Significance
Ischemic retinopathies include a diverse group of diseases in which immature retinal vasculature or damage to mature retinal vessels leads to retinal ischemia. The anticipated rise in the worldwide prevalence of diabetes will result in a concurrent increase in the number of patients with vision impairment from diabetic eye disease, the most common cause of ischemic retinopathy. We set out to identify novel hypoxia-inducible genes that promote vascular permeability and may therefore play a role in the pathogenesis of diabetic eye disease. We demonstrate that angiopoietin-like 4 (ANGPTL4) is up-regulated by the transcriptional enhancer, hypoxia-inducible factor-1 in hypoxic retinal Müller cells, and can promote vascular permeability. Our findings suggest that ANGPTL4 may be a potential therapeutic target for ischemic retinopathies.
Keywords: diabetes, retinal vein occlusion, angiogenesis, transcription factor
Abstract
Vision loss from ischemic retinopathies commonly results from the accumulation of fluid in the inner retina [macular edema (ME)]. Although the precise events that lead to the development of ME remain under debate, growing evidence supports a role for an ischemia-induced hyperpermeability state regulated, in part, by VEGF. Monthly treatment with anti-VEGF therapies is effective for the treatment of ME but results in a major improvement in vision in a minority of patients, underscoring the need to identify additional therapeutic targets. Using the oxygen-induced retinopathy mouse model for ischemic retinopathy, we provide evidence showing that hypoxic Müller cells promote vascular permeability by stabilizing hypoxia-inducible factor-1α (HIF-1α) and secreting angiogenic cytokines. Blocking HIF-1α translation with digoxin inhibits the promotion of endothelial cell permeability in vitro and retinal edema in vivo. Interestingly, Müller cells require HIF—but not VEGF—to promote vascular permeability, suggesting that other HIF-dependent factors may contribute to the development of ME. Using gene expression analysis, we identify angiopoietin-like 4 (ANGPTL4) as a cytokine up-regulated by HIF-1 in hypoxic Müller cells in vitro and the ischemic inner retina in vivo. ANGPTL4 is critical and sufficient to promote vessel permeability by hypoxic Müller cells. Immunohistochemical analysis of retinal tissue from patients with diabetic eye disease shows that HIF-1α and ANGPTL4 localize to ischemic Müller cells. Our results suggest that ANGPTL4 may play an important role in promoting vessel permeability in ischemic retinopathies and could be an important target for the treatment of ME.
Ischemic retinopathies include a diverse group of retinal diseases, in which immature retinal vasculature (e.g., retinopathy of prematurity or incontinentia pigmenti) or damage to mature retinal vessels (e.g., diabetic retinopathy, retinal vein occlusion, or sickle cell retinopathy) leads to retinal ischemia (1). Although diverse (and poorly understood) etiologies may lead to insufficient perfusion of the retina, all lead to a common sequelae: the formation of abnormal leaky blood vessels that can manifest clinically with the accumulation of fluid in the inner retina [i.e., macular edema (ME)] and often, a profound loss of vision (2). Indeed, ME in patients with ischemia-induced retinopathies remains the leading cause of vision loss in the working age population in the developed world (3).
The concept that ischemic retinopathies are driven by ischemia-induced angiogenic factors was proposed over half a century ago (4). A single transcriptional activator, hypoxia-inducible factor-1 (HIF-1), has recently emerged as the master regulator of these angiogenic mediators. HIF-1 is a heterodimeric protein composed of an exquisitely oxygen-sensitive α-subunit and a ubiquitous β-subunit. Under hypoxic conditions, degradation of the oxygen-sensitive HIF-1α subunit is reduced, whereas its transcriptional activity is enhanced (5–7). The resulting increased amount of active HIF-1α protein localizes to the nucleus and binds to HIF-1β, forming a heterodimer (HIF-1) that is capable of binding to the DNA of specific (hypoxia-inducible) genes and inducing broad changes in gene expression that mediate acclimation of cells, tissues, and the organism to conditions of low oxygen tension (8).
Although several HIF-1–dependent factors have been previously reported to stimulate retinal neovascularization, surprisingly, few have been proven to play a significant role in the promotion of vascular permeability and ME. Arguably, the most critical of the HIF-1–dependent secreted factors elaborated by hypoxic cells in ischemic retinopathies is VEGF (9). VEGF, originally identified as vascular permeability factor, is a potent inducer of vessel permeability and ME (10). The recent development of specific monoclonal antibodies directed against VEGF has revolutionized the treatment of ME in patients with ischemic retinopathies. Results from recent clinical trials using anti-VEGF therapies for diabetic ME (DME) have shown remarkable results: not only maintaining but also improving visual acuity (11). Nonetheless, although anti-VEGF treatment has shown better outcomes than alternative treatments, only a minority of patients with DME treated monthly with intravitreal injections of anti-VEGF therapies achieves a clinically significant improvement in visual acuity [i.e., a gain of at least 15 letters—or three lines—on the Early Treatment Diabetic Retinopathy Study (ETDRS) vision chart] after treatment (12). Moreover, some of these patients suffer from persistent or worsening edema and/or vision loss despite treatment.
These observations suggest that other HIF-1–dependent genes may contribute to the pathogenesis of ME in patients with ischemic retinopathies. Here, we used the oxygen-induced retinopathy (OIR) mouse model to examine the contribution of HIF-1 (and HIF-1–dependent factors that participate with VEGF) to the promotion of vascular permeability in ischemic retinopathies.
Results
HIF-1α Accumulation and Müller Glial Cell Injury/Activation Localize to the Ischemic Inner Retina in the OIR Model.
Microvascular complications in diabetic patients are caused by prolonged exposure to high glucose levels. Mouse models, in which the hyperglycemic state is replicated, have proven essential for studying the early stages of diabetic eye disease. However, these models do not adequately reproduce the retinal nonperfusion that results in the release of growth factors that, in turn, promote the vascular permeability characteristic of patients with later stages of diabetic retinopathy (1). Although no animal model has yet been found to show all of the microvascular complications associated with patients with diabetic eye disease, the OIR mouse model faithfully reproduces the inner retina ischemia (nonperfusion) observed in patients with ischemic retinopathies, including the later stages of diabetic retinopathy; it has proven to be an important tool for studying the pathogenesis of these diseases (13).
The inner retina is composed of several cell types, including neurons (retinal ganglion cells, bipolar cells, horizontal cells, and amacrine cells) and glial cells (astrocytes and Müller cells). However, the cells responsible for elaborating HIF-1 target proteins that contribute to ischemic retinal disease remain unclear. To better understand the molecular pathogenesis of ischemic retinopathies, we first set out to examine HIF-1α protein stabilization in retinal cells using the OIR mouse model. In OIR mice, vaso-obliteration of the posterior retinal vasculature during the hyperoxia phase (P; P7–P12) results in retinal hypoxia in the posterior—but not the peripheral—retina (Fig. S1) (14), thereby providing a reliable internal control (i.e., the peripheral retina) for these studies. HIF-1α protein levels were increased within 24 h of returning mice to normoxic conditions in the posterior (hypoxic) inner retina within the inner nuclear layer (Fig. 1), which has been previously described (15).
Fig. 1.
