Abstract
Islet cell transplantation has therapeutic potential to cure type 1 diabetes (T1D), which is characterized by autoimmune-mediated destruction of insulin-producing β cells. However, current success rates are limited by long-term decline in islet graft function resulting partially from poor revascularization and immune destruction. MSCs have the potential to enhance islet transplantation and prevent disease progression by a multifaceted approach. MSCs have been shown to be effective at inhibiting inflammatory-mediated immune responses and at promoting tissue regeneration. The immunomodulatory and tissue repairing properties of MSCs may benefit β cell regeneration in the context of T1D. This review will elucidate how MSCs can minimize β cell damage by providing survival signals and simultaneously modulate the immune response by inhibiting activation and proliferation of several immune cell types. In addition, MSCs can enhance islet graft revascularization, maintaining long-term β cell viability and function.
Keywords: Mesenchymal stem cells, islet transplantation, type 1 diabetes, immunomodulation
I. Introduction
Mesenchymal stem cells (MSCs), also referred to as stromal cells or mesenchymal progenitor cells, are non-hematopoietic multipotent stromal cells that can differentiate into a variety of tissues.[1] Recently, MSCs have been investigated as a treatment for a wide range of diseases due to their capacity for self-renewal and unique immunosuppressive and regenerative properties.[2] For these reasons, MSCs have been studied as therapies in the context of immunopathological disorders such as autoimmune encephalomyelitis (EAE) a model of human multiple sclerosis (MS),[3] arthritis,[4] systemic lupus erythematosus (SLE),[5] Crohn’s disease,[6] graft versus host disease,[7]. In these models, MSCs have been shown to be effective at inhibiting immune inflammation and promoting tissue regeneration. As immunomodulators, MSCs can use both direct and indirect mechanisms to modulate the immune response in settings of inflammation, autoimmune diseases, and transplantation. In addition, the inherent regenerative properties of MSCs have been demonstrated to aid in tissue repair by assisting endogenous cell function and revascularization of damaged tissue. MSC-based therapeutic strategies are now under investigation to overcome the autoimmune destruction of insulinproducing cells in type 1 diabetes (T1D) and the inflammatory mediated beta cell dysfunction and insulin resistance in type 2 diabets (T2D).[8].
T1D is characterized as an autoimmune-mediated destruction of insulin-producing β cells by proinflammatory autoreactive CD4+ and CD8+ T cells.[9] Prior to disease onset, a B cell immune response has taken place producing antibodies to β cell antigens detected in the peripheral blood.[10] These processes together result in reduced and insufficient β cell mass to maintain glucose homeostasis rendering patients dependent on exogenous insulin. In addition to insulin administration, other treatment options currently available to patients with T1D include whole pancreas transplantation and islet transplantation. Islet transplantation, primarily indicated in patients with unstable hypoglycemia, is advantageous compared to whole pancreas transplantation because it is relatively non-invasive. But significant challenges to islet transplantation still remain including: revascularization of the islet cell graft, prevention of inflammation, rejection and autoimmune destruction of the graft, requirement for lifelong immunosuppression which can be harmful to islet β cell function, and lastly the limited supply of donor islets for widespread clinical therapies. In this context, MSC-based therapies may become an alternative option in the prevention of T1D disease onset and may also enhance tolerogenicity and engraftment of allo-islet graft after transplantation.
This review first highlights the capacity of MSCs to modulate the autoimmune response during the pathogenesis of T1D and the allo-immune response in the setting of islet transplantation. Second, this review focuses on the role of MSCs in the repair of β cell mass and function in both T1D and T2D. Lastly, it illustrates the promises and potential obstacles for MSC therapies to become a clinically relevant approach to treatment of insulin dependent diabetes.
II. MSC Characterization
Although rare, MSCs can be isolated from a variety of sources including: bone-marrow, cord blood, dental pulp, adipose tissue, lung, placenta, tendons, synovial fluid, circulating peripheral blood, and fetal liver.[11] MSC isolation and purification was initially performed from bone marrow and defined by the Colony Forming Unit-Fibroblast (CFU-F) assay utilizing the tendency of MSCs to adhere to plastic.[12] Isolation of MSCs from solid tissue (ie fat, placenta) requires a collagenase digestion step. Isolated MSCs may be further purified prior to culture by Fluorescence Activated Cell Sorting (FACS), using monoclonal antibodies against common markers shared by all stromal cell precursors, such as STRO-1.[13–16]
In vitro, MSCs proliferate, display fibroblast-like morphology and are further characterized by their ability to differentiate into bone, cartilage and fat.[17] According to the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy, MSCs are identifiable based upon their ability to adhere to plastic and stain positive for the expression of surface markers CD105, CD73 and CD90.[18] Although there is growing data suggesting that MSCs express additional surface markers, such as CD54, CD44, CD29, CD49, CD71, and CD271, the expression of these markers largely depends on culturing techniques, isolation methods, tissue sources, and species differences.[2, 19, 20]
Historically, MSCs have been considered hypo-immunogenic because of their limited expression of MHC class II and costimulatory molecules and inability to stimulate T cell proliferation. However, MSCs are no longer considered immunologically silent.[21] Costimulation with IFNγ can induce MSCs to express elevated levels of MHC class II, and it has been shown that MSCs express MHC class I and other receptors that interact with immune cells.[2] While MSCs’ immunosuppressive effects are well established, these findings demonstrate the greater complexity and bi-directionality of MSCs’ interaction with the immune system.
