Abstract
HIV persists in cellular and anatomical reservoirs during Highly Active Antiretroviral Therapy (HAART). In vitro studies as well as in vivo observations have identified cytokines as important factors regulating the immunological and virological mechanisms involved in HIV persistence. Immunosuppressive cytokines might contribute to the establishment of viral latency by dampening T cell activation and HIV production, thereby creating the necessary immuno-virological condition for the establishment of a pool of latently infected cells. Other cytokines that are involved in the maintenance of memory CD4+ T cells promote the persistence of these cells during HAART. Conversely, proinflammatory cytokines may favor HIV persistence by exacerbating low levels of ongoing viral replication in lymphoid tissues even after prolonged therapy. The ability of several cytokines to interfere with the molecular mechanisms responsible for HIV latency makes them attractive candidates for therapeutic strategies aimed at reducing the pool of latently infected cells. In this article, we review the role of cytokines in HIV persistence during HAART and discuss their role as potential eradicating agents.
Keywords: HIV, Viral reservoirs, Cytokines, Memory CD4+ T cells, HAART, IL-7, IL-2
1. Introduction
Cytokines play a major role during HIV pathogenesis by regulating viral replication as well as innate and adaptive immune responses [1]. Their role during chronic untreated HIV infection has been extensively studied and a large variety of cytokine dysregulations contributing to HIV disease progression have been identified during the past 30 years [2]. The development and implementation of Highly Active Antiretroviral Therapy (HAART) at the end of the 90s demonstrated that combinational therapies are extremely efficient at reducing viral replication: up to 90% of HIV-infected individuals receiving HAART display viral RNA plasma levels below the limit of detection of commercially available tests [3]. Despite this unquestioned success, effective therapy requires life-long adherence and does not eradicate HIV. There are two well-described barriers to HIV eradication: (i) HIV persists as a free virus as a result of residual levels of viral replication that may occur in anatomical reservoirs such as the gastrointestinal tract, lymph node and central nervous system. (ii) HIV persists in long-lived cellular reservoirs as a proviral DNA integrated into the host genome [4,5]. Interestingly, cytokines may play a crucial role in our failure to eradicate HIV by promoting both mechanisms of HIV persistence in HIV-infected subjects receiving suppressive HAART. In addition, cytokines may be critically involved in the establishment of a latent viral reservoir within the first weeks of HIV infection. It is important to distinguish the role of cytokines in the establishment and in the maintenance of the viral reservoir: while the inhibition of viral replication is a prerequisite for the establishment of latency, opposite effects might contribute to HIV persistence by promoting ongoing viral replication at low levels in anatomical reservoirs, even after prolonged HAART.
2. Impact of cytokines on HIV infection of resting CD4+ T cells
Two major forms of viral latency coexist in vivo [6]: preintegration latency refers to unintegrated HIV DNA that is unstable and will either degrade or will integrate into the host cell genome, usually following cell activation [7–9]. This form of latency is established after partial or complete block of the viral life cycle at steps prior to the integration of viral DNA. Postintegration latency refers to the presence of integrated HIV DNA in cells that are not actively producing viral particles. This latent state is extremely stable and is limited only by the lifespan of the infected cell and its progeny. Cytokines may modulate the mechanisms responsible for the establishment of these two forms of viral latency.
Low levels of integrated HIV DNA can be detected following in vitro infection of resting CD4+ T cells [10]. Nevertheless, resting CD4+ T cells are considered to be relatively resistant to HIV infection in vitro when compared to activated CD4+ T cells [7,11]. Interestingly, in vitro exposure of resting CD4+ T cells to common gamma-chain (γc) cytokines (IL-2, IL-4, IL-7 and IL-15) increases the susceptibility of these cells to HIV infection with minimal impact on T cell activation [12,13]. This effect is likely to result from progression to the G1b phase of cell cycle, which may promote reverse transcription [14] and integration [9]. Overall, and in sharp contrast with the well-documented roles of several chemokines [15,16], little is known about the mechanisms by which γc cytokines may establish HIV latency in resting CD4+ T cells (Fig. 1).
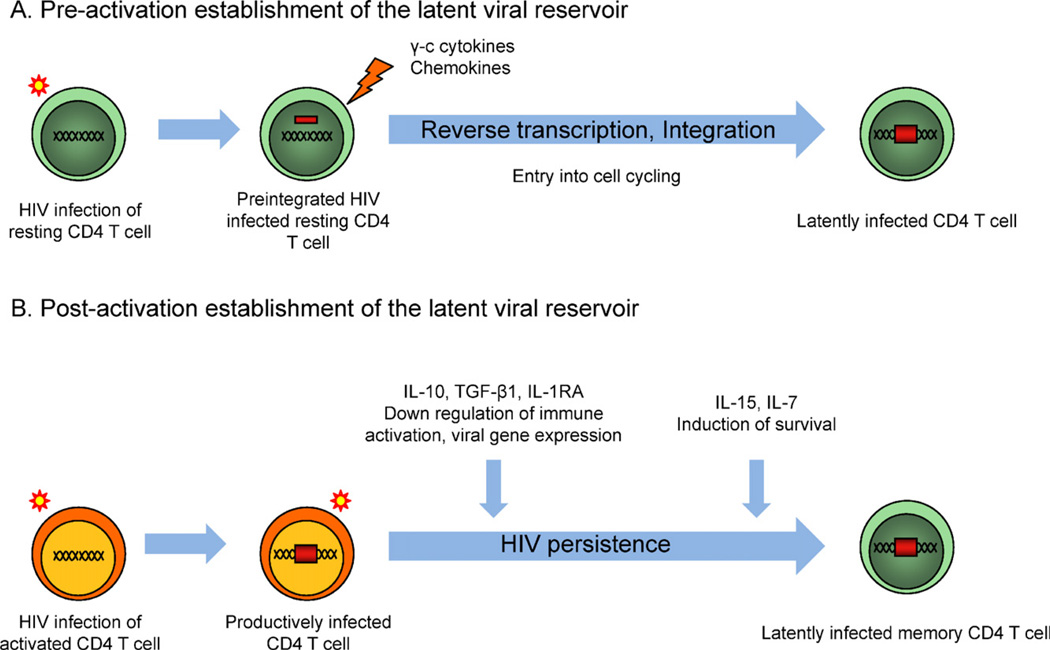
Fig. 1.
Establishment of the latent viral reservoir. (A) Pre-activation establishment of the latent viral reservoir: Resting CD4+ T cells are exposed to HIV particles in a microenvironment enriched in γc cytokines and chemokines promoting the integration of provirus in the absence of cell activation. (B) Post-activation establishment of the latent viral reservoir: immunomodulatory cytokines (IL-10, TGF-β, IL-1RA) may down regulate activation of productively infected CD4+ T cells, thereby silencing viral transcription. γc cytokines promote the survival of latently infected cells. Altogether, this cytokinic environment may favor the establishment of long-lived latently infected CD4+ T cells.
