Abstract
Human referential communication is often thought as coding–decoding a set of symbols, neglecting that establishing shared meanings requires a computational mechanism powerful enough to mutually negotiate them. Sharing the meaning of a novel symbol might rely on similar conceptual inferences across communicators or on statistical similarities in their sensorimotor behaviors. Using magnetoencephalography, we assess spectral, temporal, and spatial characteristics of neural activity evoked when people generate and understand novel shared symbols during live communicative interactions. Solving those communicative problems induced comparable changes in the spectral profile of neural activity of both communicators and addressees. This shared neuronal up-regulation was spatially localized to the right temporal lobe and the ventromedial prefrontal cortex and emerged already before the occurrence of a specific communicative problem. Communicative innovation relies on neuronal computations that are shared across generating and understanding novel shared symbols, operating over temporal scales independent from transient sensorimotor behavior.
Keywords: social interaction, theory of mind, experimental semiotics, MEG, broadband spectral change
We can modify reality by selecting either instrumental actions that change the physical state of the environment according to the mechanics of the action or communicative actions that change the mental state of other agents according to the content of the action (1, 2). For instance, we can fill a glass with a drink or ask a bartender to do that. A common language might help to achieve the latter by providing access to previously established shared symbols, but those symbols presuppose a computational mechanism powerful enough to negotiate them across interlocutors (3). Here, we study the electrophysiological correlates supporting the rapid negotiation of shared symbols, a fundamental property of human communication (4, 5).
Given the vast number of possible meanings that can be attributed to a novel communicative action (6, 7), it remains unclear how novel shared symbols can be rapidly selected and understood. General-purpose learning algorithms such as temporal difference or Hebbian learning (8, 9) do not seem suitable, because they require many trials to converge on statistically relevant features. There are brain circuits that support fast predictions on sensory inputs or consequences of planned actions (10, 11), but those circuits are geared toward a specific domain of application with well-defined priors [e.g., faces (12)]. Novel shared symbols, being novel, do not have well-defined priors (3, 13, 14). Solving this type of communicative problem requires a mechanism that supports a rapid exploration through a large search space, generating connections between different conceptual structures (15, 16).
These theoretical considerations about human communication lead to three predictions on its underlying mechanism. First, given that establishing shared symbols requires taking into account the inferred knowledge of the interlocutor [“audience design” (17–19)], the generation and comprehension of those symbols should involve neural patterns associated with flexible conceptual knowledge (20–23), rather than sensorimotor couplings with limited generalization patterns (9, 24–28). Second, cerebral activities supporting these conceptual processes during generation and comprehension of novel shared symbols should overlap, given that these processes relate to the specific conversational context shared by the interlocutors of the communicative exchange (29). Third, cerebral activity supporting this predicted overlap should predate in time the processing of the communicative stimuli themselves, given that the meaning of any stimulus arises from a conceptual space defined by the ongoing communicative interaction (19, 30), rather than by the sensory material itself.
We test these predictions by characterizing spatial, spectral, and temporal features of neural activity supporting the planning and understanding of novel communicative actions, using an absolute index of source-reconstructed magnetoencephalographic activity. In contrast to previous work largely focused on individuals perceiving instrumental actions (31, 32) or known linguistic material (30, 33), here we investigated both production and comprehension of novel communicative actions during a live interaction between pairs of participants and directly contrast those phenomena with a control interaction involving no communicative necessities (Fig. 1).
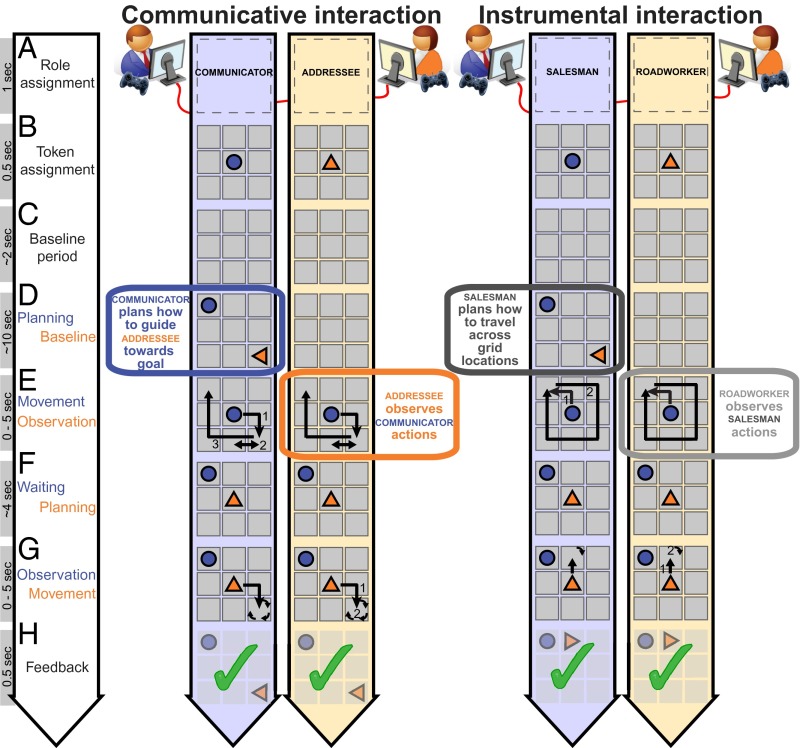
Fig. 1.
