Abstract
Peripheral arterial disease (PAD) is a common vascular disease that reduces blood flow capacity to the legs of patients. PAD leads to exercise intolerance that can progress in severity to greatly limit mobility, and in advanced cases leads to frank ischemia with pain at rest. It is estimated that 12–15 million people in the United States are diagnosed with PAD, with a much larger population that is undiagnosed. The presence of PAD predicts a 50–1500% increase in morbidity and mortality, depending on severity. Treatment of patients with PAD is limited to modification of cardiovascular disease risk factors, pharmacological intervention, surgery, and exercise therapy. Extended exercise programs that involve walking ~5 times/wk, at a significant intensity that requires frequent rest periods, are most significant. Pre-clinical studies and virtually all clinical trials demonstrate the benefits of exercise therapy, including: improved walking tolerance, modified inflammatory/hemostatic markers, enhanced vasoresponsiveness, adaptations within the limb (angiogenesis, arteriogenesis, mitochondrial synthesis) that enhance oxygen delivery and metabolic responses, potentially delayed progression of the disease, enhanced quality of life indices, and extended longevity. A synthesis is provided as to how these adaptations can develop in the context of our current state of knowledge and events known to be orchestrated by exercise. The benefits are so compelling that exercise prescription should be an essential option presented to patients with PAD in the absence of contraindications. Obviously, selecting for a life style pattern, that includes enhanced physical activity prior to the advance of PAD limitations, is the most desirable and beneficial.
1. Introduction
Peripheral arterial disease (PAD) is a fairly common degenerative vascular condition that leads to inadequate blood flow (BF), typically in the legs. PAD is due to atherosclerosis that causes chronic narrowing of arteries, which can precipitate acute thrombotic events. This atherosclerotic condition often affects a large primary conduit artery (e.g., iliofemoral/femoral artery region), but it can also be multilevel and diffuse, causing complex and generally more severe complications. The initial narrowing of an artery reduces the flow capacity to the limb. The loss of this BF reserve seems benign until the flow demands of the limb muscles require a BF that exceeds the reduced flow capacity. At this time, exercise tolerance becomes limited, with a significant but limited fraction of the patients (10–35%) exhibiting pain on exertion with an altered gait typical of intermittent claudication, while ~50% describe atypical symptoms that limit exercise (181, 336, 388). Upon resting, the pain or discomfort goes away, but returns with renewed exertion. Unfortunately, the vascular lesions often progress leading to a greater loss of flow reserve resulting in an even greater limitation to mobility. In its extreme, BF can become limiting at rest, leading to frank ischemia, ulcerations, pathological changes, gangrene, and, all too often, amputation of the distal tissues (388). As illustrated in Figure 1, the prevalence of intermittent claudication increases markedly with age, with a generally higher rate in men than women (180, 661). There is some evidence that the prevalence of PAD is influenced by race/ethnicity, with a higher rate among African American men and women and a lower rate among Hispanic women and Chinese men (601) and that heritability of PAD is real, but limited (20–45%), after adjusting for other risk factors (656). It is estimated that approximately 12–15 million people in the United States are diagnosed with PAD, with a much larger number that are not diagnosed (180). Since there is such a strong influence of age on the prevalence of PAD and the population of older individuals in the US has increased disproportionally in the past 10 years, the number with PAD must be much greater. In one study, with a population base in southern California, between 2% and 20% of individuals between the ages 38 to 82 years exhibited BF deficits in large vessel(s) of the limb (181). This is similar to the prevalence of PAD observed in other studies, again increasing dramatically with age (cf., Figure 1)(661).
Figure 1.
Prevalence of peripheral arterial disease, and the subset of patients with intermittent claudication, increases markedly with age. Reproduced from Norgren et al., (661) with permission.
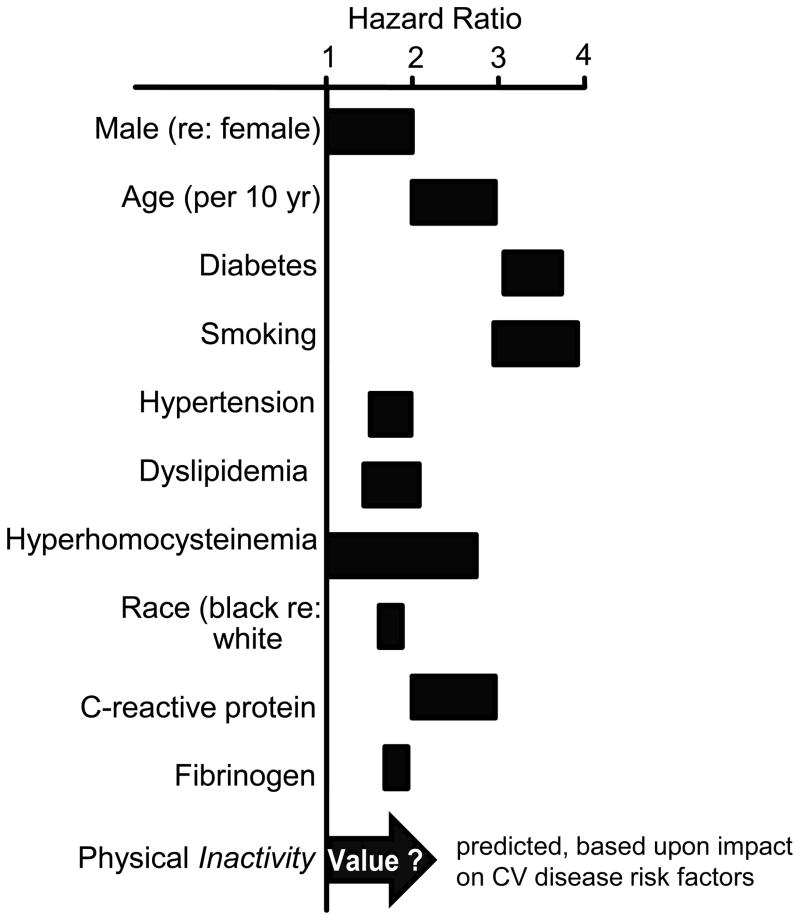
Since PAD is an atherosclerotic disease, the risk factors are numerous, predictable, and common to cardiovascular diseases in general, and associated with inflammation (94, 348, 829). Thus, the typical risk profile of smoking, dyslipidemia, hypertension, diabetes, obesity, and physical inactivity raise the prospects that numerous co-mobidities are frequent with PAD. Figure 2 (55, 661) illustrates the hazardous odds ratios for developing symptomatic PAD, as a function of various risk factors, with diabetes and smoking as the strongest modifiable risk factors. Smoking and diabetes are particularly noteworthy risks, as the ischemic limitations and dysfunction are more exaggerated, as compared to their absence (9, 315). Not considered by this Study Group [Trans-Atlantic Inter-Society Consensus Document on Management of Peripheral Arterial Disease (TASC II)] is the impact of physical inactivity. In view of the evidence that physical inactivity is a major risk factor for coronary heart disease (CHD) (511, 706), with significant impact of associated risk factors of atherosclerotic diseases (86), it stands to reason that physical activity should have a major influence in the primary prevention of PAD. Thus, physical inactivity should be listed as an additional modifiable risk factor for PAD, although the precise quantitative impact has not been studied. In addition, it is becoming recognized that PAD invokes inflammatory responses (94) that exhibit themselves as elevated biomarkers of the inflammatory process, such as C-reactive protein (483, 603). The coincidence of CHD in patients with PAD is fairly high, generally ranging from 35 to 60% of patients based upon clinical history and ECG (656, 829); however, when a more sensitive criteria of angiographic-defined coronary stenosis of >50% was applied ~90% of PAD patients were identified to have CHD (309). Similarly, the coincidence of PAD and cerebrovascular disease (CBVD) is extensive with up to 20%, up to 50%, or up to 80% of PAD patients exhibiting CBVD, based upon criteria of clinical history, bruits, or ultrasonic evaluation, respectively (309). Diabetes engenders a 1.5 to 4-fold increased risk of developing PAD (55). This comorbidity is especially difficult, since large vessel disease occurs earlier in life and appears more aggressive (661). Further, PAD patients with diabetes tend to experience more complex distal obstructions, have revascularization interventions that are less successful, and have higher rates of perioperative complications and death (470). Thus, while PAD can have serious consequences, independent of other chronic diseases, it is particularly ominous when exacerbated by the presence of CHD, CBVD, and/or diabetes.
Figure 2.
The risk factors for peripheral arterial disease are numerous, as illustrated by these hazard ratios. Figure adapted from Norgren et al., (661), with permission, and added concept from Booth et al., (80).
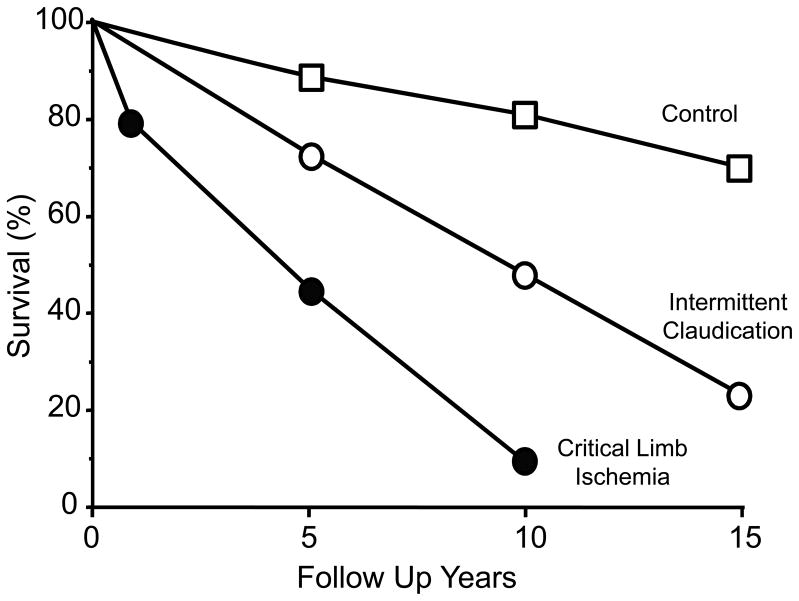
PAD leads to a reduced mobility, to a significant loss in the quality of life, and to premature death. The impact of PAD can be overwhelming, as depression occurs at a high frequency among affected patients, and is associated with reduced success of surgical intervention and recurrence of symptomatic PAD (148). Nonetheless, PAD rarely presents as the cause of death. Rather, CHD and CBVD account for the vast majority (~65%) of deaths, with other vascular diseases accounting for an additional 10% leaving the remainder of 25% due to non-vascular diseases (213). There is an increase in mortality based upon the severity of PAD. Premature death due to cardiovascular disease increases 50% to 1500%, depending upon the severity of PAD (182). As illustrated in Figure 3 the ten-year survival of patients with intermittent claudication is well below that of the normal population, but further exceeded by patients with critical limb ischemia (661). Even the diagnostic measure of the ankle-brachial index (ABI) provides clear evidence for the severity of PAD. This is illustrated in Figure 4 by the increase in CV and all-cause mortality, as the ABI approaches and declines below 0.60 (596). As would be expected from the above, the presence of PAD also predicts an increase in morbidity, independent of other comorbidities, as seen by increased cardiovascular events and complications. This high prevalence of PAD and its dire predictions of increased morbidity and mortality places an importance on primary care for detection and management of such patients (387). Interestingly, the presence of PAD, in the absence of CHD, is a more powerful predictor of cardiovascular events than is the presence of CHD, in the absence of PAD (309). As shown in Figure 4, even patients that present with borderline PAD, as defined by an ABI less than 1.0 but greater than 0.9, experience an increased risk of all-cause mortality (266). In the face of this risk and the high prevalence of ~10% of these borderline individuals in the general population older than 40 yr of age (499), has placed greater emphasis on clinical recognition of borderline PAD in the primary care setting (596). Thus, since PAD represents such a major health hazard that, unfortunately, leads to increased frequency of medical events and premature death, it is critical to establish early disease detection and appropriate treatment, even for secondary prevention measures (264).
Figure 3.
The increase in mortality with peripheral arterial disease is related to its severity. Reproduced from Norgren et al., (661) with permission.
Figure 4.
The increased mortality of peripheral arterial disease is predicted by the decline in the ankle-brachial artery pressure ratio. Reproduced from Resnick et al., (749) with permission.
2. Treatment of Peripheral Arterial Disease
There has been a relatively small arsenal available to manage patients with PAD. Typical management includes treatment for general cardiovascular risk factors, cessation of smoking, loss of body weight, pharmacological interventions, increased physical activity, and in certain candidates surgical intervention (304, 375, 388, 661, 752); however, there have been no ‘breakthroughs’ to reverse or eliminate the disease. There has been success in managing patients with PAD by pharmacological treatments to inhibit phosphodiesterase III (782) and influence blood rheology and hemostasis (618). However, the magnitude of benefit is not as great as that observed with participation in a supervised exercise program (304, 618). While surgical intervention can provide a marked improvement in distal perfusion with critical benefit to tissue oxygenation (557), the success rate has been less than optimal (689), especially if there is early surgical failure of the procedure (768), and the long term outlook has been guarded (728), owing to the complex and progressive nature of the disease. Interestingly, while vascular surgery imparts an advantage to patients, as compared to an exercise program at six months post intervention (154), the long-term benefit was observed with exercise training to increase claudication and maximal walking distance, especially in patients with superficial femoral artery obstructions (710). Indeed, surgical patients can gain an additional benefit by participation in an exercise training program (557). Thus, the treatment of enhanced physical activity is a worthy means of managing patients with PAD.
3. Influence of Exercise Training in Peripheral Arterial Insufficiency
There are several comprehensive meta analyses (69, 88, 290, 304, 326, 738, 985), exhibiting some overlap in studies evaluated, and a host of excellent reviews presenting various aspects of exercise training in patients with PAD that should be consulted (108, 127, 133, 290, 304, 336, 578, 597, 618, 699, 728, 743, 744, 748, 764, 886–888, 985). The wealth of this information and attention reveals the extensive clinical interest in the myriad of biological responses to exercise that can impart potential benefits to patients with PAD. These include: enhanced 6 min walking distance, increased walk time to pain onset, increased walk time to maximal pain, improved self-defined quality of life index, improved muscle function, enhanced metabolic response, improved inflammatory/hemostatic function, reduced morbidity and mortality risk, and possibly a reduced rate of disease progression.
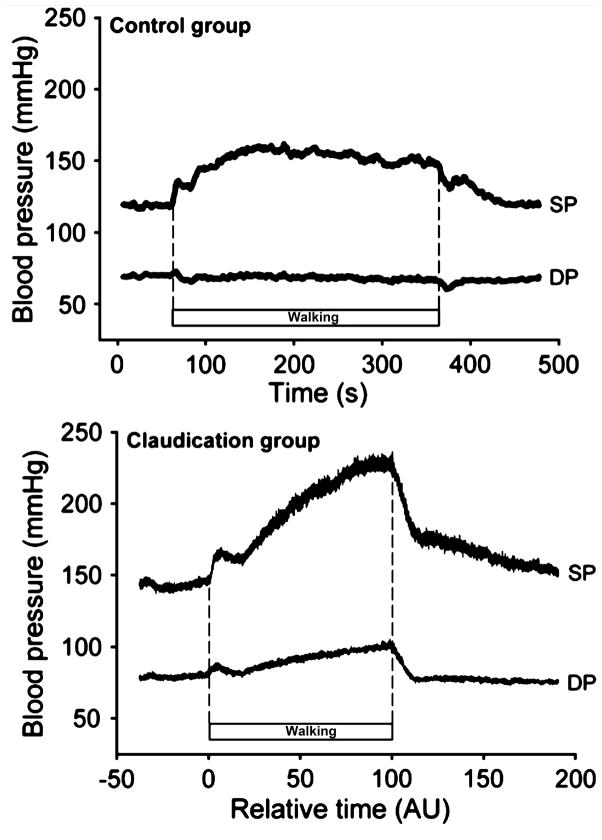
Virtually all studies that have evaluated the impact of exercise training in patients with PAD have demonstrated a benefit in exercise tolerance, a primary outcome measure (69, 88, 290, 738, 985). Exercise tolerance has been evaluated using the time of exercise or the distance walked during fairly standard treadmill conditions where the patient must conform to a defined exercise protocol. Further, the duration of walking to the onset of pain, as well as the duration of walking until maximum pain causes cessation of the exercise, have been used as valuable parameters of walking tolerance. A recent analysis has identified that progressive treadmill tests provide the best reliability for patient evaluation (658). These involve walking at a given speed and then progressively increasing the grade of the treadmill over time (291, 377). As you can appreciate, the patients must conform to the progressive intensity of the task until the onset of pain and/or until maximal tolerance. Relying on these walking tests, the improvements in exercise tolerance with training are substantial, with typical increases in walking to the onset of pain of ~180% and increases in maximal walking of ~120%, as compared to before exercise training began. In addition, numerous studies have evaluated less standard exercise conditions, for example where patients walk for a 6 min period at their own pace, or walk at their selected pace until the onset of pain or maximal pain. As illustrated in Figure 5, the duration of walking that can achieved at the patient’s selected pace can be far greater than that observed in the more rigorous conditions of a laboratory treadmill test. Thus, while the standard treadmill test is most useful for quantification of the patients’ capacity, the ‘free walking’ ability likely better characterizes the real impact of increased mobility that translates to an improved quality of life for the patient. The actual improvement in exercise tolerance realized from participation in a training program depends upon a number of parameters in the study, including: patient population, mode of exercise, intensity of exercise, duration of each exercise bout, frequency of exercise periods per week, duration of the training program, the exercise setting, and compliance to the exercise program.
Figure 5.
Typical increase in exercise tolerance, measured during a defined treadmill protocol and during free-pace walking, that was observed in patients with peripheral arterial disease who participated in an exercise program. Data taken from Carter et al., (132).
3.1. Patient population and exercise program compliance
The population of patients with PAD is rather heterogeneous, presenting from single large vessel to multiple-level vascular involvement (181). This varying degree of vascular obstruction leads to varied presentation of symptoms, from a noticeable limit to mobility during taxing locomotion, to a substantial impairment in walking tolerance, to an extremely limited mobility associated with rest ischemia. Thus, studies evaluating the influence of exercise training involve those patients who are at least mobile and able to achieve the demands of the exercise program, even when the walking task is made relative to each individual patient. The presence of co-morbidities, such as diabetes or risk biomarkers of the metabolic syndrome, is associated with worsened PAD, physical function, and peripheral circulation (285), although for the same disease presentation in diabetics a poorer exercise tolerance was attributed to obesity (315). It is well-recognized that due to the nature of PAD, patients select for a much reduced level of leisure-time physical activity (288). Indeed, the amount of leisure-time activity declines directly with the severity of the disease, as reflected in the ankle-brachial pressure index (281). For these patients, barriers to walking include the walking surfaces, uncertainty about the outcome of walking, the need to take rest breaks, and the concern about leg pain (278). Thus, it can be expected that PAD patients enrolled in controlled studies represent the near-extreme of inactive individuals. This should optimize the opportunity to realize a response, should increased physical activity impart any change. Further, as nearly all patients enrolled in studies exhibit intermittent claudication, it is relatively easy to establish changes in performance. However, it is important to recall that the majority of patients with PAD do not exhibit intermittent claudication, but rather experience a rather non-descript feeling of impaired mobility. Interestingly, McDermott and coworkers (598) have observed that the impairment in walking performance, that scales with the reduction in ankle-brachial index, is similar between PAD patients that exhibit intermittent claudication (10–35% of total (181, 336, 388)) and those that do not. This raises the expectation that the physiological response to training by PAD patients with intermittent claudication reasonably well characterize the responses of all PAD patients in the general case.
Supervised exercise therapy provides a significant and clinically relevant improvement of maximal treadmill walking distance, compared with non-supervised exercise programs (69). Obvious benefits of supervised exercise programs include potential advantages as available instruction, oversight and accountability for compliance, favorable facilities, and the availability of social interactions. However, the most successful programs are likely to be those that combine regular, supervised exercise with daily home exercise (738). This may be attributed to the development of behavior patterns that encourage exercise compliance and continuation of increased activity long after supervision has ended. Further, while short-term supervised programs typically achieve better program management, in a two year follow-up the adherence rate to the program was only 36%, compared to 68% in a home-based exercise program (37). As mentioned above, concern about the onset of pain is one reported reason for reduced leisure-time activity; however, pain intensity was not considered a dominant factor influencing walking behavior. Rather, it was the individual’s proclivity for planned behavior that favored activity (277). This implies that individual motivation for exercise and a conviction toward its merits is a critical factor in compliance to an exercise program.
3.2. Training program: type, intensity, and duration of exercise bout
Most studies evaluating exercise prescription have utilized walking as the primary or sole activity. Training by walking has been shown to impart greater increases in performance, as compared to mixed or alternative activity programs (290) that have included cycling (799) and resistance-type exercise (380). This could be due, in part, to the well-known specificity of training, wherein the best means of training is to employ the activity of the measurement outcome--walking performance. On the other hand, exercise performance can be markedly influenced by the muscle mass available to perform the task (790). Thus, the muscle atrophy that accompanies aging, which can be compounded by inactivity in PAD patients, could provide a significant impediment to patient mobility. One strategy to address this potential problem has been to utilize resistance training as the exercise prescription. While a 12 wk ‘weight’ training program increased the strength of the lower limb muscle (12–17%) in patients, there was only a modest 36% increase in maximal walking time, compared to the 74% increase observed in the group of patients who trained by treadmill walking (380). Further, introducing strength training during a subsequent 12 wk period of walking training did not further increase walking performance. Thus, while the rationale to minimize muscle atrophy was reasonable, it appears that conditioning by walking remains the most important feature of exercise to improve the condition of PAD patients. This places emphasis on endurance-type exercise training, which primarily enhances the duration of performing exercise at a reasonable intensity.
PAD patients with intermittent claudication who performed any amount of physical activity, beyond light intensity, have a lower mortality rate than similar patients who were effectively sedentary (286). This reduced risk of mortality remained evident even when the findings were adjusted for age, disease severity (ankle-brachial index), and obesity (body mass index) (286). While these findings illustrate the importance of being physically active, in the general case, there can be different viewpoints as to what intensity of exercise should be proposed for patients with PAD. There is unanimity in the need to recommend an exercise intensity that is tailored to the capacity of each individual patient. Obviously, even a modest intensity of exercise, for a normal individual, would be overwhelming for PAD patients with limited mobility. Thus, recommendations typically involve walking until the onset of pain or to the limit of when the severe pain stops exercise. In analyzing 33 earlier studies evaluating training responses in patients with PAD, Gardner and Poehlman (290) identified intensity of exercise as the most important factor that determines the improvement in walking tolerance after training. Exercise programs that involved repeated walking to the limit of maximal pain could account for 55% and 40% of the total variance in the improved performance, marked either as the onset of pain or maximal walking tolerance, respectively. This emphasis on exercise intensity is consistent with accepted training rationale, even in normal healthy people. The higher the intensity of exercise the greater the cardiovascular responses approach their limit (781), the more encompassing motor unit recruitment occurs within the active muscles (797), and the greater the metabolic responses are stressed (612). These typically lead to quantitatively greater adaptations. In the absence of severe central cardiovascular disease, patients with PAD are typically limited by vascular problems within the limbs. Thus, walking to the limit of pain may not challenge all physiological responses equally, resulting in a heterogeneity of adaptations. However, there has been some concern for PAD patients that exercise to the pain limit induce an extensive inflammatory response that may exacerbate the their condition (930). While this is true and can affect other tissues (e.g., coronary endothelium (95)), it is generally recognized that the inflammatory response to the very intense exercise attenuates with continuation in the exercise program (714). Nonetheless, the stress for the individual patient can be overwhelming due to the leg pain. This can be counterproductive, as there can be intolerable discomfort that reduces compliance with the exercise program. Rather, placing emphasis on tempering the intensity of the training bouts by, for example, walking only until the onset of pain could result in more successful physical activity. While this may not provide for an optimal stimulus for training adaptations, it can foster greater success in the exercise program. Further, even modest intensity training can enhance exercise performance. This improvement in walking performance can permit extended walking which can in turn impart greater benefit. Thus, while performing intense exercise, that may provide an optimal training stimulus and outcome, is desirable for patients with PAD, a more tempered intensity of walking may be the best, since some benefit is derived and success with the exercise program may be superior. In time, an increase in the intensity of exercise may be better tolerated, as the patient’s capacity for walking improves.
The duration of exercise performed each day is also an important determinant of the training outcome. For example, over time the muscle adaptation of an increase in mitochondrial content reaches its asymptote with exercise bout durations of ~20 -60 min, depending upon the intensity of exercise (217). In PAD patients, walking for 30 min or greater duration per session results in greater increases in exercise tolerance than walking for less that 30 min per session (290). Since patients with PAD have a limited ability to walk continuously (e.g., ~5–12 min), they must rest to permit the pain to abate. Thus, the means to extend the walking duration has been to perform repeated walking bouts, separated by sufficient rest periods. This makes it possible to achieve at least 30 min of exercise, the desired duration that is often prescribed. As exercise tolerance improves, some PAD patients increase their total walking time or sometimes introduce two walking periods, morning and afternoon.
3.3. Training program: exercise frequency and program duration
It is generally recognized that physical activity at least three times per week is essential to realize the benefits of a training program. This should be considered a minimum, as patients that exercised three or more times per week exhibited improvements in walking tolerance far greater than those patients who exercised less than three times per week (290). It is likely that benefits, other than simply increased walking performance, can be achieved with an exercise frequency greater than three times per week. For example, the activity-induced benefit of improved glucose regulation, which is needed by inactive people, is an exercise adaptation that is relatively short lived, lost within 48 h following an exercise bout (757). Thus, the improved insulin responsiveness would be lost with long intervals between daily exercise sessions. Thus, exercise programs with exercise frequency approaching five days per week are highly advisable.
The duration of the exercise program is also an important determinant for a successful outcome. An improvement in exercise tolerance can be observed within 3 months after initiating the exercise prescription. However, involvement in exercise programs greater than six months proved greater improvements in exercise tolerance than those programs that were less than six months. Indeed, length of the training program was the second most important determinant of outcome, with 22% to 28% of the variance of improved walking, depending on the time to the onset of pain or the maximal pain tolerance (290). Thus, patients who participate in an exercise program should view their involvement as long term, with benefits clearly realized in 6 months with continued improvement by 12 months. Further, they should view their exercise prescription as a lifestyle pattern whose participation would sustain their enhanced mobility.
3.4 Training versus interventional therapytheray (endovascular angioplasty or surgery)
There have been a number of randomized control trials to evaluate the merits of exercise therapy compared to surgical reconstruction and endovascular therapies (percutaneous transluminal angioplasty: PTA) (178, 298, 557, 593, 646, 710, 711, 880), including a combination of both PTA plus exercise therapy (316, 505, 557, 593). These have been nicely summarized in reports by META analyses (11, 154, 998) and reviews (267, 985). In general, these trials indicate that, in successful outcomes, surgical and endovascular interventions in patients with PAD lead to improvements in distal blood flow (557), distal perfusion pressure and thereby ABI (178, 393, 505), no change (593) or improvements (646) in Quality of Life (QoL) indices, and increases in walking performance (298, 316, 393, 505, 557, 593, 646, 668, 710, 880, 991, 992) but not always (178). While these benefits are quite demonstrable in the early months following treatment, the prolongation of these effects have been less than optimal (689, 880), especially if there is early failure of the procedure (e.g., graft) (768), and the long term outlook has been guarded (728), likely owing to the complex and progressive nature of the disease. However, this pattern has not always been seen, as significant longer-term (up to 2 years) benefits can be realized by PTA therapy (710, 991). This variable pattern of response contributes, in part, to mixed conclusions when compared to exercise therapy. For example, some studies conclude that surgery and PTA provide a better outcome as compared to exercise therapy (298, 393, 557, 880), whereas others conclude that supervised exercise prescription is better than PTA (178, 646, 710). Comparisons of effects are further obfuscated by studies that have not utilized supervised exercise prescription but have only provided advice on the benefits of exercise to the patients (666, 668, 991, 992). It is well establish that supervised exercise prescription leads to significantly greater improvements in walking performance, as compared with non-supervised exercise programs (69). Thus, these trials with an intent-to-treat for exercise prescription do not likely provide the power to assess the comparison to exercise prescription. There has even been an assessment of the costs of PTA vs supervised exercise prescription, showing no difference in outcome measures (QoL, performance), but at a higher cost for PTA (879).
The improved walking performance with exercise prescription typically persists as long as participation in the exercise program continues. On the other hand, any loss in clinical benefit from vascular interventions over time would undermine the comparison to exercise prescription. What seems clear, however, is that PTA, in combination with supervised exercise prescription, results in the greatest benefit to the patient (316, 505, 557, 593). While vascular reconstruction and PTA procedures and supervised exercise prescription can both impart clinical benefit to the PAD patient, it is presently not possible to provide a definitive conclusion as which may be superior, owing to the limited number of total patients enrolled in the published studies to date. Thus, there has been a call for larger, more encompassing clinical trails to be conducted to provide a more definitive assessment (10).
4. Improved Quality of Life with Exercise Prescription
Although indices of quality of life vary by the focus of the questionnaire, there is general consensus that patients with PAD exhibit deficits in numerous quality of life parameters. These are most easily identified as those domains related to physical health, level of independence, pain and discomfort, energy and fatigue, mobility and activities of daily living (90). Thus, patients with PAD exhibit substantial impairment, often related to the severity of disease, in: physical index, including mobility, recreation, and work deficits; body care; sleep and rest; psychosocial index, social interactions; and even a small impact on depression (326, 881, 900). The dominance of reduced quality of life index based primarily upon physical condition, with the resultant impact that can have on mobility, leisure time activities, level of independence, fatigue, potential social interactions, raises the expectation that improved activity tolerance induced by exercise training can have a major influence on the overall quality of life of the patients with PAD. Indeed, participation in an exercise program establishes significant improvement in the overall health related quality of life (481).
5. Improved Inflammatory/Hemostatic Function with Training
It is well recognized that risks of cardiovascular diseases are greater in the presence of abnormal inflammatory/hemostatic biomarkers (637, 657), including those related to: a) inflammation: elevations in circulating monocyte chemotractant protein-1 (MCP-1), interlukin-6, C-reactive protein (CPR), soluble forms of vascular cell adhesion molecule-1 (sVCAM-1) and intracellular adhesion molecule-1 (sICAM-1); and b) coagulation and fibrinolysis: enhanced coagulation, platelet aggregation, and increased plasma fibrinogen, tissue plasminogen activator (tPA), and plasminogen activator-inhibitor-1 (PAI-1) concentrations. While not all of these parameters have been measured in any single study, each one is related to enhanced risk of cardiovascular disease. Since PAD is a general atherosclerotic/inflammatory disease, with co-morbidities of cardiac and cerebrovascular disease, there is also strong evidence that these biomarkers provide insight into the risks of PAD (94, 603). Inflammatory markers such as MCP-1 and IL-6 are significantly associated with the extent of atherosclerosis, as assessed by angiographic score, and the maximum treadmill walking distance in patients with PAD (667). In addition to the risk prediction of PAD (348), elevated MCP-1, D-dimer (fibrin degradation product), CRP, IL-6, sVCAM-1, and sICAM-1, are associated with poorer 6-min walk performance (599, 602). Further, platelet aggregation and sensitivity for platelet activation, which could portend to unwanted thromboembolic events, inversely correlate with the ABI (869). These conditions raise the potential to accelerate the atherosclerotic and hemostatic processes, which could exacerbate the condition of the patients with PAD. Thus, a number of these parameters have been proposed as useful biomarkers of PAD and its severity (603).
There is a seeming paradox in the circulating inflammatory markers, observed following an acute bout of exercise, and the inflammatory state of the individual following repeated bouts of exercise, as observed with participation in an exercise training program. On one hand, an acute bout of prolonged strenuous exercise can increase some inflammatory markers, even in healthy young athletes, while at the same time participation in an exercise training program provides a long-term ‘anti-inflammatory’ effect (475). This acute response in healthy individuals typically requires prolonged strenuous exercise, as it may or may not be observed in less demanding exercise. Thus, it is generally believed that the intensity of exercise, and its resultant stress, is an important determinant of this acute phase response (24, 475). The situation with patients with peripheral arterial disease is complicated because their exercise tolerance is so limited and by the potential of an ischemia/reperfusion response that can occur in the legs when exercise is performed to the onset of pain and certainly when continued and ultimately limited by claudicant pain. Thus, even at rather slow walking conditions, that are nonetheless strenuous for patients with PAD, it has been repeatedly observed that inflammatory biomarkers are elevated in the serum following exercise to claudication or to the limit of pain tolerance (24, 93, 225, 488, 655, 929, 930, 943, 1003). It is probable that ischemia/reperfusion within the active muscle contributes significantly to this response. Neumann et al (655) exercised a group of patients with unilateral PAD to the time of pain limitation and observed an increase in neutrophil count and neutrophil activation in the venous blood from the affected limb with exercise, compared to the contralateral limb. Similarly, Nawaz et al., (652) observed increases in markers of neutrophil activation with leg exercise, but not arm exercise, in patients with claudication. Neutrophil activation, which may be related to the elevated IL-8 (488), could contribute to the increase in ROS, that is typically observed in these patients (67, 930, 943). In accordance with these global indicators of muscle oxidative stress, capillary swelling within the microvasculature of the ischemic muscle is more pronounced and leukocyte adherence to venules is augmented in rodent muscles activated by electrical stimulation (381, 427). While the capillary swelling and leukocyte adherence are seen as negative effects (i.e. capillary blockage and reduced distribution of flow), it is clear that, overall, electrical stimulation enhances recovery of muscle function. Thus, additional positive effects of the muscle activation, which may include improved arteriolar dilatation and angiogenesis, overcome any deleterious effects of the enhanced inflammatory response. However, the extent of activity must be low, as more strenuous muscle activity is associated with aggravated muscle injury without an enhancement of muscle blood flow recovery (424). These acute responses to demanding exercise could exacerbate the inflammatory risk profile that already exists in patients with PAD and potentially contribute to endothelial dysfunction, progression of atherosclerosis, and thromboembolic events. Thus, there has been some discussion on the advisability of promoting exercise in patients with PAD (930). However, as discussed in the next paragraph, chronic physical activity can produce anti-inflammatory effects (475, 705, 706, 714, 930).
It is generally recognized that physically active, as compared to sedentary older adults, exhibit an enhanced immunity (857). There is a well characterized inverse relationship between markers of inflammation and the level of physical activity or aerobic capacity of individuals (63). This relationship is observed, independent of obesity, a major contribution to chronic inflammatory state. Indeed, the reduction in inflammatory/hemostatic markers associated with higher levels of physical activity, accounted for a major portion of the reduced risk of cardiovascular disease in these individuals (637). This inverse relationship implies that repeated bouts of exercise may have a direct effect on the expression of inflammatory markers. A number of studies have not observed modifications in inflammatory markers in healthy subjects with training, possibly related to a modest exercise program (652). However, other studies observed reductions not only in healthy subjects, but especially when the markers are initially elevated, as for example in patients with chronic disease (63, 929, 1004). Exercise training can lessen the magnitude of the acute phase response (e.g., neutrophil activation, free radical production and lipid peroxidation) to an exercise bout (67, 101, 943). The enhanced anti-oxidant capacity, induced within the active muscle and vasculature (518), should provide a greater buffer to free radical production and contribute to these observed training effects. In addition, the advancing work of BK Pedersen and coworkers has provided significant insight into the integrated processes brought about by exercise training. Muscle can release copious amounts of IL-6 during exercise (705, 706), which is in turn anti-inflammatory by fostering an increase in anti-inflammatory cytokines (IL-1receptor antagonists and IL-10) and a reduction in TNFα and IL-1β (705, 706, 714). Thus, a compelling case is made that exercise is anti-inflammatory to low-grade inflammation. This benefit of chronic exercise can also be realized by patients with cardiovascular disease (977, 1004), including PAD (929, 930). Since inflammatory/hemostatic markers are predictive of disease severity, morbidity and mortality (94, 603), any reduction of these makers should provide a benefit to these patients and could contribute to the realized improvement in PAD patients that participate in an exercise program.
6. Training Improves Walking Efficiency in PAD Patients During Extended Walking
Altered gait caused by PAD has been most well characterized in patients with intermittent claudication, because it is relatively easy to identify when the limits of activity are approached by the onset of pain. These patients exhibit an altered gait (986) that can be characterized by temporal-spatial gait parameters and gait kinematics (183) especially at the ankle (136). The altered gait is seen prior to, but exacerbated by pain onset (501), and evident in both limbs, even with unilateral PAD (502, 1011). Thus, the gait pattern of claudicants may include some entrainment based upon history, since it can be evident before flow limits become manifest. However, pain slows walking velocity and increases gait asymmetry (287). While there is a shorted gait that develops in the elderly, possibly related to vibrotactile sensitivity (206), the altered gait in PAD patients can be viewed as a consequence of the limited blood flow experienced during exercise. This has been nicely demonstrated by the induction of a gait change during exercise with cuff occlusion of the legs, even in normal healthy young subjects (647). There has been the suggestion to evaluate PAD patients using a cycle ergometer to avoid the gait problems during treadmill walking. However, the outcome of limited performance and pain onset was similar to that achieved during walking, although a higher cardiopulmonary response could be elicited (940). Treatment of PAD patients with pentoxifylline or cilostazol, which improves exercise tolerance, does not improve gait abnormalities (429, 430). Thus, it appears that gait abnormality is an inherent feature in the sequelae of PAD.
The altered gait mechanics of claudicants would be expected to increase the energy costs of walking, especially after the onset of pain, since there is the marked potential for a modified muscle recruitment that could add inefficiency. This would be particularly seen with the utilization of an inordinate muscle mass or if relatively high energy-cost motor units were to become recruited, to support some fatigue in relatively low energy-cost motor units. Certainly a shortened stride length increases the energy cost of walking, when the velocity of walking is kept constant (409). However, claudicants typically slow their velocity of walking at the onset of pain (287) and this is expected to reduce slightly the energy costs of walking (39). Thus, it is presently unclear whether the energy cost of walking is inherently greater (i.e., at the onset of steady state oxygen consumption 3–5 min after the start of submaximal walking), simply due to an altered gait. Steady state oxygen cost of walking in PAD patients, measured near the onset of exercise, has been found to decrease slightly (378) or remain unchanged (380, 1005) after exercise training. However, gait abnormalities were not improved by 12 months of exercise training, even though there was an enhanced walking performance and a delay in the onset of pain (183).
There can be a marked consequence of PAD on the energy cost of walking, however, if the patient attempts an extended duration of walking at a rate that is challenging but within their capability. It is well recognized in healthy individuals that oxygen consumption increases gradually with time when the exercise intensity is fairly demanding. Muscle fatigue of some motor units is a requisite (123) and the increase cost of exercise is thought to be due to the recruitment of relatively high energy-cost of contraction, fast-twitch motor units to support the relatively low energy-cost of contraction, slow-twitch motor units that presumably have fatigued somewhat (955). Since even a modest walking pace is rather challenging for a person with PAD, the potential for fatigue of the motor units initially recruited during exercise becomes exaggerated, compared to healthy individuals. Womack and colleagues (1005) performed an interesting study in which PAD patients walked at 2 mph for nearly 20 min or until fatigue. This challenging effort was followed by a significant increase in oxygen consumption (~10%) at the end of exercise, as compared to near the beginning of exercise (at 3 min). Thus, the exercise effort was performed in a less efficient manner over time. It is easy to imagine how this increase in oxygen consumption could place the distal muscle at even greater risk of ischemia and lead to the cessation of activity. However, following a prolonged exercise training program of 4 months there was no increase in oxygen consumption over the exercise time during the same walking task, whereas the initial energy cost was unchanged from before exercise training (1005). Thus, the patients performed the prolonged exercise bout more efficiently after training than before. Since these patients realized a significant increase in maximal oxygen consumption (~12%) and a markedly improvement in endurance time (130% increase to the onset of pain, and 67% increase to maximal duration), it is likely that muscle fatigue was less profound after training, as compared to before training. This could lead to a lesser need to recruit additional motor units as time progressed, thereby contributing to the unchanged oxygen consumption. Thus, exercise training can meaningfully improve the physiological responses of muscle function in patients with PAD.
7. Improved Endothelial-mediated Vessel Dilation with Training
Dilation of the large conduit arteries occurs with muscle activity and serves to reduce the upstream resistance to optimally perfuse active muscle. It develops in response to a reduction in the downstream resistance within the active muscle, resulting in an increase in flow through the conduit artery. Absence of this dilatation can impede flow to the active muscles. Flow-mediated dilation (FMD), observed experimentally as the increase in diameter of conduit arteries established by ischemia-reperfusion, is thought to primarily reflect endothelial vasodilatation (518). This measure of endothelial ‘health’, typically obtained from the brachial or femoral artery, has become a useful index of cardiovascular health, as there is a significant reduction in FMD in patients with chronic cardiovascular diseases and it is an independent predictor of increased risk of coronary artery disease (307). Similarly, a low FMD is an independent predictor of PAD (95, 96). Indeed, most patients with PAD exhibit a significantly lower FMD, as compared to healthy individuals. This could contribute to the slower rate of perfusion (447), altered Hb saturation kinetics (58, 289), and metabolic adjustments within the active muscle (317) observed in patients with PAD. The reduced FMD is associated with both the severity and extent of atherosclerosis in the lower limb arteries of PAD patients and predicts a worsened health outcome (95). Further, FMD deficits, in a relatively small group of PAD patients, were related to the presence of the co-morbidity, coronary artery disease, as evaluated by myocardial perfusion imaging (712). This reduction in FMD in patients with PAD is likely related to an inadequate bioavailability of nitric oxide, a potent endothelial-mediated dilator of arteries. Indirect evidence comes from the experiment of Boger and colleagues (84) who administered arginine, a precursor of nitric oxide, to PAD patients. Walking time, measured to the onset of pain and the maximum tolerated, increased along with an improved FMD. However, it is possible that other dilatory processes are important, since administration of prostaglandin E1, which is expected to lead to relaxation of vascular smooth muscle, also improved walking tolerance of these same PAD patients, although it did not change FMD (84). In addition, an increase in sympathetic output, thought to be associated with PAD (cf., Section 15 Cardiovascular Control in Patients with PAD), could be a contributor to the impaired FMD, acting via an exaggerated α-sympathetic stimulation (383). The increase in the potent vasoconstrictor endothelin-1, which occurs in the circulation following exercise in PAD patients (576), could also confound the dilatory response during FMD. Interestingly, PAD patients who exercise to the maximal limit of tolerance exhibit a further reduction in FMD (23, 95, 469, 856) that is relatively short-lived with a recovery over 4 h (469). An extreme effort to maximum exercise tolerance is apparently needed, as submaximal exercise does not alter FMD (856). This distinction is thought to be due to the accompanying increase in reactive oxygen species (ROS), observed during maximal exercise (856), which can reduce nitric oxide bioavailability. Indeed, experimentally providing the antioxidant vitamin C eliminated the exercise-induced reduction in FMD (856). Thus, considerable evidence indicates that there is a dysfunction in flow-mediated dilation in the arteries of patients with PAD that likely contributes to functional limitations in muscle performance.
Exercise training can ameliorate the reduced FMD observed in patients with PAD. Supervised training programs improved exercise tolerance (time to pain onset and maximal effort) and FMD in the brachial artery (16, 23, 92); however, an unsupervised activity program was not effective (16), probably related to lack of compliance. The improvement in time to the onset of pain, established by exercise training, was correlated with the increase in plasma nitrite flux, an index of nitric oxide metabolism (16). There is a general association between higher levels of physical activity and the FMD responsiveness in a selected population of patients with PAD, even when adjusting for age, sex, race, ABI, cardiovascular risk factors and other potential confounders (704). This is similar to the general improvement in vasoresponsiveness observed with exercise training, even in healthy subjects (518). Thus, an improvement in vasoresponsiveness of the supply arteries likely contributes to the improvement in muscle perfusion, thereby enhancing walking tolerance in PAD patients who participate in an exercise training program.
8. Training Adaptations Within the Active Muscle: Increased Capillarity
One of the hallmark adaptations induced within active skeletal muscle by endurance-type exercise training, is an increase in capillarity of the active muscle brought about by the process of angiogenesis (21, 100, 441). This increase in capillary density should enhance the nutritional blood flow within the contracting muscle by increasing red blood cell transit time to exchange oxygen, by shortening the diffusion path length, and by increasing the capillary surface area for diffusion. While it is apparent that the shortened average diffusion path length for oxygen should provide an advantage, Hepple and co-workers (371) provided evidence that the greater capillarity imparts an advantage due to an enhanced capillary-to-tissue surface area, which is thought to be a major site of resistance for oxygen diffusion (411). Regardless of the precise physiological basis, an enhanced muscle capillarity is expected to result in a greater oxygen extraction and muscle performance (245, 1023, 1024). Such adaptations with training could be most significant in patients with PAD where optimizing utilization of the limited oxygen delivery to the distal muscles would be an advantage, as illustrated year ago by Zetterquist (1036) and Sorlie and Myhre (877). Even in the absence of training, Askew and co-workers (38) found a correlation between the area of high-oxidative, high-capillarity fibers in the calf muscle, indicative of well-functioning mitochondria (415), and exercise tolerance in patients with peripheral arterial disease. A reduced capillary density is found in the gastrocnemius muscle of PAD patients who experience intermittent claudication, and in this population, capillary density correlates significantly with several indicators of exercise tolerance such as peak oxygen consumption, peak walking time and claudication onset time (763). Furthermore, an exercise training program can induce increases in capillary density within gastrocnemius muscle of PAD patients, which precedes improvements in peak oxygen consumption (221). Thus, it is likely that an enhanced muscle capillarity, typical of endurance-type exercise training in normal individuals, is also an important contributor to the improvement of exercise tolerance in patients with PAD. As such, it is important to better understand the process of angiogenesis and its control.
8.1. Skeletal Muscle Capillary Morphology
The capillaries within skeletal muscle are composed of a layer of thin endothelial cells, having an average cell thickness 0.3 μm excepting at the nuclear region. These cells are tightly opposed to each other, often with overlapping or interwoven junctional regions. The endothelial cells are surrounded on the abluminal side by a continuous basement membrane, composed predominantly of the extracellular matrix proteins type IV collagen and laminin. Pericytes, which are located within the basement membrane, form processes that extend around the capillary and, at variable regions, extend directly through the basement membrane inner leaflet to form tight junctions with the abluminal endothelial cell surface (389). The advential region surrounding the basement membrane is composed predominantly of fibrillar interstitial collagens, as well as elastin fibers and some amorphous matrix materials. Perivascular cells (mast cells, macrophages, fibroblasts) also are localized intermittently within this matrix (106).
Capillaries within skeletal muscle are oriented preferentially in parallel with muscle fibers. Krogh’s pioneering studies of oxygen transport in skeletal muscle (504), whose model of oxygen diffusion often is referred to as the “Krogh cylinder”, resulted in the widely accepted portrayal of skeletal capillaries as straight unbranched structures. However, detailed morphological analyses of skeletal muscle microcirculation using corrosion casting and scanning electron microscopy reveals a highly complex capillary geometry, characterized by the presence of anastamoses formed by lateral branching of capillaries, and a high degree of capillary tortuosity (451, 486, 587). Furthermore, geometry of the capillary network is not static, because capillary orientation (degree of tortuosity) varies substantially with sarcomere length, providing an indication that skeletal muscle capillaries are subjected routinely to mechanical perturbations. Capillaries are tethered to the surrounding tissue by extracellular matrix. These tethers serve to transmit load to the abluminal capillary wall when the muscle fibers change orientation (i.e. during contraction, relaxation or lengthening)(237, 318).
8.2. Assessment of Capillarity in Muscle
Inherent in the capacity to quantify changes in capillary number is the ability to accurately detect all capillaries within the muscle. Early studies of capillary number in muscle utilized India ink-infusion to identify capillaries. However, this technique identified only the perfused vessels, and thus, would lead to under-representation of the anatomical number of capillaries if the perfusion pressure was not adequate. Direct staining of the endothelium utilizing a periodic acid Schiff reaction, or utilizing colorimetric substrates for alkaline phosphatase (an enzyme that is enriched within capillary endothelium of many animals) facilitates the detection of all capillaries within a muscle. However, alkaline phosphatase is present in all vessel types in humans, thus cannot be used reliably to detect capillaries in human tissue samples. Some types of plant-derived lectin bind with high affinity to glycoproteins on the surface of endothelial cells, and thus, are useful tools for detection of capillaries. However, lectin affinities vary between species; for instance, Griffonia simplicifolia agluttinin I-B4 (which has affinity for α-d-galactosyl and N-acetyl galactosaminyl residues) interacts strongly with glycoproteins on the surface of rodent skeletal muscle endothelial cells, but only with human endothelial cells from subjects having the B blood group (342, 509, 713). Ulex europaeus lectin interacts strongly only with endothelial cells of human origin (18, 410).
The second issue of concern regards the presentation of capillary number. Data commonly are reported as capillary density (number of capillaries per mm2 of tissue), capillaries around a muscle fiber, capillary to muscle fiber ratio, or capillary to fiber perimeter ratio (586). Capillary density may provide a realistic value of the oxygen distribution to a certain size region. However, capillary density is dependent on myofiber size, and thus changes in capillary density do not necessarily correspond to events of capillary growth or rarefaction. Measurements of capillary density also vary dependent on the fixation technique utilized, as variable levels of tissue shrinkage will skew the density values. Capillary to fiber ratio, by normalizing total number of capillaries to the number of whole myofibers within a field of view, avoids the effects of changes in myofiber size, or tissue shrinkage, and can more accurately represent changes in the structure of the capillary network. However, an implicit limitation in presentation of this normalized value is that information about capillary spacing is lacking.
By combining detection of alkaline phosphatase (for capillaries) and cytochrome oxidase (to distinguish myofiber type), Romanul (773) made the seminal observation that the density of capillaries around a muscle fiber is proportional to the oxidative activity of the fiber. This result fuelled discussion of the concept that structural adaptations generate a non-uniform capillary network within muscle, specialized to ensure matching of oxygen delivery with cellular metabolic demand. However, modeling of oxygen delivery in a way that takes into account the non-homogeneous layout of the capillary network remains a challenge (229).
8.3. Early observations of capillary remodeling in skeletal muscle of animals and humans
The earliest studies that indicated exercise-induced increases in muscle capillary number, conducted by Vanotti and Magiday and Petren and colleagues in the 1930’s, relied on detection of capillaries by dye infusion or by the presence of red blood cells (as reviewed in (329, 422)) and thus their results may have reflected differences in flow to the muscle rather than an anatomical difference in capillary number. Similarly, Carrow et al (131) reported an increase in capillary number relative to muscle fiber number following 35 days of either voluntary or forced exercise, observing also that the greatest increase in capillary number was detected in association with white, rather than red, muscle fiber. Their detection of capillaries utilized infusion of India ink, and thus, they concluded that their data provided evidence of the opening of precapillary sphincters to allow more flow to specific areas of the muscle.
Myrhage and Hudlicka, using a combination of histological and “real time” intravital recordings, provided concrete evidence of capillary angiogenesis (i.e. new capillary sprouting) in response to increased muscle activity induced by electrical stimulation (648). The type of sprouting they described concurred with descriptions of capillary growth made from other model systems (41, 159), suggesting the existence of a conserved process.
Adolfsson (8) showed that endurance training (swimming) of rats could induce significant increases in capillary to fiber number. The pattern of capillary growth varies in association with the type of motor unit recruitment. Mai and colleagues observed that endurance training of guinea pigs evoked more capillary growth around oxidative muscle fibers (569). Conversely, the greatest amount of capillary growth in rat muscle activated by electrical stimulation was observed to occur around glycolytic muscle fibers (45), fiber consistent with recruitment of these muscle fibers by neural stimulation.
Examining the musculature of humans, Hermansen and colleagues (100, 373) reported a correlation between level of endurance training (as indicated by VO2 max) and capillary to muscle fiber ratio by cross sectional comparison of untrained and trained individuals. In concordance with animal studies, Andersen and Henriksson (21) first showed that a training program induced significant increases in capillary number in human skeletal muscle, postulating growth of new capillaries in response to the exercise stimulus. Ingjer (442) replicated and extended this observation by reporting that the exercise-induced change in capillary number varied relative to the associated muscle fiber type, with the greatest increases occurring around type I fibers, and the smallest response associated with type IIb fibers (442).
8.4. Sprouting Angiogenesis
The conventional process of angiogenesis is understood to occur via a series of well-orchestrated cellular events (as reviewed in (128, 265, 708)). Angiogenic stimuli activate the endothelium, and through a cascade of intracellular signals, first cause increased endothelial cell permeability through dissolution of adherens junctions. Endothelial cell proliferation occurs early in the angiogenesis process, and continues to occur in specific locations as the new capillary sprout elongates. Proteolysis of basement membrane matrix components is necessary to promote endothelial sprout invasion into the surrounding interstitial matrix. Cellular migration is triggered and the sprouting tip of the endothelial cell proceeds into the interstitium, utilizing filopodia/or lamellipodia extensions to explore the interstitial matrix. Lumen formation occurs as the sprout forms a multi-cell structure. The new capillary channel forms an anastamosis with a pre-existing capillary, creating a new patent capillary. Ultimately, the nascent capillary is stabilized through the construction of basement membrane matrix proteins, re-establishment of adherens junctions and cessation of endothelial cell activation. Each of these stages is described below in greater detail.
8.4.1. Increased capillary permeability
Angiogenic factors commonly induce alterations in endothelial cell permeability. This occurs via re-organization of the adherens junctions, which form the major permeability barrier throughout the majority of the vascular system (as reviewed in (199, 200)). The adherens junction is formed by a dimer of Vascular Endothelial (VE-)-cadherin proteins that interact with each other through extracellular domains at sites of cell-cell contact. The cytoplasmic domain of VE-cadherin links with adaptor proteins (p120, β-catenin and plakoglobin), which in turn form a bridge between VE-cadherin and the anchoring actin cytoskeleton, through association with actin-binding proteins such as α-actinin. Tyrosine phosphorylation of VE-cadherin, p120 and β-catenin may occur in response to growth factor stimulation. This phosphorylation likely is mediated by src kinase, though inhibition of specific phosphatases such as VE-protein tyrosine phosphatase will promote similar end-effects. Phosphorylation modifies protein-protein affinities, destabilizing the binding between VE-cadherin proteins as well as between VE-cadherin-catenin complexes. As a result, the adherens junction loosens. widening the gap between adjacent endothelial cells, which promotes enhanced filtration of fluids and macromolecules from plasma to the interstitial space. Permeability also may be regulated by enhanced clathrin-dependent internalization of VE-cadherinVEcadherin or by proteolytic cleavage of the extracellular domain of VE-cadherin. It is postulated that these changes in permeability assist in promoting subsequent events in the angiogenic cascade. Plasma components that filter into the interstitial matrix (i.e. plasminogen, fibrinogen) may activate adhesion proteins and growth factor receptors on the cell surface, which further stimulate the endothelial cell proliferative and migratory phenotype. Furthermore, intracellular signals triggered by the changes to the adherens junction promote cell proliferation, migration and invasion. While it is clear that substantive increases in permeability occur in wound healing and tumor angiogenesis, the extent of change in capillary permeability that occurs during activity induced angiogenesis has not been determined.
8.4.2. Proliferation of endothelial cells
Under normal conditions within the adult, proliferation of capillary endothelial cells is extremely limited. The estimated capillary endothelial cell half-life is 1000 days (244). However, endothelial cell proliferation does occur within capillaries that have been exposed to an angiogenic stimulus. Switching from a quiescent to a proliferative phenotype requires cellular transition from Go to G1 of the cell cycle, which often is stimulated by PI3K/Akt and ras/MAPK signal pathways. MAPK (ERK1/2, JNK1/2) transmit the proliferative signals associated with growth factor stimulation of endothelial cells (691). MAPK, through activation of transcription factors such as c-fos, c-jun and c-myc, promote simultaneous upregulation of cyclins and cyclin dependent kinases and downregulation of inhibitory proteins such as p27. Electrical stimulation of muscle (10 Hz, 8 hrs per day) induces significant increases in endothelial cell proliferation within 3 days, as indicated directly by BrdU labeling or indirectly by immunodetection of markers of cell cycle progression such as proliferating cell nuclear antigen (PCNA) or Ki-67 (228, 420).
8.4.3. Proteolysis of basement membrane and interstitial matrix
The basement membrane is an uninterrupted layer of matrix proteins surrounding the capillary. Integrin-mediated adhesion of endothelial cells to these matrix components contributes structurally to capillary integrity and biochemically, via activation of intracellular survival signal pathways. Deletion or mutation of basement membrane proteins, such as laminin α4, causes development of weak, leaky vessels, resulting in embryonic lethality (926). During the process of angiogenesis, the production, secretion and activation of enzymes facilitate the cleavage of adhesion proteins and matrix proteins, enabling endothelial cells to be released from the stabilizing influence of the basement membrane (472). Sprouts protrude through breaks in the extracellular matrix (334, 341). It has been postulated that sprouts occur most frequently in the locations that are closest to perivascular cells (pericytes, fibroblasts) (227). While the process of angiogenesis in tumors or wound healing appears to involve complete dissolution of the substantial regions of basement membrane, sprouting of skeletal muscle capillaries involves circumspect proteolysis that is limited to the tip region of the sprout, while the basement membrane remains intact throughout the remainder of the capillary (1045).
Enzymes associated with basement membrane proteolysis include matrix metalloproteinases (MMPs) and plasminogen activators (PA) (333, 709). Chronic electrical stimulation or muscle overload induces expression of MMP-2 and MT1-MMP in endothelial cells (334, 762). MMPs also are produced by perivascular cells and myocytes. Inhibition of MMP activity is sufficient to block the angiogenic response to chronic electrical stimulation, although the endothelial cell proliferation response was not affected (334). This finding suggests that proteolysis of matrix bound growth factors is not required to initiate the process of endothelial cell proliferation, but that proteolysis is required to permit sprout formation.
8.4.4. Migration and extension of the sprout
Extension of the proximal end of the sprout is led by the “tip” cells which form long filopodia and are enriched with receptors for Vascular Endothelial Growth Factor A (VEGFA) and other growth factors. These filopodia are highly dynamic, and may undergo either rapid formation or regression as they “feel” for directional cues. Deposition of VEGFA165 into the matrix appears to play a predominant role in providing guidance cues for the migrating tip cell. In the retina of animals expressing only the soluble form, VEGF120, filopodia formation occurs less frequently and in a disorganized way, with loss of polarity (300). Tip cells have a differential pattern of gene expression compared to stalk cells. Tip cells are characterized by enrichment in VEGFR2 and VEGFR3, PDGFB, Unc5b and, dll4 and jagged (7, 796). Phenotypically, these cells are migratory rather than proliferative, forming more extensive filopodia/lamellipodia than can be observed on stalk cells (400). This differential phenotype is in part attained through dll4/Notch signalling. Signals from dll4 (expressed on tip cells) activate Notch on adjacent stalk cells, which represses their sprouting (366). Stalk cells express another Notch ligand, Jagged1, which has only weak capacity to activate Notch. Therefore, Jagged1 competes with dll4 for binding to Notch receptors on adjacent tip cells, effectively silences Notch signaling. This reciprocal activation and inhibition of Notch helps to establish the tip cell selection (71). Cells treated with dll4 show a reduced sensitivity to VEGFA, as evidenced by reduced capacity to activate ERK1/2 in response to VEGF165 (345). This may be a result of Notch-dependent downregulation of VEGFR2 (916) or its co-receptor neuropilin-1 (996) or by upregulation of VEGFR1 (345). VEGFA itself induces the expression of dll4 in tip cells (548, 892). Thus, cells in which extending filopodia encounter matrix-bound VEGFA165 will be stimulated to produce dll4, which will promote the maintenance of the tip cell phenotype.
It is likely that the same guidance molecules are utilized for sprouting angiogenesis in the majority of tissues. For instance, blockade of dll4 inhibits tumor growth because it promotes deregulated formation of nonfunctional vessels (660, 758). However, an important issue to consider in extrapolating the findings from models of sprouting in zebrafish or retina to understanding sprouting in skeletal muscle is that of the role of tissue density in determining permissive sprouting pathways. The myocytes create considerable spatial constraint that limits the possible routes for sprouting (229). The tightly regulated spatial organization of capillaries within the muscle suggests that new sprouts are directed in a non-random process. The role of VEGF gradients and guidance molecules remain to be investigated in this micro-environment.
8.4.5. Lumen formation and stabilization
The growing sprout must form a patent lumen in order to establish a new functional flow pathway. The process by which a lumen forms is not well established, though several potential mechanisms have been described through combination of cell culture and electron microscopy observations (reviewed in: (230, 444). Coalescence of intracellular vesicles is one mechanism by which lumena form. This is substantiated by in vivo observations of “seamless” capillaries (i.e. growing sprouts that are formed by a single endothelial cell). Sprouts formed by 2–3 co-migrating endothelial cells also are observed, and these sprouts may form intercellular lumena. In both cases, it appears likely that fusion of intracellular vesicles with the plasma membrane assists in the progressive enlargement of the lumen. It also is feasible that both events may occur within a single growing sprout, with the tip cell acting differently than those cells forming the stalk of the sprout.
Integrins, particularly α2β1, are required for successful lumen formation in 3D cultured endothelial cells (190). Ccm1, a gene product associated with cerebral cavernous malformations, regulates lumen formation through activation of Rac1 GTPase (543). Ccm1 induces production of extracellular matrix proteins and activates the dll4/notch signal pathway (1012). Ccm1 may contribute to stabilization of the newly formed sprout, through inhibition of proliferation and migration while protecting cells from apoptosis via activation of Akt (1012).
8.5. Alternate forms of capillary angiogenesis: intussusception and luminal splitting
The concept of capillary growth without sprout formation was first proposed by Short (849), who postulated that capillary growth in the lung occurred through the insertion of interstitial tissue columns into the lumen of pre-existing vessels. Caduff et al (117) extended these observations, noting that the tiny “holes” in plaster casts of the post natal rat lung were actually tissue posts inserted into the lumen of the vessel. This form of angiogenesis was termed intussusceptive microvascular growth, literally meaning that the growth occurred with “in-itself”. Since then, intussusceptive microvascular growth has been described in a variety of embryonic and adult tissues including the chick chorioallantoic membrane, the myocardium, and tumors (109, 702, 960). Intussusceptive division of capillaries is thought to occur in response to elevated hemodynamic forces. It is initiated by the inward protrusion of perivascular cells, which pinches the capillary wall, causing the opposing walls of the capillary to come into contact with each other, leading to the formation of new intercellular junctions (571, 891). The endothelial bilayer then appears to be perforated by the interstitial tissue, which creates a pillar surrounded by the endothelial cells. These cross-luminal pillars are composed of cellular projections of myofibroblasts and/or pericytes, as well as collagen fibrils and other matrix components (571).
Rigorous investigation of skeletal muscle capillary morphology by transmission electron microscopy led to the description of an alternative mechanism of capillary network expansion, through the luminal division or “splitting” of capillaries (228). In this process, filopodial extensions are seen to protrude into the lumen rather than from the abluminal surface. These filopodia often, but not exclusively, form at sites of cell-cell junctions. The extending filopodia connect with the opposite surface of the capillary, forming a cellular bridge across the lumen. This bridge continues to extend along the length of the lumen, effectively creating two parallel flow channels (1046). The internal wall between the two lumena remodels, which allows for physical separation of the channels and results in two distinct capillaries. Capillary networks remodelled by this means are characterized by fewer lateral branches, and a preferentially longitudinal orientation of the capillaries (228). This process has been observed to occur only under conditions in which capillary blood flow is elevated, indicating that it is a response to altered hemodynamics. It is postulated that this process occurs as a means to facilitate self-regulation of shear stress within the microcirculatory network (435). The addition of parallel flow paths will result in redistribution of flow, and lower shear stress within individual capillaries. During luminal splitting, endothelial cells exhibit indications of activation (cell thickening, increased number of cytoplasmic vacuoles, irregular lumen surface). However, endothelial cell proliferation is modest (228, 624). There is no upregulation of MMPs (762), and the basement membrane remains intact throughout this process (1046).
It remains possible that luminal splitting and intussusception describe different aspects of the same process, or they may be two distinct processes, both capable of remodeling the microvascular network in response to changes in flow. Ultimately, capillary remodelling by intussusception or by luminal splitting will generate two parallel capillaries from one initial capillary. One key advantage of this type of network growth is that it conserves energy, as there is little energy expended on cellular proliferation or migration.
Angiogenic stimuli are generally identified as metabolic, hypoxic, or mechanical, although there can be considerable interaction among these factors. For example, metabolic demand, typical of that that occurs in active muscle, requires cardiovascular adjustments to increase blood flow (oxygen delivery) to maintain tissue oxygenation. However, even when oxygenation of normal muscle is adequate for the metabolic demand, there is a reduction in pO2 within the muscle (65, 66, 753, 754). This relatively low muscle pO2, however, is well within the range to serve as an important signal for angiogenesis by the up-regulation of VEGF (651, 850), a powerful angiogenic regulator. This could contribute to the capillary proliferation that occurs in the active muscles of normal individuals after exercise training (cf., Section 8.3). If hypoxia is established (e.g., by inspiring 8% O2), the increases in VEGF can be amplified (907). Similarly, a limited blood flow, as can occur in PAD, causes difficulty in maintaining a sufficient pO2 of the muscle, if the metabolic demands of the activity surpass that that can supported aerobically. Thus, inadequate blood flow, ischemia, is expected to exacerbate the stimuli for angiogenesis (971). Hypoxemia would also contribute, if there were attendant pulmonary dysfunction to reduce oxygen saturation or a reduced inspired pO2 associated with altitude. Thus, exacerbating the decline in muscle pO2 can enhance the stimulus for angiogenesis, as well as alter metabolic and mechanical stimuli that might normally occur with contractions. While we will separate these factors in the discussion below, it is apparent that a low pO2 (hypoxia), metabolic factors, and mechanical stress should be viewed as a comprehensive set of stimuli for angiogenesis.
8.6.1. Metabolic factors that induce angiogenesis
Metabolites produced in exercising muscle are attractive to consider as potential inducers of angiogenesis because they could directly couple the elevation in cellular metabolic activity with signals for capillary growth (237). To address the impact of metabolic stress on skeletal muscle adaptation to exercise in humans, researchers have utilized an exercise protocol conducted under flow-restricted conditions, in which flow to the exercising leg is reduced by 15–20% (81). Venous O2 saturation decreases and plasma lactate increases, indicative of a reduction in oxygen availability (330, 896). Exercise training under these flow-restricted conditions induces a greater angiogenic response than comparative exercise under non-restricted conditions, indicated by increased labeling of proliferating cells (332) and enhanced capillary to fiber number after 4 weeks (896).
Of the metabolites produced and released by exercising skeletal muscle, adenosine has received the greatest attention as an angiogenic factor. Other metabolites such as lactate, pyruvate, hydrogen or potassium ion provide excellent indicators of metabolic status, but either have no direct effect or they exert a negative effect on endothelial cell angiogenic behaviour or on the stimulated release of angiogenic factors from other cell types (41, 128, 265). As a result, discussion will be limited to the evidence for adenosine as an angiogenic stimulus. Key to the hypothesis that adenosine mediates activity-induced angiogenesis is whether levels of adenosine increase in sufficient quantities, and in an appropriate time frame, within the active muscle. Extracellular levels of adenosine increase in the heart and other tissues during exercise and tissue ischemia (6). Interstitial adenosine accumulates in muscle during exercise, with the amount detected proportional to workload (363).
Extracellular adenosine is derived mainly from cellular release of adenosine triphosphate (ATP). Extracellular ATP is converted rapidly on the endothelial surface to adenosine, first through the enzymatic activity of the ecto-apyrase (CD39, which converts ATP to adenosine monophosphate [AMP]) and then by the ecto-5′-nucleotidase (CD73, which converts AMP to adenosine) (as reviewed in (549)). The affinity of 5′ nucleotidase for AMP increases when pH decreases from 7.4 to 6.8, resulting in greater conversion of ATP to adenosine (145). Multiple cellular sources of ATP/adenosine exist within skeletal muscle. Elevated extracellular adenosine is detectable in cultures of primary rat skeletal muscle fibers activated by electrical stimulation(361, 362, 562). Lactic acid and low intracellular pH both are associated with elevated release of adenosine from skeletal muscle cells (50, 51). Endothelial cells also release adenosine (207, 208). Furthermore, ATP is released from transiting red blood cells when the cells are exposed to low oxygen tension (as reviewed by (238)). Conversely, adenosine can be rapidly cleared from extracellular fluid through passive or active uptake by nucleoside transporters. Equilibrative nucleoside transporter 1 and 2 (ENT1,2) are expressed at high levels on vascular endothelial cells while skeletal myocytes predominantly express ENT1,2,4 (549). Adenosine is degraded rapidly to inosine by the action of the extracellular ecto-enzyme adenosine deaminase (240). Thus, extracellular adenosine concentration is regulated not only by cellular production, but also by the activity of ecto-enzymes and transporters.
In support of the role of adenosine as an angiogenic mediator, adenosine receptor blockers interfere with vascular development in chick embryos (1047), and with neovascularization associated with hind limb ischemia (792). Chronic infusion of adenosine leads to increases capillary numbers in rabbit fast twitch skeletal muscle (334). Adenosine stimulates endothelial cell proliferation (228, 609), which may involve production of VEGF (321). Infusion of adenosine into muscle interstitium results in the enhanced interstitial VEGFA protein levels (399). On the other hand, intra-arterial administration of adenosine in intact rat skeletal muscle did not enhance VEGFA expression despite initiating a strong vasodilator response (72). This may reflect the high levels of adenosine deaminase present on the endothelial surface, which could prevent elevation of interstitial adenosine levels to induce skeletal muscle VEGFA production. Thus, while adenosine has angiogenic properties, the contribution of adenosine to exercise-induced angiogenesis requires further investigation.
8.6.2. Hypoxia induces angiogenesis
N. Ashton (36) first suggested that “endothelial cells themselves are in some way directly sensitive to oxygen-multiplying at low 02 levels, resting at normal 02, levels and dying at high 02 concentrations”. Myrhage and Hudlicka postulated that relative lack of oxygen may provoke the growth of new capillaries in skeletal muscle (648). Considering that addition of new capillaries should enhance oxygen delivery to the muscle, it would be logical to conclude that low tissue pO2 is a key homeostatic regulator of the process of angiogenesis.
Measurements of oxygen tension in exercising muscle indicate that tissue pO2 is reduced after a brief delay with the onset of exercise and remains low or recovers slightly, depending upon the fiber type (66, 604). Poole and co-workers made simultaneous assessments of VO2, blood flow and red blood cell O2 saturation. They found that capillary red blood cell velocity increased after just a single muscle contraction, coinciding with the increase in VO2 (487). Similarly, using of nuclear magnetic resonance spectroscopy to assess myoglobin oxygen saturation in humans, researchers found that intramuscular pO2 levels were reduced significantly during exercise (660). Myoglobin desaturation occurred rapidly (within 20 seconds) after initiation of exercise, and accordingly, returned to pre-exercise value within 45 seconds of exercise cessation. Surprisingly, the degree of myoglobin desaturation was similar across a range of workload intensities. However, researchers who employed a different type of exercise protocol to assess myoglobin desaturation were able to demonstrate that desaturation was proportional to VO2max (635), indicating a progressive drop in myocyte pO2 with increased workload. The initial detection of capillary growth to glycolytic fibers (426), where greater declines in muscle pO2 are expected (66, 604), has been cited as evidence to support the role of hypoxia in stimulating exercise-induced angiogenesis.
Adaptive cellular responses to hypoxia are complex, as they differ dependent on whether the stimulus is intermittent or continuous. Furthermore, there are both acute (rapid) and chronic (delayed) phases to the cellular responses that are initiated to adapt to the hypoxic environment (as reviewed by (831, 832). The hypoxia inducible transcription factor (HIF)1 is considered to be a master regulator of the adaptive responses to hypoxia. HIF1, first identified by Semenza and Wang (833), is a heterodimeric protein that consists of α and β (ARNT) subunits. HIF2α (also called also called EPAS-1/HRF/HLF/MOP2) is regulated by hypoxia through similar mechanisms as HIF1α, and also dimerizes with the β subunit ARNT. In contrast to the universal expression of HIF1α, HIF2α is expressed preferentially, although not exclusively, in endothelial cells during development as well as in the adult (242, 262).
Under normoxic conditions, HIFα subunits are rapidly and efficiently targeted for degradation through the action of prolyl hydroxylase domain (PHD) proteins (76). Prolyl hydroxylation facilitates binding of von Hippel-Lindau protein, which in turn promotes the ubiquitination and proteasomal degradation of the HIF protein (reviewed by (471)). Reduction in tissue pO2 reduces the enzymatic activity of the PHD proteins, resulting in an extended half life of the HIFα subunits, enabling dimerization with HIFβ and interaction with co-activators Creb binding protein (CBP) and p300, to drive transcription of a series of oxygen sensitive gene products. Factor inhibiting HIF (FIH-1) hydroxylates an asparagine residue on HIF, which reduces its capacity to interact with p300 and CBP, thus reducing its transcriptional activity. This enzyme also is inhibited under hypoxic conditions (512). Because both HIF1 total protein level and DNA binding/transcriptional activity are enhanced rapidly upon reduction in tissue pO2, substantial changes in HIF target gene expression occur within a short period of time. “Hypoxia-sensitive” genes include growth factors (erythropoietin, VEGFA), connective tissue growth factor, stem cell factor), transcription factors (Ets1, Id2), proteins involved in redox signalling (NOS, hemoxygenase, cytochrome C oxidase, cyclooxygenase) and proteins involved in metabolic pathways (Glut1, PGK1, PFK) (reviewed in (832)).
Deletion of either HIF1α or HIF2α results in embryonic lethality, characterized by severe vascular defects (171, 457, 707), indicating a non-redundant function of these 2 transcriptional regulators. Genes required for glycolysis are exclusively regulated by HIF1α, but VEGFA is regulated by both HIF1α and HIF2α (417). HIF2α also enhances transcription of other endothelial cell genes required for angiogenesis, such as VEGFR2 (241) and Tie2 (927). Endothelial cell-specific deletion of HIF1α impairs the proliferative response to hypoxia, reduces wound healing and tumor angiogenesis, and modifies expression of VEGFR1 and 2 (911). On the other hand, endothelial cell-specific deletion of HIF2α results in loss of blood vessel integrity, high permeability, and reduced expression of VEGFR1, VEGFR2, Ang2 and Dll4, (but not VEGFA) in response to a hypoxic challenge (860). However, the role of HIF1 in skeletal muscle subjected to chronic hypoxia has been questioned. Researchers have found minimal change in HIF1α protein level in muscle biopsies from individuals exposed to environmental hypoxia either briefly or for a period of days (966).
