Abstract
Background
Executive dysfunction, distinct from other cognitive deficits in depression, has been associated with suicidal behavior. However, this dysfunction is not found consistently across samples.
Method
Medication-free subjects with DSM-IV major depressive episode (major depressive disorder and bipolar type I disorder) and a past history of suicidal behavior (n=72) were compared to medication-free depressed subjects with no history of suicidal behavior (n=80) and healthy volunteers (n=56) on a battery of tests assessing neuropsychological functions typically affected by depression (motor and psychomotor speed, attention, memory) and executive functions reportedly impaired in suicide attempters (abstract/contingent learning, working memory, language fluency, impulse control).
Results
All of the depressed subjects performed worse than healthy volunteers on motor, psychomotor and language fluency tasks. Past suicide attempters, in turn, performed worse than depressed non-attempters on attention and memory/working memory tasks [a computerized Stroop task, the Buschke Selective Reminding Task (SRT), the Benton Visual Retention Test (VRT) and an N-back task] but not on other executive function measures, including a task associated with ventral prefrontal function (Object Alternation). Deficits were not accounted for by current suicidal ideation or the lethality of past attempts. A small subsample of those using a violent method in their most lethal attempt showed a pattern of poor executive performance.
Conclusions
Deficits in specific components of attention control, memory and working memory were associated with suicidal behavior in a sample where non-violent attempt predominated. Broader executive dysfunction in depression may be associated with specific forms of suicidal behavior, rather than suicidal behavior per se.
Keywords: Bipolar disorder, cognition, depression, neuropsychology, suicidal behavior
Introduction
Neuropsychological dysfunction in the context of depression is a risk factor for suicidal behavior, with executive dysfunction thought to play a predominant role. We had previously identified a post-hoc-derived executive performance factor that discriminated subjects with past histories of highly lethal suicidal behavior (Keilp et al. 2001), that correlated with language fluency and a secondary measure (Failure to Maintain Set) from the Wisconsin Card Sorting Test (WCT). The lack of any differences on standard WCT measures (e.g. category attainment, errors, perseverative errors) and Failure to Maintain Set’s association with ventral prefrontal function (Stuss et al. 2000) led us to hypothesize that other measures sensitive to ventral prefrontal dysfunction might be useful as a way to characterize deficits associated with suicidality.
Subsequent studies have found attempter/non-attempter differences on tasks whose common feature is an association with ventral prefrontal function, including decision-making measures such as the Iowa Gambling Task (Jollant et al. 2005, 2007, 2010; Westheide et al. 2008) and the Cambridge Gambling Task (Clark et al. 2011), behavioral measures of impulse control (Swann et al. 2005; Dougherty et al. 2009; Wu et al. 2009) and measures of mental flexibility such as Reversal Learning (Dombrovski et al. 2010). However, deficits in standard WCT indices have been found in suicide ideators (Marzuk et al. 2005) and not all studies find differences in individuals at risk for suicidal behavior (e.g. self-injurers) using performance measures of impulsiveness (Janis & Nock, 2009).
In a recent review, Jollant et al. (2011) speculate that a network of brain regions implicated in the performance of decision-making tasks, which include the ventral prefrontal cortex, anterior cingulate and amygdala, are probably involved in suicidal behavior. However, this review also highlighted the diversity in patient samples that have been studied with regard to clinical state, medication status and nature of attempts, complicating any conclusions that might be drawn. There have been few studies that have examined larger samples of past attempters during a period of presumptive risk, using a comprehensive battery, to determine whether deficits on individual executive measures reflect a more general deficit, or even more fundamental impairments in basic information processing.
These basic neuropsychological functions have received less consideration in studies of suicidal behavior, despite their ability to differentiate attempters. Impaired attention control (Williams & Broadbent, 1986; Becker et al. 1999; Cha et al. 2010) has been found in suicide attempter and at-risk samples, especially if provocative distractors (i.e. suicide-related words) are used. In an interim analysis including our original sample and a portion of this sample (Keilp et al. 2008), past attempters performed more poorly than non-attempters on a Stroop task, but not a Continuous Performance Task, suggesting that conflict detection measures may be especially sensitive to an information-processing deficit associated with suicidal behavior (one aim of this study was to determine whether these deficits stand out against the back-ground of a larger neuropsychological battery). Memory performance is also deficient in suicide attempters, on both standard list learning tasks and autobiographical measures (Keilp et al. 2001; Sinclair et al. 2007; Arie et al. 2008). It is not known whether these deficits underlie, or are associated with, the executive impairments found in other studies.
It is also not known whether different types of suicidal behavior are associated with different types of neuropsychological impairment. In our previous work, for example, deficits in executive performance were found in those who had made highly lethal past attempts (Keilp et al. 2001). Deficits in decision making are reported to be most pronounced in violent past attempters (Jollant et al. 2005).
The purpose of this study was to systematically assess a new, larger sample of medication-free individuals with a past history of suicidal behavior who were currently depressed (major depressive disorder or type I bipolar disorder) and therefore in a period of risk. In our previous study (Keilp et al. 2001), a post-hoc discriminant analysis found two dimensions in our data corresponding to impairments related to depression itself, and to higher lethality past suicide attempt. A strategy to distinguish these two dimensions in our previous data was built into the design of the current assessment battery, which assessed eight domains of functioning. Four domains were expected to reflect depression-related impairments: domains assessing motor speed, psychomotor performance, attention and memory (Veiel, 1997; Zakzanis et al. 1998; Baune et al. 2010). Four additional domains were designed to assess executive functions that were most likely to be affected by past suicide attempt status, including abstract/contingent learning (Keilp et al. 2001; Marzuk et al. 2005), working memory (Keilp et al. 2001), language fluency (Bartfai et al. 1990; Keilp et al. 2001; Audenaert et al. 2002) and impulse control (Swann et al. 2005; Wu et al. 2009; Dougherty et al. 2009). Relative to the assessment in our earlier study, our assessment of abstract/contingent learning was enhanced with the addition of a computerized Object Alternation task, which, along with gambling tasks and reversal learning, is one of the best-validated measures of ventral prefrontal dysfunction (Zald & Andreotti, 2010). Specific measures of impulsiveness (Go–No Go and Time Estimation; Keilp et al. 2005) were also included.
We hypothesized that depressed attempters and non-attempters would not differ on measures of motor speed and psychomotor performance, and most aspects of attention and memory, and that both groups would perform worse than healthy volunteers on these measures. Past attempters were expected to perform worse than non-patients and non-attempters on executive measures, including abstract/contingent learning, working memory, language fluency and impulse control tasks. Because the relationship between neuropsychological performance and suicide attempt may be mediated by characteristics of suicidal behavior, we also assessed the influence of level of current suicidal ideation, severity of past attempts and the violence of past attempts in supplementary analyses, to determine whether these factors contributed to attempter/non-attempter neuropsychological differences.
