Abstract
Evidence suggests that children and adults diagnosed with autism spectrum disorders (ASD) exhibit difficulties with postural control. Retrospective video studies of infants later diagnosed with ASD indicate that infants who eventually receive an ASD diagnosis exhibit delays in postural development. This study investigates early posture development prospectively and longitudinally in 22 infants at heightened biological risk for ASD (HR) and 18 infants with no such risk (Low Risk; LR). Four HR infants received an autism diagnosis (AD infants) at 36 months. Infants were videotaped at home at 6, 9, 12, and 14 months during everyday activities and play. All infant postures were coded and classified as to whether or not they were infant-initiated. Relative to LR infants, HR infants were slower to develop skill in sitting and standing postures. AD infants exhibited substantial delays in the emergence of more advanced postures and initiated fewer posture changes. Because posture advances create opportunities for infants to interact with objects and people in new and progressively more sophisticated ways, postural delays may have cascading effects on opportunities for infant exploration and learning. These effects may be greater for infants with ASD, for whom posture delays are more significant.
Keywords: Autism Spectrum Disorders, motor development, posture, early identification, infant siblings
Over the course of the first 18 months, typically-developing (TD) infants achieve a series of milestones in postural development, progressing from postures in which the entire body is fully supported by a surface (prone, supine) to positions that require progressively greater strength, muscle coordination, and balance (e.g., unsupported sitting, standing). In addition to indexing advances in motor control and setting the stage for independent locomotion, the attainment of new postures provides infants with access to a whole set of new perceptual and social experiences that create opportunities for learning and development in other domains. Thus, for example, achievement of unsupported sitting (which results in substantial changes in respiration and the position of the speech articulators) is accompanied by changes in the characteristics of infant vocalizations; and advances in head and trunk control during sitting relate to changes in reaching skill, which in turn facilitates interaction with and exploration of objects (Soska, Adolph, & Johnson, 2010; Spencer, Vereijken, Diedrich, & Thelen, 2000; also see Iverson, 2010, for an extended discussion of this issue).
In light of the relatively rapid pace at which TD infants attain new postures and their importance for enhancing opportunities for new ways of interacting with the environment and for learning, the aim of this research was to explore postural development longitudinally in infants who are at heightened biological risk for autism spectrum disorder (ASD) because they have an affected older sibling (HR infants). The risk of ASD for these infants is more than is 200 times greater than that in the general population (Ritvo et al., 1989), and more recent reports indicate that the recurrence risk is 18.7% (Ozonoff et al., 2011). This approach has recently been adopted by a number of research teams because it guarantees the sampling of a subset of infants who will go on to receive an ASD diagnosis (e.g., Zwaigenbaum et al., 2007). In addition to identification of potential early indicators of ASD in this subgroup of infants, there is also growing evidence pointing to the existence of delays in HR infants who do not receive an ASD diagnosis (e.g., see Rogers, 2009, for a review).
The rationale for our focus on postural development stems from empirical findings indicating that: a) children and adults with ASD demonstrate difficulties with postural control; and b) infants who eventually receive an ASD diagnosis exhibit delays in postural development and atypical postures. For this initial foray into the question of postural development in HR infants, we chose to focus on longitudinal change in the number, duration, and self-initiation of postures rather than on the consequences for infant action of these postural variations. While change in movement patterns as a function of postural development is of undoubted importance and should be the target of future research, we adopted this more limited approach for two reasons: a) because the emergence of major postural milestones is highly salient and relatively easy for both parents and pediatricians to observe, delays in this domain may constitute a valuable component in a program of developmental surveillance for HR children; and b) because our observations were derived from home rather than laboratory visits, we were unable to employ the complex technology necessary for the study of subtle variations in motor patterns.
Posture in children and adults with ASD
Although postural deficits are not a primary diagnostic criterion for ASD, evidence suggests that they are characteristic of the ASD profile (see Bhat, Landa, & Galloway, 2011; Fournier et al., 2010a for recent reviews). Thus, for example, studies using force platform technology to measure postural sway in individuals diagnosed with ASD have consistently reported that relative to TD comparison groups, those diagnosed with ASD exhibit significantly greater postural sway during quiet stance. This is characteristic of both adults (Minshew, Sung, Jones, & Furman, 2004; Molloy, Dietrich, & Bhattacharya, 2003) and children (Fournier et al., 2010b; Memari et al., 2013) and is taken to indicate the greater difficulty that individuals with ASD have in maintaining postural control.
Posture in infants later diagnosed with ASD
Research using retrospective home video to compare postural behavior in infants eventually receiving an ASD diagnosis to that of TD and/or developmentally delayed (DD) infants has reported delays and atypicalities during the first two years. Thus, for example, Ozonoff et al. (2008a) found that relative to TD infants, infants later diagnosed with ASD were significantly older when they attained the most mature forms of sitting and walking postures. In addition, relative to TD infants, ASD infants have been more frequently observed in asymmetrical lying and sitting positions (Esposito & Venuti, 2009); and persistent asymmetry is thought to be a potential indicator of developmental disorder (e.g., Teitelbaum et al., 1998).
Because retrospective video research, limited as it is to available footage, has focused primarily on age of attainment of postural milestones and relative immaturity of observed postures (e.g., asymmetry), we know little about how much time infants spend in various postures, the frequency with which they move spontaneously from one posture to another, or ways in which postural durations and shifts may change over time and vary by risk status and ASD diagnosis. The present study was designed to address these limitations by gathering in-home, prospective, longitudinal behavioral data from HR infants and a comparison group of infants with a TD older sibling and no family history of ASD (Low Risk; LR). Infants were observed at four time points (6, 9, 12, and 14 months) as they engaged in everyday activities. Because these ages coincide with the emergence of new postural skills and increased mobility, we focus on longitudinal change in the diversity of infants’ posture repertoires, the amounts of time spent in each posture, and infants’ ability to move spontaneously from one posture to another.
Method
Participants
Participants included 22 infants from families in which there was at least one older sibling with an autism diagnosis (8 male, 14 female). Families of High Risk (HR) infants were recruited through the Autism Research Program at the University of Pittsburgh, parent support organizations, and local agencies and schools serving families of children with ASD. Prior to the infant’s enrollment, the older sibling was seen at Autism Center of Excellence at the University of Pittsburgh for evaluation by a trained clinician using the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000). If the older sibling scored above the threshold for Autism on the ADOS, the infant was eligible to participate in the study.