Angiogenic effects of hypoxia also may be exerted through the influence of adenosine signaling. Endothelial cell ENT1 and 2 mRNA and protein are down-regulated by hypoxia, likely involving transcriptional repression by HIF1α (239). Reduced levels of ENT would potentially raise interstitial adenosine concentration. Consistent with this effect, systemic hypoxia has been reported to increase the concentration of skeletal muscle interstitial adenosine in healthy human subjects (567). Hypoxia also induces a shift in human endothelial and smooth muscle cell expression of adenosine receptors from A2A to the more angiogenic receptor, A2B, which is associated with HRE-independent upregulation of VEGFA expression (255). Furthermore, hypoxic stimulation of VEGF mRNA production in cultured endothelial cells can be blocked by treating cells with the adenosine receptor blocker, 8-PT (259). Thus, hypoxia facilitates greater responsiveness to adenosine.
Hypoxia stimulates endothelial cell proliferation (111). Hypoxia is a stimulus for increased capillary density in skeletal muscle of hatchling Canada geese (870) and is a potent stimulus for capillary growth in chicken/quail embryos (222, 890, 1032). In contrast, numerous studies using rodents have reported that skeletal muscle capillary density is unchanged by hypoxic conditions (79, 854, 871, 872). More recently, however, Deveci et al reported that significant capillary growth can be observed in rat skeletal muscle following exposure to chronic hypoxia conditions, noting a considerably stronger response in oxidative muscle (diaphragm, soleus) compared to glycolytic muscles (EDL, TA) (209). Thus, the degree to which hypoxia stimulates angiogenesis remains controversial, and varies substantially between species and between tissue types. The variability in responsiveness to hypoxia suggests that the local or intracellular environment provides contextual information that substantially impacts capillary endothelial responsiveness. Importantly, while sustained systemic exposure to low oxygen may help to explain environmental adaptations of muscle vasculature, it does not adequately explain the response to exercise. Scenarios in which the extent of capillary growth does not vary proportionally with the decline in tissue pO2 are reported under both physiological and pathological conditions (274, 423). Recently, a longitudinal training study reported that capillary density and capillary to fiber ratio increased to the same extent (approximately 16–18% above pre-training levels) regardless of whether subjects were engaged in a low amount, moderate intensiy or a high amount, high intensity training program (220). This finding provides support for the hypothesis that workload-dependent alterations in metabolic or hypoxia-related factors on their own cannot sufficiently account for the angiogenic response to exercise.
8.6.3. Mechanical stimuli that induce angiogenesis: Tensional forces/stretch and shear stress
Experiments in which cultured endothelial cells are exposed to cyclic/static stretch or to shear stress demonstrate the direct responsiveness of these cells to tensional forces. Mechanical stimuli activate a wide range of sensors on the endothelial cell surface, referred to as “mechanosensors”, which include integrins, cell-cell adhesion proteins, tyrosine kinase receptors, G proteins and G protein-coupled receptors, ion channels and glycocalyx components (150). Mechanosensor activation is followed by recruitment of intracellular adaptor proteins (Shc, Grb2) and/or activation of signal cascades, often involving a combination of kinases (PKC, ERK1/2, JNK1/2, p38, Akt) and Rho family GTPases (42, 189, 851). Mechanotransduction is not a feature exclusive to endothelial cells, as fibroblasts, resident immune cells and skeletal myocytes all respond to mechanical activation (102, 440). In endothelial cells, major outcomes include rearrangement of actin cytoskeleton, modulation of proliferation and migration, and altered gene expression. The specific mechanosensors involved, as well as the types of intracellular signaling pathways and resultant shifts in cellular phenotype, vary dependent on the type of mechanical stimulus.
Capillary dimensions are altered significantly with changes in muscle sarcomere length (237). Attachments to the extracellular matrix effectively tether capillaries to the surrounding tissue and act to transmit load to the abluminal capillary wall when the muscle fibers change orientation (i.e. during contraction, relaxation or lengthening). Prolonged muscle overload, evoked experimentally by removal of a synergist muscle, results in lengthening of myofibers (as indicated by increased sarcomere length) induces capillary growth via abluminal sprouting (226, 1045). Overload induces expression of VEGF, which correlates with increased proliferation of endothelial cells, as well as increased production and activation of MMP-2 and MT1-MMP, correlating with basement membrane degradation and abluminal sprout formation (762). Evidence in humans that passive muscle stretch is sufficient to evoke increased adenosine and VEGF(364) provides additional support to the role of altered conformation of the tissue in transducing angiogenic signals.
Application of stretch to cultured endothelial cells elicits an angiogenic phenotype of the cells. Stretch enhances proliferation and migration of endothelial cells (659). Production of ROS via NADPH oxidase increases with endothelial cell strain, which may modulate endothelial cell proliferation, survival and migration pathways (500). Specific integrins (i.e. α2 β1) are required to transmit stretch signals that promote alterations in cell shape (386). Angiogenic genes induced by exposure of cultured endothelial cells to stretch include MMP-2, VEGF, (627), VEGFR2, VEGFR1, Tie2 (627, 1044). Endothelial cell stretch modulates the levels of pro-angiogenic transcriptional regulators such as c-jun, HIF1α and HIF2α. These factors also are elevated in response to muscle overload, and their inhibition prevents overload-induced angiogenesis (627). These findings are consistent with the hypothesis that tensile forces acting on endothelial cells contribute to the overload-induced angiogenic response.
Shear stress is generated by the flow of blood past the luminal surface of the endothelium. Both blood velocity and vessel diameter play significant physiological roles in determining shear stress. Arteries and arterioles actively modulate vessel diameter to manage elevated shear stress associated with increased blood flow, a feedback mechanism referred to as flow-mediated dilation. Long term adaptations to elevated flow involve outward remodelling of the arterial wall (815, 950). However, capillaries lack the capacity to substantially modulate diameter. Under resting conditions, it is estimated that close to 100% of capillaries within the skeletal muscle circulation are perfused (690, 871). Thus, arteriolar vasodilatation results in increased flux through the capillary network. Capillary red blood cell velocity increases 1.5–2 fold within 500 milliseconds of initiation of a skeletal muscle contraction (487). The elevation in capillary shear stress is not limited to the duration of the muscle contraction itself, but remains significantly elevated between episodes of muscle activity (231). Adaptation to chronic elevation in capillary shear stress involves addition of new capillaries via the process of luminal splitting (as described earlier). Chronic administration of the vasodilator dipyridamole induces endothelial cell proliferation and increases capillary number in skeletal and cardiac muscle (575, 591, 932). The alpha adrenergic receptor antagonist prazosin, which induces vasodilatation of skeletal muscle arterioles, stimulates significant capillary growth in skeletal muscle (191). Other vasodilators (adenosine, xanthenes analogs) also show capacity to induce capillary growth in skeletal muscle (1047). In contrast, passive hyperperfusion (1 hr) of the dog hindlimb has not been shown to increase VEGF or FGF2 mRNA, while there was a slight increase in TGFβ mRNA (770). It is possible that the time frame of stimulation or the shear stress magnitude within the microcirculation was not comparable to the vasodilator treatments.
Shear stress is sensed by luminal surface molecules, as well as those at the apical and basal surfaces of the cell. This broad response is credited to the arrangement of the cytoskeletal proteins within the cell, which forms a network that links transmembrane proteins to the nucleus. Shear sensors include glycocalyx, receptor tyrosine kinases (VEGFR2), G protein coupled receptors, cell adhesion molecules (PECAM1) and integrins (839). Elevated shear stress of cultured cells induces re-organization of junctional proteins, reducing endothelial cell permeability (595, 827). . VEGFR2 itself acts as a shear sensor, with its auto-phoshorylation stimulated by exposure to shear stress, which leads to recruitment of downstream signal molecules such as Gab1, Akt, p38MAPK (297, 463, 464). Activation of eNOS also occurs downstream of shear stress-induced VEGFR2 activation (463).
While some of the signaling intermediaries associated with shear sensitivity now are established, there is less known about the specific changes in gene expression that are required in order to support the process of shear stress-induced angiogenesis. Shear stress exposure leads to increased production of VEGFR2 in cultured endothelial cells (4) and in skeletal muscle capillaries subjected to chronic increases in shear stress (997). VEGF production is increased in response to prazosin treatment, and can be localized to capillary endothelial cells (624, 762). The shear stress-induced increase in VEGF production is regulated via activation of VEGFR2 and p38 MAPK (297). Blockade of p38MAPK signaling is sufficient to repress skeletal muscle angiogenesis induced by prazosin administration, perhaps due to the reduction in VEGF production (297). Shear stress increases NO production, which plays a key role in shear stress-induced angiogenesis. Reduction of NO levels either through pharmacological inhibition of NOS, or by genetic knockout of eNOS inhibits new capillary growth (59, 421). Shear stress inhibits production of MMP-2 (623) and MT1-MMP (1033) and also enhances production of protease inhibitors including PAI-1, TIMP1 and TIMP3 (630), all of which may contribute to capillary stabilization and the prevention of abluminal sprouting.
8.7. Angiogenic Role of Vascular Endothelial Cell Growth Factor-A (VEGFA)
Among the known growth factors present in mammals, VEGFA is considered to play the predominant role in promoting angiogenesis. Alternate splicing results in production of multiple forms of VEGFA protein, with amino acid lengths of 121, 145, 148, 165, 183, 189 or 201 (529, 692, 928). These isoforms have conserved N-terminal domains, which include sites for dimerization and for binding to VEGFR1 or VEGFR2. They differ predominantly in the C-terminal portion (exons 6 and 7), which contains domains that enable heparin binding, and sites for binding to co-receptor neuropilin 1, affecting the capacity of VEGFA protein to interact with extracellular matrix components and with VEGFR2 (1006). The predominant pro-angiogenic splice forms of VEGFA are VEGF121 and VEGF165. The significant difference between these isoforms is that VEGF121 is freely diffusible while VEGF165 predominantly is sequestered to the matrix through interaction with heparin sulphate groups on extracellular matrix and cell surface proteoglycans. Some studies have indicated that VEGF121 appears to be less angiogenic than VEGF165 (does not stimulate proliferation, weakly activates ERK1/2, but strongly induces increases in endothelial cell permeability through activation of p-src) (1040). However, other researchers have not observed significant differences in the proliferative capacity of these two isoforms (1039). Nonetheless, mice that were deficient in exons 6 and 7 (resulting in expression only of VEGF121 and no larger isoforms) were shown to be reduced in capacity for angiogenesis (130, 883). mRNA levels of Tie2 and neuropilin-1 were reduced in the muscle and myocardium of VEGF120/120 mice. While VEGF120/120 embryos could undergo vasculogenesis and angiogenesis, the resultant networks were characterized by large caliber vessels with excessive numbers of endothelial cells, and with fewer branch points. This led to the conclusion that heparin-bound forms of VEGF are required to appropriately direct filopodial extensions (784). Interestingly, these investigators also observed that expression of only a heparin binding form of VEGFA (VEGF188/188) generates vascular abnormalities, characterized by thin vessels with many, but disorganized, branches. Thus both soluble and bound forms of VEGFA appear to contribute significantly to appropriate angiogenesis.
More recently, a distal splice variant of exon 8 was reported that gives rise to VEGFAxxxb isoforms (57, 1007). These isoforms differ from their VEGFAxxxa counterparts only in the final 6 amino acids of the C-terminus. They can bind to VEGFR2, but have reduced capacity to activate the receptor, which may be the result of impaired binding to neuropilin 1. Thus, these isoforms act as competitors for VEGFAxxxa isoforms. It has been postulated that the presence of VEGFxxxb isoforms in the adult helps to maintain endothelial cells in a quiescent state, and that disruption of the ratio between VEGFxxxa and xxxb isoforms correlates with disease progression (tumour growth, diabetic retinopathy)(1006). The VEGFA165b isoform was reported to be undetectable in biopsies from healthy human skeletal muscle (331). At this time, there is no evidence that this VEGFAxxxb isoforms contributes to the homeostatic regulation of capillary networks in the skeletal muscle microcirculation.
Heparin sulfate-bound isoforms of VEGFA can be released from the matrix as a soluble protein through cleavage by the serine protease plasmin (416) and by matrix metalloproteineases (74). Plasmin cleavage results in ~17 kDa forms of VEGF165 and VEGF188. Likewise, cleavage of these isoforms by MMP-3, 7,9,19 also generates a small ~ 16 kDa fragment (VEGF113). This fragment can bind to, and induce, VEGFR2 phosphorylation, but is not as efficient at stimulating tumour growth as VEGF165 (523). Interestingly, VEGF165 promotes a migratory phenotype (sprouting and branching) while VEGF113 stimulation primarily results in increased cellular proliferation.
An emergent theme is that the means by which VEGFA is presented to endothelial cells significantly factors into the phenotypic response of the endothelial cells, thus modulating the resultant angiogenic response. This is dependent in part on which isoforms are being produced within the local environment. Isoform expression varies from tissue to tissue. However, shifts in isoform production within a tissue may favor pathological angiogenesis (1006). Interestingly, the microenvironment also may regulate VEGFA presentation to the endothelial cells. For example, VEGFA binding to heparin sulfates, and to fibronectin is pH dependent. As pH decreases, there is enhanced binding of VEGFA to cell surface HSPG and to fibronectin (305, 306).
8.7.1. Regulation of VEGFA production: transcription, splicing and translation
Transcription of VEGFA increases substantially when cells are subjected to hypoxic conditions (631, 692). This regulation is dependent in part on the presence of hypoxia response elements (HRE) in both 5′ and 3′ flanking regions of the coding sequence, which facilitate the binding of HIF1 or HIF2 transcriptional regulators (632). Despite the strong evidence of the requirement for these sites based on in vitro studies (850), researchers found that deletion of the HRE sites in the VEGFA promoter does not result in substantial physiological reduction in VEGFA production in vivo (685). Mice expressing this mutant do not have global vascular defects, and skeletal muscle VEGF levels are unaffected in normoxia (685). In fact, this study reported reduced VEGFA mRNA levels in skeletal muscle of both wildtype and HRE-mutant mice upon exposure to hypoxia. These findings indicate that HIF is not a prominent physiological regulator of VEGFA expression in muscle.
Numerous cis-elements aside from the HRE regulate VEGFA expression under both normoxic and hypoxic conditions (as reviewed by (692). PGC1α interaction with estrogen response elements within the VEGFA promoter results in enhanced, but HIF-independent, expression of VEGFA in response to hypoxia and nutrient stress. This supports a mechanism by which metabolic status of the tissue can regulate VEGF production. In support of this hypothesis, AICAR, which is an activator of energy-sensor kinase AMPK, stimulates VEGFA production in skeletal muscle. This effect does not occur in PGC1α−/− mice, providing evidence that PGC1α mediates the AMPK-induced increase in VEGFA production (526). However, the role of the AMPK signal pathway in regulation of VEGFA is not as apparent when considering data obtained from muscle specific AMPK−/− mice. These animals have lower than normal basal muscle capillarization, which is consistent with a reduced expression of VEGFA. However, exercise-stimulated VEGFA levels are greater in these mice compared to wildtype controls (1055), suggesting both that alternative regulatory mechanisms control VEGFA production, and that AMPK/PGC1α may limit VEGFA expression, under exercise conditions.
VEGFA transcription is upregulated by activation of tyrosine kinase receptors including those responsive to EGF, insulin/IGF, HGF, PDGF and FGF, which share the feature of inducing activation of Ras/MEK/ERK1/2 and PI3K/Akt pathways. Sp family, AP1 and Egr-1 transcription factors are associated with growth factor induced transcription of VEGF-A, with Sp1 and Sp3 playing predominant roles (692). ERK1/2 phosphorylates Sp1, which stimulates Sp1 binding and trans-activation of the VEGFA promoter (619). Interestingly, skeletal myocyte expression of VEGFA is regulated by MyoD, linking VEGFA production with the skeletal muscle differentiation program (107). In fact, stimulation of embryonic stem cells with VEGFA promotes differentiation of cells into myocytes (107).
Given the evidence for differential functional consequences of VEGF121 versus VEGF165 cell stimulation, the regulation of isoform splicing may be critical for understanding the physiological and pathological modulation of VEGFA signaling in skeletal muscle. There is evidence that the microenvironment can modify the production of VEGFA splice forms. Decreased cellular pH results in elevated production of VEGF121 alone. Similarly, cobalt chloride (used as a hypoxia mimetic) induces VEGF121 to a greater extent than VEGF165 (236). However, regulation of VEGFA isoform splicing remains poorly understood.
Overall, cellular rate of transcription declines upon exposure to hypoxia. Thus, in order for cells to upregulate specific hypoxia-inducible gene products such as VEGFA, they must employ posttranscriptional regulatory mechanisms. mRNA stability is regulated through interaction of RNA binding proteins to 5′ or 3′ untranslated regions of mRNA. These proteins likely function through competing for binding with degradation-inducing RNA binding proteins or microRNAs. Human antigen R (HuR) is an example of an RNA binding protein that enhances the stability of VEGFA mRNA (as reviewed by (582)). 5′ cap-dependent translation of proteins also is inhibited under hypoxic conditions. Thus, increased production of VEGFA under hypoxic conditions relies on translation from one of two internal ribosomal entry sites (IRES) (13, 87). An upstream open reading sequence acts as a negative regulator of VEGF121 transcription under normoxic conditions (56), providing evidence of a mechanism by which isoform switching may be regulated by hypoxia. Additional regulation of VEGFA translation occurs through the actions of various miRNA (418).
8.7.2. VEGF receptors and intracellular signaling
VEGFR1, VEGFR2 and VEGFR3 (restricted to lymph endothelial cells) (which correspond to flt1, flk1, flt3, respectively, in the mouse) belong to the tyrosine kinase receptor family (as reviewed in (683, 804, 844)). Their intracellular tyrosine kinase domains are highly conserved, and recruit similar activation pathways as EGF and IGF receptors. The extracellular domain consists of 7 Ig domains, classifying VEGF receptors as part of the immunoglobulin superfamily. VEGFR1 plays a significant role in development of the vasculature, however in the adult, its primary role is thought to be as a decoy receptor. VEGFR1 competes with VEGFR2 to bind VEGFA. The VEGFR1-VEGFA interaction is high affinity, but VEGFR1 possesses a much lower kinase activity than VEGFR2, and minimal intracellular signalling is initiated by this binding (804). Soluble VEGFR1 is produced by alternative splicing and by proteolytic clipping of the full length receptor. This product retains the capacity to bind VEGFA but is no longer tethered to the membrane, and cannot induce intracellular signals. This form of VEGFR1 is associated with downregulation of angiogenic signaling (976). Formation of VEGFR1-VEGFR2 heterodimers also reduces signaling associated with VEGFA stimulation. Thus, the balance between VEGFR1 and VEGFR2 modulates VEGFA efficacy, and may serve as a physiological mechanism to limit angiogenesis in the adult. Pathological expression of VEGFR1 also may be associated with impaired angiogenesis (as discussed in later section). Neuropilins (Nrp) act as co-receptors for VEGFRs. Nrp1 interacts with VEGFR2, and enhances the binding of VEGF165 (but not VEGF121) to VEGFR2 (874). Mice lacking nrp have vascular defects associated with defective VEGF signalling (903). Nrp1 may preferentially regulate cell motility signals associated with VEGFR2 signalling (982). Mice deficient in nrp1 are characterized by deficiency in cell migration, while cell proliferation is not affected (301, 468).
Binding of VEGFA to VEGFR2 causes receptor dimerization and phophorylation of a subset of the 17 tyrosine residues within the intracellular domain of the receptor. This facilitates recruitment of adaptor proteins containing SH2 (Src homology 2) or PTB (phosphotyrosine binding) domains. The specific downstream signaling molecules recruited and activated following receptor phosphorylation depends in part on which specific tyrosine residues have been phosphorylated (as reviewed in (683)). Tyrosine residue Y951 binds T-cell-specific adapter molecule (TSAd), which promotes actin reorganization and migration, but does not affect proliferation (589). Y1175 can bind to multiple SH2-domain containing molecules, including phospholipase C-γ1, and p85 of phosphatidylinositol 3 kinase (PI3K) and Shb. The downstream consequences of VEGFR2 activation will depend on which of these molecules is recruited. For instance, activation of PLC-γ1 recruits Ras-MEK1/2-ERK1/2, leading to cell proliferation (902). On the other hand, PI3K and Shb stimulate cell migration, and PI3K-dependent activation of Akt will promote cell survival and enhanced production of NO (408). Y1001 also binds to PLC-γ1, promoting cell differentiation rather than proliferation (613). Y1214 recruits adaptor protein Nck, which induces cdc42 activation and p38 phosphorylation, leading to actin reorganization and cellular migration (510). Src activation and calcium mobilization are associated with increased cell permeability. While tyrosine residue-specific activation of downstream signal pathways is recognized, there remains little knowledge about the conditions that govern the pattern of tyrosine phosphorylation upon receptor activation.
VEGFA signaling most frequently is considered in association with the induction of angiogenesis. However, it also is evident that constitutive production of VEGFA, and the associated activation of VEGFR2-dependent signal pathways, is required to maintain endothelial cell survival. Notably, this effect involves an autocrine signal loop. Endothelial cell-restricted deletion of VEGFA results in increased apoptosis, despite “normal” plasma levels of VEGF (522). Paracrine VEGF is not able to compensate for loss of endothelial cell expression of VEGF (522).
Other members of the VEGF family elicit angiogenic responses, and may play significant roles in maintaining the stable vascular network. VEGFB displays angiogenic characteristics in some cellular environments, and may be elevated in response to exercise. Placental growth factor (PlGF) also is capable of stimulating angiogenesis (194). These factors bind with highest affinity (VEGFB) or exclusively (PlGF) to VEGFR1. Neither VEGFB nor PlGF appear to be regulated by hypoxia.
8.8. Other Potential Angiogenic Growth Factors within Skeletal Muscle
8.8.1. Erythropoietin (Epo)
Epo is known to induce angiogenesis under some conditions. Activation of the Epo receptor induces JAK2/STAT phosphorylation, Akt signaling, and production of matrix metalloproteinases. Epo induces VEGF production, and may stimulate recruitment of endothelial progenitor cells (988). Sustained Epo release stimulates capillary growth in a model of hindlimb ischemia (534). Epo and the Epo receptor are present in skeletal muscle tissue. Exercise stimulates activation of the Epo receptor (788). This suggests it may play a role in exercise-induced angiogenesis. However, neither a single bolus injection nor repeated injections of Epo promote angiogenesis in healthy human skeletal muscle (556). Thus, the physiological relevance of Epo signaling within skeletal muscle remains unclear.
8.8.2. Hepatocyte growth factor (HGF)
HGF has been described as an angiogenic master trigger (3), in part due to its modulation of Ets-1, which in turn initiates transcription of numerous genes encoding angiogenic modulators (347). Angiogenesis within rabbit ischemic hindlimbs can be induced by protein-or plasmid-based delivery of HGF (29, 638). HGF also has been used to induce therapeutic angiogenesis in a diabetic hind limb ischemia model, and it was postulated that this positive effect may have been a result of reversing the diabetic reduction in Ets-1 production (913). HGF also plays significant roles in satellite cell activation (see Section 9.12.2), thus may coordinate multiple remodelling processes in skeletal muscle.
8.8.3. Fibroblast growth factor 2 (FGF2)
This 18–23 kDa mitogen is capable of inducing proliferation of a number of cell types, including endothelial cells. FGF2 (or, basic FGF) also stimulates the release of VEGF, thus further extending its angiogenic influence (884). FGF2 does not contain a secretory signal peptide, and thus, it is postulated that extracellular FGF2 may arise from cell damage, rather than routine release mechanisms. Extracellular FGF2 interacts with heparin sulphate groups on extracellular matrix proteins, and may be released from matrix by protease cleavage (665). In human muscle, FGF2 is localized to the cytosol and sarcolemma of skeletal myocytes, and is found within the interstitium, but is not localized to endothelial cells (462). FGF2 mRNA in skeletal muscle is repressed by chronic hypoxia (681).
8.8.4. Angiopoietins (Ang) 1, 2
Ang1 and Ang2 both interact with the endothelial cell specific tyrosine kinase receptor Tie2. Ang1 promotes Tie2 kinase activity and downstream intracellular signaling while Ang2 antagonizes Tie2 activation (570). Ang1-dependent activation of Tie2 is associated with promoting the later stages of sprouting (pericyte recruitment, sprout stabilization) and the maintenance of the quiescent endothelial cell phenotype that predominates in the adult vasculature (as reviewed by (40). Ang1 appears to promote events that oppose the effects of VEGFA stimulation, as it reduces capillary permeability and inhibits the expression of pro-inflammatory and pro-thrombotic cell adhesion molecules. However, sustained over-activation of Tie2 also has negative consequences, resulting in dysregulated vascular formations (967). The result of Ang2 antagonism of Ang1 signaling is vessel destabilization and regression (547, 687). The physiological roles of Ang2 now are appreciated to be more complicated than that of a competitive antagonist. For instance, Ang2 activates Tie2 in cultured endothelial cells, inducing chemotaxis and tube formation (634, 919). Thus, Ang2 may be more appropriately classified as a weak agonist of Tie2 (1031). Ang2 cellular effects also vary dependent on the existing levels of VEGFA. If VEGFA is present, Ang2 facilitates the classic angiogenic behaviors of endothelial cell sprouting, migration and proliferation (547). Ang2 also induces basement membrane remodeling and enlargement of capillary diameter. However, if VEGFA levels are reduced, then Ang2 enhances the rate of cell apoptosis, thus promoting vessel regression (547). Thus the phenotype resulting from activation of the angiopoietin-Tie signal axis depends substantially on the ratio of Ang1, Ang2 and VEGFA within the local environment, and examination of only a single molecule has limited usefulness in elucidating functional consequences.
8.8.5. Transforming growth factor β (TGFβ)
TGFβ is secreted in a latent form that requires cleavage or a change in conformation to permit function of the active 25 kDa TGFβ molecule, which then binds to TGFβRI, II (581). TGFβ modulates endothelial cell migration, proliferation, and mural cell recruitment (400). It is known to exert conflicting patterns of action, suggesting that local environment provides strongly modulatory cues that ultimately define the cellular response. The hallmark response to TGFβ is the production of extracellular matrix proteins (collagen) and PDGFB, thus it may play a role in stabilizing newly sprouted and pre-existing capillaries. However, interactions with notch signalling pathway may be necessary to realize these functions.
8.8.6. Platelet derived growth factor-BB (PDGF-BB)
PDGF-BB complements the functions of TGFβ. It plays a role in recruitment of mural cells, resulting in vessel stabilization, and it could promote the remodeling of capillaries to arterioles (77, 360). PDGF-BB also regulates the deposition and turnover of extracellular matrix proteins. In endothelial cells, PDGF-BB production is responsive to shear stress (694), thus may play role in protecting endothelial cells from excessive shear forces through promoting capillary remodeling by processes of luminal splitting, intussusception or arteriolarization.
8.9. Additional Molecular Participants in Angiogenesis
8.9.1. RhoGTPases
The Rho GTPase family includes RhoA, Cdc42 and Rac1. As regulators of major cellular processes including cytoskeletal dynamics, membrane transport, gene expression, cell cycle regulation, survival/apoptosis signalling, RhoGTPases play significant roles in all phases of the angiogenic process. These molecular machines regulate cell shape by modifying cytoskeletal tension. RhoA induces actin stress fiber formation; Cdc42 promotes formation of filopodia and Rac1 induces polymerization of cortical actin and the formation of lamellipodia (250). All three GTPases are activated subsequent to VEGF stimulation. Cellular migration requires co-ordinated and spatially distinct activities of these enzymes, as the extending endothelial tip is pushed forward by nucleating cortical actin to form filopodia (Cdc42) and lamellipodia (Rac1). Conversely, RhoA activity at the rear of the cell induces contractile forces that help to drive the cell forward. Together with these effects on cell migration, the RhoGTPases play significant roles in stabilizing and modulating endothelial cell permeability, through manipulation of the stability of the adherens junctional complexes (878). RhoA, Rac1 and Cdc42 each are implicated in activation of cell cycle progression through stimulation of cyclins D1 and A, and repression of p21 and p27 (875). Rac1/Cdc42 activity is associated with increased production, secretion and activation of matrix metalloproteinases (454), which will facilitate the proteolysis of cell adhesion proteins and extracellular matrix proteins. Cdc42 and Rac1 both are involved in endothelial lumen formation in vitro, through regulating the fusion of intracellular vacuoles (62, 261, 498). While considerable information with respect to the specific roles of these proteins has been generated using cultured cell models of angiogenesis, very little is known about the extent of their involvement in the process of sprouting in vivo.
8.9.2. Angiomotin (Amot)
Amot is a transmembrane receptor for the angiostatic peptide, angiostatin (89, 934). There are two isoforms of Amot, p80 and p130. The p80 form stimulates migration and angiogenesis (which is inhibited by angiostatin). Conversely, the p130 form tightly associates with the actin cytoskeleton, does not stimulate migration, and is associated with a stabilized, quiescent phenotype (246, 247). Amot p80 interacts with the Rho GEF syx, thus regulating focal RhoAGTPase activity at the leading front of migrating cells (247). The p80/p130 ratio correlates with training and may be an indicator of the angioadaptive responsiveness of muscle (779).
8.9.3. β-catenin
β-catenin plays an essential role in stabilization of adherens junctions, as described previously. However, β-catenin exerts additional functions by acting as a transcriptional co-activator. VEGFA stimulation induces tyrosine phosphorylation of VEcadherin and β-catenin, which provokes dissociation of the two proteins (636). Release from the VEcadherin complex allows β-catenin to translocate to the nucleus, where it binds to T cell factor (TCF) or other transcription factors, and acts as a transcriptional co-activator of numerous genes. Key transcriptional targets include c-myc and cyclinD1, two positive regulators of cell cycle progression (310, 664). β-catenin promotes the transcription of several members of the matrix metalloproteinase family. Consensus TCF-binding sites are located within the promoter regions of MT1-MMP and MMP-2, and β-catenin triggers enhanced transcription of these enzymes (214, 391). Furthermore, β-catenin is a transcriptional regulator of dll4 (175), promoting tip cell phenotype. Thus, β-catenin exerts influence at multiple steps of the angiogenesis cascade, promoting changes in permeability, and induction of the proliferative and invasive phenotype required to sustain sprouting.
8.9.4. Matrix metalloproteinases (MMPs)
The matrix metalloproteinases (MMPs) are a family of zinc and calcium dependent endopeptidases. MMPs (more than 20 in total) are divided into the following groups based on common structural elements and proteolytic specificities: matrilysins (MMP-7 and MMP-26), collagenases (MMP-1, MMP-8 and MMP-13), stromelysins (MMP-3, MMP-10 and MMP-11) gelatinases (MMP-2 and MMP-9) and membrane type (MT)-MMPs (MMP-14 (MT1-MMP), MMP-15, MMP-16, MMP-17, MMP-24 and MMP-25) (649, 1001). These enzymes have established roles in the remodeling of basement membrane and interstitial matrix proteins. Additionally, MMP cleavage of extracellular matrix proteins may result in exposure of matricryptic sites. For instance, collagen IV cleavage by MMP-2 makes available a new epitope that acts as a ligand for the integrin αvβ3 rather than α1β1, resulting in a highly pro-angiogenic phenotype (1016).
However, MMPs also modulate cellular functions independently of extracellular matrix proteolysis. For example, some MMPs cleave the ectodomain of cell surface receptors (141). For example, MT1-MMP cleavage of full length Tie2 regulates activation of the Ang-Tie2 signal pathway) (684). MMP-dependent cleavage of VE-cadherin modulates endothelial cell proliferation through enhanced accumulation of β-catenin in the nucleus (434). MMPs also modulate growth factor activity. This includes the release of active peptides from latent complexes (in the case of TGFβ1) or the cleavage of growth factors to release them from matrix binding sites. For example, MMP-9 releases VEGF165 from the extracellular matrix, which results in VEGF-dependent angiogenesis (74). As mentioned earlier, proteolytic cleavage of VEGF165 or VEGF188 to form VEGF113 alters the capacity of VEGF to activate VEGFR2 (523). MMP-2 and MMP-9 both may cleave latent TGF-β, releasing the active factor (1030).
MMP-2,-9 and MT1-MMP (MMP-14) have established pro-angiogenic roles (333). Production of these MMPs is controlled predominantly at the level of transcription. Trans-activation of MMP promoters commonly occurs via binding of transcription factors including AP1, AP2 and NFκB, in response to growth factor or cytokine stimulation (160). VEGF stimulation of endothelial cells induces increases in MMP-2 and MT1-MMP mRNA, involving transcriptional regulation by c-jun and β-catenin/Tcell factor (214, 453). Production of MMP-2 and MT1-MMP also is responsive to mechanostimulation, as stretch of endothelial cells and myocytes is capable of increasing mRNA levels of these enzymes (627).
8.9.5. Nitric oxide (NO) and reactive oxygen species (ROS)
NO is generated under physiological conditions by nNOS or eNOS, which are present in endothelium and in skeletal myocytes. The activity of both of these two NOS enzymes is regulated by a calmodulin binding domain, which separates the oxygenase and reductase domains. Calcium binding to calmodulin provides the conformational shift to allow for efficient generation of NO, making NO production sensitive to intracellular Ca2+ levels. Akt phosphorylation of ser1177 on eNOS is a major regulatory mechanism, enhancing the production of NO at any concentration of Ca2+ (210, 275, 615). VEGF induces production of NO, which then stimulates angiogenesis (696, 1048). Conversely, NO also is reported to regulate VEGF production, which would confer to it both upstream and downstream roles in the VEGF signal pathway.
The effects of NO have concentration and time dependent variations, such that opposing influences of NO on angiogenic behaviour have been reported. Elevated shear stress enhances NO production (263), however this may promote vessel stabilization rather than angiogenesis. For example, NO production contributes to the shear stress inhibition of MMP-2 production and activity (623).
ROS, including superoxide and hydrogen peroxide, are produced in endothelial cells due to activation of NADPH oxidase, xanthine oxidase, nitric oxide synthase, as well as in the mitchondrial electron transport chain. ROS production via NADPH oxidase may be stimulated by growth factor receptor signalling (VEGFR2, Ang1, Angiotensin II) as well as by mechanotransduction signal pathways (i.e. in response to shear stress or cell stretch). ROS produced by NADPH oxidase are credited with inhibition of protein tyrosine phosphatases and activation of numerous redox-sensitive cell signal pathways, including, Akt, MAPK, src, PKC, which in turn activate transcription factors such as Ets1, HIF1α, NFκB, p53 (as reviewed by (268, 952). Notably, many of these factors are established pro-angiogenic mediators. In vitro experiments indicate that ROS production promotes endothelial cell permeability, migration, proliferation and survival (268). VEGFA-stimulated tyrosine phosphorylation of VEcadherin and βcatenin, which provokes increased endothelial cell proliferation, is dependent on ROS production and rac activation (636). In agreement with angiogenic behaviours attributed to ROS in cultured endothelial cells, mice null for gp91phox (NOX2; the catalytic subunit of NADPH oxidase) exhibit a reduced recovery to hindlimb ischemia (931, 952). However, ROS effects are concentration dependent, with higher levels inducing substantial oxidative damage.
8.10. Modification of Angiogenic Factors with Activity
The capacity of exercise to induce soluble angiogenic mediators was first observed by Hudlicka et al (428), who reported that tissue samples from exercised hearts stimulated vascular growth in chick allantoic membranes more frequently than those derived from non-exercised hearts. Other researchers also used heparin binding affinity to extract factors (likely FGF2) from muscle homogenates of stimulated or control muscles, showing that the extract from stimulated muscle had an enhanced capacity to induce proliferation of fibroblasts (639). Figures 6 and Table 1 summarizes changes in key angioadaptive factors in response to acute and repeated exercise.
Figure 6.
The predominance of angiogenic factors that are induced in response to repeated exercise (upper panel) and the combination of angiogenic, inflammatory and angiostatic factors that are prevalent during muscle ischemia (lower panel). Refer to the text for additional details.
Table 1.
Modulation of Angiogenic and Angiostatic Factors with Exercise and in Ischemic Conditions
| Exercise | Ischemia | Ischemia + Exercise | ||
|---|---|---|---|---|
| Acute Exercise | Repeated Exercise or Training | |||
| Growth factors/receptors | ||||
|
| ||||
| VEGFA | ↑ mRNA and protein (80, 91, 292, 293, 296, 330, 390, 398, 755, 787) ↓ then ↔ protein (293) ↑ protein in plasma (292, 503) |
↑↑ mRNA and protein short term to ↔ mRNA long term (26, 328, 332, 340, 398, 755, 756) ↑ protein (muscle overload) (762) Deficiency blocks exercise induced angiogenesis (679) |
↑↑ mRNA(acute ischemia) (177, 621, 759, 941, 963) ↑ protein (acute ischemia) (149, 177, 351, 374, 559, 621) ↑ protein in plasma (critical limb ischemia) (258, 572) ↔ protein (intermittent claudication) (258) ↔ or ↓ mRNA, protein (chronic ischemia) (559, 621, 759, 941, 963) ↓ mRNA following chronic hypoxia (681) |
↑↑ mRNA (flow-restriction of exercising muscle) (331, 332) ↑↑ mRNA (exercise in hypoxic conditions) (91) ↑ mRNA (exercise following chronic hypoxia) (681) ↑ mRNA (ischemia + training) (545, 840) |
| VEGFR1 | ↑ mRNA (80, 293, 331, 332, 678) ↔ mRNA (292) |
↔ resting mRNA; ↑ mRNA with exercise (678) ↔ soluble VEGFR1 protein (398) |
↔ full length, ↑ soluble protein in acute ischemia (351) ↑ full length, ↑ soluble protein in acute ischemia + diabetes (351) ↔ soluble VEGFR1 protein in plasma (258, 572) ↑ full length protein (622) ↓ mRNA (chronic hypoxia) (681) |
↑↑ mRNA (flow-restriction of exercising muscle) (331) ↑↑ mRNA (exercise of ischemic muscle) (545) ↓ protein (electrical stimulation of ischemic muscle) (622) ↑ mRNA (exercise in hypoxic conditions) (678) Loss of exercise-induced ↑ mRNA (chronic hypoxia) (681) |
| VEGFR2 | ↑ mRNA and protein (292, 293, 332) ↓ mRNA (678) ↔ mRNA (80) |
↑ mRNA, protein to ↔ mRNA (332, 626) ↔ resting mRNA; ↓ mRNA with exercise (678) |
↑ mRNA (acute ischemia) (759, 941) ↑↑ protein (acute ischemia) (622) ↔ or ↓ mRNA, protein (chronic ischemia) (622, 759, 941) ↓ mRNA (chronic hypoxia) (681) |
↑ mRNA (exercise of ischemic muscle) (545, 840) ↓ mRNA (exercise in hypoxic conditions) (678) ↑ protein (electrical stimulation of ischemic muscle) (622) |
| PlGF | ↔ mRNA or protein (303) | Deletion does not affect exercise induced angiogenesis (303) | ↔ mRNA or protein; deletion does not affect ischemia-induced angiogenesis (302) | -- |
| EpoR | ↑ mRNA and receptor activation (788) | -- | -- | Required for full activation of VEGF/VEGFR2 signals (650) |
| Ang1,2 | ↔ mRNA or protein (292, 332) ↑ Ang2 mRNA (398) |
↑ Ang2:Ang1 protein (332) | ↔ Ang1,2 mRNA (545) ↑ Ang2 mRNA (695) ↑ Ang2 protein in plasma (critical limb ischemia and intermittent claudication) (258) |
↑ Ang2:Ang1 mRNA and protein (flow-restriction of exercising muscle) (332) ↑ Ang2:Ang1 mRNA (exercise post-ischemia) (545) |
| Tie2 | ↔ mRNA (332) ↑ mRNA (292, 398) |
↔ mRNA (332) Loss of acute exercise response with training (398) |
↑ mRNA (acute ischemia) (941) ↔ mRNA (545) ↑ mRNA but ↓ full length:soluble protein (684) ↑ sTie2 in plasma (critical limb ischemia) but ↔ sTie2 (intermittent claudication) (258) |
↑ mRNA (exercise post-ischemia) (545) |
| FGF2 | ↔ or small ↑ mRNA (296, 678) | ↑ or ↔ mRNA (330, 678, 755) | ↔ mRNA (chronic hypoxia) (681) ↔ protein (ischemia) (149) Deletion does not affect ischemia-induced angiogenesis (894) |
Loss of exercise-induced ↑ mRNA with hypoxia (678) ↑ mRNA (chronic hypoxia) (681) ↔ mRNA or protein (exercise post-ischemia (205, 840) |
| TGFβ1 | ↑ mRNA (91, 296, 678) | ↑ mRNA (296, 678) | ↑ mRNA (acute hypoxia), ↓ mRNA (chronic hypoxia) (91) ↔ mRNA (chronic hypoxia) (681) |
Loss of exercise - induced ↑ mRNA (chronic hypoxia) (91) Loss of exercise-induced ↑ mRNA with hypoxia (678) |
| HGF | ↑ by stretch or muscle injury (349) | -- | ↑ mRNA (350) ↑ plasma HGF (acute ischemia) (538) |
-- |
| Dll4 | -- | -- | ↑ protein (acute ischemia) (14) | -- |
| SDF1 | -- | -- | ↑ mRNA (acute ischemia) but ↓ mRNA (chronic ischemia) (963) | -- |
|
| ||||
| Transcription factors | ||||
|
| ||||
| HIF1α | ↔ mRNA (80, 330) ↑ protein (20) ↑ mRNA (555) |
↔ mRNA (328) ↑ mRNA and protein (muscle overload) (620, 625) Loss of exercise response in mRNA after 4 weeks (555); capillary growth is increased in HIF1 deficient mice (579) |
↑ mRNA (acute ischemia) (941) ↑ protein (acute ischemia) (628, 629, 963) ↔ mRNA (chronic ischemia) (941) ↔ protein (chronic ischemia) (759, 963) |
↑ protein (flow restriction of exercising muscle) (20) ↑ protein (post-ischemia) (146) |
| HIF2α | ↑ mRNA (555) | ↑ mRNA and protein (muscle overload) (625) Loss of acute exercise response in mRNA after 4 weeks (555) |
↑ mRNA (acute) (941) | -- |
| PGC1α | ↑ mRNA (662, 663, 717) | ↑↑ mRNA (717) | -- | ↑↑ mRNA (flow restriction of exercising muscle) (662, 663) |
|
| ||||
| Other Pro-Angiogenic factors | ||||
|
| ||||
| NOS | ↑ NOS activity (765) | ↑ eNOS protein (626) ↑ NOS activity (895) ↑ nNOS protein (747) |
↔ to ↓ protein (chronic ischemia) (104) eNOS deletion impairs post-ischemia angiogenesis (645, 698) |
↑ mRNA (exercise post-ischemia) (545) ↑ protein (exercise post-ischemia) (103) |
| MMP-2 | ↔ mRNA (787) | ↑ mRNA, protein and activation (334, 762, 786) ↔ mRNA (398) |
↑ protein and activation (276, 642, 643) Deficiency impairs neovascularization post-ischemia (147) |
↑ mRNA and protein (flow-restriction of exercising muscle) (786) |
| MMP-9 | ↑ mRNA, protein and activity (786, 787) | ↑ mRNA but ↔ activity (786) | ↑ mRNA (695) ↑ protein and activity (276, 642, 643) |
↑↑ mRNA and protein (flow-restriction of exercising muscle) (786) |
| MT1-MMP | ↔ mRNA (786, 787) | ↑ mRNA and protein (334, 762, 786) | ↔ protein (643) ↑ mRNA and protein (684) |
↑ mRNA (flow-restriction of exercising muscle) (786) |
|
| ||||
| Angiostatic factors/receptors | ||||
|
| ||||
| TSP1 | ↑ mRNA (677) | ↓ mRNA to ↔ mRNA (492, 677) | ↑ mRNA acute ischemia (253, 695) ↓ mRNA chronic hypoxia (677) |
Loss of exercise response with chronic hypoxia (677) |
| Endostatin | ↑ protein in plasma (322, 893) ↔ protein in muscle or plasma (787) |
↓ protein in trained muscle (323) ↓ protein in plasma following training (99) |
-- | -- |
| Vasohibin-1 | ↑ protein (490) | ↓ protein (490) | -- | -- |
↑ increased
↓ decreased
↔ no change
-- not reported in literature
8.10.1. VEGFA
Initial investigations reported that VEGFA mRNA in rat TA/EDL muscle increases significantly after 4 days of electrical stimulation, returning to control levels by 21 days of stimulation (340). Since HIF1 can enhance the transcription of VEGF expression is responsive to HIF1, the authors concluded that VEGF expression and subsequent vessel growth is triggered by a low muscle pO2, acting in a negative feedback loop to reduce the original hypoxic signal. Further investigation using rodent models demonstrated that a single intense exercise bout is sufficient to increase levels of VEGFA mRNA within the exercised muscle (80, 80, 91, 296). Exercise-induced changes in VEGFA mRNA are paralleled by increases in VEGFA protein, which localizes predominantly in the type IIb(d) fibers (80), perhaps reflecting a greater decrease in pO2 in these large fibers, as compared to slow-twitch red fibers (604).. In chronically stimulated rabbit muscles, VEGFA immunostaining is detectable in the interstitial matrix between myofibers, and also in interstitial cells and endothelial cells, but not within the myocytes themselves (26). Because the amount of increase in protein was similarly increased between 3 and 21 days, and even was sustained after 56 days of stimulation, the authors discounted a role for hypoxia in stimulating this increase in VEGFA production.
Analogous findings have been reported in humans in response to exercise. Increased VEGFA mRNA and VEGFA protein are detectable in muscle after a single submaximal exercise bout (292, 293, 330, 332, 390, 398, 755, 787). Further increases in VEGFA mRNA are observed if the exercise is conducted under flow restricted conditions (332). The VEGFA165 isoform is most abundant (~40% of total VEGFA mRNA) in human skeletal muscle, but isoforms 121, 165 and 189 all increase in response to an acute submaximal exercise bout conducted under flow-restricted conditions (331). Interestingly, the temporal dynamics varies between isoforms, with the peak increase in VEGFA121 observable immediately after exercise, while the peak in VEGFA165 occurs 2 hr after exercise, and the increase in VEGFA189 occurs only 6 hours post-exercise. It may be speculated that the delayed production of these heparin-binding forms of VEGFA contributes to the promotion of an angiogenic or chemotactic response to subsequent exercise bouts.
Together with increased transcription of VEGFA, synthesis and/or release of VEGFA protein occurs in response to exercise. The plasma VEGFA arteriovenous difference is decreased 1 hour after a single 3 hr bout of submaximal exercise. Given that arterial VEGFA levels remains constant, this indicates increased production and or release from the muscle (390). Other investigators have detected increased VEGFA in microdialysate derived from the muscle interstitial fluid during first 30 minutes of exercise (397). Similarly, VEGFA levels in venous blood are significantly elevated during a single 1 hour exercise bout (787). Overall, these measurements portray a scenario in which both rapid release of VEGFA from the interstitial matrix (potentially through increased proteolytic release of heparin-bound VEGF) and a sustained increase in the production and secretion of VEGFA contribute to the elevation of tissue and plasma VEGFA protein during, and immediately subsequent to, a single exercise bout. Interestingly, venous VEGFA levels drop below pre-exercise levels during recovery from exercise, suggesting that there may be tissue uptake of circulating VEGFA (787). In contrast to the reduced responsiveness of VEGFA mRNA to repeated bouts of exercise, the substantial increase in interstitial VEGFA protein observed within human skeletal muscle following an acute bout of exercise is not attenuated following 4 weeks of moderate intensity exercise training (398). While it is not known whether this reflects increased VEGFA protein production or secretion, or release of matrix-bound VEGFA, these data emphasize the relevance of post-transcriptional mechanisms of control for VEGF protein in exercising muscle.
The elevation in VEGF mRNA and protein observed with an acute exercise bout persists after repeated exercise sessions (26, 328, 332, 340, 762). However, in humans, the response of VEGFA mRNA to a single bout of exercise appears to be blunted by long term training (398, 756). This finding may reinforce the interpretation that the exercise-induced adaptations (capillary growth, metabolic changes within the myocytes) within the muscle result in a reduced production of pro-angiogenic factors.
Several studies support the hypothesis that VEGFA plays a significant role in the process of angiogenesis in skeletal muscle. Inhibition of VEGFA signalling through use of a blocking antibody prevents angiogenesis stimulated by shear stress and by muscle overload. In that study, endothelial cell proliferation was reduced, and morphological analysis by electron microscopy showed that capillary endothelial cells exhibited less signs of activation in the presence of VEGF-trap (993). Administration of a pharmacological inhibitor of VEGFR to rats partially blocks exercise training induced increases in capillary contacts per muscle fiber (544). Finally, disruption of the VEGF-A gene in skeletal muscle using cre/lox technology, either using injection of adenoviral expression of cre recombinase (908), or using mice with muscle-specific cre expression (Myo-Cre) substantially reduces capillary to fiber ratio under basal conditions (682). The latter mice also exhibit a reduced angiogenic response to endurance exercise (679).
8.10.2. VEGFR
VEGFR1 mRNA is reported to increase following a single bout of exercise in most studies (80, 293, 331, 332, 678). However, it may be unaffected by some exercise conditions (292), or may increase only under flow-restricted conditions (331). Studies in which VEGFR2 mRNA was assessed following a single exercise bout have reported variable outcomes, ranging from modest increases in mRNA and protein (292, 293, 332), to no change in mRNA (80), to a reduction in mRNA (678). The increases in VEGFR2 mRNA are no longer observed in response to exercise subsequent to weeks of exercise training (332, 678). In the rat, electrical stimulation of glycolytic muscle enhances VEGFR2 (flk1) protein within 2 days and this response is blocked by inhibition of nitric oxide synthase (626).
8.10.3. HIF
In rats, mRNA for HIF1α and HIF2α increase significantly in muscle samples taken 6 hours after a single exercise bout, and this response is not seen after a 4 week training program (555), which again may reflect reduced hypoxic stress following adaptation to exercise. However, because substantial regulation of HIF occurs at the protein level, it is not apparent that changes in mRNA reflect an increase in HIF protein and transcriptional activity. Other investigators have not observed a change in HIF1α mRNA with exercise (80, 330), which again may indicate that the majority of HIF regulation occurs at the level of protein. HIF1α protein does increase in rat muscle subjected to functional overload of the EDL. Furthermore, inhibition of HIF activity prevents overload induced angiogenesis (627).
In healthy humans, a single bout of exercise in normal or flow-restricted conditions does not result in a change in HIF1α mRNA (330). However, in both conditions, there is a significant and sustained post-exercise increase in HIF1α protein that correlates with increased nuclear immunolocalization of HIF1α and enhanced DNA binding activity (20). Levels of HIF target genes VEGF and EPO also increase after exercise. Interestingly, VEGF levels increase to a greater extent in the restricted flow vs. non-restricted flow exercise condition although HIF1α protein levels are comparable in each condition (20), suggesting that other transcriptional regulators participate in this induction of VEGF.
The physiological role of HIF1α in maintenance and adaptation of skeletal muscle capillary networks is unclear. Myocyte-specific deletion of HIF1α in mice actually enhances their endurance capacity (579). Baseline capillary to fiber ratio is higher in the HIF1α−/− animals compared to control, and exercise induces a greater increase in capillary density in these mice (580), which would point to an angio-repressive role of HIF1α. It is possible that enhanced HIF2α signaling results in the capillary network adaptations observed in these mice. These results do indicate that HIF1α involvement in skeletal muscle angiogenesis is not as straightforward as originally hypothesized.
8.10.4. PGC1α
Exercise transiently increases the transcription of PGC1α mRNA in human muscle, and this response is greater in men who have undergone a training routine (717). PGC1α mRNA is further enhanced by exercise under flow-restricted conditions, and this increase is not fiber type-specific (663). A variety of genetic approaches provide consistent evidence for PGC1α involvement in exercise-induced angiogenesis. PGC1α expression and activity is regulated via activation of p38γ MAPK. Mice deficient in p38γ have reduced expression of PGC1α which correlates with blunted mitochondrial biogenesis and angiogenesis in response to exercise (726). PGC1α global knockout mice exhibited normal capillary density (although lower capillary to fiber ratio due to reduction in myofiber size) as well las lower basal levels of VEGF protein and lacked a training induced increase in VEGF (526). Similarly, mice with muscle specific ablation of PGC1α (myo PGC1α−/−) have normal basal capillary to fiber ratio. However, these mice do not exhibit exercise induced angiogenesis (in response to 14 days of voluntary running exercise (151).
8.10.5. VEGF-B/PlGF
While PlGF is capable of inducing angiogenesis within an ischemic environment, PlGF levels do not increase in muscle in response to a single bout of exercise (303). Furthermore, deletion of PlGF in mice does not impact exercise induced angiogenesis (303). Interestingly, these mice do have a lower basal capillary to fiber ratio in muscle (303), similar to that observed for the myocyte-specific VEGFA−/− mice (682), implying that PlGF does play a role in the developmental formation of a complete microvascular network.
8.10.6. Ang1,2
Several studies have reported that neither Ang1 nor Ang2 mRNA change in response to a single bout of exercise, either in trained or untrained subjects, regardless of whether exercise is conducted under normal or flow-restricted conditions (295, 332). However, basal levels of Ang2 mRNA are elevated after 10 days or 5 weeks of training, which results in an increase in ratio of Ang2 to Ang1 (332). This suggests a pro-angiogenic status within the muscle, supported by the detection of elevated Ang2 protein also is detectably elevated in muscles following 10 days of training (332). In contrast, mRNA levels of Ang2 and Tie2 have been shown to increase in muscle 3 hours following an acute exercise bout (398). It is possible that exercise regimen and timing of muscle samples contribute to these different findings.
8.10.7. FGF2
FGF2 was the first pro-angiogenic molecule characterized to be present in extracts from exercised muscle (639). The release of FGF2 from cultured skeletal myocytes can be induced by electrical stimulation of the cells (462). However, in vivo experiments do not provide strong evidence of a role for FGF2 in mediating exercise-induced angiogenesis. In rats, FGF2 mRNA is not affected by an acute exercise bout (294, 678), but it does increase after 8 wk normoxic exercise training (678). Likewise, there is no observable increase in FGF2 mRNA or its receptors, is observed in rat EDL muscle subsequent to chronic electrical stimulation (105). In humans, FGF2 mRNA also does not increase either after a single bout of exercise (330, 755) or after 8 weeks of exercise training (756).
8.10.8. TGFβ
A moderate increase in TGFβ mRNA occurs after a single bout of exercise (91, 296, 678), which may be augmented following an exercise training period (296, 678). TGFβ mRNA also increases with acute exposure to hypoxia, however, this effect is not additive when combined with exercise (91). Conversely, chronic hypoxia represses TGFβ mRNA, and prevents the increase in TGFβ normally associated with exercise (678). These studies do not indicate a clear role for TGFβ in angiogenesis. The stimulation of extracellular matrix, and other known effects of TGFβ on skeletal myocyte differentiation, may be more consistent with an effect on myofiber adaptation.
8.10.9. Nitric oxide (NO)
Protein levels of nNOS and eNOS increase with sustained muscle activity (626, 747, 895). Resting levels of eNOS protein also are increased in human skeletal muscle following a 4 week period of moderate intensity exercise training (398). These data support the premise that NO plays an important role in exercise-induced adaptations within the capillary network. Consistent with this hypothesis, NO inhibition with LNNA blocks electrical stimulation-induced capillary growth, with an associated reduction in endothelial cell proliferation (421), and reduced expression of VEGF and VEGFR2 (626). LNAME treatment of rats prevents the increase in VEGF mRNA in response to an acute bout of running exercise, but does not block the exercise induced increase in TGFβ mRNA (294).
Shear stress also increases eNOS protein levels (59, 994). NO inhibition prevents prazosin-induced angiogenesis, but does not block overload induced angiogenesis (994), pointing to a differential requirement for NO in the processes underlying luminal splitting and abluminal sprouting forms of angiogenesis. However, it is interesting to note that basal capillary to fiber ratio is 20% higher in the EDL of eNOS−/− compared to wildtype mice, which would appear consistent with an angiostatic role of basal NO production. In these mice, chronic vasodilatation using prazosin treatment does not induce a further increase in capillarization (59). Elucidating the role of NO in these angiogenic processes is complicated by the direct effect that alteration in NO production exerts on blood flow. For example, inhibition of NOS with LNNA prevents exercise-induced increases in capillary shear stress (425), which itself could significantly impact VEGF production and angiogenic responsiveness. The majority of studies indicate a predominant role of NO is in mediating the shear stress induced signals that regulate both arteriogenesis and angiogenesis.
However, NO has been shown to dampen angiogenic responsiveness in some conditions. For example, treatment of cultured human myocytes with the NO donor SNAP significantly reduces the normal increase in VEGFA and FGF2 mRNA following electrical stimulation. Medium collected from these cells has a reduced capacity to induce endothelial cell proliferation (462). The apparent disparity between this reported inhibitory role of NO compared to the pro-angiogenic role of NO surmised from in vivo studies that inhibited synthesis of endogenous NO may be related to concentration differences. The levels of NO that accumulate with SNAP treatment likely greatly surpass typical endogenous levels of NO.
8.10.10. Matrix metalloproteinases
Increased levels of MMP-2 and MT1-MMP mRNA and protein are detected in rat muscle within 4 days of chronic electrical stimulation (334), correlating in timing with the physical appearance of degraded basement membrane and the formation of abluminal sprouts. Inhibition of MMP activity (using the general MMP inhibitor GM6001) prevents the normal increase in capillary to fiber ratio seen in response to chronic electrical stimulation, while not impairing endothelial cell proliferation (334). In human studies, elevation of MMP-9 (both pro and latent forms) is detectable in muscle after 1 and 3 hours of a single intense exercise bout (398, 787). Conversely, repeated exercise bouts induce increases in expression of MMP-2 and MT1-MMP in human muscle (786). The majority of cells within the muscle tissue (including endothelial cells, fibroblasts, resident immune cells and skeletal myocytes) are capable of producing and secreting MMPs. The individual contributions of these cells to the overall proteolytic landscape within the muscle tissue has have not been established.
While muscle activity or muscle overload both elicit substantive increases in MMP-2 production and activity, the lack of increase in these MMPs is a hallmark of luminal splitting angiogenesis (762). In fact, exposure of cultured microvascular endothelial cells to shear stress results in a repression of MMP-2 production, dependent both on NOS activity and p38 MAPK activation (623).
8.11. Angiostatic Molecules and Their Regulation in Skeletal Muscle
The capacity to evoke an angiogenesis response is determined not only by the presence of angiogenic mediators, but also by the levels of angiostatic signals. The local balance between angiogenic and angiostatic signals will determine whether angiogenesis occurs (as reviewed in (680). Several relevant angiostatic mediators are described below.
8.11.1. Thrombospondin 1 (TSP1)
TSP1 is a large molecular weight homotrimeric heparin binding protein. TSP1 induces anti-proliferative/apoptosis signals in endothelial cells, promotes p53 activity and inhibits nitric oxide signaling (327, 445). Consistent with these known cellular effects, TSP1−/− mice have increased basal capillary to fiber in gastrocnemius and soleus muscles, and higher VEGF protein levels (574).
A single bout of exercise induces an immediate but transient increase in TSP1 mRNA (677), which may counteract early angiogenic signals such as those elicited by the production and release of VEGFA. However, after several exercise bouts, TSP1 mRNA no longer exhibits an exercise response (677) and basal levels also may be suppressed (492), consistent with a shift towards angiogenesis. The elevation of TSP1 mRNA with an acute exercise bout is detectable once again following a longer period of exercise training (at which point, capillary remodeling may have been completed) (398, 677). Interestingly, chronic exposure to hypoxia reduces the basal level of TSP1, and reduces the increase in TSP1 mRNA in response to a single exercise bout (677), which may enhance angiogenesis.
TSP1 also affects nitric oxide production (450), thus impacting arteriolar resistance and, potentially, the capacity to dilate in response to exercise. This negative effect is greater in aged animals and impairs recovery of hindlimb blood flow post-ischemia (448, 449). This signaling also could exert a negative influence on shear stress-induced angiogenesis. More recently, TSP1 ligation of its receptor, CD47, was shown to inhibit VEGFR2-dependent intracellular signaling in microvascular endothelial cells, providing another mechanism through which TSP1 exerts angiostatic effects (478).
8.11.2. Angiostatin
Angiostatin is a 38 kDa proteolytic cleavage fragment of plasminogen. First recognized for the capacity to suppress tumor growth (670, 672), it inhibits endothelial cell proliferation and induces apoptosis (158). At least 5 membrane binding partners, including angiomotin, have been identified to date (972). Angiostatin can inhibit HGF (but not VEGFA or FGF) -stimulated proliferative and migratory signals (973). Although it is feasible that the increased production of MMPs in response to exercise may result in higher levels of angiostatin, these assessments have not been made. There is some evidence of increased angiostatin in diabetic animals, but this is not a consistent finding across studies (269, 873).
8.11.3. Endostatin
Endostatin is a 20–22 kDa fragment consisting of the heavy C-terminal fragment of collagen XVIII, which is produced by cleavage of the full length collagen by MMPs (359) or by cathepsin L (254). It is known to inhibit angiogenesis (671), in part by binding to αv and α5 integrins, thus competing with native ECM components and serving to reduce signals associated with cell migration and survival (746). It also promotes the intracellular activation of protein phosphatase PP2A, which enhances the dephosphorylation (Ser1177) of eNOS (951).
The contribution of endostatin to exercise induced angiogenic signalling is unclear. Some researchers have reported minor, time dependent fluctuations in the arterial-venous difference in plasma endostatin levels, but no change in tissue endostatin levels, following an acute exercise bout (787). Others have reported increases in circulating endostatin in response to exercise (322, 893), although muscle levels of endostatin decrease after exercise training (323). Endostatin levels are increased in skeletal muscle of diabetic patients compared to non-diabetic controls (873), suggesting that endostatin may play a role in maintaining an angiostatic environment in the muscle of diabetic patients.
8.11.4. Vasohibin-1
Vasohibin-1 is an endothelial cell-secreted protein that is induced by VEGF or FGF2 stimulation, and is thought to act as a negative feedback regulator of the VEGF signal pathway (983). It is associated with downregulation of endothelial cell proliferation and tube formation (983). Vasohibin-1 is hypothesized to promote vessel stabilization, as evidenced by its localization to stalk rather than tip cells of extending sprouts, and based on the observation that knockdown of vasohibin-1 results in excessive numbers of immature vessels (485). Interestingly, hypoxia represses the VEGF-induction of vasohibin-1 (983).
Vasohibin-1 is present in skeletal muscle. While vasohibin levels are upregulated transiently by brief exercise bout, this response is lost after short term training (490). Further evidence of the physio/pathophysiological role of vasohibin-1 is that it increases significantly in response to unloading of the soleus muscle, correlating with capillary regression. It also is elevated in the muscles of Zucker diabetic fatty rats (490). These results suggest that vasohibin-1 may regulate the process of skeletal muscle angiogenesis both under physiological and pathological conditions.
8.12. Contributions of a Multi-cellular Environment to Angiogenesis in Skeletal Muscle
Interactions between the resident cells within skeletal muscle have been proposed to facilitate the coordinated responsiveness of muscle to stressors (exercise; regeneration) (Figure 7). In recent years, numerous reports have provided some evidence in support of this hypothesis.
Figure 7.
Activating stimuli and cellular interactions within the skeletal muscle microenvironment. Arrows denote paracrine signaling crosstalk that ensures co-ordination of the processes of angiogenesis, satellite cell activation and myocyte metabolic adaptation in response to physical/mechanical or biochemical stimuli.
8.12.1. Skeletal myocyte-endothelial cell interactions
Myocyte-derived VEGFA likely is the largest source of VEGFA within muscle, and thus plays a significant paracrine function in regulating activation of the adjacent capillary endothelial cells. Electrical stimulation of isolated human myocytes induces greater VEGFA mRNA production, and the media collected from these cells enhances endothelial cell proliferation (462). Takahashi and colleagues (901) showed that hypertrophic stimuli that induce Akt activation (such as IGF-1 or insulin/dexamethasone) will elicit myocyte production of VEGFA. Notably, injection of muscle with constitutively active Akt promotes both myocyte hypertrophy and the growth of new capillaries. Thus, stimuli that activate myocyte Akt serve to elicit the production of VEGFA, leading researchers to hypothesize that Akt plays a critical role in coupling the adaptation of skeletal myocytes and their capillary network to an enhanced load.
Mice harboring a genetic deletion of VEGFA confined to the skeletal myocytes have provided compelling evidence for the requisite role of this source of VEGFA in the development and adaptive maintenance of skeletal muscle capillary networks. VEGFA protein levels in these muscles are reduced by 80% compared to wildtype littermates (682). Normal development of the capillary networks is impaired significantly within these mice, with capillary to muscle fiber ratios that are half to one third that observed in muscles from wildtype mice. Furthermore, the typical exercise training-induced increase in capillary number is not detectable in these animals (679).
8.12.2. Satellite cell-endothelial cell interactions
Satellite cells reside beneath the myocyte basal lamina. Upon activation, these cells proliferate and subsequently differentiate into myofibers. Many factors that induce satellite cell activation and proliferation also are known angiogenic stimuli, including mechanical stretch and growth factors (102, 349). For example, angiotensin II is produced locally by satellite cells/regenerating myofibers, and can exert growth and chemotactic effects on nearby endothelial cells and satellite cells (68, 465). This leads to the hypothesis that the process of angiogenesis is coordinated with muscle growth/regeneration, which would provide a means of maintaining balance between metabolic demand and the capacity to deliver oxygen to the tissue. The termination of satellite cell proliferation and angiogenesis also share common signaling. For instance, satellite cells synthesize the Tie2 receptor. Ang1 stimulation of satellite cells represses apoptosis, but also inhibits proliferation and differentiation (2). Thus, the actions of the Ang1/Tie2 signal axis may co-ordinately promote stabilization of newly formed capillaries and quiescence of the satellite cell population.
Signaling cross-talk between endothelial and satellite cells may be necessary in order to achieve full responses in both cell types. Christov et al (156) provided strong evidence that endothelial cells stimulate satellite cell proliferation, and conversely, that satellite cells stimulate endothelial cell angiogenic behavior. Satellite cell number per muscle fiber correlates strongly with the number of capillaries around a muscle fiber, either when comparing glyolytic versus oxidative muscles, or in cases of pathological or physiological muscle adaptation. They also demonstrated that co-culturing of satellite cells with endothelial cells stimulates greater endothelial cell sprouting, which could be blocked by addition of an anti-VEGF neutralizing antibody (156). Satellite cell induction of HIF1α occurs in response to stretch injury and also in response to hypoxia, suggesting that HIF/VEGF production and subsequent release by satellite cells stimulates the adjacent capillaries to induce sprouting (751).
While these studies provide provocative support for the hypothesis of co-operative and coordinated signaling between satellite and endothelial cells, definitive cause-effect relationships have yet to be established in vivo. In fact, radiation treatment of muscle, which prevents satellite cell proliferation, does not impair angiogenesis induced by long term voluntary exercise (535).
8.12.3. Perivascular cell (fibroblasts and pericytes)-endothelial cell interactions
Pericytes reside within capillary endothelial basal lamina and thus are in a privileged position to exert strong paracrine effects on the adjacent endothelial cells. In vitro, co-culturing of pericytes and endothelial cells has provided insight into such paracrine signaling (389). Co--cultured pericytes exert an anti-proliferative effect on endothelial cells, but this requires cell-cell contact (686). Pericyte production of TGFβ1 in the retina promotes capillary stabilization, both by stimulating production of basement membrane matrix components and by inhibiting endothelial cell proliferation (974). Absence of pericytes is associated with endothelial cell hyperplasia (365). Conversely, VEGFA production by pericytes contributes substantially to endothelial cell survival (187).
Analysis of the distribution of pericytes along capillaries in muscle exposed to angiogenic stimuli indicates that increased muscle activity (overload or chronic electrical stimulation) is associated with a retraction of pericyte processes, so that less of the abluminal surface of the capillary is in contact with the pericyte (232). Interestingly, angiogenesis occurring through luminal splitting (in response to prazosin administration), is associated with an increased pericyte coverage, which is consistent with the maintenance of an intact basement membrane and lack of abluminal sprouting observed in this form of angiogenesis (227). However, the nature (if any) of the modulatory role of pericytes in the physiological angiogenic process remains unclear.
8.12.4. Immune cell-endothelial cell interactions
Mast cells are present in the muscle, situated around neurovascular bundles and adjacent to capillaries (106). Accumulation of mast cells occurs subsequent to myofiber membrane damage, with the peak increase corresponding to the time of muscle fiber regeneration (311, 525). Mast cells are known to release pro-angiogenic factors (i.e. VEGFA), as well as factors that can activate satellite cells. However, a causal relationship has not been established between mast cell accumulation or activation and angiogenesis within skeletal muscle (629).
Muscle damage is associated with an influx of immune cells, first neutrophils and then macrophages and mast cells (319). VEGFA is chemotactic for macrophages (F4/80 positive), neutrophils and mast cells, likely through activation of VEGFR1 (846). In turn, the activated macrophages produce and secrete numerous pro-angiogenic factors, including FGF-2, IGF-1, IL6, TNFα, TGFβ, VEGFA (846), thereby amplifying the angiogenic signal cascade. In support of the importance of macrophages in the response to muscle damage, inhibition of chemokine receptor 2 (CCR2) reduces macrophage infiltration at sites of muscle injury, and also delays the increase in VEGFA and new capillary growth in response post-muscle injury (673). Macrophage accumulation and secretion of IGF-1 in response to skeletal muscle injury also promotes muscle regeneration (552). However, the contribution of macrophages to angiogenesis within non-injured muscle appears to be minimal.
Exercise induces an elevation of factors known to stimulate release of endothelial progenitor cells (EPCs) from bone marrow (VEGFA, interleukins). The number of circulating EPCs increases after a single bout (30 min) of moderate or intense exercise (515). Exercise training of coronary artery patients also increased number of circulating EPC (516). Exercise training (7–28 days) improves the total number of EPC residing in the bone marrow, and those in circulation. This effect is blunted severely in eNOS−/− mice (or those treated with LNNA), suggesting that NO plays a substantial role in stimulating this effect (516).
8.13. Aging and the Angiogenic Response to Exercise
The angiogenic response in exercised muscle is diminished in aged rats/mice compared to their younger counterparts (897). It was reported initially that capillary growth did not occur in response to exercise in the aged human muscle. These investigators showed that an apparent increase in capillary density was due to a decrease in myofiber size in the aged muscle, rather than an increase in capillary number (there was no change in capillary contacts) (204). The increase in interstitial VEGFA protein in response to a single exercise bout was substantially lower in aged compared to young men (295, 791). Not surprisingly, aged men had lower basal capillarization compared to young counterparts (791). However, several studies provide evidence that capillary number does increase in response to a prolonged exercise training program, albeit not as much as the increase observed in younger individuals (295, 372). The latter studies provide evidence that endothelial cells retain the capacity to undergo angiogenesis during the aging process. However, the extent of the response appears to be blunted due to a reduced amount of angiogenic factors, such as VEGFA, which decreases during aging. Fortunately, exercise training reverses this effect (526).
8.14. Inactivity and Capillary Loss
Physical inactivity is associated with a reduction in muscle capillary to fiber ratio. While this has been attributed in part to the decrease in fiber size (924), it is clear that unloading provokes unfavorable conditions for capillaries. Unloading results in significant decreases in capillary diameter and increased apoptotic labelling of endothelial cells, suggesting progression loss of endothelial cells (186, 271). Molecular mechanisms underlying this switch to apoptosis could include decreased survival signalling through the VEGFA-VEGFR2 pathways, as both VEGFA mRNA (78) and VEGFR2 protein levels (778) were reduced with muscle unloading reduces VEGFR2 protein. Fourteen days of unloading also reduced eNOS mRNA and protein expression in soleus muscle, and attenuated endothelial cell dependent vasodilatation (460, 826). These changes are consistent with a reduced capacity of endothelial cells to respond to changes in hemodynamics and growth factor stimuli. Additional factors that correlate with an angiostatic and potentially pro-apoptotic environment during muscle unloading include increased levels of p53 and TSP1 (778).
8.15. Angiogenesis in Human Critical Limb Ischemia (CLI)
Lower leg muscle biopsies from individuals with intermittent claudication are characterized by reduced capillary density compared to individuals without PAD (467, 763). In contrast, biopsies from patients with prolonged CLI show a higher density of microvessels, and increased microvessel to muscle fiber ratio within the ischemic tissue. However, microvessel structure in these biopsies is abnormal. Lack of Ki67 staining indicates that there is no longer a proliferative response, leading researchers to conclude that the angiogenic response is short-lived and does not adequately respond to the continued ischemic environment (392). The increase in capillary to fiber ratio within the medial gastrocnemius appears localized to type IIA fibers. However, this may be skewed by the reduction in type IIB fibers found in muscles of patients with peripheral artery disease compared to controls (337). Conversely, others have reported a decrease in the capillary to fiber ratio in the gastrocnemius of patients with stable intermittent claudication compared to healthy age-matched controls (38).}
Chronic ischemia is characterized by the presence of both normal and atrophic muscle fibers. Elevated levels of VEGFA and VEGFR2 mRNA (detected using in situ hybridization) is limited only to the atrophic fibers, and correlates strongly with presence of inflammatory cells (759). These authors suggest that VEGFR2 signalling may play a role in differentiation of regenerating myocytes. VEGFR1 was detectable only on endothelial cells in both chronic and acute ischemia. HIF1α expression is not prominent in chronic ischemia (963).