HIF and GFAP expressions colocalize to injured Müller cells in the OIR model. Representative images from immunofluorescent analysis showing HIF-1α protein levels are increased (purple arrow) in P13 OIR mice (24 h posthyperoxia) in the posterior (hypoxic) inner retina within the inner nuclear layer and localize to areas of increased expression of GFAP (green arrows) in injured Müller glial cells. Accumulation of HIF-1α protein is inhibited by daily i.p. injections of digoxin (2 mg/kg) to levels similar to those levels in P13 control mice. n = 6 animals in each group.
Increased HIF-1α protein levels in the inner retina localized to an area with an increase in the expression of the intermediate filament protein glial fibrillary acidic protein (GFAP) (Fig. S2A). GFAP is expressed in astrocytes but also, injured or activated retinal Müller glial cells in response to injury during different pathological conditions (including ischemia, trauma, retinal degeneration, and glaucoma). Of interest, in ischemic diseases affecting the brain (e.g., stroke), astrocytes—glial cells previously thought to contribute only a supportive or structural role—have recently emerged as central players in the response to ischemia (16). In this context, glial cells play an essential role in the angiogenic response, producing key secreted factors that act in concert to help acclimate the neurons (and brain) to conditions of reduced oxygen tension. To further explore whether Müller glial cells may play an analogous role in ischemic retinopathies, we examined expression of GFAP in the posterior (hypoxic) inner retina compared with the intermediate and peripheral inner retinas in the OIR model. We observed a marked increase in expression of GFAP within hypoxic Müller cells in the inner retina in the ischemic posterior–intermediate but not the perfused peripheral retina (Fig. S2B). Interestingly, GFAP expression was not affected by administration of digoxin, an inhibitor of HIF-1α translation (17), suggesting that GFAP expression is a result of Müller cell injury from hypoxia but independent of HIF-1α transcriptional activity (Fig. 1 and Fig. S2).
Hypoxia Up-Regulates HIF and VEGF in Injured Müller Glial Cells.
To directly assess the response of retinal Müller cells to hypoxia, we isolated primary Müller cell cultures (>95% pure) from the neurosensory retinas of P0–P5 C57BL/6 mice (Fig. 2A). These cells maintained a Müller cell phenotype for over eight passages, which was shown by the expression of key Müller cell markers, including vimentin, cellular retinaldehyde-binding protein (CRALBP), and GFAP (Fig. S3). Primary murine Müller cells responded to hypoxia (3% O2) with an increase in HIF-1α protein stability and nuclear localization (Fig. 2 B and C) and an increase in the mRNA levels of the HIF-1 target gene Vegf (Fig. 2D). To confirm a role for Müller cells in the hypoxic response in humans, we took advantage of the availability of a previously characterized immortalized human Müller (MIO-M1) cell line (18). Similar to primary murine Müller cells, exposure of MIO-M1 cells to hypoxia resulted in an increase in HIF-1α protein stability and nuclear localization (Fig. 2 E and F), resulting in an increase in VEGF mRNA levels and secreted protein (Fig. 2G).
Fig. 2.
HIF-1α protein accumulation and VEGF expression in cultured hypoxic Müller cells and patients with diabetic eye disease. (A) Primary murine Müller cells isolated from mouse retinas at age P0–P5 express key Müller cell markers (vimentin, GFAP, and CRALBP). (B–D) Exposure of primary mouse Müller cells to hypoxia (8 h) induces (B) HIF-1α protein stability by western blot (WB) and (C) nuclear localization, and (D) it results in increased Vegf mRNA. (E–G) Exposure of immortalized human Müller (MIO-M1) cells to hypoxia (8 h or indicated time) induces (E) HIF-1α protein stability and (F) nuclear localization, and (G) it results in increase VEGF mRNA and protein. (H) Representative images from immunohistochemical analysis of eyes from patients with known diabetic eye disease (n = 5 eyes) reveal the presence of activated (vimentin and GFAP-expressing) Müller cells in the ischemic (posterior) retina but not the peripheral retina (vimentin-expressing only). Similar to GFAP, HIF-1α and VEGF protein were also detected in cells in the inner retina in the posterior but not the peripheral retina. Student's t test, *P < 0.05; **P < 0.01.
We then examined retinal tissue from patients with known diabetic eye disease to determine whether ischemic (injured) Müller cells up-regulate HIF-1 and its target genes in these patients. Similar to the OIR model, injured (GFAP-expressing) Müller cells were detected in the ischemic (posterior) retina but not the perfused peripheral retina (Fig. 2H). HIF-1α and VEGF also localized to the posterior inner retina but were not detected in the peripheral retina (Fig. 2H).
Inhibition of HIF Blocks Edema in Ischemic Retinal Disease in Vivo.
We next set out to determine whether inhibition of HIF-1α could reduce edema in ischemic retinopathies in vivo. Although the OIR model has been used extensively as a model for retinal neovascularization (14), we observed that this model results in increased vascular permeability, with leakage of plasma (Fig. 3A) and the plasma protein albumin (Fig. 3 B and C) into the interstitial tissue. The administration of digoxin to inhibit HIF-1α translation resulted in a decrease in vascular permeability (Fig. 3 and Fig. S4), showing that HIF-1 is required for the promotion of vascular permeability in ischemic retinopathies.
Fig. 3.
Inhibition of HIF-1 translation with digoxin blocks vascular permeability in the OIR model. (A and B) Representative images from H&E and immunohistochemical analysis of P13 OIR mice (24 h posthyperoxia) show (A) an increase in inner retinal cysts (Inset, black arrows) and (B) extravasation of albumin (immunohistochemistry; blue arrows) compared with control animals. Treatment of OIR mice with daily i.p. injections with the HIF inhibitor, digoxin (2 mg/kg), inhibits the increase in inner retinal cysts and extravasated albumin. (C) Representative images from immunofluorescent analysis showing extravascular albumin (green arrows) colocalizing with the inner retinal capillaries (CD31; red arrows) in the P13 OIR mouse but not the control mouse or the P13 OIR mouse treated with daily i.p. injections of digoxin. n = 6 animals in each group.
VEGF Alone Is Not Sufficient to Explain the Induction of Vascular Permeability Mediated by HIF-1 in Hypoxic Müller Cells.