For purposes of comparison, 18 infants (8 males, 10 females) with a typically-developing older sibling and no family history of ASD were selected from a larger group of infants participating in a longitudinal study of vocal-motor coordination in infancy (Low Risk infants; LR). Infants in both groups were from full term, uncomplicated pregnancies and came from monolingual, English-speaking homes. Parental level of education was comparable between groups; the majority of parents either held college degrees or had completed some college. Parents’ ages ranged from 24 to 44 years (M = 34.5, SD = 4.39). All but four infants were of Caucasian descent. Two HR infants were Hispanic and one LR and one HR infant were Asian-American.
Procedure
The data to be reported here were collected as part of larger longitudinal studies in which infants and a primary caregiver were videotaped at regular, frequent intervals at home for approximately 45 minutes as they engaged in everyday activities and semi-structured play. HR infants were seen monthly from 5 to 14 months with a follow up observation at 18 months, and then at three-month intervals until the age of 36 months. LR infants were observed biweekly from 2 to 19 months; for this study, comparison data were utilized from comparable monthly visits. All study procedures received Institutional Review Board approval and informed consent was obtained from the parents of all participating infants prior to the commencement of data collection.
Observations were scheduled within three days of the monthly anniversary of the infant’s birth and at times when caregivers thought the infant would be most alert and playful (typically just after awakening and a feeding). The present study focused on the first 10 minutes of the sessions completed at infant ages 6, 9, 12, and 14 months. This portion of the visit consisted of naturalistic observation. The infant, wearing whatever clothing he/she had been dressed in by the caregiver, was typically placed on the floor with toys. Following the infant with a hand-held video camera, a trained research assistant recorded the child’s behavior as he/she engaged in everyday activities. The relatively unstructured nature of this observation permitted us to film the infant in a wide variety of naturally-occurring postures and behaviors. Although the caregiver was typically seated near the infant during the observation and was available should he/she begin to fuss, the caregiver was involved in completing an interview and not generally engaged with the infant. Thus, caregiver-infant interaction during the period of observation was relatively minimal.
At 36 months, all HR infants visited the Autism Center of Excellence for diagnostic outcome classification by a trained clinician blind to all previous study data using the ADOS and DSM-IV criteria. Of the 22 HR infants, four (3 male, 1 female) met criteria for Autistic Disorder (AD; i.e., ADOS scores above the cutoff for Autism and clinical judgment using DSM-IV criteria); all of the remaining infants scored below the threshold for ASD. Analyses involving HR infants were performed with the AD infants excluded, and data for the AD infants will be presented separately. No developmental concerns were noted by parents or experimenters for any of the LR infants.
Coding
Coding was carried out using The Observer (Noldus Information Technologies), a video-linked computer program that allowed identification of onset and offset times for each posture. Prior to initiation of the coding process, coders naïve to infant group membership were trained to a minimum criterion of 80% agreement on all coding categories on three consecutive videos. Only postures sustained for at least 1 second were coded. Descriptions of each posture category with definitions (adapted from Spencer et al., 2000) of the specific postures included in each are provided in Table 1. A posture was coded as infant-initiated when the infant spontaneously moved into the posture (e.g., previously Prone infant shifts herself into All-4). Instances in which the caregiver initiated a postural change (e.g., picking up a Prone infant and placing him/her in a Supported Sit posture) and all instances in which initiation was unclear were excluded from data analyses.
Table 1.
Coding categories for infant postures.
| Posture | Definition |
|---|---|
| Lying | |
| Prone | Infant is lying on his/her stomach |
| Supine | Infant is lying on his/her back |
| Sitting | |
| Sit Supported | Infant is seated with support provided by one or both hands, the caregiver, or objects (e.g., pillows). |
| Sit Unsupported | Infant is seated without support from the hands, caregiver, or other objects. |
| Kneeling | |
| Kneel | Infant is on one or both knees with no support. Both hands are free to move. |
| All-4 | Infant is on hands and knees. |
| Standing | |
| Stand Supported | Infant is standing with support from one or both hands, a caregiver, furniture, or an object. |
| Stand Unsupported | Infant is standing without support from the hands, caregiver, or other objects. |
| Squat | Infant is standing on both feet with knees bent at an angle less than 90 degrees. |
Reliability
Interrater reliability was assessed via independent coding of 20% (n = 30) of the video clips. Reliability videos were chosen so as to include participants from both groups and at all 4 age points. Mean percent agreement averaged across the 30 videos was 82.3% for posture identification and initiation categories (range 72–100%). Following reliability calculation, disagreements were resolved through discussion and adoption of consensus codes.
Results
The current study employed a prospective, longitudinal design to examine postural development in infants who are at heightened biological risk for ASD and compare it to data obtained from infants with no such risk. Our focus was on postures assumed by infants as they engaged in everyday activities in the home. We begin by presenting data on infants’ postural repertoires (i.e., the number of different postures observed at each session) at each observation. We then focus on the amounts of time infants spent in each posture; this is followed by data on the frequency of infant-initiated postures within the 10-minute observation.
Data were unavailable for 2 sessions for 2 infants: one due to recording equipment malfunction (1 HR infant at 12 months) and one due to a missed visit (1 HR infant at 6 months). We also excluded sessions in which infants were unable to move freely for the entire duration of the 10-minute observation period (e.g., infant was seated in a high chair or exersaucer). This was the case for 2 HR and 3 LR infants at 6 months and 2 HR infants at 9 months. Thus, the analyses reported below are based on data from 15 HR and 15 LR infants at 6 months, 16 HR and 18 LR infants at 9 months, 17 HR and 18 LR infants at 12 months, and 18 HR and 18 LR infants at 14 months. No data from any of the 4 AD infants had to be excluded.
Statistical Analysis
In the analyses presented below, we first examine age-related changes in posture in LR and HR infants. These involve 2 (Group: LR, HR) × 4 (Age: 6, 9, 12, 14 months) repeated measures analysis of variance (ANOVA) with follow up pairwise comparisons where appropriate. We then compare infants eventually diagnosed with AD to LR and HR infants (collapsed into a single comparison group when appropriate) using nonparametric Mann-Whitney U tests. Nonparametric tests were adopted due to unbalanced sample sizes (Siegel, 1956).
