Significance
Granule cells (GCs) are the most abundant neuronal type in the olfactory bulb (OB) and play a critical role in odor processing. GCs integrate bottom-up and top-down information and regulate the output of principal neurons to higher brain areas. Here, we provide direct evidence that GCs in the OB are regulated by GABAergic neurons from the basal forebrain and that disrupting this inhibition affects odor discrimination. Recent work has highlighted the role of feedback excitatory cortical inputs to the OB. Like the excitatory cortical feedback, the inhibitory input we describe could mediate fast changes in olfactory coding in the OB in response to rapid changes in environmental context.
Keywords: habituation, uncaging
Abstract
Granule cells (GCs) are the most abundant inhibitory neuronal type in the olfactory bulb and play a critical role in olfactory processing. GCs regulate the activity of principal neurons, the mitral cells, through dendrodendritic synapses, shaping the olfactory bulb output to other brain regions. GC excitability is regulated precisely by intrinsic and extrinsic inputs, and this regulation is fundamental for odor discrimination. Here, we used channelrhodopsin to stimulate GABAergic axons from the basal forebrain selectively and show that this stimulation generates reliable inhibitory responses in GCs. Furthermore, selective in vivo inhibition of GABAergic neurons in the basal forebrain by targeted expression of designer receptors exclusively activated by designer drugs produced a reversible impairment in the discrimination of structurally similar odors, indicating an important role of these inhibitory afferents in olfactory processing.
Sensory information from the external world is integrated through a series of feed-forward stages toward higher cognitive cortical areas. At these different stages, sensory perception can be regulated by an individual’s internal state to enhance meaningful information relative to less valuable information associated with different behavioral tasks (1, 2). Unlike other sensory systems, peripheral sensory input onto principal neurons in the olfactory bulb (OB) is relayed directly to olfactory cortices and subcortical nuclei, bypassing the thalamus (3). The OB is the first stage in which extensive fine-tuning and processing of olfactory information occurs (4). This processing involves integration of bottom-up and top-down information by the most abundant OB cell type, the granule cells (GCs). GCs establish most of their connections with output neurons, the mitral and tufted cells (MCs herein), through the ubiquitous dendrodendritic synapses (DDS) and with a few other subtypes of interneurons (5–8). Importantly, the interaction between MCs and GCs is thought to give rise to network oscillations in the OB that are associated with MC firing synchronization, adding an important time component to olfactory processing (9–13).
In analogy with the regulation of thalamic neurons by cortical inputs, GCs receive important feedback regulation from cortical and subcortical projection areas of MCs and afferents from neuromodulatory systems (3, 14–16). Activation of these feedback projections and neuromodulatory systems increases GC excitability (14, 17, 18), thus increasing GABA-mediated inhibition at DDS and regulating olfactory processing. For example, regulation of GC-mediated inhibition by noradrenaline in the main olfactory bulbs (MOBs) and accessory olfactory bulbs (AOBs) has been shown to affect a range of olfactory behaviors including odor discrimination and more complex behaviors such as memory formation during mating (19). In addition, other studies have indicated an extensive innervation by GABAergic fibers originating in the horizontal limb of the diagonal band of Broca (HDB) and magnocellular preoptic area (MCPO) that preferentially targets the GC layer in the OB (20, 21). Interestingly, the HDB/MCPO is also the origin of the cholinergic afferent fibers that target the OB (22), and this cholinergic input has been shown to have an important influence in olfactory processing (23). Although mechanisms that promote excitation of GCs have been studied extensively, the mechanisms that promote inhibition of GCs have received less attention. The existence of a descending inhibitory input suggests that regulation of GCs by afferent inhibition also can modulate olfactory processing. Previous studies indicated that direct stimulation of HDB/MCPO can inhibit neuronal activity in the OB (24); however, the presence of cholinergic projections from this region has confounded the interpretation of these observations. Here, we expressed channelrhodopsin (ChR2) exclusively in inhibitory neurons of the HDB/MCPO to control GABA release in the OB selectively and to determine its influence on GC function and olfactory discrimination.
