Abstract
Non-Hodgkin lymphoma symbolizes a heterogeneous group of diseases resulting from malignant transformation of lymphocytes with differing patterns of behavior and responses to treatment. The potential curability of non-Hodgkin lymphoma differs among the various histologic subtypes and is associated in part with the stage at presentation. CD19 antigen is a type I transmembrane glycoprotein belonging to the immunoglobulin Ig superfamily. CD19 is specifically expressed in normal and neoplastic B-cells. Recent study showed that in a mouse model, CD19 and c-Myc synergize functionally to accelerate B-cell lymphomagenesis, which is associated with increased disease severity. Specificity is the most important challenge in cancer therapeutics. Antibody–drug conjugates have the prospect of enhancing the therapeutic efficacy over unconjugated monoclonal antibodies through the selective delivery of cytotoxic agents to cancer cells. The ubiquitous expression of CD19 in these tumors, especially at an earlier stage and the property of efficient internalization, makes CD19 an attractive and affective target for antibody–drug conjugate therapy as compared to CD20. SAR3419 (huB4-DM4) is a novel antibody–drug conjugate that is composed of a humanized monoclonal IgG1 anti-CD19 antibody (huB4) attached to the potent cytotoxic drug, a maytansine derivative (DM4), through a cleavable disulfide cross-linking agent N-Succinimidyl-4-2-pyridyldithio butanoic acid (SPDB). The preclinical efficacy of maytansine derivative–anti-CD19 conjugate was demonstrated in our laboratory, and SAR3419 was found to be more effective than CHOP in a xenograft model. Phase I trials have also been conducted on the basis of preclinical studies that demonstrated promising antitumor activity with acceptable safety results in human B-cell lymphoma models. Additional trials are ongoing and will provide additional insight into the full potential of this novel drug.
Keywords: lymphoma, SAR3419, antibody-drug conjugates (ADC), maytansinoids, microtubule inhibitors
Introduction
Non-Hodgkin lymphoma (NHL) represents a diverse group of diseases resulting from malignant transformation of lymphocytes that vary in clinical behavior, morphologic appearance, immunologic and molecular phenotype. The various types represent neoplastic lymphoid cells arrested at different stages of normal differentiation. NHL is the seventh most commonly diagnosed cancer in men and women in the United States. In 2013, an estimated 69,740 new cases and 19,020 deaths (10,590 in males and 8,430 in females) were due to NHL. The disease accounts for approximately 4% of all cancer diagnoses in both incidence and deaths per year.1
Since the 1970s, the incidence of NHL has doubled. Eighty-five percent of NHLs are B-cell that can be broadly classified as indolent (40%), aggressive (50%) and highly aggressive (10%) NHL based on their clinical behavior. Diffuse large B-cell (DLBCL) NHL is the most common subtype (30%) of all lymphomas and is the prototype of aggressive but curable NHL. Follicular lymphoma (FL) is the second most common subtype, representing 22% and is the most common indolent NHL2,3 with a tendency for relapse after standard treatments. Burkitt lymphoma and precursor B- or T-cell lymphoblastic leukemia/lymphoma fall into the highly aggressive NHL category.
The World Health Organization classification of lymphoid neoplasms is now the most commonly used system worldwide. It has facilitated uniformity in classification and comparison of clinical trial results. According to the organization’s revised classification system, B-cell malignancies are divided into two broad categories: precursor and mature B-cell neoplasms. The precursor B-cell neoplasms include B lymphoblastic leukemia/lymphoma with or without recurrent genetic abnormalities. The mature B-cell neoplasms are broadly divided into pregerminal center neoplasm (mantle lymphoma), germinal center neoplasm (follicular lymphoma, Burkitt lymphoma, some DLBCL) and postgerminal center neoplasm (marginal zone and MALT lymphoma, lymphoplasmacytic lymphoma, chronic lymphocytic leukemia[CLL]/small lymphocytic lymphoma, some DLBCL, plasma cell myeloma). They also refined the definitions of well-recognized diseases including CLL,4 Waldenstrom macroglobulinemia,5 and plasma cell neoplasms.6
Current treatment options and patient outcomes
The treatment of NHL is determined by histologic type and stage of disease. The indolent lymphomas are considered incurable (except when they present with truly localized disease), whereas the aggressive and very aggressive types are curable. More than 80% of the indolent lymphomas have advanced stage (III or IV) at the time of diagnosis. These are considered incurable, but because of the indolent clinical course, they have a reasonably good overall prognosis with a median survival extended to 10–15 years. Treatment may therefore be deferred in asymptomatic patients but will require surveillance (watchful waiting). The minority of patients with indolent lymphoma (~20%) who have limited stage (I or II) at diagnosis are usually given involved field radiation therapy as definitive treatment and have a chance of cure (40%–50% in most series).7 The aggressive and very aggressive subtypes are characterized by rapid growth, as the name implies, and have shorter survival, measured in months unless successfully treated. Fortunately, combination chemotherapy regimens are curative. Although chemotherapy remains the cornerstone of NHL treatment,8 modern treatment regimens include a combination of cytotoxic chemotherapy agent with anti-CD20 antibody, the so called chemoimmunotherapy, which has universally produced superior results compared with chemotherapy alone. The intensity of chemotherapy varies depending on the histologic types. R-CHOP, R-FC, R-CVP, R-ICE and R-DHAP9–12 are a few of the immunochemotherapeutic combinations that are considered standard of care for lymphoma. R-CHOP is recommended as first line treatment for FL and diffuse large B-cell lymphoma (DLBCL), R-FC is used for CLL and R-CVP for FL.
For recurrent NHL, a variety of treatment options exist depending on the patient’s age, performance status, and if there are any comorbid conditions. Salvage regimens like R-ICE and R-DHAP for relapsed DLBCL followed by autologous stem cell transplantation for chemosensitive disease is a common strategy for patients up to age 70. Recently, two randomized studies13,14 have evaluated the role of highdose therapy and autologous stem cell transplantation as part of initial therapy in patients with aggressive NHL. The progression-free survival was found to be significant in these trials, but there was no difference in overall survival.