III. MSCs and T1D
MSCs-based therapies may play a role in the prevention of T1D disease onset and in islet graft survival and function in the context of islet cell transplantation. MSCs have been shown to prevent and/or delay disease onset [22] and to reduce the infiltration of autoreactive T cells into the pancreas in both T cell-transferred and spontaneous diabetic mouse models.[23] In addition to their immunomodulatory properties (Table 1), MSCs may act as “repair cells” by regulating the expression of key anti-apoptotic and regenerative genes in beta cells, and reverse β cell damage (Table 2). Lastly, MSCs can increase islet graft β cell function by the secretion of trophic factors that can enhance insulin response and graft revascularization, minimizing the loss of transplanted islets during the peritransplant period. We will next discuss evidence for each of these potential mechanisms of MSCs as “helper cells” in the context of T1D.
Table I.
Immunomodulatory Molecules Expressed or Secreted by MSCs in the Context of Type I Diabetes
| Molecule | Target Cell | Effect | Reference |
|---|---|---|---|
| PD-1 | Autoreactive T cells | Binds to PD-L1 and suppresses proliferation by direct cell contact | Jurewicz, M., et al. (2010) |
| IDO | Activated T cells NK cells |
Depletes amino acids and inhibits immune cell proliferation and function | Krampera, M., L. et al. (2006) |
| MMPs | Activated T cells | Reduces T cell responsiveness to IL-2 | Ding, Y.C., et al. (2009) |
| PGE2 | Activated T cells | Inhibits IL-2 production and proliferation by direct action on T cells | Tse, W. T., et al. (2003) |
| DC | Inhibits DC function and secretion of IL-12 and IFN-γ. | Chen, L., Zhang, W., et al. (2007) | |
| NK Cells | Suppresses IL-2–and IL-15–mediated cytotoxicity and cytokine production. | Sotiropoulou, P. A., et al. (2006) | |
| NO | Activated T cells | Inhibits T cell activation and phosphorylation of Stat5 | Sato, K., et al. (2007) |
| TGF-β | Activated T cells | Inhibits proliferation | Di Nicola, M., et al. (2002) |
| Regulatory T cells | Facilitates expansion and generation | Casiraghi, Azzollini et al. (2008) | |
| NK cells | Inhibits IL-2-induced activation of NK proliferation | Spaggiari, G.M., et al. (2006) | |
| IL-6 | DC | Inhibits differentiation and maturation | Djouad, F., et al. (2007) Jiang, X. X., et al. (2005) |
| Regulatory T cells | Increases IL-10 secretion |
Engela, A.U., et al. (2012) Crop, M.J., et al. (2010) |
|
| HGF | Activated T cells | Suppresses proliferation synergistically with TGF-β | Di Nicola, M., et al. (2002) |
| NK cells | Suppresses proliferation, cytokine secretion, and cytotoxicity | Sotiropoulou, P. A., et al. (2006) | |
| Galectins | T cells | Induces apoptosis | Sioud, M. et al. (2011) |
Abbreviations: PD-1, programmed death 1; PD-L1, programmed death ligand 1; IDO, 2,3-dioxygenasel; NK cells, natural killer cells; MMPs, matrix metalloproteinases; IL-2, interleukin-2; PGE2, prostaglandin-E2; DC, dendritic cell; IL-12, interleukin-12; TGF-α, transforming growth factor alpha; IL-15, interleukin-15; NO, nitric oxide; TGF-β, transforming growth factor beta; IL-6, interleukin-6; IL-10, interleukin-10; HGF, hepatocyte growth factor.
A. Immunomodulatory properties of MSCs in vitro
Numerous studies show that MSCs primarily modulate the effector arm of the T cell immune response through the suppression of T cell proliferation and the inhibition of dendritic cell (DC) differentiation. Additionally, MSC may modulate NK cell cytotoxic activity, B cell proliferation, and immunoglobulin production.
1. MSC interaction with immune cells
a. direct cell-cell contact
MSCs can exert immunomodulatory properties on T cells by direct cell-to-cell contact through the engagement of the inhibitory molecule programmed cell death 1 (PD-1) to its ligand PD-L1. In vitro MSCs stimulated with recombinant IFN-γ showed upregulated expression of the surface molecule PD-L1 and suppressed autoreactive T cell proliferation; the suppression was reversed in the presence of a PD-L1 siRNA knockdown.[24] Similarly in vivo, Fiornia et al. showed that PD-L1 expression levels were high on MSCs that migrated to the pancreas of prediabetic NOD mice, delaying the onset of T1D by suppressing the proliferation of autoreactive T cells.[22] These results suggest that in the context of T1D, PD-L1 ligation on the surface of MSCs results in T cell immunosuppression.
b. secretion of soluble immunomodulatory molecules
MSCs can suppress immune cell proliferation by a second mechanism the secretion of soluble molecules. In mixed lymphocyte reaction (MLR) cultures, MSCs down-regulated alloreactive T cell proliferation through soluble factors including, 2,3-dioxygenase (IDO), prostaglandin-E2 (PGE2), nitric oxide, and transforming growth factor-β (TGF-β) [25, 26].
