesicle composition, the use of temperature alone can be insufficient to induce vesicle fusion. Furthermore, this technique is not appropriate when using temperature sensitive membrane components, such as membrane embedded proteins. In such instances, researchers are referred to alternative techniques discussed below.
3.2 Effect of pH
Initial vesicle-substrate interactions are often the limiting factor in determining whether successful SLB formation occurs. Whether these interactions are sufficient for SLB formation largely depends on electrostatic forces between the lipid vesicles and substrate surface.[25] Generally, to readily achieve vesicle fusion, electrostatic attraction between vesicles and the substrate surface should be enhanced. If attractive electrostatic interactions are not possible, then repulsive electrostatic forces should be minimized. This can allow van der Waals, steric, and hydration forces to dominate surface interactions which can be sufficient to promote bilayer formation.
Electrostatic forces play an important role in creating biomimetic SLBs because most native cell membranes contain negatively charged lipids (yielding a surface charge of ~ −0.05 C/m2)[40] and SLBs are most commonly formed on negatively charged mica and silica substrates. This repulsive electrostatic interaction results in an increase in θc required for vesicle rupture and, depending on the concentration of negatively charged lipid, may prevent SLB formation entirely.[34**] An effective technique for tuning the electrostatic interactions between vesicles and the substrate surface is to adjust the buffer pH (under constant ionic strength). The buffer pH influences the state of lipid charge by affecting the degree of ionization, reflected in the lipid’s pKa. This pKa is largely determined by the lipid head group acid-base chemistry.
Here, we present a general guideline for using buffer pH (with ionic strength ≈ 100 mM NaCl) to minimize the negative charge of common lipid types.[41] For example, common negatively charged lipid head groups include phosphatidylserine (PS), phosphatidylinositol (PI), phosphatidic acid (PA), and phosphatidylglycerol (PG). PS has pKas at 2.6, 5.5, and 11.5, requiring a pH < 6 to diminish its charge of 1 and at pH < 4, PS will begin to have a net positive charge. PI, PA, and PG have a pKa at 2.5, 3.0, and 3.5, respectively. Thus, each lipid type will begin to diminish its charge of −1 at pH < 4. Furthermore, common zwitterionic lipids include those with a phosphatidylcholine (PC) or phosphatidylethanolamine (PE) head group. PE has pKas at ~1.7 and 9.8, resulting in a neutral charge between pH ≈ 3–9. PC has one pKa at ~1.0 and will be neutral for any pH > ~3.[41] Positively charged lipids (at pH = 7) are not commonly used to model native cell membranes; however, when using negatively charged surfaces (e.g., silica or mica) vesicle-substrate interactions can be enhanced by strategically incorporating positively charged membrane components (e.g., peptides, lipids, or fluorescent probes) within the vesicles. As little as 1 mol% of positively charged membrane probe can be effective at inducing SLB formation.[42]
Buffer pH can also affect the ionization of hydroxyl groups that exist on surface oxides commonly used for SLB formation (e.g., mica, silica, and titanium oxide). At a certain pH, known as the point of zero charge (pzc), the surface charge is approximately zero. At a pH above its pzc value, a surface is generally negatively charged, while at a pH below the pzc the surface is generally positively charged. For silica and mica surfaces, the pzc is ~1.5 – 3.5, while the pzc of titanium oxide is ~6.5.[43] Thus, using a pH at or below the pzc value, can diminish repulsive vesicle-substrate interactions and enhance vesicle adsorption and subsequent SLB formation for negatively charged vesicles.
Cho et al. demonstrates how oxide surfaces are affected by pH and ultimately control successful vesicle fusion. SLB formation was monitored in various buffer pH (with 200 mM NaCl) using zwitterionic POPC vesicles on silicon and titanium oxide surfaces.[33*] For POPC vesicles on silicon oxide at a pH ≥ 10.0 an irreversibly adsorbed vesicle monolayer is formed, with no subsequent vesicle fusion. At pH = 7.5, however, a SLB is formed, as expected, in two successive steps. At a pH ≤ 6.0, SLB formation approaches one-step behavior, indicating a decreasing role of vesicle-vesicle interactions required for rupture. POPC vesicles on titanium oxide, which showed weaker vesicle-substrate interactions than silicon oxide, form an irreversibly adsorbed vesicle monolayer already at pH = 7.5, again with no subsequent vesicle fusion. However, at pH = 4.0 the vesicle-substrate interactions are strong enough to generate isolated SLB patches mixed with adsorbed vesicles, and at pH = 2.5, a complete bilayer finally forms. These results demonstrate that stronger vesicle-substrate interactions often occur at low pH and that oxide surfaces have titratable OH groups that control the surface charge density, which plays an important role in the adsorption and fusion of vesicles.
3.3 Effect of ionic strength and ion type
Closely linked to surface charge is the surface potential, which also needs to be considered when evaluating vesicle fusion interactions. The lipid surface potential is best approximated by the ζ potential, which describes the charge seen at a distance from the surface, where the Stern layer and diffuse layer meet. The ionic strength of the buffer plays an essential in role in determining the ζ potential and is likely the major reason why zwitterionic vesicles fail to adsorb to and fuse on substrates in deionized water. For example, Anderson et al. demonstrated that adhesion and fusion of DMPC vesicles on silica decreased with decreasing ionic strength, and below a critical ionic strength of about 1.5 mM no longer formed SLBs.[12*] Even though DMPC is theoretically zwitterionic over a broad range of pH values, due to head group orientation[44] and hydration layers surrounding the head group surface,[45] PC has a negative ζ potential of ~ 12 mV in deionized water.[46] As the ionic strength increases, the ζ potential increases because cations adsorb to the surface of the polar lipid head group. At a high ionic strength, the ζ potential of PC becomes positive and vesicles readily adsorb and fuse on negatively charged substrates.[46]
Presence of divalent ions can also influence the formation of SLBs, especially for vesicle compositions containing charged lipid head groups or charged membrane embedded peptides. Divalent cations stabilize vesicle-substrate interactions between the negatively charged lipid head groups and negatively charged substrates by charge bridging.[47] For example, calcium (Ca2+) and magnesium (Mg2+) ions are common divalent cations used to promote SLB formation with negatively charged vesicles.[35, 48, 49] Already at concentrations as low as 25 μM, Ca2+ ions contribute significantly to vesicle fusion.[42] It is important to introduce divalent cations only during the vesicle-substrate adsorption step. If introduced earlier, divalent cations can inhibit SLB formation by promoting vesicle-vesicle interactions, which leads to vesicle aggregation and an increase in vesicle polydispersity.
An example of how ionic strength and pH have a pronounced effect on SLB formation is given by Cremer and Boxer.[42] Using positively and negatively charged vesicles on borosilicate, SLB formation was monitored while varying the pH and ionic strength of a sodium phosphate buffer. For negatively charged vesicles, they observed that SLB formation occurred at high ionic strengths and low pH, but was inhibited at low ionic strength and high pH (Fig. 3A).[42] The low pH likely reduced negative charge density of the substrate and the high ionic strength likely shielded repulsive vesicle-vesicle and vesicle-substrate electrostatic interactions. As the pH of the buffer is raised (or the ionic strength is lowered) electrostatic repulsion increases and eventually overcomes the attractive forces, thus inhibiting SLB formation.
Figure 3.
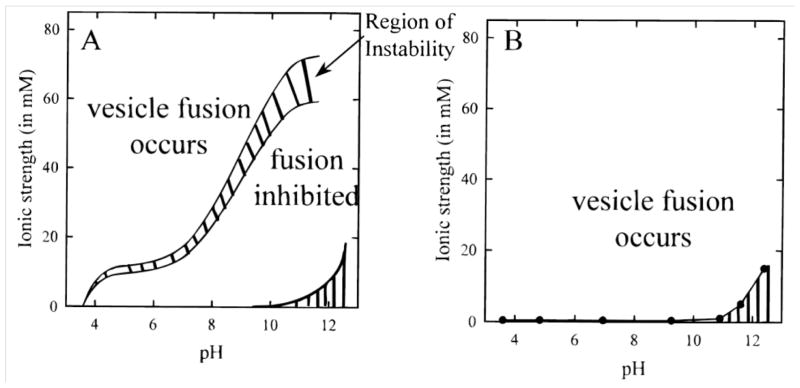
Vesicle fusion phase diagrams for egg PC vesicles containing: (A) 1 mol% of negatively charged lipid probe and (B) 1 mol% of positively charged membrane probe. Regions of vesicle instability and incomplete SLB formation are indicated by the crosshatched area in (A). The regions with vertical stripes in the lower right corner of both diagrams indicate conditions where buffer formation was not possible. Reprinted with permission from reference [42]. Copyright (1999) American Chemical Society.
