Significance
ATP is an important extracellular signal that activates P2X receptor channels. Although a large fraction of ATP is bound to divalent cations in vivo, the forms of ATP that activate P2X receptors are unknown. Here we show how the activity of homomeric P2X receptors is tuned by Mg2+ in some subtypes by preventing activation by free ATP, and in others by binding to a distinct regulatory site. We also find that both regulatory mechanisms are disengaged in heteromeric channels to form a sensitive ATP signaling pathway. These fundamental properties of P2X receptors will be valuable for investigating their physiological functions.
Keywords: magnesium ATP, magnesium regulation
Abstract
The identity and forms of activating ligands for ion channels are fundamental to their physiological roles in rapid electrical signaling. P2X receptor channels are ATP-activated cation channels that serve important roles in sensory signaling and inflammation, yet the active forms of the nucleotide are unknown. In physiological solutions, ATP is ionized and primarily found in complex with Mg2+. Here we investigated the active forms of ATP and found that the action of MgATP2− and ATP4− differs between subtypes of P2X receptors. The slowly desensitizing P2X2 receptor can be activated by free ATP, but MgATP2− promotes opening with very low efficacy. In contrast, both free ATP and MgATP2− robustly open the rapidly desensitizing P2X3 subtype. A further distinction between these two subtypes is the ability of Mg2+ to regulate P2X3 through a distinct allosteric mechanism. Importantly, heteromeric P2X2/3 channels present in sensory neurons exhibit a hybrid phenotype, characterized by robust activation by MgATP2− and weak regulation by Mg2+. These results reveal the existence of two classes of homomeric P2X receptors with differential sensitivity to MgATP2− and regulation by Mg2+, and demonstrate that both restraining mechanisms can be disengaged in heteromeric channels to form fast and sensitive ATP signaling pathways in sensory neurons.
Seven subtypes of P2X receptors have been identified in mammals that can form either homomeric (P2X1, P2X2, P2X3, P2X4, P2X5, P2X7) or heteromeric (P2X1/2, P2X1/4, P2X1/5, P2X2/3, P2X2/5, P2X2/6, P2X4/6, and possibly, P2X4/7) channels (1–8). These subtypes of P2X receptors have distinct gating properties, pharmacology, and cellular distributions. P2X1 and P2X3 receptors desensitize within a few hundred milliseconds when opened by ATP, and their distributions are restricted to either smooth muscle cells and platelets (P2X1) or a subset of sensory neurons (P2X3) (1, 9–14). P2X2 and P2X4 receptors exhibit slow desensitization during prolonged ATP application, and these receptors are the most abundant subtypes in the central nervous system (15). P2X2 subunits also express in a subset of sensory neurons; however, in these cells they only form heteromeric channels with P2X3 subunits (12, 16, 17). In sensory neurons, P2X3 homomeric channels together with P2X2/3 heteromeric channels play important roles in mediating the primary sensory effects of ATP, and knock-out animals with either P2X3 deletion or P2X2 and P2X3 double-deletions have revealed critical roles in taste, pain, oxygen sensing, and bladder filling (17–20).
A long-standing conundrum in P2X receptor-mediated signaling concerns the forms of ATP that activate these channels. In neutral solutions, ATP is ionized and exists mostly as free ATP (ATP4−), an efficient chelator of divalent cations such as Mg2+, and to a lesser extent Ca2+ (21). In extracellular biological compartments, such as the synaptic cleft, Ca2+ and Mg2+ are present in the millimolar range, and therefore only a relatively small fraction of ATP released from vesicles is present in the free form. Although a range of important studies have explored the regulatory effects of Ca2+ and Mg2+ on P2X receptor channels (22–30), the essential question of which forms of ATP serve as agonists remains unresolved. Several previous studies have reported that P2X2, P2X7, and the native P2X receptors in cilia are activated by ATP in solutions containing low concentrations of divalent cations, and that the addition of divalent cations shifts the concentration dependence for activation of the channels to higher ATP concentrations, suggesting that either ATP4− is the most active form of ATP or that divalent cations regulate those subtypes through allosteric mechanisms (27, 30–36). In the present study, we investigated the form(s) of ATP that serve as agonists for a range of subtypes of P2X receptor channels. Our primary focus was to determine whether ATP4− or MgATP2− are the principal agonists and to explore whether Mg2+ might serve specific regulatory roles. Our results demonstrate that the action of MgATP2− and ATP4− differ between subtypes of P2X receptors, and reveal that heteromeric channels can have unique hybrid phenotypes, findings that will be crucial for understanding the physiological functions of these channels in both the peripheral and central nervous systems.
Results
ATP4− Activates All Subtypes of Homomeric P2X Receptor Channels.
We began by establishing whether ATP4− can activate each subtype of P2X receptor that can be heterologously expressed as a homomeric channel. To test for activation by ATP4−, we used the patch-clamp recording technique to record ATP-activated currents when applying the nucleotide in the presence of 10 mM EDTA to remove contaminating divalent ions that are present in aqueous solutions. Because divalent ions stabilize seals between electrode glass and cell membranes that are required for patch-clamp recordings (37, 38), we first established GΩ seals with 0.5 mM Mg2+ and 2 mM Ca2+ in the extracellular solution, and only studied cells further if the seal was exceptionally tight (>10 GΩ resistance) and stable for 5 min after switching to a 10-mM EDTA solution. Using this approach to study HEK cells transfected with P2X receptor cDNA, we observed that 300 μM ATP evokes currents with kinetic properties resembling those that have been reported in solutions with divalent cations (1) (Fig. 1A). Both P2X1 and P2X3 receptors rapidly desensitized in response to ATP4−, whereas the others exhibited a more slowly desensitizing phenotype. For P2X1, P2X2, P2X3, and P2X4 receptors, the EC50 concentrations for ATP activation in the divalent-free solution were between 1 and 2 μM (Fig. 1B). In agreement with previous studies, the ATP-activated P2X5 currents are typically very small, and P2X7 receptors activate slowly and with low affinity for ATP, limitations that precluded further experiments with these subtypes (34, 39). We also did not study P2X6 receptors because they are difficult to express as homomeric channels (40, 41).
Fig. 1.
Divalent-free ATP can open P2X receptor channels. (A) ATP (300 μM) activates P2X receptors expressed in HEK cells when the agonist is applied in a divalent-free solution containing 10 mM EDTA. Macroscopic ATP-activated currents were measured using a holding voltage of −60 mV. (B) Concentration–response relations for ATP activation of P2X1, P2X2, P2X3, and P2X4 receptors in divalent-free solution. Each point is the average ± SEM of four measurements. The Hill equation was used to fit the data, and the EC50 and (n) are 1.2 ± 0.2 μM and (1.5 ± 0.2) for P2X1; 1.4 ± 0.1 μM and (1.3 ± 0.1) for P2X2; 2.0 ± 0.7 μM and (1.1 ± 0.3) for P2X3; and 1.3 ± 0.1 μM and (1.3 ± 0.1) for P2X4.
Differential Responses to MgATP2− for Homomeric P2X Receptor Channels.
The above results demonstrate that ATP can activate P2X receptor channels in the absence of divalent cations, indicating that ATP in its free anionic form is an agonist. Our next objective was to test whether MgATP2− can also activate P2X receptors. We reasoned that if Mg2+-bound ATP is also an agonist of P2X receptors, a solution containing a substantial amount of MgATP2− and a minimal amount of ATP4− would effectively activate these channels. To test this possibility, we activated P2X receptors with a saturating concentration of ATP (10 μM) in divalent-free solution, and compared the extent of activation to that observed in the presence of 5 mM MgCl2, a solution that contains ∼0.2 μM ATP4− and ∼9.8 μM MgATP2− (Fig. 2; Materials and Methods). According to the concentration dependence for activation of P2X1–4 receptors by ATP in divalent-free solution (Fig. 1B), the currents activated by a solution containing ∼0.2 μM ATP4− and ∼9.8 μM MgATP2− should be between 5% and 10% of the current activated by 10 μM ATP4− if MgATP2− does not activate the channel.
