Abstract
Humans are an exceptionally cooperative species, but there is substantial variation in the extent of cooperation across societies. Understanding the sources of this variability may provide insights about the forces that sustain cooperation. We examined the ontogeny of prosocial behavior by studying 326 children 3–14 y of age and 120 adults from six societies (age distributions varied across societies). These six societies span a wide range of extant human variation in culture, geography, and subsistence strategies, including foragers, herders, horticulturalists, and urban dwellers across the Americas, Oceania, and Africa. When delivering benefits to others was personally costly, rates of prosocial behavior dropped across all six societies as children approached middle childhood and then rates of prosociality diverged as children tracked toward the behavior of adults in their own societies. When prosocial acts did not require personal sacrifice, prosocial responses increased steadily as children matured with little variation in behavior across societies. Our results are consistent with theories emphasizing the importance of acquired cultural norms in shaping costly forms of cooperation and creating cross-cultural diversity.
Keywords: development, population differences, gene-culture coevolution
Human cooperation poses one of the great problems of the human sciences (1). Although all human groups are generally more cooperative than other primates (2), the extent and scale of cooperation varies across societies, behavioral domains, and through historical time (3). Evolutionary researchers generally agree that kinship and reciprocity have shaped our inclinations to help others in important ways, but disagree about whether these evolutionary processes are sufficient to explain the levels of cooperation in contemporary human societies or to account for the diversity in cooperation across societies (4). Some researchers have argued that the psychological mechanisms that underlie our concern for the welfare of others and motivate helpful (prosocial) behavior have been shaped by an interaction between genes and culture (3–7). In this account, gene–culture coevolution has shaped social norms, which are acquired during development, and subsequently influence individual behavior and social preferences in ways that affect the relative success of competing social groups (3).
Many models predict that there will be substantial variation in prosocial behavior across social groups, but gene–culture coevolutionary models make two distinctive predictions about this variation: (i) variation in prosocial behavior across groups will be more pronounced when the costs of cooperation (and incentives to defect) are higher, and (ii) this variation will emerge as children begin to acquire the social norms of their communities. To investigate these predictions, we conducted a study of 3- to 14-y-old children’s prosocial choices in six different societies that collectively capture a substantial amount of human environmental and cultural variation. We demonstrate that population differences in costly prosocial behavior (but not noncostly prosociality) first emerge in middle childhood, suggesting that this is when children become sensitive to cultural influences that modulate their willingness to provide costly help to others.
There is substantial variation in the levels of cooperation across societies, and this is reflected in people’s willingness to share monetary rewards with others and incur monetary costs to punish unfair behavior (5–10). Gene–culture coevolution predicts that population-level variation in sharing and punishment is linked to demographic and economic variables. Consistent with these predictions, individuals from larger societies are more willing to punish stinginess, and members of world religions and more market-oriented groups are more willing to share (6, 9). These relationships may be the result of selection for social norms and institutions (i.e., sets of norms) that maintain cooperation even under conditions in which cooperation has high costs and is thus fragile, as in large societies where many interactions are ephemeral and there are many opportunities for exchange among strangers or anonymous others (6, 9, 11). However, it is unlikely that cultural evolutionary forces generate identical norms and institutions across all societies. Instead, selective forces acting on cultural beliefs and motivations should maintain differences in cooperative behavior across populations that vary in how costly cooperation is used to overcome ecological and economic challenges. An important implication of this is that gene–culture coevolution predicts more systematic population-level variation in prosociality in contexts in which the costs of cooperation are relatively high, and less variation in contexts in which the costs of a prosocial act are lower.
It is not yet clear how population differences in prosocial behavior emerge during the life span. Many developmental studies suggest that prosocial behavior increases substantially across childhood (12, 13), but these findings are largely based on children in Western societies or urban settings [(14–18), but see refs. 19–21 for some samples from small-scale non-Western societies). This narrow focus constrains our ability to assess the extent of variation in children’s prosocial behavior across populations and to examine the relationship between the cost of helping and the development of population differences in prosocial behavior. To overcome these limitations, we conducted a set of simple choice tasks in which children had the opportunity to deliver benefits to peers, but the personal costs of delivering benefits varied across trials. We conducted these studies with 326 3- to 14-y-old children in six different populations (Table 1; see SI Appendix, Appendix 2 for age distributions), and 120 adults from five of the six populations. Participants were not immediate family members. This sample includes nomadic hunter-gatherers living in the Congo Basin (Aka), one of the last remaining societies of this kind, as well as seminomadic agro-pastoralists from Namibia (Himba), slash and burn horticulturalists from Amazonia (Shuar), sedentized foragers from Australia (Martu), marine forager-horticulturalists from Melanesia (Yasawa Island, Fiji), and urban Americans (Los Angeles). We investigated the effects of age and population of origin on children’s responses when they were presented with opportunities to provide benefits to others.
Table 1.
Populations in the current study
| Population | Location |
N (female) |
Primary subsistence | |
| Children | Adults | |||
| Los Angeles | United States | 75 (34) | 28 (23) | Urban |
| Yasawa Island | Fiji | 75 (33) | 25 (10) | Horticulture, marine foraging |
| Aka | Central African Republic | 35 (13) | 10 (6) | Hunting/gathering |
| Himba | Namibia | 82 (48) | 32 (19) | Pastoralism, horticulture |
| Shuar | Ecuador | 37 (13) | 25 (7) | Horticulture |
| Martu | Australia | 22 (10) | — | Hunting/gathering |
See SI Appendix, Appendix 2 for additional details about age distributions and demographics across samples.