However, SLBs formed from positively charged vesicles readily form on negatively charged substrates, often regardless of the pH and ionic strength used. Cremer and Boxer showed that positively charged vesicles formed SLBs at all pH values and ionic strengths measured (Fig. 3B).[42] Furthermore, with attractive electrostatic interactions, SLB formation can occur in one step, as the vesicle-substrate interaction alone can be sufficient to cause vesicle rupture.[31]
When using pH and ionic strength conditions conducive to vesicle fusion, it is important to realize that once a SLB has been formed, the buffer can be changed to one that more accurately mimics physiological conditions. This does not adversely affect bilayer adhesion or stability.[42] Thus, inducing vesicle fusion under low pH and high ionic strength conditions with a subsequent buffer change represents a practical technique to promote vesicle fusion.
3.4 Effect of osmotic stress
Increasing vesicle membrane tension is an important factor that can promote rupture of adsorbed vesicles. It is likely that the activation barrier against vesicle rupture is lowered for vesicles under high membrane tension. In addition to using small diameter vesicles between 50–100 nm (which can be controlled through vesicle extrusion), membrane tension can also be increased by creating a gradient in ionic strength across the vesicle membrane. The resulting osmotic pressure difference causes an osmotic stress that changes the volume of the vesicle, and thus creates membrane tension leading to rupture.[35] Reimhult et al. found that SLBs created from egg PC vesicles, with a fixed interior ionic strength of 150 mM, formed at all solution ionic strengths tested (115–300 mM).[9*] However, the critical vesicle coverage (θc) decreased when the vesicles were osmotically stressed, i.e., when the ionic strength of the buffer differed from that of the vesicle interior. This was also evident in the decrease of the Δf and ΔD peaks in QCM-D measurements, indicating that SLB formation occurred more quickly and required less surface adsorbed vesicles to induce vesicle rupture. For successful vesicle rupture via osmostic stress, it is thus common practice to introduce vesicles to substrates in buffers with ionic strengths between 150–500 mM NaCl (to maximize vesicle adsorption as discussed above) and to then induce an osmotic shock after their adsorption, by exchanging the buffer with deionized water. Once the SLB is formed, the deionized water can be replaced with standard buffer to maintain SLB stability.[50, 51]
4. Amphipathic α-helical (AH) peptide-induced vesicle fusion
While adjusting experimental conditions provides a successful strategy for creating complex SLBs, the approach can be tedious since conditions are chosen based on the substrate type and vesicle composition. As substrates and lipid mixtures increase in complexity it can be difficult to anticipate which optimization techniques will promote vesicle fusion. To reliably create SLBs, it is likely that multiple experimental conditions will have to be optimized, including those not discussed above (e.g., vesicle concentration, flow conditions, and chemical surface modifications). Furthermore, experimental optimization techniques can fail or become inconsistent when the vesicle cholesterol content exceeds 30 mol%. To overcome these limitations, we summarize an alternative approach that uses amphipathic, α-helical (AH) peptides as a catalyst to promote vesicle fusion and that generates complex SLBs containing as much as 45 mol% cholesterol. While this approach can be used in combination with the optimization techniques discussed above, a unique and major advantage of AH peptide-induced vesicle fusion is that is sufficient to form SLBs independently of optimizing experimental conditions. With sufficient vesicle-substrate adsorption, it is generally recommended to introduce AH peptides under standard experimental conditions (room temperature, pH = 7, ionic strength ≈ 150 mM) regardless of the substrate and vesicle composition.
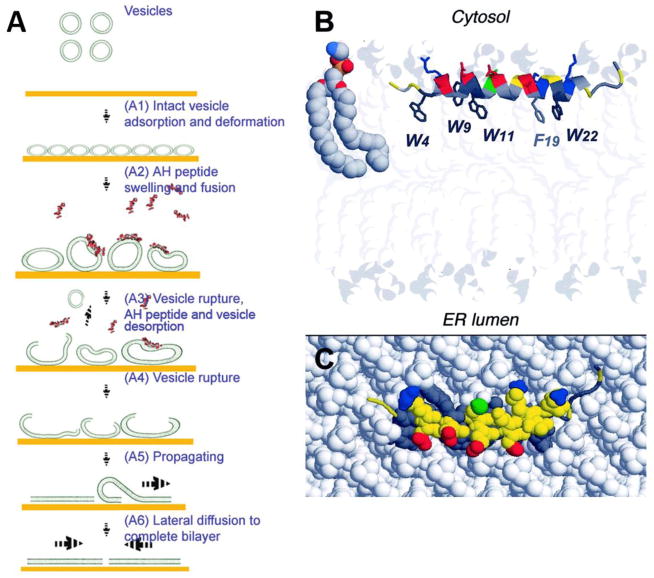
AH peptide is derived from hepatitis C virus’s (HCV) nonstructural protein 5A (NS5A), which interacts with host cell membranes and is required for HCV replication. NS5A’s membrane association is mediated by its N-terminal amphipathic α-helix, termed the AH peptide (Fig. 4B,C).[52] After realizing the AH peptide’s ability to rupture intact adsorbed vesicles, Cho et al. created SLBs from simple POPC vesicles adsorbed on gold substrates (Fig. 4A).[53*, 54] Without the use of AH peptide, gold substrates are limited to vesicle adsorption and do not induce vesicle rupture.[23] Previously, SLB formation from vesicle fusion was mainly limited to hydrophilic substrates (e.g., mica, glass, and silicon oxide). This work thus established a technique that expanded the type of substrates amenable to easy SLB formation by vesicle fusion. Recently, our group has extended the value of AH peptide-induced vesicle fusion by demonstrating a reliable and easy way to form SLBs that contain high cholesterol content (45 mol%) and multiple lipid types.[14**] For the first time, this vesicle fusion technique enables researchers to form SLBs with complex lipid compositions and high cholesterol content, without the need to optimize experimental conditions such as temperature, pH, and ionic strength.
Figure 4.
Membrane activity of the NS5A amphipathic α-helix (AH) peptide. (A) Potential mechanism of AH-peptide induced vesicle fusion on gold. (B) Expected positioning of the average structure of amphipathic α-helix membrane anchor domain of NS5A (PDB entry 1R7E) at the interface between phospholipid polar head groups and hydrophobic tails. The phospholipid bilayer was drawn using the phosphatidylethanolamine (PE) models reported in the Protein Data Bank entry 1BCC. Molecules are colored according to atom types (N, blue; O, red; P, yellow; C, H, gray). (C) Top view of AH peptide embedded in a model phospholipid membrane. Adapted with permission from references [55] and [96]. Copyright (2009) American Chemical Society.
