Significance
The causes of the sporadic form of Alzheimer’s disease (AD) are unknown. In this study we show that copper (Cu) critically regulates low-density lipoprotein receptor-related protein 1–mediated Aβ clearance across the blood–brain barrier (BBB) in normal mice. Faulty Aβ clearance across the BBB due to increased Cu levels in the aging brain vessels may lead to accumulation of neurotoxic Aβ in brains. In a mouse model of AD low levels of Cu also influences Aβ production and neuroinflammation. Our study suggests that Cu may also increase the severity of AD.
Keywords: BBB, cerebrovascular, BACE1, toxicity, environmental
Abstract
Whereas amyloid-β (Aβ) accumulates in the brain of normal animals dosed with low levels of copper (Cu), the mechanism is not completely known. Cu could contribute to Aβ accumulation by altering its clearance and/or its production. Because Cu homeostasis is altered in transgenic mice overexpressing Aβ precursor protein (APP), the objective of this study was to elucidate the mechanism of Cu-induced Aβ accumulation in brains of normal mice and then to explore Cu’s effects in a mouse model of Alzheimer’s disease. In aging mice, accumulation of Cu in brain capillaries was associated with its reduction in low-density lipoprotein receptor-related protein 1 (LRP1), an Aβ transporter, and higher brain Aβ levels. These effects were reproduced by chronic dosing with low levels of Cu via drinking water without changes in Aβ synthesis or degradation. In human brain endothelial cells, Cu, at its normal labile levels, caused LRP1-specific down-regulation by inducing its nitrotyrosination and subsequent proteosomal-dependent degradation due in part to Cu/cellular prion protein/LRP1 interaction. In APPsw/0 mice, Cu not only down-regulated LRP1 in brain capillaries but also increased Aβ production and neuroinflammation because Cu accumulated in brain capillaries and, unlike in control mice, in the parenchyma. Thus, we have demonstrated that Cu’s effect on brain Aβ homeostasis depends on whether it is accumulated in the capillaries or in the parenchyma. These findings should provide unique insights into preventative and/or therapeutic approaches to control neurotoxic Aβ levels in the aging brain.
Copper (Cu), an essential trace element, is an integral component of cuproproteins required for many physiological functions, such as energy production, scavenging of free radicals, connective tissue production, iron mobilization, and neurotransmission (1, 2). Almost all of plasma Cu (16–20 μM) is bound to ceruloplasmin (Cp), and the remainder, non-Cp bound Cu, (labile Cu) is bound to albumin, transcuprein, peptides, and amino acids (3, 4). Excess free Cu is toxic (5, 6). Imbalance in cerebral Cu homeostasis plays a role in the pathogenesis of Alzheimer’s disease (AD), and possibly other neurodegenerative diseases (7, 8). Increased Cu levels in plasma and/or brain have been associated with AD (9–12). In phase II clinical trials, PBT2, a modified 8-hydroxyquinoline analogue, and Cu chaperone, are showing promise (13). However, further studies are needed on effects of Cu on the CNS to fully understand the benefits of this potential therapy.
Amyloid-β (Aβ) accumulates around cerebral blood vessels and in brain parenchyma in rabbits dosed with low levels of Cu (0.12 mg/L) via their drinking water and cholesterol in their chow for 10 wk (14). Similar data were obtained in beagles (15), but the mechanism is unclear. However, in transgenic (Tg) mice overexpressing amyloid-β precursor protein (APP), soluble Aβ levels in brain were reduced in APP23 mice dosed with higher Cu levels (16). In TgCRND8 mice crossed with mice that had Cu toxicities (toxic-milk mice), brain Aβ levels were also reduced (17). In Tg2576 mice, Cu levels in brain were reduced (18, 19). High levels of Cu inhibited in vitro Aβ production (20). Thus, Tg mice overexpressing APP may be unsuitable to study Cu’s normal role in Aβ homeostasis. APP expression is normal in the sporadic form of AD (21). The objective of this study was to elucidate the mechanisms of Cu-induced brain Aβ accumulation in normal young mice, so as to provide a better understanding of Cu’s role in Aβ homeostasis before exploring its role in a mouse model of AD.
Brain Aβ levels are regulated by its rate of production from APP and by its rate of clearance (22). Clearance of Aβ from brain involves enzymatic degradation by proteases, including insulin-degrading enzyme (IDE) and neprilysin (NEP) (23), bulk flow of interstitial fluid (24), and transport across the blood–brain barrier (BBB) via low-density lipoprotein receptor-related protein 1 (LRP1) (25). LRP1 is expressed in brain endothelium, and reduced expression was observed in aging rodents and in patients with AD (25, 26), but the mechanism is still unclear. Herein, we uniquely show that Cu accumulated in brain capillaries but not in the parenchyma and that this was associated with reduced levels of LRP1 and increased brain Aβ levels in the aging mouse brain. Long-term exposure to low levels of Cu reproduced these effects and specifically caused LRP1 down-regulation, due, in part, to Cu/cellular prion/LRP1 interaction, LRP1 nitrotyrosination, and its proteosomal degradation. In a mouse model of AD, unlike normal mice, Cu levels were also increased in the parenchyma and caused increased Aβ production and neuroinflammation.