Results and Discussion
Adenoviral injections of flexed ChR2 were made stereotactically in the HDB/MCPO of mice expressing Cre recombinase under the promoter of one of the isoforms of glutamate decarboxylase (GAD), the enzyme responsible for GABA synthesis (GAD65-Cre mice). Double immunofluorescence against the two GAD isoforms, GAD65 and GAD67, confirmed the presence of GABAergic neurons across the HDB/MCPO axis (Fig. 1A and Fig. S1). The HDB/MCPO also is populated by cholinergic neurons that innervate the OB; 2 wk after injection, we found ChR2-positive (ChR2+) neurons intermingled with neurons expressing choline acetyl transferase (ChAT), further corroborating that the virus injection is restricted to the HDB/MCPO. More importantly, ChR2+ fibers were abundantly present in the MOB and AOB at 6 wk after injection. (Fig. 1 B, 1–3). In both regions, HDB/MCPO GABAergic fibers targeted mostly the GC layer, where the soma and proximal dendrites of GCs are found; very sparse labeling was observed in the external plexiform layer, where most of GC–MC synapses are found, or in the glomerular layer. The labeling pattern also indicates that inhibitory fibers target mostly GCs, but not MCs, in both the MOB and AOB (Fig. 1 B, 3). Similar to the excitatory feedback projections, this inhibitory input could have a modulatory role on GCs throughout the OB.
Fig. 1.
Selective labeling of GABAergic neurons of the HDB/MCPO reveals a profuse projection into the OB. (A) Confocal imaging of HDB/MCPO sections of a GAD65-Cre mouse immunostained against GAD65 (green) and GAD67 (red). GABAergic neurons express one of the markers exclusively (white arrowhead), but a few cells are positive for GAD65 and GAD67 (yellow, white arrow). (Right) After targeted injections, GABAergic neurons expressing ChR2 (red) are intermingled with cholinergic neurons stained for ChAT (green). (Scale bar: 25 μM.) (B) Confocal imaging of a sagittal section of the OB from a GAD65-Cre mouse showing extensive labeling of GABAergic fibers expressing ChR2 (B2, red) throughout the inner layers of the OB after injection of ChR2 virus into the HDB/MCPO (B1). (Scale bar: 500 μM.) (B3) The afferents mostly innervate the GC layer with only sparse fibers reaching beyond the MC layer to the external plexiform and glomerular layers (GL). (Scale bar: 100 μM.)
We next recorded from GCs surrounded by ChR2+ GABAergic fibers, which were recognized visually by the expression of tandem dimer Tomato (tdTomato). Stimulation of these fibers with blue light (hereafter, LightStim) inhibited GCs in the MOB and AOB (Fig. 2A). LightStim at 10 Hz produced a rapid and reversible decrease in spiking elicited by a depolarizing current stimulus (MOB: 45.3 ± 9.9% reduction, n = 7, P < 0.004; AOB: 70.1 ± 12% reduction, n = 4, P < 0.01). Similar experiments under voltage-clamp conditions at 0 mV (Fig. S2A) indicated the occurrence of spontaneous inhibitory postsynaptic currents (sIPSCs), which were completely abolished by the GABAA blocker GABAzine (Fig. S2B). LightStim of ChR2+ GABAergic fibers at 10 Hz evoked robust inhibitory postsynaptic currents (eIPSCs), synchronized with the LightStim pulses, in MOB GCs (20/22 cells; Fig. 2B). The eIPSCs, like the sIPSCs, were completely abolished in the presence of GABAzine (Fig. S2C). Excitation of deep short axon cells by cortical feedback projections (14) or direct excitation of another subtype of inhibitory neurons, Blanes cells, has been shown to inhibit MOB GCs (6). However, unlike these studies, our observations suggest that inhibition of GCs by HDB/MCPO GABAergic neurons does not require the activation of local interneurons. Accordingly, blockers of excitatory glutamatergic transmission [100 µM (2R)-amino-5-phosphonopentanoate (APV) and 10 µM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX)] did not affect either the amplitude or the frequency of the eIPSCs (Fig. 2B). In these experiments we also included the metabotropic glutamate receptor blocker LY367385 (100 µM). Metabotropic glutamate receptors are found in MCs and GCs and have been shown to participate in GC→MC inhibition in the MOB and AOB (25–27). To quantify the responses across different cells better, we integrated the currents produced by the LightStim sweep (SI Materials and Methods), which correspond to the total charge transfer. As shown in Fig. 2B, Right, the average charge at 10 Hz (19 ± 4 × 10−12 C, n = 11) was not reduced in the presence of blockers (16 ± 4 10−12 C, n = 9; P > 0.5). However, the charge transfer elicited by LightStim was largely abolished (0.6 ± 0.2 × 10−12 C, n = 2) by tetrodotoxin (TTX) (0.5 µM), which is known to decrease responses to ChR2 activation by inhibiting action potentials (14, 28). Similar results were obtained in recordings from AOB GCs, stimulated at 5 Hz (control: 13 ± 3 × 10−12 C, n = 10; plus blockers: 9 ± 5 × 10−12 C, n = 3, P > 0.4; blockers plus TTX: 0.2 ± 0.2 × 10−12 C, n = 3, P < 0.03). Previous work has indicated that local interneurons activated by excitatory afferent fibers are an important component of GC inhibition (14). Our results support the notion that GCs receive an important direct inhibitory feedback projection from the basal forebrain and, importantly, that olfactory behaviors mediated by the AOB and MOB could be regulated by HDB/MCPO GABAergic neurons (see Fig. 4).
