Abstract
Synapses made by local interneurons dominate the intrinsic circuitry of the mammalian visual thalamus and influence all signals traveling from the eye to cortex. Here we draw on physiological and computational analyses of receptive fields in the cat's lateral geniculate nucleus to describe how inhibition helps to enhance selectivity for stimulus features in space and time and to improve the efficiency of the neural code. Further, we explore specialized synaptic attributes of relay cells and interneurons and discuss how these might be adapted to preserve the temporal precision of retinal spike trains and thereby maximize the rate of information transmitted downstream.
Introduction
Spikes travelling from retina to the thalamus excite both relay cells, which project to cortex, and local inhibitory interneurons at the same time. Even though relay cells in the lateral geniculate nucleus outnumber interneurons three to one [1], they are only sparsely interconnected [2*]. By contrast, interneurons densely innervate relay cells and each other [3-7]. Further, relay cells send collaterals to the overlying thalamic reticular nucleus, a sheet of inhibitory cells that project to the geniculate in return [2*,8-10]. Hence all retinal information transmitted to cortex is influenced by a feedforward and a feedback inhibitory pathway [11]. Here we focus on the role of thalamic inhibition in different aspects of visual processing as well as the structure and synaptic physiology of local inhibitory circuits. For accounts of thalamic inhibition in sleep see [12] and in development [13,14].
Spatial and temporal arrangement of excitation and inhibition within the receptive field: push-pull
The receptive fields of relay cells and interneurons have the same qualitative shape, comprising two concentrically arranged subregions (a center and a surround) that have the opposite preference for luminance contrast, On or Off [15*,16*]. Further, there is a push-pull relationship (see Glossary) between stimuli of the reverse sign within each subfield [15*-17], (Figure 1a). For example, in On subregions, bright light excites whereas dark stimuli inhibit, and vice versa for Off subregions (Figure 1b, top). Moreover, simple computational models made using the spatiotemporal receptive fields of interneurons or relay cells suggest that responses of both types of neurons are roughly linear [15*].
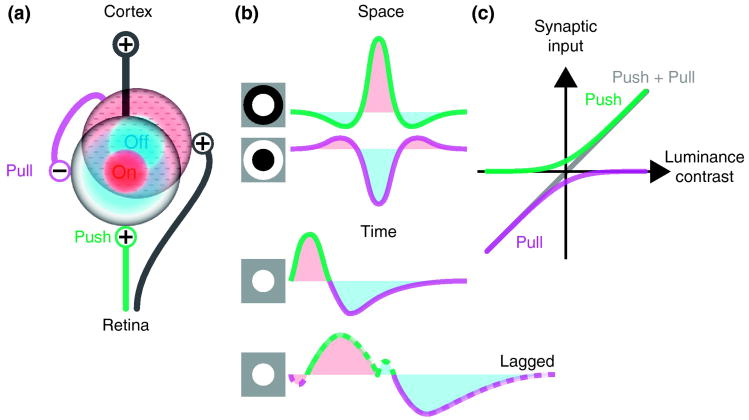
Figure 1.
Synaptic organization of spatiotemporal receptive fields in the visual thalamus. (a) Wiring diagram of connections between retinal ganglion cells and thalamic neurons. Neurons are represented by their receptive fields. An On-center (red) thalamic relay cell receives excitatory input (push, green) from an On-center retinal ganglion cell and inhibitory input (pull, purple) from an Off-center (blue) local interneuron which is driven by Off-center retinal afferents. (b) Diagrams that illustrate spatial (top) and temporal (middle) receptive fields of push and pull for a typical (On Center) relay cell and the temporal profile for a lagged (On Center) cell (bottom); the dashed lines indicate same-sign inhibition. Icons at left illustrate the stimuli that evoke the schematized patterns of push and pull. For the spatial profiles, the stimulus is presented to the center and surround. For the temporal profiles, a bright stimulus is shown in the center and then removed. (c) Illustration of how push-pull circuits extend the dynamic range of stimulus encoding.
We do not wish to imply that push and pull are mirror images of one another; they are not. The centers of the excitatory and inhibitory receptive fields are displaced by distances comparable to the spacing between presynaptic On and Off center ganglion cells [16*-18] . Also, interneurons probably have larger receptive fields than relay cells since they pool more retinal input [19,20] and may be fewer in number than ganglion cells [21].
Push-pull operates in time as well as space; excitation and inhibition are recruited sequentially when luminance contrast reverses as the image over the receptive field changes [15*-17,22], (Figure 1b, middle). There is a variation of the standard temporal pattern of push-pull; a population of “lagged” cells whose responses involve an inhibitory dip prior to excitation [23,24] (Figure 1b, bottom);lagged cells might be involved in establishing direction selectivity in cortex [25].
A universal role for push-pull in neural circuits is to extend the dynamic range of response, (Figure 1c), and to restore linearity of response following rectification through the synapse [26*]. In the geniculate, these actions are supplemented by push-pull in interneurons, which are thus able to inhibit and to disinhibit their targets [15*].
Sensitivity to visual features is enhanced by inhibition
Ideas for the value of push-pull in vision arose from studies of retina [27], but also apply to thalamus. When Kuffler [27] and Wiesel [28] mapped retinal receptive fields they found that the center and surround had an antagonistic affect on each other when illuminated together-- cells responded vigorously to discs confined to the center but poorly to a larger stimulus that spilled into the surround. This subfield antagonism reflects the influence of the pull in one subregion on the push in the other [28]. Thus, by virtue of the geometry of the receptive field and interactions between push-pull across subregions, ganglion cells become sensitive to local contrast. These features of the receptive field have been formalized mathematically as a difference of Gaussians [29]. Filtering the image through this function essentially forms the second (spatial) derivative, or Laplacian, of the image, a transformation that enhances contrast borders.