Method
Sample
Participants were 152 patients meeting DSM-IV criteria for a current major depressive episode (major depressive disorder or type I bipolar disorder; type II were excluded based on their variability and our earlier work; Harkavy-Friedman et al. 2006) and 56 non-patient comparison subjects. Characteristics of the samples are presented in Table 1. Patients were currently depressed, with a Hamilton Rating Scale for Depression (HAMD, 24-item) score >16 at the time of recruitment. Non-patients were free of current or past Axis I or Axis II disorders. All subjects were free of neurological disease and gross organic brain dysfunction by clinical history and examination, and all had an estimated IQ>80. None had current psychosis or current substance abuse/dependence. Of the participating patients, 72 had made at least one prior suicide attempt and 80 had no history of suicidal behavior. All subjects were either medication free or washed out of medications for participation in associated biological studies for at least 2 weeks prior to their assessment (6 weeks for fluoxetine). This study was approved by the local Institutional Review Board and all participants gave written informed consent.
Table 1.
Demographic and clinical rating data
| Variable | Non-patient comparison (C) (n=56) |
Depressed non-attempters (NA) (n=80) |
Depressed attempters (ATT) (n=72) |
p valuea | Contrast |
|---|---|---|---|---|---|
| Age (years) | 31.5±11.1 | 40.1±11.9 | 35.7±11.6 | <0.001 | C<ATT<NA |
| Education (years) | 16.0±2.1 | 15.8±2.3 | 15.3±2.3 | 0.20 | |
| WAIS-III Vocabulary and Matrix Reasoning (average scaled score) |
13.4±2.5 | 13.0±2.2 | 12.9±2.3 | 0.35 | |
| HAMD | 0.9±1.7 | 25.6±7.4 | 25.7±7.3 | <0.001 | C<NA, ATT |
| BDI | 1.4±2.2 | 28.0±9.4 | 28.8±11.7 | <0.001 | C<NA, ATT |
| GAF | 89.4±7.2 | 49.5±9.9 | 46.7±12.2 | <0.001 | C>NA, ATT |
| GAF without suicide item | 89.4±7.2 | 49.6±10.0 | 47.2±12.0 | <0.001 | C>NA, ATT |
| Beck Hopelessness Scale | 1.2±1.2 | 12.3±5.8 | 13.6±5.7 | <0.001 | C<NA, ATT |
| Scale for Suicide Ideation (prior to hospitalization) | 0.0±0.0 | 5.9±7.1 | 15.3±10.7 | <0.001 | C<NA<ATT |
| Scale for Suicide Ideation (current) | 0.0±0.0 | 4.6±6.0 | 8.2±8.2 | <0.001 | C<NA<ATT |
| No. of past depressive episodes | – | 7.5±10.4 (median=3.0) | 13.0±16.9 (median=5) | 0.02 | NA<ATT |
| Duration of current depressive episode (weeks) | – | 82.3±218.0 (median=24) | 61.2±117.0 (median=16.5) | 0.49 | |
| Barratt Impulsiveness Scale | 38.0±14.2 | 53.6±17.5 | 57.4±17.3 | <0.001 | C<NA, ATT |
| Buss–Durkee Hostility Inventory | 20.0±8.5 | 32.3±11.7 | 39.1±12.2 | <0.001 | C<NA<ATT |
| Brown–Goodwin Aggression History | 14.6±4.1 | 17.0±4.3 | 19.7±5.9 | <0.001 | C<NA<ATT |
| Cognitive failures | 26.5±11.6 | 51.1±15.2 | 53.2±17.4 | <0.001 | C<NA, ATT |
| No. of previous suicide attempts | – | – | 2.5±1.8 | ||
| Lethality of most recent attempt | – | – | 2.6±2.0 | ||
| Maximum lethality of attempt | – | – | 3.1±2.0 | ||
| Suicide Intent Scale, most recent attempt | – | – | 15.8±5.5 | ||
| Suicide Intent Scale, most lethal attempt | – | – | 16.2±5.6 | ||
| Violent attempt (most lethal) | 18.3 (13) | ||||
| Time since most recent attempt (months) | 44.5±94.1 (median=4.6) | ||||
| Sex (female) | 50.0 (28) | 52.5 (42) | 63.9 (46) | 0.22 | |
| Axis I diagnosis | – | ||||
| Unipolar | 78.8 (63) | 69.4 (50) | 0.19 | ||
| Bipolar I | 21.3 (17) | 30.6 (22) | |||
| Axis II diagnosis (BPD) | – | 13.8 (11) | 33.8 (24) | 0.004 | NA<ATT |
| Past substance abuse/dependence | – | 26.3 (21) | 45.8 (33) | 0.01 | NA<ATT |
| PTSD (lifetime) | – | 11.3 (9) | 27.8 (20) | 0.01 | NA<ATT |
WAIS-III, Wechsler Adult Intelligence Scale, 3rd revision ; HAMD, 24-item Hamilton Rating Scale for Depression ; BDI, Beck Depression Inventory ; GAF, Global Assessment of Functioning ; BPD, borderline personality disorder ; PTSD, post-traumatic stress disorder.
Omnibus ANOVA for continuous variables, χ2 for categorical variables.
Values given as mean ± standard deviation or % (n).
Instruments
Diagnosis was established in patients using the Structured Clinical Interview for DSM-IV, Axis I (Spitzer et al. 1990) and Axis II (First et al. 1996). Psychiatric illnesses were ruled out in non-patients using the non-patient version of the SCID (First et al. 1997). Other clinical ratings have been described previously (Mann et al. 1999) and are listed in Table 1. Premorbid intellectual ability was assessed with the Vocabulary and Matrix Reasoning subtests from the Wechsler Adult Intelligence Scale, 3rd revision (WAIS-III; Wechsler, 1997; subjects with an average scaled score <7 on these subtests were excluded). Subjective cognitive complaint was assessed with the Cognitive Failures Questionnaire (Broadbent et al. 1982). History of past suicidal behavior was assessed using the Columbia Suicide History Scale (Oquendo et al. 2003) and intent with the Suicide Intent Scale (Beck et al. 1975). Severity of past suicide attempts was quantified using Beck’s medical damage rating of physical injury resulting from an attempt (Beck et al. 1975), which ranges from 0 (no physical damage) to 8 (death).
Subjects were evaluated in eight neuropsychological domains, with the first four targeting core deficits in depression and the second four a broad array of executive functions associated with suicidal behavior in prior studies. These domains, and the tests included in them, were as follows: (1) Motor Function [Finger Tapping Test, Simple and Choice Reaction Time (RT)], (2) Psychomotor Function (Trail Making Test, WAIS-III Digit Symbol subtest), (3) Attention [Continuous Performance Test – Identical Pairs, 4-digits fast condition (CPT), computerized Stroop task], (4) Memory [Buschke Selective Reminding Test (SRT), Benton Visual Retention Test (VRT), administration D], (5) Abstract/Contingent Learning [Wisconsin Card Sorting Test (WCT), computerized Object Alternation test], (6) Working Memory (computerized N-Back Test, A, Not B Logical Reasoning Test), (7) Language Fluency (Letter and Animal/Category tasks), and (8) Impulse Control (computerized Go–No Go and Time Estimation/Production tasks). All tasks have been used in our previous studies (Keilp et al. 2001, 2005), with the exception of Object Alternation, which is a computerized adaptation of a primate task similar to that used by other investigators (Zald et al. 2005), sensitive to ventral prefrontal dysfunction (Zald et al. 2005; Zald & Andreotti, 2010), and included as a complement to the WCT, which is primarily associated with dorsolateral dysfunction (Stuss et al. 2000). A detailed description of this task is presented in the Appendix. The principal measures from each task (see Table 2) were converted to Z scores based on age-, sex- and/or education-corrected external norms (Wechsler, 1997; Keilp et al. 2005; Spreen & Strauss, 2006) and averaged to compute domain scores.