Fig. 2.
Stimulation of ChR2 expressed in HDB/MCPO GABAergic afferents inhibits GCs in the OB. (A) (Left) Diagram showing the recording array used; we recorded from GCs surrounded by ChR2+ GABAergic fibers and stimulated with blue light. (Center) Stimulation of GABAergic fibers in the vicinity of a recorded GC (50 pulses, 10 Hz) decreases the frequency of firing elicited by a depolarizing current stimulus in the MOB (16 pA, 10 s) (Upper) and in the AOB (24 pA, 15 s) (Lower). The raster plots represent the activity on three cells in each region, highlighting the reversible decrease in action potential frequency induced by LightStim. (Right) LightStim produced a frequency-dependent increase in inhibition of firing in GCs. Bar graph summarizing the reduction in GC firing produced by LightStim at 10 Hz (MOB, n = 7, *P < 0.004; AOB, n = 4, P < 0.02). (Scale bars: 1 s and 20 mV.) (B) (Upper Left) In voltage-clamp conditions, LightStim (10 Hz) elicited robust eIPSCs in a MOB GC. The Inset shows the expanded time axis; most light pulses produced a synchronized eIPSC. (Lower Left) In the same cell, the eIPSCs were not affected by the presence of blockers of glutamatergic transmission (CNQX, 10 µM; APV, 100 µM; LY367385 100 µM) but were completely abolished by the addition of TTX (0.5 µM). (Right) The integral under the area of the LightStim-induced eIPSCs at 10 Hz, or charge transfer (black), was not different in the presence of blockers (orange) but was completely abolished in the presence of TTX (gray bar; *P < 0.03).
Fig. 4.
Disruption of inhibition from the HDB/MCPO affects odor discrimination. (Upper) A virus encoding for hM4Di was injected into the HDB/MCPO of GAD65-Cre mice. Four to five weeks later, animals received an i.p. injection of either PBS (control condition) or CNO (treated condition) and were tested for odor discrimination using the habituation/dishabituation paradigm or were tested for odor-detection threshold (SI Materials and Methods). (Lower) Before the CNO injection, mice habituated to C7 (grey bar) and showed a significant increase in investigation time in the presence of the dishabituated odor, C8 (red bar). Two hours after the CNO injection, mice habituated to C7 failed to show an increase in investigation time for C8. However, the investigation time was increased significantly for a pair of odors differing by two carbons (C6/C8). The inability to discriminate between C7 and C8 was recovered completely 4 h after CNO administration. *P < 0.05; **P < 0.02.
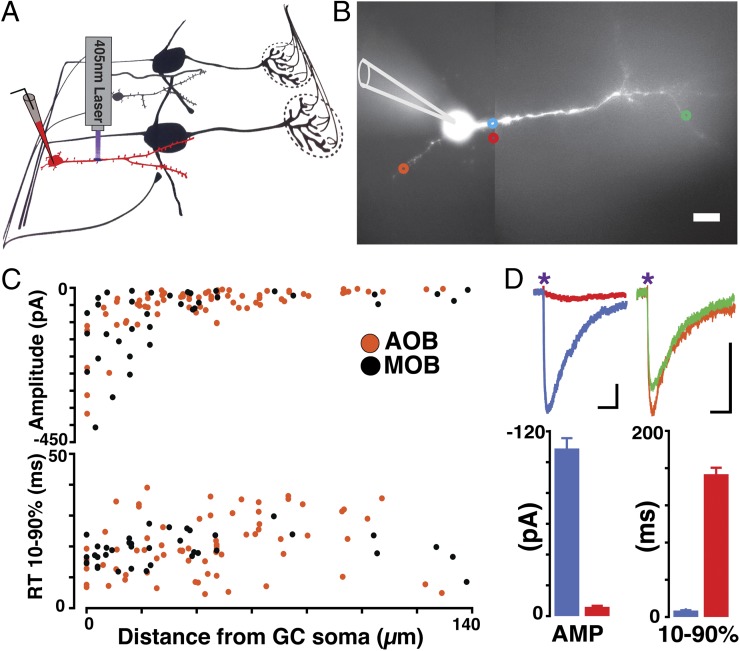
The diffuse projection pattern of GABAergic fibers from the HDB/MCPO and the high frequency of occurrence of inhibitory responses elicited by activation of these axons suggest that inhibitory synapses occur throughout the soma and dendritic tree of GCs. To explore this possibility further, we conducted single-photon uncaging of GABA onto GCs using 1-(4-aminobutanoyl)-4-[1,3-bis(dihydroxyphosphoryloxy)propan-2-yloxy]-7-nitroindoline (DNPI-GABA), while responses were recorded in GCs loaded with Alexa-594 to direct the uncaging spatially to the recorded cell. As shown in Fig. 3, uncaging of GABA revealed inhibitory responses throughout