The expression of growth factors, etc. depends substantially on the severity and duration of ischemia. Gene array has been used to examine gene expression in acute onset versus chronic critical ischemia. While acute onset ischemia induces upregulation of hundreds of genes (growth factors and receptors, particularly the HIF1α/VEGF/VEGFR2 pathway, MMPs, TNFα and inflammatory pathways, caspases), chronic critical ischemia modulates few genes, and these mostly belong to anabolic and cell survival pathways (IGF1,2) (941). Expression of VEGFA and VEGFR2 is observed in distal ischemic muscles in humans with acute-onset ischemia (for example, induced by thrombosis of the femoral artery). Strong immunostaining of HIF1α in nuclei within this region suggests that VEGFA expression is mediated by HIF1α (759). In muscle biopsies from chronic limb ischemia patients, VEGFA, SDF1 and CXCR4 mRNA levels are increased in acute-on chronic ischemia (compared to non-ischemic muscle), but these gene products tend to be decreased in those patients with chronic ischemia (963).
Muscle of patients with chronic CLI does not exhibit elevated mRNA levels of VEGFR2, HIF-1α, ephrins or Tie2 (941). In some individuals, VEGF expression appears to correlate with areas of increased microvessel density (941). However, the observed higher density of microvessels may not be the result of angiogenesis but rather be due to muscle atrophy, which reduces myocyte cross-sectional area substantially, making capillary density appear greater. This concurs with another human study showing that chronic ischemia reduces the extent of VEGFA expression in skeletal muscle (963).
Plasma VEGFA levels are elevated in humans with PAD compared to healthy controls (572), and the extent of VEGF expression appears to correlate with disease severity (258). A limitation is that it is not possible to get non-invasive measurement of interstitial skeletal muscle VEGFA in these patients. Circulating HGF, but not FGF2, is elevated and correlates with disease severity (538).
8.16. Animals Models of Ischemia
Various animal models have been developed to facilitate investigation of the pathology of peripheral artery disease, and to test therapeutic options (984, 1049). Generally, ischemia is induced through surgical ligation or removal of one or more major hindlimb feed arteries. This induces acute onset ischemia, the extent to which will depend greatly on the species and the location of surgical ligation. Muscle and vascular morphology and gene expression, blood flow recovery and limb function may then be monitored over a period of time (often up to 4 weeks) following ligation.
8.16.1. Rabbit
The model of ‘resting’ limb ischemia in rabbits was first described in 1990 (367). Surgical ligation of the common iliac artery induces moderate limb ischemia, which results in functional limb impairment. Substantial recovery of blood flow and oxygen levels to 80% of control values occurs by 17 days post-ligation, however complete recovery is not observed even after 31 days.
Femoral artery ligation (without excision) (455) generates a moderate level of ischemia that is characterized by an absence of overt muscle wasting, gangrene, or impairment of hindlimb function. There is no blood flow deficit observable at rest or after maximal dilation in the proximal muscle groups, but there is consistent flow deficit in the lower limb muscles (455). This model is associated with arteriogenesis observed in thigh region (surrounding the site of ligation) while angiogenesis occurs in the distal muscle groups (lower leg). An increased number of BrdU-positive capillary endothelial cells may be detected 1 week post-ligation, corresponding with a slight increase in total density of capillaries within glycolytic but not oxidative muscles. Monocytes (positive for FGF2 and TNFα) accumulate 7 days post-ligation, and their accumulation correlates temporally with angiogenesis. Fibroblasts and myocytes also are positive for FGF2. Investigators have observed that the greatest angiogenic response occurs in areas with the highest levels of tissue-resident macrophages, indicating they may play a key role in regulating angiogenesis in the ischemic muscle (34). Complete removal of the femoral artery (which also may involve ligation of femoral artery branches) (734) results in severe ischemic damage, and shows similarities to human acute ischemia induced by thrombosis (759). There is widespread necrosis of muscle, substantive influx of inflammatory cells, and widespread enhancement of VEGFA expression. In this model, capillary sprouting is an early post-ischemic event that correlates temporally with the inflammatory response (occurring after 5 days) while collateralization is a later response (374).
8.16.2. Rat
Ligation of the femoral artery has been reported to induce a modest reduction of blood flow at rest (25), or to have no significant effect on resting blood flow (98). It is likely that muscle ischemia at rest is minimal because collateral flow pathways maintain oxygen delivery to distal muscle groups and this may be sufficient to meet the low metabolic needs of the inactive muscle. However, during muscle contraction, blood flow to the active muscle is markedly below that of non-ligated muscle, which results in severely limited exercise performance (98, 1022, 1025). Based on this pattern of blood flow insufficiency, this model approximates the intermittent claudication observed in humans with peripheral artery disease, where symptoms are observed during activity but not at rest (585). Minimal or no angiogenesis response is observed in response to femoral artery ligation. Some reports indicate a reduction in capillary density, which is restored only partially over 28 days following ligation (436). In accordance with these observations, endothelial cell proliferation is not detected in the hindlimb muscles of sedentary ligated rats (205, 545).
Iliac artery ligation significantly reduces resting muscle blood flow acutely, with flow recovering to 40% and 60% of control levels after 1 and 5 weeks, respectively (104). Due to these altered hemodynamics, capillary shear stress is reduced following iliac artery ligation (103). Again, this deficiency in muscle flow is exaggerated greatly in active muscle. Muscle fatigue index is reduced substantially, although there is recovery to approximately 80% of control by 35 days post-ligation (427). Ischemia also results in swelling of capillary endothelial cells visible after 7 days, which results in a narrowed lumen (381, 382, 427). Notably, capillary swelling appears to be a systemic effect not limited to the region of ischemia, as it also is observed (though to a lesser extent) in non-ischemic skeletal muscle (382). The ischemic microcirculation also is characterized by increased leukocyte rolling and adherence to venules (381, 427). The adherence of leukocytes correlates with increased venular permeability, as assessed by visualizing leakage of FITC-albumin (381). Despite the inflammatory signals observed within the microvascular network, there is no increase in macrophage number within the ischemic muscle. An increase in PCNA-positive nuclei can be observed at 2 weeks post-ligation, while there is no change in capillary to fiber ratio until week 5, when it is moderately elevated above control values (104).
While the majority of ischemia studies involve acute-onset ischemia due to complete artery ligature, a chronic gradual ischemia model has been used successfully. In this approach, an ameroid constrictor placed around the iliac artery causes progressive arterial occlusion over a period of 2 weeks. This gradual onset ischemia, which may more closely mimic the human onset of ischemia, is characterized by very poor blood flow recovery (i.e. formation of collaterals), lack of inflammation, and minimal angiogenesis (910). Likewise, if iliac artery ligation is followed 2 weeks later by femoral artery ligation within the same limb, the resultant ischemic state is chronic and the blood flow recovery is limited (103). The absence of inflammatory infiltrate appears to be a defining characteristic that distinguishes acute onset and chronic ischemic states, and correlates substantially with the extent of angiogenesis that is observed.
8.16.3. Mouse
Femoral artery ligation of mice induces mild to severe ischemia depending on the type of ligation and whether it is accompanied by excision of the artery. It is important to note that the extent of muscle ischemia (based on measurements of the acute reduction in flow and on tissue ATP measurements), and the types of responses observed, also differ substantially between mouse strains (825). These differences must be recognized when interpreting data from various mouse strains. One variable that contributes significantly to the severity of ischemia is the presence and caliber of pre-existing collateral vessels, which correlate inversely with magnitude of tissue hypoxia, extent of tissue necrosis, inflammation and myocyte regeneration. The C57/Bl6 strain response to femoral artery ligation is characterized by a moderate acute hypoxic stimulation, followed by rapid recovery of flow. Conversely, BALB/c mice exhibit very poor recovery of blood flow, and there is evidence of more ischemic tissue damage (necrosis and edema) (358). Ischemic muscles in BALB/c mice are characterized by a reduced angiogenic response compared to those of C57/Bl6, which correlates with reduced expression of VEGFA (particularly isoforms 165 and 188). Bioinformatics analysis using quantitative trait loci mapping indicates that polymorphisms near the VEGFA gene, as well as associated with HIF1α, sonic hedgehog, and Src homology region 2 domain-containing phosphatase-2 (SHP-2), may be responsible for the modified responsiveness of the BALBc mice (139). A similar quantitative trait loci mapping strategy has identified chromosome 7 as harboring genes that are associated with the severity of hindlimb-ischemia post-ligation (212). These studies provide an example of the opportunity that exists to differentiate responsiveness to ischemia based on genetic polymorphisms. To date, there has been limited analysis of genetic traits underlying susceptibility to (or severity of) PAD in humans. One study has used linkage analysis to localize a gene associated with susceptibility for PAD to chromosome 1p31, providing evidence that genetic variants mapping to PAD severity can be found in the human population as well (325).
Ligation and excision of the femoral artery of C57Bl/6 mice generates a severe reduction in limb blood flow lasting up to 7 days. Blood flow is reduced sufficiently within the first week such that toe necrosis is observed in about 10 percent of animals. Blood flow gradually increases until reaching a plateau between 21 and 28 days (177). Revascularization (capillary density) correlates with blood flow recovery, increasing between 7 and 35 days. Proliferation of endothelial cells is highest at 7 and 14 days post-ligation (695).
8.17. Acute ischemia, inflammation and EPCs
In most animal models of critical limb ischemia, inflammation is an immediate and short term consequence of acute arterial ligation. Substantial infiltration of inflammatory cells occurs within 3 days of ischemia (695). The recruitment of circulating immune cells plays a crucial role in facilitating the subsequent angiogenic response. Infiltrated inflammatory/progenitor cells are sources of cytokines/growth factors and proteolytic enzymes. Macrophage production of VEGF is stimulated by nitric oxide or hypoxia (741), and also by activation of Toll Like receptors or the adenosine A2A receptor (653). Infiltrated neutrophils produce substantial amounts of MMPs, which modulate various aspects of the angiogenic process (as described in Section 8.12.4).
Acutely hypoxic cells within the ischemic region produce multiple immune cell chemo-attractants, including VEGFA and SDF-1. VEGFA is chemotactic for circulating monocytes and EPCs (34, 473). SDF-1 is a chemo-attractant for CXCR4 positive circulating cells (137). Ischemia also promotes rapid increases in expression of monocyte chemotactic peptide (MCP)1, E-selectin and ICAM-1 (675), each of which play roles in augmenting infiltration of circulating cells. Ischemia upregulates TNFαR2 on circulating cells (560), which promotes homing of these cells to the ischemic regions. TNFαR2 signaling, through activation of NFkB, results in pro-angiogenic gene expression. For example, knock out of TNFR2 reduces the ischemia-induced increase in VEGFA mRNA, due to lack of TNFR2-dependent transcription of VEGF. These mice have a greatly reduced angiogenic response to ischemia and an increased frequency of tissue necrosis and limb auto-amputation, which could be restored by bone marrow transplant from wildtype mice, providing evidence that this role for TNFR2 resides with the circulating cells (313). Endothelial expression of E-selectin and ICAM1 also are important for homing of EPCs to the ischemia tissue, as there is reduced EPC infiltration in E-selectin−/− mice (675). E-selectin-stimulated EPCs produce IL8, which stimulates endothelial cell tube formation (675). These studies provide evidence that the secretion of angiogenic molecules is an important functional consequence of EPC recruitment. There is an age dependent decline in the capacity for blood flow recovery following acute ligation, which appears to correlate with reduced EPC mobilization (146).
8.18. Balance between angiogenic and angiostatic factors in ischemia
The magnitude of angiogenic response that occurs within the ischemic environment may be considered to be a result of the balance between angiogenic and angiostatic factors. While angiogenesis may occur within ischemic muscle, it is clear that there are many scenarios, particularly in the human population with critical limb ischemia, in which the angiogenic response is inadequate. This failure in responsiveness could result from insufficient stimulus to produce angiogenic factors, inefficient signalling of these factors, or the presence of conflicting inhibitory signals.
8.18.1. Growth factors
VEGFA mRNA and protein are elevated in skeletal myocytes, endothelial cells, and infiltrating inflammatory cells within the ischemic region (149, 177, 351, 374, 559, 621, 759, 941, 963). The increase in VEGFA appears to be essential for angiogenesis, as administration of a VEGFA neutralizing antibody substantially impairs the increase in capillarity post-ligation (177). While there is a modest increase in HIF1α in response to femoral artery ligation, this can be augmented by treating animals with DMOG, which inhibits PHD and FIH, resulting in HIF1α stabilization. DMOG treatment results in enhanced levels of VEGFR2 and increased capillary to fiber ratio (628). HIF1α is not co-localized with VEGFA and VEGFR2 in regenerating myofibers, leading researchers to conclude that VEGFA expression in the regenerating myofibers is driven by alternate mechanisms. The expression of VEGFA in regenerating fibers is strikingly similar to the pattern of expression of HGF, which may imply a common regulatory mechanism (461). While hypoxia typically results in suppression of gene expression, VEGF internal ribosome entry site (IRES) activity is enhanced during ischemia, enabling enhanced cap-independent translation of VEGFA (87).
Production of the VEGF120 isoform predominates within the ischemic tissue, whereas isoforms 164 and 188 increase in the region of collateral artery development (162). This suggests that environmental cues can act to regulate VEGF A splicing. Production of the soluble form is induced by the ischemic environment, while heparin binding, matrix immobilized forms are stimulated by the high flow environment in the collaterals (162) and may contribute to formation and remodeling of collaterals. VEGF expression patterns also differ depending on the muscle type examined. Modest and short term increases in VEGFA121 and 165 are detectable in the predominantly glycolytic rabbit tibia is anterior muscle after 1 or 5 days of ligation, but levels return to control by 21 days. Interestingly, VEGF165 levels decrease in the oxidative soleus muscle after 1 day of ligation, but increase substantially by 5 days post-ligation. In both muscles, VEGFA protein primarily is localized to the periphery of fibers and to interstitial cells (149). Despite these increases in VEGFA, there was no improvement in capillary to fiber ratio or muscle oxygenation after 21 days, consistent with the concept that increasing VEGFA expression on its own may be insufficient to drive angiogenesis within the ischemic environment.
PlGF levels do not increase with ischemia. Knockdown of PlGF (Plgf–/–)) does not impact recovery from ischemia. However, combined Plgf−/−/eNos−/− mice show defective angiogenesis associated with increased oxidative stress in response to tissue ischemia (302).
FGF2 does not increase over 21 days following femoral artery ligation (149). While this suggests that FGF2 does not play a role in post-ischemic angiogenic adaptation, it is possible that pre-existing matrix-sequestered FGF2 may be released via proteolytic cleavage, which would enable it to activate cell surface receptors and exert cellular effects. Not surprisingly, targeted disruption of FGF2 does not impact the recovery of blood flow in response to hindlimb ischemia, and capillary density is not different from wildtype mice (894). It thus appears unlikely that FGF2 contributes substantially to the ischemic response.
HGF mRNA is not detectable in adult skeletal muscle, but it increases in response to ischemic stress, and during the subsequent muscle regeneration. In contrast to acute-onset ischemia, chronic hypoxia down-regulates production of many growth factors. HGF mRNA and protein levels are down-regulated by exposure of endothelial or smooth muscle cells to prolonged hypoxia, which correlates with reduced cell survival (350).
Dll4 expression is enhanced post-femoral artery ligation, and is associated with increased capillary sprouting. Inhibition of Dll4 function through overexpression of soluble Dll4 results in impaired recovery from ischemia (14). This study showed evidence that sprouting endothelial cells formed inappropriate (arteriole-arteriole) and non-functional vascular connections, indicating the requirement of Dll4 signalling for appropriate integration of neo-capillaries into the microvascular network. Interestingly, Dll4 inhibition also increased leukocyte accumulation, which may be due to relieving Dll4-mediated repression of CXCL1 (IL-8) expression (14).
8.18.2. Growth factor receptors
Levels of cell surface receptors are affected by modulation in transcription and translation, as well as by internalization and proteolysis. Despite increases in Tie2 mRNA, decreased levels of full length Tie2 receptor are detectable in mouse hindlimb muscle 1 day following induction of ischemia (684). The decline in full length Tie2 correlates with the increased production of MT1-MMPMT1MMP, which is capable of clipping Tie2 to release the extracellular domain of the receptor. In cultured endothelial cells, this leads to impaired responsiveness of cells to Ang1, and reduces cell viability (684).
Plasma levels of VEGFA and sTie2 are strong predictors of ischemia severity, with higher levels of both proteins detected in patients with critical limb ischemia compared to those with intermittent claudication. Interestingly, treatment of cultured endothelial cells with VEGFA results in increased levels of sTie2 (258), implying that the elevated levels of VEGFA may play a role in the pathogenesis of the ischemic condition. VEGFA treatment of endothelial cells is known to enhance production of MMP-2 and MT1-MMP (453), which may then contribute to clipping of Tie2 and other cell surface receptors.
Mouse studies also suggest that pathological generation of soluble VEGFR1 (sFlt1) may contribute to the reduced angiogenic response to ischemia (351). Increased Flt1 and sFlt1 may act as a “sink” for VEGFA, reducing positive signalling through the VEGFR2. Conversely, computational modeling of the relationship between VEGF and VEGFR1 in peripheral arterial disease, based on available clinical data, and extrapolations from animal studies, suggests that sufficient “free” VEGF exists to create a favourable angiogenic environment in the ischemic limb and that increases in sVEGFR1 are not likely to impair this signaling (1010). In fact, a reduced level of VEGFR1 protein was detected in skeletal muscle of PAD patients with IC, compared to age-matched controls, while VEGFA levels were similar between groups (467). Considering that the extent of free VEGF does not correlate well with the clinically described outcomes (i.e. poor angiogenic responsiveness), it may be concluded that disruptions in signaling downstream of VEGFR2 or additional angiostatic factors modify the capacity of the endothelial cells to respond to the ischemic environment.
The angiogenic response to ischemia is significantly impaired in mice deficient in EPOR in all cells except the erythroid cells (650). The angiogenic response cannot be restored by bone marrow transplant from wildtype mice, providing strong evidence that the phenotype is associated with the impaired vascular cell expression of EPOR. Reduced levels of VEGFA and VEGFR2 in these mice led authors to conclude that EPOR plays a significant role in promoting activation of the VEGFA/VEGFR2 signal axis in response to ischemia (650).
These growth factor receptors generally involve Akt activation as a potential component of the downstream signal pathways. Thus, deficiencies in the activation of these receptors will result in reduced Akt activation, which may exert negative effects on cell survival signalling as well as nitric oxide synthase activity. Not surprisingly, targeted deletion of Akt1 results in reduced post-ischemia angiogenesis, and impaired recruitment of EPCs to the ischemic site (5). Reduced phosphorylation of endogenous Akt has been reported in response to iliac artery ligation, correlating with increased levels of cytostatic/pro-apopotic transcription factor FoxO1 (629). Conversely, enhanced expression of Akt promotes enhanced angiogenesis and recovery from ischemia (901), indicating that direct Akt activation can by-pass potential deficiencies in upstream signaling events.
8.18.3. MMPs
MMP-2 and 9 levels within the ischemic muscle increase as early as 1 day post-ligation, peaking at day 3, and remaining elevated for up to 14 days. This is associated with increased infiltration of neutrophils, which are the major cellular sources of MMP-9 (643). Depletion of neutrophils significantly reduces both MMP-9 and active levels of MMP-2 in the ischemic muscle (642).
Some functions of MMPs may promote angiogenesis within the ischemic muscle. For example, the increase in MMP-9 correlates temporally with exposure of a cryptic site from collagen IV (recognized by mAb HU177)(276). This site is associated with stimulation of angiogenesis through promoting endothelial cell adhesion, migration and proliferation (179). Reduced exposure of this site is seen in MMP-9−/− mice, corresponding with reduced recovery post-ischemia (276). MMP-9−/− also have reduced collateral artery formation and capillary growth in response to hind limb ischemia, which appears to be the result of reduced proliferation and invasion of both endothelial cells and EPCs (147). Notably exercise training could rescue the effect of hindlimb ischemia in wildtype, but not in MPP-2−/− mice, leading authors to suggest that MMP-2 production/activation is an important component in the exercise-induced adaptations to ischemia (146). Conversely, MMPs may exert a negative influence on angiogenesis through shedding of the extracellular domains of growth factor receptors (684), as mentioned previously. MMP-9 also may exert a negative role on angiogenesis through enhanced production of angiostatic cleavage products such as angiostatin (727), although this has not been observed within the ischemic environment.
8.18.4. Nitric oxide
eNOS plays critical role in promoting angiogenesis within the ischemic environment. Mice deficient in eNOS have an impaired angiogenic response (698) and reduced recruitment of pericytes, an indicator of stabilized capillaries (1029). A reduced expression of PDGFRβ may underlie the reduced recruitment of pericytes. These mice also have lower expression of VEGFA,B and C in response to ischemia, with an increased ratio of Flt1 to Flk1 compared to wildtype mice (1029). Conversely, eNOS−/− mice do not exhibit impairment in the mobilization of EPCs in response to ischemia (1029).
Consistent with the pro-angiogenic role of NO, dietary supplementation with L-arginine enhances the capillary density in ischemic skeletal muscle of rabbits (645), while LNAME treatment reduces the recovery of blood flow and capillary growth post-ischemia (645, 698). Nitrite therapy also enhances angiogenesis and blood flow recovery following induction of hindlimb ischemia (507). It has been reported that eNOS protein level does not decrease in ischemia (622), which suggests that eNOS activity and/or NO bioavailability are altered in the ischemic state. This may be attributed to reduced Akt activity as well as increased levels of ROS, which would scavenge NO. Oxidative stress is enhanced within ischemic skeletal muscle of PAD patients (719). ROS can contribute to cellular senescence, decreased responsiveness of growth factor pathways, and increased senescence and/or apoptosis of ischemic endothelial cells (953).
8.18.5. Angiostatic factors
Elevated levels of TSP1 mRNA and protein (CD47) are detectable in the distal ischemic (amputated) muscle of patients with CLI, which correlates with lower capillary density (253). The level of Tsp1 is increased in aged animals, which has been associated with limited recovery post-ischemia (448, 449).
Endothelin levels are elevated in patients with CLI (188). Endothelin receptor antagonism (at subpressor levels) in rat femoral artery ligation is associated with enhanced capillary growth within the ischemic limb (436). These positive effects are prevented in animals co-treated with anti-VEGFA antibodies or with L-NAME. Endothelin receptor blockade results in enhanced production of VEGFA and eNOS, suggesting that endothelin signalling represses NO-VEGF pathways.
While low levels of AngII have been associated with capillary growth (19), high levels of AngII impart negative consequences to the microvasculature. Angiotensin converting enzyme (ACE) inhibition using quinaprilat improves capillary density in ischemic tissue, equivalent to treatment with recombinant VEGFA (252). Authors propose that tissue levels of ACE activity, rather than plasma levels, are the key negative factor, as the ACE inhibitor captopril (which has limited access to extra-vascular compartments) does not induce a significant improvement in angiogenesis within the ischemic limb, and did not control ACE tissue levels (252). This also suggests that the improvements seen with ACE inhibition are not simply a result of increased vasodilatation and limb blood flow, as these systemic effects are similar with both inhibitors.
Post-transcriptional production of the anti-inflammatory cytokine IL-10 is increased by elevated adenosine levels (653), which may serve to limit angiogenesis. Correspondingly, mice deficient for IL-10 have enhanced levels of tissue VEGFA and increased capillary density, while mice with elevated IL-10 levels have an impaired response to femoral artery ligation (855).
8.19. Diabetes and Peripheral Artery Disease
The microcirculation is impaired in type 2 diabetes. Capillary rarefaction is associated with diabetes and metabolic syndrome (70, 269). Goto rats, a model of type II diabetes, have reduced perfusion of capillaries within the skeletal muscle under resting conditions (66% compared to 93% in non-diabetic controls) (690). Thus, if combined with arteriosclerosis of the iliac or femoral arteries, ischemia may be more severe in these individuals. Hematocrit and red blood cell velocities also are lower, resulting in substantively reduced oxygen delivery capacity to the muscle in these animals. This enhances the likeliness of ischemia, and also generates a local environment that disadvantages the appropriate cellular responses to ischemia. Endothelial cell apoptosis is increased, and capillary density reduced, in 3 month old leptin receptor deficient mice (db/db) compared to WT controls (243). Diabetic mice subjected to 1 hr of running exercise have a reduced response of VEGFA mRNA, and a reduction in VEGFR2 mRNA. Conversely, thrombospondin1 is significantly increases in these mice, independent of exercise (491).
Patients with type II diabetes have elevated plasma levels of VEGFA (82). Conversely, tissue levels of VEGFA are reduced (157), which may suggest reduced production of heparin binding isoforms of VEGFA.
Db/db mice display significantly reduced angiogenesis and blood flow recovery following ischemia induced by femoral artery ligation (243). Diet-induced type II diabetic mice have an elevated level of VEGFA mRNA and protein in ischemic muscle (351). Elevated levels of full length and soluble VEGFR1 are detected in these mice (351), leading researchers to postulate that VEGFR1 interferes with appropriate VEGFA signaling. Alternatively, decreased cellular responsiveness to VEGF (particularly Akt-dependent signals) may be a result of enhanced intracellular phospatase signaling (PTP-1B, PTEN, SHIP2), in line with the mechanisms responsible for insulin insensitivity (351). Increased angiostatin levels also exist within vessels from type II diabetic patients (correlating with elevated activity of matrix metalloproteinases 2 and 9), which may inhibit angiogenesis signaling. MicroRNA 503 is enhanced in endothelial cells under conditions mimicking diabetes. It also is elevated in ischemic muscle from streptozotocin -diabetic mice and in the muscle and plasma from patients with peripheral artery disease (126). miR503 is associated with inhibition of endothelial cell proliferation. Blocking miR503 by injection of a decoy results in enhanced angiogenesis and blood flow recovery in response to femoral artery ligation (126).
8.20. Pharmacological Treatments for PAD: A Role for Angiogenesis?
Of the two drugs approved for use to treat PAD, cilostazol (phosphodiesterase 3 inhibitor) and pentoxifylline (phosphodiesterase 1–5 inhibitor), only cilostazol consistently improves maximal walking distance of patients (782). However, some studies find evidence for improved ankle-brachial index with cilostazol treatment, while others do not (192). These results suggest that the major effect of cilostazol is not at the level of collateral arterialization. Similarly, it is known that pentoxifylline increases exercise performance in rats with femoral artery stenosis, but does not enhance maximual blood flow to the working muscles, leading authors to suggest that pentoxifylline may enhance oxygen extraction by improving flow distribution within the muscle (211). While little is known about the cellular effects of these drugs, it is possible that they may enhance angiogenesis within the ischemic muscle. Cilostazol treatment is associated with an increase in circulating VEGFA (particularly in non-diabetic patients), and the extent of VEGFA production correlates well with the percent change in patient mean walking distance (524). This may be consistent with enhanced angiogenesis within the ischemic muscle. However, VEGFA levels alone do not indicate increased angiogenesis (as discussed earlier). Interestingly, significant increases in circulating VEGFA were not observed with pentoxifylline treatment in the same study, despite the drug resulting in increased in maximum walking distance (524). Cilostazol has been reported to enhance NO production (346, 438). Recently, cilostazol was reported to enhance the phosphorylation of eNOS in a mouse model of limb ischemia. Further, the improved blood flow recovery post-ischemia seen with cilostazol treatment was prevented by treatment with LNAME (414). Thus, through activation of the eNOS/NO pathway, cilostazol may improve vasodilation and contribute to local increases in VEGFA production, as well as promote anti-thrombosis, and protect against endothelial cell senescence (688).
8.21. Exercise Training, Angiogenesis and Peripheral Artery Disease
Exercise training is associated with enhanced recovery of muscle blood flow, improving exercise tolerance and muscle performance following arterial ligation in animal models (585). Even modest exercise such as treadmill walking is able to facilitate improved muscle function in rats (1023). The majority of animal studies indicate that exercise training increases capillary to fiber ratio in ischemic muscle. In rats subjected to bilateral femoral artery ligation followed by progressive exercise training, endothelial cell proliferation is observed in the ischemic muscle after 3–7 days of exercise, followed by significant increases in capillary contacts per fiber compared to sedentary ligated after 12 days of exercise. Over the same time period, there is no evidence of endothelial cell proliferation and capillary contacts per fiber remains constant within the hindlimb muscles of sedentary ligated rats (205). However, in models in which femoral artery ligation itself induces a compensatory increase in capillary to fiber ratio, exercise training may not generate further improvements. For example, exercise training (7–9 weeks of progressively moderate to high intensity treadmill running) did not stimulate further enhancement of capillary growth in plantaris muscle (despite increasing the oxidative capacity of the muscle)(766).
Exercise induces responses within the ischemic limb that parallel those observed in healthy exercised muscle. Within 4–8 days of exercise, increased levels of VEGFA, VEGFR2 and eNOS mRNA are detectable (545). Muscle activity induced by electrical stimulation reduces expression of VEGFR1 and increases expression of VEGFR2, suggesting enhanced responsiveness to VEGFA (622). Interestingly, VEGFR2 antagonism only partially prevents the exercise-training induced angiogenesis post-femoral artery ligation in rats, despite complete abrogation of improvements in muscle blood flow (544). This finding points to redundancy of mechanisms capable of inducing angiogenesis, as well as providing support for the diversity in function of VEGFA across multiple cell types. Exercise training also is well established to improve NO bioavailability. However, treatment of rats with LNAME does not affect the exercise-induced increase in capillary contacts per fiber within ischemic muscle (546), indicating NO-independent mechanisms of capillary growth. Tie2 and Ang2 levels also are upregulated within 4–8 days of exercise post-ligation while Ang1 tends to decrease at the same time (545). This results in a higher Ang2 to Ang1 ratio, which is indicative of an angiogenic phenotype.
In contrast, there is no change in FGF2 mRNA levels in ischemic muscle following arterial ligation (149), or in response to exercise training or electrical stimulation of the ischemic muscle (205, 840). While this suggests that FGF2 does not play a role in post-ischemic angiogenic adaptation, it is possible that pre-existing matrix-sequestered FGF2 may be released via proteolytic cleavage, which would enable it to activate cell surface receptors and exert cellular effects.
There is evidence that an exercise training program can augment levels of circulating EPCs in patients with ischemic syndromes or PAD (800, 821). Increased circulating EPCs may participate in the process of capillary growth, or may promote enhanced endothelial cell function, thus contributing to the observed improvements in ABI and maximal walking distance for these patients.
Exercise training of mice has been shown to reduce levels of prolyl hydroxylase-3 (PHD3) and factor inhibiting HIF (FIH), which would result in the enhanced the stabilization of HIF1α and greater production of VEGFA (146). While training improves blood flow recovery of wildtype mice, it does not rescue the effect of ischemia on MMP-2−/− mice, leading authors to suggest that MMP--2MMP2 production and/or activation is an important downstream consequence of VEGFA signaling (146).
Low intensity chronic electrical stimulation also has been used successfully to enhance the angiogenic response within an ischemic limb. Intermittent electrical stimulation of lower limb muscles in humans with peripheral artery disease over a period of 4 weeks results in a significant increase in pain free walking distance and maximum walking distance, combined with a reduced fatigue index within the muscle (drop in muscle tension over time (935). Investigators hypothesize that capillary growth contributes to this increase in muscle performance. Intermittent electrical stimulation (5 minutes stimulation, followed by 5 minutes rest, repeated 8 times daily) over 4 weeks does result in an increase in capillary density in ischemic rabbit skeletal muscle, with corresponding increases in VEGFA and VEGFR2 mRNA (840). The stimulation frequency is important, as 10 and 40, but not 1, Hz stimulation resulted in improved capillary density. However, as discussed earlier (Section 8.16.2), increased muscle activity leads to a greater incidence of capillary swelling and leukocyte adherence to venules (381, 382, 427), and thus strenuous activity may induce muscle damage and interfere with blood flow recovery (424).
Another interesting avenue for future development is the concept of remote conditioning to enhance angiogenesis within ischemic muscle. One research group has shown that high intensity electrical stimulation of a non-ischemic limb over a period of 4 weeks promotes increased VEGFA levels and angiogenesis within the contralateral ischemic limb. This suggests that exercise-induced release of angiogenic factors (such as IL-6, VEGFA) into the circulation can promote angiogenesis at distant sites (841).
9. Training Adaptations Within the Active Muscle: Redistribution of Blood Flow
A training-induced redistribution of blood flow within the limb affected by PAD has the potential of improving oxygen delivery to the active muscle without an overall increase in blood flow to the entire limb. This could occur by one or both of two processes: 1) there could be a redistribution of blood flow from the upper, less affected region of the limb, to the lower limb muscles; and 2) there could be a redistribution of blood flow within the distal muscles to better perfuse the regions of active muscle fiber recruitment.
A redistribution of blood flow from the upper to the lower limb regions is possible in conditions with more proximal obstructions. Collateral vessels that develop in this condition arise from non-obstructed vessels that are normally designed to perfuse the proximal muscles of the upper thigh. While these collateral vessels circumvent the obstruction, they derive blood flow from circuits not originally intended for the distal muscles. Thus, any flow that proceeds distally has the potential to ‘steal’ flow from the intended proximal region of the vascular circuit. Alternatively, any increase in flow to the intended proximal muscles would lessen the potential for collateral flow distally. This potential ‘competition’ for the upstream perfusion pressure is established by the relative resistances of these vascular circuits that are effectively in parallel with each other. If exercise training were to make a more efficient motor unit recruitment, combined with a more responsive and selective vascular distribution of flow during a given modest intensity activity such as walking, then the upper limb muscles may be better able to function with a relatively reduced blood flow. The required oxygen demand could be met by an expanded oxygen extraction, a response quite capable of trained muscle (767, 769, 1023). The net result is that a greater blood flow would be available to be perfuse distally. Evidence for this prediction has been observed in a pre-clinical model of PAD where the vascular obstruction was introduced in the femoral artery. The relative fraction of total limb blood flow that went to the distal limb muscles became greater in trained rats (584). Whether this occurs in patients that are trained and contributed to their increased exercise tolerance is not possible to determine. Appropriate blood flow measurements cannot be made. However, if this happened there would be a greater oxygen extraction across the entire limb of patients who were trained, an observation made some 30–40 yrs ago ({Zetterquist, 878, 1037).
A redistribution of blood flow within an active muscle could occur after training to better support an improved exercise tolerance. Even with a limited oxygen delivery to the calf muscle in patients with PAD, a better utilization of this oxygen delivery could be optimized to effect a benefit. We have seen that patients with PAD can exhibit an altered gait while walking, a response that would alter motor unit recruitment. A potentially inefficient recruitment of muscle fibers would lead to a broad requirement of the limited flow to the muscle. This could lead to undue fatigue of some motor units and an inefficient use of the flow that remains to the affected fiber regions (565). If exercise training helped normalize gait and motor unit recruitment, there is the potential to better perfuse the lesser number of motor units that are now needed for the locomotion. Exercise tolerance would be improved with this optimal use of the oxygen delivered. Further, training-induced adaptations of an increased mitochondrial content and an increase capillarity should also optimize oxygen utilization and enhance the performance of individual motor units that are recruited, as observed in rats with stenosis of the femoral artery that were trained by treadmill running (585). If this occurred in patients with PAD, there would be a greater oxygen extraction across the limb, as observed previously (877, 1036).
10. Training Adaptations Within the Active Muscle: Increased Mitochondrial Content
Another hallmark adaptation induced, within active skeletal muscle by endurance-type exercise training, is an increase in mitochondrial content (401). Each of the different skeletal muscle fiber types will increase their mitochondrial density as long as they are recruited (49) in a manner related to the intensity of the training program (217). This increase in mitochondrial content is thought to underpin significant increases in endurance performance through metabolic changes in fatty acid oxidation and reduced glycogen utilization (404). In deed, cellular signals that influence substrate selection are meaningfully altered in trained muscle (218, 401) to favor a beneficial selection of substrate source to fatty acids during prolonged sub maximal exercise. As described above, in addition to these biochemical changes, individual muscle fibers within the trained muscles are surrounded by more capillaries (21, 100, 441). Further, muscle fibers regions that contain high-mitochondrial, high-capillarity fibers also receive high blood flows (33, 517, 565). Thus, there appears to be an important coordination in the design of muscle for the convective delivery of oxygen, for the diffusive capacity for oxygen exchange, and for the biochemical capacity to utilize oxygen, thereby establishing a variety of oxidative capacities among the different muscle fiber types and the influence of exercise training.
10.1. Training adaptations in muscle of patients with PAD
Interestingly, the adaptations of an increased capillarity and increased mitochondrial content can be found in patients with intermittent claudication. An increase in mitochondrial enzyme activity (116, 405, 407) and an increase in capillary density (164, 337, 573, 606, 921) have been found in patients, even without participation in an exercise program. The appearance of these changes has not always been found (163), possibly due to reductions in the high-oxidative, high-capillary type I fiber number and area (38, 164). Further, their appearance is likely dependent upon the severity of the occlusive disease, since below normal mitochondrial contents are found in advanced stages of PAD (116, 405, 407), possibly indicative of muscle disuse atrophy and/or tissue pathology. In cases of successful vascular surgery that improved blood flow, the initially elevated muscle mitochondrial content reverted back to normal (407). Whereas, those patients whose surgery was unsuccessful with no improvement in blood flow, did not demonstrate a change in mitochondrial content (407). Thus, the stimulus for enhanced mitochondrial content appears related to ischemia. Since blood flow, and thereby oxygen delivery, had been improved by surgery, it has been suggested that hypoxia is an important stimulus (235, 406) that serves to ameliorate the tissue insult caused by peripheral arterial insufficiency. If this is the case, then it would be expected that any involvement in an exercise program that fosters continued muscle ischemia would further enhance angiogenesis. Indeed, patients with intermittent claudication who participate in an exercise program demonstrate a significant increase in mitochondrial content, above that of inactive control claudicates (235, 406, 557, 558), although this has not always been found (379). However, as developed in the section on Angiogenesis, hypoxia is not the sole, nor possibly even the most important, factor stimulating angiogenesis in the active muscle.
11. Coordination in Mitochondrial Biogenesis and Angiogenesis in Muscle by Exercise Training
11.1. Control of mitochondrial biogenesis
The control of mitochondrial content within muscle is subject to a complex set of variables including, muscle fiber type composition (797), aging (513), inactivity (384), training status (49, 402), and factors that alter muscle fiber composition (e.g., nerve firing pattern (715), thyroid status (433, 999)). Yet to be fully understood, there are keen differences in mitochondrial contents among adult skeletal muscle fiber types that occur during development. The greatest contrast in muscle fiber mitochondrial content is observed in non-primate mammals between the low-oxidative white fibers (fast-twitch, type IIb) and the high-oxidative red fibers (slow-twitch and fast-twitch, type I, IIa and IIx) which can be as much as a 4-fold difference. While individual fibers of human muscle can exhibit large variations in some enzyme activities (551), there is generally a much smaller difference in mitochondrial enzyme activity between low-oxidative and high-oxidative muscle fibers (248, 797). Thus, in the absence of other influences the mitochondrial content of a whole muscle will be determined by its muscle fiber composition. The metabolic and functional implications of differences in muscle fiber mitochondrial contents are generally the same across species, when quantitative variations are taken into consideration. For example, in contrast to the low-oxidative white fibers, muscle fibers that have the greatest mitochondrial content, within a species, exhibit the greatest relative capacity for aerobic metabolism and are highly fatigue resistant during prolonged submaximal contractions. Further, there is an impressive design in skeletal muscle, as a tissue, to coordinate aspects of vascular support to match the oxygen demand of each mitochondrial content. In addition to the variation in capillary densities among muscle fiber types, there is a corresponding variation in blood flow to these muscle fiber areas (517, 565). Thus, there is a coordinated relationship among mitochondrial content (biochemical capacity for oxygen consumption), blood flow (absolute oxygen delivery), and capillarity (oxygen diffusion capacity) for the different skeletal muscle fiber types. We will now consider how this coordination in the design of mitochondrial content and vascular capacity is controlled, particularly from the perspective of muscle use and the adaptations that it establishes.
There are many excellent reviews covering mitochondrial biosynthesis that provide details beyond the scope of this article (30, 402, 403, 412, 413, 466, 513, 539, 605, 676, 772, 806, 807, 1017). Thus, only a general overview will be provided and that in the context of exercise responses in muscle. A major break through in our understanding of the control of mitochondrial biosynthesis came by the discovery of peroxisome proliferative-activated receptor-γ coactivator1α (PGC-1α)(736), which binds to transcriptional factors that modify gene expression. Indeed, over expression of PGC-1α leads to a striking increase in red, high mitochondrial content muscle within the transgenic mouse (537) and an improvement in exercise performance and increased oxygen uptake (121). While PGC-1 was first recognized to be important in transcriptional regulation of mitochondria, it has been viewed as a ‘master’ regulator, since it has wide-spread effects on numerous processes important within cells (338, 339, 536).
The activity of PGC-1α can increase by a dual process. Phosphorylations, along with other post-translational modifications, of PGC-1α enhance activity and thereby nuclear gene transcription. In addition, there can be an increased in PGC-1α expression, thereby increasing its abundance within the cell making more for available for activation. In particular, enhancing PGC1α activity increases the expression of two nuclear regulatory factors (NRF-1 and NRF-2) (806), which are imported into the nucleus to increase transcription of nuclear-derived mitochondrial proteins (251). In addition, NRF-1 and NRF-2 promote gene transcription of transcription factor A mitochondrial (TFAM) (969), which promotes mitochondrial gene transcription. Thus, activation of PGC-1α by phosphorylation has downstream effects to promote the coordinated production of both mitochondrial-and nuclear-coded proteins needed for mitochondrial biosynthesis. It then remains for the nuclear coded proteins to be imported into the mitochondria and assembled with relevant mitochondrial protein components into mature, functioning mitochondrial elements (412, 413). PGC-1α is significantly elevated in muscle by individual exercise bouts (27, 43, 312, 443, 717, 789, 899, 920). Thus, the actions of PGC-1α are thought to be an important, but likely not the only (299, 437, 945, 1017) feature that leads to the greater mitochondrial content in muscle after exercise training. We now turn our attention to what is thought to control PGC-1 expression in active skeletal muscle.
11.2. Factors initiating muscle adaptations
Factors that could serve as initiators of muscle adaptations are expansive, since muscle contractions instigate a multitude of changes that modify the quiescent condition within resting muscle, including: altered tension (radial, longitudinal), myocyte shorting (lengthening), membrane events, ion redistribution, energy expenditure, heat production, signaling pathway activation/inhibition, a myriad of metabolic processes, vascular responses, cytokine influences, etc. However, among these, increases in cytoplasmic [Ca2+] and responses to the ‘energy state’ of the fiber have been the two features that have gained the most attention with extensive evidence for their importance (30, 402, 412, 413, 466, 539, 605, 676, 772, 1017). Increases in [Ca2+] is a logical candidate since it is essential for muscle contraction, varies over time as a function of contraction intensity, and has such a potent influence in activating signaling pathways. Thus, increases in [Ca2+] over time within the muscle fiber has the expectation of satisfying the known influence of exercise intensity and duration on the magnitude of mitochondrial increases with training (217). An increase in [Ca2+] within the myocyte activates calcium/calmodulin dependent protein kinase (e.g., CAMKII)(774), which sets into motion the p38 mitagen-activated protein kinase (p38MAPK) pathway (1008, 1009) and subsequent activation of transcription factors, activating transcription factor 2 (ATF2)(12) and myocyte enhancing factor 2 (MEF2)(1041) which promote PGC-1α transcription. This, in turn, leads to enhanced protein accumulation of PGC-1α, increasing the availability of PGC-1α to effect promotion of mitochondrial biogenesis following exercise (cf., Figure 8). In addition to this important calcium-dependent signaling pathway, muscle contractions stimulate mitochondrial biogenesis via a pathway that is sensitive to the energy demands within the myocyte. An increase in the freely available adenosine monophosphate concentration [AMPfree] within the fiber activates a protein kinase (AMP-activated protein kinase (AMPK))(1000) which phosphorylates PGC-1α (458) to effect the sequelae of events in mitochondrial biogenesis (402, 794, 1000). AMP activation of AMPK is two fold, first by a concentration-dependent allosteric effect and second, by activation of an upstream AMPK kinase (LBK1(431)). This influence of AMP is exquisitely sensitive, since the [AMPfree] is extremely low within the cymosely and subject to significant increases as a function of the rate of energy expenditure (218) due to the equilibrium reactions exchanging ATP, ADP and AMP (cf., (612)). Thus, the [AMPfree]-dependent pathway also fulfills the expectation of a driving stimulus for mitochondrial production that is influenced by both the intensity and duration of exercise (217). Further, it also fulfills the requirement that the stimulus for mitochondrial biogenesis be self-limiting, since as the mitochondrial content within a fiber increases to its asymptotic value for a training program, the increase in [AMPfree] within the fiber during the training bout is expected to progressively decrease (218, 403). A number of other factors that change with exercise have been shown to influence PGC-1α activity (e.g., nitric oxide (540)), but are less well studied compared to the p38MAPK and AMPK pathways. Interestingly, changes in some microns within active muscle (27, 793) may contribute to the increase in PGC-1α with exercise. However, at this time, the Ca2+-and AMP-dependent pathways stimulating PGC-1α activity within the active muscle are likely the primary factors causing the increase in mitochondrial content within active muscle after exercise training.
Figure 8.
An overview of signaling pathways that coordination exercise-induced angiogenesis and mitochondrial biogenesis. Information obtained from (12,31,403, 413, 513, 750, 1009).
11.3. Role of PGC-1 in coordinating mitochondrial biogenesis and angiogenesis
We have seen from the section on angiogenesis, the process enhancing capillary density in active muscle is rather complex to orchestrate, but likely involves the influence of powerful cytokines like vascular endothelial growth factor (VEGF). VEGF is highly up regulated in active muscle by exercise by a process that includes regulatory control by the hypoxia inducible factor (HIF-1α). In addition and as illustrated in Figure 8, there is transcriptional regulation of VEGF by an HIF-1α independent process, that of control by PGC-1α (31, 151). Thus, coactivation of transcription factors by PGC-1 serve as a common feature in the signaling of both mitochondrial biogenesis and capillary expansion by angiogenesis (299, 847, 1017). This action of PGC-1α to up regulate VEGF expression occurs by co activating the gene expression of the estrogen related receptor alpha (ERRα) which in turn is thought to activate a promoter region on the VEGF gene (31, 151, 1038). A reduced increase in VEGF expression, in PGC-1 knockout mice following exercise, demonstrates the functional relevance of this non-HIF-1 pathway (151, 299, 526) in coordinating the mitochondrial and angiogenic processes to exercise. As apparent in Figure 8, VEGF is common to both upstream signals of HIF-1α and ERRα and appears to be essential for the training-induced increase in capillarity. Thus, in myocyte-specific VEGF gene-deleted mice, exercise training increased mitochondrial enzymes but did not change capillary density (679). These recent findings have meaningfully advanced our understanding of how signals prompted by exercise effect adaptations which enhance the functioning of muscle. Such adaptations could impact patients with PAD, even if the limited blood flow to the ischemic muscles is not increased, since there could be a more effective use of the oxygen that is delivered to the active muscles (877, 1023, 1036).
12. Improved Muscle Metabolism with Exercise Training
An inadequate blood flow to the limbs of patients with PAD can cause greater consequences than the obvious rapid onset of fatigue during activity. As the severity of PAD increases there is a great potential for pathological changes to develop. This is most apparent in conditions of rest ischemia where tissue necrosis, muscle fiber pathology, ulceration, and gangrene can be found (661). However, even when the flow capacity to the muscle is sufficient for rest, there can be a challenge to the muscle as ischemia occurs during physical activity, such as extended walking or climbing up flights of stairs. For example, there is the potential for ischemia-reperfusion injury of muscle following such a bout of severe exercise. As described above in Section 5, this challenge of free radical production and an acute phase inflammatory response can be viewed as detrimental (930). However, it is apparent from numerous studies that repeated bouts of exercise, that lead to a trained condition, temper this inflammatory response to exercise, resulting in a lessened inflammatory state (929, 930). Further, PAD patients, who participated in an exercise-training program, exhibited a reversal of the abnormal metabolic response initially observed following exercise to maximal claudication pain (379).
Nonetheless, challenges to effective utilization of muscle can be found in patients with PAD (718, 719). During muscle contractions the rate of energy expenditure must be matched by an adequate rate of ATP provision in order to avoid fatigue. This is established through mitochondrial respiration, in the steady-state, and by the addition of glycogenolysis, in the transition from rest to exercise and with relatively intense or during ischemic exercise. While the ischemic contractions of patients exercising to point of maximum pain tolerance is expected to prompt a high rates of lactate production, increases in circulating levels of lactate are relative modest (887), probably owing to the relatively small muscle mass that precipitates the cessation of activity. A metabolic consequence of insufficient oxygen limiting mitochondrial respiration is a build up of reducing equivalents and carbon sources that would normally be better oxidized by mitochondria to synthesize ATP (e.g., short-chain acylcarnitine). This was recognized by Hiatt and coworkers (376, 378, 379) who observed excessive alterations in muscle carnitine metabolism when patients exercised to maximal claudication pain. A potential contributor to this inadequate energy supply could be dysfunctional mitochondrial (718), owing to morphological evidence and where substrate oxidation and electron flux capacity was reduced in PAD patients (720, 721). Such mitochondrial dysfunction could contribute to several findings observed in PAD patients in the response to exercise. For example, muscle oxygen desaturation kinetics were slowed during easy, but not more intense, exercise (58), as was its recovery following exercise (289). Similarly, the time-constants for PCr change at the onset of exercise and during recovery, reflecting the transitions in energy expenditure (611, 612), were delayed in patients with PAD (317, 446, 446). While these changes are consistent with a mitochondrial dysfunction, and the metabolic inertia that would result (1050), there are also a number of other factors that should be considered.
The incidence of PAD markedly increases with age. Further, for understandable reasons, patients with PAD tend to be relatively inactive. Besides the profound effect that inactivity and aging has on muscle morphology and function (fiber type, mitochondrial content, muscle capillarity)(122, 925), there are significant repercussions in the vasoresponsiveness of the vasculature leading to a dulled vasodilatation of conduit and small resistance vessels (518, 644) involving endothelial dysfunction and nitric oxide bioavailability. Indeed, nitric oxide bioavailability significantly impacts the oxygen exchange transition at the onset of muscle contractions (174, 385). Thus, such an impaired vasodilatory response could have contributed to the slower rate of perfusion at the onset of contractions measured in patients with PAD (447), as well as the delayed PCr kinetics (317, 446, 446). Interestingly, the vascular/tissue oxygen exchange transition is also influence by alpha sympathetic activity (439), owing to the elevated sympathetic outflow that can be dominant during contractions. As described in Section 15.5, there is a hypersympathetic response to exercise in patients with PAD. This could profoundly impede the vascular response to contractions in these patients. Fortunately, exercise training has been shown to improve mitochondrial dysfunction (172), reverse the decrease in aerobic function of aging (922), improve vascular/muscle oxygen exchange (769, 1023), and is expected to improve the reduced PCr transition observed in inactivity patients with PAD (249). Thus, it is likely that the muscle-specific adaptations of an improved mitochondrial function and an enhanced capillarity, and improved vasoresponsiveness are features that underpin the uniform improvement in exercise tolerance experienced by patients with PAD that are more physically active.
13. Training Adaptations Within the Active Muscle: Increased collateral blood flow
Collateral arteries are small arterial vessels that connect the perfusion territory of one supply artery with the perfusion territory of another supply artery. Under normal conditions, little flow passes through these vessels, due to their narrow diameter and correspondingly high resistance. However, in response to occlusion of one of the upstream supply arteries, collaterals can enlarge and accommodate a significant level of blood flow. This process is known as arteriogenesis, to distinguish it from the related process of capillary proliferation (angiogenesis). Arteriogenesis can preserve tissue distal to an arterial occlusion by providing an alternate route for blood flow. Thus, stimulation of this process is an attractive potential treatment for the complications of ischemic cardiovascular diseases such as peripheral artery disease. The reader is directed to a number of excellent reviews (112, 115, 118, 125, 193, 352, 353, 353, 354, 357, 785, 808, 810, 822, 830, 923, 961, 964) and an entire monograph (810) on arteriogenesis. It is well established that exercise training serves as a physiological stimulus for arteriogenesis in the peripheral circulation. An overview of the arteriogenic process in the peripheral vasculature and its functional consequences for blood flow to the distal skeletal muscle tissue is shown in Figure 9.
Figure 9.
Summary of some key events in the remodeling of a collateral artery in response to upstream occlusion. The approximate time course is shown moving from upper left (with events occurring within hours/days of occlusion) to the bottom right (completion of remodeling after >1 month). Increased shear stress and vessel stretch following upstream occlusion leads to endothelial cell activation, adhesion molecule expression, and monocyte infiltration, followed by reorganization of the extracellular matrix. Phenotypic shift, migration, and proliferation of vascular smooth muscle cells leads to neointima formation and an increase in the number of smooth muscle cell layers. The process is complete when vascular smooth muscle cells have returned to a contractile phenotype and the vessel structure has regained a relatively normal appearance. (Not all cell types are shown at each time point, and the number of smooth muscle cell layers is limited for clarity).
13.1. Key early studies on the collateral circulation
The collateral circulation and its ability to remodel have been the object of study for many decades. An early question asked by investigators was whether collateral arteries were present in normal human tissues. Matas studied collateral-dependent limb blood flow in human subjects by occluding main supply arteries (brachial and femoral) with a tourniquet. This sudden obstruction of the blood supply to the limb caused it to turn pale; however, some faint color eventually returned in some subjects, although the main artery remained occluded. This observation was interpreted to mean that collateral circulation existed below the level of the occlusion (583). Prinzmetal and Simkin addressed this question in the coronary circulation by more quantitative post-mortem studies in which they perfused human hearts with glass spheres of varying sizes. Based on their studies, they concluded that collaterals with a diameter of 70–180 m existed in the coronary circulation (729).
Another early observation made in humans was that collateral blood flow can increase over time. (cf., Figure 10). By using bath calorimeters to calculate blood flow in the hands of human subjects, Stewart found that blood flow to the hand gradually increased over ~1 month following ligation of major supply arteries during surgery for aneurysms (889). Later researchers pioneered the use of animal models to explore the mechanisms by which collateral arteries enlarge. Eckstein demonstrated that experimental stenosis of the left circumflex coronary artery (LCX) in dogs induced coronary collateral enlargement. A further key observation was that collateral enlargement was proportional to the degree of stenosis (224). Sewell further characterized the relationship between degree of stenosis and collateral enlargement in the dog heart and concluded that collaterals only enlarge when the upstream stenosis is severe enough to create a significant pressure gradient between the stenosed and non-stenosed supply arteries, and that collateral size is proportional to this pressure gradient (836). These findings were later confirmed in humans in studies showing low levels of collateral blood flow and high collateral resistance in normal hearts, compared to hearts of patients with atherosclerosis. This work suggested that collaterals exist but are not well developed in the normal human heart and that upstream occlusion is a key stimulus for collateral development (308).
Figure 10.
Diagram of forces acting on peripheral collateral vasculature and the resulting changes in collateral-dependent blood flow in response to upstream arterial occlusion. Top: simplified representation of the peripheral vasculature. Collateral vessels are present under normal conditions (left). However, there is no pressure gradient across the collaterals. Moreover, collateral resistance is high due to the narrow vessel diameter. Thus, collateral blood flow is low under normal conditions. Following an acute occlusion (center), a pressure gradient is created across the collaterals, driving flow through the vessels. Vasodilation produces a further limited increase in collateral blood flow. Since the vessel diameter remains relatively small and the pressure gradient for flow is large, shear stress levels in the collaterals are high. High shear stress initiates structural remodeling, which is evident following chronic occlusion (right). Smooth muscle cell proliferation occurs, resulting in increased vascular wall thickness. Since the ends of the vessel are fixed, vascular growth also produces an increase in tortuosity of the collaterals. Eventually, the diameter of the vessel increases to a point where shear stress is reduced to non-stimulatory levels, and remodeling ceases. Middle: the events described above, seen at the level of the individual collateral artery. A limited number of smooth muscle layers is shown for clarity. Bottom: functional consequences of arterial occlusion and collateral remodeling in skeletal muscle of the distal limb. Vasodilation of collaterals following acute occlusion may provide sufficient flow for tissue needs under resting conditions depending on the location of the occlusion (center), but is insufficient for active skeletal muscle demands. Thus, distal skeletal muscle is at risk of ischemia and may become hypoxic. (The area of collateral remodeling in the proximal limb is itself well-perfused and non-hypoxic). Reduced tissue pO2 leads to opening of capillaries within the muscle. After structural remodeling of the collateral vasculature (right), blood flow capacity to the distal limb is improved and may suffice to support the demands of active skeletal muscle. In conjunction with arteriogenesis in the proximal limb, capillary proliferation (angiogenesis) occurs in distal tissue, in response to hypoxia and other factors.
Although studies of the peripheral collateral circulation in human patients were being published 100 years ago (as discussed above), most of the early mechanistic studies that were subsequently published focused on the coronary collateral circulation. However, remodeling of peripheral collaterals appears to occur via similar mechanisms to those described in the coronary circulation. Similarly to the findings in the coronary collateral circulation, peripheral collateral-dependent blood flow was demonstrated to be higher in dogs with a chronic iliac artery ligation than in acutely ligated dogs, showing that peripheral collaterals also remodel in response to upstream occlusion (166). Matolo et al demonstrated that peripheral collateralization could be enhanced by creation of an arteriovenous fistula, suggesting the role of mechanical influences on arteriogenesis (588). Arteriogenesis in the periphery is locally regulated and occurs independently of arteriogenesis in the coronary circulation, as shown by studies in which occlusion of the femoral artery induced peripheral collateralization without any effect on the distant, nonoccluded coronary circulation (368).
Key early methodological developments in the study of arteriogenesis included the use of radioisotopes and radiolabeled microspheres to quantitatively assess collateral dependent blood flow (64, 143) and the development of ameroid constrictors to produce more physiological, gradual arterial occlusions that better mimicked human ischemic cardiovascular disease (968). These early experiments and techniques laid the groundwork for the following several decades of study of arteriogenesis.
13.2. Number and size of pre-existing collaterals varies across and within species
Although collateral arteries have been demonstrated in normal human and animal tissue, they are small and carry very little flow under normal conditions. The majority of pre-existing collaterals in the dog hindlimb are than 100 μm in diameter, with a total cross-sectional area equal to 7.5% of the normal arterial supply and calculated conductance of 1.5% of normal arterial conductance (173). Similarly, collateral conductance in the dog heart following acute occlusion was reported to be only 5% of normal coronary artery conductance (814).
Considerable variation in the number of pre-existing collaterals has been reported across species. This variation is reflected by the level of collateral-dependent blood flow following acute occlusion, and with the functional consequences of acute occlusion. Thus, it was soon observed that while acute coronary ligation is generally lethal in pigs (553, 554), which have sparse collaterals, it is somewhat less so in dogs (143), which have abundant collaterals. In a comparative study of eight species, Maxwell and coworkers found that collateral flow to acutely ischemic myocardium (as a percentage of normal blood flow) ranged from a high of 15.9% in dogs (excepting guinea pigs, which showed no impairment of flow following acute occlusion) to a low of 0.6% in pigs (592). Similar findings of greater collateralization in dogs than other species have been reported in other studies (344).
Recent work has also demonstrated significant variation across strains within a species in both the flow capacity of the pre-existing collateral vasculature, and the extent of remodeling in response to occlusion. Strain differences are particularly noticeable in mice. In a comparison of three commonly used mouse strains, collateral blood flow to the hindlimb following acute occlusion of the peripheral arterial supply was found to be highest in C57BL/6 mice and lowest in BALB/c mice, with intermediate level of flow in 129S2/Sv mice. Likewise, C57BL/6 mice had a greater number of pre-existing collateral vessels than 129S2/Sv mice (358). These strain differences are reflected by more severe clinical signs of ischemia in the poorly collateralized strains following acute occlusion (140, 358, 963). In addition to a better development of pre-existing collaterals, the C57BL/6 strain also exhibits greater improvement in collateral blood flow in response to occlusion than other strains examined (273, 358, 825). The genetic basis for this difference has been partially localized (140, 981). Similar studies in Fischer 344 rats, brown Norway rats, and Fischer 344/brown Norway crosses showed that brown Norway rats have much greater ability to develop mesenteric collaterals than either Fisher 344 rats or the crosses (842). Thus, strain differences should be considered when designing studies on collateral remodeling in rodents.
These observations suggest that the abundance of pre-existing collaterals has a strong genetic basis. Few studies to date have investigated the genetic basis for determination of pre-existing collateral number. However, recent research implicates endothelial nitric oxide synthase (eNOS) (185) and vascular endothelial growth factor (VEGF) (139, 162, 273) as determinants of collateral extent and remodeling. Interestingly, the diameter of pre-existing collaterals also seems to have some genetic basis, and has been linked to expression of platelet-endothelial cell adhesion molecule (PECAM) (144). Finally, some evidence exists that variations in collateralization may have an immune component (965).
13.3. Collaterals enlarge and become tortuous in response to upstream arterial occlusion
Although native (unstimulated) collaterals have limited blood flow capacity, gradual occlusion of an upstream supply artery stimulates them to enlarge and results in significant increases in collateral blood flow. Early studies demonstrated that chronic occlusion resulted in higher collateral blood flow than acute occlusion in the dog heart (224) and hindlimb (166).The degree of collateral enlargement is strongly associated with the degree of stenosis in the upstream artery. Eckstein showed that collateral flow to dog myocardium increased in proportion to the degree of stenosis of the LCX (224). Sewell found that collaterals did not enlarge in dog heart unless the diameter of the LAD was reduced by at least 45% (836). Likewise, in an angiographic study of human patients with single-vessel coronary artery disease, the presence of collaterals was strongly correlated with the degree of vessel stenosis. No collaterals seen in patients with <70% stenosis, whereas collaterals were visible in 97% of patients with 100% stenosis (654). Similarly, another angiographic study in humans found that collateral filling score during brief balloon occlusion of the coronary artery during angioplasty was correlated with percent stenosis of the upstream supply artery, with 86% of patients with 80% stenosis having a collateral score of 0 or 1, and 100% of patients with 95% stenosis having a collateral score of 2 or higher (167).
In addition to increasing in diameter with increasing time since upstream occlusion, collaterals increase in tortuosity over time (432). Tortuosity is a hallmark of remodeling collateral vessels and is easily visualized by angiography. The development of tortuosity is attributed to the increase in collateral length, which occurs as they remodel. Since the two ends of the collateral vessel are fixed to the donor and recipient arterial vessels, any increase in length produces tortuosity.
13.4. Morphological changes in collaterals during arteriogenesis
Several studies have demonstrated active DNA synthesis in EC and SMC of remodeling collaterals, especially during the first 2–4 wk post-ameroid placement (701, 809, 990). Electron and light microscope studies provided further evidence that collateral enlargement is an active process involving cellular proliferation, rather than a passive vasodilatation. In general, these changes are most prominent in the collateral midsole. Structural reorganization of peripheral collaterals begins soon after an upstream occlusion. Fragmentation of the internal elastic lamina is visible within 2–3 d post-occlusion in rabbit and mouse hindlimb collaterals (824, 825). This reorganization of the extracellular matrix is accompanied by evidence of proliferation in endothelial cells and a subpopulation of smooth muscle cells, and the beginnings of neointima formation (824, 825). Monocyte infiltration is also an early event, occurring during the first 1–2 wk post-occlusion (722, 824, 825). Infiltration of monocytes into the adventitial layers is associated with changes in expression of adhesion molecules within the adventitia, suggesting that the adventitia plays an important role in collateral remodeling (722). By ~2 wk post-occlusion, a well-developed neointima is present in rabbit hindlimb collaterals and some SMC have returned to a contractile phenotype, although the extracellular matrix continues to show signs of active reorganization and many cells retain a synthetic phenotype (824). At later time points, the increased number of SMC results in the formation of new SMC layers; SMC regain a contractile phenotype, and their orientation begins to return to a more typical pattern (812, 824, 1002). When collateral remodeling is complete, the SMC are seen to be arranged in a fairly normal orientation, although collaterals may still have a somewhat abnormal appearance 1 yr post-occlusion (812).
Although the time course of early ultrastructural events in peripheral collateral remodeling appears to be similar between species, the later time course varies. In the mouse ischemic hindlimb, collaterals can double in diameter within the first week post-occlusion, and the vessels have a relatively normal appearance by 2 weeks post-occlusion, although some further increase in size occurs up to 3 wk (825). In contrast, the number of SMC layers in rabbit peripheral collaterals continues to increase for up to 6 wk post-occlusion, at which time most cells have regained a contractile phenotype (824). Remodeling in the rabbit hindlimb appears to be fairly complete at this time, with no further major structural changes noted 6–8 mo post-occlusion (824).
13.5. Time course of arteriogenesis following occlusion
Enlarged collateral arteries become angiographically visible 7–10 d post-occlusion in the rabbit hindlimb and continue to enlarge over at least the next 2–3 wk, with corresponding increases in collateral-dependent blood flow (374, 395, 455, 824). A similar time course has been reported in rat hindlimb, whereas the process is slightly faster in mice (700, 825). Some species variation exists in the extent of collateral enlargement in response to an upstream occlusion. Collaterals in the rabbit ischemic hindlimb have been found to increase their diameter as much as 4–5 fold over 21 d (824). In the C57BL/6 mouse, hindlimb collateral diameter increases more than 2 fold over 21 d (825).
A range of values has been reported for the improvement in resting collateral-dependent blood flow over time in response to occlusion or stenosis. Conrad et al reported that collateral conductance had reached 59% of normal conductance in the dog hindlimb 11 wk after femoral artery ligation (173). Although varying numbers have been reported, 40% is a generally accepted figure for the typical improvement in conductance produced by arteriogenesis (353). Although the total cross-sectional area of the remodeled collateral vasculature may be equal to or even larger than the cross-sectional area of the artery it replaces, there is an increased resistance and tortuosity of the collateral network of quadrupeds that results in lower blood flow than that provided by the original arterial supply (824, 825). As discussed below, an exception can be demonstrated, with a surgical intervention to elevate and sustain luminal shear stress within the collateral arteries, to increase collateral vascular conductance equal to or above that of normal blood flow (233, 732).
13.6. Role of shear stress in arteriogenesis
Early studies of arteriogenesis generally assumed that the key signal for collateral artery enlargement was some signal related to tissue “need” following occlusion of an upstream supply vessel. This assumption appeared to be strengthened by the discovery of VEGF, a hypoxia-inducible endothelial cell mitogen that was shown to be present in ischemic regions of tumors (850). However, some of the observations made in studies of arteriogenesis were inconsistent with this hypothesis. For instance, Paskins-Hurlburt and Hollenberg studied collateral growth in relation to indices of blood flow and skeletal muscle function in rat hindlimb and found that although blood flow and muscle contractile function had returned to relatively normal levels by 3 wk (indicating that tissue needs were being adequately supplied), collateral growth continued for up to 3 mo (700). In a landmark editorial, Schaper made the additional key point that arteriogenesis is separated from ischemia not only by time, but also by distance, and usually occurs in areas which are not ischemic themselves (813). Schaper presented the hypothesis that the altered pressure gradient created by an upstream occlusion increases flow through collaterals, which in turn increases shear stress. The increased shear stress causes endothelial cell activation and recruitment of monocytes to the vascular wall, where they release growth factors and other mediators that induce EC and SMC proliferation. Thus, Schaper envisioned that arteriogenesis is primarily stimulated by changes in shear stress, not ischemia or hypoxia, and that it has a strong inflammatory component.
The shear stress hypothesis has been well supported by subsequent studies. Unthank et al studied collateral remodeling in a rat intestinal model of arterial insufficiency and found that enlargement occurred only in arteries located between the normal region and the ischemic region. Induction of arterial insufficiency led to an approximate doubling of wall shear stress in these vessels. In contrast, arteries within the ischemic region itself did not enlarge, demonstrating that hypoxia is not a requirement for arteriogenesis (946). Several studies have used arteriovenous shunts to chronically increase shear stress. These experiments have shown that collaterals can continue to enlarge beyond the upper limit seen in more physiological model systems if shear stress remains high (233, 722, 817, 818). Interestingly, a recent study suggests that shear stress in collaterals may be transiently decreased immediately following occlusion of an upstream vessel, and that this brief low-shear period facilitates the adhesion of monocytes to the endothelium (795).
In keeping with a primary role for shear stress in regulation of arteriogenesis, the evidence for hypoxia as a trigger of collateral growth is weak. Deindl and coauthors found no elevation in numerous markers of ischemia in quadriceps muscle from rabbit ischemic hindlimb, although collateral growth was well defined in this region (198). Likewise, Hershey et al showed that the time course of collateral growth in rabbit ischemic hindlimb was not associated with tissue ischemia or VEGF expression (374). Studies in mouse strains with varying degrees of ischemia following femoral artery ligation have also shown that the extent of collateral artery remodeling does not correlate well with the severity of ischemia (825).
13.7. Arteriogenic growth factors
A number of growth factors have been reported to have pro-arteriogenic activity in pre-clinical and/or clinical models, including (but not limited to) VEGF, PLGF, FGF2, FGF1, PDGF, MCP-1, and GM-CSF. The results of selected pre-clinical studies of growth factor administration are summarized in Table 2. Several observations can be made from this overview. First, most studies of single growth factor administration demonstrate fairly limited effects of growth factor treatment on indices of arteriogenesis such as collateral-dependent blood flow, number of visible collaterals, ischemic/normal limb blood flow ratio, or hindlimb collateral conductance. Of the single factors shown in Table 2, MCP-1 and FGF-2 show the most marked and consistent effects, whereas VEGF is among the least consistent and effective agents. Second, combined growth factor administration tends to be more effective than single factor administration, although the effectiveness of combined growth factor administration varies widely depending on the factors used. Third, most studies where the growth factor was administered following a significant delay post-induction of ischemia demonstrated little or no beneficial effect of treatment. Many studies also showed that growth factor administration increases the speed of the arteriogenic remodeling process, rather than the final overall extent of remodeling. Thus, single growth factor therapy may only be useful at certain times, or may be most useful for accelerating the arteriogenic process. Finally, it can be appreciated from the table that a variety of routes of administration, dosing protocols, and assessment methods have been used in preclinical studies.
Table 2.