The potential curability of NHL differs among the various histologic subtypes and is associated in part to the stage at presentation. The 5-year relative survival rate of patients with NHL increased from 47% between 1975 and 1977 to about 70% between 2001 and 2007. These improvements in survival occurred primarily in patients with intermediate- to high-grade histologic appearance.2 The International Prognostic Index is a clinical tool developed to aid in predicting the prognosis of patients with aggressive NHL. There are two other modified prognostic indexes: Follicular Lymphoma International Prognostic Index (FLIPI) and Mantle Cell Lymphoma International Prognostic Index (MIPI), identified and adapted for follicular and mantle lymphoma respectively.15–17
Monoclonal antibody therapy in cancer management
Specificity is the most important challenge in cancer therapeutics. Monoclonal antibody-based treatment of cancer has been attractive since it has a favorable therapeutic index compared with cytotoxic agents. Monoclonal antibodies can effectively recognize and kill cancer cells through different mechanisms such as direct stimulation or blockage of cell surface receptors, inhibition of tumor-related signal transduction leading to apoptosis, or indirectly through induction of host immune response. Antibodies can also exhibit antitumor effects by impeding angiogenesis in tumor or supporting stroma. Antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity appear to play a major role in immune-mediating antitumor effects of monoclonal antibodies. ADCC is a cell-mediated, innate immunity mechanism whereby an effector cell of the immune system, such as natural killer cells or macrophages, bind to the Fc portion of the monoclonal antibody through Fcγ receptors. This binding is followed by the release of effector molecules such as perforin by the effector cells leading to cell lysis and death. Complement-dependent cytotoxicity involves the fixation of complement via the Fc portion of the antibody and activation of the complement cascade resulting in membrane and cell destruction.18,19
Cancer treatment has been further revolutionized by monoclonal antibodies conjugated with the chemotherapeutic agents. These antibody–drug conjugates (ADCs) are being developed in the quest for potentially more clinically efficacious drugs and to confer higher tumor selectivity. ADCs provide a potentially new way to treat cancer by combining monoclonal antibodies that have antigen-specific selectivity and antitumor activity of potent cytotoxic molecules.20,21 It can be considered a form of “targeted chemotherapy” that should have higher efficacy than unconjugated antibody and can limit systemic side effects of cytotoxic agents.22,23 The US Food and Drug Administration has approved several antibodies for the treatment of a variety of hematologic malignancies. These are outlined in Table 1. They are available as unconjugated antibodies like rituximab, alemtuzumab, and ofatumumab as well as conjugated antibodies for delivery of radioisotope (yttrium Y90-ibritumomab tiuxetan, iodine I131-tositumomab), or cytotoxic agents (brentuximab vedotin, gemtuzumab ozogamicin) to the cancer cells. Similarly, bevacizumab, catumaxomab, ipilimumab, denosumab, trastuzumab, cetuximab, and panitumumab are among numerous other monoclonal antibodies, which have now received regulatory authorization for a variety of solid tumors.
Table 1.
FDA-approved monoclonal antibodies of lymphoid malignancies
| Antibody | Brand name | Type | Approval year | Target | Malignancies |
|---|---|---|---|---|---|
| Rituximab | Rituxan, mabthera | Chimeric | 1997 | CD20 | B-NHL |
| Alemtuzumab | Campath | Humanized | 2001 | CD52 | CLL |
| Ofatumumab | Arzerra | Human | 2009 | CD20 | CLL |
| I131-tositumomab | Bexxar | Murine | 2003 | CD20 | B-NHL |
| Y90-ibritumomab tiuxetan | Zevalin | Murine | 2002 | CD20 | B-NHL |
| Brentuximab vedotin | Adcetris | Chimeric | 2011 | CD30 | ALCL, HL |
Abbreviations: ALCL, anaplastic large cell lymphoma; B-NHL, B-cell non-Hodgkin’s lymphoma; CLL, chronic lymphocytic leukemia; HL, Hodgkin lymphoma.
Rituximab is a chimeric monoclonal antibody (incorporating human immunoglobulin G1 heavy-chain sequences and murine immunoglobulin variable regions) that recognizes the human CD20 antigen. CD20 is found on both normal B-cells and on most low-grade and some higher grade B-cell lymphomas.24 November 1997 was a milestone for rituximab when it became the first monoclonal antibody approved specifically for cancer therapy.25 It has been investigated as a single agent in induction and maintenance therapy; however, it is primarily used in combination with standard chemotherapies in the treatment of patients with B-cell NHL (R-CHOP) and CLL.26–29
Alemtuzumab is a humanized monoclonal antibody used primarily for CLL.30 It targets the CD52 antigen found on B lymphocytes and is effective for induction and maintenance therapy. Alemtuzumab is typically not combined with chemotherapy due to increased risk of infection30 as compared with anti-CD20.
Radioimmunotherapy 31–33 is a kind of targeted radio-nuclide therapy for relapsed/refractory indolent B-cell lymphomas that utilizes a monoclonal antibody to deliver localized radiation. It is the most suitable alternative treatment modality for multiple site relapse that cannot be readily irradiated. BEXXAR® (GlaxoSmithKline, Research Triangle Park, NC, USA), (Iodine I 131-tositumomab) is composed of the monoclonal antibody tositumomab radiolabeled with 131I. It is a murine IgG2a lambda monoclonal antibody directed against the CD20 antigen.32,34 On the other hand, ZEVALIN (Spectrum Pharmaceuticals, Inc., Irvine, CA, USA) (ibritumomab tiuxetan) is composed of a monoclonal antibody that is linked with a radioactive substance Yttrium.90 The antibody moiety of Zevalin is ibritumomab, a murine IgG1 kappa monoclonal antibody directed against the CD20 antigen.31,32
Brentuximab vedotin ADCETRIS (Seattle Genetics, Inc., Bothell, WA, USA) comprises the chimeric CD30 targeted monoclonal antibody, brentuximab, linked to three to five units of the antimitotic agent monomethyl auristatin E. Brentuximab vedotin is approved for treatment of anaplastic large cell lymphoma and Hodgkin lymphoma.35
Role of CD19 in B-cell lymphoma
CD19 antigen is a type I transmembrane glycoprotein belonging to the immunoglobulin Ig superfamily. CD19 is specifically expressed in normal B-cells and neoplastic B-cells.36–38
It is considered a pan B-cell marker expressed throughout B-cell development but with threefold higher expression in mature cells as compared to immature B-cells. CD19 expression however, is lost in the terminally differentiated plasma cells.
