Significance
ATP, a ubiquitous source of energy for all cells, also serves as an important messenger for intercellular communication. This role as a signal molecule is especially crucial for taste buds, which release ATP to trigger purinergic receptors on the taste nerves. Normally, the released ATP is degraded by a specific enzyme, nucleoside triphosphate diphosphohydrolase-2 (NTPDase2). We find that genetic elimination of NTPDase2 results in accumulation of ATP in extracellular space, thereby desensitizing the receptors on nerve fibers. The result is a loss of transmission of taste information from taste buds to the taste nerves. Disruption of taste function may be an unintended consequence of pharmaceutical agents now being developed to target purinergic receptors and enzymes as treatments for chronic pain and other illnesses.
Keywords: purinergic signaling, synaptic function, E-NTPDase, mouse, gustatory
Abstract
Taste buds are unusual in requiring ATP as a transmitter to activate sensory nerve fibers. In response to taste stimuli, taste cells release ATP, activating purinergic receptors containing the P2X2 and P2X3 subunits on taste nerves. In turn, the released ATP is hydrolyzed to ADP by a plasma membrane nucleoside triphosphate previously identified as nucleoside triphosphate diphosphohydrolase-2 (NTPDase2). In this paper we investigate the role of this ectonucleotidase in the function of taste buds by examining gene-targeted Entpd2-null mice globally lacking NTPDase2. RT-PCR confirmed the absence of NTPDase2, and ATPase enzyme histochemistry reveals no reaction product in taste buds of knockout mice, suggesting that NTPDase2 is the dominant form in taste buds. RT-PCR and immunocytochemistry demonstrated that in knockout mice all cell types are present in taste buds, even those cells normally expressing NTPDase2. In addition, the overall number and size of taste buds are normal in Entpd2-null mice. Luciferin/luciferase assays of circumvallate tissue of knockout mice detected elevated levels of extracellular ATP. Electrophysiological recordings from two taste nerves, the chorda tympani and glossopharyngeal, revealed depressed responses to all taste stimuli in Entpd2-null mice. Responses were more depressed in the glossopharyngeal nerve than in the chorda tympani nerve and involved all taste qualities; responses in the chorda tympani were more depressed to sweet and umami stimuli than to other qualities. We suggest that the excessive levels of extracellular ATP in the Entpd2-knockout animals desensitize the P2X receptors associated with nerve fibers, thereby depressing taste responses.
Taste buds, the sensory end organs of gustation, are unique among the special senses in using ATP as a key transmitter to activate their sensory nerve fibers (1). The gustatory nerves express the purinergic receptor subunits P2X2 and P2X3 (2), which rapidly depolarize the nerve terminal when exposed to ATP. An important feature of neurotransmission is the removal of transmitter from extracellular space to prevent desensitization of the receptors by prolonged exposure to the ligand. In the case of taste buds, removal of ATP is accomplished largely by one of the eight known ectonucleotidases (for a review, see ref. 3), nucleoside triphosphate diphosphohydrolase-2 (NTPDase2), a highly specific nucleoside triphosphate diphosphohydrolase (4, 5), which preferentially degrades ATP over ADP (6), (i.e., an ectoATPase, as defined histochemically by specificity for ATP).
The NTPDase of taste buds is expressed by only one of the three principal types of cells within the bud. Each taste bud contains an onion-shaped cluster of 50–100 elongate taste cells, comprising morphologically and molecularly distinct cell types (for review, see ref. 7): type I, type II, and type III. The detection and transduction of different tastants is accomplished by type II and type III cells (for review, see refs. 7 and 8). Type II cells, also called “receptor” cells, express the G protein-coupled taste receptors and downstream effectors for bitter, sweet, and umami qualities. When activated, type II cells release ATP via a nonvesicular mechanism, likely involving ion channels (9–12). Type III cells, also called “presynaptic” cells, are implicated in transduction of sour and salty substances and are the only taste cells that possess conventional synaptic contacts with afferent nerve fibers (13–17). Type I cells, generally considered to have a support or “glial-like” function because they wrap around the other cell types, are the cells that express NTPDase2 (18-19).
Once ATP is released into the extracellular space within a taste bud, it activates purinergic receptors on other taste cells as well as the ionotropic purinergic receptors on the taste nerves composed of P2X2 and P2X3 subunits. Various taste cells express ionotropic P2X2 and P2X7 receptors (20, 21) and metabotropic P2Y1 (22, 23), P2Y2, and P2Y4 (24) receptors. After release, ATP is degraded via NTPDase2 to ADP, which itself can activate the P2Y receptors. The ADP then is further degraded to AMP and finally to adenosine via other and less specific ectonucleotidases including ecto-5′-nucleotidase expressed in type III cells (25).
In this study we have focused on the role of NTPDase2 in regulating synaptic function in taste buds. We report that genetic deletion of NTPDase2 results in the accumulation of ATP in the taste tissues and a concomitant significant decrease of neural taste responses, likely because of receptor desensitization.
Results
Characterization of Entpd2-Null Mice.
