Abstract
The periaqueductal gray (PAG) and amygdala are known to be important for defensive responses, and many contemporary fear-conditioning models present the PAG as downstream of the amygdala, directing the appropriate behavior (i.e., freezing or fleeing). However, empirical studies of this circuitry are inconsistent and warrant further examination. Hence, the present study investigated the functional relationship between the PAG and amygdala in two different settings, fear conditioning and naturalistic foraging, in rats. In fear conditioning, electrical stimulation of the dorsal PAG (dPAG) produced unconditional responses (URs) composed of brief activity bursts followed by freezing and 22-kHz ultrasonic vocalization. In contrast, stimulation of ventral PAG and the basolateral amygdalar complex (BLA) evoked freezing and/or ultrasonic vocalization. Whereas dPAG stimulation served as an effective unconditional stimulus for fear conditioning to tone and context conditional stimuli, neither ventral PAG nor BLA stimulation supported fear conditioning. The conditioning effect of dPAG, however, was abolished by inactivation of the BLA. In a foraging task, dPAG and BLA stimulation evoked only fleeing toward the nest. Amygdalar lesion/inactivation blocked the UR of dPAG stimulation, but dPAG lesions did not block the UR of BLA stimulation. Furthermore, in vivo recordings demonstrated that electrical priming of the dPAG can modulate plasticity of subiculum–BLA synapses, providing additional evidence that the amygdala is downstream of the dPAG. These results suggest that the dPAG conveys unconditional stimulus information to the BLA, which directs both innate and learned fear responses, and that brain stimulation-evoked behaviors are modulated by context.
Keywords: fear circuitry, learning and memory, long-term depression, long-term potentiation, synaptic plasticity
Decades of research involving various techniques have identified that the amygdala is essential for both innate and learned fear (1). Evidence indicates that neurons in the basolateral amygdalar complex (BLA) (basal and lateral nuclei) (2) are responsive to both the conditional stimulus (CS) and unconditional stimulus (US) (3, 4), undergo plastic changes during fear conditioning (5), and are necessary for producing fear responses (6, 7). Indeed, a recent study has shown that optogenetically induced depolarization of pyramidal neurons in the lateral amygdala (LA) can elicit a fear unconditional response (UR) and, when repeatedly paired with auditory CS, supports fear conditioning via Hebbian-like synaptic plasticity (8).
However, stimulation-induced fear conditioning is not only achievable through the amygdala. Other studies have found that stimulation of the dorsal periaqueductal gray (dPAG) is an effective US in fear conditioning (9, 10). The PAG has long been implicated in generating defensive behaviors (11), and it has been suggested that its stimulation can support fear conditioning to a CS because it transmits the aversive US information to the LA (9, 12). Some have also argued that the dPAG and the amygdala elicit URs independently, based on findings that inactivation of amygdalar nuclei do not affect dPAG thresholds for eliciting freezing and escape behaviors in a small circular arena (ref. 13; but see ref. 14). However, it is most commonly proposed that the PAG is downstream of the amygdala, directing motor outputs toward the appropriate defensive behavior (8, 15–18). This has been supported by findings that ventral (v)PAG lesions impair conditioned freezing behavior while sparing other fear responses, whereas dPAG lesions do not block fear conditioning to a footshock US (19). Anatomically, the PAG consists of dorsolateral and dorsomedial columns (dPAG), which are separated from the ventrolateral column (vPAG) by the lateral column (20). Whereas the PAG is involved in numerous functions such as regulating cardiovascular function, nociception, and vocalizations, the dorsal and ventral columns seem geared toward oppositional forms of defensive behavior: escape and freezing, respectively (11).
Although amygdalar and PAG systems have been well-investigated in terms of providing footshock US information with respect to fear conditioning, not much is known about the effects of stimulating these structures in more naturalistic settings. Questions about the ecological validity of the results obtained using the standard Pavlovian fear-conditioning paradigm, in which the animal is placed in a small chamber and given footshocks, have existed for several decades, since findings that conditional responses depend upon the properties of the US and its contextual cues (7, 21–23). Despite these concerns, many claims about the functional organization of the brain have been based solely on the results of fear-conditioning procedures. Here we investigated the functions of the amygdala and dPAG specifically in the development of learned fear responses during fear conditioning and in the expression of innate fear responses during foraging in a seminaturalistic environment (Fig. 1). The functional connection between the amygdala and the dPAG was examined by combining stimulation and lesion/inactivation of these structures and confirmed by in vivo electrophysiology.
Fig. 1.
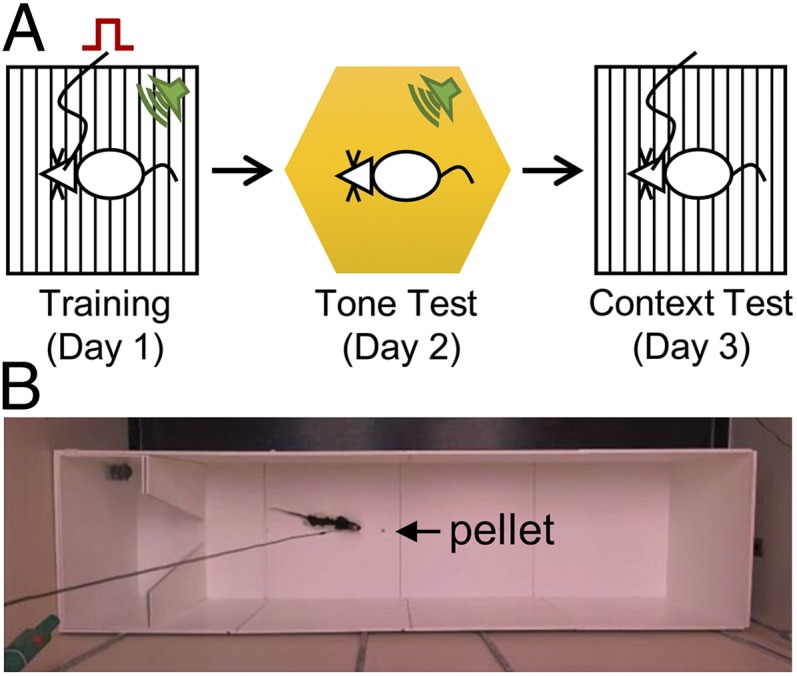
Experimental designs for fear-conditioning and foraging tasks. (A) Tone-brain stimulation pairings occurred in training context on the first day of conditioning. On the second day, the animal was exposed to the tone in a different context. On the third day, the animal was exposed to the original context without a tone. (B) The seminaturalistic foraging apparatus. The arrow points to the placement of the pellet in all trials. Video clips are available at http://faculty.washington.edu/jeansokk/Brain_stimulation.html.
Results
Amygdalar and dPAG Stimulation and Fear Conditioning.
