Significance
In several critically ill patients the homeostatic regulation of adrenocortical hormone secretion is impaired. Toll-like receptors (TLR) play a substantial role in HPA axis activation in the course of systemic inflammation. Here, using mice with conditional deletion of a crucial TLR adapter protein, MyD88, we investigated the role of systemic and local adrenal TLR signaling in the activation of adrenal glucocorticoid responses to stress and regulation of immune-adrenal crosstalk during systemic inflammatory response syndrome. Our results suggest that immune cells, rather than adrenal cells, are major regulators of the systemic and intra-adrenal inflammatory response to LPS. However, the full HPA axis activation in SIRS is not entirely dependent on systemic TLR signaling.
Keywords: adrenal gland insufficiency, Toll-like receptors, the HPA axis
Abstract
Inflammation-related dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis is central to the course of systemic inflammatory response syndrome or sepsis. The underlying mechanisms, however, are not well understood. Initial activation of adrenocortical hormone production during early sepsis depends on the stimulation of hypothalamus and pituitary mediated by cytokines; in late sepsis, there is a shift from neuroendocrine to local immune–adrenal regulation of glucocorticoid production. Therefore, the modulation of the local immune–adrenal cross talk, and not of the neuroendocrine circuits involved in adrenocorticotropic hormone production, may be more promising in the prevention of the adrenal insufficiency associated with prolonged sepsis. In the present work, we investigated the function of the crucial Toll-like receptor (TLR) adaptor protein myeloid differentiation factor 88 (MyD88) in systemic and local activation of adrenal gland inflammation and glucocorticoid production mediated by lipopolysachharides (LPSs). To this end, we used mice with a conditional MyD88 allele. These mice either were interbred with Mx1 Cre mice, resulting in systemic MyD88 deletion, predominantly in the liver and hematopoietic system, or were crossed with Akr1b7 Cre transgenic mice, resulting thereby in deletion of MyD88, which was adrenocortical-specific. Although reduced adrenal inflammation and HPA-axis activation mediated by LPS were found in Mx1Cre+-MyD88fl/fl mice, adrenocortical-specific MyD88 deletion did not alter the adrenal inflammation or HPA-axis activity under systemic inflammatory response syndrome conditions. Thus, our data suggest an important role of immune cell rather than adrenocortical MyD88 for adrenal inflammation and HPA-axis activation mediated by LPS.
Sepsis and septic shock are major causes of death in intensive-care units worldwide and show an increasing incidence (1). In sepsis, excessive, uncontrolled activation of the immune system is harmful to the host and leads to multiorgan failure and death. Adrenal glucocorticoid production plays a beneficial role in response to systemic inflammation by counteracting hyperactivation of the immune system. However, in many critically ill patients, this homeostatic activation of adrenocortical hormone secretion is impaired (2). It has been estimated that 60% of critically ill patients show an abnormal adrenal glucocorticoid response to administration of exogenous adrenocorticotropic hormone (ACTH) (3).
Adrenal hormone production in sepsis is thought to be regulated by cytokines that elevate hypothalamic corticotropin releasing hormone (CRH) levels. CRH, in turn, produces the release of pituitary ACTH—the main regulator of synthesis of adrenal glucocorticoid hormones (4). It is generally accepted that pattern-recognition receptors such as Toll-like receptors (TLRs) play a substantial role in hypothalamic–pituitary–adrenal (HPA) axis activation induced by pathogens. This activation, in turn, may be attributable to functions of these receptors on both myeloid and nonmyeloid cells (e.g., endothelial cells of the blood–brain barrier) (5). Furthermore, besides activation of immune cells, bacterially derived TLR ligands, such as lipopolysaccharides (LPSs), can directly affect both neuronal cells (6) and steroid-producing cells in the adrenal gland (7).
Although most LPS actions on adrenal-gland glucocorticoid production have been attributed to activation of HPA axis by cytokines at the level of the hypothalamus and pituitary gland, a critical involvement of an intrinsically regulated immune–adrenal cross talk has also been implicated. In fact, we and others have demonstrated that adrenocortical cells express several TLRs and secrete multiple proinflammatory cytokines such as tumor necrosis factor (TNF) and interleukin (IL)6 in response to bacterial endotoxin stimulation (7). Using mice deficient in either TLR2 or TLR4, we demonstrated a critical function of these receptors as regulators of the immune–adrenal cross talk during LPS-mediated systemic inflammation. The absence of these receptors correlates with increased basal levels of either ACTH (for TLR2) or corticosterone (for TLR4) and impaired adrenal glucocorticoid responses to injection of LPS (8, 9). However, the exact contributions of the TLRs on different cell types to inflammation-mediated adrenal dysfunction as well as to activation of HPA axis are not entirely clear. Furthermore, because both TLR2 and TLR4 were shown to be involved in early neurogenesis (10), the deficiency of these receptors from the very early developmental stage might potentially influence the basal function of the HPA axis. Myeloid differentiation factor 88 (MyD88) is a central component of the signaling pathway of TLRs, promoting nuclear factor kappa B (NF-κB) activation in response to LPS or IL1 β. Thus, blocking of MyD88-dependent signaling in a cell-specific manner represents an attractive experimental tool to address the aforementioned issue.
