Significance
There is increasing interest in developing pharmacological strategies to inhibit the allosteric regulatory mechanisms of signaling enzymes. The Abl tyrosine kinase is a prominent target, due to its ubiquitous cellular role and its involvement in cancer. Here, computational and experimental methods are used in synergy to probe the mechanism of the regulatory unit of Abl, whose dual function is to inhibit the enzyme and to mediate its interaction with other signaling proteins. Our results provide insights into the thermodynamic basis for the mechanism of Abl autoinhibition and activation and expand our understanding of the principles that govern modular domain organization.
Keywords: allosteric inhibitors, protein–protein interactions, macromolecular assembly, population shift, leukemia
Abstract
The regulation and localization of signaling enzymes is often mediated by accessory modular domains, which frequently function in tandems. The ability of these tandems to adopt multiple conformations is as important for proper regulation as the individual domain specificity. A paradigmatic example is Abl, a ubiquitous tyrosine kinase of significant pharmacological interest. SH3 and SH2 domains inhibit Abl by assembling onto the catalytic domain, allosterically clamping it in an inactive state. We investigate the dynamics of this SH3–SH2 tandem, using microsecond all-atom simulations and differential scanning calorimetry. Our results indicate that the Abl tandem is a two-state switch, alternating between the conformation observed in the structure of the autoinhibited enzyme and another configuration that is consistent with existing scattering data for an activated form. Intriguingly, we find that the latter is the most probable when the tandem is disengaged from the catalytic domain. Nevertheless, an amino acid stretch preceding the SH3 domain, the so-called N-cap, reshapes the free-energy landscape of the tandem and favors the interaction of this domain with the SH2-kinase linker, an intermediate step necessary for assembly of the autoinhibited complex. This allosteric effect arises from interactions between N-cap and the SH2 domain and SH3–SH2 connector, which involve a phosphorylation site. We also show that the SH3–SH2 connector plays a determinant role in the assembly equilibrium of Abl, because mutations thereof hinder the engagement of the SH2-kinase linker. These results provide a thermodynamic rationale for the involvement of N-cap and SH3–SH2 connector in Abl regulation and expand our understanding of the principles of modular domain organization.
Tyrosine kinases are involved in a wide variety of key signaling processes and are therefore tightly regulated by the cell. Indeed, numerous pathologies, ranging from cancer to neurodegeneration, result from or are associated with deficiencies in kinase regulation. Consequently, these enzymes are a prominent target for novel pharmacological strategies against human disease.
This study focuses on Abelson murine-leukemia viral-oncogene homolog-1 (Abl), which is one of the most ubiquitously conserved tyrosine kinases. Abl is present in all metazoa, where it plays an essential role in processes as diverse as cytoskeleton reorganization, DNA repair, and regulation of apoptosis (1, 2). Accordingly, when constitutively active forms of Abl are present in normal cells, these are transformed into cancer cells (3). For example, in human white blood cells, a chromosomal abnormality leads to the fusion of the bcr and abl genes, which together encode for a cytoplasm-targeted deregulated form of Abl; Bcr-Abl interferes with the cell cycle, resulting in uncontrolled cell proliferation, and is the principal cause of chronic myeloid leukemia (CML) (4).
The architecture of Abl kinases resembles that of other tyrosine kinase families such as Src and Tec (5). It consists of a Src-homology-3 (SH3) module, which is an interaction domain specialized for recognition of xPxxP sequence motifs; a Src-homology-2 (SH2) module, which recognizes phosphorylated tyrosines; and the catalytic domain, which binds and cleaves ATP, and mediates tyrosine phosphorylation in protein targets. Additional elements in Abl are not conserved in Src or Tec and vary among isoforms. The Abl-1b splice variant, which is our focus, features a long, seemingly unstructured N-terminal extension preceding the SH3 domain, known as the N-cap, which becomes myristoylated posttranslationally. Following the catalytic domain is another long stretch, containing various sequence motifs for DNA or cytoskeleton recognition, among others.
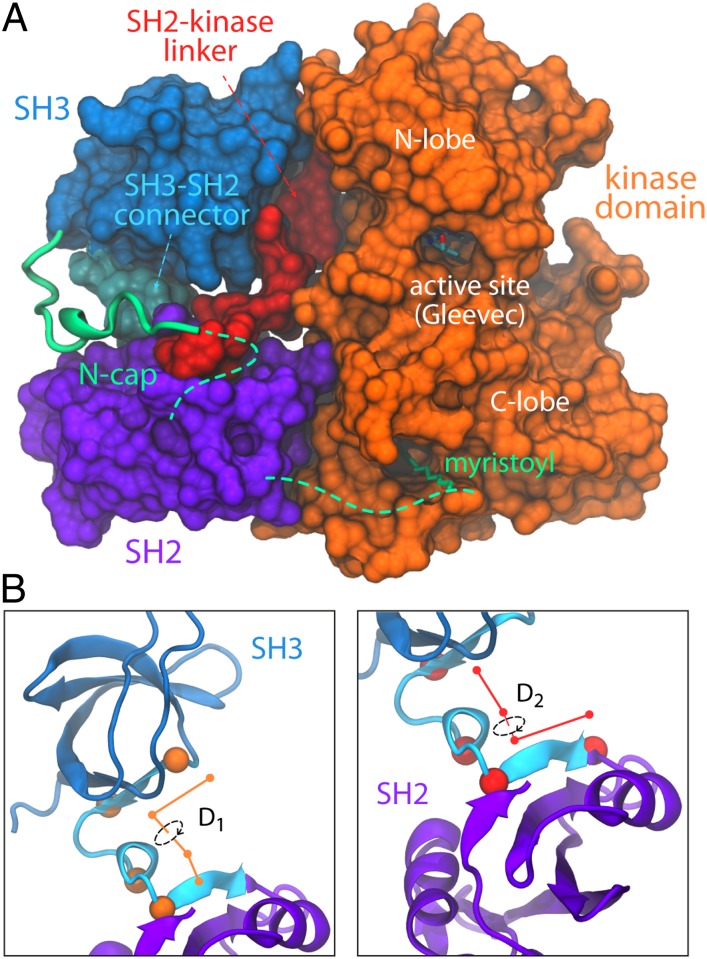
The SH3 and SH2 domains not only function as interaction modules, but also as allosteric inhibitors of the catalytic domain. In autoinhibited Abl, the three domains are assembled into a compact arrangement, in which the SH3–SH2 tandem appears to mechanically clamp the N- and C-lobes of the catalytic domain in an inactive configuration (Fig. 1A) (6, 7), without directly occluding the active site. The domain-domain linkers appear to be key elements in this assembly. The SH2 domain docks directly onto the C-lobe of the catalytic domain, whereas its linker to the N-lobe engages the SH3 domain. Simultaneously, the portion of N-cap most proximal to the SH3 domain binds to the short SH3–SH2 connector, while the myristoyl group, at the very N terminus, inserts itself into a hydrophobic cavity within the C-lobe of the catalytic domain. Interestingly, Src-family kinases adopt the same compact domain organization in their down-regulated form (8), although their equivalent to N-cap serves as a membrane anchor and is not involved in autoinhibition. Instead, a C-terminal extension from the catalytic domain containing a phosphorylated tyrosine associates with the SH2 module and seemingly locks the complex in the autoinhibited state. Reversible phosphorylation of this C-terminal tail is a major regulatory mechanism of Src family kinases (9, 10).
Fig. 1.