the soma and basal and apical dendrites in GCs of the MOB and AOB (n = 4 slices each). Larger current amplitudes were observed in the proximity of the soma (∼30 µm); however, the amplitude of the responses in distal regions of the apical dendrites could be as large as the responses elicited in basal dendrites (Fig. 3D). The latency and time course of the responses did not vary significantly across different regions, in agreement with the current kinetics observed in other inhibitory synapses stimulated using DNPI-GABA (29). These results indicate that GABA inhibitory responses are very prominent throughout the GCs. To corroborate these findings further, we determined the expression and location of postsynaptic GABA receptors clusters using antibodies against gephyrin, an anchoring protein associated with postsynaptic clusters of GABAA receptors (30, 31). GCs were labeled by electroporation of GFP in the subventricular zone. Confocal analysis of immunostaining against GFP and gephyrin indicated that GABA receptor clusters can be found throughout the GCs’ soma and proximal and apical dendrites (Fig. S3). Importantly, the site of inhibition can have different consequences for GC excitability and information processing. Spatially restricted excitation of GC dendrites can produce localized inhibition of MCs through recurrent inhibition, whereas global activation of GCs (i.e., somatic excitation) can affect a larger number of MCs through lateral inhibition (17, 32). Similarly, we propose that GABAergic inhibitory inputs from the HDB/MCPO in the soma and proximal dendrites could exert a more global effect on GCs’ activity, regulating not only recurrent inhibition of MCs, but lateral inhibition as well.
Fig. 3.
GABA responses occur throughout the soma and dendritic tree of GCs. (A) Experimental setup used for GABA uncaging experiments. We recorded from the soma of GCs filled with Alexa 594 (red cell) while the slices were perfused with DPNI-GABA (2 mM) and photolysis was elicited by single-photon activation with a 405-nm laser. (B) GC filled with Alexa 594 (20 µM). Colored circles show representative uncaging spots: The blue circle is 10 µm from soma; the red circle indicates a control uncaging event 4 µm from the blue spot; the orange circle indicates a basal dendrite 28 µm from soma; and the green circle indicates a distal dendrite 99 µm from soma. (Scale bars: 10 µm.) (C) Scatter plot of the amplitude (Upper) and rise times (10–90%) (Lower) of laser-evoked GABA IPSC on GC dendrites, as a function of distance from soma, for AOB (n = 4, orange) and MOB (n = 4, black) (107 spots, 1,070 events). Larger IPSC amplitudes were observed within the proximity of the soma (∼30 µm), but the rise time did not vary significantly. (D) (Upper) Representative laser-evoked IPSCs at the specified colored spots shown in B (average of 10 traces per spot). The purple asterisk indicates the time of photolysis. (Scale bars: 20 pA and 100 ms.) (Lower) Bar graph comparing amplitude and kinetics of the representative photolysis-evoked IPSCs shown above. Note that the response evoked at 4 µm from the dendrite (red) has a significantly lower amplitude and larger rise time.
