Abstract
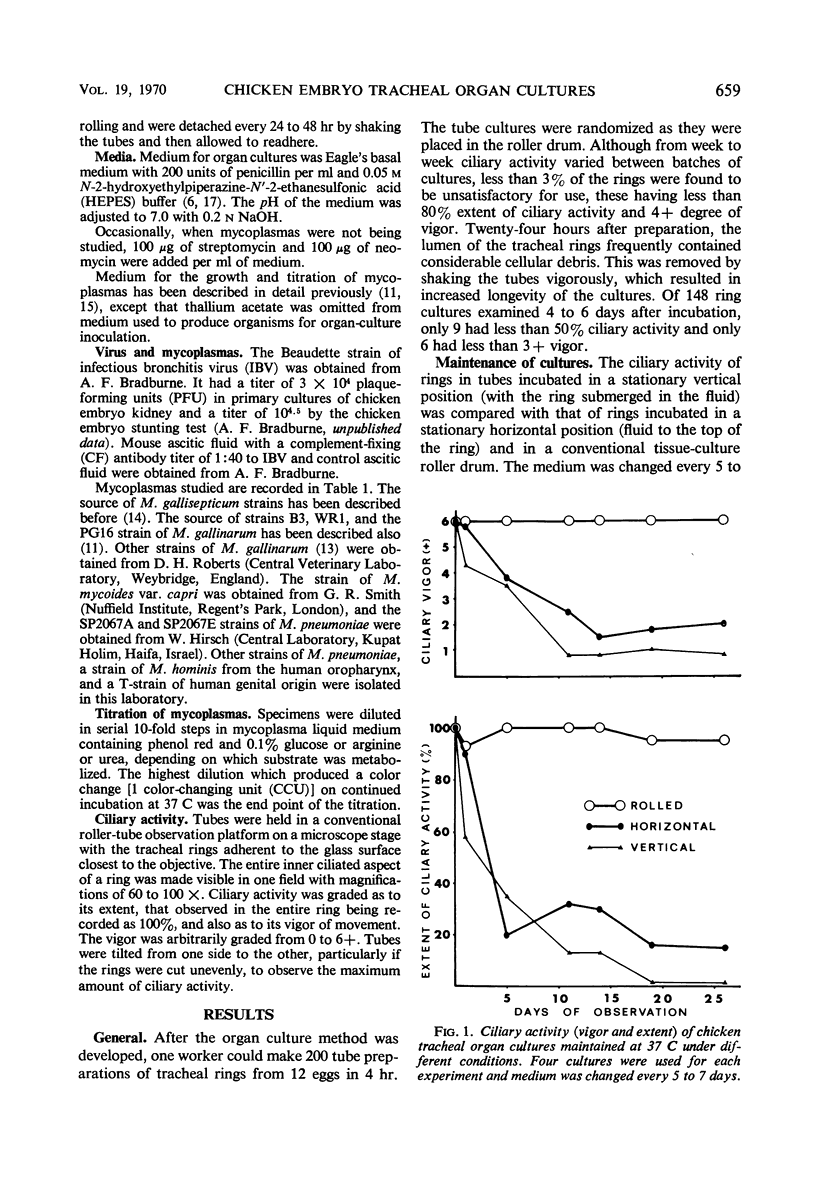
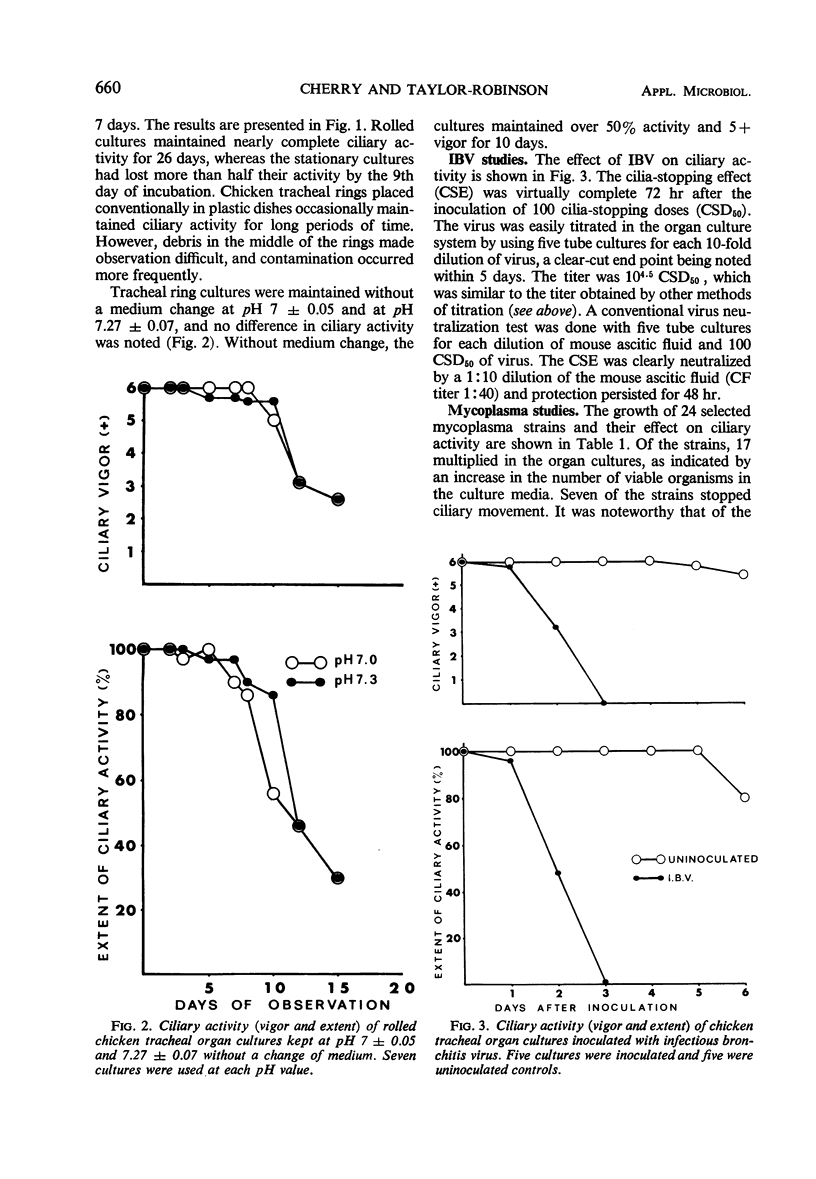
Chicken tracheal organ cultures were made from embryos which were 19 to 20 days old. Transversely cut rings of trachea were placed in screw-capped tissue-culture tubes with Eagle's-N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) medium and incubated in roller drums. The method had advantages over other organ culture systems in that these cultures were prepared in numbers similar to conventional tissue cultures, ciliary activity was quickly and accurately evaluated, and contamination occurred less frequently than with organ cultures in petri dishes. Ciliary activity persisted for at least 1 month when the medium was changed at 5-to 7-day intervals and for 10 to 15 days without a change. Infectious bronchitis virus stopped ciliary movement, and this effect was used as a basis for titrating the virus and for determining the neutralizing capacity of immune mouse ascitic fluid. Twenty-four Mycoplasma strains were tested. Organisms of 17 strains, both avian and mammalian, multiplied in the organ cultures, and 7 strains, belonging to the species M. gallisepticum and M. mycoides var. capri, inhibited ciliary activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler M. Isolation and growth of mycoplasma in human embryo trachea cultures. Nature. 1969 Nov 8;224(5219):605–606. doi: 10.1038/224605a0. [DOI] [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A., Jr, Denny F. W. Biologic effects of Mycoplasma pneumoniae and other mycoplasmas from man on hamster tracheal organ culture. Proc Soc Exp Biol Med. 1969 Dec;132(3):1153–1158. doi: 10.3181/00379727-132-34385. [DOI] [PubMed] [Google Scholar]
- Fisk A., Pathak S. HEPES-buffered medium for organ culture. Nature. 1969 Dec 6;224(5223):1030–1031. doi: 10.1038/2241030a0. [DOI] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Harnett G. B., Hooper W. L. Test-tube organ cultures of ciliated epithelium for the isolation of respiratory viruses. Lancet. 1968 Feb 17;1(7538):339–340. doi: 10.1016/s0140-6736(68)90800-3. [DOI] [PubMed] [Google Scholar]