We used a standardized task to assess the generosity of a subject actor when it was personally costly for them to deliver rewards to a peer recipient [costly sharing game (CSG)] and when it cost them nothing to confer benefits on recipients [prosocial game (PG)]. This task was based on methods used by Thompson et al. (22) and Fehr et al. (23), along with several other studies (24–27). Following previous work on chimpanzees (28, 29) and children (24, 26), we included a social condition in which children were paired with a familiar peer in a face-to-face interaction and an asocial condition in which no recipient was present, and rewards were not allocated to anyone except for the actor (see Materials and Methods and SI Appendix, Appendix 1 for details and SI Appendix, Appendix 1 for variations in methods across sites). If participants were prosocial, they were expected to deliver more rewards in the social condition than the asocial condition.
Both the asocial and social conditions included the same four choice tasks: two familiarization (FAM) tasks followed by two test tasks. In each of the four choice tasks, actors were presented with a pair of different payoff options to choose between (option 1 and option 2), where each pair differed in the distribution of payoffs to the actor and recipient.
In the test trials, actors chose between a prosocial outcome and a selfish outcome. In the CSG (a simplified dictator game), actors chose between one option that provided two real and visible food rewards to themselves and nothing to their partner (2/0) and a second option that provided one reward to themselves and one reward to their partner (1/1). In the PG, actors chose between one option that provided one reward for themselves and nothing for their partner (1/0) and another that provided one reward to both themselves and their partners (1/1).
The FAM trials were designed to provide actors with the experience of recipients obtaining a reward and the experience of recipients obtaining nothing. In FAM1, actors chose between one option that provided two rewards to both themselves and to their partner (2/2) and a second option that provided one reward to themselves and one reward to their partner (1/1). In FAM2, actors chose between one option that provided two rewards for themselves and nothing for their partner (2/0) and another option that provided one reward to themselves and nothing to their partners (1/0). Thus, after both FAM trials, actors had seen a recipient obtain a reward in one trial and not obtain a reward in another trial.
We can use actors’ choices in the FAM1 social condition as a measure of participants’ comprehension of the basic choice task by investigating the development of children’s tendency to select 2/2 (the income-maximizing outcome) over 1/1. The other FAM trials (FAM1 asocial, FAM2 asocial, and FAM2 social) cannot be used for such an analysis because they are potentially confounded by inequity aversion. Children who understand our choice task might choose 2/2 or 2/0 on these trials because it maximizes their income, but they might also choose 1/1 or 1/0 because it minimizes inequity between themselves and the recipient. Due to this, we focus only on FAM1 social in our analyses because it is not confounded in this way (see SI Appendix, Appendix 7 for more details and an analysis of all FAM trials).
Results
Unlike chimpanzees tested in a similar version of the PG (28, 29), children and adults across societies differentiated between the asocial and social conditions in both the PG and CSG. In the PG, children chose the prosocial outcome (1/1) more in the social condition when another child was present to receive rewards [mean number of trials (SE) = 0.60 (0.03)] than in the asocial condition when no one was present to receive rewards [mean (SE) = 0.51 (0.03)]. In the CSG, the likelihood of choosing the prosocial option (1/1) over the selfish option (2/0) was lower than the likelihood of choosing the prosocial option in the PG, reflecting the increased cost of prosocial behavior in this game. However, in the CSG, children still chose the prosocial outcome more in the social condition [mean (SE) = 0.34 (0.03)] than in the asocial condition [mean (SE) = 0.22 (0.02)]. Similarly, adults also selected 1/1 more frequently in the PG social [mean (SE) = 0.73 (0.08)] than in the PG asocial [mean (SE) = 0.43 (0.09)] and more frequently in the CSG social [mean (SE) = 0.57 (0.09)] than the CSG asocial [mean (SE) = 0.33 (0.08)] (see SI Appendix, Appendix 3 for analysis of the effect of condition and population membership on adults’ behavior).
To model the effects of children’s age on the likelihood of choosing the prosocial option in the CSG and PG (and the income-maximizing option in FAM1), we centered participants’ ages to create an age parameter called centered age (CA) and created a second age parameter by squaring CA (CA2; see Materials and Methods for details). Including both CA and CA2 permits our regression models to create either monotonic or nonmonotonic age functions, allowing for a range of developmental trajectories.
To investigate whether the development of children’s choices in the CSG and PG varied across our conditions (social and asocial) and populations, we compared how well these choices were fit by a set of generalized linear multilevel logistic regression models (Table 2). These models either included a single age function (CA and CA2) for all populations and both conditions (model A), separate age functions for the social and asocial conditions but collapsed across populations (model B), separate age functions for each population but collapsed across conditions (model C), or separate age functions for each condition for each population (model D). We also investigated whether the development of children’s choices in FAM1 social varied across populations and thus focus on models A and C for this task.
Table 2.