Table 2.
Neuropsychological performance measures
| Variable | Non-patient comparison (C) |
Depressed non-attempters (NA) |
Depressed attempters (ATT) |
p valuea | Contrast |
|---|---|---|---|---|---|
| Depression-related domains | |||||
| Motor functioning | 0.14±0.76 | −0.29±1.12 | −0.33±1.01 | 0.03 | C>NA, ATT |
| Tapping dominant | −0.07±1.23 | −0.26±1.45 | −0.13±1.51 | 0.75 | |
| Tapping non-dominant | −0.38 ±0.98 | −0.38±1.34 | −0.35±1.42 | 0.87 | |
| Simple reaction time | 0.12±1.29 | −0.47±2.10 | −0.63±1.67 | 0.06 | |
| Choice reaction time | 0.51±1.00 | 0.07±1.36 | −0.12±1.46 | 0.01 | C>NA, ATT |
| Psychomotor function | 0.04±0.96 | −0.27±0.97 | −0.33±0.81 | 0.05 | C>NA, ATT |
| Trails A | −0.39±1.12 | −0.36±1.10 | −0.49±0.95 | 0.83 | |
| Trails B | −0.07±1.19 | −0.11±1.06 | −0.34±1.07 | 0.30 | |
| WAIS-III Digit Symbol | 0.31±1.22 | −0.31±1.19 | −0.24±1.09 | 0.004 | C>NA, ATT |
| Attention | 0.02±0.78 | −0.10±0.82 | −0.35±0.91 | 0.04 | C, NA>ATT |
| CPT (d’) | 0.03±0.96 | −0.08±1.08 | −0.17±1.07 | 0.51 | |
| Stroop interference | 0.01±1.05 | −0.11±1.11 | −0.54±1.31 | 0.03 | C, NA>ATT |
| Memory | −0.06±0.75 | −0.31±1.07 | −0.72±1.05 | <0.001 | C, NA>ATT |
| Buschke SRT (total) | −0.01±1.03 | −0.32±1.46 | −0.75±1.30 | 0.006 | C, NA>ATT |
| Benton VRT (error) | −0.11±0.72 | −0.29±1.09 | −0.70±1.20 | 0.009 | C, NA>ATT |
| Executive domains | |||||
| Abstract/contingent learning | −0.19±0.85 | −0.35±0.71 | −0.39±0.87 | 0.19 | |
| WCT (error) | −0.32±1.09 | −0.31±0.98 | −0.50±1.05 | 0.47 | |
| Object alternation (error) | −0.05±1.21 | −0.39±1.14 | −0.24±1.21 | 0.16 | |
| Working memory | −0.12±0.83 | −0.36±0.93 | −0.40±1.05 | 0.12 | |
| N-back | −0.33±1.02 | −0.29±0.92 | −0.57±1.19 | 0.05 | C, NA>ATT |
| A, not B timed reasoning | 0.09±1.15 | −0.40±1.35 | −0.23±1.31 | 0.11 | |
| Language fluency | 0.07±0.81 | −0.37±0.91 | −0.38±0.92 | 0.003 | C>NA, ATT |
| Letter fluency | 0.23±1.05 | −0.21±1.08 | −0.26±1.06 | 0.002 | C>NA, ATT |
| Category fluency | −0.08±0.96 | −0.54±1.07 | −0.49±1.05 | 0.05 | C>NA, ATT |
| Impulse control | −0.18±0.57 | 0.06±0.60 | 0.05±0.77 | 0.30 | |
| Go–no go commission error (log) | −0.50±0.73 | −0.27±0.76 | −0.16±1.10 | 0.33 | |
| Time production (deviation) | 0.16±0.76 | 0.39±0.92 | 0.26±0.96 | 0.36 |
CPT, Continuous Performance Test ; WCT, Wisconsin Card Sorting Test ; SRT, Selective Reminding Task; VRT, Visual Retention Test.
ANCOVA with main effect for group, covarying presence of borderline personality disorder.
Statistical analyses
Demographic and clinical data were compared using a one-way ANOVA and post-hoc Neuman–Keuls tests for continuous variables and χ2 tests for categorical variables. Analyses of neuropsychological scores proceeded in a hierarchical fashion to control experiment-wise error rate. Neuropsychological domain scores were compared simultaneously among groups in a repeated-measures General Linear Model with neuropsychological domain (eight levels) and subject group (three levels) as factors. Covariates for clinical variables that might affect group differences were tested together in the first step of the analysis; only those having a significant effect on test performance were retained for the final model. A significant effect for subject grouping in this final model led to evaluation of individual domain scores, followed by evaluation of individual tests. An α level of 0.05 was maintained at each level of the analysis. Supplemental analyses were conducted covarying suicidal ideation, comparing subjects with high versus low lethality past suicide attempts (high=medical damage rating >4, injury requiring major medical intervention), and comparing subjects who had used a violent method in their most lethal attempt (firearm, drowning, cutting, jumping, or hanging) to those who had used a non-violent method (overdose, substance ingestion).
Correlations (non-parametric, to minimize distributional effects) were computed between domain or test scores that distinguished past attempters and clinical variables.
Results
Demographic and clinical characteristics
Depressed non-attempters were older than past attempters, and both patient groups were older than non-patients. However, groups were equivalent in education level and estimated intelligence, and all test scores were adjusted for normative age effects. Non-attempters and past attempters were both comparably depressed with comparable levels of functional impairment [Global Assessment of Functioning (GAF) score]. Suicide attempters had more past major depressive episodes, in addition to higher levels of current suicidal ideation, self-reported hostility and past aggressive behavior. Subjective complaints of cognitive impairment were equally elevated in both patient groups compared with non-patients. Median time since most recent attempt was approximately 5 months (range 4 days to 37 years). For attempters, approximately half of the most recent attempts were within 1 year of evaluation (n=39). There were significantly more individuals with a past history of substance use disorder, borderline personality disorder (BPD) and post-traumatic stress disorder (PTSD) among suicide attempters relative to non-attempters, all conditions that might affect cognitive performance (and that were tested as covariates). The percentage of unipolar and bipolar subjects did not differ between attempters and non-attempters, and no significant performance differences were found between the groups in any domain. The suicide attempter group had, on average, made 2.5 prior attempts of moderate lethality.
Neuropsychological performance
In the first step of the analysis of neuropsychological performance, dichotomous covariates for presence of bipolar disorder, history of substance use disorder, BPD and PTSD were entered simultaneously. Age was not included as a covariate because all test scores were adjusted for normative age effects. The number of past depressive episodes was tested separately as a covariate in patients alone as detailed below. A covariate effect was found for the presence of BPD (F1,200=3.89, p=0.05) because of their paradoxically better performance on impulse control tasks (t146=3.00, p=0.003). All other co-morbidity covariates were non-significant (all p>0.10), so that only presence/absence of BPD was retained as a covariate in both the main analysis and all subsequent lower-level analyses.