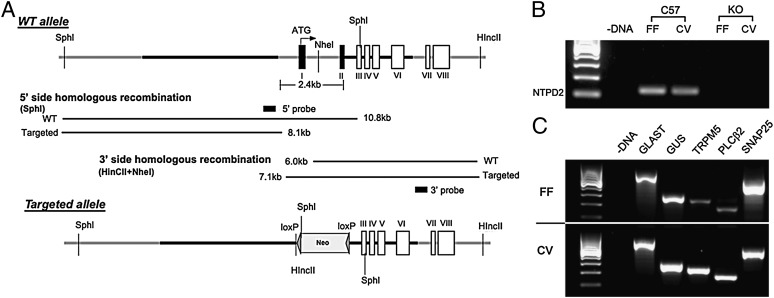
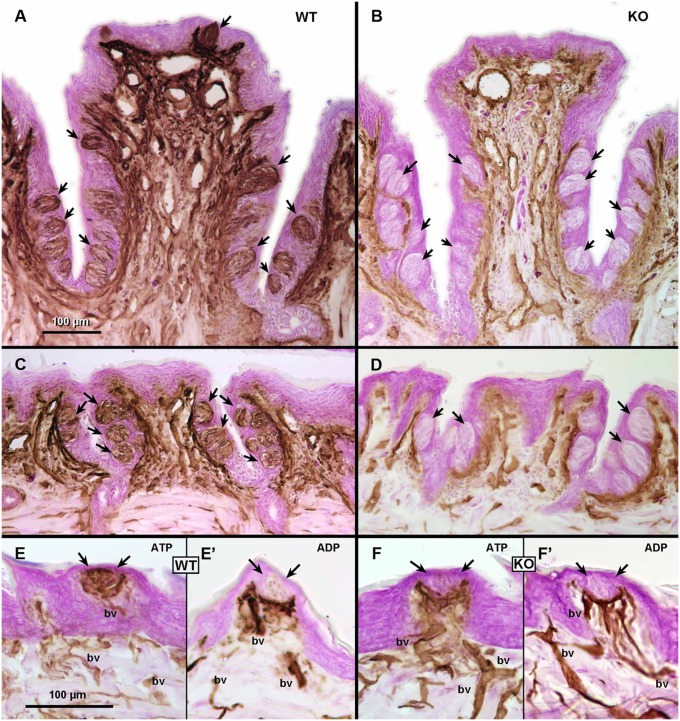
We used molecular, histochemical, and anatomical measures to determine the effects of the genetic deletion of Entpd2 on taste epithelia (Fig. 1). First, we used RT-PCR to test for expression of NTPDase2 mRNA in pooled taste buds isolated from fungiform and circumvallate papillae. As shown in Fig. 1B, genetic deletion of Entpd2 effectively eliminated expression of NTPDase2 mRNA in taste buds. To determine whether all three types of taste cells were present in the taste buds of the KO mice, we used RT-PCR to test for expression of taste cell-specific markers: glutamate aspartate transporter (GLAST) for type I cells; α-gustducin, transient receptor potential melastatin 5 (TRPM5), and phospholipase C β2 (PLCβ2) for type II cells; and synaptosomal-associated protein 25 (SNAP25) for type III cells. As shown in Fig. 1C, PCR products representing all three cell types are present in both circumvallate and fungiform taste buds of the KO mice. In addition, to verify that NTPDase2 is the only ectoATPase present and functional in taste buds, we compared ectoATPase activity in circumvallate papillae of WT and Entpd2-KO animals. Use of two different substrates, ADP and ATP, allowed us to distinguish specific ectoATPase staining representing NTPDase2 or NTPDase8 (26) from less specific nucleotidases that degrade ADP as well as ATP. As expected, in WT animals, dense reaction product forms within taste buds when ATP but not ADP is used as substrate, demonstrating the high specificity for the ectonucleotidase in taste buds, commensurate with the expression of NTPDase2. In contrast, in Entpd2-KO animals, no ectoATPase activity was detected in the taste buds (Fig. 2 A–D) with ATP as a substrate, confirming the major role of NTPDase2 in the degradation of ATP in this system. In both WT and Entpd2 KO lines, the nerve bundles beneath taste buds exhibited nucleotidase activity when ADP was used as a substrate, indicating the presence of a different nucleotidase in and around these nerve bundles (Fig. 2 E–H).
Fig. 1.
Targeting strategy for generation of Entpd2-null mice. (A) ES targeting of Entpd2. Thick black lines are homologous fragments used for recombination. (B) GelRed (Biotium)-stained RT-PCR product for NTPDase2 (NTPD2) in C57 and KO mice. Expected sizes for NTPDase2 (124 bp) were seen in the C57BL/6 fungiform (FF) and circumvallate (CV) taste papillae, whereas mRNA expression was effectively eliminated in the Entpd2-KO tissues. (C) GelRed-stained RT-PCR products for cell-type markers in Entpd2-KO taste tissue. The first lane represents a 100-bp ladder, and no expression was observed in the zero template controls (− DNA). Expected sizes for GLAST (727 bp), α-gustducin (GUS; 286 bp), TRPM5 (234 bp), PLCβ2 (163 bp), and SNAP25 (521 bp) were seen in the Entpd2-KO fungiform and circumvallate taste papillae.
Fig. 2.
Ectonucleotidase staining (brown) and Giemsa counterstaining (magenta) in taste papillae from WT and KO mice. (A and B) EctoATPase staining in the circumvallate papilla from WT (A) and Entpd2-KO (B) animals. Arrows indicate the taste buds, which are heavily reactive in WT mice but not reactive in KO mice. (C and D) EctoATPase staining in foliate papillae from WT (C) and KO (D) mice. Arrows indicate the location of taste buds. Similar to the circumvallate papilla, taste buds are heavily reactive in WT animals and are nonreactive in KO mice. (E and F) Comparison of ectoATPase staining (E and F) and nonspecific nucleotidase staining (ADP substrate) (E′ and F′) in fungiform papillae of WT (E and E′ ) and KO (F and F′) animals. Arrows indicate the position of taste buds. In WT (E) but not KO (F) mice, dense reaction product occurs within the taste buds with ATP as a substrate. With ADP substrate, to reveal nonspecific nucleotidase activity, similar patterns of staining occur in blood vessels (bv) and in the nerve bundle beneath the taste buds in WT and KO animals.