Results
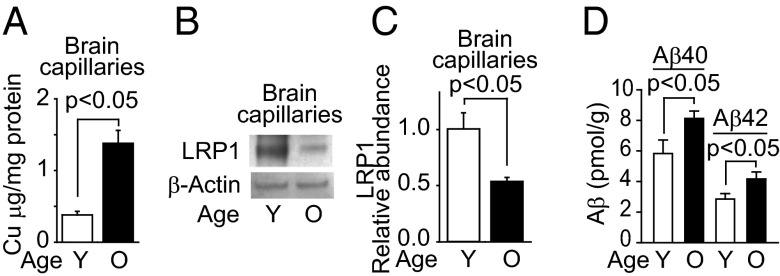
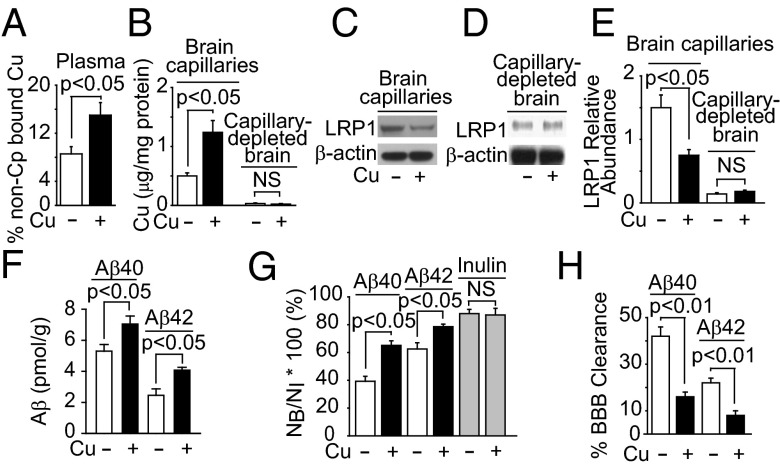
First we found a fourfold increase in Cu levels (Fig. 1A) in brain capillaries isolated from aging mice (2–3 mo old and 25–28 mo old), which was associated with a twofold decrease in LRP1 levels (Fig. 1 B and C) and a 1.5-fold increase in levels of brain endogenous Aβ40 and Aβ42 (Fig. 1D). These effects were recapitulated in mice chronically dosed via their drinking water with low levels of Cu (0.13 mg/L) for 90 d. Compared with vehicle treatment, Cu-dosed mice had a twofold increase in non-Cp bound Cu levels in plasma (Fig. 2A), a 2.6-fold increase in Cu levels in brain capillaries without significant changes in the parenchyma (Fig. 2B), a twofold reduction in LRP1 levels in brain capillaries without significant changes in the parenchyma (Fig. 2 C–E), and a 1.2- to 1.6-fold increase in brain endogenous mouse Aβ40 and Aβ42 levels, respectively (Fig. 2F). No significant change was obtained for Cu bound to >100 kDa compounds (mainly Cp) in plasma (Fig. S1A) or in brain levels of APP, IDE, or NEP (Fig. S1B). Cu increased the retention of human 125I-Aβ40 and 125I-Aβ42 in brain (Fig. 2F), due to a 2.5-fold reduction in BBB 125I-Aβ40 and 125I-Aβ42 transport into blood (Fig. 2H). There was no significant change in the retention of 14C-inulin (Fig. 2G) nor in brain capillary levels of APP, β-site APP cleaving enzyme 1 (BACE1), IDE, NEP, very low density lipoprotein receptor (VLDLR), low density lipoprotein receptor (LDLR), receptor for advanced glycation endproducts (RAGE), P-glycoprotein (Pgp), or transferrin receptor in mice treated with and without Cu (Fig. S1 C–E). As in wild-type (WT) mice, Cu reduced BBB 125I-Aβ40 transport in Ldlr−/− and Vldlr−/− mice (Fig. S1G), which have normal LRP1 levels in brain capillaries (27). In contrast, receptor-associated protein null (Rap−/−) mice, with reduced LRP1 levels (25), had a smaller effect on BBB 125I-Aβ40 transport (Fig. S1G). Sex had no effect on Cu-induced reduction in BBB transport of Aβ (Fig. S1H). No significant changes occurred in physiological parameters, such as blood pressure, heart rate, breathing rate, pH, and blood gases in mice dosed with and without Cu (Table S1). These results suggest that chronic treatment with low levels of Cu might selectively reduce LRP1 levels in brain capillaries in wild-type mice.
Fig. 1.
Aging increased Cu levels in brain capillaries and Aβ levels in mice brains. Levels of Cu (A), representative Western blot analysis of LRP1 (B), and quantification of relative LRP1 levels from data as in B (C) in isolated brain microvessels from 2- to 3-mo-old (Y) and 25- to 28-mo-old mice (O). (D) Mouse endogenous Aβ40 and Aβ42 levels in the cerebrum: 2- to 3-mo-old mice (clear column) and 25- to 28-mo-old mice (black column). β-Actin was the reference molecule for each Western blot analysis. Values are mean ± SEM, n = 5 mice per group.
Fig. 2.
Cu reduced LRP1-mediated 125I-Aβ clearance across the BBB. (A) Nonceruloplasmin (Cp) bound Cu levels in plasma. (B) Cu levels in brain capillaries and capillary depleted brain. (C and D) Representative LRP1 Western blot analysis for isolated brain capillaries and capillary-depleted brain and their relative quantification (E), (F) Mouse cerebrum endogenous Aβ40 and Aβ42 levels, (G) Brain recovery of human 125I-Aβ40, 125I-Aβ42 and 14C-inulin (gray column) 30 min after their simultaneous injection into the caudate nucleus, and (H) BBB 125I-Aβ clearance from data in G. Mice were treated with low levels of Cu (+) or vehicle (−) in their drinking water for 90 d starting at 2 mo of age. β-Actin was the reference molecule for each Western blot analysis. Values are mean ± SEM, n = 5 mice per group.
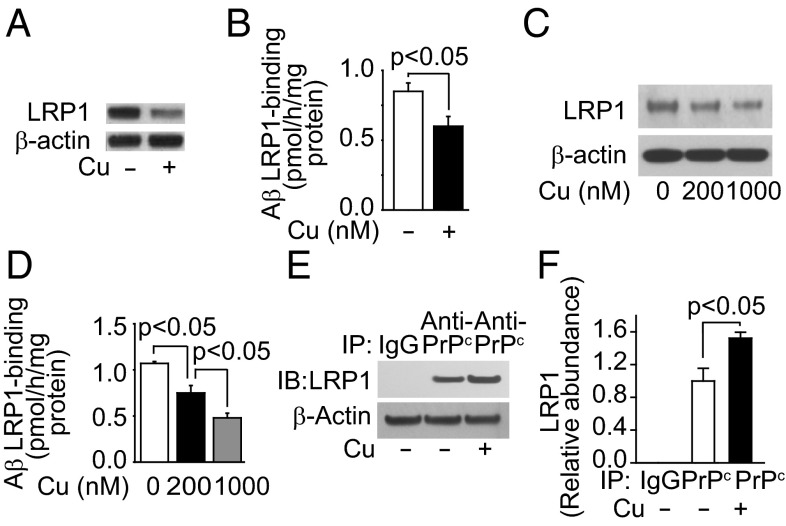
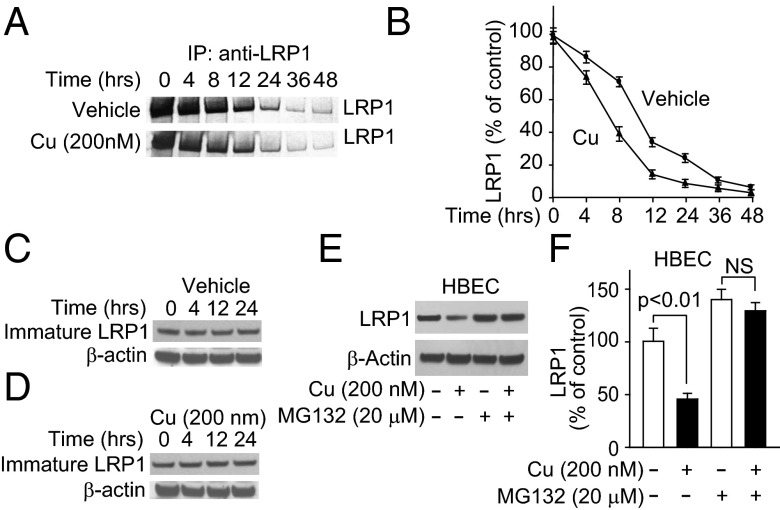
We then tested cultured primary mouse brain endothelial cells (MBECs), and showed that Cu (200 nM), at levels normally found for non–Cp-bound Cu in plasma, reduced LRP1 levels (Fig. 3A) and Aβ42 binding (Fig. 4B). Next we tested an established cultured primary human brain endothelial cells (HBECs) (25) to elucidate possible mechanisms of the Cu-mediated LRP1 down-regulation. The viability of HBECs was unaffected by Cu levels within the nanomole range (Fig. S2A). Cu induced oxidative stress in HBECs (Fig. S2 B and C). The 125I-Aβ binding to HBEC was inhibited (>70%) by anti-LRP1 antibody (25). Cu levels at 200 nM and 1 μM progressively reduced LRP1 levels (Fig. 3C) and decreased 125I-Aβ42 binding (Fig. 3D). In contrast to Cu, 200 nM Al3+, Zn2+, or Fe2+ had no effect on LRP1 levels (Fig. S2 D–F) or cell viability (Fig. S2G). We reasoned that Cu’s specific effect on LRP1 might be due, in part, to its interaction with cellular prion protein (PrPc), because Cu-bound PrPc binds LRP1 (28, 29). After incubation of HBECs with Cu, immunoprecipitated PrPc was associated with 1.5-fold increased levels of LRP1 (Fig. 3 E and F). Cu (200 nM) had no effect on the density of brain endothelial cells (Fig. S3 A and B) or on angiogenesis (Fig. S3 C and D).