During lymphopoiesis, CD19 directs B-cell fate and differentiation by modulating B-cell receptor signaling. It is critically involved in establishing the optimal immune response through its roles in the antigen-independent development as well as the immunoglobulin-induced activation of B-cells. CD19 deficiency in humans and mice leads to an overall impaired humoral response with increased susceptibility to infection.36,39,40
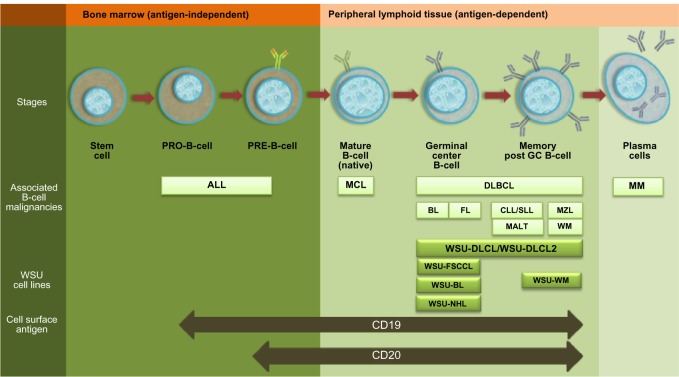
The pattern of CD19 expression is maintained among B-cell malignancies where it is expressed in indolent and aggressive subtypes of NHL, B-cell CLL, and non-T acute lymphoblastic leukemia.41–43 CD19 is expressed in the B-cell lineage at an earlier stage compared with CD20. This fact therefore, may provide an advantage to CD19 targeted drugs over rituximab, especially for early B-cell neoplasms like acute lymphoblastic leukemia (Figure 1).44 Moreover, CD19 is shown to be internalized efficiently in lymphoma tumor models with the use of different monoclonal antibodies (huB4, hBU12),45,46 making it an attractive and effective target for ADC therapy as compared to CD20.
Figure 1.
Expression of CD19 and CD20 in B-cell lineage.
Notes: Illustrative representation of B-cell differentiation, maturation, antigen expression and B-cell neoplasm associated with different stages of B-cell development. Cell lines used in the research study.47–51
Abbreviations: GC, germinal center; ALL, acute lymphoblastic leukemia; MCL, Mantle cell lymphoma; FL, follicular lymphoma; BL, Burkitt lymphoma; DLBCL, Diffuse Large B-Cell Lymphoma; MZL, Marginal Zone Lymphoma; CLL/SLL, Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma; MALT, Mucosa-Associated lymphoid tissue; WM, Waldenstrom macroglobulinemia; MM, plasma cell myeloma; WSU-BL, Wayne State University-Burkitt lymphoma cell line; WSU-FSCCL, Wayne State University-follicular small cleaved cell lymphoma Cell line; WSU-NHL, Wayne State University-FL grade 3 Cell line; WSU-DLCL and WSU-DLCL2, Wayne State University-Diffuse large B-Cell lymphoma cell line; WSU-WM, Wayne State University-Waldenstrom macroglobulinemia Cell line.
Lymphomagenesis is a multistep process which involves accumulation of multiple genetic and epigenetic aberrations over a period of time. Despite the complexity of this process, it has been observed that inactivation of a single oncogene can significantly impede the progression of cancer cells, supporting the notion of “oncogene addiction”.52 c-MYC is one of the oncoproteins that is mutated in both Burkitt lymphoma and some DLBCLs.53,54 PAX5 (paired box transcription factor 5) is a B-cell-specific transcription factor involved in B-cell lymphogenesis.55,56 PAX5 is required for the normal expression of CD19. Using in both in vivo and in vitro models, it was shown that MYC protein levels are regulated by both PAX5 and CD19 through a posttranscriptional mechanism. CD19 signaling activates the PI3K pathway in the context of B-cell receptor signaling, crucial for expansion of the B-cell population. This posttranslational mechanism of c-MYC regulation was found to be independent of B-cell receptor activity and acting through the PI3K pathway. The higher expression of c-MYC causes uncontrolled cell growth and tumor development.57 This c-Myc-CD19 regulatory loop consequently enhances the B-cell transformation and lymphomagenesis. CD19 deficient-c-Myc transgenic mice have a relatively reduced chance of malignancies and more than 80% increase in median life span and survival.39
SAR3419: targeting CD19
Exploitation of tumor immunology for therapy has been a major undertaking over the past few decades. It has resulted in identification of potential receptor targets, generation of optimized antibodies, and exploration of the role of sophisticated interactions between the immune system and the targeted cancer cells.58,59 ADCs have the prospect of enhancing therapeutic efficacy over that of unconjugated monoclonal antibodies through the selective delivery of cytotoxic agents to cancer cells.22,23 In order to obtain optimum efficacy, an ADC target must be internalized to permit transfer of the cytotoxic drug inside cancer cells.22,60
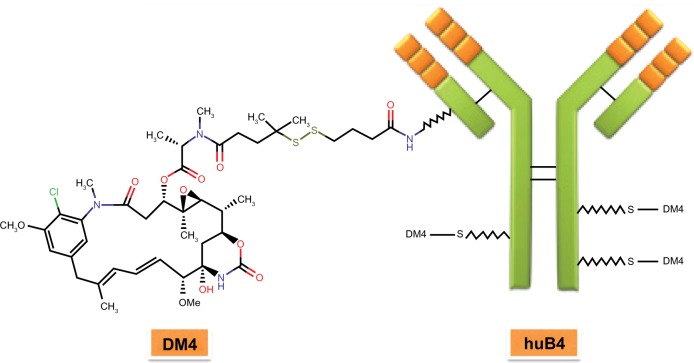