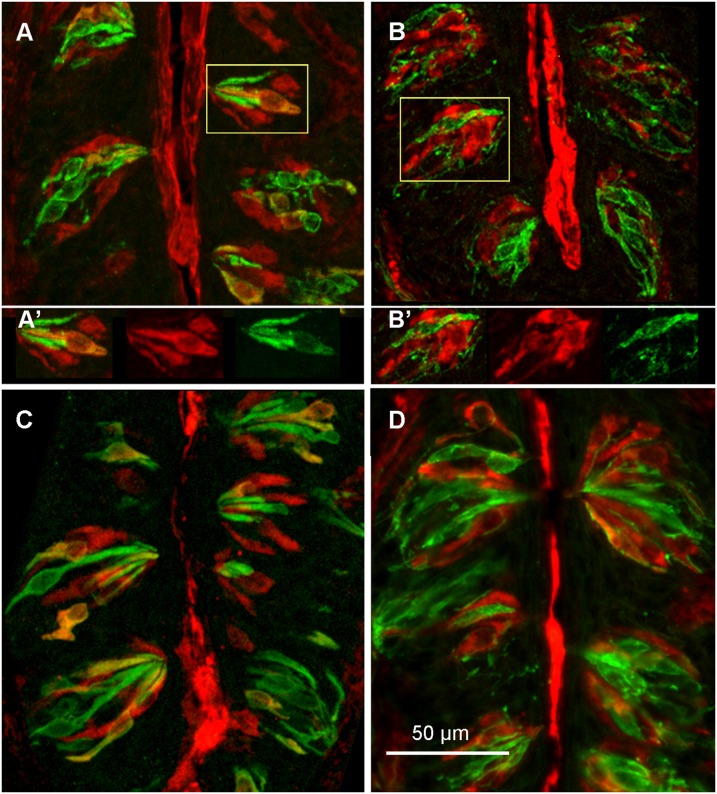
To determine if genetic deletion of Entpd2 affected the morphology of gustatory papillae, we measured the size of the circumvallate papillae of four KO and four WT mice. The overall papilla size was ∼15% smaller (t test; P < 0.05) in the KO mice than in their WT counterparts (Table S1). In two individuals of each genotype from this group, we also measured the size and total number of taste buds. Despite the difference in size of the papillae, the size of taste buds (average diameter 37.1 µm for WT and 37.48 µm for KO) and total number of taste buds (WT = 121–137; KO = 126–148) was not different between genotypes (Table S1). To determine if the KO and WT mice have a similar complement of cell types, we used immunohistochemistry with antibodies specific to individual taste cell types (GLAST for type I taste cells, α-gustducin for type II cells, and SNAP25 for type III cells). All markers were present in taste buds of the two strains (Fig. 3), indicating that, despite the genetic deletion of NTPDase2 from type I cells, taste buds in both WT and KO lines still contain all three major taste cell types. In summary, the morphology of taste buds is similar in the WT and KO lines, so differences in function cannot be attributed to gross differences in taste bud number or structure.
Fig. 3.
Micrographs showing the presence of all major taste cell types within the circumvallate taste buds of WT (A and B) and Entpd2-KO (C and D) mice. (A) GLAST (red) for type I cells and gustducin (green) for type II cells. A few cells exhibit double labeling, suggesting that GLAST labels some type II cells as well as type I cells (19). An off-tissue region showing bright red fluorescence was eliminated by replacement with adjacent pixels. (A′) Color separation images from the boxed portion of A demonstrate the presence of single-labeled (red only) GLAST-positive cells as well as scattered double-labeled cells. (B) Double labeling for GLAST (red) and SNAP25 (green) for type III cells and nerve fibers shows mutually exclusive patterns of labeling. (B′) Color separation images from the taste bud in the boxed area in B. (C) Staining for GLAST (red) in type I cells and gustducin (green) in type II cells in KO mice. The images are very similar to the corresponding panel in A from WT mice. (D) Staining for GLAST (red) and SNAP25 (green) in type III cells and nerve fibers is similar to the image in B from WT mice. In this image, a single double-labeled cell is apparent at top right (orange-yellow), but such rare double-labeled cells also occur in the WT animals.
Tissue Levels of ATP.
Because NTPDase2 degrades extracellular ATP, we used a luciferin/luciferase assay to test whether mice lacking this enzyme have elevated levels of ATP in epithelium isolated from the circumvallate taste papillae. Indeed, significantly more ATP was present in the extracellular solution surrounding the Entpd2-null taste tissue than in the WT tissues (P < 0.05; t test) (Table 1). These data suggest that in the absence of NTPDase2 to degrade ATP, this nucleotide accumulates significantly in the extracellular microenvironment. Stimulation of the apical membrane with a mixture of bitter tastants (20 mM denatonium + 100 µM cycloheximide) evokes ATP release in WT mice, but the KO mice fail to release detectable levels of ATP over background levels (Table 1).
Table 1.
Luciferase assay of ATP concentration in circumvallate papillae of WT and Entpd2-KO mice
| Tyrode’s bathing solution (nM ATP) | Taste (nM ATP) | |
| WT | 2.3 ± 0.3 | 3.2 ± 0.4 |
| KO | 8.1 ± 1.8 | 7.2 ± 1.4 |
Data shown are mean ± SEM of three 1-min measurements; n = 15 KO and 10 WT mice. KO Tyrode’s bathing solution > WT Tyrode’s bathing solution, P < 0.05, unpaired t test. WT taste > WT Tyrode’s bathing solution, P < 0.05, paired t test.
Nerve Recordings.