3. Role of cytokines in the establishment of the latent viral reservoir
Although the majority of viral DNA is non integrated during untreated HIV infection [17], integrated HIV DNA is readily detected in PBMCs of subjects recruited at the earliest stage of the disease [18]. The generation of a small pool of latently infected cells harbouring replication competent HIV occurs very early after HIV infection [19–22]. The precise mechanisms responsible for the establishment of this small pool of latently infected CD4+ T cells are not fully elucidated. Cytokines may contribute to this phenomenon, as the steep rise in the HIV viral load coincides with a large burst of inflammatory cytokines during acute HIV infection [2]. IFN-α and IL-15 are the first cytokines elevated within 5 days after detection of viremia, followed by TNF-α, CXCL10, and IFN-γ, and then by IL-12 [23]. Following this initial burst of proinflammatory cytokines, an anti-inflammatory response is observed with a delayed peak of IL-10 production [23,24], along with the upregulation of the IL-1 receptor antagonist (IL-1RA) [23]. The development of this anti-inflammatory response, which characterizes the transition from the acute to the chronic phase of the infection, may contribute to the generation of a reservoir of longlived latently infected cells: negative signals, notably mediated by immunosuppressive cytokines, may allow the generation of quiescent memory cells harbouring HIV integrated DNA by reducing the availability of cellular transcription factors essential for active viral gene expression, thereby establishing viral latency in long-lived memory CD4+ T cells. The key players during this immunosuppressive phase are immunomodulatory cytokines such as IL-10 and transforming growth factor-beta (TGF-β) secreted by regulatory T cells, NKT cells and myeloid cells [25]. Both cytokines have the ability to inhibit HIV transcription by reducing T cell activation but can also dampen virus-specific immunity, which may contribute to viral persistence: in the LCMV model, up regulation of IL-10 production by antigen-presenting cells is associated with impairment of T cell functions allowing viral persistence [26,27]. In addition to its immunomodulatory effect, IL-10 may directly contribute to HIV latency by inhibiting activation and viral production in infected CD4+ T cells. Although the administration of IL-10 to chronically HIV-infected subjects results in little or no impact on viral replication [28], inhibition of HIV replication by IL-10 has been clearly demonstrated in vitro [29]. Furthermore, high IL-10 plasma levels have been associated with the control of viral replication during pregnancy [30]. Altogether, these studies suggest that IL-10 may contribute to viral persistence, although the potential role of IL-10 on the establishment of the latent viral reservoir has not been formally demonstrated yet. Finally, as TGF-β also plays an important role in the suppression of activation of CD8+ T cells during acute infection [31], this other well-described immunosuppressive cytokine may also favor the establishment of the latent viral reservoir.
Additional cytokines present at high levels in the plasma of recently HIV infected subjects may contribute to the early establishment of the HIV reservoir. Among those, IL-1RA, which has been shown to inhibit IL-1-mediated HIV replication [32], may suppress viral replication during acute infection and promote the establishment of HIV latency. Finally, the γc cytokine IL-15, which is involved in the maintenance of the memory T cell pool, is also up regulated during acute HIV infection and may play a role in the establishment of the latent reservoir. If a possible role of IL-15 in that process still needs to be determined, IL-7, another γc cytokine, has been used to establish HIV latency in vitro [33]. Since both cytokines are known to promote the generation and maintenance of memory T cells through the up-regulation of Bcl-2 expression [34], they may also counteract the pro-apoptotic signals characteristic of infected CD4+ T cells and promote the establishment and maintenance of long-lived latently infected cells. Of note, the role of Bcl-2 in HIV latency is the cornerstone of a recently developed in vitro model of HIV latency [35], suggesting that IL-7 and IL-15 may contribute to the persistence of HIV-infected cells through the upregulation of pro-survival pathways.
4. Role of cytokines in the maintenance of latently infected CD4+ T cells during HAART
The timing of HAART initiation has a critical impact on the extent of CD4+ T cell reconstitution and on the size of the latent reservoir: individuals who start HAART at an early stage of HIV infection, when CD4+ T cell depletion is limited, usually display preserved immune function and harbour a latent reservoir of limited magnitude [36]. Conversely, HIV infected subjects who initiate HAART at a later stage of the disease often display incomplete restoration of the CD4 compartment despite viral suppression, higher levels of immune activation and a higher frequency of latently infected cells after prolonged HAART [37,38]. Up to 30% of HIV-infected subjects receiving HAART do not recover normal CD4 counts even after prolonged treatment [39]. This persistent lymphopenia is accompanied by a variety of immune alterations that likely contribute to HIV persistence in spite of undetectable viremia. In particular, high levels of IL-7, a cytokine that plays an important role in the homeostasis and survival of CD4+ T cells [40,41] have been measured in the plasma of immunological non-responders [37]. The impact of IL-7 on HIV persistence during HAART is still unclear. In vitro studies have shown that IL-7 may induce HIV replication in PBMC from virally suppressed subjects [42] and in thymocytes from a SCID-hu mouse model of HIV persistence [43], suggesting that IL-7 may disrupt viral latency during HAART. However, using an in vitro model of viral latency in central memory cells, Bosque et al. recently demonstrated that IL-7 induces homeostatic proliferation of latently infected cells in the absence of viral reactivation or cell differentiation [44]. This observation is consistent with our previous study in which we reported that the proliferation of CD4+ T cells isolated from virally suppressed subjects in response to IL-7 stimulation led to the maintenance of the HIV latent reservoir in its size and genetic diversity [36]. Altogether, these observations suggest that IL-7 may have a dual role on HIV persistence by promoting both viral production and proliferation of latently infected cells. Studies aimed at evaluating the immunological and virological impacts of IL-7 on distinct cellular reservoirs (central and transitional memory CD4+ T cells) are warranted.
5. Role of cytokines in residual viral replication during HAART
Residual levels of viral replication during HAART constitute another potential mechanism for HIV persistence. Incomplete restoration of the CD4 compartment as well as persistent levels of immune activation are suspected to promote viral production during HAART [38,45–50]. The origin of these residual levels of immune activation has not been clearly identified yet, but may result from the continuous translocation of microbial products from the gut, even after prolonged therapy [45,51]. Persistent microbial translocation during HAART may result from incomplete restoration of Th17 CD4+ T cells [52], which are directly targeted and depleted by HIV [53], thereby leading to insufficient productions of IL-17 and IL-22, two cytokines involved in mucosal defences of the lung and intestines [54,55]. Taken together, these studies suggest an attractive model in which IL-17 and IL-22 produced by Th17 cells may play a major role by maintaining the integrity of the intestinal barrier, thereby limiting microbial translocation, immune activation and viral persistence. However, in virally suppressed subjects achieving effective CD4+ T cells restoration associated with enhanced Th17 cells accumulation, persistent CD4+ T cell proviral burden and residual immune activation in the gut are still observed [56], strongly suggesting that other mechanisms are at play.