To determine the impact of HDB/MCPO inhibition in olfactory processing, we used designer receptors exclusively activated by designer drugs (DREADDs) technology to silence GABAergic neurons selectively. DREADDs are muscarinic receptors that have been mutated to respond selectively to the exogenous compound clozapine-N-oxide (CNO) but not to the endogenous ligand acetylcholine (33). We injected a Cre-dependent expression virus encoding the inhibitory DREADD, AAV8/hSyn-DIO-HA-hM4D(Gi)-IRES-mCitrine (hM4Di), into the HDB/MCPO of GAD65-Cre mice. Animals injected with hM4Di (n = 6) were tested 4 wk after injection for olfactory discrimination, after PBS (control mice) or CNO injections (treated mice) (Fig. 4). We tested structurally similar odors (e.g., esters differing in one carbon moiety) using the habituation/dishabituation paradigm. Animals habituated to an odor (ethyl heptanoate; C7) show a reduction in the sniffing time. Under control conditions (i.e., before the CNO injection), presentation of the novel odor (ethyl octanoate; C8) resulted in a significant increase in investigation time, indicating that the mice are able to discriminate between these odors (1.1 ± 0.2 s vs. 4.1 ± 0.4 s; P < 0.01). More importantly, the same animals were unable to discriminate the odor pair 2 h after CNO injection (0.5 mg/kg) (C7 0.7 ± 0.3 s vs. C8 0.3 ± 0.2 s; P < 0.4) but had no difficulty discriminating these odors when tested 2 h after a PBS injection (C7, 0.7 ± 0.3 vs. C8, 0.3 ± 0.2, P < 0.02). Previous behavioral studies have shown that the maximal effect of CNO, acting on the DREADDs, occurs within a window of a few hours (34, 35). Accordingly, 4 h after CNO injection, mice begin to recover their ability to discriminate the C7/C8 pair, indicating that the effect of CNO is reversible (C7 0.5 ± 0.2 s vs. C8 3.0 ± 0.7 s; P < 0.02). The deficit in olfactory discrimination was limited to closely related odors, and discrimination of esters differing by two carbons, e.g., between ethyl hexanoate (C6) and C8, was normal 2 h after the CNO injection (1.25 ± 1.0 s vs. 4.6 ± 0.7 s; P < 0.01) (Fig. 4). We note that these mice exhibited a significant increase in investigation time for the C8 odor after habituation to C6, even in the presence of CNO. Previous studies have shown that lesions in the HDB, impair the animal’s ability to habituate to consecutive presentations of an odor (36); however, our behavioral test showed that these mice had no deficit in habituation to repetitive odor presentations. Furthermore, the olfactory discrimination deficit observed is not caused by a change in the odor-detection threshold: The detection threshold of the C7 ester was not different in mice injected with PBS or CNO (Fig. S4A). Similarly, like control mice, hM4Di-injected mice learned to discriminate between stereoisomers of carvone in an associative learning paradigm, suggesting that cortical processing is not affected (Fig. S4B).
Together our results indicate that afferent inhibition from the HDB/MPOC onto GCs is required for proper olfactory discrimination. We hypothesize that the inhibitory inputs, like excitatory inputs, act to maintain a proper balance for the degree of GC→MC inhibition in the OB, which is necessary for fine discrimination. Future experiments are needed to determine the impact of this inhibitory input in the processing of information about social cues mediated by the AOB. Furthermore, proper olfactory coding depends on several properties such as local synaptic connectivity and also on network properties. Oscillations in the MOB are known to be particularly important for information processing. There is evidence that γ oscillations arise from the interaction between cortical structures and bulbar components (14) and that GABA inhibition onto GCs is required to maintain proper levels of γ oscillations (12). The HDB/MCPO receives inputs from several regions of the brain, including the OB, amygdala, hypothalamus, and brainstem (37, 38). Therefore, the GABAergic inhibitory input from the basal forebrain could channel integrated information from other brain areas to the OB. This information, like the excitatory cortical feedback, could mediate fast changes in olfactory coding in the OB in response to rapid changes in environmental context.
Materials and Methods
Animals.
All experiments were conducted following the guidelines of the Institutional Animal Care and Use Committee of the University of Maryland, College Park. Electrophysiological and behavioral experiments were performed on C57/BL6 or GAD65-Cre 30- to 180-d-old female and male mice obtained from breeding pairs housed in our animal facility. Animals were kept on a 12-h light/dark cycle with access to food and water ad libitum.
Viral Injections.
Expression of ChR2 in GAD65-Cre mice was achieved by targeted injection (1 µL) of the AAV2/5.CAGGS.flex.ChR2tdTomato adenovirus into the HDB/MCPO (University of Pennsylvania vector core; SI Materials and Methods). Similarly, in GAD65-Cre mice used for behavior studies the HDB/MCPO was injected bilaterally with 0.5 µL of the hM4Di adenovirus (University of North Carolina vector core). Animals were allowed to recover and were used for electrophysiological recordings at least 6 wk after injection. For behavioral studies, animals were used 4–5 wk after injection of hM4Di.
Confocal Imaging and Double-Labeling Immunofluorescence.