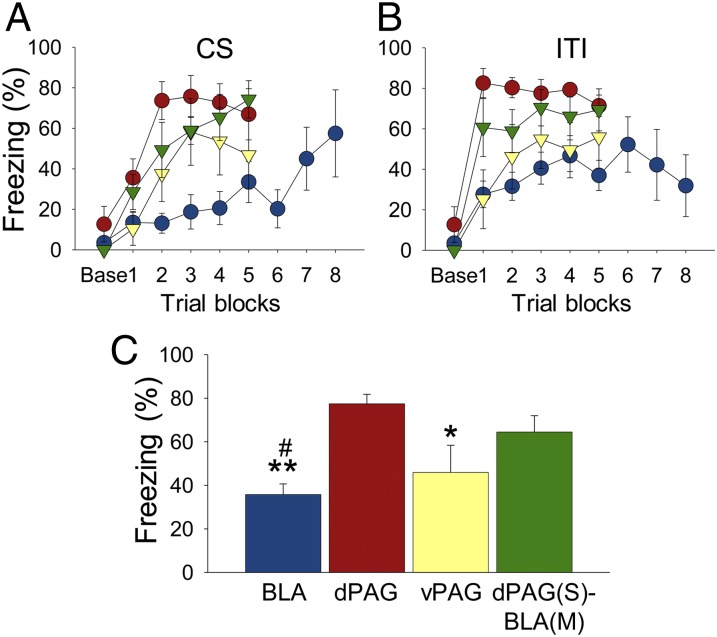
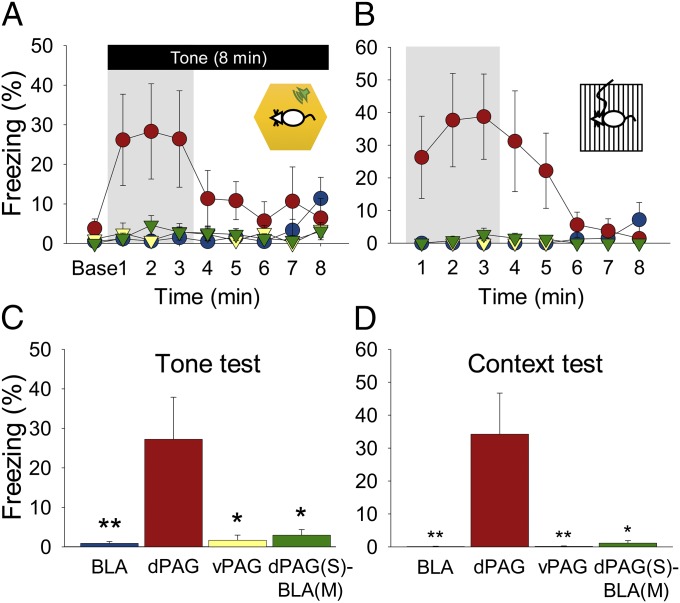
Stimulation intensity needed to evoke aversive behavior was calibrated for each rat before all experiments (BLA: 275.0 ± 24.44 µA, mean ± SEM; dPAG: 65.0 ± 6.85 µA; vPAG: 97.9 ± 7.55 µA; Materials and Methods). Bilateral stimulation of the BLA (n = 10; six rats underwent 10 trials and four rats underwent 16 trials), dPAG (n = 9; 10 trials), and vPAG (n = 7; 10 trials) in the operant chamber elicited 22-kHz ultrasonic vocalization (USV) distress calls as occurs after footshock (Fig. 2). To examine whether each area could substitute for a US, stimulation coincided with a tone CS (Fig. 1A). As shown in Fig. 3 A and B, all animals displayed freezing behavior during the conditioning trials and/or intertrial intervals (ITIs), although to different degrees [one-way ANOVA; F(3, 29) = 7.207, P = 0.001; Fig. 3C]. Tukey’s HSD post hoc tests revealed significant differences in poststimulation freezing between the dPAG-stimulated rats and the vPAG-stimulated (P < 0.05) and BLA-stimulated (P < 0.01) rats but not the dPAG-stimulated rats that had muscimol infused bilaterally into the BLA before conditioning (P > 0.05). When tested for retention on tone test day 2 and context test day 3, the dPAG-stimulated rats, but not BLA-stimulated or vPAG-stimulated rats, displayed freezing behavior to both the tone and the context. However, dPAG-stimulated rats that had muscimol infused into the BLA during conditioning day 1 (n = 7) failed to freeze to both the tone and the context (Fig. 4 A and B). A one-way ANOVA with Tukey’s Honest Significant Difference (HSD) post hoc tests confirmed that the dPAG-stimulated rats froze significantly more during exposure to the tone [F(3, 29) = 4.706, P = 0.009] and context [F(3, 29) = 6.480, P = 0.002] compared with the other groups (Fig. 4 C and D). The absence of fear conditioning in BLA-stimulated rats cannot be attributed to inadequate CS–brain stimulation trials because those (n = 4) given extended 16 pairings did not differ from those (n = 6) that received 10 pairings [tone test, t(8) = 0.133, P > 0.8; context test, t(8) = 0.142, P > 0.8]. Note also that because BLA-stimulated rats displayed no evidence of conditioned fear responses, a combined BLA stimulation+dPAG inactivation manipulation was not necessary. In summary, whereas stimulation of all structures reflexively elicited a UR, only stimulation of the dPAG, and not the BLA or vPAG, served as an effective US for fear conditioning. Furthermore, the BLA was necessary for dPAG stimulation-produced fear conditioning.
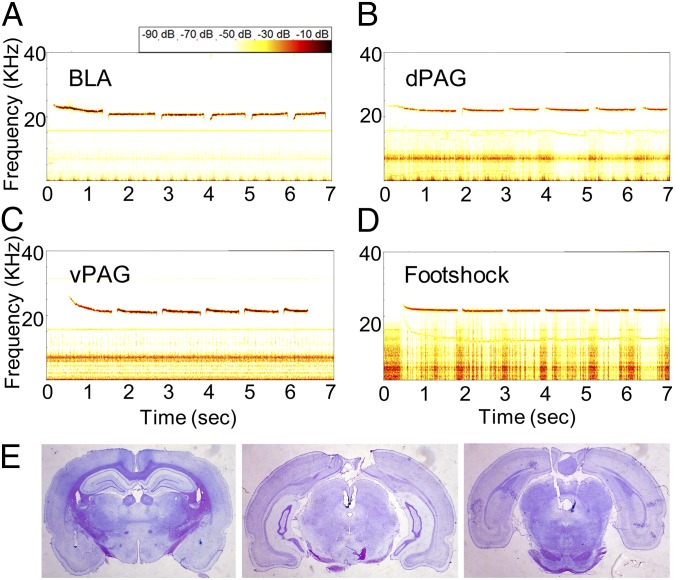
Fig. 2.