Posture Repertoire
Our first set of analyses focused on the number of different postures in which infants were observed in the course of the 10-minute observation. To be conservative, we credited infants with a posture if they assumed it spontaneously (i.e., it had to be infant-initiated) and were able to maintain it without external support (i.e., for Supported Sitting and Supported Standing, infants had to use their own bodies to maintain the posture, i.e., the hands and arms). For this analysis, each posture was only counted once: thus, if an infant was observed Unsupported Sitting, then moved to All-4, and then moved back to Unsupported Sitting, s/he would be credited with two postures. For each infant, we totaled the number of different postures observed and then averaged these across infants in each group at each age. These data are presented in Table 2.
Table 2.
Means, standard deviations, and ranges of infant-initiated posture repertoire sizes for infants in the LR, HR, and AD groups at 6, 9, 12, and 14 months.
| 6 months | 9 months | 12 months | 14 months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LR (n=15) | HR (n=15) | AD (n=4) | LR (n=18) | HR (n=16) | AD (n=4) | LR (n=18) | HR (n=17) | AD (n=4) | LR (n=18) | HR (n=18) | AD (n=4) | |
| Mean | 1.93 | 1.93 | 0 | 4.17 | 4.25 | 2.25 | 6.28 | 6.05 | 3.75 | 6.72 | 6.38 | 5.75 |
| SD | 1.22 | 1.22 | 0 | 1.92 | 1.61 | 0.96 | 1.53 | 1.95 | 1.50 | 1.07 | 1.79 | 0.96 |
| Range | 0–4 | 0–4 | 0 | 0–7 | 0–7 | 1–3 | 2–9 | 1–9 | 3–6 | 5–9 | 2–9 | 5–7 |
The data in the table indicate that for LR and HR infants, the size of the posture repertoire increased in a relatively linear fashion from 6 to 14 months. A two-way repeated measures analysis of variance (ANOVA) with Age (6, 9, 12, 14 months) as the within subjects factor and Group (LR, HR) as the between-subjects factor revealed a main effect of Age, F (3,78) = 65.966, p =.000, partial η2 = .717. Pairwise comparisons indicated that the number of different postures increased significantly from 6 to 9 and 9 to 12 months (ps = .000); the difference from 12 to 14 months was not statistically reliable. No other effects were significant.
Infants with AD were seen in about half as many different postures as were HR and LR infants at 6, 9, and 12 months, but by 14 months this difference was no longer apparent. Mann-Whitney U tests confirmed that at 6, 9, and 12 months, but not at 14 months, AD infants’ posture repertoires were significantly smaller than those of infants in the HR and LR groups combined (at 6 months, U = 8, p = .004; at 9 months, U = 21, p = .023; at 12 months, U = 18.5, p = .014).
In light of these findings, we decided to examine individual posture types within infants’ repertoires at each age. The proportions of LR, HR, and AD infants in each group who were credited with each posture type at each observation are presented in Table 3. As is apparent in the table, at 6 months, as a group, LR and HR infants were seen in five different postures: Prone, Supine, Sit Supported, Sit Unsupported, and All-4. There were no group differences in the numbers of infants observed in each of these postures. By contrast, none of the AD infants was seen in any infant-initiated postures at 6 months: all were placed in postures by a caregiver and remained in them until they were moved by the caregiver.
Table 3.
Proportions of infants in the LR, HR, and AD groups observed in infant-initiated postures at 6, 9, 12, and 14 months.
| 6 months | 9 months | 12 months | 14 months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LR (n=15) | HR (n=15) | AD (n=4) | LR (n=18) | HR (n=16) | AD (n=4) | LR (n=18) | HR (n=17) | AD (n=4) | LR (n=18) | HR (n=18) | AD (n=4) | |
| Prone | .40 | .47 | 0 | .22 | .31 | .50 | .22 | .44 | .50 | .17 | .38 | 0 |
| Supine | .53 | .47 | 0 | .22 | .38 | .50 | .22 | .44 | 0 | .22 | .33 | 0 |
| Sit Supported | .40 | .47 | 0 | .94 | .94 | .25 | .94 | .94 | 1.0 | .94 | 1.0 | 1.0 |
| Sit Unsupported | .33 | .27 | 0 | .94 | .88 | .50 | .94 | .88 | 1.0 | .89 | .78 | 1.0 |
| All-4 | .07 | .27 | 0 | .78 | .88 | .50 | .94 | .76 | .75 | 1.0 | .89 | 1.0 |
| Kneel | 0 | 0 | 0 | .28 | .31 | 0 | .67 | .59 | 1.0 | .56 | .44 | 1.0 |
| Stand Supported | 0 | 0 | 0 | .50 | .63 | 0 | .94 | .82 | 0 | 1.0 | .83 | 1.0 |
| Stand Unsupported | 0 | 0 | 0 | 0 | .06 | 0 | .67 | .71 | 0 | 1.0 | .83 | .50 |
| Squat | 0 | 0 | 0 | .28 | .06 | 0 | .67 | .53 | 1 | .83 | .83 | .25 |
By 9 months, over 75% of LR and HR infants were seen in Sit Supported, Sit Unsupported, and All-4 postures, and approximately half of the infants in the two groups had begun to Stand Supported. The AD infants were observed in roughly half as many different postures as comparison infants, and none were seen in Stand Supported.
At 12 months, the numbers of different postures observed among LR and HR infants had again increased. A majority of infants in each group assumed Kneeling, Stand Supported, Stand Unsupported, and Squat. AD infants were once again seen in fewer different postures, and those that were observed were developmentally less advanced. All 4 infants sat independently, and 3 assumed an All-4 posture, but only 1 was observed Kneeling and Squatting. Both of these postures require greater balance control due to the need to maintain an upright position on two points of contact without the hands for support (Kneel) or with the center of mass in a low, unstable position (Squat). None of the AD infants was observed in either of the Stand postures.
Finally, all of the LR and almost all (83%) of the HR infants were standing without support at 14 months. Interestingly, the AD infants appeared to have caught up to comparison infants at this age. Only 2 stood independently, but all 4 were observed in Stand Supported.
Posture Duration
We next focused on the amount of time infants spent in specific postures at the four age points. For purposes of clarity, data are presented for the three most commonly observed broader posture categories: Lying, Sitting, and Standing. For each infant and 10-minute observation, posture durations within each category were summed to yield total durations for that category (e.g., lying = prone + supine). These were then averaged across infants separately for each group.