- Hoorn B., Tyrrell D. A. Organ cultures in virology. Prog Med Virol. 1969;11:408–450. [PubMed] [Google Scholar]
- Johnson R. B., Newman J. A., Wills F. K. Titration of infectious bronchitis virus in tracheas of susceptible and immune chickens. Avian Dis. 1969 Aug;13(3):632–635. [PubMed] [Google Scholar]
- LIU C. Studies on primary atypical pneumonia. I. Localization, isolation, and cultivation of a virus in chick embryos. J Exp Med. 1957 Oct 1;106(4):455–466. doi: 10.1084/jem.106.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchee R. J., Taylor-Robinson D. Haemadsorption and haemagglutination by mycoplasmas. J Gen Microbiol. 1968 Mar;50(3):465–478. doi: 10.1099/00221287-50-3-465. [DOI] [PubMed] [Google Scholar]
- ROBERTS D. H. SEROTYPES OF AVIAN MYCOPLASMA. J Comp Pathol. 1964 Oct;74:447–456. doi: 10.1016/s0368-1742(64)80051-5. [DOI] [PubMed] [Google Scholar]
- Razin S. Mycoplasma taxonomy studiedy electrophoresis of cell proteins. J Bacteriol. 1968 Sep;96(3):687–694. doi: 10.1128/jb.96.3.687-694.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson D., Berry D. M. The evaluation of the metabolic-inhibition technique for the study of Mycoplasma gallisepticum. J Gen Microbiol. 1969 Jan;55(1):127–137. doi: 10.1099/00221287-55-1-127. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson D., Williams M. H., Haig D. A. The isolation and comparative biological and physical characteristics of T-mycoplasmas of cattle. J Gen Microbiol. 1968 Nov;54(1):33–46. doi: 10.1099/00221287-54-1-33. [DOI] [PubMed] [Google Scholar]
- Tyrrell D. A., Bynoe M. L., Hoorn B. Cultivation of "difficult" viruses from patients with common colds. Br Med J. 1968 Mar 9;1(5592):606–610. doi: 10.1136/bmj.1.5592.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. D., Cox P. Use of a new buffer in the culture of animal cells. J Gen Virol. 1968 Mar;2(2):309–312. doi: 10.1099/0022-1317-2-2-309. [DOI] [PubMed] [Google Scholar]