These data and observations prompted us to investigate the role of systemic and local adrenal TLR signaling in the activation of the adrenal glucocorticoid response to stress and regulation of the immune–adrenal cross talk during the systemic inflammatory response syndrome (SIRS) phenomenon. Our results suggest that immune cells, rather than adrenal cells, are the major regulators of the systemic and intraadrenal inflammatory response to LPS. However, the full HPA axis activation in SIRS is not entirely dependent on systemic TLR signaling.
Results
Generation of Mice with Adrenocortical Cell-Specific and General Inactivation of TLR Signaling.
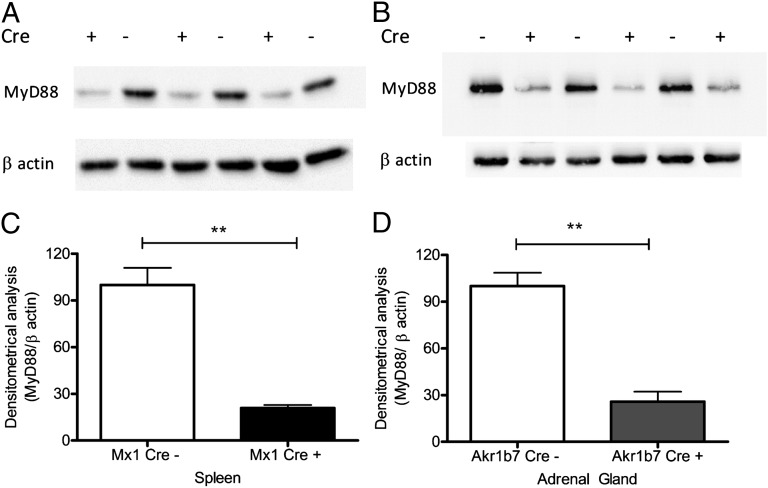
To investigate the role of the intact TLR signaling required for proper activation of adrenal steroidogenesis in the course of SIRS mediated by LPS, within different compartments of the adrenal microenvironment, we crossed mice with the myd88flox allele with mice carrying either aldo-keto reductase 1B7 (Akr1b7)Cre transgene (which allows MyD88 deletion in adrenocortical cells) (11) or myxovirus resistance 1 (Mx1)Cre transgene (which results in major MyD88 deletion in the liver and hematopoietic cells, with minor deletion in other tissues) (12). The reduction of MyD88 expression was assessed by Western blot of splenic tissue of Cre-positive and littermate Cre-negative Mx1Cre-MyD88fl/fl mice (Fig. 1A) as well as adrenal tissue of Cre-positive and littermate Cre-negative Akr1b7Cre-MyD88fl/fl mice (Fig. 1B). This analysis reveals successful deletion of MyD88 in splenocytes of the Mx1Cre+-MyD88fl/fl mice (Fig. 1A) and in the adrenocortical cells of Akr1b7Cre+-MyD88fl/fl mice (Fig. 1B).
Fig. 1.
For the adrenocortical cell-specific and general deletion of MyD88, mice with myd88flox allele were crossed with mice carrying either Akr1b7Cre transgene (preferentially expressed in adrenocortical cells) or mice with Mx1Cre transgene (expressed in the liver and hematopoietic system with minor expression in other tissues). The deletion of MyD88 protein was measured by Western blot in (A) spleen tissue lysates of Cre recombinase positive and negative Mx1Cre-MyD88fl/fl mice and in (B) adrenal glands of Cre recombinase positive and negative Akr1b7Cre-MyD88fl/fl mice. Densitometric analysis of Western blot results revealed a successful deletion of MyD88 in (C) the splenocytes of the Mx1Cre-MyD88fl/fl mice (black bars) and in (D) the adrenal glands of Akr1b7Cre-MyD88fl/fl mice (gray bars) compared with respective controls (open bars). **P < 0.01.
Systemic (Not Adrenocortical Cell-Specific) MyD88 Deficiency Results in Impaired Activity of HPA Axis During SIRS Induced by LPS.