Fig. 2.
MgATP2− activates P2X1 and P2X3, but not P2X2 and P2X4 receptors. Macroscopic currents activated by 10 µM ATP in the absence and presence of Mg2+ were recorded at −60 mV from HEK cells expressing P2X1 (A), P2X3 (B), P2X2 (C), or P2X4 (D). (A–D) The solution of 10 μM ATP plus 5 mM MgCl2 contains ∼0.2 μM ATP4− and ∼9.8 μM MgATP2−. Between ATP pulses, cells were perfused with divalent-free solution. To estimate rundown, 10 μM ATP was applied twice at 90-s intervals in the divalent-free solution, and all subsequent ATP applications were given at 90-s intervals. Similar experiments were repeated on 3–4 different cells. (Right) Summary of normalized currents in response to each ATP pulse. Numbering indicates the sequence of ATP applications from first (1) to last (4). P2X1–P2X3 subtypes were studied with conventional whole-cell recording, and P2X4 was studied with the perforated-patch configuration. IR observed in P2X2 and P2X4 are indicated with an asterisk. (E and F) Enlarged views of IR for P2X2 (E) or P2X4 (F). Scale bars on the left show the amplitude of currents normalized to that activated by 10 μM ATP4−.
In the case of P2X1 and P2X3 receptor channels, >90% of maximum activation was observed in the presence of 5 mM MgCl2 (Fig. 2 A and B), suggesting that MgATP2− is an agonist for these two subtypes. However, in the case of P2X2, this Mg-ATP solution activated very little steady-state current (Fig. 2C), and for P2X4, the fractional activation was ∼10% (Fig. 2D). Notably, upon exchanging the test extracellular solution containing both Mg2+ and ATP with the control solution containing EDTA, we detected a rapid increase in the inward current for cells expressing either P2X2 or P2X4, which we termed a resurgent current (Fig. 2 C and D, asterisk, and Fig. 2 E and F, expanded views). This resurgent current (IR) required solution exchange times of <50 ms to be consistently detected, and it decays with similar kinetics for deactivation of these channels following removal of ATP4−. We therefore hypothesized that MgATP2− can bind to P2X2 and P2X4 receptor channels, but that MgATP2− cannot open P2X receptor channels as efficiently as ATP4−. During external application of the Mg-ATP solution, both ATP4− and MgATP2− bind to the channel (with occupancies determined by their relative affinities and concentrations), but the steady-state current is dominated by the relatively small fraction of channels with ATP4− bound. In this scenario, upon removal of Mg2+ and ATP, IR is observed because Mg2+ unbinds before ATP4−, enabling a larger fraction of channels to open than would be possible if only ATP4− bound to the receptor when applied in the presence of Mg2+. In the following sections, we further explore this hypothesis, focusing on the P2X2 subtype, a channel that displays the least desensitization and most stable ATP-activated currents.
MgATP2− Binds to P2X2 Receptors.
If IR results from Mg2+ unbinding from channels that already have MgATP2− bound, then the amplitude of that current should vary with the concentration of MgATP2− in the external test solution. To test whether this is the case, we varied the concentrations of MgATP2− in the external solution from ∼0.9 to 29.9 μM by increasing the concentration of both Mg2+ and total ATP while maintaining ATP4− at 0.1 μM, a concentration that by itself would cause minimal activation of P2X2 receptor channels (Fig. 1B). As the concentration of Mg2+ and ATP increase, we observed steady-state currents that were consistently small when the test solution was applied; however, the amplitude of IR increased along with the concentration of MgATP2− (Fig. 3 A and B), in line with the idea that MgATP2− can bind to P2X2 receptors and that this complex is not effective at opening the channel.
Fig. 3.
MgATP2− can bind to P2X2 receptors. (A) P2X2 currents activated by 10 μM free ATP or 0.1 μM ATP4− and varying concentrations of MgATP2−. EDTA solution was applied to the cell between each ATP application. Resurgent currents (IR) are marked with an asterisk. The four test MgATP2− solutions were made using the following concentrations of ATP and MgCl2: 0.1 μM ATP4−/0.9 μM MgATP2− (1 μM ATP plus 1 mM MgCl2); 0.1 μM ATP4−/2.9 μM MgATP2− (3 μM ATP plus 3 mM MgCl2); 0.1 μM ATP4−/9.9 μM MgATP2− (10 μM ATP plus 10 mM MgCl2); and 0.1 μM ATP4−/29.9 μM MgATP2− (30 μM ATP plus 30 mM MgCl2). (B) Normalized resurgent current (IR/IMax) plotted against MgATP2− concentration (n = 4). Imax is the current activated by saturating ATP4−. (C) Comparison of currents activated with an EC20 concentration of ATP4− with either low (0.6 µM) or high (10 µM) MgATP2−. The concentrations of ATP4−, MgATP2−, and Mg2+ are 0.4 μM, 0.6 μM, and 199.4 μM in 1 μM ATP plus 200 μM MgCl2 solution, and 0.4 μM, 9.6 μM, and 2.99 mM in 10 μM ATP plus 3 mM MgCl2 solution. (D) Superimposed activation of P2X2 receptor channel currents shown in C (second trace in green and third trace in orange). (E) Kinetics of MgATP2− binding to P2X2 receptors. A 10-μM ATP plus 5-mM MgCl2 solution was applied to cells expressing P2X2 receptors for 20 ms, 100 ms, 500 ms, or 5 s, and then switched to EDTA solution. IR was normalized to the maximal IR value obtained after 5 s ATP plus MgCl2 application (n = 3). (F) Kinetics of resurgent current activation. P2X2 receptor channels were activated by 10 μM ATP plus 5 mM MgCl2 before returning to an external EDTA solution to evoke IR. The red trace shows currents measured when an open tip is switched between two solutions with different ionic composition. Similar results were obtained from three cells. (G) Enlargement of the part of the current in purple box shown in F. A piezoelectric perfusion system was used for all experiments shown in C–G.
To confirm these results, we performed additional experiments using a piezoelectric-driven solution exchange system that can routinely achieve solution exchange within 4 ms. We designed solutions to contain approximately an EC20 concentration of ATP4− (0.4 µM) together with either low (0.6 μM) or high (10 μM) MgATP2− (Fig. 3C). According to the titration curve shown in Fig. 3B, binding of MgATP2− to P2X2 receptors should be minimal at 0.6 μM, but substantial at 10 μM. The results show that the solution with 0.4 μM ATP4− and 0.6 μM MgATP2− elicited only steady-state current (without a measurable IR) that is 13 ± 2% (n = 6) of maximum current activated by saturating concentration of ATP4− (Fig. 3C). In contrast, the solution containing 0.4 μM ATP4− and 10 μM MgATP2− activates a smaller steady-state current, which is only 6 ± 1% (n = 6) of the maximum current, and when Mg2+ and ATP are removed IR is now observed (Fig. 3C). All of these observations are consistent with the idea that ATP4− binds with higher affinity compared with MgATP2−, and that the former promotes channel opening much more effectively. The activation kinetics for the steady-state current activated by 0.4 μM ATP4− and 0.6 μM MgATP2− are slower than that activated by 0.4 μM ATP4− and 10 μM MgATP2− (Fig. 3D), which is consistent with the notion that both ATP4− and MgATP2− bind to the receptor. We also investigated the amplitude of IR as a function of how long MgATP2− is applied to the external solution, and determined that 100 ms is sufficient for IR to reach its maximum (Fig. 3E), confirming that binding of MgATP2− is rapid. After the MgATP2− solution is removed, IR develops within a few milliseconds (Fig. 3 F and G), suggesting that Mg2+ unbinds very rapidly when it is bound together with ATP.
The Site of Mg2+ Action on P2X2 Receptors.
