Abstract
Background & Aims
Although patients with Barrett's esophagus commonly undergo endoscopic surveillance, its effectiveness in reducing mortality from esophageal/gastroesophageal junction adenocarcinomas has not been evaluated rigorously.
Methods
We performed a case-control study in a community-based setting. Among 8272 members with Barrett's esophagus, we identified 351 esophageal adenocarcinoma: 70 in persons who had a prior diagnosis of Barrett's esophagus (who were eligible for surveillance); 51 of these patients died, 38 as a result of the cancers (cases). Surveillance histories were contrasted with a sample of 101 living persons with Barrett's esophagus (controls), matched for age, sex, and duration of follow-up evaluation.
Results
Surveillancei within 3 years was not associated with a decreased risk of death from esophageal adenocarcinoma (adjusted odds ratio, 0.99; 95% confidence interval, 0.36–2.75). Fatal cases were nearly as likely to have received surveillance (55.3%) as were controls (60.4%). A Barrett's esophagus length longer than 3 cm and prior dysplasia each were associated with subsequent mortality, but adjustment for these did not change the main findings. Although all patients should be included in evaluations of effectiveness, excluding deaths related to cancer treatment and patients who failed to complete treatment, changed the magnitude, but not the significance, of the association (odds ratio, 0.46; 95% confidence interval, 0.13–1.64).
Conclusions
Endoscopic surveillance of patients with Barrett's esophagus was not associated with a substantially decreased risk of death from esophageal adenocarcinoma. The results do not exclude a small to moderate benefit. However, if such a benefit exists, our findings indicate that it is substantially smaller than currently estimated. The effectiveness of surveillance was influenced partially by the acceptability of existing treatments and the occurrence of treatment-associated mortality.
Keywords: BE, EAC, Esophageal Cancer, Prevention
Endoscopic surveillance of persons with Barrett's esophagus is considered a relatively safe and sensitive method for the early detection of treatable esophageal adenocarcinomas and for the detection of precancerous low- and high-grade dysplasia that may warrant closer surveillance, resection, or ablation.1–3 Although surveillance is recommended by specialty society guidelines, a national expert panel in the United States determined that no controlled studies exist to support its widespread use.1,4–6 In the absence of definitive data, influential cost-effectiveness analyses assume that almost all cancers detected during surveillance are resectable for potential cure.5 However, a national governmental review in the United Kingdom concluded that surveillance every 3 years may do more harm than good, while noting a “major gap in the evidence is the lack of…data on the effectiveness of surveillance program[s] in reducing morbidity and mortality from adenocarcinoma.”7–9
The incidence of esophageal adenocarcinoma, whichhas a 5-year survival of less than 15%, has increased more than 6-fold in the past 3 decades in the UnitedStates.10–12 Barrett s esophagus is a metaplastic change from the esophagus' usual squamous epithelial lining to a specialized columnar epithelium; its presence signifies a markedly increased risk of esophageal/gastroesophageal junction adenocarcinoma.13,14 Because at least 6% of asymptomatic US adults are estimated to have Barrett's esophagus,15 surveillance recommendations for this condition represent a substantial public health question.
Optimally, the effectiveness of surveillance would be evaluated through a randomized trial of patients with Barrett's esophagus, which would randomize patients with known Barrett's esophagus to surveillance vs no surveillance and then follow them up until an important outcome, such as death from cancer. The study then would contrast the proportions in each arm who died of cancer, using a risk ratio; however, the practicality of such a trial in the United States is challenging given current clinical practices, specialty society guidelines recommending surveillance, and the large sample sizes needed to detect a mortality benefit.1,4 Alternatively, case-control studies efficiently can evaluate the effectiveness of cancer screening interventions.16–19 The odds ratio in a case-control study of screening/surveillance is a valid estimate of the risk ratio that might be obtained from a randomized trial.20 Such studies contrast screening/surveillance histories of subjects who die of cancer or its treatment with those from a sample of persons at risk for cancer death. Thus, for esophageal adenocarcinoma, a case-control study of Barrett's esophagus surveillance would contrast patients with known Barrett's esophagus who subsequently die of esophageal adenocarcinoma to a sample of similar Barrett's esophagus patients who do not die of esophageal adenocarcinoma. This control group may contain some patients with nonfatal cancers. If surveillance were effective, we would expect that patients dying of cancer would be less likely to have received surveillance examinations than the average population of Barrett's esophagus patients.
This case-control study evaluated whether endoscopic surveillance of Barrett's esophagus is associated with a lower risk of death from esophageal/gastroesophageal junction adenocarcinoma. The methods were similar to those of a case-control study of screening sigmoidoscopy and death from colorectal cancer within Kaiser Permanente; the protective association shown in that study was confirmed recently by randomized trials.18,19 Its conduct in a community-based population provides an approximation of surveillance effectiveness as currently practiced in a general population.
Materials and Methods
Source Population and Data Sources
The underlying study population was all adult (≥18 y) members of Kaiser Permanente, Northern California (KPNC) during the years 1995–2009. KPNC is an integrated health care delivery system with approximately 3.3 million current members who are approximately representative of the age, sex, and ethnic distributions of the underlying regional population.21 Patients with Barrett's esophagus receive surveillance examinations through physician-directed recommendations.