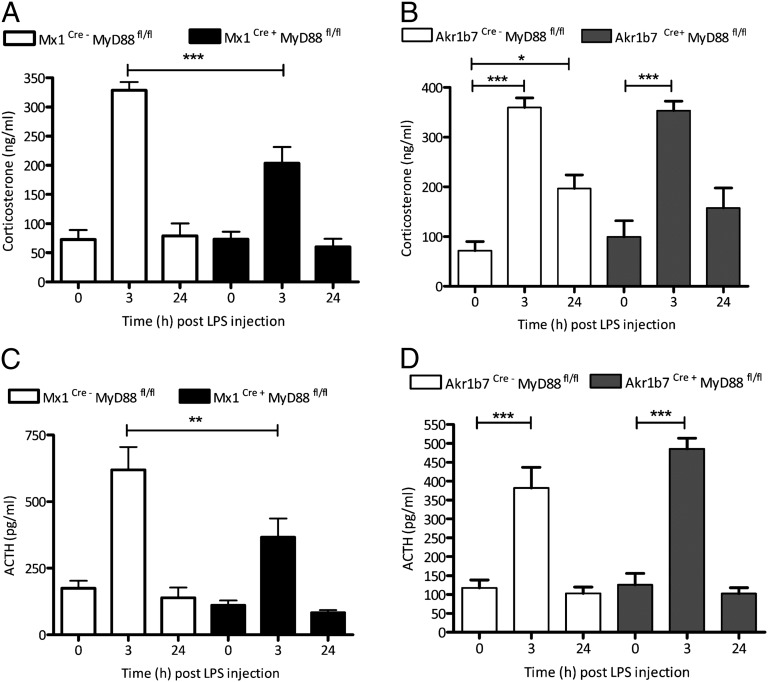
Previous studies demonstrated a critical involvement of TLR2, TLR4, and MyD88 in adrenal glucocorticoid production and in the regulation of immune–adrenal cross talk in response to bacterial endotoxin. However, these studies did not delineate the contribution of the immune system versus the steroid-producing cells of the adrenal gland in endotoxin-mediated induction of glucocorticoid production (9, 13). LPS (1 mg per kg of body weight, i.p.) was administered to both Mx1Cre-MyD88fl/fl and Akr1b7Cre-MyD88fl/fl mice and their corresponding Cre-negative littermates. Three and 24 h after injection, mice were killed, and activation of HPA axis was elucidated by the determination of plasma levels of both ACTH and corticosterone. Our results revealed that systemic (Fig. 2 A and C), but not adrenocortical cell-specific (Fig. 2 B and D), TLR signaling is critical for proper activation of corticosterone and ACTH production at 3 h post LPS administration. Interestingly, the LPS-mediated activation of the HPA axis, although significantly reduced, was not completely abolished in Mx1Cre-MyD88fl/fl animals (Fig. 2 A and C).
Fig. 2.
Systemic but not adrenocortical cell-specific TLR signaling is involved in regulation of the HPA axis during endotoxin challenge. Mice with general inactivation of MyD88 signaling (Mx1Cre+-MyD88fl/fl; black bars) and specific adrenocortical cell inactivation (Akr1b7Cre+-MyD88fl/fl; gray bars) together with respective Cre-negative littermates (open bars) received a single i.p. injection of LPS (1 mg/kg). Three hours and 24 h after LPS injection, mice were killed and plasma (A and B) corticosterone and (C and D) ACTH levels were determined. The results showed that, 3 h after LPS injection, mice with general deletion of MyD88 (A and C) but not mice with specific adrenocortical cell deletion of MyD88 (B and D) demonstrate an impaired glucocorticoid and ACTH response to LPS injection. ***P < 0.001; **P < 0.01; *P < 0.05. n ≥ 6 animals were used in each group. Results are presented as means ± SEM.
Systemic but Not Local Adrenal TLR Signaling Is Involved in the LPS-Mediated Neutrophil Infiltration into Adrenal Gland.
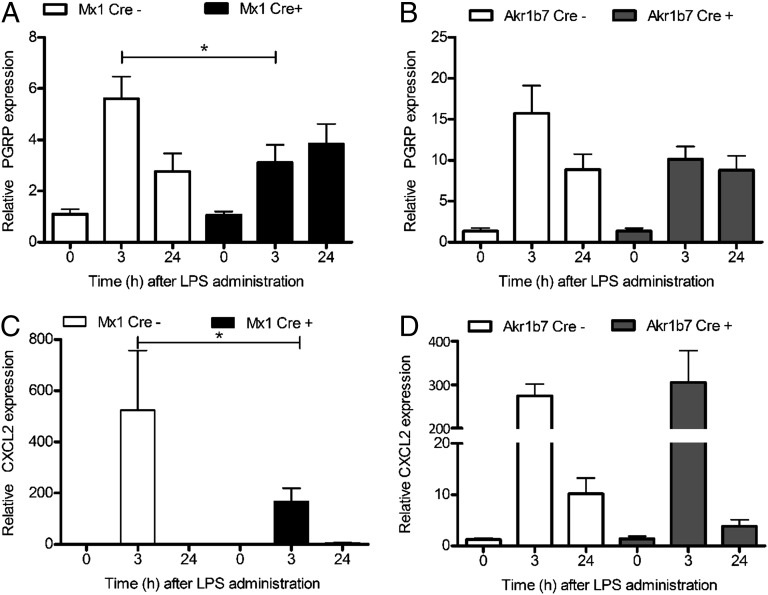
We then gauged neutrophil infiltration into adrenal glands during LPS-induced systemic inflammation by performing real-time PCR analysis of the neutrophil gene marker peptidoglycan recognition protein (PGRP) (14). LPS induced a rapid infiltration of neutrophils into the adrenal gland. Interestingly, systemic (Fig. 3A) but not adrenocortical-cell specific (Fig. 3B) deletion of MyD88 was associated with significantly reduced neutrophil infiltration into the adrenal gland at 3 h after LPS injection. Previously, we had demonstrated that neutrophil infiltration into the adrenal gland is associated with an elevation of the granulocyte-attracting chemokine (C-X-C motif) ligand 2 (CXCL2) (14). Consistent with the reduced LPS-induced neutrophil infiltration to the adrenal gland of Mx1Cre+-MyD88fl/fl mice, CXCL2 was also significantly decreased in these mice (Fig. 3C). In contrast, adrenocortical deletion of MyD88 in Cre-positive Akr1b7Cre-MyD88fl/fl mice did not alter either neutrophil infiltration (Fig. 3B) or CXCL2 expression (Fig. 3D) in their adrenal glands after LPS injection. In addition, the expression of the monocyte-specific chemokine CCL2 was not affected by inactivation of either systemic or adrenocortical cell specific MyD88 (Fig. S1). Taking these together, and given that the predominant MyD88 deletion in Mx1Cre transgenic mice is in the liver and hematopoietic cells, and that reduced neutrophil recruitment to the adrenal glands is seen in Mx1Cre+-MyD88fl/fl mice, our data imply that the MyD88 in immune cells rather than that in adrenocortical cells is responsible for the immune–adrenal cross talk within the adrenal microenvironment.