Selected pre-clinical studies of the effects of exogenous growth factor administration on peripheral arteriogenesis
| Growth factor | Dose | Specie s | Delivery method | Method(s) of assessing arteriogenesis | Key findings | Reference |
|---|---|---|---|---|---|---|
| FGF-2 | 1 μg/d, 3 μg/d | Rabbit | im injection, daily for 2 wk | Angiography | ~3 fold increase in number of visible vessels on angiography; reduction in clinical signs of ischemia; dose-dependent | (46, 153) |
| FGF-2 | 1 μg/d | Rat | ia infusion (osmotic pump), 1–4 wk | Microspheres, vascular casts | ~3 fold increase in collateral-dependent blood flow to calf muscles after 2 wk, no further improvement at 4 wk; increased vascularity of arterial casts | (1018) |
| FGF-2 | 20 μg/d | Dog | iv injection x 3 (every 2 d x 1 wk) | Angiography, flow probe | ~4.5 fold increase in collateral blood flow after 7 d; ~130% increase in visible collateral numbers at 7 d | (740) |
| FGF-2 | 11.3 μg | Rat | Combination of slow release polymer and repeated im injections | Angiography, histology, LDPI | ~2–3 fold increase in visible collaterals at 42 d post-ligation; <1.5 fold increase in paw blood flow at 42 d | (1037) |
| FGF-1 | 4 mg/d | Rabbit | im injection, daily x 10 d (starting 10 d post-occlusion) | Angiography | ~2.5 fold increase in number of visible collaterals at day 40 | (735) |
| FGF-1 | 1 μg | Rat | sc injection, daily x 10 d | Histology | ~2.5 fold more vessels per histologic field; further improvement when coadministered with heparin; ~9.5 fold increase in number of PCNA-positive vascular cells | (775) |
| VEGF165 | 500–1000 μg | Rabbit | ia injection, single dose | Angiography | ~1.5 fold higher angiographic score in treated animals after 20 d; no further improvement at 40 d; not dose-dependent | (905) |
| VEGF165 | 1 mg, 5 mg | Rabbit | iv, single dose | Angiography, Doppler flow wire | <1.5 fold increase in calf blood pressure ratio, angiographic score, and flow reserve at 30 d; not dose dependent | (61) |
| VEGF165 | Not stated | Rabbit | Plasmid delivered via angioplasty balloon | Angiography | <1.5 fold increase in calf blood pressure ratio and angiographic score vs controls at 30 d | (904) |
| VEGF | 20 μg/d | Dog | iv injection x 3 (every 2 d x 1 wk) | Angiography, flow probe | ~5 fold increase in collateral blood flow after 7 d; ~80% increase in visible collateral numbers at 7 d | (740) |
| VEGF165 | 500 μg | Rabbit | ia injection, single dose | Angiography | <1.5 fold higher angiographic score and calf blood pressure ratio in treated animals; effects of VEGF similar in young and old rabbits | (760) |
| VEGF121 | 106–108 pfu | Rabbit | Adenovirus; injection into thigh muscles at 4 sites 2 wk prior to inducing ischemia | Angiography, microspheres | ~5 fold higher calf blood pressure ratio at 4 wk in rabbits given 108 pfu, no effect with 106 pfu; <1.5 fold increase in blood flow to gastrocnemius muscle in treated rabbits (not dose dependent); more visible vessels | (314) |
| VEGF | 1.5 μg/d | Mouse | Osmotic pump, 7 d | Angiography, LDPI, microspheres | No significant effect on “total perfusion area” of collateral side branches or blood flow to gastrocnemius muscle; ~1.5 fold increase in total hindlimb blood flow; reduced performance on endurance exercise test | (561) |
| VEGF164 | 50 μg | Mouse (C57B L/6, BALB/c) | Plasmid; injected into thigh muscles followed by electroporation | LDPI | No effect to rescue blood flow recovery in BALB/c; slight effect on blood flow in C57BL/6 (not quantified) | (273) |
| VEGF | 1.5–3.0 μg/kg | Rabbit | Osmotic pump near femoral artery, 7 d infusion | Angiography, ultrasonic flow probe | <1.5 fold increase in number of visible collaterals 7 d post-femoral ligation; <1.5 fold increase in hindlimb collateral conductance at 7 d | (723) |
| VEGF165 | 500 μg cDNA, 109 micro-bubbles | Rat | Plasmid; targeted to adductor region by ultrasound mediated microbubble destruction | Ultrasound, microangiography | ~2 fold increase in microvascular blood volume and <2 fold increase in microvascular density at 28 d, partially returning towards control level at 8 wk | (528) |
| VEGF | 0.5 × 1011 particles | Rabbit | Adenovirus; infused into peripheral vasculature 7 d post-occlusion | Angiography, microspheres | No effect on collateral growth or blood flow at 35 d despite a marked effect on angiogenesis (capillary to fiber ratio) | (508 |
| PLGF | 1.5 μg/d | Mouse | Osmotic pump, 7 d | Angiography, LDPI, microspheres | ~1.5 fold increase in “total perfusion area” of collateral side branches, ~1.5 fold increase in total hindlimb blood flow, and ~3 fold increase in blood flow to gastrocnemius muscle 7 d post-ligation; improved performance on endurance exercise test; more effective than VEGF | (561) |
| PLGF | 1.5–3.0 μg/kg | Rabbit | Osmotic pump near femoral artery, 7 d infusion | Angiography, ultrasonic flow probe | ~1.5 fold increase in number of visible collaterals 7 d post-femoral ligation; collaterals larger than in VEGF-treated rabbits; ~1.5 fold increase in hindlimb collateral conductance at 7 d | (723) |
| PLGF | 108 pfu | Mouse (BALB/c) | Adenovirus; delivered by 3 injections into adductor muscle near ligation site | LDPI, micro CT | ~2 fold increase in number of vessels >30 μm in lower limb; ~2 fold increase in volume of vessels 96–136 μm in diameter; <1.5 fold increase in ischemic/normal hindlimb blood flow at 28 d post-ligation | (532) |
| MCP-1 | 3 μg | Rabbit | Osmotic pump near femoral artery, 7 d infusion | Microspheres, angiography | ~3 fold increase in collateral conductance at 7 d; increased number of visible collaterals | (456) |
| MCP-1 | 0.2 μg/kg/d | Rabbit | Osmotic pump near femoral artery, 7 d starting either at induction of ischemia or 3 wk later | Microspheres, angiography | ~8 fold increase in hindlimb conductance after 7 d when MCP-1 administered immediately, but no effect when treatment started 3 wk post-occlusion; ~2 fold increase in visible collaterals with immediate treatment, no effect with delayed treatment | (395) |
| MCP-1 | 0.5 μg/d | Rabbit | ia via osmotic pump in femoral artery for 7 d, starting immediately post-occlusion or 3 wk post-occlusion | Microspheres | ~7 fold increase in hindlimb collateral conductance after 7 d when administered immediately; no effect when administered 3 wk post-occlusion | (114) |
| MCP-1 | 0.5 μg/kg | Rabbit | Osmotic pump near femoral artery, 7 d infusion | Angiography, ultrasonic flow probe | >1.5 fold increase in number of visible collaterals at 7 d; >2 fold increase in hindlimb collateral conductance at 7 d; more effective than either VEGF or PLGF | (723) |
| HGF | 1.5 mg | Rabbit | ia injection 10 d post-ischemia + iv injection 12 and 14 post-ischemia (500 μg each) | Angiography, microspheres | >1.5 fold increase in angiographic score and ischemic/nonischemic limb blood flow ratio at 30 d post-ischemia; more angiographically visible collaterals at 30 d; more effective than VEGF | (957) |
| HGF | 1–15 mg | Rabbit | ia injection at 10 and 12 d post-induction of ischemia (500 μg/d) or iv infusion (5 d starting 10 d post-ischemia, 3 mg/d) | Angiography | ~40–50% improvement in angiographic score compared to vehicle 10 d post-treatment by either delivery route | (638) |
| HGF | 100–500 μg (rat); 1–2 mg (rabbit) | Rat, rabbit | Plasmid delivered via im injection into ischemic limb at 0 d (rat) or 10 d (rabbit) post-ischemia | LDPI, angiography | ~2 fold increase in ischemic/nonischemic blood limb flow ratio at 5 wk in rats; ~2.5 fold increase in angiographic score at 5 wk in rabbits | (912) |
| GM-CSF | 100 μg/d | Rabbit | ia via osmotic pump in femoral artery for 7 d, starting immediately post-occlusion or 3 wk post-occlusion | Microspheres | ~5 fold increase in collateral conductance after 7 d when administered immediately; no effect when administered 3 wk after occlusion | (114) |
| GM-CSF | 5 μg/kg/d | Pig | ia, via implanted catheter and infusion pump for 7 d (continuous or intermittent) | Ultrasonic flow probe | No effect on ankle/brachial index; ~2 fold increase in maximal collateral conductance with either dosing protocol | (320) |
| PDGF-AA | 22.6 μg | Rat | Combination of slow release polymer and repeated im injections | Angiography, histology, LDPI | <1.5 fold increase in number of visible collaterals, no significant effect on paw blood flow at 6 wk post-ligation | (1037) |
| PDGF-AB | 22.6 μg | Rat | Combination of slow release polymer and repeated im injections | Angiography, histology, LDPI | ~1.5–2 fold increase in number of visible collaterals, no significant effect on paw blood flow at 6 wk post-ligation | (1037) |
| PDGF-BB | 22.6 μg | Rat | Combination of slow release polymer and repeated im injections | LDPI | ~2 fold increase in ischemic/normal hindlimb blood flow ratio after 3 wk, not different from vehicle at 9 wk | (124) |
| PDGF-BB | 10 μg/kg/d | Rabbit | Osmotic pump near occlusion site for 7 d | Angiography, microspheres | ~1.5 fold increase in number of visible collaterals in hindlimb at 1 wk; <1.5 fold increase in collateral-dependent blood flow at 1 wk | (124) |
| VEGF165 + FGF-2 | 500 μg VEGF165, 10 μg FGF-2 | Rabbit | ia injection, single dose | Angiography | ~2 fold increase in calf blood pressure ratio and <1.5 fold increase in diameter of stem collateral artery at 30 d; either factor alone produced <1.5 fold increase in calf blood pressure ratio and no change in stem collateral artery diameter | (35) |
| VEGF + angiopoietin 1 | 500 μg each | Rabbit | Plasmid delivered by im injection in thigh muscles (4 sites) | Doppler flow wire, angiography | ~2 fold increase in number of angiographically visible vessels; ~1.5 fold increase in maximal hindlimb blood flow at 40 d; either factor alone or combination produced similar effects | (138) |
| GM-CSF + MCP-1 | 100 μg/d GM-CSF, 0.5 μg/d MCP-1 | Rabbit | ia via osmotic pump in femoral artery for 7 d, starting immediately post-occlusion or 3 wk post-occlusion | Microspheres | ~15 fold increase in collateral conductance after 7 d; ~1.5 fold increase with delayed treatment; more effective than either factor alone; either factor alone not effective with delayed administration | (114) |
| PDGF-BB + FGF-2 | 22.6 μg PDGF-BB, 11.3 μg FGF-2 | Rat | Combination of slow release polymer and repeated im injections | LDPI | ~2 fold increase in ischemic/normal hindlimb blood flow ratio at 3 wk; sustained at 9 wk; more effective and long lasting than either factor alone | (124) |
| PDGF-BB + FGF-2 | 10 μg/kg | Rabbit | Osmotic pump near occlusion site for 7 d | Angiography, microspheres | ~2.5 fold increase in number of visible collaterals at 7 d; ~2 fold increase in collateral dependent blood flow at 7 d; more effective than either factor alone | (124) |
| VEGF + FGF-2 | 10 μg total | Mouse (C57B L/6) | Plasmids delivered via im injection in adductor muscle (4 sites) followed by electroporation | LDPI, histology | ~2.5 fold increase in ischemic/nonischemic limb blood flow ratio at 14 d; ~3 fold increase in number of smooth muscle α-actin positive vessels in adductor; ~2 fold increase in both parameters with either factor alone | (521) |
| PDGF-AB + FGF-2 | 22.6 μg PDGF-AB, 11.3 μg FGF-2 | Rat | Combination of slow release polymer and repeated im injections | Angiography, histology, LDPI | ~2.5–3.5 fold increase in number of visible collaterals and ~2 fold increase in ischemic/normal limb blood flow ratio at 6 wk; more effective than either factor alone | (1037) |
| PDGF-AA + FGF-2 | 22.6 μg PDGF-AA, 11.3 μg FGF-2 | Rat | Combination of slow release polymer and repeated im injections | Angiography, histology, LDPI | ~2–3 fold increase in number of visible collaterals and ~1.5 fold increase in ischemic/normal limb blood flow ratio at 6 wk; no improvement over FGF-2 alone | (1037) |
We will discuss on VEGF and PLGF in more detail as arteriogenic growth factors, which have been suggested as potential therapeutic agents, as there are interesting contrasts in the effects and mechanism of action of these two related proteins. Of the two, VEGF has received by far the most attention. Leung et al identified VEGF as a secreted factor mediating endothelial cell proliferation (529). Two major receptors for VEGF with a distribution largely restricted to endothelium (VEGFR-1, or Flt-1; VEGFR-2, or KDR) were characterized (197, 737). For a detailed review of VEGF signaling via these receptors in endothelial cells, see (1034).
The cell biology of VEGF is complex. In addition to the original ligand (VEGF-A, referred to in this review as VEGF), several other members of the family have now been described: VEGF-B, VEGF-C, VEGF-D, VEGF-E, and PLGF. Furthermore, some of the family members (especially VEGF-A) have multiple splice variants with differing ability to bind to heparin. Additional receptors have been described, including soluble variants that have been proposed to function as “traps” for circulating VEGF-family ligands. Finally, the VEGF family ligands and receptors are capable of heterodimerization, further complicating the signaling pathways. An excellent comprehensive review of the complexity of VEGF family ligands and receptors was recently published by Mac Gabhann and Popel (563).
Soon after its initial description, VEGF was found to be upregulated in cancer cells from hypoxic regions of tumors, while VEGF receptor 1 (VEGFR-1, Flt-1) was upregulated in tumor endothelium (725, 850). Studies showing that VEGF was hypoxia-inducible in cardiac myocytes in vitro and in vivo suggested that VEGF might play a role in adaptive vascular growth (53, 530). The ability of VEGF to induce arteriogenesis was immediately tested in animal models and reported to produce modest improvements in collateral-dependent blood flow (52, 343) and angiographic score (61, 761, 904). However, study results were inconsistent, with other researchers finding little or no effect of VEGF to enhance collateral dependent blood flow (508, 519).
VEGF exerts its effects on arteriogenesis by several different mechanisms. VEGFR-1 is expressed by monocytes as well as endothelial cells, and VEGF induces monocyte chemotaxis by activating this receptor (54, 161). In vitro experiments have demonstrated that endothelial cells release NO in response to VEGF (958), and that the mitogenic effect of VEGF on endothelial cells is NO-dependent (696, 697). The arteriogenic effects of VEGF in vivo require downstream NO production as well (590, 645, 1027). VEGF treatment also results in NO-dependent vasodilatation (343, 506), which is enhanced in collateral-dependent regions of the myocardium (828).
Although there was much early optimism about the potential of VEGF as a therapeutic agent, preclinical studies of VEGF soon revealed the potential for serious side effects. In a study of VEGF for therapeutic arteriogenesis in pig myocardium, VEGF administration caused severe hypotension resulting in the deaths of 50% of the treated animals (343). In keeping with the originally described function of VEGF as a vascular permeability factor (834), adenoviral over expression of VEGF was also reported to cause peripheral edema in some studies (954). Finally, there are concerns that VEGF overexpression could promote diabetic retinopathy or the development of latent tumors.
Despite the incomplete picture of the true role of VEGF in arteriogenesis and the possibility for side effects, clinical studies of VEGF administration in humans were begun very soon after the first pre-clinical studies. Isner and colleagues reported an improved angiographic appearance of the limb of a patient with severe peripheral artery disease following treatment with a VEGF-encoding plasmid (452). However, the patient developed spider angiomas and limb edema, and the increased vascularization prompted by VEGF administration failed to salvage the limb. Further small, uncontrolled studies in humans followed. VEGF therapy had mixed results in patients with critical limb ischemia (60) and reduced angina and improved angiographic score in patients with coronary artery disease (370, 898). Despite these initially encouraging results, subsequent double-blind placebo-controlled trials demonstrated little or no beneficial effect of VEGF administration in humans. The VIVA trial of recombinant VEGF in 178 patients with stable exertional angina found a modest improvement in angina score in patients treated with the higher of 2 doses studied, but only a very slight improvement in treadmill exercise time at 120 d post--treatment and no improvement in indices of myocardial function (369). Similarly, the RAVE trial of adenoviral VEGF gene therapy in 105 patients with peripheral arterial disease failed to show any improvement in VEGF-treated patients in exercise treadmill test time, ankle/brachial index, or time to claudication, and a higher incidence of limb edema was reported in treated patients (739).
There are a variety of possible reasons why the results of VEGF therapy have been disappointing. In patients with ischemic cardiovascular disease, there may be a window in which growth factors can enhance arteriogenesis, and this window may have been missed in clinical trials, which tended to focus on patients with late-stage disease that was untreatable by other means. A single growth factor may also not adequately reconstitute the complex physiological signaling that drives arteriogenesis. Indeed, Schierling et al reported that administration of single growth factors (MCP-1, FGF2, PDGF, or VEGF) did not induce arteriogenesis as effectively as an artificial increase in shear stress (818). Alternatively, VEGF may not be well suited for use as an arteriogenic therapy, as it primarily mediates capillary proliferation (angiogenesis). The difficulties that have been encountered in translating the results of promising pre-clinical studies of angiogenic/arteriogenic growth factor treatment have been recently reviewed (820).
Placenta growth factor (PLGF) is a VEGF-family ligand, which preferentially induces arteriogenesis (as opposed to angiogenesis). PLGF was isolated soon after VEGF (568) and was shown to bind to VEGFR-1 but not VEGFR-2 (as opposed to VEGF, which binds to both receptors) (54, 161). Carmeliet and coauthors reported that arteriogenesis in mouse ischemic hindlimb, but not embryonic vasculogenesis, was inhibited by PLGF gene knockout (129). In contrast, PLGF overexpression resulted in a striking increase in the number and size of blood vessels, although overexpression was also reported to increase vascular permeability (674). Micro-CT studies of mouse ischemic hindlimb showed that PLGF selectively increased the volume of 96–136 μm-diameter vessels (532). Luttun and colleagues compared the efficacy of PLGF and VEGF to induce arteriogenesis in mouse ischemic hindlimb and found that PLGF treatment had a more pronounced effect on collateral growth and produced greater functional improvement in the muscle than VEGF treatment (561).
Studies on the mechanism of PLGF action revealed that the arteriogenic effect of PLGF is dependent on monocytes (723) and that the inhibition of arteriogenesis produced by PLGF gene knockout could be rescued by transplant of wild-type bone marrow (823), demonstrating the role of circulating bone-marrow derived cells in PLGF-mediated arteriogenesis. Furthermore, although PLGF is non-mitogenic for endothelial cells, it has been shown to enhance the effect of VEGF (129). Similarly, the presence of PLGF is required in order for VEGF to induce proliferation of smooth muscle cells (561). The strong arteriogenic activity of PLGF, its pleiotropic effects, and its apparent specificity for adaptive remodeling have led to the suggestion that it may represent an arteriogenic “master switch” (195). To date, however, the potential usefulness of PLGF as a pro-arteriogenic therapy has not been evaluated in human clinical trials. Additional studies are needed to better define how PLGF expression is regulated in the vasculature and to characterize its downstream effects. The experience with VEGF has demonstrated that moving into clinical trials before the underlying processes are fully understood is likely to produce disappointing results.
13.8. Involvement of monocytes in arteriogenesis
The involvement of monocytes in arteriogenesis has been demonstrated in many studies. Monocyte recruitment to remodeling collaterals is one of the earliest events in arteriogenesis. In rabbit ischemic hindlimb, monocytes were seen to adhere to collaterals within 12–48 h of upstream occlusion (34, 824); furthermore, monocyte adherence was seen only in collaterals, not in nearby vessels or in similarly-sized vessels from distant regions (34).
The mechanism of monocyte recruitment to growing collaterals has been of much interest. Many studies have focused on monocyte chemoattractant protein-1 (MCP-1), which has been reported to be upregulated by both shear in endothelial cells (852) and stretch in smooth muscle cells (203). MCP-1 binds to CC-chemokine receptor-2 (CCR-2 ) on monocytes. Administration of exogenous MCP-1 increases monocyte recruitment and collateral conductance in the rabbit ischemic hindlimb (456, 824), whereas recovery of blood flow to mouse ischemic hindlimb is moderately reduced by genetic deletion of MCP-1 (970) or by dominant-negative MCP-1 (270). Granulocyte-macrophage colony stimulating factor (GM-CSF) administered in combination with MCP-1 enhances arteriogenesis more than MCP-1 alone, perhaps by inhibition of monocyte apoptosis (114). Consistent with these studies, Heil et al reported that gene knockout of CCR-2 delays arteriogenesis in mouse models of hindlimb ischemia, although these authors found strain-dependent differences in the magnitude of inhibition (356). In contrast, Tang and coworkers were not able to show inhibition of arteriogenesis in the ischemic hindlimb of CCR-2 mice (909). Thus, the MCP-1/CCR-2 pathway appears to be only one aspect of the mechanism by which monocytes are recruited to actively remodeling collaterals.
Several adhesion molecules that can also facilitate monocyte recruitment are upregulated in the setting of arteriogenesis, including the integrin/focal adhesion kinase (FAK) pathway (120), PECAM-1 (144), ICAM-1 (396, 722), and VCAM-1 (722). Increased ICAM and VCAM mRNA levels are detectable in collateral arteries of rabbit ischemic hindlimb at 12 h post-occlusion, with elevated protein detectable at 12–48 h post-occlusion (824). Furthermore, gene knockout of either ICAM-1 or its receptor on the monocyte (Mac-1) inhibits arteriogenesis in mouse ischemic hindlimb (396). Thus, ICAM-1/Mac-1 interaction may be another important pathway for monocyte recruitment during arteriogenesis.
In addition to the MCP-1/CCR-2 and ICAM-1/Mac-1 pathways, growth factors can also facilitate recruitment of monocytes to remodeling collaterals. Monocytes express VEGF receptor 1 (VEGFR-1, Flt-1). Both VEGF-A and placenta growth factor (PLGF) are ligands for VEGFR-1 and are chemotactic for monocytes (54, 161, 917). Indeed, the arteriogenic effects of PLGF have been shown to be dependent on monocytes (723).
Monocyte dysfunction or depletion can attenuate arteriogenesis. Mutant mice with a genetically-based ~87% reduction in circulating monocyte level develop fewer collaterals and have reduced hindlimb perfusion in response to hindlimb ischemia (75). Conversely, the number of visible collaterals and collateral conductance in ischemic hindlimb is enhanced by artificial elevation of monocyte levels in mice and rabbits (355). Further evidence for a specific role for monocytes in arteriogenesis (as opposed to other leukocyte subtypes) was presented by Hoefer and colleagues, who studied the effect of chemo attractants for monocytes, granulocytes, and lymphocytes on arteriogenesis and concluded that only monocytes enhance collateral remodeling (394). However, studies by other groups have demonstrated a pro-arteriogenic action of lymphocytes in the mouse ischemic hindlimb (882, 965) and thus this issue remains open to debate.
13.9. Nitric oxide and arteriogenesis
It is clear that nitric oxide contributes to acute vasodilatation in collateral arteries. The role of nitric oxide in arteriogenesis, however, has been the subject of much debate. Several studies have shown that arteriogenesis induced by a variety of stimuli can be blocked by inhibition of NO production. Matsuyama et al found that L-NAME treatment blocked the increase in collateral-dependent blood flow that occurred over time in a dog model of repetitive coronary occlusion (590). Lloyd and coauthors assessed both angiogenesis and arteriogenesis in rat ischemic hindlimb in response to exercise training and found that L-NAME blocked the time-dependent increase in collateral-dependent blood flow, but did not prevent the increase in skeletal muscle capillarity (546). This study clearly demonstrated that differences exist in the signaling mechanisms regulating angiogenesis and arteriogenesis. Yang et al showed that VEGF-and FGF2-induced increase in collateral-dependent blood flow to rat ischemic hindlimb could also be prevented by L-NAME treatment (1027). Yu and colleagues presented angiographic evidence for decreased arteriogenesis in the ischemic hindlimb of eNOS−/− mice and demonstrated that impaired arteriogenesis in this model could be rescued by adenoviral expression of constitutively active eNOS (1029).
Enhancement of NO levels has been shown to have a stimulatory effect on arteriogenesis. Murohara and colleagues found that L-arginine supplementation improved angiographic score in rabbit ischemic hindlimb, and that recovery of blood flow in ischemic hindlimb of eNOS−/− mice was impaired compared to wild type mice (645). Brevetti et al found that adenoviral overexpression of eNOS in rat ischemic hindlimb increased various indices of arteriogenesis (97). In agreement with these results, eNOS has been shown to be upregulated in growing collaterals. Cai et al demonstrated increased eNOS immunofluorescence in the endothelium of growing canine coronary collateral arteries, compared to unstimulated or mature collaterals (119), and Prior et al showed that eNOS mRNA is increased in a rat hindlimb collateral by exercise training throughout the period of remodeling (730). These findings suggested that eNOS-derived NO was an important mediator of structural remodeling in collaterals, as well as acute vasodilatation. However, this view was challenged by a recent study by Meets et al. These authors found that overexpression of eNOS enhanced acute vasodilatation, but not arteriogenesis, in mouse ischemic hindlimb. Furthermore, collaterals were found to enlarge similarly in eNOS−/− mice compared to wild type mice. Thus, Meets et al concluded that reduced flow recovery in ischemic hindlimb of eNOS−/− mice is due to impaired vasodilatation, not impaired arteriogenesis (608).However, in a follow-up study, Troidl and coauthors reported that combining eNOS gene knockout with iNOS inhibition did block structural remodeling of collaterals, supporting an essential role for NO in arteriogenesis (933). The authors hypothesized that a significant fraction of the NO released during arteriogenesis comes not from endothelial NOS, but from iNOS in monocytes/macrophages. Indeed, earlier studies demonstrated that iNOS is not upregulated in collaterals themselves during the remodeling process (119).
It is well established that NO regulates a variety of key cellular processes involved in vascular remodeling, including endothelial cell migration, proliferation, and differentiation. The signaling mechanisms by which NO regulates these processes during arteriogenesis are not fully defined. However, it has been demonstrated that NO lies both upstream (294) and downstream (590, 645, 876, 1027) of key angiogenic growth factors such as VEGF. For a further review of the role of NO signaling in arteriogenesis, see Prior et al (731).
13.10. Oxidative stress and arteriogenesis
A recent focus of attention has been the role of reactive oxygen species (ROS) in regulation of arteriogenesis. The ROS of most interest in vascular biology are superoxide (O2−) and hydrogen peroxide (H2O2). Superoxide can be generated in vascular cells by a variety of enzymatic and non-enzymatic processes. Major sources of O2− production in vascular cells include NADPH oxidase, xanthine oxidase, uncoupled nitric oxide synthase, and leakage from the mitochondrial electron transport chain. Superoxide is short-lived and is converted to the more stable compound hydrogen peroxide by superoxide dismutase (SOD) and other mechanisms. For an extensive review on the cell biology of ROS generation and signaling, see (215).
Overproduction of ROS (“oxidative stress”) causes endothelial dysfunction, in part by scavenging NO, and has been implicated in the pathogenesis of atherosclerosis, hypertension, ischemia-reperfusion injury, and heart failure. Several excellent comprehensive reviews of cardiovascular ROS production and its role in cardiovascular pathophysiology are available (134, 268, 533).
Although excessive ROS production is damaging to the cardiovascular system, a large body of evidence now demonstrates that low, physiological levels of ROS are beneficial for adaptive vascular remodeling processes such as angiogenesis and arteriogenesis. The antioxidant N-acetylcysteine inhibited time-dependent increases in collateral blood flow in the dog coronary circulation following repetitive coronary occlusion, and in the ischemic hindlimb of wild-type mice (223, 324). Similarly, the antioxidants ebselen and Tempol delayed recovery of blood flow to ischemic hindlimb of wild-type mice (484, 931). Genetic deletion or insufficiency of gp91phox (which encodes the Nox2 subunit of NADPH oxidase) also delayed recovery of blood flow in ischemic hindlimbs of mice, relative to wild-type mice (223, 931). These findings demonstrate that a certain basal level of ROS production is required in order for arteriogenesis to proceed normally.
Interestingly, ROS appear to be a double-edged sword in terms of their effect on collateral remodeling; low amounts are required for arteriogenesis, but higher amounts are inhibitory. Kim et al found that collateral growth and recovery of blood flow were impaired in ischemic hindlimbs of mice lacking endothelial superoxide dismutase (ecSOD, which converts O2− into H2O2). The reduced arteriogenesis in this model was associated with elevated O2 levels and rescued by the antioxidant Tempol (484). Likewise, Rocic and coworkers found that both inhibition of NADPH oxidase (to reduce O2− )-) and inhibition of SOD (to increase O2−) had a similar effect to reduce the development of collateral-dependent blood flow in rat myocardium in response to repetitive ischemia. Thus, these authors concluded that arteriogenesis is inhibited when O2− levels are either too low or too high (771). Since inhibition of SOD causes decreased H2O2 levels in addition to increased O2−, another interpretation of these results is that H2O2 is the ROS species required for arteriogenesis. In agreement with this hypothesis, Shaw et al recently demonstrated that the arteriogenic mediator placenta growth factor (PLGF) is upregulated by H2O2 in vascular smooth muscle cells (838). The role of H2O2 in vascular physiology has been recently reviewed (32, 783).
13.11. Effect of disease states and aging on arteriogenesis
The primary therapeutic application for pro-arteriogenic therapies would be enhancement of blood flow in patients with ischemic cardiovascular disease. Since a pharmacological method of improving blood flow would be much less invasive than current treatments such as coronary artery bypass grafting, angioplasty, and stent placement, arteriogenesis is a highly attractive therapeutic target. Mechanistic information regarding arteriogenic signaling is required in order to develop such treatments. The majority of studies to identify the signaling pathways involved in arteriogenesis and test the efficacy of growth factors as pro-arteriogenic agents have been conducted in young, healthy animals. However, the human patient population for which such treatments would be indicated is older and has pre-existing disease, which necessitates the treatment. Thus, the patient group to which pro-arteriogenic treatments would be targeted is not well mimicked in many studies.
Several studies have suggested that the process of arteriogenesis may be impaired by conditions such as the metabolic syndrome and aging. However, different studies have reported conflicting results. Abaci and colleagues compared diabetic and non-diabetic patients with a similar extent of coronary artery disease and concluded that collateral score was significantly reduced in the diabetic group (1). Similarly, De Vivo and coauthors studied patients with claudication due to superficial femoral artery occlusion and found that diabetes was associated with the presence of fewer collaterals in the limb (196). Celik et al reported that coronary collateralization was poorer in diabetics with proliferative diabetic retinopathy than diabetics without this microvascular complication, possibly associated with the presence of more longstanding and severe diabetes in this patient group (135). Yilmaz and coauthors studied patients with total occlusion of the right coronary artery and found that diabetes and metabolic syndrome were associated with poorer collateral score in this group (1028). Likewise, Turhan et al found that patients with metabolic syndrome had lower collateral scores (942). However, Fujita et al found no association between various factors including diabetes, age, smoking, hypertension, and hypercholesterolemia with coronary collateral score in patients presenting with acute MI (272). Zbinden and colleagues measured collateral flow index in diabetic and nondiabetic patient groups, where the clinical characteristics of the groups were carefully matched, and found no difference between diabetics and nondiabetics (1035). Melidonis et al even reported increased collateral score in patients with diabetes (610). In the latter study, the apparent pro-arteriogenic effect of diabetes was likely due to the presence of more extensive coronary artery disease (and thus a greater stimulus for arteriogenesis) in the diabetic group. Thus, although the evidence generally supports a diabetes-associated inhibition of arteriogenesis in humans, studies in humans are complicated by a variety of factors including variation in the patient population (both between and within studies).
Animal studies have been somewhat more consistent in reporting inhibition of arteriogenesis by diabetes, and have attempted to identify which specific facets of this complex condition influence collateral growth. Hyperlipidemia and hyperglycemia have been assessed separately in several studies. The case for an inhibitory effect of hyperlipidemia on arteriogenesis appears to be fairly strong. Blood flow recovery and collateral development in the ischemic hindlimb is impaired in hyperlipidemic mice (176), rats (216), and rabbits (956). Similarly, blood flow to collateral-dependent myocardium is reduced in hypercholesterolemic pigs (85). In contrast, no effect of a high fat/cholesterol diet on collateral conductance was seen in cynomolgus monkeys (550); however, the lack of apparent inhibitory effect could be due to the time point which was studied (16 mo post-iliac occlusion). Many studies, which report inhibition of arteriogenesis by hyperlipidemia or other factors, show that indices of collateral function eventually reach levels similar to that seen in normal animals, but with a slower time course.
Hyperglycemia has also been shown to inhibit arteriogenesis in animal models. Recovery of hindlimb blood flow is inhibited in nonobese diabetic mice (NOD), a model for insulin-dependent or type I diabetes, compared to C57BL/6 mice (760). Collateral arteries are less numerous and smaller in ischemic hindlimb of rabbits made hyperglycemic with alloxan than in the hindlimb of normal rabbits (959). Infusion of glucose (to raise blood levels to 350–400 mg/dL, 8 h/d for 21 d) prevented collateral-dependent blood flow from increasing in response to repetitive coronary occlusion in dog myocardium (987). Angiographic score and hindlimb blood flow were lower in ischemic hindlimb of mice with streptozotocin-induced hyperglycemia than in control mice (906). Finally, in a useful direct comparison of hyperglycemia and hyperlipidemia, van Weel and coauthors studied several mouse strains and found that both hyperlipidemia and hyperglycemia inhibited recovery of limb perfusion after induction of ischemia, although hyperlipidemia had a greater effect to reduce angiographic score than hyperglycemia (962). Thus, the evidence supports a role for both hyperlipidemia and hyperglycemia in inhibition of arteriogenesis by diabetes. However, these two factors may act by different underlying mechanisms.
A limited number of studies have addressed the mechanism(s) underlying diabetic inhibition of arteriogenesis in humans. Monocyte migration in response to chemotactic agents such as VEGF, PLGF, and MCP-1 has been shown to be reduced in diabetic humans (918, 975) as well as rabbits (959), suggesting that inhibition of monocyte function could contribute to reduced collateral growth in diabetes. Both altered gene expression (in response to stimulation with lipopolysaccharide) (819) and abnormal adhesion to collagen and fibrinogen (843) have been reported in monocytes from patients with poor collateralization compared to monocytes of patients with good collateralization, suggesting that monocyte function may indeed be a key determinant of collateral growth in humans. Altered VEGF signaling may also contribute to impaired arteriogenesis in human patients with diabetes. Sasso et al found that although VEGF protein levels are increased in human diabetic myocardium, both VEGFR-1 and VEGFR-2 protein are decreased, and phosphorylation of downstream effectors is likewise reduced (803).
Studies in animal models of both hyperlipidemia and hyperglycemia have also implicated reduced VEGF expression and/or signaling activity in the diabetes-associated inhibition of arteriogenesis. Rivard and coauthors found that VEGF mRNA and protein levels were reduced in skeletal muscle of nonobese diabetic mice (760). Similarly, Chou et al found that VEGF and VEGFR-1 and -2 mRNA was decreased in myocardium of streptozotocin-treated rats and Zucker fatty rats, although the effect was greater in the hyperglycemic group (155). Interestingly, VEGF expression in aorta did not change, suggesting that VEGF is differentially regulated in cardiac muscle and vascular smooth muscle. An opposite pattern of expression was seen in retina and glomerulus, with an increase in VEGF and VEGFR expression. These data are consistent with the observation that although diabetes appears to inhibit arteriogenesis in tissues such as myocardium and skeletal muscle, it increases angiogenesis in retina (219). Impaired recovery of blood flow in ischemic hindlimb of ApoE−/− mice has also been associated with reduced VEGF expression (176).
Altered expression of other factors that promote or inhibit arteriogenesis likely also contributes to the anti-arteriogenic effects of diabetes. For example, an increase in the levels of anti-angiogenic factors such as endostatin and angiostatin has been associated with experimental diabetes (85, 987). Dysfunctional extracellular matrix remodeling may also play a role. Advanced glycation end product (AGE)-mediated inhibition of extracellular matrix remodeling is associated with inhibition of arteriogenesis by hyperglycemia (906).
Although it may seem reasonable to conclude from these studies that altered VEGF signaling plays a major role in inhibiting arteriogenesis in diabetes, it is important to remember that VEGF is primarily an angiogenic factor, which induces capillary proliferation. As the first hypoxia-inducible, secreted angiogenic factor to be identified, VEGF was an attractive target for study and thus many early studies examined only VEGF and its receptors rather than screening a panel of potentially involved factors. Thus, although the VEGF pathway may play a role, it is likely that hyperlipidemia and hyperglycemia have additional effects on arteriogenesis, which are yet to be revealed. For a detailed recent review of the effects of diabetes on arteriogenesis, see Ruiter et al (785).
Aging has also been demonstrated to impair arteriogenesis. As discussed above, most patients with ischemic cardiovascular disease who would be candidates for a pro-arteriogenic therapy would be older persons. Thus, it is important to determine how aging affects arteriogenesis. Indices of arteriogenesis are reduced in aged rabbits and mice, in association with apparent endothelial dysfunction (761, 845). Interestingly, the effect of age on arteriogenesis is influenced by genetic factors, as age-related impairment of arteriogenesis occurs in Wistar rats (944) but not in brown Norway rats (842). Similarly to diabetes, a recent report suggests that aging may impair arteriogenesis, but not angiogenesis in ischemic hindlimb (989).
13.12. Stimulation of arteriogenesis by exercise training
Exercise training was recognized early as a key physiological stimulus for collateral enlargement in the presence of an upstream arterial stenosis or occlusion. Eckstein presented perhaps the first evidence that exercise training could induce arteriogenesis when he showed that 7–9 wk of treadmill exercise increased collateral blood flow in hearts of dogs with experimental stenosis of the LCX (224). Importantly, the presence of a flow-limiting stenosis seems to be required in order for a training effect on collateral remodeling to be seen. This requirement is not surprising, since collateral flow would not be expected to increase greatly during exercise in the absence of a stenosis (due to the higher resistance of the collateral circuit relative to the normal arterial pathway). Burt and Jackson assessed the effect of exercise (4–5 wk of running on a track) on coronary collateral blood flow in dogs with non-occluded coronary arteries and reported that training did not improve collateral flow in the absence of a stenosis (110). Likewise, no significant effects of training were seen in subsequent studies of collateral-dependent blood flow in normal pig (798) or dog (168, 816) myocardium. In contrast, Leon et al showed that coronary artery cross-sectional area increased in rats that were swim-trained daily for 10 wk, but not in rats that trained less frequently, and that vessel area declined when the animals returned to a sedentary lifestyle (527). A modest improvement in blood flow to the collateral-dependent region of acutely ischemic myocardium in response to treadmill training was also shown in the rat heart (497). Thus, although most studies reported little or no improvement in collateral blood flow in response to exercise in the absence of an upstream occlusion or stenosis, some species, training method, or tissue-related differences may exist.
Studies in animals with experimental coronary or peripheral artery occlusion have consistently shown enhanced collateral development in response to exercise training. Sanne and Sivertsson studied hindlimb blood flow in cats with femoral artery ligation following 5 wk of treadmill training and demonstrated that collateral resistance was lower in trained cats than sedentary cats. They concluded that “physical exercise is a potent additional stimulus for the development of collateral vessels” (801). Similarly, Scheel and coauthors reported that 6 wk of treadmill training significantly reduced collateral resistance and increased collateral conductance in the LCX-occluded dog myocardium (816). These results were in good agreement with studies showing that 3–5 mo of treadmill training increased collateral-dependent blood flow in hearts of dogs (169) and pigs (83) with experimental stenosis of the LCX.
More recently, significantly improved blood flow to the distal hindlimb following 6 wk of treadmill exercise training was demonstrated in rats with femoral artery stenosis, consistent with training-induced collateral artery remodeling (584). The hypothesis that exercise training induces arteriogenesis was subsequently strengthened by further studies in rats with complete occlusion of the femoral artery (which renders distal hindlimb blood flow collateral-dependent) which definitively showed that treadmill exercise training increased collateral dependent blood flow (544, 546, 730, 1020, 1025). Improvement in collateral-dependent blood flow was associated with functional improvement in the distal skeletal muscle tissue at risk of ischemia (1025).
The mechanism by which training stimulates arteriogenesis has not yet been fully described, but certainly involves upregulation of growth factors and other mediators of angiogenesis. Angiogenic growth factor expression has been shown to be influenced by exercise training or electrical stimulation of skeletal muscle in numerous studies. Chronic electrical stimulation of rat skeletal muscle induced an early increase in VEGF mRNA which declined over the 21 d of study (340). An immediate increase in VEGF mRNA was also seen in rat skeletal muscle in response to a single exercise bout (91, 294) and in rat (296) and human (328) skeletal muscle in response to short-term exercise training. TGF-β1 mRNA has also been reported to be upregulated by a single bout of exercise or by short-term training, although the upregulation is not as striking as that of VEGF mRNA (91, 294, 296). Lloyd and colleagues assessed the time course of gene expression of a panel of eight angiogenesis-associated factors in rat skeletal muscle in response to 1–24 d of exercise training. These studies demonstrated that a variety of angiogenic growth factors and receptors are affected by exercise training, and that the temporal pattern of expression varies between factors. Upregulation of VEGF and monocyte chemoattractant protein1 (MCP-1) were the earliest events, followed by upregulation of VEGFR-1, VEGFR-2 and eNOS. An increase in the angiopoietin 2:angiopoietin 1 ratio and upregulation of the angiopoietin receptor Tie-2 followed a similar time course to that of the VEGF receptors (545). A similar study by Prior et al assessed angiogenic factor gene expression within rat peripheral collateral arteries in response to 2–25 d of treadmill training. Although alterations in gene expression with training were less marked in collaterals themselves than in skeletal muscle, this study also demonstrated an increase in eNOS and VEGFR-1 mRNA (730).
Exercise is a complex stimulus, since it induces changes in numerous parameters in active skeletal muscle and skeletal muscle vasculature, including increased blood flow, changes in the levels of metabolic substrates and products, and altered mechanical forces. It is likely that these various stimuli act on different signaling pathways to induce arteriogenesis. For instance, Roca and colleagues compared the effects of electrical stimulation and passive hyperperfusion of skeletal muscle on angiogenic growth factor expression and found no apparent effect of an acute increase in blood flow to induce VEGF, FGF2, or TGF-β1 gene expression (770).
14. Collateral Development and Function in Humans
14.1. Development of a structural collateral circuit
Extensive preclinical studies demonstrate that a significant collateral vessel circuit develops in the hind limb of animals to circumvent obstructions of the femoral arteries. A modest change occurs spontaneously with no intervention (730, 733), while more significant increases in collateral-dependent blood flow are observed following interventions such as delivery of angiogenic growth factors (808, 1021, 1027) and/or daily physical exercise (730, 733). The improvement in collateral-dependent blood flow is physiologically significant, as the animals exhibit a marked improvement in exercise tolerance. The uniform nature of these studies raised keen expectations that similar adaptations would be prolific in patients with PAD that are involved in exercise training programs. While clinical studies are almost uniform in demonstrating that exercise training leads to an improvement in exercise tolerance in these patients, this cannot typically be attributed to an increase in collateral blood flow (699). First, only a relatively few of these studies have attempted to measure blood flow to the limb, and many of those do not find any significant change (184, 234, 514, 861, 877). This may not be surprising, since the techniques for measuring blood flow to the limb in humans are fairly imprecise, regional in nature, and/or not dynamic in real time. The problem becomes even more difficult in patients with PAD, where minimizing the resistance of the distal muscle (e.g., typically by contractions), in order to ensure a valid measure of maximal blood flow, is burdensome and often not possible. There are other, possibly even more compelling, reasons that make it very difficult for collateral vessel development in humans to become substantial enough to recover large flow deficits back to normal. A brief consideration of vascular hemodynamics will suffice. Illustrated in Figure 11 is the decline in blood flow of a major conduit artery of 5 mm (e.g., femoral artery), as an obstruction reduces its caliber. Note that resistance to blood flow increases geometrically, based upon Poiseuille’s relationship where resistance is a function to the fourth power of the radius. Thus, a reduction in vessel radius to one-half its initial increases resistance by 16-fold, leading to a reduction in flow capacity to ~6% of initial! It becomes apparent why vascular obstructions can have such a profound consequence in producing ischemia, especially when the flow demands of the down stream muscle are elevated. Any such upstream obstruction that reduces vessel caliber leads to a loss in perfusion pressure down stream, since in a hydraulic system there is a pressure decline that occurs across any resistance. This is illustrated in Figure 12 where the decline in distal pressure is plotted against vessel diameter for flow demands typical for 1 kg of muscle (e.g., calf) at rest (780). Note that a reduction in diameter to one-half normal leads to an apparently small, but real, reduction in distal pressure to <90% of normal, the recognized standard used to identify patients with PAD (213, 257, 600, 661). While this decrease in diameter (to 2.5 mm) presents no risk of ischemia at rest, it only takes a further reduction in diameter to 1.5 mm to reach the limit of flow reserve for resting flow needs. At this diameter of 1.5 mm the upstream resistance is so high that no matter how low the resistance of the distal muscle becomes, flow to the calf muscle becomes inadequate. Even if the muscle reduced it resistance to its minimum possible (e.g., where resistance = 0.0417 at a blood flow = 3,000 ml/min/kg (22), the resistance of the entire circuit would be inadequate to support the 40 ml/min resting needed for the calf muscle. The resultant condition of ‘ischemia at rest’ has dire consequences for clinical management and leads to inordinate increases in morbidity risks and premature death (607). If, on the other hand, the obstruction remained at 2.5 mm, the absence of problems at rest changes markedly upon walking at a modest rate (e.g., 2.5 mph). Also shown in Figure 12 is the impact of increasing flow needs to the calf muscle by a very modest 4-fold. Note that approximately 50% of the arterial pressure is lost across this upstream resistance. This increased blood flow need (160 ml/min) to the muscle could be met, since the resistance of the muscle can decrease extensively due it its high conductance. However, any significant increase in flow demands by the calf muscle, for example by walking up 2 flights of stairs, could lead to frank ischemia and potentially cause claudication. It is easy to see, with this illustration, how restrictions in arterial supply can be so debilitating in patients with PAD.
Figure 11.
Relationships between vessel size and blood flow (right axis) and resistance (left axis) for a typical femoral artery of 5 mm diameter. Note the precipitous decline in blood flow, and increase in vascular resistance, as vessel caliber decreases, since these are a 4th-power function of vessel radius. Thus, blood flow capacity is only ~6% of normal, if the size of the vessel declines to one-half. The insert is an expanded region of interest.
Figure 12.
Calculated pressure to the distal calf muscles as a function of the reduction in caliber of the upstream vessel when blood flow to the distal limb is sufficient for resting tissue needs of 40 ml/min (circles) or during walking at a slow pace where blood flow needs increase to 160 ml/min (squares). Note that a reduction in upstream vessel caliber to one-half initial leads to a reduction in distal pressure to < 90% normal, a value that defines the presence of PAD. At the same time, this individual would experience a marked reduction in distal perfusion pressure to <50% of normal during walking. Note that it would take the development of ~3500 500μ or 5 2.5 mm diameter collateral vessels to recover distal perfusion pressure to above 90% during the mild walking rate.
A pertinent question can be asked, how easy is it then for patients to develop a collateral vessel circuit sufficient to compensate for the above-calculated level of conduit vessel obstruction (i.e., 5.0 mm down to 2.5 mm)? An answer requires two considerations, knowing: a) the caliber of the collateral vessel(s) and b) the flow demands of the distal limb muscles. For simplicity, we will consider the same reduction in vessel caliber from 5.0 mm to 2.5 mm, as well as the two conditions, rest and modest walking at 2.5 mph, as discussed above. As developed in the section on arteriogenesis, the primary means of collateral vessel development thus far described is the enlargement of the small, near-resistance size vessels that are found in the tissues surrounding the arterial obstruction. These 50–100 μ vessels are expected to enlarge to 250–500 μ conduits that deliver collateral flow circumventing the obstruction. As illustrated in Figure 12, it would take the formation of 672 collateral vessels 500 μ in diameter in order to take a distal pressure in the PAD range (<0.9 ankle/brachial index) to above and into the borderline PAD range (ABI between 0.9–1.0) during the resting condition. In contrast, it would take more than 3,477 collateral vessels 500 μ in diameter to return the low distal pressure during walking to above 90% of normal. Even if rather larger 2.5 mm diameter collateral vessels developed, it would take more than 5 to recover the flow potential back to that of a 4.0 mm artery (cf., Figure 12). It is obvious that expansive collateral development is essential if patients with advanced obstructions are to recover their capacity for ambulatory activities. Further, these calculations simply take into account differences in resistance established by changes in vessel caliber and not the added resistance due to the length of the vessels, the extent of tortuosity, or the presence of multilevel obstructions. The relatively long distances in patients, that exist between common sites of vascular obstructions (eg., ilio-femoral/femoral) and the distal limb muscles in need of oxygen, adds further complexity. Indeed, the relative high flows, due to the mass of the limbs, and the long distance of the conduits in patients, establish a marked distinction between humans and animals, where substantial collateral vessels develop over relatively short distances in the hindquarter vasculature of the quadrupeds. Thus, it is not surprising that few studies find significant increases in blood flow to the limbs of patients after participation in an exercise program. On the other hand, it is documented that significant collateral vessels can develop, even spontaneously as illustrated in Figure 13 (249). Further, some studies show improvement in limb flow (17, 92, 282–284, 378, 859) and there is even an anecdotal report of an extensive collateral development in one conscientious patient that was sufficient to permit him to finish walking a marathon (335). Interestingly, even in the rodent model of peripheral arterial insufficiency, prolonged daily running markedly increased, but did not return blood flow capacity to the calf muscle back to normal, as could be achieved by surgically increasing shear stress within the collateral vessels combined with concurrent administration of angiogenic growth factor, VEGF (732). It may be likely, however, that a higher incidence of improved collateral blood flow might be found in patients, if a more profound arteriogenic stimulus were established, for example by an extended program of sufficiently intense physical exercise. Additional factors may intervene, as there is even the potential that limb collateral vessel development could be genetically determined, if predilection in the limb is similar to that observed in coronary collateral development (152).
Figure 13.

Magnetic Resonance angiograph illustrating that collateral vessels can develop to circumvent a short-segment occlusion (right superficial femoral artery) and long-segment occlusion (left femoral artery) of patients with PAD. Reproduced from Esterhammer et al with permission from (249).
14.2. Functional behavior of vessels distal to an obstruction
While there is relatively little known about the vasoresponsiveness of vessels affected by a major obstruction of an artery, there is enough information to know that they probably do not function normally. For example, the low luminal pressure fostered on the down stream vasculature, by an upstream obstruction, would reduce radial wall tension of even otherwise normal vessels. A structural increase in the diameter of conduit vessels (saphenous a, soleus feed a. and calf muscle feed a.) ensues over time (915), possibly attempting to recover some of the lost radial wall tension that comes with a reduction in luminal pressure. This reduction in radial wall tension has the potential to diminish the vasodilator response of the vessel (914). A dulled flow-mediated dilation of the popliteal artery was observed after ischemic exercise (leg occlusion), but not following the same exercise without leg occlusion, even in normal healthy 17 yr old volunteers (73). Further, a dulled vasodilator response was observed in the small arterioles of a distal limb muscle following upstream occlusion of the iliac artery (480). This could have contributed to the reduced conductance of the calf muscles following occlusion of the femoral artery, as compared to that of the normal flow, contralateral calf muscle (914). Whether an up regulation of alpha-sympathetic receptors (802) in the distal vessels contributed to this response is not known. Similarly, exaggerated responses to norepinephrine (165, 459) have been observed in vessels obtained from patients with critical limb ischemia. However, this potential for an exaggerated vasoconstriction of the limb vasculature seems inconsistent with the reduced sympathetic orthostatic response observed in patients with PAD (202). Interestingly, the dulled vasodilator response of the vasculature was improved by daily contractions of the ischemic muscle, either by muscle stimulation (480) or by treadmill walking (915). The evidence to date suggests that there is the potential to exacerbate the blood flow deficits caused by the upstream arterial obstruction, if these aberrant responses occur in patients with PAD. On the other hand, the recovery from vasomotor dysfunction with muscle contractions (480, 915) appears to offer the potential for improvement by participation in an exercise program.
14.3. Functional behavior of collateral vessels that circumvent an obstruction
The hemodynamic function of collateral vessels becomes unique among the traditional roles of vessels within the vasculature. For example, large conduit vessels are responsible to deliver flow downstream at a high rate, without significant resistance, and be responsive to luminal flow events. Smaller resistance vessels, which are close to the tissue being perfused, are highly innervated to regulate caliber, thereby exerting a high resistance, to limit flow until local tissue demands initiate dilatation. Finally, capillaries function to ‘bathe the tissue in blood’ to optimize diffusion of metabolites and gases between blood and cells. On the other hand, to be effective collateral vessels must function as ‘low’ resistance conduits, albeit relatively small ones, but arise from even smaller arteriolar size vessels (113, 118, 811, 1018). This raises the potential that these small vessels, that are the substrate for the collateral circuit, retain their vasoconstrictor properties as the vessels enlarge. Even the potential that local tissue conditions exist to foster dilatation of the collateral vessels may not occur, since the site of collateral circuit is often within tissue that is not collateral-dependent. This appears to be the case with proximal obstructions of large vessels (e.g., femoral artery), where the collateral circuit develops in the thigh region which enjoys a much higher blood flow than the collateral dependent muscles of the lower limb (455, 1018, 1019). Thus, inordinate vasoconstriction of the collateral circuit could exacerbate the difficulty in delivering blood flow downstream, well beyond that established by the limited physical caliber of the collateral vessels themselves.
While very little is known about the vasomotor function of the collateral circuit, there are animal studies that have evaluated the functional behavior of the collateral circuit in vivo, and the vasoresponsiveness of individual collateral vessels in vitro. Opening of a nascent collateral circuit, following acute occlusion of the femoral artery, can occur within minutes (776, 777) and requires nitric oxide to be present (947–949, 1026), likely related to flow-mediated dilation that must occur as these vessels begin to function. Further, nitric oxide continues to be important during the process of collateral vessel remodeling (947, 949). Collateral flow increases in response to the dilatory effect of adenosine (577). On the other hand, serotonin has been shown to cause collateral vasoconstriction (550). Further, a dominant sympathetic influence remains that tempers flow delivery down stream. Blocking either alpha1-, alpha2-adrenergic receptors, or in combination, increases the conductance of the collateral circuit during exercise (914, 915). In addition, inhibition of neuropeptide Y (NPY)-1 receptors, that would be responsive to the NPY released concurrent with release of norepinephrine (1054), increases the conductance of the collateral circuit (915). Thus, sympathetic output, which is expected to be increased in PAD (44, 47) due to the muscle pressor reflex, likely serves to limit collateral blood flow during activity, by more than that caused by the small structural diameter of the collateral vessels (e.g., the collateral plumbing). Interestingly, it may be that exercise training has the potential of ameliorating this detrimental condition in patients, since the muscle pressor reflex is less robust after training even in normal healthy subjects (640, 742) and collateral circuit conductance was improved by exercise training in experimental peripheral arterial insufficiency (915).
Evaluating the vasoresponsiveness of individual collateral vessels in vitro provided consistent evidence with the in vivo assessment discussed above, in demonstrating that an exaggerated vasoconstriction to increasing luminal flow was reversed by exercise training (170). This assessment was performed on the re-entry portion of a collateral vessel, which delivers collateral blood flow into the distal vasculature to complete the circuit bypassing the obstruction. Thus, it may not represent the intermediate collateral vessels segments that connect the entry vessels to these re-entry vessels. It is apparent that this pre-existing vessel, that was co-opted to serve as a collateral vessel, remodeled following occlusion of the femoral artery. As illustrated in Figure 14, inhibition of the cyclooxygenase pathway with indomethacin reversed the inherent flow-mediated vasoconstriction to a modest vasodilatation, compared to normal vessels perfused at the low luminal pressure common to this vessel (170). Further, removing the influence of this cyclooxygenase product, putatively thromboxane A2, in the vessels from trained animals resulted in a robust vasodilatation. Indeed, this marked flow-mediated vasodilatation was impervious to nitric oxide removal with L-Name or by loss of both NOS and cyclooxygenase products. This indicates that exercise training causes a remodeling of the collateral vessel to favor vasodilatation via enhanced production of the putative endothelium-derived hyperpolarizing factor(s) (170). If these findings were applicable to patients with PAD, there could be an improved collateral vessel function and enhanced exercise tolerance following participation in a program of routine physical activity.
Figure 14.
Influence of exercise training on the vasoresponsiveness of a collateral vessel as a function of shear stress. An initial modest dilatation to low shear stress in control animals (open circles) reverted to a dominant vasoconstriction at high shear stress. This response was eliminated in the presence of indomethacin, L-NAME, and in combination, as illustrated (filled circles), to a modest vasodilatation at very high shear stress. In contrast, collateral vessels from trained animals exhibited a marked vasodilation in the presence of indomethacin, L-NAME, and in combination, as illustrated (filled squares). This implies that exercise training induces a cyclooxygenase- and NOS-independent stimulus for vasodilatation. Data taken from Colleran et al (170) with permission.
15. Cardiovascular Control in Patients with Peripheral Arterial
Peripheral arterial disease is thought to be caused by a general atherosclerotic condition exacerbated by a chronic inflammatory state (55, 483, 835). There are a number of expected health consequences associated with this condition that appear as co-morbidities, including ischemic heart and cerebral vascular disease (213, 661, 829), hypertension (55), hyperlipidemia (55), diabetes (745, 837), renal disease (55), and even obesity (724), and often with attendant elevated biomarkers (e.g., PAI-1, CRP)(483). Thus, it is common that patients with PAD will exhibit cardiac, vascular, and metabolic dysfunction, and resulting in a reduced activity level (55, 596). Many of these co-morbidities may be directly related to cardiovascular dysfunction and thereby contribute to the increased morbidity and shorter life span of PAD patients. However, PAD patients have an increased risk for cardiovascular morbidity and mortality that is independent of the typical risk factors for atherosclerosis (596, 862), implying that features other than generalized atherosclerotic disease are consequential. Further, there is a subset of PAD patients that do not present with ischemic heart disease or cerebral vascular disease. Estimates place this population at approximately 35–50% of all patients with PAD (596, 656). Yet, the risk of premature death and increased morbidity is just the same as those patients with attendant cardiac and cerebral vascular disease (180, 862). Thus, the presence of ostensibly ‘pure’ PAD is not without its ominous risk of premature death. It is presently unclear what factor or factors contribute to this unfortunate outcome, but some insight can be gained by understanding cardiovascular dysregulation that is likely promulgated by ischemia to the legs.
There is a reflex neural input from the periphery that contributes to cardiovascular regulation during exercise, termed the exercise pressor reflex (476, 482, 669, 858, 865). During aerobic-type, rhythmical exercise afferent nerve traffic, arising from within active muscles, stimulates the sympathetic centers in the medulla. This neural input serves to activate sympathetic output contributing to heighten cardiac function and vascular control to support exercise. This, along with higher center activation, increases cardiac output and the drive for vasoconstriction throughout the body, especially the skin, kidney and gastrointestinal tract. While this vasoconstrictor outflow also extends to all muscles, there is lysis of this effect in the contracting muscles where local vasodilatation greatly enhances blood flow, thereby decreasing peripheral resistance and tempering any significant increase in blood pressure. Thus, the importance of the muscle pressor response is to contribute to sympathetic control during exercise and help redistribute cardiac output to the active muscles (780). Interestingly, if exercise is static (isometric), with only high force development by the muscle, there is little to no decrease in vascular resistance, nor elevation in blood flow through the muscle. As a result, blood pressure increases inordinately--ergo, the term -ischemic pressor response -is sometime used. Under this condition, the afferent input from the active muscle is greatly increased resulting in even greater elevation in blood pressure. An extreme in elevated blood pressure is realized during exceptionally high-load weight lifting of large muscle groups (564).
15.1. Components of the exercise pressor reflex
There are at least two features established within active muscle that prompt afferent traffic centrally: mechanoreceptors, driven by tension within the muscle caused by either active force development or passive stretch, and metaboreceptors, acting as ‘chemical’ sensors that are responsive to a wide variety of stimuli created within the environment of ischemic muscle (476, 482, 669, 858, 865). These two sources were originally distinguished in experiments where a large fraction ( 50%) of the hypertension developed during prolonged ischemic isometric contractions, continued as long as the tourniquet remained in place even though the contractions were stopped (15). Mechanosensors within the muscle are responsive to muscle force, initiating afferent nerve traffic to the brain, predominantly via group III nerves (477), in a manner proportional to the force developed (864). On the other hand, metaboreceptors within the muscle initiate afferent input primarily via group IV nerves and are responsive a wide variety of factors (e.g., lactate, H+, ATP, ROS, bradykinin, capsaicin) via a variety of receptors and sensitive membrane channels (494, 496, 858, 980). Interestingly, the intensity of neural activation depends upon the type of muscle fiber contracting. Force development in the high-oxidative slow-twitch (type I) motor units prompt a lesser degree of afferent activation than when the low-oxidative fast-twitch (type IIb) motor units are contracting (38, 1015). Thus, the muscle pressor reflex should become more exaggerated as the intensity of contraction increases in severity, as the low-oxidative fast-twitch motor units become more highly recruited. This likely contributed to the exceptionally high blood pressures, in excess of 300/200 mmHg, observed in experienced weight lifters who performed near-maximally intense contractions of large muscle groups (564, 693).
15.2. Exercise pressor reflex in heart failure
Germane to the present consideration are elements of the exercise pressor reflex that change in conditions typical of chronic disease, such as heart disease (858, 865) and hypertension (201, 633). For example, an exaggerated exercise pressor reflex, measured as hypertension and/or an elevated sympathetic nerve output, is observed in patients with heart failure (716, 848, 853). Studies evaluating the physiological bases for this exaggerated response, in animal models of heart failure (279, 280, 482, 495, 496, 978, 979, 995), have found that the mechanically sensitive fibers are overactive (531, 616, 617, 863, 866), whereas the metabolically sensitive afferents are less responsive to activation (531, 868, 885). The reasons for the increased responsiveness of the mechanoreflex remains unclear, whereas the loss in the metaboreflex is due, in part, to less active receptors on group IV afferent neurons, the transient receptor potential vanilloid 1 (TRPv1) (531, 868) and cannabinoid 1 (CB1) receptors (995)). It is possible that this exaggerated exercise pressor reflex contributes to the hyper-sympathetic state that typifies heart failure patients (260). Interestingly, animals with heart failure that are exercise trained reverse their low metaboreflex and elevated mechanoreflex back toward normal (979).
15.3. Exercise pressor reflex in hypertension
The exercise pressor reflex is exaggerated in hypertensive patients and animals models of hypertension. Hypertensive patients exhibit an enhanced elevation in blood pressure (28, 479, 805) and sympathetic nerve output (201) during ischemic handgrip exercise. It is likely that the metaboreflex contributed to this response in patients, since the blood pressure and sympathetic nerve activity remain elevated following contractions when the tourniquet cuff was left intact (201). Smith and co-workers also found that the exercise pressor reflex was exaggerated in spontaneously hypertensive rats (SHR)(867), due to changes in both the mechano-and metaboreflexes (520, 633). The exaggerated exercise pressor reflex in the condition of hypertension, seems rather predictable, since there is already dysregulation in blood pressure control. On the other hand, as with heart failure, differences arising from the active muscle are intriguing. While there are many contributors to hypertension, alterations in the central neural control centers can be important (260, 614). Interestingly, even with long-term blood pressure correction of hypertension in patients, the exercise pressor reflex remained somewhat elevated, as compared to normal healthy subjects (479). This raises the probability that the exaggerated exercise pressor reflex observed in the hypertensive condition, involves more than just an enhanced afferent neural traffic from the contracting muscles. Future research will, no doubt, reveal some of the complex interactions among the mechano-and metaboreflex inputs and alterations among the central cardiovascular control elements.
15.4 Exercise pressor reflex in peripheral arterial insufficiency
It is likely that the exercise pressor response contributes to hypertension in patients with PAD. However, it is not possible to make a definitive assignment of the hypertension, commonly observed is these patients, to muscle afferent traffic, since hypertension is its own pathology and a common co-morbidity with PAD (55). On the other hand, as illustrated in Figure 15, the exercise pressor reflex could contribute to the frank hypertension that was induced during exercise in patients with PAD, as compared to age-matched controls (44, 47). The elevated blood pressure was evident early during the treadmill walk, well before the time of pain-onset or when the patients had to stop walking (47). As expected the hypertension in PAD patients is a function of exercise intensity (44). This could invoke another possible contributor to the elevated exercise pressor reflex. PAD patients often exhibit a loss of muscle mass, which could foster earlier recruitment of the more reflex-responsive fast-twitch motor units. Thus, probable activation of these fast-twitch motor units, to achieve even a modest intensity of locomotion, would exaggerate the afferent nerve traffic from the muscle (38, 1015). It is apparent, however, that differences in the muscle reflex occur, since even young healthy subjects will demonstrate an exercise hypertension while walking when cuffs occlude limb blood flow (73). Interestingly, the absence of an exaggerated exercise pressor reflex between PAD patients and healthy control subjects, when induced by handgrip exercise (48), implies that the changes in the exercise pressor response with PAD are not generalized, but dependent upon afferent nerve traffic derived from a diseased ischemic limb. Indeed, animal experiments have shown this specificity, since the afferent neurons in the dorsal root ganglion remodel only at the cord level innervating the muscles that are ischemic (541), the elevated exercise pressor reflex was observed only in the ischemic limb and not the free-flow perfused contralateral limb of the same animal (936), and the dilatory capacity of ischemic muscle during treadmill exercise was markedly less than that of the normal flow, non-ischemic muscle (914, 915). Further, the presence of the exaggerated exercise pressor reflex was dependent upon muscle contractions, as neuromuscular blockade and denervation of the afferent loop of the reflex completely eliminated the pressor response.
Figure 15.
Example of hypertension during exercise in a group of patients with PAD who exhibit claudication. Note that the elevation in blood pressure in the claudicant group is greater than that of aged-matched control group well prior to the cessation of walking. Figure reproduced from Baake et al (47) with permission.
Recent evidence has provided significant insights on how the exercise pressor response is exaggerated in experimental animals with peripheral arterial insufficiency caused by femoral artery occlusion (541, 936–938, 1013, 1014). Femoral artery occlusion induces an increase in the exercise pressor response prompted by both muscle contractions and passive stretch of the muscle, implicating both mechano-and metaboreceptors in the response. The time-course post-occlusion needed to observe this increase is surprising brief, within 24 hr leading to a near-maximal response in 72 hr (541); yet, the exaggerated exercise pressor reflex is not observed with acute occlusion (3 min) of the femoral artery (936). Further, the enhanced exercise pressor reflex is observed without frank ischemia within the limb muscles. Occlusion of the femoral artery, as done in these studies, removes approximately 75–85% of the flow reserve to the calf muscle (1026); however, nascent collateral blood capacity to the limb is sufficient to supply the calf muscle with blood flow (1022, 1026) that is approximately 3-fold greater than ‘resting’ blood flow observed in muscle of quiescent anesthetized animals (565, 566). Thus, while the exercise capacity of these animals is greatly limited, there should be little stress on the muscle during limited cage activity. Nonetheless, factors must be promulgated within the muscle by femoral artery occlusion that initiate fairly immediate remodeling of the neuronal sensing and/or propagation processes responsive to tissue tension and/or metabolites, including: an increased response to acid-sensing ion channels (e.g., responsive to lactic acid) (541, 939); transient receptor potential vanilloid type I receptor (nociception) up regulation in the afferent dorsal root neurons (1013), possibly driven by up regulation of nerve growth factor (542, 1014) although inhibition of TRPV1 receptors did not lessen the elevated exercise pressor response (936); inhibition of the exaggerated pressor response by activating micro-opioid receptors, likely implicating metaboreceptors (937); and sensitivity of the exaggerated exercise pressor reflex to tetrodotoxin inhibition of sodium channels, but not TTX-resistant sodium channels in dorsal root ganglia (938); and the action of tempol, but not likely due to its ROS buffering capacity (594). These important papers demonstrate that critical changes occur in the afferent arm of the exercise pressor reflex in the condition of peripheral arterial insufficiency, leading to an enhanced afferent traffic to the central cardiovascular control centers. Whether there are also adaptive changes induced within the central cardiovascular control centers, that could ameliorate or exacerbate conditions in PAD patients, is unknown. Thus, it may be hypothesized that PAD patients are at risk of hypertension during activity and that this elevated afferent nerve traffic could cause neural adaptations which ‘bias’ central neural cardiovascular control and lead to a hyper sympathetic state even at rest, which has been observed (419). The added risks of premature death and morbidity, due to a hypersympathetic condition, have been well recognized (260). Such a causative link could help explain the equivalent risks of morbidity and mortality in PAD patients, even in the absence of cardiac or cerebral vascular disease (180, 862).
15.5. Central cardiovascular control centers influence sympathetic output
Recent research has established that critical areas within the brain contribute to the hypersympathetic output that appears common to cardiovascular diseases (641). While the complex interactions among the numerous important loci within the brain are beyond the scope of this review, a couple of brain areas have received the most attention and merit comment. The paraventricular nucleus (PVN) in the hypothalamus and the rostral ventrolateral medulla (RVLM) have become a major focus for evaluating experimental heart failure (1051, 1053) and hypertension (256). In each case, substantial derangements of these centers, related to inherent alterations of excitatory/inhibitor input, have been observed. For example, in experimental heart failure there is a modified behavior of the PVN related to a reduced bioavailability of nitric oxide (703) and enhanced angiotensin II activation (1042), possible due to a down regulation of local angiotensin converting enzyme (474), that contributes to the hypersympathetic state. Similarly, a deficit in nitric oxide bioavailability in the RVLM contributes to the hypertension characteristic of spontaneous hypertensive rats (142, 489). Interestingly, exercise training reverses the hypersympathetic state (1052) in heart failure models, apparently by improving the antioxidant condition (280), nitric oxide bioavailability (1043), and by reducing the excitatory influence of glutamate in the PVN (493). Even in the absence of imputed disease conditions, normal animals that are exercise trained exhibit a smaller response to excitatory stimuli (glutamate) in the RVLM (641). Thus, recent work has developed compelling evidence that central cardiovascular centers are subject to dysregulation, found to be plastic, and potentially responsive to afferent nerve traffic to modify their behavior. This raises the potential that significant changes could be promulgated onto these control centers by a barrage of afferent nerve traffic arising from the exaggerated exercise pressor reflex observed in the condition of peripheral arterial insufficiency. Further, the above-mentioned training responses in experimental heart failure and hypertension raises the potential that exercise training in patients with PAD could induce beneficial central neural adaptations. However, these hypotheses remain to be tested.
Conclusion
It is apparent that involvement in recommended exercise prescription brings about a multitude of adaptations that are beneficial to the patient with PAD. While much is known about these adaptations, the signals that bring them about, and the realized impact of these changes, there is much to be learned of the impact of exercise in the condition of PAD. New advances in technology are needed to assist in the management of these patients. For example, there is difficulty in assessing the extent of blood flow deficit to the diseased limb, in identifying the sites of vulnerability within the limb, and in evaluating the extent of therapeutic intervention. Further, while exercise prescription is one of the most effective means of managing PAD, in the absence of contraindications, achieving compliance is a significant clinical problem. Thus, there are many avenues for advances in scientific inquiry, application of technology, clinical management of PAD patients, and in educating the public of the need for life style changes to enhance the amount of daily physical activity.
Cross-References.
| Exercise and peripheral arterial insufficiency Exercise as preventive medicine for chronic disease |
| Molecular mechanisms and muscle plasticity with acute and chronic exercise Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle: implications for health and disease |
| Circulation to skeletal muscle (legacy) Control of microcirculation and blood-tissue exchange (legacy) |
| Skeletal muscle adaptability: significance for metabolism and performance (legacy) |
| Central/peripheral circulatory integration Central neural control of respiration and circulation during exercise (legacy) |
Acknowledgments
Cited work by the authors was supported by the following grants:
TLH: Canadian Institutes of Health Research Grant #107537, Heart and Stroke Foundation of Canada Grant in Aid #NA 7059, and National Science and Engineering Council of Canada Grant #222865; PGL: NHLBI F32-HL10406, NHLBI R01-HL084494; HTY and RLT: NIAMS R37-AR21617, NHLBI R01-HL37387, NHLBI P01-HL52490, NIAMS T32-AR048523, and NHLBI F32-HL10485.