Multilevel logistic regression models
| Model | Parameters included | Hypothesis | CSG [DIC (weight)] | PG [DIC (weight)] | FAM1 social [DIC (weight)] |
| A | FE: CA, CA2 | One overall developmental trajectory | 765.12 (0.00) | 904.61 (0.06) | 321.53 (0.05) |
| RE: Actor ID | |||||
| B | FE: CA, CA2, condition, CA × condition, CA2 × condition | One trajectory for each condition | 746.86 (0.00) | 899.10 (0.94) | |
| RE: Actor ID | |||||
| C | FE: CA, CA2 | One trajectory for each population | 747.30 (0.00) | 914.97 (0.00) | 315.58 (0.95) |
| RE: Actor ID | |||||
| Population | CA, CA2 | |||||
| D | FE: CA, CA2, condition, CA × condition, CA2 × condition | One trajectory for each condition, for each population | 734.49 (1.00) | 916.52 (0.00) | |
| RE: Actor ID | |||||
| Population | CA, CA2, condition, CA x condition, CA2 x condition |
This table includes details about the fixed effect (FE) and random effect (RE) parameters included in each model, the developmental hypothesis reflected in each model, and the DIC and DIC weight values associated with each model when it is applied to the CSG, PG, and FAM1 social. Best-fit models are in bold.
All models for the CSG and PG include random effects for actor identity (actor ID) to compensate for the fact that each actor contributed two observations (one from each of the social and asocial conditions). Where we modeled age functions for each condition, we included a fixed effect parameter for condition (social coded as 1; asocial as 2). As we focused only on the social conditions for FAM1, we did not consider models that included the condition parameter (models B and D), nor did we include actor ID as a random effect.
Where we modeled age functions for each population, we included population as a random effect, which estimates parameters for each population in relation to our entire dataset (rather than just the observations from that sample). By using a random effect analysis to estimate parameters for each population using the entire dataset, these models are robust to variation in sample sizes across populations, and they also provide a conservative test of population differences by tending to shrink estimates for each population toward the grand mean of the data.
We determined model fit using deviance information criterion (DIC) (30) and DIC weights, which adds a more appropriate penalty to the deviance of multilevel models than does Akaike information criterion (AIC) (31). DIC estimates the out-of-sample prediction error of a model by penalizing a model for its flexibility in fitting. As a result, smaller values of DIC indicate better-expected out-of-sample predictions (i.e., better predictions about new participants from our populations), and complex models must overcome a large penalty to be deemed better than simpler models. DIC weight is a transformation of DIC that can be thought of as the probability that an individual model is the best out of the set of models being considered. These values allow a group of models to be compared rather than requiring that individual models be accepted or rejected, and they permit the comparison of models with different structures without the concern that more complex models might appear better due to overfitting.
Model B has a DIC weight of 0.94 for the PG, meaning it has a 0.94 probability of being the best model for this task. This model indicates that development very likely differed across the social and asocial conditions but that differences across populations were likely small. In contrast, model D is the best fit for the CSG, indicating that development likely varied substantially across the social and asocial conditions and also across our six populations. Model C (without including actor ID as a random effect) had a 0.95 probability of being the best model for FAM1 social, suggesting that here, too, development varied substantially across populations. We obtain the same best-fit models when we include actor sex as a covariate in our regression models and when we focus only on children 5–10 y of age (SI Appendix, Appendix 4, Table S4b).
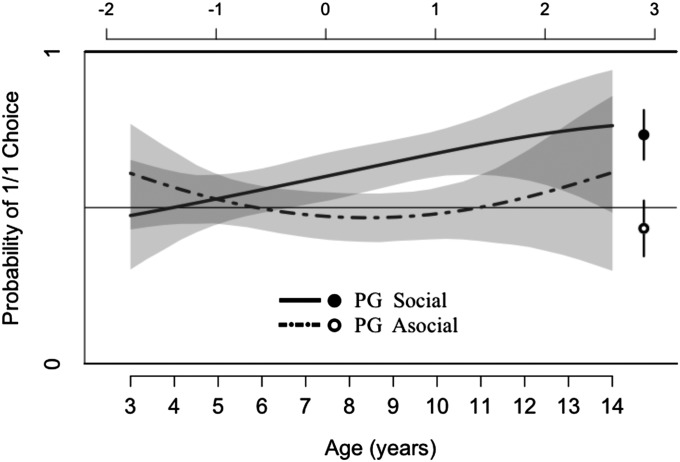
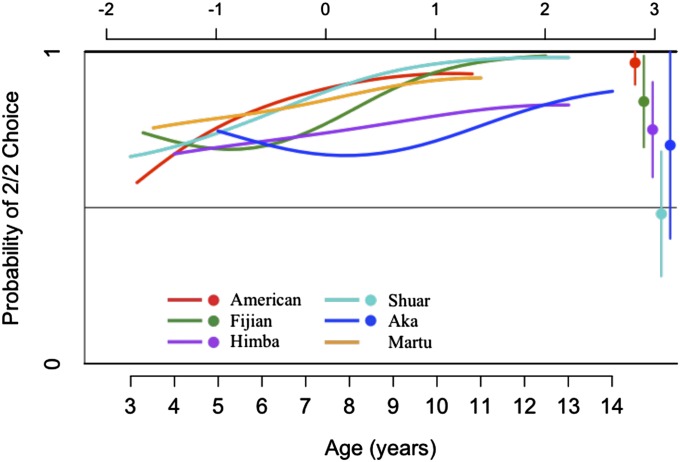
Fig. 1 plots how model B estimates the probability that children select the 1/1 outcome in the PG as a function of age, for both the social and asocial conditions. Fig. 2 plots how model D estimates the probability that children from different populations select the 1/1 outcome in the CSG as a function of age, for both the social condition (Fig. 2A) and the asocial condition (Fig. 2B). Fig. 3 plots how model C estimates the probability that children from different populations select the 2/2 outcome in FAM1 social as a function of age. Fully specified models used to plot Figs. 1–3 are provided in SI Appendix, Appendix 4.