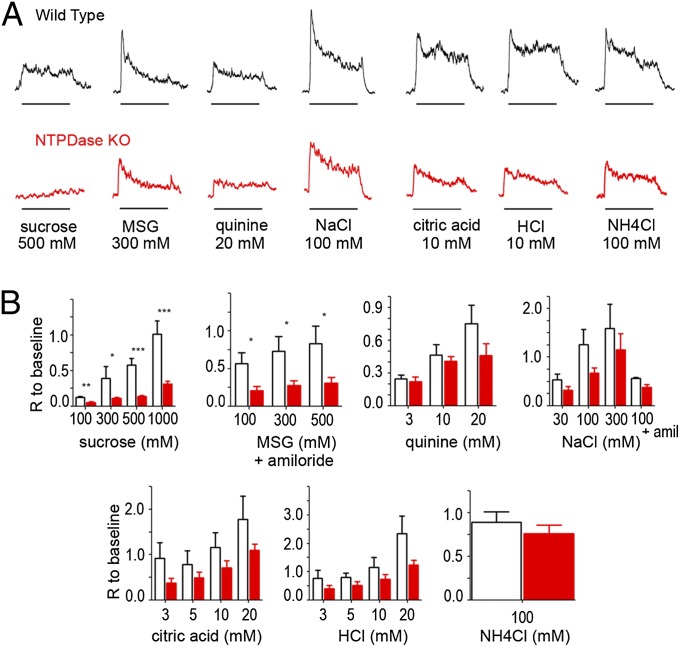
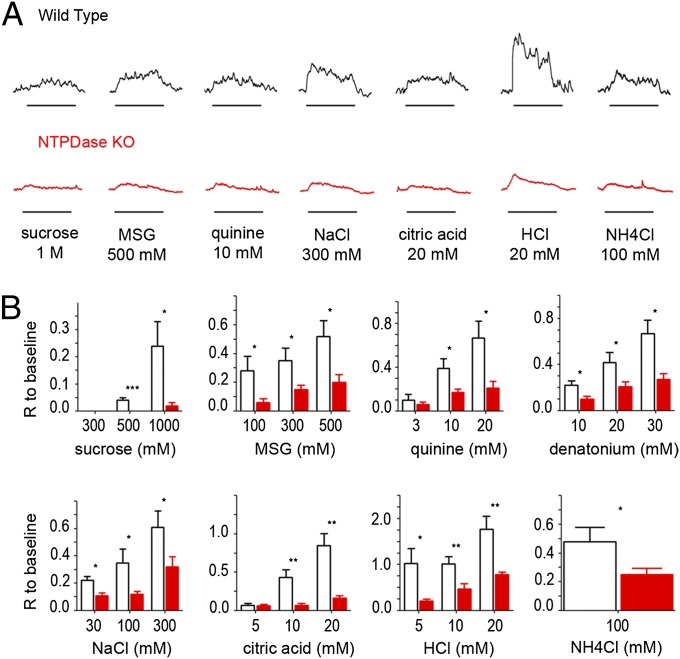
To determine if genetic deletion of Entpd2 affects synaptic function in the taste bud, we measured responses to taste stimuli with whole-nerve recordings from chorda tympani and glossopharyngeal nerves in WT and Entpd2-null animals. In both nerves, KO animals showed reduced responses to all taste qualities. Because the response to 100 mM NH4Cl, which often is used as a reference, was decreased also, we normalized responses to the baseline immediately before stimulation to compare responses in different mice. In the chorda tympani (Fig. 4), the genotype factor is significant for all tastants except NaCl, quinine, and NH4Cl (two-way ANOVA, P < 0.05). Taken individually, the responses to sucrose (300 mM, 500 mM, and 1 M), and monosodium glutamate (MSG) with amiloride (100 mM, 300 mM, and 500 mM) were significantly smaller in KO (62–77% decrease) than in WT animals (Student t test, P < 0.05). Responses to HCl and citric acid also were decreased in KO mice (28–59% of WT response), but the decrease was not statistically significant. In the glossopharyngeal nerve (Fig. 5), the genotype factor is significant for all tastants (two-way ANOVA, P < 0.05), and individual concentrations of all taste qualities except 3 mM quinine and 5 mM citric acid) were reduced significantly in the KO animals (51–100% decrease compared with WT), including acids and NaCl. These results suggest that the lack of degradation of ATP and its accumulation in the taste tissue of Entpd2-KO mice decreases responses to all taste qualities.
Fig. 4.
(A) Integrated chorda tympani nerve responses from WT (Upper) and Entpd2-KO (Lower) mice to 500 mM sucrose, 300 mM MSG (with amiloride to block the Na+ component), 20 mM quinine, 100 mM NaCl, 10 mM citric acid, 10 mM HCl, and 100 mM NH4Cl. Taste stimuli were applied for 30 s with 50-s intervening washes; bars beneath each recording denote the onset and duration of the stimulus. (B) Bar graphs showing the amplitude of the integrated response in WT (open bars) and Entpd2 KO (red bars) mice (n = 4–9 mice for each stimulus). Each response was normalized to the baseline so that different mice could be compared. Two-way ANOVA revealed a significant difference between the KO and WT mice for all qualities except NaCl, quinine, and NH4Cl (P < 0.05), but responses to sucrose and MSG were more affected than responses to citric acid and HCl. The Student t test revealed that all concentrations of sucrose and MSG were significantly depressed relative to WT mice, but only the highest concentrations of acids were significantly depressed. Data are shown as mean ± SEM. *P < 0.05; ** < 0.01; *** < 0.001.
Fig. 5.
(A) Integrated glossopharyngeal nerve responses to taste stimulation in WT (Upper) and Entpd2-KO (Lower) mice. Taste stimuli were applied for 60 s with 50-s intervening washes; bars beneath each recording denote onset and duration of the stimulus. (B) Bar graph showing amplitude (relative to baseline) of the integrated response in WT (open bars) and Entpd2-KO mice (red bars) to the same stimuli as in Fig. 4 plus denatonium (n = 4–9 mice for each stimulus). Each integrated taste response was normalized to the baseline. Two-way ANOVA was significant for all qualities, although sweet stimuli and acids were affected more than the other stimuli. Data are shown as mean ± SEM.
Discussion
The principal finding in this study is that genetic deletion of NTPDase2, the only ectoATPase expressed in taste buds, results in decreased neural responses to taste stimuli. Because taste bud numbers and taste cell types were unaffected by the knockout, the decrease in responsiveness presumably reflects the lack of degradation and elevated tissue levels of ATP. Because ATP activation of P2X receptors on the gustatory nerve fibers is required for neurotransmission in the taste system, we suggest that genetic deletion of the ectoATPase results in a disruption of purinergic transmission at this crucial synapse.