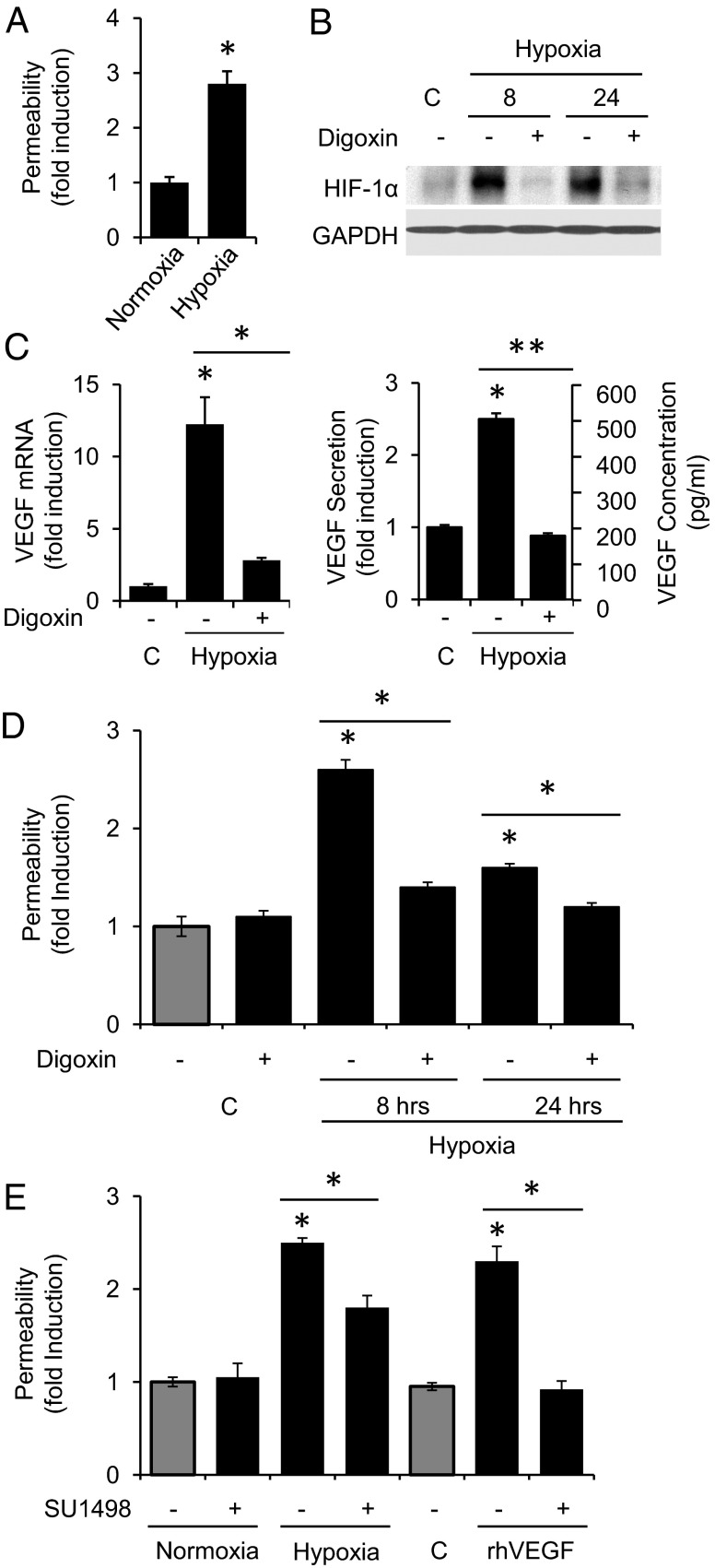
To further examine the contribution of secreted factors elaborated by hypoxia-treated Müller cells to vessel leakage, we next treated monolayers of human dermal microvascular endothelial cells (HMVECs) with conditioned medium from MIO-M1 cells exposed to hypoxia and assessed the promotion of endothelial cell permeability as determined by passage of FITC-dextran. Conditioned medium from the MIO-M1 cells exposed to hypoxia increased endothelial cell permeability by almost threefold compared with MIO-M1 cells cultured under nonhypoxic conditions (Fig. 4A).
Fig. 4.
HIF-1–dependent promotion of endothelial cell permeability by hypoxic Müller cells. (A) Exposure of a monolayer of HMVECs to conditioned media from MIO-M1 cells exposed to hypoxia (8 h) promotes endothelial cell permeability (as measured by passage of FITC-dextran through the monolayer). (B and C) Pretreatment with digoxin (100 nM; 2 h before exposure to hypoxia) inhibits (B) hypoxia-induced HIF-1α protein stabilization, (C) VEGF mRNA, and VEGF secretion (8 h hypoxia) in MIO-M1 cells. (D) Pretreatment with 100 nM digoxin (2 h before 8-h hypoxia exposure) inhibits the promotion of endothelial cell permeability by hypoxic MIO-M1 cells. (E) Pretreatment (2 h before hypoxia) with the VEGF receptor (KDR) inhibitor (SU1498; 5 μM) only partially blocks the promotion of endothelial cell permeability by MIO-M1 cells exposed to 8 h of hypoxia but abolishes the promotion of endothelial cell permeability by 100 ng rhVEGF. Student's t test, *P < 0.05; **P < 0.01.
HIF-1 plays a major role in regulating the ubiquitous transcriptional response to hypoxia. Nonetheless, a number of other transcription factors (e.g., NF-κB, CREB, AP-1, p53, and SP-1 and -3) are also activated either directly or indirectly by hypoxia. We, therefore, set out to confirm that HIF-1–dependent gene expression in hypoxic MIO-M1 cells was primarily responsible for the promotion of endothelial cell permeability. Pretreatment of MIO-M1 cells with digoxin blocked hypoxic induction of HIF-1α protein accumulation (Fig. 4B), which in turn, inhibited VEGF mRNA expression and protein secretion (Fig. 4C) and potently blocked the property of the conditioned media to promote an increase in endothelial cell permeability (Fig. 4D).
To assess whether the effect of digoxin on the promotion of endothelial cell leakage by hypoxic Müller cells was mediated mainly by its inhibition of HIF-1 up-regulation of VEGF, we pretreated endothelial cells with the VEGF receptor-2 or kinase insert domain receptor (KDR) inhibitor SU1498. Although effective doses of SU1498 completely blocked the induction of endothelial cell permeability promoted by 100 ng recombinant human (rh) VEGF—two orders of magnitude higher than the levels secreted by hypoxic MIO-M1 cells—it resulted in only a partial inhibition of endothelial cell permeability by conditioned media from hypoxia-treated MIO-M1 cells (Fig. 4E). These results suggest that inhibition of HIF-1α may be a more potent therapeutic approach for the treatment of ME than inhibition of VEGF alone. Our findings also suggest that, in addition to VEGF, other HIF-dependent secreted factors may participate in the promotion of vascular permeability by hypoxic Müller cells.
Angiopoietin-Like 4 Is Up-Regulated by HIF-1 in Hypoxic Cultures of Müller Cells.
To address the relative contribution of other HIF-1–dependent secreted factors to the pathogenesis of vascular permeability in ischemic retinopathies, we analyzed changes in mRNA expression induced by exposure of MIO-M1 cells to hypoxia using an Affymetrix Gene Array with over 25,000 gene sequences. Transcripts of several known HIF-1 target genes that play key roles in cell survival (DDIT4 and HSP70-2), angiogenesis (VEGF and EDN1), metabolism (PFKFB4 and ALDOC), and pH regulation (CA9 and CA12) were up-regulated in hypoxic MIO-M1 cells (Table S1). Expression of mRNA encoding the angiogenic cytokine, angiopoietin-like 4 (ANGPTL4), was also increased in hypoxic cells (19, 20). Indeed, of over 25,000 genes screened in the hypoxia-treated MIO-M1 cells, ANGPTL4 was among the most highly induced genes (up-regulated more than ninefold). We confirmed that exposure of MIO-M1 cells to hypoxia induced ANGPTL4 mRNA and protein and that ANGPTL4 mRNA was inhibited by digoxin and therefore, HIF-dependent (Fig. 5 A–C). Similar results were observed in primary murine Müller cells (Fig. 5 D and E). These results were confirmed using a second HIF inhibitor rapamycin (Fig. S5 A and B) and further corroborated using an RNAi approach targeting HIF-1β, thereby preventing the formation of the functional HIF-1 (or HIF-2) heterodimer (Fig. S5 C and D).