References
- 1.Abaci A, Oguzhan A, Kahraman S, Eryol NK, Unal S, Arinc H, Ergin Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation. 1999;99:2239–2242. doi: 10.1161/01.cir.99.17.2239. [DOI] [PubMed] [Google Scholar]
- 2.Abou-Khalil R, Le GF, Pallafacchina G, Valable S, Authier FJ, Rudnicki MA, Gherardi RK, Germain S, Chretien F, Sotiropoulos A, Lafuste P, Montarras D, Chazaud B. Autocrine and paracrine angiopoietin 1/Tie-2 signaling promotes muscle satellite cell self-renewal. Cell Stem Cell. 2009;5:298–309. doi: 10.1016/j.stem.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abounader R, Laterra J. Scatter factor/hepatocyte growth factor in brain tumor growth and angiogenesis. Neuro Oncol. 2005;7:436–451. doi: 10.1215/S1152851705000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abumiya T, Sasaguri T, Taba Y, Miwa Y, Miyagi M. Shear Stress Induces Expression of Vascular Endothelial Growth Factor Receptor Flk-1/KDR Through the CT-Rich Sp1 Binding Site. Arterioscler Thromb Vasc Biol. 2002;22:907–913. doi: 10.1161/01.atv.0000018300.43492.83. [DOI] [PubMed] [Google Scholar]
- 5.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, Walsh K, Sessa WC. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115:2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adair TH. Growth regulation of the vascular system: an emerging role for adenosine. Am J Physiol. 2005;289:R283–R296. doi: 10.1152/ajpregu.00840.2004. [DOI] [PubMed] [Google Scholar]
- 7.Adams RH, Eichmann A. Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol. 2010;2:a001875. doi: 10.1101/cshperspect.a001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adolfsson J, Ljungqvist A, Tornling G, Unge G. Capillary increase in the skeletal muscle of trained young and adult rats. J Physiol. 1981;310:529–532. doi: 10.1113/jphysiol.1981.sp013565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Afaq A, Montgomery PS, Scott KJ, Blevins SM, Whitsett TL, Gardner AW. The effect of current cigarette smoking on calf muscle hemoglobin oxygen saturation in patients with intermittent claudication. Vasc Med. 2007;12:167–173. doi: 10.1177/1358863X07081317. [DOI] [PubMed] [Google Scholar]
- 10.Agency for Healthcare Research and Quality. Treatment strategies for patients with peripheral areterial disease (PAD) Effective Health Care Program. 2012:1–35. www.effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?productid=948&pageaction=displayproduct:
- 11.Ahimastos AA, Pappas EP, Buttner PG, Walker PJ, Kingwell BA, Golledge J. A meta-analysis of the outcome of endovascular and noninvasive therapies in the treatment of intermittent claudication. J Vasc Surg. 2011;54:1511–1521. doi: 10.1016/j.jvs.2011.06.106. [DOI] [PubMed] [Google Scholar]
- 12.Akimoto T, Pohnert S, Li P, Zhang M, Gumbs C, Rosenberg P, Williams RS, Yan Z. Exercise stimuluates PGC-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem. 2005;280:19587–19593. doi: 10.1074/jbc.M408862200. [DOI] [PubMed] [Google Scholar]
- 13.Akiri G, Nahari D, Finkelstein Y, Le SY, Elroy-Stein O, Levi BZ. Regulation of vascular endothelial growth factor (VEGF) expression is mediated by internal initiation of translation and alternative initiation of transcription. Oncogene. 1998;17:227–236. doi: 10.1038/sj.onc.1202019. [DOI] [PubMed] [Google Scholar]
- 14.Al H, Zen A, Oikawa A, Bazan-Peregrino M, Meloni M, Emanueli C, Madeddu P. Inhibition of delta-like-4-mediated signaling impairs reparative angiogenesis after ischemia. Circ Res. 2010;107:283–293. doi: 10.1161/CIRCRESAHA.110.221663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol. 1937;146:372–383. doi: 10.1113/jphysiol.1937.sp003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen JD, Stabler T, Kenjale A, Ham KL, Robbins JL, Duscha BD, Dobrosielski DA, Annex BH. Plasma nitrite flux predicts exercise performance in peripheral arterial disease after 3 months of exercise trainng. Free Radic Biol Med. 2010;49:1138–1144. doi: 10.1016/j.freeradbiomed.2010.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alpert JS, Larsen OA, Lassen NA. Exercise and intermittent claudication Blood flow in the calf muscle during walking studied by the xenon-133 clearance method. Circulation. 1969;39:353–359. doi: 10.1161/01.cir.39.3.353. [DOI] [PubMed] [Google Scholar]
- 18.Alroy J, Goyal V, Skutelsky E. Lectin histochemistry of mammalian endothelium. Histochemistry. 1987;86:603–607. doi: 10.1007/BF00489554. [DOI] [PubMed] [Google Scholar]
- 19.Amaral SL, Linderman RJ, Morse MM, Greene AS. Angiogenesis induced by elecrical stimulation is mediated by angiotensis II and VEGF. Microcirculation. 2001;8:57–67. [PubMed] [Google Scholar]
- 20.Ameln H, Gustafsson T, Sundberg CJ, Okamoto K, Jansson E, Poellinger L, Makino Y. Physiological activation of hypoxia inducible factor-1 in human skeletal muscle. FASEB J. 2005;19:1009–1011. doi: 10.1096/fj.04-2304fje. [DOI] [PubMed] [Google Scholar]
- 21.Andersen P, Henriksson J. Capillary supply of the quadriceps femoris muscle of man: adaptive response to exercise. J Physiol. 1977;270:677–690. doi: 10.1113/jphysiol.1977.sp011975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andreozzi GM, Leone A, Laudani R, Deinite G, Martini R. Acute impairment of the endothelial function by maximal treadmill exercise in patients with intermittent claudication, and its improvement after supervised physical training. Int Angiol. 2007;26:12–17. [PubMed] [Google Scholar]
- 24.Andreozzi GM, Martini R, Cordova R, D’Eri A, Salmistraro G, Mussap M, Plebani M. Circulating levels of cytokines (IL-6 and IL-1beta) in patients with intermittent claudication, at rest, after maximal exercise treadmill test and during restore phase. Could they be progression markers of the disease? International Angiology. 2007;26:245–252. [PubMed] [Google Scholar]
- 25.Angersbach D, Jukna JJ, Nicholson CD, Ochlich P, Wilke R. The effect of short-term and long-term femoral artery ligation on rat calf muscle oxygen tension, blood flow, metabolism and function. Int J Microcirc Clin Exp. 1988;7:15–30. [PubMed] [Google Scholar]
- 26.Annex BH, Torgan CE, Lin P, Taylor DA, Thompson MA, Peters KG, Kraus WE. Induction and maintenance of increased VEGF protein by chronic motor nerve stimulation in skeletal muscle. Am J Physiol. 1998;274:H860–H867. doi: 10.1152/ajpheart.1998.274.3.H860. [DOI] [PubMed] [Google Scholar]
- 27.Aoi W, Naito Y, Mizushima K, Takanami Y, Kawai Y, Ishikawa H, Yoshikawa T. The microRNA miR-696 regulates PGC-1alpha in mouse skeletal muscle in response to physical activity. Am J Physiol. 2010;298:E799–E806. doi: 10.1152/ajpendo.00448.2009. [DOI] [PubMed] [Google Scholar]
- 28.Aoki K, Sato K, Kondo S, Pyon CB, Yamamoto M. Increased response of blood pressure to rest and handgrip in subjects with essential hypertension. Jpn Circ J. 1983;47:802–809. doi: 10.1253/jcj.47.802. [DOI] [PubMed] [Google Scholar]
- 29.Aoki M, Morishita R, Taniyama Y, Kida I, Moriguchi A, Matsumoto K, Nakamura T, Kaneda Y, Higaki J, Ogihara T. Angiogenesis induced by hepatocyte growth factor in non-infarcted myocardium and infarcted myocardium: up-regulation of essential transcription factor for angiogenesis, ets. Gene Ther. 2000;7:417–427. doi: 10.1038/sj.gt.3301104. [DOI] [PubMed] [Google Scholar]
- 30.Arany Z. PGC-1 coactivators and skeletal muscle adaptations in health and disease. Curr Opin Genet Dev. 2008;18:426–434. doi: 10.1016/j.gde.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arany Z, Foo SY, Ma Y, Raus JL, Bommi-Reddy A, Gimun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 32.Ardanaz N, Pagano PJ. Hydrogen peroxide as a paracrine vascular mediator: regulation and signaling leading to dysfunction. Exp Biol Med. 2006;231:237–251. doi: 10.1177/153537020623100302. [DOI] [PubMed] [Google Scholar]
- 33.Armstrong RB, Laughlin MH. Rat muscle blood flows during high speed locomotion. J Appl Physiol. 1985;59:1322–1328. doi: 10.1152/jappl.1985.59.4.1322. [DOI] [PubMed] [Google Scholar]
- 34.Arras M, Ito WD, Scholz D, Winkler B, Schaper J, Schaper W. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest. 1998;101:40–50. doi: 10.1172/JCI119877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asahara T, Bauters C, Zheng LP, Takeshita S, Bunting S, Ferrara N, Symes JF, Isner JM. Synergistic effect of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in vivo. Circulation. 1995;92:II365–II371. doi: 10.1161/01.cir.92.9.365. [DOI] [PubMed] [Google Scholar]
- 36.Ashton N. Neovascularization in ocular disease. Trans Ophthalmol Soc UK. 1961;81:145–161. [PubMed] [Google Scholar]
- 37.Ashworth NL, Chad KE, Harrison EL, Reeder BA, Marshall SC. Home versus center based physical activity programs in older adults. Cochrane Database Syst Rev. 2005 Jan 25;:CD004017. doi: 10.1002/14651858.CD004017.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Askew CD, Green S, Walker PJ, Kerr GK, Green AA, Williams AD, Febbraio MA. Skeletal muscle phenotype is associated with exercise intolerance in patients with peripheral arterial disease. J Vasc Surg. 2005;41:802–807. doi: 10.1016/j.jvs.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 39.Astrand PO, Rodahl K. Textbook of Work Physiology. New York: McGraw-Hill Book Company; 1970. [Google Scholar]
- 40.Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 41.Ausprunk DH, Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res. 1977;14:53–65. doi: 10.1016/0026-2862(77)90141-8. [DOI] [PubMed] [Google Scholar]
- 42.Azuma N, Duzgun SA, Ikeda M, Kito H, Akasaka N, Sasajima T, Sumpio BE. Endothelial cell response to different mechanical forces. J Vasc Surg. 2000;32:789–794. doi: 10.1067/mva.2000.107989. [DOI] [PubMed] [Google Scholar]
- 43.Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002;16:1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- 44.Baccelli G, Reggiani P, Mattioli A, Corbellini E, Garducci S, Catalano M. The exercise pressor reflex and changes in radial arterial pressure and heart rate during walking in patients with arteriosclerosis obliterans. Angiology. 1999;50:31–374. doi: 10.1177/000331979905000502. [DOI] [PubMed] [Google Scholar]
- 45.Badr I, Brown MD, Egginton S, Hudlicka O, Milkiewicz M, Verhaeg J. Differences in local environment determine the site of physiological angiogenesis in rat skeletal muscle. Exp Physiol. 2003;88:565–568. doi: 10.1113/eph8802601. [DOI] [PubMed] [Google Scholar]
- 46.Baffour R, Berman J, Garb JL, Rhee SW, Kaufman J, Friedmann P. Enhanced angiogenesis and growth of collaterals by in vivo administration of recombinant basic fibroblast growth factor in a rabbit model of acute lower limb ischemia: dose-response effect of basic fibroblast growth factor. J Vasc Surg. 1992;16:181–191. [PubMed] [Google Scholar]
- 47.Bakke EF, Hisdal J, Jorgensen JJ, Kroese A, Stranden E. Blood pressure in patients with intermittent claudication increases continuously during walking. Eur J Vasc Endovasc Surg. 2007;33:20–25. doi: 10.1016/j.ejvs.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 48.Bakke EF, Hisdal J, Kroese AJ, Jorgensen JJ, Stranden E. Blood pressure response to isometric exercise in patients with peripheral atherosclerotic disease. Clin Physiol Funct Imaging. 2007;27:109–115. doi: 10.1111/j.1475-097X.2007.00720.x. [DOI] [PubMed] [Google Scholar]
- 49.Baldwin KM, Klinkerfuss GH, Terjung RL, Mole PA, Holloszy JO. Respiratory capacity of white, red, and intermediate muscle: adaptive response to exercise. Am J Physiol. 1972;222:373–378. doi: 10.1152/ajplegacy.1972.222.2.373. [DOI] [PubMed] [Google Scholar]
- 50.Ballard HJ. The influence of lactic acid on adenosine release from skeletal muscle in anaesthetized dogs. J Physiol. 1991;433:95–108. doi: 10.1113/jphysiol.1991.sp018416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ballard HJ. The role of intracellular pH in the control of adenosine output from red skeletal muscle. Biol Signals. 1995;4:168–173. doi: 10.1159/000109437. [DOI] [PubMed] [Google Scholar]
- 52.Banai S, Shweiki D, Pinson A, Chandra M, Lazarovici G, Keshet E. Upregulation of vascular endothelial growth factor expression induced by myocardial ischaemia: implications for coronary angiogenesis. Cardiovasc Res. 1994;28:1176–1179. doi: 10.1093/cvr/28.8.1176. [DOI] [PubMed] [Google Scholar]
- 53.Banai S, Jaklitsch MT, Shou M, Lazarous DF, Scheinowitz M, Biro S, Epstein SE, Unger EF. Angiogenic-induced enhancement of collateral blood flow to ischemic myocardium by vascular endothelial growth factor in dogs. Circulation. 1994;89:2183–2189. doi: 10.1161/01.cir.89.5.2183. [DOI] [PubMed] [Google Scholar]
- 54.Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336–3343. [PubMed] [Google Scholar]
- 55.Bartholomew JR, Olin JW. Pathophysiology of peripheral arterial disease and risk factors for its development. Cleveland Clin J Med. 2006;73:S8–S14. doi: 10.3949/ccjm.73.suppl_4.s8. [DOI] [PubMed] [Google Scholar]
- 56.Bastide A, Karaa Z, Bornes S, Hieblot C, Lacazette E, Prats H, Touriol C. An upstream open reading frame within an IRES controls expression of a specific VEGF-A isoform. Nucleic Acids Res. 2008;36:2434–2445. doi: 10.1093/nar/gkn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bates DO, MacMillan PP, Manjaly JG, Qiu Y, Hudson SJ, Bevan HS, Hunter AJ, Soothill PW, Read M, Donaldson LF, Harper SJ. The endogenous anti-angiogenic family of splice variants of VEGF, VEGFxxxb, are down-regulated in pre-eclamptic placentae at term. Clin Sci (Lond) 2006;110:575–585. doi: 10.1042/CS20050292. [DOI] [PubMed] [Google Scholar]
- 58.Bauer TA, Brass EP, Barstow TJ, Hiatt WR. Skeletal muscle StO2 kinetics are slowed during low work rate calf exercise in peripheral arterial disease. Eur J Appl Physiol. 2007;100:143–151. doi: 10.1007/s00421-007-0412-0. [DOI] [PubMed] [Google Scholar]
- 59.Baum O, Silva-Azevedo L, Willerding G, Wockel A, Planitzer G, Gossrau R, Pries AR, Zakrzewicz A. Endothelial NOS is main mediator for shear stress-dependent angiogenesis in skeletal muscle after prazosin administration. Am J Physiol. 2004;287:H2300–H2308. doi: 10.1152/ajpheart.00065.2004. [DOI] [PubMed] [Google Scholar]
- 60.Baumgartner I, Pieczek Manor O, Blair R, Kearney M, Walsh K, Isner JM. Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation. 1998;97:1114–1123. doi: 10.1161/01.cir.97.12.1114. [DOI] [PubMed] [Google Scholar]
- 61.Bauters C, Asahara T, Zheng LP, Takeshita S, Bunting S, Ferrara N, Symes JF, Isner JM. Site-specific therapeutic angiogenesis after systemic administration of vascular endothelial growth factor. J Vasc Surg. 1995;21:314–324. doi: 10.1016/s0741-5214(95)70272-5. [DOI] [PubMed] [Google Scholar]
- 62.Bayless KJ, Davis GE. The Cdc42 and Rac1 GTPases are required for capillary lumen formation in three-dimensional extracellular matrices. J Cell Sci. 2002;115:1123–1136. doi: 10.1242/jcs.115.6.1123. [DOI] [PubMed] [Google Scholar]
- 63.Beavers KM, Brinkley TE, Nicklas BJ. Effect of exercise training on chronic inflammation. Clin Chim Acta. 2010;411:785–793. doi: 10.1016/j.cca.2010.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Becker LC, Pitt B. Collateral blood flow in conscious dogs with chronic coronary artery occlusion. Am J Physiol. 1971;221:1507–1510. doi: 10.1152/ajplegacy.1971.221.5.1507. [DOI] [PubMed] [Google Scholar]
- 65.Behnke BJ, Kindig CA, Musch TI, Koga S, Poole DC. Dynamics of microvascluar oxygen pressuer across the rest-exercise transition in rat skeletal muscle. Respir Physiol. 2001;126:53–63. doi: 10.1016/s0034-5687(01)00195-5. [DOI] [PubMed] [Google Scholar]
- 66.Behnke BJ, McDonough P, Padilla DJ, Musch TI, Poole DC. Oxygen exchange profile in rat muscles of ontrol of microvasc. J Physiol. 2003;549:597–605. doi: 10.1113/jphysiol.2002.035915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Belch JJF, McLaren M, Kahn F, Hickman P, Muir A, Stonebridge P. The inflammatory process in intermittent claudication. Eur Heart J. 2002;4:B31–B34. [Google Scholar]
- 68.Bellamy LM, Johnston AP, De LM, Parise G. Skeletal muscle-endothelial cell cross talk through angiotensin II. Am J Physiol. 2010;299:C1402–C1408. doi: 10.1152/ajpcell.00306.2010. [DOI] [PubMed] [Google Scholar]
- 69.Bendermacher BL, Willigendael EM, Teijink JA, Prins MH. Supervised exercise therapy versus non-supervised exercise therapy for intermittent claudication. Cochrane Database Syst Rev. 2006 Apr 19;:CD005263. doi: 10.1002/14651858.CD005263.pub2. [DOI] [PubMed] [Google Scholar]
- 70.Benedict KF, Coffin GS, Barrett EJ, Skalak TC. Hemodynamic systems analysis of capillary network remodeling during the progression of type 2 diabetes. Microcirculation. 2011;18:63–73. doi: 10.1111/j.1549-8719.2010.00069.x. [DOI] [PubMed] [Google Scholar]
- 71.Benedito R, Roca C, Sörensen I, Adams S, Gossler A, Fruttiger M, Adams RH. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 72.Benoit H, Jordan M, Wagner H, Wagner PD. Effect of NO, vasodilator prostaglandins, and adenosine on skeletal muscle angiogenic growth factor gene expression. J Appl Physiol. 1999;86:1513–1518. doi: 10.1152/jappl.1999.86.5.1513. [DOI] [PubMed] [Google Scholar]
- 73.Benzi CP, Tanaka H, Sugawara J. Effects of leg blood flow restriction during walking on cardiovascular function. Med Sci Sports Exer. 2010;42:726–732. doi: 10.1249/MSS.0b013e3181bdb454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bergmann CE, Hoefer IE, Meder B, Roth H, van RN, Breit SM, Jost MM, Aharinejad S, Hartmann S, Buschmann IR. Arteriogenesis depends on circulating monocytes and macrophage accumulation and is severely depressed in op/op mice. J Leukoc Biol. 2006;80:59–65. doi: 10.1189/jlb.0206087. [DOI] [PubMed] [Google Scholar]
- 76.Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouyssegur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J. 2003;22:4082–4090. doi: 10.1093/emboj/cdg392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Betsholtz C, Lindblom P, Gerhardt H. Role of pericytes in vascular morphogenesis. EXS. 2005:115–125. doi: 10.1007/3-7643-7311-3_8. [DOI] [PubMed] [Google Scholar]
- 78.Bey L, Akunuri N, Zhao P, Hoffman EP, Hamilton DG, Hamilton MT. Patterns of global gene expression in rat skeletal muscle during unloading and low-intensity ambulatory activity. Physiol Genomics. 2003;13:157–167. doi: 10.1152/physiolgenomics.00001.2002. [DOI] [PubMed] [Google Scholar]
- 79.Bigard AX, Brunet A, Guezennec CY, Monod H. Effects of chronic hypoxia and endurance training on muscle capillarity in rats. Pflugers Arch. 1991;419:225–229. doi: 10.1007/BF00371099. [DOI] [PubMed] [Google Scholar]
- 80.Birot OJG, Koulmann N, Peinnequin A, Bigard XA. Exercise-Induced Expression of Vascular Endothelial Growth Factor mRNA in Rat Skeletal Muscle is Dependent on Fibre Type. J Physiol. 2003;552:213–221. doi: 10.1113/jphysiol.2003.043026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bjurstedt H, Eiken O. Graded restriction of blood flow in exercising leg muscles: a human model. Adv Exp Med Biol. 1995;381:147–156. doi: 10.1007/978-1-4615-1895-2_14. [DOI] [PubMed] [Google Scholar]
- 82.Blann AD, Belgore FM, McCollum CN, Silverman S, Lip PL, Lip GY. Vascular endothelial growth factor and its receptor, Flt-1, in the plasma of patients with coronary or peripheral atherosclerosis, or Type II diabetes. Clin Sci (Lond) 2002;102:187–194. [PubMed] [Google Scholar]
- 83.Bloor CM, White FC, Sanders TM. Effects of exercise on collateral development in myocardial ischemia in pigs. J Appl Physiol. 1984;56:656–665. doi: 10.1152/jappl.1984.56.3.656. [DOI] [PubMed] [Google Scholar]
- 84.Boger RH, Bode-Boger SM, Thiele W, Creutzig A, Alexander K, Frolich JC. Restoring vascular nitric oxide formation by L-arginine improves the symptoms of intermittent claudication in patients with peripheral arterial occlusive disease. J Am Coll Cardiol. 1998;32:1336–1344. doi: 10.1016/s0735-1097(98)00375-1. [DOI] [PubMed] [Google Scholar]
- 85.Boodhwani M, Nakai Y, Mieno S, Voisine P, Bianchi C, Araujo EG, Feng J, Michael K, Li J, Sellke FW. Hypercholesterolemia impairs the myocardial angiogenic response in a swine model of chronic ischemia: role of endostatin and oxidative stress. Ann Thorac Surg. 2006;81:634–641. doi: 10.1016/j.athoracsur.2005.07.090. [DOI] [PubMed] [Google Scholar]
- 86.Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic disease. Compr Physiol. 2012;2:000–000. doi: 10.1002/cphy.c110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bornes S, Prado-Lourenco L, Bastide A, Zanibellato C, Iacovoni JS, Lacazette E, Prats AC, Touriol C, Prats H. Translational induction of VEGF internal ribosome entry site elements during the early response to ischemic stress. Circ Res. 2007;100:305–308. doi: 10.1161/01.RES.0000258873.08041.c9. [DOI] [PubMed] [Google Scholar]
- 88.Brandsma JW, Robeer BG, van den Heuvel S, Smit B, Wittens CH, Oostendorp RA. The effect of exercises on walking distance of patients with intermittent claudication: a study of randomized clinical trials. Phys Ther. 1998;78:278–286. doi: 10.1093/ptj/78.3.278. [DOI] [PubMed] [Google Scholar]
- 89.Bratt A, Birot O, Sinha I, Veitonmaki N, Aase K, Ernkvist M, Holmgren L. Angiomotin regulates endothelial cell-cell junctions and cell motility. J Biol Chem. 2005;280:34859–34869. doi: 10.1074/jbc.M503915200. [DOI] [PubMed] [Google Scholar]
- 90.Breek JC, Hamming JF, DeVries J, Aquarius AE, Van Berge Henegouwen DP. Quality of life in patients with intermittent claudication using the World Health Organisation (WHO) questionnaire. Eur J Vasc Endovasc Surg. 2001;21:118–122. doi: 10.1053/ejvs.2001.1305. [DOI] [PubMed] [Google Scholar]
- 91.Breen EC, Johnson EC, Wagner H, Tseng HM, Sung LA, Wagner PD. Angiogenic growth factor mRNA responses in muscle to a single bout of exercise. J Appl Physiol. 1996;81:355–361. doi: 10.1152/jappl.1996.81.1.355. [DOI] [PubMed] [Google Scholar]
- 92.Brendle DC, Joseph LJ, Corretti MC, Gardner AW, Katzel LI. Effects of exercise rehabilitation on endothelial reqctivity in older patients with peripheral arterial disease. Am J Cardiol. 2001;87:324–329. doi: 10.1016/s0002-9149(00)01367-9. [DOI] [PubMed] [Google Scholar]
- 93.Brevetti G, De Caterina M, Martone VD, Ungaro B, Corrado F, Silvestro A, de Cristofaro T, Scopacasa F. Exercise increases soluble adhesion molecules ICAM-1 and VCAM-1 in patients with intermittent claudication. Clin Hemorheo Microcirc. 2001;24:193–199. [PubMed] [Google Scholar]
- 94.Brevetti G, Giugliano G, Brevetti L, Hiatt WR. Inflammation in peripheral artery disease. Circulation. 2010;122:1862–1875. doi: 10.1161/CIRCULATIONAHA.109.918417. [DOI] [PubMed] [Google Scholar]
- 95.Brevetti G, Schiano V, Chiariello M. Endothelial dysfunction: A key to the pathophysiology and natural history of peripheral arterial disease? Atherosclerosis. 2008;197:1–11. doi: 10.1016/j.atherosclerosis.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 96.Brevetti G, Silvestro A, Schiano V, Chiariello M. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease. Additive value of flow-mediated dilation to ankle-brachial pressure index. Circulation. 2003;108:2093–2098. doi: 10.1161/01.CIR.0000095273.92468.D9. [DOI] [PubMed] [Google Scholar]
- 97.Brevetti LS, Chang DS, Tang GL, Sarkar R, Messina LM. Overexpression of endothelial nitric oxide synthase increases skeletal muscle blood flow and oxygenation in severe rat hind limb ischemia. Journal of Vascular Surgery. 2003;38:820–826. doi: 10.1016/s0741-5214(03)00555-x. [DOI] [PubMed] [Google Scholar]
- 98.Brevetti LS, Paek R, Brady SE, Hoffman JI, Sarkar R, Messina LM. Exercise-induced hyperemia unmasks regional blood flow deficit in experimental hindlimb ischemia. J Surg Res. 2001;98:21–26. doi: 10.1006/jsre.2001.6161. [DOI] [PubMed] [Google Scholar]
- 99.Brixius K, Schoenberger S, Ladage D, Knigge H, Falkowski G, Hellmich M, Graf C, Latsch J, Montie GL, Prede GL, Bloch W, Bloch W. Long-term endurance exercise decreases antiangiogenic endostatin signalling in overweight men aged 50–60 years. Br J Sports Med. 2008;42:126–129. doi: 10.1136/bjsm.2007.035188. [DOI] [PubMed] [Google Scholar]
- 100.Brodal P, Ingjer F, Hermansen L. Capillary supply of skeletal muscle fibers in untrained and endurance-trained men. Am J Physiol. 1977;232:H705–H712. doi: 10.1152/ajpheart.1977.232.6.H705. [DOI] [PubMed] [Google Scholar]
- 101.Brooks SV, Vasilaki A, Larkin LM, McArdle A. Repeated bouts of aerobic exercise lead to reductions in skeletal muscle free radical generation and nuclear factor kB activation. J Physiol. 2008;586:3979–3990. doi: 10.1113/jphysiol.2008.155382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brosig M, Ferralli J, Gelman L, Chiquet M, Chiquet-Ehrismann R. Interfering with the connection between the nucleus and the cytoskeleton affects nuclear rotation, mechanotransduction and myogenesis. Int J Biochem Cell Biol. 2010;42:1717–1728. doi: 10.1016/j.biocel.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 103.Brown MD, KELSALL CJ, Milkiewicz M, ANDERSON S, Hudlicka O. A new model of peripheral arterial disease: Sustained impairment of nutritive microcirculation and its recovery by chronic electrical stimulation. Microcirc. 2005;12:373–381. doi: 10.1080/10739680590934817. [DOI] [PubMed] [Google Scholar]
- 104.Brown MD, Kent J, KELSALL CJ, Milkiewicz M, Hudlicka O. Remodeling in the microcirculation of rat skeletal muscle during chronic ischemia. Microcirc. 2003;10:179–191. doi: 10.1038/sj.mn.7800183. [DOI] [PubMed] [Google Scholar]
- 105.Brown MD, Walter H, Hansen-Smith FM, Hudlicka O, Egginton S. Lack of involvement of basic fibroblast growth factor (FGF-2) in capillary growth in skeletal muscle exposed to long-term contractile activity. Angiogenesis. 1998;2:81–91. doi: 10.1023/a:1009058511532. [DOI] [PubMed] [Google Scholar]
- 106.Bruns RR, Palade GE. Studies on blood capillaries I General organization of blood capillaries in muscle. J Cell Biol. 1968;37:244–276. doi: 10.1083/jcb.37.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bryan BA, Walshe TE, Mitchell DC, Havumaki JS, Saint-Geniez M, Maharaj AS, Maldonado AE, D’Amore PA. Coordinated vascular endothelial growth factor expression and signaling during skeletal myogenic differentiation. Mol Biol Cell. 2008;19:994–1006. doi: 10.1091/mbc.E07-09-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bulmer AC, Coombes JS. Optimising exercise training in peripheral arterial disease. Sports Med. 2004;34:983–1003. doi: 10.2165/00007256-200434140-00004. [DOI] [PubMed] [Google Scholar]
- 109.Burri PH, Hlushchuk R, Djonov V. Intussusceptive angiogenesis: its emergence, its characteristics, and its significance. Dev Dyn. 2004;231:474–488. doi: 10.1002/dvdy.20184. [DOI] [PubMed] [Google Scholar]
- 110.Burt JJ, Jackson R. The effects of physical exercise on the coronary collateral circulation of dogs. J Sports Med Phys Fitness. 1965;5:203–206. [PubMed] [Google Scholar]
- 111.Burton HW, Barclay JK. Metabolic factors from exercising muscle and the proliferation of endothelial cells. Med Sci Sports Exerc. 1986;18:390–395. [PubMed] [Google Scholar]
- 112.Buschmann I, Heil M, Jost M, Schaper W. Influence of inflammatory cytokines on arteriogenesis. Microcirculation. 2003;10:371–379. doi: 10.1038/sj.mn.7800199. [DOI] [PubMed] [Google Scholar]
- 113.Buschmann I, Schaper W. Arteriogenesis Versus Angiogenesis: Two Mechanisms of Vessel Growth. News Physiol Sci. 1999;14:121–125. doi: 10.1152/physiologyonline.1999.14.3.121. [DOI] [PubMed] [Google Scholar]
- 114.Buschmann IR, Hoefer IE, van RN, Katzer E, Braun-Dulleaus R, Heil M, Kostin S, Bode C, Schaper W. GM-CSF: a strong arteriogenic factor acting by amplification of monocyte function. Atherosclerosis. 2001;159:343–356. doi: 10.1016/s0021-9150(01)00637-2. [DOI] [PubMed] [Google Scholar]
- 115.Busti C, Falcinelli E, Momi S, Gresele P. Matrix metalloproteinases and peripheral arterial disease. Intern Emerg Med. 2010;5:13–25. doi: 10.1007/s11739-009-0283-y. [DOI] [PubMed] [Google Scholar]
- 116.Bylund AC, Hammarsten J, Holm J, Schersten T. Enzyme activities in skeletal muscles from patients with peripheral arterial insufficiency. Europ J Clin Invest. 1976;6:425–429. doi: 10.1111/j.1365-2362.1976.tb00538.x. [DOI] [PubMed] [Google Scholar]
- 117.Caduff JH, Fischer LC, Burri PH. Scanning electron microscope study of the developing microvasculature in the postnatal rat lung. Anat Rec. 1986;216:154–164. doi: 10.1002/ar.1092160207. [DOI] [PubMed] [Google Scholar]
- 118.Cai W, Schaper W. Mechanisms of arteriogenesis. Acta Biochim Biophys Sin. 2008;40:681–692. [PubMed] [Google Scholar]
- 119.Cai WJ, Kocsis E, Luo X, Schaper W, Schaper J. Expression of endothelial nitric oxide synthase in the vascular wall during arteriogenesis. Mol Cell Biochem. 2004;264:193–200. doi: 10.1023/b:mcbi.0000044388.27953.a0. [DOI] [PubMed] [Google Scholar]
- 120.Cai WJ, Li MB, Wu X, Wu S, Zhu W, Chen D, Luo M, Eitenmuller I, Kampmann A, Schaper J, Schaper W. Activation of the integrins alpha 5beta 1 and alpha v beta 3 and focal adhesion kinase (FAK) during arteriogenesis. Mol Cell Biochem. 2009;322:161–169. doi: 10.1007/s11010-008-9953-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Calvo JA, Daniels TG, Wang X, Paul A, Spiegelman BM, Stevenson SC, Rangwala SM. Muscle-specific expression of PPARgamma coactivator-1alpha improves exercise performance and increases peak oxygen uptake. J Appl Physiol. 2008;104:1304–1312. doi: 10.1152/japplphysiol.01231.2007. [DOI] [PubMed] [Google Scholar]
- 122.Canepari M, Pellegrino MA, D’Antona G, Bottinelli R. Single muscle fiber progerties in aging and disuse. Scand J Med Sci Sports. 2010;20:10–19. doi: 10.1111/j.1600-0838.2009.00965.x. [DOI] [PubMed] [Google Scholar]
- 123.Cannon DT, White AC, Andriano MF, Kolkhorst FW, Rossiter HB. Skeletal muscle fatigue precedes the slow component of oxygen uptake kinetics during exercise in humans. J Physiol. 2011;589:727–739. doi: 10.1113/jphysiol.2010.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cao R, Brakenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E, Leboulch P, Cao Y. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med. 2003;9:604–613. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- 125.Cao Y. Monotherapy versus combination therapy of angiogenic and arteriogenic factors for the treatment of ischemic disorders. Curr Mol Med. 2009;9:967–972. doi: 10.2174/156652409789712747. [DOI] [PubMed] [Google Scholar]
- 126.Caporali A, Meloni M, Vollenkle C, Bonci D, Sala-Newby GB, Addis R, Spinetti G, Losa S, Masson R, Baker AH, Agami R, le SC, Condorelli G, Madeddu P, Martelli F, Emanueli C. Deregulation of microRNA-503 contributes to diabetes mellitus-induced impairment of endothelial function and reparative angiogenesis after limb ischemia. Circulation. 2011;123:282–291. doi: 10.1161/CIRCULATIONAHA.110.952325. [DOI] [PubMed] [Google Scholar]
- 127.Carman TL, Fernandez BBJ. Contemporary management of peripheral arterial disease: II Improving walking distance and quality of life. Cleveland Clinic J Med. 2006;73:S38–S44. doi: 10.3949/ccjm.73.suppl_4.s38. [DOI] [PubMed] [Google Scholar]
- 128.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 129.Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, Scholz D, Acker T, DiPalma T, Dewerchin M, Noel A, Stalmans I, Barra A, Blacher S, Vandendriessche T, Ponten A, Eriksson U, Plate KH, Foidart JM, Schaper W, Charnock-Jones DS, Hicklin DJ, Herbert JM, Collen D, Persico MG. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 130.Carmeliet P, Ng YS, Nuyens D, Theilmeier G, Brusselmans K, Cornelissen I, Ehler E, Kakkar VV, Stalmans I, Mattot V, Perriard JC, Dewerchin M, Flameng W, Nagy A, Lupu F, Moons L, Collen D, D’Amore PA, Shima DT. Impaired myocardial angiogenesis and ischemic cardiomyopathy in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Nat Med. 1999;5:495–502. doi: 10.1038/8379. [DOI] [PubMed] [Google Scholar]
- 131.Carrow RE, Brown RE, Van Huss WD. Fiber sizes and capillary to fiber ratios in skeletal muscle of exercised rats. Anat Rec. 1967;159:33–39. doi: 10.1002/ar.1091590106. [DOI] [PubMed] [Google Scholar]
- 132.Carter SA, Hamel ER, Paterson JM, Snow CJ, Mymin D. Walking ability and ankle systolic pressures: observations in patients with intermittent claudication in a short-term walking exercise program. J Vasc Sur. 1989;10:642–649. [PubMed] [Google Scholar]
- 133.Casillas JM, Gremeaux V, Damak S, Felki A, Perennou FD. Exercise training for patients with cardiovascular disease. Annales de Readaptation et de Medecine Physique. 2007;50:403–418. doi: 10.1016/j.annrmp.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 134.Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, Shah AM. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal. 2006;8:691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- 135.Celik T, Berdan ME, Iyisoy A, Kursaklioglu H, Turhan H, Kilic S, Gulec M, Ozturk S, Isik E. Impaired coronary collateral vessel development in patients with proliferative diabetic retinopathy. Clin Cardiol. 2005;28:384–388. doi: 10.1002/clc.4960280808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Celis R, Pipinos II, Scott-Pandorf MM, Myers SA, Stergiou N, Johanning JM. Peripheral arterial disease affects kinematics during walking. J Vasc Surg. 2009;49:127–132. doi: 10.1016/j.jvs.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 137.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 138.Chae JK, Kim I, Lim ST, Chung MJ, Kim WH, Kim HG, Ko JK, Koh GY. Coadministration of angiopoietin-1 and vascular endothelial growth factor enhances collateral vascularization. Arterioscler Thromb Vasc Biol. 2000;20:2573–2578. doi: 10.1161/01.atv.20.12.2573. [DOI] [PubMed] [Google Scholar]
- 139.Chalothorn D, Clayton JA, Zhang H, Pomp D, Faber JE. Collateral density, remodeling, and VEGF-A expression differ widely between mouse strains. Physiol Genomics. 2007;30:179–191. doi: 10.1152/physiolgenomics.00047.2007. [DOI] [PubMed] [Google Scholar]
- 140.Chalothorn D, Faber JE. Strain-dependent variation in collateral circulatory function in mouse hindlimb. Physiol Genomics. 2010;42:469–479. doi: 10.1152/physiolgenomics.00070.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997;89:1260–1270. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- 142.Chan JY, Wang LL, Wu KL, Chan SH. Reduced functional expression and molecular synthesis of inducible nitric oxide synthase in rostral ventrolateral medulla of spontaneously hypertensive rats. Circulation. 2001;104:1676–1681. doi: 10.1161/hc3901.095767. [DOI] [PubMed] [Google Scholar]
- 143.Chansky M, Levy MN. Collateral circulation to myocardial regions supplied by anterior descending and right coronary arteries in the dog. Circ Res. 1962;11:414–417. doi: 10.1161/01.res.11.3.414. [DOI] [PubMed] [Google Scholar]
- 144.Chen Z, Rubin J, Tzima E. Role of PECAM-1 in Arteriogenesis and Specification of Preexisting Collaterals. Circ Res. 2010;107:1355–1363. doi: 10.1161/CIRCRESAHA.110.229955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cheng B, Essackjee HC, Ballard HJ. Evidence for control of adenosine metabolism in rat oxidative skeletal muscle by changes in pH. J Physiol. 2000;522(Pt 3):467–477. doi: 10.1111/j.1469-7793.2000.t01-1-00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cheng XW, Kuzuya M, Kim W, Song H, Hu L, Inoue A, Nakamura K, Di Q, Sasaki T, Tsuzuki M, Shi GP, Okumura K, Murohara T. Exercise training stimulates ischemia-induced neovascularization via phosphatidylinositol 3-kinase/Akt-dependent hypoxia-induced factor-1 alpha reactivation in mice of advanced age. Circulation. 2010;122:707–716. doi: 10.1161/CIRCULATIONAHA.109.909218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cheng XW, Kuzuya M, Nakamura K, Maeda K, Tsuzuki M, Kim W, Sasaki T, Liu Z, Inoue N, Kondo T, Jin H, Numaguchi Y, Okumura K, Yokota M, Iguchi A, Murohara T. Mechanisms underlying the impairment of ischemia-induced neovascularization in matrix metalloproteinase 2-deficient mice. Circ Res. 2007;100:904–913. doi: 10.1161/01.RES.0000260801.12916.b5. [DOI] [PubMed] [Google Scholar]
- 148.Cherr GS, Wang J, Zimmerman PM, Dosluoglu HH. Depression is associated with worse patency and recurrent leg symptoms after lower extremity revascularization. J Vasc Surg. 2007;45:744–750. doi: 10.1016/j.jvs.2006.11.057. [DOI] [PubMed] [Google Scholar]
- 149.Cherwek DH, Hopkins MB, Thompson MJ, Annex BH, Taylor DA. Fiber type-specific differential expression of angiogenic factors in response to chronic hindlimb ischemia. Am J Physiol. 2000;279:H932–H938. doi: 10.1152/ajpheart.2000.279.3.H932. [DOI] [PubMed] [Google Scholar]
- 150.Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol. 2007;292:H1209–H1224. doi: 10.1152/ajpheart.01047.2006. [DOI] [PubMed] [Google Scholar]
- 151.Chinsomboon J, Raus J, Gupta RK, Thom R, Shoag J, Rowe GC, Sawada N, Raghuram S, Arany Z. The transcriptional coactivator PGC-1 alpha mediates exercise-induced angiogenesis. Proc Natl Acad Sci USA. 2009;106:21401–21406. doi: 10.1073/pnas.0909131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chittenden TW, Sherman JA, Xiong F, Hall AE, Lanahan AA, Taylor JM, Duan H, Pearlman JD, Moore JH, Schwartz SM, Simons M. Transcriptional profiling in coronary artery disease. Indications for novel markers of coronary collateralizatioin. Circulation. 2006;114:1811–1820. doi: 10.1161/CIRCULATIONAHA.106.628396. [DOI] [PubMed] [Google Scholar]
- 153.Chleboun JO, Martins RN, Mitchell CA, Chirila TV. bFGF enhances the development of the collateral circulation after acute arterial occlusion. Biochem Biophys Res Commun. 1992;185:510–516. doi: 10.1016/0006-291x(92)91654-9. [DOI] [PubMed] [Google Scholar]
- 154.Chong PF, Golledge J, Greenhalgh RM, Davies AH. Exercise therapy or angioplasty? A summation analysis. Eur J Vasc Endovasc Surg. 2000;20:4–12. doi: 10.1053/ejvs.2000.1112. [DOI] [PubMed] [Google Scholar]
- 155.Chou E, Suzuma I, Way KJ, Opland D, Clermont AC, Naruse K, Suzuma K, Bowling NL, Vlahos CJ, Aiello LP, King GL. Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic states: a possible explanation for impaired collateral formation in cardiac tissue. Circulation. 2002;105:373–379. doi: 10.1161/hc0302.102143. [DOI] [PubMed] [Google Scholar]
- 156.Christov C, Chretien F, Abou-Khalil R, Bassez G, Vallet G, Authier FJ, Bassaglia Y, Shinin V, Tajbakhsh S, Chazaud B, Gherardi RK. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell. 2007;18:1397–1409. doi: 10.1091/mbc.E06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chung AW, Hsiang YN, Matzke LA, McManus BM, van BC, Okon EB. Reduced expression of vascular endothelial growth factor paralleled with the increased angiostatin expression resulting from the upregulated activities of matrix metalloproteinase-2 and -9 in human type 2 diabetic arterial vasculature. Circ Res. 2006;99:140–148. doi: 10.1161/01.RES.0000232352.90786.fa. [DOI] [PubMed] [Google Scholar]
- 158.Claesson-Welsh L, Welsh M, Ito N, Anand-Apte B, Soker S, Zetter B, O’Reilly M, Folkman J. Angiostatin induces endothelial cell apoptosis and activation of focal adhesion kinase independently of the integrin-binding motif RGD. Proc Natl Acad Sci USA. 1998;95:5579–5583. doi: 10.1073/pnas.95.10.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Clark ER, Clark EL. Microscopic observations on the growth of blood capillaries in the living mammal. Am J Anat. 1939;64:251–301. [Google Scholar]
- 160.Clark IM, Swingler TE, Sampieri CL, Edwards DR. The regulation of matrix metalloproteinases and their inhibitors. Int J Biochem Cell Biol. 2008;40:1362–1378. doi: 10.1016/j.biocel.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 161.Clauss M, Weich H, Breier G, Knies U, Rockl W, Waltenberger J, Risau W. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J Biol Chem. 1996;271:17629–17634. doi: 10.1074/jbc.271.30.17629. [DOI] [PubMed] [Google Scholar]
- 162.Clayton JA, Chalothorn D, Faber JE. Vascular endothelial growth factor-A specifies formation of native collaterals and regulates collateral growth in ischemia. Circ Res. 2008;103:1027–1036. doi: 10.1161/CIRCRESAHA.108.181115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Clyne CA, Mears H, Weller RO, O’Donnell TF. Calf muscle adaptation to peripheral arterial disease. Cardiovasc Res. 1985;19:507–512. doi: 10.1093/cvr/19.8.507. [DOI] [PubMed] [Google Scholar]
- 164.Clyne CAC, Weller RO, Bradley WG, Silber DI, O’Donnell TF, Callow AD. Ultrastructural and capillary adaptation of gastrocnemius muscle to occlusive peripheral vascular disease. Surgery. 1982;92:434–440. [PubMed] [Google Scholar]
- 165.Coats P, Hillier C. Differential responses in human subcutaneous and skeletal muscle vascular beds to critical limb ischaemia. Eur J Vasc Endovasc Surg. 2000;19:387–395. doi: 10.1053/ejvs.1999.1023. [DOI] [PubMed] [Google Scholar]
- 166.Coffman JD. Peripheral collateral blood flow and vascular reactivity in the dog. J Clin Invest. 1966;45:923–931. doi: 10.1172/JCI105407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cohen M, Sherman W, Rentrop KP, Gorlin R. Determinants of collateral filling observed during sudden controlled coronary artery occlusion in human subjects. J Am Coll Cardiol. 1989;13:297–303. doi: 10.1016/0735-1097(89)90502-0. [DOI] [PubMed] [Google Scholar]
- 168.Cohen MV, Yipintsoi T, Malhotra A, Penpargkul S, Scheuer J. Effect of exercise on collateral development in dogs with normal coronary arteries. J Appl Physiol. 1978;45:797–805. doi: 10.1152/jappl.1978.45.5.797. [DOI] [PubMed] [Google Scholar]
- 169.Cohen MV, Yipintsoi T, Scheuer J. Coronary collateral stimulation by exercise in dogs with stenotic coronary arteries. J Appl Physiol. 1982;52:664–671. doi: 10.1152/jappl.1982.52.3.664. [DOI] [PubMed] [Google Scholar]
- 170.Colleran PN, Li Z, Yang HT, Laughlin MH, Terjung RL. Vasoresponsiveness of collateral vessels in the rat hindlimb: influence of training. J Physiol. 2010;588:1293–1307. doi: 10.1113/jphysiol.2009.186247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, Nemery B, Dewerchin M, Van VP, Plate K, Moons L, Collen D, Carmeliet P. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med. 2002;8:702–710. doi: 10.1038/nm721. [DOI] [PubMed] [Google Scholar]
- 172.Conley KE, Jubrias SA, Amara CE, Marcinek DJ. Mitochondrial dysfunction: Impact on exercise performance and cellular aging. Exerc Sport Sci Rev. 2007;35:43–49. doi: 10.1249/JES.0b013e31803e88e9. [DOI] [PubMed] [Google Scholar]
- 173.Conrad MC, Anderson JL, Garrett JB. Chronic collateral growth after femoral artery occlusion in the dog. J Appl Physiol. 1971;31:550–555. doi: 10.1152/jappl.1971.31.4.550. [DOI] [PubMed] [Google Scholar]
- 174.Copp SW, Hiral DM, Ferguson SK, Musch TI, Poole DC. Role of neuronal nitric oxide synthase in modulating microvascular and contractile function in rat skeletal muscle. Microcirc. 2011;18:501–511. doi: 10.1111/j.1549-8719.2011.00111.x. [DOI] [PubMed] [Google Scholar]
- 175.Corada M, Nyqvist D, Orsenigo F, Caprini A, Giampietro C, Taketo MM, Iruela-Arispe ML, Adams RH, Dejana E. The Wnt/beta-catenin pathway modulates vascular remodeling and specification by upregulating Dll4/Notch signaling. Dev Cell. 2010;18:938–949. doi: 10.1016/j.devcel.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Couffinhal T, Silver M, Kearney M, Sullivan A, Witzenbichler B, Magner M, Annex B, Peters K, Isner JM. Impaired collateral vessel development associated with reduced expression of vascular endothelial growth factor in ApoE−/− mice. Circulation. 1999;99:3188–3198. doi: 10.1161/01.cir.99.24.3188. [DOI] [PubMed] [Google Scholar]
- 177.Couffinhal T, Silver M, Zheng LP, Kearney M, Witzenbichler B, Isner JM. Mouse model of angiogenesis. Am J Pathol. 1998;152:1667–1679. [PMC free article] [PubMed] [Google Scholar]
- 178.Creasy TS, McMillan PJ, Fletcher EW, Collin J, Morris PJ. Is percutaneous transluminal angioplasty better than exercies for claudication? Preliminary results from a prospective randomised trial. Eur J Vasc Surg. 1990;4:135–140. doi: 10.1016/s0950-821x(05)80427-x. [DOI] [PubMed] [Google Scholar]
- 179.Cretu A, Roth JM, Caunt M, Akalu A, Policarpio D, Formenti S, Gagne P, Liebes L, Brooks PC. Disruption of endothelial cell interactions with the novel HU177 cryptic collagen epitope inhibits angiogenesis. Clin Cancer Res. 2007;13:3068–3078. doi: 10.1158/1078-0432.CCR-06-2342. [DOI] [PubMed] [Google Scholar]
- 180.Criqui MH. Peripheral arterial disease - epidemiological aspects. Vasc Med. 2001;6:3–7. doi: 10.1177/1358836X0100600i102. [DOI] [PubMed] [Google Scholar]
- 181.Criqui MH, Fronek A, Barrett-Connor E, Klauber MR, Gabriel S, Goodman D. The prevalence of pheripheral arterial disease in a defined population. Circulation. 1985;71:510–515. doi: 10.1161/01.cir.71.3.510. [DOI] [PubMed] [Google Scholar]
- 182.Criqui MH, Langer RD, Fronek A, Feigelson HS, Kaluber MR, McCann TJ, Browner D. Mortality over a periof of 10 years in pateints with peripheral arterial disease. N Engl J Med. 1992;326:381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 183.Crowther RG, Spinks WL, Leicht AS, Sangla K, Quigley F, Golledge J. Effects of a long-term exercise program on lower limb mobility, physiological responses, walking performance, and physical activity levels in patients with peripheral arterial disease. J Vasc Surg. 2008;47:303–309. doi: 10.1016/j.jvs.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 184.Dahllof AG, Holm J, Schersten T, Sivertsson R. Peripheral arterial insufficiency, effect of physical training on walking tolerance, calf blood flow, and blood flow resistance. Scand J Rehab Med. 1976;8:19–26. [PubMed] [Google Scholar]
- 185.Dai X, Faber JE. Endothelial nitric oxide synthase deficiency causes collateral vessel rarefaction and impairs activation of a cell cycle gene network during arteriogenesis. Circ Res. 2010;106:1870–1881. doi: 10.1161/CIRCRESAHA.109.212746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Dapp C, Schmutz S, Hoppeler H, Fluck M. Transcriptional reprogramming and ultrastructure during atrophy and recovery of mouse soleus muscle. Physiol Genomics. 2004;20:97–107. doi: 10.1152/physiolgenomics.00100.2004. [DOI] [PubMed] [Google Scholar]
- 187.Darland DC, Massingham LJ, Smith SR, Piek E, Saint-Geniez M, D’Amore PA. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev Biol. 2003;264:275–288. doi: 10.1016/j.ydbio.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 188.Dashwood M, Tsui J. Further evidence for a role of endothelin-1 (ET-1) in critical limb ischaemia. J Cell Comm Signaling. 2011;5:45–49. doi: 10.1007/s12079-010-0109-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Davis GE, Bayless KJ, Mavila A. Molecular basis of endothelial cell morphogenesis in three-dimensional extracellular matrices. Anat Rec. 2002;268:252–275. doi: 10.1002/ar.10159. [DOI] [PubMed] [Google Scholar]
- 191.Dawson JM, Hudlicka O. The effects of long term administration of prazosin on the microcirculation in skeletal muscles. Cardiovasc Res. 1989;23:913–920. doi: 10.1093/cvr/23.11.913. [DOI] [PubMed] [Google Scholar]
- 192.Dawson DL, Cutler BS, Meissner MH, Strandness DE., Jr Cilostazol has beneficial effects in treatment of intermittent claudication: results from a multicenter, randomized, prospective, double-blind trial. Circulation. 1998;98:678–686. doi: 10.1161/01.cir.98.7.678. [DOI] [PubMed] [Google Scholar]
- 193.de Groot D, Pasterkamp G, Hoefer IE. Cardiovascular risk factors and collateral artery formation. Eur J Clin Invest. 2009;39:1036–1047. doi: 10.1111/j.1365-2362.2009.02205.x. [DOI] [PubMed] [Google Scholar]
- 194.De FS, Gigante B, Persico MG. Structure and function of placental growth factor. Trends Cardiovasc Med. 2002;12:241–246. doi: 10.1016/s1050-1738(02)00168-8. [DOI] [PubMed] [Google Scholar]
- 195.De MED, Simons M. Re-evaluating therapeutic neovascularization. J Mol Cell Cardiol. 2004;36:25–32. doi: 10.1016/j.yjmcc.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 196.De VS, Palmer-Kazen U, Kalin B, Wahlberg E. Risk factors for poor collateral development in claudication. Vasc Endovascular Surg. 2005;39:519–524. doi: 10.1177/153857440503900609. [DOI] [PubMed] [Google Scholar]
- 197.De VC, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255:989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]
- 198.Deindl E, Buschmann I, Hoefer IE, Podzuweit T, Boengler K, Vogel S, van RN, Fernandez B, Schaper W. Role of ischemia and of hypoxia-inducible genes in arteriogenesis after femoral artery occlusion in the rabbit. Circ Res. 2001;89:779–786. doi: 10.1161/hh2101.098613. [DOI] [PubMed] [Google Scholar]
- 199.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008;121:2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- 200.Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell. 2009;16:209–221. doi: 10.1016/j.devcel.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 201.Delaney EP, Greaney JL, Edwards DG, Rose WC, Fadel PH, Farquhar WB. Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: role of the muscle metabolreflex. Am J Physiol. 2010;299:H1318–H1327. doi: 10.1152/ajpheart.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Delis KT, Lennox AF, Nicolaides AN, Wolfe JH. Sympathetic autoregulation is peripheral vascular disease. Br J Surg. 2001;88:523–528. doi: 10.1046/j.1365-2168.2001.01735.x. [DOI] [PubMed] [Google Scholar]
- 203.Demicheva E, Hecker M, Korff T. Stretch-induced activation of the transcription factor activator protein-1 controls monocyte chemoattractant protein-1 expression during arteriogenesis. Circ Res. 2008;103:477–484. doi: 10.1161/CIRCRESAHA.108.177782. [DOI] [PubMed] [Google Scholar]
- 204.Denis C, Chatard JC, Dormois D, Linossier MT, Geyssant A, Lacour JR. Effects of endurance training on capillary supply of human skeletal muscle on two age groups (20 and 60 years) J Physiol (Paris) 1986;81:379–383. [PubMed] [Google Scholar]
- 205.Deschenes MR, Ogilvie RW. Exercise stimulates neovascularization in occluded muscle without affecting bFGF content. Med Sci Sports Exerc. 1999;31:1599–1604. doi: 10.1097/00005768-199911000-00016. [DOI] [PubMed] [Google Scholar]
- 206.Deshpande N, Ferrucci L, Metter J, Faulkner KA, Strotmeyer E, Satterfield S, Schwartz A, Simonsick E. Association of lower limb cutaneous sensitivity with gain speed in the elderly. Am J Phys Med Rehabil. 2008;87:921–928. doi: 10.1097/PHM.0b013e31818a5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Deussen A, Bading B, Kelm M, Schrader J. Formation and salvage of adenosine by macrovascular endothelial cells. Am J Physiol. 1993;264:H692–H700. doi: 10.1152/ajpheart.1993.264.3.H692. [DOI] [PubMed] [Google Scholar]
- 208.Deussen A, Moser G, Schrader J. Contribution of coronary endothelial cells to cardiac adenosine production. Pflugers Arch. 1986;406:608–614. doi: 10.1007/BF00584028. [DOI] [PubMed] [Google Scholar]
- 209.Deveci D, Marshall JM, Egginton S. Chronic hypoxia induces prolonged angiogenesis in skeletal muscles of rat. Exp Physiol. 2002;87:287–291. doi: 10.1113/eph8702377. [DOI] [PubMed] [Google Scholar]
- 210.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 211.Dinn RF, Yang HT, Terjung RL. The influence of pentoxifylline and torbafylline on muscle blood flow in animals with peripheral arterial insufficiency. J Clin Pharmacol. 1990;30:704–710. doi: 10.1002/j.1552-4604.1990.tb03630.x. [DOI] [PubMed] [Google Scholar]
- 212.Dokun AO, Keum S, Hazarika S, Li Y, Lamonte GM, Wheeler F, Marchuk DA, Annex BH. A quantitative trait locus (LSq-1) on mouse chromosome 7 is linked to the absence of tissue loss after surgical hindlimb ischemia. Circulation. 2008;117:1207–1215. doi: 10.1161/CIRCULATIONAHA.107.736447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Dormandy JA, Rutherford RB. Management of periperal arterial disease (PAD): TASC Working Group. J Vasc Surg. 2000;31:S1–S34. [PubMed] [Google Scholar]
- 214.Doyle JL, Haas TL. Differential role of beta-catenin in VEGF and histamine-induced MMP-2 production in microvascular endothelial cells. J Cell Biochem. 2009;107:272–283. doi: 10.1002/jcb.22123. [DOI] [PubMed] [Google Scholar]
- 215.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 216.Duan J, Murohara T, Ikeda H, Katoh A, Shintani S, Sasaki K, Kawata H, Yamamoto N, Imaizumi T. Hypercholesterolemia inhibits angiogenesis in response to hindlimb ischemia: nitric oxide-dependent mechanism. Circulation. 2000;102:370–376. doi: 10.1161/01.cir.102.suppl_3.iii-370. [DOI] [PubMed] [Google Scholar]
- 217.Dudley GA, Abraham WM, Terjung RL. Influence of exercise intensity and duration on biochemical adaptations in skeletal muscle. J Appl Physiol. 1982;53:844–850. doi: 10.1152/jappl.1982.53.4.844. [DOI] [PubMed] [Google Scholar]
- 218.Dudley GA, Tullson PC, Terjung RL. Influence of mitochondrial content on the sensitivity of respiratory control. J Biol Chem. 1987;262:9109–9114. [PubMed] [Google Scholar]
- 219.Duh E, Aiello LP. Vascular endothelial growth factor and diabetes: the agonist versus antagonist paradox. Diabetes. 1999;48:1899–1906. doi: 10.2337/diabetes.48.10.1899. [DOI] [PubMed] [Google Scholar]
- 220.Duscha BD, Annex BH, Johnson JL, Huffman K, Houmard J, Kraus WE. Exercise dose response in muscle. Int J Sports Med. 2012;33:218–223. doi: 10.1055/s-0031-1291323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Duscha BD, Robbins JL, Jones WS, Kraus WE, Lye RJ, Sanders JM, Allen JD, Regensteiner JG, Hiatt WR, Annex BH. Angiogenesis in skeletal muscle precede improvements in peak oxygen uptake in peripheral artery disease patients. Arterioscler Thromb Vasc Biol. 2011;31:2742–2748. doi: 10.1161/ATVBAHA.111.230441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Dusseau JW, Hutchins PM. Hypoxia-induced angiogenesis in chick chorioallantoic membranes: a role for adenosine. Respir Physiol. 1988;71:33–44. doi: 10.1016/0034-5687(88)90113-2. [DOI] [PubMed] [Google Scholar]
- 223.Ebrahimian TG, Heymes C, You D, Blanc-Brude O, Mees B, Waeckel L, Duriez M, Vilar J, Brandes RP, Levy BI, Shah AM, Silvestre JS. NADPH oxidase-derived overproduction of reactive oxygen species impairs postischemic neovascularization in mice with type 1 diabetes. Am J Pathol. 2006;169:719–728. doi: 10.2353/ajpath.2006.060042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Eckstein RW. Effect of exercise and coronary artery narrowing on coronary collateral circulation. Circ Res. 1957;5:230–235. doi: 10.1161/01.res.5.3.230. [DOI] [PubMed] [Google Scholar]
- 225.Edwards AT, Biann AD, Suarez-Mendez VJ, Lardi AM, McCollum CN. Systemic responses in patients with intermittent claudication after treadmill exercise. Br J Surg. 1994;81:1738–1741. doi: 10.1002/bjs.1800811211. [DOI] [PubMed] [Google Scholar]
- 226.Egginton S, Hudlicka O, Brown MD, Walter H, Weiss JB, Bate A. Capillary growth in relation to blood flow and performance in overloaded rat skeletal muscle. J Appl Physiol. 1998;85:2025–2032. doi: 10.1152/jappl.1998.85.6.2025. [DOI] [PubMed] [Google Scholar]
- 227.Egginton S, Zhou AL, Brown MD, Hudlicka O. The role of pericytes in controlling angiogenesis in vivo. Adv Exp Med Biol. 2000;476:81–99. doi: 10.1007/978-1-4615-4221-6_7. [DOI] [PubMed] [Google Scholar]
- 228.Egginton S, Zhou AL, Brown MD, Hudlicka O. Unorthodox angiogenesis in skeletal muscle. Cardiovasc Res. 2001;49:634–646. doi: 10.1016/s0008-6363(00)00282-0. [DOI] [PubMed] [Google Scholar]
- 229.Egginton S, Gaffney E. Tissue capillary supply--it’s quality not quantity that counts! Exp Physiol. 2010;95:971–979. doi: 10.1113/expphysiol.2010.053421. [DOI] [PubMed] [Google Scholar]
- 230.Egginton S, Gerritsen M. Lumen formation - In vivo versus in vitro observations. Microcirc. 2003;10:45–61. doi: 10.1038/sj.mn.7800174. [DOI] [PubMed] [Google Scholar]
- 231.Egginton S, Hudlicka O. Early changes in performance, blood flow and capillary fine structure in rat fast muscles induced by electrical stimulation. J Physiol. 1999;515:265–275. doi: 10.1111/j.1469-7793.1999.265ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Egginton S, Hudlicka O, Brown MD, Graciotti L, Granata AL. In vivo pericyte-endothelial cell interaction during angiogenesis in adult cardiac and skeletal muscle. Microvasc Res. 1996;51:213–228. doi: 10.1006/mvre.1996.0022. [DOI] [PubMed] [Google Scholar]
- 233.Eitenmuller I, Volger O, Kluge A, Troidl K, Barancik M, Cai WJ, Heil M, Pipp F, Fischer S, Horrevoets AJ, Schmitz-Rixen T, Schaper W. The range of adaptation by collateral vessels after femoral artery occlusion. Circ Res. 2006;99:656–662. doi: 10.1161/01.RES.0000242560.77512.dd. [DOI] [PubMed] [Google Scholar]
- 234.Ekroth R, Dahllof AG, Gundevall B, Holm J, Schersten T. Physical training of patients with intermittent claudication: indications, methods, and results. Surgery. 1978;84:640–643. [PubMed] [Google Scholar]
- 235.Elander A, Idstrom JP, Schersten T, Bylund-Fellenius AC. Metabolic adaptation to reduced muscle blood flow I Enzyme and metabolite alterations. Am J Physiol. 1985;249:E63–E69. doi: 10.1152/ajpendo.1985.249.1.E63. [DOI] [PubMed] [Google Scholar]
- 236.Elias AP, Dias S. Microenvironment changes (in pH) affect VEGF alternative splicing. Cancer Microenviron. 2008;1:131–139. doi: 10.1007/s12307-008-0013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ellis CG, Mathieucostello O, Potter RF, Macdonald IC, Groom AC. Effect of sarcomere-length on Total capillary length in skeletal-muscle - in vivo evidence for longitudinal stretching of capillaries. Microvasc Res. 1990;40:63–72. doi: 10.1016/0026-2862(90)90008-f. [DOI] [PubMed] [Google Scholar]
- 238.Ellsworth ML, Ellis CG, Goldman D, Stephenson AH, Dietrich HH, Sprague RS. Erythrocytes: oxygen sensors and modulators of vascular tone. Physiol (Bethesda) 2009;24:107–116. doi: 10.1152/physiol.00038.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Eltzschig HK, Abdulla P, Hoffman E, Hamilton KE, Daniels D, Schonfeld C, Loffler M, Reyes G, Duszenko M, Karhausen J, Robinson A, Westerman KA, Coe IR, Colgan SP. HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J Exp Med. 2005;202:1493–1505. doi: 10.1084/jem.20050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Eltzschig HK, Faigle M, Knapp S, Karhausen J, Ibla J, Rosenberger P, Odegard KC, Laussen PC, Thompson LF, Colgan SP. Endothelial catabolism of extracellular adenosine during hypoxia: the role of surface adenosine deaminase and CD26. Blood. 2006;108:1602–1610. doi: 10.1182/blood-2006-02-001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Elvert G, Kappel A, Heidenreich R, Englmeier U, Lanz S, Acker T, Rauter M, Plate K, Sieweke M, Breier G, Flamme I. Cooperative interaction of hypoxia-inducible factor-2alpha (HIF-2alpha) and Ets-1 in the transcriptional activation of vascular endothelial growth factor receptor-2 (Flk-1) J Biol Chem. 2003;278:7520–7530. doi: 10.1074/jbc.M211298200. [DOI] [PubMed] [Google Scholar]
- 242.Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci USA. 1997;94:4273–4278. doi: 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Emanueli C, Caporali A, Krankel N, Cristofaro B, Van LS, Madeddu P. Type-2 diabetic Lepr(db/db) mice show a defective microvascular phenotype under basal conditions and an impaired response to angiogenesis gene therapy in the setting of limb ischemia. Front Biosci. 2007;12:2003–2012. doi: 10.2741/2205. [DOI] [PubMed] [Google Scholar]
- 244.Engerman RL, Pfaffenbach D, Davis MD. Cell turnover of capillaries. Lab Invest. 1967;17:738–743. [PubMed] [Google Scholar]
- 245.Erney TP, Mathien GM, Terjung RL. Muscle adaptations in trained rats with peripheral arterial insufficiency. Am J Physiol. 1991;260:H445–H452. doi: 10.1152/ajpheart.1991.260.2.H445. [DOI] [PubMed] [Google Scholar]
- 246.Ernkvist M, Birot O, Sinha I, Veitonmaki N, Nystrom S, Aase K, Holmgren L. Differential roles of p80- and p130-angiomotin in the switch between migration and stabilization of endothelial cells. Biochim Biophys Acta. 2008;1783:429–437. doi: 10.1016/j.bbamcr.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 247.Ernkvist M, Luna PN, Audebert S, Lecine P, Sinha I, Liu M, Schlueter M, Horowitz A, Aase K, Weide T, Borg JP, Majumdar A, Holmgren L. The Amot/Patj/Syx signaling complex spatially controls RhoA GTPase activity in migrating endothelial cells. Blood. 2009;113:244–253. doi: 10.1182/blood-2008-04-153874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Essen B, Jansson E, Henriksson J, Taylor AW, Saltin B. Metabolic characteristics of fibre types in human skeletal muscle. Acta Physiol Scand. 1975;95:153–165. doi: 10.1111/j.1748-1716.1975.tb10038.x. [DOI] [PubMed] [Google Scholar]
- 249.Esterhammer R, Schocke MF, Gorny O, Posch L, Messner H, Jaschke W, Fraedrich G, Greiner A. Phosphocreatine kinetics in the calf muscle of patients with bilateral symptomatic peripheral arterial disease during exhaustive incremental exercise. Mol Imaging Biol. 2008;10:30–39. doi: 10.1007/s11307-007-0118-z. [DOI] [PubMed] [Google Scholar]
- 250.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 251.Evans MJ, Scarpulla RC. Interaction of nuclear factors with multiple sites in the somatic cytochrome c promoter Characterization of upstream NRF-1, ATF, and intron Sp1 recognition sequences. J Biol Chem. 1989;264:14361–14368. [PubMed] [Google Scholar]
- 252.Fabre JE, Rivard A, Magner M, Silver M, Isner JM. Tissue inhibition of angiotensin-converting enzyme activity stimulates angiogenesis in vivo. Circulation. 1999;99:3043–3049. doi: 10.1161/01.cir.99.23.3043. [DOI] [PubMed] [Google Scholar]
- 253.Favier J, Germain S, Emmerich J, Corvol P, Gasc JM. Critical overexpression of thrombospondin 1 in chronic leg ischaemia. J Pathol. 2005;207:358–366. doi: 10.1002/path.1833. [DOI] [PubMed] [Google Scholar]
- 254.Felbor U, Dreier L, Bryant RA, Ploegh HL, Olsen BR, Mothes W. Secreted cathepsin L generates endostatin from collagen XVIII. EMBO J. 2000;19:1187–1194. doi: 10.1093/emboj/19.6.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Feoktistov I, Ryzhov S, Zhong H, Goldstein AE, Matafonov A, Zeng D, Biaggioni I. Hypoxia modulates adenosine receptors in human endothelial and smooth muscle cells toward an A2B angiogenic phenotype. Hypertension. 2004;44:649–654. doi: 10.1161/01.HYP.0000144800.21037.a5. [DOI] [PubMed] [Google Scholar]
- 256.Ferguson AV, Latchford KJ, Samson WK. The paraventricular nucleus of the hypothalamus - a potential target for integrative treatment of autonomic dysfuction. Expert Opin Ther Targets. 2008;12:7117–7727. doi: 10.1517/14728222.12.6.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Feringa HH, Bax JJ, van Waning VH, Boersma E, Elhendy A, Schouten O, Tangelder MJ, van Sambeek MH, van den Meiracker AH, Poldermans D. The long-term prognostic value of the resting and postexercise ankle-brachial index. Arch Internal Med. 2006;166:529–535. doi: 10.1001/archinte.166.5.529. [DOI] [PubMed] [Google Scholar]
- 258.Findley CM, Mitchell RG, Duscha BD, Annex BH, Kontos CD. Plasma levels of soluble Tie2 and vascular endothelial growth factor distinguish critical limb ischemia from intermittent claudication in patients with peripheral arterial disease. J Am Coll Cardiol. 2008;52:387–393. doi: 10.1016/j.jacc.2008.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Fischer S, Knoll R, Renz D, Karliczek GF, Schaper W. Role of adenosine in the hypoxic induction of vascular endothelial growth factor in porcine brain derived microvascular endothelial cells. Endothelium. 1997;5:155–165. doi: 10.3109/10623329709053395. [DOI] [PubMed] [Google Scholar]
- 260.Fisher JP, Young CN, Fadel PJ. Central sympathetic overactivity: Maladies and mechanisms. Autonomic Neurosci-Basic & Clin. 2009;148:8–15. doi: 10.1016/j.autneu.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Fisher KE, Sacharidou A, Stratman AN, Mayo AM, Fisher SB, Mahan RD, Davis MJ, Davis GE. MT1-MMP- and Cdc42-dependent signaling co-regulate cell invasion and tunnel formation in 3D collagen matrices. J Cell Sci. 2009;122:4558–4569. doi: 10.1242/jcs.050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Flamme I, Frohlich T, von RM, Kappel A, Damert A, Risau W. HRF, a putative basic helix-loop-helix-PAS-domain transcription factor is closely related to hypoxia-inducible factor-1 alpha and developmentally expressed in blood vessels. Mech Dev. 1997;63:51–60. doi: 10.1016/s0925-4773(97)00674-6. [DOI] [PubMed] [Google Scholar]
- 263.Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol. 2003;284:R1–R12. doi: 10.1152/ajpregu.00323.2002. [DOI] [PubMed] [Google Scholar]
- 264.Flu HC, Tamsma JT, Lindeman JH, Hamming JF, Lardenoye JH. A systematic review of implementation of established recommended secondary prevention measures in patients with PAOD. Eur J Vasc Endovasc Surg. 2010;39:7–86. doi: 10.1016/j.ejvs.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 265.Folkman J. Angiogenesis and apoptosis. Sem Cancer Biol. 2003;13:159–167. doi: 10.1016/s1044-579x(02)00133-5. [DOI] [PubMed] [Google Scholar]
- 266.Fowkes FGR, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, Wautrecht JC, Kornitzer M, Newman AB, Cushman M, Sutton-Tyrrell K, Lee AJ, Price JF, d’Agostino RB, Murabito J, Norman PE, Jamrozik K, Curb JD, Masaki KH, Rodriguez BL, Dekker JM, Bouter LM, Heine RJ, Nijpels G, Stehauwer CDA, Ferucci L, McDermott MM, Stoffers HE, Hooi JD, Knottnerus JA, Ogren M, Hedblad B, Witteman JC, Breteler MMB, Hunink MGM, Hofman A, Criqui MH, Langer RD, Fronek A, Hiatt WR, Hmman R, Resnick HE, Guralnik J. Ankle brachial index combined with Framingham risk score to predict cardiovascular events and mortality. A meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Frans FA, Bipat S, Reekers JA, Legemate DA, Koelemay MJW. Systematic review of exercises training or percutaneous transluminal angioplasty for intermittent claudication. Brit J Surg. 2012;99:16–28. doi: 10.1002/bjs.7656. [DOI] [PubMed] [Google Scholar]
- 268.Frey RS, Ushio-Fukai M, Malik AB. NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid Redox Signal. 2009;11:791–810. doi: 10.1089/ars.2008.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Frisbee C. Obesity, insulin resistance, and microvessel density. Microcirc. 2007;14:289–298. doi: 10.1080/10739680701282945. [DOI] [PubMed] [Google Scholar]
- 270.Fujii T, Yonemitsu Y, Onimaru M, Tanii M, Nakano T, Egashira K, Takehara T, Inoue M, Hasegawa M, Kuwano H, Sueishi K. Nonendothelial mesenchymal cell-derived MCP-1 is required for FGF-2-mediated therapeutic neovascularization: critical role of the inflammatory/arteriogenic pathway. Arterioscler Thromb Vasc Biol. 2006;26:2483–2489. doi: 10.1161/01.ATV.0000244684.23499.bf. [DOI] [PubMed] [Google Scholar]
- 271.Fujino H, Kohzuki H, Takeda I, Kiyooka T, Miyasaka T, Mohri S, Shimizu J, Kajiya F. Regression of capillary network in atrophied soleus muscle induced by hindlimb unweighting. J Appl Physiol. 2005;98:1407–1413. doi: 10.1152/japplphysiol.00961.2004. [DOI] [PubMed] [Google Scholar]
- 272.Fujita M, Nakae I, Kihara Y, Hasegawa K, Nohara R, Ueda K, Tamaki S, Otsuka K, Sasayama S. Determinants of collateral development in patients with acute myocardial infarction. Clin Cardiol. 1999;22:595–599. doi: 10.1002/clc.4960220911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Fukino K, Sata M, Seko Y, Hirata Y, Nagai R. Genetic background influences therapeutic effectiveness of VEGF. Biochem Biophys Res Commun. 2003;310:143–147. doi: 10.1016/j.bbrc.2003.08.134. [DOI] [PubMed] [Google Scholar]