What is the mechanism of the decreased responsiveness? Desensitization of purinergic receptors on the taste nerve fibers seems a likely explanation. The nerve fibers express purinergic receptors containing P2X2 and P2X3 subunits (2), both of which adapt to prolonged (∼60 s) exposure to ATP, with P2X3 adapting more rapidly than P2X2 (27). Although both P2X2 and P2X3 can form homotrimeric receptors, they also tend to form heteromers when coexpressed in a single cell. P2X3 is expressed by nearly all ganglionic neurons of the chorda tympani nerve, whereas P2X2 is expressed on only 50–60% of these ganglion cells (28). Thus, ganglion cells expressing only P2X23 produce P2X3 homomeric receptors, whereas other cells expressing both P2X2 and P2X3 generate mostly heteromeric receptors (29). In either case, P2X3 is a component of the receptors expressed on gustatory afferent fibers. Responses elicited by P2X3 homotrimers show rapid activation and desensitization (30), with nanomolar concentrations of ATP capable of causing chronic desensitization (31). In contrast, P2X2/3 heteromers show less desensitization, with the degree of desensitization being determined by the number of P2X3-containing subunits in the receptor (30).
The extracellular solution surrounding the unstimulated taste tissue in the Entpd2-null mice contains high nanomolar concentrations of ATP—levels consistent with the desensitization of P2X3 homomers, suggesting that chronic desensitization of P2X3 (and concurrently P2X2) may cause the decreased responsiveness observed in the Entpd2-null mice. The differential distribution of P2X2 and P2X3 subunits between the two taste nerves may explain the taste specificity of the effect. For example, if P2X3 homotrimers were expressed selectively on nerve fibers that contact sweet- and umami-sensitive taste cells, then these fibers would be more affected by high levels of extracellular ATP than would fibers expressing P2X2/3 heteromers. Interestingly, in P2X3 single-KO mice responses to sweet stimuli are more impaired than responses to bitter stimuli (1), suggesting that any differential distribution of P2X subunits may correlate with specificity of taste cell innervation.
Similarly, the effects of the NTPDase2 knockout on taste responsiveness were not uniform across the two taste nerves studied. In the chorda tympani nerve, which innervates fungiform papillae of the anterior tongue, responses to sweet and umami (MSG) qualities were most impaired, with little or no effect on salts, the bitter stimulus quinine, and acids. In contrast, in the glossopharyngeal nerve, which innervates posterior lingual taste fields, i.e., circumvallate and foliate papillae, most taste qualities were severely impacted. One possible explanation for the differences in response of chorda tympani and glossopharyngeal nerves relates to the packing density of taste buds within the respective taste epithelia. Taste buds in glossopharyngeal-innervated taste fields are packed more densely than the taste buds sitting singly in fungiform papillae. Assuming that each taste bud releases a similar amount of ATP, the denser packing of taste buds in posterior taste fields would likely produce higher levels of extracellular ATP than in the epithelium surrounding the isolated fungiform taste buds. Thus, the higher impact of the knockout on glossopharyngeal nerve responses from posterior taste fields is not surprising.
The marked decrease in taste nerve responses to all taste qualities is itself noteworthy. Direct ATP release in response to taste stimuli has been noted only for the cells mediating responses to sweet, umami, and bitter qualities, i.e., type II cells (9, 11). So the effects of the knockout on transmission of these qualities are easily understood by the interference with purinergic transmission from type II cells to the nerve fibers. Less explicable is the loss of responses to sour (acid) stimuli in the glossopharyngeal nerve of the KO mice. Sour is transduced by type III cells (7); no one has yet succeeded in measuring ATP release from this cell type. Furthermore, the mechanism of neurotransmission from type III cells to nerve fibers is not well understood. Nonetheless, the loss of acid responsiveness in the Entpd2-null mice is consistent with loss of responsiveness to all taste qualities, including sour, in mice with genetic deletion of the purinergic receptors (P2X2/3 double knockout) (1). Taken together, the data strongly suggest that ATP is required for the transmission of all taste qualities via P2X receptors on the nerve fibers. However, the cellular source of ATP required for transmission of salty and sour information remains unclear.
The lack of degradation of ATP in the NTPDase2-KO mice results not only in high levels of ATP in extracellular space but also in a concomitant decrease in the breakdown products ADP and adenosine. We suggest that the decrease in taste nerve responses in the KO mice also may reflect the absence of ADP that normally results from the NTPDase2-mediated degradation of ATP. Purinergic P2Y receptors are present on taste cell membranes, with P2Y1 expressed by type II cells and P2Y4 expressed by type III cells. Inhibitors of P2Y1, which prefers ADP to ATP, decrease taste-evoked ATP release from type II cells (23). Presumably, ADP resulting from the hydrolysis of ATP stimulates P2Y1, causing an increase in intracellular Ca2+ and an enhanced taste-evoked release of ATP via a positive feedback mechanism. Thus, the loss of ATP hydrolysis in the NTPDase2-KO mice, and consequent reduction in available ADP to activate the P2Y1 receptors, should decrease ATP release from type II cells. We confirmed a lack of taste-evoked ATP release from circumvallate taste buds, but the high levels of ATP in the extracellular solution surrounding the taste tissue of the KO mice made it difficult to detect taste-evoked ATP release over background levels.
The reduction of extracellular ADP in the KO mice not only reduces the activation of P2Y receptors but also decreases the production of adenosine generated from ADP by nonspecific ectonucleotidases including ecto-5′-nucleotidase (25). Adenosine acting via the A2B receptor, which is expressed selectively on sweet-responsive taste cells of the posterior tongue, is required for normal sweet taste responses in circumvallate taste buds (25, 32). Activation of the A2B receptor elevates intracellular Ca2+, thereby enhancing ATP release to sweet stimuli. Thus, lowered levels of extracellular adenosine in the KO mice may contribute to the decrease in sweet responsiveness in glossopharyngeal-innervated taste fields. However, A2B is not expressed in anterior tongue, so this mechanism cannot account for the decreased sweet responses in the chorda tympani nerve.
All neurotransmitter systems rely on the effective removal of synaptically released transmitter for maintaining proper synaptic function. However, in many cases failure to remove transmitter, either by inhibiting the uptake mechanism or by blocking the degradative enzyme, enhances postsynaptic responses. For example, serotonin reuptake inhibitors prolong the lifetime of serotonin at the synapse, thereby increasing the effectiveness of the serotonin. In the case of ATP as a transmitter in taste buds, our data suggest that rather than enhancing synaptic transmission, failure to remove ATP results in depressed synaptic transmission.