CD19 is considered an attractive target for B-cell malignancies due to its ubiquitous expression in these tumors.12,37 SAR3419 (huB4-DM4) is a novel ADC that is composed of a humanized monoclonal IgG1 anti-CD19 antibody (huB4) attached to the potent cytotoxic drug, a maytansine derivative (N2′-deacetyl-N2′-[4-mercapto-4-methyl-1-oxopentyl] [DM4]), through a cleavable disulfide cross-linking agent, N-Succinimidyl-4-2-pyridyldithio butanoic acid (SPDB) (Figure 2). DM4 is a thiol-containing derivative of maytansine, an agent originally isolated from the bark of the African shrub Maytenus ovatus.61 It is an antimitotic agent that binds to tubulin and inhibits the microtubule assembly in amanner similar to but 100–1000-fold more potent than Vinca alkaloids,62 leaving a majority of the cells arrested in G2/M phase of the cell cycle, which leads to apoptotic cell death.63 Although maytansine is a highly cytotoxic natural compound and is found to be very effective in various hematologic and oncologic malignancies,62,64–68 it has failed as an anticancer agent in human clinical trials because of unacceptable systemic toxicity. Conjugating this compound with antibody that enables selective delivery to CD19-positive cells is one way of limiting its systemic toxicity. SAR3419 and its huB4 antibody component do not show any complement-dependent toxicity. The unconjugated huB4 antibody induces ADCC,63 an activity that was preserved following conjugation to the maytansinoid, DM4; however, huB4 alone has not been shown to demonstrate any significant antitumor activity in cell lines in vitro.
Figure 2.
Structure of SAR3419.
Abbreviations: DM4, N2′-deacetyl-N2′-(4-mercapto-4-methyl-1-oxopentyl); huB4, a humanized IgG1 anti-CD19 monoclonal antibody.
Two different linkers have been used to conjugate the maytansinoids, DM4 and DM1, to antibodies: SPDP (SPDB-DM4; Figure 2) and SMCC (SMCC-DM1).69 SPDP is a disulfide linker which is cleavable, and SMCC is the thioether linker. Lysosomal processing is vital for the activity of antibody-maytansinoid conjugates, irrespective of the linker. Inside the lysosomes, SPDB-DM4 is degraded and releases intact maytansinoid drug and linker attached to lysine. The lysine-SPDB-DM4 is then reduced and S-methylated to yield S-methyl-DM4, a potent lipophilic and stable cytotoxic metabolite. Kovtun et al have shown that SPDB-DM4, ADC with the disulfide linker, has a “bystander” effect,70 where antigen-positive and antigen-negative tumor cells are effectively killed. The presence of antigen-positive cells is required for such effect.
The preclinical efficacy of DM4-anti-CD19 conjugate was demonstrated in our laboratory (Table 2);71 it was evaluated in subcutaneous and systemic models of B-cell NHL including diffuse large B-cell and follicular lymphoma, respectively. The huB4-DM4 conjugate (SAR3419) was found to be more effective than the CHOP (cyclophosphamide-Adriamycin-vincristine-prednisone) regimen or rituximab in these chemotherapy-resistant models. The entire group of animals that received SAR3419 survived to the end of the experiment (150–155 days) in both models. Higher doses of SAR3419 (15 and 30 mg/kg) were more effective than a lower dose of 7.5 mg/kg. The immunoconjugation was critical for efficacy since neither huB4 nor DM4 alone had significant antitumor activity against these models to induce tumor shrinkage.
Table 2.
Preclinical and clinical development of SAR3419
| Preclinical studies | Year | Models | ||||
|---|---|---|---|---|---|---|
| Superior antitumor activity of SAR3419 to rituximab in xenograf models for non-Hodgkin’s lymphoma.71 | 2009 | WSU-DLCL/WSU-FSCCL | ||||
| Preclinical evaluation of SAR3419 (huB4-DM4), an anti-CD19-maytansinoid immunoconjugate, for the treatment of B-cell lymphoma.45 | 2006 | Burkitt lymphomas/diffuse large cell lymphoma (ramos lymphoma xenograft model) | ||||
| The anti-CD19 antibody-drug conjugate SAR3419 prevents hematolymphoid relapse postinduction therapy in preclinical models of pediatric acute lymphoblastic leukemia.75 | 2013 | BCP-ALL, T-ALL, and MLL-ALL xenograft model | ||||
| Trial | Phase | Status | Year | Condition | Protocol ID | |
|
| ||||||
| SAR3419 as single agent in relapsed-refractory diffuse large B-celI lymphoma patients | Phase II | Active | 2012 | DLBCL | ARD10248, 2011-003657-26, U1111-1115-3349, NCT01472887 | |
| SAR3419 in acute lymphoblastic leukemia (ALL) | Phase II | Active | 2011 | ALL | EFC11603 U1111-1118-0642, NCT01440179 | |
| Combination of SAR3419 and rituximab in relapsed/refractory diffuse large B-cell lymphoma | Phase II | Active | 2011 | DLBCL | TCD123332011-002865-39, U1111-1120-0315, NCT01470456 | |
| SAR3419 administered weekly in patients with relapsed/refractory CD19-positive B-cell non-Hodgkin’s lymphoma.74 | Phase I | Completed | 2008–2012 | B-NHL | TED6829 EudraCT2007-004868-41, NCT00796731 | |
| Multi-dose-escalation safety and pharmacokinetic study of SAR3419 as single agent in relapsed/refractory B-cell non-Hodgkin’s lymphoma.73 | Phase I | Completed | 2007–2012 | B-NHL | TED6828, NCT00549185 | |
Abbreviations: ALL, acute lymphoblastic leukemia; WSU-DLCL, Wayne State University-diffuse large B-cell lymphoma cell line; WSU-FSCCL, Wayne State University-follicular small cleaved cell lymphoma cell line; B-NHL, B-cell non-Hodgkin’s lymphoma; DLBCL, diffuse large B-cell lymphoma; BCP-ALL, B-cell precursor acute lymphoblastic leukemia; MLL-ALL, mixed lineage acute lymphoblastic leukemia; T-ALL, T-cell acute lymphoblastic leukemia; CD, cluster of differentiation.