4.1 Mechanism of AH peptide-induced vesicle fusion
The mechanism of AH peptide vesicle fusion is not yet fully understood. It has been hypothesized that AH peptide membrane integration may cause the vesicle membrane to swell and greatly expand, possibly into microvilli folds.[53*, 55, 56] This hypothesis is supported by findings from Cho et al. who showed that the interaction of AH fusion peptides with POPC vesicles (~60 nm in diameter) on gold substrates caused vesicle swelling, which in turn enhanced vesicle-vesicle interactions and led to vesicle fusion and SLB formation.[55] However, this vesicle fusion activity was diminished for vesicles greater than 100 nm in diameter.[57*, 58] Furthermore, at AH peptide concentrations that rupture vesicles, no peptide binding to planar bilayers was observed.[59**] This functional dependence on vesicle diameter/membrane curvature is unique and suggests that the mechanism of AH peptide-induced vesicle fusion is not analogous to that of other membrane disruptive peptides interacting with large diameter membranes (e.g., cells, bacteria, and giant unilamellar vesicles). For these other membrane disruptive peptides, membrane disruption often occurs via pore formation by barrel-stave or toroidal pore mechanisms.[60–62]
To study the unique fusion mechanism of AH-peptide, Jackman and Cho studied the initial binding of the AH peptide to vesicles and found that binding saturation occurred before completion of vesicle swelling.[59**] This lag time suggests that membrane association alone is insufficient to directly cause complete vesicle swelling. It is likely that associated peptides may first rearrange to achieve pore formation, which leads to solvent uptake and subsequent vesicle deformation and SLB formation. It was also observed that the onset of pore formation is almost a factor of 10 slower for larger vesicles (~200 nm in diameter) compared to that of smaller vesicles (~70 nm in diameter), however, the rate of peptide binding had no significant dependence on the vesicle diameters studied.[57*] Typically, pore formation increases with vesicle diameter due to an increase in the number of bound peptides per vesicle (given a constant peptide/lipid (P/L) ratio).[63] However, this is not the case for the AH peptide which suggests that pore formation, rather than the rate of peptide binding, is dependent on high membrane curvature.[57*]
To investigate how AH peptide action depends on nanoscale membrane curvature, Tabaei et al. observed the release of encapsulated vesicle content, induced by AH peptide pore formation and membrane disruption.[57*] They found that as little as four AH peptides per vesicle were required to cause pore formation, which translates into an unusually low effective P/L ratio of ~1/1000.[57*] Furthermore, their results suggest that the inability of AH peptides to rupture large vesicles and planar bilayers is due to insufficient line tension, related to reduced or nonexistent membrane curvature. They also suggest that AH peptide’s pore formation process may be facilitated by curvature-induced defects in lipid packing.
4.2 SLB applications of the NS5A-derived AH peptide
While Cho and others are focused on realizing antiviral clinical benefits of this AH peptide[57*, 58, 64, 65], our group is focused on promoting the use of AH peptides to create SLB platforms under conditions that are generally unfavorable for vesicle fusion. Examples of such conditions include vesicles containing high amounts of cholesterol, the presence of membrane-embedded proteins, strong lipid-substrate interactions, and formation of SLBs on hydrophobic or irregular/patterned substrate surfaces. The extent to which AH peptides can overcome these and other barriers limiting SLB formation are subject to current investigation.
Using AH peptides, we have previously established formation of complex biomimetic SLBs that contain cholesterol concentrations of up to 45 mol% on mica and silica substrates.[14**] Our chosen SLB system is a five-component lipid bilayer that models the native lipid envelope of human immunodeficiency virus-1 (HIV-1).[66] We chose to model this lipid envelope due to the membrane’s significance in viral infection and its potential use as a target in next-generation vaccine designs.[67–70] Furthermore, the native viral envelope is of interest as it contains a unique composition of heterogeneous membrane components that likely represents a mosaic of lipid rafts,[71] protein and antigen clustering,[72] and various gradients of lipid diffusivity.[73] Generating a complex SLB that models the native HIV-1 envelope also provides a proof-of-concept for modeling other complex native biological membranes.
The lipid composition of the model HIV-1 membrane consists of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE), 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-L-serine (POPS), brain sphingomyelin, and cholesterol in a molar ratio of 9.35 : 19.25 : 8.25 : 18.15 : 45.00. SLB formation from vesicles with this composition cannot be achieved by conventional vesicle fusion. In part, this is due to the structural rigidity of the model HIV-1 vesicles. This rigidity arises from the high cholesterol content and presence of sphingomyelin, which are known to order membrane lipids. Furthermore, the negatively charged POPS and negatively charged silica give rise to repulsive lipid-substrate interactions that further resist vesicle fusion. The use of AH peptide-induced vesicle fusion allows us to overcome these obstacles and to more accurately recapitulate the composition of the HIV-1 lipid envelope.[74]
We used QCM-D to study the effect of AH peptide on the fusion of HIV-1 mimetic vesicles. With vesicles that reflect the high cholesterol content and complex lipid composition of the HIV-1 envelope, vesicle fusion did not occur (Fig. 5A). Rather, the model HIV-1vesicles adsorbed to the silica surface, and formed a monolayer of un-ruptured vesicles. This is shown by the leveled-off frequency response (Δf = −150 Hz) of the QCM-D (Fig. 5A). Figure 5B demonstrates the ability of AH peptide to induce SLB formation from a monolayer of model HIV-1 vesicles. Vesicles are first added to the QCM-D chamber to achieve monolayer saturation on the substrate. After excess vesicles are removed by buffer washes, AH peptide (15 μM) is added to cause vesicle rupture which then leads to SLB formation. This process results in a steady increase in the QCM-D frequency response. After frequency equilibration, AH peptide is flushed from the bilayer, leaving behind a complete SLB (Fig. 5B). The apparently complete removal of AH peptide from the SLB after flushing is indicated from neutron reflection studies,[14**] QCM-D measurements,[34**] and simultaneous QCM-D and reflectometry measurements.[55] The ability of AH peptide to be removed from the completed SLB is essential for preventing its influence on SLB properties.
Figure 5.
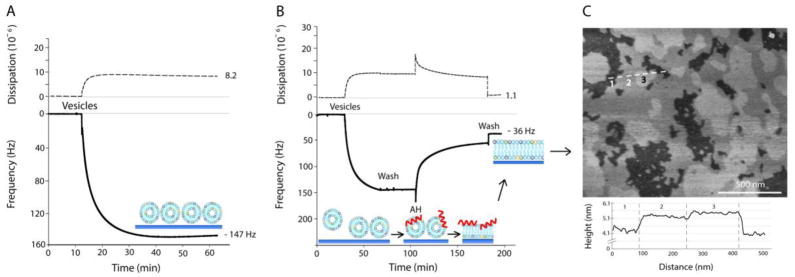
QCM-D plots of energy dissipation (dashed) and third overtone frequency (solid) plotted versus time, and representative AFM image of the resulting SLB. (A) Failure of model HIV-1 vesicles to undergo spontaneous vesicle fusion on silica. (B) Successful model HIV-1 SLB formation by AH peptide-induced vesicle fusion on silica. (C) Atomic force microscopy height image of the model HIV-1 SLB on mica. The height cross-section was taken along the three domains indicated by the position of the dashed line. The three domains labeled in the height cross-section correspond to the numbers labeled on the AFM height image. Ordered membrane domains are taller and appear brighter compared to the more disordered membrane domains which are lower and appear darker. Adapted with permission from reference [14**].
The final Δf for the model HIV-1 SLB was −35.4 Hz with a ΔD of 1.91 × 10−6.[14**] These values are significantly different than those of SLBs formed from pure POPC vesicles which result in a final Δf of −26 Hz and a ΔD of 0.2 × 10−6.[23] The frequency difference between these SLBs corresponds to a 32% mass increase for the model HIV-1 SLB. These results agree closely, however, with a previous publication that formed SLBs from vesicles with a similar cholesterol content.[15] Although this mass increase is still under investigation, it likely arises from the increase in lipid packing density due to the presence of cholesterol and sphingomyelin, and the minor presence of intact vesicles on the bilayer surface. There is also a possibility that upon AH peptide-induced vesicle fusion, the amount of liposomes fused into the bilayer exceeds the lipid content necessary to form a planar SLB. This could give rise to small undulations in the SLB,[75] which would contribute to lower Δf and higher ΔD final values.