Fig. 3.
Systemic but not local adrenocortical cell-specific TLR signaling is involved in the LPS-mediated neutrophil infiltration into adrenal gland. A real-time PCR analysis of neutrophil gene marker PGRP and neutrophil-specific chemokine CXCL2 revealed that (A) systemic but not (B) adrenocortical cell-specific inactivation of TLR signaling within the adrenal microenvironment resulted in decreased leukocyte infiltration into adrenal glands during LPS-induced systemic inflammation. Furthermore, reduced neutrophil infiltration into adrenal glands of (C) Mx1Cre+-MyD88fl/fl mice (black bars) was associated with reduced LPS-mediated up-regulation of granulocyte-specific CXCL2 chemokine in the adrenal glands from these animals. In contrast, in mice with (D) adrenocortical-specific deletion of MyD88 (gray bars) and in control animals (open bars), no difference was found. Results are presented as means ± SEM; *P < 0.05.
General but Not Adrenocortical-Specific MyD88 Deficiency Is Associated with Reduced Adrenal Gland Inflammation After LPS Administration.
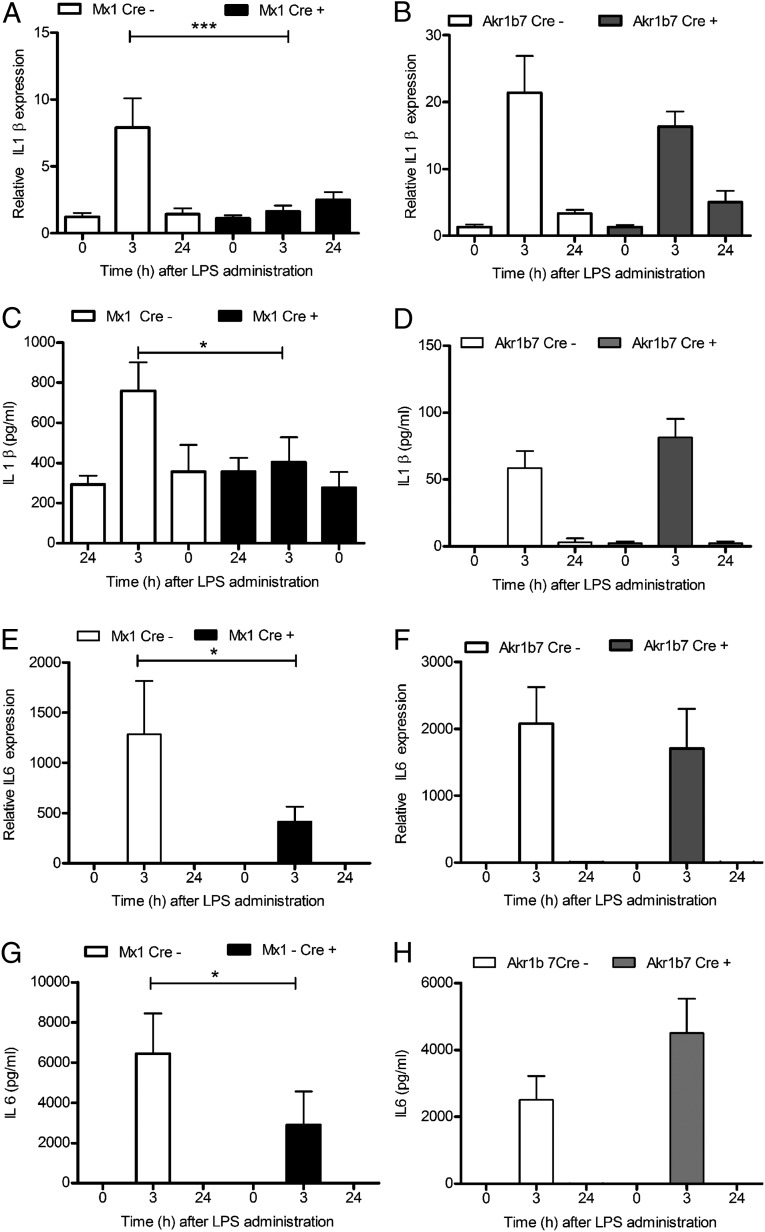
Immune–adrenal cross talk may regulate LPS-induced adrenal steroidogenesis. In particular, a critical role for macrophage-derived IL1 β and prostaglandin E2 (PGE2) in the initial, and IL6 in the sustained, activation of adrenal glucocorticoid production has been demonstrated (15). Therefore, we next examined the expression of IL1 β and IL6 in the adrenal glands of those mice carrying an adrenocortical-specific MyD88 deletion (Akr1b7Cre-MyD88fl/fl) and also those mice carrying MyD88 deletion predominantly in the immune and liver cells (Mx1Cre-MyD88fl/fl). At 3 h and 24 h after LPS administration, up-regulation of both cytokines mediated by LPS was significantly reduced in Cre-positive Mx1Cre-MyD88fl/fl mice compared with Cre-negative animals (Fig. 4 A and C). Similar to the results with the studies on IL1 β or IL6 gene expression, the peripheral plasma levels of IL1 β and IL6 were reduced in Cre-positive Mx1Cre-MyD88fl/fl mice (Fig. 4 C and G). In contrast, no differences in adrenal IL1 β and IL6 expression, or in the plasma levels of these cytokines, were observed to result from adrenocortical MyD88 deficiency (Fig. 4 B, D, F, and H). Thus, we conclude that an intact MyD88 signaling in immune cells mediates the local immune–adrenal cross talk and adrenal inflammation.
Fig. 4.
Immune cells are the main regulators involved in the LPS-mediated systemic and intraadrenal inflammation. Real-time PCR results demonstrating almost complete abrogation of LPS-mediated (A) adrenal and (C) plasma IL1 β elevation and a significant decrease in (E) adrenal and (G) plasma IL6 expression at 3 h following LPS injection in mice with general deletion of MyD88 (Mx1Cre+-MyD88fl/fl; black bars). In contrast, LPS-modulated (B and F) adrenal and (D and H) plasma IL1 β and IL6 expression did not differ between mice with specific inactivation of TLR signaling in the adrenocortical cells (gray bars) and the respective Cre-negative littermates (open bars). Results are presented as means ± SEM; ***P < 0.001; *P < 0.05.
Discussion