Efficacy of SAR3419 was also demonstrated by exposing it to different CD19-positive lymphoma cell lines in vitro, with EC50 values in the nanomolar to subnanomolar range. The majority of cells were arrested at the G2/M phase of the cell cycle within 24 hours of exposure, followed by an increase in apoptotic cells from 24 to 48 hours.63 SAR3419 was also shown to display potent antitumor activity in vivo against Burkitt lymphoma using the Ramos xenograft model.45,63
In our study, SAR3419 was effective when given 7 days after a large cell dose (107) of inoculum. Similar results were demonstrated using CMC-544-DM1 (anti-CD22 ADC) in the Ramos lymphoma xenograft model.72 In that study, the treatment was effective when given up to 15 days after cell dose injection, though the number of cells used (106) was lower than ours.
Clinical development of SAR3419
Phase I/II (Table 2) trials have been conducted on the basis of the aforementioned preclinical studies71,45 and have demonstrated promising antitumor activity, with acceptable safety results in human B-cell lymphoma models. Two Phase I clinical trials conducted with SAR3419 in patients with refractory/relapsed B-cell NHL expressing CD19 have been completed.
In one trial, patients with relapsed CD19 B-cell lymphoma were treated with escalating doses of SAR3419 given by intravenous infusion once every 3 weeks for six cycles with the maximum tolerated dose of 160 mg/m2. A total of 35 patients were evaluated for tumor response. Twenty-six patients (74%) demonstrated a reduction from baseline in their tumor sizes, of which six patients achieved partial or complete remissions. Tumor shrinkage was observed in approximately half of 15 patients (seven of 15 [47%]) with rituximab-refractory disease.
The clinical pharmacokinetic profile of SAR3419 showed linear kinetics with an estimated termination halflife of 7 days. The free DM4 were quantifiable at doses of 40 mg/m2 as compared to S-Methyl-DM4 (the product of cellular metabolism of SAR3419) whose levels were measureable at doses of 80 mg/m2. The exposure of both agents increased with the dose; however, S-Methyl-DM4 exposure was found to be higher than DM4 exposure with respect to the same dose.
The dose-limiting toxicities were reversible: severe blurred vision associated with microcystic epithelial corneal changes and neuropathy. The only grade 3 or 4 adverse events observed and considered related to the study drug were ocular toxicity in six patients (15%), neutropenia in four patients (10%), peripheral sensory neuropathy in three patients (8%), and thrombocytopenia in one patient (3%). The study showed that the SAR3419 can be safely administered to patients with relapsed B-cell lymphoma and demonstrated promising clinical activity, including patients who were refractory to rituximab.73
The other phase I/II trial was conducted with a different schedule of administration. SAR3419 was administered by intravenous infusion, at a weekly dose for eight to 12 doses, to patients with relapsed/refractory B-cell NHL expressing CD19. The maximum tolerated dose was defined at 55 mg/m2. An objective response was observed in eight of 22 patients (36%) including three who underwent complete remission [complete response (CR)/complete response, unconfirmed(CRu)]. Results of this trial also showed evidence of clinical activity and manageable toxicity profiles without any clinically significant myelosuppression.74
Other Phase II trials are currently underway which will further our understanding of SAR3419 and provide additional insight into the full potential of this novel drug. In these ongoing trials, SAR3419 is being evaluated as a single agent in patients with relapsed/refractory DLBCL and acute lymphoblastic leukemia as well as in combination with rituximab in patients with relapsed/refractory DLBCL.
Conclusion
CD19 is an attractive target for B-cell malignancies due to its broad expression in the B-cell lineage. SAR3419 is a novel ADC that has shown its efficacy, not only in preclinical models for NHL, but also holds promise as a novel and well-tolerated therapy in clinical trials. The clinical efficacy of SAR3419 in NHL validates our preclinical xenografts to be clinically relevant and an applicable experimental therapeutic model. Future direction in SAR3419 development for lymphoma therapy is to rationally combine it with other antilymphoma agents to maximize its therapeutic efficacy.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al.SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) [webpage on the Internet]Bethesda, MD: National Cancer Institute; 2011[updated Apr 2012]. Available from: http://seer.cancer.gov/csr/1975_2009_pops09/Assessed July 10, 2013 [Google Scholar]
- 3.Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IARC; 2008. [Google Scholar]
- 4.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owen RG, Treon SP, Al-Katib A, et al. Clinicopathological definition of Waldenstrom’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom’sMacroglobulinemia. Semin Oncol. 2003;30(2):110–115. doi: 10.1053/sonc.2003.50082. [DOI] [PubMed] [Google Scholar]
- 6.The International Myeloma Working Group Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121(5):749–757. [PubMed] [Google Scholar]