Live interactions. During a communicative interaction (Movie S1), pairs of participants had to jointly reproduce a spatial configuration of two tokens shown only to the Communicator (epoch D). This required the Communicator to use the movements of his token (in blue) (epoch E) to indicate to the Addressee how she should configure her token (in orange). In this game, shared meanings of the behaviors had to be constructed and inferred. During an instrumental interaction (Movie S2), the same pair interacted by moving their tokens on the board according to preassigned rules. The Salesman implemented his rules on a visually presented configuration. The Roadworker implemented her rules according to the behavior of the Salesman in epoch E. The critical epochs for the analysis of neural activity are the planning phase (epoch D) for the Communicator/Salesman and the observation phase (epoch E) for the Addressee/Roadworker.
Results
Task Manipulation.
We studied 24 pairs of participants engaged in real-time controlled interactions (18) and measured neural activity with magnetoencephalography (MEG) from one participant within each pair. Each pair of participants played an interactive game that requires the generation and understanding of novel, mutually negotiated communicative actions (i.e., communicative interactions between a “Communicator” and an “Addressee” pair; Fig. 1 and Movie S1). We distinguished neural activity specifically associated with those communicative actions from activity evoked during another interactive game that involved the same stimuli, responses, attention, and between-participant dependencies but no communicative necessities (i.e., instrumental interactions between a “Salesman” and a “Roadworker” pair; Fig. 1 and Movie S2). Within each task, participants alternated between those two task-specific roles on a trial-by-trial basis (80 trials in each task). We further distinguished neural activity common to both generating (epoch D: planning; Fig. 1) and understanding communicative actions (epoch E: observation; Fig. 1) from activity uniquely evoked by either task component by means of conjunction analyses (34). An absolute index of neural activity was quantified by estimating (“beamforming”) time-resolved spectral power of the signals recorded with MEG before and during task performance (35).
The communicative and instrumental tasks are explained in detail in SI Materials and Methods. Here, we highlight their overlapping and differing features relevant for labeling and interpreting the results. In both tasks, pairs of participants were instructed to move their token on a visually presented 3 × 3 digital grid (Fig. 1). In the communicative task, the goal of the Communicator was to make sure that both his token (e.g., a circle) and that of the Addressee (e.g., a triangle) were arranged according to a configuration visually presented to the Communicator only. This required the Communicator to use the movements of his token to indicate to the Addressee how she should configure her token on the grid. This task has proven effective in encouraging the generation of pair-specific communicative behaviors (18, 36, 37). The same movements could be used by different pairs to negotiate different meanings, and the same meaning could be conveyed by different movements across different pairs (Movie S3). The same movement could even be used to convey different goal states by the same pair in different trials (Movie S4) and vice versa (Movie S5). The latter observation emphasizes how, in this game, a movement acquires meaning by virtue of the history of the communicative interactions within a given pair, rather than by virtue of its sensory attributes. In the instrumental task, the goal of the Salesman was to move his token across the board following a learned rule, according to a visually presented configuration. The coplayer, labeled Roadworker, was instructed to place her token on the board following a learned rule, according to the movements of the Salesman on the board. Stimuli, movements, and between-player dependencies were matched between the two types of interactions, but the necessity to construct and infer shared movement-meaning mappings differed. In the communicative task, the success of a trial relied on the Communicator designing an action that can be understood by the Addressee, and on the Addressee inferring the Communicator’s intentions. In the instrumental task, the success of a trial relied on each of the two players implementing preestablished rules, without communicative requirements, despite the fact that the actions of the Roadworker were determined by those of the Salesman.
Behavioral Characteristics of Communicative Interactions.
Participants solved both tasks well above chance level (communicative trials: 71 ± 3% correct; instrumental trials: 73 ± 4% correct; mean ± SEM; Fig. S1E; estimate of chance level: 1/32th; eight locations with four potential orientations). The communicative interactions evoked stronger mutual adjustments between pairs than the instrumental interactions. First, during the communicative interactions, Communicators spent longer times at the grid location where the Addressee should place her token (Addressee “target”), compared with other visited locations on the board [“nontargets”; location × task interaction: F(1,23) = 108.0, P < 0.001; Fig. S1F]. This pausing behavior was adjusted to the inferred knowledge of the communicative partner on a trial-by-trial basis (36), a quantitative indication of recipient design (38). Second, during the communicative interactions, Communicators made repeated movements from and to the target location to indicate the desired orientation of the Addressee’s token (2.09 ± 0.49 visits per trial; mean ± SD; see action 2, epoch E in Fig. 1, communicative task). This behavior was not observed in the instrumental task, and it follows the general principle of using a patently dysfunctional action to ostensively mark the action as being communicative in nature (14). Third, in the communicative interactions, the within-trial coupling between Communicator and Addressee planning times (r = 0.29 ± 0.17; z-transformed cross-correlations) was stronger than in the instrumental interactions [i.e., between Salesman and Roadworker planning times; r = 0.09 ± 0.22; t(23) = 4.2; P < 0.001]. This observation suggests that a difficult communicative problem was concomitantly more difficult for both Communicators and Addressees (18, 39). Fourth, in the instrumental task, the number of executed movements explained a larger portion of planning time variance than in the communicative task [instrumental: r = 0.63 ± 0.12; communicative: r = 0.42 ± 0.17; t(23) = 5.5, P < 0.001]. This finding suggests that, in the instrumental task, planning times increase almost linearly with an increasing number of movement steps to plan. In contrast, in the communicative task, planning times were governed by cognitive operations less directly related to the mechanics of the individual movement steps.
Neural Characteristics of Communicative Interactions: Spatial and Spectral Features.