TGF-β, IL-10 and HGF are the most commonly described MSC-secreted cytokines mediating suppression of T cell activation and proliferation both in vitro and in vivo.[27–29] TGF-β secretion was further increased in the presence of IFN-γ, supporting the notion that inflammatory signals enhance MSCs immunosuppressive activity [29, 30]. In the context of T1D, serum levels of TGF-β and IL-10 were increased in intravenous MSC-treated NOD mice.[31] β cell-specific T cells harvested from diabetic NOD mice also showed decreased proliferation in vitro in the presence of MSCs, and the effect was reversed by neutralization of TGF-β.[31]
PGE2 is constitutively expressed and secreted by MSCs and may be associated with their immune suppressive effect in an MLR setting.[32] Whether the immune suppressive effects of MSCs are mediated by PGE2 has been debated; however, mounting evidence suggests a strong role of PGE2 in inhibition of T cell proliferation by MSCs, [33–36] and the contradictory evidence [32] may be due to differences in isolation techniques[36]. A recent study by Duffy, et al. established that MSCs inhibit Th17 cell proliferation and differentiation from naïve and memory precursors through PGE2 via the EP4 receptor.[37] In addition to the inhibition of T cell proliferation, MSCs inhibit DC differentiation and reduce DCs ability to produce IL-12 and IFN-γ.[35]
MSCs also secrete IDO, a tryptophan-catabolizing enzyme that causes amino acid depletion and the inhibition of proliferation and function of immune cells. MSCs do not constitutively express IDO; however, IFN-γ triggered by paracrine signaling induces upregulation of IDO expression by MSCs. [38] This upregulation of IDO expression has been shown to be partially responsible for MSCs suppression of T cell proliferation.[39]
Similarly, nitric oxide is not constitutively expressed by MSCs but is induced and secreted upon direct cell contact with activated T cells. [40] NO inhibits T cell proliferation by reducing phosphorylation of the tyrosine residues of the Stat5 transcription factor.[41]. In this way, NO blocks the Jak3/Stat5 signaling pathway, which locks the T cells in G0/G1 phase.[42]
Matrix metalloproteinases (MMPs) secreted by MSCs may also play a role in inhibiting T cell proliferation. In vitro, MMP-2 and MMP-9 cleave CD25 from T cell surfaces rendering them unresponsive to IL-2 and thus impeding activation and expansion of alloreactive T cells.[43] In vivo, islet co-transplanted with MSCs into a diabetic mouse model showed rapid reversal of hyperglycemia, however MMP inhibitor administration overturned the protective effects afforded by the MSCs and resulted in islet graft failure [43].
MSCs also secrete galectins, carbohydrate-binding proteins that contribute to MSCs immunsuppressive effects by inducing T cell apoptosis and interfering with immune cells activation and secretory function.[44] Galectin-1 induces T cell apoptosis by binding to CD45, CD43, and CD7,[45] and galectin-3 by binding to either CD7 and CD29 or CD45 and CD71.[46] The secretion of galectins by MSCs affects T cell development and activation, apoptosis, cytokine secretion and regulatory T cell function.[47]
MSCs also have a suppressive effect on B lymphocyte proliferation, chemotactic behavior, and immunoglobulin production. Soluble factors involved in B lymphocyte inhibition are secreted by MSCs upon a paracrine signal from B cells, as shown in a transwell assay by Corcione et al. [48]. As well, MSCs interact with NK cells through a combination of direct cell-to-cell contact and soluble molecules (ie TGF-ß, PGE2). The MSC-NK cell interaction has immunosuppressive effects resulting in decreased NK cell proliferation, IFN-γ production, and cytotoxicity activity. [49]
2. MSC interaction with dendritic cells (DC)
a. DC maturation
DC and macrophage infiltration initiates general immune responses and perinsulitis of the pancreas in which β cell mass is decreased. MSCs have suppressive effects both on the maturation and function of DCs, suggesting that MSCs may be beneficial in an islet-MSC co-transplant setting by protecting the β cells from DC infiltration. MSCs may suppress the generation of inflammatory DCs through IL-6 secretion.[24]. In vitro, MSCs have been shown to inhibit the differentiation of monocytes into immature DC.[21, 50, 51] In the context of islet transplantation, DC isolated from allo-islet-MSC co-transplant recipients showed inhibited maturation, impaired antigen-presentation capabilities, and suppressed IL-12 secretion, which plays an important role in DC maturation and function.[52] In addition to inhibiting DC maturation, MSCs may modulate the secretory function of DCs. Co-culture of DCs and MSCs resulted in changes in the cytokine secretion profile of DC with upregulated expression of regulatory cytokines (IL-10) and reduced expression of inflammatory cytokines (TNF-α, IFN-γ, IL-12).[51, 53, 54]
b. DC migration
In the islet allograft setting, co-administration of MSCs may also enhance graft survival by minimizing DC migration. DCs are involved in initiating cell-mediated immunity by migrating to local lymph nodes and presenting allo-islet antigens to the T cells. DC migration is controlled in part by upregulation of CCR7, a chemokine receptor important for migration to lymph nodes, and downregulation of tissue anchoring proteins such as E-cadherin.[55] English et al. demonstrated in vitro, that co-culture of DC and MSCs resulted in low CCR7 expression on DC and decreased DC migration in response to CCL19 (chemokine for CCR7).[55] Thus the co-localization of MSCs with an islet graft may reduce DC migration and recruitment to the peripheral draining lymph nodes, limiting allogeneic antigen presentation and immune rejection.