Similar modulation of purinergic receptor function occurs after deletion of another related ectonucleotidase, as in Entpd1-null (Cd39-/-) mice (33). NTPDase1 is expressed at the sympathetic neuromuscular junction, where ATP is co-released with norepinephrine. Entpd1 null mice exhibit decreased synaptic efficacy, presumably due to desensitization P2X1 receptors (34, 35). However in the cochlea, Entpd1 gene deletion had little effect, if any, on hearing, but this lack of effect may result from the coexpression of other ectonucleotidases –NTPDase2 and NTPDase8 –normally present in that epithelium (36). Unlike the situation in the ear, taste buds express only NTPDase2. Our histochemical results in taste tissues show no evidence of up-regulation or de novo expression of any other ectoATPase(s) in the face of genetic elimination of NTPDase2, but we cannot rule out changes in expression of other, less specific nucleotidases.
Although the crucial role for purinergic signaling in rodent taste systems is well established, clinical evidence for its role in human gustation is scanty. A clinical study testing the effects of the broad-spectrum P2X receptor antagonist suramin reports taste disturbances in more than two-thirds of the patients tested (37). These data implicate a role for receptors containing the P2X subunit in human taste, but whether disrupted NTPDase2 function in a patient population would lead to similar taste disturbances remains to be tested.
In summary, our results show that elimination of NTPDase2, the predominant ectoATPase in taste buds, substantially impairs transmission of taste information from the taste buds to the taste nerves. We suggest that lack of NTPDase2 allows the accumulation of extracellular ATP within the taste bud and consequent desensitization of the P2X receptors on the taste nerves. Because of the crucial role of P2X receptors in taste transmission, the desensitization of the P2X receptors, as does their genetic deletion, prevents normal transmission of taste information to the gustatory nerves.
Materials and Methods
Animals.
All experimental procedures were approved by the Animal Care and Use Committees at the University of Colorado Denver School of Medicine. Experiments included the use of WT and Entpd2-null mice (both in C57BL6 background) generated at GenOway.
Generation of Mutant Mice.
Entpd2-null mice were created using a targeting construct that deletes exons I and II including the promoter region by homologous recombination in murine ES cells derived from 129sv mice (Fig. 1). Recombination in the ES cells was confirmed by Southern blot analysis both on 5′ and 3′ regions using probes generated by PCR (Fig. S1). The primer sets used to generate probes were ggtccttggccatgagtgtc and gaacaggcaaggacaaraggc for the 5′ side and aagacacaggagagactcagcag and ccaagaaaggcaggaaatacac for the 3′ side. The targeted ES cells were used to create chimeric mice that transmit the targeted allele through their germ lines. The resultant mutant mice were screened by PCR and backcrossed six times onto the C57BL6tac background to create homozygous deletion mice in which the gene deletion was validated by PCR and immunohistochemistry. The Entpd2-null mice are viable and fertile; no gross morphological or behavioral abnormalities are obvious. Interbreeding the heterozygote mouse yielded offspring at a WT:heterozygous:KO ratio of 1:1.9:1.1 (n = 125), suggesting Mendelian inheritance of the deletion.
RT-PCR.
RT-PCR was used to validate the absence of NTPDase2 mRNA and to test whether expression of other taste-related genes was affected in the Entpd2-null mouse. Tissue was isolated from the fungiform and circumvallate papillae pooled from six adult C57/Bl6 mice and six Entpd2-null mice. RNA was extracted using the RNeasy Mini kit from Qiagen according to the manufacturer’s instructions. Reverse transcription was performed using the iScript cDNA Synthesis kit from Bio-Rad. All primers spanned at least one intron to control for genomic DNA contamination (Table S2). PCR products were sequenced and compared with published sequences using BLAST (National Center for Biotechnology Information). Over the regions sequenced, there was 100% identity between our PCR product and published sequences for NTPDase2, GLAST, α-gustducin, TRPM5, PLCβ2, and SNAP25.
ATPase Histochemistry.
Entpd2-KO and C57/Bl6 mice were anesthetized and perfused transcardially with saline followed by fixative consisting of 2% (wt/vol) paraformaldehyde, 0.2% glutaraldehyde in 0.1 M Tris-maleate buffer (pH7.4) with 0.2 mM CaCl2. After cryoprotection overnight, cryostat sections of the tongue (12–16 µm) were processed for specific ATPase histochemistry using a reaction solution containing 1 mM levamisole, 1 mM ouabain, 50 uM αβ-methylene ADP, 2 mM CaCl2, in Tris-maleate buffer (pH 7.4) along with either 0.94 mM ATP or 0.85 mM ADP as substrates. After exposure to ammonium sulfide, the tissue was stained with a Giemsa counterstain, dehydrated, and coverslipped with Permount (Fisher Scientific).
Morphometrics.
For quantitative anatomy, all sections were cut at 12 µm and collected in a series with spacing of 144 µm between sections. Measurements of staining intensity and papillary size were performed using ImageJ software v. 1.46r (National Institutes of Health) on sections from four different individuals of each strain. Because small but significant differences in papillary size existed between the two lines, we tested whether the taste buds were different sizes and whether there were different numbers of taste buds in the two lines. For these counts, we carried out detailed morphometric measures on two individuals of each line. We calculated the total number of taste buds per papilla by applying the Abercrombie correction factor to the raw counts of taste buds in the samples. Calculated values were similar to figures obtained previously by other investigators (38). See SI Materials and Methods for technical details.
Immunohistochemistry.