Fig. 3.
Cu down-regulated LRP1 and reduced Aβ binding in brain endothelial cells. (A) Representative Western blot analysis of LRP1. (B) The 125I-Aβ42 binding in primary mouse endothelial cells treated with (+, 200 nM) and without Cu (−). (C) Representative LRP1 Western blot analysis. (D) The 125I-Aβ42 binding with (200 nM, black column; 1 μM, gray column) and without Cu (clear column) in human brain endothelial cells (HBECs). (E) Representative LRP1 immunoblotting after immunoprecipitation with IgG or antiprion antibody in the presence (200 nM) and absence of Cu. (F) Relative levels of LRP1 from data as in E. β-Actin was the reference molecule for each Western blot analysis. Values are mean ± SEM, n = 3–5 independent experiments per group.
Fig. 4.
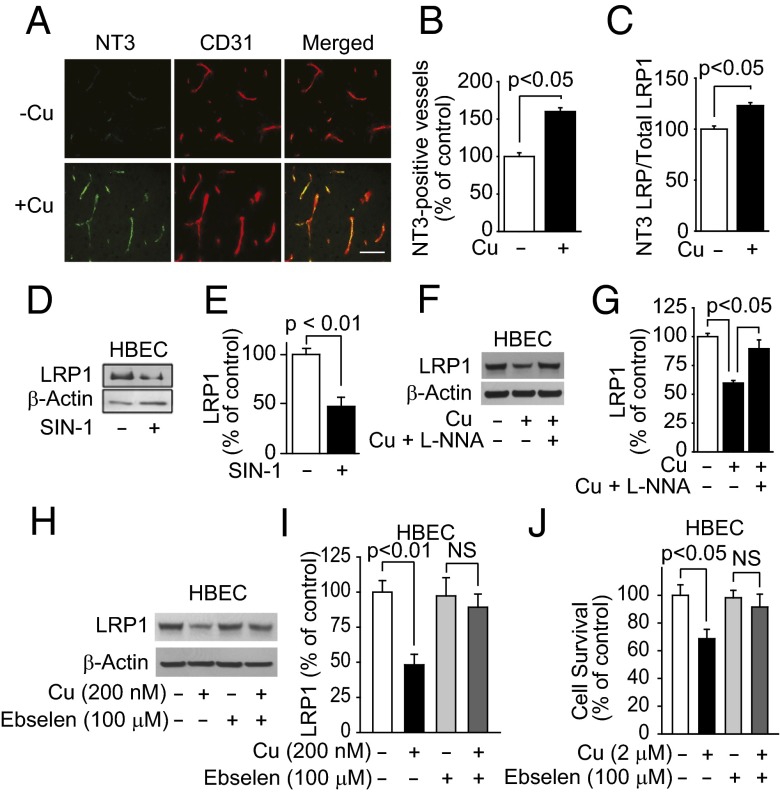
Cu increased LRP1 nitrotyrosination. (A) Representative immunostained brain sections for nitrotyrosine (NT3), CD31 (endothelial marker), and merged images in mice treated with tracer levels of Cu or vehicle in their drinking water. (Scale bar, 50 μm.) (B) Quantification of nitrotyrosine-positive vessels in A. (C) NT3-LRP1/ total LRP1 ratio in HBECs treated with (200 nM) and without Cu. (D) Representative LRP1 Western blot analysis and its quantification (E) in HBECs treated with and without nitric oxide donor (sodium nitroprusside; 1 μM). (F) Representative LRP1 Western blot analysis and its quantification (G) in HBECs treated with vehicle, Cu (200 nM), and with Cu (200 nM) and 10−4 M l-NNA (nitro-l-arginine). (H) Representative LRP1 Western blot analysis and its quantification (I) in HBECs treated with and without Cu or with ebselen, a decomposition catalyst of peroxynitrite. (J) HBEC survival with and without Cu or ebselen. β-Actin was the reference molecule for each Western blot analysis. Values are mean ± SEM, n = 4–5 independent experiment or mice per group.
We then showed that compared with vehicle, levels of oxidative stress in cerebral cortex were increased fivefold (Fig. S4 A and B) and levels of thiobarbituric acid reactive substances (TBARS) increased twofold (Fig. S4C) in mice treated with Cu. Because nitric oxide (NO) is increased in oxidative stress, brain sections were immunostained for nitrotyrosinated proteins (NT3). Mice treated with Cu had a 1.6-fold increase in NT3-positive vessels compared with vehicle treatment (Fig. 4 A and B). In HBECs, Cu caused a 1.5-fold increase in NT3 LRP1/total LRP1 ratio (Fig. 4C). Sodium nitroprusside, a nitric oxide (NO) donor, decreased LRP1 levels by 2.5-fold (Fig. 4 D and E), whereas nitro-l-arginine (l-NNA), a NO synthase inhibitor, rescued LRP1 from Cu-induced nitrotyrosination (Fig. 4 F and G). A decomposition catalyst of peroxynitrite prevented Cu-induced LRP1 reduction (Fig. 4 H and I) and Cu-induced toxicity in HBECs (Fig. 4J). Because reduction of Cu2+ to Cu1+ is associated with its toxicity (30), we demonstrated that mouse brain capillaries reduced Cu2+ to Cu1+ (Fig. S4D). The antioxidant, N-acetyl cysteine (NAC), almost completely rescued LRP1 from Cu-induced down-regulation (Fig. S5 A and B).