CD4+ T cells residing in lymphoid tissues are naturally exposed to proinflammatory cytokines and this phenomenon is amplified during HIV infection. For instance, exposure of intestinal cells to HIV particles induces the expression of the proinflammatory cytokines IL-6 and TNF-α [57]. In line with this observation, HIV infected subjects displaying high levels of viral replication in the mucosa are characterized by increased levels of IL-6, TNF-α, IFN-γ and IL-12 at this site [58]. Abnormally high levels of several cytokines associated with HIV disease progression during untreated HIV infection may contribute to HIV persistence during HAART by promoting residual levels of viral production. For instance, IL-6 and TNF-α have been independently shown to reactivate HIV replication in several in vitro models of viral latency [59,60]. More importantly, the combination of the proinflammatory cytokines TNF-α, IL-2 and IL-6 induces viral reactivation in resting CD4+ T cells isolated from virally suppressed subjects [61], suggesting that these cytokines may contribute to the residual levels of viral production observed in virally suppressed subjects. In addition, successful HAART is associated with persistent CD8+ T cell activation along with IL-12 production [62], a cytokine that enhances viral replication in mitogen stimulated PBMCs [63,64].
IL-18, another proinflammatory cytokine that is present at high levels in the serum of chronically infected individuals [65], may also contribute to HIV persistence during HAART. IL-18 is secreted mainly by monocytes/macrophages and plays an important role in innate and adaptive immune response against viruses and intracellular pathogens [66]. IL-18 enhances HIV replication in monocytic and T cell lines infected with HIV [67,68] as well as in human CD4+ T cells [69]. Viral suppression following HAART initiation is accompanied by a marked decline in the levels of IL-18, while virological treatment failure is associated with persistently increased levels of IL-18 during therapy [70], suggesting that this cytokine may promote viral replication during HAART.
IFN-γ, which is produced by NK cells and T cells in response to a combination of cytokines such as IL-12 and IL-18, is detected at high levels in subjects displaying high levels of viral replication in the mucosa [58]. Interestingly, IFN-γ levels increase in some subjects and remain high after HAART initiation [71]. Despite its well-characterized antiviral activity, IFN-γ can indirectly favour HIV replication: as a potent pro-differentiation agent, IFN-γ promotes the differentiation of CD4+ T cells, thereby generating more targets for HIV infection, which may in turn enhance viral replication in lymphoid tissues.
Altogether these results suggest that the continuous production of proinflammatory cytokines in lymphoid tissues creates an environment that may favour residual levels of viral replication even after prolonged HAART (Fig. 2).
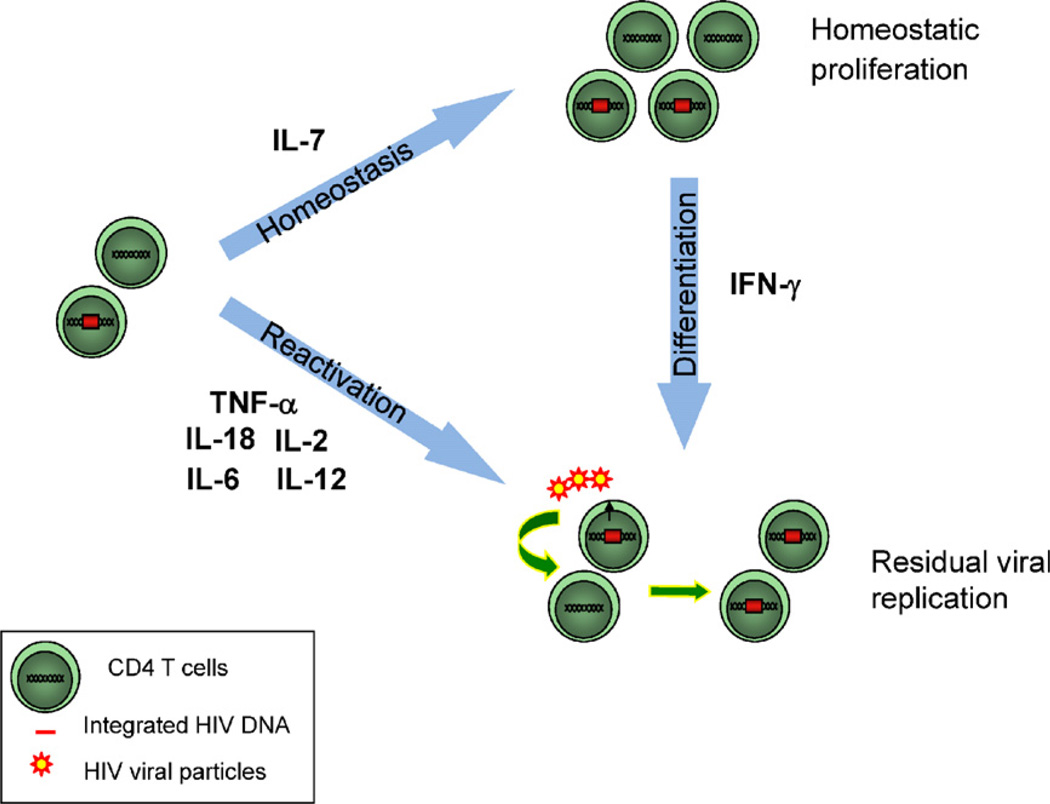
Fig. 2.
Role of cytokines in HIV persistence. IL-7 plays an important role in the homeostasis of CD4+ T cells and may ensure the long-term persistence of latently infected cells through homeostatic proliferation during HAART. Several cytokines expressed at high levels in the plasma and lymphoid tissues of HIV infected subjects such as TNF-α, IL-2, IL-12 and IL-18 may induce HIV reactivation from latently infected cells and sustain ongoing viral replication during HAART. As a potent pro-differentiation agent, IFN-γ may induce viral production in latently infected cells concomitant to cell-differentiation. In addition, by generating more targets for HIV infection, IFN-γ may in turn enhance viral replication.
6. Cytokines and HIV eradication
Cytokines have been proposed as potential therapeutic agents to eradicate HIV for almost 15 years [61]. The capacity of several cytokines to simultaneously regulate HIV replication and restore immune functions makes them attractive candidate to be used in eradication strategy. A limited number of studies conducted in non-human primates and humans have evaluated the impact of cytokine administration on HIV persistence during HAART.