Entpd2-KO and WT mice were deeply anesthetized and perfused using periodate-lysine-paraformaldehyde fixative (75 mM lysine, 1.6% paraformaldehyde, 10 mM NaIO4 in 0.1 M phosphate buffer, pH 7.4). After 1.5 h postfix and cryoprotection, 12- to 16-µm cryostat sections were cut and mounted onto slides. After buffer washes, antigen retrieval was preformed as necessary using 10 mM sodium citrate (pH 9.0) at 80 °C for 25 min followed by exposure to 3% H2O2. Specific primary antibodies for each cell type then were applied to the sections. We used guinea pig anti-GLAST (1:1,000) (AB1782; Chemicon) for type I taste cells; rabbit anti-Gα-gustducin (1:2,500) (SC-395; Santa Cruz Biotechnology) for type II cells; and rabbit anti-SNAP25 (1:5,000) (Sigma) for type III cells. Primary antibody binding was revealed using an avidin-biotin-peroxidase complex followed by a tyramide detection system; double labeling was performed using indirect immunofluorescence after the tyramide reaction. Images were collected using a confocal laser-scanning microscope FV300 (Olympus Fluoview) and were adjusted with Photoshop for brightness and gamma. See SI Materials and Methods for details.
Luciferin/Luciferase Assay.
To measure the concentration of ATP present surrounding the taste tissue, a piece of lingual epithelium containing the circumvallate papilla was peeled after enzymatic treatment with 3 mg/mL Dispase II (Roche) and 3 mg/mL elastase for 18 min. The epithelium then was placed in a modified Ussing chamber made of a plastic sheet with the basal part of the circumvallate papilla coming through a small hole in contact with the Tyrode’s bathing solution (45 µL). The apical part of the epithelium was stimulated for three consecutive 1-min periods with (5 µL) Tyrode’s bathing solution as a control, followed by three consecutive 1-min stimulations with a tastant bitter mix (20 mM denatonium + 100 µM cycloheximide in Tyrode’s bathing solution). The bathing solution then was collected and transferred to a 96-well plate for luminescence reading. A plate reader (Synergy HT; Biotek) equipped with an injector added the same amount of luciferase reagent (ATP Bioluminescence Assay Kit HS II; Roche) to each well and performed the reading. In parallel, an ATP curve was obtained from known ATP concentrations and was used to convert the arbitrary values obtained with the taste tissue to ATP concentrations. The Tyrode’s bathing solution contained (in mM) 140 NaCl, 5 KCl, 4 CaCl2, 1 MgCl2, 10 glucose, 1 Na-Pyruvate, 10 Hepes, adjusted to pH 7.4 with NaOH. ATP release in WT and KO animals was compared using a Student t test (P < 0.05) (GraphPad Prism5).
Nerve Recordings.
Mice were anesthetized with an i.p. injection of sodium pentobarbital (50 mg/kg). The animals were maintained in a head holder, and the trachea was cannulated to facilitate breathing. The chorda tympani nerve was exposed using a ventral approach free from surrounding tissue and was cut near the tympanic bulla. The glossopharyngeal nerve was exposed after removal of the digastric muscle and was cut near its entrance to the posterior foramen. For recording, the nerve was placed on a platinum-iridium wire electrode, and a reference electrode was placed in the nearby tissue. Responses were fed to an amplifier, integrated (time constant 0.5 s) and were recorded using AcqKnowledge software (Biopac) for offline analysis. For stimulation, fungiform papillae or circumvallate papillae were exposed to different taste stimuli applied with a medium constant flow pump (Fisher Scientific). The taste stimuli were prepared in water: NH4Cl 100 mM, sucrose 300–1,000 mM, MSG 100–500 mM + amiloride (100 µM), quinine 3–20 mM, denatonium 10–30 mM, NaCl 30–300 mM, citric acid 3–20 mM, HCl 3–20 mM. The stimuli were applied for 30 s (chorda tympani nerve) or 60 s (glossopharyngeal nerve), and the tongue was rinsed with water for 50 s between successive stimulations. For data analysis, the amplitude of the integrated response for each stimulation was averaged for 30 s (chorda tympani nerve) or 60 s (glossopharyngeal nerve) using AcqKnowledge software and was normalized to the baseline to reduce the variability across animals. The normalized nerve responses were compared between WT and NTPDase-KO mice using two-way ANOVA, and each individual concentration was compared with an unpaired Student t test (P < 0.05) (GraphPad Prism 5).
Supplementary Material
Acknowledgments
We thank Dr. Anthony Ford for helpful comments on the manuscript, Nicole Schultz for technical assistance with taste bud anatomy, and Matthew Steritz for breeding and genotyping the KO mice. This work was supported by National Institutes of Health Grants R01 DC012555 (to S.C.K.), P30 DC004657 (to D. Restrepo), and R01 HL094400 and P01 HL087203 (to S.C.R.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1309468110/-/DCSupplemental.