Barrett's Esophagus
Persons with Barrett's esophagus were identified using physician-assigned electronic diagnoses followed by manual confirmation using endoscopy and pathology records. Electronic diagnoses used the International Classification of Diseases, 9th revision code 530.2, which at KPNC is uniquely coded as Barrett's esophagitis, International Classification of Diseases, 9th revision code 530.85 (Barrett's esophagus), or the College of American Pathologists' Systematized Nomenclature of Human and Veterinary Medicine code 73330 (Barrett's esophagus). Manual physician review for confirmation required the presence of visible endoscopic changes consistent with Barrett's esophagus and the histologic presence of esophageal intestinal metaplasia. Patients were excluded if they had only gastric-type metaplasia of the esophagus, had columnar metaplasia without intestinal metaplasia, lacked endoscopic changes indicating Barrett's esophagus; or lacked an esophageal biopsy. Prior validation studies by our group showed that these combined methods (electronic coding, manual review, and pathology review) were accurate and highly reproducible.22,23
Index Date
The index date for cases was the cancer diagnosis date. The index date for controls was the corresponding date for their matched case.
Cases
Cases were adult KPNC members who were diagnosed with esophageal or gastroesophageal junction adenocarcinoma before September 2007; had a Barrett's esophagus diagnosis (as defined earlier) 6 months or more before their cancer diagnosis; and subsequently died of esophageal/gastroesophageal junction adenocarcinoma or its complications before December 31, 2009. Cancers were identified using the region's Surveillance, Epidemiology, and End Results (SEER) cancer registry; audits confirm the registry captures 99% or more of cancer diagnoses among KPNC members. Site and histology definitions used the International Classification of Disease for Oncology, 2nd edition, codes for esophageal carcinoma (C15.0–C15.9) and the gastroesophageal junction (C16.0)24; both locations were included to permit identification of all pertinent cancers.
Survival status was ascertained from mortality files that concatenate data from the SEER registry, membership files, death certificates, and the Social Security Death Index; cause of death was determined by manual record review.
Controls
Controls were KPNC members with a diagnosis of Barrett's esophagus (confirmed as described earlier) who did not die of esophageal or gastroesophageal junction adenocarcinoma through the end of the follow-up evaluation. Controls were matched to cases by age at Barrett's esophagus diagnosis, year of Barrett's esophagus diagnosis, medical center of Barrett's esophagus diagnosis, sex, and race (Table 1). For patients who lacked an exact diagnosis year match, we expanded the diagnosis year to within 1 or 2 years, and for patients without an exact race match, we modified the race category to white vs nonwhite. We did not exclude living patients with a cancer diagnosis from the control group so that we could include patients who may have benefited from surveillance. This matching scheme provided similar time periods and durations for surveillance opportunities between cases and controls.
Table 1. Patient Characteristics.
| Demographics | Cases (N = 38) | Controls (N = 101) |
|---|---|---|
| Male sexa (%) | 34 (89.5) | 93 (92.1) |
| Mean age, y, at index datea (SD) | 73.5 (8.2) | 73.8 (8.1) |
| Mean age, y, at Barrett's esophagus diagnosis (SD)a | 69.6 (7.8) | 69.9 (7.6) |
| Age (by 5-year groupings) (%)a | ||
| 50–54 | 1 (2.6) | 4 (4.0) |
| 55–59 | 3 (7.9) | 9 (8.9) |
| 60–64 | 8 (21.1) | 15 (14.9) |
| 65–69 | 9 (23.7) | 20 (19.8) |
| 70–74 | 7 (18.4) | 26 (25.7) |
| 75–79 | 7 (18.4) | 20 (19.8) |
| ≥80 | 3 (7.9) | 7 (6.9) |
| Race/ethnicity (non-Hispanic white) (%)a | 36 (94.7) | 95 (94.1) |
| Barrett's esophagus characteristics Length of Barrett's esophagus | N (%) | N (%) |
| <3 cm | 1 (2.6) | 15 (14.9) |
| ≥3 cm | 31 (81.6) | 79 (78.2) |
| Not defined | 6 (15.8) | 7 (6.9) |
| Intervals from diagnosis date to index date; y (SD)a | ||
| <1 | 2 (5.3) | 6 (5.9) |
| 1–1.9 | 6 (15.8) | 16 (15.8) |
| 2–2.9 | 7 (18.4) | 21 (20.8) |
| 3–3.9 | 6 (15.8) | 15 (14.9) |
| 4–4.9 | 4 (10.5) | 7 (6.9) |
| 5–5.9 | 5 (13.2) | 17 (16.8) |
| 6–6.9 | 7 (18.4) | 16 (15.8) |
| 7–7.9 | 0 (0) | 1 (1.0) |
| 8–8.9 | 0 (0) | 0 (0) |
| 9–9.9 | 1 (2.6) | 2. (2.0) |
| Mean | 3.9 (2.1) | 3.8 (2.1) |
| Median (10%–90% range) number of endoscopies with biopsy between diagnosis date up to and including index date | 3 (2-8) | 2 (1–4) |
Subsequent cancer with metastatic disease.
Matching factors to cases.
Exposure Status: Surveillance
A surveillance endoscopy was any esophagogastroduodenoscopy performed principally for cancer surveillance of a previously documented Barrett's esophagus, not for symptoms. A patient in surveillance was someone who had at least 1 surveillance endoscopy within the 3 years before the index date. We included as surveillance examinations those that diagnosed the index cancer, if the examination was performed only for surveillance and not for symptoms. A 3-year interval was selected a priori because it is the shortest recommended interval in guidelines for persons without dysplasia and, thus, the one most likely to be associated with a mortality benefit.1,4,5 Assignment of surveillance status used endoscopy reports, pathology requests, and outpatient visits; prior research by our group determined the surveillance status assignment was 97% reproducible between blinded reviewers.25
Data Collection
After institutional review board approval, trained physicians, blinded to case status, abstracted medical records from the earliest date available to the index date. Double abstraction was performed to confirm surveillance status, Barrett's esophagus diagnosis, and cause of death.