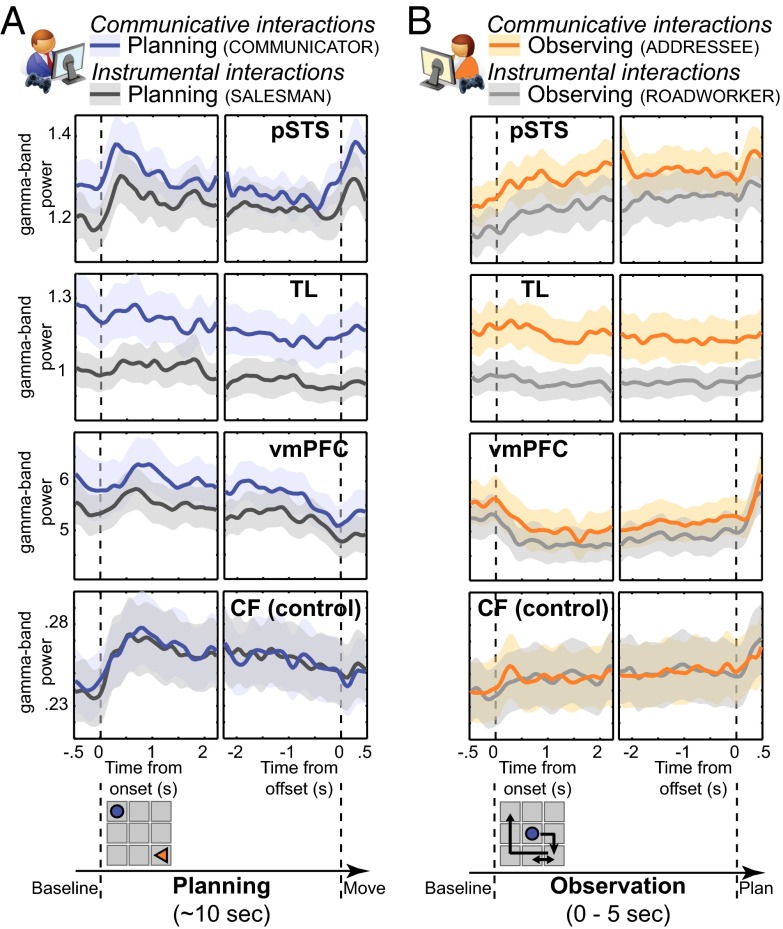
Having shown the relevance of the communicative task for studying novel communicative actions, we also verified that the neural activity evoked by performance of the communicative and instrumental tasks was largely matched (Fig. S2) and devoid of eye-movement confounds (Fig. S3). Having satisfied these preconditions, we proceeded to test the three hypotheses of this study. First, we isolated neural activity evoked by the communicative task over and above the instrumental task, testing whether those neural differences were present in the sensorimotor system or in higher-order cortical areas. We considered the whole time interval covered by the planning and observation epochs (epochs D and E in Fig. 1), a conservative approach that intrinsically focuses toward neural effects spanning both epochs. Two brain regions [right temporal lobe (TL) and ventromedial prefrontal cortex (vmPFC); Fig. 2 A and C] falling outside the core sensorimotor systems exhibited significantly stronger power over a broad frequency range (Fig. 2 B and D) during the processing of communicative actions than during instrumental actions. There were no significant clusters where planning or observing instrumental actions evoked stronger responses than communicative actions.
Fig. 2.
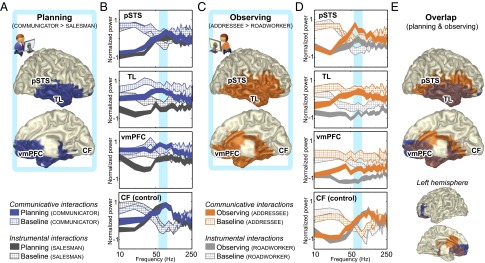
Spatial and spectral characteristics of neural activity evoked during communicative and instrumental interactions. Brain regions exhibiting stronger γ-band activity (55–85 Hz) when participants planned (A) and observed (C) communicative actions compared with instrumental actions. The spatial distribution of the conjunction (in brown) (E) was lateralized to the right hemisphere, covering most of the TL and vmPFC. Power spectral densities of neural activity (±1 SEM, striped for baseline epochs, filled for task epochs) (B and D) indicate that the task differences were broadband in nature (compare blue vs. dark gray and orange vs. light gray ribbons), statistically most pronounced in the 55- to 85-Hz frequency range (in cyan). Spectral power is mean-centered (i.e., the average across the eight experimental epochs was set to zero) and variance-normalized (SD = 1) over subsequent 10-Hz frequency bins. When averaged over the whole task epochs, the differences in the right pSTS were present only during the observation epoch (but see Fig. 3). The CF is presented for control purposes, showing only band-limited modulations from baseline.
The second hypothesis of this study predicts an overlap in the cognitive processes evoked during generation and comprehension of novel shared symbols. Accordingly, we tested whether those task-dependent neural differences are shared between planning (epoch D) and observing (epoch E) communicative actions. We used a minimum-statistic conjunction analysis (34) to isolate neural effects shared across communicative roles, and different from the corresponding instrumental roles (40), effectively filtering out between-tasks differences that are not consistent across paired roles within each task (Fig. S1 C and D). The overlap in neural effects across communicative roles was statistically most pronounced in the 55–85 Hz γ-band (Fig. S4) and spatially encompassed the vmPFC and the right TL (Fig. 2E, in brown).
Neural Characteristics of Communicative Interactions: Temporal Features.