(A) Crystal structure of Abl in the autoinhibited state (PDB ID code 2FO0). The SH2 domain docks onto the C-lobe of the catalytic domain, whereas the SH3 domain engages the SH2-kinase linker. This inhibitory configuration of the SH3–SH2 tandem is referred to as the on conformer throughout the text. (B) Pseudodihedral angles used to describe the relative orientation of the regulatory domains during the simulations, encompassing residues in the C-terminal β-strand of the SH3 domain, the SH3–SH2 connector, and the N-terminal β-strand of the SH2 domain (Methods).
Activation of both Src and Abl is enabled by the disengagement or reconfiguration of the intramolecular interactions just described (7, 11, 12). Regulation can therefore be thought as a reversible equilibrium whereby the SH3, SH2, and catalytic domains are either dissociated or self-assembled in one or more configurations. External factors, such as competing interactions involving one or both regulatory domains, will bias this equilibrium in one or the other direction (13). For Abl in particular, the significance of this mechanism is underscored by the fact that mutations that impair the correct assembly of the autoinhibited complex, either in the SH3–SH2 tandem or in the domain-domain linkers, confer CML cells with resistance against inhibitory drugs designed to target the catalytic domain of the Bcr-Abl oncogene (14). This outcome has prompted considerable interest in the mechanisms of allosteric regulation of Abl and other tyrosine kinases and in the development of compounds designed to interfere with these mechanisms (15–19).
In this paper, we use molecular simulations, free energy calculations, and differential scanning calorimetry (DSC) to study the determining factors of a necessary step in the assembly of the autoinhibited form of Abl, namely the organization of the SH3 and SH2 domains into a conformation conducive to its association with the SH2–kinase linker (KL). Our results show that the conformational dynamics of the Abl SH3–SH2 tandem are clearly distinct from those of Src and related kinases. This finding enables us to reconcile seemingly contradictory structural and biophysical data on the mechanism of Abl regulation. Our analysis also enables us to formulate a mechanistic hypothesis for the role of the N-cap. Finally, we also examine the impact of activating mutations within the SH3–SH2 unit, particularly in the short connector between the domains.
Results and Discussion
Isolated SH3–SH2 Tandem Switches Between Two Main Conformers.
Experimental and computational studies of SH3–SH2 tandems from several Src family tyrosine kinases, such as Fyn or Hck, have concluded that these constructs can retain the conformation observed in the down-regulated, assembled enzyme, even in the absence of other elements from the kinase (13, 20, 21). To assess whether the SH3–SH2 tandem from Abl is similar in this regard, we calculated two 500-ns molecular dynamics trajectories, both starting in the inhibitory conformation of the tandem observed in the crystal structure of the inactivated complex—hereafter referred to as the “on” state of the SH3–SH2 clamp (Table S1). In the course of these simulations, the internal structure of each domain was very well preserved; on average, the conformation of the backbone deviated from the X-ray structure [root-mean-square-difference (RMSD)] by only ∼0.6 Å. Nonetheless, significant changes in the relative orientation of the two domains were observed throughout the simulations. We monitored these with the two pseudodihedral angles defined in Fig. 1B. The variations in these dihedral angles in the simulation time scale were both very significant (up to ∼180°) and reversible (Fig. 2). Thus, the initial “on” conformation was not sustained beyond the first 20–50 ns of the simulations, although it could be transiently observed thereafter. Instead, we observed that the tandem frequently adopted an alternative, well-defined arrangement, markedly different from the on conformation.
Fig. 2.
Conformational dynamics of the isolated SH3–SH2 tandem in two independent simulation trajectories of 500 ns, initiated in the on conformation. Time series of the pseudodihedral angles defined in Fig. 1 are represented as probability histograms in blocks of 20, 100, and 500 ns (red color equals greater probability). In both trajectories, the tandem switches to the noninhibitory off conformation within 100 ns; in one of them, the tandem switches back to the inhibitory on state, after 400 ns.
Further analysis of this alternative arrangement, which we refer to as the “off” or noninhibitory state of the SH3–SH2 clamp, reveals that it closely resembles (RMSD < 3 Å) an existing crystal structure of the isolated tandem (22), reported several years before that of the inactive, complete enzyme (6). In this isolated tandem structure (Fig. 3A), the orientation of the two domains differs drastically (RMSD ∼10 Å) from that seen in the assembled kinase complex. The tandem is also slightly more compact, with several direct contacts between the SH3 and SH2 domains, specifically involving the distal loop of the SH3 domain and the βB-βc, βD′-βc loops of the SH2 domain (Fig. S1). By contrast, in the on conformation, there are no direct interactions between these domains; instead, this conformation seems to be stabilized by interactions between the SH3 domain and the SH3–SH2 connector (Fig. S1). Two additional trajectories of 500 ns, now initiated in the crystal structure of the off conformer, confirm that the Abl SH3–SH2 tandem exchanges between off and on states within the simulation time scale (Fig. 3B), with a preference for the former.
Fig. 3.
(A) Comparison of the on and off conformers of the SH3–SH2 tandem (blue and red, respectively), overlaid only using the SH2 domain; the on conformer is shown as seen in the autoinhibited configuration of the Abl complex and viewed from two perspectives. The catalytic domain is shown as orange spheres, and the SH2-kinase linker is shown in cyan. The RMSD between the two conformations of the tandem (considering regions with secondary structure only) is ∼8 Å. The off conformer sampled in our simulations closely resembles a crystal structure of the isolated SH3–SH2 tandem (RMSD, ∼2–3 Å; PDB ID code 2ABL). This alternate conformation does not interfere with the interaction between the SH2 and catalytic domains but appears to be incompatible with the engagement of the SH2-kinase linker by the SH3 domain. (B) Conformational dynamics of the SH3–SH2 tandem in two independent simulation trajectories of 500 ns, initiated in the off conformation. In one of the simulations, the tandem switches to the on conformation after 50 ns, returning to the off state after 100 ns, and switching again to the on conformer after 450 ns. In the second trajectory, the tandem remains in the off state throughout the simulation.
To further evaluate the switch-like dynamics of the Abl SH3–SH2 tandem, we set out to verify that the on and off states are minima in its conformational free energy landscape. To calculate this landscape, we used an advanced sampling technique known as bias-exchange metadynamics (Methods). This calculation, based on ∼6 μs of simulation time, confirms that the on and off conformers correspond to the two main energetic minima (Fig. 4A; Fig. S2A), separated by a relatively small barrier, of about 4 kcal/mol (Fig. S3). Moreover, it reveals that the exchange between these two states correlates with a conformational change within the SH3–SH2 connector (Fig. 4A). Importantly, the off state is more probable than the on state, by approximately fivefold (see Methods for formal definitions); the population of the on state is only ∼8% (±2%).
Fig. 4.
Conformational free-energy landscapes of (A) the SH3–SH2 tandem, (B) the tandem engaged to the SH2-kinase linker (red), and (C) the tandem engaged to N-cap (green). Each of the free-energy landscapes derives from ∼6 μs of all-atom bias-exchange metadynamics simulations, using five collective variables (Methods). The free-energy landscapes shown derive from projections of the unbiased probability density on two of these variables; i.e., the probability density function is integrated along the other variables. S1 represents the relative orientation of the SH3 and SH2 domains; S2 describes the conformation of the SH3–SH2 connector. The integration excludes values in S3 larger than 1 nm2, because those are more poorly sampled (Fig. S2).