- 7.Ganem G, Cartron G, Girinsky T, Haas RL, Cosset JM, Solal-Celigny P. Localized low-dose radiotherapy for follicular lymphoma: history, clinical results, mechanisms of action, and future outlooks. Int J Radiat Oncol Biol Phys. 2010;78(4):975–982. doi: 10.1016/j.ijrobp.2010.06.056. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network [homepage on the Internet] NCCN Clinical Practice Guidelines in Oncology: NCCN Non-Hodgkin’s lymphoma Guidelines Version 1.2013 Available from: http://www.nccn.org/professionals/physician%20gls/f%20guidelines.aspAccessed July 10, 2013
- 9.Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106(12):3725–3732. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 10.Persky DO, Unger JM, Spier CM, et al. Phase II study of rituximab plus three cycles of CHOP and involved-field radiotherapy for patients with limited-stage aggressive B-cell lymphoma: Southwest Oncology Group study 0014. J Clin Oncol. 2008;26(14):2258–2263. doi: 10.1200/JCO.2007.13.6929. [DOI] [PubMed] [Google Scholar]
- 11.Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24(19):3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 12.Hagberg H, Gisselbrecht C, CORAL study group Randomized phase III study of R-ICE versus R-DHAP in relapsed patients with CD20 diffuse large B-cell lymphoma (DLBCL) followed by high-dose therapy and a second randomization to maintenance treatment with rituximab or not: an update of the CORAL study. Ann Oncol. 2006;17(Suppl 4):iv31–iv32. doi: 10.1093/annonc/mdj996. [DOI] [PubMed] [Google Scholar]
- 13.Stiff PJ, Unger JM, Cook J, et al. Randomized phase III US/Canadian intergroup trial (SWOG S9704) comparing CHOP ± R for eight cycles to CHOP ± R for six cycles followed by autotransplant for patients with high-intermediate (H-int) or high IPI grade diffuse aggressive non-Hodgkin lymphoma. J Clin Oncol. 2011;29(Suppl):a8001. abstract. [Google Scholar]
- 14.Vitolo U, Chiappella A, Brusamolino E, et al. A randomized multicentre phase III study for first line treatment of young patients with high-risk (aaIPI 2-3) diffuse large B-cell lymphoma (DLBCL): Rituximab ®plus dose-dense chemotherapy CHOP14/megaCHOP14 with or without intensified high-dose chemotherapy (HDC) and autologous stem cell transplantation (ASCT). Results of DLCL04 trial of Italian Lymphoma Foundation (FIL) Annals of Oncology. 2011;22(Suppl 4):a072–iv106. [Google Scholar]
- 15.A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329(14):987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 16.Solal-Céligny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood. 2004;104(5):1258–1265. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 17.Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111(2):558–565. doi: 10.1182/blood-2007-06-095331. [DOI] [PubMed] [Google Scholar]
- 18.Carter P. Improving the efficacy of antibody-based cancer therapies. Nat Rev Cancer. 2001;1(2):118–129. doi: 10.1038/35101072. [DOI] [PubMed] [Google Scholar]
- 19.Glennie MJ, van de Winkel JG. Renaissance of cancer therapeutic antibodies. Drug Discov Today. 2003;8(11):503–510. doi: 10.1016/s1359-6446(03)02714-4. [DOI] [PubMed] [Google Scholar]
- 20.Trail PA, Bianchi AB. Monoclonal antibody drug conjugates in the treatment of cancer. Curr Opin Immunol. 1999;11(5):584–588. doi: 10.1016/s0952-7915(99)00012-6. [DOI] [PubMed] [Google Scholar]
- 21.Wu AM, Senter PD. Arming antibodies: prospects and challenges for immunoconjugates. Nat Biotechnol. 2005;23(9):1137–1146. doi: 10.1038/nbt1141. [DOI] [PubMed] [Google Scholar]
- 22.Teicher BA, Chari RV. Antibody conjugate therapeutics: challenges and potential. Clin Cancer Res. 2011;17(20):6389–6397. doi: 10.1158/1078-0432.CCR-11-1417. [DOI] [PubMed] [Google Scholar]
- 23.Alley SC, Okeley NM, Senter PD. Antibody-drug conjugates: targeted drug delivery for cancer. Curr Opin Chem Biol. 2010;14(4):529–537. doi: 10.1016/j.cbpa.2010.06.170. [DOI] [PubMed] [Google Scholar]
- 24.Reff ME, Carner K, Chambers KS, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83(2):435–445. [PubMed] [Google Scholar]
- 25.Dillman RO. Magic bullets at last! Finally – approval of a monoclonal antibody for the treatment of cancer!!! Cancer Biother Radiopharm. 1997;12(4):223–225. doi: 10.1089/cbr.1997.12.223. [DOI] [PubMed] [Google Scholar]
- 26.Coiffer B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 27.Ghielmini M, Schmitz SF, Cogliatti SB, et al. Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly ×4 schedule. Blood. 2004;103(12):4416–4423. doi: 10.1182/blood-2003-10-3411. [DOI] [PubMed] [Google Scholar]
- 28.Marcus R, Imrie K, Belch A, et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood. 2005;105(4):1417–1423. doi: 10.1182/blood-2004-08-3175. [DOI] [PubMed] [Google Scholar]
- 29.Byrd JC, Peterson BL, Morrison VA, et al. Randomized phase 2 study of fudarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-cell chronic lymphocytic leukemia: results from Cancer and Leukemia Group B 9712 (CALGB 9712) Blood. 2003;101(1):6–14. doi: 10.1182/blood-2002-04-1258. [DOI] [PubMed] [Google Scholar]
- 30.Keating MJ, Flinn I, Jain V, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood. 2002;99(10):3554–3561. doi: 10.1182/blood.v99.10.3554. [DOI] [PubMed] [Google Scholar]