Studies using the framework of generalized linear models to analyze retinal and thalamic spike trains are consistent with a role for push-pull inhibition generated de novo in thalamus. [30*], (Figure 2a). The models suggest that excitatory stimuli that extend beyond the center into the surround recruit inhibition. This suppression can be explained by subfield antagonism mediated through the surrounds of (opposite-sign) local interneurons that supply the pull (Figure 2b) and/or input from the reticular nucleus [31]. Last, potential contributions of inhibition to various types of gain control and adaptation [32,33] have yet to be investigated.
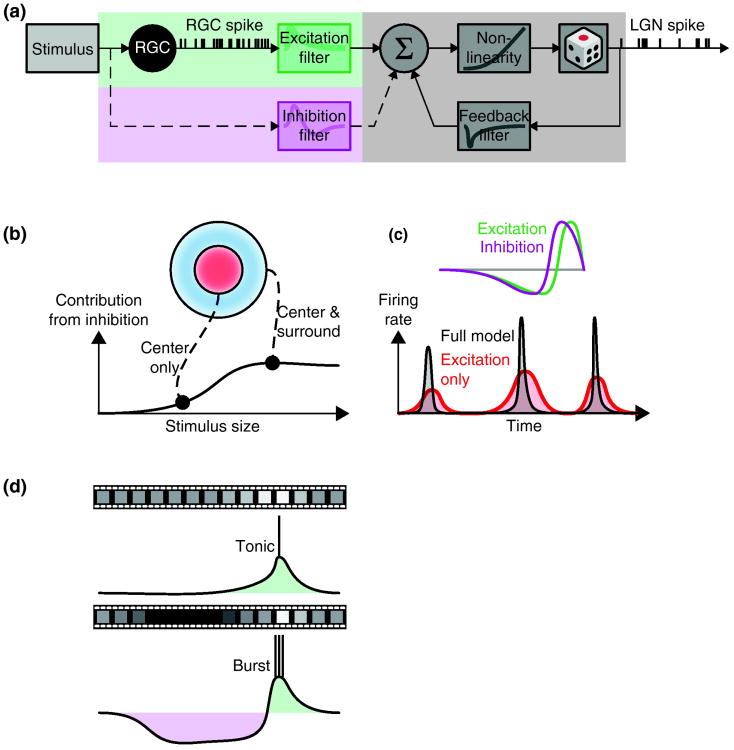
Figure 2.
How feedforward inhibition influences thalamic output. (a) Schematic of a generalized linear model used to describe retinothalamic visual processing [30*]. Here a general nonlinear model with excitatory inputs is expanded to include an inhibitory pathway (dashed line), RGC (retinal ganglion cell) LGN (lateral geniculate nucleus). (b) The inhibitory component improves the predictive power of the model when the stimulus covers both the center and surround of the receptive field [30*]. (c) A temporal delay between excitation and inhibition increases the temporal precision of thalamic spike trains [39*]. (d) Feedforward inhibition evoked by natural movies is able to drives relay cells between tonic (top) and burst (bottom) modes [16*] via a push-pull mechanism.
Inhibition contributes to efficient coding in thalamus
Barlow considered the retinal receptive field from an alternative vantage point, information theory [34]. From this perspective, processes like subfield antagonism that weaken responses to spatially uniform patterns reduce redundant information about the stimulus and improve the efficiency of the neural code. Barlow's ideas have gained increasing attention because of observations about the statistics of natural images and about differences in the amount of information encoded by single retinal versus thalamic spikes.
Mathematical analyses show that visual patterns in the environment are, themselves, highly redundant [35]. This can be described intuitively; neighboring points in an image often have similar intensities, forming large regions of homogeneous contrast and, thus, extensive spatial correlations. More formally, the structure of the visual world has 1/f statistics, which means that the power spectrum of a given natural image is skewed towards low spatial frequencies [35,36]. Since, as above, the receptive field essentially computes the second derivative of the image, higher frequencies (e.g. edges) are enhanced and redundancy in the stimulus reduced.
As a consequence of spatial correlations and smooth movement within the environment, there are also temporal correlations in natural stimuli; luminance values at any given point remain similar for long times [35]. For neurons with push-pull, changes in stimulus polarity over time elicit commensurate reversals in the sign of the synaptic response [15*,22,26*], (Figure 1b, middle). Stimuli with pronounced transitions from the nonpreferred to the preferred sign drive relay cells best [16*,22], presumably because the pull readies the membrane to fire, analogous to anode break excitation first described by Hodgkin and Huxley [37]. This selection for biphasic stimuli corresponds to forming the first (temporal) derivative of the image, thus reducing redundancy of signals with 1/f temporal structure such as natural stimuli [38].
Recent computational analyses of thalamic spike trains provide evidence for a second type of inhibition different from push-pull that is evoked by optimal stimuli but is delayed relative to excitation [39*] – delayed same-sign inhibition (see Glossary). Since the authors did not compare pre-and postsynaptic spike trains, it is not clear whether this form of suppression originates in retina [40*], thalamus or both. Like sequential push-pull, delayed same-sign-inhibition can mediate preference for the temporal derivative of the stimulus (Figure 2c). Further, delayed inhibition of any sort increases response precision by shortening the time window in which spikes can fire [39*].
A separate line of support for efficient coding in thalamus comes from analyses of simultaneous recordings from ganglion and relay cells. These experiments show that only a fraction of presynaptic impulses trigger the postsynaptic cell, such that each thalamic action potential contains more information than a retinal one [15*,41,42]. This process of down-sampling across the synapse reduces redundancy in information transmitted about slowly changing signals such as natural stimuli. In addition to inhibition [30*,39*,43] processes such as temporal summation [44-46] help increase the economy of the neural code.