The third hypothesis of this study predicts that selecting and understanding novel shared symbols relies on a cognitive set implemented through ongoing neural activity that predates the occurrence of the communicative stimulus material itself. Therefore, we explored the temporal dynamics of an absolute index of neural activity [i.e., source-reconstructed time-resolved estimates of γ-band power (35)]. This index is appropriate for isolating tonic state-dependent effects that are temporally stable and not exclusively bound to the occurrence of task events. We observed up-regulated neural activity in three regions (Fig. 3). A ventrolateral portion of the right TL showed a tonic up-regulation of γ-band power during both planning and observation of communicative actions (TL; Fig. 3 A and B), without transient responses time-locked to the sensorimotor events occurring during those epochs. This temporal dynamics indicate that neural activity in the right TL is modulated by the communicative task but over a timescale decoupled from within-trial events. A different neural dynamics was found in the vmPFC. This region showed a sustained decrease in γ-band power during the observation epochs of both tasks, again with stronger γ-band power in the communicative task, and a sharp power increase when participants started selecting their actions on the basis of the observed movements of their coplayer (vmPFC; Fig. 3). These temporal dynamics indicate that neural activity in the vmPFC is tonically up-regulated during performance of the communicative task, with planning and observation of actions evoking opposite computational loads in this region with respect to the pre- and postepoch phases. A third temporal profile of γ-band activity was found in the right posterior superior temporal sulcus (pSTS), a region previously reported to increase its metabolic demands as a function of communicative difficulty, both for Communicators generating novel communicative actions and Addressees trying to decode those signals (41). Differently from the ventral portions of the right TL and the vmPFC, the right pSTS is sensitive to computational demands that occur early in planning and that rise during action observation (pSTS; Fig. 3).
Fig. 3.
Temporal characteristics of neural activity evoked during communicative and instrumental interactions. γ-Band activity (55–85 Hz; in arbitrary units ± 1 SEM) in pSTS, TL, vmPFC, and CF, time-locked to planning (A) and observation (B) onset and offset. The graphic panel at the bottom highlights the characteristics of the relevant task epochs. Both tasks induced transient changes in neural activity in pSTS, vmPFC, and CF. During the communicative task, neural activity was tonically up-regulated in TL, vmPFC, and pSTS but not in CF.
Communicative Consequences of Tonic Up-Regulation of γ-Band Power.
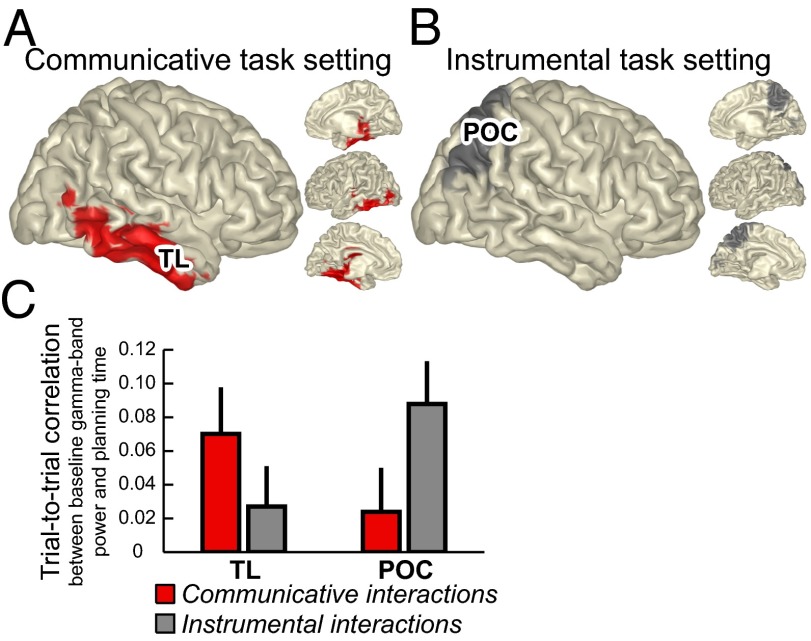
The γ-band power changes described above were spatially and functionally specific, as illustrated by the absence of a tonic up-regulation of γ-band power during the same task epochs in a primary sensory area [right calcarine fissure (CF); Fig. 3, bottom row], despite strong transient changes in γ-band power time-locked to the presentation of the visual stimuli. A fourth analysis tested whether those tonic γ-band power changes are behaviorally relevant, with measurable consequences on the performance of the communicative task. We assessed trial-by-trial correlations of neural activity and behavioral performance (SI Materials and Methods). γ-Band activity measured during the baseline period preceding the occurrence of observable events predicted the planning time of the subsequent trial epoch both when solving communicative and instrumental problems (both as Communicator/Salesman, or as Addressee/Roadworker). Critically, the spatial distribution and magnitude of the baseline neural activity predicting task performance differed as a function of the current cognitive set. During the communicative task, tonic baseline activity in the right TL [of both Communicator (epoch C) and Addressee (epoch D); Fig. 1] predicted planning time in the same trial [Communicator: epoch D; Addressee: epoch F; r = 0.07 ± 0.02; t(23) = 2.5; P < 0.03; Fig. 4 A and C]. The spatial distribution of this effect overlaps with the changes in γ-band activity shared across the two communicators (Fig. 2E). In contrast, during the instrumental task, tonic baseline activity in the parietooccipital cortex (of both Salesman and Roadworker) predicted planning time in the same trial [r = 0.09 ± 0.02; t(23) = 3.5; P < 0.05; Fig. 4 B and C]. The spatial distribution of this effect overlaps with the known contribution of the parietooccipital cortex in supporting visuospatial transformations during action planning (42) and with the observation that planning time during the instrumental task was linearly related to the number of movement steps performed by the subjects in the subsequent task epoch. In both tasks, there were no significant correlations between tonic baseline activity and planning time in the preceding trial (r = 0.02 ± 0.02 for each task).