- 274.Fukumura D, Duda DG, Munn LL, Jain RK. Tumor microvasculature and microenvironment: novel insights through intravital imaging in pre-clinical models. Microcirc. 2010;17:206–225. doi: 10.1111/j.1549-8719.2010.00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Gagne PJ, Tihonov N, Li X, Glaser J, Qiao J, Silberstein M, Yee H, Gagne E, Brooks P. Temporal exposure of cryptic collagen epitopes within ischemic muscle during hindlimb reperfusion. Am J Pathol. 2005;167:1349–1359. doi: 10.1016/S0002-9440(10)61222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Galea MN, Bray SR. Determinants of walking exercise among individuals with intermittent claudication: does pain play a role? J Cardiopul Rehab Prevent. 2007;27:107–113. doi: 10.1097/01.HCR.0000265045.36725.97. [DOI] [PubMed] [Google Scholar]
- 278.Galea MN, Bray SR, Ginis KA. Barriers and facilitators for walking in individuals with intermittent claudication. J Aging Physical Activity. 2008;16:69–83. doi: 10.1123/japa.16.1.69. [DOI] [PubMed] [Google Scholar]
- 279.Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Superoxide mediates sympathoexcitation in heart failure: roles of angiotensin II and NAD(P)H oxidase. Circ Res. 2004;95:937–944. doi: 10.1161/01.RES.0000146676.04359.64. [DOI] [PubMed] [Google Scholar]
- 280.Gao L, Wang W, Liu D, Zucker IH. Exercise training normalizes sympathetic outflow by central antioxidant mechanisms in rabbits with pacing-induced chronic heart failure. Circulation. 2007;115:3095–3102. doi: 10.1161/CIRCULATIONAHA.106.677989. [DOI] [PubMed] [Google Scholar]
- 281.Gardner AW, Clancy RJ. The relationship between ankle-brachial index and leisure-time physical activity in patients with intermittent claudication. Angiology. 2006;57:539–545. doi: 10.1177/0003319706293114. [DOI] [PubMed] [Google Scholar]
- 282.Gardner AW, Katzel LI, Sorkin JD, Bradham DD, Hochberg MC, Flinn WR, Goldberg AP. Exercise rehabilitation improves functional outcomes and peripheral circulation in patients with intermittent claudication: a randomized controlled trial. J Am Geriatrics Soc. 2001;49:755–762. doi: 10.1046/j.1532-5415.2001.49152.x. [DOI] [PubMed] [Google Scholar]
- 283.Gardner AW, Katzel LI, Sorkin JD, Goldberg AP. Effects of long-term exercise rehabilitation on claudication distances in patients with peripheral arterial disease: a randomized controlled trial.[see comment] J Cardiopul Rehab. 2002;22:192–198. doi: 10.1097/00008483-200205000-00011. [DOI] [PubMed] [Google Scholar]
- 284.Gardner AW, Katzel LI, Sorkin JD, Lillewich LA, Ryan A, Flinn WR, Goldberg AP. Improved functional outcomes following exercise rehabilitation in patients with intermittent claudication. J Gerontol A Biol Sci Med Sci. 2000;55:M70–M577. doi: 10.1093/gerona/55.10.m570. [DOI] [PubMed] [Google Scholar]
- 285.Gardner AW, Montgomery PS. The effect of metabolic syndrome components on exercise performance in patients with intermittent claudication. J Vasc Surg. 2008;47:1251–1258. doi: 10.1016/j.jvs.2008.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Gardner AW, Montgomery PS, Parker DE. Physical activity is a predictor of all-cause mortality in patients with intermittent claudication. J Vasc Surg. 2008;47:117–122. doi: 10.1016/j.jvs.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Gardner AW, Montgomery PS, Ritti-Dias RM, Forrester L. The effect of claudication pain on temporal and spatial gait measures during self-paced ambulation. Vasc Med. 2010;15:21–26. doi: 10.1177/1358863X09106836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Gardner AW, Montgomery PS, Scott KJ, Afaq A, Blevins SM. Patterns of ambulatory activity in subjects with and without intermittent claudication. J Vasc Surg. 2007;46:1208–1214. doi: 10.1016/j.jvs.2007.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Gardner AW, Parker DE, Webb N, Montgomery PS, Scott KJ, Blevins SM. Calf muscle hemoglobin oxygen saturation characteristics and exercise performance in patients with intermittent claudication. J Vasc Surg. 2008;48:644–649. doi: 10.1016/j.jvs.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain A meta-analysis. JAMA. 1995;274:975–980. [PubMed] [Google Scholar]
- 291.Gardner AW, Skinner JS, Cantwell BW, Smith LK. Progressive vs single-stage treadmill tests for evaluation of claudication. Med Sci Sports Exer. 1991;23:402–408. [PubMed] [Google Scholar]
- 292.Gavin TP, Drew JL, Kubik CJ, Pofahl WE, Hickner RC. Acute resistance exercise increases skeletal muscle angiogenic growth factor expression. Acta Physiol. 2007;191:139–146. doi: 10.1111/j.1748-1716.2007.01723.x. [DOI] [PubMed] [Google Scholar]
- 293.Gavin TP, Robinson CB, Yeager RC, England JA, Nifong LW, Hickner RC. Angiogenic growth factor response to acute systemic exercise in human skeletal muscle. J Appl Physiol. 2004;96:19–24. doi: 10.1152/japplphysiol.00748.2003. [DOI] [PubMed] [Google Scholar]
- 294.Gavin TP, Spector DA, Wagner H, Breen EC, Wagner PD. Nitric oxide synthase inhibition attenuates the skeletal muscle VEGF mRNA response to exercise. J Appl Physiol. 2000;88:1192–1198. doi: 10.1152/jappl.2000.88.4.1192. [DOI] [PubMed] [Google Scholar]
- 295.Gavin TP, Ruster RS, Carrithers JA, Zwetsloot KA, Kraus RM, Evans CA, Knapp DJ, Drew JL, McCartney JS, Garry JP, Hickner RC. No difference in the skeletal muscle angiogenic response to aerobic exercise training between young and aged men. J Physiol. 2007;585:231–239. doi: 10.1113/jphysiol.2007.143198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Gavin TP, Wagner PD. Effect of short-term exercise training on angiogenic growth factor gene responses in rats. J Appl Physiol. 2001;90:1219–1226. doi: 10.1152/jappl.2001.90.4.1219. [DOI] [PubMed] [Google Scholar]
- 297.Gee E, Milkiewicz M, Haas TL. p38 MAPK Activity is Stimulated by Vascular endothelial growth factor receptor 2 activation and is essential for shear stress-induced angiogenesis. J Cell Physiol. 2010;222:120–126. doi: 10.1002/jcp.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Gelin J, Jivegard L, Taft C, Karlsson J, Sullivan M, Dahllof AG, Sandstrom R, Arfvidsson B, Lundholm K. Treatment efficacy of intermittent claudication by surgical intervention, supervised physical exercise training compared to no treatment in unselected randomised patients I: one year results of functional and physiological improvements. Eur J Vasc Endovasc Surg. 2001;22:107–113. doi: 10.1053/ejvs.2001.1413. [DOI] [PubMed] [Google Scholar]
- 299.Geng T, Li P, Okutsu M, Yin X, Kwek J, Zhang M, Yan Z. PGC-1alpha plays a functional role in exercise-induced mitochondrial biogenesis and angiogenesis but not fiber-type transformation in mouse skeletal muscle. Am J Physiol. 2010;298:C572–C579. doi: 10.1152/ajpcell.00481.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Gerhardt H, Ruhrberg C, Abramsson A, Fujisawa H, Shima D, Betsholtz C. Neuropilin-1 is required for endothelial tip cell guidance in the developing central nervous system. Dev Dyn. 2004;231:503–509. doi: 10.1002/dvdy.20148. [DOI] [PubMed] [Google Scholar]
- 302.Gigante B, Morlino G, Gentile MT, Persico MG, De FS. Plgf−/− eNos−/− mice show defective angiogenesis associated with increased oxidative stress in response to tissue ischemia. FASEB J. 2006;20:970–972. doi: 10.1096/fj.05-4481fje. [DOI] [PubMed] [Google Scholar]
- 303.Gigante B, Tarsitano M, Cimini V, De FS, Persico MG. Placenta growth factor is not required for exercise-induced angiogenesis. Angiogenesis. 2004;7:277–284. doi: 10.1007/s10456-004-4179-1. [DOI] [PubMed] [Google Scholar]
- 304.Girolami B, Bernardi E, Prins MH, Ten Cate JW, Hettiarachchi R, Prandoni P, Girolami A, Buller HR. Treatment of intermittent claudication with physical training, smoking cessation, pentoxifylline, or nafronyl: a meta-analysis. Arch Int Med. 1999;159:337–345. doi: 10.1001/archinte.159.4.337. [DOI] [PubMed] [Google Scholar]
- 305.Goerges AL, Nugent MA. Regulation of vascular endothelial growth factor binding and activity by extracellular pH. J Biol Chem. 2003;278:19518–19525. doi: 10.1074/jbc.M211208200. [DOI] [PubMed] [Google Scholar]
- 306.Goerges AL, Nugent MA. pH regulates vascular endothelial growth factor binding to fibronectin: a mechanism for control of extracellular matrix storage and release. J Biol Chem. 2004;279:2307–2315. doi: 10.1074/jbc.M308482200. [DOI] [PubMed] [Google Scholar]
- 307.Gokce N, Keaney JF, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41:1769–1775. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 308.Goldstein RE, Michaelis LL, Morrow AG, Epstein SE. Coronary collateral function in patients without occlusive coronary artery disease. Circulation. 1975;51:118–125. doi: 10.1161/01.cir.51.1.118. [DOI] [PubMed] [Google Scholar]
- 309.Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease Morbidity and mortality implications. Circulation. 2006;114:688–699. doi: 10.1161/CIRCULATIONAHA.105.593442. [DOI] [PubMed] [Google Scholar]
- 310.Goodwin AM, Sullivan KM, D’Amore PA. Cultured endothelial cells display endogenous activation of the canonical Wnt signaling pathway and express multiple ligands, receptors, and secreted modulators of Wnt signaling. Dev Dyn. 2006;235:3110–3120. doi: 10.1002/dvdy.20939. [DOI] [PubMed] [Google Scholar]
- 311.Gorospe JR, Nishikawa BK, Hoffman EP. Recruitment of mast cells to muscle after mild damage. J Neurol Sci. 1996;135:10–17. doi: 10.1016/0022-510x(95)00255-z. [DOI] [PubMed] [Google Scholar]
- 312.Goto M, Terada S, Kato M, Katoh M, Yokozeki T, Tabata I, Shimokawa T. cDNA cloning and mRNA analysis of PGC-1 in epitrochlearis muscle in swimming-exercised rats. Biochem Biophys Res Commun. 2000;274:350–354. doi: 10.1006/bbrc.2000.3134. [DOI] [PubMed] [Google Scholar]
- 313.Goukassian DA, Qin G, Dolan C, Murayama T, Silver M, Curry C, Eaton E, Luedemann C, Ma H, Asahara T, Zak V, Mehta S, Burg A, Thorne T, Kishore R, Losordo DW. Tumor necrosis factor-alpha receptor p75 is required in ischemia-induced neovascularization. Circulation. 2007;115:752–762. doi: 10.1161/CIRCULATIONAHA.106.647255. [DOI] [PubMed] [Google Scholar]
- 314.Gowdak LH, Poliakova L, Wang X, Kovesdi I, Fishbein KW, Zacheo A, Palumbo R, Straino S, Emanueli C, Marrocco-Trischitta M, Lakatta EG, Anversa P, Spencer RG, Talan M, Capogrossi MC. Adenovirus-mediated VEGF(121) gene transfer stimulates angiogenesis in normoperfused skeletal muscle and preserves tissue perfusion after induction of ischemia. Circulation. 2000;102:565–571. doi: 10.1161/01.cir.102.5.565. [DOI] [PubMed] [Google Scholar]
- 315.Green S, Askew CD, Walker PJ. Effect of type 2 diabetes mellitus on exercise intolerance and the physiological responses to exercise in peripheral arterial disease. Diabetologia. 2007;50:859–866. doi: 10.1007/s00125-006-0587-7. [DOI] [PubMed] [Google Scholar]
- 316.Greenhalgh RM, Belch JJ, Brown LC, Gaines PA, Gao L, Reise JA, Thompson SG Participants aMT. The adjuvant benefit of angioplasty in patients with mild to moderate intermittent claudication (MIMIC) managed by supervised exercise, smoking cessation advice and best medical therapy: results from two randomised trials for stenotic femoropopliteal and aortoiliac arterial disease. Eur J Vasc Endovas Surg. 2008;36:680–688. doi: 10.1016/j.ejvs.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 317.Greiner A, Esterhammer R, Messner H, Biebl M, Muhlthaler H, Fraedrich G, Jaschke WR, Schocke MF. High-energy phosphate metabolism during incremental calf exercise in patients with unilaterally symptomatic peripheral arterial disease measured by phosphor 31 magnetic resonance spectroscopy. J Vasc Surg. 2006;43:978–986. doi: 10.1016/j.jvs.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 318.Groom AC, Ellis CG, Potter RF. Microvascular geometry in relation to modeling oxygen transport in contracted skeletal muscle. Am Rev Respir Dis. 1984;129:S6–S9. doi: 10.1164/arrd.1984.129.2P2.S6. [DOI] [PubMed] [Google Scholar]
- 319.Grounds MD. Age-associated changes in the response of skeletal muscle cells to exercise and regeneration. Ann NY Acad Sci. 1998;854:78–91. doi: 10.1111/j.1749-6632.1998.tb09894.x. [DOI] [PubMed] [Google Scholar]
- 320.Grundmann S, Hoefer I, Ulusans S, Bode C, Oesterle S, Tijssen JG, Piek JJ, Buschmann I, van Royen N. Granulocyte-macrophage colony-stimulating factor stimulates arteriogenesis in a pig model of peripheral artery disease using clinically applicable infusion pumps. J Vasc Surg. 2006;43:1263–1269. doi: 10.1016/j.jvs.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 321.Gu JW, Brady AL, Anand V, Moore MC, Kelly WC, Adair TH. Adenosine upregulates VEGF expression in cultured myocardial vascular smooth muscle cells. Am J Physiol. 1999;277:H595–H602. doi: 10.1152/ajpheart.1999.277.2.H595. [DOI] [PubMed] [Google Scholar]
- 322.Gu JW, Gadonski G, Wang J, Makey I, Adair TH. Exercise increases endostatin in circulation of healthy volunteers. BMC Physiol. 2004;4:2. doi: 10.1186/1472-6793-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Gu JW, Shparago M, Tan W, Bailey AP. Tissue endostatin correlates inversely with capillary network in rat heart and skeletal muscles. Angiogenesis. 2006;9:93–99. doi: 10.1007/s10456-006-9035-z. [DOI] [PubMed] [Google Scholar]
- 324.Gu W, Weihrauch D, Tanaka K, Tessmer JP, Pagel PS, Kersten JR, Chilian WM, Warltier DC. Reactive oxygen species are critical mediators of coronary collateral development in a canine model. Am J Physiol. 2003;285:H1582–H1589. doi: 10.1152/ajpheart.00318.2003. [DOI] [PubMed] [Google Scholar]
- 325.Gudmundsson G, Matthiasson SE, Arason H, Johannsson H, Runarsson F, Bjarnason H, Helgadottir K, Thorisdottir S, Ingadottir G, Lindpaintner K, Sainz J, Gudnason V, Frigge ML, Kong A, Gulcher JR, Stefansson K. Localization of a gene for peripheral arterial occlusive disease to chromosome 1p31. Am J Hum Genet. 2002;70:586–592. doi: 10.1086/339251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Guidon M, McGee H. Exercise-based interventions and health-related quality of life in intermittentclaudication: a 20-year (1989–2008) review. Eur J Cardiovasc Prev Rehabil. 2010;17:140–154. doi: 10.1097/HJR.0b013e3283377f08. [DOI] [PubMed] [Google Scholar]
- 327.Guo N, Krutzsch HC, Inman JK, Roberts DD. Thrombospondin 1 and type I repeat peptides of thrombospondin 1 specifically induce apoptosis of endothelial cells. Cancer Res. 1997;57:1735–1742. [PubMed] [Google Scholar]
- 328.Gustafsson T, Knutsson A, Puntschart A, Kaijser L, Nordqvist AC, Sundberg CJ, Jansson E. Increased expression of vascular endothelial growth factor in human skeletal muscle in response to short-term one-legged exercise training. Pflugers Archiv - European Journal of Physiology. 2002;444:752–759. doi: 10.1007/s00424-002-0845-6. [DOI] [PubMed] [Google Scholar]
- 329.Gustafsson T, Kraus WE. Exercise-induced angiogenesis-related growth and transcription factors in skeletal muscle, and their modification in muscle pathology. Front Biosci. 2001;6:D75–D89. doi: 10.2741/gustafss. [DOI] [PubMed] [Google Scholar]
- 330.Gustafsson T, Puntschart A, Kaijser L, Jansson E, Sundberg CJ. Exercise-induced expression of angiogenesis-related transcription and growth factors in human skeletal muscle. Am J Physiol. 1999;276:H679–H685. doi: 10.1152/ajpheart.1999.276.2.H679. [DOI] [PubMed] [Google Scholar]
- 331.Gustafsson T, Ameln H, Fischer H, Sundberg CJ, Timmons JA, Jansson E. VEGF-A splice variants and related receptor expression in human skeletal muscle following submaximal exercise. J Appl Physiol. 2005;98:2137–2146. doi: 10.1152/japplphysiol.01402.2004. [DOI] [PubMed] [Google Scholar]
- 332.Gustafsson T, Rundqvist H, Norrbom J, Rullman E, Jansson E, Sundberg CJ. The influence of physical training on the angiopoietin and VEGF-A systems in human skeletal muscle. J Appl Physiol. 2007;103:1012–1020. doi: 10.1152/japplphysiol.01103.2006. [DOI] [PubMed] [Google Scholar]
- 333.Haas TL. Endothelial cell regulation of matrix metalloproteinases. Can J Physiol Pharmacol. 2005;83:1–7. doi: 10.1139/y04-120. [DOI] [PubMed] [Google Scholar]
- 334.Haas TL, Milkiewicz M, Davis SJ, Zhou AL, Egginton S, MDB, Madri JA, Hudlicka O. Matrix metalloproteinase activity is required for activity-induced angiogenesis in rat skeletal muscle. Am J Physiol. 2000;279:H1540–H1547. doi: 10.1152/ajpheart.2000.279.4.H1540. [DOI] [PubMed] [Google Scholar]
- 335.Hall JA, Dixon GH, Barnard RJ, Pritikin N. Effect of diet and exercise on peripheral vascular disease. Physician Sports Medicine. 1982;10:90–101. doi: 10.1080/00913847.1982.11947226. [DOI] [PubMed] [Google Scholar]
- 336.Hamburg NM, Balady GJ. Exercise rehabilitation in peripheral artery disease Functional impact and mechanisms of benefits. Circulation. 2011;123:87–97. doi: 10.1161/CIRCULATIONAHA.109.881888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Hammarsten J, Bylund-Fellenius AC, Holm J, Schersten T, Krotkiewski M. Capillary supply and muscle fibre types in patients with intermittent claudication: relationships between morphology and metabolism. Eur J Clin Invest. 1980;10:301–305. doi: 10.1111/j.1365-2362.1980.tb00037.x. [DOI] [PubMed] [Google Scholar]
- 338.Handschin C, Spiegelman BM. Peroxisome Proliferator-Activated Receptor γ Coactivator 1 Coactivators, Energy Homeostasis, and Metabolism. Endocr Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- 339.Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454:463–469. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Hang J, Kong L, Gu JW, Adair TH. VEGF gene expression is upregulated in electrically stimulated rat skeletal muscle. Am J Physiol. 1995;269:H1827–H1831. doi: 10.1152/ajpheart.1995.269.5.H1827. [DOI] [PubMed] [Google Scholar]
- 341.Hansen-Smith FM, Hudlicka O, Egginton S. In vivo angiogenesis in adult rat skeletal muscle: early changes in capillary network architecture and ultrastructure. Cell Tissue Res. 1996;286:123–136. doi: 10.1007/s004410050681. [DOI] [PubMed] [Google Scholar]
- 342.Hansen-Smith FM, Watson L, Lu DY, Goldstein I. Griffonia simplicifolia I: fluorescent tracer for microcirculatory vessels in nonperfused thin muscles and sectioned muscle. Microvasc Res. 1988;36:199–215. doi: 10.1016/0026-2862(88)90022-2. [DOI] [PubMed] [Google Scholar]
- 343.Hariawala MD, Horowitz JR, Esakof D, Sheriff DD, Walter DH, Keyt B, Isner JM, Symes JF. VEGF improves myocardial blood flow but produces EDRF-mediated hypotension in porcine hearts. J Surg Res. 1996;63:77–82. doi: 10.1006/jsre.1996.0226. [DOI] [PubMed] [Google Scholar]
- 344.Harken AH, Simson MBHaselgrove. Wetstein L, Harden WR, Barlow CH Early ischemia after complete coronary ligation in the rabbit, dog, pig, and monkey. Am J Physiol. 1981;241:H202–H210. doi: 10.1152/ajpheart.1981.241.2.H202. [DOI] [PubMed] [Google Scholar]
- 345.Harrington LS, Sainson RC, Williams CK, Taylor JM, Shi W, Li JL, Harris AL. Regulation of multiple angiogenic pathways by Dll4 and Notch in human umbilical vein endothelial cells. Microvasc Res. 2008;75:144–154. doi: 10.1016/j.mvr.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 346.Hashimoto A, Miyakoda G, Hirose Y, Mori T. Activation of endothelial nitric oxide synthase by cilostazol via a cAMP/protein kinase A- and phosphatidylinositol 3-kinase/Akt-dependent mechanism. Atherosclerosis. 2006;189:350–357. doi: 10.1016/j.atherosclerosis.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 347.Hashiya N, Jo N, Aoki M, Matsumoto K, Nakamura T, Sato Y, Ogata N, Ogihara T, Kaneda Y, Morishita R. In vivo evidence of angiogenesis induced by transcription factor ets-1 - Ets-1 is located upstream of angiogenesis cascade. Circulation. 2004;109:3035–3041. doi: 10.1161/01.CIR.0000130643.41587.DB. [DOI] [PubMed] [Google Scholar]
- 348.Haugen S, Casserly IP, Regensteiner JG, Hiatt WR. Risk assessment in the patient with established periperal arterial disease. Vasc Med. 2007;12:343–350. doi: 10.1177/1358863X07083278. [DOI] [PubMed] [Google Scholar]
- 349.Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol. 2001;91:534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- 350.Hayashi S, Morishita R, Nakamura S, Yamamoto K, Moriguchi A, Nagano T, Taiji M, Noguchi H, Matsumoto K, Nakamura T, Higaki J, Ogihara T. Potential role of hepatocyte growth factor, a novel angiogenic growth factor, in peripheral arterial disease: downregulation of HGF in response to hypoxia in vascular cells. Circulation. 1999;100:II301–II308. doi: 10.1161/circ.100.suppl_2.Ii-301. [DOI] [PubMed] [Google Scholar]
- 351.Hazarika S, Dokun AO, Li Y, Popel AS, Kontos CD, Annex BH. Impaired angiogenesis after hindlimb ischemia in type 2 diabetes mellitus: differential regulation of vascular endothelial growth factor receptor 1 and soluble vascular endothelial growth factor receptor 1. Circ Res. 2007;101:948–956. doi: 10.1161/CIRCRESAHA.107.160630. [DOI] [PubMed] [Google Scholar]
- 352.Heil M, Eitenmueller I, Schmitz-Rixen T, Schaper W. Arteriogenesis versus angiogenesis: similarities and differences. J Cell Mol Med. 2006;10:45–55. doi: 10.1111/j.1582-4934.2006.tb00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Heil M, Schaper W. Influence of mechanical, cellular, and molecular factors on collateral artery growth (arteriogenesis) Circ Res. 2004;95:449–458. doi: 10.1161/01.RES.0000141145.78900.44. [DOI] [PubMed] [Google Scholar]
- 354.Heil M, Schaper W. Insights into pathways of arteriogenesis. Curr Pharm Biotechnol. 2007;8:35–42. doi: 10.2174/138920107779941408. [DOI] [PubMed] [Google Scholar]
- 355.Heil M, Ziegelhoeffer T, Pipp F, Kostin S, Martin S, Clauss M, Schaper W. Blood monocyte concentration is critical for enhancement of collateral artery growth. Am J Physiol. 2002;283:2411–2419. doi: 10.1152/ajpheart.01098.2001. [DOI] [PubMed] [Google Scholar]
- 356.Heil M, Ziegelhoeffer T, Wagner S, Fernandez B, Helisch A, Martin S, Tribulova S, Kuziel WA, Bachmann G, Schaper W. Collateral artery growth (arteriogenesis) after experimental arterial occlusion is impaired in mice lacking CC-chemokine receptor-2. Circ Res. 2004;94:671–677. doi: 10.1161/01.RES.0000122041.73808.B5. [DOI] [PubMed] [Google Scholar]
- 357.Helisch A, Schaper S. Arteriogenesis: the development and growth of collateral arteries. Microcirculation. 2003;10:83–97. doi: 10.1038/sj.mn.7800173. [DOI] [PubMed] [Google Scholar]
- 358.Helisch A, Wagner S, Khan N, Drinane M, Wolfram S, Heil M, Ziegelhoeffer T, Brandt U, Pearlman JD, Swartz HM, Schaper W. Impact of mouse strain differences in innate hindlimb collateral vasculature. Arterioscler Thromb Vasc Biol. 2006;26:520–526. doi: 10.1161/01.ATV.0000202677.55012.a0. [DOI] [PubMed] [Google Scholar]
- 359.Heljasvaara R, Nyberg P, Luostarinen J, Parikka M, Heikkila P, Rehn M, Sorsa T, Salo T, Pihlajaniemi T. Generation of biologically active endostatin fragments from human collagen XVIII by distinct matrix metalloproteases. Exp Cell Res. 2005;307:292–304. doi: 10.1016/j.yexcr.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 360.Hellberg C, Ostman A, Heldin CH. PDGF and vessel maturation. Recent Results Cancer Res. 2010;180:103–114. doi: 10.1007/978-3-540-78281-0_7. [DOI] [PubMed] [Google Scholar]
- 361.Hellsten Y, Skadhauge L, Bangsbo J. Effect of ribose supplementation on resynthesis of adenine nucleotides after intense intermittent training in humans. Am J Physiol. 2004;286:R182–R188. doi: 10.1152/ajpregu.00286.2003. [DOI] [PubMed] [Google Scholar]
- 362.Hellsten Y, Frandsen U. Adenosine formation in contracting primary rat skeletal muscle cells and endothelial cells in culture. J Physiol. 1997;504:695–704. doi: 10.1111/j.1469-7793.1997.695bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Hellsten Y, Maclean D, Radegran G, Saltin B, Bangsbo J. Adenosine concentrations in the interstitium of resting and contracting human skeletal muscle. Circulation. 1998;98:6–8. doi: 10.1161/01.cir.98.1.6. [DOI] [PubMed] [Google Scholar]
- 364.Hellsten Y, Rufener N, Nielsen JJ, Hoier B, Krustrup P, Bangsbo J. Passive leg movement enhances interstitial VEGF protein, endothelial cell proliferation, and eNOS mRNA content in human skeletal muscle. Am J Physiol. 2008;294:R975–R982. doi: 10.1152/ajpregu.00677.2007. [DOI] [PubMed] [Google Scholar]
- 365.Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalen M, Gerhardt H, Betsholtz C. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 367.Hendricks DL, Pevec WC, Shestak KC, Rosenthal MC, Webster MW, Steed DL. A model of persistent partial hindlimb ischemia in the rabbit. J Surg Res. 1990;49:453–457. doi: 10.1016/0022-4804(90)90195-8. [DOI] [PubMed] [Google Scholar]
- 368.Hengy B, Watanabe N, Williams AG, Downey HF. Does peripheral collateralization also cause collateralization in the canine heart? Clin Exp Pharmacol Physiol. 1989;16:429–432. doi: 10.1111/j.1440-1681.1989.tb01581.x. [DOI] [PubMed] [Google Scholar]
- 369.Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, Shah PK, Willerson JT, Benza RL, Berman DS, Gibson CM, Bajamonde A, Rundle AC, Fine J, McCluskey ER. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003;107:1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 370.Henry TD, Rocha-Singh K, Isner JM, Kereiakes DJ, Giordano FJ, Simons M, Losordo DW, Hendel RC, Bonow RO, Eppler SM, Zioncheck TF, Holmgren EB, McCluskey ER. Intracoronary administration of recombinant human vascular endothelial growth factor to patients with coronary artery disease. Am Heart J. 2001;142:872–880. doi: 10.1067/mhj.2001.118471. [DOI] [PubMed] [Google Scholar]
- 371.Hepple RT, Hogan MC, Stary C, Bebout DE, Mathieu-Costello O, Wagner PD. Structural basis of muscle O2 diffusing capacity: evidence from muscle function in situ. J Appl Physiol. 2000;88:560–566. doi: 10.1152/jappl.2000.88.2.560. [DOI] [PubMed] [Google Scholar]
- 372.Hepple RT, Mackinnon SL, Thomas SG, Goodman JM, Plyley MJ. Quantitating the capillary supply and the response to resistance training in older men. Pflugers Arch. 1997;433:238–244. doi: 10.1007/s004240050273. [DOI] [PubMed] [Google Scholar]
- 373.Hermansen L, Wachtlova M. Capillary density of skeletal muscle in well-trained and untrained men. J Appl Physiol. 1971;30:860–863. doi: 10.1152/jappl.1971.30.6.860. [DOI] [PubMed] [Google Scholar]
- 374.Hershey JC, Baskin EP, Glass JD, Hartman HA, Gilberto DB, Rogers IT, Cook JJ. Revascularization in the rabbit hindlimb: dissociation between capillary sprouting and arteriogenesis. Cardiovasc Res. 2001;49:618–625. doi: 10.1016/s0008-6363(00)00232-7. [DOI] [PubMed] [Google Scholar]
- 375.Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Eng J Med. 2001;344:1608–1621. doi: 10.1056/NEJM200105243442108. [DOI] [PubMed] [Google Scholar]
- 376.Hiatt WR, Nawaz D, Brass EP. Carnitine metabolism during exercise in patients with peripheral vascular disease. J Appl Physiol. 1987;62:2383–2387. doi: 10.1152/jappl.1987.62.6.2383. [DOI] [PubMed] [Google Scholar]
- 377.Hiatt WR, Nawaz D, Regensteiner JG, Hossack KF. The evaluation of exercise performance in patients with peripheral vascular disease. J Cardiopul Rehabil. 1988;12:525–532. [Google Scholar]
- 378.Hiatt WR, Regensteiner JG, Hargarten ME, Wolfel EE, Brass EP. Benefit of exercise conditioning for patients with peripheral arterial disease. Circulation. 1990;81:602–609. doi: 10.1161/01.cir.81.2.602. [DOI] [PubMed] [Google Scholar]
- 379.Hiatt WR, Regensteiner JG, Wolfel EE, Carry MR, Brass EP. Effect of exercise training on skeletal muscle histology and metabolism in peripheral arterial disease. J Appl Physiol. 1996;81:780–788. doi: 10.1152/jappl.1996.81.2.780. [DOI] [PubMed] [Google Scholar]
- 380.Hiatt WR, Wolfel EE, Meier RH, Regensteiner JG. Superiority of treadmill walking exercise versus strength training for patients with peripheral arterial disease Implications for the mechanism of the training response. Circulation. 1994;90:1866–1874. doi: 10.1161/01.cir.90.4.1866. [DOI] [PubMed] [Google Scholar]
- 381.Hickey NC, Hudlicka O, Gosling P, Shearman CP, Simms MH. Intermittent claudication incites systemic neutrophil activation and increased vascular permeability. Br J Surg. 1993;80:181–184. doi: 10.1002/bjs.1800800215. [DOI] [PubMed] [Google Scholar]
- 382.Hickey NC, Hudlicka O, Simms MH. Claudication induces systemic capillary endothelial swelling. Eur J Vasc Surg. 1992;6:36–40. doi: 10.1016/s0950-821x(05)80092-1. [DOI] [PubMed] [Google Scholar]
- 383.Hijmering ML, Stoes ESG, Olijhoek J, Hutten BA, Blankestijn PJ, Rabelink TJ. Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. J Am Coll Cardiol. 2002;39:683–688. doi: 10.1016/s0735-1097(01)01786-7. [DOI] [PubMed] [Google Scholar]
- 384.Hikida R, Gollnick PD, Dudley GA, Convertino V, Buchanan P. Structural and metabolic characteristics of human skeletal muscle following 30 days of simulated microgravity. Aviat Space Environ Med. 1989;60:664–670. [PubMed] [Google Scholar]
- 385.Hiral DM, Copp SW, Ferreira LF, Musch TI, Poole DC. Nitric oxide bioavailability modulates the dynamics of microvascular oxygen exchange during recovery from contractions. Acta Physiol. 2010;200:159–169. doi: 10.1111/j.1748-1716.2010.02137.x. [DOI] [PubMed] [Google Scholar]
- 386.Hirayama Y, Sumpio BE. Role of ligand-specific integrins in endothelial cell alignment and elongation induced by cyclic strain. Endothelium. 2007;14:275–283. doi: 10.1080/10623320701746248. [DOI] [PubMed] [Google Scholar]
- 387.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, McDermott MM, Hiatt WR. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 388.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin J, Hiratzka LF, Murphy WR, JW O, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor L, White CJ, White J, White RA, Antman EM, Smith SC, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Halperin J, Hiratzka LF, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Guidelines for the management of patients with peripheral arterial disease: Executive summary. J Am Coll Cardiol. 2006;47:1239–1312. doi: 10.1016/j.jacc.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 389.Hirschi KK, D’Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32:687–698. [PubMed] [Google Scholar]
- 390.Hiscock N, Fischer CP, Pilegaard H, Pedersen BK. Vascular endothelial growth factor mRNA expression and arteriovenous balance in response to prolonged, submaximal exercise in humans. Am J Physiol. 2003;285:H1759–H1763. doi: 10.1152/ajpheart.00150.2003. [DOI] [PubMed] [Google Scholar]
- 391.Hlubek F, Spaderna S, Jung A, Kirchner T, Brabletz T. beta-catenin activates a coordinated expression of the proinvasive factors laminin-5 gamma 2 chain and MT1-MMP in colorectal carcinomas. Int J Cancer. 2004;108:321–326. doi: 10.1002/ijc.11522. [DOI] [PubMed] [Google Scholar]
- 392.Ho TK, Rajkumar V, Black CM, Abraham DJ, Baker DM. Increased angiogenic response but deficient arteriolization and abnormal microvessel ultrastructure in critical leg ischaemia. Br J Surg. 2006;93:1368–1376. doi: 10.1002/bjs.5496. [DOI] [PubMed] [Google Scholar]
- 393.Hobbs SD, Marshall T, Fegan C, Adam DJ, Bradbury AW. The constitutive procoagulant and hypofibrinolytic state in patients with intermittent claudication due to infrainguinal disease significantly improves with percutaneous transluminal balloon angioplasty. J Vasc Surg. 2006;43:40–46. doi: 10.1016/j.jvs.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 394.Hoefer IE, Grundmann S, van RN, Voskuil M, Schirmer SH, Ulusans S, Bode C, Buschmann IR, Piek JJ. Leukocyte subpopulations and arteriogenesis: specific role of monocytes, lymphocytes and granulocytes. Atherosclerosis. 2005;181:285–293. doi: 10.1016/j.atherosclerosis.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 395.Hoefer IE, van RN, Buschmann IR, Piek JJ, Schaper W. Time course of arteriogenesis following femoral artery occlusion in the rabbit. Cardiovasc Res. 2001;49:609–617. doi: 10.1016/s0008-6363(00)00243-1. [DOI] [PubMed] [Google Scholar]
- 396.Hoefer IE, van RN, Rectenwald JE, Deindl E, Hua J, Jost M, Grundmann S, Voskuil M, Ozaki CK, Piek JJ, Buschmann IR. Arteriogenesis proceeds via ICAM-1/Mac-1- mediated mechanisms. Circ Res. 2004;94:1179–1185. doi: 10.1161/01.RES.0000126922.18222.F0. [DOI] [PubMed] [Google Scholar]
- 397.Hoffner L, Nielsen JJ, Langberg H, Hellsten Y. Exercise but not prostanoids enhance levels of vascular endothelial growth factor and other proliferative agents in humans skeletal muscle interstitium. J Physiol. 2003;550:217–225. doi: 10.1113/jphysiol.2002.037051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Hoier B, Nordsborg N, Andersen S, Jensen L, Nybo L, Bangsbo J, Hellsten Y. Pro- and anti-angiogenic factors in human skeletal muscle in response to acute exercise and training. J Physiol. 2012;590:595–606. doi: 10.1113/jphysiol.2011.216135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Hoier B, Olsen K, Nyberg M, Bangsbo J, Hellsten Y. Contraction-induced secretion of VEGF from skeletal muscle cells is mediated by adenosine. Am J Physiol. 2010;299:H857–H862. doi: 10.1152/ajpheart.00082.2010. [DOI] [PubMed] [Google Scholar]
- 400.Holderfield MT, Hughes CCW. Crosstalk between vascular endothelial growth factor, notch, and transforming growth factor-beta in vascular morphogenesis. Circ Res. 2008;102:637–652. doi: 10.1161/CIRCRESAHA.107.167171. [DOI] [PubMed] [Google Scholar]
- 401.Holloszy JO. Biochemical adaptations in muscle Effects of exercise on itochondrial oxygen uptake and respiratory exzyme activity in skeletal muscle. J Biol Chem. 1967;242:2278–2282. [PubMed] [Google Scholar]
- 402.Holloszy JO. Regulation by exercise of skeletal muscle content of mitochondria nad GLUT4. J Physiol Pharmacol. 2008;59:5–18. [PubMed] [Google Scholar]
- 403.Holloszy JO. Regulatioin of mitochondrial biogenesis and GLUT4 expression by exercise. Compr Physiol. 2011;1:921–940. doi: 10.1002/cphy.c100052. [DOI] [PubMed] [Google Scholar]
- 404.Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol. 1984;56:831–838. doi: 10.1152/jappl.1984.56.4.831. [DOI] [PubMed] [Google Scholar]
- 405.Holm J, Bjorntorp P, Schersten T. Metabolic activity in human skeletal muscle. Effect of peripheral arterial insufficiency. Eur J Clin Invest. 1972;2:321–325. doi: 10.1111/j.1365-2362.1972.tb00657.x. [DOI] [PubMed] [Google Scholar]
- 406.Holm J, Dahllof AG, Bjorntorp P, Schersten T. Enzyme studies in muscles of patients with intermittent claudication. Effect of training. Scand J Clin Lab Invest. 1973;128:201–205. [PubMed] [Google Scholar]
- 407.Holm J, Dahllof AG, Schersten T. Metabolic activity of skeletal muscle in patients with peripheral arterial insufficiency. Scand J Clin Invest. 1975;35:81–86. [PubMed] [Google Scholar]
- 408.Holmqvist K, Cross MJ, Rolny C, Hagerkvist R, Rahimi N, Matsumoto T, Claesson-Welsh L, Welsh M. The adaptor protein shb binds to tyrosine 1175 in vascular endothelial growth factor (VEGF) receptor-2 and regulates VEGF-dependent cellular migration. J Biol Chem. 2004;279:22267–22275. doi: 10.1074/jbc.M312729200. [DOI] [PubMed] [Google Scholar]
- 409.Holt KG, Hamill J, Andres RO. Predicting the minimal energy costs of human walking. Med Sci Sports Exer. 1991;23:491–498. [PubMed] [Google Scholar]
- 410.Holthofer H, Virtanen I, Kariniemi AL, Hormia M, Linder E, Miettinen A. Ulex europaeus I lectin as a marker for vascular endothelium in human tissues. Lab Invest. 1982;47:60–66. [PubMed] [Google Scholar]
- 411.CR H, Connett RJ, Gayeski TE. O2 transport and its interaactioin with metabolism: a systems view of aerobic capacity. Med Sci Sports Exer. 1992;24:47–53. [PubMed] [Google Scholar]
- 412.Hood DA. Contractile activity-induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol. 2001;90:1137–1157. doi: 10.1152/jappl.2001.90.3.1137. [DOI] [PubMed] [Google Scholar]
- 413.Hood DA. Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle. Appl Physiol Nutr Metab. 2009;34:465–472. doi: 10.1139/H09-045. [DOI] [PubMed] [Google Scholar]
- 414.Hori A, Shibata R, Morisaki K, Murohara T, Komori K. Cilostazol stimulates revascularisation in response to ischaemia via an eNOS-dependent mechanism. Eur j Vasc Endovasc Surg. 2012;43:62–65. doi: 10.1016/j.ejvs.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 415.Hou XY, Green S, Askew CD, Barker G, Green A, Walker PJ. Skeletal muscle mitochondrial ATP production rate and walking performance in peripheral arterial disease. Clin Physiol Funct Imaging. 2002;22:226–232. doi: 10.1046/j.1475-097x.2002.00423.x. [DOI] [PubMed] [Google Scholar]
- 416.Houck KA, Leung DW, Rowland AM, Winer J, Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem. 1992;267:26031–26037. [PubMed] [Google Scholar]
- 417.Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361–9374. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Hua Z, Lv Q, Ye W, Wong CK, Cai G, Gu D, Ji Y, Zhao C, Wang J, Yang BB, Zhang Y. MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS ONE. 2006;1:e116. doi: 10.1371/journal.pone.0000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Huber KH, Rexroth W, Werle E, Koeth T, Weicker H, Hild R. Sympathetic neuronal activity in diabetic and non-diabetic subjects with peripheral arterial occlusive disease. Klinische Wochenschrift. 1991;69:233–238. doi: 10.1007/BF01666848. [DOI] [PubMed] [Google Scholar]
- 420.Hudlicka O, Brown MD, Egginton S. Angiogenesis in skeletal and cardiac muscle. Physiol Rev. 1992;72:369–417. doi: 10.1152/physrev.1992.72.2.369. [DOI] [PubMed] [Google Scholar]
- 421.Hudlicka O, Brown MD, Silgram H. Inhibition of capillary growth in chronically stimulated rat muscles by N(G)-nitro-1-arginine, nitric oxide synthase inhibitor. Microvasc Res. 2000;59:45–51. doi: 10.1006/mvre.1999.2193. [DOI] [PubMed] [Google Scholar]
- 422.Hudlicka O. Growth of capillaries in skeletal and cardiac muscle. Circ Res. 1982;50:451–461. doi: 10.1161/01.res.50.4.451. [DOI] [PubMed] [Google Scholar]
- 423.Hudlicka O. What makes blood vessels grow? J Physiol. 1991;444:1–24. doi: 10.1113/jphysiol.1991.sp018863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Hudlicka O, Brown MD, Egginton S, Dawson JM. Effect of long-term electrical stimulation on vascular supply and fatigue in chronically ischemic muscles. J Appl Physiol. 1994;77:1317–1324. doi: 10.1152/jappl.1994.77.3.1317. [DOI] [PubMed] [Google Scholar]
- 425.Hudlicka O, Brown MD, May S, Zakrzewicz A, Pries AR. Changes in capillary shear stress in skeletal muscles exposed to long-term activity: role of nitric oxide. Microcirc. 2006;13:249–259. doi: 10.1080/10739680600556951. [DOI] [PubMed] [Google Scholar]
- 426.Hudlicka O, Dodd L, Renkin EM, Gray SD. Early changes in fiber profile and capillary density in long-term stimulated muscles. Am J Physiol. 1982;243:H528–H535. doi: 10.1152/ajpheart.1982.243.4.H528. [DOI] [PubMed] [Google Scholar]
- 427.Hudlicka O, Garnham A, Shiner R, Egginton S. Attenuation of changes in capillary fine structure and leukocyte adhesion improves muscle performance following chronic ischaemia in rats. J Physiol. 2008;586:4961–4975. doi: 10.1113/jphysiol.2008.158055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Hudlicka O, West D, Kumar S, el KF, Wright AJ. Can growth of capillaries in the heart and skeletal muscle be explained by the presence of an angiogenic factor? Br J Exp Pathol. 1989;70:237–246. [PMC free article] [PubMed] [Google Scholar]
- 429.Huisinga JM, Pipinos II, Johanning JM, Stergiou N. The effect of pharmacological treatment on gait biomechanics in peripheral arterial disease patients. J Neuroeng Rehabil. 2010;7:7–25. doi: 10.1186/1743-0003-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Huisinga JM, Pipinos II, Stergiou N, Johanning JM. Treatment with pharmacologial agents in peripheral arterial disease patients does not result in biomechanical gait changes. J Appl Biomech. 2010;26:341–348. doi: 10.1123/jab.26.3.341. [DOI] [PubMed] [Google Scholar]
- 431.Hurst D, Taylor EB, Cline TD, Greenwood LJ, Compton CL, Lamb JD, Winder WW. AMP-activated protein kinase kinase activity and phosphorylation of AMP-activated protein kinase in contracting muscle of sedentary and endurance-trained rats. Am J Physiol. 2005;289:E710–E715. doi: 10.1152/ajpendo.00155.2005. [DOI] [PubMed] [Google Scholar]
- 432.Hutchins GM, Miner MM, Bulkley BH. Tortuosity as an index of the age and diameter increase of coronary collateral vessels in patients after acute myocardial infarction. Am J Cardiol. 1978;41:210–215. doi: 10.1016/0002-9149(78)90158-3. [DOI] [PubMed] [Google Scholar]
- 433.Ianuzzo D, Patel P, Chen V, O’brien P, Williams C. Thyroidal trophic influence on skeletal muscle myosin. Nature. 1977;270:74–76. doi: 10.1038/270074a0. [DOI] [PubMed] [Google Scholar]
- 434.Ichikawa Y, Ishikawa T, Momiyama N, Kamiyama M, Sakurada H, Matsuyama R, Hasegawa S, Chishima T, Hamaguchi Y, Fujii S, Saito S, Kubota K, Hasegawa S, Ike H, Oki S, Shimada H. Matrilysin (MMP-7) degrades VE-cadherin and accelerates accumulation of beta-catenin in the nucleus of human umbilical vein endothelial cells. Oncol Rep. 2006;15:311–315. [PubMed] [Google Scholar]
- 435.Ichioka S, Shibata M, Kosaki K, Sato Y, Harii K, Kamiya A. In VivoMeasurement of Morphometric and Hemodynamic Changes in the Microcirculation during Angiogenesis under Chronic [alpha]1-Adrenergic Blocker Treatment. Microvasc Res. 1998;55:165–174. doi: 10.1006/mvre.1998.2069. [DOI] [PubMed] [Google Scholar]
- 436.Iglarz M, Silvestre JS, Duriez M, Henrion D, Levy BI. Chronic blockade of endothelin receptors improves ischemia-induced angiogenesis in rat hindlimbs through activation of vascular endothelial growth factor-no pathway. Arterioscler Thromb Vasc Biol. 2001;21:1598–1603. doi: 10.1161/hq1001.097065. [DOI] [PubMed] [Google Scholar]
- 437.Ikeda S, Kawamoto H, Kasaoka K, Hitomi Y, Kizaki T, Sankai Y, Ohno H, Haga S, Takemasa T. Muscle tpe-specific responsse of PGC-1alpha and oxidative exzymes during voluntary wheel running in mouse skeletal muscle. Acta Physiol. 2006;188:217–223. doi: 10.1111/j.1748-1716.2006.01623.x. [DOI] [PubMed] [Google Scholar]
- 438.Ikeda U, Ikeda M, Kano S, Kanbe T, Shimada K. Effect of cilostazol, a cAMP phosphodiesterase inhibitor, on nitric oxide production by vascular smooth muscle cells. Eur J Pharmacol. 1996;314:197–202. doi: 10.1016/s0014-2999(96)00551-1. [DOI] [PubMed] [Google Scholar]
- 439.Inagaki T, Sonobe T, Poole DC, Kano Y. Arteriolar vasomotor control and contractile performance during fatiguing tetanic contractions in rat skeletal muscle: role of sympathetic system. Adv Exp Med Biol. 2010;662:309–315. doi: 10.1007/978-1-4419-1241-1_44. [DOI] [PubMed] [Google Scholar]
- 440.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 441.Ingjer F. Maximal aerobic power related to the capillary supply of the quadriceps femoris muscle in man. Acta Physiol Scand. 1978;104:238–240. doi: 10.1111/j.1748-1716.1978.tb06273.x. [DOI] [PubMed] [Google Scholar]
- 442.Ingjer F. Effects of endurance training on muscle fibre ATP-ase activity, capillary supply and mitochondrial content in man. J Physiol. 1979;294:419–432. doi: 10.1113/jphysiol.1979.sp012938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Irrcher I, Adhihetty PJ, Sheehan T, Joseph AM, Hood DA. PPARgama coactivator-1alpha expression during thyroid hormone- and contractile activity-induced mitochondrial adaptations. Am J Physiol. 2003;284:C1669–C1677. doi: 10.1152/ajpcell.00409.2002. [DOI] [PubMed] [Google Scholar]
- 444.Iruela-Arispe ML, Davis GE. Cellular and molecular mechanisms of vascular lumen formation. Dev Cell. 2009;16:222–231. doi: 10.1016/j.devcel.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Iruela-Arispe ML, Lombardo M, Krutzsch HC, Lawler J, Roberts DD. Inhibition of angiogenesis by thrombospondin-1 is mediated by 2 independent regions within the type 1 repeats. Circulation. 1999;100:1423–1431. doi: 10.1161/01.cir.100.13.1423. [DOI] [PubMed] [Google Scholar]
- 446.Isbell DC, Berr SS, Toledano AY, Epstein FH, Meyer CH, Rogers WJ, Harthun NL, Hagspiel KD, Weltman A, Kramer CM. Delayed calf muscle phosphocreatine recovery after exercise identifies peripheral arterial disease. J Am Coll Cardiol. 2006;47:2289–2295. doi: 10.1016/j.jacc.2005.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Isbell DC, Epstein FH, Zhong X, DiMaria JM, Berr SS, Meyer CH, Rogers WJ, Harthun NL, Hagspiel KD, Weltman A, Kramer CM. Calf muscle perfusion at peak exercise in peripheral arterial disease: measurement by first-pass contrast-enhanced magnetic resonance imaging. J Mag Res Imaging. 2007;25:1013–1020. doi: 10.1002/jmri.20899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Isenberg JS, Romeo MJ, Abu-Asab M, Tsokos M, Oldenborg A, Pappan L, Wink DA, Frazier WA, Roberts DD. Increasing survival of ischemic tissue by targeting CD47. Circ Res. 2007;100:712–720. doi: 10.1161/01.RES.0000259579.35787.4e. [DOI] [PubMed] [Google Scholar]
- 449.Isenberg JS, Hyodo F, Matsumoto K, Romeo MJ, Abu-Asab M, Tsokos M, Kuppusamy P, Wink DA, Krishna MC, Roberts DD. Thrombospondin-1 limits ischemic tissue survival by inhibiting nitric oxide-mediated vascular smooth muscle relaxation. Blood. 2007;109:1945–1952. doi: 10.1182/blood-2006-08-041368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Isenberg JS, Jia Y, Fukuyama J, Switzer CH, Wink DA, Roberts DD. Thrombospondin-1 inhibits nitric oxide signaling via CD36 by inhibiting myristic acid uptake. J Biol Chem. 2007;282:15404–15415. doi: 10.1074/jbc.M701638200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Ishikawa H. Fine structure of skeletal muscle. Cell Muscle Motil. 1983;4:1–84. [PubMed] [Google Scholar]
- 452.Isner JM, Pieczek A, Schainfeld R, Blair R, Haley L, Asahara T, Rosenfield K, Razvi S, Walsh K, Symes JF. Clinical evidence of angiogenesis after arterial gene transfer of phVEGF165 in patient with ischaemic limb. Lancet. 1996;348:370–374. doi: 10.1016/s0140-6736(96)03361-2. [DOI] [PubMed] [Google Scholar]
- 453.Ispanovic E, Haas TL. JNK and PI3K differentially regulate MMP-2 and MT1-MMP mRNA and protein in response to actin cytoskeleton reorganization in endothelial cells. Am J Physiol. 2006;291:C579–C588. doi: 10.1152/ajpcell.00300.2005. [DOI] [PubMed] [Google Scholar]
- 454.Ispanovic E, Serio D, Haas TL. Cdc42 and RhoA have opposing roles in regulating membrane type 1-matrix metalloproteinase localization and matrix metalloproteinase-2 activation. Am J Physiol. 2008;295:C600–C610. doi: 10.1152/ajpcell.00460.2007. [DOI] [PubMed] [Google Scholar]
- 455.Ito WD, Arras M, Scholz D, Winkler B, Htun P, Schaper W. Angiogenesis but not collateral growth is associated with ischemia after femoral artery occlusion. Am J Physiol. 1997;273:H1255–H1265. doi: 10.1152/ajpheart.1997.273.3.H1255. [DOI] [PubMed] [Google Scholar]
- 456.Ito WD, Arras M, Winkler B, Scholz D, Schaper J, Schaper W. Monocyte chemotactic protein-1 increases collateral and peripheral conductance after femoral artery occlusion. Circ Res. 1997;80:829–837. doi: 10.1161/01.res.80.6.829. [DOI] [PubMed] [Google Scholar]
- 457.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes and Development Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Jager S, Handschin CSt. Pierre J, Spiegelman BM AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. PNAS. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Jarajapu YP, Coats P, McGrath JC, MacDonald A, Hillier C. Increased alpha(1)- and alpha(2)-adrenoceptor-mediated contractile responses of human skeletal muscle resistance arteries in chronic limb ischemia. Cardiovasc Res. 2001;49:218–225. doi: 10.1016/s0008-6363(00)00224-8. [DOI] [PubMed] [Google Scholar]
- 460.Jasperse JL, Woodman CR, Price EM, Hasser EM, Laughlin MH. Hindlimb unweighting decreases ecNOS gene expression and endothelium-dependent dilation in rat soleus feed arteries. J Appl Physiol. 1999;87:1476–1482. doi: 10.1152/jappl.1999.87.4.1476. [DOI] [PubMed] [Google Scholar]
- 461.Jennische E, Ekberg S, Matejka GL. Expression of hepatocyte growth factor in growing and regenerating rat skeletal muscle. Am J Physiol. 1993;265:C122–C128. doi: 10.1152/ajpcell.1993.265.1.C122. [DOI] [PubMed] [Google Scholar]
- 462.Jensen L, Schjerling P, Hellsten Y. Regulation of VEGF and bFGF mRNA expression and other proliferative compounds in skeletal muscle cells. Angiogenesis. 2004;7:255–267. doi: 10.1007/s10456-004-4184-4. [DOI] [PubMed] [Google Scholar]
- 463.Jin ZG, Ueba H, Tanimoto T, Lungu AO, Frame MD, Berk BC. Ligand-independent activation of vascular endothelial growth factor receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthase. Circ Res. 2003;93:354–363. doi: 10.1161/01.RES.0000089257.94002.96. [DOI] [PubMed] [Google Scholar]
- 464.Jin ZGen, Wong C, Wu J, Berk BC. Flow Shear Stress Stimulates Gab1 Tyrosine Phosphorylation to Mediate Protein Kinase B and Endothelial Nitric-oxide Synthase Activation in Endothelial Cells. J Biol Chem. 2005;280:12305–12309. doi: 10.1074/jbc.M500294200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Johnston AP, Baker J, Bellamy LM, McKay BR, De LM, Parise G. Regulation of muscle satellite cell activation and chemotaxis by angiotensin II. PLoS ONE. 2010;5:e15212. doi: 10.1371/journal.pone.0015212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Jomayvaz FR, Shulman GI. Regulation of mitochondrial biogenesis. Essays Biochem. 2010;47:69–84. doi: 10.1042/bse0470069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Jones WS, Duscha BD, Robbins JL, Duggan NN, Regensteiner JG, Kraus WE, Hiatt WR, Dokun AO, Annex BH. Alteration in angiogenic and anti-angiogenic forms of vascular endothelial growth factor-A in skeletal muscle of patients with intermittent claudication following exercise training. Vasc Med. 2012;17:000–000. doi: 10.1177/1358863X11436334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Jones EA, Yuan L, Breant C, Watts RJ, Eichmann A. Separating genetic and hemodynamic defects in neuropilin 1 knockout embryos. Development. 2008;135:2479–2488. doi: 10.1242/dev.014902. [DOI] [PubMed] [Google Scholar]
- 469.Joras M, Poredos P. The association of acute exercise-induced ischaemia with systemic vasodilator function in patients with peripheral arterial disease. Vasc Med. 2008;13:255–262. doi: 10.1177/1358863X08096347. [DOI] [PubMed] [Google Scholar]
- 470.Jude EB, Eleftheriadou I, Tentolouris N. Peripheral arterial disease in diabetes--a review. Diabet Med. 2010;27:4–14. doi: 10.1111/j.1464-5491.2009.02866.x. [DOI] [PubMed] [Google Scholar]
- 471.Kaelin WG., Jr Ratcliffe PJ Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 472.Kalebic T, Garbisa S, Glaser B, Liotta LA. Basement-Membrane Collagen - Degradation by Migrating Endothelial-Cells. Science. 1983;221:281–283. doi: 10.1126/science.6190230. [DOI] [PubMed] [Google Scholar]
- 473.Kalka C, Tehrani H, Laudenberg B, Vale PR, Isner JM, Asahara T, Symes JF. VEGF gene transfer mobilizes endothelial progenitor cells in patients with inoperable coronary disease. Ann Thorac Surg. 2000;70:829–834. doi: 10.1016/s0003-4975(00)01633-7. [DOI] [PubMed] [Google Scholar]
- 474.Kar S, Gao L, Zucker IH. Exercise training normalizes ACE and ACE2 in the brain of rabbits with pacing-induced heart failure. J Appl Physiol. 2010;108:923–932. doi: 10.1152/japplphysiol.00840.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Kasapis C, Thompson PD. The effects of physical activity on serum c-reactive protein and inflammatory markers. J Am Coll Cardiol. 2005;45:1563–1569. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 476.Kaufman MP, Hayes SG. The exercise pressor reflex. Clin Auton Res. 2002;12:429–439. doi: 10.1007/s10286-002-0059-1. [DOI] [PubMed] [Google Scholar]
- 477.Kaufman MP, Waldrop TG, Rybicki KJ, Ordway GA, Mitchell JH. Effects of static and rhythmic twitch contractions on the discharge of group II an IV muscle afferents. Cardiovasc Res. 1984;18:663–668. doi: 10.1093/cvr/18.11.663. [DOI] [PubMed] [Google Scholar]
- 478.Kaur S, Martin-Manso G, Pendrak ML, Garfield SH, Isenberg JS, Roberts DD. Thrombospondin-1 inhibits VEGF receptor-2 signaling by disrupting its association with CD47. J Biol Chem. 2010;285:38923–38932. doi: 10.1074/jbc.M110.172304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Kazatani Y, Hamada M, Shigematsu Y, Hiwanda K, Kokubu T. Beneficial effect of a long-term antihypertensive therapy on blood pressuer response to isometric handgrip exercise in patients with essential hypertension. Am J Ther. 1995;2:165–169. doi: 10.1097/00045391-199503000-00003. [DOI] [PubMed] [Google Scholar]
- 480.Kelsall CJ, Brown MD, Hudlicka O. Alterations of small arterioles in rat skeletal muscle as a result of chronic ischaemia. J Vasc Res. 2001;38:212–218. doi: 10.1159/000051049. [DOI] [PubMed] [Google Scholar]
- 481.Keo H, Grob E, Guggisberg F, Widmer J, Baumgartner I, Schmid JP, Kalka C, Saner H. Long-term effects of supervised exercise training on walking capacity and quality of life in patients with intermittent claudication. Vasa. 2008;37:250–256. doi: 10.1024/0301-1526.37.3.250. [DOI] [PubMed] [Google Scholar]
- 482.Khan MH, Sinoway LI. Muscle reflex control of sympathetic nerve activity in heart failure: the role of exercise conditioning. Heart Fail Rev. 2000;5:87–100. doi: 10.1023/A:1009802308872. [DOI] [PubMed] [Google Scholar]
- 483.Khawaja FJ, Kullo IJ. Novel markers of peripheral arterial disease. Vasc Med. 2009;14:381–392. doi: 10.1177/1358863X09106869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Kim HW, Lin A, Guldberg RE, Ushio-Fukai M, Fukai T. Essential role of extracellular SOD in reparative neovascularization induced by hindlimb ischemia. Circ Res. 2007;101:409–419. doi: 10.1161/CIRCRESAHA.107.153791. [DOI] [PubMed] [Google Scholar]
- 485.Kimura H, Miyashita H, Suzuki Y, Kobayashi M, Watanabe K, Sonoda H, Ohta H, Fujiware T, Shimosegawa T, Sato Y. Distinctive localization and opposed roles of vasohibin-1 and vasohibin-2 in the regulation of angiogenesis. Blood. 2009;113:4810–4118. doi: 10.1182/blood-2008-07-170316. [DOI] [PubMed] [Google Scholar]
- 486.Kindig CA, Poole DC. Effects of skeletal muscle sarcomere length on in vivo capillary distensibility. Microvasc Res. 1999;57:144–152. doi: 10.1006/mvre.1998.2123. [DOI] [PubMed] [Google Scholar]
- 487.Kindig CA, Richardson TE, Poole DC. Skeletal muscle capillary hemodynamics from rest to contractions: implications for oxygen transfer. J Appl Physiol. 2002;92:2513–2520. doi: 10.1152/japplphysiol.01222.2001. [DOI] [PubMed] [Google Scholar]
- 488.Kirk G, Kickman P, McLaren M, Stonebridge PA, Belch JJ. Interleukin-8 (IL-8) may contribute to the activation of neutrophils in patients with peripheral occlusive disease (PAOD) Eur J Vasc Endovasc Surg. 1999;18:434–438. doi: 10.1053/ejvs.1999.0927. [DOI] [PubMed] [Google Scholar]
- 489.Kishi T, Hirooka Y, Ito K, Sakai K, Shhimokawa H, Takeshita A. Cardiovascular effects of overexpresion of endothelial nitric oxide synthase in the rostral ventrolateral medulla in stroke-prone syponteaneously hypertensive rats. Hypertension. 2002;39:264–268. doi: 10.1161/hy0202.102701. [DOI] [PubMed] [Google Scholar]
- 490.Kishlyansky M, Vojnovic J, Roudier E, Gineste C, Decary S, Forn P, Bergeron R, Desplanches D, Birot O. Striated muscle angio-adaptation requires changes in Vasohibin-1 expression pattern. Biochem Biophys Res Commun. 2010;399:359–364. doi: 10.1016/j.bbrc.2010.07.076. [DOI] [PubMed] [Google Scholar]
- 491.Kivela R, Silvennoinen M, Lehti M, Jalava S, Vihko V, Kainulainen H. Exercise-induced expression of angiogenic growth factors in skeletal muscle and in capillaries of healthy and diabetic mice. Cardiovasc Diabetol. 2008;7:13. doi: 10.1186/1475-2840-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Kivela R, Silvennoinen M, Touvra AMaria, Lehti TM, Kainulainen H, Vihko V. Effects of experimental type 1 diabetes and exercise training on angiogenic gene expression and capillarization in skeletal muscle. FASEB J. 2006;20:1570–1572. doi: 10.1096/fj.05-4780fje. [DOI] [PubMed] [Google Scholar]
- 493.Kleiber AC, Zheng H, Schultz D, Peuler JD, Patel KP. Exercise training normalizes enhanced glutamate-mediated sympathetic activation from the PVN in heart failure. Am J Physiol. 2008;294:R1863–R1872. doi: 10.1152/ajpregu.00757.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Koba S, Hayes SG, Sinoway LI. Transient receptor potential A1 channel contributes to activation of the muscle reflex. Am J Physiol. 2011;300:H201–H213. doi: 10.1152/ajpheart.00547.2009. [DOI] [PubMed] [Google Scholar]
- 495.Koba S, Xing J, Sinoway LI, Li J. Sympathetic nerve responses to muscle contraction and stretch in ischemic heart failure. Am J Physiol. 2008;294:H311–H321. doi: 10.1152/ajpheart.00835.2007. [DOI] [PubMed] [Google Scholar]
- 496.Koba S, Xing J, Sinoway LI, Li J. Bradykinin receptor blockade reduces symmpathetic nerve response to muscle contraction in rats with ischemic heart failure. Am J Physiol. 2010;298:H1438–H144. doi: 10.1152/ajpheart.00558.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Koerner JE, Terjung RL. Effect of physical training on coronary collateral circulation of the rat. J Appl Physiol. 1982;52:376–387. doi: 10.1152/jappl.1982.52.2.376. [DOI] [PubMed] [Google Scholar]
- 498.Koh W, Mahan RD, Davis GE. Cdc42- and Rac1-mediated endothelial lumen formation requires Pak2, Pak4 and Par3, and PKC-dependent signaling. J Cell Sci. 2008;121:989–1001. doi: 10.1242/jcs.020693. [DOI] [PubMed] [Google Scholar]
- 499.Korhonen P, Aarnio P. Borderline pheripheral arterial disease. Int J Angiol. 2008;17:175–177. doi: 10.1055/s-0031-1278304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Kou B, Zhang J, Singer DR. Effects of cyclic strain on endothelial cell apoptosis and tubulogenesis are dependent on ROS production via NAD(P)H subunit p22phox. Microvasc Res. 2009;77:125–133. doi: 10.1016/j.mvr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 501.Koutakis P, Johanning JM, Haynatzki GR, Myers SA, Stergiou N, Longo GM, Pipinos II. Abnormal joint powers before and after the onset of claudication symptoms. J Vasc Surg. 2010;52:340–347. doi: 10.1016/j.jvs.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Koutakis P, Pipinos II, Myers SA, Stergiou N, Lynch TG, Johanning JM. Jiont torques and powers are reduced during ambulation for both limbs in patients with unilateral claudication. J Vasc Med. 2010;51:80–88. doi: 10.1016/j.jvs.2009.07.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Kraus RM, Stallings HW, Yeager RC, Gavin TP. Circulating plasma VEGF response to exercise in sedentary and endurance-trained men. J Appl Physiol. 2004;96:1445–1450. doi: 10.1152/japplphysiol.01031.2003. [DOI] [PubMed] [Google Scholar]
- 504.Krogh A. The number and distribution of capillaries in muscles with calculations of the oxygen pressure head necessary for supplying the tissue. J Physiol. 1919;52:409–415. doi: 10.1113/jphysiol.1919.sp001839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Kruidenier LM, Nicolai SP, Rouwet EV, Peters RJ, Prins MH, Teijink JA. Additional supervised exercise therapy after a percutaneous vascular intervention for peripheral arterial disease: a randomized clinical trial. J Vasc Interv Radiol. 2011;22:961–968. doi: 10.1016/j.jvir.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 506.Ku DD, Zaleski JK, Liu S, Brock TA. Vascular endothelial growth factor induces EDRF-dependent relaxation in coronary arteries. Am J Physiol. 1993;265:H586–H592. doi: 10.1152/ajpheart.1993.265.2.H586. [DOI] [PubMed] [Google Scholar]
- 507.Kumar D, Branch BG, Pattillo CB, Hood J, Thoma S, Simpson S, Illum S, Arora N, Chidlow JH, Langston W, Teng X, Lefer DJ, Patel RP, Kevil CG. Chronic sodium nitrite therapy augments ischemia-induced angiogenesis and arteriogenesis. Proc Natl Acad Sci. 2008;105:7540–7545. doi: 10.1073/pnas.0711480105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Kupatt C, Hinkel R, Pfosser A, El-Aouni C, Wuchrer A, Fritz A, Globisch F, Thormann M, Horstkotte J, Lebherz C, Thein E, Banfi A, Boekstegers P. Cotransfection of vascular endothelial growth factor-A and platelet-derived growth factor-B via recombinant adeno-associated virus resolves chronic ischemic malperfusion role of vessel maturation. J Am Coll Cardiol. 2010;56:414–422. doi: 10.1016/j.jacc.2010.03.050. [DOI] [PubMed] [Google Scholar]
- 509.Laitinen L. Griffonia simplicifolia lectins bind specifically to endothelial cells and some epithelial cells in mouse tissues. Histochem J. 1987;19:225–234. doi: 10.1007/BF01680633. [DOI] [PubMed] [Google Scholar]
- 510.Lamalice L, Houle F, Jourdan G, Huot J. Phosphorylation of tyrosine 1214 on VEGFR2 is required for VEGF-induced activation of Cdc42 upstream of SAPK2/p38. Oncogene. 2004;23:434–445. doi: 10.1038/sj.onc.1207034. [DOI] [PubMed] [Google Scholar]
- 511.LaMonte MJ, Blair SN, Church TS. Physical activity and diabetes prevention. J Appl Physiol. 2005;99:1205–1213. doi: 10.1152/japplphysiol.00193.2005. [DOI] [PubMed] [Google Scholar]
- 512.Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes and Development Genes Dev. 2002;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Lanza IR, Nair SK. Regulation of skeletal muscle mitochondrial function: genes to proteins. Acta Physiol. 2010;199:529–547. doi: 10.1111/j.1748-1716.2010.02124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Larsen OA, Lassen NA. Effect of daily muscular exercise in patients with intermittent claudication. Lancet. 1966;2:1093–1096. doi: 10.1016/s0140-6736(66)92191-x. [DOI] [PubMed] [Google Scholar]
- 515.Laufs U, Urhausen A, Werner N, Scharhag J, Heitz A, Kissner G, Bohm M, Kindermann W, Nickenig G. Running exercise of different duration and intensity: effect on endothelial progenitor cells in healthy subjects. Eur J Cardiovasc Prev Rehabil. 2005;12:407–414. doi: 10.1097/01.hjr.0000174823.87269.2e. [DOI] [PubMed] [Google Scholar]
- 516.Laufs U, Werner N, Link A, Endres M, Wassmann S, Jurgens K, Miche E, Bohm M, Nickenig G. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation. 2004;109:220–226. doi: 10.1161/01.CIR.0000109141.48980.37. [DOI] [PubMed] [Google Scholar]
- 517.Laughlin MH, Armstrong RB. Muscular blood flow distribution patterns as a function of running speed in rats. Am J Physiol. 1982;243:H296–H306. doi: 10.1152/ajpheart.1982.243.2.H296. [DOI] [PubMed] [Google Scholar]
- 518.Laughlin MH, Davis MJ, Secher NH, van Lieshout JJ, Arce-Esquivel AA, Simmons GH, Bender SB, Padilla J, Bache RJ, Merkus D, Duncker DJ. Peripheral circulation. Compr Physiol. 2012;2:321–447. doi: 10.1002/cphy.c100048. [DOI] [PubMed] [Google Scholar]
- 519.Lazarous DF, Shou M, Scheinowitz M, Hodge E, Thirumurti V, Kitsiou AN, Stiber JA, Lobo AD, Hunsberger S, Guetta E, Epstein SE, Unger EF. Comparative effects of basic fibroblast growth factor and vasecular endothelial growth factor on coronary collateral development and the arterial response to injury. Circ Res. 1996;94:1074–1082. doi: 10.1161/01.cir.94.5.1074. [DOI] [PubMed] [Google Scholar]
- 520.Leal AK, MA W, Garry MG, Mitchell JH, Smith SA. Evidence for functional alterations in the skeletal muscle mechanoreflex and metaboreflex in hypertensive rats. Am J Physiol. 2008;295:H1429–H1438. doi: 10.1152/ajpheart.01365.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Lee JS, Kim JM, Kim KL, Jang HS, Shin IS, Jeon ES, Suh W, Byun J, Kim DK. Combined administration of naked DNA vectors encoding VEGF and bFGF enhances tissue perfusion and arteriogenesis in ischemic hindlimb. Biochem Biophys Res Commun. 2007;360:752–758. doi: 10.1016/j.bbrc.2007.06.120. [DOI] [PubMed] [Google Scholar]
- 522.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF Signaling Is Required for Vascular Homeostasis 1. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169:681–691. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Lee TM, Su SF, Tsai CH, Lee YT, Wang SS. Differential effects of cilostazol and pentoxifylline on vascular endothelial growth factor in patients with intermittent claudication. Clin Sci (Lond) 2001;101:305–311. [PubMed] [Google Scholar]
- 525.Lefaucheur JP, Gjata B, Sebille A. Factors inducing mast cell accumulation in skeletal muscle. Neuropathol Appl Neurobiol. 1996;22:248–255. [PubMed] [Google Scholar]
- 526.Leick L, Hellsten Y, Fentz J, Lyngby SS, Wojtaszewski JFP, HIdalgo J, Pilegaard H. PGC-1α mediates exercise-induced skeletal muscle VEGF expression in mice. Am J Physiol. 2009;297:E92–E103. doi: 10.1152/ajpendo.00076.2009. [DOI] [PubMed] [Google Scholar]
- 527.Leon AS, Bloor CM. Effects of exercise and its cessation on the heart and its blood supply. J Appl Physiol. 1968;24:485–490. doi: 10.1152/jappl.1968.24.4.485. [DOI] [PubMed] [Google Scholar]
- 528.Leong-Poi H, Kuliszewski MA, Lekas M, Sibbald M, Teichert-Kuliszewska K, Klibanov AL, Stewart DJ, Lindner JR. Therapeutic arteriogenesis by ultrasound-mediated VEGF165 plasmid gene delivery to chronically ischemic skeletal muscle. Circ Res. 2007;101:295–303. doi: 10.1161/CIRCRESAHA.107.148676. [DOI] [PubMed] [Google Scholar]
- 529.Leung W, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 530.Levy AP, Levy NS, Loscalzo J, Calderone A, Takahashi N, Yeo KT, Koren G, Colucci WS, Goldberg MA. Regulation of vascular endothelial growth factor in cardiac myocytes. Circ Res. 1995;76:758–766. doi: 10.1161/01.res.76.5.758. [DOI] [PubMed] [Google Scholar]
- 531.Li J, Sinoway AN, Gao Z, Maile MD, Pu M, Sinoway LI. Muscle mechoreflex and metaboreflex responses after myocardial infarction in rats. Circulation. 2004;110:3049–3054. doi: 10.1161/01.CIR.0000147188.46287.1B. [DOI] [PubMed] [Google Scholar]
- 532.Li W, Shen W, Gill R, Corbly A, Jones B, Belagaje R, Zhang Y, Tang S, Chen Y, Zhai Y, Wang G, Wagle A, Hui K, Westmore M, Hanson J, Chen YF, Simons M, Singh J. High-resolution quantitative computed tomography demonstrating selective enhancement of medium-size collaterals by placental growth factor-1 in the mouse ischemic hindlimb. Circulation. 2006;113:2445–2453. doi: 10.1161/CIRCULATIONAHA.105.586818. [DOI] [PubMed] [Google Scholar]
- 533.Li JM, Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol. 2004;287:R1014–R1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- 534.Li L, Okada H, Takemura G, Esaki M, Kobayashi H, Kanamori H, Kawamura I, Maruyama R, Fujiwara T, Fujiwara H, Tabata Y, Minatoguchi S. Sustained release of erythropoietin using biodegradable gelatin hydrogel microspheres persistently improves lower leg ischemia. J Am Coll Cardiol. 2009;53:2378–2388. doi: 10.1016/j.jacc.2009.02.056. [DOI] [PubMed] [Google Scholar]
- 535.Li P, Akimoto T, Zhang M, Williams RS, Yan Z. Resident stem cells are not required for exercise-induced fiber-type switching and angiogenesis but are necessary for activity-dependent muscle growth. Am J Physiol. 2006;290:C1461–C1468. doi: 10.1152/ajpcell.00532.2005. [DOI] [PubMed] [Google Scholar]
- 536.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 537.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Pulgserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibers. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 538.Lin JW, Sheu WH, Lee WJ, Chen YT, Liu TJ, Ting CT, Lee WL. Circulating hepatocyte growth factor level but not basic fibroblast growth factor level is elevated in angiography-proven symptomatic peripheral artery disease. Angiology. 2007;58:420–428. doi: 10.1177/0003319706294556. [DOI] [PubMed] [Google Scholar]