3. MSC expand regulatory T cells
MSCs are known to induce regulatory T cell expansion both in vitro and in vivo.[34, 56] The differentiation and generation of regulatory T cells is dependent in part on TGF-β [57, 58]. TGF-β gene therapy was shown to enhance β cell function in diabetic NOD mice, [59–61] and this protection may be partially attributed to the effect of TGF-β on generation of regulatory T cells. MSCs administered into a rat model of streptozotocin-induced β cell injury also shifted peripheral T cells toward a Th2 phenotype with IL-10/IL-13 production and higher frequencies of CD4+/CD8+ Foxp3+ regulatory T cells.[62] The MSC-mediated upregulation of IL-10 secretion [63, 64] may facilitate T cell differentiation toward a tolerogenic regulatory T cell phenotype. [65]
In an NOD T1D model Fiornia et al. observed only a marginal increase in regulatory T cells in the pancreatic draining lymph nodes and no significant increase in these cells in the spleens of NOD mice treated with MSCs compared to control NOD mice.[22] The MSC-mediated shift toward the generation of regulatory T cells is more consistently confirmed when MSCs are co-administered with the islets rather than injected systemically and allowed to migrate to the β cells. This is supported by the finding that diabetic non-human primate recipients presented with increased numbers of regulatory T cells in their peripheral blood following allo-islet-MSC co-transplantation. [66]
Similarly, Wood demonstrated MSCs ability to modulate immune cells in a mouse islet allograft model in which MSCs, co-localized with the islets, prevented islet allo-graft rejection. Additionally, T cells isolated from the spleen of islet-MSC co-transplant mouse recipients showed low levels of IFN-γ and TNF-α secretion upon ex-vivo activation compared to T cells isolated from islet alone transplant recipients.[67]. The mechanism of MSCs’ ability to regulate the cytokine profile of inflammatory cells remains under investigation as the suppression of T cell responses either at T1D onset or after islet transplantation will be important to protect β cell mass and function.
B. Regenerative and repairing properties of MSCs as a therapeutic approach for T1D treatment
1. Migration to site of injury
MSCs selectively migrate to sites of injury and participate in repair as shown in lung injury[68] and myocardial infarction.[69] In vitro, Lin et al. used a microfluidic device to show that freshly isolated islets secrete attractant signals that encourage MSC migration towards them, resulting in improved islet cell survival and function.[70] In T1D MSCs may use their migratory properties to localize at the site of islet cell damage and aid in cellular repair as shown by Prockop et al.[71] Similarly, in a streptozotocin-induced β cell injury rodent model, MSCs injected intravenously appeared in the pancreas within 7 days and reversed hyperglycemia.[62] These observations suggest that in vivo recruitment of MSCs to injured pancreatic islets might contribute in β cell repair. Some of the functional chemokine receptor/ligand pairs involved in MSC migration were identified as CX3CL1-CX3CR1 and CXCL12-CXCR4.[72]
2. Supporting ß cellular regeneration and function at the site of injury
Once the MSCs have reached the site of islet cell injury, they may aid in islet regeneration, as shown in experimental animal models. Human MSCs injected into NOD-SCID mice reduced hyperglycemia by increasing pancreatic islet β cell mass.[71, 73] Furthermore, Lee et al. found that new islets formed off of pancreatic ducts, suggesting that MSCs promote islet regeneration.[71] As well, Ezquer et al. observed a significantly increased β cell mass in streptozotocin-induced diabetic mice treated with a single injection of MSCs compared to non-treated animals.[74] Although MSC-treated recipients did not perform better against a high glucose challenge, the increased number of insulin-producing cells suggests that MSC treatment contributes to newly regenerated β cells through induced proliferation and differentiation of endogenous progenitors.
Another potential mechanism for MSCs therapeutic effect in T1D is the modulation of islet gene expression. Islets co-cultured with MSCs showed increased β cell expression of anti-apoptotic signaling molecules, XIAP,[75, 76] Bcl-2, and Bcl-xL.[77] In addition, islets isolated from streptozotocin-induced diabetic animals treated with MSCs expressed high levels of PDX-1, a transcription factor that regulates insulin gene expression and plays a role in pancreatic development and differentiation.[62] The expression and activation of PDX-1 can potentially stimulate growth, survival, and differentiation of β cells, resulting in enhanced β cell function.
MSCs secrete many bioactive growth factors and cytokines with paracrine and autocrine activities that may be responsible for the observed increase in expression of PDX-1 and anti-apoptotic molecules.[78] Freshly isolated islets were susceptible to β cell loss from apoptosis, but when co-cultured with cord-blood derived MSCs, these islets showed improved viability and function.[79] This enhanced viability and function was due to anti-apoptotic proteins and active trophic agents secreted by the MSCs, [79] such as HO-1,[80] IL-6, hepatocyte growth factor (HGF), and SDF-1.[62, 79] IL-6 may induce expression of β cell anti-apoptotic signaling molecules Bcl-2 and Bcl-xL.[81–84] HGF, a β cell growth factor, [85] may prevent primary nonfunction in islet grafts by inhibiting apoptosis, inducing β cell proliferation [86], and improving β cell insulin response to high glucose.[87] SDF-1 is involved in islet generation and differentiation from endocrine stem cells within the pancreas [88] and enhances the β cell regeneration potential provided by HGF. The secretion of trophic molecules may be a key mechanism in the ability of MSCs to minimize β cell loss during T1D onset and protect islet cell engraftment after transplantation.