Whereas the first studies investigating the administration of IL-2 in HIV infected patients focused on the immunological consequences of this intervention, Chun et al. determined the impact of IL-2 therapy on the size of the pool of resting CD4+ T cells harbouring replication competent virus [72]. IL-2 was administered intravenously or subcutaneously to 12 subjects receiving suppressive HAART in doses of 3 to 18 million units per day for 5 days followed by a rest period of at least 8 weeks. Results from this non-randomized study indicated that using the standard micro-culture assay, replication competent virus was isolated from the resting CD4+ T cells from all 12 patients who were receiving HAART alone, whereas replication competent virus was not detected in 6 out of 12 patients who received HAART plus IL-2. Using a larger number of CD4+ T cells, replication competent virus was still undetectable in 3 of these subjects. Importantly, a similar analysis conducted with lymph nodes obtained from 2 of these patients revealed that replication competent could not be isolated from this compartment either. These provocative findings suggested that the intermittent administration of IL-2 with continuous HAART may lead to a substantial reduction in the pool of resting CD4+ T cells harbouring replication competent HIV. Disappointingly, HAART interruption in these subjects led to a rapid rebound in HIV viremia [73], indicating that HIV was not eradicated, even though replication competent virus could not be isolated before HAART withdrawal by using extremely sensitive assays. These results were in line with those from larger studies concluding that intermittent IL-2 administration during HAART does not prevent viral rebound upon treatment discontinuation [74] and does not translate into clinical benefits [75].
The possibility that IL-2 used in combination with the anti-CD3 antibody OKT3 could deplete the latent HIV reservoir during HAART was examined in small study enrolling 3 subjects [76,77]. The administration of OKT3 and IL-2 induced a strong and transient increase in the plasma levels of IL-6, TNF-α and IFN-γ. The percentage of blood CD4+ and CD8+ T cells increased to almost 100% and the frequencies of cells expressing the proliferation marker Ki67 increased 10-fold in lymph nodes. Although an increase in plasma HIV viremia was observed in one of the study subjects upon treatment, this extremely potent T-cell stimulation method failed to reduce viral reservoirs and depleted the pool of CD4+ T cells, thereby disrupting T-cell homeostasis [77,78]. Even when intensification therapy with didanosine and hydroxyurea was added to baseline suppressive HAART before treatment with OKT3 and IL-2, all patients developed plasma virus rebound after treatment interruption [79].
In a pilot study combining HAART and cytokine therapy during early HIV infection, the co-administration of IL-2 and IFN-γ resulted in a reduction on the proviral load and contributed to immune reconstitution [80]. Nevertheless, this aggressive treatment was not able to eradicate HIV as demonstrated by evidences of low levels of viral replication in the lymph nodes of the subjects and a rapid viral rebound in those who interrupted HAART.
Altogether, these studies indicate that IL-2 used either alone or in combination with an anti CD3 antibody or with IFN-γ failed at eradicating HIV. There are several potential reasons for these findings. First, it is possible that such therapies did not reactivate all latently infected cells. Second, the HAART regimen may not be potent enough to completely inhibit ongoing viral replication, which should be a pre-requisite to any therapeutic intervention aimed at eradicating HIV through a reactivation strategy. In addition, potential HIV sanctuary sites such as the central nervous system might not have been affected by these interventions. Finally it is now well described that IL-2 does not necessarily promote antiviral immune responses: the expansion of regulatory T (Treg) cells during IL-2 therapy might indeed compromise the elimination of HIV infected cells by inhibiting the cytotoxic capacity of CD8+ T cells [81].
IL-7, another member of the γc cytokine family, constitutes an alternate and attractive candidate to achieve HIV eradication. Using the SCID-hu model, Scripture Adams et al. demonstrated that IL-7 induces substantial expression of latent HIV while having minimal effects on the cell phenotype [43]. While their study directly pertained to activation of latent virus in the naive quiescent cell subset, their observations suggested that latent virus in memory CD4+ T cells would behave similarly. Using resting CD4+ T cells isolated from virally suppressed subjects, Wang et al. confirmed these findings and found that IL-7 was significantly more effective at enhancing HIV proviral reactivation in CD8-depleted PBMCs than either IL-2 alone, or IL-2 combined with PHA [42]. Importantly, the phylogenetic analyses of the viral particles released after stimulation indicated that IL-7 induces distinct proviral quasispecies than those recovered upon treatment with phytohemagglutinin and IL-2. Two clinical trials aimed at evaluating the safety and efficacy of IL-7 administration in HIV infected individuals brought more insight into the role of IL-7 in HIV persistence [82,83]. Both studies reported that IL-7 increases the number of circulating CD4+ and CD8+ T cells, predominantly of central memory phenotype. Interestingly, both studies also reported the occurrence of viral blips in virally suppressed subjects, particularly in those who received the highest doses of IL-7 (more than 10 µg/kg). This observation suggested that IL-7 can induce the production from latently infected cells, although the possibility that IL-7 administration enhances viral production from productively infected cells persisting in cryptic reservoirs could not be excluded. Indeed, a recent study by Imamichi et al. aimed at identifying the sources of HIV detected during transient viremic episodes following IL-7 administration demonstrated that the HIV sequences detected during these viral blips were closely related to those present before and after cytokine administration [84]. This suggested that the low level viremia induced by IL-7 likely reflects predominantly transient induction of virus from a preexisting pool of productively infected cells rather than activation of silent quasispecies.
In a recent report, Levy et al. observed that the administration of IL-7 to virally suppressed subjects results in modest increases in total levels of intracellular HIV DNA, proportional to CD4+ T cell expansions [85]. In line with these observations, Bosque et al. recently demonstrated using an in vitro model of HIV latency that IL-7-induced homeostatic proliferation of central memory latently infected CD4+ T cells fails to efficiently reactivate HIV production [44]. These results suggest that IL-7 may expand or at least maintain the pool of latently infected cells during HAART. Indeed, we previously reported that incomplete T-cell recovery and elevated IL-7 levels are associated with increased levels of T-cell proliferation, and with stability of the HIV reservoir in its size and genetic diversity [36]. These findings suggest that T cell division of latently infected cells without viral production is likely to occur in virally suppressed subjects receiving IL-7 therapy and challenge the use of IL-7 as an eradication agent.
7. Modulation of antiviral immune response during HAART
It is becoming more and more evident that an efficient eradication strategy should not only rely on the reactivation of HIV production in latently infected cells but should also promote the elimination of these cells. Past studies have mostly focused on the capacity of cytokines to reactivate HIV production from latent reservoirs but the ability of such therapies to enhance the capacity of the immune system to eliminate infected cells during HAART has often been neglected. In a recent study, Shan et al. reported that CD8+ T cells from virally suppressed subjects are generally inefficient at eliminating latently infected cells after viral reactivation [86]. This important finding suggests that the capacity of the immune system to eliminate infected CD4+ T cells should be taken into account when evaluating the efficacy of an eradication strategy. In that context, the usage of IL-2 as a purging agent is questionable, as it has been demonstrated that IL-2 expands Treg cells, which might inhibit the killing capacity of HIV-specific CD8+ T cells and impede viral clearance [81]. IL-15 preferentially induces the proliferation of CD8+ T cells rather than Treg cells and, in contrast to IL-2, is not expected to induce increased tolerance. Moreover, IL-15 has stronger effects than IL-2 on the activity of NK cells and cytotoxic T lymphocytes [87], making it a promising candidate for immunotherapies aimed at enhancing the capacity of the immune system to eliminate infected cells during HAART.