Power Calculations
A priori power calculations indicated that 34 cases (matched to up to 4 controls) would provide more than 99% power to detect a 40% difference in surveillance between cases vs controls (eg, 65% of controls in surveillance vs 25% of cases) and more than 80% power to detect a 30% difference. These proportions were considered conservative because prior studies suggested that few patients dying from esophageal adenocarcinoma (ie, cases) had received recent surveillance.25–28
Statistical Methods
The relationship between case status and surveillance status was evaluated using conditional logistic regression. The main model adjusted for dysplasia status. Dysplasia status was defined as the most advanced level found before the 3-year surveillance period or, for persons with fewer than 3 years between their diagnosis and index dates, the dysplasia status from the first endoscopy (the first examination that diagnosed Barrett's esophagus, by definition, was not itself considered a surveillance examination). This definition was used because dysplasia before the 3-year surveillance window could influence both the likelihood of receiving surveillance in the 3-year surveillance window and the patient's cancer risk. In addition to adjusting for dysplasia status, sensitivity analyses were performed to determine whether dropping some dysplasia patients (and their matched controls) influenced results. Analyses were performed using Stata version 10.1 (Stata Corporation, College Station, TX).
Results
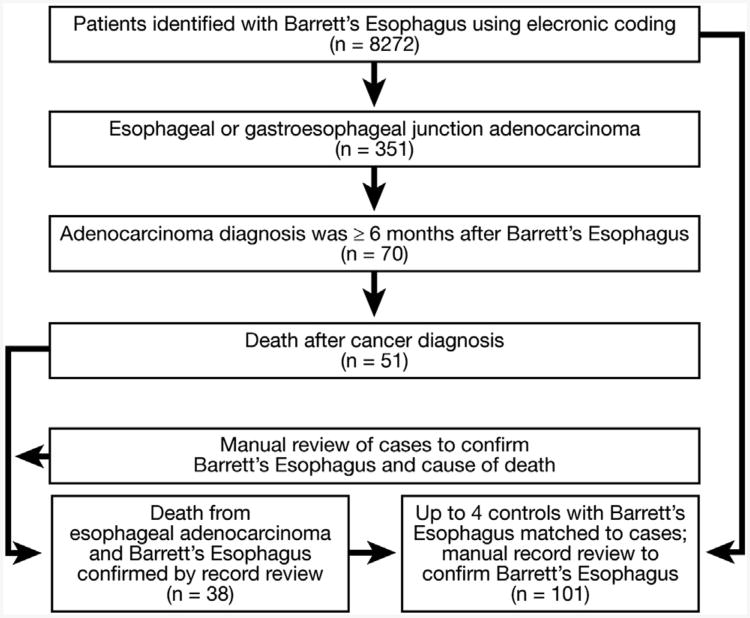
We identified 8272 patients with a Barrett's esophagus diagnosis using electronic coding (Figure 1), 351 of whom simultaneously or subsequently were diagnosed with an esophageal or gastroesophageal junction adenocarcinoma. Of these 351, 70 had their cancer diagnosed 6 months or more after their Barrett's esophagus diagnosis, and 51 died during follow-up evaluation. Manual review excluded 13 patients: 5 patients whose cause of death could not be determined, 2 with deaths from other cancers, 3 with fatal strokes or lung disease, and 3 patients who did not meet criteria for Barrett's esophagus. This provided 38 case patients for the primary analyses (31 esophageal and 7 gastroesophageal junction cancers); each was paired with up to 4 controls (as available with matching criteria), yielding 101 controls (Tables 1 and 2).
Figure 1.
Patient identification and flow diagram.
Table 2. Indications for Examination That Diagnosed Cancer.
| Indicationa | Cases | Controls |
|---|---|---|
| Barrett's esophagus surveillanceb | 19 | 1 |
| Dysphagia | 10 | 0 |
| Gastrointestinal bleeding | 4 | 0 |
| Pain | 4 | 0 |
| Gastroesophageal reflux disease | 3 | 0 |
| Weight loss | 1 | 0 |
| Diagnosed at surgery for achalasia shortly after negative endoscopy | 1 | 0 |
| Other or indication not listed | 5 | 0 |
| Total cancers | 38 | 1 |
May be >1 indication per examination.
Surveillance was the only indication for 14 of the cases at the time of cancer diagnosis.
Surveillance Status Versus Case Status
Surveillance within 3 years was not associated with a decreased risk of death from esophageal adenocarcinoma (odds ratio [OR], 0.99; 95% confidence interval [CI], 0.36–2.75), controlling for dysplasia status; adjustment for Barrett's esophagus length provided similar results (Tables 3 and 4). Cases were slightly (but not significantly) less likely to receive surveillance within the prior 3 years (55.3%) than controls (60.4%) (Table 3); similar results were found for surveillance within the previous 5 years (63.2% for cases and 73.3% for controls).