A reduced model was then applied, including group (attempter/non-attempter/non-patient) as a factor and presence/absence of BPD as a control variable. Effects for group (F2,203=7.08, p=0.001) and the group by domain interaction (F14,1421=1.94, p=0.02) were statistically significant.
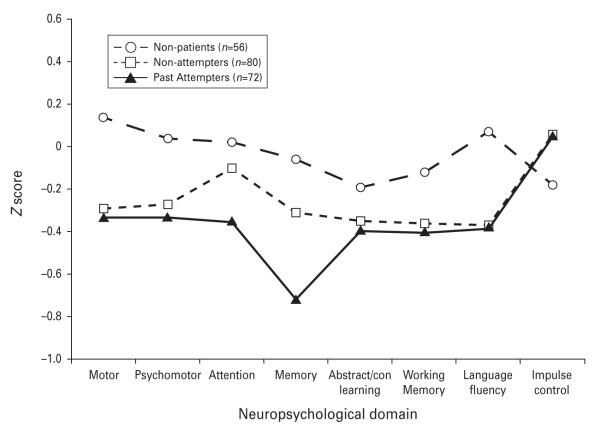
In comparisons of individual domain scores (Fig. 1), significant group differences were found in the Motor (F2,203=3.77, p=0.03), Psychomotor (F2,203=3.02, p=0.05), Attention (F2,203=3.33, p=0.04), Memory (F2,202=7.11, p=0.001), and Language Fluency (F2,203=6.07, p=0.003) domains. No group differences were found for the Abstract/Contingent Learning (F2,201=1.68, p=0.19), Working Memory (F2,203=2.18, p=0.12) and Impulse Control (F2,200=1.20, p=0.30) domain scores. [Groups differences in Abstract/Contingent Learning domain were non-significant if based on Fail to Maintain rather than error scores (F2,201=1.29, p=0.28).]
Fig. 1.
Average standardized neuropsychological performance across eight domains of function in non-patients, depressed non-attempters and depressed past suicide attempters.
In the Motor, Psychomotor and Language Fluency domains, both depressed groups performed significantly worse than non-patients (Table 2). Differences in these domains were attributable to poorer patient performance on Choice RT (F2,202=4.50, p=0.01), WAIS-III Digit Symbol (F2,203=5.60, p=0.004), and both letter (F2,203=6.30, p=0.002) and category fluency (F2,202=3.14, p=0.05) tasks. Simple RT approached significance (F2,199=2.85, p=0.06), contributing to the overall Motor domain difference.
In the Attention and Memory domains, past attempters performed worse than both depressed non-attempters and non-patients. On individual tests, past attempters performed worse than both other groups on the Stroop interference measure (F2,203=3.75, p=0.03), Buschke SRT (F2,202=5.31, p=0.006) and Benton VRT (F2,199=4.88, p=0.009). Although there were no differences in the Working Memory domain overall, N-back performance was significantly poorer in past attempters (F2,195=3.07, p=0.05).
In an additional analysis to evaluate the effect of number of past episodes of depression on these group differences, non-patients were excluded and non-attempters compared directly to past attempters. Non-attempter/attempter differences on the Stroop (F1,145=6.62, p=0.01), Benton VRT (F1,141=4.75, p=0.03) and N-back (F1,137=5.67, p=0.02) were maintained even when the number of past depressive episodes (log transformed to normalize distribution) was included as a covariate, along with BPD. The difference in Buschke SRT (F1,144=3.55, p=0.06) became marginal, even though number of past depressive episodes was not a significant covariate (F1,144=0.37, p=0.54).
Including primary diagnosis (unipolar versus bipolar) as an additional factor did not alter the significance of any attempter/non-attempter difference. This variable and its interactions were not significant in any comparison.
Current suicidal ideation
When current suicidal ideation was included as a covariate in group comparisons (in addition to BPD), the subject group effect (F2,198=6.87, p=0.001) and group by domain interaction (F14,1386=1.90, p=0.02) in the main analysis remained significant. Covariate effects for current suicidal ideation (F1,198=0.40, p=0.53) and the ideation by domain interaction (F7,1386=0.70, p=0.67) were not significant. Differences in the Attention (F2,198=4.00, p=0.02) and Memory (F2,197=8.40, p<0.001) domains remained significant with ideation as a covariate, as did differences in Stroop interference (F2,198=3.47, p=0.03), Buschke SRT (F2,197=7.15, p=0.001), Benton VRT (F2,194=4.88, p=0.009) and N-back (F2,190=4.11, p=0.02). Current suicidal ideation was not a significant covariate in any of these comparisons, nor was it significant in any other domain, including executive function domains.
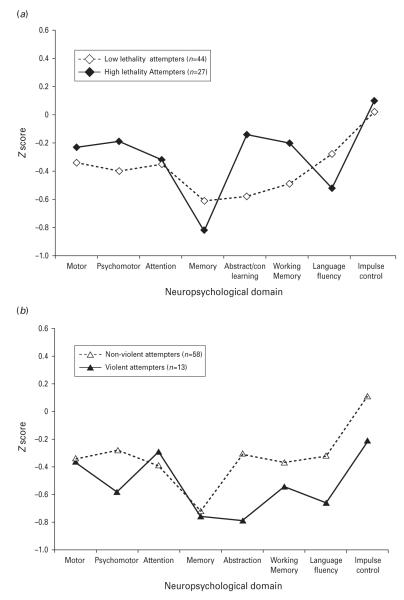
Attempt lethality
When past attempters are divided into those with high (n=27) versus low (n=44) lethality past attempts (Fig. 2a), there were no significant differences in domain scores between them, although high lethality attempters paradoxically outperformed low lethality attempters at a trend level in the Abstract/Contingent Learning domain (F1,66=3.51, p=0.07). Within that domain, high lethality attempters performed significantly better on Object Alternation (F1,57=6.01, p=0.02). High lethality attempters also performed better on Trail Making Part B (F1,67=5.52, p=0.02), a psychomotor tests with executive components. Differences on Attention and Memory measures, or on N-back, were not accounted for by markedly poorer performance in the high lethality group.
Fig. 2.
Average standardized domain scores in depressed past attempters, according to (a) lethality of past attempt and (b) violence of method of attempt.
Violent method in most severe past attempt
High lethality attempters’ comparable or better performance in most domains, relative to low lethality attempters, was partially explained by the distribution of participants who used a violent method in their most severe attempt. In this sample, those who used a violent method (n=13: attempted drowning, n=1; cutting, n=7; jumping, n=3; hanging, n=2) tended to make low lethality attempts (10/13, 76.9% of violent attempters; mean lethality 2.3±2.5 for violent attempters versus 3.3±1.9 in non-violent). Despite the small size of this sample, violent attempters (Fig. 2b) performed significantly worse in Abstract/Contingent Learning (F1,66=3.84, p=0.05) with a similar trend in Impulse Control (F1,67=3.55, p=0.06). On individual tasks, Go–No Go performance was significantly poorer in violent attempters (F1,65=5.52, p=0.02), with a similar trend in Object Alternation (F1,57=3.13, p=0.08; no other differences with p<0.10). These were the types of differences expected in the attempter group as a whole, but only found in this subgroup. They were not a function of an excess of patients with BPD (46.2% of violent attempters versus 31.6% of non-violent; , p=0.32). Differences between attempters and non-attempters on Attention and Memory measures, then, were not accounted for by poorer performance in the violent attempters group.