- 31.Zinzani PL, Tani M, Pulsoni A, et al. A phase II trial of short course fludarabine, mitoxantrone, rituximab followed by 90Y-ibritumomab tiuxetan in untreated intermediate/high-risk follicular lymphoma. Ann Oncol. 2012;23(2):415–420. doi: 10.1093/annonc/mdr145. [DOI] [PubMed] [Google Scholar]
- 32.Zinzani PL, Rossi G, Franceschetti S, et al. Phase II trial of short course R-CHOP followed by 90Y-ibritumomab tiuxetan in previously untreated high-risk elderly diffuse large B-cell lymphoma patients. Clin Cancer Res. 2010;16(15):3998–4004. doi: 10.1158/1078-0432.CCR-10-0162. [DOI] [PubMed] [Google Scholar]
- 33.Kaminski MS, Tuck M, Estes J, et al. 131I-tositumomab therapy as initial treatment for follicular lymphoma. N Engl J Med. 2005;352(5) doi: 10.1056/NEJMoa041511. [DOI] [PubMed] [Google Scholar]
- 34.Kaminski MS, Tuck M, Estes J, et al. Tositumomab and iodine I-131 tositumomab for previously untreated, advanced stage, follicular lymphoma: median 10 year follow-up results. Blood (ASH Annual Meeting Abstracts) 2009;114(22):3759. [Google Scholar]
- 35.Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363(19):1812–1821. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 36.Tedder TF. CD19: a promising B cell target for rheumatoid arthritis. Nat Rev Rheumatol. 2009;5(10):572–577. doi: 10.1038/nrrheum.2009.184. [DOI] [PubMed] [Google Scholar]
- 37.Haas KM, Tedder TF. Role of the CD19 and CD21/35 receptor complex in innate immunity, host defense and autoimmunity. Adv Exp Med Biol. 2005;560:125–139. doi: 10.1007/0-387-24180-9_16. [DOI] [PubMed] [Google Scholar]
- 38.Bradbury LE, Kansas GS, Levy S, Evans RL, Tedder TF. The CD19/CD21 signal transducing complex of human B lymphocytes includes the target of antiproliferative antibody-1 and Leu-13 molecules. J Immunol. 1992;149(9):2841–2850. [PubMed] [Google Scholar]
- 39.Poe JC, Minard-Colin V, Kountikov EI, Haas KM, Tedder TF. A c-Myc and Surface CD19 signaling amplification loop promotes B cell lymphoma development and progression in mice. J Immunol. 2012;189(5):2318–2325. doi: 10.4049/jimmunol.1201000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Zelm MC, Reisli I, van der Burg M, et al. An antibody-deficiency syndrome due to mutations in the CD19 gene. N Engl J Med. 2006;354(18):1901–1912. doi: 10.1056/NEJMoa051568. [DOI] [PubMed] [Google Scholar]
- 41.Nadler LM, Anderson KC, Marti G, et al. B4, a human B lymphocyte-associated antigen expressed on normal, mitogen-activated, and malignant B lymphocytes. J Immunol. 1983;131(1):244–250. [PubMed] [Google Scholar]
- 42.Scheuermann RH, Racila E. CD19 antigen in leukemia and lymphoma diagnosis and immunotherapy. Leuk Lymphoma. 1995;18(5–6):385–397. doi: 10.3109/10428199509059636. [DOI] [PubMed] [Google Scholar]
- 43.Anderson KC, Bates MP, Slaughenhoupt BL, Pinkus GS, Schlossman SF, Nadler LM. Expression of human B cell-associated antigens on leukemias and lymphomas: a model of human B cell differentiation. Blood. 1984;63(6):1424–1433. [PubMed] [Google Scholar]
- 44.Carter RH, Myers R. Germinal center structure and function: lessons from CD19. Semin Immunol. 2008;20(1):43–48. doi: 10.1016/j.smim.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lutz RJ, Zuany-Amorim C, Vrignaud P, Mayo MF, Guerif S, Xie H, et al. Preclinical evaluation of SAR3419 (huB4-DM4), an anti-CD19-maytansinoid immunoconjugate, for the treatment of B-cell lymphoma. Proc Am Assoc Cancer Res. 2006;47:3731. [Google Scholar]
- 46.Gerber HP, Kung-Sutherland M, Stone I, et al. Potent antitumor activity of the anti-CD19 auristatin antibody drug conjugate hBU12-vcMMAE against rituximab-sensitive and -resistant lymphomas. Blood. 2009;113(18):4352–4361. doi: 10.1182/blood-2008-09-179143. [DOI] [PubMed] [Google Scholar]
- 47.Mohammad RM, Mohamed AN, Smith MR, Jawadi NS, Al-Katib A. A unique EBV-negative low-grade lymphoma line (WSU-FSCCL) exhibiting both t(14;18) and t(8;11) Cancer Genet Cytogenet. 1993;70(1):62–67. doi: 10.1016/0165-4608(93)90132-6. [DOI] [PubMed] [Google Scholar]
- 48.Mohamed AN, Al-Katib A. Establishment and characterization of a human lymphoma cell line (WSU-NHL) with 14; 18 translocation. Leuk Res. 1988;12(10):833–843. doi: 10.1016/0145-2126(88)90037-9. [DOI] [PubMed] [Google Scholar]
- 49.Mohammad RM, Mohamed AN, KuKuruga M, Smith MR, Al-Katib A. A human B-cell lymphoma line with de novo multidrug resistance phenotype. Cancer. 1992;69(6):1468–1474. doi: 10.1002/1097-0142(19920315)69:6<1468::aid-cncr2820690626>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 50.Al-Katib A, Mohammad RM, Mohamed AN. WSU-BL: a new Burkitt’s lymphoma cell line with capacity to differentiate in vitro. Ann NY Acad Sci. 1989;567:317–319. [Google Scholar]
- 51.Mohamed AN, Mohammad RM, Koop BF, Al-Katib A. Establishment and characterization of a new human Burkitt’s lymphoma cell line (WSU-BL) Cancer. 1989;64(5):1041–1048. doi: 10.1002/1097-0142(19890901)64:5<1041::aid-cncr2820640514>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 52.Weinstein IB, Joe A. Oncogene addiction. Cancer Res. 2008;68(9):3077–3080. doi: 10.1158/0008-5472.CAN-07-3293. [DOI] [PubMed] [Google Scholar]