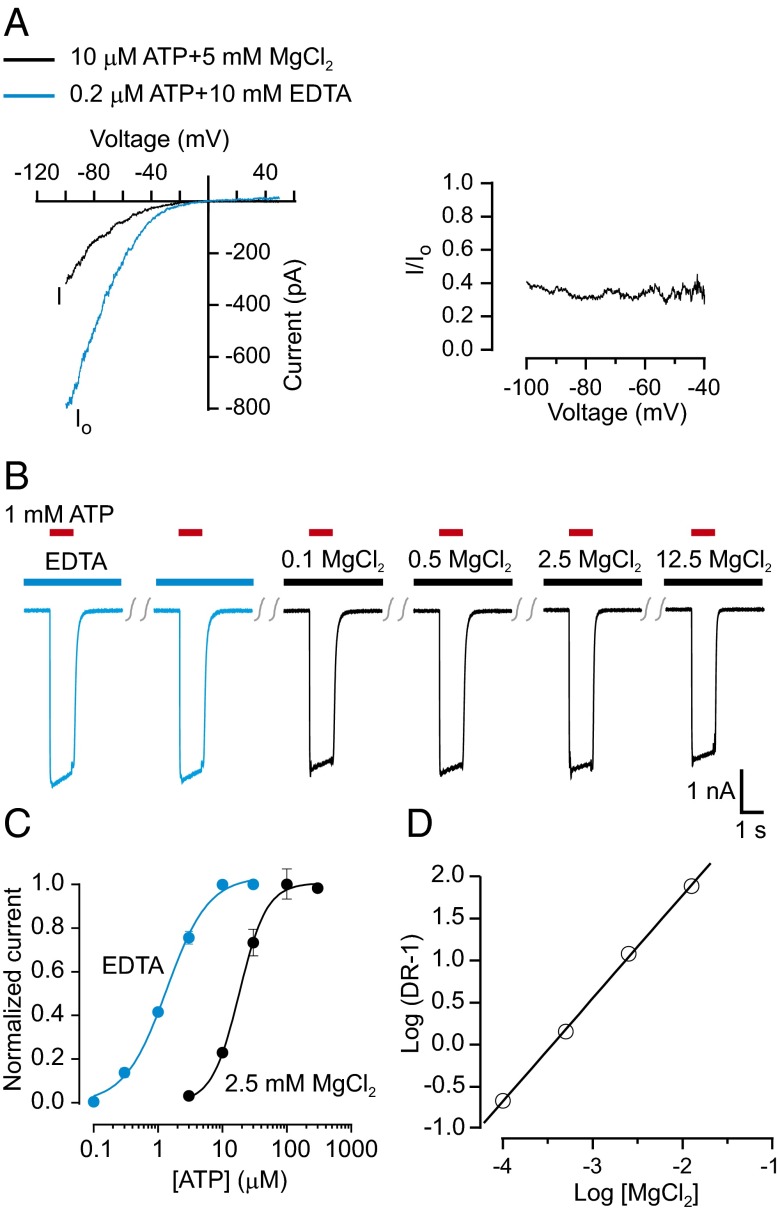
Our results thus far for P2X2 receptor channels are consistent with the idea that Mg2+ binds to ATP (in solution and on the receptor), but that MgATP2− binds to the channel with much lower affinity than ATP4− (compare Fig. 1B with Fig. 3 A and B) and promotes opening less effectively than ATP4− (Fig. 3 A and B); in essence, this is an inhibitory mechanism by which Mg2+ would appear to behave as a simple competitive antagonist. To investigate this possibility, we obtained concentration-response relations for ATP activation of P2X2 over a range of Mg2+ concentrations and used Schild analysis to determine whether the effects of Mg2+ obey a simple competitive mechanism (42). Because P2X receptors might be blocked by Mg2+, we determined the range of MgCl2 concentrations over which block was not significant. We did not observe any obvious voltage dependence under conditions where Mg2+ produced robust inhibition of the P2X2 receptor channel (Fig. 4A), arguing against pore block as the primary mechanism of inhibition. In experiments in which the channels are activated with 1 mM ATP to ensure that ATP4− is saturating in the presence of all tested Mg2+ concentrations, increasing MgCl2 from 0.1 mM to 12.5 mM has only modest effects on P2X2 currents (Fig. 4B). Next, we constructed concentration–response relations for ATP activation in solutions with four different concentrations of MgCl2 (0.1, 0.5, 2.5, and 12.5 mM), and observed that although the relations are similar in shape, they shift to higher ATP concentrations as the concentration of Mg2+ increases (Fig. 4C). The slope of the Schild plot for the EC50 concentrations obtained in different concentrations of Mg2+ is 1.1 ± 0.1 (Fig. 4D), suggesting that Mg2+ behaves as a simple competitive inhibitor of P2X2 receptors. It is notable that the log(Kd) obtained from the Schild plot for Mg2+ shifting the concentration–response relation for ATP activation of the channel is −4.2, which is indistinguishable from the stability constant of 4.1 for formation of MgATP2− in aqueous solution (where the stability constant is logKa) (21, 43). Taken together, these results demonstrate that MgATP is not an effective agonist for P2X2 receptor channels, and that ATP4− is the principal agonist that activates this channel subtype.
Fig. 4.
The site of Mg2+ action on P2X2 receptors. (A) Magnesium inhibition is not voltage dependent. (Left) Macroscopic P2X2 receptor channel currents recorded with 1.5-s voltage ramps from −100 mV to +50 mV. The solution of 10 μM ATP plus 5 mM MgCl2 contains ∼0.2 μM ATP4−, 4.9 mM Mg2+, and ∼9.8 μM MgATP2−. (Right) Current recorded in 10 μM ATP plus 5 mM MgCl2 was normalized to that recorded in 0.2 µM ATP plus EDTA. (B) Effects of magnesium on P2X2 currents activated by 1 mM ATP. The lowest ATP4− concentration in these solutions is 9.6 μM (12.5 mM MgCl2 plus 1 mM ATP). All currents were recorded from the same cell. The amplitudes of ATP-activated currents for the second through sixth ATP applications were 99 ± 1%, 94 ± 2%, 95 ± 2%, 95 ± 4%, and 88 ± 5% of the first application (n = 4). (C) Dose–response curves for ATP activation of P2X2 receptors obtained in EDTA solutions and in solutions with 2.5 mM MgCl2. Each point is the average ± SEM of four measurements. The Hill equation was fit to the data, and the EC50 and (n) are 1.4 ± 0.1 μM and (1.3 ± 0.1) for that in EDTA solution and 17.9 ± 0.9 μM and (1.9 ± 0.2) for that in 2.5 mM MgCl2. (D) Schild plot for Mg antagonism of P2X2 activation with ATP. Dose ratio (DR) was calculated by dividing the EC50 in the presence of MgCl2 with the EC50 in the absence of MgCl2. The slope of the Schild plot is 1.1 ± 0.1.
Mg2+ Inhibits P2X3 Receptors by Binding to the Extracellular Central Cavity.