8. Conclusion
In addition to their role in the seeding of the latent reservoir during the earliest stages of HIV infection, cytokines can promote long-term viral persistence during HAART by exacerbating ongoing HIV replication and by maintaining a small pool of latently infected cells. The complexity of the mechanisms of viral persistence and the heterogeneity of the cellular subsets in which HIV persists reinforce the need for more comprehensive and detailed studies aimed at determining the effect of cytokines on viral production and HIV latency at the cell-population level.
Because HIV persists through a variety of mechanisms, an eradication strategy relying on a single approach is unlikely to be successful. For instance, the use of proinflammatory cytokines to disrupt viral latency may have detrimental effect by promoting residual levels of viral replication in cryptic anatomical reservoirs. In addition to these potential opposite effects on viral persistence, the impact of cytokine-based therapies on the quality of the CD8+ T cell response needs to be evaluated. The recently developed models of viral suppression in non-human primates [88–90] constitute a unique tool to evaluate the efficacy of such curative strategies as they allow to simultaneously assess the virological and immunological perturbations induced by the administration of cytokine in vivo. These animal models will be useful to identify the reasons underlying the failure of past attempts, and will help in the design of more successful cytokine-based eradication strategies.
Biographies

Dr. Claire Vandergeeten is a postdoctoral researcher at VGTI-Florida. She obtained her PhD in biochemistry at University of Liège (Belgium) in 2009 where she studied the effect of HDAC inhibitors on HIV-1 reactivation in vitro and in vivo in a humanized mouse model. At VGTI-Florida, she is currently studying the differential impact of IL-7 and IL-15 on HIV-1 persistence during HAART.

Dr. RIémi Fromentin is a postdoctoral researcher at VGTI-Florida. He obtained his PhD in microbiology-immunology at University Laval (Canada) in 2010 where he studied both HCV morphogenesis and HIV interactions with liver cells. He joined VGTI-Florida to develop a novel assay aimed at quantifying and analyzing HIV latency in biological samples from virally suppressed subjects. He is also evaluating the potential impact of HIV immunization on viral latency.

Dr. Nicolas Chomont is an assistant member at VGTI-Florida. He obtained his PhD in medical virology at University Paris VI (France) in 2004 where he extensively studied the interactions between HIV and the genital mucosa. In 2009, he described for the first time distinct immunological mechanisms that contribute to the persistence of latently infected cells in HIV-infected subjects receiving suppressive HAART. At VGTI Florida, Dr. Chomont is studying the molecular mechanisms involved in HIV latency to develop novel therapeutic strategies aimed at reducing the size of the HIV reservoir.
References
- 1.Clerici M. Beyond IL-17: new cytokines in the pathogenesis of HIV infection. Current Opinion in HIV and AIDS. 2010;5:184–188. doi: 10.1097/COH.0b013e328335c23c. [DOI] [PubMed] [Google Scholar]
- 2.Katsikis PD, Mueller YM, Villinger F. The cytokine network of acute HIV infection: a promising target for vaccines and therapy to reduce viral setpoint? PLoS Pathogens. 2011;7:e1002055. doi: 10.1371/journal.ppat.1002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carr A, Amin J. Efficacy and tolerability of initial antiretroviral therapy: a systematic review. AIDS. 2009;23:343–353. doi: 10.1097/QAD.0b013e32831db232. discussion 355–356. [DOI] [PubMed] [Google Scholar]
- 4.Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- 5.Trono D, Van Lint C, Rouzioux C, Verdin E, Barre-Sinoussi F, Chun TW, et al. HIV persistence and the prospect of long-term drug-free remissions for HIV-infected individuals. Science. 2010;329:174–180. doi: 10.1126/science.1191047. [DOI] [PubMed] [Google Scholar]
- 6.Colin L, Van Lint C. Molecular control of HIV-1 postintegration latency: implications for the development of new therapeutic strategies. Retrovirology. 2009;6:111. doi: 10.1186/1742-4690-6-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevenson M, Stanwick TL, Dempsey MP, Lamonica CA. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO Journal. 1990;9:1551–1560. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zack JA, Arrigo SJ, Weitsman SR, Go AS, Haislip A, Chen IS. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Y, Zhang H, Siliciano JD, Siliciano RF. Kinetics of human immunodeficiency virus type 1 decay following entry into resting CD4+ T cells. Journal of Virology. 2005;79:2199–2210. doi: 10.1128/JVI.79.4.2199-2210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai J, Agosto LM, Baytop C, Yu JJ, Pace MJ, Liszewski MK, et al. Human immunodeficiency virus integrates directly into naive resting CD4+ T cells but enters naive cells less efficiently than memory cells. Journal of Virology. 2009;83:4528–4537. doi: 10.1128/JVI.01910-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swiggard WJ, Baytop C, Yu JJ, Dai J, Li C, Schretzenmair R, et al. Human immunodeficiency virus type 1 can establish latent infection in resting CD4+ T cells in the absence of activating stimuli. Journal of Virology. 2005;79:14179–14188. doi: 10.1128/JVI.79.22.14179-14188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Unutmaz D, KewalRamani VN, Marmon S, Littman DR. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. The Journal of Experimental Medicine. 1999;189:1735–1746. doi: 10.1084/jem.189.11.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ducrey-Rundquist O, Guyader M, Trono D. Modalities of interleukin-7-induced human immunodeficiency virus permissiveness in quiescent T lymphocytes. Journal of Virology. 2002;76:9103–9111. doi: 10.1128/JVI.76.18.9103-9111.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korin YD, Zack JA. Progression to the G1b phase of the cell cycle is required for completion of human immunodeficiency virus type 1 reverse transcription in T cells. Journal of Virology. 1998;72:3161–3168. doi: 10.1128/jvi.72.4.3161-3168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saleh S, Solomon A, Wightman F, Xhilaga M, Cameron PU, Lewin SR. CCR7 ligands CCL19 and CCL21 increase permissiveness of resting memory CD4+ T cells to HIV-1 infection: a novel model of HIV-1 latency. Blood. 2007;110:4161–4164. doi: 10.1182/blood-2007-06-097907. [DOI] [PubMed] [Google Scholar]