Fig. 1.
Best-fit model of actors’ choices of 1/1 in the PG. Vertical axis is the estimated probability that children will choose the prosocial (1/1) outcome. Bottom horizontal axis is children’s age (in years), and top horizontal axis is the equivalent value of CA. Age functions capture the estimated probability that children will select the 1/1 outcome as a function of age, with estimates extracted from the best-fit model for the PG (Model B, Table 2) for both the social condition (solid line) and the asocial condition (dotted line). Wide CIs above age 13 are due to small samples above this age. The dot and hollow circle on the right side of the plot reflects the proportion of 1/1 choices actually made by adults in the PG social and PG asocial (respectively). The lines above and below the dot and circle correspond to 95% CIs. See SI Appendix, Appendix 4 for models and a comparable plot for the CSG.
Fig. 2.
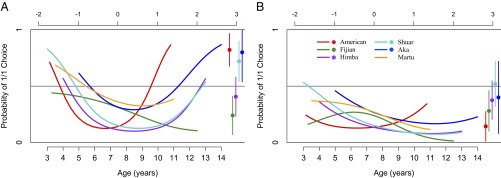
Best-fit model of actors’ choices of 1/1 in the CSG. Vertical axis is the estimated probability that children will choose the prosocial (1/1) outcome. Bottom horizontal axis is children’s age (in years), and top horizontal axis is the equivalent value of CA. Colored lines represent estimates for child participants’ choices in each population. Dots on the right side of the plot represent data from adults’ actual choices in these populations, and lines above and below the dots correspond to 95% CIs. A plots choices in the CSG social, whereas B plots choices from the CSG asocial. Estimates are all extracted from the best-fit model for the CSG (Model D, Table 2). See SI Appendix, Appendix 4 for models and SI Appendix, Appendix 6 for CIs.
Fig. 3.
Best-fit model of actors’ choices of 2/2 in FAM1 social. Vertical axis is the estimated probability that children will choose the income-maximizing (2/2) outcome. Bottom horizontal axis is children’s age (in years), and top horizontal axis is the equivalent value of CA. Colored lines represent the age functions for child participants’ choices in each population. Dots on the right side of the plot represent data from adults’ actual choices in these populations, and lines above and below the dots correspond to 95% CIs. Estimates for each population were extracted from the best-fit model for FAM1 social (Model C, Table 2). See SI Appendix, Appendix 4 for models, and SI Appendix, Appendix 7 for CIs.
Figs. 1–3 reveal a number of important developmental patterns. Fig. 1 depicts the effects of condition in the PG, showing that across all populations there is a general tendency for children to select 1/1 more frequently in the social condition than in the asocial condition and that this effect of condition increases with age. In the PG asocial, the probability of selecting 1/1 doesn’t deviate from 0.5 (chance) across the entire age range, whereas in the PG social, the probability of 1/1 choices increases monotonically across the age range, rising above the probability of 1/1 choices in the PG asocial by about age 6–7.
For the CSG, our model selection procedure indicates that we must consider both condition and population to fully understand children’s behavior, and Fig. 2 plots separate age functions for each population in both conditions. In the CSG social (Fig. 2A) there is considerable conformity across populations before middle childhood, with children in all groups becoming less likely to select the 1/1 outcome up until about middle childhood. After this point, the societies begin to diverge, as children in some groups become relatively more likely to select 1/1. The extent of the increase in prosociality among older children varies considerably across populations, but the model’s predictions about the behavior of older children roughly corresponds to the actual behavior of adults from the same population in the same task (colored dots on the right side of Fig. 2A). Variation across groups in adults’ behavior in the CSG also matches population-level variation in anonymous dictator games (6), and this suggests that children’s behavior in the CSG reflects the same kinds of preferences that are captured by other allocation games with adults and children (SI Appendix, Appendix 8). See SI Appendix, Appendix 5 for additional discussion and analyses showing that these effects are not due to small samples and for a version of Fig. 2A that provides means and SEs for children’s choices for each population across three age groups (3–5 y, 6–8 y, 9–14 y).
In the CSG asocial (Fig. 2B), across populations, the probability of selecting 1/1 is below 0.5 for almost the entire age range, and either drops or remains generally flat as age increases. There are some differences in the shape of the age functions across populations, but they are qualitatively similar in that they reveal a common tendency to choose 2/0 across our age range, a pattern that starkly contrasts both with the greater likelihood of 1/1 choices in the CSG social and with the substantial population variation in the development of children’s likelihood of selecting 1/1 (Fig. 2A).