As expected for SLBs that contain cholesterol and sphingomyelin, lo domains/rafts formed within the ld phase (Fig. 5C), and could be imaged with atomic force microscopy (AFM).[76] As cholesterol and sphingolipids order a lipid’s acyl chains, the lipid is presented in a more upright position. This organization facilitates a high lipid packing density and results in height differences between lo domains and the surrounding ld phase. With SLBs containing cholesterol, sphingomyelin, and one other lipid type, a single height difference is typically observed for the lo domain areas. However, for more complex SLBs, that contain several lipid types in the presence of cholesterol and sphingomyelin, there are domains which form within domains. For example, for the model HIV-1 SLB, three visually-distinct SLB height differences are observed in high-resolution AFM images (Fig. 5C). The height difference between the lowest and middle domain (i.e., domains 1 and 2) was ~10 Å, and between the middle and tallest domain (i.e., domains 2 and 3) was ~2–3 Å. The 10 Å height measurement between domains 1 and 2 is consistent with height differences between three component SLBs with well-defined lo/ld phases. For example, SLBs prepared from DOPC/sphingomyelin/cholesterol, the height difference between the lo and ld phase is ~8–12 Å in 10–35 mol% cholesterol.[77]
5. Bilayer edge-induced vesicle fusion
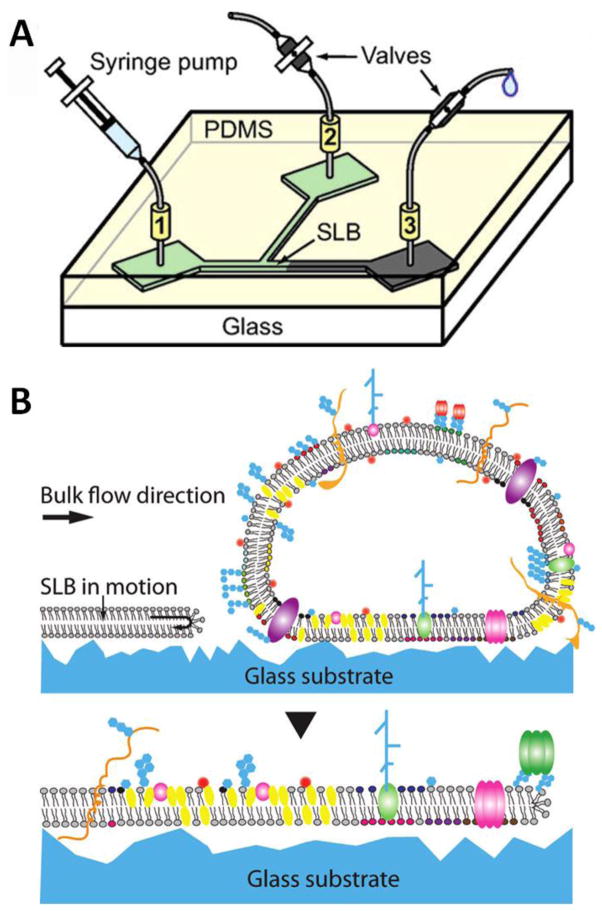
An additional technique to create complex, biomimetic SLBs is the use of a moving SLB edge that collides with substrate-adhered vesicles.[78, 79**] In this approach, the hydrodynamic forces from a liquid flowing in a microfluidic channel (Fig. 6A), drive SLB formation in the direction of fluid flow.[80] As the SLB edge encounters adsorbed vesicles, the energetically unstable SLB edge fuses vesicles into the advancing SLB front (Fig. 6B). As with AH-peptide induced vesicle fusion, this technique does not require optimization of experimental variables. However, it is perhaps a more robust technique as it has been shown to create SLBs with 50 mol% cholesterol and from native cell-derived vesicles.[78] Furthermore, bilayer edge-induced vesicle fusion is a physical-based phenomenon and does not rely on introduction of peptides, thus eliminating any potential for unintentional, collateral peptide interactions.
Figure 6.
(A) Example of the microfluidic device used to drive a SLB moving edge. (B) Concept of SLB driven forward from hydrodynamic force (top). Upon contract, the surface adhered vesicle ruptures and integrates into the leading edge of the moving SLB (bottom). Adapted with permission from reference [78] and [80]. Copyright (2008, 2011) American Chemical Society.
In the original microfluidic device used to form SLBs (Fig. 6A), the final SLB composition results in a mixture of the composition of the initial SLB edge and that of the adsorbed vesicles (due to lipid diffusion). This limited the precise control of the SLB composition. Simonsson and Hook overcame this limitation by using a four-armed microfluidic cross-channel to create a small lipid patch which was used to initiate the SLB leading edge.[79**] Specifically, sodium dodecyl sulfate (SDS) was used to selectively remove lipids in the microfluidic channels to create a small SLB patch adjacent to the adsorbed target vesicles. Using the hydrodynamic force from the flowing buffer, this small lipid patch was then driven against the adsorbed vesicles to initiate vesicle rupture and SLB formation. This process creates a final SLB whose composition essentially reflects that of the adsorbed vesicles. In addition to testing SLB diffusivities with and without cholesterol, Simonsson and Hook used this technique to create phase-separated cholesterol-rich lo domains. Formation of an SLB containing DOPC/DOPE/cholesterol (39:21:40 mol%) and rhodamine-DHPE resulted in bright spots likely indicating the existence of lo domains within the ld phase.
Bilayer edge-induced vesicle fusion can also be used to create SLBs from vesicles derived from native cell membranes.[78] Cell membrane-derived vesicles were made by extruding 3T3 fibroblast cells and removing water soluble proteins by ultracentrifugation.[78] The microfluidic cross channel was used to drive a POPC bilayer edge against the cell membrane-derived vesicles to create the cell-derived SLB. Furthermore, this technique also allows natively bound membrane molecules to be isolated based on differences in SLB drift velocity. This technique provides a more biomimetic environment to study membrane molecules rather than using detergents to extract and isolate molecules from the cell membrane.
6. Creating SLBs on non-siliceous surfaces
In addition to the lipid composition of vesicles, the substrate also plays a crucial role in creating SLBs via vesicle fusion. Vesicle fusion is typically dominated by surface adhesion energy between vesicles and the substrate. Traditional substrates used for vesicle fusion include hydrophilic silica, glass, mica, and quartz. These siliceous substrates are commonly used because they provide the necessary balance between adhesion, repulsion, and hydration forces that results in vesicle rupture and a hydration layer between the substrate and SLB. This hydration layer allows SLBs to mimic the lateral fluidity of native cell membranes. However, as applications of SLBs continue to evolve, there is a need to create SLBs on a wider range of surfaces. Progress has been made to form SLBs on many solid non-siliceous surfaces including chrome,[81] indium tin oxide,[82] gold,[53*] titanium oxide,[83] and alumina.[84] In many cases these surfaces have vesicle-substrate interactions that do not enable conventional vesicle fusion, and thus surface-specific techniques must be used to promote SLB formation.
Although optimization techniques, including surface functionalization[85, 86] and the use of charged lipids,[87] have been successfully applied to create SLBs on solid non-siliceous surfaces, these techniques are often specific to a particular substrate and not universally applicable. AH peptide-induced vesicle fusion may overcome this limitation as it can offer SLB formation on a broad range of substrates. For example, AH peptide-induced vesicle fusion was successfully used on gold and titanium oxide surfaces.[53*] Furthermore, our group is currently using AH peptides to create SLBs on chromium and experimenting with its use on a polymeric, Nafion surface.
While AH peptide-induced vesicle fusion may be harnessed to form SLBs under a range of conditions on a variety of substrates, it requires vesicle-substrate interactions that are strong enough to (i) form a monolayer of vesicles on the surface, and (ii) resist vesicle desorption upon a buffer wash. A vesicle monolayer is required to provide sufficient vesicle-vesicle interactions, while a buffer wash is required to remove excess vesicles from the surface and the bulk solution. Excess vesicles need to be removed from the system before AH peptide is introduced, so that it can interact exclusively with the surface adsorbed vesicles. For surfaces with especially weak vesicle-substrate interactions these requirements may not be met, and AH peptides may fail to form a complete SLB. In this case, the so-called bubble-collapse deposition (BCD) technique may overcome this limitation.[7*]
BCD provides an innovative approach that requires a lower vesicle-substrate adhesion energy then traditional vesicle fusion. For example, Mager et al. use this technique to form a POPC SLB on alumina; a substrate which usually does not provide sufficient vesicle adhesion to induce fusion.[84] BCD uses an air bubble that is blown underwater at the end of a needle. This bubble is “inked” with a lipid monolayer by contacting it with a previously formed sacri cial bilayer. The bubble is then brought into contact with the substrate at the desired deposition site. The needle then withdraws air from the bubble causing the bubble to shrink until the surface-supported monolayer folds back on itself, forming a bilayer patch on the substrate surface.
To date, BCD can form a SLB with simple lipid composition on substrates that otherwise are unable to induce vesicle rupture due to weak vesicle-substrate interactions. It is thus conceivable that if a complex SLB, created by one of the techniques discusses above, is used as the sacrificial inking bilayer, then BCD may be able to form SLBs with complex lipid composition on substrates that have weak vesicle interactions.