To our knowledge, the mechanistic significance of the off configuration of the Abl SH3–SH2 tandem has not been readdressed since the determination of the structures of the complete enzyme in the inactive (6) and active (7) states. It is worth noting, however, that diffusion anisotropy measurements by solution NMR had previously indicated that the SH3 and SH2 domains in the Abl tandem have a greater propensity to be in contact than those in tandems from Src kinases (20, 23), in agreement with our results. Moreover, the off conformation sampled in our simulations is highly consistent with small-angle X-ray scattering (SAXS) measurements obtained for a constitutively active form of Abl, where the regulatory unit is partially disassembled from the catalytic domain (7) (Fig. 5). Thus, even though this alternative off conformation of the Abl SH3–SH2 regulatory unit might have been perceived as an artifact induced by the crystallization process, this and previous studies actually indicate that it is mechanistically relevant.
Fig. 5.
Putative model of fully activated Abl, with the SH3–SH2 tandem in the proposed off conformation. The model is based on a partial X-ray structure (cyan) of a deregulated, mutagenized form of Abl (PDB ID code 1OPL, chain B), in which the SH2 domain docks onto the N-lobe of the catalytic domain (7). The SH3–SH2 tandem is overlaid using the SH2 domain as reference, either in the on or off conformers explored in the simulations (blue and red, respectively). Both models are fitted into a low-resolution envelope (gray mesh) constructed on the basis of SAXS measurements for the same deregulated Abl mutant (7). It is apparent that the experimental scattering data very likely reflects the SH3–SH2 tandem in the off conformation predicted to be the most probable by our free-energy calculations (when the tandem is disengaged from the SH2-kinase linker and the N-cap).
The structural dynamics of the Abl SH3–SH2 tandem and those in Src-type kinases, therefore, differ significantly. Free energy simulations of the SH3–SH2 tandem from Hck (a close homolog of cSrc) identified a single major conformer, which closely resembles the on state (13); previous studies of Fyn using X-ray crystallography and NMR led to an analogous conclusion (20, 21). In these kinases, the SH3–SH2 tandem is thus predisposed to adopt conformations conducive to the inactive, assembled form of the enzyme, in which the SH3 domain can readily engage the SH2–KL. The fact that the regulatory tandem of Abl differs in this regard suggests that the assembly of the autoinhibited state involves other elements, as we discuss below.
Engagement of the SH2–KL Requires the On Conformation.
Experimental studies of the Abl SH3–SH2 tandem using hydrogen/deuterium exchange (HDX) measurements have shown that the SH3 domain may bind the SH2–KL even in the absence of other elements of the kinase (24). Here, we showed that the isolated tandem alternates between two conformational states, referred to as on and off conformations. The former is known to be compatible with the interaction between the SH3 domain and SH2–KL (Fig. 1A); however, it is unclear whether this interaction is also feasible in the off conformation, which, as mentioned, is inherently preferred by the tandem.
To address this question, we calculated two additional 500-ns molecular dynamics trajectories of an SH3–SH2 construct that also includes the SH2–KL, bound to the SH3 domain, with the on conformation as the starting point (Table S1). As Fig. 6A shows, the tandem remained in the initial conformation throughout these simulations (note that spontaneous dissociation of the linker from the SH3 is unlikely to be observed in this time scale). This result is in clear contrast to what we observe for the isolated tandem (Fig. 3) and suggests that the interaction with the SH2-linker would not be viable in the alternate off conformation of the SH3–SH2 unit. Bias-exchange metadynamics calculations of the conformational free energy landscape of this construct support this observation. The calculated landscape, based again on ∼6 μs of simulation time, clearly demonstrates that the off conformer is energetically prohibited (Fig. 4B; Fig. S2B). Instead, we observe a single free energy minimum (population 98%), which corresponds precisely to the on conformation observed in the autoinhibited state of the enzyme (Fig. 1A). These results clearly indicate that engagement of the SH2–KL has a profound effect on the energetic landscape of the Abl SH3–SH2 tandem.
Fig. 6.
(A) Conformational dynamics of the SH3–SH2 tandem when bound to the SH2-kinase linker, in terms of a histogram of the D1/D2 time series for two independent simulation trajectories of 500 ns each, both of which were initiated in the on conformation. (B) Analysis of the interaction between the Abl SH2-kinase linker and the SH3 domain, via differential scanning calorimetry, for constructs of the SH3–SH2 tandem with and without the linker and the individual domains.
DSC experiments carried out with SH3–SH2 and SH3–SH2–KL constructs further support this observation. DSC provides direct experimental access to the folding/unfolding partition function, and consequently, the shape of the calorimetric trace provides insights not only on the energetics of the unfolding equilibrium, but also on the mechanism underlying the process. Specifically, the shape of the calorimetric profile of a multidomain protein can provide information on the degree of interaction and conformational cooperativity between domains and on the influence of ligand binding (25, 26). Thus, to experimentally probe the effect of the kinase linker on the conformational equilibrium of the SH3–SH2 tandem, we measured the temperature dependence of the partial heat capacity of the individual SH3 and SH2 domains, the SH3–SH2 tandem, and the SH3–SH2–KL construct.
Unfortunately, with the exception of the Abl SH3 domain, all other constructs resulted in irreversible thermodynamic transitions under all conditions studied, precluding a quantitative evaluation of the linker binding affinity. Nonetheless, from a qualitative perspective, we observed very revealing differences between the heat capacity profiles of the SH3–SH2 and SH3–SH2–KL constructs (Fig. 6B). The thermogram obtained with the SH3–SH2 tandem features two peaks, each of which likely corresponding to the unfolding of one domain, based on a comparison with the single-domain thermograms. The SH2 domain appears to be more stable if isolated; nonetheless, we clearly discern two transitions for the tandem, indicating that even though there might be some cooperativity, it is fairly weak. We can conclude, therefore, that in this construct the two domains unfold largely independently of each other.
As mentioned, binding of the SH2–KL to the SH3 domain can take place in the absence of the kinase domain, as previously demonstrated via HDX measurements (24). Simulated DSC thermograms (SI Text, Table S2) show that if this process was noncooperative, binding would be expected to result in a displacement of the peak corresponding to the SH3 unfolding transition to higher temperatures, whereas the SH2 unfolding transition would be either unaffected, if the linker were to bind to the off conformer of the tandem (Fig. S4A), or shifted toward lower temperatures, if the linker binds to the on conformer (Fig. S4B). As Fig. 6B shows, however, this is clearly not what is observed experimentally for SH3–SH2–KL construct. The measured unfolding thermogram shows instead a single sharp peak, characteristic of highly cooperative transitions, centered around 55 °C, between the peaks corresponding to the isolated domains.
Highly cooperative transitions can be expected only if we assume that the SH3 domain cannot engage the KL when the SH2 domain is unfolded. However, as illustrated in Fig. S4 C and D, these peaks are characterized by Tm values much higher than that of the SH3–SH2–KL construct (Fig. 6B). Highly cooperative peaks with Tm values comparable to those observed experimentally can be rationalized only in a highly cooperative scenario, as shown in Fig. S4 E and F, in which partially unfolded states of the SH3–SH2 tandem are not significantly populated. Nevertheless, the theoretical range of Tm values for these cooperative peaks depends on whether the SH2–KL is assumed to bind to on or off conformation, i.e., the high or low free energy states of the tandem, according to our bias-exchange simulations. In particular, Tm values comparable to the experimental observation (Fig. 6B) are only compatible with two possible scenarios: (i) the SH2–KL binds to the off conformer, albeit with extremely low dissociation constants, in the 10- to 100-mM range (Fig. S4E) or (ii) the linker binds to the on conformation with dissociation constants in the millimolar to micromolar range (Fig. S4F). Only the latter scenario seems probable, in view of the binding affinities typically observed for poly-proline recognition by SH3 domains (27).