Fig. 5.
HIF-1–dependent up-regulation of ANGPTL4 by hypoxic Müller cells in vitro. (A–C) ANGPTL4 (A) mRNA and (B and C) protein (arrow points to the top band in WB that corresponds to ANGPTL4) increase with exposure of MIO-M1 cells to hypoxia but are inhibited by pretreatment with the HIF inhibitor, digoxin (100 nM; 2 h before hypoxia). (D and E) Exposure of primary murine Müller cells to hypoxia (8 h) results in (D) an increase in HIF-1α protein accumulation and (E) a corresponding increase in Angptl4 mRNA. (F–J) Infection of MIO-M1 cells with adenovirus expressing a constitutively active deletion mutant of HIF-1α (Ad-CA5) results in (F) accumulation of the stable CA5-HIF-1α mutant and an increase in (G and I) VEGF and Angptl4 mRNA as well as (H and J) protein under normoxic (20% oxygen) conditions. Student's t test, *P < 0.05; **P < 0.01.
ANGPTL4 has previously been shown to be up-regulated by hypoxic stabilization of HIF (21–25). To confirm that stabilization of HIF-1α was sufficient to promote ANGPTL4 expression in retinal Müller cells, we infected MIO-M1 cells with a recombinant adenovirus expressing a constitutively active HIF-1α mutant (Ad-CA5) (26). Infection of MIO-M1 cells with Ad-CA5 showed that forced HIF-1α expression was sufficient to increase ANGPTL4 mRNA levels and protein secretion in nonhypoxic cells (Fig. 5 F–J).
ANGPTL4 Is Up-Regulated by HIF-1 in the Hypoxic Inner Retina in Vivo.
We next returned to the OIR model to examine the induction of ANGPTL4 in the ischemic retina in vivo. We observed that Angptl4 mRNA was induced more than 50-fold in the ischemic retina—two times the effect seen with Vegf (paralleling the results observed in vitro)—and that the up-regulation of Angptl4 was sustained for 72 h after ischemia (Fig. 6A). Immunohistochemical analysis of eyes from OIR mice showed strong expression of ANGPTL4 in the inner retinal layers in the posterior retina in these animals, similar to the expression pattern observed for VEGF (Fig. 6B). Only light expression of ANGPLT4 was detected in age-matched non-OIR mice. Inhibition of HIF-1α protein accumulation in the ischemic inner retina with daily treatment with digoxin completely abolished the induction of Angptl4 but only partially inhibited the induction of Vegf mRNA expression (Fig. 6 C and D).
Fig. 6.
HIF-1–dependent up-regulation of ANGPTL4 in ischemic inner retina in vivo. (A) RT-PCR of Angptl4 and Vegf RNA from the neurosensory retina of OIR animals at P12–P15 normalized to cyclophilin A mRNA and reported as fold induction compared with P12. (B) Representative images from H&E or immunohistochemical analysis of VEGF and ANGPTL in the retina of P13 OIR animals showing expression in the inner posterior retina (Insets). Immunohistochemical analysis of ANGPTL4 levels in age-matched non-OIR pups was low. No primary antibody and IgG as primary antibody were used for negative controls. (C) Representative Western blot of HIF-1α protein accumulation in OIR model with (+) or without (−) daily i.p. injection of digoxin. (D) RT-PCR of Angptl4 and Vegf mRNA from the neurosensory retina of OIR animals at P12–P14 with (+ dig) or without daily i.p. injection of digoxin normalized to cyclophilin A mRNA and reported as fold induction compared with P12. (E) Schematic showing intravitreal injection of Ad-LacZ or Ad-CA5. (F) Western blot showing accumulation of stable HIF-1α in the neurosensory retina in animals infected with Ad-CA5. (G) RT-PCR of Angptl4 mRNA from the neurosensory retina of animals infected with Ad-LacZ (LacZ) or Ad-CA5 (HIF) normalized to cyclophilin A mRNA and reported as fold induction compared with uninfected eyes. n = 6 animals in each group. Student's t test, *P < 0.05. INL, inner nuclear layer; RGC, retinal ganglion cell layer.
We next examined whether forced HIF-1α expression in the nonischemic retina was sufficient to promote an increase in Angptl4 transcription in mice. Intravitreal injection of Ad-CA5 (Fig. 6E) resulted in an accumulation of stable HIF-1α protein within 48 h (Fig. 6F) and an increase of Angptl4 mRNA by almost twofold (Fig. 6G).
ANGPTL4 Promotes Vascular Permeability in Vitro and in Vivo.
The role of ANGPTL4 in endothelial cell function remains controversial and possibly tissue-specific (20). To directly assess whether ANGPTL4 was sufficient to promote vascular permeability in vitro, we treated monolayers of HMVECs with rhANGPTL4 and assessed the promotion of endothelial cell permeability as determined by passage of FITC-dextran. ANGPTL4 potently induced endothelial cell permeability, similar to VEGF (Fig. 7A). We next assessed whether ANGPLT4 could promote vascular permeability in vivo. To this end, we used the modified Miles assay to measure vascular permeability in the mouse ear after intradermal injection with PBS or ANGPTL4. We observed a remarkable increase in vascular permeability after treatment with ANGPTL4, similar to the increase observed with VEGF (Fig. 7B).
Fig. 7.
ANGPTL4 promotes endothelial cell permeability in vitro and vascular permeability in the inner retina in vivo. (A) Exposure of a monolayer of HMVECs to rhANGPTL4 (2.5 or 5 μg/mL) or VEGF (100 ng/mL) promotes endothelial cell permeability (as measured by passage of FITC-dextran through the monolayer). (B) Modified Miles assay was used to measure vascular permeability in the mouse ear after intradermal injection with 20 μL PBS, ANGPTL4 (0.2 μg), or VEGF (0.2 μg) in 20 μL PBS. Representative photos showing leakage of dye at the injection site minutes after injection. Quantitation of Evans blue dye using spectrophotometry (610 nm) shows a marked increase in dye in ears injected with ANGPTL4 compared with PBS (similar to VEGF). n = 3 animals in each group. (C) RNAi targeting ANGPTL4 blocks ANGPTL4 but not VEGF mRNA expression and protein secretion in transfected MIO-M1 cells, and it inhibits the promotion of endothelial cell permeability by conditioned media from MIO-M1 cells exposed to hypoxia for 8 h. (D) Representative H&E-stained sections after intravitreal injection with 200 ng rmANGPTL4 into the mouse eye, showing increased inner retinal cysts (black arrows) compared with control (PBS-treated) eyes. (E) Representative images from immunohistochemical analysis shows extravasation of albumin (blue arrows) in mouse eyes after intravitreal injection of 200 ng rmANGPTL4 compared with control animals. (F) Representative images from immunofluorescent analysis showing albumin (green arrows) extravasation colocalizing with the inner retinal capillaries (CD31; red arrows) in mouse eyes after intravitreal injection of 200 ng rmANGPTL4 compared with control animals. n = 6 animals in each group. Student's t test, *P < 0.05, ***P < 0.001.
We next examined the potential contribution of ANGPTL4 to the promotion of vascular permeability by hypoxic Müller cells. To this end, we used RNAi to specifically inhibit the expression of ANGPTL4. We treated monolayers of HMVECs with conditioned medium from hypoxic MIO-M1 cells pretreated with ANGPTL4 RNAi. Inhibition of ANGPTL4 expression significantly reduced the endothelial cell permeability promoted by hypoxic Müller cells (Fig. 7C).