To establish whether Cu increased proteosomal degradation of LRP1, we performed pulse chase studies and showed that it decreased the half-life of LRP1 from 9.8 to 6.9 h (Fig. 5 A and B) without affecting the levels of immature (600 kDa) newly synthesized LRP1 (Fig. 5 C and D). MG132, a potent inhibitor of proteosomes, completely rescued the Cu-induced LRP1 down-regulation (Fig. 5 E and F). Cu did not affect LRP1 synthesis (Fig. S5C).
Fig. 5.
Cu increased LRP1 proteosomal-dependent degradation in HBECs. (A) Representative autoradiogram of [35S]methionine-labeled LRP1 in HBECs after 1-h pulse with [35S]methionine and chased for the indicated time. (B) Quantification of LRP1 levels from the data in A. (C and D) Immature 600 kDa LRP1 (LRP-600) with and without Cu at the indicated chase times. (E and F) Representative immunoblots and quantification of LRP1 in the presence and absence of Cu and with Cu and MG132. Values are mean ± SEM, n = 5 independent experiments per group.
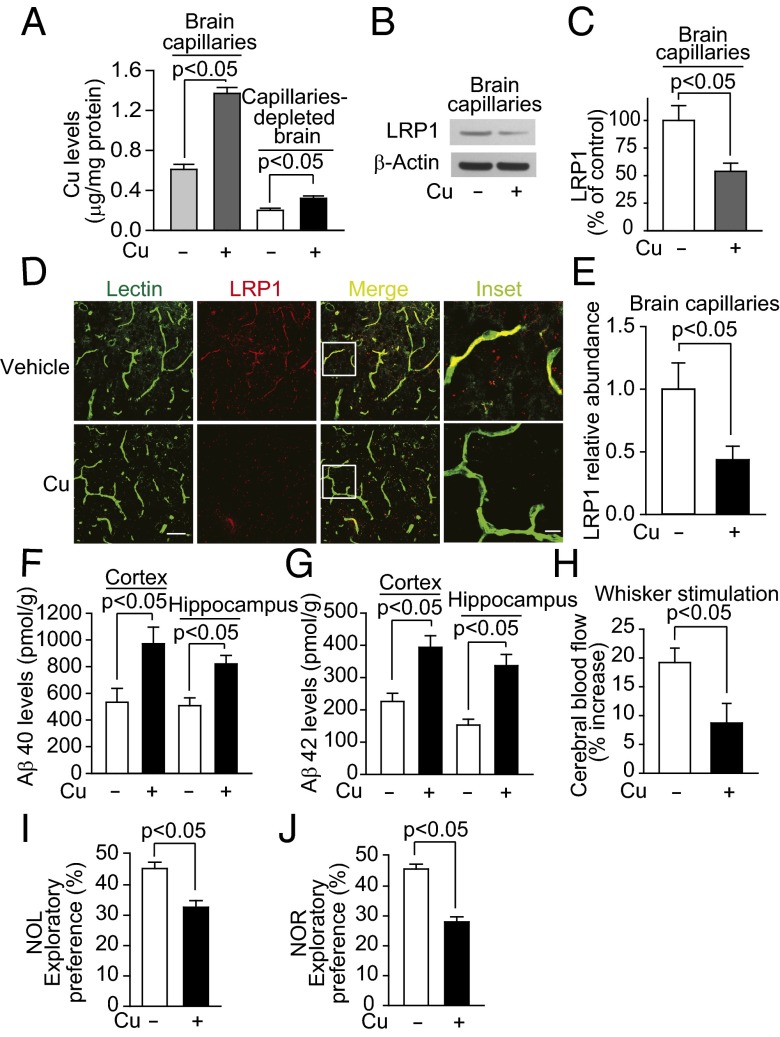
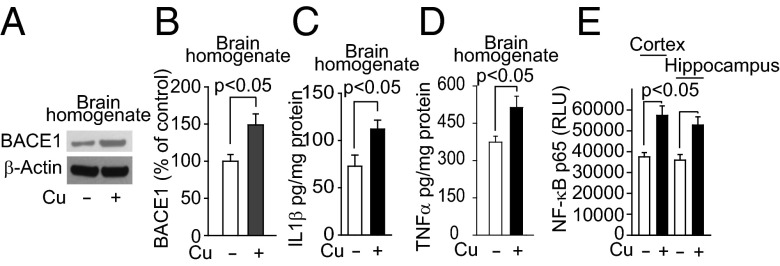
Next, we explored the effect of Cu in a mouse model of AD. APPsw/0 mice treated with Cu had a 2.3-fold increase in Cu levels in brain capillaries, and unlike WT mice, a 1.6-fold increase in Cu levels in brain parenchyma (Fig. 6A). Cu treatment also reduced levels of LRP1 in brain capillaries (Fig. 6 B–E), increased Aβ40 and Aβ42 levels in the cortex and hippocampus 1.6–2.2-fold (Fig. 6 F and G), reduced cerebral blood flow responses to whisker stimulation 2.5-fold (Fig. 6H) and exploratory preferences for novel object location (NOL) and novel object recognition (NOR) 1.4–1.7-fold (Fig. 6 I and J). In addition, in the Barnes maze test, the latency to escape into the hole was increased in Cu-treated mice (Fig. S6A). Because oxidative stress is increased in the Cu-treated mice (Fig. S6B), and associated with enhanced Aβ production and secretion of proinflammatory cytokines (31), we reasoned that the increased Cu levels in brain parenchyma could contribute to these conditions by increasing nuclear factor-kappa B (NF-κB) and BACE1 levels. We showed that BACE1 levels were increased in brains of Cu-dosed APPsw/0 mice by 1.5-fold (Fig. 7 A and B) and similarly in capillary-depleted brain (Fig. S6 C and D). Levels of proinflammatory cytokines, interleukin 1β (IL1β), and tumor necrosis factor α (TNFα) were increased by 1.5- to twofold (Fig. 7 C and D). We then showed that Cu increased NF-κB levels in cortex and hippocampus (Fig. 7E). Cu increased the levels of BACE1 in primary mouse neuronal cells in culture (Fig. S7 A and B) and the secretion of IL1β and TNFα by microglia cells (Fig. S8 A and B). Thus, in APPsw/0 mice, Cu reduced LRP1 levels in brain capillaries and increased Aβ production and levels of neuroinflammation, all of which will cause accumulation of neurotoxic Aβ in the brain.