- 539.Lira VA, Benton CR, Yan Z, Bonen A. PGC-1alpha regulation by exercise training and its influences on muscle function and insulin sensitivity. Am J Physiol. 2010;299:E145–E161. doi: 10.1152/ajpendo.00755.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Lira VA, Brown DL, Lira AK, Kavazis AN, Soltow QA, Zeanah EH, Criswell DS. Nitric oxide and AMPK cooperatively regulate PGC-1 in skeletal muscle cells. J Physiol. 2010;588:3551–3566. doi: 10.1113/jphysiol.2010.194035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Liu J, Gao Z, Li J. Femoral artery occlusion increases expression of ASIC3 in dorsal root ganglion neurons. Am J Physiol. 2010;299:H1357–H1364. doi: 10.1152/ajpheart.00612.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Liu J, Li D, Lu J, Xing J, Li J. Contribution of nerve growth factor to upregulation of P2X3 expression in DRG neurons of rats with femoral artery occlusion. Am J Physiol. 2011;301:H1070–H1079. doi: 10.1152/ajpheart.00188.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Liu H, Rigamonti D, Badr A, Zhang J. Ccm1 regulates microvascular morphogenesis during angiogenesis. J Vasc Res. 2011;48:130–140. doi: 10.1159/000316851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Lloyd PG, Prior BM, Li H, Yang HT, Terjung RL. VEGF receptor antagonism blocks arteriogenesis, but only partially inhibits angiogenesis, in skeletal muscle of exercise-trained rats. Am J Physiol. 2005;288:H759–H768. doi: 10.1152/ajpheart.00786.2004. [DOI] [PubMed] [Google Scholar]
- 545.Lloyd PG, Prior BM, Yang HT, Terjung RL. Angiogenic growth factor expression in rat skeletal muscle in response to exercise training. Am J Physiol. 2003;284:H1668–H1678. doi: 10.1152/ajpheart.00743.2002. [DOI] [PubMed] [Google Scholar]
- 546.Lloyd PG, Yang HT, Terjung RL. Arteriogenesis and angiogenesis in rat ischemic hindlimb: role of nitric oxide. Am J Physiol. 2001;281:H2528–H2538. doi: 10.1152/ajpheart.2001.281.6.H2528. [DOI] [PubMed] [Google Scholar]
- 547.Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci USA. 2002;99:11205–11210. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci USA. 2007;104:3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Loffler M, Morote-Garcia JC, Eltzschig SA, Coe IR, Eltzschig HK. Physiological roles of vascular nucleoside transporters. Arterioscler Thromb Vasc Biol. 2007;27:1004–1013. doi: 10.1161/ATVBAHA.106.126714. [DOI] [PubMed] [Google Scholar]
- 550.Lopez A, Armstrong ML, Harrison DG, Piegors DJ, Heistad DD. Responsiveness of iliac collateral vessels to constrictor stimuli in atherosclerotic primates. Circ Res. 1988;63:1020–1028. doi: 10.1161/01.res.63.6.1020. [DOI] [PubMed] [Google Scholar]
- 551.Lowry CV, Kimmey JS, Felder S, Chi MY, Kaiser KK, Passonneau PN, Kirk KA, Lowry OH. Enzyme paterns in single human muscle fibers. J Biol Chem. 1978;253:8260–8277. [PubMed] [Google Scholar]
- 552.Lu H, Huang D, Saederup N, Charo IF, Ransohoff RM, Zhou L. Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury. FASEB J. 2011;25:358–369. doi: 10.1096/fj.10-171579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Lumb G, Hardy LB. Collaterals and coronary artery narrowing I The effect of coronary artery narrowing on collateral channels in swine. Lab Invest. 1964;13:1530–1540. [PubMed] [Google Scholar]
- 554.Lumb G, Singletary HP, Hardy LB. Collateral circulation following experimental gradual narrowing of the coronary arteries. Angiology. 1962;13:463–465. doi: 10.1177/000331976201301004. [DOI] [PubMed] [Google Scholar]
- 555.Lundby C, Gassmann M, Pilegaard H. Regular endurance training reduces the exercise induced HIF-1alpha and HIF-2alpha mRNA expression in human skeletal muscle in normoxic conditions. Eur J Appl Physiol. 2006;96:363–369. doi: 10.1007/s00421-005-0085-5. [DOI] [PubMed] [Google Scholar]
- 556.Lundby C, Hellsten Y, Jensen MB, Munch AS, Pilegaard H. Erythropoietin receptor in human skeletal muscle and the effects of acute and long-term injections with recombinant human erythropoietin on the skeletal muscle. J Appl Physiol. 2008;104:1154–1160. doi: 10.1152/japplphysiol.01211.2007. [DOI] [PubMed] [Google Scholar]
- 557.Lundgren F, Dahllof AG, Lundholm K, Schersten T, Volkmann R. Intermittent claudication--surgical reconstruction or physical training? A prospective randomized trial of treatment efficiency. Annal Surg. 1989;209:346–355. doi: 10.1097/00000658-198903000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Lundgren F, Dahllof AG, Schersten T, Bylund-Fellenius AC. Muscle enzyme adaptation in patients with peripheral arterial insufficiency: spontaneous adaptation, effect of different treatments and consequences on walking performance. Clin Sci. 1989;77:485–493. doi: 10.1042/cs0770485. [DOI] [PubMed] [Google Scholar]
- 559.Luo F, Wariaro D, Lundberg G, Blegen H, Wahlberg E. Vascular growth factor expression in a rat model of severe limb ischemia. J Surg Res. 2002;108:258–267. doi: 10.1006/jsre.2002.6551. [DOI] [PubMed] [Google Scholar]
- 560.Luo Y, Xu Z, Wan T, He Y, Jones D, Zhang H, Min W. Endothelial-Specific Transgenesis of TNFR2 Promotes Adaptive Arteriogenesis and Angiogenesis. Arterioscler Thromb Vasc Biol. 2010;30:1307–1314. doi: 10.1161/ATVBAHA.110.204222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F, Nagy JA, Hooper A, Priller J, De Klerck B, Compernolle V, Daci E, Bohlen P, Dewerchin M, Herbert JM, Fava R, Matthys P, Carmeliet G, Collen D, Dvorak HF, Hicklin DJ, Carmeliet P. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med. 2002;8:831–840. doi: 10.1038/nm731. [DOI] [PubMed] [Google Scholar]
- 562.Lynge J, Juel C, Hellsten Y. Extracellular formation and uptake of adenosine during skeletal muscle contraction in the rat: role of adenosine transporters. J Physiol. 2001;537:597–605. doi: 10.1111/j.1469-7793.2001.00597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Mac GF, Popel AS. Systems biology of vascular endothelial growth factors. Microcirc. 2008;15:715–738. doi: 10.1080/10739680802095964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.MacDougall JD, Tuxen D, Sale DG, Moroz JR, Sutton JR. Arterial blood pressure response to heavy resistance exercise. J Appl Physiol. 1985;58:785–790. doi: 10.1152/jappl.1985.58.3.785. [DOI] [PubMed] [Google Scholar]
- 565.Mackie BG, Terjung RL. Blood flow to different skeletal muscle fiber types during contraction. Am J Physiol. 1983;245:H265–H275. doi: 10.1152/ajpheart.1983.245.2.H265. [DOI] [PubMed] [Google Scholar]
- 566.Mackie BG, Terjung RL. Influence of training on blood flow to different skeletal muscle fiber types. Journal of Applied Physiology: Respiratory, Environmental & Exercise Physiology. 1983;55:1072–1078. doi: 10.1152/jappl.1983.55.4.1072. [DOI] [PubMed] [Google Scholar]
- 567.MacLean DA, Sinoway LI, Leuenberger U. Systemic hypoxia elevates skeletal muscle interstitial adenosine levels in humans. Circulation. 1998;98:1990–1992. doi: 10.1161/01.cir.98.19.1990. [DOI] [PubMed] [Google Scholar]
- 568.Maglione D, Guerriero V, Viglietto G, li-Bovi P, Persico MG. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc Nat Acad Sci. 1991;88:9267–9271. doi: 10.1073/pnas.88.20.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Mai JV, Edgerton VR, Barnard RJ. Capillarity of red, white and intermediate muscle fibers in trained and untrained guinea pigs. Experientia. 1970;26:1222–1223. doi: 10.1007/BF01897977. [DOI] [PubMed] [Google Scholar]
- 570.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 571.Makanya AN, Hlushchuk R, Djonov VG. Intussusceptive angiogenesis and its role in vascular morphogenesis, patterning, and remodeling. Angiogenesis. 2009;12:113–123. doi: 10.1007/s10456-009-9129-5. [DOI] [PubMed] [Google Scholar]
- 572.Makin AJ, Chung NA, Silverman SH, Lip GY. Vascular endothelial growth factor and tissue factor in patients with established peripheral artery disease: a link between angiogenesis and thrombogenesis? Clin Sci (Lond) 2003;104:397–404. doi: 10.1042/CS20020182. [DOI] [PubMed] [Google Scholar]
- 573.Makitie J. Skeletal muscle capillaries in intermittent claudication. Arch Pathol Lab Med. 1977;101:500–503. [PubMed] [Google Scholar]
- 574.Malek MH, Olfert IM. Global deletion of thrombospondin-1 increases cardiac and skeletal muscle capillarity and exercise capacity in mice. Exp Physiol. 2009;94:749–760. doi: 10.1113/expphysiol.2008.045989. [DOI] [PubMed] [Google Scholar]
- 575.Mall G, Schikora I, Mattfeldt T, Bodle R. Dipyridamole-induced neoformation of capillaries in the rat heart. Quantitative stereological study on papillary muscles. Lab Invest. 1987;57:86–93. [PubMed] [Google Scholar]
- 576.Mangiafico RA, Malatino LS, Spada RS, Santonocito M, Messina R, Dell’Srte S, Attina T. Treadmill exercise-induced release of endothelin-1 in patients with peripheral arterial occlusive disease at Fontaine stage IIb. Int Angiol. 2000;19:14–17. [PubMed] [Google Scholar]
- 577.Manthey J, Gaehtgens P, Hirche H, Hombach V, Steinhagen C. Collateral flow in canine skeletal muscle. Pflugers Arch. 1974;352:303–313. doi: 10.1007/BF00585684. [DOI] [PubMed] [Google Scholar]
- 578.Marcus BH, Williams DM, Dubbert PM, Sallis JF, King AC, Yancey AK, Franklin BA, Buchner D, Daniels SR, Claytor RP. Physical activity intervention studies. Circulation. 2006;114:2739–2752. doi: 10.1161/CIRCULATIONAHA.106.179683. [DOI] [PubMed] [Google Scholar]
- 579.Mason SD, Howlett RA, Kim MJ, Olfert IM, Hogan MC, McNulty W, Hickey RP, Wagner PD, Kahn CR, Giordano FJ, Johnson RS. Loss of skeletal muscle HIF-1alpha results in altered exercise endurance. PLoS Biol. 2004;2:e288. doi: 10.1371/journal.pbio.0020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Mason SD, Rundqvist H, Papandreou I, Duh R, McNulty WJ, Howlett RA, Olfert IM, Sundberg CJ, Denko NC, Poellinger L, Johnson RS. HIF-1alpha in endurance training: suppression of oxidative metabolism. Am J Physiol. 2007;293:R2059–R2069. doi: 10.1152/ajpregu.00335.2007. [DOI] [PubMed] [Google Scholar]
- 581.Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 582.Masuda K, Abdelmohsen K, Gorospe M. RNA-binding proteins implicated in the hypoxic response. J Cell Mol Med. 2009;13:2759–2769. doi: 10.1111/j.1582-4934.2009.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Matas R. Testing the efficiency of the collateral circulation as a preliminary to the occlusion of the great surgical arteries. Ann Surg. 1911;53:1–43. doi: 10.1097/00000658-191101000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Mathien GM, Terjung RL. Muscle blood flow in trained rats with peripheral arterial insufficiency. Am J Physiol. 1990;258:H759–H765. doi: 10.1152/ajpheart.1990.258.3.H759. [DOI] [PubMed] [Google Scholar]
- 585.Mathien GM, Terjung RL. Influence of training following bilateral stenosis of the femoral artery in rats. Am J Physiol. 1986;250:H1050–H1059. doi: 10.1152/ajpheart.1986.250.6.H1050. [DOI] [PubMed] [Google Scholar]
- 586.Mathieu-Costello O, Hepple RT. Muscle structural capacity for oxygen flux from capillary to fiber mitochondria. Exerc Sport Sci Rev. 2002;30:80–84. doi: 10.1097/00003677-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 587.Mathieu-Costello O, Potter RF, Ellis CG, Groom AC. Capillary configuration and fiber shortening in muscles of the rat hindlimb: correlation between corrosion casts and stereological measurements. Microvasc Res. 1988;36:40–55. doi: 10.1016/0026-2862(88)90037-4. [DOI] [PubMed] [Google Scholar]
- 588.Matolo NM, Cohen SE, Wolfman EF. Use of an arteriovenous fistula for treatment of the severely ischemic extremity: experimental evaluation. Ann Surg. 1976;184:622–625. doi: 10.1097/00000658-197611000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Matsumoto T, Bohman S, Dixelius J, Berge T, Dimberg A, Magnusson P, Wang L, Wikner C, Qi JH, Wernstedt C, Wu J, Bruheim S, Mugishima H, Mukhopadhyay D, Spurkland A, Claesson-Welsh L. VEGF receptor-2 Y951 signaling and a role for the adapter molecule TSAd in tumor angiogenesis. EMBO J. 2005;24:2342–2353. doi: 10.1038/sj.emboj.7600709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Matsunaga T, Warltier DC, Weihrauch DW, Moniz M, Tessmer J, Chilian WM. Ischemia-induced coronary collateral growth is dependent on vascular endothelial growth factor and nitric oxide. Circulation. 2000;102:3098–3103. doi: 10.1161/01.cir.102.25.3098. [DOI] [PubMed] [Google Scholar]
- 591.Mattfeldt T, Mall G. Dipyridamole-induced capillary endothelial cell proliferation in the rat heart--a morphometric investigation. Cardiovasc Res. 1983;17:229–237. doi: 10.1093/cvr/17.4.229. [DOI] [PubMed] [Google Scholar]
- 592.Maxwell MP, Hearse DJ, Yellon DM. Species variation in the coronary collateral circulation during regional myocardial ischaemia: a critical determinant of the rate of evolution and extent of myocardial infarction. Cardiovasc Res. 1987;21:737–746. doi: 10.1093/cvr/21.10.737. [DOI] [PubMed] [Google Scholar]
- 593.Mazari FAK, Gulati S, Rahman MNA, Lee HDL, Mehta TA, McCollum PT, Chetter IC. Early outcomes from a randomized, controlled trial of supervised exercise, angioplasty, and combined therapy in intermittent claudication. Ann Vasc Surg. 2010;24:69–79. doi: 10.1016/j.avsg.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 594.McCord JL, Tsuchimochi H, Yamauchi K, Leal AK, Kaufman MP. Tempol attenuates the exercise presor reflex independently of neutralizing reactive oxygen species in femoral arterial ligated rats. J Appl Physiol. 2011;111:971–979. doi: 10.1152/japplphysiol.00535.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.McCue S, Dajnowiec D, Xu F, Zhang M, Jackson MR, Langille BL. Shear Stress Regulates Forward and Reverse Planar Cell Polarity of Vascular Endothelium In Vivo and In Vitro. Circ Res. 2006;98:939–946. doi: 10.1161/01.RES.0000216595.15868.55. [DOI] [PubMed] [Google Scholar]
- 596.McDermott MM. The magnitude of the problem of peripheral arterial disease: Epidemiology and clinical significance. Cleveland Clin J Med. 2006;73:S2–S7. doi: 10.3949/ccjm.73.suppl_4.s2. [DOI] [PubMed] [Google Scholar]
- 597.McDermott MM, Ades P, Guralnik JM, Dyer A, Ferrucci L, Liu K, Nelson M, Lloyd-Jones D, Van Horn L, Garside D, Kibbe M, Domanchuk K, Stein JH, Liao Y, Tao H, Green D, Pearce WH, Schneider JR, McPherson D, Laing ST, McCarthy WJ, Shroff A, Criqui MH. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA. 2009;301:165–174. doi: 10.1001/jama.2008.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.McDermott MM, Ferrucci L, Guralnik JM, Dyer AR, Liu K, Pearce WH, Clark E, Liao Y, Criqui MH. The ankle-brachial index is associated with the magnitude of impaired walking endurance among men and women with peripheral arterial disease. Vasc Med. 2010;15:251–257. doi: 10.1177/1358863X10365181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.McDermott MM, Greenland P, Green D, Guralnik JM, Criqui MH, Liu K, Chan C, Pearce WH, Taylor L, Ridker PM, Schneider JR, Martin GJ, Rifai N, Quann M, Fornage M. D-Dimer, inflammatory markers, and lower extremity functioning in patients with and without peripheral arterial disease. Circulation. 2003;107:3191–3198. doi: 10.1161/01.CIR.0000074227.53616.CC. [DOI] [PubMed] [Google Scholar]
- 600.McDermott MM, Greenland P, Liu K, Guralnik JM, Celic L, Criqui MH, Chan C, Martin GJ, Schneider J, Pearce WH, Taylor LM, Clark E. The ankle brachial index is associated with leg function and physical activity: the Walking and Leg Circulation Study. Ann Intern Med. 2002;136:873–883. doi: 10.7326/0003-4819-136-12-200206180-00008. [DOI] [PubMed] [Google Scholar]
- 601.McDermott MM, Liu K, Criqui MH, Ruth K, Goff D, Saad MF, Wu C, Homma S, Sharrett AR. Ankle-brachial index and subclinical cardiac and carotid disease: the multi-ethnic study of artherosclerosis. Am J Epidemiol. 2005;162:33–41. doi: 10.1093/aje/kwi167. [DOI] [PubMed] [Google Scholar]
- 602.McDermott MM, Liu K, Ferrucci L, Tian L, Guralnik JM, Green D, Tan J, Liao Y, Pearce WH, Schneider JR, McCue K, Ridker PM, Rifai N, Criqui MH. Circulating blood markers and functional impairment in peripheral arterial disease. J Am Geriatric Soc. 2008;56:1504–1510. doi: 10.1111/j.1532-5415.2008.01797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.McDermott MM, Lloyd-Jones DM. The role of biomarkers and genetics in peripheral arterial disease. J Am Coll Cardiol. 2009;54:1228–1237. doi: 10.1016/j.jacc.2009.04.081. [DOI] [PubMed] [Google Scholar]
- 604.McDohnough P, Behnke BJ, Pazdilla DJ, TI M, Poole DC. Control of microvascular oxygen pressuers in rat muscles comprised of different fibre types. J Physiol. 2005;563:903–913. doi: 10.1113/jphysiol.2004.079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.McGee SL, Hargreaves M. AMPK-mediated regulation of transcription in skeletal muscle. Clin Sci. 2010;118:507–518. doi: 10.1042/CS20090533. [DOI] [PubMed] [Google Scholar]
- 606.McGuigan MR, Bronks R, Newton RU, Sharman MJ, Graham JC, Cody DV, Kraemer WJ. Muscle fiber characteristics in patients with peripheral arterial disease. Med Sci Sports Exerc. 2001;33:2016–2021. doi: 10.1097/00005768-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 607.McKenna M, Wolfson SK, Kuller L. The ratio of ankle and arem arterial pressure as an independent predictor of mortality. Atherosclerosis. 1991;87:119–128. doi: 10.1016/0021-9150(91)90014-t. [DOI] [PubMed] [Google Scholar]
- 608.Mees B, Wagner S, Ninci E, Tribulova S, Martin S, van HR, Kostin S, Heil M, de CR, Schaper W. Endothelial nitric oxide synthase activity is essential for vasodilation during blood flow recovery but not for arteriogenesis. Arterioscler Thromb Vasc Biol. 2007;27:1926–1933. doi: 10.1161/ATVBAHA.107.145375. [DOI] [PubMed] [Google Scholar]
- 609.Meininger CJ, Granger HJ. Mechanisms leading to adenosine-stimulated proliferation of microvascular endothelial cells. Am J Physiol. 1990;258:H198–H206. doi: 10.1152/ajpheart.1990.258.1.H198. [DOI] [PubMed] [Google Scholar]
- 610.Melidonis A, Tournis S, Kouvaras G, Baltaretsou E, Hadanis S, Hajissavas I, Tsatsoulis A, Foussas S. Comparison of coronary collateral circulation in diabetic and nondiabetic patients suffering from coronary artery disease. Clin Cardiol. 1999;22:465–471. doi: 10.1002/clc.4960220706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Meyer RA. A linear model of muscle respiration explains monoexponential phosphorcreative changes. Am J Physiol. 1988;254:C548–C553. doi: 10.1152/ajpcell.1988.254.4.C548. [DOI] [PubMed] [Google Scholar]
- 612.Meyer RA, Foley JM. Cellular processes integrating the metabolic response to exercise. Compr Physiol. 2011:841–869. Supplement 29: Handbook of Physiology, Exercise: Regulation and Integration of Multiple Systems. [Google Scholar]
- 613.Meyer RD, Latz C, Rahimi N. Recruitment and activation of phospholipase Cgamma1 by vascular endothelial growth factor receptor-2 are required for tubulogenesis and differentiation of endothelial cells. J Biol Chem. 2003;278:16347–16355. doi: 10.1074/jbc.M300259200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Michelini LC, Stern JE. Exercise-induced neuronal plasticity in central autonomic networks: role in cardiovascular control. Exp Physiol. 2009;94:947–960. doi: 10.1113/expphysiol.2009.047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Michell BJ, Griffiths JE, Mitchelhill KI, Rodriguez-Crespo I, Tiganis T, Bozinovski S, de MPR, Kemp BE, Pearson RB. The Akt kinase signals directly to endothelial nitric oxide synthase. Curr Biol. 1999;9:845–848. doi: 10.1016/s0960-9822(99)80371-6. [DOI] [PubMed] [Google Scholar]
- 616.Middlekauff HR, Chiu J, Hamilton MA, Fonarow GC, Maclellan WR, Hage A, Moriguchi JD, Patel J. Muscle mechanoreceptor sensitivity in heart failure. Am J Physiol. 2004;287:H1937–H1943. doi: 10.1152/ajpheart.00330.2004. [DOI] [PubMed] [Google Scholar]
- 617.Middlekauff HR, Nitzsche EU, Hoh CK, Hamilton MA, Fonarow GC, Hage A, Moriguchi JD. Exaggerated muscle mechanoreflex control of reflex renal vasoconstriction in heart failure. J Appl Physiol. 2001;90:1714–1719. doi: 10.1152/jappl.2001.90.5.1714. [DOI] [PubMed] [Google Scholar]
- 618.Milani RV, Lavie CJ. The role of exercise training in peripheral arterial disease. Vasc Med. 2007;12:351–358. doi: 10.1177/1358863X07083177. [DOI] [PubMed] [Google Scholar]
- 619.Milanini-Mongiat J, Pouyssegur J, Pages G. Identification of two Sp1 phosphorylation sites for p42/p44 mitogen-activated protein kinases: their implication in vascular endothelial growth factor gene transcription. J Biol Chem. 2002;277:20631–20639. doi: 10.1074/jbc.M201753200. [DOI] [PubMed] [Google Scholar]
- 620.Milkiewicz M, Haas TL. Effect of mechanical stretch on HIF-1{alpha} and MMP-2 expression in capillaries isolated from overloaded skeletal muscles: laser capture microdissection study. Am J Physiol. 2005;289:H1315–H1320. doi: 10.1152/ajpheart.00284.2005. [DOI] [PubMed] [Google Scholar]
- 621.Milkiewicz M, Hudlicka O, Shiner R, Egginton S, Brown M. Vascular endothelial growth factor mRNA and protein do not change in parallel during non-inflammatory skeletal muscle ischaemia in rat. J Physiol. 2006;577:671–678. doi: 10.1113/jphysiol.2006.113357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622.Milkiewicz M, Hudlicka O, Verhaeg J, Egginton S, Brown MD. Differential expression of Flk-1 and Flt-1 in rat skeletal muscle in response to chronic ischaemia: favourable effect of muscle activity. Clin Sci. 2003;105:473–482. doi: 10.1042/CS20030035. [DOI] [PubMed] [Google Scholar]
- 623.Milkiewicz M, Kelland C, Colgan S, Haas TL. Nitric oxide and p38 MAP Kinase mediate shear stress-dependent inhibition of MMP-2 production in microvascular endothelial cells. J Cell Physiol. 2006;208:229–237. doi: 10.1002/jcp.20658. [DOI] [PubMed] [Google Scholar]
- 624.Milkiewicz M, Brown MD, Egginton S, Hudlicka O. Association between shear stress, angiogenesis, and VEGF in skeletal muscles in vivo. Microcirc. 2001;8:229–241. doi: 10.1038/sj/mn/7800074. [DOI] [PubMed] [Google Scholar]
- 625.Milkiewicz M, Doyle JL, Fudalewski T, Ispanovic E, Aghasi M, Haas TL. HIF-1{alpha} and HIF-2{alpha} play a central role in stretch-induced but not shear stress-induced angiogenesis in rat skeletal muscle. J Physiol. 2007;583:753–766. doi: 10.1113/jphysiol.2007.136325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Milkiewicz M, Hudlicka O, Brown MD, Silgram H. Nitric oxide, VEGF, and VEGFR-2: interactions in activity-induced angiogenesis in rat skeletal muscle. Am J Physiol. 2005;289:H336–H343. doi: 10.1152/ajpheart.01105.2004. [DOI] [PubMed] [Google Scholar]
- 627.Milkiewicz M, Mohammedzadeh F, Ispanovic E, Gee E, Haas TL. Static Strain stimulates expression of matrix metalloproteinase-2 and VEGF in microvascular endothelium via JNK and ERK dependent pathways. J Cell Biochem. 2007;100:750–761. doi: 10.1002/jcb.21055. [DOI] [PubMed] [Google Scholar]
- 628.Milkiewicz M, Pugh CW, Egginton S. Inhibition of endogenous HIF inactivation induces angiogenesis in ischaemic skeletal muscles of mice. J Physiol (London) 2004;560:21–26. doi: 10.1113/jphysiol.2004.069757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Milkiewicz M, Roudier E, Doyle JL, Trifonova A, Birot O, Haas TL. Identification of a mechanism underlying regulation of the anti-angiogenic forkhead transcription factor FoxO1 in cultured endothelial cells and ischemic muscle. Am J Pathol. 2011;178:935–944. doi: 10.1016/j.ajpath.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630.Milkiewicz M, Uchida C, Gee E, Fudalewski T, Haas TL. Shear stress-induced Ets-1 modulates protease inhibitor expression in microvascular endothelial cells. J Cell Physiol. 2008;217:502–510. doi: 10.1002/jcp.21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 631.Minchenko A, Bauer T, Salceda S, Caro J. Hypoxic stimulation of vascular endothelial growth factor expression in vitro and in vivo. Lab Invest. 1994;71:374–379. [PubMed] [Google Scholar]
- 632.Minchenko A, Salceda S, Bauer T, Caro J. Hypoxia regulatory elements of the human vascular endothelial growth factor gene. Cell Mol Biol Res. 1994;40:35–39. [PubMed] [Google Scholar]
- 633.Mizuno M, Murphy MN, Mitchell JH, Smith SA. Skeletal muscle reflex-mediated changes in sympathetic nerve activity are abnormal in spontaneously hypertensive rats. Am J Physiol. 2011 Jan; doi: 10.1152/ajpheart.01145.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Mochizuki Y, Nakamura T, Kanetake H, Kanda S. Angiopoietin 2 stimulates migration and tube-like structure formation of murine brain capillary endothelial cells through c-Fes and c-Fyn. J Cell Sci. 2002;115:175–183. doi: 10.1242/jcs.115.1.175. [DOI] [PubMed] [Google Scholar]
- 635.Mole PA, Chung Y, Tran TK, Sailasuta N, Hurd R, Jue T. Myoglobin desaturation with exercise intensity in human gastrocnemius muscle. Am J Physiol. 1999;277:R173–R180. doi: 10.1152/ajpregu.1999.277.1.R173. [DOI] [PubMed] [Google Scholar]
- 636.Monaghan-Benson E, Burridge K. The regulation of vascular endothelial growth factor-induced microvascular permeability requires Rac and reactive oxygen species. J Biol Chem. 2009;284:25602–25611. doi: 10.1074/jbc.M109.009894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events. Circulation. 2007;116:2110–2118. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Morishita R, Nakamura S, Hayashi S, Taniyama Y, Moriguchi A, Nagano T, Taiji M, Noguchi H, Takeshita S, Matsumoto K, Nakamura T, Higaki J, Ogihara T. Therapeutic angiogenesis induced by human recombinant hepatocyte growth factor in rabbit hind limb ischemia model as cytokine supplement therapy. Hypertension. 1999;33:1379–1384. doi: 10.1161/01.hyp.33.6.1379. [DOI] [PubMed] [Google Scholar]
- 639.Morrow NG, Kraus WE, Moore JW, Williams RS, Swain JL. Increased expression of fibroblast growth factors in a rabbit skeletal muscle model of exercise conditioning. J Clin Invest. 1990;85:1816–1820. doi: 10.1172/JCI114640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Mostoufi-Moab S, Widmaier EJ, Cornett JA, Gray KS, Sinoway LI. Forearm training reduces the exercise pressor reflex during ischemic rhythmic handgrip. J Appl Physiol. 1998;84:277–283. doi: 10.1152/jappl.1998.84.1.277. [DOI] [PubMed] [Google Scholar]
- 641.Mueller H. Physical (in)activity-dependent alterations at the rostral ventrolateral medulla: influence on sympathetic nervous system regulation. Am J Physiol. 2010;298:R1468–R1474. doi: 10.1152/ajpregu.00101.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.Muhs BE, Gagne P, Plitas G, Shaw JP, Shamamian P. Experimental hindlimb ischemia leads to neutrophil-mediated increases in gastrocnemius MMP-2 and -9 activity: a potential mechanism for ischemia induced MMP activation. J Surg Res. 2004;117:249–254. doi: 10.1016/j.jss.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 643.Muhs BE, Plitas G, Delgado Y, Ianus I, Shaw JP, Adelman MA, Lamparello P, Shamamian P, Gagne P. Temporal expression and activation of matrix metalloproteinases-2, -9, and membrane type 1-matrix metalloproteinase following acute hindlimb ischemia. J Surg Res. 2003;111:8–15. doi: 10.1016/s0022-4804(02)00034-3. [DOI] [PubMed] [Google Scholar]
- 644.Muller-Delp JM. Aging-induced adaptations of microvascular reactivity. Microcirc. 2006;13:301–314. doi: 10.1080/10739680600619023. [DOI] [PubMed] [Google Scholar]
- 645.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, Huang PL, Isner JM. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Murphy TP, Cutlip DE, Regensteiner JG, Mohler ER, Cohen DJ, Reynolds MR, Massaro JM, Lewis BA, Cerezo J, Oldenburg NC, Thum CC, Goldberg S, Jaff MR, Steffes MW, Comerota AJ, Ehrman JK, Treat-Jacobson D, Walsh ME, Collins T, Badenhop DT, Bronas UG, Hirsch AT. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the Claudication: Exercise Versus Endoluminal Revascularization (CLEVER) Study. Circulation. 2012;125:130–139. doi: 10.1161/CIRCULATIONAHA.111.075770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647.Myers SA, Stergiou N, Pipinos II, Johanning JM. Gait variability patterns are altered in healthy young individuals during the acute reperfusion phase of ischemia-reperfusion. J Surg Res. 2010;164:6–12. doi: 10.1016/j.jss.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 648.Myrhage R, Hudlicka O. Capillary growth in chronically stimulated adult skeletal muscle as studied by intravital microscopy and histological methods in rabbits and rats. Microvasc Res. 1978;16:73–90. doi: 10.1016/0026-2862(78)90046-8. [DOI] [PubMed] [Google Scholar]
- 649.Nagase H, Woessner JF. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 650.Nakano M, Satoh K, Fulkumoto Y, Ito Y, Kagaya Y, Ishii N, Sugamura K, Shimokawa H. Important role of erythropoietin receptor in promoting vascular endothelial growth factor expression and angiogenesis in peripheral ischemia in mice. Arterioscler Thromb Vasc Biol. 2007;27:E101–E101. doi: 10.1161/01.RES.0000260179.43672.fe. [DOI] [PubMed] [Google Scholar]
- 651.Namiki A, Brogi E, Kearney M, Kim EA, Wu T, Couffinhal T, Varticovski L, Isner JM. Hypoxia induces vascular endothelial growth factor in cultured human endothelial cells. J Biol Chem. 1995;270:31189–31195. doi: 10.1074/jbc.270.52.31189. [DOI] [PubMed] [Google Scholar]
- 652.Nawaz S, Walker RD, Wilkinson CH, Saxton JM, Pockley AG, Wood RF. The inflammatory response to upper and lower limb exercise and the effects of exercise training in patients with claudication. Journal of Vascular Surgery. 2001;33:392–399. doi: 10.1067/mva.2001.111988. [DOI] [PubMed] [Google Scholar]
- 653.Nemeth ZH, Lutz CS, Csoka B, Deitch EA, Leibovich SJ, Gause WC, Tone M, Pacher P, Vizi ES, Hasko G. Adenosine augments IL-10 production by macrophages through an A2B receptor-mediated posttranscriptional mechanism. J Immunol. 2005;175:8260–8270. doi: 10.4049/jimmunol.175.12.8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 654.Nestico PF, Hakki AH, Meissner MD, Bemis CE, Kimbiris D, Mintz GS, Segal BL, Iskandrian AS. Effect of collateral vessels on prognosis in patients with one vessel coronary artery disease. J Am Coll Cardiol. 1985;6:1257–1263. doi: 10.1016/s0735-1097(85)80211-4. [DOI] [PubMed] [Google Scholar]
- 655.Neumann FJ, Waas W, Diehm C, Weiss T, Haupt HM, Zimmermann R, Tillmanns H, Kubler W. Activation and decreased deformability of neutrophils after intermittent claudication. Circulation. 1990;82:922–929. doi: 10.1161/01.cir.82.3.922. [DOI] [PubMed] [Google Scholar]
- 656.Newman AB, Siscovick DS, Manolio TA, Polak J, Fried LP, Borhani NO, Wolfson SK. Ankle-arm index as a marker of atherosclerosis in the cardiovascular health study. Circulation. 1993;88:837–845. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 657.Nicklas BJ, You T, Pahor M. Behavioural treatments for chronic systemic inflammation: effects of dietary weight loss and exercise training. Can Med Assoc J. 2005;172:1199–1209. doi: 10.1503/cmaj.1040769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Nicolai SP, Viechtbauer W, Kriudenier LM, Candel MJ, Prins MH, Teijink JA. Reliability of treadmill testing in peripheral arterial disease: a meta-regression analysis. J Vasc Surg. 2009;50:322–329. doi: 10.1016/j.jvs.2009.01.042. [DOI] [PubMed] [Google Scholar]
- 659.Nishimura K, Li W, Hoshino Y, Kadohama T, Asada H, Ohgi S, Sumpio BE. Role of AKT in cyclic strain-induced endothelial cell proliferation and survival. Am J Physiol. 2006;290:C812–C821. doi: 10.1152/ajpcell.00347.2005. [DOI] [PubMed] [Google Scholar]
- 660.Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, Lin HC, Yancopoulos GD, Thurston G. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 661.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR. Inter-society consensus for the management of peripheral arterial disease (TASC II) J Vasc Surg. 2007;45:S5A–S67A. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 662.JN, Sällstedt EK, Fischer H, Sundberg CJ, Rundqvist H, Gustafsson T. Alternative splice variant PGC-1α-b is strongly induced by exercise in human skeletal muscle. Am J Physiol. 2011;301:E1092–E1098. doi: 10.1152/ajpendo.00119.2011. [DOI] [PubMed] [Google Scholar]
- 663.Norrbom J, Sundberg CJ, Ameln H, Kraus WE, Jansson E, Gustafsson T. PGC-1alpha mRNA expression is influenced by metabolic perturbation in exercising human skeletal muscle. J Appl Physiol. 2004;96:189–194. doi: 10.1152/japplphysiol.00765.2003. [DOI] [PubMed] [Google Scholar]
- 664.Novak A, Dedhar S. Signaling through beta-catenin and Lef/Tcf. Cell Mol Life Sci. 1999;56:523–537. doi: 10.1007/s000180050449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 665.Nugent MA, Iozzo RV. Fibroblast growth factor-2. Int J Biochem Cell Biol. 2000;32:115–120. doi: 10.1016/s1357-2725(99)00123-5. [DOI] [PubMed] [Google Scholar]
- 666.Nylaende M, Abdelnoor M, Stranden E, Morken BWMR, Fowkes FGR, Kerracher EMG, Gillespie IN, Lee AJ, Housley E, Ruckley C. The Oslo balloon angioplasty versus conservative treatment study (OBACY)--the 2-years results of a single centre, prospective, randomised study in patients with intermittent claudication. Eur J Vasc Endovas Surg. 2007;33:3–12. doi: 10.1016/j.ejvs.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 667.Nylaende M, Kroese A, Stranden E, Morken B, Sandbaek G, Lindahl AK, Arnesen H, Seljeflot I. Markers of vascular inflammation re associated with the extent of atherosclerosis assessed as angiographic score and treadmill walking distances in patients with peripheral arterial occlusive disease. Vasc Med. 2006;11:21–28. doi: 10.1191/1358863x06vm662oa. [DOI] [PubMed] [Google Scholar]
- 668.Nylaende M, Kroese AJ, Morken B, Stranden E, Sandbaek G, Lindahl AK, Armesen H, Seljeflot I. Beneficial effect of 1-year optimal medical treatment with and without aditional PTA on inflammatory markers of atherosclerosis in patients with PAD. Results from the Oslo Balloon Angioplasty versus Conservative Treatment (OBACT) study. Vasc Med. 2007;12:275–283. doi: 10.1177/1358863X07082720. [DOI] [PubMed] [Google Scholar]
- 669.O’Leary DS. Altered reflex cardiovascular control during exercise in heart failure: animal studies. Exp Physiol. 2006;91:73–77. doi: 10.1113/expphysiol.2005.031179. [DOI] [PubMed] [Google Scholar]
- 670.O’Reilly MS. Angiostatin: an endogenous inhibitor of angiogenesis and of tumor growth. EXS. 1997;79:273–294. [PubMed] [Google Scholar]
- 671.O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 672.O’Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 673.Ochoa O, Sun D, Reyes-Reyna SM, Waite LL, Michalek JE, McManus LM, Shireman PK. Delayed angiogenesis and VEGF production in CCR2−/− mice during impaired skeletal muscle regeneration. Am J Physiol. 2007;293:R651–R661. doi: 10.1152/ajpregu.00069.2007. [DOI] [PubMed] [Google Scholar]
- 674.Odorisio T, Schietroma C, Zaccaria ML, Cianfarani F, Tiveron C, Tatangelo L, Failla CM, Zambruno G. Mice overexpressing placenta growth factor exhibit increased vascularization and vessel permeability. J Cell Sci. 2002;115:2559–2567. doi: 10.1242/jcs.115.12.2559. [DOI] [PubMed] [Google Scholar]
- 675.Oh IY, Yoon CH, Hur J, Kim JH, Kim TY, Lee CS, Park KW, Chae IH, Oh BH, Park YB, Kim HS. Involvement of E-selectin in recruitment of endothelial progenitor cells and angiogenesis in ischemic muscle. Blood. 2007;110:3891–3899. doi: 10.1182/blood-2006-10-048991. [DOI] [PubMed] [Google Scholar]
- 676.Olesen J, Killerich K, Pilegaard H. PGC-1alpha-mediated adaptations in skeletal muscle. Pflugers Arch. 2010;460:153–162. doi: 10.1007/s00424-010-0834-0. [DOI] [PubMed] [Google Scholar]
- 677.Olfert IM, Breen EC, Gavin TP, Wagner PD. Temporal thrombospondin-1 mRNA response in skeletal muscle exposed to acute and chronic exercise. Growth Factors. 2006;24:253–259. doi: 10.1080/08977190601000111. [DOI] [PubMed] [Google Scholar]
- 678.Olfert IM, Breen EC, Mathieu-Costello O, Wagner PD. Skeletal muscle capillarity and angiogenic mRNA levels after exercise training in normoxia and chronic hypoxia. J Appl Physiol. 2001;91:1176–1184. doi: 10.1152/jappl.2001.91.3.1176. [DOI] [PubMed] [Google Scholar]
- 679.Olfert IM, Howlett RA, Wagner RD, Breen EC. Myocyte vascular endothelial growth factor is required for exercise-induced skeletal muscle angiogenesis. Am J Physiol. 2010;299:R1059–R1067. doi: 10.1152/ajpregu.00347.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680.Olfert IM, Birot O. Importance of anti-angiogenic factors in the regulation of skeletal muscle angiogenesis. Microcirc. 2011;18:316–330. doi: 10.1111/j.1549-8719.2011.00092.x. [DOI] [PubMed] [Google Scholar]
- 681.Olfert IM, Breen EC, Mathieu-Costello O, Wagner PD. Chronic hypoxia attenuates resting and exercise-induced VEGF, flt-1, and flk-1 mRNA levels in skeletal muscle. J Appl Physiol. 2001;90:1532–1538. doi: 10.1152/jappl.2001.90.4.1532. [DOI] [PubMed] [Google Scholar]
- 682.Olfert IM, Howlett RA, Tang K, Dalton ND, Gu Y, Peterson KL, Wagner PD, Breen EC. Muscle-specific VEGF deficiency greatly reduces exercise endurance in mice. J Physiol. 2009;587:1755–1767. doi: 10.1113/jphysiol.2008.164384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 683.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 684.Onimaru M, Yonemitsu Y, Suzuki H, Fujii T, Sueishi K. An autocrine linkage between matrix metalloproteinase-14 and Tie-2 via ectodomain shedding modulates angiopoietin-1-dependent function in endothelial cells. Arterioscler Thromb Vasc Biol. 2010;30:818–826. doi: 10.1161/ATVBAHA.109.201111. [DOI] [PubMed] [Google Scholar]
- 685.Oosthuyse B, Moons L, Storkebaum E, Beck H, Nuyens D, Brusselmans K, Van DJ, Hellings P, Gorselink M, Heymans S, Theilmeier G, Dewerchin M, Laudenbach V, Vermylen P, Raat H, Acker T, Vleminckx V, Van D, Bosch L, Cashman N, Fujisawa H, Drost MR, Sciot R, Bruyninckx F, Hicklin DJ, Ince C, Gressens P, Lupu F, Plate KH, Robberecht W, Herbert JM, Collen D, Carmeliet P. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet. 2001;28:131–138. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- 686.Orlidge A, D’Amore PA. Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J Cell Biol. 1987;105:1455–1462. doi: 10.1083/jcb.105.3.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 687.Oshima Y, Oshima S, Nambu H, Kachi S, Takahashi K, Umeda N, Shen J, Dong A, Apte RS, Duh E, Hackett SF, Okoye G, Ishibashi K, Handa J, Melia M, Wiegand S, Yancopoulos G, Zack DJ, Campochiaro PA. Different effects of angiopoietin-2 in different vascular beds: new vessels are most sensitive. FASEB J. 2005;19:963–965. doi: 10.1096/fj.04-2209fje. [DOI] [PubMed] [Google Scholar]
- 688.Ota H, Eto M, Kano MR, Ogawa S, Iijima K, Akishita M, Ouchi Y. Cilostazol inhibits oxidative stress-induced premature senescence via upregulation of Sirt1 in human endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:1634–1639. doi: 10.1161/ATVBAHA.108.164368. [DOI] [PubMed] [Google Scholar]
- 689.Owens CD, Ho KJ, Conte MS. Lower extremity vein graft failure: a translational approach. Vasc Med. 2008;13:63–74. doi: 10.1177/1358863X07083432. [DOI] [PubMed] [Google Scholar]
- 690.Padilla DJ, McDonough P, Behnke BJ, Kano Y, Hageman KS, Musch TI, Poole DC. Effects of Type II diabetes on capillary hemodynamics in skeletal muscle. Am J Physiol. 2006;291:H2439–H2444. doi: 10.1152/ajpheart.00290.2006. [DOI] [PubMed] [Google Scholar]
- 691.Pages G, Milanini J, Richard DE, Berra E, Gothie E, Vinals F, Pouyssegur J. Signaling Angiogenesis via p42/p44 MAP Kinase Cascade. Ann NY Acad Sci. 2000;902:187–200. doi: 10.1111/j.1749-6632.2000.tb06313.x. [DOI] [PubMed] [Google Scholar]
- 692.Pages G, Pouyssegur J. Transcriptional regulation of the Vascular Endothelial Growth Factor gene--a concert of activating factors. Cardiovasc Res. 2005;65:564–573. doi: 10.1016/j.cardiores.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 693.Palatini P, Mos L, Munari L, Valle F, Del Torre M, Rossi A, Varotto L, Macor F, Martina S, Pessina AC. Blood pressure changes during heavy-resistance exercise. J Hypertens. 1989;7:S72–S73. doi: 10.1097/00004872-198900076-00032. [DOI] [PubMed] [Google Scholar]
- 694.Palumbo R, Gaetano C, Antonini A, Pompilio G, Bracco E, Ronnstrand L, Heldin CH, Capogrossi MC. Different effects of high and low shear stress on platelet-derived growth factor isoform release by endothelial cells: consequences for smooth muscle cell migration. Arterioscler Thromb Vasc Biol. 2002;22:405–411. doi: 10.1161/hq0302.104528. [DOI] [PubMed] [Google Scholar]
- 695.Paoni NF, Peale F, Wang F, Errett-Baroncini C, Steinmetz H, Toy K, Bai W, Williams PM, Bunting S, Gerritsen ME, Powell-Braxton L. Time course of skeletal muscle repair and gene expression following acute hind limb ischemia in mice. Physiol Genomics. 2002;11:263–272. doi: 10.1152/physiolgenomics.00110.2002. [DOI] [PubMed] [Google Scholar]
- 696.Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 1997;100:3131–3139. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 697.Parenti A, Morbidelli L, Cui XL, Douglas JG, Hood JD, Granger HJ, Ledda F, Ziche M. Nitric oxide is an upstream signal of vascular endothelial growth factor-induced extracellular signal-regulated kinase1/2 activation in postcapillary endothelium. J Biol Chem. 1998;273:4220–4226. doi: 10.1074/jbc.273.7.4220. [DOI] [PubMed] [Google Scholar]
- 698.Park B, Hoffman A, Yang Y, Yan J, Tie G, Bagshahi H, Nowicki PT, Messina LM. Endothelial nitric oxide synthase affects both early and late collateral arterial adaptation and blood flow recovery after induction of hind limb ischemia in mice. J Vasc Surg. 2010;51:165–173. doi: 10.1016/j.jvs.2009.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 699.Parmenter BJ, Raymond J, Fiatarone Singh MA. The effect of exercise on haemodynamics in intermittent claudication: A systemic review of randomized controlled trials. Sports Med. 2010;40:433–447. doi: 10.2165/11531330-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 700.Paskins-Hurlburt AJ, Hollenberg NK. “Tissue need” and limb collateral arterial growth Skeletal contractile power and perfusion during collateral development in the rat. Circ Res. 1992;70:546–553. doi: 10.1161/01.res.70.3.546. [DOI] [PubMed] [Google Scholar]
- 701.Pasyk S, Schaper W, Schaper J, Pasyk K, Miskiewicz G, Steinseifer B. DNA synthesis in coronary collaterals after coronary artery occlusion in conscious dog. Am J Physiol. 1982;242:H1031–H1037. doi: 10.1152/ajpheart.1982.242.6.H1031. [DOI] [PubMed] [Google Scholar]
- 702.Patan S, Alvarez MJ, Schittny JC, Burri PH. Intussusceptive microvascular growth: a common alternative to capillary sprouting. Arch Histol Cytol. 1992;55 (Suppl):65–75. doi: 10.1679/aohc.55.suppl_65. [DOI] [PubMed] [Google Scholar]
- 703.Patel KP. Role of paraventricular nucleus in mediating sympathetic outflow in heart failure. Heart Fail Rev. 2000;5:73–86. doi: 10.1023/A:1009850224802. [DOI] [PubMed] [Google Scholar]
- 704.Payvandi L, Dyer A, McPherson D, Ades P, Stein JH, Liu K, Ferrucci L, Criqui MH, Guralnik JM, Lloyd-Jones D, Kibbe MR, Liang ST, Kane B, Pearce WH, Verta M, McCarthy WJ, Schneider JR, Shroff A, McDermott MM. Physical activity during daily life and brachial artery flow-mediated dilation in peripheral arterial disease. Vasc Med. 2009;14:193–201. doi: 10.1177/1358863X08101018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 705.Pedersen BK. Health benefits related to exercise in patients with chronic low-grade systemic inflammation. Am J Lifestyle Med. 2007;1:289–298. [Google Scholar]
- 706.Pedersen BK. The diseaseome of physical inactivity--and the role of myokines in muscle--fat cross talk. J Physiol. 2009;587:5559–5568. doi: 10.1113/jphysiol.2009.179515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 707.Peng J, Zhang L, Drysdale L, Fong GH. The transcription factor EPAS-1/hypoxia-inducible factor 2alpha plays an important role in vascular remodeling. Proc Natl Acad Sci USA. 2000;97:8386–8391. doi: 10.1073/pnas.140087397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 708.Pepper MS. Manipulating angiogenesis. Arterioscler Thromb Vasc Biol. 1997;17:605–619. doi: 10.1161/01.atv.17.4.605. [DOI] [PubMed] [Google Scholar]
- 709.Pepper MS. Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler Thromb Vasc Biol. 2001;21:1104–1117. doi: 10.1161/hq0701.093685. [DOI] [PubMed] [Google Scholar]
- 710.Perkins JM, Collin J, Creasy TS, Fletcher EW, Morris PJ. Exercise training versus angioplasty for stable claudication Long and medium term results of a prospective, randomised trial. Eur J Vasc Endovasc Surg. 1996;11:409–413. doi: 10.1016/s1078-5884(96)80171-7. [DOI] [PubMed] [Google Scholar]
- 711.Perkins JMT, Collin J, Creasy TS, Fletcher EW, Morris PJ. Exercise training versus angioplaty for stable claudication Long and medium term results of a prospective, randomised trial. Eur J Vasc Endovas Surg. 2011;42:S41–S45. doi: 10.1016/j.ejvs.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 712.Perrone-Filardi P, Cuocolo A, Brevetti G, Silvestro A, Storto G, Dellegrottaglie S, Corrado L, Cafiero M, Camerino R, Polimeno M, Zarrilli A, Caiazzo G, Maglione A, Petretta A, Chiariello M. Relation of brachial artery flow-mediated vasodilation to significant coronary artery disease in patients with peripheral arterial disease. Am J Cardiol. 2005;96:1337–1341. doi: 10.1016/j.amjcard.2005.06.084. [DOI] [PubMed] [Google Scholar]
- 713.Peters BP, Goldstein IJ. The use of fluorescein-conjugated Bandeiraea simplicifolia B4-isolectin as a histochemical reagent for the detection of alpha-D-galactopyranosyl groups Their occurrence in basement membranes. Exp Cell Res. 1979;120:321–334. doi: 10.1016/0014-4827(79)90392-6. [DOI] [PubMed] [Google Scholar]
- 714.AMW P, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 715.Pette D. Historical Perspectives: plasticity of mammalian skeletal muscle. J Appl Physiol. 2001;90:1119–1124. doi: 10.1152/jappl.2001.90.3.1119. [DOI] [PubMed] [Google Scholar]
- 716.Piepoli M, Clark AL, Volterrani M, Adamopoulos S, Sleight P, Coats AJS. Contribution of muscle afferents to the hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure. Circulation. 1996;93:940–952. doi: 10.1161/01.cir.93.5.940. [DOI] [PubMed] [Google Scholar]
- 717.Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol. 2003;546:851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 718.Pipinos II, Judge AR, Selsby JT, Zhu Z, Swanson SA, Nella AA, Dodd SL. The myopathy of peripheral arterial occlusive disease: Part 1 Functional and histomorphological changes and evidence for mitochondrial dysfunction. Vasc Endovasc Surg. 2008;41:481–489. doi: 10.1177/1538574407311106. [DOI] [PubMed] [Google Scholar]
- 719.Pipinos II, Judge AR, Selsby JT, Zhu Z, Swanson SA, Nella AA, Dodd SL. The myopathy of peripheral occlusive disease: Part 2 Oxidative stress, neuropathy, and shift in muscle fiber type. Vasc Endovasc Surg. 2008;42:101–112. doi: 10.1177/1538574408315995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 720.Pipinos II, Judge AR, Zhu Z, Selsby JT, Swanson SA, Johanning JM, Bascter BT, Lynch TG, Dodd SL. Mitochondrial defects and oxidative damage in patients with peripheral arterial disease. Free Rad Biol Med. 2006;41:262–269. doi: 10.1016/j.freeradbiomed.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 721.Pipinos II, Sharow VG, Shepard AD, Anagnostopoulos PV, Katsamouris A, Takor A, Filis KA, Sabbah HN. Abnormal mitochondrial respiration in skeletal muscle in patients with peripheral arterial disease. J Vasc Sur. 2003;38:827–832. doi: 10.1016/s0741-5214(03)00602-5. [DOI] [PubMed] [Google Scholar]
- 722.Pipp F, Boehm S, Cai WJ, Adili F, Ziegler B, Karanovic G, Ritter R, Balzer J, Scheler C, Schaper W, Schmitz-Rixen T. Elevated fluid shear stress enhances postocclusive collateral artery growth and gene expression in the pig hind limb. Arterioscler Thromb Vasc Biol. 2004;24:1664–1668. doi: 10.1161/01.ATV.0000138028.14390.e4. [DOI] [PubMed] [Google Scholar]
- 723.Pipp F, Heil M, Issbrucker K, Ziegelhoeffer T, Martin S, van dH J, Weich H, Fernandez B, Golomb G, Carmeliet P, Schaper W, Clauss M. VEGFR-1-selective VEGF homologue PlGF is arteriogenic: evidence for a monocyte-mediated mechanism. Circ Res. 2003;92:378–385. doi: 10.1161/01.RES.0000057997.77714.72. [DOI] [PubMed] [Google Scholar]
- 724.Planas A, Clara A, Pou JM, Vidal-Barraquer F, Gasol A, deMoner A, Contreras C, Marrugat J. Relationship of obesity distribution and preipheral arterial occlusive disease in elderly men. Int J Obes Relat Metab Disord. 2001;25:1068–1070. doi: 10.1038/sj.ijo.0801638. [DOI] [PubMed] [Google Scholar]
- 725.Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 726.Pogozelski AR, Geng T, Li P, Yin X, Lira VA, Zhang M, Chi JT, Yan Z. p38gamma mitogen-activated protein kinase is a key regulator in skeletal muscle metabolic adaptation in mice. PLoS ONE. 2009;4:e7934. doi: 10.1371/journal.pone.0007934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 727.Pozzi A, LeVine WF, Gardner HA. Low plasma levels of matrix metalloproteinase 9 permit increased tumor angiogenesis. Oncogene. 2002;21:272–281. doi: 10.1038/sj.onc.1205045. [DOI] [PubMed] [Google Scholar]
- 728.Price JF, Leng GC, Fowkes FG. Should claudicants receive angioplasty or exercise training? Cardiovasc Surg. 1997;5:463–470. doi: 10.1016/s0967-2109(97)00054-9. [DOI] [PubMed] [Google Scholar]
- 729.Prinzmetal M, Simkin B. Studies on the coronary circulation; the collateral circulation of the normal human heart by coronary perfusion with radioactive erythrocytes and glass spheres. Am Heart J. 1947;33:420–442. doi: 10.1016/0002-8703(47)90090-2. [DOI] [PubMed] [Google Scholar]
- 730.Prior BM, Lloyd PG, Ren J, Li H, Yang HT, Laughlin MH, Terjung RL. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. Am J Physiol. 2004;287:H2434–H2447. doi: 10.1152/ajpheart.00398.2004. [DOI] [PubMed] [Google Scholar]
- 731.Prior BM, Lloyd PG, Ren J, Li Z, Yang HT, Laughlin MH, Terjung RL. Arteriogenesis: role of nitric oxide. Endothelium: Journal of Endothelial Cell Research. 2003;10:207–216. doi: 10.1080/10623320390246388. [DOI] [PubMed] [Google Scholar]
- 732.Prior BM, Ren J, Terjung RL, Yang HT. Significant, but limited collateral blood flow increases occur with prolonged training in rats with femoral artery occlusion. J Physiol Pharmacol. 2011;62:000–000. [PubMed] [Google Scholar]
- 733.Prior BM, Yang HT, Terjung RL. What makes vessels grow with exercise training? J Appl Physiol. 2004;97:1119–1128. doi: 10.1152/japplphysiol.00035.2004. [DOI] [PubMed] [Google Scholar]
- 734.Pu LQ, Jackson S, Lachapelle KJ, Arekat Z, Graham AM, Lisbona R, Brassard R, Carpenter S, Symes JF. A persistent hindlimb ischemia model in the rabbit. J Invest Surg. 1994;7:49–60. doi: 10.3109/08941939409018282. [DOI] [PubMed] [Google Scholar]
- 735.Pu LQ, Sniderman AD, Brassard R, Lachapelle KJ, Graham AM, Lisbona R, Symes JF. Enhanced revascularization of the ischemic limb by angiogenic therapy. Circulation. 1993;88:208–215. doi: 10.1161/01.cir.88.1.208. [DOI] [PubMed] [Google Scholar]
- 736.Pulgserver P, Wu Z, Park CW, Graves R, Wright M, Spiegeelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 737.Quinn TP, Peters KG, De VC, Ferrara N, Williams LT. Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. Proc Natl Acad Sci. 1993;90:7533–7537. doi: 10.1073/pnas.90.16.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 738.Radack K, Wyderski RJ. Conservative management of intermittent claudication. Ann Intern Med. 1990;113:135–146. doi: 10.7326/0003-4819-113-2-135. [DOI] [PubMed] [Google Scholar]
- 739.Rajagopalan S, Mohler ER, Lederman RJ, Mendelsohn FO, Saucedo JF, Goldman CK, Blebea J, Macko J, Kessler PD, Rasmussen HS, Annex BH. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation. 2003;108:1933–1938. doi: 10.1161/01.CIR.0000093398.16124.29. [DOI] [PubMed] [Google Scholar]
- 740.Rakue H, Nakajima H, Katoh T, Usui M, Amemiya T, Miyagi M, Hara T, Tamura K, Sasame A, Naito Y, Nagai Y, Ibukiyama C. Low-dose basic fibroblast growth factor and vascular endothelial growth factor for angiogenesis in canine acute hindlimb insufficiency. Jpn Circ J. 1998;62:933–939. doi: 10.1253/jcj.62.933. [DOI] [PubMed] [Google Scholar]
- 741.Ramanathan M, Giladi A, Leibovich SJ. Regulation of vascular endothelial growth factor gene expression in murine macrophages by nitric oxide and hypoxia. Exp Biol Med (Maywood) 2003;228:697–705. doi: 10.1177/153537020322800608. [DOI] [PubMed] [Google Scholar]
- 742.Ray CA. Sympathetic adaptations to one-legged training. J Appl Physiol. 1999;86:1583–1587. doi: 10.1152/jappl.1999.86.5.1583. [DOI] [PubMed] [Google Scholar]
- 743.Regensteiner JG. Exercise in the treatment of claudication: assessment and treatment of functional impairment. Vascular Medicine. 1997;2:238–242. doi: 10.1177/1358863X9700200313. [DOI] [PubMed] [Google Scholar]
- 744.Regensteiner JG. Exercise rehabilitation for the patient with intermittent claudication: a highly effective yet underutilized treatment. Cur Drug Targets - Cardiovasc Haemato Disorders. 2004;4:233–239. doi: 10.2174/1568006043336195. [DOI] [PubMed] [Google Scholar]
- 745.Regensteiner JG, Hiatt WR. Current medical therapies for patients with peripheral arterial disease: a critical review. Am J Med. 2002;112:49–57. doi: 10.1016/s0002-9343(01)01034-8. [DOI] [PubMed] [Google Scholar]
- 746.Rehn M, Veikkola T, Kukk-Valdre E, Nakamura H, Ilmonen M, Lombardo C, Pihlajaniemi T, Alitalo K, Vuori K. Interaction of endostatin with integrins implicated in angiogenesis. Proc Natl Acad Sci USA. 2001;98:1024–1029. doi: 10.1073/pnas.031564998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 747.Reiser PJ, Kline WO, Vaghy PL. Induction of neuronal type nitric oxide synthase in skeletal muscle by chronic electrical stimulation in vivo. J Appl Physiol. 1997;82:1250–1255. doi: 10.1152/jappl.1997.82.4.1250. [DOI] [PubMed] [Google Scholar]
- 748.Remijnse-Tamerius HC, Duprez D, De Buyzere M, Oeseburg B, Clement DL. Why is training effective in the treatment of patients with intermittent claudication? Int Angiol. 1999;18:103–112. [PubMed] [Google Scholar]
- 749.Resnick HE, Lindsay RS, McDermott MM, Devereax RB, Jones KL, Fabsitz RR, Howard BV. Relationship of high and low ankle brachial index to all-causse and cardiovascular disease mortality The Strong Heart Study. Circulation. 2004;109:733–739. doi: 10.1161/01.CIR.0000112642.63927.54. [DOI] [PubMed] [Google Scholar]
- 750.Rey S, Semenza GL. Hypoxia-inducible factor-1-dependent mechanisms of vascular ization and vascular remodelling. Cardiovasc Res. 2010;86:236–242. doi: 10.1093/cvr/cvq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 751.Rhoads RP, Johnson RM, Rathbone CR, Liu X, Temm-Grove C, Sheehan SM, Hoying JB, Allen RE. Satellite cell-mediated angiogenesis in vitro coincides with a functional hypoxia-inducible factor pathway. Am J Physiol. 2009;296:C1321–C1328. doi: 10.1152/ajpcell.00391.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 752.Rice TW, Lumsden AB. Optimal medical management of peripheral arterial disease. Vasc Endovasc Surg. 2006;40:312–327. doi: 10.1177/1538574406291835. [DOI] [PubMed] [Google Scholar]
- 753.Richardson RS, Duteil S, Wary C, Wray DW, Hoff J, Carlier PG. Human skeletal msucle intracellular oxygenation: the impact of ambient oxygen availability. J Physiol. 2006;571:415–424. doi: 10.1113/jphysiol.2005.102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 754.Richardson RS, Noyszewski EA, Kendrick KF, Leigh JS, Wagner PD. Myoglobin O2 desaturation during exercise Evidence of limited O2 transport. J Clin Invest. 1995;96:1916–1926. doi: 10.1172/JCI118237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 755.Richardson RS, Wagner H, Mudaliar SR, Henry R, Noyszewski EA, Wagner PD. Human VEGF gene expression in skeletal muscle: effect of acute normoxic and hypoxic exercise. Am J Physiol. 1999;277:H2247–H2252. doi: 10.1152/ajpheart.1999.277.6.H2247. [DOI] [PubMed] [Google Scholar]
- 756.Richardson RS, Wagner H, Mudaliar SRD, Saucedo E, Henry R, Wagner PD. Exercise adaptation attenuates VEGF gene expression in human skeletal muscle. Am J Physiol. 2000;279:H772–H778. doi: 10.1152/ajpheart.2000.279.2.H772. [DOI] [PubMed] [Google Scholar]
- 757.Richter EA. Glucose utilization. Compr Physiol Suppl. 2011;29:912–951. [Google Scholar]
- 758.Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, Kowalski J, Watts RJ, Callahan C, Kasman I, Singh M, Chien M, Tan C, Hongo JA, de SF, Plowman G, Yan M. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 759.Rissanen TT, Vajanto I, Hiltunen MO, Rutanen J, Kettunen MI, Niemi M, Leppanen P, Turunen MP, Markkanen JE, Arve K, Alhava E, Kauppinen RA, Yla-Herttuala S. Expression of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 (KDR/Flk-1) in ischemic skeletal muscle and its regeneration. Am J Pathol. 2002;160:1393–1403. doi: 10.1016/S0002-9440(10)62566-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 760.Rivard A, Fabre JE, Silver M, Chen D, Murohara T, Kearney M, Magner M, Asahara T, Isner JM. Age-dependent impairment of angiogenesis. Circulation. 1999;99:111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 761.Rivard A, Silver M, Chen D, Kearney M, Magner M, Annex B, Peters K, Isner JM. Rescue of diabetes-related impairment of angiogenesis by intramuscular gene therapy with adeno-VEGF. Am J Path. 1999;154:355–363. doi: 10.1016/S0002-9440(10)65282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 762.Rivilis I, Milkiewicz M, Boyd P, Goldstein J, Brown MD, Egginton S, Hansen FM, Hudlicka O, Haas TL. Differential involvement of MMP-2 and VEGF during muscle stretch-versus shear stress-induced angiogenesis. Am J Physiol. 2002;283:H1430–H1438. doi: 10.1152/ajpheart.00082.2002. [DOI] [PubMed] [Google Scholar]
- 763.Robbins JL, Jones WS, Duscha BD, Allen JD, Kraus WE, Regensteiner JG, Hiatt WR, Annex BH. Relationship between leg muscle capillary density and peak hyperemic blood flow with endurance capacity in peripheral artery disease. J Appl Physiol. 2011;111:81–86. doi: 10.1152/japplphysiol.00141.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 764.Robeer GG, Brandsma JW, van den Heuvel SP, Smit B, Oostendorp RA, Wittens CH. Exercise therapy for intermittent claudication: a review of the quality of randomised clinical trials and evaluation of predictive factors. Eur J Vasc Endovasc Surg. 1998;15:36–43. doi: 10.1016/s1078-5884(98)80070-1. [DOI] [PubMed] [Google Scholar]
- 765.Roberts CK, Barnard RJ, Jasman A, Balon TW. Acute exercise increases nitric oxide synthase activity in skeletal muscle. Am J Physiol. 1999;277:E390–E394. doi: 10.1152/ajpendo.1999.277.2.E390. [DOI] [PubMed] [Google Scholar]
- 766.Roberts KC, Nixon C, Unthank JL, Lash JM. Femoral artery ligation stimulates capillary growth and limits training-induced increases in oxidative capacity in rats. Microcirc. 1997;4:253–260. doi: 10.3109/10739689709146788. [DOI] [PubMed] [Google Scholar]
- 767.Robinson DM, Ogilvie RW, Tullson PC, Terjung RL. Increased peak oxygen consumption of trained muscle requires increased electron flux capacity. J Appl Physiol. 1994;77:1941–1952. doi: 10.1152/jappl.1994.77.4.1941. [DOI] [PubMed] [Google Scholar]
- 768.Robinson WP, Nguyen LL, Bafford R, Belkin M. Results of second-time angioplasty and stenting for femoropopliteal occlusive disease and factors affecting outcomes. J Vasc Surg. 2011;53:651–657. doi: 10.1016/j.jvs.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 769.Roca J, Agusti AG, Alonso A, Poole DC, Viegas C, Barbera JA, Rodriguez-Roisin R, Ferrer A, Wagner PD. Effects of training on muscle O2 transport at VO2max. J Appl Physiol. 1992;73:1067–1076. doi: 10.1152/jappl.1992.73.3.1067. [DOI] [PubMed] [Google Scholar]
- 770.Roca J, Gavin TP, Jordan M, Siafakas N, Wagner H, Benoit H, Breen E, Wagner PD. Angiogenic growth factor mRNA responses to passive and contraction-induced hyperperfusion in skeletal muscle. J Appl Physiol. 1998;85:1142–1149. doi: 10.1152/jappl.1998.85.3.1142. [DOI] [PubMed] [Google Scholar]
- 771.Rocic P, Kolz C, Reed R, Potter B, Chilian WM. Optimal reactive oxygen species concentration and p38 MAP kinase are required for coronary collateral growth. Am J Physiol. 2007;292:H2729–H2736. doi: 10.1152/ajpheart.01330.2006. [DOI] [PubMed] [Google Scholar]
- 772.Rockl KS, Witczak CA, Goodyear LJ. Signaling mechanisms in skeletal muscle: acute responses and chronic adaptations to exercise. IUBMB Life. 2008;60:145–153. doi: 10.1002/iub.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 773.Romanul FC. Distribution of capillaries in relation to oxidative metabolism of skeletal muscle fibers. Nature. 1964;201:307–308. doi: 10.1038/201307a0. [DOI] [PubMed] [Google Scholar]
- 774.Rose AJ, Hargreaves M. Exercise increases Ca2+-calmodulin-dependent protein kinase II activity in human skeletal muscle. J Physiol. 2003;553:303–309. doi: 10.1113/jphysiol.2003.054171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 775.Rosengart TK, Budenbender KT, Duenas M, Mack CA, Zhang QX, Isom OW. Therapeutic angiogenesis: a comparative study of the angiogenic potential of acidic fibroblast growth factor and heparin. J Vasc Surg. 1997;26:302–312. doi: 10.1016/s0741-5214(97)70193-9. [DOI] [PubMed] [Google Scholar]
- 776.Rosenthal SL, Guyton AC. Hemodynamics of collateral vasodilatation following femoral artery occlusion in anesthetized dogs. Circ Res. 1968;23:239–248. doi: 10.1161/01.res.23.2.239. [DOI] [PubMed] [Google Scholar]
- 777.Rosenthal SL. Effects of collateral flow on muscle gas exchange after femoral artery occlusion. Am J Physiol. 1972;223:461–465. doi: 10.1152/ajplegacy.1972.223.2.461. [DOI] [PubMed] [Google Scholar]
- 778.Roudier E, Gineste C, Wazna A, Dehghan K, Desplanches D, Birot O. Angio-adaptation in unloaded skeletal muscle: new insights into an early and muscle type-specific dynamic process. J Physiol. 2010;588:4579–4591. doi: 10.1113/jphysiol.2010.193243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 779.Roudier E, Chapados N, Decary S, Gineste C, Le BCatherina, Lavoie JMarc, Bergeron R, Birot O. Angiomotin p80/p130 ratio: a new indicator of exercise-induced angiogenic activity in skeletal muscles from obese and non-obese rats? J Physiol. 2009;587:4105–4119. doi: 10.1113/jphysiol.2009.175554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 780.Rowell LB. Human circulation regulation during physical stress. New York: Oxford University Press; 1986. [Google Scholar]
- 781.Rowell LB, Saltin B, Kiens B, Christensen NJ. Is peak quadriceps blood flow in humans even higher during exercise with hypoxemia? Am J Physiol. 1986;251:H1038–H1044. doi: 10.1152/ajpheart.1986.251.5.H1038. [DOI] [PubMed] [Google Scholar]
- 782.Rowlands TE, Donnelly R. Medical therapy for intermittent claudication. Eur J Vasc Endovasc Surg. 2007;34:314–321. doi: 10.1016/j.ejvs.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 783.Roy S, Khanna S, Sen CK. Redox regulation of the VEGF signaling path and tissue vascularization: Hydrogen peroxide, the common link between physical exercise and cutaneous wound healing. Free Radic Biol Med. 2008;44:180–192. doi: 10.1016/j.freeradbiomed.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 784.Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, Betsholtz C, Shima DT. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes and Development Genes Dev. 2002;16:2684–2698. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 785.Ruiter MS, van GJM, Schaper NC, Stehouwer CD, Huijberts MS. Diabetes impairs arteriogenesis in the peripheral circulation: review of molecular mechanisms. Clin Sci. 2010;119:225–238. doi: 10.1042/CS20100082. [DOI] [PubMed] [Google Scholar]
- 786.Rullman E, Norrbom J, Stromberg A, Wagsater D, Rundqvist H, Haas T, Gustafsson T. Endurance exercise activates matrix metalloproteinases in human skeletal muscle. J Appl Physiol. 2009;106:804–812. doi: 10.1152/japplphysiol.90872.2008. [DOI] [PubMed] [Google Scholar]
- 787.Rullman E, Rundqvist H, Wagsater D, Fischer H, Eriksson P, Sundberg CJ, Jansson E, Gustafsson T. A single bout of exercise activates matrix metalloproteinase in human skeletal muscle. J Appl Physiol. 2007;102:2346–2351. doi: 10.1152/japplphysiol.00822.2006. [DOI] [PubMed] [Google Scholar]
- 788.Rundqvist H, Rullman E, Sundberg CJ, Fischer H, Eisleitner K, Stahlberg M, Sundblad P, Jansson E, Gustafsson T. Activation of the erythropoietin receptor in human skeletal muscle. Eur J Endocrinol. 2009;161:427–434. doi: 10.1530/EJE-09-0342. [DOI] [PubMed] [Google Scholar]
- 789.Russell AP, Fellchenfeldt J, Schreiber S, Praz M, Crettenand A, Gobelet C, Meier CA, Bell DR, Kralli A, Giacobino JP, Deriaz O. Endurance training in humans leads to fiber type=specific increases in levels of perixisome proliferator-activated receptor-gamma coactivator-1 and perioxisome proliferator-activate receptor-alha in skeletal muscle. Diabetes. 2003;52:2874–2881. doi: 10.2337/diabetes.52.12.2874. [DOI] [PubMed] [Google Scholar]
- 790.Ryan AS, Katzel LI, Gardner AW. Determinants of peak V(O2) in peripheral arterial occlusive disease patients. J Geron Series A-Biol Sci Med Sci. 2000;55:B302–B306. doi: 10.1093/gerona/55.6.b302. [DOI] [PubMed] [Google Scholar]
- 791.Ryan NA, Zwetsloot KA, Westerkamp LM, Hickner RC, Pofahl WE, Gavin TP. Lower skeletal muscle capillarization and VEGF expression in aged vs young men. J Appl Physiol. 2006;100:178–185. doi: 10.1152/japplphysiol.00827.2005. [DOI] [PubMed] [Google Scholar]
- 792.Ryzhov S, McCaleb JL, Goldstein AE, Biaggioni I, IF Role of adenosine receptors in the regulation of angiogenic factors and neovascularization in hypoxia. J Pharmacol Exp Ther. 2007;320:565–572. doi: 10.1124/jpet.106.114850. [DOI] [PubMed] [Google Scholar]
- 793.Safdar A, Abadi A, Akhtar M, Hettinga BP, Tarnopolsky MA. miRNA in the regulation of skeletal muscle adaptation to acute endurance exercise in C57Bl/6J male mice. PLoS ONE. 2009;4:e5610. doi: 10.1371/journal.pone.0005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 794.Safdar A, Little JP, Stokl AJ, Hettinga BP, Akhtar M, Tarnopolsky MA. Exercise increases mitochondrial PGC-1alpha content and promotes nuclear-mitochondrial cross-talk to coordinate mitochondrial biogenesis. J Biol Chem. 2011;286:10605–10617. doi: 10.1074/jbc.M110.211466. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 795.Sager HB, Middendorff R, Rauche K, Weil J, Lieb W, Schunkert H, Ito WD. Temporal patterns of blood flow and nitric oxide synthase expression affect macrophage accumulation and proliferation during collateral growth. J Angiogenes Res. 2010;2:18. doi: 10.1186/2040-2384-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 796.Sainson RC, Johnston DA, Chu HC, Holderfield MT, Nakatsu MN, Crampton SP, Davis J, Conn E, Hughes CC. TNF primes endothelial cells for angiogenic sprouting by inducing a tip cell phenotype. Blood. 2008;111:4997–5007. doi: 10.1182/blood-2007-08-108597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 797.Saltin B, Gollnick PD. Skeletal msucle adaptability: significance for metabolism and performance. Compr Physiol. 2011:555–631. Supplement 27: Handbook of Physiology, Skeletal Muscle. [Google Scholar]