References
- 1.Finger TE, et al. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310(5753):1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- 2.Bo X, et al. Localization of ATP-gated P2X2 and P2X3 receptor immunoreactive nerves in rat taste buds. Neuroreport. 1999;10(5):1107–1111. doi: 10.1097/00001756-199904060-00037. [DOI] [PubMed] [Google Scholar]
- 3.Robson SC, Sévigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: Structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2(2):409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barry MA. Ecto-calcium-dependent ATPase activity of mammalian taste bud cells. J Histochem Cytochem. 1992;40(12):1919–1928. doi: 10.1177/40.12.1453008. [DOI] [PubMed] [Google Scholar]
- 5.Iwayama T, Nada O. Histochemically demonstrable ATPase activity in the taste buds of the rat. Exp Cell Res. 1967;46(3):607–608. doi: 10.1016/0014-4827(67)90388-6. [DOI] [PubMed] [Google Scholar]
- 6.Kukulski F, et al. Comparative hydrolysis of P2 receptor agonists by NTPDases 1, 2, 3 and 8. Purinergic Signal. 2005;1(2):193–204. doi: 10.1007/s11302-005-6217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhari N, Roper SD. The cell biology of taste. J Cell Biol. 2010;190(3):285–296. doi: 10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444(7117):288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- 9.Huang YJ, et al. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci USA. 2007;104(15):6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romanov RA, et al. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J. 2007;26(3):657–667. doi: 10.1038/sj.emboj.7601526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murata Y, et al. Action potential-enhanced ATP release from taste cells through hemichannels. J Neurophysiol. 2010;104(2):896–901. doi: 10.1152/jn.00414.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taruno A, et al. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature. 2013;495(7440):223–226. doi: 10.1038/nature11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang AL, et al. The cells and logic for mammalian sour taste detection. Nature. 2006;442(7105):934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinnamon JC, Taylor BJ, Delay RJ, Roper SD. Ultrastructure of mouse vallate taste buds. I. Taste cells and their associated synapses. J Comp Neurol. 1985;235(1):48–60. doi: 10.1002/cne.902350105. [DOI] [PubMed] [Google Scholar]
- 15.Yee CL, Yang R, Böttger B, Finger TE, Kinnamon JC. “Type III” cells of rat taste buds: Immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 9.5, and serotonin. J Comp Neurol. 2001;440(1):97–108. doi: 10.1002/cne.1372. [DOI] [PubMed] [Google Scholar]
- 16.Kataoka S, et al. The candidate sour taste receptor, PKD2L1, is expressed by type III taste cells in the mouse. Chem Senses. 2008;33(3):243–254. doi: 10.1093/chemse/bjm083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oka Y, Butnaru M, von Buchholtz L, Ryba NJ, Zuker CS. High salt recruits aversive taste pathways. Nature. 2013;494(7438):472–475. doi: 10.1038/nature11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pumplin DW, Yu C, Smith DV. Light and dark cells of rat vallate taste buds are morphologically distinct cell types. J Comp Neurol. 1997;378(3):389–410. doi: 10.1002/(sici)1096-9861(19970217)378:3<389::aid-cne7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 19.Bartel DL, Sullivan SL, Lavoie EG, Sévigny J, Finger TE. Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. J Comp Neurol. 2006;497(1):1–12. doi: 10.1002/cne.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayato R, Ohtubo Y, Yoshii K. Functional expression of ionotropic purinergic receptors on mouse taste bud cells. J Physiol. 2007;584(Pt 2):473–488. doi: 10.1113/jphysiol.2007.138370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang YA, et al. Knocking out P2X receptors reduces transmitter secretion in taste buds. J Neurosci. 2011;31(38):13654–13661. doi: 10.1523/JNEUROSCI.3356-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kataoka S, Toyono T, Seta Y, Ogura T, Toyoshima K. Expression of P2Y1 receptors in rat taste buds. Histochem Cell Biol. 2004;121(5):419–426. doi: 10.1007/s00418-004-0647-3. [DOI] [PubMed] [Google Scholar]
- 23.Huang YA, Dando R, Roper SD. Autocrine and paracrine roles for ATP and serotonin in mouse taste buds. J Neurosci. 2009;29(44):13909–13918. doi: 10.1523/JNEUROSCI.2351-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bystrova MF, Yatzenko YE, Fedorov IV, Rogachevskaja OA, Kolesnikov SS. P2Y isoforms operative in mouse taste cells. Cell Tissue Res. 2006;323(3):377–382. doi: 10.1007/s00441-005-0098-8. [DOI] [PubMed] [Google Scholar]
- 25.Dando R, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. Adenosine enhances sweet taste through A2B receptors in the taste bud. J Neurosci. 2012;32(1):322–330. doi: 10.1523/JNEUROSCI.4070-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimmermann H, Zebisch M, Sträter N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012;8(3):437–502. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82(4):1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 28.Ishida Y, et al. P2X(2)- and P2X(3)-positive fibers in fungiform papillae originate from the chorda tympani but not the trigeminal nerve in rats and mice. J Comp Neurol. 2009;514(2):131–144. doi: 10.1002/cne.22000. [DOI] [PubMed] [Google Scholar]
- 29.Radford KM, Virginio C, Surprenant A, North RA, Kawashima E. Baculovirus expression provides direct evidence for heteromeric assembly of P2X2 and P2X3 receptors. J Neurosci. 1997;17(17):6529–6533. doi: 10.1523/JNEUROSCI.17-17-06529.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu M, et al. Coexpression of P2X(3) and P2X(2) receptor subunits in varying amounts generates heterogeneous populations of P2X receptors that evoke a spectrum of agonist responses comparable to that seen in sensory neurons. J Pharmacol Exp Ther. 2001;296(3):1043–1050. [PubMed] [Google Scholar]
- 31.Grote A, et al. Nanomolar ambient ATP decelerates P2X3 receptor kinetics. Neuropharmacology. 2008;55(7):1212–1218. doi: 10.1016/j.neuropharm.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 32.Kataoka S, et al. A2BR adenosine receptor modulates sweet taste in circumvallate taste buds. PLoS ONE. 2012;7(1):e30032. doi: 10.1371/journal.pone.0030032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Enjyoji K, et al. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999;5(9):1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 34.Mulryan K, et al. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature. 2000;403(6765):86–89. doi: 10.1038/47495. [DOI] [PubMed] [Google Scholar]
- 35.Schaefer U, Machida T, Broekman MJ, Marcus AJ, Levi R. Targeted deletion of ectonucleoside triphosphate diphosphohydrolase 1/CD39 leads to desensitization of pre- and postsynaptic purinergic P2 receptors. J Pharmacol Exp Ther. 2007;322(3):1269–1277. doi: 10.1124/jpet.107.125328. [DOI] [PubMed] [Google Scholar]
- 36.Vlajkovic SM, et al. Preservation of cochlear function in Cd39 deficient mice. Hear Res. 2009;253(1-2):77–82. doi: 10.1016/j.heares.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Eisenberger MA, et al. Phase I and clinical evaluation of a pharmacologically guided regimen of suramin in patients with hormone-refractory prostate cancer. J Clin Oncol. 1995;13(9):2174–2186. doi: 10.1200/JCO.1995.13.9.2174. [DOI] [PubMed] [Google Scholar]
- 38.Mistretta CM, Goosens KA, Farinas I, Reichardt LF. Alterations in size, number, and morphology of gustatory papillae and taste buds in BDNF null mutant mice demonstrate neural dependence of developing taste organs. J Comp Neurol. 1999;409(1):13–24. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.