Outside of using solid supports, there is a large body of research focused on creating SLBs on soft, polymer cushions. There is a major advantage of using polymer cushions over solid supports when studying SLBs with transmembrane proteins.[88] A typical solid supported SLB will have a hydration layer of 1–3 nm separating the SLB from the substrate. This is often insufficient space for SLBs containing transmembrane proteins. The cytosolic domain of the protein will often contact the substrate resulting in adhesion, deformation, and eventually denaturation. The use of polymer cushioned SLBs can overcome this problem by providing a low friction and more inert environment for transmembrane proteins. McCabe and Forstner provide a recent review on their fabrication and advantages.[89]
7. Vesicle fusion considerations
Many questions exist concerning how preparation methods affect properties of SLBs, and to what extent these properties accurately mimic native cell membranes. For the techniques discussed above, the conditions that are manipulated to induce vesicle fusion (e.g., temperature, buffer type, fusion peptides, etc.) can be replaced with the desired experimental conditions once the SLB has formed. However, after the conditions have been changed, it is important to take into consideration the possibility of residual effects on SLB properties that originate from the SLB preparation conditions. For example, zwitterionic lateral lipid-lipid interactions are believe to be promoted by ions bound into the membrane. Strong interaction between Na+ and Ca2+ ions and the carbonyl oxygens of the lipids form tight ion-lipid complexes.[46] This increases membrane organization and can affect bilayer cohesion and lipid diffusivities. Thus, using high ionic strength for SLB formation, and then changing to a physiological ionic strength may have a lasting effect on lateral lipid-lipid interactions. Furthermore, temperature changes are known to affect the ζ potential and orientation of lipid head groups[45] and it is unclear if the resulting effects, both direct and indirect, are reversible.
Compositional asymmetry between the leaflets of the SLB must also be taken into consideration. Bilayer asymmetry is a common property in native cell membranes and is believed to contribute to many biological functions by mediating membrane protein distribution and functionality.[88] Thus, there is a large focus on controlling and characterizing asymmetry in deposited bilayers.[90, 91] Typically, Langmuir-Blodgett/Schäfer deposition offers the most control of SLB asymmetry since each leaflet can be deposited independently, allowing lipid selection of each initial leaflet composition. Asymmetry is harder to control with vesicle fusion techniques since it must originate from the vesicle or occur from lipid re-arrangement once the SLB has formed. However, it is possible to achieve and control bilayer asymmetry using vesicle fusion. Studies have suggested that the solid support can induce preferential distribution of certain lipid types. In the presence of Ca2+ ions, it has been observed that negatively charged DOPS preferentially resides in the leaflet closest to mica and titanium oxide surfaces.[47, 92] This suggests that lipid head group chemistry leads to membrane asymmetry.[93] SLBs have also shown leaflet organizational asymmetry between gel- and fluid-phases[94] and with cholesterol induced domain formations.[95] Furthermore, it has been shown that SLB leaflet asymmetry can be controlled by vesicle deposition temperature and the salt concentration of the vesicle solution.[91]
This brief discussion is meant to demonstrate that details in preparation methods can affect SLB properties, and emphasizes that such details need to be considered and perhaps accounted for in experimental design and data interpretation.
8. Conclusions
Common criticism against SLB formation via vesicle fusion states that it is limited to few select surfaces and vesicle compositions. In this review, we have attempted to prove this criticism to be outdated by summarizing vesicle fusion techniques that are able to readily form SLBs with multiple lipid types, high cholesterol content, and on non-siliceous substrates. These techniques are simple and reliable, and thus maintain the major advantage of forming SLBs via vesicle fusion.
In the past, optimization of experimental conditions has been widely used to induce vesicle fusion and has provided a successful strategy for creating simple SLBs. With increasing SLB complexity and emerging applications for SLBs, such optimization techniques will play an even more important role for their formation. Here, we have summarized the strategies that are used to tune the most important conditions to make vesicle fusion applicable to a larger range of SLB compositions and substrates. Important strategies include elevating the temperature, increasing buffer ionic strength, adding divalent cations, and lowering buffer pH. By optimizing these conditions it is possible to create SLBs from vesicles that ordinarily resist vesicle fusion, and to access substrates that are generally not amenable to SLB formation.
In cases where such optimization strategies fail to provide easy SLB formation, the optimization efforts can be supplemented with novel vesicle fusion techniques such as AH peptide- and bilayer edge-induced vesicle fusion. Finally, bubble collapse deposition can be combined with these vesicle fusion techniques to create complex SLBs on surfaces with especially weak vesicle-substrate interactions. Together, these techniques allow researchers to easily create SLBs that contain high cholesterol content and multiple lipid types on a wide range of substrates. Thus, SLB formation via vesicle fusion is no longer restricted to a few select surfaces and a limited range of lipid compositions.
Highlights.
Formation of complex, biomimetic SLBs using simple vesicle fusion techniques
Optimization of experimental conditions form complex SLBs via vesicle fusion
α-helical peptides act as catalyst to induce vesicle rupture and SLB formation
Bilayer-edge induced vesicle fusion uses micro-fluidics to form native-derived SLB
Acknowledgments
We acknowledge funding from the NIH, grant AI102814-01 (S.Z.). G.J.H gratefully acknowledges the financial and training support from The Center for Biomolecular and Tissue Engineering, the Structural Biology and Biophysics program (Duke University), and the Karlsruhe House of Young Scientists (Karlsruhe Institute of Technology). We thank Dr. Thomas J. McIntosh and Dr. S. Munir Alam for discussions regarding SLB formation and applications.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tamm LK, Mcconnell HM. Supported Phospholipid-Bilayers. Biophysical journal. 1985;47:105–13. doi: 10.1016/S0006-3495(85)83882-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mennicke U, Salditt T. Preparation of solid-supported lipid bilayers by spin-coating. Langmuir : the ACS journal of surfaces and colloids. 2002;18:8172–77. [Google Scholar]
- 3.Jung SY, Holden MA, Cremer PS, Collier CP. Two-component membrane lithography via lipid backfilling. Chemphyschem. 2005;6:423–26. doi: 10.1002/cphc.200400540. [DOI] [PubMed] [Google Scholar]
- 4.Hohner AO, David MPC, Radler JO. Controlled solvent-exchange deposition of phospholipid membranes onto solid surfaces. Biointerphases. 2010;5:1–8. doi: 10.1116/1.3319326. [DOI] [PubMed] [Google Scholar]
- 5.Tiberg F, Harwigsson I, Malmsten M. Formation of model lipid bilayers at the silica-water interface by co-adsorption with non-ionic dodecyl maltoside surfactant. Eur Biophys J Biophy. 2000;29:196–203. doi: 10.1007/pl00006646. [DOI] [PubMed] [Google Scholar]
- 6.Brinker CJ, Lu YF, Sellinger A, Fan HY. Evaporation-induced self-assembly: Nanostructures made easy. Adv Mater. 1999;11:579. [Google Scholar]
- 7.Mager MD, Melosh NA. Lipid bilayer deposition and patterning via air bubble collapse. Langmuir: the ACS journal of surfaces and colloids. 2007;23:9369–77. doi: 10.1021/la701372b. Bubble collapse deposition is a novel technique that allows the creation of SLBs on substrates that have weak vesicle interactions. [DOI] [PubMed] [Google Scholar]