To directly assess whether ANGPTL4 was sufficient to promote vascular permeability in vivo, we injected recombinant murine (rm) ANGPTL4 intravitreally into the mouse eye and examined the retinas after 48 h. We observed a marked and statistically significant increase in vascular permeability, resulting in leakage of plasma (Fig. 7D) and albumin (Fig. 7 E and F) into the interstitial tissue, similar to the increase in vascular permeability seen with intravitreal injections with rmVEGF (Figs. S6 and S7). Taken together, these results strongly support a role for ANGPTL4 in the promotion of vascular permeability in ischemic retinal disease.
ANGPTL4 Is Expressed in the Inner Retina of Patients with Diabetic Eye Disease.
Examination of retinal tissue from five individuals with known diabetic eye disease revealed that ANGPTL4 was consistently expressed in the posterior ischemic inner retina adjacent to areas of retinal edema, but it was not observed in the peripheral (perfused) retina in all tissues examined (Fig. 8). This expression pattern was similar to the pattern observed for VEGF, and it was identical to the expression pattern of HIF-1α in ischemic (injured) Müller cells (Fig. 2H). Expression of ANGPTL4 was not detected in retinal tissue from normal (age-matched) control patients without a known diagnosis of an ischemic retinopathy (Fig. 8).
Fig. 8.
ANGPTL4 specifically expressed in the ischemic retina of patients with diabetic eye disease. Representative H&E staining and immunohistochemical analysis of eyes from patients with diabetic eye disease (n = 5 eyes) or age-matched nondiabetic controls (n = 5) reveals the presence of ANGPTL4 in the ischemic (posterior) retina but not the perfused (peripheral) retina in diabetic patients and nondiabetic control patients, similar to the expression of VEGF protein. INL, inner nuclear layer; IPL, inner plexiform layer; ONL, outer nuclear layer; OPL, outer plexiform layer; RGC, retinal ganglion cell layer; RPE, retinal pigment epithelium.
Discussion
By 2050, the prevalence of diabetes will more than triple globally, dramatically increasing the burden of this disease worldwide (27). The increase in the diabetic population will result in a concurrent rise in the number of patients with vision impairment from diabetic eye disease, the most common cause of severe vision loss in the working age population in the developed world (9). Despite the recent introduction of therapies targeting VEGF, the majority of patients with DME do not respond with a clinically significant gain in vision (11). An alternative approach for those patients who fail current anti-VEGF agents is to design treatment modalities that more efficiently inhibit VEGF; however, these efforts may have unwanted consequences. VEGF has been shown to play an important role as a neurotrophic factor, and long-term inhibition of VEGF may potentially damage the neurosensory retina (28, 29). The observation that loss of a single copy of Vegf is embryonically lethal in mice shows the importance of this potent growth factor in development (30). Collectively, these considerations support the rationale for the identification and targeting of other factors that participate in the pathogenesis of vascular permeability in patients with ischemic retinopathies.
We provide evidence here that HIF-1 may be a target for the treatment of ME in ischemic retinopathies. In animal models of ischemic retinopathies, inhibition of HIF-1α has been previously shown to also prevent retinal neovascularization (31). These observations argue in favor of therapies directed against HIF-1 as a broad spectrum approach to target multiple hypoxia-inducible genes that promote vascular permeability. However, HIF-1 plays a fundamental role in acclimating cells to ischemia: HIF-1 regulates the metabolic shift from respiration to aerobic glycolysis and lactic acid production, stimulates nutrient supply by influencing adaptive survival mechanisms (e.g., autophagy and lipid and glycogen synthesis and storage), protects cells from oxidative stress, and safeguards cells from acidosis (32, 33). In concert with the angiogenic genes regulated by HIF-1, the responsible genes work together to collectively promote the survival of cells/tissue exposed to chronic ischemia. Inhibition of HIF-1 may, therefore, have undesirable effects on the highly metabolically active retina. Additional studies examining the sequelae of chronic HIF inhibition on the retina are necessary before this approach could be brought to the clinic.
An alternative strategy for the treatment of patients with ischemic retinopathies may be to identify and inhibit only the specific HIF-1–dependent target gene products that promote ME, which has been shown by anti-VEGF therapies. In this regard, we show here that ANGPTL4 is a key HIF-regulated gene expressed in the inner retina by hypoxic Müller cells that promotes vascular permeability in ischemic retinal disease. Angiopoietins have been described as critical factors in vascular development (34). Angiopoietin-1 promotes vessel maturation, whereas angiopoietin-2 antagonizes its effect on vessel stabilization. ANGPTL4 is a secreted glycoprotein that—unlike angiopoietin-1 and -2—does not bind to the TIE-2 receptor and remains an orphan ligand (19, 20, 35). Although ANGPTL4 secretion has been shown to modulate the disposition of circulating triglycerides by inhibiting lipoprotein lipase (20, 36), its role in vascular biology is less clear.
Initial studies on the role of ANGPLT4 in cancer showed that this cytokine may inhibit angiogenesis and tumor metastasis (37–41). However, more recent reports suggest that ANGPTL4 may be proangiogenic and provascular permeability (23, 25, 42–46). ANGPTL4 has been shown to disrupt vascular endothelial cell–cell (tight and adherens) junctions, facilitating cellular transendothelial passage and tumor dissemination (47). ANGPTL4 has also been found to promote the angiogenic and exudative phenotypes characteristic of the unique vascular tumor Kaposi’s sarcoma and activate the rho-associated kinase (ROCK) pathway (48). More recently, it has been shown that ANGPTL4 may promote the disruption of vascular integrity by directly interacting with integrin α5β1, vascular endothelial-cadherin, and claudin-5 in a temporally sequential manner (49).
In the context of the eye, this multifaceted cytokine has also been shown to be both pro- and antiangiogenic (37, 50). Recently, examination of the eyes of homozygous Angptl4 null mice suggested that ANGPTL4 is proangiogenic (50). However, contrary to the observations described here, Perdiguero et al. (50) also found that loss of expression of ANGPTL4 resulted in an increase in vascular permeability in the developing retina. These disparate results may be a consequence of compensatory changes in the levels of other factors that also affect vascular permeability (e.g., VEGF) during development of the retina in the Angptl4 null mice. It may also be that ANGPTL4 plays different roles in vascular development and pathological angiogenesis in the eye. Additional studies to delineate the mechanisms (and context) in which ANGPTL4 exerts its varied effects are clearly necessary.
In addition to VEGF, the list of cytokines and growth factors that have been proposed to participate in the pathogenesis of diabetic eye disease is long. This list includes—but is not limited to—angiopoietins, ILs, PDGF, FGF, hepatocyte growth factor, TGF, placental endothelial cell growth factor, connective tissue growth factor, angiotensin, and monocyte chemotactic protein. However, to date, only VEGF has proven to be an effective target for the treatment of vascular hyperpermeability in DME. Our results suggest that, like VEGF, ANGPTL4 may play an important role in promoting vessel permeability in patients with ischemic retinopathies. We propose that ANGPTL4 may, therefore, be an important mediator of ME in ischemic retinopathies. Our findings provide the foundation for studies to assess a role for ANGPTL4 in DME.