Correlations
There were few correlations between clinical variables and either the domain scores (Attention, Memory) or specific test scores (Stroop interference, Buschke SRT recall, Benton VRT errors, N-back d-prime) that distinguished past attempters from non-attempters. The Attention domain score and Stroop score were modestly correlated with the HAMD (ρ=−0.26, p=0.001 and ρ=−0.27, p=0.001 respectively), BDI (ρ=−0.17, p=0.03 and ρ=−0.23, p=0.003 respectively) and GAF score (ρ=0.21, p=0.01 and ρ=0.19, p=0.03 respectively). Memory domain score was weakly correlated with the HAMD score (ρ=−0.17, p=0.05) and GAF (ρ=0.20, p=0.02) but not the BDI (ρ=0.07, p=0.38). The Buschke score correlated with GAF (ρ=0.20, p=0.01).
Stroop performance correlated negatively, but weakly, with suicidal ideation prior to admission (ρ=−0.17, p=0.05), Barratt Impulsiveness (ρ=−0.21, p=0.02) and Buss–Durkee Hostility (ρ=−0.21, p=0.02). Memory domain score and Benton VRT correlated modestly with Hostility (ρ=−0.20, p=0.03 and ρ=−0.21, p=0.02 respectively).
Stroop correlated negatively with the number of past suicide attempts (ρ=−0.35, p=0.003), but no other test score was associated with suicidal behavior measures.
Discussion
In contrast to our expectations, depressed individuals with a history of suicidal behavior did not show any greater impairment of abstract/contingent learning, language fluency or impulse control relative to non-attempters in this acutely ill, medication-free sample. However, other deficits associated with suicidal behavior that we had reported previously in a separate sample (Keilp et al. 2001), in selective attention, memory and working memory, were again observed here. Past attempters’ poorer performance on the Stroop task and memory/working memory measures was not a function of depression severity or suicidal ideation, suggesting it represents a relatively independent marker of suicide risk within the context of depression, one that is not captured in standard clinical ratings. Although both the Stroop and memory measures were weakly associated with ratings of impulsiveness and/or hostility, it is difficult to attribute poor Stroop and memory/working memory performance in suicide attempters to failures of inhibition (see MacLeod et al. 2003). Other measures such as Go-No Go are clearly more direct measures of disinhibition, and did not differ among the groups unless violent attempters were analyzed separately. Interference effects on the Stroop in particular have been tied to attention control rather than impulse control networks in the brain (Botvinik et al. 2001; Egner & Hirsch, 2005). Finally, error rates on Stroop conditions did not differ among groups in this study (data not reported; available on request), as in our previous report, which included a portion of this sample (Keilp et al. 2008). Poorer performance on these tasks, then, seems to reflect an information processing deficit rather than a failure of inhibition.
Depressed patients, regardless of past attempt history, exhibited slowed reaction times, psychomotor performance and fluency. These patient/non-patient differences were less extensive than expected, partly due to splitting the depressed group by attempter status (analyzed as a single group, depressed patients differed from non-patients in all domains except abstract/contingent learning) and to the intelligence level of the sample. Nonetheless, the most consistent differences between depressed patients and healthy volunteers were found in two of the domains where they were expected: motor function and psychomotor performance. Differences in fluency reflect deficits on another set of speeded tasks, ones in which suicide attempters were expected to perform more poorly. Violent attempters showed a trend toward poorer performance in fluency relative to all other patients (F1,147=2.22, p=0.14), but this did not reach significance.
The small subsample of violent attempters in this study exhibited a pattern of performance more closely approximating the pattern of broad executive impairment expected in all attempters. Although consistent with studies of violent attempter samples (Jollant et al. 2005; Dougherty et al. 2009), these data raise questions about the specificity of the relationship of this type of executive impairment to suicidal behavior, as opposed to violent behavior more generally. Violence directed toward others is associated with executive dysfunction (Morgan & Lilienfield, 2000; Brower & Price, 2001; Hanlon et al. 2010), and violent suicidal behavior may simply be a subset of this general class of behavior. Non-violent suicidal behavior may not be associated with these same impairments. For example, the small subsample of violent attempters in this study performed worse than the non-violent attempters (and also non-attempters; F3,207=4.41, p=0.005) on Object Alternation (Freedman et al. 1998; Zald et al. 2005; Zald & Andreotti, 2010). Conversely, the mostly non-violent high lethality attempters outperformed low lethality attempters on this task. In the initial study of Iowa Gambling Task performance in past suicide attempters (Jollant et al. 2005), only violent attempters differed statistically from psychiatric controls, and no information was provided about the lethality of their attempts. It is important to note that violence and lethality are somewhat independent dimensions of suicidal behavior, and the mechanisms underlying these dimensions may be different. Models of suicidal behavior appropriate to violent attempts at any level of lethality may not apply to very serious non-violent suicide attempts, especially those that are planned over time. Specific types of executive dysfunction may play a role in determining the form of suicidal behavior, but may not account for the initial self-destructive nature of the behavior itself.
Our data suggest that specific deficits in attention control, memory and working memory may be prevalent across all types of attempters when assessed in a depressed, unmedicated state. Deficits in attention control do not encompass all aspects of attention, but seem to be specific to interference processing, which has an executive component, albeit one that is distinct from other executive capacities.
Although deficits in attention control and working memory have been noted in our previous work, the prominence of memory deficits on both verbal and visual–spatial tasks was less expected. In our previous work, however, the visual memory task used (Rey–Osterrieth Complex Figure) allowed substantial encoding time in the initial learning phase (at least 3.5 min for the complex visual stimulus). On the memory tasks used in this study, exposure to stimuli was relatively brief. Attempters’ poorer memory task performance may therefore reflect disorganization of initial encoding rather than a defect in storage capacity. Prefrontal involvement in attention control (Carter & van Veen, 2007), along with both the acquisition and retrieval of information from memory (Badre & Wagner, 2007; Kuhl et al. 2007), suggests a role for this brain region in suicidal behavior, but through different subregions than those related to behavioral inhibition. The degree of overlap between these fundamental aspects of information processing and deficits on decision-making or set-switching measures is unknown. Elderly suicide attempters who exhibited deficits on reversal learning (Dombrovski et al. 2010) and gambling tasks (Clark et al. 2011) also exhibited deficits on attention and memory subscales of a mental status examination (Dombrovski et al. 2008).
Because our results are not as initially hypothesized, they do not fit neatly into most existing theories regarding neuropsychological dysfunction in suicidal behavior. The consistency of our empirical results over two samples, however, indicate that these functions play some role in the suicidal process. Functional imaging studies indicate a great deal of overlap between activation related to Stroop performance and activation related to emotion regulation, in dorsal and lateral prefrontal cortex, in addition to the dorsal cingulate (Ochsner & Gross, 2008; Van Snellenberg & Wager, 2009). These regulatory systems may play a role in managing the ‘psychic pain’ experienced by suicidal individuals (Olie et al. 2010) or in the flexible control of attention that allows someone to redirect thinking from an acute sense of despondency or hopelessness and to manage suicidal urges. Targeted therapies for suicidality, such as dialectical behavior therapy (Lynch et al. 2007; Linehan & Dexter-Mazza, 2008) or mindfulness therapy (Baer, 2003; Bishop et al. 2004), train individuals to manage their feeling states through distraction, an apparent exercise of the same capacities evident in performance on selective attention and/or working memory tasks. Other types of neurocognitive impairment, especially that related to inhibitory control, may then make suicide attempts more likely (Burton et al. 2011) or perhaps more violent.