Fig. 4.
Ongoing neural activity associated with the cognitive set. (A and B) Spatial distribution of cortical regions showing trial-by-trial correlation between baseline neural activity and task performance. Baseline γ-band (55–85 Hz) power in the TL accounted for variation in planning time of Communicators and Addressees; baseline γ-band power in the parietooccipital cortex (POC) accounted for variation in planning time of Salesmen and Roadworkers. (C) Group-averaged correlations for each of the two tasks and cortical regions (±1 SEM).
Discussion
This study describes the spectral, spatial, and temporal features of neural activity evoked during the selection and comprehension of novel shared symbols, two processes essential for understanding the flexibility of human communication (3, 5). There are three main findings. First, solving novel communicative problems up-regulated local neural activity in the right ventrolateral TL and the vmPFC, two regions necessary for processing conceptual knowledge and mental models of other agents (23, 43, 44). Second, the same up-regulation of neural activity was found across Communicator and Addressee, irrespectively of whether a communicative action was being selected or comprehended. This finding indicates that the overlapping neural up-regulation was driven by abstract task features shared across interlocutors, rather than sensorimotor events which differed between interlocutors. Third, the overlapping neural up-regulation was present well before the occurrence of a specific communicative problem. This finding provides a neural counterpart to the notion that the meaning of novel communicative actions is inferred by embedding those stimuli in a conceptual space whose activation predates in time the processing of the communicative stimuli themselves (45). Taken together, these observations indicate that the brain solves the computational challenges evoked by creating novel shared symbols by up-regulating the same neuronal mechanism in the same brain regions across pairs of communicators, and over temporal scales independent from transient sensorimotor events (46).
Tonically Increased Neural Activity During Communicative Interactions.
The up-regulation of neural activity evoked by the presence of communicative demands had specific spatial, spectral, and temporal characteristics. First, the spatial distribution of differential neural activity between the communicative and the instrumental task was confined to the right temporal and medial prefrontal regions. These two areas have been shown to be necessary for accessing conceptual knowledge and mental models of other agents (23, 43, 44). Second, the spectral profile of this differential source-reconstructed neural activity was extremely broad. Physiologically, broadband shifts of local neural activity are functionally distinct from band-limited neuronal oscillations (47), and they are thought to reflect changes in mean firing rates of neuronal populations (48–51). Population-level firing rates have been shown to be affected by internal cortical states as much as by external stimuli (52, 53), and they are instrumental for integrating driving afferences with contextual information (54–56). Third, the temporal profile of the broadband shift of neural activity started already during the baseline epoch, before the presentation of a particular communicative problem and well before the observation of communicative actions. This baseline-related local neural activity had measurable behavioral consequences on communicative performance during a subsequent epoch in the same trial (Fig. 4), and it fits with the behavioral observation that these subjects displayed audience design during trials following a communicative error (36). Taken together, these observations suggest that the tonic up-regulation of broadband neural activity evoked by communicative challenges reflects increased firing rates of neuronal populations in the right ventrolateral TL and the vmPFC. Those increased firing rates might provide a neurophysiological mechanism for integrating the current communicative problem with conceptual knowledge. Crucially, the present data suggest that this integration is not temporally bound to the presentation of a specific communicative problem in the course of a trial. In fact, the current findings support the notion that conceptual knowledge during a communicative interaction needs to be continuously aligned to the conversational context and to the interlocutor’s behavior (19). The tonic up-regulation of broadband activity observed in this study during communicative interactions might be a neural marker of this cognitive phenomenon.
Shared Tonic Computations Between Production and Comprehension of Communicative Actions.
A large portion of the right TL showed a sustained increase in broadband activity during both planning and understanding of communicative actions. This finding qualifies the characteristics of the coarse spatiotemporal cerebral overlaps between communicators reported in previous studies (30, 33, 41, 57). Namely, the presence of a spectral overlap between communicators suggests that the human brain uses the same neurophysiological mechanisms when planning and understanding communicative actions. Given that those two epochs had considerable sensorimotor differences, and that the spectral overlap arose from brain regions necessary for processing conceptual knowledge and mental models of other agents, it is conceivable that Communicators and Addressees might share a basic conceptual mechanism that supports a rapid exploration through a large search space (41).
Shared Phasic Computations During Social and Nonsocial Behaviors.
This study shows that solving complex communicative and instrumental problems relies on computational processes with surprisingly matched phasic neural dynamics. For instance, γ-band power in the vmPFC transiently increased during the selection of complex action sequences, irrespectively of the communicative characteristics of those actions. The within-trial fluctuations of γ-band power in pSTS also showed a strikingly similar pattern when solving communicative compared with instrumental problems. These findings suggests that vmPFC and pSTS are involved in selecting communicative actions using neural dynamics similar to those involved in selecting noncommunicative actions (8). This observation argues against the notion that these two regions are exclusively dedicated to social cognition (12).
Conclusions
Humans are surprisingly effective at creating novel shared symbols (6, 18), an evolutionary anomaly at the root of human communication (3, 5). This study describes the spectral, temporal, and spatial characteristics of neural activity evoked during planning and understanding of novel communicative actions. The computational challenges evoked by solving communicative problems result in tonically up-regulated neural activity over right temporal and ventromedial prefrontal regions. The phasic temporal dynamics of those regions was sensitive to the occurrence of transient sensory or motor events, but it was indifferent to the communicative characteristics of the problems. These findings define the neurophysiological characteristics of a mechanism supporting human communicative innovation, opening the way for understanding the neural implementation of human symbolic communication.