The results above for P2X1 and P2X3 receptors show that MgATP2− is an effective agonist for these subtypes (Fig. 2). However, previous studies have shown that Mg2+ can also inhibit the P2X3 subtype when the divalent is given sufficient time to equilibrate with the receptor (26). The duration of Mg2+ application in our experiments thus far was relatively brief because we applied Mg2+ together with ATP; we therefore investigated the actions of Mg2+ when it was preequilibrated with the channel for 90 s before application of ATP. For these experiments, we tested the effects of 1 mM MgCl2 on P2X3 currents activated with 300 μM ATP, a solution predicted to contain ∼40 μM ATP4− (a saturating concentration for activating P2X1–4 receptors according to Fig. 1B), ∼260 μM MgATP2−, and ∼740 μM Mg2+. Consistent with previous results, we observed that Mg2+ inhibited P2X3 under these conditions (Fig. 5A). Even more pronounced inhibition by Mg2+ was observed for P2X1 receptors, whereas relatively modest inhibition was evident for P2X2 or P2X4 receptors (Fig. S1). Next we constructed concentration–response relations for ATP activation of P2X3 receptors in the continuous presence of 0.1, 0.5, or 2.5 mM MgCl2 (Fig. 5B), and observed that currents activated by saturating concentrations of ATP decrease as the concentration of Mg2+ increases, and that near maximal inhibition is obtained at submillimolar Mg2+ concentrations. In contrast to the inhibitory mechanism discussed above for P2X2 receptors, Mg2+ inhibition of P2X3 cannot be overcome by increasing the concentration of ATP, suggesting a distinct regulatory mechanism. Interestingly, the concentration–response relationship is detectably shifted toward lower ATP concentrations in the presence of 0.1 mM MgCl2, suggesting that MgATP2− has either a higher binding affinity or efficacy for activating P2X3 receptors compared with ATP4−.
Fig. 5.

Mg2+ inhibition of P2X3 receptor channels. (A) Effects of preincubation with 1 mM MgCl2 on P2X3 receptors activated with 300 μM ATP. Macroscopic ATP-activated currents were measured using a holding voltage of −60 mV. To estimate rundown, 300 μM ATP was applied twice in EDTA solution with a 90-s interval between repeated ATP applications. Five seconds after the second ATP pulse, the external solution was switched to one containing 1 mM MgCl2, and ATP was applied again 90 s later to estimate the effects of MgCl2. In control experiments in divalent-free solution, the rundown to repeated ATP applications was estimated to be 7 ± 4%. Compared with currents in divalent-free solutions, the currents in solutions with 1 mM MgCl2 are 66 ± 7% smaller (n = 4). (B) Dose–response curves for ATP activation of P2X3 WT receptors in the presence of EDTA or different concentrations of MgCl2. Each point is the average ± SEM of 3–4 measurements. The Hill equation was fit to the data, and the EC50 and (n) are 2.0 ± 0.7 μM and (1.1 ± 0.3) for that in EDTA solution, 0.6 ± 0.1 μM and (1.1 ± 0.2) for that in 0.1 mM MgCl2, 0.7 ± 0.1 μM and (1.1 ± 0.2) for that in 0.5 mM MgCl2, and 0.6 ± 0.1 μM and (1.2 ± 0.2) for that in 2.5 mM MgCl2. (C) Sequence alignment of two extracellular segments of P2X receptors. (D) Effects of 1 mM MgCl2 on P2X3 T87A mutant receptors activated with 300 μM ATP. Experimental protocol is the same as that shown in A. In control experiments in divalent-free solution, the rundown to repeated ATP applications was estimated to be 5 ± 3%. Compared with currents in divalent-free solutions, the currents in solutions with 1 mM MgCl2 are 11 ± 5% smaller (n = 3). (E) P2X3 T87A mutant channel dose–response curves for ATP activation in the presence of EDTA or different concentrations of MgCl2. Each point is the average ± SEM of 3–4 measurements. The Hill equation was used to fit the data, and the EC50 and (n) are 1.1 ± 0.2 μM and (1.3 ± 0.2) for that in EDTA solution, 0.7 ± 0.1 μM and (1.1 ± 0.1) for that in 0.5 mM MgCl2, and 0.9 ± 0.3 and (1.1 ± 0.3) for that in 2.5 mM MgCl2. (F) Summary of the effect of 1 mM MgCl2 on P2X3 WT and mutant channels. The experiments were carried out in the same way as that shown in A and E. Currents after 1 mM MgCl2 application were normalized to the currents activated by 300 μM ATP4−. (G) Homology models of hP2X3 receptors constructed with SWISS-MODEL using the apo and ATP-bound zfP2X4 crystal structures PDB ID codes (3H9V and 4DW1). Residues E167 and N170 are colored green, and T87 is colored red. The apo P2X3 model (Upper) is viewed from the side, whereas the apo (closed) and ATP-bound (open) models (Lower) are viewed from the intracellular side.
To identify the binding site for Mg2+ in P2X3 receptors, we examined the Gd3+ sites identified crystallographically in zebrafish P2X4 (zfP2X4) (44). Gd3+ is commonly used to localize putative divalent cation binding sites in X-ray structures of proteins, and two types of Gd3+ binding sites have been identified in the closed state zfP2X4 crystal structure—a singular site at the threefold axis within the central chamber of the extracellular domain, and three identical peripheral sites within that domain (Fig. 5 C and G) (44). As shown in that structure, E98 from each of the three zfP2X4 subunits form a single binding site in the central chamber, and D184 and N187 from each subunit form the three sites at the periphery of the trimeric receptor (Fig. S2). We mutated E167 and N170 in P2X3, positions equivalent to D184 and N187 in the peripheral Gd3+ sites of zfP2X4, and T87 in P2X3, a residue located within the central chamber nearby E98 in zfP2X4, and investigated whether preincubation with 1 mM MgCl2 would affect the channel currents activated by saturating concentrations of ATP (Fig. 5 C–G). Although Mg2+ inhibition was unaffected in the E167A and N170A mutants in the peripheral sites (Fig. 5 C, F, and G), inhibition was greatly attenuated in the case of the T87A mutant in the central chamber (Fig. 5 C–F). We also constructed concentration–responses relations for ATP activation in the presence of either 0.5 or 2.5 mM MgCl2 (Fig. 5E) for the T87A mutant. No inhibition was observed at 0.5 mM MgCl2, compared with nearly maximal inhibition at that concentration for WT P2X3. Indeed, at this concentration of MgCl2, we observed a modest shift of the concentration–response relationship for activation by ATP to lower ATP concentrations, consistent with MgATP2− having a higher affinity or efficacy for the P2X3 receptor. At 2.5 mM, MgCl2 inhibited the maximum activation by 30 ± 1%, which is significantly reduced compare with the 73 ± 1% inhibition for the WT, suggesting that this mutant displays a diminished affinity for Mg2+.
To rule out the possibility that Mg2+ also binds to the central chamber in the P2X2 receptor channel or to the peripheral sites to produce inhibition, we looked for residues in P2X2 that might be related to those forming the two types of Gd3+ sites in zfP2X4. The Glu in the central cavity and the Asp in the periphery are not conserved in rP2X2, but we identified nearby residues that might play similar roles. We mutated E91 and D315 (rP2X2) in the central cavity, and M179 plus N182 at the peripheral site to Ala, and tested if Mg-ATP solution could activate these mutant channels (Fig. S3). Although the concentration dependence for activation by ATP4− was altered to varying degrees in these mutant channels (in particular for D315A mutant), all were weakly activated in the Mg-ATP solution and display IR when removing Mg2+ and ATP (Fig. S3).
Regulatory Constraints of Mg2+ Are Disengaged in Heteromeric P2X2/3 Receptor Channels.