Natural stimuli recruit inhibition that drives the membrane between tonic and burst firing
So far, we have only discussed synaptic contributions to sensory integration. However, inhibition also influences neural responses by interacting with membrane dynamics. Relay cells fire either tonic trains or rapid bursts of spikes. Cells fire in tonic mode when the membrane is depolarized, as during waking. Bursts are produced when the membrane voltage is low, as during sleep; they are generated by T-type calcium channels that cannot respond to fresh excitatory input unless de-inactivated by strong hyperpolarization [47]. Mounting evidence suggests that bursts play an active role in vision [47,48], not only sleep, and are predictably driven by natural stimuli [16*,49,50]. This matters because bursts preferentially excite cortex [45,51] and also because they can convey information separate from that encoded by single impulses [49,52].
Intracellular studies have illustrated how moving natural scenes drive the membrane from tonic to burst mode [16*]. Namely, spatiotemporal correlations in the visual environment lead to instances in which a stimulus of the nonpreferred sign lingers over the receptive field, evoking prolonged inhibition that de-inactivates T-channels. When the stimulus shifts to the preferred sign, retinal input from triggers a burst (Figure 2d). Reconstruction of the receptive fields for the inhibition and excitation evoked by moving scenes suggests that the transitions between firing modes rely on push-pull interactions in large part. Specifically, the receptive fields for both excitation and inhibition overlap and are circular, consistent with excitation from retina and inhibition supplied by local interneurons, e.g. (Figure 1a).
Connections of interneurons and of relay cells
The inhibitory cells in the geniculate are unusual in many regards; for example both their axons and dendrites form contacts with postsynaptic targets. The axonal synapses are made with all types of relay cells (X, Y and W) and with interneurons [3-5]. The axonal connections can, in principle, serve multiple purposes. They might contact targets with the opposite selectivity for luminance contrast to mediate push-pull. Alternatively they might link to cells with the same stimulus preference to supply same-sign inhibition, such as seen in lagged cells.
Dendrodendritic contacts allow cells to communicate even in the absence of action potentials [53*]. They often involve triads[53*], structures in which a retinal bouton synapses with the apposed dendrites of a relay cell and of an interneuron that synapses onto the relay cell in turn [53*]. Triads typically involve X cells, but dendrodendritic synapses onto interneurons or other types of relay cells are present but less frequent [7,20,54]. Further, there is evidence that collaterals of relay cells synapse in the geniculate [55,56], sometimes through triadic synapses [2*] where the terminal of a relay cell substitutes for a retinal bouton; these profiles concentrate at interlaminar zones [2*] and might promote synchrony or oscillations [2*,56].
Triads are unlikely to mediate push-pull since common input yokes inhibition to excitation, but are suited for same-sign inhibition. One might hope that comparing X with Y or W cells would give insight into the role of triads, however differences among relay cells seem inherited from retina. Also the timecourses of retinogeniculate EPSPs seem similar across relay cells in vivo [16*,44,46]. Work in vitro, however, shows that activation of a single retinal input produces an EPSP immediately followed by an IPSP in some cells [57], consistent with triadic or other mechanisms of same-sign inhibition. Thus, the purpose of triads remains unresolved, with a leading hypothesis suggesting a role in gain control [53*]. Notably the rodent somatosensory thalamus lacks interneurons [58]. Perhaps this species difference can be exploited to form new hypotheses about roles of interneurons in general and triads in particular.
Neurons in the reticular nucleus connect with each other via electrical [59] or conventional GABAergic synapses [60-62] and project selectively to relay cells in a topographic fashion [8,10]. Since reticular cells have varied preferences for the stimulus, ranging from On, On-Off to Off, their contributions to visual processing are probably diverse [10], but remain poorly understood. There is, however, compelling evidence that the reticular nucleus modulates relay cells as a function of spatial attention [63], presumably through descending cortical pathways. Whether attention regulates local interneurons, which also receive cortical feedback [64,65] and other sources of modulatory input [11], is unknown.
Patterns of synaptic inputs to relay cells and interneurons and the transmission of information
Even though relay cells and interneurons have similar receptive fields, recent evidence suggests that the they use different but complementary forms of synaptic integration to process retinal input during vision [15*]. The push in relay cells is generated by series of large, peaked EPSPs while the pull is seamlessly graded -- under standard conditions single IPSPs are not visible. On the contrary, the push in interneurons is smooth and the pull is jagged, formed by trains of unitary IPSPs (Figure 3a).
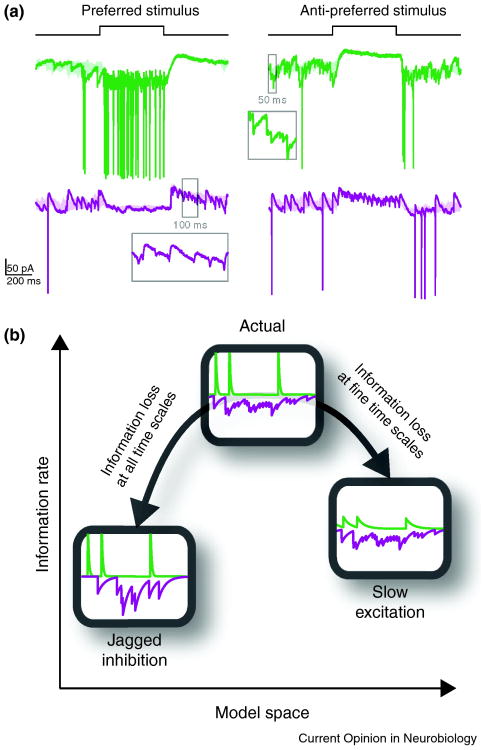
Figure 3.