Table 3. Proportions of Patients in Surveillance by Dysplasia Status.
| Cases | Controls | |||
|---|---|---|---|---|
|
|
|
|||
| Dysplasia status | Total n (%)a | In surveillance n (%)b | Total n (%)a | In surveillance (%)b |
| No dysplasia | 17 (44.7) | 9 (52.9) | 80 (79.2) | 46 (57.5) |
| Indeterminate | 3 (7.9) | 1 (33.3) | 7 (6.9) | 6 (85.7) |
| Low-grade dysplasia | 9 (23.7) | 6 (66.7) | 13 (12.9) | 9 (69.2) |
| High-grade dysplasia | 9 (23.7) | 5 (55.6) | 1 (1.0) | 0 (0) |
| Total | 38 (100) | 21 (55.3) | 101 (100) | 61 (60.4) |
NOTE. The most advanced dysplasia found before the 3-year surveillance period or, for persons with <3 years between their diagnosis date and the index date, the dysplasia status from their first endoscopy.
Denominator is total cases or total controls.
Denominator is the relevant population (eg, cases: no dysplasia in surveillance for 9 of 17; 52.9%).
Table 4. Associations Between Surveillance Endoscopy and Fatal Adenocarcinomas.
| Cases | Controls | ||
|---|---|---|---|
|
|
|
||
| In surveillance n (%) | In surveillance n (%) | OR (95% CI)a | |
| Unadjusted1 | |||
| Surveillance examination within 3 years | 21 (55.3) | 61 (60.4) | 0.82 (0.35–2.00) |
| Controlling factors | |||
| Dysplasia status (main model)b | 21 (55.3) | 61 (60.4) | 0.99 (0.36–2.75) |
| Barrett's esophagus lengthc | 21 (55.3) | 61 (60.4) | 0.97 (0.38–2.50) |
| Dysplasia status and Barrett's esophagus lengthb,c | 21 (55.3) | 61 (60.4) | 1.14 (0.39–3.32) |
| Excluding cases with 7–12 months between Barrett's esophagus and cancer diagnoses, adjusted for dysplasia statusb,d | 19 (52.8) | 57 (60.0) | 0.95 (0.32–2.70) |
| Excluding cases with high-grade dysplasia before 3-year surveillance interval, adjusted for other dysplasia statusb,d | 16 (55.2) | 38 (52.8) | 1.00 (0.34–2.94) |
| Excluding cases with gastroesophageal junction adenocarcinomas, adjusted for dysplasia statusb,d | 19 (61.3) | 57 (67.1) | 0.88 (0.29–2.67) |
| Excluding cases unable to be treated, adjusted for dysplasia statusb,d | 18 (56.3) | 54 (64.3) | 0.80 (0.27–2.34) |
| Excluding cases with treatment-related mortality or unable to be treated, adjusted for dysplasia statusb,d | 14 (51.9) | 47 (65.3) | 0.46 (0.13–1.64) |
| Total | 38 (100) | 101 (100) |
ORs and 95% CIs from conditional logistic regression. Controls were matched to cases by age at Barrett's esophagus diagnosis, year of Barrett's esophagus diagnosis, sex, race, and medical center of Barrett's esophagus diagnosis.
Categories of dysplasia in models include the following: none, indeterminate, low grade, and high grade. The main model adjusted for dysplasia status before the 3-year surveillance window because this could influence both the likelihood of receiving surveillance in the 3-year surveillance window and the patient's cancer risk. Thus, dysplasia status was defined as the most advanced level found before the 3-year surveillance period or, for persons with fewer than 3 years between their diagnosis and index dates, the dysplasia status from the first endoscopy (the first examination, which diagnosed Barrett's esophagus, by definition was not itself considered a surveillance examination).
More than 3 cm vs less than 3 cm vs not defined.
Excluded cases and their matched controls.
Because high-grade dysplasia may require more frequent surveillance, analyses were repeated with cases diagnosed with high-grade dysplasia before the 3-year surveillance period excluded; results did not change much (Table 4). Sample size precluded detailed evaluations of multiple surveillance patterns; however, among the 11 cases in surveillance who also were diagnosed with low- or high-grade dysplasia (at any time), all had multiple prior endoscopies without cancer and 8 had at least 1 negative endoscopy in the 10 months before the examination that diagnosed their cancer.
Dysplasia Status, Barrett's Esophagus Length, and Cancer Stage
Cases were more likely to have dysplasia (low grade or high grade) on either the first examination or any examination before the 3-year surveillance period (47.4% of cases vs 13.9% of controls; Table 3), and prior dysplasia was associated with cancer mortality (OR, 4.68; 95% CI, 1.75-12.51), independent of Barrett's esophagus length. Two controls ultimately were diagnosed with high-grade dysplasia and 1 was diagnosed with cancer.
Cases were more likely than controls to have longer segments of Barrett's esophagus (≥3 cm in 97% of cases vs 84% of controls, among persons with a defined length; P = .05). Among patients with a segment length of 3 cm or longer, somewhat fewer cases than controls were in surveillance (58.1% of cases vs 67.1% of controls, among persons with a defined length); few cases had short segment lengths (Table 1).
Among the cases, initial SEER cancer stages were intramucosal (n = 4), limited (no lymph node involvement; n = 15), lymph node involvement (n = 9), distant disease (n = 8), and insufficient data for staging (n = 2). Consistent with prior observations, there was a nonsignificant trend for early stage disease (intramucosal or limited) found more often among the cases in surveillance (12 patients; 57%) than among the cases not in surveillance (7 patients, 41%; P = .33). The control patient with cancer had lymph node involvement.