In summary, the DSC data indicate that the presence of the linker has a profound effect on the structural dynamics of the tandem, coupling the unfolding of the two domains in a fully cooperative manner. Also, from a qualitative perspective, the low Tm value of the SH3–SH2–KL transitions suggests a preferential interaction of the linker with the high free energy on conformation, in agreement with the molecular dynamics simulations.
N-Cap Extension Promotes Inactive-Like Conformations.
The fact that the SH3–SH2 unit may engage the SH2–KL, as demonstrated by HDX and DSC experiments, indicates that the affinity of this interaction must be sufficiently large to overcome the energy difference between on and off conformers, i.e., to displace the conformational equilibrium of the tandem toward the on conformation. Nevertheless, the formation of this interaction would entail a conformational strain in the tandem, because the off conformer is energetically preferred when the SH3–SH2 unit is disengaged from other elements in the kinase (Fig. 4A). Such strain is probably minimal in other tightly regulated kinases such as Src or Fyn, in which the SH3–SH2 tandem is biased toward binding-competent conformations. The lack of this conformational drive in Abl suggests a different strategy for the stabilization of the autoinhibited complex relative to other kinases, e.g., a higher affinity between SH3 domain and SH2-kinase linker, the involvement of additional elements in the kinase, or both.
As mentioned above, a key element in the autoinhibition of Src-type tyrosine kinases is a C-terminal extension of the catalytic domain, featuring a tyrosine phosphorylation site. This site is recognized by the SH2 domain in the assembled form of the enzyme, and thus the short C-tail seems to mechanically lock the down-regulated state (8). Functional studies indicate that the myristoylated N terminus of the Abl N-cap region (in the 1b splice variant) might have a comparable role, because mutant forms that cannot be myristoylated are constitutively active (28). Indeed, a comparison of crystal structures of autoinhibited and active Abl suggests that binding of the myristoyl moiety to the catalytic domain is required for its C-lobe to adopt a conformation complementary with the SH2 domain interface (6, 29).
The role of the N-cap, however, is not to provide a tight physical link between the SH3 domain and the myristoyl-binding pocket. Most of the N-cap can be deleted without impairing the autoinhibition of the enzyme (28), consistent with the fact that this region is highly disordered in crystal structures (6, 7). However, a ∼20 amino acid fragment of N-cap immediately preceding the SH3 domain in Abl-1b is crucial for proper regulation. In one of the crystal structures of the autoinhibited complex (7), this fragment establishes several direct interactions with the SH2 domain and the SH3–SH2 connector, one of which is mediated by a phosphorylated serine (Ser69). Consistent with this finding, mutation of Ser69 and neighboring residues increases the activation level of the enzyme (28). We therefore hypothesized that the mechanistic role of this N-cap fragment might be to modulate the structural dynamics of the SH3–SH2 unit, in a manner that promotes the engagement of the SH2–KL and further stabilizes the autoinhibited state.
To assess this hypothesis, we carried out two 500-ns molecular dynamics simulations of an SH3–SH2 construct including the N-cap fragment visible in the experimental structure of the autoinhibited enzyme (Fig. 1A) (7), and a bias-exchange metadynamics calculation of the free energy landscape of the construct. As illustrated in Figs. 4C and 7A, the results indeed show that the N-cap reshapes the conformational landscape of the tandem so as to favor the on configuration, relative to the off conformer, approximately by a factor of ∼2 (Fig. 4C; Fig. S2C) compared with the tandem alone (Fig. 4A; Fig. S2A). Specifically, the population of the on state in this construct is now ∼20% (±5%), i.e., approximately two times more likely than in the SH3–SH2 construct with no N-cap. The most likely pathway followed by the domains in the transitions between the states is also not identical, although the calculated free energy barrier is largely unchanged (Fig. S3).
Fig. 7.
Conformational dynamics of the SH3–SH2 tandem, either when bound to WT N-cap (A), with a mutated form of N-cap that readily dissociates from the tandem (B), or to both N-cap and the SH2-kinase linker (C). The trajectory data are presented as in Fig. 6. In A and B, the histograms combine D1/D2 time series for two independent simulation trajectories of 500 ns each, both of which were initiated in the on conformation. A single trajectory was calculated in C.
In the course of these simulations, the interactions mediated by pSer69 with residues Ser145 and His144 (which is positively charged in this case) in the SH3–SH2 connector are preserved. In a second set of two 500-ns simulations, in which we induced the dissociation of the N-cap region by replacing Ser69 with alanine, the SH3–SH2 tandem departed from the on configuration, adopting predominantly the off conformer (Fig. 7B), consistent with the simulations of the SH3–SH2 construct lacking this N-cap segment (Fig. 2). By contrast, the structural dynamics of the tandem in a construct including both N-cap (WT) and the SH2–KL is very similar to that observed when only the SH2-kinase linker is bound (Fig. 7C).
The mechanistic implication of these results is that binding of the N-cap to the SH3–SH2 connector ought to facilitate the engagement of the SH2–KL by the SH3 domain and thus the formation of the autoinhibited complex. This allosteric role, not previously recognized, is in our view not only consistent with the abovementioned functional assays of mutant forms of Abl (28), but also with existing HDX measurements designed to probe the influence of N-cap on the conformational dynamics of the SH3 domain, when in tandem with the SH2 domain (30). Specifically, Chen et al. (30) reported a twofold increase in the unfolding half-life of the SH3 domain (i.e., an approximate twofold decrease in the rate of deuterium incorporation) when the tandem was coexpressed with the SH2–KL. An almost identical shift (1.9-fold) was measured for a construct containing the N-cap region instead. Inclusion of both N-cap and the SH2–KL, however, extended the half-life 4.6 times. This result was interpreted as evidence of noncooperativity between the interactions with N-cap and the SH2–KL; however, our interpretation of this measurement differs. If the HDX measurements are analyzed using a suitable thermodynamic model (SI Text), it can be shown that an increase in the SH3 domain half-life by a factor of 4.6 could actually imply that N-cap enhances the affinity for the SH2–KL by about twofold (Fig. S5). This interpretation would be consistent with the calculated increase in the population of the on state when N-cap is included in our simulations.
In summary, our analysis supports a model in which the region of N-cap most proximal to the SH3 domain may bind the SH3–SH2 connector in either of the two main conformers of the tandem, although its affinity for the on conformer is slightly higher. Either way, binding of N-cap introduces a conformational bias in the SH3–SH2 tandem toward the on state, thus relieving the strain associated with the engagement of the SH2–KL, which as mentioned opposes the intrinsic conformational propensity of the tandem. The conformational bias induced by N-cap on the SH3–SH2 tandem is admittedly subtle; however, it should be noted that mutations that disrupt the interaction of N-cap with the SH3–SH2 connector result in kinase activity levels that are only two to six times greater than those of the fully regulated enzyme (7). Thus, we envisage that these mutations diminish the thermodynamic stability of the assembled complex, i.e., they result in an increased population of partially disassembled, and thus uninhibited, Abl. This subtle perturbation of the regulatory mechanism is not negligible; by comparison, impairment of the interaction between the SH3 domain and the SH2–KL, or deletion of the myristoylated N terminus, lead to an approximate eightfold increase in kinase activity (28). Thus, the magnitude of the allosteric effect that we propose for N-cap is, in our view, significant.
Mutations in the SH3–SH2 Connector Influence Assembly Equilibrium.
From the data presented thus far and from previous studies (13, 21, 31), it can be inferred that the connector between the SH3 and SH2 domains in tyrosine kinases is key to determining the relative arrangements of these domains in space, rather than being a simple flexible linker. Indeed, our calculated free energy landscapes for the cAbl SH3–SH2 tandem reveal a clear correlation between the two-state dynamics of the tandem (S1 variable in Fig. 4) and alternate, well-defined conformations of the connector (S2 variable in Fig. 4).