Stimulation- and footshock-induced 22-kHz ultrasonic vocalizations and electrode placement. Stimulation of the (A) BLA, (B) dPAG, and (C) vPAG elicited USVs similar to (D) postfootshock USVs. (E) Photomicrographs of stimulating electrode tips in the BLA (Left), dPAG (Center), and vPAG (Right).
Fig. 3.
Freezing during tone-stimulation conditioning. Mean percentage (±SEM) freezing during tone-stimulation conditioning (day 1): (A) tone CS and (B) intertrial interval. Groups are designated by colored symbols: blue circle, BLA; red circle, dPAG; yellow triangle, vPAG; green triangle, dPAG(S)-BLA(M). Data are shown as two trials per block. (C) Group differences in freezing during training. BLA, BLA stimulation group; dPAG, dPAG stimulation group; vPAG, vPAG stimulation group; dPAG(S)-BLA(M), dPAG stimulation and BLA muscimol group. *P < 0.05 and **P < 0.01 compared with the dPAG group. #P < 0.05 compared with the dPAG(S)-BLA(M) group.
Fig. 4.
Freezing during the retention tests. (A) Mean percentage (±SEM) freezing during 8-min tone testing (day 2). (B) Mean percentage (±SEM) freezing during 8-min context testing (day 3). Groups are designated by colored symbols: blue circle, BLA; red circle, dPAG; yellow triangle, vPAG; green triangle, dPAG(S)-BLA(M). (C and D) Group differences in freezing during the tone and context retention tests (first 3 min). *P < 0.05 and **P < 0.01 compared with the dPAG group.
Amygdalar and dPAG Stimulation and Foraging Behavior.
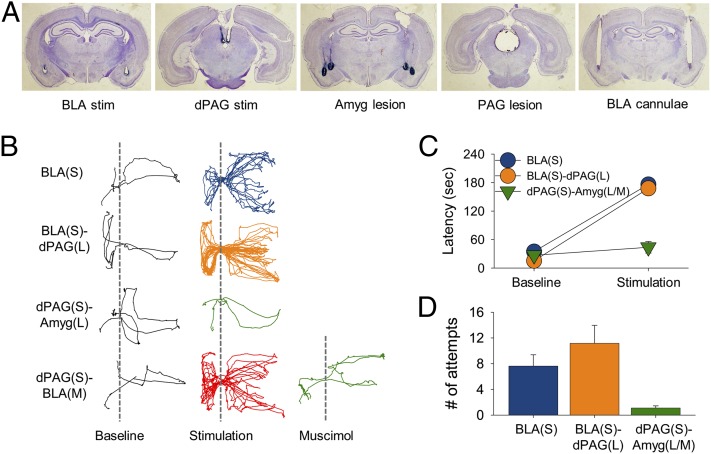
Food-restricted rats quickly learned to search for food pellets placed 25.4, 50.8, or 76.2 cm away from the acclimated nesting area in a large, open field (Fig. 1B). Upon procuring the pellet, the animals instinctively returned to the nest for consumption. After 3–5 d of baseline foraging, stimulation of either the amygdala or dPAG (BLA: 244.3 ± 21.83 µA; dPAG: 105.0 ± 22.39 µA; Fig. 5A) occurred when the rat approached a food pellet placed 76.2 cm away from the nest. All rats performed similarly during the prestimulation baseline trials (Fig. 5 B and C). Rats receiving BLA stimulation with intact dPAG (n = 8) almost always ran back to the nest and therefore were unable to obtain the pellet. Rats receiving amygdalar stimulation with dPAG lesions (n = 6) similarly retreated to the nest and therefore were unsuccessful in securing the pellet. Rats with amygdalar (BLA+central nucleus) lesions (n = 6) or BLA inactivations (n = 3) receiving dPAG stimulation, data pooled because they did not differ in the latency to retrieve the pellet, were able to retrieve the pellet. Note that all rats that received dPAG stimulation with cannulated amygdalae failed to retrieve the pellet when tested with no drug (Fig. 5B). A repeated-measures ANOVA on the latency to retrieve the pellet during baseline and stimulation trials revealed significant trial [F(1, 20) = 177.917, P < 0.0001] and group [F(2, 20) = 21.109, P < 0.0001] effects and a trial × group interaction [F(2, 20) = 34.237, P < 0.0001] (Fig. 5C). Multiple comparisons with Bonferroni correction confirmed that the dPAG-stimulated rats with amygdalar lesions/BLA inactivation were unaffected by stimulation compared with the other two groups (P < 0.0001 for each comparison). In terms of the number of attempts to retrieve the pellet during stimulation trials (Fig. 5D), a one-way ANOVA revealed significant group differences [F(2, 22) = 9.678, P = 0.001]. Tukey’s HSD post hoc tests indicated that the dPAG-stimulated rats with amygdalar lesions/BLA inactivation required significantly fewer attempts to retrieve the pellet compared with the BLA-stimulated rats with intact dPAG (P = 0.020) and the BLA-stimulated rats with dPAG lesions (P = 0.001). Whereas stimulation of both the dPAG and BLA reliably elicited fleeing, thereby preventing the rats from retrieving the pellet, lesion/inactivation of the amygdala blocked this effect of dPAG stimulation but dPAG lesions did not block fleeing elicited by BLA stimulation. These results indicate that the BLA is downstream of the dPAG in mediating fleeing behaviors during foraging.
Fig. 5.
Effects of stimulation during the foraging experiment. (A) Photomicrographs of stimulating electrode tips (BLA and dPAG), lesions (amygdala and PAG), and cannulae implantation (BLA). (B) Representative track plots from the baseline, stimulation, and muscimol trials. Dotted vertical lines demarcate the nest–foraging area boundary. (C) Averaged group latencies (s) in time to retrieve the pellet and return to the nest area. (D) Averaged number of attempts to retrieve the pellet by condition. BLA(S), BLA stimulation group; BLA(S)-dPAG(L), BLA stimulation and dPAG lesion group; dPAG(S)-Amyg(L), dPAG stimulation and BLA/CeA lesion group; dPAG(S)-Amyg(L/M), dPAG stimulation and amygdala lesion/BLA muscimol group. Error bars, SEM.
dPAG Priming and Synaptic Plasticity in the BLA.