Treatment-Related Deaths and Patients Not Treated
Five patients had treatment-related mortality: 3 had fatal pneumonias attributed to aspirations from esophagectomy, 1 had sepsis related to chemotherapy, and 1 died postoperatively; 4 of these patients were in surveillance. Six cases were unable or unwilling to receive apotentially curative resection; 3 of whom were in surveillance. Although all 11 of these patients should be included in the analyses of surveillance effectiveness, their exclusion decreased the odds ratio between surveillance and survival(OR, 0.46; 95% CI, 0.13–1.64).
Exclusion of 3 cases (and their matched controls) who had only 7–12 months between their Barrett's esophagus and cancer diagnoses had little impact on the results (OR, 0.95; 95% CI, 0.32-2.70). Analyses restricted to cancer cases diagnosed after the year 2000 or that excluded gastroesophageal junction cancers also provided similar results.
Discussion
Surveillance for persons at high risk of a disease offers the potential to detect preclinical disease amenable to early treatment. To provide this benefit, the surveillance method must fulfill certain criteria.20 First, the test must be able to detect a condition before it would present with symptoms. Second, treatment of this preclinical condition should yield a superior outcome to treatment of disease detected because of symptoms. Third, it should be feasible, available, and economically sound. The current study used a case-control design to evaluate the first 2 criteria; however, despite the detection of early stage disease in the majority of case patients in surveillance, the use of surveillance was not associated with an overall reduced risk of death from esophageal/gastroesophageal junction adenocarcinoma.
There were several strengths to the current study. It evaluated a large group of Barrett's esophagus patients and a large number of cancer-related deaths reported in a surveillance study. A case-control design provides the closest approximation to date of a randomized trial and the study's performance within a large, integrated health system permitted capture of virtually all patients diagnosed with Barrett's esophagus and an evaluation less biased by socioeconomic differences in receipt of care.29,30
The current study was much less vulnerable to bias than prior studies; in particular, prior reports that evaluated survival time, which can introduce lead time and length time biases.9,31 Lead time bias is the finding of a longer survival time in the screened group solely owing to early recognition of preclinical disease.20,25 Length time bias represents the tendency for screening tests to identify more slower-growing cancers, which have a more favorable course after diagnosis.20,25 The current study design is much less prone to these potential biases because it examines the absolute mortality from adenocarcinoma, rather than the survival times.25 Finally, a priori calculations indicated the study would be powered adequately to look at surveillance and mortality, even using conservative estimates from the literature.5
This study had several limitations. It cannot exclude the possibility of a small to moderate benefit from surveillance; however, if present, the benefit would be much smaller than those incorporated into widely used cost-effectiveness analyses, which assume that 85%–99% of surveillance-detected cancers would be resectable and 60%–90% of all cancers would be cured.5 Even under such optimistic conditions, some analyses have concluded that surveillance may not be cost effective for most patients with nondysplastic Barrett's esophagus.32 A smaller potential benefit would be more consistent with other screening modalities such as mammography for breast cancer, which is estimated to reduce cancer deaths by approximately 25%–30%.33 We also cannot exclude a benefit from more intensive surveillance methods. Second, endoscopic surveillance performed in the community may not be performed optimally, even if it is performed at appropriate intervals. However, if this were the sole explanation for the results shown, we would expect surveyed patients to present with advanced cancers, but case patients frequently were diagnosed with asymptomatic, limited stage disease, suggesting that surveillance did, as predicted, detect earlier-stage cancers than might be expected. Patients found to have dysplasia are recommended to have examinations more frequently than patients without dysplasia. Although sample size precluded detailed evaluations of multiple dysplasia surveillance patterns, more intensive surveillance in this group was found: among the 11 fatal cases with any history of dysplasia who were in surveillance, all had multiple prior endoscopies without cancer and 8 had 1 or more negative endoscopies in the 10 months before the examination that diagnosed their cancer. Although exact biopsy counts are not available, manual evaluation of pathology reports showed that a substantial number of biopsy specimens were taken throughout multiple regions in the esophagus in case patients. Third, there likely were differences between cases and controls that impacted cancer risk and that could not be fully adjusted for by the case-control design. Cases, for example, were more likely than controls to have been diagnosed with dysplasia at some point, although analyses adjusting for dysplasia status and analyses that excluded diagnoses of high-grade dysplasia before entering the surveillance window did not influence the findings. Fourth, misclassification of examination indication could influence the results. Although there was a high level of agreement between medical record abstractors, medical record review is imperfect: if symptomatic examinations were misclassified as surveillance and if this happened preferentially among the cancer patients, this would diminish a protective association with surveillance.
The current study adds higher-quality evidence to the literature on whether surveillance markedly decreases the risk of death from esophageal adenocarcinoma.7,26,34–36 Some prior studies found that, among esophageal adenocarcinoma patients, those with a history of surveillance for Barrett's esophagus had lower-stage disease and longer survival times than did other cancer patients. In carbone et al,37 for example, reported 97 patients with esophageal adenocarcinoma. Twelve patients (12.4%) had prior Barrett's esophagus with regular surveillance, 9 (75%). of whom had early stage adenocarcinomas. In contrast, only 9 cancer patients (10.6%) not in surveillance had early stage disease.38 Similarly, our group previously reported that among 23 Barrett's esophagus patients with esophageal or gastroesophageal junction adenocarcinomas, cancers detected in patients in surveillance had lower-stage disease than patients without recent surveillance.25 The study reinforces prior reports that relatively few patients with esophageal adenocarcinoma are diagnosed precancer with Barrett's esophagus.25 Thus, if effective methods of surveillance are established, improving methods for detecting patients with Barrett's esophagus would be important for decreasing total mortality.