- 798.Sanders M, White FC, Peterson TM, Bloor CM. Effects of endurance exercise on coronary collateral blood flow in miniature swine. Am J Physiol. 1978;234:H614–H619. doi: 10.1152/ajpheart.1978.234.5.H614. [DOI] [PubMed] [Google Scholar]
- 799.Sanderson B, Askew C, Stewart I, Walker P, Gibbs H, Green S. Short-term effects of cycle and treadmill training on exercise tolerance in peripheral arterial disease. J Vasc Surg. 2006;44:119–127. doi: 10.1016/j.jvs.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 800.Sandri M, Adams V, Gielen S, Linke A, Lenk K, Krankel N, Lenz D, Erbs S, Scheinert D, Mohr FW, Schuler G, Hambrecht R. Effects of exercise and ischemia on mobilization and functional activation of blood-derived progenitor cells in patients with ischemic syndromes: results of 3 randomized studies. Circulation. 2005;111:3391–3399. doi: 10.1161/CIRCULATIONAHA.104.527135. [DOI] [PubMed] [Google Scholar]
- 801.Sanne H, Sivertsson R. The effect of exercise on the development of collateral circulation after experimental occlusion of the femoral artery in the cat. Acta Physiol Scand. 1968;73:257–263. doi: 10.1111/j.1748-1716.1968.tb04104.x. [DOI] [PubMed] [Google Scholar]
- 802.Sapienza P, Edwards JD, Mingoli A, McGregor PE, Cavallari N, Agrawal DK. Ischemia-induced peripheral arterial vasospasm role of alpha1- and alpha2-adrenoceptors. J Surg Res. 1996;62:192–196. doi: 10.1006/jsre.1996.0194. [DOI] [PubMed] [Google Scholar]
- 803.Sasso FC, Torella D, Carbonara O, Ellison GM, Torella M, Scardone M, Marra C, Nasti R, Marfella R, Cozzolino D, Indolfi C, Cotrufo M, Torella R, Salvatore T. Increased vascular endothelial growth factor expression but impaired vascular endothelial growth factor receptor signaling in the myocardium of type 2 diabetic patients with chronic coronary heart disease. J Am Coll Cardiol. 2005;46:827–834. doi: 10.1016/j.jacc.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 804.Sato Y, Kanno S, Oda N, Abe M, Ito M, Shitara K, Shibuya M. Properties of two VEGF receptors, Flt-1 and KDR, in signal transduction. Ann NY Acad Sci. 2000;902:201–205. doi: 10.1111/j.1749-6632.2000.tb06314.x. [DOI] [PubMed] [Google Scholar]
- 805.Sausen MT, Delaney EP, Stillabower ME, Farquhar WB. Exhanced metabolreflex sensitivity in hypertensive humans. Eur J Appl Physiol. 2008;105:351–356. doi: 10.1007/s00421-008-0910-8. [DOI] [PubMed] [Google Scholar]
- 806.Scarpulla RC. Nuclear control of respiratory chain expression by nuclear respiratory factors and PGC-1-related coactivator. Ann NY Acad Sci. 2008;1147:321–334. doi: 10.1196/annals.1427.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 807.Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev. 2008;88:611–638. doi: 10.1152/physrev.00025.2007. [DOI] [PubMed] [Google Scholar]
- 808.Schaper W. Collateral circulation: Past and present. Basic Res Cardiol. 2009;104:5–21. doi: 10.1007/s00395-008-0760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 809.Schaper W, De Brabander M, Lewi P. DNA synthesis and mitoses in coronary collateral vessesls of the dog. Circ Res. 1971;28:671–679. doi: 10.1161/01.res.28.6.671. [DOI] [PubMed] [Google Scholar]
- 810.Schaper W, Schaper J. Arteriogenesis. Kluwer Academic Pub; Boston: 2004. [Google Scholar]
- 811.Schaper W, Scholz D. Factors regulating arteriogenesis. Arterioscl Throm Vasc Biol. 2003;23:1143–1151. doi: 10.1161/01.ATV.0000069625.11230.96. [DOI] [PubMed] [Google Scholar]
- 812.Schaper J, Borgers M, Schaper W. Ultrastructure of ischemia-induced changes in the precapillary anastomotic network of the heart. Am J Cardiol. 1972;29:851–859. doi: 10.1016/0002-9149(72)90506-1. [DOI] [PubMed] [Google Scholar]
- 813.Schaper W. New paradigms for collateral vessel growth. Basic Res Cardiol. 1993;88:193–198. doi: 10.1007/BF00794992. [DOI] [PubMed] [Google Scholar]
- 814.Schaper W, Flameng W, Winkler B, Wusten B, Turschmann W, Neugebauer G, Carl M, Pasyk S. Quantification of collateral resistance in acute and chronic experimental coronary occlusion in the dog. Circ Res. 1976;39:371–377. doi: 10.1161/01.res.39.3.371. [DOI] [PubMed] [Google Scholar]
- 815.Schaper W, Eitenmueller I, Volger O, Troidl K, Schmitz-Rixen T. Factors influencing arteriogenesis. J Mol Cell Cardiol. 2005;38:1064–1064. [Google Scholar]
- 816.Scheel KW, Ingram LA, Wilson JL. Effects of exercise on the coronary and collateral vasculature of beagles with and without coronary occlusion. Circ Res. 1981;48:523–530. doi: 10.1161/01.res.48.4.523. [DOI] [PubMed] [Google Scholar]
- 817.Schierling W, Troidl K, Mueller C, Troidl C, Wustrack H, Bachmann G, Kasprzak PM, Schaper W, Schmitz-Rixen T. Increased intravascular flow rate triggers cerebral arteriogenesis. J Cereb Blood Flow Metab. 2009;29:726–737. doi: 10.1038/jcbfm.2008.165. [DOI] [PubMed] [Google Scholar]
- 818.Schierling W, Troidl K, Troidl C, Schmitz-Rixen T, Schaper W, Eitenmuller IK. The role of angiogenic growth factors in arteriogenesis. J Vasc Res. 2009;46:365–374. doi: 10.1159/000189797. [DOI] [PubMed] [Google Scholar]
- 819.Schirmer SH, Fledderus JO, Bot PT, Moerland PD, Hoefer IE, Baan J, Henriques JP, van dS RJ, Vis M, Horrevoets AJ, Piek JJ, van RN. Interferon-beta signaling is enhanced in patients with insufficient coronary collateral artery development and inhibits arteriogenesis in mice. Circ Res. 2008;102:1286–1294. doi: 10.1161/CIRCRESAHA.108.171827. [DOI] [PubMed] [Google Scholar]
- 820.Schirmer SH, van NFC, Piek JJ, van RN. Stimulation of collateral artery growth: travelling further down the road to clinical application. Heart. 2009;95:191–197. doi: 10.1136/hrt.2007.136119. [DOI] [PubMed] [Google Scholar]
- 821.Schlager O, Giurgea A, Schuhfried O, Seidinger D, Hammer A, MG, Fialka-Moser V, Gschwandtner M, Koppensteiner R, Steiner S. Exercise training increases endothelial progenitor cells and decreases asymmetric dimethylarginine in peripheral arterial disease: a randomized controlled trial. Atherosclerosis. 2011;217:240–248. doi: 10.1016/j.atherosclerosis.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 822.Scholz D, Cai WJ, Schaper W. Arteriogenesis, a new concept of vascular adaptation in occlusive disease. Angiogenesis. 2001;4:247–257. doi: 10.1023/a:1016094004084. [DOI] [PubMed] [Google Scholar]
- 823.Scholz D, Elsaesser H, Sauer A, Friedrich C, Luttun A, Carmeliet P, Schaper W. Bone marrow transplantation abolishes inhibition of arteriogenesis in placenta growth factor (PlGF)−/− mice. J Mol Cell Cardiol. 2003;35:177–184. doi: 10.1016/s0022-2828(02)00304-8. [DOI] [PubMed] [Google Scholar]
- 824.Scholz D, Ito W, Fleming I, Deindl E, Sauer A, Wiesnet M, Busse R, Schaper J, Schaper W. Ultrastructure and molecular histology of rabbit hind-limb collateral artery growth (arteriogenesis) Virchows Arch. 2000;436:257–270. doi: 10.1007/s004280050039. [DOI] [PubMed] [Google Scholar]
- 825.Scholz D, Ziegelhoeffer T, Helisch A, Wagner S, Friedrich C, Podzuweit T, Schaper W. Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. J Mol Cell Cardiol. 2002;34:775–787. doi: 10.1006/jmcc.2002.2013. [DOI] [PubMed] [Google Scholar]
- 826.Schrage WG, Woodman CR, Laughlin MH. Hindlimb unweighting alters endothelium-dependent vasodilation and ecNOS expression in soleus arterioles. J Appl Physiol. 2000;89:1483–1490. doi: 10.1152/jappl.2000.89.4.1483. [DOI] [PubMed] [Google Scholar]
- 827.Seebach J, Dieterich P, Luo F, Schillers H, Vestweber D, Oberleithner H, Galla HJ, Schnittler HJ. Endothelial barrier function under laminar fluid shear stress. Lab Invest. 2000;80:1819–1831. doi: 10.1038/labinvest.3780193. [DOI] [PubMed] [Google Scholar]
- 828.Sellke FW, Wang SY, Stamler A, Lopez JJ, Li J, Li J, Simons M. Enhanced microvascular relaxations to VEGF and bFGF in chronically ischemic porcine myocardium. Am J Physiol. 1996;271:H713–H720. doi: 10.1152/ajpheart.1996.271.2.H713. [DOI] [PubMed] [Google Scholar]
- 829.Selvin E, Hirsch AT. Contemporary risk factor control and walking dysfunction in individuals with peripheral arterial disease: NHANES 1999–2004. Atherosclerosis. 2008;201:425–433. doi: 10.1016/j.atherosclerosis.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 830.Semenza GL. Vasculogenesis, angiogenesis, and arteriogenesis: mechanisms of blood vessel formation and remodeling. J Cell Biochem. 2007;102:840–847. doi: 10.1002/jcb.21523. [DOI] [PubMed] [Google Scholar]
- 831.Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol. 2001;13:167–171. doi: 10.1016/s0955-0674(00)00194-0. [DOI] [PubMed] [Google Scholar]
- 832.Semenza GL. Vascular responses to hypoxia and ischemia. Arterioscler Thromb Vasc Biol. 2010;30:648–652. doi: 10.1161/ATVBAHA.108.181644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 833.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 834.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 835.Sethi A, Arora RR. Medical management and cardiovascular risk reduction in peripheral arterial disease. Exp Clin Cardiol. 2008;13:113–119. [PMC free article] [PubMed] [Google Scholar]
- 836.Sewell WH. Coronary cinearteriography for recognition of ‘demand’ for collateral arteries. JAMA. 1963;186:224–228. doi: 10.1001/jama.1963.63710030006010a. [DOI] [PubMed] [Google Scholar]
- 837.Shammas NW. Epidemiology, classification, and modifiable risk factors of peripheral arterial disease. Vasc Health Risk Manag. 2007;3:229–234. doi: 10.2147/vhrm.2007.3.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 838.Shaw JH, Xiang L, Shah A, Yin W, Lloyd PG. Placenta growth factor expression is regulated by hydrogen peroxide in vascular smooth muscle cells. Am J Physiol. 2011;300:C349–C355. doi: 10.1152/ajpcell.00374.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 839.Shay-Salit A, Shushy M, Wolfovitz E, Yahav H, Breviario F, Dejana E, Resnick N. VEGF receptor 2 and the adherens junction as a mechanical transducer in vascular endothelial cells. Proc Natl Acad Sci USA. 2002;99:9462–9467. doi: 10.1073/pnas.142224299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 840.Shen M, Gao J, Li J, Su J. Effect of stimulation frequency on angiogenesis and gene expression in ischemic skeletal muscle of rabbit. Can J Physiol Pharmacol. 2009;87:396–401. doi: 10.1139/y09-007. [DOI] [PubMed] [Google Scholar]
- 841.Shen M, Gao J, Li J, Su J. Effect of ischaemic exercise training of a normal limb on angiogenesis of a pathological ischaemic limb in rabbits. Clin Sci (Lond) 2009;117:201–208. doi: 10.1042/CS20080212. [DOI] [PubMed] [Google Scholar]
- 842.Sheridan KM, Ferguson MJ, Distasi MR, Witzmann FA, Dalsing MC, Miller SJ, Unthank JL. Impact of genetic background and aging on mesenteric collateral growth capacity in Fischer 344, Brown Norway, and Fischer 344 x Brown Norway hybrid rats. Am J Physiol. 2007;293:H3498–H3505. doi: 10.1152/ajpheart.00040.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 843.Sherman JA, Hall A, Malenka DJ, De MED, Simons M. Humoral and cellular factors responsible for coronary collateral formation. Am J Cardiol. 2006;98:1194–1197. doi: 10.1016/j.amjcard.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 844.Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549–560. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 845.Shimada T, Takeshita Y, Murohara T, Sasaki K, Egami K, Shintani S, Katsuda Y, Ikeda H, Nabeshima Y, Imaizumi T. Angiogenesis and vasculogenesis are impaired in the precocious-aging klotho mouse. Circulation. 2004;110:1148–1155. doi: 10.1161/01.CIR.0000139854.74847.99. [DOI] [PubMed] [Google Scholar]
- 846.Shireman PK. The chemokine system in arteriogenesis and hind limb ischemia. J Vasc Surg. 2007;45 (Suppl A):A48–A56. doi: 10.1016/j.jvs.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 847.Shoag J, Arany Z. Regulation of hyposia-inducible genes by PGC-1alpha. ATVB. 2010;30:662–666. doi: 10.1161/ATVBAHA.108.181636. [DOI] [PubMed] [Google Scholar]
- 848.Shoemaker JK, Kunselman AR, Siler DH, Sinoway LI. Maintained exercise pressor response in heart failure. J Appl Physiol. 1998;85:1793–1799. doi: 10.1152/jappl.1998.85.5.1793. [DOI] [PubMed] [Google Scholar]
- 849.Short RHD. Alveolar Epithelium in Relation to Growth of the Lung. Phil Trans Royal Soc London Series B, Biol Sci. 1950;235:35–86. doi: 10.1098/rstb.1950.0014. [DOI] [PubMed] [Google Scholar]
- 850.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 851.Shyy JY, Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ Res. 2002;91:769–775. doi: 10.1161/01.res.0000038487.19924.18. [DOI] [PubMed] [Google Scholar]
- 852.Shyy YJ, Hsieh HJ, Usami S, Chien S. Fluid shear stress induces a biphasic response of human monocyte chemotactic protein 1 gene expression in vascular endothelium. Proc Natl Acad Sci. 1994;91:4678–4682. doi: 10.1073/pnas.91.11.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 853.Silber DH, Sutliff G, Yang QX, Smith MB, Sinoway LI, Leuenberger UA. Altered mechanism of sympathetic activation during rhythmic forarm exercise in heart failure. J Appl Physiol. 1998;84:1551–1559. doi: 10.1152/jappl.1998.84.5.1551. [DOI] [PubMed] [Google Scholar]
- 854.Sillau AH, Aquin L, Bui MV, Banchero N. Chronic hypoxia does not affect guinea pig skeletal muscle capillarity. Pflugers Arch. 1980;386:39–45. doi: 10.1007/BF00584185. [DOI] [PubMed] [Google Scholar]
- 855.Silvestre JS, Mallat Z, Duriez M, Tamarat R, Bureau MF, Scherman D, Duverger N, Branellec D, Tedgui A, Levy BI. Antiangiogenic effect of interleukin-10 in ischemia-induced angiogenesis in mice hindlimb. Circ Res. 2000;87:448–452. doi: 10.1161/01.res.87.6.448. [DOI] [PubMed] [Google Scholar]
- 856.Silvestro A, Scopacasa F, Oliva G, de Cristofaro T, Iuliano L, Brevetti G. Vitamin C prevents endothelial dysfunction induced by acute exercise in patients with intermittent claudication. Atherosclerosis. 2002;165:277–283. doi: 10.1016/s0021-9150(02)00235-6. [DOI] [PubMed] [Google Scholar]
- 857.Simpson RJ, Guy K. Coupling aging immunity with a sedentary lifestyle: Has the damage already beeen done? Gerontology. 2010;56:449–458. doi: 10.1159/000270905. [DOI] [PubMed] [Google Scholar]
- 858.Sinoway LI, Li J. A perspective on the muscle reflex: Implication for congestive heart failure. J Appl Physiol. 2005;99:5–22. doi: 10.1152/japplphysiol.01405.2004. [DOI] [PubMed] [Google Scholar]
- 859.Skinner JS, Strandness DE. Exercise and intermittent claudication II Effect of physical training. Circulation. 1967;36:23–29. doi: 10.1161/01.cir.36.1.23. [DOI] [PubMed] [Google Scholar]
- 860.Skuli N, Liu L, Runge A, Wang T, Yuan L, Patel S, Iruela-Arispe L, Simon MC, Keith B. Endothelial deletion of hypoxia-inducible factor-2alpha (HIF-2alpha) alters vascular function and tumor angiogenesis. Blood. 2009;114:469–477. doi: 10.1182/blood-2008-12-193581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 861.Slordahl SA, Wang E, Hoff J, Kemi OJ, Amundsen BH, Helgerud J. Effective training for patients with intermittent claudication. Scand Cardiovasc J. 2005;39:244–249. doi: 10.1080/14017430510035844. [DOI] [PubMed] [Google Scholar]
- 862.Smith GD, Shipley MJ, Rose G. Intermittent claudication, heart disease risk factors, and mortality. The Whitehall Study. Circulation. 1990;82:1925–1931. doi: 10.1161/01.cir.82.6.1925. [DOI] [PubMed] [Google Scholar]
- 863.Smith SA, Mammen PPA, Mitchell JH, Garry MG. Role of the exercise pressor reflex in rats with dilated cardiomyopathy. Circulation. 2003;108:1126–1132. doi: 10.1161/01.CIR.0000084538.40542.56. [DOI] [PubMed] [Google Scholar]
- 864.Smith SA, Mitchell JH, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol. 2001;537:961–979. doi: 10.1111/j.1469-7793.2001.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 865.Smith SA, Mitchell JH, Garry MG. The mammalian exercise pressor reflex in health and disease. Exp Physiol. 2006;91:89–102. doi: 10.1113/expphysiol.2005.032367. [DOI] [PubMed] [Google Scholar]
- 866.Smith SA, Mitchell JH, Naseem RH, Garry MG. Mechanoreflex mediates the exaggerated exercise pressor reflex in heart failure. Circulation. 2005;112:2293–2300. doi: 10.1161/CIRCULATIONAHA.105.566745. [DOI] [PubMed] [Google Scholar]
- 867.Smith SA, Williams MA, Leal AK, Mitchell JH, Garry MG. Exercise pressor reflex function is altered in spontaneously hypertensive rats. J Physiol. 2006;577:1009–1020. doi: 10.1113/jphysiol.2006.121558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 868.Smith SA, Williams MA, Mitchell JH, Mammen PPA, Garry MG. The capsaicin-sensitive afferent neuron in skeletal muscle is abnormal in heart failure. Circulation. 2005;111:2056–2065. doi: 10.1161/01.CIR.0000162473.10951.0A. [DOI] [PubMed] [Google Scholar]
- 869.Smith T, Dhunnoo G, Mohan I, Charlton-Menys V. A pilot study showing an association between platelet hyperactivity and the severity of peripheral arterial disease. Platelets. 2007;18:245–248. doi: 10.1080/09537100601078091. [DOI] [PubMed] [Google Scholar]
- 870.Snyder GK, Byers RL, Kayar SR. Effects of hypoxia on tissue capillarity in geese. Respir Physiol. 1984;58:151–160. doi: 10.1016/0034-5687(84)90144-0. [DOI] [PubMed] [Google Scholar]
- 871.Snyder GK, Farrelly C, Coelho JR. Adaptations in skeletal muscle capillarity following changes in oxygen supply and changes in oxygen demands. Eur J Appl Physiol. 1992;65:158–163. doi: 10.1007/BF00705074. [DOI] [PubMed] [Google Scholar]
- 872.Snyder GK, Wilcox EE, Burnham EW. Effects of hypoxia on muscle capillarity in rats. Respir Physiol. 1985;62:135–140. doi: 10.1016/0034-5687(85)90057-x. [DOI] [PubMed] [Google Scholar]
- 873.Sodha NR, Clements RT, Boodhwani M, Xu SH, Laham RJ, Bianchi C, Sellke FW. Endostatin and angiostatin are increased in diabetic patients with coronary artery disease and associated with impaired coronary collateral formation. Am J Physiol. 2009;296:H428–H434. doi: 10.1152/ajpheart.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 874.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor 2. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 875.Somlyo AV, Phelps C, Dipierro C, Eto M, Read P, Barrett M, Gibson JJ, Burnitz MC, Myers C, Somlyo AP. Rho kinase and matrix metalloproteinase inhibitors cooperate to inhibit angiogenesis and growth of human prostate cancer xenotransplants. FASEB J. 2003;17:223–234. doi: 10.1096/fj.02-0655com. [DOI] [PubMed] [Google Scholar]
- 876.Sonveaux P, Martinive P, DeWever J, Batova Z, Daneau G, Pelat M, Ghisdal P, Gregoire V, Dessy C, Balligand JL, Feron O. Caveolin-1 expression is critical for vascular endothelial growth factor-induced ischemic hindlimb collateralization and nitric oxide-mediated angiogenesis. Circ Res. 2004;95:154–161. doi: 10.1161/01.RES.0000136344.27825.72. [DOI] [PubMed] [Google Scholar]
- 877.Sorlie D, Myhre K. Effects of physical training in intermittent claudication. Scand J Clin Lab Invest. 1978;38:217–222. [PubMed] [Google Scholar]
- 878.Spindler V, Schlegel N, Waschke J. Role of GTPases in control of microvascular permeability. Cardiovasc Res. 2010;87:243–253. doi: 10.1093/cvr/cvq086. [DOI] [PubMed] [Google Scholar]
- 879.Spronk S, Bosch JL, den Hoed PT, Veen HF, Pattynama PM, Hunink MG. Cost-effectiveness of endovascular revascularization compared to supervised hospital-based exercise training in patients with intermittent claudication: a randomized controlled trial. J Vasc Surg. 2008;48:1472–1480. doi: 10.1016/j.jvs.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 880.Spronk S, Bosch JL, den Hoed PT, Veen HF, Pattynama PM, Hunink MG. Intermittent claudication: clinical effectiveness of endovascular revascularization versus supervised hospital-based exercise training--randomized controlled trial. Radiology. 2009;250:586–595. doi: 10.1148/radiol.2501080607. [DOI] [PubMed] [Google Scholar]
- 881.Spronk S, White JV, Bosch JL, Hunink MG. Impact of claudication and its treatment on quality of life. Semin Vasc Surg. 2007;20:3–9. doi: 10.1053/j.semvascsurg.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 882.Stabile E, Kinnaird T, La SA, Hanson SK, Watkins C, Campia U, Shou M, Zbinden S, Fuchs S, Kornfeld H, Epstein SE, Burnett MS. CD8+ T lymphocytes regulate the arteriogenic response to ischemia by infiltrating the site of collateral vessel development and recruiting CD4+ mononuclear cells through the expression of interleukin-16. Circulation. 2006;113:118–124. doi: 10.1161/CIRCULATIONAHA.105.576702. [DOI] [PubMed] [Google Scholar]
- 883.Stalmans I, Ng YS, Rohan R, Fruttiger M, Bouche A, Yuce A, Fujisawa H, Hermans B, Shani M, Jansen S, Hicklin D, Anderson DJ, Gardiner T, Hammes HP, Moons L, Dewerchin M, Collen D, Carmeliet P, D’Amore PA. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J Clin Invest. 2002;109:327–336. doi: 10.1172/JCI14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 884.Stavri GT, Zachary IC, Baskerville PA, Martin JF, Erusalimsky JD. Basic fibroblast growth facor upregulates the expression of vascular endothelial growth factor in vascular smooth muscle cells: Synergistic interaction with hypoxia. Circulation. 1995;92:11–14. doi: 10.1161/01.cir.92.1.11. [DOI] [PubMed] [Google Scholar]
- 885.Sterns DA, Ettinger SM, Gray KS, Whisler SK, Mosher TJ, Smith MB, Sinoway LI. Skeletal muscle metaboreceptor exercise responses are attenuated in heart failure. Circulation. 1991;84:2034–2–39. doi: 10.1161/01.cir.84.5.2034. [DOI] [PubMed] [Google Scholar]
- 886.Stewart AH, Lamont PM. Exercise training for claudication. The Surgeon. 2007;5:291–299. doi: 10.1016/s1479-666x(07)80028-x. [DOI] [PubMed] [Google Scholar]
- 887.Stewart AHR, Smith FC, Baird RN, Lamont PM. Local versus systemic mechanisms underlying supervised exercise training for intermittent claudication. Vasc Endovasc Surg. 2008;42:314–320. doi: 10.1177/1538574408314442. [DOI] [PubMed] [Google Scholar]
- 888.Stewart KJ, Hiatt WR, Regensteiner JG, Hirsch AT. Exercise training for claudication. N Engl J Med. 2002;347:1941–1951. doi: 10.1056/NEJMra021135. [DOI] [PubMed] [Google Scholar]
- 889.Stewart GN. Studies on the circulation in man : XVI A study of the development of the collateral circulation in the right hand after ligation of the innominate artery for subclavian aneurysm. J Exp Med. 1915;22:694–700. doi: 10.1084/jem.22.6.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 890.Strick DM, Waycaster RL, Montani JP, Gay WJ, Adair TH. Morphometric measurements of chorioallantoic membrane vascularity: effects of hypoxia and hyperoxia. Am J Physiol. 1991;260:H1385–H1389. doi: 10.1152/ajpheart.1991.260.4.H1385. [DOI] [PubMed] [Google Scholar]
- 891.Styp-Rekowska B, Hlushchuk R, Pries AR, Djonov V. Intussusceptive angiogenesis: pillars against the blood flow. Acta Physiol. 2011;202:213–223. doi: 10.1111/j.1748-1716.2011.02321.x. [DOI] [PubMed] [Google Scholar]
- 892.Suchting S, Freitas C, le N Ferdinand, Benedito R, Br+©ant C, Duarte A, Eichmann A. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. PNAS. 2007;104:3225–3230. doi: 10.1073/pnas.0611177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 893.Suhr F, Brixius K, de MM, Bolck B, Kleinoder H, Achtzehn S, Bloch W, Mester J. Effects of short-term vibration and hypoxia during high-intensity cycling exercise on circulating levels of angiogenic regulators in humans. J Appl Physiol. 2007;103:474–483. doi: 10.1152/japplphysiol.01160.2006. [DOI] [PubMed] [Google Scholar]
- 894.Sullivan CJ, Doetschman T, Hoying JB. Targeted disruption of the Fgf2 gene does not affect vascular growth in the mouse ischemic hindlimb. J Appl Physiol. 2002;93:2009–2017. doi: 10.1152/japplphysiol.00451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 895.Sun D, Huang A, Koller A, Kaley G. Short-term daily exercise activity enhances endothelial NO synthesis in skeletal muscle arterioles. J Appl Physiol. 1994;76:2241–2247. doi: 10.1152/jappl.1994.76.5.2241. [DOI] [PubMed] [Google Scholar]
- 896.Sundberg CJ. Exercise and training during graded leg ischaemia in healthy man with special reference to effects on skeletal muscle. Acta Physiol Scand Suppl. 1994;615:1–50. [PubMed] [Google Scholar]
- 897.Suzuki J, Gao M, Batra S, Koyama T. Effects of treadmill training on the arteriolar and venular portions of capillary in soleus muscle of young and middle-aged rats. Acta Physiol Scand. 1997;159:113–121. doi: 10.1046/j.1365-201X.1997.582353000.x. [DOI] [PubMed] [Google Scholar]
- 898.Symes JF, Losordo DW, Vale PR, Lathi KG, Esakof DD, Mayskiy M, Isner JM. Gene therapy with vascular endothelial growth factor for inoperable coronary artery disease. Ann Thorac Surg. 1999;68:830–836. doi: 10.1016/s0003-4975(99)00807-3. [DOI] [PubMed] [Google Scholar]
- 899.Tadaishi M, Miura S, Kai Y, Kawasaki E, Koshinaka K, Kawanaka K, Nagata J, Oishih Y, Ezaki O. Effect of exercise intensity and AICAR on isoform-specific expressions of murine skeletal msucle PGC-1alpha mRNA: a role of beta2-adrenergic receptor activation. Am J Physiol. 300 doi: 10.1152/ajpendo.00400.2010. [DOI] [PubMed] [Google Scholar]
- 900.Taft C, Karlsson J, Gelin J, Jivegard L, Sandstrom R, Arfvidsson B, Dahllof AG, Lundholm K, Sullivan M. Treatment efficacy of intermittent claudication by invasive therapy, supervised physical exercise training compared to no treatment in unselected randomised patients II: one-year results of health-related quality of life. Eur J Vasc Endovasc Surg. 2001;22:114–123. doi: 10.1053/ejvs.2001.1406. [DOI] [PubMed] [Google Scholar]
- 901.Takahashi A, Kureishi Y, Yang J, Luo Z, Guo K, Mukhopadhyay D, Ivashchenko Y, Branellec D, Walsh K. Myogenic Akt signaling regulates blood vessel recruitment during myofiber growth. Mol Cell Biol. 2002;22:4803–4814. doi: 10.1128/MCB.22.13.4803-4814.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 902.Takahashi T, Yamaguchi S, Chida K, Shibuya M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-{{gamma}} and DNA synthesis in vascular endothelial cells. EMBO J. 2001;20:2768–2778. doi: 10.1093/emboj/20.11.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 903.Takashima S, Kitakaze M, Asakura M, Asanuma H, Sanada S, Tashiro F, Niwa H, Miyazaki JJ, Hirota S, Kitamura Y, Kitsukawa T, Fujisawa H, Klagsbrun M, Hori M. Targeting of both mouse neuropilin-1 and neuropilin-2 genes severely impairs developmental yolk sac and embryonic angiogenesis. Proc Natl Acad Sci USA. 2002;99:3657–3662. doi: 10.1073/pnas.022017899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 904.Takeshita S, Weir L, Chen D, Zheng LP, Riessen R, Bauters C, Symes JF, Ferrara N, Isner JM. Therapeutic angiogenesis following arterial gene transfer of vascular endothelial growth factor in a rabbit model of hindlimb ischemia. Biochem Biophys Res Commun. 1996;227:628–635. doi: 10.1006/bbrc.1996.1556. [DOI] [PubMed] [Google Scholar]
- 905.Takeshita S, Zheng LP, Brogi E, Kearney M, Pu LQ, Bunting S, Ferrara N, Symes JF, Isner JM. Therapeutic angiogenesis A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin Invest. 1994;93:662–670. doi: 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 906.Tamarat R, Silvestre JS, Huijberts M, Benessiano J, Ebrahimian TG, Duriez M, Wautier MP, Wautier JL, Levy BI. Blockade of advanced glycation end-product formation restores ischemia-induced angiogenesis in diabetic mice. Proc Natl Acad Sci. 2003;100:8555–8560. doi: 10.1073/pnas.1236929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 907.Tang K, Breen EC, Wagner H, Brutsaert TD, Gassmann M, Wagner PD. HIF and VEGF relationships in response to hypoxia and sciatic nerve stimulation in rat gastrocnemius. Respir Physiol Neurobiol. 2004;144:71–80. doi: 10.1016/j.resp.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 908.Tang K, Breen EC, Gerber HP, Ferrara NM, Wagner PD. Capillary regression in vascular endothelial growth factor-deficient skeletal muscle. Physiol Genomics. 2004;18:63–69. doi: 10.1152/physiolgenomics.00023.2004. [DOI] [PubMed] [Google Scholar]
- 909.Tang G, Charo DN, Wang R, Charo IF, Messina L. CCR2−/− knockout mice revascularize normally in response to severe hindlimb ischemia. J Vasc Surg. 2004;40:786–795. doi: 10.1016/j.jvs.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 910.Tang GL, Chang DS, Sarkar R, Wang R, Messina LM. The effect of gradual or acute arterial occlusion on skeletal muscle blood flow, arteriogenesis, and inflammation in rat hindlimb ischemia. J Vasc Surg. 2005;41:312–320. doi: 10.1016/j.jvs.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 911.Tang N, Wang L, Esko J, Giordano FJ, Huang Y, Gerber HP, Ferrara N, Johnson RS. Loss of HIF-1alpha in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell. 2004;6:485–495. doi: 10.1016/j.ccr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 912.Taniyama Y, Morishita R, Aoki M, Nakagami H, Yamamoto K, Yamazaki K, Matsumoto K, Nakamura T, Kaneda Y, Ogihara T. Therapeutic angiogenesis induced by human hepatocyte growth factor gene in rat and rabbit hindlimb ischemia models: preclinical study for treatment of peripheral arterial disease. Gene Ther. 2001;8:181–189. doi: 10.1038/sj.gt.3301379. [DOI] [PubMed] [Google Scholar]
- 913.Taniyama Y, Morishita R, Hiraoka K, Aoki M, Nakagami H, Yamasaki K, Matsumoto K, Nakamura T, Kaneda Y, Ogihara T. Therapeutic angiogenesis induced by human hepatocyte growth factor gene in rat diabetic hind limb ischemia model: molecular mechanisms of delayed angiogenesis in diabetes. Circulation. 2001;104:2344–2350. doi: 10.1161/hc4401.098470. [DOI] [PubMed] [Google Scholar]
- 914.Taylor JC, Li Z, Yang HT, Laughlin MH, Terjung RL. Alpha-adrenergic inhibition increases collateral circuit conductance in rats following acute occlusion of the femoral artery. J Physiol. 2008;586:1649–1667. doi: 10.1113/jphysiol.2007.149567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 915.Taylor JC, Yang HT, Laughlin MH, Terjung RL. Alpha-adrenergic and neuropeptide Y Y1 receptor control of collateral circuit conductance: influence of exercise training.[see comment] J Physiol. 2008;586:5984–5998. doi: 10.1113/jphysiol.2008.160101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 916.Taylor KL, Henderson AM, Hughes CC. Notch activation during endothelial cell network formation in vitro targets the basic HLH transcription factor HESR-1 and downregulates VEGFR-2/KDR expression. Microvasc Res. 2002;64:372–383. doi: 10.1006/mvre.2002.2443. [DOI] [PubMed] [Google Scholar]
- 917.Tchaikovski V, Fellbrich G, Waltenberger J. The molecular basis of VEGFR-1 signal transduction pathways in primary human monocytes. Arterioscler Thromb Vasc Biol. 2008;28:322–328. doi: 10.1161/ATVBAHA.107.158022. [DOI] [PubMed] [Google Scholar]
- 918.Tchaikovski V, Olieslagers S, Bohmer FD, Waltenberger J. Diabetes mellitus activates signal transduction pathways resulting in vascular endothelial growth factor resistance of human monocytes. Circulation. 2009;120:150–159. doi: 10.1161/CIRCULATIONAHA.108.817528. [DOI] [PubMed] [Google Scholar]
- 919.Teichert-Kuliszewska K, Maisonpierre PC, Jones N, Campbell AI, Master Z, Bendeck MP, Alitalo K, Dumont DJ, Yancopoulos GD, Stewart DJ. Biological action of angiopoietin-2 in a fibrin matrix model of angiogenesis is associated with activation of Tie2. Cardiovasc Res. 2001;49:659–670. doi: 10.1016/s0008-6363(00)00231-5. [DOI] [PubMed] [Google Scholar]
- 920.Terada S, Goto M, Kato M, Kawanaka K, Shimokawa T, Tabata I. Effects of low-intensity prolonged exercise on PGC-1 mRNA expression in rat epitrochlearis muscle. Biochem Biophys Res Commun. 2002;296:350–354. doi: 10.1016/s0006-291x(02)00881-1. [DOI] [PubMed] [Google Scholar]
- 921.Teravainen H, Makitie J. Striated muscle ultrastructure in intermittent claudication. Arch Pathol Lab Med. 1977;101:230–235. [PubMed] [Google Scholar]
- 922.Terjung RL, Zarzeczny R, Yang HT. Muscle blood flow and mitochondrial function: influence of aging. Int J Sport Nutr Exer Metabol. 2002;12:368–378. doi: 10.1123/ijsnem.12.3.368. [DOI] [PubMed] [Google Scholar]
- 923.Testa U, Pannitteri G, Condorelli GL. Vascular endothelial growth factors in cardiovascular medicine. J Cardiovasc Med. 2008;9:1190–1221. doi: 10.2459/JCM.0b013e3283117d37. [DOI] [PubMed] [Google Scholar]
- 924.Thomason DB, Booth FW. Atrophy of the soleus muscle by hindlimb unweighting. J Appl Physiol. 1990;68:1–12. doi: 10.1152/jappl.1990.68.1.1. [DOI] [PubMed] [Google Scholar]
- 925.Thompson LV. Skeletal muscle adapttions with age, inactivity, and therapeutic exercise. J Orthop Sports Phys Ther. 2002;32:44–57. doi: 10.2519/jospt.2002.32.2.44. [DOI] [PubMed] [Google Scholar]
- 926.Thyboll J, Kortesmaa J, Cao R, Soininen R, Wang L, Iivanainen A, Sorokin L, Risling M, Cao Y, Tryggvason K. Deletion of the laminin alpha4 chain leads to impaired microvessel maturation. Mol Cell Biol. 2002;22:1194–1202. doi: 10.1128/MCB.22.4.1194-1202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 927.Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes and Development Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- 928.Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA. The human gene for vascular endothelial growth factor Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947–11954. [PubMed] [Google Scholar]
- 929.Tisi PV, Hulse M, Chulakadabba A, Gosling P, Shearman CP. Exercise training for intermittent claudication: does it adversely affect biochemical markers of the exercise-induced inflammatory response? Eur J Vasc Endovasc Surg. 1997;14:344–350. doi: 10.1016/s1078-5884(97)80283-3. [DOI] [PubMed] [Google Scholar]
- 930.Tisi PV, Shearman CP. The evidence for exercise-induced inflammation in intermittent claudication: should we encourage patients to stop walking? Eur J Vasc Endovasc Surg. 1998;15:7–17. doi: 10.1016/s1078-5884(98)80066-x. [DOI] [PubMed] [Google Scholar]
- 931.Tojo T, Ushio-Fukai M, Yamaoka-Tojo M, Ikeda S, Patrushev N, Alexander RW. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation. 2005;111:2347–2355. doi: 10.1161/01.CIR.0000164261.62586.14. [DOI] [PubMed] [Google Scholar]
- 932.Tornling G, Unge G, Skoog L, Ljungqvist A, Carlsson S, Adolfsson J. Proliferative Activity of Myocardial Capillary Wall Cells in Dipyridamole-Treated Rats. Cardiovasc Res. 1978;12:692–695. doi: 10.1093/cvr/12.11.692. [DOI] [PubMed] [Google Scholar]
- 933.Troidl K, Tribulova S, Cai WJ, Ruding I, Apfelbeck H, Schierling W, Troidl C, Schmitz-Rixen T, Schaper W. Effects of endogenous nitric oxide and of DETA NONOate in arteriogenesis. J Cardiovasc Pharmacol. 2010;55:153–160. doi: 10.1097/FJC.0b013e3181c9556f. [DOI] [PubMed] [Google Scholar]
- 934.Troyanovsky B, Levchenko T, Mansson G, Matvijenko O, Holmgren L. Angiomotin: an angiostatin binding protein that regulates endothelial cell migration and tube formation. J Cell Biol. 2001;152:1247–1254. doi: 10.1083/jcb.152.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 935.Tsang GM, Green MA, Crow AJ, Smith FC, Beck S, Hudlicka O, Shearman CP. Chronic muscle stimulation improves ischaemic muscle performance in patients with peripheral vascular disease. Eur J Vasc Surg. 1994;8:419–422. doi: 10.1016/s0950-821x(05)80960-0. [DOI] [PubMed] [Google Scholar]
- 936.Tsuchimochi H, McCord JL, Hayes sG, Koba S, Kaufman MP. Chronic femoral artery occlusion augments exercise pressor reflex in decerbrated rats. Am J Physiol. 2010;299:H106–H113. doi: 10.1152/ajpheart.00141.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 937.Tsuchimochi H, McCord JL, Kaufman MP. Peripheral μ-opioid receptors attenuate the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. Am J Physiol. 2010;299:H557–H565. doi: 10.1152/ajpheart.00387.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 938.Tsuchimochi H, McCord JL, Leal AK, Kaufman MP. Dorsal root tetrodotoxin-resistant sodium channels do not contribute to the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. Am J Physiol. 2011;300:H652–H663. doi: 10.1152/ajpheart.00859.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 939.Tsuchimochi H, Yamauchi K, McCord JL, Kaufman MP. Blockade of acid sensing ion channels attenuates the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. J Physiol. 2011;589:6173–6189. doi: 10.1113/jphysiol.2011.217851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 940.Tuner SL, Easton C, Wilson J, Byrne DS, Rogers P, Kilduff LP, Kingsmore DB, Pitsiladis YP. Cardioulmonary responses to treadmill and cycle ergometry exercise in patients with peripheral arterial disease. J Vasc Surg. 2008;47:123–130. doi: 10.1016/j.jvs.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 941.Tuomisto TT, Rissanen TT, Vajanto I, Korkeela A, Rutanen J, Yla-Herttuala S. HIF-VEGF-VEGFR-2, TNF-alpha and IGF pathways are upregulated in critical human skeletal muscle ischemia as studied with DNA array. Atherosclerosis. 2004;174:111–120. doi: 10.1016/j.atherosclerosis.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 942.Turhan H, Yasar AS, Erbay AR, Yetkin E, Sasmaz H, Sabah I. Impaired coronary collateral vessel development in patients with metabolic syndrome. Coron Artery Dis. 2005;16:281–285. doi: 10.1097/00019501-200508000-00004. [DOI] [PubMed] [Google Scholar]
- 943.Turton EP, Coughlin PA, Kester RC, Scott DJ. Exercise training reduces the acute inflammatory response associatedd with claudication. Eur J Vasc Endovasc Surg. 2002;23:309–316. doi: 10.1053/ejvs.2002.1599. [DOI] [PubMed] [Google Scholar]
- 944.Tuttle JL, Hahn TL, Sanders BM, Witzmann FA, Miller SJ, Dalsing MC, Unthank JL. Impaired collateral development in mature rats. Am J Physiol. 2002;283:H146–H155. doi: 10.1152/ajpheart.00766.2001. [DOI] [PubMed] [Google Scholar]
- 945.Uguccioni G, Hood DA. The importance of PGC-1α in contractile activity-induced mitochondrial adaptations. Am J Physiol. 2011;300:E361–E371. doi: 10.1152/ajpendo.00292.2010. [DOI] [PubMed] [Google Scholar]
- 946.Unthank JL, Nixon JC, Burkhart HM, Fath SW, Dalsing MC. Early collateral and microvascular adaptations to intestinal artery occlusion in rat. Am J Physiol. 1996;271:H914–H923. doi: 10.1152/ajpheart.1996.271.3.H914. [DOI] [PubMed] [Google Scholar]
- 947.Unthank JL, Nixon JC, Daising MC. Nitric oxide maintains dilation of immature and mature collaterals in rat hindlimb. J Vasc Surg. 1996;33:471–479. doi: 10.1159/000159186. [DOI] [PubMed] [Google Scholar]
- 948.Unthank JL, Nixon JC, Dalsin MC. Acute compensation to abrupt occlusion of rat femoral artery is prevented by NO synthase inhibitors. Am J Physiol. 1994;267:H2523–H2530. doi: 10.1152/ajpheart.1994.267.6.H2523. [DOI] [PubMed] [Google Scholar]
- 949.Unthank JL, Nixon JC, Dalsing MC. Inhibition of NO sythase prevents acute collateral artery dilation in the rat hindlimb. J Surg Res. 1996;61:463–468. doi: 10.1006/jsre.1996.0147. [DOI] [PubMed] [Google Scholar]
- 950.Unthank JL, Fath SW, Burkhart HM, Miller SC, Dalsing MC. Wall Remodeling During Luminal Expansion of Mesenteric Arterial Collaterals in the Rat. Circ Res. 1996;79:1015–1023. doi: 10.1161/01.res.79.5.1015. [DOI] [PubMed] [Google Scholar]
- 951.Urbich C, Reissner A, Chavakis E, Dernbach E, Haendeler J, Fleming I, Zeiher AM, Kaszkin M, Dimmeler S. Dephosphorylation of endothelial nitric oxide synthase contributes to the anti-angiogenic effects of endostatin. FASEB J. 2002;16:706–708. doi: 10.1096/fj.01-0637fje. [DOI] [PubMed] [Google Scholar]
- 952.Ushio-Fukai M, Alexander RW. Reactive oxygen species as mediators of angiogenesis signaling: role of NAD(P)H oxidase. Mol Cell Biochem. 2004;264:85–97. doi: 10.1023/b:mcbi.0000044378.09409.b5. [DOI] [PubMed] [Google Scholar]
- 953.Ushio-Fukai M, Urao N. Novel role of NADPH oxidase in angiogenesis and stem/progenitor cell function. Antioxid Redox Signal. 2009;11:2517–2533. doi: 10.1089/ars.2009.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 954.Vajanto I, Rissanen TT, Rutanen J, Hiltunen MO, Tuomisto TT, Arve K, Narvanen O, Manninen H, Rasanen H, Hippelainen M, Alhava E, Yla-Herttuala S. Evaluation of angiogenesis and side effects in ischemic rabbit hindlimbs after intramuscular injection of adenoviral vectors encoding VEGF and LacZ. J Gene Med. 2002;4:371–380. doi: 10.1002/jgm.287. [DOI] [PubMed] [Google Scholar]
- 955.Vamjatalo A, Poole DC, DiMenna FJ, Bailey SJ, Jones AM. Muscle fiber recruitment and the slow component of O2 uptake: constant work rate vs all-out sprint exercise. Am J Physiol. 2011;300:R700–R707. doi: 10.1152/ajpregu.00761.2010. [DOI] [PubMed] [Google Scholar]
- 956.Van Belle E, Rivard A, Chen D, Silver M, Bunting S, Ferrara N, Symes JF, Bauters C, Isner JM. Hypercholesterolemia attenuates angiogenesis but does not preclude augmentation by angiogenic cytokines. Circulation. 1997;96:2667–2674. doi: 10.1161/01.cir.96.8.2667. [DOI] [PubMed] [Google Scholar]
- 957.Van Belle E, Witzenbichler B, Chen D, Silver M, Chang L, Schwall R, Isner JM. Potentiated angiogenic effect of scatter factor/hepatocyte growth factor via induction of vascular endothelial growth factor: the case for paracrine amplification of angiogenesis. Circulation. 1998;97:381–390. doi: 10.1161/01.cir.97.4.381. [DOI] [PubMed] [Google Scholar]
- 958.van der Zee R, Murohara T, Luo Z, Zollmann F, Passeri J, Lekutat C, Isner JM. Vascular endothelial growth factor/vascular permeability factor augments nitric oxide release from quiescent rabbit and human vascular endothelium. Circulation. 1997;95:1030–1037. doi: 10.1161/01.cir.95.4.1030. [DOI] [PubMed] [Google Scholar]
- 959.van Golde JM, Ruiter MS, Schaper NC, Voo S, Waltenberger J, Backes WH, Post MJ, Huijberts MS. Impaired collateral recruitment and outward remodeling in experimental diabetes. Diabetes. 2008;57:2818–2823. doi: 10.2337/db08-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 960.van Groningen JP, Wenink AC, Testers LH. Myocardial capillaries: increase in number by splitting of existing vessels. Anat Embryol (Berl) 1991;184:65–70. doi: 10.1007/BF01744262. [DOI] [PubMed] [Google Scholar]
- 961.van Oostrom MC, van Oostrom O, Quax PH, Verhaar MC, Hoefer IE. IOnsights into mechanisms behing arteriogeneisis: what does the future hold? J Leukoc Biol. 2008;84:1379–1391. doi: 10.1189/jlb.0508281. [DOI] [PubMed] [Google Scholar]
- 962.van Weel V, de VM, Voshol PJ, Verloop RE, Eilers PH, van HVW, van BJH, Quax PH. Hypercholesterolemia reduces collateral artery growth more dominantly than hyperglycemia or insulin resistance in mice. Arterioscler Thromb Vasc Biol. 2006;26:1383–1390. doi: 10.1161/01.ATV.0000219234.78165.85. [DOI] [PubMed] [Google Scholar]
- 963.van Weel V, Seghers L, de Vries MR, Kuiper EJ, Schlingemann RO, Bajema IM, Lindeman JH, Delis-van Diemen PM, van Hinsbergh VW, van Bockel JH, Quax PH. Expression of vascular endothelial growth factor, stromal cell-derived factor-1, and CXCR4 in human limb muscle with acute and chronic ischemia. Arterioscler Thromb Vasc Biol. 2007;27:1426–1432. doi: 10.1161/ATVBAHA.107.139642. [DOI] [PubMed] [Google Scholar]
- 964.van Weel V, van Tongeren RB, van Hinsbergh VW, van Bockel JH, Quax PH. Vascular growth in ischemic limbs: a review of mechanisms and possible therapeutic stimulation. Ann Vasc Surg. 2008;22:582–597. doi: 10.1016/j.avsg.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 965.van Weel V, Toes RE, Seghers L, Deckers MM, de VMR, Eilers PH, Sipkens J, Schepers A, Eefting D, van HVW, van BJH, Quax PH. Natural killer cells and CD4+ T-cells modulate collateral artery development. Arterioscler Thromb Vasc Biol. 2007;27:2310–2318. doi: 10.1161/ATVBAHA.107.151407. [DOI] [PubMed] [Google Scholar]
- 966.Vigano A, Ripamonti M, De PS, Capitanio D, Vasso M, Wait R, Lundby C, Cerretelli P, Gelfi C. Proteins modulation in human skeletal muscle in the early phase of adaptation to hypobaric hypoxia. Proteomics. 2008;8:4668–4679. doi: 10.1002/pmic.200800232. [DOI] [PubMed] [Google Scholar]
- 967.Vikkula M, Boon LM, Carraway KLIII, Calvert JT, Diamonti AJ, Goumnerov B, Pasyk KA, Marchuk DA, Warman ML, Cantley LC, Mulliken JB, Olsen BR. Vascular dysmorphogenesis caused by an activating mutation in the receptor tyrosine kinase TIE2. Cell. 1996;87:1181–1190. doi: 10.1016/s0092-8674(00)81814-0. [DOI] [PubMed] [Google Scholar]
- 968.Vineberg AM, Chari RS, Pifarre R, Mercier C. The effect of persantin on intercoronary collateral circulation and survival during gradual experimental coronary occlusion. A preliminary report. Can Med Assoc J. 1962;87:336–345. [PMC free article] [PubMed] [Google Scholar]
- 969.Virbasius JV, Scarpulla RC. Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in orgnaelle biogenesis. Proc Natl Acad Sci USA. 1994;91:1309–1313. doi: 10.1073/pnas.91.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 970.Voskuil M, Hoefer IE, van RN, Hua J, de GS, Bode C, Buschmann IR, Piek JJ. Abnormal monocyte recruitment and collateral artery formation in monocyte chemoattractant protein-1 deficient mice. Vasc Med. 2004;9:287–292. doi: 10.1191/1358863x04vm571oa. [DOI] [PubMed] [Google Scholar]
- 971.Wagner PD. Skeletal muscle angiogenesis A possible role for hypoxia. Adv Exp Med Biol. 2001;502:21–38. [PubMed] [Google Scholar]
- 972.Wahl ML, Kenan DJ, Gonzalez-Gronow M, Pizzo SV. Angiostatin’s molecular mechanism: aspects of specificity and regulation elucidated. J Cell Biochem. 2005;96:242–261. doi: 10.1002/jcb.20480. [DOI] [PubMed] [Google Scholar]
- 973.Wajih N, Sane DC. Angiostatin selectively inhibits signaling by hepatocyte growth factor in endothelial and smooth muscle cells. Blood. 2003;101:1857–1863. doi: 10.1182/blood-2002-02-0582. [DOI] [PubMed] [Google Scholar]
- 974.Walshe TE, Saint-Geniez M, Maharaj AS, Sekiyama E, Maldonado AE, D’Amore PA. TGF-beta is required for vascular barrier function, endothelial survival and homeostasis of the adult microvasculature. PLoS ONE. 2009;4:e5149. doi: 10.1371/journal.pone.0005149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 975.Waltenberger J, Lange J, Kranz A. Vascular endothelial growth factor-A-induced chemotaxis of monocytes is attenuated in patients with diabetes mellitus: A potential predictor for the individual capacity to develop collaterals. Circulation. 2000;102:185–190. doi: 10.1161/01.cir.102.2.185. [DOI] [PubMed] [Google Scholar]
- 976.Waltenberger J, Claessonwelsh L, Siegbahn A, Shibuya M, Heldin CH. Different Signal-Transduction Properties of Kdr and Flt1, 2 Receptors for Vascular Endothelial Growth-Factor. J Biol Chem. 1994;269:26988–26995. [PubMed] [Google Scholar]
- 977.Walther C, Mobius-Winkler S, Linke A, Bruegel M, Thiery J, Schuler G, Halbrecht R. Regular exercise training compared with percutaneous intervention leads to a reduction of inflammatory markers and cardiovascular events in patients with coronary artery disease. Eur J Cardiovasc Prev Rehabil. 2008;15:107–112. doi: 10.1097/HJR.0b013e3282f29aa6. [DOI] [PubMed] [Google Scholar]
- 978.Wang HJ, Li YL, Gao L, Zucker IH, Wang W. Alteration in skeletal muscle afferents in rats with chronic heart failure. J Physiol. 2010;588:5033–5047. doi: 10.1113/jphysiol.2010.199562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 979.Wang HJ, Pan YX, Wang WZ, Gao L, Zimmerman MC, Zucker IH, Wang W. Exercise training prevents the exaggerated exercise pressor reflex in rats with chronic heart failure. J Appl Physiol. 2010;108:1365–1375. doi: 10.1152/japplphysiol.01273.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 980.Wang HJ, Pan YX, Wang WZ, Zucker IH, Wang W. NADPH oxidaase-derived reactive oxygen species in skeletal muscle modulates the exercise pressor reflex. J Appl Physiol. 2009;107:450–459. doi: 10.1152/japplphysiol.00262.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 981.Wang SH, Zhang HT, Dai XZ, Sealock R, Faber JE. Genetic architecture underlying variation in extent and remodeling of the collateral circulation. Circ Res. 2010;300:558–568. doi: 10.1161/CIRCRESAHA.110.224634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 982.Wang L, Zeng H, Wang P, Soker S, Mukhopadhyay D. Neuropilin-1-mediated vascular permeability factor/vascular endothelial growth factor-dependent endothelial cell migration. J Biol Chem. 2003;278:48848–48860. doi: 10.1074/jbc.M310047200. [DOI] [PubMed] [Google Scholar]
- 983.Watanabe K, Hasegawa Y, Yamashita H, Shimizu K, Ding Y, Abe M, Ohta H, Imagawa K, Hojo K, Maki H, Sonoda H, Sato Y. Vasohibin as an endothelium-derived negative feedback regulator of angiogenesis. J Clin Invest. 2004;114:898–907. doi: 10.1172/JCI21152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 984.Waters RE, Terjung RL, Peters KG, Annex BH. Preclinical models of human peripheral arterial occlusive disease: implications for investigation of therapeutic agents [Review] [64 refs] J Appl Physiol. 2004;97:773–780. doi: 10.1152/japplphysiol.00107.2004. [DOI] [PubMed] [Google Scholar]
- 985.Watson L, Ellis B, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2008 Oct 8;:CD000990. doi: 10.1002/14651858.CD000990.pub2. [DOI] [PubMed] [Google Scholar]
- 986.Watson NL, Sutton-Tyrrell K, Youk AO, Boudreau RM, Mackey RH, Simonsick EM, Rosano C, Hardy SE, BG W, Harris TB, Nijjar SS, Lakatta EG, Atkinson HH, Johnson KC, Bauer DC, Nemwan AB. Arterial stiffness and gait speed in older adults with and without peripheral arterial disease. Am J Hypertens. 2011;24:90–95. doi: 10.1038/ajh.2010.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 987.Weihrauch D, Lohr NL, Mraovic B, Ludwig LM, Chilian WM, Pagel S, Warltier DC, Kersten JR. Chronic hyperglycemia attenuates coronary collateral development and impairs proliferative properties of myocardial interstitial fluid by production of angiostatin. Circulation. 2004;109:2343–2348. doi: 10.1161/01.CIR.0000129225.67353.1F. [DOI] [PubMed] [Google Scholar]
- 988.Westenbrink BD, Lipsic E, van dM Peter, van dH Pim, Oeseburg H, Du MS, Gideon J, Koster J, Voors AA, van V, Dirk J, van G, Wiek H, Schoemaker RG. Erythropoietin improves cardiac function through endothelial progenitor cell and vascular endothelial growth factor mediated neovascularization. Eur Heart J. 2007;28:2018–2027. doi: 10.1093/eurheartj/ehm177. [DOI] [PubMed] [Google Scholar]
- 989.Westvik TS, Fitzgerald TN, Muto A, Maloney SP, Pimiento JM, Fancher TT, Magri D, Westvik HH, Nishibe T, Velazquez OC, Dardik A. Limb ischemia after iliac ligation in aged mice stimulates angiogenesis without arteriogenesis. J Vasc Surg. 2009;49:464–473. doi: 10.1016/j.jvs.2008.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 990.White FC, Carroll SM, Magnet A, Bloor CM. Coronary collateral development in swine after coronary artery occlusion. Circ Res. 1992;71:1490–1500. doi: 10.1161/01.res.71.6.1490. [DOI] [PubMed] [Google Scholar]
- 991.Whyman MR, Fowkes FG, Kerracher EM, Gillespie IN, Lee AJ, Housley E, Ruckley CV. Is intermittent claudication improved by percutaneous transluminal angioplasty? A randomized controlled trial. J Vasc Surg. 1997;26:551–557. doi: 10.1016/s0741-5214(97)70052-1. [DOI] [PubMed] [Google Scholar]
- 992.Whyman MR, Fowkes FGR, Kerracher EMG, Gillespie IN, Lee AJ, Housley E, Ruckley CV. Randomised controlled trial of percutaneous transluminal angioplasty for intermittent claudication. Eur J Vasc Endovas Surg. 1996;12:167–172. doi: 10.1016/s1078-5884(96)80102-x. [DOI] [PubMed] [Google Scholar]
- 993.Williams J, Cartland D, Rudge J, Egginton S. VEGF Trap Abolishes Shear Stress- and Overload-Dependent Angiogenesis in Skeletal Muscle. Microcirc. 2006;13:499–509. doi: 10.1080/10739680600785717. [DOI] [PubMed] [Google Scholar]
- 994.Williams JL, Cartland D, Hussain A, Egginton S. A differential role for nitric oxide in two forms of physiological angiogenesis in mouse. J Physiol. 2006;570:445–454. doi: 10.1113/jphysiol.2005.095596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 995.Williams MA, Smith SA, DE OB, Mitchell JH, Garry MG. The group IV afferent neuron expresses multiple receptor alterations in cardiomyopathic rats: evidence at the cannabinoid CB1 receptor. J Physiol. 2008;586:835–845. doi: 10.1113/jphysiol.2007.140392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 996.Williams CK, Li JL, Murga M, Harris AL, Tosato G. Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood. 2006;107:931–939. doi: 10.1182/blood-2005-03-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 997.Williams JL, Weichert A, Zakrzewicz A, Silva-Azevedo L, Pries AR, Baum O, Egginton S. Differential gene and protein expression in abluminal sprouting and intraluminal splitting forms of angiogenesis. Clin Sci. 2006;110:587–595. doi: 10.1042/CS20050185. [DOI] [PubMed] [Google Scholar]
- 998.Wilson SE. Trials of endovascular treatment for superficial femoral artery occlusive lesions: a call for medically managed control patients. Ann Vasc Surg. 2010;24:498–502. doi: 10.1016/j.avsg.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 999.Winder WW, Holloszy JO. Response of mitochondria of different types of skeletal muscle to thyrotoxicosis. Am J Physiol. 1977;232:C180–C184. doi: 10.1152/ajpcell.1977.232.5.C180. [DOI] [PubMed] [Google Scholar]
- 1000.Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol. 2000;88:2219–2226. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]
- 1001.Woessner JF. The family of matrix metalloproteinases. Ann NY Acad Sci. 1994;732:11–21. doi: 10.1111/j.1749-6632.1994.tb24720.x. [DOI] [PubMed] [Google Scholar]
- 1002.Wolf C, Cai WJ, Vosschulte R, Koltai S, Mousavipour D, Scholz D, Afsah-Hedjri A, Schaper W, Schaper J. Vascular remodeling and altered protein expression during growth of coronary collateral arteries. J Mol Cell Cardiol. 1998;30:2291–2305. doi: 10.1006/jmcc.1998.0790. [DOI] [PubMed] [Google Scholar]
- 1003.Womack CJ, Ivey FM, Gardner AW, Macko RF. Fibrinolytic response to acute exercise in patients with peripheral arterial disease. Med Sci Sports Exer. 2001;33:214–219. doi: 10.1097/00005768-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 1004.Womack CJ, Nagelkirk PR, Coughlin AM. Exercise-induced chanegs in coagulation and fibrinolysis in healthy populations and patients with cardiovascular disease. Sports Med. 2003;33:795–807. doi: 10.2165/00007256-200333110-00002. [DOI] [PubMed] [Google Scholar]
- 1005.Womack CJ, Sieminski DJ, Katzel LI, Yataco A, Gardner AW. Improved walking economy in patients with peripheral arterial occlusive disease. Med Sci Sports Exer. 1997;29:1286–1290. doi: 10.1097/00005768-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 1006.Woolard J, Bevan HS, Harper SJ, Bates DO. Molecular diversity of VEGF-A as a regulator of its biological activity. Microcirc. 2009;16:572–592. doi: 10.1080/10739680902997333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1007.Woolard J, Wang WY, Bevan HS, Qiu Y, Morbidelli L, Pritchard-Jones RO, Cui TG, Sugiono M, Waine E, Perrin R, Foster R, Digby-Bell J, Shields JD, Whittles CE, Mushens RE, Gillatt DA, Ziche M, Harper SJ, Bates DO. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. 2004;64:7822–7835. doi: 10.1158/0008-5472.CAN-04-0934. [DOI] [PubMed] [Google Scholar]
- 1008.Wright DC, Geiger PC, Han DH, Jones TE, Holloszy JO. Calcium induces increases in peroxisome proliferator-activated receptor gamma coactivator-1alpha and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J Biol Chem. 2007;282:18793–18799. doi: 10.1074/jbc.M611252200. [DOI] [PubMed] [Google Scholar]
- 1009.Wright DC, Han DH, Garcia-Roves PM, Geiger PC, Jones TE, Holloszy JO. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1alpha expression. J Biol Chem. 2007;282:194–199. doi: 10.1074/jbc.M606116200. [DOI] [PubMed] [Google Scholar]
- 1010.Wu FT, Stefanini MO, Mac GF, Kontos CD, Annex BH, Popel AS. VEGF and soluble VEGF receptor-1 (sFlt-1) distributions in peripheral arterial disease: an in silico model. Am J Physiol. 2010;298:H2174–H2191. doi: 10.1152/ajpheart.00365.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1011.Wurdeman SR, Myers SA, Johanning JM, Pipinos II, Stergiou N. External work is deficient in both limgs of patients with unilateral PAD. Med Eng Phys. 2012;34:000–000. doi: 10.1016/j.medengphy.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1012.Wustehube J, Bartol A, Liebler SS, Brutsch R, Zhu Y, Felbor U, Sure U, Augustin HG, Fischer A. Cerebral cavernous malformation protein CCM1 inhibits sprouting angiogenesis by activating DELTA-NOTCH signaling. Proc Natl Acad Sci USA. 2010;107:12640–12645. doi: 10.1073/pnas.1000132107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1013.Xing J, Gao Z, Lu J, Sinoway LI, Li J. Femoral artery occlusion augments TRPV1-mediated sympathetic responsiveness. Am J Physiol. 2008;295:H1262–H1269. doi: 10.1152/ajpheart.00271.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1014.Xing J, Lu J, Li J. Contribution of nerve growth factor to augmented TRPV1 responses of muscle sensory neurons by femoral artery occlusion. Am J Physiol. 2009;296:H1380–H1387. doi: 10.1152/ajpheart.00063.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1015.Xing J, Sinoway LI, Li J. Differential responses of sensory neurones innervating glycolytic and oxidative muscle to protons and capsaicin. J Physiol. 2008;586:3245–3252. doi: 10.1113/jphysiol.2008.154450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1016.Xu J, Rodriguez D, Petitclerc E, Kim JJ, Hangai M, Moon YS, Davis GE, Brooks PC, Yuen SM. Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J Cell Biol. 2001;154:1069–1079. doi: 10.1083/jcb.200103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1017.Yan Z, Okutsu M, Akhtar YN, Lira VA. Regulation of exercise-induced fiber type transformation, mitochondrial biogenesis, and angiogenesis in skeletal muscle. J Appl Physiol. 2011;110:264–274. doi: 10.1152/japplphysiol.00993.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1018.Yang HT, Deschenes MR, Ogilvie RW, Terjung RL. Basic fibroblast growth factor increases collateral blood flow in rats with femoral arterial ligation. Circ Res. 1996;79:62–69. doi: 10.1161/01.res.79.1.62. [DOI] [PubMed] [Google Scholar]
- 1019.Yang HT, Feng Y, Allen LA, Protter A, Terjung RL. Efficacy and specificity of bFGF increased collateral flow in experimental peripheral arterial insufficiency. Am J Physiol. 2000;278:H1966–H1973. doi: 10.1152/ajpheart.2000.278.6.H1966. [DOI] [PubMed] [Google Scholar]
- 1020.Yang HT, Ogilvie RW, Terjung RL. Training increases collateral-dependent muscle blood flow in aged rats. Am J Physiol. 1995;268:H1174–H1180. doi: 10.1152/ajpheart.1995.268.3.H1174. [DOI] [PubMed] [Google Scholar]
- 1021.Yang HT, Feng Y. bFGF increases collateral blood flow in aged rats with femoral artery ligation. Am J Physiol. 2000;278:H85–H93. doi: 10.1152/ajpheart.2000.278.1.H85. [DOI] [PubMed] [Google Scholar]
- 1022.Yang HT, Laughlin MH, Terjung RL. Prior exercise training increases collateral-dependent blood flow in rats after acute femoral artery occlusion. Am J Physiol. 2000;279:H1890–H1897. doi: 10.1152/ajpheart.2000.279.4.H1890. [DOI] [PubMed] [Google Scholar]
- 1023.Yang HT, Ogilvie RW, Terjung RL. Low-intensity training produces muscle adaptations in rats with femoral artery stenosis. J Appl Physiol. 1991;71:1822–1829. doi: 10.1152/jappl.1991.71.5.1822. [DOI] [PubMed] [Google Scholar]
- 1024.Yang HT, Ogilvie RW, Terjung RL. Peripheral adaptations in trained aged rats with femoral artery stenosis. Circ Res. 1994;74:235–243. doi: 10.1161/01.res.74.2.235. [DOI] [PubMed] [Google Scholar]
- 1025.Yang HT, Ogilvie RW, Terjung RL. Heparin increases exercise-induced collateral blood flow in rats with femoral artery ligation. Circ Res. 1995;76:448–456. doi: 10.1161/01.res.76.3.448. [DOI] [PubMed] [Google Scholar]
- 1026.Yang HT, Ren J, Laughlin MH, Terjung RL. Prior exercise training produces NO-dependent increases in collateral blood flow after acute arterial occlusion. Am J Physiol. 2002;282:H301–H310. doi: 10.1152/ajpheart.00160.2001. [DOI] [PubMed] [Google Scholar]
- 1027.Yang HT, Yan Z, Abraham JA, Terjung RL. VEGF(121)- and bFGF-induced increase in collateral blood flow requires normal nitric oxide production. Am J Physiol. 2001;280:H1097–H1104. doi: 10.1152/ajpheart.2001.280.3.H1097. [DOI] [PubMed] [Google Scholar]
- 1028.Yilmaz MB, Caldir V, Guray Y, Guray U, Altay H, Demirkan B, Cay S, Kisacik HL, Korkmaz S. Relation of coronary collateral vessel development in patients with a totally occluded right coronary artery to the metabolic syndrome. Am J Cardiol. 2006;97:636–639. doi: 10.1016/j.amjcard.2005.09.103. [DOI] [PubMed] [Google Scholar]
- 1029.Yu J, deMuinck ED, Zhuang Z, Drinane M, Kauser K, Rubanyi GM, Qian HS, Murata T, Escalante B, Sessa WC. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc Natl Acad Sci. 2005;102:10999–11004. doi: 10.1073/pnas.0501444102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1030.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes and Development Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 1031.Yuan HT, Khankin EV, Karumanchi SA, Parikh SM. Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in the endothelium. Mol Cell Biol. 2009;29:2011–2022. doi: 10.1128/MCB.01472-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1032.Yue X, Tomanek RJ. Stimulation of coronary vasculogenesis/angiogenesis by hypoxia in cultured embryonic hearts. Dev Dyn. 1999;216:28–36. doi: 10.1002/(SICI)1097-0177(199909)216:1<28::AID-DVDY5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 1033.Yun S, Dardik A, Haga M, Yamashita A, Yamaguchi S, Koh Y, Madri JA, Sumpio BE. Transcription Factor Sp1 Phosphorylation induced by shear stress inhibits membrane type 1-matrix metalloprotinase expression in endothelium. J Biol Chem. 2002;277:34808–34814. doi: 10.1074/jbc.M205417200. [DOI] [PubMed] [Google Scholar]
- 1034.Zachary I, Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc Res. 2001;49:568–581. doi: 10.1016/s0008-6363(00)00268-6. [DOI] [PubMed] [Google Scholar]
- 1035.Zbinden R, Zbinden S, Billinger M, Windecker S, Meier B, Seiler C. Influence of diabetes mellitus on coronary collateral flow: an answer to an old controversy. Heart. 2005;91:1289–1293. doi: 10.1136/hrt.2004.041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1036.Zetterquist S. The effect of active training on the nutritive blood flow in exercising ischemic legs. Scand J Clin Lab Invest. 1970;25:101–111. doi: 10.3109/00365517009046196. [DOI] [PubMed] [Google Scholar]
- 1037.Zhang J, Cao R, Zhang Y, Jia T, Cao Y, Wahlberg E. Differential roles of PDGFR-alpha and PDGFR-beta in angiogenesis and vessel stability. FASEB J. 2009;23:153–163. doi: 10.1096/fj.08-113860. [DOI] [PubMed] [Google Scholar]
- 1038.Zhang K, Lu J, Mori T, Smith-Powell L, Synold TW, Chen S, Wen W. Baicalin increases VEGF expression and angiogenesis by activating the ERR(alpha)/PGC-1(alpha) pathway. Cardiovasc Res. 2011;89:426–435. doi: 10.1093/cvr/cvq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1039.Zhang HT, Scott PA, Morbidelli L, Peak S, Moore J, Turley H, Harris AL, Ziche M, Bicknell R. The 121 amino acid isoform of vascular endothelial growth factor is more strongly tumorigenic than other splice variants in vivo. Br J Cancer. 2000;83:63–68. doi: 10.1054/bjoc.2000.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1040.Zhang Y, Furumura M, Morita E. Distinct signaling pathways confer different vascular responses to VEGF 121 and VEGF 165. Growth Factors. 2008;26:125–131. doi: 10.1080/08977190802105909. [DOI] [PubMed] [Google Scholar]
- 1041.Zhao M, New L, Kravchenko VV, Kato Y, Gram H, diPadova F, Olson EN, Ulevitch RJ, Han J. Regulation of the MEF2 family of transcription factors by p38. Mol Cell Biol. 1999;19:21–30. doi: 10.1128/mcb.19.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1042.Zheng H, Li YF, Wang W, Patel KP. Enhanced angiotensin-mediated excitation of renal sympathetic nerve actvity within the paraventricular nucleus of anesthetized rats with heart failure. Am J Physiol. 2009;297:R1364–R1374. doi: 10.1152/ajpregu.00149.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1043.Zheng H, Yi YF, Cornish KG, Zucker IH, Patel KP. Exercise training improves endogenous nitric oxide mechanisms within the paraventricular nucleus in rats with heart failure. Am J Physiol. 2005;288:H2332–H2341. doi: 10.1152/ajpheart.00473.2004. [DOI] [PubMed] [Google Scholar]
- 1044.Zheng W, Christensen LP, Tomanek RJ. Differential effects of cyclic and static stretch on coronary microvascular endothelial cell receptors and vasculogenic/angiogenic responses. Am J Physiol. 2008;295:H794–H800. doi: 10.1152/ajpheart.00343.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1045.Zhou AL, Egginton S, Brown MD, Hudlicka O. Capillary growth in overloaded, hypertrophic adult rat skeletal muscle: An ultrastructural study. The Anatomical Record. 1998;252:49–63. doi: 10.1002/(SICI)1097-0185(199809)252:1<49::AID-AR6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 1046.Zhou AL, Egginton S, Hudlicka O, Brown MD. Internal division of capillaries in rat skeletal muscle in response to chronic vasodilator treatment with α1-antagonist prazosin. Cell Tissue Res. 1998;293:293–303. doi: 10.1007/s004410051121. [DOI] [PubMed] [Google Scholar]
- 1047.Ziada AM, Hudlicka O, Tyler KR, Wright AJ. The effect of long-term vasodilatation on capillary growth and performance in rabbit heart and skeletal muscle. Cardiovasc Res. 1984;18:724–732. doi: 10.1093/cvr/18.12.724. [DOI] [PubMed] [Google Scholar]
- 1048.Ziche M, Morbidelli L, Choudhuri R, Zhang HT, Donnini S, Granger HJ, Bicknell R. Niric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest. 1997;99:2625–2634. doi: 10.1172/JCI119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1049.Ziegler MA, Distasi MR, Bills RG, Miller SJ, Alloosh M, Murphy MP, George AA, Sturek M, Dalsing MC, Unthank JL. Marvels, mysteries, and misconceptions of vascular compensation to peripheral artery occlusion. Microcirc. 2010;17:3–20. doi: 10.1111/j.1549-8719.2010.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1050.Zoladz JA, Korzeniewski B, Grassi B. Training-induced acceleration of oxygen uptake kinetics in skeletal muscle: the underlying mechanisms. J Physiol Pharmacol. 2006;57:67–84. [PubMed] [Google Scholar]
- 1051.Zucker IH. Novel mechanisms of sympathetic regulation on chronic heart failure. Hypertension. 2006;48:1005–1011. doi: 10.1161/01.HYP.0000246614.47231.25. [DOI] [PubMed] [Google Scholar]
- 1052.Zucker IH, Patel KP, Schultz HD, Li YF, Wang W, Pliquett RU. Exercise training and sympathetic regulation in experimental heart failure. Exerc Sport Sci Rev. 2004;32:107–111. doi: 10.1097/00003677-200407000-00006. [DOI] [PubMed] [Google Scholar]
- 1053.Zucker IH, Wang W, Pliquett R, Liu JL, Patel KP. The regulation of sympathetic outflow in heart failure The roles of angiotensin II, nitric oxide, and exercise training. Ann NY Acad Sci. 2001;940:431–443. [PubMed] [Google Scholar]
- 1054.Zukowska Z, Pons J, Lee EW, Li L. Neuropeptide Y: a new mediator linking sympathetic nerves, blood vessels and immune system? Can J Physiol Pharmacol. 2003;81:89–94. doi: 10.1139/y03-006. [DOI] [PubMed] [Google Scholar]
- 1055.Zwetsloot KA, Westerkamp LM, Holmes BF, Gavin TP. AMPK regulates basal skeletal muscle capillarization and VEGF expression, but is not necessary for the angiogenic response to exercise. J Physiol. 2008;586:6021–6035. doi: 10.1113/jphysiol.2008.159871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further Reading
- 1056.Schaper W, Schaper J. Arteriogenesis. Boston: Kluwer Academic Publishers; 2004. pp. 1–377. [Google Scholar]
- 1057.Charkravarthy MV, Booth FW. Exercise. Philadelphia: Hanley & Belfus; 2003. pp. 1–326. [Google Scholar]