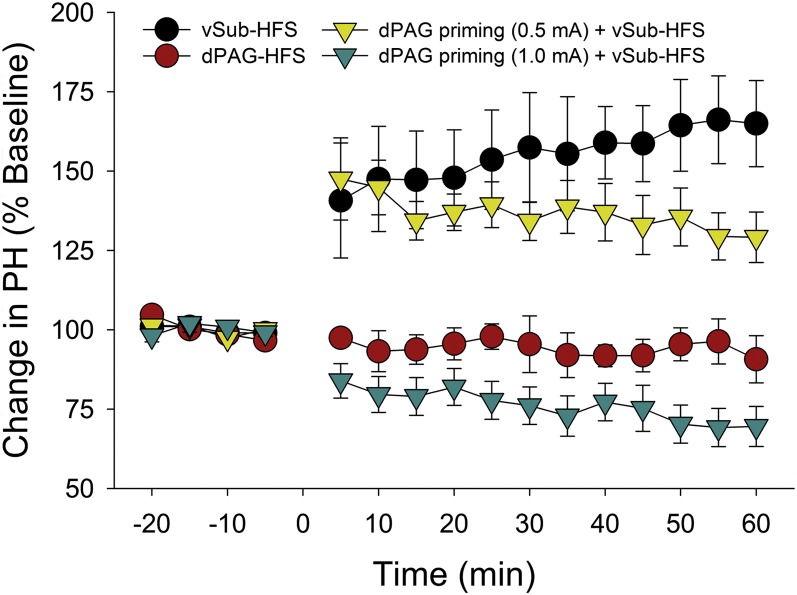
To further explore whether the dPAG functionally modulates BLA activity, in vivo electrophysiology tests were performed. Anesthetized rats received high-frequency stimulation (HFS) to the ventral subiculum (vSub) or to the dPAG, and some were given either 1.0-mA or 0.5-mA priming stimulation to the dPAG before applying HFS to the vSub. Before priming and HFS, recordings from the BLA did not demonstrate any significant differences between groups [repeated-measures ANOVA; F(3, 31) = 0.75, P > 0.05] (Fig. 6). After applying HFS and priming stimulation, however, there were significant group differences in long-term potentiation (LTP) induction [F(3, 31) = 10.62, P < 0.01] (Fig. 6). Further post hoc Bonferroni comparisons revealed a significant difference between the vSub HFS group (n = 8) and the dPAG HFS group (n = 8) (P < 0.05), and between the vSub HFS group and the 1.0-mA dPAG priming+vSub HFS group (n = 8). In addition, a significant difference was found between the 0.5-mA dPAG priming+vSub HFS group (n = 8) and the 1.0-mA dPAG priming+vSub HFS group (P < 0.05). Priming dPAG at 1.0 mA resulted in a form of long-term depression (LTD), as indicated by a significant reduction in the BLA response of that group compared with the dPAG HFS group (P < 0.05). These results further demonstrate that dPAG neurons project to the BLA and that activity in these neurons can modulate synaptic plasticity in the BLA.
Fig. 6.
Priming of the dPAG and ensuing long-term potentiation in the BLA. Applying HFS to the vSub reliably induced LTP in the BLA (vSub HFS group). Stimulation of the dPAG alone had no significant effect on BLA response (dPAG HFS group). Priming the dPAG just before HFS to vSub resulted in suppression of LTP; priming of the dPAG at 1.0 mA in fact induced a form of LTD. A milder priming stimulation did not prevent vSub-BLA LTP (dPAG priming 0.5 mA). PH, peak-to-peak amplitude, ratio peak high. Error bars, SEM.
Discussion
Previous studies have shown that electrical stimulation of the amygdala evokes fear-associated responses such as cardiovascular changes (24, 25), potentiated startle (26), and freezing (27). Contemporary fear models posit that the basolateral amygdalar (basal and lateral nuclei) complex is interconnected with the central nucleus (CeA), which is thought to be the main amygdaloid output structure sending efferent fibers to various autonomic and somatomotor centers involved in mediating specific fear responses (7, 28, 29). In the present study, although freezing and 22-kHz USV were robustly elicited, the BLA stimulation was an ineffective US in supporting fear conditioning to tone and context CSs. This finding seemingly contrasts with a recent study that demonstrated fear conditioning to a tone CS that was paired with optical stimulation of pyramidal neurons in the LA virally transfected and expressing light-responsive channelrhodopsin (ChR2) (8). Because electrical stimulation evoked stronger fear responses than optogenetic stimulation, this difference may be due to antidromic interference with the CS inputs to the amygdala during electrical stimulation. Similarly, electrical stimulation of the cerebellar interpositus nucleus, a locus of eyeblink conditioning (30), does not support eyeblink conditioning in rabbits (31) despite the fact that stimulation of its inputs can substitute as the CS [i.e., lateral pons (32)] and US [i.e., inferior olive (33)]. Alternatively, whereas both electrical and optogenetic stimulation of the BLA produced freezing, the former (but not latter) manipulation may have also disrupted the amygdala-dependent processing of long-term fear memory.
The stimulation of the dPAG also elicited (after brief activity bursts) robust freezing and USV in rats. In contrast to the amygdala, however, dPAG stimulation supported fear conditioning, as indicated by the animals’ conditioned freezing to the CS. Importantly, this conditioning effect was completely blocked when the BLA was inactivated during dPAG stimulation, indicating that the dPAG-BLA circuit plays a role in the US pathway. This is consistent with a previous study showing that PAG inactivation disrupted amygdala activity signaling US information (12). Furthermore, the present findings are similar to a previous study that found that reversible inactivation of the medial nucleus of the amygdala (MeA) increased the threshold of current applied to the dPAG needed to elicit aversive responses (ref. 14; but see ref. 13). Although it is possible that the muscimol infused into the BLA in the present study spread to the MeA, these results nonetheless suggest a more complex role for amygdalar nuclei in directing fear responses. Supporting this idea, another study (34) found that the CeA is necessary for the expression of conditioned freezing but not active avoidance, whereas the BLA is critically involved in conditioned avoidance and the conditioned reinforcement of novel behaviors that terminate an aversive stimulus. Although dPAG stimulation was sufficient to support fear conditioning, the finding that fear conditioning occurs in rats with dPAG lesions (19) suggests that the dPAG projection to the amygdala (35–37) is one component of the US pathways critical in fear conditioning.
When the BLA and the dPAG were stimulated in a foraging apparatus, animals displayed completely different responses from those exhibited in the conditioning chamber. Each time the rats foraged for food, BLA stimulation reliably caused the animals to run back to the nesting area (a fleeing response) instead of freezing and emitting USVs, as they did in the conditioning chamber. Similarly, dPAG stimulation, which evoked activity bursts [i.e., jumping, running (6)] in the conditioning chamber, caused a fleeing response toward the safety of the nest. Whereas dPAG (as well as entire PAG) lesions did not alter the effects of amygdalar stimulation, amygdalar lesions/BLA inactivation completely blocked the effects of dPAG stimulation, paralleling the findings of the fear-conditioning experiment that dPAG stimulation served as an effective US and that this was disrupted by BLA inactivation. These findings are inconsistent with the contemporary fear model of the PAG directing motor output toward the appropriate response after activation by the amygdala (7, 16), and instead suggest that the BLA is downstream of the dPAG in mediating fleeing responses during foraging, a more likely natural scenario for rats. A recent diffusion tensor imaging study in humans (36) has found neurons originating from the PAG that terminate in the amygdala as well. The unexpected findings that BLA and dPAG stimulation produced dissimilar effects in the conditioning chamber from in the foraging apparatus further highlight the importance of the context in which brain stimulation occurs in the expression of fear responses. In other words, the environmental setting can significantly influence the behavioral readout. This contextual modulation may rely on cortical and/or hippocampal input (38). Future studies need to use advanced, spatially resolved techniques (e.g., optogenetics) to investigate whether the BLA–CeA dichotomy in conditioned avoidance vs. freezing (34) applies to the present foraging paradigm and to characterize the cell types involved in the dPAG-amygdala fear circuitry.