- 8.Lenhert S, Mirkin CA, Fuchs H. In Situ Lipid Dip-Pen Nanolithography Under Water. Scanning. 2010;32:15–23. doi: 10.1002/sca.20166. [DOI] [PubMed] [Google Scholar]
- 9.Reimhult E, Hook F, Kasemo B. Intact vesicle adsorption and supported biomembrane formation from vesicles in solution: Influence of surface chemistry, vesicle size, temperature, and osmotic pressure. Langmuir: the ACS journal of surfaces and colloids. 2003;19:1681–91. Adsorption kinetics of egg-yolk phosphatidylcholine vesicles was investigated by QCM-D, as a function of surface chemistry (SiO2, Si3N4, Au, TiO2, and Pt) and important experimental conditions. Results provide insight to determine experimental conditions when attempting to form more complex SLBs on a range of substrate types. [Google Scholar]
- 10.Eeman M, Deleu M. From biological membranes to biomimetic model membranes. Biotechnol Agron Soc. 2010;14:719–36. [Google Scholar]
- 11.Czolkos I, Jesorka A, Orwar O. Molecular phospholipid films on solid supports. Soft Matter. 2011;7:4562–76. A comprehensive review is presented on the current state of molecular thin lipid layer fabrication with an emphasis on support materials, film formation mechanisms, characterisation methods, and applications. [Google Scholar]
- 12.Anderson TH, Min YJ, Weirich KL, Zeng HB, Fygenson D, Israelachvili JN. Formation of Supported Bilayers on Silica Substrates. Langmuir: the ACS journal of surfaces and colloids. 2009;25:6997–7005. doi: 10.1021/la900181c. Provides mechanistic information on SLB formation via vesicle fusion in various aqueous solutions and temperatures using a number of complementary surface-diagnostic techniques. [DOI] [PubMed] [Google Scholar]
- 13.Dopico AM, Tigyi GJ. A glance at the structural and functional diversity of membrane lipids. Methods in molecular biology. 2007;400:1–13. doi: 10.1007/978-1-59745-519-0_1. [DOI] [PubMed] [Google Scholar]
- 14.Hardy GJ, Nayak R, Alam SM, Shapter JG, Heinrich F, Zauscher S. Biomimetic supported lipid bilayers with high cholesterol content formed by alpha-helical peptide-induced vesicle fusion. Journal of Materials Chemistry. 2012;22:19506–13. doi: 10.1039/C2JM32016A. AH peptide-induced vesicle fusion was used to form a SLB that models the native composition of the human immunodeficiency virus-1 lipid envelope. In the absence of AH peptides, these biomimetic vesicles fail to form a complete SLB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sundh M, Svedhem S, Sutherland DS. Influence of phase separating lipids on supported lipid bilayer formation at SiO2 surfaces. Physical chemistry chemical physics : PCCP. 2010;12:453–60. doi: 10.1039/b912598a. [DOI] [PubMed] [Google Scholar]
- 16.Owen DM, Magenau A, Williamson D, Gaus K. The lipid raft hypothesis revisited--new insights on raft composition and function from super-resolution fluorescence microscopy. BioEssays : news and reviews in molecular, cellular and developmental biology. 2012;34:739–47. doi: 10.1002/bies.201200044. This review describes new microscopy techniques that circumvent the resolution limit of light and, for the first time, allow the fluorescence imaging of membrane structures on length scales below 200 nm. Such techniques, provide newly emerging facets of lipid raft biology. [DOI] [PubMed] [Google Scholar]
- 17.Block RC, Dorsey ER, Beck CA, Brenna JT, Shoulson I. Altered cholesterol and fatty acid metabolism in Huntington disease. Journal of clinical lipidology. 2010;4:17–23. doi: 10.1016/j.jacl.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Paolo G, Kim TW. Linking lipids to Alzheimer’s disease: cholesterol and beyond. Nature reviews Neuroscience. 2011;12:284–96. doi: 10.1038/nrn3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao Z, Graham DR, Hildreth JE. Lipid rafts and HIV pathogenesis: virion-associated cholesterol is required for fusion and infection of susceptible cells. AIDS research and human retroviruses. 2003;19:675–87. doi: 10.1089/088922203322280900. [DOI] [PubMed] [Google Scholar]
- 20.Honda A, Matsuzaki Y. Cholesterol and chronic hepatitis C virus infection. Hepatology research : the official journal of the Japan Society of Hepatology. 2011;41:697–710. doi: 10.1111/j.1872-034X.2011.00838.x. [DOI] [PubMed] [Google Scholar]
- 21.Maguy A, Hebert TE, Nattel S. Involvement of lipid rafts and caveolae in cardiac ion channel function. Cardiovascular research. 2006;69:798–807. doi: 10.1016/j.cardiores.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Sotgia F, Williams TM, Schubert W, Medina F, Minetti C, Pestell RG, et al. Caveolin-1 deficiency (−/−) conveys premalignant alterations in mammary epithelia, with abnormal lumen formation, growth factor independence, and cell invasiveness. The American journal of pathology. 2006;168:292–309. doi: 10.2353/ajpath.2006.050429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keller CA, Kasemo B. Surface specific kinetics of lipid vesicle adsorption measured with a quartz crystal microbalance. Biophysical journal. 1998;75:1397–402. doi: 10.1016/S0006-3495(98)74057-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seifert U, Berndl K, Lipowsky R. Shape Transformations of Vesicles - Phase-Diagram for Spontaneous-Curvature and Bilayer-Coupling Models. Phys Rev A. 1991;44:1182–202. doi: 10.1103/physreva.44.1182. [DOI] [PubMed] [Google Scholar]
- 25.Richter RP, Berat R, Brisson AR. Formation of solid-supported lipid bilayers: an integrated view. Langmuir : the ACS journal of surfaces and colloids. 2006;22:3497–505. doi: 10.1021/la052687c. [DOI] [PubMed] [Google Scholar]
- 26.Speight RE, Cooper MA. A Survey of the 2010 Quartz Crystal Microbalance Literature. J Mol Recognit. 2012;25:451–73. doi: 10.1002/jmr.2209. [DOI] [PubMed] [Google Scholar]
- 27.Sauerbrey G. Verwendung Von Schwingquarzen Zur Wagung Dunner Schichten Und Zur Mikrowagung. Z Phys. 1959;155:206–22. [Google Scholar]
- 28.Reviakine I, Brisson A. Formation of supported phospholipid bilayers from unilamellar vesicles investigated by atomic force microscopy. Langmuir. 2000;16:1806–15. [Google Scholar]
- 29.Zhdanov VP, Kasemo B. Van der Waals interaction during protein adsorption on a solid covered by a thin film. Langmuir. 2001;17:5407–09. [Google Scholar]
- 30.Reimhult E, Hook F, Kasemo B. Vesicle adsorption on SiO2 and TiO2: Dependence on vesicle size. J Chem Phys. 2002;117:7401–04. [Google Scholar]
- 31.Richter R, Mukhopadhyay A, Brisson A. Pathways of lipid vesicle deposition on solid surfaces: A combined QCM-D and AFM study. Biophysical journal. 2003;85:3035–47. doi: 10.1016/S0006-3495(03)74722-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reimhult E, Hook F, Kasemo B. Temperature dependence of formation of a supported phospholipid bilayer from vesicles on SiO2. Physical review E, Statistical, nonlinear, and soft matter physics. 2002;66:051905. doi: 10.1103/PhysRevE.66.051905. [DOI] [PubMed] [Google Scholar]
- 33.Cho NJ, Jackman JA, Liu M, Frank CW. pH-Driven Assembly of Various Supported Lipid Platforms: A Comparative Study on Silicon Oxide and Titanium Oxide. Langmuir : the ACS journal of surfaces and colloids. 2011;27:3739–48. doi: 10.1021/la104348f. Provides evidence that stronger vesicle-substrate interactions occur at low pH and that oxide surfaces have titratable OH groups that control the surface charge density, which plays an important role in the adsorption and fusion of zwitterionic vesicles. [DOI] [PubMed] [Google Scholar]
- 34.Cho NJ, Frank CW, Kasemo B, Hook F. Quartz crystal microbalance with dissipation monitoring of supported lipid bilayers on various substrates. Nat Protoc. 2010;5:1096–106. doi: 10.1038/nprot.2010.65. Essential vesicle fusion protocols are presented to form zwitterionic SLBs supported on silicon oxide and titanium oxide, and alpha-helical (AH) peptide-induced vesicle fusion is summarized to form SLBs on gold and titanium oxide substrates. Technical issues that arise when working with charged vesicle compositions are also discussed. [DOI] [PubMed] [Google Scholar]