Lying
The upper left panel of Figure 1 presents data on total duration of Lying postures for LR, HR, and AD infants. As is apparent in the figure, Lying durations were longest at 6 months for both LR (M = 292.114s, SD = 252.57) and HR infants (M = 306.59, SD = 213.89) and decreased continuously through 14 months. A two-way repeated measures analysis of variance (ANOVA) with Age (6, 9, 12, 14 months) as the within-subjects factor and Group (LR, HR) as the between-subjects factor revealed a significant main effect of Age, F (3,81) = 45.373, p =.000, partial η2 = .627. Pairwise comparisons indicated that overall infants spent significantly more time in Lying postures at 6 months than at any of the three subsequent ages (all ps = .000), which did not differ reliably from one another. Neither the main effect of Group nor the interaction was statistically reliable.
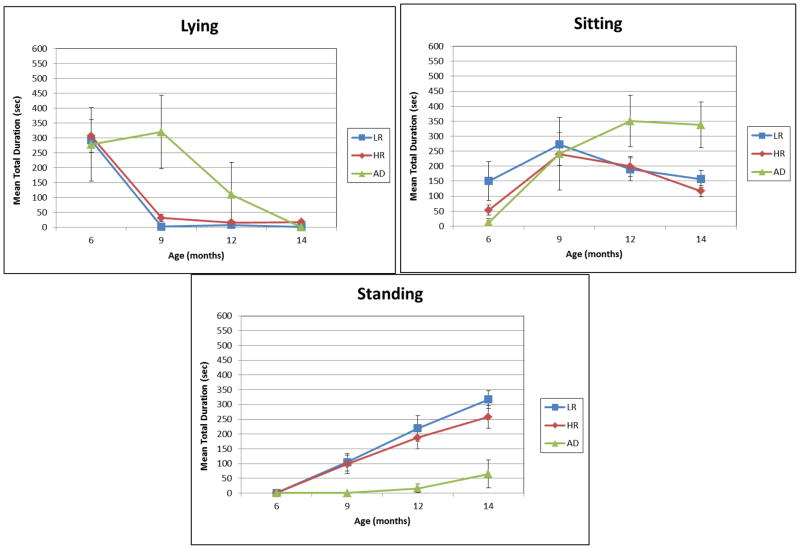
Figure 1.
Mean total duration of Lying, Sitting, and Standing postures for LR, HR, and AD infants at 6, 9, 12, and 14 months of age. Error bars indicate standard errors.
In contrast to LR and HR infants, the amount of time spent Lying remained high for AD infants at 9 months and then declined. Indeed, 3 of the 4 AD infants spent approximately half of the 9-month observation period in Lying postures, with total durations falling more than 6, 11, and 14 standard deviations respectively above the combined mean. A Mann-Whitney U test indicated that this group difference was statistically significant, U = 111.5, p = .025.
Sitting
Data on Sitting posture durations are presented in the upper right panel of Figure 1. As is apparent, time spent Sitting peaked at 9 months and declined steadily to 14 months for LR and HR infants. ANOVA results indicated a significant effect of Age, F (3,81) = 9.570, p =.000, partial η2 = .262, but no significant Group or interaction effects. Pairwise comparisons revealed that mean total Sitting duration at 9 months differed significantly from that at 6, 9, and 14 months (all ps < .002); but durations at these ages did not differ reliably from one another.
Although the Age x Group interaction was not significant, the data in the figure suggest that at 6 months, the total duration of sitting for HR infants was on average about one-third that for LR infants. In an exploratory followup analysis, we therefore looked within the subgroups of 6 month-old LR and HR infants who were observed sitting to determine whether relative durations of Supported vs. Unsupported Sitting varied by group. Mann-Whitney U tests indicated that relative to LR infants who were observed sitting at 6 months (n = 7), HR sitters (n = 9) spent significantly more time in Supported Sitting (MLR = 16.102, SD = 14.608; MHR = 38.079, SD = 29.378; U = 40, p = .020) and significantly less time in Unsupported Sitting (MLR = 305.838, SD = 283.262; MHR = 45.994, SD = 39.266; U = 3, p = .006).
AD infants displayed a different developmental pattern: while Sitting durations increased from 6 to 9 months, they did not decline thereafter, instead remaining relatively high at both 12 and 14 months. This difference tended toward significance at 12 months, U = 109.0, p = .075, and was statistically reliable by 14 months, U = 125.0, p = .017. At both of these ages, total Sitting durations for 3 of the 4 AD infants were more than double the combined LR/HR group means.
Standing
Mean total durations of Standing postures at each of the four observations are presented in the lower panel of Figure 1. Note that these durations included both time spent standing in a stationary posture and time spent locomoting (i.e., cruising or walking). As is apparent, time spent in Standing postures increased consistently and linearly for LR and HR infants across the 6- to 14-month period. An ANOVA revealed no significant effect of Group, but there was a significant effect of Age, F (3, 81) = 41.365, p =.000, partial η2 = .605. Pairwise comparisons indicated that Standing durations at each age point differed significantly from one another, all ps < .012.
As noted above, none of the AD infants were seen Standing prior to 14 months. Differences in Standing durations between the AD and combined LR/HR groups grew over time and were statistically significant at 9, 12, and 14 months of age (all ps < .020). Surprisingly, even at 14 months, the AD infants as a group spent relatively little time in Standing postures. Indeed, 3 of the 4 AD infants spent only about 10% as much time Standing (range 12.04–24.71s) as did LR and HR infants, nearly 2 standard deviations below the combined LR/HR group mean.
Frequency of Infant-Initiated Postures
Our final set of analyses examined the numbers of infant-initiated postures observed at each session. For these analyses, we identified all instances of infant-initiated postures during the 10-minute session (regardless of posture category). These were totaled separately for each infant at each session and then averaged across infants in each group. These data are presented in Figure 2.