Although BLA stimulation and dPAG stimulation produced the similar fleeing behavior in the foraging apparatus, the current intensity was significantly lower for the dPAG animals. This difference may be due to the amygdala’s non–fear-related functions, such as appetitive and sexual behaviors (39). Electrical stimulation will then activate amygdalar neurons indiscriminately and cause different amygdala-mediated behaviors to interfere with each other. Although optogenetics techniques can be fairly selective (e.g., the CaMKII promoter of amygdalar pyramidal cells), it remains to be determined whether specific forms of learning, such as fear conditioning, are supported exclusively by neurons expressing particular genetic promoters. The fact that dPAG stimulation required much less intensity to elicit activity bursts, freezing, and USV responses (in the conditioning chamber) and a fleeing response (in the foraging arena) than BLA stimulation suggests that dPAG neurons may be dedicated to the function of defensive behavior, and therefore the stimulation of dPAG selectively stimulates those downstream amygdalar neurons involved in fear-related behaviors. This possibility presents an interesting avenue for future fear research. Recently, a conditioned fear memory was erased by specifically ablating virally targeted amygdalar neurons overexpressing cAMP response element-binding protein (CREB), a genetic transcription factor involved in long-term potentiation, during fear conditioning with diphtheria toxin (40). Thus, instead of using fear conditioning to overexpress CREB and ablate amygdalar neurons involved in fear conditioning post hoc, dPAG stimulation should be effective for expressing CREB in the amygdala and ablating those neurons specifically involved in mediating fear behavior pre hoc.
Additionally, the present study found that dPAG priming stimulation altered vSub-BLA LTP and even induced a form of LTD depending on the stimulation intensity, confirming the BLA is downstream of the dPAG. The dPAG stimulation intensity of 1.0 mA (but not 0.5 mA) affecting the BLA LTP is comparable to the dPAG stimulation intensity used in fear-conditioning and foraging experiments. Furthermore, the requirement for stronger dPAG priming intensity supports the hypothesis that whereas moderate threatening stimuli inhibit the dPAG, this inhibition may be overcome with extreme danger (41, 42). In addition, the fact that dPAG HFS did not change synaptic efficacy in the BLA raises a possibility that the dPAG indirectly modulates synaptic plasticity in the BLA induced by multiple afferents from other brain regions such as the hippocampal formation (43) and medial geniculate nucleus (44), without direct effects on the BLA. Because the dPAG priming stimulation differently affected synaptic efficacy in the vSub-BLA pathway depending on the stimulation intensities, further studies are required to identify how the dPAG modulates BLA plasticity by using different intensities, including the low intensities used for our behavioral experiments.
In summary, the finding that amygdalar lesion/BLA inactivation blocked the freezing in fear conditioning and the fleeing behavior in the foraging environment produced by dPAG stimulation, whereas dPAG lesions coupled with BLA stimulation did not, provides evidence that the BLA is downstream of the dPAG and directs the motor output of fear responses during stimulation-induced fear conditioning and foraging. Corroborating this conclusion, the electrophysiological experiment showed that the dPAG can modulate plasticity in the BLA. The present data then suggest that the processing and expression of fear by the amygdala involve a bottom-up influence from the PAG. Thus, it is possible that aberrant activity in dPAG afferents to the amygdala contributes to fear-related psychopathologies such as anxiety, phobic, panic, and posttraumatic disorders. Looking beyond the amygdala and toward a circuit-level understanding of fear behavior will provide more power to the treatment of fear-related disorders, but it is imperative that future studies use diverse and representative experimental designs to best converge upon the functions of fear circuitry.
Materials and Methods
Subjects.
Naïve male Charles River Sprague-Dawley (used in the fear-conditioning experiment) and Long-Evans rats (initially weighing 275–300 g) were individually housed in the Department of Psychology animal care facility at the University of Washington (accredited by the Association for Assessment and Accreditation of Laboratory Animal Care) and maintained on a reverse 12-h light/dark cycle (lights on at 19:00 hours). Long-Evans rats were placed on a standard food-deprivation schedule, with free access to water, to gradually reach and maintain 80–85% of their normal weight. Experiments were conducted during the dark phase of the cycle, in strict compliance with University of Washington Institutional Animal Care and Use Committee guidelines.
For the electrophysiology experiment, 32 adult (postnatal day 50 at arrival) Sprague-Dawley rats (Harlan Jerusalem) were used. Following delivery, rats were group-housed (four per cage) under a 12-h light/dark cycle with free access to food and water. All experimental procedures were conducted during the light phase, and were approved by the Institutional Animal Care Committee (University of Haifa) and adhered to the guidelines of the US Institute of Laboratory Animal Research’s Guide for the Care and Use of Laboratory Animals.
Surgery.
Fear-conditioning and foraging experiments.
Under anesthesia (30 mg/kg ketamine and 2.5 mg/kg xylazine, i.p.), rats were mounted in a stereotaxic instrument and implanted chronically with stimulating electrodes (MS303/3-B; Plastics One) bilaterally into the BLA, dPAG, or vPAG. During the surgery, some rats also received either electrolytic lesions (i.e., BLA-stimulating electrodes+PAG lesions; dPAG-stimulating electrodes+amygdala lesions) or guide cannulae (26 gauge; Plastics One) implanted bilaterally (i.e., dPAG-stimulating electrodes+BLA cannulae). Stereotaxic coordinates were as follows (referenced from bregma): (i) stimulating electrodes: anteroposterior (AP) −2.8, mediolateral (ML) ±5.0, and dorsoventral (DV) −8.4; (ii) guide cannulae: AP −2.8, ML ±5.0, and DV −7.4; and (iii) lesion electrodes: AP −6.8, ML ±1.1 at a 10° angle, DV −5.0 (dPAG), and AP −7.8, ML ±1.7 at a 10° angle, DV −6.0 (vPAG). Amygdala and PAG lesions were made by passing constant current (1 mA, 10 s; Grass Medical Instruments) through epoxy-coated stainless steel insect pins (no. 00) except for ∼0.5 mm at the tip. Stimulating electrodes and guide cannulae were cemented to the skull with four anchoring screws. All rats were given 7 d of surgical recovery and daily handling before the experimental procedures began.
Electrophysiology experiment.