Shapes of synaptic inputs and relay of information. (a) Membrane current of a relay cell (green) and an interneuron (purple) in response to preferred (left) and anti-preferred (right) stimuli in the center of the receptive field. Insets show magnified synaptic events in time windows marked on the traces. Excitatory (green) and inhibitory (purple) inputs to a relay cell have distinctive shapes: excitation is composed of fast, jagged changes in conductance whereas inhibition is smooth in time. (b) The relayed information about the stimulus is optimized with this synaptic configuration: if excitation is made slower, information at fine temporal resolution will be lost; if inhibition is made jagged, transmitted information will suffer loss at all temporal resolutions [15].
The large, sharp EPSPs originate in retina and evoke spikes from relay cells with millisecond precision [66,67]. Simulations with conductance based models of relay cells indicate that the shape of these EPSPs promote the rapid and reliable generation of thalamic spikes and thus the ability to transmit information at fine temporal resolution [15*]. The simulations further suggest that smooth inhibition does not interfere with this tight coupling between ganglion and relay cells. By contrast, replacing smooth with jagged inhibition reduces the amount of information relayed to cortex across all time scales (Figure 3b).
The graded inhibition that relay cells receive, that seems optimal for transmitting information downstream, is likely formed by several mechanisms. For example, retinal input to interneurons often targets distal, electronically remote dendrites [53*]. Studies in vitro show that retinal drive activates L-type calcium currents [68,69*] that transmit depolarization to the soma and evoke spikes at variable delay [69*]. . Also, synapses between retina and interneurons include metabotropic glutamate receptors that might contribute lasting excitation [70]. Thus, EPSPs from ganglion cells might be variously blurred by slow synaptic potentials, passive attenuation and/or intrinsic regenerative currents, thereby decoupling the timing of presynaptic (retinal) and postsynaptic (interneuronal) spikes. Remarkably, work completed over forty years ago supports this idea of jittered transmission. Cross-correlations made from spike trains of ganglion cells and putative interneurons are broader than those for relay cells [31]. Thus, convergent inhibitory inputs to a relay cell [14,71,72] could arrive asynchronously and average to form smooth pull. It is unclear how the jagged pull in interneurons is formed. One clue is that the frequency of the individual IPSPs matches retinal firing rates; it is possible that they are generated by dendrodendritic synapses between On and Off center interneurons [15*]. Why should the pull in interneurons retain the high-frequency structure of retinal spike trains; perhaps to disinhibit relay cells on the timescale of single EPSPs.
Comparison between the lateral geniculate nucleus and cortical layer 4
In cat, thalamic afferents project to simple cells in cortex. Like relay cells, simple cells have receptive fields built of segregated On and Off subregions [73] with push-pull [74,75]. Unlike the concentric arrangement in retina and thalamus, however, cortical subfields are elongated and lie side by side, a change in geometry that confers selectivity to oriented contours. The circuits that construct push-pull in relay cells and cortical simple cells share commonalities. For example, the push can be explained by feedforward drive and the pull by interneurons with receptive fields opposite in sign but similar in shape to those of their postsynaptic partners [76]. There is a salient difference between the thalamus and cortex, however. In the cat's cortex, the shapes of synaptic responses recorded from excitatory and inhibitory cells are similar, formed by graded excitation and inhibition [76-78] (see [79,80] for review of cortical inhibition in various species and modalities and [81,82] for studies of rodent cortex, which seems to lack true simple cells). While there are many subtle variations in the synaptic physiology of excitatory and inhibitory cortical neurons (e.g. [83]), the dramatic differences between the responses of relay cells and neighboring interneurons seem unique to the demands of thalamic processing.
Conclusions
This review focused on inhibition in the visual thalamus, with the aim of integrating perspectives from computation to synaptic physiology. The center-surround structure of receptive fields in the lateral geniculate, coupled with the push-pull arrangement of excitation and inhibition within subregions extends the dynamic range of response, enhances selectivity to luminance contrast in space and time and serves to reduce redundant information about the stimulus, thereby improving the efficiency of the neural code. Further, the synaptic physiology of interneurons and relay cells are highly specialized and operate in concert to increase the amount of information encoded at fine time scales and improve the overall rate of information transmitted downstream.
Highlights.
Thalamic interneurons and relay cells have receptive fields with push-pull excitation and inhibition in both the center and surround.
Push-pull increases the dynamic range of response, sharpens feature selectivity, removes redundancy and drives firing between burst and tonic modes.
Thalamic receptive fields compute the second (spatial) derivative of the image and the first (temporal) derivative
Thalamic inhibition, whether push-pull or same-sign, increases the temporal precision of response.
Relay cells and interneurons have complementary patterns of synaptic response that optimize the efficiency of neural coding.
Acknowledgments
We thank Kilian Koepsell, Luis M. Martinez, Cristina Soto Sanchez, Vishal Vaingankar and Qingbo Wang for contributions to experiments and for discussion. The work was supported by NIH Grant EY09593 to JAH and NSF Grant (IIS-0713657) to FTS.