- 35.Seantier B, Kasemo B. Influence of Mono- And Divalent Ions on the Formation of Supported Phospholipid Bilayers via Vesicle Adsorption. Langmuir : the ACS journal of surfaces and colloids. 2009;25:5767–72. doi: 10.1021/la804172f. [DOI] [PubMed] [Google Scholar]
- 36.Simons K, Sampaio JL. Membrane Organization and Lipid Rafts. Csh Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Almeida RF, Fedorov A, Prieto M. Sphingomyelin/phosphatidylcholine/cholesterol phase diagram: boundaries and composition of lipid rafts. Biophysical journal. 2003;85:2406–16. doi: 10.1016/s0006-3495(03)74664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Redondo-Morata L, Giannotti MI, Sanz F. Influence of cholesterol on the phase transition of lipid bilayers: a temperature-controlled force spectroscopy study. Langmuir: the ACS journal of surfaces and colloids. 2012;28:12851–60. doi: 10.1021/la302620t. SLBs containing elevated cholesterol content were formed using temperatures 15 °C above the highest lipid transition temperature. [DOI] [PubMed] [Google Scholar]
- 39.Lawrence JC, Saslowsky DE, Edwardson JM, Henderson RM. Real-time analysis of the effects of cholesterol on lipid raft behavior using atomic force microscopy. Biophysical journal. 2003;84:1827–32. doi: 10.1016/s0006-3495(03)74990-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cevc G. Membrane Electrostatics. Biochimica et biophysica acta. 1990;1031:311–82. doi: 10.1016/0304-4157(90)90015-5. [DOI] [PubMed] [Google Scholar]
- 41.Marsh D. CRC handbook of lipid bilayers. Boca Raton: CRC press; 1990. [Google Scholar]
- 42.Cremer PS, Boxer SG. Formation and spreading of lipid bilayers on planar glass supports. J Phys Chem B. 1999;103:2554–59. [Google Scholar]
- 43.Kosmulski M. The pH-dependent surface charging and points of zero charge: V. Update Journal of colloid and interface science. 2011;353:1–15. doi: 10.1016/j.jcis.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 44.Egawa H, Furusawa K. Liposome adhesion on mica surface studied by atomic force microscopy. Langmuir : the ACS journal of surfaces and colloids. 1999;15:1660–66. [Google Scholar]
- 45.Makino K, Yamada T, Kimura M, Oka T, Ohshima H, Kondo T. Temperature-Induced and Ionic Strength-Induced Conformational-Changes in the Lipid Head Group Region of Liposomes as Suggested by Zeta-Potential Data. Biophys Chem. 1991;41:175–83. doi: 10.1016/0301-4622(91)80017-l. [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Manyes S, Oncins G, Sanz F. Effect of ion-binding and chemical phospholipid structure on the nanomechanics of lipid bilayers studied by force spectroscopy. Biophysical journal. 2005;89:1812–26. doi: 10.1529/biophysj.105.064030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rossetti FF, Textor M, Reviakine I. Asymmetric distribution of phosphatidyl serine in supported phospholipid bilayers on titanium dioxide. Langmuir: the ACS journal of surfaces and colloids. 2006;22:3467–73. doi: 10.1021/la053000r. [DOI] [PubMed] [Google Scholar]
- 48.Richter RP, Brisson AR. Following the formation of supported lipid bilayers on mica: A study combining AFM, QCM-D, and ellipsometry. Biophysical journal. 2005;88:3422–33. doi: 10.1529/biophysj.104.053728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramirez DM, Ogilvie WW, Johnston LJ. NBD-cholesterol probes to track cholesterol distribution in model membranes. Biochimica et biophysica acta. 2010;1798:558–68. doi: 10.1016/j.bbamem.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 50.Schonherr H, Johnson JM, Lenz P, Frank CW, Boxer SG. Vesicle adsorption and lipid bilayer formation on glass studied by atomic force microscopy. Langmuir: the ACS journal of surfaces and colloids. 2004;20:11600–6. doi: 10.1021/la049302v. [DOI] [PubMed] [Google Scholar]
- 51.Reich C, Horton MR, Krause B, Gast AP, Radler JO, Nickel B. Asymmetric structural features in single supported lipid bilayers containing cholesterol and GM1 resolved with synchrotron X-Ray reflectivity. Biophysical journal. 2008;95:657–68. doi: 10.1529/biophysj.107.113068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brass V, Bieck E, Montserret R, Wolk B, Hellings JA, Blum HE, et al. An amino-terminal amphipathic alpha-helix mediates membrane association of the hepatitis C virus nonstructural protein 5A. The Journal of biological chemistry. 2002;277:8130–9. doi: 10.1074/jbc.M111289200. [DOI] [PubMed] [Google Scholar]
- 53.Cho NJ, Cho SJ, Cheong KH, Glenn JS, Frank CW. Employing an amphipathic viral peptide to create a lipid bilayer on Au and TiO2. Journal of the American Chemical Society. 2007;129:10050–1. doi: 10.1021/ja0701412. AH peptide-induced vesicle fusion was used to create SLBs on gold and titanium oxide substrates. Results indicate vesicle expansion and potential creation of microvilli from AH peptide interactions. [DOI] [PubMed] [Google Scholar]
- 54.Cho NJ, Cheong KH, Lee C, Frank CW, Glenn JS. Binding dynamics of hepatitis C virus’ NS5A amphipathic peptide to cell and model membranes. Journal of virology. 2007;81:6682–9. doi: 10.1128/JVI.02783-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cho NJ, Wang G, Edvardsson M, Glenn JS, Hook F, Frank CW. Alpha-helical peptide-induced vesicle rupture revealing new insight into the vesicle fusion process as monitored in situ by quartz crystal microbalance-dissipation and reflectometry. Analytical chemistry. 2009;81:4752–61. doi: 10.1021/ac900242s. [DOI] [PubMed] [Google Scholar]
- 56.Gidalevitz D, Ishitsuka Y, Muresan AS, Konovalov O, Waring AJ, Lehrer RI, et al. Interaction of antimicrobial peptide protegrin with biomembranes. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6302–7. doi: 10.1073/pnas.0934731100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tabaei SR, Rabe M, Zhdanov VP, Cho NJ, Hook F. Single vesicle analysis reveals nanoscale membrane curvature selective pore formation in lipid membranes by an antiviral alpha-helical peptide. Nano letters. 2012;12:5719–25. doi: 10.1021/nl3029637. Effective AH-peptide membrane pore formation and vesicle destabilization was found to occur at low concentrations (10 nM to 1 μM) and a low peptide-to-lipid ratio (~1/1000). The AH peptide showed selective rupturing of vesicles with nanometer diameters. These results demonstrate AH peptide’s potential as an anti-viral agent. [DOI] [PubMed] [Google Scholar]
- 58.Cho NJ, Dvory-Sobol H, Xiong A, Cho SJ, Frank CW, Glenn JS. Mechanism of an amphipathic alpha-helical peptide’s antiviral activity involves size-dependent virus particle lysis. ACS chemical biology. 2009;4:1061–7. doi: 10.1021/cb900149b. [DOI] [PubMed] [Google Scholar]
- 59.Jackman JA, Cho NJ. Model membrane platforms for biomedicine: case study on antiviral drug development. Biointerphases. 2012;7:18. doi: 10.1007/s13758-011-0018-2. Current review that summarizes the progress and advantages for using model membrane platforms for developing effective clinical therapies. Uses AH peptides applied towards hepatitis C virus therapy as a promising example. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–95. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 61.He K, Ludtke SJ, Huang HW, Worcester DL. Antimicrobial peptide pores in membranes detected by neutron in-plane scattering. Biochemistry. 1995;34:15614–8. doi: 10.1021/bi00048a002. [DOI] [PubMed] [Google Scholar]
- 62.Henzler Wildman KA, Lee DK, Ramamoorthy A. Mechanism of lipid bilayer disruption by the human antimicrobial peptide, LL-37. Biochemistry. 2003;42:6545–58. doi: 10.1021/bi0273563. [DOI] [PubMed] [Google Scholar]