Rats were anesthetized with urethane (5 mg/1 kg, i.p.) and mounted to a stereotaxic apparatus (Stoelting). The scalp was incised and retracted, and the head position was adjusted to place bregma and lambda in the same horizontal plane. Small burr halls (2-mm diameter) were drilled unilaterally in the skull for the placement of bipolar concentric stimulating (125-μm diameter; Kopf) and glass recording (2- to 5-μm tip diameter; 2 M NaCl internal solution) electrodes. Reference electrodes were affixed to the skull in the area overlapping the nasal sinus. Stimulation electrodes were positioned in the dPAG (AP −6.05; ML ±0.65; DV −5.72) and in the vSub (AP −6.3; ML ±5.0; DV −7.5). A recording electrode was positioned in the BLA (AP −3.2; ML 5.0; DV −7.0). During the course of experiments, body temperature was maintained at 36.4–36.9 °C with a feedback-regulated temperature controller (FHC).
Drug Infusion.
Muscimol-free base (Sigma-Aldrich), dissolved in artificial cerebrospinal fluid (pH ∼7.4), was microinfused into the BLA (bilaterally) by backloading the drug up a 33-gauge infusion cannula into polyethylene (PE 20) tubing connected to 10-µL microsyringes (Hamilton). The infusion cannula protruded 1 mm beyond the guide cannula. An infusion volume of 0.3 µL (per side) was delivered using a PHD2000 syringe pump (Harvard Apparatus) at a rate of 0.1 µL/min for 3 min. The infusion cannula remained in place for 30 s after the infusions before being pulled out. The dosage and volume of muscimol (2.628 nmol, 0.3 µL per side) used are within the intraamygdalar infusion parameters used in typical fear-conditioning studies (45, 46) as well as in recent fluorescent imaging of muscimol spread in the amygdala (47).
Fear-Conditioning Apparatus.
The brain stimulation-induced fear conditioning took place in an operant test chamber equipped with a speak module and located in a sound-attenuated chest (Coulbourn Instruments). The conditioning chamber was rectangular (27-cm width × 28-cm length × 30.5-cm height) with front and back walls made of clear Plexiglas and two side walls made of metal plates. The grid floor was composed of 17 stainless steel bars (5-mm diameter) spaced 15 mm center-to-center. The tone testing occurred in another chamber placed in a different room with novel visual and olfactory cues that minimized generalized fear (Fig. 4A). A 24-cell infrared activity monitor was used to assess freezing (cf. 48), and a D1000X ultrasound detector and BatSound (version 4.03) real-time spectrogram software (Pettersson Elektronik) were used to analyze brain stimulation- or footshock-induced USV calls (cf. 49).
Foraging Apparatus.
A custom-built “seminaturalistic” apparatus (Fig. 1B) consisted of a nesting area (29.21-cm length × 57.12-cm width × 59.69-cm height; equipped with a water bottle; 16.2 Lux luminance) with a remotely controlled, horizontally opening gateway to an adjacent foraging area (201.93-cm length × 58.42-cm width × 60.96-cm height; 56.7 Lux; 60-dB white noise, A scale). The ANY-maze video tracking system (Stoelting), with video feed from an overhead ultradigital wireless camera (LW2101; Lorex Technology) connected to a Sony HD DVD recorder (RDR-HX900), was used to record and automatically track the animal’s movement (30 frames per s) from both nesting and foraging areas.
Fear-Conditioning Procedures.
Sprague-Dawley rats given free access to food and water underwent initial stimulation calibration, drug infusion, and tone-stimulation conditioning over 3 d (Fig. 1A).
Stimulation calibration.
At least 1 h before testing, electrical stimulation (0.1-ms pulse, 100 Hz, 2-s train) applied to rats with electrodes implanted in their BLA, dPAG, or vPAG was calibrated for each animal. Stimulation intensity began at 30 µA and increased by 5 µA until USVs, freezing, and/or activity bursts were robustly elicited by the stimulation. The final stimulation intensity reached for each rat was then used for tone-brain stimulation fear conditioning.
Brain-stimulation conditioning.
On day 1, rats were placed in the conditioning chamber and baseline behavior was recorded for 1 min. Following this baseline period, the rats underwent 10 trials of a tone CS (2.9 kHz, 85 dB, 20 s) paired with stimulation of the relevant brain area (0.1-ms pulses, 100 Hz, 2 s), which coterminated (2-min ITI). Four of the BLA-stimulated rats were given extended CS–brain stimulation US-paired trials (16 trials). The next day, rats were placed in a novel context (nonlighted, hexagonal chamber with a Plexiglas floor) and an 8-min tone was presented continuously following a 1-min baseline period. On day 3, rats were placed back into the original conditioning context for 8 min. Upon completion of the retention test, animals received footshocks (1 mA, 1 s; Coulbourn Precision-Regulated Animal Shocker) to ascertain whether the brain stimulation-evoked USV calls were comparable to fear-evoked USV calls (Fig. 2 A–D).
Foraging Procedures.
Rats maintained between 80% and 85% of their normal body weight underwent successive stages of habituation, baseline foraging, and brain-stimulation trials.
Habituation.
Animals were placed in the nesting area for 30 min/d for 2 consecutive days with 10 food pellets (F0173; Bio-Serv; grain-based, 1 g) in the nest to acclimatize to the nesting area.
Baseline days.
After ∼2 min in the nesting area (no food), the gateway to the foraging area opened, and the animal was allowed to explore and search for a food pellet placed 25.4 cm from the nest area (first trial). As soon as the animal took the pellet back inside the nest, the gateway closed. Once the animal finished consuming the pellet, the second foraging trial (with the pellet placed 50.8 cm from the nest area) and then the third foraging trial (with the pellet placed 76.2 cm from the nest area) started in the same manner. All animals met the criterion of retrieving the pellets and returning back to the nest within 60 s for three successive trials between 3 and 5 consecutive days of baseline foraging.
Brain-stimulation testing.
The food pellet was placed at the 76.2-cm location on the first trial. After the gateway opened, the animal was allowed to explore and search for a food pellet (prestimulation baseline trial). On the second foraging trial, each time the animal approached the vicinity of the pellet (∼25 cm), the rat received brain stimulation (with the pellet placed 76.2 cm from the nest area). Animals were permitted 3 min to procure the pellet. If the rat was unsuccessful, the gate was closed with the animal inside the nest area, and the food pellet was placed 25.4 cm closer to the nest on the following trial. If the rat was again unsuccessful, the pellet was placed 25.4 cm closer to the nest on the following trial, and again 12.7 cm closer (12.7 cm from the nest) if still unsuccessful. The number of attempts and the latency required for the animal to procure the pellet successfully (i.e., the time from the gate opening to the rat’s returning to the nest with the pellet) served as dependent variables.
Electrophysiological Procedures.