A hallmark of experimental models of endotoxemia is the activation of the HPA axis, which leads to release of glucocorticoids (4). A large part of this response of the CNS to LPS has been attributed to the action of cytokines and other acute phase reactants; however, the cellular origin of these inflammatory mediators is still controversial (5, 16, 17). In addition to the central activation of HPA axis in the course of SIRS and sepsis, a local immune–adrenocortical cross talk may also contribute to modulation of systemic levels of glucocorticoids (18). In this context, we have demonstrated in our previous studies that either systemic TLR2 or TLR4 deficiency is associated with an enlargement of the adrenal cortex and results in a reduced response to bacterial endotoxin by adrenal glucocorticoids. This observation suggests that TLRs not only are involved in the initial HPA axis activation, but also are critically involved in the regulation of the immune–adrenocortical cross talk within the adrenal gland (8, 9).
A major, yet unanswered, question resulting from these observations is whether TLR signaling in adrenocortical cells or in other cells of the adrenal microenvironment, including recruited leukocytes, regulates adrenal steroidogenesis, adrenal inflammation, and immune–adrenal cross talk during SIRS. Our present study addresses this question by using mice with adrenocortical cell-specific inactivation of MyD88, the major component of TLR signaling (19), and mice wherein MyD88 is deleted by using the Mx1 Cre transgene, which leads to inactivation of TLR signaling, predominantly in hematopoietic cells and the liver. Surprisingly, and partially in contrast to previous studies in adrenocortical cell cultures in vitro that have implicated TLR-signaling in the regulation of steroidogenesis (20), this study revealed no contribution of adrenocortical MyD88 to the systemic HPA activation or to the local immune–adrenal cross talk within the adrenal microenvironment in LPS-induced inflammation. On the other hand, our findings with these Mx1Cre-MyD88fl/fl mice strongly suggests that MyD88 in immune cells recruited to the adrenal gland dictates the local immune–adrenal cross talk, whereas MyD88 in immune cells and likely also in the liver regulates HPA axis activity during SIRS. These conclusions are supported by the following observations: (i) MyD88 inactivation by the Mx1 Cre transgene, but not the adrenocortical-specific Cre transgene, resulted in a decreased secretion of both ACTH and corticosterone in response to bacterial endotoxin. That is, inactivation of MyD88 reduced HPA axis activity. (ii) Mice without MyD88 in their hematopoietic cells displayed reduced LPS-mediated, inflammation-triggered neutrophil recruitment to the adrenal gland and also displayed decreases in both the levels of proinflammatory cytokines and chemokines in the adrenal gland, and also in the circulation. None of these processes was altered by the absence of MyD88 in adrenocortical cells. In other words, MyD88 in hematopoietic cells drives inflammatory cell recruitment in the adrenal gland in the course of SIRS and instigates the local immune–adrenal cross talk that may modulate the adrenal dysfunction occurring during systemic inflammation. However, generation of mice with MyD88 deletion only in the hematopoietic cells would be necessary to fully ensure the exclusive involvement of the immune cells in this process.