- 53.Taub R, Kirsch I, Morton C, et al. Translocation of the c-myc gene into the immunoglobulin heavy chain locus in human Burkitt lymphoma and murine plasmacytoma cells. Proc Natl Acad Sci U S A. 1982;79(24):7837–7841. doi: 10.1073/pnas.79.24.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dalla-Favera R, Bregni M, Erikson J, Patterson D, Gallo RC, Croce CM. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci U S A. 1982;79(24):7824–7827. doi: 10.1073/pnas.79.24.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Busslinger M. Transcriptional control of early B cell development. Annu Rev Immunol. 2004;22:55–79. doi: 10.1146/annurev.immunol.22.012703.104807. [DOI] [PubMed] [Google Scholar]
- 56.Monroe JG. ITAM-mediated tonic signaling through pre-BCR and BCR complexes. Nat Rev Immunol. 2006;6(4):283–294. doi: 10.1038/nri1808. [DOI] [PubMed] [Google Scholar]
- 57.Chung EY, Psathas JN, Yu D, Li Y, Weiss MJ, Thomas-Tikhonenko A. CD19 is a major B cell receptor-independent activator of MYC-driven B-lymphomagenesis. J Clin Invest. 2012;122(6):2257–2266. doi: 10.1172/JCI45851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pillay V, Gan HK, Scott AM. Antibodies in oncology. N Biotechnol. 2011;28(5):518–529. doi: 10.1016/j.nbt.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 59.Pillay V, Allaf L, Wilding AL, et al. The plasticity of oncogene addiction: implications for targeted therapies directed to receptor tyrosine kinases. Neoplasia. 2009;11(5):448–458. doi: 10.1593/neo.09230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Younes A. Beyond chemotherapy: new agents for targeted treatment of lymphoma. Nat Rev Clin Oncol. 2011;8(2):85–96. doi: 10.1038/nrclinonc.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nat Rev Clin Oncol. 2011;8(3):124. doi: 10.1038/nrclinonc.2011.11. Erratum in: [DOI] [PubMed] [Google Scholar]
- 61.Kupchan SM, Komoda Y, Court WA, et al. Maytansine, a novel anti-leukemic ansa macrolide from Maytenus ovatus. J Am Chem Soc. 1972;94(4):1354–1356. doi: 10.1021/ja00759a054. [DOI] [PubMed] [Google Scholar]
- 62.Remillard S, Rebhun LI, Howie GA, Kupchan SM. Antimitotic activity of the potent tumor inhibitor maytansine. Science. 1975;189(4207):1002–1005. doi: 10.1126/science.1241159. [DOI] [PubMed] [Google Scholar]
- 63.Blanc V, Bousseau A, Caron A, Carrez C, Lutz RJ, Lambert JM. SAR3419: an anti-CD19-maytansinoid immunoconjugate for the treatment of B-cell malignancies. Clin Cancer Res. 2011;17(20):6448–6458. doi: 10.1158/1078-0432.CCR-11-0485. [DOI] [PubMed] [Google Scholar]
- 64.Chabner BA, Levine AS, Johnson BL, Young RC. Initial clinical trials of maytansine, an antitumor plant alkaloid. Cancer Treat Rep. 1978;62(3):429–433. [PubMed] [Google Scholar]
- 65.Blum RH, Kahlert T. Maytansine: a phase I study of an ansa macrolide with antitumor activity. Cancer Treat Rep. 1978;62(3):435–438. [PubMed] [Google Scholar]
- 66.Cabanillas F, Rodriguez V, Hall SW, Burgess MA, Bodey GP, Freireich EJ. Phase I study of maytansine using a 3-day schedule. Cancer Treat Rep. 1978;62(3):425–428. [PubMed] [Google Scholar]
- 67.Eagan RT, Ingle JN, Rubin J, Frytak S, Moertel CG. Early clinical study of an intermittent schedule for maytansine (NSC-153858): brief communication. J Natl Cancer Inst. 1978;60(1):93–96. doi: 10.1093/jnci/60.1.93. [DOI] [PubMed] [Google Scholar]
- 68.Issell BF, Crooke ST. Maytansine. Cancer Treat Rev. 1978;5(4):199–207. doi: 10.1016/s0305-7372(78)80014-0. [DOI] [PubMed] [Google Scholar]
- 69.Erickson HK, Park PU, Widdison WC, et al. Antibody-maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing. Cancer Res. 2006;66(8):4426–4433. doi: 10.1158/0008-5472.CAN-05-4489. [DOI] [PubMed] [Google Scholar]
- 70.Kovtun YV, Audette CA, Ye Y, et al. Antibody-drug conjugates designed to eradicate tumors with homogeneous and heterogeneous expression of the target antigen. Cancer Res. 2006;66(6):3214–3221. doi: 10.1158/0008-5472.CAN-05-3973. [DOI] [PubMed] [Google Scholar]
- 71.Al-Katib AM, Aboukameel A, Mohammad R, Bissery MC, Zuany-Amorim C. Superior antitumor activity of SAR3419 to rituximab in xenograft models for non-Hodgkin’s lymphoma. Clin Cancer Res. 2009;15(12):4038–4045. doi: 10.1158/1078-0432.CCR-08-2808. [DOI] [PubMed] [Google Scholar]
- 72.DiJoseph JF, Goad ME, Dougher MM, et al. Potent and specific antitumor efficacy of CMC-544, aCD22-targeted immunoconjugate of calicheamicin, against systemically disseminated B-cell lymphoma. Clin Cancer Res. 2004;10(24):8620–8629. doi: 10.1158/1078-0432.CCR-04-1134. [DOI] [PubMed] [Google Scholar]
- 73.Younes A, Kim S, Romaguera J, et al. Phase I multi-dose escalation study of the anti-CD19 maytansinoid immunoconjugate SAR3419 administered by intravenous infusion every 3 weeks to patients with relapsed/refractory B-cell lymphoma. J Clin Oncol. 2012;30(22):2776–2782. doi: 10.1200/JCO.2011.39.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coiffer B, Ribrag V, Dupuis J, et al. Phase I/II study of the anti-CD19 maytansinoid immunoconjugate SAR3419 administered weekly to patients with relapsed/refractory B-cell non-Hodgkin’s lymphoma (NHL) J Clin Oncol. 2011;29(suppl):8017. abstract. [Google Scholar]
- 75.Carol H, Szymanska B, Evans K, et al. The anti-CD19 antibody-drug conjugate SAR3419 prevents hematolymphoid relapse postinduction therapy in preclinical models of pediatric acute lymphoblastic leukemia. Clin Cancer Res. 2013;19(7):1795–1805. doi: 10.1158/1078-0432.CCR-12-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]