The HFS train consisted of stimulating the vSub or dPAG for 10 brief bursts (200 ms, 100 Hz) delivered at 1 Hz (a total of 200 pulses). For each rat, a 20-min pre-HFS baseline was collected at the stimulation intensity that elicited a field potential with at least a 200-µV negative component in the BLA (ramped from 0.2 mA up to 1.8 mA at 0.2-mA intervals). Immediately following the baseline, rats received four HFS trains (5-min ITI). Responses were collected once every 20 s during baseline and for 60 min following the last stimulation session (partially adapted from ref. 39). Priming was composed of a single HFS train to the dPAG delivered in one of two intensities: 1.0 mA or 0.5 mA, and was given 30 s before the HFS to the vSub. In the BLA, the principal measure of size of the averaged evoked field potentials was the peak-to-peak amplitude (ratio peak high).
Histology.
At the completion of behavioral testing, animals were overdosed with Beuthanasia and perfused intracardially with 0.9% saline followed by 10% (vol/vol) buffered formalin. The brains were removed and stored in 10% (vol/vol) formalin overnight and then kept in 30% (mass/vol) sucrose solution until they sank. Transverse sections (50-µm) were taken through the extent of the lesion and cannulae, mounted on gelatin-coated slides, and stained with cresyl violet and Prussian blue dyes.
Statistical Analyses.
All statistics were conducted using SPSS (versions 18.0 and 19.0). A one-way ANOVA and Tukey’s HSD post hoc tests were used to test for statistical significance of freezing in the fear-conditioning experiment. In the foraging and electrophysiology experiments, a repeated-measures ANOVA and post hoc Bonferroni tests were used. Graphs were made using SigmaPlot (version 11.0).
Acknowledgments
We thank Truc T. Hang for assistance with the experiments. This study was supported by National Institutes of Health Grants MH64457, MH099073, and The James McKeen Cattell Sabbatical Award (to J.J.K.), and by a research award from the Hope for Depression Research Foundation (to G.R.-L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: A critical review. Neurosci Biobehav Rev. 2006;30(2):188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: Anatomy and physiology. Physiol Rev. 2003;83(3):803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- 3.Barot SK, Chung A, Kim JJ, Bernstein IL. Functional imaging of stimulus convergence in amygdalar neurons during Pavlovian fear conditioning. PLoS One. 2009;4(7):e6156. doi: 10.1371/journal.pone.0006156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pascoe JP, Kapp BS. Electrophysiological characteristics of amygdaloid central nucleus neurons during Pavlovian fear conditioning in the rabbit. Behav Brain Res. 1985;16(2-3):117–133. doi: 10.1016/0166-4328(85)90087-7. [DOI] [PubMed] [Google Scholar]
- 5.Applegate CD, Frysinger RC, Kapp BS, Gallagher M. Multiple unit activity recorded from amygdala central nucleus during Pavlovian heart rate conditioning in rabbit. Brain Res. 1982;238(2):457–462. doi: 10.1016/0006-8993(82)90123-8. [DOI] [PubMed] [Google Scholar]
- 6.Choi JS, Kim JJ. Amygdala regulates risk of predation in rats foraging in a dynamic fear environment. Proc Natl Acad Sci USA. 2010;107(50):21773–21777. doi: 10.1073/pnas.1010079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LeDoux JE. Rethinking the emotional brain. Neuron. 2012;73(4):653–676. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansen JP, et al. Optical activation of lateral amygdala pyramidal cells instructs associative fear learning. Proc Natl Acad Sci USA. 2010;107(28):12692–12697. doi: 10.1073/pnas.1002418107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castilho VM, Macedo CE, Brandão ML. Role of benzodiazepine and serotonergic mechanisms in conditioned freezing and antinociception using electrical stimulation of the dorsal periaqueductal gray as unconditioned stimulus in rats. Psychopharmacology (Berl) 2002;165(1):77–85. doi: 10.1007/s00213-002-1246-4. [DOI] [PubMed] [Google Scholar]
- 10.Di Scala G, Mana MJ, Jacobs WJ, Phillips AG. Evidence of Pavlovian conditioned fear following electrical stimulation of the periaqueductal grey in the rat. Physiol Behav. 1987;40(1):55–63. doi: 10.1016/0031-9384(87)90185-5. [DOI] [PubMed] [Google Scholar]
- 11.Bandler R, Depaulis A. Midbrain periaqueductal gray control of defensive behavior in the cat and the rat. In: Depaulis A, Bandler R, editors. The Midbrain Periaqueductal Gray Matter. New York: Plenum; 1991. pp. 175–198. [Google Scholar]
- 12.Johansen JP, Tarpley JW, LeDoux JE, Blair HT. Neural substrates for expectation-modulated fear learning in the amygdala and periaqueductal gray. Nat Neurosci. 2010;13(8):979–986. doi: 10.1038/nn.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez RC, de Oliveira AR, Brandão ML. Conditioned and unconditioned fear organized in the periaqueductal gray are differentially sensitive to injections of muscimol into amygdaloid nuclei. Neurobiol Learn Mem. 2006;85(1):58–65. doi: 10.1016/j.nlm.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Herdade KC, Strauss CV, Zangrossi Júnior H, Viana MB. Effects of medial amygdala inactivation on a panic-related behavior. Behav Brain Res. 2006;172(2):316–323. doi: 10.1016/j.bbr.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 15.Depaulis A, Keay KA, Bandler R. Longitudinal neuronal organization of defensive reactions in the midbrain periaqueductal gray region of the rat. Exp Brain Res. 1992;90(2):307–318. doi: 10.1007/BF00227243. [DOI] [PubMed] [Google Scholar]
- 16.Fanselow MS. The midbrain periaqueductal gray as a coordinator of action in response to fear and anxiety. In: Depaulis A, Bandler R, editors. The Midbrain Periaqueductal Gray Matter. New York: Plenum; 1991. pp. 151–173. [Google Scholar]
- 17.Gray TS, Magnuson DJ. Peptide immunoreactive neurons in the amygdala and the bed nucleus of the stria terminalis project to the midbrain central gray in the rat. Peptides. 1992;13(3):451–460. doi: 10.1016/0196-9781(92)90074-d. [DOI] [PubMed] [Google Scholar]
- 18.Lavond DG, Kim JJ, Thompson RF. Mammalian brain substrates of aversive classical conditioning. Annu Rev Psychol. 1993;44:317–342. doi: 10.1146/annurev.ps.44.020193.001533. [DOI] [PubMed] [Google Scholar]
- 19.Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behav Neurosci. 1993;107(6):1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- 20.Bandler R, Carrive P, Depaulis A. Introduction: Emerging principles of organization of the midbrain periaqueductal gray matter. In: Depaulis A, Bandler R, editors. The Midbrain Periaqueductal Gray Matter. New York: Plenum; 1991. pp. 1–8. [Google Scholar]