Experimentally, mutations in this region have been shown to result in increased activation levels in both Src and Abl kinases (6, 28). A plausible interpretation of these observations that has been put forward is that in these mutants the SH3–SH2 unit has a diminished ability to clamp the two lobes of the catalytic domain in the inactive state, even if the complex is assembled (6, 31). An alternative, possibly complementary interpretation is that in the mutants the equilibrium between assembled and disassembled forms of the enzyme is shifted toward the latter, i.e., the uninhibited form (13).
To assess this second interpretation, we set out to study the impact of mutations in the Abl SH3–SH2 connector on the ability of the tandem to engage the SH2–KL. The specific residues to be mutated were selected on the basis of Ramachandran plots extracted from the simulations of the SH3–SH2 tandem presented previously (Fig. S6). This analysis shows that the exchange between on and off conformations of the tandem correlates with ϕ/ψ transitions at positions Asn139, Ser140, and Leu141. Thus, two different constructs were studied experimentally and computationally: a triple mutant with Gly residues at these three positions and a single proline mutant at position S140.
The triple glycine substitution leads to a significant decrease in the melting temperature of the SH3–SH2–KL construct, by about 3.5 °C, without much change in the overall shape of the thermogram (Fig. 8A). We interpret this shift to reflect an increased entropic penalty on association of the SH3 domain with the KL, shifting the binding equilibrium toward unbound conformations. Consistent with this interpretation, molecular simulations of an SH3–SH2 construct carrying these mutations demonstrate a marked increase in the conformational entropy of the tandem, relative to the WT, although conformations compatible with the engagement of the SH2–KL by the SH3 domain are still significantly populated (Fig. 8B; Fig. S6).
Fig. 8.
Effect of SH3–SH2 connector mutations on the conformational dynamics of the Abl tandem. (A) Analysis of the interaction between the SH2-kinase linker and the SH3 domain, via differential scanning calorimetry, for WT and mutated SH3–SH2-kinase linker constructs. (B) Molecular dynamics trajectories of the SH3–SH2 tandem, on mutation of the SH3–SH2 connector.
The substitution of Ser140 by proline has an even more drastic effect. The thermogram for this construct features two peaks and closely resembles the sum of the thermograms measured for the individual domains, suggesting that the association between the SH3 domain and the SH2–KL is more severely impaired (Fig. 8A). Accordingly, molecular simulations of the tandem reveal that the Ser140Pro mutation induces the tandem to adopt essentially a unique conformation (Fig. 8B) that is apparently incompatible with the engagement of the SH2–KL (the RMSD relative to the on conformer is >6 Å).
In summary, DSC and simulation data provide support to the notion that mutations in the SH3–SH2 connector significantly impair one of the necessary intermediate steps in the assembly of the autoinhibited form of Abl, namely the association of the SH2–KL with the regulatory tandem. It is therefore logical to conclude that this impairment accounts, at least in part, for the increased activation levels observed for such mutants (28).
Conclusions
Modular domains play a central role in the organization and propagation of signals in protein interaction networks. These modules may function as scaffolds to facilitate the colocalization of signaling enzymes and thus contribute to amplify the relay of signals with the necessary specificity (32). In many cases, such as tyrosine kinases and phosphatases, tandems of modular domains also function as intramolecular regulatory units, assembling onto the catalytic components and allosterically inhibiting their mechanisms (33). The emerging paradigm from studies of the structural dynamics of these complexes is that the modular regulatory units have evolved to adopt multiple, well-defined conformations, consistent with their dual functional roles (34). As for other multidomain proteins, the nature of these conformational states seems to be encoded, at least in part, by seemingly nonstructured linkers between individual modules (35); thus, mutations or posttranslational modifications of these linkers often have a significant effect on activity.
Here, we showed that the SH3–SH2 tandem of the Abl tyrosine kinase is a dynamic two-state switch, alternating between the well-known inhibitory conformation and another configuration in which both the SH3 domain and the PxxP motifs in the SH2–KL are exposed and that seems to correspond to the uninhibited state, according to available SAXS data. We envisage that this alternate conformer, which is also consistent with an existing crystal structure, is likely to mediate interactions of Abl with other proteins. For example, the so-called Abl interactor protein Abi-2, widely expressed in human tissues, is believed to interact with Abl concurrently via its proline-rich amino terminus and its C-terminal SH3 domain, which would engage the Abl SH3 domain and SH2–KL, respectively (36). Likewise, the SH3 domain of the adaptor protein Crk targets PxxP motifs in Abl (37), while simultaneously, the Abl SH3 domain recognizes a PxxP motif within the Crk SH2 domain (38).
Our data also indicate that the specific amino acid sequence of the connector between the SH3 and SH2 domains has evolved to restrict the conformational flexibility of the regulatory unit, so that a well-defined set of conformations is favored; mutations in this connector, therefore, are likely to influence the thermodynamic equilibrium between autoinhibited and uninhibited forms of Abl, as was previously proposed for Src family kinases (13), whose domain architecture is similar to that of Abl. Nevertheless, our simulations reveal subtle differences between Abl and Src kinases. In particular, we find that in Abl, the so-called N-cap complements the SH3–SH2 connector in stabilizing the SH3–SH2 unit in a conformation conducive to the autoinhibited state. This allosteric role is consistent with comparative activity measurements of WT and mutant forms of Abl, as well as with unfolding experiments specifically probing the stabilizing role of the N-cap, according to our own independent analysis of the published data.
In summary, we gained detailed structural and thermodynamic information on the molecular mechanism of regulation of a signaling enzyme of significant biomedical importance, which is also paradigmatic among modular domain proteins. From a methodological standpoint, our study also underscores the potential of computationally distributed, enhanced-sampling simulation methods for the quantitative analysis of conformational exchange processes. We envisage that such methods will surely complement conventional simulation approaches focused on sampling ever-increasing time scales.
Methods
Unbiased Simulations.
Molecular dynamics trajectories were calculated with NAMD 2.7b1 (39) and the CHARMM22/CMAP protein force field (40, 41). The simulation systems (Table S1) were prepared with CHARMM (42). Each of the constructs was immersed in a truncated-octahedral water box, 75 Å in size, which also included 0.15 M KCl and counter ions to neutralize the net charge of the protein. To optimize the protein–solvent interface, each system was energy minimized with harmonic restraints on protein atoms and crystallographic water molecules and subsequently equilibrated via conventional molecular dynamics simulations, with gradually weaker restraints, over 4 ns. All restraints were then released, and the simulations were continued for up to 500 ns per repeat. All trajectories were calculated at 298 K and 1 atm, using a stochastic thermostat/barostat, and periodic boundary conditions. The integration time step was 2 fs. Electrostatic interactions were computed using the Particle-Mesh-Ewald (PME) algorithm with a real-space cutoff distance at 12 Å; the same cutoff distance was used for van der Waals interactions, modeled with a Lennard-Jones function.
Processing of SAXS Data.
The X-ray scattering data reported in ref. 7 for a deregulated, mutant form of Abl was scanned from the published figure (Fig. 3C, red curve) and used to construct a 3D, low-resolution shape reflecting the most probable conformation of the enzyme. Specifically, we used the simulated annealing procedure in GASBOR (43) to construct 34 alternative shapes consistent with the probability distribution of interatomic distances derived from the scattering data. These shapes were aligned and averaged using DAMAVER (44). The normalized space discrepancy among these shapes is 1.46 ± 0.045, which is only slightly higher than the value originally reported in ref. 7 (1.362 ± 0.032). The final SAXS envelope shown in Fig. 5 was then obtained from the average shape, using DAMFILT. Alternative conformations of the atomic structure of the SH3–SH2-kinase complex were fitted into the envelope using DAMSUP.