Fig. 6.
Cu reduced LRP1levels in brain capillaries and increased brain Aβ levels in APPsw/o mice. (A) Cu levels in brain capillaries and capillary-depleted brain. (B) Representative LRP1 Western blot analysis for isolated brain capillaries and its quantification (C). (D) Representative immunostained images of brain section for lectin, LRP1, and merged images. (Scale bar, 50 µm.) and relative quantification of LRP1-positive vessels (E). (F and G) Aβ40 and Aβ42 levels. (H) Cerebral blood flow responses to brain stimulation: (I) NOL and (J) NOR responses. APPsw/0 mice were treated with tracer levels of Cu (+) or vehicle (−) in their drinking water for 90 d starting at 6 mo old. β-Actin was the reference molecule for each Western blot analysis. Values are mean ± SEM, n = 8 mice per group.
Fig. 7.
Cu increased levels of BACE1 and proinflammatory cytokines in APPsw/o mice. (A) Representative BACE1 Western blot analysis in brain homogenates and its quantification (B). (C) Levels of IL1β and TNFα (D), and NF-κB p65 nuclear levels (E) in the cortex and hippocampus. APPsw/0 mice were treated with tracer levels of Cu (+) or vehicle (−) in their drinking water for 90 d starting at 6 mo old. β-Actin was the reference molecule for each Western blot analysis. Values are mean ± SEM, n = 4 mice per group.
Discussion
The unique findings are as follows: (i) Cu accumulated in brain capillaries but not in the parenchyma of aging mice and selectively reduced levels of LRP1, a transporter of Aβ, which in turn contributed to increased levels of Aβ in brain. (ii) Mice chronically dosed with low levels of Cu (0.13 mg/L) in their drinking water for 90 d, starting at 2 mo of age, had the same effects on LRP1 and brain Aβ as in the aging mice. Levels of APP, IDE, and NEP were unchanged compared with vehicle-treated mice. (iii) In HBECs, Cu treatment, at levels (200 nM) similar to normal non–Cp-bound Cu in plasma, induced PrPc binding to LRP1, which in turn caused LRP1 nitrotyrosination and its subsequent proteosomal-dependent degradation. This leads to Cu-induced LRP1 down-regulation and decreased Aβ clearance in the aging brain. (iv) In APPsw/0 mice, Cu levels were increased in brain capillaries and, unlike WT mice, in the parenchyma. Increased Cu levels in brain parenchyma enhanced Aβ production and neuroinflammation, which potentiated Aβ accumulation. Faulty Aβ clearance across the BBB, due to increased Cu levels in the aging brain endothelium may lead to neurotoxic Aβ accumulation in normal brains, which may contribute to the development of the sporadic form of AD.
The CNS vascular barriers protect the brain from blood-borne toxins (32). In the case of heavy metal ions, such as lead and iron, these are accumulated in brain capillaries, and transport into the parenchyma is rate limiting (33, 34). Our data show that Cu is also accumulated in brain capillaries, corroborated by data obtained in rats in which brain capillaries accumulated Cu (about 10 times) compared with brain parenchyma (35). Age-dependent increase in brain copper has been reported (18). Thus, the normal BBB functioning in its protective roles restricts the transport of Cu into brain parenchyma. However, this may contribute significantly to the age-dependent down-regulation of LRP1 in the endothelium, leading to decreased Aβ clearance across the BBB into blood and accumulation of Aβ peptides in brain.
In these studies, mice were dosed with low levels of Cu (0.13 mg/L), 10% of the maximum contaminant level goal set by the Environment Protection Agency (EPA) and 52-fold higher levels of Cu compared with that in the animals’ normal drinking water. Although Cu absorption is controlled (36), the non–Cp-bound Cu (labile Cu) levels in plasma were increased but not levels of Cu associated with proteins >100 kDa, mainly Cp-bound Cu (37, 38). Cu absorption is controlled (36). Thus, a substantial fraction of the absorbed Cu bypasses the liver and enters the blood (11, 39), as demonstrated with orally administered 64Cu (40). This increases Cu availability for transport into brain. Cu slowly enters brain parenchyma and cerebrospinal fluid (CSF) when the cerebrovasculature is perfused, in situ, with free Cu (41). Cu transport presumably occurs via Cu transporter 1 (Ctr1) and/or divalent metal transporter 1 (DMT1) on the luminal surface, and ATP7A, a Cu ATPase transporter, on the abluminal surface of the BBB (42) or presenilins (43). In our in vitro studies, we used levels of Cu comparable to the normal non–Cp-bound Cu fraction in plasma and twofold lower than Cu levels normally found in the human CSF (3, 11). High levels of Cu (in millimoles) were shown to induce angiogenesis in peripheral endothelial cells (44). The low levels of Cu used in our studies had no effect on angiogenesis.
The accumulation of Cu in brain capillaries generates hydroxyl free radical by the Fenton/Haber-Weiss reactions (45, 46). Cu2+ is reduced to Cu1+ by suitable electron donor (superoxide anion, NADPH, ascorbate, APP, Aβ), and Cu1+ in turn reacts with hydrogen peroxide to generate hydroxyl radical, which can cause protein oxidation and lipid peroxidation (47). Metal-catalyzed oxidation of proteins is a selective reaction that occurs mainly at sites on protein with a transition metal binding domain. APP, Aβ, and PrPc possess Cu binding domains (28, 46, 48). APP has two Cu binding domains, one in the N-terminal and the other within the Aβ sequence (48). Cu binding domain in APP also has Cu2+ reductase activity that requires methionine170 or cysteine144 (30). In addition, Cu-bound APP could be processed because LRP1 binds the Kunitz protease inhibitor domain of the longer APP isoforms (APP751 and 770) (49). In vitro studies have shown that Cu avidly binds Aβ via mainly histidine and tyrosine residues (50, 51). Aβ is also a Cu reductase (42 > 40), that generates reactive oxygen species consequently to Cu/Aβ interaction (46). Cu binds PrPc in its N-terminal domain (28), which facilitates PrPc/LRP1 interaction and subsequent internalization (29). Cu binding to APP and PrPc, and their interaction with LRP1, may explain Cu’s selective effect on LRP1. Aluminum, iron, and zinc, at the same molar concentrations as Cu, did not affect LRP1 levels in HBECs, nor did they affect Aβ accumulation in brain (15).