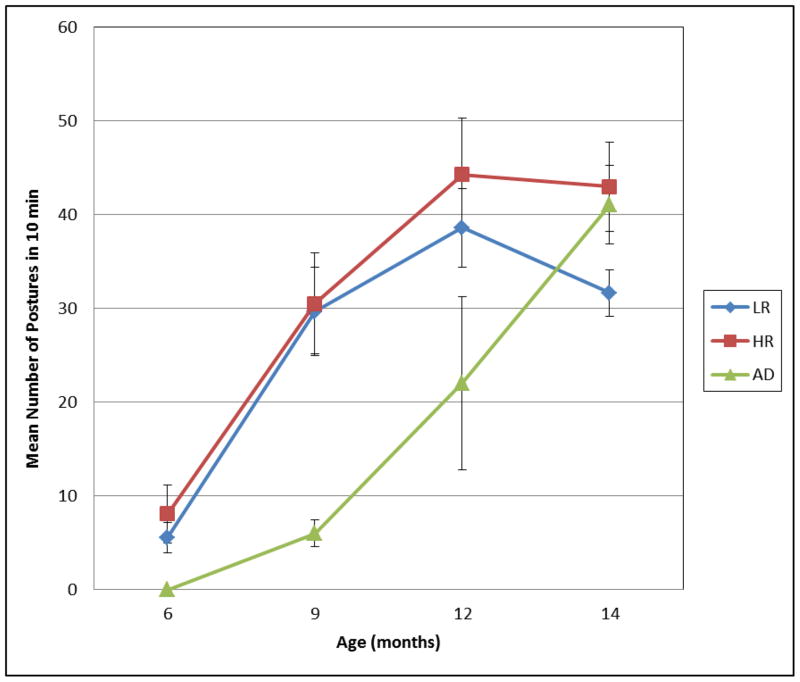
Figure 2.
Mean number of infant-initiated postures for LR, HR, and AD infants at 6, 9, 12, and 14 months of age. Error bars indicate standard errors.
As is evident in the figure, the number of infant-initiated postures observed in 10 min increased steadily from 6 to 12 months for both LR and HR infants. An ANOVA revealed significant main effects of Age, F (3,81) = 28.211, p =.000, partial η2 = .511, and Group, F (1,27) = 7.957, p =.009, partial η2 = .228. Pairwise comparisons conducted to follow up the Age effect indicated this was due to significant increases from 6 to 9 (p = .000) and 9 to 12 months (p = .009); the difference between 12 and 14 months was not statistically reliable. With regard to Group, HR infants (M = 34.592, SD = 9.096) initiated more postures overall than did LR infants (M = 25.058, SD = 9.093). Inspection of the data indicated that the Group difference was especially evident at 12 and 14 months and for the All-4 posture, which was much more frequent among HR than LR infants. Although difference in frequency of All-4 was not reliable at 12 months, at 14 months HR infants assumed the All-4 posture significantly more frequently than LR peers, t(34) = 2.422, p = .024. No other effects were significant.
Data for the AD infants are also presented in Figure 2. As is apparent, AD infants initiated many fewer postures than comparison infants at 6, 9, and 12 months, but this difference had virtually disappeared by 14 months. As previously discussed, at 6 months none of the AD infants produced any infant-initiated postures; and at 9 and 12 months, AD infants were observed in one-fifth and half as many infant-initiated postures respectively as infants in the comparison groups. A series of Mann-Whitney U tests confirmed that these differences were significant at 6 (U = 12, p = .009) and 9 months (U = 19, p = .020), and approached significance at 12 months (U = 31.5, p = .075). By 14 months, however, frequencies of infant-initiated postures for infants with AD (range 29–48) were much closer to the HR group mean (M = 42.94).
Discussion
Posture development in infants at heightened risk for ASD
The primary goal of the present study was to gather prospective, longitudinal data on posture development from infants at heightened biological risk for ASD and compare it to that of infants with no such risk. The broad finding was that posture repertoires and durations for global posture categories were generally similar for HR and LR infants. Despite this general similarity, however, two intriguing differences were observed, one at 6 months and the other at 14 months.
At 6 months, HR infants spent significantly more time in Supported Sitting and significantly less time in Unsupported Sitting than LR infants. Given the fact that time spent performing an emergent behavior typically indexes the extent to which the behavior is becoming well-established (e.g., see Iverson & Thelen, 1999), this difference suggests the hypothesis that HR infants may have greater difficulty than LR infants in maintaining a stable sitting posture at 6 months.
At 14 months, although almost all of the HR infants stood independently, they were observed in a significantly greater number of biomechanically less demanding All-4 postures than their LR peers. The tendency in HR infants to transition more frequently back to All-4 suggests the hypothesis that relative to LR infants, HR infants as a group may be less stable when standing at 14 months.
It is noteworthy that the emergence of Unsupported Sitting and the emergence of Unsupported Standing make new and substantially different demands on infants’ balance and coordination skills. Unsupported Sitting requires control and coordination of trunk (from shoulders to hips) and neck muscles (for control of head position) and continuous integration of vestibular and proprioceptive information with ongoing motor activity to control postural sway. Unsupported Standing makes additional demands on balance and strength due to the need to support the raised center of mass and expand the locus of postural sway to the legs and feet. While onsets of unsupported sitting and standing are major developmental milestones, there is substantial research indicating that skills required to sustain these postures consolidate relatively slowly and only after an extended period of instability and inflexibility following the appearance of the new posture (e.g., see Adolph & Berger, 2005).
Unfortunately, our data, limited as they are to postural frequency and duration, do not allow us directly to address questions concerning either relative stability or progressive consolidation over time of Unsupported Sitting and Unsupported Standing. The possibility that subtle delays may occur in the development of postural control in some HR infants and that the process of consolidation may be more protracted for HR than for LR infants as a group suggests the importance of carrying out future research focused on postural stability and consolidation with this population.
Infants later diagnosed with AD
The four infants who eventually received an AD diagnosis exemplified unique trajectories in postural development that did not parallel those of HR infants with no such diagnosis. The emergence of new postures was delayed among the AD infants, and from 9 months on, they consistently spent more time than comparison infants in less developmentally advanced postures (e.g., lying, sitting). Although our data cannot speak directly to this possibility, it is possible that these delays, particularly in the progression from lying to sitting postures, are related to delays or atypicalities in the development of head and trunk control. Along these lines, Flanagan, Landa, Bhat, and Bauman (2012) noted that in their sample of HR infants, those who eventually received an ASD diagnosis were more likely to exhibit head lag when pulled to a sit at 6 months than HR infants with no ASD symptoms.
This general pattern of results is consistent with work to date on postural control in older children and adults with ASD highlighting greater postural instability and delays in the development of postural control relative to matched comparison individuals (e.g., Minshew et al., 2004). They also suggest that a pattern of postural delays emerges relatively early in development in infants with AD, well before the end of the first year of life. The mechanisms underlying postural delays in infants with ASD await future investigation; but a critical next step will be the collection of kinematic data permitting the examination of postural sway and stability from infants who later receive an ASD diagnosis.