A strength of the current study was its use of a case-control design that attempted to mimic what would happen if a cohort of patients with known Barrett's esophagus were randomized to surveillance vs no surveillance and followed up for development of an important outcome (ie, death from cancer or its treatment). Similar to a randomized study, the group at risk, which served as the comparison group, was a sample of all patients eligible for randomization (ie, all patients diagnosed with Barrett's esophagus). For this reason, the study did not include other patients, such as the large number of esophageal adenocarcinoma patients who were not diagnosed with Barrett's esophagus before their cancer diagnosis. Such patients were not eligible for surveillance before their cancer diagnosis; similarly, they would not have been eligible for inclusion in a randomized trial at the time their Barrett's esophagus was diagnosed.
The current results, at first, may seem incongruent with some prior studies; however, the finding that a screening or surveillance program can find earlier stages of disease, identify some curable cancers, and yet not produce a substantial net mortality benefit is well recognized from large randomized screening studies for lung cancer and ovarian cancer.39–41 For esophageal adenocarcinoma, a study within a veterans affairs population found that a history of any esophagogastroduodenoscopy at least 1 year before a diagnosis of esophageal adenocarcinoma was not associated with a mortality benefit; subgroup analyses of patients with a diagnosis of Barrett's esophagus who had additional endoscopy examinations suggestive of surveillance also did not show a survival benefit, despite the detection of earlier stage disease.42 Prior esophageal adenocarcinoma studies differed in several ways from the current report, including comparing patients with Barrett's esophagus with cancer patients without known Barrett's esophagus; including patients without cancer (ie, who had only dysplasia) in the comparison group; most studies were performed at tertiary centers and therefore subject to referral bias; and almost all studies only evaluated cancer patients, which de facto excludes Barrett's esophagus patients who lack cancer or who die of competing causes of disease before their cancer became symptomatic: both of these groups may have experienced adverse effects of surveillance without a potential for benefit.7,26,34–36 Macdonald et al,43 for example, found that surveillance detected only 1 of the 5 cancers that developed during surveillance of 409 patients with Barrett's esophagus over 10 years, and concluded that most deaths were from causes other than esophageal carcinoma.
The diagnosis of early stage cancer also does not necessarily lead to cure. The majority of cases in surveillance in the current study had early stage disease, but the treatments, in some patients, were either ineffective, harmful, or declined. Such patients should be included in studies because they represent cancer deaths and are required to determine the overall effectiveness of a surveillance program.
The current results provide higher-quality data to inform discussions regarding the use of surveillance in low-risk groups, while highlighting the need to develop more accurate methods to identify persons at high risk of progression to cancer, to test cancer prevention methods in such persons, and to evaluate lower-risk methods for cancer treatment. The results also reinforce the finding that few patients with esophageal adenocarcinoma have a pre-existing diagnosis of Barrett's esophagus; thus, even if it were effective, surveillance of patients with known Barrett's esophagus alone would have only a small impact on the deaths from esophageal adenocarcinoma in the total population.
In conclusion, endoscopic surveillance of Barrett's esophagus was not associated with any substantial decrease in the risk of death from esophageal adenocarcinoma, within a large, community-based population. The results cannot exclude a small to moderate benefit or a benefit from more intensive surveillance (eg, annual); however, many patients had cancer-related deaths and some were not able to be treated despite detection of early stage disease, a finding at least partially influenced by the risks, acceptability, and effectiveness of standard existing treatments. Randomized trials are needed to evaluate whether commonly used surveillance methods, combined with follow-up treatments, provide any overall mortality benefits to patients with Barrett's esophagus. The results also suggest that alternative approaches, such as chemoprevention or ablation, warrant further evaluation to determine, in controlled studies, if they may provide other approaches to decrease cancer mortality.
Supplementary Material
Acknowledgments
Funding: Supported by US National Institutes of Health grant RO1 DK63616; the Kaiser Permanente Research Project on Genes, Environment and Health; and a Kaiser Permanente Community Benefits Grant.
Abbreviations used in this paper
- CI
confidence interval
- KPNC
Kaiser Permanente, Northern California
- OR
odds ratio
- SEER
Surveillance, Epidemiology, and End Results
Appendix 1. Cause of Death
Survival status was assigned using research-specific mortality files created by the strategic programming group at the Kaiser Permanente Division of Research. The mortality file concatenates data from the SEER program, Kaiser Permanente membership files, California state death certificates, and the Social Security Death Index. It uses probabilistic matching on data elements such as name, date of birth, and social security number to assign mortality status.
Cause of death assignment used physician medical record review; the reviewer was blinded to the surveillance status of the patient. In-hospital deaths were attributed to esophageal cancer if the patient had documented widely metastatic disease without another apparent cause of death or if, after review, the death was reported to result from a cancer-related treatment (eg, sepsis during chemotherapy). Out-of-hospital deaths were attributed to esophageal cancer using data from recent admissions and outpatient notes in the electronic medical record. For example, if a patient with advanced cancer was discharged home on hospice, a diagnosis of a cancer-related death was assigned. Patients with deaths attributable to other causes (eg, stroke, myocardial infarction, chronic obstructive pulmonary disease) or patients in whom a cause of death was unclear were assigned as non–cancerrelated deaths.