Materials and Methods
Participants.
Fifty-two participants (22 males and 30 females; ages, 18–40 y), were recruited to take part in this study. They were screened for a history of psychiatric and neurological problems and had normal or corrected-to-normal vision. Participants gave informed consent according to institutional guidelines of the local ethics committee (Committee on Research Involving Human Subjects, region Arnhem-Nijmegen, The Netherlands; approved by Radboud University Nijmegen) and were either offered a financial payment or given credits toward completing a course requirement. Magnetoencephalographic activity was acquired from one member of each pair. Two pairs of participants were excluded from data analysis because of MEG-system failure and muscle artifacts, leaving 24 pairs of participants for data analysis.
Tasks.
The communicative and the instrumental tasks are described in detail in SI Materials and Methods.
MEG and MRI Data Acquisition.
Brain activity was recorded over two sessions using a whole-head MEG with 275 axial gradiometers (CTF275; VSM MedTech; 1,200-Hz sampling rate; 300-Hz analog low-pass filter). Before the second session, each participant repositioned his or her head in the same location and orientation as the position measured before the first session, using a real-time head localizer tool (58). Anatomical images of the brain for forward model generation (voxel size, 1 mm3) were acquired using a 1.5T Siemens Avanto scanner. During MR acquisition, identical earplugs (with a vitamin E capsule in place of the MEG localization coils) were used for coregistration of the MRI and MEG data.
MEG Data Analysis.
Data were analyzed offline using the FieldTrip toolbox (59) and custom MATLAB code (MathWorks). Trials with muscle and MEG artifacts were removed from the MEG time series, resulting in 91 ± 5% of the original trials being included for further analysis. Following our experimental rationale, we focused the analysis of the MEG data on the trial epochs during which the Communicator and Salesman planned their actions (epoch D: planning; Fig. 1), and the Addressee and Roadworker observed the other player’s movements (epoch E: observation). For each epoch, we also considered the preceding baseline period (1 s), during which only the empty grid was visible. We analyzed these task epochs in two ways, differing in the time scale at which the inferences can be drawn.
In analysis 1, we considered the whole time interval covered by the planning and observation events. Accordingly, we extracted the overall changes in cerebral neural activity evoked during those events, using adaptive spatial filtering (beamforming; SI Materials and Methods) to estimate local neural population activity throughout the brain as a function of frequency. We matched the signal-to-noise ratios of the different conditions within each participant by ensuring that each condition contributed the same number of samples to the data analysis. To achieve this, each trial was segmented into multiple consecutive nonoverlapping windows of 500 ms. For each participant, windows were randomly selected and excluded from subsequent analyses until the different conditions provided the same number of windows. Then, the windowed time series from each trial epoch were tapered with a set of 4 orthogonal Slepian tapers before spectral estimation and the resulting estimates of the (cross-)spectral densities were averaged across tapers. This resulted in a spectral smoothing of ±5 Hz.
In analysis 2, we extracted the fine-grained temporal dynamics of power changes during the task epochs mentioned above, performing a time-frequency analysis at the source level. This analysis was time-locked to the moments the Communicator and Salesman started and finished planning (epochs D: planning) and the Addressee and Roadworker started and finished observing (epochs E: observation), extending over a time window of 2.75 s (range: −0.5 to +2.25 s and −2.25 to +0.5 s, respectively; resolution: 50 ms). We applied an adaptive spatial filtering approach within a set of frequencies (55–85 Hz) shown to contain task-relevant neural activity by analysis 1. Here, 200-ms windows were tapered with three orthogonal Slepian tapers (±10-Hz smoothing) before applying the Fourier transforms. Projection of the sensor-level data through the spatial filters, and subsequently computing the magnitude squared, yielded a location-specific (absolute) estimate of the time course of spectral power at the frequency of interest.
Statistical Model and Inference.
We considered differential effects evoked during corresponding trial epochs in participants playing the Communicator or the Salesman role (epoch D: planning in Fig. 1) and the Addressee or the Roadworker role (epoch E: observation). First, we estimated participant-specific effects (independent samples t tests) on signal power at the source level (obtained from analysis 1) for each of these two sets of temporally independent comparisons. Second, these participant-specific effects were z-normalized to account for differences in degrees of freedom and entered into a second-level random effects analysis correcting for multiple comparisons at the cluster level (P < 0.05; 10,000 randomizations) (60). Third, the resulting group statistics of the two contrasts were entered into a conjunction analysis (34), effectively implementing a logical AND relation between the individual contrasts.
Supplementary Material
Acknowledgments
This research was supported by VICI Grant 453-08-002 from Netherlands Organisation for Scientific Research (to I.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.T.K. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1303170110/-/DCSupplemental.