To visualize ChR-tdTomato expression, brains harvested from previously injected mice were fixed with 4% (wt/vol) paraformaldehyde (SI Materials and Methods), and fixed brain slices were mounted with Vectashield (Vector Laboratories) and visualized with a Leica SP5 × confocal microscope. For double-labeling immunofluorescence, we used free-floating sections incubated with 10% donkey serum (Sigma Aldrich) in PBS with 0.1% Triton X-100 (PBS-T) for 2 h at room temperature. Slices then were incubated overnight at room temperature with one or more of the following primary antibodies: mouse anti-GAD65 (1:300), rabbit anti-GAD67 (1:100), and/or goat anti-ChAT (1:500). After the incubation with the primary antibodies, the samples were incubated for 2 h at room temperature with the secondary antibodies: donkey anti-mouse Alexa-488, donkey anti-rabbit Alexa-594, and donkey anti-goat Alexa-488, all diluted at 1:750 in PBS-T with 2.5% of donkey serum. The samples were mounted with Vectashield and visualized with a Leica SP5 × confocal microscope.
Electrophysiological Recordings in OB Slices.
Brain slice recordings were conducted as previously described (SI Materials and Methods) (39). We used sagittal sections containing the MOB and AOB, respectively. After sectioning, the slices were placed in normal artificial cerebrospinal fluid [ACSF; in mM, 125 NaCl, 25 NaHCO3, 1.25 NaH2PO4, 3 KCl, 2 CaCl2, 1 MgCl2, 3 myo-inositol, 0.3 ascorbic acid, 2 Na-pyruvate and 15 glucose, continuously oxygenated (95% O2 and 5% CO2)] and were left to recuperate for 30–45 min. For LightStim at 473 nm, 5–50 pulses of 10 ms at 5 or 10 Hz were delivered through the 40× objective, using a Lambda LS Xenon Lamp (Sutter) controlled by a Lambda SC Smart Shutter. The area of infection was assessed visually to confirm that the injection was restricted to the HDB/MCPO (Fig. 1 B, 1). eIPSCs were recorded at a holding potential of 0 mV and were analyzed offline using the Synaptasoft Mini analysis program. Events were detected using an amplitude threshold of 5–6 pA. Only events with fast kinetics were considered for the analysis; for older ChR2-injected animals, the cutoff was 4.5 ms.
Uncaging of GABA.
We performed GABA uncaging as previously described (29). To correlate the physiological responses with the cell's morphology, we included Alexa 594 (20 μM) in the recording pipette, so that we were able to aim the uncaging laser spot to spatially restricted areas of the recorded GC. The collimated output of a 405 nm laser (Coherent, LLC) was expanded to 60% of the back aperture of a 63× Olympus objective. The spot has a Gaussian profile in the focal plane with a 1/e2 radius = 0.87 μm. Fluorescence illumination was achieved using a green LED (exciter 594 nm center wave length) (Chroma), and the emitted light was collected by a CCD camera (Hamamatsu). The concentration of DPNI-GABA was 2 mM (Tocris). Laser flashes usually were of 100-μs duration with power intensities at the surface of the slice up to 2 mW⋅μm−2.
Habituation/Dishabituation Test.
Odor discrimination was assessed using the habituation/dishabituation test (SI Materials and Methods). Control mice and animals injected 4–5 wk previously with hM4Di received a single i.p. injection of either PBS (control group) or 0.5 mg/kg CNO (treated group). Both groups were tested for their ability to discriminate between C7 and C8 or between C6 and C8. A clean standard mouse cage without bedding was used for the tests, and mice were allowed to become familiar with the test environment for 30 min. Each mouse was presented with three exposures to a wooden block scented with 100 µL of the test odor at a 1:1,000 dilution. Exposures lasted 2 min with a 1-min intertrial interval. Each trial was videotaped, and the time the mouse spent investigating the block was quantified offline. Investigation was defined as the time during which the mouse’s nose was within a 2-cm radius from the block.
Data analysis was performed using the Igor Pro software (WaveMetrics, Inc), and image analysis was performed using Image J software (National Institutes of Health). Data are shown as the mean ± SEM. Statistical significance was determined using the Student t test. Statistical differences were considered significant at P < 0.05.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health-National Institute on Deafness and Other Communication Disorders Grant DCR01-DC-009817 (to R.C.A.). A.N.-P. was supported by a Chilean government fellowship (Becas Chile); R.K.M. was supported by an undergraduate Howard Hughes Medical Institute research fellowship; R.S.S. was supported by an National Science Foundation predoctoral fellowship and a Chateaubriand Fellowship from the French Embassy.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1310686110/-/DCSupplemental.