- 16.Cameron PU, Saleh S, Sallmann G, Solomon A, Wightman F, Evans VA, et al. Establishment of HIV-1 latency in resting CD4+ T cells depends on chemokine-induced changes in the actin cytoskeleton. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16934–16939. doi: 10.1073/pnas.1002894107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koelsch KK, Liu L, Haubrich R, May S, Havlir D, Gunthard HF, et al. Dynamics of total, linear nonintegrated, and integrated HIV-1 DNA in vivo and in vitro. The Journal of Infectious Diseases. 2008;197:411–419. doi: 10.1086/525283. [DOI] [PubMed] [Google Scholar]
- 18.Ananworanich J, Schuetz A, Vandergeeten C, Sereti I, de Souza M, Rerknimitr R, et al. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PloS One. 2012;7:e33948. doi: 10.1371/journal.pone.0033948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chun TW, Engel D, Berrey MM, Shea T, Corey L, Fauci AS. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nature Medicine. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 21.Blankson JN, Siliciano JD, Siliciano RF. The effect of early treatment on the latent reservoir of HIV-1. The Journal of Infectious Diseases. 2005;191:1394–1396. doi: 10.1086/428783. [DOI] [PubMed] [Google Scholar]
- 22.Strain MC, Little SJ, Daar ES, Havlir DV, Gunthard HF, Lam RY, et al. Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. The Journal of Infectious Diseases. 2005;191:1410–1418. doi: 10.1086/428777. [DOI] [PubMed] [Google Scholar]
- 23.Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. Journal of Virology. 2009;83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nilsson J, Kinloch-de-Loes S, Granath A, Sonnerborg A, Goh LE, Andersson J. Early immune activation in gut-associated and peripheral lymphoid tissue during acute HIV infection. AIDS. 2007;21:565–574. doi: 10.1097/QAD.0b013e3280117204. [DOI] [PubMed] [Google Scholar]
- 25.Brockman MA, Kwon DS, Tighe DP, Pavlik DF, Rosato PC, Sela J, et al. IL-10 is upregulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood. 2009;114:346–356. doi: 10.1182/blood-2008-12-191296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nature Medicine. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, et al. Resolution of a chronic viral infection after interleukin-10 receptor blockade. Journal of Experimental Medicine. 2006;203:2461–2472. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Angel JB, Jacobson MA, Skolnik PR, Giordano M, Shapiro L, LeBeaut A, et al. A multicenter, randomized, double-blind, placebo-controlled trial of recombinant human interleukin-10 in HIV-infected subjects. AIDS. 2000;14:2503–2508. doi: 10.1097/00002030-200011100-00012. [DOI] [PubMed] [Google Scholar]
- 29.Arias JF, Nishihara R, Bala M, Ikuta K. High systemic levels of interleukin-10, interleukin-22 and C-reactive protein in Indian patients are associated with low in vitro replication of HIV-1 subtype C viruses. Retrovirology. 2010;7:15. doi: 10.1186/1742-4690-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bento CA, Hygino J, Andrade RM, Saramago CS, Silva RG, Silva AA, et al. IL-10-secreting T cells from HIV-infected pregnant women downregulate HIV-1 replication: effect enhanced by antiretroviral treatment. AIDS. 2009;23:9–18. doi: 10.1097/QAD.0b013e328317461e. [DOI] [PubMed] [Google Scholar]
- 31.Petitjean G, Chevalier MF, Tibaoui F, Didier C, Manea ME, Liovat AS, et al. Level of double negative T cells, which produce TGF-beta and IL-10, predicts CD8 T-cell activation in primary HIV-1 infection. AIDS. 2012;26:139–148. doi: 10.1097/QAD.0b013e32834e1484. [DOI] [PubMed] [Google Scholar]
- 32.Poli G, Kinter AL, Fauci AS. Interleukin 1 induces expression of the human immunodeficiency virus alone and in synergy with interleukin 6 in chronically infected U1 cells: inhibition of inductive effects by the interleukin 1 receptor antagonist. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:108–112. doi: 10.1073/pnas.91.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marini A, Harper JM, Romerio F. An in vitro system to model the establishment and reactivation of HIV-1 latency. Journal of Immunology. 2008;181:7713–7720. doi: 10.4049/jimmunol.181.11.7713. [DOI] [PubMed] [Google Scholar]
- 34.Marrack P, Kappler J. Control of T cell viability. Annual Review of Immunology. 2004;22:765–787. doi: 10.1146/annurev.immunol.22.012703.104554. [DOI] [PubMed] [Google Scholar]
- 35.Yang HC, Xing S, Shan L, O’Connell K, Dinoso J, Shen A, et al. Small-molecule screening using a human primary cell model of HIV latency identifies compounds that reverse latency without cellular activation. Journal of Clinical Investigation. 2009;119:3473–3486. doi: 10.1172/JCI39199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nature Medicine. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marchetti G, Gori A, Casabianca A, Magnani M, Franzetti F, Clerici M, et al. Comparative analysis of T-cell turnover and homeostatic parameters in HIV-infected patients with discordant immune-virological responses to HAART. AIDS. 2006;20:1727–1736. doi: 10.1097/01.aids.0000242819.72839.db. [DOI] [PubMed] [Google Scholar]
- 38.Mavigner M, Delobel P, Cazabat M, Dubois M, L’Faqihi-Olive FE, Raymond S, et al. HIV-1 residual viremia correlates with persistent T-cell activation in poor immunological responders to combination antiretroviral therapy. PLoS One. 2009;4:e7658. doi: 10.1371/journal.pone.0007658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelley CF, Kitchen CM, Hunt PW, Rodriguez B, Hecht FM, Kitahata M, et al. Incomplete peripheral CD4+ cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clinical Infectious Diseases An Official Publication of the Infectious Diseases Society of America. 2009;48:787–794. doi: 10.1086/597093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nature Immunology. 2003;4:680–686. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 42.Wang FX, Xu Y, Sullivan J, Souder E, Argyris EG, Acheampong EA, et al. IL-7 is a potent and proviral strain-specific inducer of latent HIV-1 cellular reservoirs of infected individuals on virally suppressive HAART. Journal of Clinical Investigation. 2005;115:128–137. doi: 10.1172/JCI22574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scripture-Adams DD, Brooks DG, Korin YD, Zack JA. Interleukin-7 induces expression of latent human immunodeficiency virus type 1 with minimal effects on T-cell phenotype. Journal of Virology. 2002;76:13077–13082. doi: 10.1128/JVI.76.24.13077-13082.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bosque A, Famiglietti M, Weyrich AS, Goulston C, Planelles V. Homeostatic proliferation fails to efficiently reactivate HIV-1 latently infected central memory CD4+ T cells. PLoS Pathogens. 2011;7:e1002288. doi: 10.1371/journal.ppat.1002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marchetti G, Bellistri GM, Borghi E, Tincati C, Ferramosca S, La Francesca M, et al. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS. 2008;22:2035–2038. doi: 10.1097/QAD.0b013e3283112d29. [DOI] [PubMed] [Google Scholar]