In contrast to mice with complete MyD88 deficiency (13), deletion of MyD88 only in the hematopoietic and liver compartments did not show complete abolition of HPA axis activation by LPS. This observation suggests an additional contribution of other cells, possibly endothelial cells, in this process (5). This fact is in accord with studies evaluating HPA axis activity mediated by LPS in irradiated chimeric mice having bone marrow transfer either from wild-type or TLR4-insufficient HeJ mice (21). Another possible explanation for these findings, wherein MyD88 is deleted only in the liver and hematopoietic compartments, is that, despite the high degree of MyD88 deletion in the liver and immune cells by the Mx1 Cre transgene, this deletion is incomplete (i.e., a small level of MyD88 expression was still detected) (12, 22). This result thus demands extra care when interpreting these data.
We and others have shown the critical involvement of proinflammatory cytokines (23, 24), TLRs (25), immune cells, and endothelial cells (14, 15) in the regulation of the immune–adrenal cross talk during endotoxemia. Within the local adrenal microenvironment, the immune and steroid-producing cells may influence each other´s function either through direct cell–cell interactions or, in a paracrine fashion, through secreted factors (26). Our present data show that MyD88-dependent signaling in immune cells, likely recruited to the adrenal gland in the course of endotoxemia, regulates the levels of IL1 β (27) or of IL6 (28) in the adrenal gland. The attenuated expression of both IL1 β and IL6 cytokines in the adrenal glands of Cre-positive Mx1Cre-MyD88fl/fl mice in the course of SIRS is in accordance with previous studies demonstrating that these immune cells are the primary cell types, within the adrenal microenvironment, that secrete and respond to IL1 β during LPS challenge (15).
In the present study, using genetically modified mice with hepatic and myeloid-specific inactivation of TLR signaling or mice with adrenocortical-specific MyD88 deletion, we found that systemic, but not adrenocortical, MyD88 deletion affected HPA axis activity in SIRS. We also showed that immune cells, rather than steroid-producing cells, are the major regulators of the immune–adrenal cross talk. However, activation of HPA axis in the course of SIRS is not fully dependent on systemic TLR signaling, an observation that requires further investigation to elucidate the exact and additional underlying mechanisms of the cross talk between the immune system and HPA axis.
Materials and Methods
Animals.
To dissect the tissue-specific role of TLR signaling within the adrenal microenvironment, we crossed mice with myd88flox allele (from The Jackson Laboratory) with mice carrying either Akr1b7Cre transgene (preferentially expressed in adrenocortical cells) (11) or with mice having the Mx1Cre transgene (predominantly expressed in hematopoietic system and in the liver with minor expression in other tissues) (12). For Mx1 Cre activation, polyadenylic-polyuridylic acid (Poly I:C; 0.2 mg disolved in 200 mL of water) was given to Mx1Cre-MyD88fl/fl mice intraperitoneally every second day for 5 consecutive days. LPS injection was carried out 2 wk after the last Poly I:C injection.
SIRS.
Animal work was approved by the Ethical Committee of the Landesdirektion Dresden. To induce SIRS, ultra-pure lipopolysaccharide from Escherichia coli strain 0111:B4 (1 mg/kg body weight; Invivogen), dissolved in PBS, was injected intraperitoneally into 8- to 11-wk-old Akr1b7Cre-MyD88fl/fl and Mx1Cre-MyD88fl/fl mice. At 0, 3, or 24 h following LPS administration, mice were killed, and blood and both adrenal glands were removed. Plasma concentrations of corticosterone and ACTH were determined using commercial ELISA kits (corticosterone from Demeditec Diagnostics; ACTH from IBL-International).
Western Blot.
Spleens and adrenal glands of Mx1Cre-MyD88fl/fl and Akr1b7Cre-MyD88fl/fl mice, respectively, were lysed in M-tissue cell lytic reagent (Sigma-Aldrich), and total protein content was determined using the BCA method (Pierce, Thermo Scientific). Equal amounts of these proteins were separated on 10% SDS/PAGE gels and then transferred onto nitrocellulose membranes. After blocking, these membranes were incubated overnight at 4 °C with an antibody specific for MyD88 (Cell Signal Technology). After washing, the membranes were incubated for 1 h with HRP-conjugated goat anti-rabbit IgG. Signals were measured using SuperSignal West Femto substrate (Pierce, Thermo Scientific). After visualization, the membranes were stripped with 2% NaOH and incubated overnight with monoclonal anti–β-actin antibody (Merck/Millipore). Densitometric analysis of identified Western blot bands was performed using Image J software (National Institutes of Health).