3. Angiogenesis
MSCs secrete angiogenic and arteriogenic cytokines including vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), angiopoietin-1 (Ang-1), and transforming growth factor–β (TGF-β). While each of these signaling proteins plays a role in angiogenesis, MSCs may promote islet vascularization primarily through VEGF secretion.[89] Increased VEGF expression at the islet transplant site significantly increased graft survival and function.[90] Similarly, a marginal islet cell mass that reversed hyperglycemia within 2 weeks in mice co-transplanted with islets and MSCs showed enhanced expression of VEGF.[91] In diabetic rodent models the co-transplantation of MSCs with pancreatic islets improves graft function significantly through islet remodeling and revascularization mediated by the MSCs.[92, 93]
Johansson et al. utilized MSCs to enhance islet revascularization by co-culturing MSCs and endothelial cells with islets in vitro.[94] The MSCs may promote islet engraftment by initiating the formation of vessel-like structures through secretion of proteases that degrade the islet extracellular matrix and allow the migration of endothelial cells into the islet. The ability for MSCs to encourage revascularization might allow for exploration of alternative sites for islet transplantation. Currently, the preferred transplantation site is the portal vein of the liver, which is not optimal due to venous hypoxemia and the potential risk of thrombosis.[95] Islet transplantation in the subcutaneous site would be more accessible and minimally invasive; however, the lack of early vascularization of the graft may result in loss of function and inability to restore normoglycemia due to poor graft oxygen supply. [96] [97–99]
An emerging therapeutic strategy involves the use of biomaterials to encapsulate islets and overcome these obstacles. Biomaterials may enhance islet function by providing a three-dimensional cellular support and delivering proteins, growth factors, and immunosuppressive agents [100, 101]. Some approaches are focused on islet encapsulation platforms with prevascularization of the device prior to islet implantation.[102] Our laboratory is developing a silk hydrogel-based biomaterial in which islets are encapsulated with ECM proteins (laminin and collagen IV) and bone marrow-derived MSCs to enhance islet cell graft revascularization survival and function (Figure 1). The use of a biomaterial-based approach in MSC-islet co-transplantation aims to reestablish the islet microenvironment, enhance islet function and provide the protective and angiogenic effects of MSC therapy.
Figure 1. Silk hydrogel-based biomaterial.
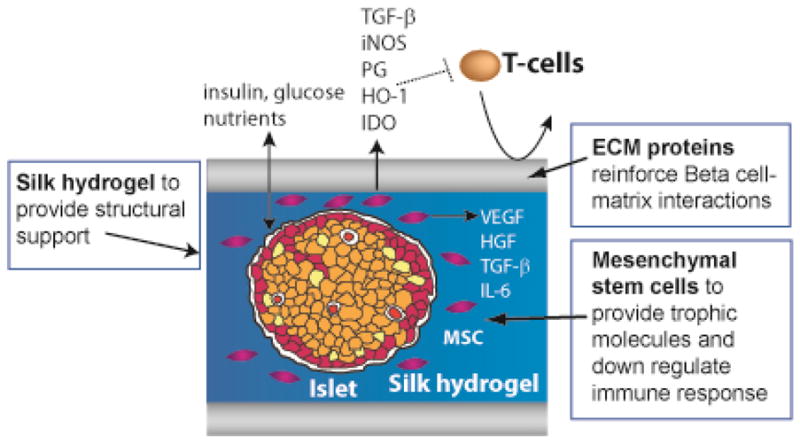
Islets are co-encapsulated in a silk hydrogel with extracellular matrix proteins (ECM) and with bone marrow-derived mesenschymal stem cells (MSC) to enhance islet cell graft revascularization survival and function.
IV. MSC and T2D
T2D is characterized clinically by uncontrolled hyperglycemia resulting from both insulin resistance and pancreatic beta-cell dysfunction[103]. Interestingly, inflammation seems to play a role in impairing beta cell insulin response to high glucose.[104]. Thus the immunomodulatory properties of MSCs may also be beneficial for the treatment of patients with T2D.[8] Recent randomized clinical trials in T2D suggest a beneficial effect of bone marrow–mononuclear cells containing MSCs on glycemic control with reduction in Hemoglobin A1C levels following intra arterial infusion into the vasculature of the pancreas. Thus B cell function is most likely improved as a result of the MSC infusion.
But a recent study by Si et al elucidates the mechanisms through which MSCs may improve insulin sensitivity in a rat model of T2D. [105] including promoting β-cell function, improving insulin sensitivity possibly by upregulating GLUT4 expression, and elevating phosphorylated IRS-1 and Akt levels in insulin target tissues. Specifically MSCs were able to restore the expression of total GLUT4 protein peripheral tissues (skeletal muscle, adipose tissue, and liver) through an insulin-independent pathway[105]…..
V. Future Challenges
MSCs have been studied as therapies in many immunopathological disorders, including: autoimmune encephalomyelitis, a model of human multiple sclerosis, arthritis, systemic lupus erythematosus, Crohn’s disease, graft-versus-host-disease, and T1D.[2] MSCs are now in clinical trials as therapies for more than 30 indications (http://clinicaltrials.gov). But some key issues need to be addressed before MSC based therapies become a safe and viable option.
Most importantly, the standardization of MSC isolation, characterization and culture in vitro needs to be addressed as MSC characteristics may vary according to culture conditions and passage number. [25] Even though cultured MSCs show progressive senescence and growth arrest without tumor transformation, [106] these may still acquire genetic abnormality and become tumorigenic in vivo.[107] In the context of islet transplantation, it is unclear if co-transplanted MSCs engraft and differentiate at the implantation site. Thus the long-term stability of MSC activity and function in vivo after transplantation needs to be assessed and safety criteria need to be defined prior to transplantation.