In addition to the pattern of delay described above, we also noted that infants who eventually received an AD diagnosis spontaneously initiated new postures less frequently than comparison infants. Although this is consistent with descriptions of infants with ASD as hypoactive (e.g., Adrien et al., 1991, 1992), a more intriguing possibility is that it is another manifestation of difficulties with spontaneous initiation of behavior that have been reported for older children with ASD in several different behavioral domains (e.g., joint attention, Mundy, Sigman, & Kasari, 1993; spontaneous communication, Winder, Wozniak, Parladé, & Iverson, in press; symbolic play, Hobson, Lee, & Brown, 1999; and imitation, Kurtz, Wozniak, & Iverson, 2011). Lower frequencies of infant-initiated postures among infants with AD may reflect a more general problem with the organization and initiation of spontaneous behavior that goes beyond the well-documented impairments in initiation of social and communicative behavior characteristic of ASD.
Posture development and delay: Cascading effects
There is mounting evidence from the normative developmental literature that posture advances create opportunities for infants to interact with objects and people in new and progressively more sophisticated ways. Thus, for example, when independent sitting emerges, hands previously needed to support the body are now free to manipulate objects and bring them into a relatively stable field of vision (e.g., Rochat & Goubet, 1995). In comparison to infants who cannot yet sit alone, self-sitting infants more frequently explore objects manually while looking at them. This permits infants to control the multiple perspectives from which an object can be viewed and thereby and obtain perceptual information about a variety of object characteristics (e.g., 3D form; Soska et al., 2010). The new upright head and trunk positions also have implications for infant vocalization: the speech articulators are repositioned in a way that sets up prime conditions for production of consonant-vowel (CV) units (Yingling, 1981; see Iverson, 2010, for further discussion).
Postures such as All-4 and standing set the stage for the emergence of crawling and walking respectively. The impact of the onset of both forms of independent locomotion is substantial and far-reaching. Crawling allows infants to move away from their caregivers to explore the environment. As infants encounter new conditions and objects (some of which are risky), caregivers respond by increasing communication in an effort to regulate infants’ activities. Because this communication comes from a distally-located caregiver and is likely to be about distally-located objects, it provides infants with experience prerequisite to the emergence of communicative pointing. Indeed, relative to same-aged precrawling infants (for whom communication is typically from a proximal caregiver and largely about the proximal environment), crawling infants are more likely to follow a pointing gesture in order to look at a target (Campos et al., 2000).
Walking is associated with new object-related social behaviors. Thus, for example, Karasik and colleagues (2011) have reported that relative to 13 month-old crawling infants, same-aged walking infants more frequently accessed distally-located objects and then initiated object sharing while carrying an object and moving toward the parent. This represents a new means for engaging in object-mediated social interaction: walking infants can select an object of interest from a broader array of possibilities (not just those that happen to be nearby), and then, with object in hand, travel to the caregiver. These moving bids are salient and highly likely to elicit a response from the caregiver, thereby setting the stage for continued interaction around the object. Occurrences of this sort are likely to be rich sources of timely linguistic input linked to the infants’ immediate focus of attention – precisely the type of input that is optimal for word learning (e.g., Tomasello & Farrar, 1986).
The implication here is clear. If postural development sets the stage for later acquisitions, postural delays might be expected to exert negative cascading effects not only on later motor skills but in a variety of other domains, including object exploration, vocalization, and social and communicative behavior.1 It is not surprising, therefore, given our finding of subtle delays among HR infants in the development of stable unsupported sitting at 6 months and of stable unsupported standing at 14 months, that delays in object exploration (e.g., reduced grasping and mouthing; Bhat, Downing, Galloway, & Landa, 2009; Koterba, Leezenbaum, & Iverson, 2012), delayed onset of reduplicated babble (i.e., vocalizations characterized by repeated CV units, e.g., [babababa]; Iverson & Wozniak, 2007) and reduced production of vocalizations containing CV syllables (Paul, Fuerst, Ramsay, Chawarska, & Klin, 2011) have all been reported for HR infants. In addition, many HR infants exhibit delays in vocabulary and language development (e.g., Iverson & Wozniak, 2007; Yirmiya et al., 2006). While word learning clearly requires the coming together of a whole set of skills from multiple developmental domains, delays in unsupported standing and walking reduce infants’ opportunities to produce the moving bids that lead to moments of joint attention accompanied by finely-tuned linguistic input known to be important in word learning.
Infants later diagnosed with AD
Consider next the case of the infants who were later diagnosed with AD, who as a group exhibited delays in the achievement of new postures, had more restricted posture repertoires, and spent more time in developmentally less advanced postures (e.g., Lying at 9 months; Sitting at 12 and 14 months) relative to comparison infants. Thus, for example, at 9 months, infants with AD spent roughly a third of the observation in Lying postures (prone or supine). To the extent that action possibilities for object exploration are constrained by the biomechanics of these postures (e.g., one or both arms must be used for support in prone; gravity restricts range of motion for object exploration in supine; Soska & Adolph, 2009), infants’ experiences with objects may be limited. Specifically, opportunities to look at objects while manipulating them (which are facilitated by upright sitting) and the development of skills that undergird effective visual-manual object exploration may be negatively impacted. Under these conditions, infants may continue to rely on well-established, developmentally-prior forms of object exploration, e.g., looking. Interestingly, two recent investigations have reported unusually long visual fixation times during object manipulation in independent samples of HR infants later diagnosed with ASD (Koterba et al., 2012; Ozonoff et al., 2008b).
In conclusion, we recognize that our findings are limited by a number of factors. Our samples are not large and, although our observations are longitudinal, all single observations are of relatively limited duration. We did not specifically code failed attempts to achieve a given posture nor were we able to trace the gradual and progressive increase in postural stability that follows the emergence of new postures. Because we were collecting data in the home rather than the laboratory, we were unable to control microcontextual variations. Finally, because we focus solely on the coding of static postures, our data do not speak specifically to variations in infant actions influenced by these postures. Despite these limitations, however, we believe that our findings point to the need for more serious consideration of the development of neuromotor control in infants at-risk for developmental concerns in general, and ASD in particular. The motor system is the principal means by which young infants explore and experience their surroundings; and postural and motor advances create opportunities for new ways to engage with the environment. Disruptions in basic postural and motor development can influence the ways in which a child interacts with the environment, thereby impacting the unfolding of these new opportunities and potentially setting the stage for atypical experiences that may, in turn, lead to further delays and/or atypicalities in development, both within and across domains (Thelen, 2004).