References
- 1.Lee SH, Dan Y. Neuromodulation of brain states. Neuron. 2012;76(1):209–222. doi: 10.1016/j.neuron.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmer CR, Kristan WB., Jr Contextual modulation of behavioral choice. Curr Opin Neurobiol. 2011;21(4):520–526. doi: 10.1016/j.conb.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kay LM, Sherman SM. An argument for an olfactory thalamus. Trends Neurosci. 2007;30(2):47–53. doi: 10.1016/j.tins.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Gire DH, et al. Temporal processing in the olfactory system: Can we see a smell? Neuron. 2013;78(3):416–432. doi: 10.1016/j.neuron.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bardy C, Alonso M, Bouthour W, Lledo PM. How, when, and where new inhibitory neurons release neurotransmitters in the adult olfactory bulb. J Neurosci. 2010;30(50):17023–17034. doi: 10.1523/JNEUROSCI.4543-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pressler RT, Strowbridge BW. Blanes cells mediate persistent feedforward inhibition onto granule cells in the olfactory bulb. Neuron. 2006;49(6):889–904. doi: 10.1016/j.neuron.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Schoppa NE, Urban NN. Dendritic processing within olfactory bulb circuits. Trends Neurosci. 2003;26(9):501–506. doi: 10.1016/S0166-2236(03)00228-5. [DOI] [PubMed] [Google Scholar]
- 8.Shepherd GM, Chen WR, Willhite D, Migliore M, Greer CA. The olfactory granule cell: From classical enigma to central role in olfactory processing. Brain Res Brain Res Rev. 2007;55(2):373–382. doi: 10.1016/j.brainresrev.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Beshel J, Kopell N, Kay LM. Olfactory bulb gamma oscillations are enhanced with task demands. J Neurosci. 2007;27(31):8358–8365. doi: 10.1523/JNEUROSCI.1199-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kay LM. Theta oscillations and sensorimotor performance. Proc Natl Acad Sci USA. 2005;102(10):3863–3868. doi: 10.1073/pnas.0407920102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lagier S, et al. GABAergic inhibition at dendrodendritic synapses tunes gamma oscillations in the olfactory bulb. Proc Natl Acad Sci USA. 2007;104(17):7259–7264. doi: 10.1073/pnas.0701846104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nusser Z, Kay LM, Laurent G, Homanics GE, Mody I. Disruption of GABA(A) receptors on GABAergic interneurons leads to increased oscillatory power in the olfactory bulb network. J Neurophysiol. 2001;86(6):2823–2833. doi: 10.1152/jn.2001.86.6.2823. [DOI] [PubMed] [Google Scholar]
- 13.Ravel N, et al. Olfactory learning modifies the expression of odour-induced oscillatory responses in the gamma (60-90 Hz) and beta (15-40 Hz) bands in the rat olfactory bulb. Eur J Neurosci. 2003;17(2):350–358. doi: 10.1046/j.1460-9568.2003.02445.x. [DOI] [PubMed] [Google Scholar]
- 14.Boyd AM, Sturgill JF, Poo C, Isaacson JS. Cortical feedback control of olfactory bulb circuits. Neuron. 2012;76(6):1161–1174. doi: 10.1016/j.neuron.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Markopoulos F, Rokni D, Gire DH, Murthy VN. Functional properties of cortical feedback projections to the olfactory bulb. Neuron. 2012;76(6):1175–1188. doi: 10.1016/j.neuron.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsutani S, Yamamoto N. Centrifugal innervation of the mammalian olfactory bulb. Anat Sci Int. 2008;83(4):218–227. doi: 10.1111/j.1447-073X.2007.00223.x. [DOI] [PubMed] [Google Scholar]
- 17.Balu R, Pressler RT, Strowbridge BW. Multiple modes of synaptic excitation of olfactory bulb granule cells. J Neurosci. 2007;27(21):5621–5632. doi: 10.1523/JNEUROSCI.4630-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zimnik NC, Treadway T, Smith RS, Araneda RC. 1A-Adrenergic regulation of inhibition in the olfactory bulb. J Physiol. 2013;591(Pt 7):1631–1643. doi: 10.1113/jphysiol.2012.248591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brennan PA, Kendrick KM. Mammalian social odours: Attraction and individual recognition. Philos Trans R Soc Lond B Biol Sci. 2006;361(1476):2061–2078. doi: 10.1098/rstb.2006.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaykema RP, Luiten PG, Nyakas C, Traber J. Cortical projection patterns of the medial septum-diagonal band complex. J Comp Neurol. 1990;293(1):103–124. doi: 10.1002/cne.902930109. [DOI] [PubMed] [Google Scholar]