- 46.Anthony KB, Yoder C, Metcalf JA, DerSimonian R, Orenstein JM, Stevens RA, et al. Incomplete CD4 T cell recovery in HIV-1 infection after 12 months of highly active antiretroviral therapy is associated with ongoing increased CD4 T cell activation and turnover. Journal of Acquired Immune Deficiency Syndromes. 2003;33:125–133. doi: 10.1097/00126334-200306010-00002. [DOI] [PubMed] [Google Scholar]
- 47.Zhang L, Ramratnam B, Tenner-Racz K, He Y, Vesanen M, Lewin S, et al. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. New England Journal of Medicine. 1999;340:1605–1613. doi: 10.1056/NEJM199905273402101. [DOI] [PubMed] [Google Scholar]
- 48.Chun TW, Nickle DC, Justement JS, Large D, Semerjian A, Curlin ME, et al. HIV-infected individuals receiving effective antiviral therapy for extended periods of time continually replenish their viral reservoir. Journal of Clinical Investigation. 2005;115:3250–3255. doi: 10.1172/JCI26197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chun TW, Murray D, Justement JS, Hallahan CW, Moir S, Kovacs C, et al. Relationship between residual plasma viremia and the size of HIV proviral DNA reservoirs in infected individuals receiving effective antiretroviral therapy. The Journal of Infectious Diseases. 2011;204:135–138. doi: 10.1093/infdis/jir208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buzon MJ, Massanella M, Llibre JM, Esteve A, Dahl V, Puertas MC, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nature Medicine. 2010;16:460–465. doi: 10.1038/nm.2111. [DOI] [PubMed] [Google Scholar]
- 51.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature Medicine. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 52.Mavigner M, Cazabat M, Dubois M, L’Faqihi FE, Requena M, Pasquier C, et al. Altered CD4+ T cell homing to the gut impairs mucosal immune reconstitution in treated HIV-infected individuals. Journal of Clinical Investigation. 2012;122:62–69. doi: 10.1172/JCI59011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elhed A, Unutmaz D. Th17 cells and HIV infection. Current Opinion in HIV and AIDS. 2010;5:146–150. doi: 10.1097/COH.0b013e32833647a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aujla SJ, Dubin PJ, Kolls JK. Th17 cells and mucosal host defense. Seminars in Immunology. 2007;19:377–382. doi: 10.1016/j.smim.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aujla SJ, Dubin PJ, Kolls JK. Interleukin-17 in pulmonary host defense. Experimental Lung Research. 2007;33:507–518. doi: 10.1080/01902140701756604. [DOI] [PubMed] [Google Scholar]
- 56.Macal M, Sankaran S, Chun TW, Reay E, Flamm J, Prindiville TJ, et al. Effective CD4+ T-cell restoration in gut-associated lymphoid tissue of HIV-infected patients is associated with enhanced Th17 cells and polyfunctional HIV-specific T-cell responses. Mucosal Immunol. 2008;1:475–488. doi: 10.1038/mi.2008.35. [DOI] [PubMed] [Google Scholar]
- 57.Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, et al. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathogens. 2010;6:e1000852. doi: 10.1371/journal.ppat.1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McGowan I, Elliott J, Fuerst M, Taing P, Boscardin J, Poles M, et al. Increased HIV-1 mucosal replication is associated with generalized mucosal cytokine activation. Journal of Acquired Immune Deficiency Syndromes. 2004;37:1228–1236. doi: 10.1097/01.qai.0000131846.12453.29. [DOI] [PubMed] [Google Scholar]
- 59.Oguariri RM, Brann TW, Imamichi T. Hydroxyurea and interleukin-6 synergistically reactivate HIV-1 replication in a latently infected promonocytic cell line via SP1/SP3 transcription factors. The Journal of Biological Chemistry. 2007;282:3594–3604. doi: 10.1074/jbc.M608150200. [DOI] [PubMed] [Google Scholar]
- 60.Saleh S, Wightman F, Ramanayake S, Alexander M, Kumar N, Khoury G, et al. Expression and reactivation of HIV in a chemokine induced model of HIV latency in primary resting CD4+ T cells. Retrovirology. 2011;8:80. doi: 10.1186/1742-4690-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chun TW, Engel D, Mizell SB, Ehler LA, Fauci AS. Induction of HIV-1 replication in latently infected CD4+ T cells using a combination of cytokines. Journal of Experimental Medicine. 1998;188:83–91. doi: 10.1084/jem.188.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Byrnes AA, Harris DM, Atabani SF, Sabundayo BP, Langan SJ, Margolick JB, et al. Immune activation and IL-12 production during acute/early HIV infection in the absence and presence of highly active, antiretroviral therapy. Journal of Leukocyte Biology. 2008;84:1447–1453. doi: 10.1189/jlb.0708438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Al-Harthi L, Roebuck KA, Landay A. Induction of HIV-1 replication by type 1- like cytokines, interleukin (IL)-12 and IL-15: effect on viral transcriptional activation, cellular proliferation, and endogenous cytokine production. Journal of Clinical Immunology. 1998;18:124–131. doi: 10.1023/a:1023246800353. [DOI] [PubMed] [Google Scholar]
- 64.Foli A, Saville MW, Baseler MW, Yarchoan R. Effects of the Th1 and Th2 stimulatory cytokines interleukin-12 and interleukin-4 on human immunodeficiency virus replication. Blood. 1995;85:2114–2123. [PubMed] [Google Scholar]
- 65.Song W, Wilson CM, Allen S, Wang C, Li Y, Kaslow RA, et al. Interleukin 18 and human immunodeficiency virus type I infection in adolescents and adults. Clinical and Experimental Immunology. 2006;144:117–124. doi: 10.1111/j.1365-2249.2006.03050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Torre D, Pugliese A. Interleukin-18: a proinflammatory cytokine in HIV-1 infection. Current HIV Research. 2006;4:423–430. doi: 10.2174/157016206778559993. [DOI] [PubMed] [Google Scholar]
- 67.Shapiro L, Puren AJ, Barton HA, Novick D, Peskind RL, Shenkar R, et al. Interleukin 18 stimulates HIV type 1 in monocytic cells. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:12550–12555. doi: 10.1073/pnas.95.21.12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klein SA, Klebba C, Kauschat D, Pape M, Ozmen L, Hoelzer D, et al. Interleukin-18 stimulates HIV-1 replication in a T-cell line. European Cytokine Network. 2000;11:47–52. [PubMed] [Google Scholar]