The results presented thus far demonstrate that physiological concentrations of Mg2+ inhibit the function of both P2X2 and P2X3 receptors. Although the inhibitory mechanisms for P2X2 and P2X3 are distinct, in both cases the binding sites for Mg2+ are likely located at subunit interfaces. For P2X2 receptors, inhibition results because MgATP2− binds to the ligand binding site with lower affinity than ATP4− and promotes opening less efficiently than ATP4−. In the X-ray structure of the zfP2X4 receptor, the ATP binding site is formed by a pocket at the interface between adjacent subunits (45). For P2X3 receptors, inhibition appears to result from Mg2+ binding to the central chamber formed at the interface between all three subunits; this raises an intriguing possibility that both inhibitory mechanisms might be altered in P2X2/3 heterotrimeric receptors, a specific combination of subunits involved in forming P2X receptor channels in sensory neurons. Heteromeric P2X2/3 receptor channels exhibit slow desensitization similar to P2X2, yet can be activated by αβ-methylene ATP (αβ-MeATP), an ATP analog that activates P2X3 with much higher affinity and efficacy compared with P2X2, together providing the means for distinguishing P2X2/3 heteromeric channels from P2X2 or P2X3 homomeric channels (12, 46). When P2X2 and P2X3 receptors are coexpressed in HEK cells, we observed that 10 μM ATP in divalent-free solution only activates slow-desensitizing currents that are similar to those activated by αβ-MeATP (Fig. 6 A and B), suggesting that under these expression conditions, heteromeric P2X2/3 channels are more abundant than homomeric P2X2 or P2X3 channels. Application of 10 μM ATP with 5 mM MgCl2 (a solution that contains ∼0.2 μM ATP4− and ∼9.8 μM MgATP2−) produces activation of the slowly desensitizing current that is much larger than that activated by 0.2 μM ATP4− (Fig. 6A). This current could not arise from P2X3 receptors because it desensitizes slowly, and it could not arise from P2X2 receptors because that subtype is not efficiently activated by MgATP2− (Fig. 2). These results demonstrate that heterologously expressed P2X2/3 heteromeric channels can be activated by both ATP4− and MgATP2−. To investigate whether Mg2+ inhibits P2X2/3 channels through the central chamber site identified in P2X3 receptors, we tested whether currents activated by saturating concentrations of ATP are inhibited by 1 mM MgCl2 when preequilibrated for 90 s (Fig. 6B), the same protocol we used with homomeric P2X3 channels. The results show that P2X2/3 channel currents activated by 100 μM ATP are not detectably inhibited by Mg2+, suggesting that central chamber Mg2+ site is not robustly engaged in these heteromeric channels.
Fig. 6.
Characterization of P2X2/3 heteromeric channels in HEK cells and DRG neurons. (A) Testing the sensitivity of P2X2/3 heteromeric channels to ATP4−, MgATP2−, and αβ-MeATP. Macroscopic current traces recorded from a HEK cell transfected with P2X2 and P2X3 with a cDNA ratio of 5:1 using a holding voltage of −60 mV. The solution of 10 μM ATP plus 5 mM MgCl2 contains ∼0.2 μM ATP4− and ∼9.8 μM MgATP2−. In all experiments, αβ-MeATP was applied in the standard extracellular solution with 2 mM CaCl2, 0.5 mM MgCl2, and 10 mM glucose. (Right) Summary of currents in response to each ATP pulse from four cells normalized to the first ATP application. Numbering indicates the sequence of ATP applications from first (1) to last (4). (B) P2X2/3 heteromeric channels in HEK cells are not inhibited after 90 s preincubation with 1 mM MgCl2 (n = 3). (C) Three types of ATP-activated currents observed in small- and medium-sized DRG neurons. (Left) The fast desensitizing current is likely the P2X3 channel current and was seen in four neurons. (Center) This current trace has two components corresponding to a mixture of P2X3 and P2X2/3 channel populations and was observed in three neurons. (Right) The slow activating and desensitizing current is likely the P2X2/3 channel current and was seen in 15 neurons. The slower activation and deactivation kinetics observed in this population of cells may be the consequence of their larger size, which slows solution exchange compared with the smaller P2X3-expressing neurons and HEK cells. (D) The properties of P2X2/3 heteromeric channels in a medium-sized DRG neuron are similar to P2X2/3 receptors expressed in HEK cells (n = 6).
To examine whether the properties of P2X2/3 heteromeric channels expressed in native dorsal root ganglion (DRG) neurons are similar to those in HEK cells, we initially applied ATP in a physiological solution containing divalent cations and observed detectable currents in 22 of 38 neurons tested. The responsive neurons exhibited three types of currents, resembling those of P2X3 only (Fig. 6C, Left; four cells), P2X3 together with P2X2/3 (Fig. 6C, Center; three cells), or P2X2/3 only (Fig. 6C, Right; 15 cells), consistent with previous studies (16, 47). The neurons that were nonresponsive to ATP in solution containing divalent cations did not respond to ATP in divalent-free solution, suggesting that homomeric P2X2 receptors are not expressed in these cells. Also consistent with previous results, the slowly desensitizing P2X receptors in DRG neurons could be activated by αβ-MeATP, further suggesting that they are P2X2/3 heteromeric channels (Fig. 6D). In this latter class of cells, 0.2 μM ATP4− only activated small currents that are <10% of those activated by 10 μM ATP4−, and a solution containing 0.2 μM ATP4− and 9.8 μM MgATP2− activated currents that are comparable to those activated by 10 μM ATP4−, indicating that P2X2/3 in DRG neurons can be activated by MgATP2− (Fig. 6D).
Discussion
The objective of the present study was to determine which forms of ATP can serve as agonists for different subtypes of P2X receptor channels and to explore whether Mg2+ serves any regulatory roles. Collectively, our results demonstrate that ATP in its free ionic form is an active ligand for all subtypes of homomeric P2X receptors, and that the activity of P2X1–P2X4 receptors are inhibited by the presence of physiological concentrations of Mg2+ (Table S1). For P2X2 receptors, Mg2+ constrains receptor activation because MgATP2− can bind to the agonist binding site, but its affinity and efficacy are lower than ATP4− (Figs. 2 and 3). In contrast to what was observed for P2X2 and P2X4, both P2X1 and P2X3 were strongly activated by MgATP2−, demonstrating that there are two classes of P2X receptors that differ in the forms of ATP that can serve as agonists (Table S1). Because Ca2+ can also chelate ATP4−, and is abundant in physiological solutions, it is interesting to consider whether it would behave similarly to Mg2+. Although quantitative comparison with Mg2+ is complicated by the fact that Ca2+ permeates P2X receptor channels to different degrees, and thus can change the unitary conductance, we obtained qualitatively comparable results with Ca2+ that are consistent with the general idea that divalent-bound ATP can serve as an agonist for P2X3, but not P2X2 receptor channels (Fig. S4).
The structural differences between the two classes of ATP binding sites in P2X receptor channels are likely subtle; however, it is interesting that their sensitivities to MgATP2− are paralleled by other pharmacological distinctions. P2X1 and P2X3 receptor channels can be activated by αβ-MeATP with an EC50 concentration about 1 μM, but P2X2 and P2X4 receptor channels can only partially be activated by 100 μM αβ-MeATP. In addition, P2X1 and P2X3 receptors are inhibited by nanomolar concentrations of trinitrophenyl-ATP, whereas P2X2 and P2X4 are much less sensitive (8, 46, 48). It appears that those receptors that can trigger opening with Mg2+ bound to ATP in the agonist binding pockets also have room for the larger agonists and antagonists. These pharmacological distinctions imply that the agonist binding site of P2X4 is most similar to P2X2, raising the possibility that the mechanism of Mg2+ inhibition is similar for these two subtypes. We observed resurgent currents for P2X4 that resemble those of P2X2 (Fig. 2), and the apparent affinities of ATP for P2X2 and P2X4 receptors are similar in physiological solutions containing divalent ions, and lower than observed for P2X1 and P2X3 receptors (1, 2). In addition, Mg2+ produces only modest inhibition of P2X4 in the presence of high concentrations of ATP (Fig. S2), demonstrating that much of the Mg2+ inhibition can be overcome by increasing the concentration of ATP. Thus, the available evidence is most consistent with the idea that both P2X2 and P2X4 are most efficiently activated by ATP4−. One important implication of this selective activation of P2X2 and P2X4 receptors by the physiologically scarce form of ATP is that these subtypes will require substantially higher concentration of released ATP for efficient signaling to occur when they are expressed as homomeric receptors. Perhaps in these instances, P2X2 and P2X4 receptors primarily serve to detect high concentrations of ATP, as might be expected with repetitive ATP release or with inflammation where cells are dying and releasing cytoplasmic ATP.