Materials and Methods
Cell Culture and Reagents.
MIO-M1 cells were a gift from Astrid Limb (University College London, Institute of Ophthalmology, London, United Kingdom). Isolation of primary Müller cells was performed as previously described (51). Primary HMVECs were obtained from Lonza and cultured according the manufacturer’s protocols with DMEM (Invitrogen) containing 1g/L glucose with 10% (vol/vol) FBS (Quality Biological) and 1% penicillin/streptomycin (Cellgro). Before treatment (hypoxia or hypoxia mimics), the growth media was replaced with serum starvation media containing 1% FBS. Recombinant ANGPTL4 and VEGF as well as ANGPTL4 and VEGF ELISA kits were obtained from R&D Systems. The KDR inhibitor, SU1498, and rapamycin were obtained from Calbiochem. Digoxin was obtained from Sigma. Predesigned control (Scrambled) and ANGPTL4 and HIF-1β siRNA sequences were obtained from Qiagen. Adenovirus expressing LacZ control (Ad-LacZ) and Ad-CA5 have been previously described (26). Hypoxia chambers were used to expose MIO-M1 cells (1% oxygen) and primary murine Müller cells (3% oxygen; exposure of primary murine Müller cells to lower oxygen concentrations resulted in cell death).
Mice.
Eight-week-old pathogen-free female C57BL/6 mice (Jackson Laboratory), female athymic nu/nu mice (Harlan Sprague–Dawley), and timed pregnant C57BL/6 mice [embryonic day 14(E14); Charles River Laboratories] were treated in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and the guidelines of the Johns Hopkins University Animal Care and Use Committee.
OIR.
OIR experiments were performed as previously described (13). In brief, C57BL/6 mice were placed in 75% O2 at postnatal day P7. At P12, the mice were returned to room air. A subset of mice was given daily i.p. injection of vehicle or 2 mg/kg digoxin. Retinas were collected from mice at P12 (2 h after return to normoxia), P13, P14, and/or P17. Representative images from specified days are shown in the figures.
Intraocular Injections.
Intraocular injections with 1 µL rmVEGF or rmANGPTL4 (200 ng/μL) were performed with a nanofil syringe (World Precision Instruments) using a 36-gauge beveled needle. Intraocular injections with 1 µL Ad-LacZ or Ad-CA5 (2 × 1012 VP/mL) were performed with pulled-glass micropipettes and a Harvard pump microinjection apparatus as previously described (52).
Western Blot and ELISA.
Cell and neurosensory retina lysates were subjected to 4–15% gradient SDS/PAGE (Invitrogen). Immunoblot assays were performed with primary antibodies specifically recognizing HIF-1α [molecular mass of 120 kDa for endogenous HIF-1α (Ab2185; Abcam) and 100 kDa for CA5 HIF deletion mutant (610959; BD Transduction Laboratories)], ANGPTL4 (Millipore), and GAPDH (Fitzgerald). Levels of secreted VEGF and ANGPTL4 were measured in media conditioned by MIO-M1 cells using DuoSet human VEGF or ANGPTL4 ELISA kits (R&D System).
Quantitative Real-Time RT-PCR.
RNA was isolated from culture cells or retinas with RNeasy Mini Kit (Qiagen), and cDNA was repaired using MuLV Reverse Transcriptase (Applied Biosystems). Quantitative real-time PCR was performed with Power SYBR Green PCR Master Mix (Applied Biosystems) and MyiQ Real-Time PCR Detection System (Bio Rad). Normalization was done using cyclophilin A for mouse tissue and cell lines and β-actin for human cell lines. Primers for quantitative PCR include human VEGF, forward: GGGCAGAATCATCACGAAGT and reverse: TGGTGATGTTGGACTCCTCA; human ANGPTL4, forward: GGACACGGCCTATAGCCTG and reverse: CTCTTGGCGCAGTTCTTGTC; human β-actin, forward: CTCTTCCAGCCTTCCTTCCT and reverse: AGCACTGTGTTGGCGTACAG; mouse VEGF, forward: TTACTGCTGTACCTCCACC and reverse: ACAGGACGGCTTGAAGATG; mouse ANGPTL4, forward: TTGGTACCTGTAGCCATTCC and reverse: GAGGCTAAGAGGCTGCTGTA; mouse cyclophilin A, forward: AGCATACAGGTCCTGGCATC and reverse: TTCACCTTCCCAAAGACCAC.
Permeability Assays.
In vitro permeability assay was performed as previously described (48). Briefly, HMVECs were seeded on collagen-coated transwells (3-mm-size pore; PTFE; Corning) and allowed to grow as a 3-d-old mature monolayer. After overnight starvation, 500 and 100 μL conditioned medium were added for 30 min (37 °C) to the bottom and top chamber, respectively. A total of 100 μL 1 mg/mL FITC-dextran (molecular weight = 40,000; Invitrogen) was added for 30 min. Fluorescence was quantified using a SpectraMax M5 Microplate Reader (Molecular Devices) with excitation at 494 nm and emission at 521 nm.
In vivo permeability was assessed using a modified Miles assay as previously described (48). Briefly, Evan Blue dye was injected into mouse tail veins (200 μL 12 mg/mL solution in PBS). After 5 min, animals were anesthetized using Tribromoethanol; 20 μL 0.2 μg VEGF or 0.2 μg ANGPTL4 (in PBS) were injected intradermally into the right ear (PBS was injected into the left ear as a control). After 8 min, photographs were taken. Levels of Evans blue dye were then extracted from mouse ear in 1 mL formamide at 55 °C for 16 h, and dye content was quantified at 610 nm using a spectrophotometer (48).
Quantitation of vascular permeability in the retina was assessed by examining four high-powered fields of the posterior retina from three animals and counting the number of CD31-labled inner retina vessels in the posterior retina that had visible adjacent extravascular albumin (detected by immunofluorescence) as a percent of the total vessels in each field.
Microarray.
Briefly, MIO-M1 cells were treated with or without hypoxia for 8 h, and the mRNA was extracted with the RNeasy Mini Kit (Qiagen); 300–500 ng mRNA were used for microarray assay. The MicroArray assay was performed using the Affymetix Human Gene 1.0ST MicroArray by the Johns Hopkins Deep Sequencing and Microarray Core Facility. The fold of increase of an individual gene expression under hypoxia treatment was calculated using the equation 2(n2 − n1), where n2 is the reading of the hypoxia sample and n1 is the reading of the normoxia sample.
Immunohistochemistry and Immunofluorescence.
Immunohistochemical detection of extravascular albumin (Cedarlane-Nordic) was performed on cryopreserved mouse tissue sections using a nitroblue tetrazolium development system using Streptavidin alkaline phosphatase as previously described (53). Immunohistochemical detection of HIF-1α (Abcam), human and murine ANGPTL4 (Abcam and Lifespan BioSciences, respectively), VEGF (Santa Cruz), and GFAP (Sigma) was performed in paraffin-embedded human tissue (obtained from the Wilmer Eye Institute Ocular Pathology Archives with approval from the Johns Hopkins School of Medicine Internal Review Board) and mice cryopreserved tissue using the ABC System (Dako) as previously described (54).