This study was limited in that suicide attempters were not necessarily evaluated close in time to a recent attempt, although all were actively depressed with elevated levels of suicidal ideation. Effect sizes for differences were not large, suggesting the need for more refined measures. Patients with BPD in this study outperformed other patient subjects on impulse control tasks, suggesting possible inconsistencies in sampling. However, we had previously found that individuals with BPD do not necessarily perform more poorly than other depressed individuals on impulse control tasks when in a depressed state if not in acute distress at the time of testing (Fertuck et al. 2006). The violent attempter sample was small, and missing those subjects who would be most theoretically useful for our understanding of the role of executive dysfunction in suicidal behavior; namely, highly lethal violent attempters. With respect to causality, this was a cross-sectional, retrospective study with regard to attempts, and the causal relationships between neurocognitive impairment and suicidal behavior remain to be established. Finally, participants with bipolar II disorder were excluded from this analysis because of their variability, and they need to be more systematically sampled in future studies. With bipolar II included, differences in N-back are no longer significant, although other attempter/non-attempter differences are maintained (data available on request).
Overall, disinhibition and poor decision making may be characteristic of certain types of suicide attempt, but lapses in attention control and information encoding, particularly in the context of suicidal thoughts or environmental triggers, may be a more general correlate of suicidal behavior. Executive dysfunction in the context of depression is clearly a risk factor for dangerous, but possibly more impetuous, attempts but may not be present among those who make equally dangerous but more deliberative attempts. Thus, general models of suicidal behavior based on disinhibition, poor decision making and ventral prefrontal circuitry (to the extent that these tasks are valid measures of this circuitry in the absence of imaging) may not be applicable to all types of attempt. On the contrary, certain information processing deficits may be more widespread among attempters. Stroop tasks and modified Stroop tasks using emotional, suicide-related distractors (Becker et al. 1999; Janis & Nock, 2009; Cha et al. 2010) or implicit association measures using suicide-related probes (Nock et al. 2010) have worked well in discriminating attempters from other groups (Jollant et al. 2011). The interaction of clinical risk factors with neurocognitive impairment (Dour et al. 2011), and also the relationships among the various neurocognitive measures that have been associated with suicide risk, warrant further study.
Acknowledgements
This work was supported by grants from the National Institute of Mental Health (MH62155, 5 P50 MH062185, MH59710) and the American Foundation for Suicide Prevention.
Appendix
The Object Alternation task is a computerized version of a paradigm first developed in primate studies, where it was found to be specifically sensitive to lesions of ventral prefrontal cortex (Pribram & Mishkin, 1956; Mishkin et al. 1969). It is an extension of the delayed alternation paradigm, and typically involves presenting two objects, one of which is baited with a reward. The subject must find the reward, and learn that the reward will be switched between objects on successive trials (the subject is given no information that this switching is the basis of the task; learning is by trial, error and insight). The task has been adapted for use in humans (Freedman et al. 1998) and computerized versions have been developed for use in both clinical (Blair et al. 2006; González-Blanch et al. 2008) and imaging studies (Zald et al. 2005). Object Alternation was included in this study to complement the WCT, whose primary measures are most sensitive (although not exclusively so) to dorsolateral prefrontal cortical dysfunction (Stuss et al. 2000).
In the Object Alternation task itself, two symbols – a red triangle and a blue circle – were presented on a computer screen, arranged either with the triangle on the left or the triangle on the right, with these orders presented randomly. Subjects were instructed to select the object that they thought was correct on any given trial, and told there was a pattern to determining which item was correct on any given trial (but given no hint regarding the nature of that pattern). The subjects responded by keypress to designate the location of the object they were selecting. Correct responses were reinforced with a computer beep; incorrect responses received a buzz. The subject’s first response, to either symbol, was correct by default. Thereafter, the opposite figure that the subject responded to correctly was designated as correct on the next trial. To respond correctly on each trial, then, the subject was required to alternate between the objects from trial to trial, regardless of which side the alternate object was presented on. The intertrial interval was 500 ms. The test was stopped if the subject made 12 correct responses in a row (12 alternations without an error). If the subject did not complete the test to criterion, it was discontinued after 180 presentations of the stimuli. Subjects were scored on their ability to reach the criterion of 12 correct in a row, on the number of errors made, on the number of perseverative errors (errors following errors), and on failures to maintain a response set (achieving 5 or more correct responses in a row and making an error before completing the test to criterion). The error score was used in the computation of the Abstract/Contingent Learning domain score, along with the WCT error score, as the best continuous measure of task performance (Freedman et al. 1998).
Footnotes
Declaration of Interest None.
References
- Arie M, Apter A, Orbach I, Yefet Y, Zalzman G. Autobiographical memory, interpersonal problem solving, and suicidal behavior in adolescent inpatients. Comprehensive Psychiatry. 2008;49:22–29. doi: 10.1016/j.comppsych.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Audenaert K, Goethals I, Van Laere K, Lahorte P, Brans B, Versijpt J, Vervaet M, Beelaert L, Van Heeringen K, Dierckx R. SPECT neuropsychological activation procedure with the Verbal Fluency Test in attempted suicide patients. Nuclear Medicine Communications. 2002;23:907–916. doi: 10.1097/00006231-200209000-00015. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Baer RA. Mindfulness training as a clinical intervention: a conceptual and empirical review. Clinical Psychology: Science and Practice. 2003;10:125–143. [Google Scholar]
- Bartfai A, Winborg IM, Nordström P, Asberg M. Suicidal behavior and cognitive flexibility: design and verbal fluency after attempted suicide. Suicide and Life-Threatening Behavior. 1990;20:254–266. [PubMed] [Google Scholar]
- Baune BT, Miller R, McAfoose J, Johnson M, Quirk F, Mitchell D. The role of cognitive impairment in general functioning in major depression. Psychiatry Research. 2010;176:183–189. doi: 10.1016/j.psychres.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Beck AT, Beck R, Kovacs M. Classification of suicidal behaviors: I. Quantifying intent and medical lethality. American Journal of Psychiatry. 1975;132:285–287. doi: 10.1176/ajp.132.3.285. [DOI] [PubMed] [Google Scholar]