- 21.Gracia-Llanes FJ, et al. GABAergic basal forebrain afferents innervate selectively GABAergic targets in the main olfactory bulb. Neuroscience. 2010;170(3):913–922. doi: 10.1016/j.neuroscience.2010.07.046. [DOI] [PubMed] [Google Scholar]
- 22.Záborszky L, Carlsen J, Brashear HR, Heimer L. Cholinergic and GABAergic afferents to the olfactory bulb in the rat with special emphasis on the projection neurons in the nucleus of the horizontal limb of the diagonal band. J Comp Neurol. 1986;243(4):488–509. doi: 10.1002/cne.902430405. [DOI] [PubMed] [Google Scholar]
- 23.Devore S, Linster C. Noradrenergic and cholinergic modulation of olfactory bulb sensory processing. Front Behav Neurosci. 2012;6:52. doi: 10.3389/fnbeh.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunze WA, Shafton AD, Kem RE, McKenzie JS. Intracellular responses of olfactory bulb granule cells to stimulating the horizontal diagonal band nucleus. Neuroscience. 1992;48(2):363–369. doi: 10.1016/0306-4522(92)90496-o. [DOI] [PubMed] [Google Scholar]
- 25.Castro JB, Hovis KR, Urban NN. Recurrent dendrodendritic inhibition of accessory olfactory bulb mitral cells requires activation of group I metabotropic glutamate receptors. J Neurosci. 2007;27(21):5664–5671. doi: 10.1523/JNEUROSCI.0613-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong HW, Heinbockel T, Hamilton KA, Hayar A, Ennis M. Metabotropic glutamate receptors and dendrodendritic synapses in the main olfactory bulb. Ann N Y Acad Sci. 2009;1170:224–238. doi: 10.1111/j.1749-6632.2009.03891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sahara Y, Kubota T, Ichikawa M. Cellular localization of metabotropic glutamate receptors mGluR1, 2/3, 5 and 7 in the main and accessory olfactory bulb of the rat. Neurosci Lett. 2001;312(2):59–62. doi: 10.1016/s0304-3940(01)02184-x. [DOI] [PubMed] [Google Scholar]
- 28.Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature. 2009;457(7233):1142–1145. doi: 10.1038/nature07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trigo FF, Papageorgiou G, Corrie JE, Ogden D. Laser photolysis of DPNI-GABA, a tool for investigating the properties and distribution of GABA receptors and for silencing neurons in situ. J Neurosci Methods. 2009;181(2):159–169. doi: 10.1016/j.jneumeth.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 30.Craig AM, Banker G, Chang W, McGrath ME, Serpinskaya AS. Clustering of gephyrin at GABAergic but not glutamatergic synapses in cultured rat hippocampal neurons. J Neurosci. 1996;16(10):3166–3177. doi: 10.1523/JNEUROSCI.16-10-03166.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sassoè-Pognetto M. Molecular and functional heterogeneity of neural circuits: An example from the olfactory bulb. Brain Res Brain Res Rev. 2011;66(1-2):35–42. doi: 10.1016/j.brainresrev.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Isaacson JS, Strowbridge BW. Olfactory reciprocal synapses: Dendritic signaling in the CNS. Neuron. 1998;20(4):749–761. doi: 10.1016/s0896-6273(00)81013-2. [DOI] [PubMed] [Google Scholar]
- 33.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA. 2007;104(12):5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krashes MJ, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121(4):1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sasaki K, et al. Pharmacogenetic modulation of orexin neurons alters sleep/wakefulness states in mice. PLoS ONE. 2011;6(5):e20360. doi: 10.1371/journal.pone.0020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paolini AG, McKenzie JS. Effects of lesions in the horizontal diagonal band nucleus on olfactory habituation in the rat. Neuroscience. 1993;57(3):717–724. doi: 10.1016/0306-4522(93)90017-a. [DOI] [PubMed] [Google Scholar]
- 37.Linster C, Hasselmo ME. Neural activity in the horizontal limb of the diagonal band of broca can be modulated by electrical stimulation of the olfactory bulb and cortex in rats. Neurosci Lett. 2000;282(3):157–160. doi: 10.1016/s0304-3940(00)00885-5. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Swann JM. The magnocellular medial preoptic nucleus I. Sources of afferent input. Neuroscience. 2006;141(3):1437–1456. doi: 10.1016/j.neuroscience.2006.04.079. [DOI] [PubMed] [Google Scholar]
- 39.Smith RS, Weitz CJ, Araneda RC. Excitatory actions of noradrenaline and metabotropic glutamate receptor activation in granule cells of the accessory olfactory bulb. J Neurophysiol. 2009;102(2):1103–1114. doi: 10.1152/jn.91093.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.