- 69.Iannello A, Boulassel MR, Samarani S, Tremblay C, Toma E, Routy JP, et al. HIV-1 causes an imbalance in the production of interleukin-18 and its natural antagonist in HIV-infected individuals: implications for enhanced viral replication. The Journal of Infectious Diseases. 2010;201:608–617. doi: 10.1086/650314. [DOI] [PubMed] [Google Scholar]
- 70.Stylianou E, Bjerkeli V, Yndestad A, Heggelund L, Waehre T, Damas JK, et al. Raised serum levels of interleukin-18 is associated with disease progression and may contribute to virological treatment failure in HIV-1-infected patients. Clinical and Experimental Immunology. 2003;132:462–466. doi: 10.1046/j.1365-2249.2003.02179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Watanabe D, Uehira T, Yonemoto H, Bando H, Ogawa Y, Yajima K, et al. Sustained high levels of serum interferon-gamma during HIV-1 infection: a specific trend different from other cytokines. Viral Immunology. 2010;23:619–625. doi: 10.1089/vim.2010.0065. [DOI] [PubMed] [Google Scholar]
- 72.Chun TW, Engel D, Mizell SB, Hallahan CW, Fischette M, Park S, et al. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nature Medicine. 1999;5:651–655. doi: 10.1038/9498. [DOI] [PubMed] [Google Scholar]
- 73.Chun TW, Davey RT, Jr, Engel D, Lane HC, Fauci AS. Re-emergence of HIV after stopping therapy. Nature. 1999;401:874–875. doi: 10.1038/44755. [DOI] [PubMed] [Google Scholar]
- 74.Stellbrink HJ, van Lunzen J, Westby M, O’Sullivan E, Schneider C, Adam A, et al. Effects of interleukin-2 plus highly active antiretroviral therapy on HIV-1 replication and proviral DNA (COSMIC trial) AIDS. 2002;16:1479–1487. doi: 10.1097/00002030-200207260-00004. [DOI] [PubMed] [Google Scholar]
- 75.Abrams D, Levy Y, Losso MH, Babiker A, Collins G, Cooper DA, et al. Interleukin-2 therapy in patients with HIV infection. New England Journal of Medicine. 2009;361:1548–1559. doi: 10.1056/NEJMoa0903175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prins JM, Jurriaans S, van Praag RM, Blaak H, van Rij R, Schellekens PT, et al. Immuno-activation with anti-CD3 and recombinant human IL-2 in HIV-1-infected patients on potent antiretroviral therapy. AIDS. 1999;13:2405–2410. doi: 10.1097/00002030-199912030-00012. [DOI] [PubMed] [Google Scholar]
- 77.van Praag RM, Prins JM, Roos MT, Schellekens PT, Ten Berge IJ, Yong SL, et al. OKT3 and IL-2 treatment for purging of the latent HIV-1 reservoir in vivo results in selective long-lasting CD4+ T cell depletion. Journal of Clinical Immunology. 2001;21:218–226. doi: 10.1023/a:1011091300321. [DOI] [PubMed] [Google Scholar]
- 78.Fraser C, Ferguson NM, Ghani AC, Prins JM, Lange JM, Goudsmit J, et al. Reduction of the HIV-1-infected T-cell reservoir by immune activation treatment is dose-dependent and restricted by the potency of antiretroviral drugs. AIDS. 2000;14:659–669. doi: 10.1097/00002030-200004140-00005. [DOI] [PubMed] [Google Scholar]
- 79.Kulkosky J, Nunnari G, Otero M, Calarota S, Dornadula G, Zhang H, et al. Intensification and stimulation therapy for human immunodeficiency virus type 1 reservoirs in infected persons receiving virally suppressive highly active antiretroviral therapy. The Journal of Infectious Diseases. 2002;186:1403–1411. doi: 10.1086/344357. [DOI] [PubMed] [Google Scholar]
- 80.Lafeuillade A, Poggi C, Chadapaud S, Hittinger G, Chouraqui M, Pisapia M, et al. Pilot study of a combination of highly active antiretroviral therapy and cytokines to induce HIV-1 remission. Journal of Acquired Immune Deficiency Syndromes. 2001;26:44–55. doi: 10.1097/00126334-200101010-00006. [DOI] [PubMed] [Google Scholar]
- 81.Sereti I, Imamichi H, Natarajan V, Imamichi T, Ramchandani MS, Badralmaa Y, et al. In vivo expansion of CD4CD45RO-CD25 T cells expressing foxP3 in IL-2- treated HIV-infected patients. Journal of Clinical Investigation. 2005;115:1839–1847. doi: 10.1172/JCI24307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Levy Y, Lacabaratz C, Weiss L, Viard JP, Goujard C, Lelievre JD, et al. Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. Journal of Clinical Investigation. 2009;119:997–1007. doi: 10.1172/JCI38052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sereti I, Dunham RM, Spritzler J, Aga E, Proschan MA, Medvik K, et al. IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood. 2009;113:6304–6314. doi: 10.1182/blood-2008-10-186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Imamichi H, Degray G, Asmuth DM, Fischl MA, Landay AL, Lederman MM, et al. HIV-1 viruses detected during episodic blips following interleukin-7 administration are similar to the viruses present before and after interleukin-7 therapy. AIDS. 2011;25:159–164. doi: 10.1097/QAD.0b013e328340a270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Levy Y, Sereti I, Tambussi G, Routy JP, Lelievre JD, Delfraissy JF, et al. Effects of rhIL-7 on T Cell recovery and thymic output in HIV-infected patients receiving antiretroviral therapy: results of a phase I/IIa randomized, placebo controlled, multicenter study. Clinical Infectious Diseases An Official Publication of the Infectious Diseases Society of America. 2012 doi: 10.1093/cid/cis383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shan L, Deng K, Shroff NS, Durand CM, Rabi SA, Yang HC, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nature Reviews Immunology. 2009;9:480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.North TW, Higgins J, Deere JD, Hayes TL, Villalobos A, Adamson L, et al. Viral sanctuaries during highly active antiretroviral therapy in a nonhuman primate model for AIDS. Journal of Virology. 2010;84:2913–2922. doi: 10.1128/JVI.02356-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lewis MG, Norelli S, Collins M, Barreca ML, Iraci N, Chirullo B, et al. Response of a simian immunodeficiency virus (SIVmac251) to raltegravir: a basis for a new treatment for simian AIDS and an animal model for studying lentiviral persistence during antiretroviral therapy. Retrovirology. 2010;7:21. doi: 10.1186/1742-4690-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dinoso JB, Rabi SA, Blankson JN, Gama L, Mankowski JL, Siliciano RF, et al. A simian immunodeficiency virus-infected macaque model to study viral reservoirs that persist during highly active antiretroviral therapy. Journal of Virology. 2009;83:9247–9257. doi: 10.1128/JVI.00840-09. [DOI] [PMC free article] [PubMed] [Google Scholar]