Immunofluorescence detection of CD31 (BD Pharmingen), Hypoxy-probe (HPI), GFAP, (Sigma), HIF-1α (Abcam), vimentin (Abcam), and albumin (Cedarlane-Nordic) was performed on retina flat mounts or cryopreserved mouse tissue sections as previously described (55–57). Immunodetection was performed using goat anti-mouse Alexa F 555, goat anti-rabbit Alexa F 488, and goat anti-rat Alexa F 647 (Invitrogen) associated with DAPI (Invitrogen). Images were captured using the Zeiss confocal microscope meta 710 LSM (Carl Zeiss Inc.).
Statistical Analysis.
In all cases, results are shown as a mean value ± SD from at least three independent experiments. Western blot scans are representative of at least three independent experiments. Statistical analysis was performed with Prism 4.2 software (GraphPad). Student's t test: ***P < 0.001; **P < 0.01; *P < 0.05.
Supplementary Material
Acknowledgments
We thank Dr. Astrid Limb for providing the MIO-M1 cells, Dr. Morton F. Goldberg for careful review of the manuscript, and the late Dr. Stephen J. Ryan, Jr., for productive scientific discussions. A.S. acknowledges support from the Research to Prevent Blindness Foundation Career Development Award, from a William and Ella Owens Medical Research Foundation research grant, and as a Stephen J. Ryan, Jr., M.D. Scholar at the Wilmer Eye Institute. A.S. also acknowledges the support of his Chairman, Dr. Peter J. McDonnell. This work was supported by Microscopy and Imaging Core Module of the Wilmer Core Grant EY001765 (to G.L.), Animal Core Grant EY001765 (to G.L.), National Eye Institute, National Institutes of Health Grant K08- EY021189 (to A.S.), and an unrestricted grant from Research to Prevent Blindness.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1217091110/-/DCSupplemental.
References
- 1.Bek T. Inner retinal ischaemia: Current understanding and needs for further investigations. Acta Ophthalmol. 2009;87(4):362–367. doi: 10.1111/j.1755-3768.2008.01429.x. [DOI] [PubMed] [Google Scholar]
- 2.Scholl S, Augustin A, Loewenstein A, Rizzo S, Kupperman B. General pathophysiology of macular edema. Eur J Ophthalmol. 2011;21(Suppl 6):10–19. doi: 10.5301/EJO.2010.6050. [DOI] [PubMed] [Google Scholar]
- 3.Crawford TN, Alfaro DV, 3rd, Kerrison JB, Jablon EP. Diabetic retinopathy and angiogenesis. Curr Diabetes Rev. 2009;5(1):8–13. doi: 10.2174/157339909787314149. [DOI] [PubMed] [Google Scholar]
- 4.Michaelson IC. The mode of development of the vascular system of the retina. With some observations on its significance for certain retinal diseases. Trans Ophthalmol Soc U K. 1948;68:137–180. [Google Scholar]
- 5.Maxwell PH. Hypoxia-inducible factor as a physiological regulator. Exp Physiol. 2005;90(6):791–797. doi: 10.1113/expphysiol.2005.030924. [DOI] [PubMed] [Google Scholar]
- 6.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5(5):343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 7.Semenza GL. Hydroxylation of HIF-1: Oxygen sensing at the molecular level. Physiology (Bethesda) 2004;19:176–182. doi: 10.1152/physiol.00001.2004. [DOI] [PubMed] [Google Scholar]
- 8.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang GE. Diabetic macular edema. Ophthalmologica. 2012;227(Suppl 1):21–29. doi: 10.1159/000337156. [DOI] [PubMed] [Google Scholar]
- 10.Nagy JA, Benjamin L, Zeng H, Dvorak AM, Dvorak HF. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis. 2008;11(2):109–119. doi: 10.1007/s10456-008-9099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Javey G, Schwartz SG, Flynn HW., Jr Emerging pharmacotherapies for diabetic macular edema. Exp Diabetes Res. 2012;2012:548732. doi: 10.1155/2012/548732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Witkin AJ, Brown GC. Update on nonsurgical therapy for diabetic macular edema. Curr Opin Ophthalmol. 2011;22(3):185–189. doi: 10.1097/ICU.0b013e3283459724. [DOI] [PubMed] [Google Scholar]
- 13.Smith LE, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35(1):101–111. [PubMed] [Google Scholar]
- 14.Montezuma SR, Vavvas D, Miller JW. Review of the ocular angiogenesis animal models. Semin Ophthalmol. 2009;24(2):52–61. doi: 10.1080/08820530902800017. [DOI] [PubMed] [Google Scholar]
- 15.Mowat FM, et al. HIF-1alpha and HIF-2alpha are differentially activated in distinct cell populations in retinal ischaemia. PLoS One. 2010;5(6):e11103. doi: 10.1371/journal.pone.0011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xing C, Hayakawa K, Lok J, Arai K, Lo EH. Injury and repair in the neurovascular unit. Neurol Res. 2012;34(4):325–330. doi: 10.1179/1743132812Y.0000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, et al. Digoxin and other cardiac glycosides inhibit HIF-1alpha synthesis and block tumor growth. Proc Natl Acad Sci USA. 2008;105(50):19579–19586. doi: 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Limb GA, Salt TE, Munro PM, Moss SE, Khaw PT. In vitro characterization of a spontaneously immortalized human Müller cell line (MIO-M1) Invest Ophthalmol Vis Sci. 2002;43(3):864–869. [PubMed] [Google Scholar]
- 19.Hato T, Tabata M, Oike Y. The role of angiopoietin-like proteins in angiogenesis and metabolism. Trends Cardiovasc Med. 2008;18(1):6–14. doi: 10.1016/j.tcm.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Zhu P, Goh YY, Chin HF, Kersten S, Tan NS. Angiopoietin-like 4: A decade of research. Biosci Rep. 2012;32(3):211–219. doi: 10.1042/BSR20110102. [DOI] [PubMed] [Google Scholar]
- 21.Burkitt K, Chun SY, Dang DT, Dang LH. Targeting both HIF-1 and HIF-2 in human colon cancer cells improves tumor response to sunitinib treatment. Mol Cancer Ther. 2009;8(5):1148–1156. doi: 10.1158/1535-7163.MCT-08-0944. [DOI] [PubMed] [Google Scholar]
- 22.Knowles HJ, Cleton-Jansen AM, Korsching E, Athanasou NA. Hypoxia-inducible factor regulates osteoclast-mediated bone resorption: Role of angiopoietin-like 4. FASEB J. 2010;24(12):4648–4659. doi: 10.1096/fj.10-162230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Jan S, et al. Angiopoietin-like 4 is a proangiogenic factor produced during ischemia and in conventional renal cell carcinoma. Am J Pathol. 2003;162(5):1521–1528. doi: 10.1016/S0002-9440(10)64285-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, et al. Hypoxia-inducible factor 1 alpha-activated angiopoietin-like protein 4 contributes to tumor metastasis via vascular cell adhesion molecule-1/integrin β1 signaling in human hepatocellular carcinoma. Hepatology. 2011;54(3):910–919. doi: 10.1002/hep.24479. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, et al. HIF-1-dependent expression of angiopoietin-like 4 and L1CAM mediates vascular metastasis of hypoxic breast cancer cells to the lungs. Oncogene. 2012;31(14):1757–1770. doi: 10.1038/onc.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Kelly BD, et al. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93(11):1074–1081. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- 27.Lam DW, LeRoith D. The worldwide diabetes epidemic. Curr Opin Endocrinol Diabetes Obes. 2012;19(2):93–96. doi: 10.1097/MED.0b013e328350583a. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Bao S, Hambly BD, Gillies MC. Vascular endothelial growth factor-A: A multifunctional molecular player in diabetic retinopathy. Int J Biochem Cell Biol. 2009;41(12):2368–2371. doi: 10.1016/j.biocel.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Saint-Geniez M, et al. Endogenous VEGF is required for visual function: Evidence for a survival role on müller cells and photoreceptors. PLoS One. 2008;3(11):e3554. doi: 10.1371/journal.pone.0003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrara N, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380(6573):439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida T, et al. Digoxin inhibits retinal ischemia-induced HIF-1alpha expression and ocular neovascularization. FASEB J. 2010;24(6):1759–1767. doi: 10.1096/fj.09-145664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semenza GL. HIF-1: Upstream and downstream of cancer metabolism. Curr Opin Genet Dev. 2010;20(1):51–56. doi: 10.1016/j.gde.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mucaj V, Shay JE, Simon MC. Effects of hypoxia and HIFs on cancer metabolism. Int J Hematol. 2012;95(5):464–470. doi: 10.1007/s12185-012-1070-5. [DOI] [PubMed] [Google Scholar]
- 34.Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10(3):165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 35.Grootaert C, Van de Wiele T, Verstraete W, Bracke M, Vanhoecke B. Angiopoietin-like protein 4: Health effects, modulating agents and structure-function relationships. Expert Rev Proteomics. 2012;9(2):181–199. doi: 10.1586/epr.12.12. [DOI] [PubMed] [Google Scholar]
- 36.Kadomatsu T, Tabata M, Oike Y. Angiopoietin-like proteins: Emerging targets for treatment of obesity and related metabolic diseases. FEBS J. 2011;278(4):559–564. doi: 10.1111/j.1742-4658.2010.07979.x. [DOI] [PubMed] [Google Scholar]
- 37.Ito Y, et al. Inhibition of angiogenesis and vascular leakiness by angiopoietin-related protein 4. Cancer Res. 2003;63(20):6651–6657. [PubMed] [Google Scholar]
- 38.Galaup A, et al. Angiopoietin-like 4 prevents metastasis through inhibition of vascular permeability and tumor cell motility and invasiveness. Proc Natl Acad Sci USA. 2006;103(49):18721–18726. doi: 10.1073/pnas.0609025103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cazes A, et al. Extracellular matrix-bound angiopoietin-like 4 inhibits endothelial cell adhesion, migration, and sprouting and alters actin cytoskeleton. Circ Res. 2006;99(11):1207–1215. doi: 10.1161/01.RES.0000250758.63358.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang YH, et al. Suppression of the Raf/MEK/ERK signaling cascade and inhibition of angiogenesis by the carboxyl terminus of angiopoietin-like protein 4. Arterioscler Thromb Vasc Biol. 2008;28(5):835–840. doi: 10.1161/ATVBAHA.107.157776. [DOI] [PubMed] [Google Scholar]
- 41.Chomel C, et al. Interaction of the coiled-coil domain with glycosaminoglycans protects angiopoietin-like 4 from proteolysis and regulates its antiangiogenic activity. FASEB J. 2009;23(3):940–949. doi: 10.1096/fj.08-115170. [DOI] [PubMed] [Google Scholar]
- 42.Hermann LM, et al. Angiopoietin-like-4 is a potential angiogenic mediator in arthritis. Clin Immunol. 2005;115(1):93–101. doi: 10.1016/j.clim.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Gealekman O, et al. Enhanced angiogenesis in obesity and in response to PPARgamma activators through adipocyte VEGF and ANGPTL4 production. Am J Physiol Endocrinol Metab. 2008;295(5):E1056–E1064. doi: 10.1152/ajpendo.90345.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakayama T, et al. Expression of angiopoietin-like 4 in human gastric cancer: ANGPTL4 promotes venous invasion. Oncol Rep. 2010;24(3):599–606. doi: 10.3892/or_00000897. [DOI] [PubMed] [Google Scholar]
- 45.Nakayama T, et al. Expression of angiopoietin-like 4 (ANGPTL4) in human colorectal cancer: ANGPTL4 promotes venous invasion and distant metastasis. Oncol Rep. 2011;25(4):929–935. doi: 10.3892/or.2011.1176. [DOI] [PubMed] [Google Scholar]
- 46.Akishima-Fukasawa Y, et al. Histopathological predictors of regional lymph node metastasis at the invasive front in early colorectal cancer. Histopathology. 2011;59(3):470–481. doi: 10.1111/j.1365-2559.2011.03964.x. [DOI] [PubMed] [Google Scholar]
- 47.Padua D, et al. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133(1):66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma T, et al. Viral G protein-coupled receptor up-regulates Angiopoietin-like 4 promoting angiogenesis and vascular permeability in Kaposi’s sarcoma. Proc Natl Acad Sci USA. 2010;107(32):14363–14368. doi: 10.1073/pnas.1001065107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang RL, et al. ANGPTL4 modulates vascular junction integrity by integrin signaling and disruption of intercellular VE-cadherin and claudin-5 clusters. Blood. 2011;118(14):3990–4002. doi: 10.1182/blood-2011-01-328716. [DOI] [PubMed] [Google Scholar]
- 50.Perdiguero EG, et al. Alteration of developmental and pathological retinal angiogenesis in angptl4-deficient mice. J Biol Chem. 2011;286(42):36841–36851. doi: 10.1074/jbc.M111.220061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wahlin KJ, et al. A method for analysis of gene expression in isolated mouse photoreceptor and Müller cells. Mol Vis. 2004;10:366–375. [PubMed] [Google Scholar]
- 52.Mori K, et al. Intraocular adenoviral vector-mediated gene transfer in proliferative retinopathies. Invest Ophthalmol Vis Sci. 2002;43(5):1610–1615. [PubMed] [Google Scholar]
- 53.Bhutto IA, et al. Localization of collagen XVIII and the endostatin portion of collagen XVIII in aged human control eyes and eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004;45(5):1544–1552. doi: 10.1167/iovs.03-0862. [DOI] [PubMed] [Google Scholar]
- 54.Montaner S, et al. Endothelial infection with KSHV genes in vivo reveals that vGPCR initiates Kaposi’s sarcomagenesis and can promote the tumorigenic potential of viral latent genes. Cancer Cell. 2003;3(1):23–36. doi: 10.1016/s1535-6108(02)00237-4. [DOI] [PubMed] [Google Scholar]
- 55.Edwards MM, et al. Lama1 mutations lead to vitreoretinal blood vessel formation, persistence of fetal vasculature, and epiretinal membrane formation in mice. BMC Dev Biol. 2011;11:60. doi: 10.1186/1471-213X-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McLeod DS, et al. Retinal and optic nerve head pathology in Susac’s syndrome. Ophthalmology. 2011;118(3):548–552. doi: 10.1016/j.ophtha.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McLeod DS, Hasegawa T, Prow T, Merges C, Lutty G. The initial fetal human retinal vasculature develops by vasculogenesis. Dev Dyn. 2006;235(12):3336–3347. doi: 10.1002/dvdy.20988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.