Glossary
- Push-pull excitation and inhibition
This term refers to an arrangement, within an On or an Off subregion of the receptive field, in which stimuli of the preferred contrast elicit excitation and stimuli of the opposite contrast evoke inhibition. For example, a bright stimulus confined to an On subregion excites whereas a dark stimulus shown in the same place inhibits. If the bright stimulus in the On subregion is enlarged to cover the adjacent Off subregion as well, it recruits push and pull at the same time to produce a lesser response. This interaction between push and pull leads to a mutually antagonist relationship between subregions in the receptive field
- Same-sign excitation and inhibition
This term refers to an arrangement in which a stimulus of the optimal sign and size evokes both excitation and inhibition within a single subregion. For example, a bright stimulus shown within an On subregion evokes both excitation and inhibition. This term does not speak to the relative timing of the excitation or inhibition. In lagged cells, same-sign inhibition precedes excitation whereas it is presumably delayed relative to excitation at the triad. Same-sign inhibition differs from subfield antagonism because it arises within a single subregion
References
- 1.Fitzpatrick D, Penny GR, Schmechel DE. Glutamic acid decarboxylase-immunoreactive neurons and terminals in the lateral geniculate nucleus of the cat. Journal of Neuroscience. 1984;4:1809–1829. doi: 10.1523/JNEUROSCI.04-07-01809.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2*.Bickford ME, Wei H, Eisenback MA, Chomsung RD, Slusarczyk AS, Dankowsi AB. Synaptic organization of thalamocortical axon collaterals in the perigeniculate nucleus and dorsal lateral geniculate nucleus. Journal of Comparative Neurology. 2008;508:264–285. doi: 10.1002/cne.21671. The authors conduct ultrastructural analyses to explore connections made by putative collaterals of relay cells in the visual thalamus. In the lateral geniculate nucleus, these collaterals concentrate in the zones between the eye specific layers and synapse, in triadic structures, with both interneurons and relay cells. See [55,56] and for physiological evidence of intranuclear excitation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guillery RW. A quantitative study of synaptic interconnections in the dorsal lateral geniculate nucleus of the cat. Z Zellforsch Mikrosk Anat. 1969;96:39–48. doi: 10.1007/BF00321474. [DOI] [PubMed] [Google Scholar]
- 4.Hamos JE, Van Horn SC, Raczkowski D, Uhlrich DJ, Sherman SM. Synaptic connectivity of a local circuit neurone in lateral geniculate nucleus of the cat. Nature. 1985;317:618–621. doi: 10.1038/317618a0. [DOI] [PubMed] [Google Scholar]
- 5.Montero VM. Ultrastructural identification of synaptic terminals from the axon of type 3 interneurons in the cat lateral geniculate nucleus. Journal of Comparative Neurology. 1987;264:268–283. doi: 10.1002/cne.902640210. [DOI] [PubMed] [Google Scholar]
- 6.Montero VM. Localization of gamma-aminobutyric acid (GABA) in type 3 cells and demonstration of their source to F2 terminals in the cat lateral geniculate nucleus: a Golgi-electron-microscopic GABA-immunocytochemical study. Journal of Comparative Neurology. 1986;254:228–245. doi: 10.1002/cne.902540207. [DOI] [PubMed] [Google Scholar]
- 7.Pasik P, Pasik T, Hámori J. Synapses between interneurons in the lateral geniculate nucleus of monkeys. Experimental Brain Research. 1976;25:1–13. doi: 10.1007/BF00237322. [DOI] [PubMed] [Google Scholar]
- 8.Cucchiaro JB, Uhlrich DJ, Sherman SM. Electron-microscopic analysis of synaptic input from the perigeniculate nucleus to the A-laminae of the lateral geniculate nucleus in cats. Journal of Comparative Neurology. 1991;310:316–336. doi: 10.1002/cne.903100304. [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Bickford ME, Van Horn SC, Erisir A, Godwin DW, Sherman SM. Synaptic targets of thalamic reticular nucleus terminals in the visual thalamus of the cat. Journal of Comparative Neurology. 2001;440:321–341. doi: 10.1002/cne.1389. [DOI] [PubMed] [Google Scholar]
- 10.Sillito AM, Jones HE. The role of the thalamic reticular nucleus in visual processing. Thalamus & Related Systems. 2008;4:1–12. [Google Scholar]
- 11.Sherman SM, Guillery RW. The role of the thalamus in the flow of information to the cortex. Philosophical Transactions of the Royal Society of London B Biological Sciences. 2002;357:1695–1708. doi: 10.1098/rstb.2002.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crunelli V, Hughes SW. The slow (<1 Hz) rhythm of non-REM sleep: a dialogue between three cardinal oscillators. Nature Neuroscience. 2010;13:9–17. doi: 10.1038/nn.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hooks BM, Chen C. Critical Periods in the Visual System: Changing Views for a Model of Experience-Dependent Plasticity. Neuron. 2007;56:312–326. doi: 10.1016/j.neuron.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Bickford ME, Slusarczyk A, Dilger EK, Krahe TE, Kucuk C, Guido W. Synaptic development of the mouse dorsal lateral geniculate nucleus. The Journal of Comparative Neurology. 2010;518:622–635. doi: 10.1002/cne.22223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15*.Wang X, Vaingankar V, Sanchez CS, Sommer FT, Hirsch JA. Thalamic interneurons and relay cells use complementary synaptic mechanisms for visual processing. Nature Neuroscience. 2011;14:224–231. doi: 10.1038/nn.2707. The authors make whole-cell recording in vivo from both relay cells and interneurons and find that each type of cell processes its inputs in distinct but complementary ways. They explore the results using conductance based models and information theory to show that these different forms of synaptic integration are optimized to transmit information at fine time scales and improve the amount of information transmitted to cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Wang X, Wei Y, Vaingankar V, Wang Q, Koepsell K, Sommer FT, Hirsch JA. Feedforward excitation and inhibition evoke dual modes of firing in the cat's visual thalamus during naturalistic viewing. Neuron. 