In oxidative stress, NO synthesis is increased, and in the presence of superoxide anion forms peroxynitrite, which causes nitrotyrosination of proteins (52). In brains of patients with AD there is widespread protein nitrotyrosination (53, 54). These proteins include enzymes of glucose metabolism (enolase, lactate dehydrogenase, and triosephosphate isomerase). In endothelial cells, nitrotyrosinated proteins include triosephosphate, antioxidant defense nonselenium glutathione, eukaryotic translation elongation factor 2, and HSP75 (55). The proteosomal system degrades almost all (about 90%) of the intracellular oxidative damaged/nitrotyrosinated proteins (52). We have shown that Cu selectively induced nitrotyrosination of LRP1 in brain capillaries and consequently its degradation by the proteosomal-dependent system.
In APPsw/0 mice, LRP1 levels in brain endothelium are reduced and the BBB may be compromised (25, 32). Treating these mice with Cu increased Cu levels in brain capillaries and in the parenchyma possibly via the compromised BBB. Because LRP1 levels are already reduced in these mice, further Cu-induced down-regulation of LRP1 may potentiate its deleterious effects. In addition, Cu in brain parenchyma could interact with Aβ and promote β-sheet conformation, aggregation, and toxicity (46, 56, 57). Thus, Cu may modulate the progressive transition from soluble monomeric Aβ to soluble oligomerized and aggregated insoluble Aβ forms. Low levels of Cu may elevate Aβ levels in lipid rafts (58). Cu may cause Aβ neurotoxicity by interacting with PrPc and N-methyl-d-aspartate (NMDA) receptor (59). In addition, oxidative stress may increase Aβ production via NF-κB and BACE1 (60). Therefore, it would be difficult to separate the effect of Cu on LRP1-dependent BBB clearance and Aβ production in brains of mouse models of AD.
Currently, there are no treatments for AD, and all clinical trials have so far failed. Alternative approaches are urgently needed to delay/prevent the onset of this devastating disease. Whereas the role of environmental factors in the development of the sporadic form of AD is controversial, long-term exposure to higher levels of Cu may contribute to this process, at least in some cases. d-penicillamine, a Cu chelator that does not cross the BBB, reduced oxidative stress in patients with AD (10). PBT2, a hydrophilic copper–zinc chaperone, which crosses the BBB, improved cognition in clinical trials (13, 61). Intracellular translocation of Zn and Cu via PBT2 may be an important mechanism of action for PBT2 (62). Our data suggest that Cu critically regulates LRP1-mediated Aβ clearance across the BBB and contributes to increased Aβ production and neuroinflammation in a mouse model of AD. Thus, Cu-specific chelating agents, chaperones, or antioxidants may reduce Cu-induced Aβ accumulation in brain, which should, by extension, prevent Aβ accumulation in brain and improve cognition in AD (63).
Materials and Methods
Mice (2-mo-old C57BL6) were dosed with Cu (0.13 mg/L) as copper sulfate or double-distilled water (vehicle) for 90 d. Brain capillaries and capillary-depleted brain were prepared, as described (25). The levels of Cu in brain capillaries, capillary-depleted brain, plasma filtrate (100 kDa cutoff filter), plasma, and drinking water of the mice were determined using a PerkinElmer Analyst 600 Graphite Furnace Atomic Absorption spectrophotometer. Brain capillaries, capillary-depleted brain, and HBECs were lysed with RIPA lysis buffer and used for Western blot analysis (25, 60). Mouse and human Aβ levels were determined by ELISA. Because there was no significant difference between the clearance of Aβ from brain using radio-iodinated Aβ and nonradio-labeled Aβ (ELISA), iodinated Aβ was used in these studies (25). MBECs were isolated and cultured, as described (64). HBECs were used as reported (25). APPsw/0 mice overexpressing human APP transgene with the K670M/N671L double mutation under control of the hamster prion promoter were dosed for 90 d, starting at 6 mo old, with and without double-distilled drinking water containing Cu (0.13 mg/L) as copper sulfate. See SI Materials and Methods for further details.
Supplementary Material
Acknowledgments
We thank Dr. Maiken Nedergaard (Center for Translational Neuromedicine, University of Rochester) for critical comments, Dr. Constance D. Baldwin (Department of Pediatrics, University of Rochester) for editing the manuscript, and members of the former Center for Neurodegenerative and Vascular Brain Disorders (Department of Neurosurgery, University of Rochester). Human brain endothelial cells (HBECs) were kindly provided by Socratech. We thank the Alzheimer’s Association (IIRG-06-26118), National Institutes of Health (AG029481), and National Institute of Environmental Health Sciences Pilot Project Grant (to R.D.) for support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1302212110/-/DCSupplemental.
References
- 1.Prohaska JR. Role of copper transporters in copper homeostasis. Am J Clin Nutr. 2008;88(3):826S–829S. doi: 10.1093/ajcn/88.3.826S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lutsenko S, Bhattacharjee A, Hubbard AL (2010) Copper handling machinery of the brain. Metallomics 2(9):596–608. [DOI] [PubMed]
- 3. Chutkow JG (1978) Evidence for uptake of nonceruloplasminic copper in the brain: Effect of ionic copper and amino acids. Proc Soc Exp Biol Med. 158(1):113–116. [DOI] [PubMed]
- 4.Harris ED. Cellular copper transport and metabolism. Annu Rev Nutr. 2000;20:291–310. doi: 10.1146/annurev.nutr.20.1.291. [DOI] [PubMed] [Google Scholar]
- 5.Brewer GJ. Copper excess, zinc deficiency, and cognition loss in Alzheimer’s disease. Biofactors. 2012;38(2):107–113. doi: 10.1002/biof.1005. [DOI] [PubMed] [Google Scholar]
- 6. Squitti R (2012) Copper dysfunction in Alzheimer's disease: From meta-analysis of biochemical studies to new insight into genetics. J Trace Elem Med Biol 26(2–3):93–96. [DOI] [PubMed]
- 7.Bush AI, Tanzi RE. Therapeutics for Alzheimer’s disease based on the metal hypothesis. Neurotherapeutics. 2008;5(3):421–432. doi: 10.1016/j.nurt.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rose F, Hodak M, Bernholc J (2011) Mechanism of copper(II)-induced misfolding of Parkinson's disease protein. Sci Rep 1:11. [DOI] [PMC free article] [PubMed]