Delayed motor development is also a prime target for intervention providing enhanced motor experience designed to address impoverished exploratory opportunities. Programs of motor intervention for infants manifesting a delay need to be developed and implemented. These interventions need not be complex or particularly intensive; for example, advances in reaching and object manipulation have been reported both for full-term and preterm infants whose caregivers spent 15 minutes per day for 3 weeks engaging the infant in activities designed to improve postural stability and encouraging reaching and object exploration (e.g., Heathcock, Lobo, & Galloway, 2008; Lobo & Galloway, 2008). Nonetheless, given the importance of the ability to maintain a stable posture and the likelihood that improvements in posture will set the stage for enhancement of skills in other domains, additional research is needed to help us better understand the nature and developmental implications of postural delays and to design and evaluate developmentally appropriate interventions for young infants.
Acknowledgments
This research was supported by grants from Autism Speaks and the National Institutes of Health (R01 HD41677 and R01 HD054979) to JMI. Additional support was provided by HD35469 and HD055748 to N.J. Minshew. Portions of these data were presented at the 2010 International Conference on Infant Studies, Baltimore, MD, and the 2010 International Meeting for Autism Research, Philadelphia, PA. We thank members of the Infant Communication Lab at the University of Pittsburgh for help with data collection and coding and Nancy Minshew and Diane Williams for valuable contributions at various stages of the project. Special thanks to the infants and their families, without whose enthusiastic and dedicated participation this study could not have been completed.
Footnotes
Note that this does not imply that motor development is either necessary or sufficient for development in these other domains. The emergence of any new behavior involves the coming together of many skills and abilities. Motor development can (and typically does) serve as an agent of change in this process, but should a given developmental pathway be obstructed, there is sufficient flexibility in the system to yield a myriad of possible developmental trajectories leading to the emergence of the new skill (see Iverson, 2010, for further discussion).
References
- Adolph KE, Berger SE. Physical and motor development. In: Bornstein MH, Lamb ME, editors. Developmental science: An advanced textbook. 5. Hillsdale, NJ: Erlbaum; 2005. pp. 223–281. [Google Scholar]
- Adrien JL, Faure M, Perrot A, Hameury L, Garreau B, Barthelemy C, Sauvage D. Autism and family home movies: Preliminary Findings. Journal of Autism and Developmental Disorders. 1991;21:43–49. doi: 10.1007/BF02206996. [DOI] [PubMed] [Google Scholar]
- Adrien JL, Perrot A, Sauvage D, Leddet I, Larmande C, Hameury L, Barthelemy C. Early symptoms in autism from family home movies. Acta Psychopaediatrica. 1992;55:71–75. [PubMed] [Google Scholar]
- Bhat AN, Downing K, Galloway JC, Landa RL. A Comparison of Object Exploration Strategies between Infant Siblings of Children with Autism and Typically-Developing Infants at 6 Months of Age. Poster presented at the International Meeting for Autism Research; Chicago, IL. 2009. May, [Google Scholar]
- Bhat AN, Galloway JAC, Landa RJ. Relationship between early motor delay and later communication delay in infants at risk for autism. Infant Behavior and Development. 2012;35:838–846. doi: 10.1016/j.infbeh.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat AN, Landa RL, Galloway JC. Current perspectives on motor functioning in infants, children, and adults with Autism Spectrum Disorders. Physical Therapy. 2011;91:1–14. doi: 10.2522/ptj.20100294. [DOI] [PubMed] [Google Scholar]
- Campos JJ, Anderson DI, Barbu-Roth MA, Hubbard EM, Hertenstein MJ, Witherington D. Travel broadens the mind. Infancy. 2000;1:149–219. doi: 10.1207/S15327078IN0102_1. [DOI] [PubMed] [Google Scholar]
- Esposito G, Venuti P. Symmetry in infancy: Analysis of motor development in Autism Spectrum Disorders. Symmetry. 2009;1:215–225. [Google Scholar]
- Flanagan JE, Landa RL, Bhat AN, Bauman M. Head lag in infants at risk for autism: A preliminary study. American Journal of Occupational Therapy. doi: 10.5014/ajot.2012.004192. in press. [DOI] [PubMed] [Google Scholar]
- Fournier KA, Naik SK, Hass CJ, Lodha N, Cauraugh JH. Motor coordination in Autism Spectrum Disorders: A synthesis and meta-analysis. Journal of Autism and Developmental Disorders. 2010a;40:1227–1240. doi: 10.1007/s10803-010-0981-3. [DOI] [PubMed] [Google Scholar]
- Fournier KA, Kimberg CI, Radonovich KJ, Tillman MD, Chow JW, Lewis MH, Bod sh JA, Hass CJ. Decreased static and dynamic postural control in children with Autism Spectrum Disorders. Gait & Posture. 2010b;32:6–9. doi: 10.1016/j.gaitpost.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heathcock JC, Lobo M, Galloway JC. Movement training advances the emergence of reaching in infants born at less than 33 weeks of gestational age: A randomized clinical trial. Physical Therapy. 2008;3:1–13. doi: 10.2522/ptj.20070145. [DOI] [PubMed] [Google Scholar]
- Hobson RP, Lee A, Brown R. Autism and congenital blindness. Journal of Autism and Developmental Disorders. 1999;29:45–56. doi: 10.1023/a:1025918616111. [DOI] [PubMed] [Google Scholar]
- Iverson JM. Developing language in a developing body: The relationship between motor development and language development. Journal of Child Language. 2010;37:229–261. doi: 10.1017/S0305000909990432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson JM, Thelen E. Hand, mouth, and brain: The dynamic emergence of speech and gesture. Journal of Consciousness Studies. 1999;6:19–40. [Google Scholar]