Footnotes
Supplementary Material: Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dx.doi.org/10.1053/j.gastro.2013.05.004.
Conflicts of interest: The authors disclose no conflicts.
References
- 1.Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett's esophagus. Am J Gastroenterol. 2008;103:788–797. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 2.Levine DS, Blount PL, Rudolph RE, et al. Safety of a systematic endoscopic biopsy protocol in patients with Barrett's esophagus. Am J Gastroenterol. 2000;95:1152–1157. doi: 10.1111/j.1572-0241.2000.02002.x. [DOI] [PubMed] [Google Scholar]
- 3.Schnell TG, Sontag SJ, Chejfec G, et al. Long-term nonsurgical, management of Barrett's esophagus with high-grade dysplasia. Gastroenterology. 2001;120:1607–1619. doi: 10.1053/gast.2001.25065. [DOI] [PubMed] [Google Scholar]
- 4.Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology. 2011;140:1084–1091. doi: 10.1053/j.gastro.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 5.Inadomi JM, Sampliner R, Lagergren J, et al. Screening and surveillance for Barrett esophagus in high-risk groups: a cost-utility analysis. Ann Intern Med. 2003;138:176–186. doi: 10.7326/0003-4819-138-3-200302040-00009. [DOI] [PubMed] [Google Scholar]
- 6.Sharma P, McQuaid K, Dent J, et al. A critical review of the diagnosis and management of Barrett's esophagus: the AGA Chicago Workshop. Gastroenterology. 2004;127:310–330. doi: 10.1053/j.gastro.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Garside R, Pitt M, Somerville M, et al. Surveillance of Barrett's oesophagus: exploring the uncertainty through systematic review, expert workshop and economic modelling. Health Technol Assess. 2006;10:1–142. iii–iv. doi: 10.3310/hta10080. [DOI] [PubMed] [Google Scholar]
- 8.Anderson LA, Murray LJ, Murphy SJ, et al. Mortality in Barrett's oesophagus: results from a population based study. Gut. 2003;52:1081–1084. doi: 10.1136/gut.52.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Burgh A, Dees J, Hop WC, et al. Oesophageal cancer is an uncommon cause of death in patients with Barrett's oesophagus. Gut. 1996;39:5–8. doi: 10.1136/gut.39.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubo A, Corley DA. Marked regional variation in adenocarcinomas of the esophagus and the gastric cardia in the United States. Cancer. 2002;95:2096–2102. doi: 10.1002/cncr.10940. [DOI] [PubMed] [Google Scholar]
- 11.Pera M, Cameron AJ, Trastek VF, et al. Increasing incidence of adenocarcinoma of the esophagus and esophagogastric junction. Gastroenterology. 1993;104:510–513. doi: 10.1016/0016-5085(93)90420-h. [DOI] [PubMed] [Google Scholar]
- 12.Eloubeidi MA, Mason AC, Desmond RA, et al. Temporal trends (1973-1997) in survival of patients with esophageal adenocarcinoma in the United States: a glimmer of hope? Am J Gastroenterol. 2003;98:1627–1633. doi: 10.1111/j.1572-0241.2003.07454.x. [DOI] [PubMed] [Google Scholar]
- 13.Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med. 2011;365:1375–1383. doi: 10.1056/NEJMoa1103042. [DOI] [PubMed] [Google Scholar]
- 14.Sikkema M, de Jonge PJ, Steyerberg EW, et al. Risk of esophagea adenocarcinoma and mortality in patients with Barrett's esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2010;8:235–244. doi: 10.1016/j.cgh.2009.10.010. quiz e32. [DOI] [PubMed] [Google Scholar]
- 15.Modiano N, Gerson LB. Barrett's esophagus: incidence, etiology, pathophysiology, prevention and treatment. Ther Clin Risk Manag. 2007;3:1035–1145. [PMC free article] [PubMed] [Google Scholar]
- 16.Collette HJ, Day NE, Rombach JJ, et al. Evaluation of screening for breast cancer in a non-randomised study (the DOM project) by means of a case-control study. Lancet. 1984;1:1224–1226. doi: 10.1016/s0140-6736(84)91704-5. [DOI] [PubMed] [Google Scholar]
- 17.Yang B, Morrell S, Zuo Y, et al. A case-control study of the protective benefit of cervical screening against invasive cervica cancer in NSW women. Cancer Causes Control. 2008;19:569–576. doi: 10.1007/s10552-008-9118-9. [DOI] [PubMed] [Google Scholar]
- 18.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624–1633. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 19.Selby JV, Friedman GD, Quesenberry CP, Jr, et al. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med. 1992;326:653–657. doi: 10.1056/NEJM199203053261001. [DOI] [PubMed] [Google Scholar]
- 20.Morrison AS. Screening in chronic disease. Oxford University Press; 1992. [Google Scholar]
- 21.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corley DA, Kubo A, DeBoer J, et al. Diagnosing Barrett's esophagus: reliability of clinical and pathologic diagnoses. Gastrointest Endosc. 2009;69:1004–1010. doi: 10.1016/j.gie.2008.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corley DA, Kubo A, Levin TR, et al. Abdominal obesity and body mass index as risk factors for Barrett's esophagus. Gastroenterology. 2007;133:34–41. doi: 10.1053/j.gastro.2007.04.046. quiz 311. [DOI] [PubMed] [Google Scholar]