- 9.Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markesbery WR. Copper, iron and zinc in Alzheimer’s disease senile plaques. J Neurol Sci. 1998;158(1):47–52. doi: 10.1016/s0022-510x(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 10.Squitti R, et al. Elevation of serum copper levels in Alzheimer’s disease. Neurology. 2002;59(8):1153–1161. doi: 10.1212/wnl.59.8.1153. [DOI] [PubMed] [Google Scholar]
- 11.Squitti R, et al. Excess of nonceruloplasmin serum copper in AD correlates with MMSE, CSF [beta]-amyloid, and h-tau. Neurology. 2006;67(1):76–82. doi: 10.1212/01.wnl.0000223343.82809.cf. [DOI] [PubMed] [Google Scholar]
- 12.James SA, et al. Elevated labile Cu is associated with oxidative pathology in Alzheimer disease. Free Radic Biol Med. 2012;52(2):298–302. doi: 10.1016/j.freeradbiomed.2011.10.446. [DOI] [PubMed] [Google Scholar]
- 13.Faux NG, et al. PBT2 rapidly improves cognition in Alzheimer’s Disease: Additional phase II analyses. J Alzheimers Dis. 2010;20(2):509–516. doi: 10.3233/JAD-2010-1390. [DOI] [PubMed] [Google Scholar]
- 14.Sparks DL, Schreurs BG. Trace amounts of copper in water induce beta-amyloid plaques and learning deficits in a rabbit model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2003;100(19):11065–11069. doi: 10.1073/pnas.1832769100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sparks DL, et al. Trace copper levels in the drinking water, but not zinc or aluminum influence CNS Alzheimer-like pathology. J Nutr Health Aging. 2006;10(4):247–254. [PMC free article] [PubMed] [Google Scholar]
- 16.Bayer TA, et al. Dietary Cu stabilizes brain superoxide dismutase 1 activity and reduces amyloid Abeta production in APP23 transgenic mice. Proc Natl Acad Sci USA. 2003;100(24):14187–14192. doi: 10.1073/pnas.2332818100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phinney AL, et al. In vivo reduction of amyloid-beta by a mutant copper transporter. Proc Natl Acad Sci USA. 2003;100(24):14193–14198. doi: 10.1073/pnas.2332851100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maynard CJ, et al. Overexpression of Alzheimer’s disease amyloid-beta opposes the age-dependent elevations of brain copper and iron. J Biol Chem. 2002;277(47):44670–44676. doi: 10.1074/jbc.M204379200. [DOI] [PubMed] [Google Scholar]
- 19. Bayer TA, Multhaup G (2005) Involvement of amyloid beta precursor protein (AbetaPP) modulated copper homeostasis in Alzheimer's disease. J Alzheimers Dis 8(2):201–206; discussion 209–215. [DOI] [PubMed]
- 20.Borchardt T, et al. Copper inhibits beta-amyloid production and stimulates the non-amyloidogenic pathway of amyloid-precursor-protein secretion. Biochem J. 1999;344(Pt 2):461–467. [PMC free article] [PubMed] [Google Scholar]
- 21.Selkoe DJ. Clearing the brain’s amyloid cobwebs. Neuron. 2001;32(2):177–180. doi: 10.1016/s0896-6273(01)00475-5. [DOI] [PubMed] [Google Scholar]
- 22.Deane R, Zlokovic BV. Role of the blood-brain barrier in the pathogenesis of Alzheimer’s disease. Curr Alzheimer Res. 2007;4(2):191–197. doi: 10.2174/156720507780362245. [DOI] [PubMed] [Google Scholar]
- 23.Iwata N, Higuchi M, Saido TC. Metabolism of amyloid-beta peptide and Alzheimer’s disease. Pharmacol Ther. 2005;108(2):129–148. doi: 10.1016/j.pharmthera.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 24. Iliff JJ, et al. (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Trans Med 4(147):147ra111. [DOI] [PMC free article] [PubMed]
- 25.Deane R, et al. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004;43(3):333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 26.Donahue JE, et al. RAGE, LRP-1, and amyloid-beta protein in Alzheimer’s disease. Acta Neuropathol. 2006;112(4):405–415. doi: 10.1007/s00401-006-0115-3. [DOI] [PubMed] [Google Scholar]
- 27.Deane R, et al. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008;118(12):4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stöckel J, Safar J, Wallace AC, Cohen FE, Prusiner SB. Prion protein selectively binds copper(II) ions. Biochemistry. 1998;37(20):7185–7193. doi: 10.1021/bi972827k. [DOI] [PubMed] [Google Scholar]
- 29.Taylor DR, Hooper NM. The low-density lipoprotein receptor-related protein 1 (LRP1) mediates the endocytosis of the cellular prion protein. Biochem J. 2007;402(1):17–23. doi: 10.1042/BJ20061736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruiz FH, González M, Bodini M, Opazo C, Inestrosa NC. Cysteine 144 is a key residue in the copper reduction by the beta-amyloid precursor protein. J Neurochem. 1999;73(3):1288–1292. doi: 10.1046/j.1471-4159.1999.0731288.x. [DOI] [PubMed] [Google Scholar]
- 31.Terwel D, et al. Neuroinflammatory and behavioural changes in the Atp7B mutant mouse model of Wilson’s disease. J Neurochem. 2011;118(1):105–112. doi: 10.1111/j.1471-4159.2011.07278.x. [DOI] [PubMed] [Google Scholar]
- 32.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Deane R, Bradbury MW. Transport of lead-203 at the blood-brain barrier during short cerebrovascular perfusion with saline in the rat. J Neurochem. 1990;54(3):905–914. doi: 10.1111/j.1471-4159.1990.tb02337.x. [DOI] [PubMed] [Google Scholar]
- 34.Deane R, Zheng W, Zlokovic BV. Brain capillary endothelium and choroid plexus epithelium regulate transport of transferrin-bound and free iron into the rat brain. J Neurochem. 2004;88(4):813–820. doi: 10.1046/j.1471-4159.2003.02221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tayarani I, Cloëz I, Clément M, Bourre JM. Antioxidant enzymes and related trace elements in aging brain capillaries and choroid plexus. J Neurochem. 1989;53(3):817–824. doi: 10.1111/j.1471-4159.1989.tb11778.x. [DOI] [PubMed] [Google Scholar]
- 36.Wapnir RA. Copper absorption and bioavailability. Am J Clin Nutr. 1998;67(5, Suppl):1054S–1060S. doi: 10.1093/ajcn/67.5.1054S. [DOI] [PubMed] [Google Scholar]
- 37.Weiss KC, Linder MC. Copper transport in rats involving a new plasma protein. Am J Physiol. 1985;249(1 Pt 1):E77–E88. doi: 10.1152/ajpendo.1985.249.1.E77. [DOI] [PubMed] [Google Scholar]
- 38.Wirth PL, Linder MC. Distribution of copper among components of human serum. J Natl Cancer Inst. 1985;75(2):277–284. [PubMed] [Google Scholar]