2007;55:465–478. doi: 10.1016/j.neuron.2007.06.039. The authors combine whole-cell recording in vivo from relay cells with computational modeling to illustrate how feedforward inhibition drives the membrane between burst and tonic modes during natural vision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez LM, Wang Q, Reid RC, Pillai C, Alonso JM, Sommer FT, Hirsch JA. Receptive field structure varies with layer in the primary visual cortex. Nature Neuroscience. 2005;8:372–379. doi: 10.1038/nn1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wässle H, Boycott BB. Functional architecture of the mammalian retina. Physiological Reviews. 1991;71:447–480. doi: 10.1152/physrev.1991.71.2.447. [DOI] [PubMed] [Google Scholar]
- 19.Van Horn SC, Erisir A, Sherman SM. Relative distribution of synapses in the A-laminae of the lateral geniculate nucleus of the cat. Journal of Comparative Neurology. 2000;416:509–520. [PubMed] [Google Scholar]
- 20.Datskovskaia A, Carden WB, Bickford ME. Y retinal terminals contact interneurons in the cat dorsal lateral geniculate nucleus. Journal of Comparative Neurology. 2001;430:85–100. doi: 10.1002/1096-9861(20010129)430:1<85::aid-cne1016>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 21.Peters A, Payne BR. Numerical relationships between geniculocortical afferents and pyramidal cell modules in cat primary visual cortex. Cereb Cortex. 1993;3:69–78. doi: 10.1093/cercor/3.1.69. [DOI] [PubMed] [Google Scholar]
- 22.Alitto HJ, Weyand TG, Usrey WM. Distinct properties of stimulus-evoked bursts in the lateral geniculate nucleus. Journal of Neuroscience. 2005;25:514–523. doi: 10.1523/JNEUROSCI.3369-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mastronarde DN. Two classes of single-input X-cells in cat lateral geniculate nucleus. II. Retinal inputs and the generation of receptive-field properties. Journal of Neurophysiology. 1987;57:381–413. doi: 10.1152/jn.1987.57.2.381. [DOI] [PubMed] [Google Scholar]
- 24.Mastronarde DN. Nonlagged relay cells and interneurons in the cat lateral geniculate nucleus: receptive-field properties and retinal inputs. Visual Neuroscience. 1992;8:407–441. doi: 10.1017/s0952523800004934. [DOI] [PubMed] [Google Scholar]
- 25.Saul AB, Humphrey AL. Spatial and temporal response properties of lagged and nonlagged cells in cat lateral geniculate nucleus. Journal of Neurophysiology. 1990;64:206–224. doi: 10.1152/jn.1990.64.1.206. [DOI] [PubMed] [Google Scholar]
- 26*.Werblin FS. Six different roles for crossover inhibition in the retina: Correcting the nonlinearities of synaptic transmission. Visual Neuroscience. 2010;27:1–8. doi: 10.1017/S0952523810000076. An excellent review of cross-over inhibition in retina. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuffler S. Discharge patterns and functional organization of the mammalian retina. Journal of Neurophysiology. 1953;16:37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- 28.Wiesel TN. Recording inhibition and excitation in the cat's retinal ganglion cells with intracellular electrodes. Nature. 1959;183:264–265. doi: 10.1038/183264a0. [DOI] [PubMed] [Google Scholar]
- 29.Marr D, Hildreth E. Theory of Edge Detection. Proceedings of the Royal Society of London Series B, Biological Sciences. 1980;207:187–217. doi: 10.1098/rspb.1980.0020. [DOI] [PubMed] [Google Scholar]
- 30*.Babadi B, Casti A, Xiao Y, Kaplan E, Paninski L. A generalized linear model of the impact of direct and indirect inputs to the lateral geniculate nucleus. Journal of Vision. 2010;10:22. doi: 10.1167/10.10.22. The authors use a generalized linear nonlinear model to analyze simultaneously recorded retinal and thalamic spikes trains of connected cells to separate retinal from extraretinal influences on thalamic responses to visual stimuli. They find that intrathalamic inhibition contributes to responses evoked by large stimuli that cover the center and surround. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubin MW, Cleland BG. Organization of visual inputs to interneurons of lateral geniculate nucleus of the cat. Journal of Neurophysiology. 1977;40:410–427. doi: 10.1152/jn.1977.40.2.410. [DOI] [PubMed] [Google Scholar]
- 32.Mante V, Frazor RA, Bonin V, Geisler WS, Carandini M. Independence of luminance and contrast in natural scenes and in the early visual system. Nature Neuroscience. 2005;8:1690–1697. doi: 10.1038/nn1556. [DOI] [PubMed] [Google Scholar]
- 33.Mante V, Bonin V, Carandini M. Functional mechanisms shaping lateral geniculate responses to artificial and natural stimuli. Neuron. 2008;58:625–638. doi: 10.1016/j.neuron.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Barlow HB. The coding of sensory messages. In: Thorpe WH, Zangwill OL, editors. In Current Problems in Animal Behaviour. Cambridge University Press; 1961. pp. 331–360. [Google Scholar]
- 35.Simoncelli EP, Olshausen BA. Natural image statistics and neural representation. Annual Review of Neuroscience. 2001;24:1193–1216. doi: 10.1146/annurev.neuro.24.1.1193. [DOI] [PubMed] [Google Scholar]
- 36.Geisler WS. Visual perception and the statistical properties of natural scenes. Annual Review of Psychology. 2008;59:167–192. doi: 10.1146/annurev.psych.58.110405.085632. [DOI] [PubMed] [Google Scholar]