References
- 1.Noordzij ML, et al. Neural correlates of intentional communication. Front Neurosci. 2010;4:188. doi: 10.3389/fnins.2010.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Searle S. Making the Social World: The Structure of Human Civilization. USA: Oxford University Press; 2010. [Google Scholar]
- 3.Levinson SC. On the human “interactional engine”. In: Enfield NJ, Levinson SC, editors. Roots of Human Sociality: Culture Cognition, and Interaction. Oxford: Berg; 2006. [Google Scholar]
- 4.Evans N, Levinson SC. With diversity in mind: Freeing the language sciences from Universal Grammar. Behav Brain Sci. 2009;32(5):472–492. doi: 10.1017/S0140525X0999094X. [DOI] [PubMed] [Google Scholar]
- 5.Tomasello M. Origins of Human Communication. Cambridge, MA: MIT Press; 2008. [Google Scholar]
- 6.Galantucci B. An experimental study of the emergence of human communication systems. Cogn Sci. 2005;29(5):737–767. doi: 10.1207/s15516709cog0000_34. [DOI] [PubMed] [Google Scholar]
- 7.Jablonka E. Information: Its interpretation, its inheritance, and its sharing. Philos Sci. 2002;69(4):578–605. [Google Scholar]
- 8.Behrens TE, Hunt LT, Rushworth MF. The computation of social behavior. Science. 2009;324(5931):1160–1164. doi: 10.1126/science.1169694. [DOI] [PubMed] [Google Scholar]
- 9.Keysers C, Perrett DI. Demystifying social cognition: A Hebbian perspective. Trends Cogn Sci. 2004;8(11):501–507. doi: 10.1016/j.tics.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Giese MA, Poggio T. Neural mechanisms for the recognition of biological movements. Nat Rev Neurosci. 2003;4(3):179–192. doi: 10.1038/nrn1057. [DOI] [PubMed] [Google Scholar]
- 11.Peelen MV, Fei-Fei L, Kastner S. Neural mechanisms of rapid natural scene categorization in human visual cortex. Nature. 2009;460(7251):94–97. doi: 10.1038/nature08103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adolphs R. The social brain: Neural basis of social knowledge. Annu Rev Psychol. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fodor JA. The Mind Doesn’t Work That Way. Cambridge, MA: MIT Press; 2000. [Google Scholar]
- 14.Sperber D, Wilson D. Relevance: Communication and Cognition. Oxford: Blackwell; 2001. [Google Scholar]
- 15.Goldstone RL, Rogosky BJ. Using relations within conceptual systems to translate across conceptual systems. Cognition. 2002;84(3):295–320. doi: 10.1016/s0010-0277(02)00053-7. [DOI] [PubMed] [Google Scholar]
- 16.Gentner D. In: Why We’re so Smart. Language in Mind: Advances in the Study of Language and Thought. Gentner D, Goldin-Meadow S, editors. Cambridge, MA: MIT Press; 2003. pp. 195–235. [Google Scholar]
- 17.Galantucci B, Garrod S. Experimental semiotics: A review. Front Hum Neurosci. 2011;5:11. doi: 10.3389/fnhum.2011.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Ruiter J, et al. Exploring the cognitive infrastructure of communication. Interact Stud. 2010;11(1):51–77. [Google Scholar]
- 19.Clark HH. Using Language. Cambridge, UK: Cambridge Univ Press; 1996. [Google Scholar]
- 20.Kumaran D, Summerfield JJ, Hassabis D, Maguire EA. Tracking the emergence of conceptual knowledge during human decision making. Neuron. 2009;63(6):889–901. doi: 10.1016/j.neuron.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siegal M, Varley R. Neural systems involved in “theory of mind”. Nat Rev Neurosci. 2002;3(6):463–471. doi: 10.1038/nrn844. [DOI] [PubMed] [Google Scholar]
- 22.Derix J, Iljina O, Schulze-Bonhage A, Aertsen A, Ball T. “Doctor” or “darling”? Decoding the communication partner from ECoG of the anterior temporal lobe during non-experimental, real-life social interaction. Front Hum Neurosci. 2012;6:251. doi: 10.3389/fnhum.2012.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lambon Ralph MA, Sage K, Jones RW, Mayberry EJ. Coherent concepts are computed in the anterior temporal lobes. Proc Natl Acad Sci USA. 2010;107(6):2717–2722. doi: 10.1073/pnas.0907307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasson U, Ghazanfar AA, Galantucci B, Garrod S, Keysers C. Brain-to-brain coupling: A mechanism for creating and sharing a social world. Trends Cogn Sci. 2012;16(2):114–121. doi: 10.1016/j.tics.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pickering MJ, Garrod S. Toward a mechanistic psychology of dialogue. Behav Brain Sci. 2004;27(2):169–190. doi: 10.1017/s0140525x04000056. discussion 190–226. [DOI] [PubMed] [Google Scholar]
- 26.Orban de Xivry JJ, et al. Stimulation of the human motor cortex alters generalization patterns of motor learning. J Neurosci. 2011;31(19):7102–7110. doi: 10.1523/JNEUROSCI.0273-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang J, et al. Neural synchronization during face-to-face communication. J Neurosci. 2012;32(45):16064–16069. doi: 10.1523/JNEUROSCI.2926-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hari R, Himberg T, Nummenmaa L, Hämäläinen M, Parkkonen L. Synchrony of brains and bodies during implicit interpersonal interaction. Trends Cogn Sci. 2013;17(3):105–106. doi: 10.1016/j.tics.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Menenti L, Pickering MJ, Garrod SC. Toward a neural basis of interactive alignment in conversation. Front Hum Neurosci. 2012;6:185. doi: 10.3389/fnhum.2012.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stephens GJ, Silbert LJ, Hasson U. Speaker-listener neural coupling underlies successful communication. Proc Natl Acad Sci USA. 2010;107(32):14425–14430. doi: 10.1073/pnas.1008662107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Lange FP, Spronk M, Willems RM, Toni I, Bekkering H. Complementary systems for understanding action intentions. Curr Biol. 2008;18(6):454–457. doi: 10.1016/j.cub.2008.02.057. [DOI] [PubMed] [Google Scholar]