The majority of research to date has focused on bone marrow-derived MSCs, but MSCs derived from other tissue sources, such as umbilical cord and adipose, may also have immunomodulatory properties. [108, 109] The regenerative and immunomodulatory properties of MSCs most likely will vary with the tissue source. For example, adipose-derived MSCs show a greater angiogenic potential than bone marrow-derived MSCs in a prevascularized biomaterial implanted in the subcutaneous tissue of diabetic rats [110]. In addition, the question of appropriate donor source needs to be clarified on whether autologous or allogeneic MSCs should be used. The ability to transplant autologous MSCs, if viable, may be advantageous to further prevent an alloimmune response against MSCs.[21] But in the case of T1D, autologous MSCs are less functional and of less therapeutic value, as observed with allogeneic rather than autologous MSCs reversing hyperglycemia in an NOD mouse model.[22] Thus the criteria for choosing a specific tissue and/or donor MSC source may differ with the indication and whether the treatment is aimed at modulating the autoimmune disease or enhancing pancreatic islet engraftment and vascularization.
VI. Conclusions
MSC have the potential to aid in the treatment of T1D and overcome some of the current limitations to islet transplantation. The immunomodulatory properties of MSCs may assist in reducing inflammatory damage to the islets in the early peri-transplant period. MSCs may also attenuate autoimmunity through their immunomodulatory properties while secreting regulatory cytokines to control autoreactive T cells and alloreactive effector CD4+ and CD8+ T cells. Thus MSCs may be a viable alternative to harmful immunosuppressive drugs that can damage islets. The ability of MSCs to secrete trophic and angiogenic factors may also prevent early islet damage and assist in engraftment. Together, MSCs may potentially establish a microenvironment that stimulates growth, survival and differentiation of β cells and minimizes apoptosis and necrosis. The multiple beneficiary roles that MSCs play could help in alleviating donor shortages by reducing the number of islets needed per transplant, decreasing early islet cell death and maintaining longterm graft function.
Table II.
Chemoattractants and Regenerative Molecules Support β Cell Repair and Function
| Regenerative and Repairing Properties | Chemoattractant Factor (cell of origin) | Target Receptor/Cell type/Effect | Reference |
|---|---|---|---|
| Migration to sites of injury | CX3CL-1 (β cell) | CX3CR1/MSC/Induces migratory activity. | Sordi, V., et al. (2005) |
| CXCL12 (intra islet endothelial cell) | CXCR4/MSC/Attracts MSCs to islets | ||
| Beta-cell Repair and Protection | Protein/Cytokine secreted by MSCs | Target Molecule or Receptor/Effect | Reference |
| Anti-apoptosis | HO-1 | Free Heme/counteracts inflammatory reactions of heme metabolites | Lu, Y., et al (2010) |
| IL-6 | XIAP/inhibits caspase-3 and caspase-9 to interrupt the apoptosis pathway |
Lu, S., et al. (2011) Caja, L., et al. (2011) |
|
| Bcl-2/modulates Bax and down regulates apoptosis |
Lu, S., et al. (2011) Park, K. S., et al. (2009) |
||
| Bcl-xL/interacts with Raf-1 and prevents apoptosis. | |||
| β cell function | Unknown | PDX-1/β cell differentiation and function | Boumaza, I., et al. (2009) |
| HGF | HGF/SF receptor (c-met proto-oncogene product)/β cell growth and insulinotropic factor that inhibits apoptosis | Nakano, M., et al. (2000) | |
| Angiogenesis | VEGF | VEGFR1 and VEGFR2/initiates formation of vessel-like structures and increases vascularization. |
Park, K.S. et al. (2010) Figliuzzi, M., et al. (2009) |
| TGF-β | TGF-β/modulates angiogenic processes via vascular cell receptors ALK-1 and AKL-5 and enhances VEGF synthesis | Park, K., et al. (2009) |
Abbreviations: CX3CL-1, chemokine (C-X3-C motif) ligand 1; CX3CR1, CX3C chemokine receptor 1; CXCL12, CXCL12 chemokine (C-X-C motif) ligand 12; CXCR4, C-X-C chemokine receptor type 4; HO-1, heme oxygenase-1; IL-6, interleukin-6; XIAP, X-linked inhibitor of apoptosis protein; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2–associated X protein; Bcl-xL, B-cell lymphoma-extra large; PDX-1, pancreatic and duodenal homeobox 1; HGF/SF, hepatocyte growth factor/scatter factor; VEGF, vascular endothelial growth factor; VEGFR1, vascular endothelial growth factor receptor 1; VEGFR2, vascular endothelial growth factor receptor 2; TGF-β, transforming growth factor-beta; ALK-1, activin receptor-like kinase 1; ALK-5, activin receptor-like kinase 5
Contributor Information
Nicolynn E Davis, Email: nicolynn.davis@gmail.com, Department of Pathology Stanford University School of Medicine.
Diana Hamilton, Email: dianah1@stanford.edu, Department of Pathology Stanford University School of Medicine.
Magali J Fontaine, Email: magalif@stanford.edu, Department of Pathology Stanford University School of Medicine.