- 37.Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. Journal of Physiology. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dan Y, Atick JJ, Reid RC. Efficient coding of natural scenes in the lateral geniculate nucleus: experimental test of a computational theory. Journal of Neuroscience. 1996;16:3351–3362. doi: 10.1523/JNEUROSCI.16-10-03351.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39*.Butts DA, Weng C, Jin J, Yeh CI, Lesica NA, Alonso JM, Stanley GB. Temporal precision in the neural code and the timescales of natural vision. Nature. 2007;449:92–95. doi: 10.1038/nature06105. The authors find that the temporal reliability of thalamic spikes evoked by stimuli such as natural movies is greater than predicted from the spatiotemporal receptive field and propose that inhibitory mechanisms account for this increased precision. [DOI] [PubMed] [Google Scholar]
- 40*.Alitto HJ, Usrey WM. Origin and dynamics of extraclassical suppression in the lateral geniculate nucleus of the macaque monkey. Neuron. 2008;57:135–146. doi: 10.1016/j.neuron.2007.11.019. The authors compare recordings from ganglion cells and relay cells and find that spatially extensive suppression from beyond the classical (antagonistic) receptive field in thalamus is fed forward from retina rather than fed back from the perigeniculate sector of the reticular nucleus, as had previously been thought. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rathbun DL, Warland DK, Usrey WM. Spike timing and information transmission at retinogeniculate synapses. Journal of Neuroscience. 2010;30:13558–13566. doi: 10.1523/JNEUROSCI.0909-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sincich LC, Horton JC, Sharpee TO. Preserving information in neural transmission. Journal of Neuroscience. 2009;29:6207–6216. doi: 10.1523/JNEUROSCI.3701-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casti A, Hayot F, Xiao Y, Kaplan E. A simple model of retina-LGN transmission. Journal of Computational Neuroscience. 2008;24:235–252. doi: 10.1007/s10827-007-0053-7. [DOI] [PubMed] [Google Scholar]
- 44.Wang X, Hirsch JA, Sommer FT. Recoding of sensory information across the retinothalamic synapse. Journal of Neuroscience. 2010;30:13567–13577. doi: 10.1523/JNEUROSCI.0910-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Usrey WM, Reppas JB, Reid RC. Paired-spike interactions and synaptic efficacy of retinal inputs to the thalamus. Nature. 1998;395:384–387. doi: 10.1038/26487. [DOI] [PubMed] [Google Scholar]
- 46.Carandini M, Horton JC, Sincich LC. Thalamic filtering of retinal spike trains by postsynaptic summation. Journal of Vision. 2007;7:20 21–11. doi: 10.1167/7.14.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sherman SM. Tonic and burst firing: dual modes of thalamocortical relay. Trends in Neurosciences. 2001;24:122–126. doi: 10.1016/s0166-2236(00)01714-8. [DOI] [PubMed] [Google Scholar]
- 48.Alitto HJ, Usrey WM, VA Casagrande RWG, Sherman SM. In Progress in Brain Research. Vol. 149. Elsevier; 2005. Dynamic properties of thalamic neurons for vision; pp. 83–90. [DOI] [PubMed] [Google Scholar]
- 49.Denning KS, Reinagel P. Visual control of burst priming in the anesthetized lateral geniculate nucleus. Journal of Neuroscience. 2005;25:3531–3538. doi: 10.1523/JNEUROSCI.4417-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lesica NA, Stanley GB. Encoding of natural scene movies by tonic and burst spikes in the lateral geniculate nucleus. Journal of Neuroscience. 2004;24:10731–10740. doi: 10.1523/JNEUROSCI.3059-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swadlow HA, Gusev AG. The impact of ‘bursting’ thalamic impulses at a neocortical synapse. Nat Neurosci. 2001;4:402–408. doi: 10.1038/86054. [DOI] [PubMed] [Google Scholar]
- 52.Lesica NA, Weng C, Jin J, Yeh CI, Alonso JM, Stanley GB. Dynamic encoding of natural luminance sequences by LGN bursts. PLoS Biology. 2006;4:e209. doi: 10.1371/journal.pbio.0040209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53*.Sherman SM. Interneurons and triadic circuitry of the thalamus. Trends in Neuroscience. 2004;27:670–675. doi: 10.1016/j.tins.2004.08.003. The most comprehensive review of triadic circuitry in thalamus. [DOI] [PubMed] [Google Scholar]
- 54.Dankowski A, Bickford ME. Inhibitory circuitry involving Y cells and Y retinal terminals in the C laminae of the cat dorsal lateral geniculate nucleus. Journal of Comparative Neurology. 2003;460:368–379. doi: 10.1002/cne.10640. [DOI] [PubMed] [Google Scholar]
- 55.Cox CL, Reichova I, Sherman SM. Functional synaptic contacts by intranuclear axon collaterals of thalamic relay neurons. Journal of Neuroscience. 2003;23:7642–7646. doi: 10.1523/JNEUROSCI.23-20-07642.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lorincz ML, Kekesi KA, Juhasz G, Crunelli V, Hughes SW. Temporal framing of thalamic relay-mode firing by phasic inhibition during the alpha rhythm. Neuron. 2009;63:683–696. doi: 10.1016/j.neuron.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blitz DM, Regehr WG. Timing and specificity of feed-forward inhibition within the LGN. Neuron. 2005;45:917–928. doi: 10.1016/j.neuron.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 58.Arcelli P, Frassoni C, Regondi MC, Biasi SD, Spreafico R. GABAergic Neurons in Mammalian Thalamus: A Marker of Thalamic Complexity? Brain Research Bulletin. 1997;42:27–37. doi: 10.1016/s0361-9230(96)00107-4. [DOI] [PubMed] [Google Scholar]
- 59.Landisman CE, Long MA, Beierlein M, Deans MR, Paul DL, Connors BW. Electrical synapses in the thalamic reticular nucleus. Journal of Neuroscience. 2002;22:1002–1009. doi: 10.1523/JNEUROSCI.22-03-01002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Montero VM, Singer W. Ultrastructure and synaptic relations of neural elements containing glutamic acid decarboxylase (GAD) in the perigeniculate nucleus of the cat. A light and electron microscopic immunocytochemical study Experimental Brain Research. 1984;56:115–125. doi: 10.1007/BF00237447. [DOI] [PubMed] [Google Scholar]