Fig. 3 depicts the probability of children’s choices of 2/2 (the income-maximizing option) in FAM1 social, which is our proxy measure of children’s comprehension of the task. There are some differences in the age trajectories across societies, but the overall pattern is that children are likely to select 2/2, and this likelihood increases with age. Looking across groups, children show systematic preferences for 2/2 over 1/1 by about age 5 (see SI Appendix, Appendix 7 for details and CIs). Thus, we assume that children across societies understand this task by at least this age, and when we repeat our model selection procedure with those subjects who selected 2/2 in FAM1 social, we obtain the same qualitative patterns as obtained from the full sample (SI Appendix, Appendix 4, Table S4b). We also note that because our methods use face-to-face interactions and the immediate distribution of real food rewards, our task is likely more easily understood by children than tasks that use participants that are anonymous or present only in photographs.
These analyses point to four important developmental patterns. First, in the CSG, children in all six populations are relatively unlikely to choose the prosocial option as they approach middle childhood. Second, beginning in middle childhood participants in the CSG show population-specific developmental shifts toward adult levels of prosocial behavior in their own groups. Third, in the PG children from all populations show a common shift toward more prosocial behavior with age, but there is markedly less variation across populations in the development of prosocial behavior when generosity is less costly (PG) relative to when it is more costly (CSG). Fourth, there is evidence that in all six societies, children understand the choice task substantially before middle childhood and the emergence of population variation.
The steep decline in prosocial behavior in the CSG social among children before middle childhood in all six populations (Fig. 2A; SI Appendix, Appendix 6, Fig. S4g) suggests a developmental trend toward progressively stronger preferences for self-interested outcomes as children approach middle childhood. This pattern is somewhat surprising because most studies of prosocial behavior indicate that children become more generous as they mature (12, 13). Thus, it is important to consider the possibility that this pattern is the product of young children’s confusion about the task or the relative value of the two options that they were presented with. Our analysis of data from FAM1 social suggests that children understood the choice task by at least age 5, but they may have understood the task even earlier. If our youngest subjects were confused, they should have made similar choices in the asocial and social conditions. However, there is some evidence that children younger than about 6 y of age (n = 100; 3.0–5.96 y) were more likely to select 1/1 in the CSG social condition than in the CSG asocial condition (see SI Appendix, Appendix 6, Fig. S6 a–g for age functions with CIs). By showing that children discriminated the conditions, this result suggests that our youngest participants understood the choice task, and it implies that the developmental trajectory we observe before middle childhood reflects a shift in children’s preferences away from outcomes that benefit others and toward outcomes that benefit themselves.
Discussion
These patterns have important implications for understanding the ontogeny of prosocial behavior and for the study of child development more broadly. Our results suggest that the prosocial behavior of young children may develop through a different process than does the prosocial behavior of older children. This finding highlights the importance of considering nonmonotonic developmental patterns, which have been documented for a number of behaviors and cognitive competencies (32). Very young children are certainly shaped by social learning, but the similarity in the prosocial behavior of our youngest subjects across the very diverse populations we studied suggests that social learning in early childhood does not shape prosocial behavior in a population-specific manner. However, during middle childhood the development of prosociality begins to diverge along population lines, suggesting that children are beginning to become sensitive to society-specific information about how to behave in costly cooperative situations.
The fact that children begin to show increasing rates of prosocial behavior in middle childhood in the CSG social is consistent with evidence from relatively similar studies showing that Swiss (23) and American (33) children become substantially more averse to inequity after 7–8 y of age. This increasing shift toward egalitarianism in Western children beginning at age 7–8 is consistent with our sample of children from Los Angeles but is not as consistent with samples from several of our non-Western populations. This pattern supports the idea that variation in egalitarian motives underlies some of the population-based variation in prosocial behavior in these games. Interestingly, in a recent study of American children, 3–4 y olds reported that both they and others should distribute payoffs equally in a dictator game, yet children in the study failed to actually do so until about 7–8 y of age (34). This finding suggests that middle childhood may be when children begin to conform to cooperative social norms, even if they may have learned these norms years prior. Overall, the timing of the shift in the developmental trajectory of prosocial behavior is consistent with claims that middle childhood―a period with unique features in humans that begins around age 6 and ends with sexual maturity (35)―is an important developmental stage across human societies in which children are incorporated into the larger cultural community outside their households (36). This period would therefore be a particularly important time during development for individuals to conform to local social norms.
Group-specific differences emerged in the CSG but not the PG, suggesting that population-specific influences on the development of prosocial behavior are most pronounced when prosocial outcomes are costly. This result fits predictions from gene–culture coevolutionary models, which hypothesize that social norms and institutions will be most influential when group beneficial behavior is costly and therefore more difficult to maintain. Further work should explore specific cultural beliefs and institutions that influence cooperative behavior and how their acquisition and application shapes children’s behavior across development.