In contrast, P2X1 and P2X3 receptor channels can be activated by lower concentrations of ATP in the presence of Mg2+ because MgATP2− is an effective agonist for these channels (Fig. 2 A and B). In the case of P2X3 receptors, we observed that the concentration dependence for activation by ATP was shifted to lower ATP concentrations in the presence of low Mg2+ concentrations, suggesting that MgATP2− has either higher affinity or efficacy compared with ATP4−. However, both P2X1 and P2X3 receptors are partially inhibited by Mg2+ through a distinct mechanism that likely involves binding of the metal to the central chamber in the extracellular domain (Fig. 5). In the X-ray structure of the apo zfP2X4 receptor, Gd3+ binds to the central chamber and is coordinated intimately by E98 (Fig. S2A). Interestingly, in both apo and ATP-bound structures solved in the absence of Gd3+, the side chain of E98 swings away from the central axis (Fig. S2 B and C), demonstrating that Gd3+ binding induces a reorientation of the E98 side chain. In a P2X3 homology model made from apo zfP2X4, the critical threonine (Thr) we identified (T87) is located within the central chamber and its side chain projects toward the threefold axis of symmetry (Fig. 5G). The hydroxyl moieties in this homology model are positioned within 7 Å of each other, compatible with a role in coordinating Mg2+ (Fig. 5G). In contrast, the expansion of the central chamber that occurs in the open-state zfP2X4 structure (and open P2X3 homology model) would be less compatible with stable coordination of Mg2+ (Fig. 5G and Fig. S2) (45, 49). Thus, we speculate that the Mg2+ inhibits both P2X1 and P2X3 receptors by stabilizing the extracellular domain in a nonconductive conformation. One possibility is that Mg2+ inhibits P2X3 receptors by stabilizing a desensitized state of the receptor, as previously proposed (26).
One intriguing common feature of the mechanisms for Mg2+ inhibition of homomeric P2X2 and P2X3 receptors is that the sites of Mg2+ action are localized to subunit interfaces. In the case of P2X2 receptors, Mg2+ binds to ATP in the agonist binding pocket formed by adjacent subunits, and in the case of P2X3, Mg2+ likely binds to the central chamber at the threefold axis. P2X2 and P2X3 receptors have been demonstrated to form heteromeric channels in sensory neurons, and in this instance the heteromeric channels contain one P2X2 subunit and two P2X3 subunits (50, 51). Thus, the Mg2+ regulatory site in the central chamber would be expected to be weakened because it is missing one of three Thr residues. Similarly, none of the three agonist binding sites would be formed purely by P2X2 subunits; two would be formed at the interface between P2X2 and P2X3 subunits, and one would be constructed at a purely P2X3/P2X3 interface. In particular for heteromeric P2X2/3 receptors, it is not surprising that these heteromeric channels would be robustly activated by MgATP2− given that all three agonist binding sites have contributions from P2X3 subunits.
The key discoveries described here are that (i) the active forms of ATP are not the same for different subtypes of P2X receptors, with P2X2 and likely P2X4 requiring free ATP, whereas P2X1 and P2X3 are also sensitive to MgATP2−; (ii) the MgATP2−-sensitive P2X1 and P2X3 receptors are partially inhibited by Mg2+ binding to the central chamber; and (iii) both of these mechanisms that constrain activation of the receptor can be disengaged when heteromeric receptors form. These findings likely have a range of important implications for understanding the physiological functions of P2X receptor channels in both the central and peripheral nervous systems. In particular, the slowly desensitizing homomeric P2X2 and P2X4 receptor channels would be well-suited for signaling roles when the concentration of extracellular ATP is sufficiently high, whereas the rapidly desensitizing homomeric P2X1 and P2X3 receptors would be more sensitive to lower concentrations of ATP. However, heteromerization of these two classes of P2X receptors channels allows the creation of hybrid channels that are sensitive to MgATP2−, slowly desensitizing and relatively insensitive to Mg2+ inhibition involving the central chamber. It will be fascinating to explore whether the present studies with heteromeric P2X2/3 receptor channels can be generalized to other complexes to provide a common logic for heteromerization. Our collective results would predict similar hybrid behavior for previously documented heteromeric P2X1/2 and P2X1/4 receptor channels, and it will be exciting to investigate the role of the P2X6 subunits when it forms heteromeric channels with P2X2 and P2X4, all three of which are widely expressed within the central nervous system (15, 26, 52–55).
Materials and Methods
Channel Constructs.
Human P2X1 (hP2X1) in pcDNA3 was generously provided by Richard Evans (University of Leicester, Leicester, United Kingdom). rP2X2 in pcDNA1 was generously provided by David Julius (University of California, San Francisco, San Francisco). hP2X2 and hP2X3 in pCMV6-XL4 were purchased from OriGENE. rP2X4 and rP2X7 in pcDNA3 were generously provided by Florentina Soto (Washington University in St. Louis, St. Louis). rP2X5 was generously provided by Alan North (University of Manchester, Manchester, United Kingdom) and was cloned into pcDNA1. rP2X2 was used in most experiments; however, the key results shown in Figs. 2C and 6A were also repeated with hP2X2 and similar results were obtained. rP2X3 and hP2X3 share 94% sequence identity, and none of the minor differences localize to the ATP binding sites observed in zfP2X4, suggesting that these two species will share the properties described here for hP2X3.
Cell Culture and Transfection.
HEK cells were cultured in DMEM supplemented with 10% (vol/vol) FBS and 10 mg/L gentamicin. All cell culture reagents were obtained from GIBCO. Trypsin-treated HEK293 cells were seeded onto glass coverslips in six-well plates 1 d before transfection and placed in a 37 °C incubator with 95% air and 5% CO2. Transfections were performed using FuGENE6 Transfection Reagent (Roche Applied Science). P2X receptors were cotransfected with a GFP cDNA construct in pGreen-Lantern (Invitrogen) at a ratio of 2:1. Cells were used for whole-cell recording 18–30 h after transfection.
DRG Preparation.
Isolated DRG neurons were prepared from 7- to 14-d-old rats using procedures similar to what has been previously described (56). After anesthetizing rats with isoflurane and decapitation, segments of the spinal cord (thoracic and lumbar segments) were removed and placed in a cold Ca2+, Mg2+-free HBSS (Gibco) containing (in mM) 137 NaCl, 5.3 KCl, 0.33 Na2HPO4, 0.44 KH2PO4, 4.1 NaHCO3, 5 Hepes, and 5.5 glucose (pH 7.4) with NaOH. The bone surrounding the spinal cord was cut away to expose DRG, which were removed with forceps. Ganglia were cut in half and incubated in a Ca2+, Mg2+-free HBSS containing 20 U/mL papain (Worthington Biochemical Corporation) and 5 mM cysteine for 20 min at 37 °C. After incubation with papain, ganglia were washed and transferred into a Ca2+, Mg2+-free HBSS containing 0.1 U/mL collagenase and 0.8 U/mL Dispase (Roche Applied Science), and incubated at 37 °C for 20 min. After two successive incubations in enzymes, ganglia were transferred into Leibovitz’s L-15 medium (Invitrogen) supplemented with 10% FCS, 5 mM Hepes, and 50 ng/mL NGF (Invitrogen). Individual cells were dispersed by mechanical trituration using a fire-polished Pasteur pipette and plated on poly-d-lysine/laminin-coated glass coverslips (BD Biosciences). Cells were incubated in a 37 °C incubator with 95% air and 5% CO2 for 3 h, after which cells were stored at room temperature and used for patch-clamping within 24 h.
Electrophysiology.