- 39.Brewer GJ. The risks of copper toxicity contributing to cognitive decline in the aging population and to Alzheimer’s disease. J Am Coll Nutr. 2009;28(3):238–242. doi: 10.1080/07315724.2009.10719777. [DOI] [PubMed] [Google Scholar]
- 40.Hill GM, Brewer GJ, Juni JE, Prasad AS, Dick RD. Treatment of Wilson’s disease with zinc. II. Validation of oral 64copper with copper balance. Am J Med Sci. 1986;292(6):344–349. doi: 10.1097/00000441-198612000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Choi BS, Zheng W. Copper transport to the brain by the blood-brain barrier and blood-CSF barrier. Brain Res. 2009;1248:14–21. doi: 10.1016/j.brainres.2008.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qian Y, Tiffany-Castiglioni E, Welsh J, Harris ED. Copper efflux from murine microvascular cells requires expression of the menkes disease Cu-ATPase. J Nutr. 1998;128(8):1276–1282. doi: 10.1093/jn/128.8.1276. [DOI] [PubMed] [Google Scholar]
- 43.Greenough MA, et al. Presenilins promote the cellular uptake of copper and zinc and maintain copper chaperone of SOD1-dependent copper/zinc superoxide dismutase activity. J Biol Chem. 2011;286(11):9776–9786. doi: 10.1074/jbc.M110.163964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu GF. Copper stimulates proliferation of human endothelial cells under culture. J Cell Biochem. 1998;69(3):326–335. doi: 10.1002/(sici)1097-4644(19980601)69:3<326::aid-jcb10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 45.Smith MA, et al. Advanced Maillard reaction end products, free radicals, and protein oxidation in Alzheimer’s disease. Ann N Y Acad Sci. 1994;738:447–454. doi: 10.1111/j.1749-6632.1994.tb21836.x. [DOI] [PubMed] [Google Scholar]
- 46.Huang X, et al. Cu(II) potentiation of alzheimer abeta neurotoxicity. Correlation with cell-free hydrogen peroxide production and metal reduction. J Biol Chem. 1999;274(52):37111–37116. doi: 10.1074/jbc.274.52.37111. [DOI] [PubMed] [Google Scholar]
- 47.Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: Potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic Biol Med. 2002;32(11):1050–1060. doi: 10.1016/s0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- 48.Kong GK, et al. Copper binding to the Alzheimer’s disease amyloid precursor protein. Eur Biophys J. 2008;37(3):269–279. doi: 10.1007/s00249-007-0234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y, Cam J, Bu G. Low-density lipoprotein receptor family: Endocytosis and signal transduction. Mol Neurobiol. 2001;23(1):53–67. doi: 10.1385/MN:23:1:53. [DOI] [PubMed] [Google Scholar]
- 50.Syme CD, Nadal RC, Rigby SE, Viles JH. Copper binding to the amyloid-beta (Abeta) peptide associated with Alzheimer’s disease: Folding, coordination geometry, pH dependence, stoichiometry, and affinity of Abeta-(1-28): Insights from a range of complementary spectroscopic techniques. J Biol Chem. 2004;279(18):18169–18177. doi: 10.1074/jbc.M313572200. [DOI] [PubMed] [Google Scholar]
- 51.Atwood CS, et al. Copper mediates dityrosine cross-linking of Alzheimer’s amyloid-beta. Biochemistry. 2004;43(2):560–568. doi: 10.1021/bi0358824. [DOI] [PubMed] [Google Scholar]
- 52.Guix FX, Uribesalgo I, Coma M, Muñoz FJ. The physiology and pathophysiology of nitric oxide in the brain. Prog Neurobiol. 2005;76(2):126–152. doi: 10.1016/j.pneurobio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 53. Smith MA, Richey Harris PL, Sayre LM, Beckman JS, Perry G (1997) Widespread peroxynitrite-mediated damage in Alzheimer's disease. J Neurosci 17(8):2653–2657. [DOI] [PMC free article] [PubMed]
- 54.Sultana R, et al. Identification of nitrated proteins in Alzheimer’s disease brain using a redox proteomics approach. Neurobiol Dis. 2006;22(1):76–87. doi: 10.1016/j.nbd.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 55. Coma M, et al. (2005) Lack of oestrogen protection in amyloid-mediated endothelial damage due to protein nitrotyrosination. Brain 128(Pt 7):1613–1621. [DOI] [PubMed]
- 56.Yoburn JC, et al. Dityrosine cross-linked Abeta peptides: Fibrillar beta-structure in Abeta(1-40) is conducive to formation of dityrosine cross-links but a dityrosine cross-link in Abeta(8-14) does not induce beta-structure. Chem Res Toxicol. 2003;16(4):531–535. doi: 10.1021/tx025666g. [DOI] [PubMed] [Google Scholar]
- 57.House E, et al. Aluminium, iron, zinc and copper influence the in vitro formation of amyloid fibrils of Abeta42 in a manner which may have consequences for metal chelation therapy in Alzheimer’s disease. J Alzheimers Dis. 2004;6(3):291–301. doi: 10.3233/jad-2004-6310. [DOI] [PubMed] [Google Scholar]
- 58.Hung YH, et al. Paradoxical condensation of copper with elevated beta-amyloid in lipid rafts under cellular copper deficiency conditions: Implications for Alzheimer disease. J Biol Chem. 2009;284(33):21899–21907. doi: 10.1074/jbc.M109.019521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.You H, et al. Aβ neurotoxicity depends on interactions between copper ions, prion protein, and N-methyl-D-aspartate receptors. Proc Natl Acad Sci USA. 2012;109(5):1737–1742. doi: 10.1073/pnas.1110789109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deane R, et al. A multimodal RAGE-specific inhibitor reduces amyloid β-mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest. 2012;122(4):1377–1392. doi: 10.1172/JCI58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lannfelt L, et al. PBT2-201-EURO study group Safety, efficacy, and biomarker findings of PBT2 in targeting Abeta as a modifying therapy for Alzheimer’s disease: A phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2008;7(9):779–786. doi: 10.1016/S1474-4422(08)70167-4. [DOI] [PubMed] [Google Scholar]
- 62.Crouch PJ, et al. The Alzheimer’s therapeutic PBT2 promotes amyloid-β degradation and GSK3 phosphorylation via a metal chaperone activity. J Neurochem. 2011;119(1):220–230. doi: 10.1111/j.1471-4159.2011.07402.x. [DOI] [PubMed] [Google Scholar]
- 63.Deane R, Bell RD, Sagare A, Zlokovic BV. Clearance of amyloid-beta peptide across the blood-brain barrier: Implication for therapies in Alzheimer’s disease. CNS Neurol Disord Drug Targets. 2009;8(1):16–30. doi: 10.2174/187152709787601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu Z, et al. Role of the MEOX2 homeobox gene in neurovascular dysfunction in Alzheimer disease. Nat Med. 2005;11(9):959–965. doi: 10.1038/nm1287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.