- Iverson JM, Wozniak RH. Variation in vocal-motor development in infant siblings of children with autism. Journal of Autism and Developmental Disorders. 2007;37:158–170. doi: 10.1007/s10803-006-0339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasik LB, Tamis-LeMonda CS, Adolph KE. Transition from crawling to walking and infants’ actions with objects and people. Child Development. 2011;82:1199–1209. doi: 10.1111/j.1467-8624.2011.01595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koterba EA, Leezenbaum NB, Iverson JM. Object exploration at 6 and 9 months in infants with and without risk for autism. Autism. 2012 doi: 10.1177/1362361312464826. Published online before print November 22, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz N, Wozniak RH, Iverson JM. Spontaneous and Elicited Immediate Imitation in Toddlers at Low- and Heightened-Risk for Autism Spectrum Disorders. Poster presented at the Biennial Meetings of the Society for Research in Child Development; Montreal, Quebec, Canada. 2011. Apr, [Google Scholar]
- Lobo MA, Galloway JC. Postural and Object-Oriented Experiences Advance Early Reaching, Object Exploration, and Means – End Behavior. Child Development. 2008;79:1869–1890. doi: 10.1111/j.1467-8624.2008.01231.x. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The Autism Diagnostic Schedule – Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Memari AH, Ghanouni P, Gharibzadeh S, Eghlidi J, Ziaee V, Moshayedi P. Postural sway patterns in children with autism spectrum disorder compared with typically developing children. Research in Autism Spectrum Disorders. 2013;7:325–332. [Google Scholar]
- Minshew NJ, Sung K, Jones BL, Furman JM. Underdevelopment of the postural control system in autism. Neurology. 2004;63:2056–2061. doi: 10.1212/01.wnl.0000145771.98657.62. [DOI] [PubMed] [Google Scholar]
- Molloy CA, Dietrich KN, Bhattacharya A. Postural stability in children with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders. 2003;33(6):643–652. doi: 10.1023/b:jadd.0000006001.00667.4c. [DOI] [PubMed] [Google Scholar]
- Mundy P, Sigman M, Kasari C. Understanding other minds: perspectives form Autism. New York: Oxford University Press; 1994. The theory of mind and joint attention deficits in Autism; pp. 181–203. [Google Scholar]
- Ozonoff S, Young GS, Goldring S, Greiss-Hess L, Herrera AM, Steele J, Macari S, Hepburn S, Rogers SJ. Gross motor development, movement abnormalities, and early identification of autism. Journal of Autism and Developmental Disorders. 2008a;38:644–656. doi: 10.1007/s10803-007-0430-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Macari S, Young GS, Goldring S, Thompson M, Rogers SJ. Atypical object exploration at 12 months of age is associated with autism in a prospective sample. Autism. 2008b;12:457–472. doi: 10.1177/1362361308096402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young G, Carter AS, Messinger D, Yirmiya N, Zwaigenbaum L, Bryson SE, Carver L, Constantino J, Dobkins K, Hutman T, Iverson J, Landa R, Rogers S, Sigman M, Stone W. Recurrence risk for Autism Spectrum Disorders: A Baby Siblings Research Consortium Study. Pediatrics. 2011;128:e488–e495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Fuerst Y, Ramsay G, Chawarska K, Klin A. Out of the mouths of babes: vocal production in infant siblings of children with ASD. Journal of Child Psychology and Psychiatry. 2011;52:588–598. doi: 10.1111/j.1469-7610.2010.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritvo ER, Jorde LB, Mason-Brothers A, Freeman BJ, Pingree C, Jones MB, McMahon WM, Petersen PB. The UCLA-University of Utah epidemiologic survey of autism: Recurrence risk estimates and genetic counseling. American Journal of Psychiatry. 1989;146:1032–1036. doi: 10.1176/ajp.146.8.1032. [DOI] [PubMed] [Google Scholar]
- Rochat P, Goubet N. Development of sitting and reaching in 5- to 6-month-old infants. Infant Behavior and Development. 1995;18:53–68. [Google Scholar]
- Rogers SJ. What are infant siblings teaching us about autism in infancy? Autism Research. 2009;2:125–137. doi: 10.1002/aur.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel S. Nonparametric statistics for the behavioral sciences. New York: McGraw-Hill Book Company, Inc; 1956. [Google Scholar]
- Soska KC, Adolph KE. Infants’ posture affects visual and haptic exploration of objects. Poster presented at the Biennial Meetings of the Society for Research in Child Development; Denver, CO. 2009. Apr, [Google Scholar]
- Soska KC, Adolph KE, Johnson SP. Systems in development: Motor skill acquisition facilitates 3D object completion. Developmental Psychology. 2010;46:129–138. doi: 10.1037/a0014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JP, Vereijken B, Diedrich FJ, Thelen E. Posture and the emergence of manual skills. Developmental Science. 2000;3:216–233. [Google Scholar]
- Teitelbaum P, Teitelbaum O, Nye J, Fryman J, Maurer RG. Movement analysis in infancy may be useful for early diagnosis of autism. Proceedings of the National Academy of Science. 1998;95:13982–13987. doi: 10.1073/pnas.95.23.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen E. The central role of action in typical and atypical development: A dynamic systems perspective. In: Stockman IJ, editor. Movement and action in learning and development: Clinical implications for pervasive developmental disorders. San Diego, CA: Elsevier Academic Press; 2004. pp. 49–73. [Google Scholar]
- Tomasello M, Farrar M. Joint attention and early language. Child Development. 1986;57:1454–1463. [PubMed] [Google Scholar]
- Winder BM, Wozniak RH, Parladé MV, Iverson JM. Spontaneous initiation of communication in infants at low and heightened risk for Autism Spectrum Disorders. Developmental Psychology. doi: 10.1037/a0031061. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yingling J. Unpublished doctoral dissertation. University of Denver; 1981. Temporal features of infant speech: A description of babbling patterns circumscribed by postural achievement. [Google Scholar]
- Yirmiya N, Gamliel I, Pilowsky T, Feldman R, Baron-Cohen S, Sigman M. The development of siblings of children with autism at 4 and 14 months: Social engagement, communication, and cognition. Journal of Child Psychology and Psychiatry. 2006;47:511–523. doi: 10.1111/j.1469-7610.2005.01528.x. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Thurm A, Stone W, Baranek G, Bryson S, Iverson JM, Kau A, Klin A, Landa R, Lord C, Rogers S, Sigman M. Studying the emergence of Autism Spectrum Disorders in high-risk infants: Methodological and practical issues. Journal of Autism and Developmental Disorders. 2007;37:466–480. doi: 10.1007/s10803-006-0179-x. [DOI] [PubMed] [Google Scholar]