Standard whole-cell patch-clamp recording was used to record most P2X receptor channel currents from transiently transfected HEK293 cells and isolated DRG neurons. Perforated patch-clamp recording was used to record P2X4 receptor channel currents to minimize the rundown in response to repeated ATP applications (57). Membrane currents were recorded under voltage clamp using an Axopatch 200B patch-clamp amplifier (Axon Instruments, Inc.) and digitized online using a Digidata 1322A interface board and pCLAMP 9.2 software (Axon Instruments, Inc.). Currents were filtered at 2 kHz using eight-pole Bessel filters, and digitized at 5 or 10 kHz. The pipette solution for standard whole-cell recording on HEK cells contained (in mM) 140 NaCl, 10 EGTA, and 10 Hepes, adjusted to pH 7.0 with NaOH. The pipette solution for standard whole-cell recording on DRG neurons contained (in mM) 65 CsCl, 65 CsF, 10 NaCl, 2 MgCl2, 10 EGTA, 10 Hepes, 14 Tris⋅creatine PO4, 4 Mg-ATP, and 0.3 Na-GTP (pH 7.2) with CsOH. The pipette solution for perforated patch-clamp recording on HEK cells contained (in mM) 65 K2SO4, 10 NaCl, 1 CaCl2, 1 MgCl2, 10 Hepes (pH 7.2) with KOH. The divalent-free extracellular solution contained (in mM) 140 NaCl, 10 EDTA, and 10 Hepes, adjusted to pH 7.3 with NaOH. Assuming that divalent ion contaminations from reagents and water is less than 1 μM, any possible free divalent cations in the 10 mM EDTA solution would be less than 0.5 nM. The extracellular solutions with different concentrations of Mg2+ contained (in mM) 140 NaCl, 10 Hepes, and desired concentration of MgCl2, adjusted to pH 7.3. K2ATP (referred to as ATP) was used in all of the experiments. The stability constants of ATP binding to cations present in our solutions are Mg2+ 104.10, Ca2+ 103.76, Na+ 100.83, and K+ 101.17 (National Institute of Standards and Technology Critically Selected Stability Constants of Metal Complexes). Max Chelator (http://maxchelator.stanford.edu/) was used to calculate concentrations of Mg2+, MgATP2−, and ATP4− in solutions with different concentrations of MgCl2 and ATP. Recording solutions were adjusted to the correct pH after adding MgCl2 and ATP. Solution exchange was achieved using either the Rapid Solution Changer RSC-200 (BioLogic), which has the capacity of switching between nine solutions, or a Piezo-driven system (AutoMate Scientific), which can change two solutions within 1 to 4 ms, depending on the size of the cell.
Data Analysis.
To generate the dose–response relationship, a reference concentration of ATP was given every time before a test concentration was applied, as previously described (31). The Hill equation was fit to the data according to
where I is the normalized current at a given concentration of ATP, Imax is the maximum normalized current, EC50 is the concentration of ATP ([ATP]) producing half-maximal currents, and n is the Hill coefficient.
Supplementary Material
Acknowledgments
We thank Joe Mindell, Miguel Holmgren, Jeff Diamond, Dmitriy Krepkiy, Jeet Kalia, Gilman Toombes, and other members of the K.J.S. laboratory for helpful discussions. We thank Pin Liu and Bruce Bean for graciously teaching us how to prepare DRG neurons. This work was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke, National Institutes of Health (NIH) (K.J.S.), and NIH Pathway to Independence Award NS070954 (to M.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1308088110/-/DCSupplemental.
References
- 1.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82(4):1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 2.Surprenant A, North RA. Signaling at purinergic P2X receptors. Annu Rev Physiol. 2009;71:333–359. doi: 10.1146/annurev.physiol.70.113006.100630. [DOI] [PubMed] [Google Scholar]
- 3.Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, Stojilkovic SS. Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev. 2011;63(3):641–683. doi: 10.1124/pr.110.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaczmarek-Hájek K, Lörinczi E, Hausmann R, Nicke A. Molecular and functional properties of P2X receptors—recent progress and persisting challenges. Purinergic Signal. 2012;8(3):375–417. doi: 10.1007/s11302-012-9314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Compan V, et al. (2012) P2X2 and P2X5 subunits define a new heteromeric receptor with P2X7-like properties. J Neurosci 32(12):4284–4296. [DOI] [PMC free article] [PubMed]
- 6.Guo C, Masin M, Qureshi OS, Murrell-Lagnado RD. Evidence for functional P2X4/P2X7 heteromeric receptors. Mol Pharmacol. 2007;72(6):1447–1456. doi: 10.1124/mol.107.035980. [DOI] [PubMed] [Google Scholar]
- 7.Boumechache M, Masin M, Edwardson JM, Górecki DC, Murrell-Lagnado R. Analysis of assembly and trafficking of native P2X4 and P2X7 receptor complexes in rodent immune cells. J Biol Chem. 2009;284(20):13446–13454. doi: 10.1074/jbc.M901255200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicke A. Homotrimeric complexes are the dominant assembly state of native P2X7 subunits. Biochem Biophys Res Commun. 2008;377(3):803–808. doi: 10.1016/j.bbrc.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 9.Khakh BS, North RA. Neuromodulation by extracellular ATP and P2X receptors in the CNS. Neuron. 2012;76(1):51–69. doi: 10.1016/j.neuron.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brederson JD, Jarvis MF. Homomeric and heteromeric P2X3 receptors in peripheral sensory neurons. Curr Opin Investig Drugs. 2008;9(7):716–725. [PubMed] [Google Scholar]
- 11.Vial C, Rolf MG, Mahaut-Smith MP, Evans RJ. A study of P2X1 receptor function in murine megakaryocytes and human platelets reveals synergy with P2Y receptors. Br J Pharmacol. 2002;135(2):363–372. doi: 10.1038/sj.bjp.0704486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis C, et al. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377(6548):432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- 13.Mulryan K, et al. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature. 2000;403(6765):86–89. doi: 10.1038/47495. [DOI] [PubMed] [Google Scholar]
- 14.Hu H, Hoylaerts MF. The P2X1 ion channel in platelet function. Platelets. 2010;21(3):153–166. doi: 10.3109/09537101003599549. [DOI] [PubMed] [Google Scholar]
- 15.Nörenberg W, Illes P. Neuronal P2X receptors: Localisation and functional properties. Naunyn Schmiedebergs Arch Pharmacol. 2000;362(4-5):324–339. doi: 10.1007/s002100000311. [DOI] [PubMed] [Google Scholar]
- 16.Grubb BD, Evans RJ. Characterization of cultured dorsal root ganglion neuron P2X receptors. Eur J Neurosci. 1999;11(1):149–154. doi: 10.1046/j.1460-9568.1999.00426.x. [DOI] [PubMed] [Google Scholar]
- 17.Cook SP, Vulchanova L, Hargreaves KM, Elde R, McCleskey EW. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature. 1997;387(6632):505–508. doi: 10.1038/387505a0. [DOI] [PubMed] [Google Scholar]
- 18.Cockayne DA, et al. P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J Physiol. 2005;567(Pt 2):621–639. doi: 10.1113/jphysiol.2005.088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eddy MC, et al. Double P2X2/P2X3 purinergic receptor knockout mice do not taste NaCl or the artificial sweetener SC45647. Chem Senses. 2009;34(9):789–797. doi: 10.1093/chemse/bjp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Souslova V, et al. Warm-coding deficits and aberrant inflammatory pain in mice lacking P2X3 receptors. Nature. 2000;407(6807):1015–1017. doi: 10.1038/35039526. [DOI] [PubMed] [Google Scholar]