References
- 1.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nature Reviews Immunology. 2008;8(9):726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 3.Rafei M, et al. Mesenchymal Stromal Cells Ameliorate Experimental Autoimmune Encephalomyelitis by Inhibiting CD4 Th17 T Cells in a CC Chemokine Ligand 2-Dependent Manner. The Journal of Immunology. 2009;182(10):5994–6002. doi: 10.4049/jimmunol.0803962. [DOI] [PubMed] [Google Scholar]
- 4.Papadopoulou A, et al. Mesenchymal stem cells are conditionally therapeutic in preclinical models of rheumatoid arthritis. Annals of the Rheumatic Diseases. 2012 doi: 10.1136/annrheumdis-2011-200985. [DOI] [PubMed] [Google Scholar]
- 5.Choi EW, et al. Reversal of serologic, immunologic, and histologic dysfunction in mice with systemic lupus erythematosus by long-term serial adipose tissue–derived mesenchymal stem cell transplantation. Arthritis & Rheumatism. 2012;64(1):243–253. doi: 10.1002/art.33313. [DOI] [PubMed] [Google Scholar]
- 6.Ciccocioppo R, et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn’s disease. Gut. 2011;60(6):788–798. doi: 10.1136/gut.2010.214841. [DOI] [PubMed] [Google Scholar]
- 7.Aksu AE, et al. Co-infusion of donor bone marrow with host mesenchymal stem cells treats GVHD and promotes vascularized skin allograft survival in rats. Clinical Immunology. 2008;127(3):348–358. doi: 10.1016/j.clim.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Fotino C, et al. Bone marrow-derived stem cell transplantation for the treatment of insulin-dependent diabetes. Rev Diabet Stud. 2010;7(2):144–57. doi: 10.1900/RDS.2010.7.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358(9277):221–229. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- 10.Schlosser M, et al. In insulin-autoantibody-positive children from the general population, antibody affinity identifies those at high and low risk. Diabetologia. 2005;48(9):1830–1832. doi: 10.1007/s00125-005-1864-6. [DOI] [PubMed] [Google Scholar]
- 11.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. Journal of Cell Science. 2006;119(11):2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 12.Friedenstein AJ, Gorskaja UF, Kulagina NN. Fibroblasts precursors in normal and irradiated mouse hematopoietic organs. Experimental Hematology. 1976;4(5):267–274. [PubMed] [Google Scholar]
- 13.Sakaguchi Y, et al. Suspended cells from trabecular bone by collagenase digestion become virtually identical to mesenchymal stem cells obtained from marrow aspirates. Blood. 2004;104(9):2728–2735. doi: 10.1182/blood-2003-12-4452. [DOI] [PubMed] [Google Scholar]
- 14.Gronthos S, Zannettino ACW. A method to isolate and purify human bone marrow stromal stem cells. In: Prockop DJ, Phinney DG, Bunnell BA, editors. Methods in Molecular Biology. Humana Press Inc; 2008. pp. 45–57. [DOI] [PubMed] [Google Scholar]
- 15.Walsh S, et al. Expression of the developmental markers STRO-1 and alkaline phosphatase in cultures of human marrow stromal cells: Regulation by fibroblast growth factor (FGF)-2 and relationship to the expression of FGF receptors 1–4. Bone. 2000;27(2):185–195. doi: 10.1016/s8756-3282(00)00319-7. [DOI] [PubMed] [Google Scholar]
- 16.Ning H, et al. Mesenchymal stem cell marker Stro-1 is a 75kd endothelial antigen. Biochemical and Biophysical Research Communications. 2011;413(2):353–357. doi: 10.1016/j.bbrc.2011.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horwitz EM, et al. Clarification of the nomenclature for MSC: The international society for cellular therapy position statement. Cytotherapy. 2005;7(5):393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 18.Dominici M, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 19.Chamberlain G, et al. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25(11):2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 20.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110(10):3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 21.Nauta AJ, et al. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108(6):2114–2120. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiorina P, et al. Immunomodulatory Function of Bone Marrow-Derived Mesenchymal Stem Cells in Experimental Autoimmune Type 1 Diabetes. Journal of Immunology. 2009;183(2):993–1004. doi: 10.4049/jimmunol.0900803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madec AM, et al. Mesenchymal stem cells protect NOD mice from diabetes by inducing regulatory T cells. Diabetologia. 2009;52(7):1391–1399. doi: 10.1007/s00125-009-1374-z. [DOI] [PubMed] [Google Scholar]
- 24.Jurewicz M, et al. Congenic Mesenchymal Stem Cell Therapy Reverses Hyperglycemia in Experimental Type 1 Diabetes. Diabetes. 2010;59(12):3139–3147. doi: 10.2337/db10-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdi R, et al. Immunomodulation by mesenchymal stem cells - A potential therapeutic strategy for type 1 diabetes. Diabetes. 2008;57(7):1759–1767. doi: 10.2337/db08-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yagi H, et al. Mesenchymal Stem Cells: Mechanisms of Immunomodulation and Homing. Cell Transplantation. 2010;19(6–7):667–679. doi: 10.3727/096368910X508762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartholomew A, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Experimental Hematology. 2002;30(1):42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 28.Le Blanc K, et al. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scandinavian Journal of Immunology. 2003;57(1):11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 29.Di Nicola M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 30.Ryan JM, et al. Interferon-γ does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clinical & Experimental Immunology. 2007;149(2):353–363. doi: 10.1111/j.1365-2249.2007.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao W, et al. TGF-beta expression by allogeneic bone marrow stromal cells ameliorates diabetes in NOD mice through modulating the distribution of CD4+ T cell subsets. Cellular Immunology. 2008;253(1–2):23–30. doi: 10.1016/j.cellimm.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Tse WT, et al. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: Implications in transplantation. Transplantation. 2003;75(3):389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 33.Yanez R, et al. Prostaglandin E2 plays a key role in the immunosuppressive properties of adipose and bone marrow tissue-derived mesenchymal stromal cells. Exp Cell Res. 2010;316(19):3109–23. doi: 10.1016/j.yexcr.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 35.Chen L, et al. Effects of human mesenchymal stem cells on the differentiation of dendritic cells from CD34+ cells. Stem Cells Dev. 2007;16(5):719–31. doi: 10.1089/scd.2007.0065. [DOI] [PubMed] [Google Scholar]