RNA Isolation and RT-PCR.
RNA from different mouse and human tissues was isolated using RNeasy Plus Mini kit (Qiagen) according to the manufacturer’s instructions. One microgram of RNA was then reverse transcribed using iScript RT kit (Bio-Rad) according to the manufacturer’s protocol. One microliter of cDNA per reaction was used for further PCR analysis.
Real-Time PCR.
Mouse IL1 β, IL6, PGRP, CXCL2, and CCL2 mRNA expression was determined using the Quantitec SYBR PCR Master Mix (Qiagen) and was expressed relative to 18S RNA levels as internal control. The amplification protocol consisted of a denaturation step at 95 °C for 7 min, followed by 40–45 cycles with a 95 °C denaturation step for 15 s, an annealing step at 60 °C for 20 s, and the extension step at 72 °C for 15 s. The expression of all genes was determined using Bio-Rad CFX 384 Real-Time System (Bio-Rad) and was analyzed using the comparative CT method (29). Primer sequences were adopted from Kanczkowski et al. (14, 30).
Statistical Analysis.
Data were evaluated by t test for independent samples (Fig. 1 C and D) or with two-way ANOVA (Figs. 2–4 and Fig. S1) using the GraphPad Prism program version 5.03 (GraphPad Software Inc.). P values of less than 0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank Uta Lehnert for help with experiments involving RT-PCR. This work was supported by Deutsche Forschungsgemeinschaft Grant KFO 252 “Microenvironment of the Adrenal” (to S.R.B. and K.Z.). Studies in Miami were supported by the Medical Research Service of the Veterans Affairs Department and the University of Miami Miller School of Medicine Department of Medicine (A.V.S.) and the Austin Weeks Family Endowment for Urologic Research (N.L.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1313945110/-/DCSupplemental.
References
- 1.Angus DC, Wax RS. Epidemiology of sepsis: An update. Crit Care Med. 2001;29(7) Suppl:S109–S116. doi: 10.1097/00003246-200107001-00035. [DOI] [PubMed] [Google Scholar]
- 2.Bornstein SR. Predisposing factors for adrenal insufficiency. N Engl J Med. 2009;360(22):2328–2339. doi: 10.1056/NEJMra0804635. [DOI] [PubMed] [Google Scholar]
- 3.Annane D, et al. Diagnosis of adrenal insufficiency in severe sepsis and septic shock. Am J Respir Crit Care Med. 2006;174(12):1319–1326. doi: 10.1164/rccm.200509-1369OC. [DOI] [PubMed] [Google Scholar]
- 4.Beishuizen A, Thijs LG. Endotoxin and the hypothalamo-pituitary-adrenal (HPA) axis. J Endotoxin Res. 2003;9(1):3–24. doi: 10.1179/096805103125001298. [DOI] [PubMed] [Google Scholar]
- 5.Gosselin D, Rivest S. MyD88 signaling in brain endothelial cells is essential for the neuronal activity and glucocorticoid release during systemic inflammation. Mol Psychiatry. 2008;13(5):480–497. doi: 10.1038/sj.mp.4002122. [DOI] [PubMed] [Google Scholar]
- 6.Tichomirowa M, et al. Bacterial endotoxin (lipopolysaccharide) stimulates interleukin-6 production and inhibits growth of pituitary tumour cells expressing the toll-like receptor 4. J Neuroendocrinol. 2005;17(3):152–160. doi: 10.1111/j.1365-2826.2005.01286.x. [DOI] [PubMed] [Google Scholar]
- 7.Kanczkowski W, Zacharowski K, Wirth MP, Ehrhart-Bornstein M, Bornstein SR. Differential expression and action of Toll-like receptors in human adrenocortical cells. Mol Cell Endocrinol. 2009;300(1-2):57–65. doi: 10.1016/j.mce.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 8.Bornstein SR, et al. Impaired adrenal stress response in Toll-like receptor 2-deficient mice. Proc Natl Acad Sci USA. 2004;101(47):16695–16700. doi: 10.1073/pnas.0407550101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zacharowski K, et al. Toll-like receptor 4 plays a crucial role in the immune-adrenal response to systemic inflammatory response syndrome. Proc Natl Acad Sci USA. 2006;103(16):6392–6397. doi: 10.1073/pnas.0601527103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rolls A, et al. Toll-like receptors modulate adult hippocampal neurogenesis. Nat Cell Biol. 2007;9(9):1081–1088. doi: 10.1038/ncb1629. [DOI] [PubMed] [Google Scholar]
- 11.Lambert-Langlais S, et al. A transgenic mouse line with specific Cre recombinase expression in the adrenal cortex. Mol Cell Endocrinol. 2009;300(1-2):197–204. doi: 10.1016/j.mce.2008.10.045. [DOI] [PubMed] [Google Scholar]