Free Energy Landscapes.
All bias-exchange metadynamics calculations were carried out with GROMACS/PLUMED (45, 46) and the CHARMM22/CMAP protein force field, using analogous simulation systems and conditions as above, except for the introduction of a virtual hydrogen scheme, which enable us to use a time step of 4 fs. In bias-exchange metadynamics, a series of concurrent simulations or replicas are carried out in which the protein is adiabatically driven from one conformer to another, through history-dependent biasing forces that oppose sampling of conformations previously explored (47, 48). At a given frequency, attempts are made to exchange the biases accumulated in each replica, using a Metropolis Monte Carlo acceptance criterion, as in other Hamiltonian exchange schemes (49). Effectively, this procedure results in the flattening of the conformational free energy landscape of the molecular system, and therefore, the protein is eventually able to interexchange between the reference states easily, in all replicas. At convergence, the accumulated biases may be combined and transformed into an unbiased probability distribution, i.e., a free energy landscape. Each of the calculations (Table S1) involved 32 simulation replicas and biasing potentials on five collective variables, referred to as D1, D2, and S1–S3. Twelve replicas included a metadynamics bias on S1 and S2; 15 replicas on S1 and S3; and 4 replicas on D1 and D2. The last replica was unbiased. D1 and D2 are pseudodihedral angles defined by the centers-of-mass of the backbone atoms of residues 137, 141, 146, and 150 and residues 134, 137, 141, and 146, respectively (Fig. 1B). S1 and S2 are so-called path collective variables (50), defined in terms of the RMSD values of a given conformation relative to the two alternate structures of the SH3–SH2 tandem, namely those in PDB ID codes 2ABL and 2FO0. Finally, S3 measures the orthogonal distance to the straight path between these two conformations (50). By construction, S1 and S3 monitor the relative orientation of the two domains, each defined by the Cα trace of the regions with secondary structure within these domains; S2 specifically monitors the conformation of the SH3–SH2 connector, defined by the Cα trace of residues 136–145. The normalization factor λ was set to 4 nm−2 for S1 and S2, and to 30 nm−2 for S3. Boundary quadratic potentials were used to restrict S1 and S3 to values between 1 and 2 and S3 to values between 0 and 3 nm2. To avoid systematic errors in the calculated free energies, a boundary correction scheme was also used at these values, as described previously (51). In the metadynamics simulations that included the N-cap region or the SH2–KL, distance restraints were applied to a subset of residue pairs (or centers-of-mass) to ensure that these remained bound to the SH3–SH2 tandem. The reference value and for each restrain was derived from the unbiased simulations previously carried out for these constructs. For SH3–SH2–KL, we used the following pairs: Pro149 and Trp118/Trp129; Pro242 and Tyr89/Tyr134; and Val244 and Phe91/Pro131. For NCap–SH3–SH2, we used Ser69 and His144; Trp67 and Trp146/Phe168/Val218; and Leu73 and Val218/Trp146.
The time-dependent metadynamics bias was progressively developed over the first 100 ns of simulation time per replica (the filling time), in which the height of the biasing-Gaussians, added every 2 ps, was gradually reduced from 0.5 to 0.1 kJ⋅mol−1. The widths of the biasing Gaussians was, however, constant, namely 0.03 (or 0.05 for NCap–SH3–SH2), 0.07 (0.015), and 0.07 nm2 for S1, S2, and S3, respectively, and 0.3 rad for both D1 and D2. Exchanges between pairs of replica were attempted every 2 ps. On average, the exchange acceptance ratio was ∼30–40% among biased replicas and ∼2–10% between these and the unbiased replica, depending on the construct.
The free energy landscapes in Fig. 4 and Fig. S2 were derived from the sampling subsequent to the filling time, namely 106 ns for SH3–SH2, 102 ns for SH3–SH2–KL, and 58 ns for NCap–SH3–SH2, per replica. The derivation followed a methodology previously described (52), using METAGUI (53). Briefly, the weighted histogram analysis method was used to combine the sampling gathered in all replicas and to derive the global, unbiased free energy landscapes. This calculation requires, for each replica, the biased probability distribution of all microstates in collective-variable space, the time-averaged bias potential accumulated during the simulation (after the filling time) on each microstate, and the variance of this average. The latter was defined as the mean-squared difference between two time averages, each spanning one half of the simulation. For a given microstate and a given replica, the larger the variance, the smaller the contribution of that replica to the unbiased probability of that microstate; for computational convenience, a cutoff in the variance of (8 kBT)2 was used in the calculation of the weights. To compute the populations of the on and off states cited in the text, the unbiased probability distribution in S1–S3/D1–D2 was first recalculated in terms of the RMSD with respect to each reference structure, i.e., PDB ID codes 2ABL (RMSD1) and 2FO0 (RMSD2). The probability of the on state is the integral of this 2D function for 0 < RMSD2 < 4 Å and 6 < RMSD1 < 10 Å, whereas for the off state is 0 < RMSD1 < 4 Å and 6 < RMSD2 < 10 Å. Error estimates were computed as described previously (52).
Protein Samples.
The Abl SH3 domain was expressed and purified as previously described (27). The full-length Abl-1b gene encoded for Escherichia coli was synthesized and cloned into the pETM-28a plasmid by GeneArt. DNA fragments corresponding to the Abl SH2 domains and to constructs SH3–SH2 (Ser78 to Pro237) and SH3–SH2–KL (Ser78 to Trp253) were subsequently subcloned by Topgenetech into the pETM-11 plasmid (Protein Expression and Purification Core Facility, EMBL), containing an N-terminal 6xHis tag and a tobacco etch virus (TEV) protease cleavage site. SH3–SH2–KL mutants were constructed using the QuikChange site-directed mutagenesis kit from Stratagene. Plasmid-encoded SH2, SH3–SH2, SH3–SH2–KL, and its mutant constructs were expressed in E. coli BL21 (DE3) strain (Novagen). Cells were grown on Luria-Bertani medium at 37 °C. Protein expression was induced at OD 600 nm ∼ 0.6–0.8 with 1 mM isopropyl-D-1-thiogalactopyranoside for 12–20 h at 37 °C. Cells were resuspended after 10-min centrifugation at 8,346 × g and 4 °C in 50 mM phosphate and 0.3 M NaCl, pH 8.0, buffer [column buffer (CB)] and broken with two passes in a French pressure cell. After 30-min centrifugation at 70,409 × g and 4 °C, the supernatant was loaded onto a 5-mL Ni-Sepharose column (Pharmacia) equilibrated with CB. The protein was eluted with a solution of CB + 500 mM imidazole. Protein-containing fractions were extensively dialyzed against TEV buffer (50 mM Tris⋅HCl, pH 8.0, 0.5 mM EDTA, and 1 mM DTT) to eliminate imidazole. The His-tag was hydrolyzed by overnight incubation with TEV protease in the presence of 1 mM ditiothreitol at room temperature. The cleaved proteins were recovered by a second chromatography step on a Ni-Sepharose resin (Pharmacia). Protein purity was checked by SDS/PAGE to be >95%. Finally, the identity and purity of the purified proteins were confirmed by matrix-assisted laser desorption/ionization time of flight experiments carried out at the Center of Scientific Instrumentation of the University of Granada. Samples were concentrated to 0.6–0.8 mg⋅mL−1 in 50 mM Pipes buffer, frozen in liquid nitrogen, and stored at −80 °C. Under these conditions, protein samples were stable for several months. Protein concentration was determined from absorbance measurements at 280 nm using an extinction coefficient of 39,880 M−1⋅cm−1 for SH3–SH2–KL (19.9 kDa), 31,400 M-1⋅cm−1 for SH3–SH2 (17.8 kDa), and 16,894 and 17,420 M−1⋅cm−1 for the SH3 Abl (7.4 kDa) and SH2 Abl (11.4 kDa) domains, respectively.