- 63.Rapaport D, Peled R, Nir S, Shai Y. Reversible surface aggregation in pore formation by pardaxin. Biophysical journal. 1996;70:2502–12. doi: 10.1016/S0006-3495(96)79822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bobardt MD, Cheng G, de Witte L, Selvarajah S, Chatterji U, Sanders-Beer BE, et al. Hepatitis C virus NS5A anchor peptide disrupts human immunodeficiency virus. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5525–30. doi: 10.1073/pnas.0801388105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheng G, Montero A, Gastaminza P, Whitten-Bauer C, Wieland SF, Isogawa M, et al. A virocidal amphipathic {alpha}-helical peptide that inhibits hepatitis C virus infection in vitro. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3088–93. doi: 10.1073/pnas.0712380105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brugger B, Glass B, Haberkant P, Leibrecht I, Wieland FT, Krausslich HG. The HIV lipidome: a raft with an unusual composition. Proc Natl Acad Sci U S A. 2006;103:2641–6. doi: 10.1073/pnas.0511136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alam SM, McAdams M, Boren D, Rak M, Scearce RM, Gao F, et al. The role of antibody polyspecificity and lipid reactivity in binding of broadly neutralizing anti-HIV-1 envelope human monoclonal antibodies 2F5 and 4E10 to glycoprotein 41 membrane proximal envelope epitopes. J Immunol. 2007;178:4424–35. doi: 10.4049/jimmunol.178.7.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alam SM, Morelli M, Dennison SM, Liao HX, Zhang R, Xia SM, et al. Role of HIV membrane in neutralization by two broadly neutralizing antibodies. Proc Natl Acad Sci U S A. 2009;106:20234–9. doi: 10.1073/pnas.0908713106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alving CR, Beck Z, Karasavva N, Matyas GR, Rao M. HIV-1, lipid rafts, and antibodies to liposomes: implications for anti-viral-neutralizing antibodies (Review) Molecular Membrane Biology. 2006;23:453–U1. doi: 10.1080/09687860600935348. [DOI] [PubMed] [Google Scholar]
- 70.Hardy GJ, Lam Y, Stewart SM, Anasti K, Alam SM, Zauscher S. Screening the interactions between HIV-1 neutralizing antibodies and model lipid surfaces. J Immunol Methods. 2012;376:13–9. doi: 10.1016/j.jim.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aloia RC, Tian HR, Jensen FC. Lipid-Composition and Fluidity of the Human-Immunodeficiency-Virus Envelope and Host-Cell Plasma-Membranes. P Natl Acad Sci USA. 1993;90:5181–85. doi: 10.1073/pnas.90.11.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu P, Liu J, Bess J, Chertova E, Lifson JD, Grise H, et al. Distribution and three-dimensional structure of AIDS virus envelope spikes. Nature. 2006;441:847–52. doi: 10.1038/nature04817. [DOI] [PubMed] [Google Scholar]
- 73.Engelman DM. Membranes are more mosaic than fluid. Nature. 2005;438:578–80. doi: 10.1038/nature04394. [DOI] [PubMed] [Google Scholar]
- 74.Franquelim HG, Chiantia S, Veiga AS, Santos NC, Schwille P, Castanho MARB. Anti-HIV-1 antibodies 2F5 and 4E10 interact differently with lipids to bind their epitopes. Aids. 2011;25:419–28. doi: 10.1097/QAD.0b013e328342ff11. [DOI] [PubMed] [Google Scholar]
- 75.Merz C, Knoll W, Textor M, Reimhult E. Formation of supported bacterial lipid membrane mimics. Biointerphases. 2008;3:Fa41–Fa50. doi: 10.1116/1.2896119. [DOI] [PubMed] [Google Scholar]
- 76.Janshoff A, Steinem C. Scanning force microscopy of artificial membranes. Chembiochem. 2001;2:799–808. doi: 10.1002/1439-7633(20011105)2:11<798::AID-CBIC798>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 77.Sullan RM, Li JK, Hao C, Walker GC, Zou S. Cholesterol-dependent nanomechanical stability of phase-segregated multicomponent lipid bilayers. Biophysical journal. 2010;99:507–16. doi: 10.1016/j.bpj.2010.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Simonsson L, Gunnarsson A, Wallin P, Jonsson P, Hook F. Continuous lipid bilayers derived from cell membranes for spatial molecular manipulation. Journal of the American Chemical Society. 2011;133:14027–32. doi: 10.1021/ja204589a. [DOI] [PubMed] [Google Scholar]
- 79.Simonsson L, Hook F. Formation and Diffusivity Characterization of Supported Lipid Bilayers with Complex Lipid Compositions. Langmuir: the ACS journal of surfaces and colloids. 2012;28:10528–33. doi: 10.1021/la301878r. A hydronomically moved SLB patch composed of POPC is used to initiate vesicle fusion and SLB formation from vesicles containing complex compositions. This technique can be used to create SLBs that more accurately mimic native membranes. [DOI] [PubMed] [Google Scholar]
- 80.Jonsson P, Beech JP, Tegenfeldt JO, Hook F. Shear-driven motion of supported lipid bilayers in microfluidic channels. Journal of the American Chemical Society. 2009;131:5294–7. doi: 10.1021/ja809987b. [DOI] [PubMed] [Google Scholar]
- 81.Groves JT, Ulman N, Cremer PS, Boxer SG. Substrate-membrane interactions: Mechanisms for imposing patterns on a fluid bilayer membrane. Langmuir : the ACS journal of surfaces and colloids. 1998;14:3347–50. [Google Scholar]
- 82.Kumar K, Tang CS, Rossetti FF, Textor M, Keller B, Voros J, et al. Formation of supported lipid bilayers on indium tin oxide for dynamically-patterned membrane-functionalized microelectrode arrays. Lab on a chip. 2009;9:718–25. doi: 10.1039/b814281e. [DOI] [PubMed] [Google Scholar]
- 83.Rossetti FF, Bally M, Reviakine I, Falconnet D, Michel R, Textor M. Formation of supported phospholipid bilayers on titanium oxide surfaces. Biophysical journal. 2005;88:7A–7A. [Google Scholar]
- 84.Mager MD, Almquist B, Melosh NA. Formation and Characterization of Fluid Lipid Bilayers on Alumina. Langmuir: the ACS journal of surfaces and colloids. 2008;24:12734–37. doi: 10.1021/la802726u. [DOI] [PubMed] [Google Scholar]
- 85.Steltenkamp S, Muller MM, Deserno M, Hennesthal C, Steinem C, Janshoff A. Mechanical properties of pore-spanning lipid bilayers probed by atomic force microscopy. Biophysical journal. 2006;91:217–26. doi: 10.1529/biophysj.106.081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cha T, Guo A, Zhu XY. Formation of supported phospholipid bilayers on molecular surfaces: Role of surface charge density and electrostatic interaction. Biophysical journal. 2006;90:1270–74. doi: 10.1529/biophysj.105.061432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rossetti FF, Bally M, Michel R, Textor M, Reviakine I. Interactions between titanium dioxide and phosphatidyl serine-containing liposomes: Formation and patterning of supported phospholipid bilayers on the surface of a medically relevant material. Langmuir: the ACS journal of surfaces and colloids. 2005;21:6443–50. doi: 10.1021/la0509100. [DOI] [PubMed] [Google Scholar]
- 88.Hussain NF, Siegel AP, Ge Y, Jordan R, Naumann CA. Bilayer asymmetry influences integrin sequestering in raft-mimicking lipid mixtures. Biophysical journal. 2013;104:2212–21. doi: 10.1016/j.bpj.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ian P. McCabe MBF. Polymer Supported Lipid Bilayers Open Journal of Biophysics. 2013;3:59–69. [Google Scholar]
- 90.Varma S, Teng M, Scottt HL. Nonintercalating Nanosubstrates Create Asymmetry between Bilayer Leaflets. Langmuir : the ACS journal of surfaces and colloids. 2012;28:2842–48. doi: 10.1021/la204623u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wacklin HP. Composition and Asymmetry in Supported Membranes Formed by Vesicle Fusion. Langmuir : the ACS journal of surfaces and colloids. 2011;27:7698–707. doi: 10.1021/la200683e. [DOI] [PubMed] [Google Scholar]
- 92.Richter RP, Maury N, Brisson AR. On the effect of the solid support on the interleaflet distribution of lipids in supported lipid bilayers. Langmuir: the ACS journal of surfaces and colloids. 2005;21:299–304. doi: 10.1021/la0478402. [DOI] [PubMed] [Google Scholar]
- 93.Reinl HM, Bayerl TM. Lipid Transfer between Small Unilamellar Vesicles and Single Bilayers on a Solid Support - Self-Assembly of Supported Bilayers with Asymmetric Lipid Distribution. Biochemistry. 1994;33:14091–99. doi: 10.1021/bi00251a018. [DOI] [PubMed] [Google Scholar]
- 94.Wacklin HP, Thomas RK. Spontaneous formation of asymmetric lipid bilayers by adsorption of vesicles. Langmuir : the ACS journal of surfaces and colloids. 2007;23:7644–51. doi: 10.1021/la063476q. [DOI] [PubMed] [Google Scholar]
- 95.Stottrup BL, Veatch SL, Keller SL. Nonequilibrium behavior in supported lipid membranes containing cholesterol. Biophysical journal. 2004;86:2942–50. doi: 10.1016/S0006-3495(04)74345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Penin F, Brass V, Appel N, Ramboarina S, Montserret R, Ficheux D, et al. Structure and function of the membrane anchor domain of hepatitis C virus nonstructural protein 5A. The Journal of biological chemistry. 2004;279:40835–43. doi: 10.1074/jbc.M404761200. [DOI] [PubMed] [Google Scholar]