- Becker ES, Strohbach D, Rinck M. A specific attentional bias in suicide attempters. Journal of Nervous and Mental Disease. 1999;187:730–735. doi: 10.1097/00005053-199912000-00004. [DOI] [PubMed] [Google Scholar]
- Bishop SR, Lau M, Shapiro S, Carlson L, Anderson ND, Carmody J, Segal ZV, Abbey S, Speca M, Velting D, Devins G. Mindfulness: a proposed operational definition. Clinical Psychology: Science and Practice. 2004;11:230–241. [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Broadbent DE, Cooper PF, FitzGerald P, Parkes KR. The Cognitive Failures Questionnaire (CFQ) and its correlates. British Journal of Clinical Psychology. 1982;21:1–16. doi: 10.1111/j.2044-8260.1982.tb01421.x. [DOI] [PubMed] [Google Scholar]
- Brower MC, Price BH. Neuropsychiatry of frontal lobe dysfunction in violent and criminal behaviour: a critical review. Journal of Neurology, Neurosurgery and Psychiatry. 2001;71:720–726. doi: 10.1136/jnnp.71.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton CZ, Vella L, Weller JA, Twamley EW. Differential effects of executive functioning on suicide attempts. Journal of Neuropsychiatry and Clinical Neurosciences. 2011;23:173–179. doi: 10.1176/appi.neuropsych.23.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cognitive, Affective, and Behavioral Neuroscience. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Cha CB, Najmi S, Park JM, Finn C, Nock MK. Attentional bias toward suicide related stimuli predicts suicidal behavior. Journal of Abnormal Psychology. 2010;119:616–622. doi: 10.1037/a0019710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Dombrovski AY, Siegle GJ, Butters MA, Shollenberger CL, Sahakian BJ, Szanto K. Impairment in risk-sensitive decision-making in older suicide attempters with depression. Psychology and Aging. 2011;26:321–330. doi: 10.1037/a0021646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrovski AY, Butters MA, Reynolds CF, 3rd, Houck PR, Clark L, Mazumdar S, Szanto K. Cognitive performance in suicidal depressed elderly: preliminary report. American Journal of Geriatric Psychiatry. 2008;16:109–115. doi: 10.1097/JGP.0b013e3180f6338d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrovski AY, Clark L, Siegle GJ, Butters MA, Ichikawa N, Sahakian BJ, Szanto K. Reward/Punishment reversal learning in older suicide attempters. American Journal of Psychiatry. 2010;167:699–707. doi: 10.1176/appi.ajp.2009.09030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Mathias CW, Marsh-Richard DM, Prevette KN, Dawes MA, Hatzis ES, Palmes G, Nouvion SO. Impulsivity and clinical symptoms among adolescents with non-suicidal self-injury with or without attempted suicide. Psychiatry Research. 2009;169:22–27. doi: 10.1016/j.psychres.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dour HJ, Cha CB, Nock MK. Evidence for an emotion-cognition interaction in the statistical prediction of suicide attempts. Behavior Research and Therapy. 2011;49:294–298. doi: 10.1016/j.brat.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nature Neuroscience. 2005;8:1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- Fertuck EA, Marsano-Jozefowicz S, Stanley B, Tryon WW, Oquendo M, Mann JJ, Keilp JG. The impact of borderline personality disorder and anxiety on neuropsychological performance in major depression. Journal of Personality Disorders. 2006;20:55–70. doi: 10.1521/pedi.2006.20.1.55. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version. Non-Patient Edition (SCID-I/NP) New York State Psychiatric Institute; New York, NY: 1997. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, Loma B. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) New York State Psychiatric Institute, Biometric Research Department; New York, NY: 1996. [Google Scholar]
- Freedman M, Black S, Ebert P, Binns M. Orbitofrontal function, object alternation and perseveration. Cerebral Cortex. 1998;8:18–27. doi: 10.1093/cercor/8.1.18. [DOI] [PubMed] [Google Scholar]
- Hanlon RE, Rubin LH, Jensen M, Daoust S. Neuropsychological features of indigent murder defendants and death row inmates in relation to homicidal aspects of their crimes. Archives of Clinical Neuropsychology. 2010;25:1–13. doi: 10.1093/arclin/acp099. [DOI] [PubMed] [Google Scholar]
- Harkavy-Friedman JM, Keilp JG, Grunebaum MF, Sher L, Printz D, Burke AK, Mann JJ, Oquendo MA. Are BP I and BP II suicide attempters distinct neuropsychologically? Journal of Affective Disorders. 2006;94:255–259. doi: 10.1016/j.jad.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Janis IB, Nock MK. Are self-injurers impulsive? Results from two behavioral laboratory studies. Psychiatry Research. 2009;169:261–267. doi: 10.1016/j.psychres.2008.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jollant F, Bellivier F, Leboyer M, Astruc B, Torres S, Verdier R, Castelnau D, Malafosse A, Courtet P. Impaired decision making in suicide attempters. American Journal of Psychiatry. 2005;162:304–310. doi: 10.1176/appi.ajp.162.2.304. [DOI] [PubMed] [Google Scholar]
- Jollant F, Guillaume S, Jaussent I, Bellivier F, Leboyer M, Castelnau D, Malafosse A, Courtet P. Psychiatric diagnoses and personality traits associated with disadvantageous decision-making. European Psychiatry. 2007;22:455–461. doi: 10.1016/j.eurpsy.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Jollant F, Lawrence NL, Olié E, Guillaume S, Courtet P. The suicidal mind and brain: a review of neuropsychological and neuroimaging studies. World Journal of Biological Psychiatry. 2011;12:319–339. doi: 10.3109/15622975.2011.556200. [DOI] [PubMed] [Google Scholar]
- Jollant F, Lawrence NS, Olie E, O’Daly O, Malafosse A, Courtet P, Phillips ML. Decreased activation of lateral orbitofrontal cortex during risky choices under uncertainty is associated with disadvantageous decision-making and suicidal behavior. NeuroImage. 2010;51:1275–1281. doi: 10.1016/j.neuroimage.2010.03.027. [DOI] [PubMed] [Google Scholar]
- Keilp JG, Gorlyn M, Oquendo MA, Burke AK, Mann JJ. Attention deficit in depressed suicide attempters. Psychiatry Research. 2008;159:7–17. doi: 10.1016/j.psychres.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilp JG, Sackeim HA, Brodsky BS, Oquendo MA, Malone KM, Mann JJ. Neuropsychological dysfunction in depressed suicide attempters. American Journal of Psychiatry. 2001;158:735–741. doi: 10.1176/appi.ajp.158.5.735. [DOI] [PubMed] [Google Scholar]
- Keilp JG, Sackeim HA, Mann JJ. Correlates of trait impulsiveness in performance measures and neuropsychological tests. Psychiatry Research. 2005;135:191–201. doi: 10.1016/j.psychres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Kuhl BA, Dudukovic NM, Kahn I, Wagner AD. Decreased demands on cognitive control reveal the neural processing benefits of forgetting. Nature Neuroscience. 2007;10:908–914. doi: 10.1038/nn1918. [DOI] [PubMed] [Google Scholar]
- Linehan MM, Dexter-Mazza ET. Dialectical behavior therapy for borderline personality disorder. In: Barlow DH, editor. Clinical Handbook of Psychological Disorders: A Step-by-Step Treatment Manual. 4th edn Guilford Press; New York: 2008. pp. 365–420. [Google Scholar]