DSC.
The heat capacities of all samples were measured as a function of temperature with a high-precision differential scanning VP-DSC microcalorimeter (MicroCal). Samples were prepared by extensive dialysis against a large volume of the appropriate buffer (50 mM Pipes, pH 7.0). All DSC experiments were performed at a scan rate of 1.5 K⋅min−1 using a protein concentration around 0.6 mg⋅mL−1. Protein samples and reference solutions were properly degassed and carefully loaded into the cell to avoid bubble formation. Thermal denaturation scans were recorded from 5–100 °C. Samples were cooled down inside the calorimeter and reheated to check the reversibility of the unfolding process at each experimental condition. The DSC thermograms were systematically corrected for the time response of the calorimeter and for the instrumental baseline obtained with both calorimeter cells filled with the corresponding dialysis buffer. After normalization for protein concentration, the partial molar heat capacity curves (Cp) were calculated from the resulting thermograms, assuming a value of 0.73 mL⋅g−1 for the partial specific volume of all proteins.
Supplementary Material
Acknowledgments
We thank Dr. José C. Martínez for many helpful discussions. This project was funded in part by Project EXC115 of the German Research Foundation (J.F.G.) and Grants BIO2009-13261-CO2 and BIO2012-39922-CO2 of the Spanish Ministry of Science and Innovation (to I.L.), as well as by the Fondo Europeo de Desarrollo Regional and Grant CVI-05915 from the Andalusian Government (to I.L.). C.C.-V. and A.Z.-R. were recipients of a Formación de Personal Investigador fellowship from the Spanish Ministry of Science and Innovation. Computing resources were provided in part by the node of the Andalusian Network for Scientific Supercomputing at the University of Granada. Mass spectrometry measurements were performed at the Center of Scientific Instrumentation of the University of Granada.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1303966110/-/DCSupplemental.
References
- 1.Colicelli J. ABL tyrosine kinases: Evolution of function, regulation, and specificity. Sci Signal. 2010;3(139):re6. doi: 10.1126/scisignal.3139re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang JY. Regulation of cell death by the Abl tyrosine kinase. Oncogene. 2000;19(49):5643–5650. doi: 10.1038/sj.onc.1203878. [DOI] [PubMed] [Google Scholar]
- 3.Raitano AB, Whang YE, Sawyers CL. Signal transduction by wild-type and leukemogenic Abl proteins. Biochim Biophys Acta. 1997;1333(3):F201–F216. doi: 10.1016/s0304-419x(97)00023-1. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Neriah Y, Daley GQ, Mes-Masson AM, Witte ON, Baltimore D. The chronic myelogenous leukemia-specific P210 protein is the product of the bcr/abl hybrid gene. Science. 1986;233(4760):212–214. doi: 10.1126/science.3460176. [DOI] [PubMed] [Google Scholar]
- 5.Hantschel O, Superti-Furga G. Regulation of the c-Abl and Bcr-Abl tyrosine kinases. Nat Rev Mol Cell Biol. 2004;5(1):33–44. doi: 10.1038/nrm1280. [DOI] [PubMed] [Google Scholar]
- 6.Nagar B, et al. Structural basis for the autoinhibition of c-Abl tyrosine kinase. Cell. 2003;112(6):859–871. doi: 10.1016/s0092-8674(03)00194-6. [DOI] [PubMed] [Google Scholar]
- 7.Nagar B, et al. Organization of the SH3-SH2 unit in active and inactive forms of the c-Abl tyrosine kinase. Mol Cell. 2006;21(6):787–798. doi: 10.1016/j.molcel.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 8.Sicheri F, Kuriyan J. Structures of Src-family tyrosine kinases. Curr Opin Struct Biol. 1997;7(6):777–785. doi: 10.1016/s0959-440x(97)80146-7. [DOI] [PubMed] [Google Scholar]
- 9.Boggon TJ, Eck MJ. Structure and regulation of Src family kinases. Oncogene. 2004;23(48):7918–7927. doi: 10.1038/sj.onc.1208081. [DOI] [PubMed] [Google Scholar]
- 10.Levinson NM, Seeliger MA, Cole PA, Kuriyan J. Structural basis for the recognition of c-Src by its inactivator Csk. Cell. 2008;134(1):124–134. doi: 10.1016/j.cell.2008.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowan-Jacob SW, et al. The crystal structure of a c-Src complex in an active conformation suggests possible steps in c-Src activation. Structure. 2005;13(6):861–871. doi: 10.1016/j.str.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Bernadó P, Pérez Y, Svergun DI, Pons M. Structural characterization of the active and inactive states of Src kinase in solution by small-angle X-ray scattering. J Mol Biol. 2008;376(2):492–505. doi: 10.1016/j.jmb.2007.11.066. [DOI] [PubMed] [Google Scholar]
- 13.Faraldo-Gómez JD, Roux B. On the importance of a funneled energy landscape for the assembly and regulation of multidomain Src tyrosine kinases. Proc Natl Acad Sci USA. 2007;104(34):13643–13648. doi: 10.1073/pnas.0704041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barouch-Bentov R, Sauer K. Mechanisms of drug resistance in kinases. Expert Opin Investig Drugs. 2011;20(2):153–208. doi: 10.1517/13543784.2011.546344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iacob RE, Zhang J, Gray NS, Engen JR. Allosteric interactions between the myristate- and ATP-site of the Abl kinase. PLoS ONE. 2011;6(1):e15929. doi: 10.1371/journal.pone.0015929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu X-L, Cao X, Liu X-Y, Jiao B-H. Recent progress of Src SH2 and SH3 inhibitors as anticancer agents. Curr Med Chem. 2010;17(12):1117–1124. doi: 10.2174/092986710790827861. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, et al. Targeting Bcr-Abl by combining allosteric with ATP-binding-site inhibitors. Nature. 2010;463(7280):501–506. doi: 10.1038/nature08675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hantschel O, Grebien F, Superti-Furga G. Targeting allosteric regulatory modules in oncoproteins: “Drugging the undruggable”. Oncotarget. 2011;2(11):828–829. doi: 10.18632/oncotarget.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grebien F, et al. Targeting the SH2-kinase interface in Bcr-Abl inhibits leukemogenesis. Cell. 2011;147(2):306–319. doi: 10.1016/j.cell.2011.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ulmer TS, Werner JM, Campbell ID. SH3-SH2 domain orientation in Src kinases: NMR studies of Fyn. Structure. 2002;10(7):901–911. doi: 10.1016/s0969-2126(02)00781-5. [DOI] [PubMed] [Google Scholar]
- 21.Arold ST, et al. The role of the Src homology 3-Src homology 2 interface in the regulation of Src kinases. J Biol Chem. 2001;276(20):17199–17205. doi: 10.1074/jbc.M011185200. [DOI] [PubMed] [Google Scholar]
- 22.Nam HJ, Haser WG, Roberts TM, Frederick CA. Intramolecular interactions of the regulatory domains of the Bcr-Abl kinase reveal a novel control mechanism. Structure. 1996;4(9):1105–1114. doi: 10.1016/s0969-2126(96)00116-5. [DOI] [PubMed] [Google Scholar]