- 61.Ide LS. The fine structure of the perigeniculate nucleus in the cat. The Journal of Comparative Neurology. 1982;210:317–334. doi: 10.1002/cne.902100402. [DOI] [PubMed] [Google Scholar]
- 62.Pinault D, Smith Y, Deschenes M. Dendrodendritic and Axoaxonic Synapses in the Thalamic Reticular Nucleus of the Adult Rat. Journal of Neuroscience. 1997;17:3215–3233. doi: 10.1523/JNEUROSCI.17-09-03215.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McAlonan K, Cavanaugh J, Wurtz RH. Guarding the gateway to cortex with attention in visual thalamus. Nature. 2008 doi: 10.1038/nature07382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Govindaiah G, Cox CL. Excitatory actions of synaptically released catecholamines in the rat lateral geniculate nucleus. Neuroscience. 2006;137:671–683. doi: 10.1016/j.neuroscience.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 65.Montero VM. A quantitative study of synaptic contacts on interneurons and relay cells of the cat lateral geniculate nucleus. Experimental Brain Research. 1991;86:257–270. doi: 10.1007/BF00228950. [DOI] [PubMed] [Google Scholar]
- 66.Koepsell K, Wang X, Hirsch JA, Sommer FT. Exploring the function of neural oscillations in early sensory systems. Front Neurosci. 2010;4:53. doi: 10.3389/neuro.01.010.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koepsell K, Wang X, Vaingankar V, Wei Y, Wang Q, Rathbun DL, Usrey WM, Hirsch J, Sommer FT. Retinal oscillations carry visual information to cortex. Frontiers in Systems Neuroscience. 2009;3 doi: 10.3389/neuro.06.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dilger EK, Shin H-S, Guido W. Requirements for synaptically evoked plateau potentials in relay cells of the dorsal lateral geniculate nucleus of the mouse. The Journal of Physiology. 2011;589:919–937. doi: 10.1113/jphysiol.2010.202499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69*.Acuna-Goycolea C, Brenowitz SD, Regehr WG. Active dendritic conductances dynamically regulate GABA release from thalamic interneurons. Neuron. 2008;57:420–431. doi: 10.1016/j.neuron.2007.12.022. Using whole-cell recording and two-photon imaging of thalamic interneurons in vitro, the authors demonstrate that L-type calcium channels propagate excitatory input across the full length of the dendrite. Thus, the authors illustrate a mechanism by which retinal input to electronically remote regions of the dendritic arbor can reach the soma to evoke spikes. [DOI] [PubMed] [Google Scholar]
- 70.Govindaiah G, Cox CL. Metabotropic glutamate receptors differentially regulate GABAergic inhibition in thalamus. Journal of Neuroscience. 2006;26:13443–13453. doi: 10.1523/JNEUROSCI.3578-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crunelli V, Haby M, Jassik-Gerschenfeld D, Leresche N, Pirchio M. Cl- - and K+-dependent inhibitory postsynaptic potentials evoked by interneurones of the rat lateral geniculate nucleus. Journal of Physiology. 1988;399:153–176. doi: 10.1113/jphysiol.1988.sp017073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ziburkus J, Lo FS, Guido W. Nature of inhibitory postsynaptic activity in developing relay cells of the lateral geniculate nucleus. Journal of Neurophysiology. 2003;90:1063–1070. doi: 10.1152/jn.00178.2003. [DOI] [PubMed] [Google Scholar]
- 73.Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. Journal of Physiology. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hirsch JA, Martinez LM. Circuits that build visual cortical receptive fields. Trends in Neuroscience. 2006;29:30–39. doi: 10.1016/j.tins.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 75.Hirsch JA, Martinez LM, Pillai C, Alonso JM, Wang Q, Sommer FT. Functionally distinct inhibitory neurons at the first stage of visual cortical processing. Nature Neuroscience. 2003;6:1300–1308. doi: 10.1038/nn1152. [DOI] [PubMed] [Google Scholar]
- 76.Hirsch JA. Synaptic physiology and receptive field structure in the early visual pathway of the cat. Cerebral Cortex. 2003;13:63–69. doi: 10.1093/cercor/13.1.63. [DOI] [PubMed] [Google Scholar]
- 77.Cardin JA, Palmer LA, Contreras D. Stimulus Feature Selectivity in Excitatory and Inhibitory Neurons in Primary Visual Cortex. Journal of Neuroscience. 2007;27:10333–10344. doi: 10.1523/JNEUROSCI.1692-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nowak LG, Sanchez-Vives MV, McCormick DA. Lack of orientation and direction selectivity in a subgroup of fast-spiking inhibitory interneurons: cellular and synaptic mechanisms and comparison with other electrophysiological cell types. Cerebral Cortex. 2008;18:1058–1078. doi: 10.1093/cercor/bhm137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alitto HJ, Dan Y. Function of inhibition in visual cortical processing. Current Opinion in Neurobiology. 2010;20:340–346. doi: 10.1016/j.conb.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hirsch JA, Martinez LM. Laminar processing in the visual cortical column. Current Opinion in Neurobiology. 2006;16:377–384. doi: 10.1016/j.conb.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 81.Kerlin AM, Andermann ML, Berezovskii VK, Reid RC. Broadly tuned response properties of diverse inhibitory neuron subtypes in mouse visual cortex. Neuron. 2010;67:858–871. doi: 10.1016/j.neuron.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu BH, Li P, Sun YJ, Li YT, Zhang LI, Tao HW. Intervening inhibition underlies simple-cell receptive field structure in visual cortex. Nature Neuroscience Reviews. 13:89–96. doi: 10.1038/nn.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thomson AM, Jovanovic JN. Mechanisms underlying synapse-specific clustering of GABAA receptors. European Journal of Neuroscience. 2010;31:2193–2203. doi: 10.1111/j.1460-9568.2010.07252.x. [DOI] [PubMed] [Google Scholar]