- 21.O’Sullivan WJ, Perrin DD. The stability constants of metal-adenine nucleotide complexes. Biochemistry. 1964;3:18–26. doi: 10.1021/bi00889a005. [DOI] [PubMed] [Google Scholar]
- 22.Acuña-Castillo C, Coddou C, Bull P, Brito J, Huidobro-Toro JP. Differential role of extracellular histidines in copper, zinc, magnesium and proton modulation of the P2X7 purinergic receptor. J Neurochem. 2007;101(1):17–26. doi: 10.1111/j.1471-4159.2006.04343.x. [DOI] [PubMed] [Google Scholar]
- 23. Cook SP, Rodland KD, McCleskey EW (1998) A memory for extracellular Ca2+ by speeding recovery of P2X receptors from desensitization. J Neurosci 18(22):9238–9244. [DOI] [PMC free article] [PubMed]
- 24.Ding S, Sachs F. Ion permeation and block of P2X(2) purinoceptors: Single channel recordings. J Membr Biol. 1999;172(3):215–223. doi: 10.1007/s002329900598. [DOI] [PubMed] [Google Scholar]
- 25.Evans RJ, et al. Ionic permeability of, and divalent cation effects on, two ATP-gated cation channels (P2X receptors) expressed in mammalian cells. J Physiol. 1996;497(Pt 2):413–422. doi: 10.1113/jphysiol.1996.sp021777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giniatullin R, Sokolova E, Nistri A. Modulation of P2X3 receptors by Mg2+ on rat DRG neurons in culture. Neuropharmacology. 2003;44(1):132–140. doi: 10.1016/s0028-3908(02)00338-6. [DOI] [PubMed] [Google Scholar]
- 27.Virginio C, Church D, North RA, Surprenant A. Effects of divalent cations, protons and calmidazolium at the rat P2X7 receptor. Neuropharmacology. 1997;36(9):1285–1294. doi: 10.1016/s0028-3908(97)00141-x. [DOI] [PubMed] [Google Scholar]
- 28.Virginio C, North RA, Surprenant A. Calcium permeability and block at homomeric and heteromeric P2X2 and P2X3 receptors, and P2X receptors in rat nodose neurones. J Physiol. 1998;510(Pt 1):27–35. doi: 10.1111/j.1469-7793.1998.027bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wildman SS, et al. Sensitization by extracellular Ca(2+) of rat P2X(5) receptor and its pharmacological properties compared with rat P2X(1) Mol Pharmacol. 2002;62(4):957–966. doi: 10.1124/mol.62.4.957. [DOI] [PubMed] [Google Scholar]
- 30.Yan Z, Khadra A, Sherman A, Stojilkovic SS. Calcium-dependent block of P2X7 receptor channel function is allosteric. J Gen Physiol. 2011;138(4):437–452. doi: 10.1085/jgp.201110647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li M, Chang TH, Silberberg SD, Swartz KJ. Gating the pore of P2X receptor channels. Nat Neurosci. 2008;11(8):883–887. doi: 10.1038/nn.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li M, Kawate T, Silberberg SD, Swartz KJ (2010) Pore-opening mechanism in trimeric P2X receptor channels. Nat Commun 1:44, 10.1038/ncomms1048. [DOI] [PMC free article] [PubMed]
- 33.Korngreen A, Ma W, Priel Z, Silberberg SD. Extracellular ATP directly gates a cation-selective channel in rabbit airway ciliated epithelial cells. J Physiol. 1998;508(Pt 3):703–720. doi: 10.1111/j.1469-7793.1998.703bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272(5262):735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- 35.Jiang LH. Inhibition of P2X(7) receptors by divalent cations: Old action and new insight. Eur Biophys J. 2009;38(3):339–346. doi: 10.1007/s00249-008-0315-y. [DOI] [PubMed] [Google Scholar]
- 36.Cockcroft S, Gomperts BD. Activation and inhibition of calcium-dependent histamine secretion by ATP ions applied to rat mast cells. J Physiol. 1979;296:229–243. doi: 10.1113/jphysiol.1979.sp013002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391(2):85–100. [DOI] [PubMed]
- 38.Priel A, Gil Z, Moy VT, Magleby KL, Silberberg SD. Ionic requirements for membrane-glass adhesion and giga seal formation in patch-clamp recording. Biophys J. 2007;92(11):3893–3900. doi: 10.1529/biophysj.106.099119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Collo G, et al. (1996) Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci 16(8):2495–2507. [DOI] [PMC free article] [PubMed]
- 40. Le KT, Babinski K, Seguela P (1998) Central P2X4 and P2X6 channel subunits coassemble into a novel heteromeric ATP receptor. J Neurosci 18(18):7152–7159. [DOI] [PMC free article] [PubMed]
- 41.Jones CA, et al. Functional regulation of P2X6 receptors by N-linked glycosylation: Identification of a novel alpha beta-methylene ATP-sensitive phenotype. Mol Pharmacol. 2004;65:979–985. doi: 10.1124/mol.65.4.979. [DOI] [PubMed] [Google Scholar]
- 42.Schild HO. pA, a new scale for the measurement of drug antagonism. Br Pharmacol Chemother. 1947;2(3):189–206. doi: 10.1111/j.1476-5381.1947.tb00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pecoraro VL, Hermes JD, Cleland WW. Stability constants of Mg2+ and Cd2+ complexes of adenine nucleotides and thionucleotides and rate constants for formation and dissociation of MgATP and MgADP. Biochemistry. 1984;23(22):5262–5271. doi: 10.1021/bi00317a026. [DOI] [PubMed] [Google Scholar]
- 44.Kawate T, Michel JC, Birdsong WT, Gouaux E. Crystal structure of the ATP-gated P2X(4) ion channel in the closed state. Nature. 2009;460(7255):592–598. doi: 10.1038/nature08198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hattori M, Gouaux E. Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature. 2012;485(7397):207–212. doi: 10.1038/nature11010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- 47.Burgard EC, et al. P2X receptor-mediated ionic currents in dorsal root ganglion neurons. J Neurophysiol. 1999;82(3):1590–1598. doi: 10.1152/jn.1999.82.3.1590. [DOI] [PubMed] [Google Scholar]
- 48.Virginio C, Robertson G, Surprenant A, North RA. Trinitrophenyl-substituted nucleotides are potent antagonists selective for P2X1, P2X3, and heteromeric P2X2/3 receptors. Mol Pharmacol. 1998;53(6):969–973. [PubMed] [Google Scholar]
- 49.Roberts JA, et al. Agonist binding evokes extensive conformational changes in the extracellular domain of the ATP-gated human P2X1 receptor ion channel. Proc Natl Acad Sci USA. 2012;109(12):4663–4667. doi: 10.1073/pnas.1201872109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hausmann R, et al. ATP binding site mutagenesis reveals different subunit stoichiometry of functional P2X2/3 and P2X2/6 receptors. J Biol Chem. 2012;287(17):13930–13943. doi: 10.1074/jbc.M112.345207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jiang LH, et al. (2003) Subunit arrangement in P2X receptors. J Neurosci 23(26):8903–8910. [DOI] [PMC free article] [PubMed]
- 52. Khakh BS, Proctor WR, Dunwiddie TV, Labarca C, Lester HA (1999) Allosteric control of gating and kinetics at P2X(4) receptor channels. J Neurosci 19(17):7289–7299. [DOI] [PMC free article] [PubMed]
- 53.Khakh BS. Molecular physiology of P2X receptors and ATP signalling at synapses. Nat Rev Neurosci. 2001;2(3):165–174. doi: 10.1038/35058521. [DOI] [PubMed] [Google Scholar]
- 54. Rubio ME, Soto F (2001) Distinct localization of P2X receptors at excitatory postsynaptic specializations. J Neurosci 21(2):641–653. [DOI] [PMC free article] [PubMed]
- 55. Sim JA, et al. (2006) Altered hippocampal synaptic potentiation in P2X4 knock-out mice. J Neurosci 26(35):9006–9009. [DOI] [PMC free article] [PubMed]
- 56.Bosmans F, Puopolo M, Martin-Eauclaire MF, Bean BP, Swartz KJ. Functional properties and toxin pharmacology of a dorsal root ganglion sodium channel viewed through its voltage sensors. J Gen Physiol. 2011;138(1):59–72. doi: 10.1085/jgp.201110614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lippiat JD. Whole-cell recording using the perforated patch clamp technique. Methods Mol Biol. 2008;491:141–149. doi: 10.1007/978-1-59745-526-8_11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.