- 24.Percy C, Van Holten V, Muir C. International classification of diseases for oncology. 2nd. Geneva: World Health Organization; 1990. [Google Scholar]
- 25.Corley DA, Levin TR, Habel LA, et al. Surveillance and survival in Barrett's adenocarcinomas: a population-based study. Gastroenterology. 2002;122:633–640. doi: 10.1053/gast.2002.31879. [DOI] [PubMed] [Google Scholar]
- 26.van Sandick JW, van Lanschot JJ, Kuiken BW, et al. Impact of endoscopic biopsy surveillance of Barrett's oesophagus on pathological stage and clinical outcome of Barrett's carcinoma. Gut. 1998;43:216–222. doi: 10.1136/gut.43.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peters JH, Clark GW, Ireland AP, et al. Outcome of adenocarcinoma arising in Barrett's esophagus in endoscopically surveyed and non-surveyed patients. J Thorac Cardiovasc Surg. 1994;108:813–821. discussion 821–822. [PubMed] [Google Scholar]
- 28.Streitz JM, Jr, Andrews CW, Jr, Ellis FH., Jr Endoscopic surveillance of Barrett's esophagus. Does it help? J Thorac Cardiovasc Surg. 1993;105:383–387. discussion 387–388. [PubMed] [Google Scholar]
- 29.Lee SL, Shekherdimian S, Chiu VY. Effect of race and socioeconomic status in the treatment of appendicitis in patients with equal health care access. Arch Surg. 2011;146:156–161. doi: 10.1001/archsurg.2010.328. [DOI] [PubMed] [Google Scholar]
- 30.Silverberg MJ, Leyden W, Quesenberry CP, Jr, et al. Race/ethnicity and risk of AIDS and death among HIV-infected patients with access to care. J Gen Intern Med. 2009;24:1065–1072. doi: 10.1007/s11606-009-1049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gudlaugsdottir S, van Blankenstein M, Dees J, et al. A majority of patients with Barrett's oesophagus are unlikely to benefit from endoscopic cancer surveillance. Eur J Gastroenterol Hepatol. 2001;13:639–645. doi: 10.1097/00042737-200106000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Hirst NG, Gordon LG, Whiteman DC, et al. Is endoscopic surveillance for non-dysplastic Barrett's esophagus cost-effective? Review of economic evaluations. J Gastroenterol Hepatol. 2011;26:247–254. doi: 10.1111/j.1440-1746.2010.06506.x. [DOI] [PubMed] [Google Scholar]
- 33.Moss SM, Cuckle H, Evans A, et al. Effect of mammographic screening from age 40 years on breast cancer mortality at 10 years' follow-up: a randomised controlled trial. Lancet. 2006;368:2053–2060. doi: 10.1016/S0140-6736(06)69834-6. [DOI] [PubMed] [Google Scholar]
- 34.Roberts KJ, Harper E, Alderson D, et al. Long-term survival and cost analysis of an annual Barrett's surveillance programme. Eur J Gastroenterol Hepatol. 2010;22:399–403. doi: 10.1097/MEG.0b013e328331fc9c. [DOI] [PubMed] [Google Scholar]
- 35.Switzer-Taylor V, Schlup M, Lubcke R, et al. Barrett's esophagus: a retrospective analysis of 13 years surveillance. J Gastroenterol Hepatol. 2008;23:1362–1367. doi: 10.1111/j.1440-1746.2008.05311.x. [DOI] [PubMed] [Google Scholar]
- 36.Aldulaimi DM, Cox M, Nwokolo CU, et al. Barrett's surveillance is worthwhile and detects curable cancers. A prospective cohort study addressing cancer incidence, treatment outcome and survival. Eur J Gastroenterol Hepatol. 2005;17:943–950. doi: 10.1097/00042737-200509000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Dellon ES, Shaheen NJ. Does screening for Barrett's esophagus and adenocarcinoma of the esophagus prolong survival? J Clin Oncol. 2005;23:4478–4482. doi: 10.1200/JCO.2005.19.059. [DOI] [PubMed] [Google Scholar]
- 38.Incarbone R, Bonavina L, Saino G, et al. Outcome of esophageal adenocarcinoma detected during endoscopic biopsy surveillance for Barrett's esophagus. Surg Endosc. 2002;16:263–266. doi: 10.1007/s00464-001-8161-3. [DOI] [PubMed] [Google Scholar]
- 39.Buys SS, Partridge E, Black A, et al. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA. 2011;305:2295–2303. doi: 10.1001/jama.2011.766. [DOI] [PubMed] [Google Scholar]
- 40.Oken MM, Hocking WG, Kvale PA, et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA. 2011;306:1865–1873. doi: 10.1001/jama.2011.1591. [DOI] [PubMed] [Google Scholar]
- 41.Infante M, Cavuto S, Lutman FR, et al. A randomized study of lung cancer screening with spiral computed tomography: three-year results from the DANTE trial. Am J Respir Crit Care Med. 2009;180:445–453. doi: 10.1164/rccm.200901-0076OC. [DOI] [PubMed] [Google Scholar]
- 42.Rubenstein JH, Sonnenberg A, Davis J, et al. Effect of a prior endoscopy on outcomes of esophageal adenocarcinoma among United States veterans. Gastrointest Endosc. 2008;68:849–855. doi: 10.1016/j.gie.2008.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macdonald CE, Wicks AC, Playford RJ. Final results from 10 year cohort of patients undergoing surveillance for Barrett's oesophagus: observational study. BMJ. 2000;321:1252–1255. doi: 10.1136/bmj.321.7271.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.