- 23.Fushman D, Xu R, Cowburn D. Direct determination of changes of interdomain orientation on ligation: Use of the orientational dependence of 15N NMR relaxation in Abl SH(32) Biochemistry. 1999;38(32):10225–10230. doi: 10.1021/bi990897g. [DOI] [PubMed] [Google Scholar]
- 24.Chen S, Brier S, Smithgall TE, Engen JR. The Abl SH2-kinase linker naturally adopts a conformation competent for SH3 domain binding. Protein Sci. 2007;16(4):572–581. doi: 10.1110/ps.062631007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Straume M, Freire E. Two-dimensional differential scanning calorimetry: simultaneous resolution of intrinsic protein structural energetics and ligand binding interactions by global linkage analysis. Anal Biochem. 1992;203(2):259–268. doi: 10.1016/0003-2697(92)90311-t. [DOI] [PubMed] [Google Scholar]
- 26.Luque I, Leavitt SA, Freire E. The linkage between protein folding and functional cooperativity: Two sides of the same coin? Annu Rev Biophys Biomol Struct. 2002;31:235–256. doi: 10.1146/annurev.biophys.31.082901.134215. [DOI] [PubMed] [Google Scholar]
- 27.Palencia A, Cobos ES, Mateo PL, Martínez JC, Luque I. Thermodynamic dissection of the binding energetics of proline-rich peptides to the Abl-SH3 domain: Implications for rational ligand design. J Mol Biol. 2004;336(2):527–537. doi: 10.1016/j.jmb.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 28.Hantschel O, et al. A myristoyl/phosphotyrosine switch regulates c-Abl. Cell. 2003;112(6):845–857. doi: 10.1016/s0092-8674(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 29.Nagar B, et al. Crystal structures of the kinase domain of c-Abl in complex with the small molecule inhibitors PD173955 and imatinib (STI-571) Cancer Res. 2002;62(15):4236–4243. [PubMed] [Google Scholar]
- 30.Chen S, Dumitrescu TP, Smithgall TE, Engen JR. Abl N-terminal cap stabilization of SH3 domain dynamics. Biochemistry. 2008;47(21):5795–5803. doi: 10.1021/bi800446b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young MA, Gonfloni S, Superti-Furga G, Roux B, Kuriyan J. Dynamic coupling between the SH2 and SH3 domains of c-Src and Hck underlies their inactivation by C-terminal tyrosine phosphorylation. Cell. 2001;105(1):115–126. doi: 10.1016/s0092-8674(01)00301-4. [DOI] [PubMed] [Google Scholar]
- 32.Cesareni G. Modular Protein Domains. Weinheim, Germany: Wiley-VCH; 2005. p. xxii. [Google Scholar]
- 33.Superti-Furga G, Barila D, Dorey K, Gonfloni S. Regulation of the SRC and ABL protein tyrosine kinases. FASEB J. 1998;12(8):A1324. [Google Scholar]
- 34.Bond PJ, Faraldo-Gómez JD. Molecular mechanism of selective recruitment of Syk kinases by the membrane antigen-receptor complex. J Biol Chem. 2011;286(29):25872–25881. doi: 10.1074/jbc.M111.223321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma B, Tsai CJ, Haliloğlu T, Nussinov R. Dynamic allostery: Linkers are not merely flexible. Structure. 2011;19(7):907–917. doi: 10.1016/j.str.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dai Z, Pendergast AM. Abi-2, a novel SH3-containing protein interacts with the c-Abl tyrosine kinase and modulates c-Abl transforming activity. Genes Dev. 1995;9(21):2569–2582. doi: 10.1101/gad.9.21.2569. [DOI] [PubMed] [Google Scholar]
- 37.Antoku S, Saksela K, Rivera GM, Mayer BJ. A crucial role in cell spreading for the interaction of Abl PxxP motifs with Crk and Nck adaptors. J Cell Sci. 2008;121(Pt 18):3071–3082. doi: 10.1242/jcs.031575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donaldson LW, Gish G, Pawson T, Kay LE, Forman-Kay JD. Structure of a regulatory complex involving the Abl SH3 domain, the Crk SH2 domain, and a Crk-derived phosphopeptide. Proc Natl Acad Sci USA. 2002;99(22):14053–14058. doi: 10.1073/pnas.212518799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phillips JC, et al. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26(16):1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacKerell AD, et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J Phys Chem B. 1998;102(18):3586–3616. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]
- 41.Mackerell AD, Jr, Feig M, Brooks CL., 3rd Extending the treatment of backbone energetics in protein force fields: Limitations of gas-phase quantum mechanics in reproducing protein conformational distributions in molecular dynamics simulations. J Comput Chem. 2004;25(11):1400–1415. doi: 10.1002/jcc.20065. [DOI] [PubMed] [Google Scholar]
- 42.Brooks BR, et al. CHARMM: The biomolecular simulation program. J Comput Chem. 2009;30(10):1545–1614. doi: 10.1002/jcc.21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Svergun DI, Petoukhov MV, Koch MH. Determination of domain structure of proteins from X-ray solution scattering. Biophys J. 2001;80(6):2946–2953. doi: 10.1016/S0006-3495(01)76260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volkov VV, Svergun DI. Uniqueness of ab initio shape determination in small-angle scattering. J Appl Cryst. 2003;36(3):860–864. doi: 10.1107/S0021889809000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hess B, Kutzner C, van der Spoel D, Lindahl E. GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comput. 2008;4(3):435–447. doi: 10.1021/ct700301q. [DOI] [PubMed] [Google Scholar]
- 46.Bonomi M, Branduardi D, Bussi G, Pietrucci F, Parrinello M. PLUMED: A portable plugin for free-energy calculations with molecular dynamics. Comput Phys Commun. 2009;180(10):1961–1972. [Google Scholar]
- 47.Piana S, Laio A. A bias-exchange approach to protein folding. J Phys Chem B. 2007;111(17):4553–4559. doi: 10.1021/jp067873l. [DOI] [PubMed] [Google Scholar]
- 48.Marinelli F, et al. Evidence for an allosteric mechanism of substrate release from membrane-transporter accessory binding proteins. Proc Natl Acad Sci USA. 2011;108(49):E1285–E1292. doi: 10.1073/pnas.1112534108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Faraldo-Gómez JD, Roux B. Characterization of conformational equilibria through Hamiltonian and temperature replica-exchange simulations: Assessing entropic and environmental effects. J Comput Chem. 2007;28(10):1634–1647. doi: 10.1002/jcc.20652. [DOI] [PubMed] [Google Scholar]
- 50.Branduardi D, Gervasio FL, Parrinello M. From A to B in free energy space. J Chem Phys. 2007;126(5):054103. doi: 10.1063/1.2432340. [DOI] [PubMed] [Google Scholar]
- 51.Crespo Y, Marinelli F, Pietrucci F, Laio A. Metadynamics convergence law in a multidimensional system. Phys Rev E Stat Nonlin Soft Matter Phys. 2010;81(5 Pt 2):055701. doi: 10.1103/PhysRevE.81.055701. [DOI] [PubMed] [Google Scholar]
- 52.Marinelli F, Pietrucci F, Laio A, Piana S. A kinetic model of trp-cage folding from multiple biased molecular dynamics simulations. PLOS Comput Biol. 2009;5(8):e1000452. doi: 10.1371/journal.pcbi.1000452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Biarnes X, Pietrucci F, Marinelli F, Laio A. METAGUI. A VMD interface for analyzing metadynamics and molecular dynamics simulations. Comput Phys Commun. 2012;183(1):203–211. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.