- 12.Kühn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269(5229):1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- 13.Ogimoto K, Harris MK, Jr, Wisse BE. MyD88 is a key mediator of anorexia, but not weight loss, induced by lipopolysaccharide and interleukin-1 beta. Endocrinology. 2006;147(9):4445–4453. doi: 10.1210/en.2006-0465. [DOI] [PubMed] [Google Scholar]
- 14.Kanczkowski W, et al. Role of the endothelial-derived endogenous anti-inflammatory factor Del-1 in inflammation-mediated adrenal gland dysfunction. Endocrinology. 2013;154(3):1181–1189. doi: 10.1210/en.2012-1617. [DOI] [PubMed] [Google Scholar]
- 15.Engström L, et al. Systemic immune challenge activates an intrinsically regulated local inflammatory circuit in the adrenal gland. Endocrinology. 2008;149(4):1436–1450. doi: 10.1210/en.2007-1456. [DOI] [PubMed] [Google Scholar]
- 16.Webster JI, Sternberg EM. Role of the hypothalamic-pituitary-adrenal axis, glucocorticoids and glucocorticoid receptors in toxic sequelae of exposure to bacterial and viral products. J Endocrinol. 2004;181(2):207–221. doi: 10.1677/joe.0.1810207. [DOI] [PubMed] [Google Scholar]
- 17.Derijk R, et al. Selective depletion of macrophages prevents pituitary-adrenal activation in response to subpyrogenic, but not to pyrogenic, doses of bacterial endotoxin in rats. Endocrinology. 1991;129(1):330–338. doi: 10.1210/endo-129-1-330. [DOI] [PubMed] [Google Scholar]
- 18.Mazzocchi G, Gottardo G, Nussdorfer GG. A local immuno-endocrine interaction may mediate rat adrenal glucocorticoid response to bacterial endotoxins. Life Sci. 1998;62(19):1783–1787. doi: 10.1016/s0024-3205(98)00140-4. [DOI] [PubMed] [Google Scholar]
- 19.Takeuchi O, et al. Cellular responses to bacterial cell wall components are mediated through MyD88-dependent signaling cascades. Int Immunol. 2000;12(1):113–117. doi: 10.1093/intimm/12.1.113. [DOI] [PubMed] [Google Scholar]
- 20.Vakharia K, Hinson JP. Lipopolysaccharide directly stimulates cortisol secretion by human adrenal cells by a cyclooxygenase-dependent mechanism. Endocrinology. 2005;146(3):1398–1402. doi: 10.1210/en.2004-0882. [DOI] [PubMed] [Google Scholar]
- 21.Chakravarty S, Herkenham M. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J Neurosci. 2005;25(7):1788–1796. doi: 10.1523/JNEUROSCI.4268-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Behrens A, et al. Impaired postnatal hepatocyte proliferation and liver regeneration in mice lacking c-jun in the liver. EMBO J. 2002;21(7):1782–1790. doi: 10.1093/emboj/21.7.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Judd AM, et al. (2000) Possible function of IL-6 and TNF as intraadrenal factors in the regulation of adrenal steroid secretion. Ann N Y Acad Sci 917:628–637. [DOI] [PubMed]
- 24.Marx C, Ehrhart-Bornstein M, Scherbaum WA, Bornstein SR. Regulation of adrenocortical function by cytokines—relevance for immune-endocrine interaction. Horm Metab Res. 1998;30(6-7):416–420. doi: 10.1055/s-2007-978907. [DOI] [PubMed] [Google Scholar]
- 25.Bornstein SR, et al. The role of toll-like receptors in the immune-adrenal crosstalk. Ann N Y Acad Sci. 2006;1088:307–318. doi: 10.1196/annals.1366.027. [DOI] [PubMed] [Google Scholar]
- 26.Ehrhart-Bornstein M, Hinson JP, Bornstein SR, Scherbaum WA, Vinson GP. Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr Rev. 1998;19(2):101–143. doi: 10.1210/edrv.19.2.0326. [DOI] [PubMed] [Google Scholar]
- 27.Fantuzzi G, et al. Effect of endotoxin in IL-1 beta-deficient mice. J Immunol. 1996;157(1):291–296. [PubMed] [Google Scholar]
- 28.Lenczowski MJ, Van Dam AM, Poole S, Larrick JW, Tilders FJ. Role of circulating endotoxin and interleukin-6 in the ACTH and corticosterone response to intraperitoneal LPS. Am J Physiol. 1997;273(6 Pt 2):R1870–R1877. doi: 10.1152/ajpregu.1997.273.6.R1870. [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Kanczkowski W, et al. Characterization of the LPS-induced inflammation of the adrenal gland in mice. Mol Cell Endocrinol. 2013;371(1-2):228–235. doi: 10.1016/j.mce.2012.12.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.