- 32.Iacoboni M, et al. Grasping the intentions of others with one’s own mirror neuron system. PLoS Biol. 2005;3(3):e79. doi: 10.1371/journal.pbio.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lerner Y, Honey CJ, Silbert LJ, Hasson U. Topographic mapping of a hierarchy of temporal receptive windows using a narrated story. J Neurosci. 2011;31(8):2906–2915. doi: 10.1523/JNEUROSCI.3684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25(3):653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Gross J, et al. Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc Natl Acad Sci USA. 2001;98(2):694–699. doi: 10.1073/pnas.98.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blokpoel M, et al. Recipient design in human communication: Simple heuristics or perspective taking? Front Hum Neurosci. 2012;6:253. doi: 10.3389/fnhum.2012.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volman I, Noordzij ML, Toni I. Sources of variability in human communicative skills. Front Hum Neurosci. 2012;6:310. doi: 10.3389/fnhum.2012.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newman-Norlund SE, et al. Recipient design in tacit communication. Cognition. 2009;111(1):46–54. doi: 10.1016/j.cognition.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 39.van Rooij I, et al. Intentional communication: Computationally easy or difficult? Front Hum Neurosci. 2011;5:52. doi: 10.3389/fnhum.2011.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Price CJ, Friston KJ. Cognitive conjunction: A new approach to brain activation experiments. Neuroimage. 1997;5(4 Pt 1):261–270. doi: 10.1006/nimg.1997.0269. [DOI] [PubMed] [Google Scholar]
- 41.Noordzij ML, et al. Brain mechanisms underlying human communication. Front Hum Neurosci. 2009;3:14. doi: 10.3389/neuro.09.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verhagen L, Dijkerman HC, Medendorp WP, Toni I. Cortical dynamics of sensorimotor integration during grasp planning. J Neurosci. 2012;32(13):4508–4519. doi: 10.1523/JNEUROSCI.5451-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sabbagh MA. Communicative intentions and language: Evidence from right-hemisphere damage and autism. Brain Lang. 1999;70(1):29–69. doi: 10.1006/brln.1999.2139. [DOI] [PubMed] [Google Scholar]
- 44.Milne E, Grafman J. Ventromedial prefrontal cortex lesions in humans eliminate implicit gender stereotyping. J Neurosci. 2001;21(12):RC150. doi: 10.1523/JNEUROSCI.21-12-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Berkum JJ, van den Brink D, Tesink CM, Kos M, Hagoort P. The neural integration of speaker and message. J Cogn Neurosci. 2008;20(4):580–591. doi: 10.1162/jocn.2008.20054. [DOI] [PubMed] [Google Scholar]
- 46.Hasson U, Yang E, Vallines I, Heeger DJ, Rubin N. A hierarchy of temporal receptive windows in human cortex. J Neurosci. 2008;28(10):2539–2550. doi: 10.1523/JNEUROSCI.5487-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buzsáki G, Wang XJ. Mechanisms of gamma oscillations. Annu Rev Neurosci. 2012;35:203–225. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller KJ. Broadband spectral change: Evidence for a macroscale correlate of population firing rate? J Neurosci. 2010;30(19):6477–6479. doi: 10.1523/JNEUROSCI.6401-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller KJ, Sorensen LB, Ojemann JG, den Nijs M. Power-law scaling in the brain surface electric potential. PLOS Comput Biol. 2009;5(12):e1000609. doi: 10.1371/journal.pcbi.1000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manning JR, Jacobs J, Fried I, Kahana MJ. Broadband shifts in local field potential power spectra are correlated with single-neuron spiking in humans. J Neurosci. 2009;29(43):13613–13620. doi: 10.1523/JNEUROSCI.2041-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buzsáki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 2012;13(6):407–420. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: Explanation of the large variability in evoked cortical responses. Science. 1996;273(5283):1868–1871. doi: 10.1126/science.273.5283.1868. [DOI] [PubMed] [Google Scholar]
- 53.Luczak A, Bartho P, Harris KD. Gating of sensory input by spontaneous cortical activity. J Neurosci. 2013;33(4):1684–1695. doi: 10.1523/JNEUROSCI.2928-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Larkum M. A cellular mechanism for cortical associations: An organizing principle for the cerebral cortex. Trends Neurosci. 2013;36(3):141–151. doi: 10.1016/j.tins.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 55.Jarsky T, Roxin A, Kath WL, Spruston N. Conditional dendritic spike propagation following distal synaptic activation of hippocampal CA1 pyramidal neurons. Nat Neurosci. 2005;8(12):1667–1676. doi: 10.1038/nn1599. [DOI] [PubMed] [Google Scholar]
- 56.Behabadi BF, Polsky A, Jadi M, Schiller J, Mel BW. Location-dependent excitatory synaptic interactions in pyramidal neuron dendrites. PLOS Comput Biol. 2012;8(7):e1002599. doi: 10.1371/journal.pcbi.1002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schippers MB, Roebroeck A, Renken R, Nanetti L, Keysers C. Mapping the information flow from one brain to another during gestural communication. Proc Natl Acad Sci USA. 2010;107(20):9388–9393. doi: 10.1073/pnas.1001791107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stolk A, Todorovic A, Schoffelen JM, Oostenveld R. Online and offline tools for head movement compensation in MEG. Neuroimage. 2013;68:39–48. doi: 10.1016/j.neuroimage.2012.11.047. [DOI] [PubMed] [Google Scholar]
- 59.Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164(1):177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.