Previous work on the ontogeny of prosocial behavior in Western subjects has suggested a trajectory of increasingly prosocial behavior throughout childhood. By tracing the ontogeny of prosociality across a wide age range and in diverse populations, our study shows that this picture is incomplete in several important respects and suggests a more complex role of culture in the ontogeny of prosocial behavior. Although there is an important phase of prosocial development before middle childhood that appears to be largely independent of society-specific information, it is one characterized by low and perhaps decreasing rates of costly prosociality in our choice task. Beginning in middle childhood, costly prosociality generally increases but the extent of the increase is highly variable and moves toward population-specific levels of mature adult prosociality, developmental diversity that conforms to a distinctive set of predictions derived from gene–culture coevolution models. We note that in daily life there is substantial cooperation in all of these groups, and our study likely does not capture all of the factors that influence prosociality (e.g., institutions, social norms, evolved biases). We caution that behavior in this study may not be sufficient to predict naturalistic prosocial behavior by individuals in these groups. However, our data show that population membership is one factor that influences cooperative behavior, and this influence emerges in middle childhood.
The fact that our youngest participants (<5–6 y) were relatively more likely to engage in costly prosociality than immediately older children (7–9 y) is a surprising finding, although it is consistent with the considerable evidence that children aged 3 y and younger act prosocially (22, 37–44). Indeed, prosociality has been found as early as 25 mo of age in choice tasks similar to the one used here (26). A focused investigation of very young children’s understanding of these tasks is needed, but our primary results concerning the emergence of population variation are not tied to this issue, as our results show that task comprehension clearly precedes the emergence of population variation in prosociality.
We also note that the specific age predictions that emerge from our models should be interpreted in light of our methods. Differences between our results in the CSG and those obtained with other tasks based on the dictator game may be partly due to the fact that, unlike most of these other studies, interactions in our study were not anonymous (as this was unnecessary for our primary questions about population variation). Our subjects’ behavior could have been influenced by reciprocity, reputational concerns, and other factors.
The emergence in middle childhood of population differences in costly prosociality, together with a population-independent pattern of monotonically increasing noncostly prosociality, suggests that human prosocial behavior develops through a complex interaction with acquired local culture. Our findings contribute to ongoing discussions of the processes that underlie both uniformity and diversity in social behavior across societies, and highlight the importance of expanding the scope of developmental studies to encompass a wider range of extant human diversity. This expansion is particularly important given the growing evidence for considerable population variation in experimental studies of human behavior (45).
Materials and Methods
Setup.
Actors and recipients were seated across from one another, with a primary experimenter seated on one side. At some sites, a secondary experimenter observed from nearby. Using an apparatus based on prior studies (23), two 8.5 × 14-in. paper trays were placed on the floor between the actor and recipient (SI Appendix, Appendix 1, Fig. S1 A–C). Each tray had one red circle and one blue circle printed on it. For each trial, payoffs were placed in the circles, and the actor was permitted to choose one of the two trays. In the social condition, actors received the payoff in the circle closest to them on the tray that they chose, whereas recipients received the payoff in the circle closest to them on the same tray. In the asocial condition, when there was no recipient, actors still only received the payoff in the circle closest to them on the tray that they chose (payoffs in the other circle were retrieved by the experimenter). Payoffs were real, visible, food items and immediately edible (see SI Appendix, Appendix 1, Table S1b for rewards used across sites).
Procedure.
Each actor was presented with two FAM trials (FAM1 and FAM2) and two test trials (CSG and PG) in each of two different conditions (social and asocial), for a total of eight different trials. In the asocial condition, actors made choices without a recipient obtaining rewards (SI Appendix, Appendix 1, Fig. S1b), whereas in the social condition, a recipient was seated across from them and received rewards (SI Appendix, Appendix 1, Fig. S1c). In each condition, FAM trials were always presented before test trials. FAM trials were always presented in the same order (FAM1 then FAM2), but the side of presentation for each payoff was counterbalanced across subjects. The order of the test trials (PG and CSG) was also counterbalanced, as was the order of the two conditions (social and asocial). Before all four FAM trials, the actor was given the full set of instructions. Instructions were not given during the test trials (CSG and PG). See SI Appendix, Appendix 1 for protocols and scripts. Data were recorded live on paper datasheets by the experimenter, as video recording was not reliably available at all sites. Datasheets were coded twice (once by a researcher naïve to hypotheses), and inconsistencies in coding were resolved before analysis.
Analysis.
We used multilevel logistic regressions to analyze our binary outcome variable: whether or not participants selected the 1/1 payoff distribution in the CSG and PG or the 2/2 payoff in FAM1. We center participants’ age (PA) to create an age parameter CA, and we create a second age parameter by squaring CA:
We analyzed the data in the R Environment for Statistical Computing (46). We fit the models using Stan (47), a Hamiltonian Monte Carlo sampler. Results are based on 5,000 samples each from four chains, after 5,000 adaptation steps in each. Convergence was assessed by both trace plots and the R-hat Gelman and Rubin statistic. Model code was generated and DIC calculated using glmer2stan (48), a convenience package for Rstan. We analyzed the data using both uninformative (flat) priors, as well as weakly informative variance priors, without any substantive change in inferences.