- Lynch TR, Trost WT, Salsman N, Linehan MM. Dialectical behavior therapy for borderline personality disorder. Annual Review of Clinical Psychology. 2007;3:181–205. doi: 10.1146/annurev.clinpsy.2.022305.095229. [DOI] [PubMed] [Google Scholar]
- MacLeod CM, Dodd MD, Sheard ED, Wilson DE, Bibi U. In opposition to inhibition. In: Ross BH, editor. The Psychology of Learning and Motivation. vol. 43. Elsevier Science; New York: 2003. pp. 163–214. [Google Scholar]
- Mann JJ, Waternaux C, Haas GL, Malone KM. Toward a clinical model of suicidal behavior in psychiatric patients. American Journal of Psychiatry. 1999;156:181–189. doi: 10.1176/ajp.156.2.181. [DOI] [PubMed] [Google Scholar]
- Marzuk PM, Hartwell N, Leon AC, Portera L. Executive functioning in depressed patients with suicidal ideation. Acta Psychiatrica Scandinavica. 2005;112:294–301. doi: 10.1111/j.1600-0447.2005.00585.x. [DOI] [PubMed] [Google Scholar]
- Morgan AB, Lilienfield SO. A meta-analytic review of the relation between antisocial behavior and neuropsychological measures of executive function. Clinical Psychology Review. 2000;20:113–136. doi: 10.1016/s0272-7358(98)00096-8. [DOI] [PubMed] [Google Scholar]
- Nock MK, Park JM, Finn CT, Deliberto TL, Dour HJ, Banaji MR. Measuring the suicidal mind: implicit cognition predicts suicidal behavior. Psychological Science. 2010;21:511–517. doi: 10.1177/0956797610364762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive emotion regulation: insights from social cognitive and affective neuroscience. Current Directions in Psychological Science. 2008;17:153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olie E, Guillaume S, Jaussent I, Courtet P, Jollant F. Higher psychological pain during major depressive episode may be a factor of vulnerability to suicidal ideation and act. Journal of Affective Disorders. 2010;120:226–230. doi: 10.1016/j.jad.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Halberstam B, Mann JJ. Risk factors of suicidal behavior: the utility and limitations of research instruments. In: First M, editor. Standardized Evaluation in Clinical Practice: Review of Psychiatry. vol. 22. American Psychiatric Publishing; Washington, DC: 2003. pp. 103–130. [Google Scholar]
- Sinclair JM, Crane C, Hawton K, Williams JM. The role of autobiographical memory specificity in deliberate self-harm: correlates and consequences. Journal of Affective Disorders. 2007;102:11–18. doi: 10.1016/j.jad.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. Instruction Manual for the Structured Clinical Interview for the DSM-IV (SCID-P) American Psychiatric Press; Washington, DC: 1990. [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd edn Oxford University Press; New York, NY: 2006. [Google Scholar]
- Stuss DT, Levine B, Alexander MP, Hong J, Palumbo C, Hamer L, Murphy KJ, Izukawa D. Wisconsin Card Sorting Test performance in patients with focal frontal and posterior brain damage: effects of lesion location and test structure on separable cognitive processes. Neuropsychologia. 2000;38:388–402. doi: 10.1016/s0028-3932(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Swann AC, Dougherty DM, Pazzaglia PJ, Pham M, Steinberg JL, Moeller FG. Increased impulsivity associated with severity of suicide attempt history in patients with bipolar disorder. American Journal of Psychiatry. 2005;162:1680–1687. doi: 10.1176/appi.ajp.162.9.1680. [DOI] [PubMed] [Google Scholar]
- Van Snellenberg JX, Wager TD. Cognitive and motivational functions of the human prefrontal cortex. In: Christensen AL, Bougakov D, Goldberg E, editors. Luria’s Legacy in the 21st Century. Oxford University Press; New York, NY: 2009. pp. 30–61. [Google Scholar]
- Veiel HO. A preliminary profile of neuropsychological deficits associated with major depression. Journal of Clinical and Experimental Neuropsychology. 1997;19:587–603. doi: 10.1080/01688639708403745. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. Third Edition The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Westheide J, Quednow BB, Kuhn KU, Hoppe C, Cooper-Mahkorn D, Hawellek B, Eichler P, Maier W, Wagner M. Executive performance of depressed suicide attempters: the role of suicidal ideation. European Archives of Psychiatry and Clinical Neuroscience. 2008;258:414–421. doi: 10.1007/s00406-008-0811-1. [DOI] [PubMed] [Google Scholar]
- Williams JM, Broadbent K. Distraction by emotional stimuli: use of a Stroop task with suicide attempters. British Journal of Clinical Psychology. 1986;25:101–110. doi: 10.1111/j.2044-8260.1986.tb00678.x. [DOI] [PubMed] [Google Scholar]
- Wu CS, Liao SC, Lin KM, Tseng MM, Wu EC, Liu SK. Multidimensional assessments of impulsivity in subjects with history of suicidal attempts. Comprehensive Psychiatry. 2009;50:315–321. doi: 10.1016/j.comppsych.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Zakzanis KK, Leach L, Kaplan E. On the nature and pattern of neurocognitive function in major depressive disorder. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1998;11:111–119. [PubMed] [Google Scholar]
- Zald DH, Andreotti C. Neuropsychological assessment of the orbital and ventromedial prefrontal cortex. Neuropsychologia. 2010;48:3377–3391. doi: 10.1016/j.neuropsychologia.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Zald DH, Curtis C, Chernitsky LA, Pardo JV. Frontal lobe activation during object alternation acquisition. Neuropsychology. 2005;19:97–105. doi: 10.1037/0894-4105.19.1.97. [DOI] [PubMed] [Google Scholar]
References
- Blair KS, Newman C, Mitchell DG, Richell RA, Leonard A, Morton J, Blair RJ. Differentiating among prefrontal substrates in psychopathy: neuropsychological test findings. Neuropsychology. 2006;20:153–165. doi: 10.1037/0894-4105.20.2.153. [DOI] [PubMed] [Google Scholar]
- Freedman M, Black S, Ebert P, Binns M. Orbitofrontal function, object alternation and perseveration. Cerebral Cortex. 1998;8:18–27. doi: 10.1093/cercor/8.1.18. [DOI] [PubMed] [Google Scholar]
- González-Blanch C, Vázquez-Barquero JL, Carral-Fernández L, Rodríguez-Sánchez JM, Alvarez-Jiménez M, Crespo-Facorro B. Preserved orbitofrontal function in first-episode schizophrenia : further evidence from the object alternation paradigm. Journal of Nervous and Mental Disease. 2008;196:67–70. doi: 10.1097/NMD.0b013e318160ea17. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Vest B, Waxler M, Rosvold HE. A re-examination of the effects of frontal lesions on object alternation. Neuropsychologia. 1969;7:357–363. [Google Scholar]
- Pribram KH, Mishkin M. Analysis of the effects of frontal lesions in monkey: III. Object Alternation. Journal of Comparative and Physiological Psychology. 1956;49:41–45. doi: 10.1037/h0046248. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Levine B, Alexander MP, Hong J, Palumbo C, Hamer L, Murphy KJ, Izukawa D. Wisconsin Card Sorting Test performance in patients with focal frontal and posterior brain damage: effects of lesion location and test structure on separable cognitive processes. Neuropsychologia. 2000;38:388–402. doi: 10.1016/s0028-3932(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Zald DH, Curtis C, Chernitsky LA, Pardo JV. Frontal lobe activation during object alternation acquisition. Neuropsychology. 2005;19:97–105. doi: 10.1037/0894-4105.19.1.97. [DOI] [PubMed] [Google Scholar]