- 21.Blanchard RJ, Fukunaga KK, Blanchard DC. Environmental control of defensive reactions to footshock. Bull Psychon Soc. 1976;8:129–130. [Google Scholar]
- 22.Bolles RC, Collier AC. The effect of predictive cues on freezing in rats. Anim Learn Behav. 1976;4:6–8. [Google Scholar]
- 23.Maren S. Pavlovian fear conditioning as a behavioral assay for hippocampus and amygdala function: Cautions and caveats. Eur J Neurosci. 2008;28(8):1661–1666. doi: 10.1111/j.1460-9568.2008.06485.x. [DOI] [PubMed] [Google Scholar]
- 24.Applegate CD, Kapp BS, Underwood MD, McNall CL. Autonomic and somatomotor effects of amygdala central N. stimulation in awake rabbits. Physiol Behav. 1983;31(3):353–360. doi: 10.1016/0031-9384(83)90201-9. [DOI] [PubMed] [Google Scholar]
- 25.Iwata J, Chida K, LeDoux JE. Cardiovascular responses elicited by stimulation of neurons in the central amygdaloid nucleus in awake but not anesthetized rats resemble conditioned emotional responses. Brain Res. 1987;418(1):183–188. doi: 10.1016/0006-8993(87)90978-4. [DOI] [PubMed] [Google Scholar]
- 26.Rosen JB, Davis M. Enhancement of acoustic startle by electrical stimulation of the amygdala. Behav Neurosci. 1988;102(2):195–202. doi: 10.1037//0735-7044.102.2.195. [DOI] [PubMed] [Google Scholar]
- 27.Weingarten H, White N. Exploration evoked by electrical stimulation of the amygdala of rats. Physiol Psychol. 1978;6(2):229–235. [Google Scholar]
- 28.Ciocchi S, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468(7321):277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- 29.Maren S. Seeking a spotless mind: Extinction, deconsolidation, and erasure of fear memory. Neuron. 2011;70(5):830–845. doi: 10.1016/j.neuron.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCormick DA, Thompson RF. Cerebellum: Essential involvement in the classically conditioned eyelid response. Science. 1984;223(4633):296–299. doi: 10.1126/science.6701513. [DOI] [PubMed] [Google Scholar]
- 31.Chapman PF, Steinmetz JE, Thompson RF. Classical conditioning does not occur when direct stimulation of the red nucleus or cerebellar nuclei is the unconditioned stimulus. Brain Res. 1988;442(1):97–104. doi: 10.1016/0006-8993(88)91436-9. [DOI] [PubMed] [Google Scholar]
- 32.Steinmetz JE, Lavond DG, Thompson RF. Classical conditioning of the rabbit eyelid response with mossy fiber stimulation as the conditioned stimulus. Bull Psychon Soc. 1985;23(3):245–248. [Google Scholar]
- 33.Mauk MD, Steinmetz JE, Thompson RF. Classical conditioning using stimulation of the inferior olive as the unconditioned stimulus. Proc Natl Acad Sci USA. 1986;83(14):5349–5353. doi: 10.1073/pnas.83.14.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amorapanth P, LeDoux JE, Nader K. Different lateral amygdala outputs mediate reactions and actions elicited by a fear-arousing stimulus. Nat Neurosci. 2000;3(1):74–79. doi: 10.1038/71145. [DOI] [PubMed] [Google Scholar]
- 35.Rizvi TA, Ennis M, Behbehani MM, Shipley MT. Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: Topography and reciprocity. J Comp Neurol. 1991;303(1):121–131. doi: 10.1002/cne.903030111. [DOI] [PubMed] [Google Scholar]
- 36.Hadjipavlou G, Dunckley P, Behrens TE, Tracey I. Determining anatomical connectivities between cortical and brainstem pain processing regions in humans: A diffusion tensor imaging study in healthy controls. Pain. 2006;123(1-2):169–178. doi: 10.1016/j.pain.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 37.Vianna DM, Borelli KG, Ferreira-Netto C, Macedo CE, Brandão ML. Fos-like immunoreactive neurons following electrical stimulation of the dorsal periaqueductal gray at freezing and escape thresholds. Brain Res Bull. 2003;62(3):179–189. doi: 10.1016/j.brainresbull.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 38.Yoon T, Graham LK, Kim JJ. Hippocampal lesion effects on occasion setting by contextual and discrete stimuli. Neurobiol Learn Mem. 2011;95(2):176–184. doi: 10.1016/j.nlm.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.deCampo DM, Fudge JL. Where and what is the paralaminar nucleus? A review on a unique and frequently overlooked area of the primate amygdala. Neurosci Biobehav Rev. 2012;36(1):520–535. doi: 10.1016/j.neubiorev.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han JH, et al. Selective erasure of a fear memory. Science. 2009;323(5920):1492–1496. doi: 10.1126/science.1164139. [DOI] [PubMed] [Google Scholar]
- 41.Deakin JF, Graeff FG. 5-HT and mechanisms of defence. J Psychopharmacol. 1991;5(4):305–315. doi: 10.1177/026988119100500414. [DOI] [PubMed] [Google Scholar]
- 42.Graeff FG, Silveira MC, Nogueira RL, Audi EA, Oliveira RM. Role of the amygdala and periaqueductal gray in anxiety and panic. Behav Brain Res. 1993;58(1-2):123–131. doi: 10.1016/0166-4328(93)90097-a. [DOI] [PubMed] [Google Scholar]
- 43.Maren S, Fanselow MS. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J Neurosci. 1995;15(11):7548–7564. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clugnet MC, LeDoux JE. Synaptic plasticity in fear conditioning circuits: Induction of LTP in the lateral nucleus of the amygdala by stimulation of the medial geniculate body. J Neurosci. 1990;10(8):2818–2824. doi: 10.1523/JNEUROSCI.10-08-02818.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maren S, Yap SA, Goosens KA. The amygdala is essential for the development of neuronal plasticity in the medial geniculate nucleus during auditory fear conditioning in rats. J Neurosci. 2001;21(6):RC135. doi: 10.1523/JNEUROSCI.21-06-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilensky AE, Schafe GE, LeDoux JE. Functional inactivation of the amygdala before but not after auditory fear conditioning prevents memory formation. J Neurosci. 1999;19(24):RC48. doi: 10.1523/JNEUROSCI.19-24-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graham LK, Yoon T, Kim JJ. Stress impairs optimal behavior in a water foraging choice task in rats. Learn Mem. 2010;17(1):1–4. doi: 10.1101/lm.1605510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee H, Kim JJ. Amygdalar NMDA receptors are critical for new fear learning in previously fear-conditioned rats. J Neurosci. 1998;18(20):8444–8454. doi: 10.1523/JNEUROSCI.18-20-08444.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim EJ, Kim ES, Covey E, Kim JJ. Social transmission of fear in rats: The role of 22-kHz ultrasonic distress vocalization. PLoS One. 2010;5(12):e15077. doi: 10.1371/journal.pone.0015077. [DOI] [PMC free article] [PubMed] [Google Scholar]