Supplementary Material
Acknowledgments
We thank Nancy Eisenberg, Dan Fessler, Daniel Haun, Keith Jensen, Laurie Santos, Felix Warneken, and two anonymous reviewers for comments and suggestions. Thanks also to all of our participants, and to those who made our research possible at our field sites. Funding for this research was provided by a grant from the UK’s Arts and Humanities Research Council (for the Culture and the Mind project) and from the Hang Seng Centre for Cognitive Studies, University of Sheffield. J.H. thanks the Canadian Institute for Advanced Research (CIFAR). B.S.H. was supported by the Interdisciplinary Relationship Science Program funded by the National Science Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data described in this paper is available on the website of the Culture and the Mind project, at http://www.philosophy.dept.shef.ac.uk/culture&mind/Data.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221217110/-/DCSupplemental.
References
- 1.Axelrod R, Hamilton WD. The evolution of cooperation. Science. 1981;211(4489):1390–1396. doi: 10.1126/science.7466396. [DOI] [PubMed] [Google Scholar]
- 2.Silk JB, House BR. Evolutionary foundations of human prosocial sentiments. Proc Natl Acad Sci USA. 2011;108(Suppl 2):10910–10917. doi: 10.1073/pnas.1100305108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chudek M, Henrich J. Culture-gene coevolution, norm-psychology and the emergence of human prosociality. Trends Cogn Sci. 2011;15(5):218–226. doi: 10.1016/j.tics.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Richerson PJ, Boyd R. Not by Genes Alone: How Culture Transformed Human Evolution. Chicago: Univ of Chicago Press; 2005. [Google Scholar]
- 5.Henrich J, et al. “Economic man” in cross-cultural perspective: Behavioral experiments in 15 small-scale societies. Behav Brain Sci. 2005;28(6):795–815, discussion 815–855. doi: 10.1017/S0140525X05000142. [DOI] [PubMed] [Google Scholar]
- 6.Henrich J, et al. Costly punishment across human societies. Science. 2006;312(5781):1767–1770. doi: 10.1126/science.1127333. [DOI] [PubMed] [Google Scholar]
- 7.Henrich J, et al. Markets, religion, community size, and the evolution of fairness and punishment. Science. 2010;327(5972):1480–1484. doi: 10.1126/science.1182238. [DOI] [PubMed] [Google Scholar]
- 8.Herrmann B, Thöni C, Gächter S. Antisocial punishment across societies. Science. 2008;319(5868):1362–1367. doi: 10.1126/science.1153808. [DOI] [PubMed] [Google Scholar]
- 9.Marlowe FW, Berbesque JC. More ‘altruistic’ punishment in larger societies. Proc Biol Sci. 2008;275(1634):587–590. doi: 10.1098/rspb.2007.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roth AE, Prasnikar V, Okuno-Fujiwara M, Zamir S. Bargaining and market behavior in Jerusalem, Ljubljana, Pittsburgh, and Tokyo: An experimental study. Am Econ Rev. 1991;81(5):1068–1095. [Google Scholar]
- 11.Henrich J. Social science. Cooperation, punishment, and the evolution of human institutions. Science. 2006;312(5770):60–61. doi: 10.1126/science.1126398. [DOI] [PubMed] [Google Scholar]
- 12. Silk JB, House BR (2012) The phylogeny and ontogeny of prosocial behavior. The Oxford Handbook of Comparative Evolutionary Psychology, eds Vonk J, Shackelford T (Oxford University Press, New York), pp 381–397.
- 13. Eisenberg N, Fabes RA, Spinrad TL (2006) Prosocial Development. Handbook of Child Psychology, Social, Emotional, and Personality Development, eds Damon W, Lerner RM, Eisenberg N (Wiley, New York), pp 646–718.
- 14.Kärtner J, Keller H, Chaudhary N. Cognitive and social influences on early prosocial behavior in two sociocultural contexts. Dev Psychol. 2010;46(4):905–914. doi: 10.1037/a0019718. [DOI] [PubMed] [Google Scholar]
- 15.Rao N, Stewart SM. Cultural influences on sharer and recipient behavior sharing in Chinese and Indian preschool children. J Cross Cult Psychol. 1999;30(2):219–241. [Google Scholar]
- 16.Takagishi H, Kameshima S, Schug J, Koizumi M, Yamagishi T. Theory of mind enhances preference for fairness. J Exp Child Psychol. 2010;105(1–2):130–137. doi: 10.1016/j.jecp.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Stewart SM, McBride-Chang C. Influences on children’s sharing in a multicultural setting. J Cross Cult Psychol. 2000;31(3):333–348. [Google Scholar]
- 18.Madsen MC, Shapira A. Cooperation and challenge in four cultures. J Soc Psychol. 1977;102(2):189–195. [Google Scholar]
- 19.Rochat P, et al. Fairness in distributive justice by 3- and 5-year-olds across seven cultures. J Cross Cult Psychol. 2009;40(3):416–442. [Google Scholar]
- 20.Callaghan T, et al. Early social cognition in three cultural contexts. Monogr Soc Res Child Dev. 2011;76(2):vii–viii, 1–142. doi: 10.1111/j.1540-5834.2011.00603.x. [DOI] [PubMed] [Google Scholar]
- 21.Madsen MC, Lancy DF. Cooperative and competitive behavior experiments related to ethnic identity and urbanization in Papua New Guinea. J Cross Cult Psychol. 1981;12(4):389–408. [Google Scholar]
- 22.Thompson C, Barresi J, Moore C. The development of future-oriented prudence and altruism in preschoolers. Cogn Dev. 1997;12(2):199–212. [Google Scholar]
- 23.Fehr E, Bernhard H, Rockenbach B. Egalitarianism in young children. Nature. 2008;454(7208):1079–1083. doi: 10.1038/nature07155. [DOI] [PubMed] [Google Scholar]
- 24.House BR, Henrich J, Brosnan SF, Silk JB. The ontogeny of human prosociality: Behavioral experiments with children aged 3 to 8. Evol Hum Behav. 2012;33(4):291–308. [Google Scholar]
- 25.House B, Henrich J, Sarnecka B, Silk JB. The development of contingent reciprocity in children. Evol Hum Behav. 2013;34(2):86–93. [Google Scholar]
- 26.Brownell CA, Svetlova M, Nichols S. To share or not to share: When do toddlers respond to another’s needs? Infancy. 2009;14(1):117–130. doi: 10.1080/15250000802569868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore C. Fairness in children’s resource allocation depends on the recipient. Psychol Sci. 2009;20(8):944–948. doi: 10.1111/j.1467-9280.2009.02378.x. [DOI] [PubMed] [Google Scholar]
- 28.Silk JB, et al. Chimpanzees are indifferent to the welfare of unrelated group members. Nature. 2005;437(7063):1357–1359. doi: 10.1038/nature04243. [DOI] [PubMed] [Google Scholar]
- 29.Jensen K, Hare B, Call J, Tomasello M. What’s in it for me? Self-regard precludes altruism and spite in chimpanzees. Proc Biol Sci. 2006;273(1589):1013–1021. doi: 10.1098/rspb.2005.3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lunn D, Jackson C, Best N, Spiegelhalter DJ, Thomas A. The BUGS Book: A Practical Introduction to Bayesian Analysis. Boca Raton, FL: CRC Press; 2012. [Google Scholar]
- 31.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. New York: Springer Verlag; 2002. [Google Scholar]
- 32.Siegler RS. U-shaped interest in U-shaped development and what it means. J Cogn Dev. 2004;5(1):1–10. [Google Scholar]
- 33.Blake PR, McAuliffe K. “I had so much it didn’t seem fair”: Eight-year-olds reject two forms of inequity. Cognition. 2011;120(2):215–224. doi: 10.1016/j.cognition.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Smith CE, Blake PR, Harris PL. I should but I won’t: Why young children endorse norms of fair sharing but do not follow them. PLoS ONE. 2013;8(3):e59510. doi: 10.1371/journal.pone.0059510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson JL, Nelson AJ. Middle childhood and modern human origins. Hum Nat. 2011;22(3):249–280. doi: 10.1007/s12110-011-9119-3. [DOI] [PubMed] [Google Scholar]
- 36.Lancy DF, Grove MA. Getting noticed. Middle childhood in cross-cultural perspective. Hum Nat. 2011;22(3):281–302. doi: 10.1007/s12110-011-9117-5. [DOI] [PubMed] [Google Scholar]
- 37.Hay DF, Castle J, Davies L, Demetriou H, Stimson CA. Prosocial action in very early childhood. J Child Psychol Psychiatry. 1999;40(6):905–916. [PubMed] [Google Scholar]
- 38.Warneken F, Tomasello M. Varieties of altruism in children and chimpanzees. Trends Cogn Sci. 2009;13(9):397–402. doi: 10.1016/j.tics.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 39.Birch LL, Billman J. Preschool children’s food sharing with friends and acquaintances. Child Dev. 1986;57(2):387–395. [Google Scholar]
- 40.Dunfield K, Kuhlmeier VA, O’Connell L, Kelley E. Examining the diversity of prosocial behavior: Helping, sharing, and comforting in infancy. Infancy. 2011;16(3):227–247. doi: 10.1111/j.1532-7078.2010.00041.x. [DOI] [PubMed] [Google Scholar]
- 41.Warneken F. Young children proactively remedy unnoticed accidents. Cognition. 2013;126(1):101–108. doi: 10.1016/j.cognition.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 42.Svetlova M, Nichols SR, Brownell CA. Toddlers’ prosocial behavior: From instrumental to empathic to altruistic helping. Child Dev. 2010;81(6):1814–1827. doi: 10.1111/j.1467-8624.2010.01512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sommerville JA, Schmidt MFH, Yun J, Burns M. The development of fairness expectations and prosocial behavior in the second year of life. Infancy. 2013;18(1):40–66. [Google Scholar]
- 44.Vaish A, Carpenter M, Tomasello M. Sympathy through affective perspective taking and its relation to prosocial behavior in toddlers. Dev Psychol. 2009;45(2):534–543. doi: 10.1037/a0014322. [DOI] [PubMed] [Google Scholar]
- 45.Henrich J, Heine SJ, Norenzayan A. The weirdest people in the world? Behav Brain Sci. 2010;33(2–3):61–83. doi: 10.1017/S0140525X0999152X. [DOI] [PubMed] [Google Scholar]
- 46. R Development Core Team (2013) R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria)
- 47. Stan Development Team (2013) Stan: A C++ Library for Probability and Sampling, Version 1.3. Available at http://mc-stan.org/
- 48. McElreath R (2013) glmer2stan: Rstan Models Defined by glmer Formulas (Univ of California, Davis, CA)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.