- 36.English K, et al. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009;156(1):149–60. doi: 10.1111/j.1365-2249.2009.03874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duffy MM, et al. Mesenchymal stem cell inhibition of T-helper 17 cell- differentiation is triggered by cell-cell contact and mediated by prostaglandin E2 via the EP4 receptor. Eur J Immunol. 2011;41(10):2840–51. doi: 10.1002/eji.201141499. [DOI] [PubMed] [Google Scholar]
- 38.Krampera M, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24(2):386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 39.Meisel R, et al. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3- dioxygenase†“mediated tryptophan degradation. Blood. 2004;103(12):4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 40.Sato K, et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109(1):228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 41.Bingisser RM, et al. Macrophage-derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. Journal of Immunology. 1998;160(12):5729–5734. [PubMed] [Google Scholar]
- 42.Glennie S, et al. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105(7):2821–7. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 43.Ding YC, et al. Mesenchymal Stem Cells Prevent the Rejection of Fully Allogenic Islet Grafts by the Immunosuppressive Activity of Matrix Metalloproteinase-2 and-9. Diabetes. 2009;58(8):1797–1806. doi: 10.2337/db09-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sioud M. New insights into mesenchymal stromal cell-mediated T-cell suppression through galectins. Scand J Immunol. 2011;73(2):79–84. doi: 10.1111/j.1365-3083.2010.02491.x. [DOI] [PubMed] [Google Scholar]
- 45.Perillo NL, et al. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378(6558):736–9. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- 46.Hsu DK, Chen HY, Liu FT. Galectin-3 regulates T-cell functions. Immunol Rev. 2009;230(1):114–27. doi: 10.1111/j.1600-065X.2009.00798.x. [DOI] [PubMed] [Google Scholar]
- 47.Sioud M, et al. Mesenchymal stem cell-mediated T cell suppression occurs through secreted galectins. Int J Oncol. 2011;38(2):385–90. doi: 10.3892/ijo.2010.869. [DOI] [PubMed] [Google Scholar]
- 48.Corcione A, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107(1):367–72. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 49.Sotiropoulou PA, et al. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24(1):74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 50.Jiang XX, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105(10):4120–6. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 51.Nauta AJ, et al. Mesenchymal stem cells inhibit generation and function of both CD34(+)-derived and monocyte-derived dendritic cells. Journal of Immunology. 2006;177(4):2080–2087. doi: 10.4049/jimmunol.177.4.2080. [DOI] [PubMed] [Google Scholar]
- 52.Li FR, et al. Immune modulation of co-transplantation mesenchymal stem cells with islet on T and dendritic cells. Clinical and Experimental Immunology. 2010;161(2):357–363. doi: 10.1111/j.1365-2249.2010.04178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ryan JM, et al. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond) 2005;2:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beyth S, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105(5):2214–9. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 55.English K, Barry FP, Mahon BP. Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation. Immunol Lett. 2008;115(1):50–8. doi: 10.1016/j.imlet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 56.Augello A, et al. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis and Rheumatism. 2007;56(4):1175–1186. doi: 10.1002/art.22511. [DOI] [PubMed] [Google Scholar]
- 57.Yamazaki S, et al. Dendritic cells are specialized accessory cells along with TGF-beta for the differentiation of Foxp3(+) CD4(+) regulatory T cells from peripheral Foxp3(−) precursors. Blood. 2007;110(13):4293–4302. doi: 10.1182/blood-2007-05-088831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peng YF, et al. TGF-beta regulates in vivo expansion of Foxp3-expressing CD4(+)CD25(+) regulatory T cells responsible for protection against diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(13):4572–4577. doi: 10.1073/pnas.0400810101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tarbell KV, et al. Dendritic cell-expanded, islet-specific CD4(+) CD25(+) CD62L(+) regulatory T cells restore normoglycemia in diabetic NOD mice. Journal of Experimental Medicine. 2007;204(1):191–201. doi: 10.1084/jem.20061631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tonkin DR, et al. Regulatory T cells prevent transfer of type 1 diabetes in NOD mice only when their antigen is present in vivo. Journal of Immunology. 2008;181(7):4516–4522. doi: 10.4049/jimmunol.181.7.4516. [DOI] [PubMed] [Google Scholar]
- 61.Luo X, et al. Systemic transforming growth factor-beta I gene therapy induces Foxp3+regulatory cells, restores self-tolerance, and facilitates regeneration of beta cell function in overtly diabetic nonobese diabetic mice. Transplantation (Hagerstown) 2005;79(9):1091–1096. doi: 10.1097/01.tp.0000161223.54452.a2. [DOI] [PubMed] [Google Scholar]
- 62.Boumaza I, et al. Autologous bone marrow-derived rat mesenchymal stem cells promote PDX-1 and insulin expression in the islets, alter T cell cytokine pattern and preserve regulatory T cells in the periphery and induce sustained normoglycemia. Journal of Autoimmunity. 2009;32(1):33–42. doi: 10.1016/j.jaut.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 63.Engela AU, et al. Interaction between adipose-tissue derived mesenchymal stem cells and regulatory T cells. Cell Transplant. 2012 doi: 10.3727/096368912X636984. [DOI] [PubMed] [Google Scholar]
- 64.Crop MJ, et al. Human adipose tissue-derived mesenchymal stem cells induce explosive T-cell proliferation. Stem Cells Dev. 2010;19(12):1843–53. doi: 10.1089/scd.2009.0368. [DOI] [PubMed] [Google Scholar]
- 65.Casiraghi F, et al. Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells. Journal of Immunology. 2008;181(6):3933–3946. doi: 10.4049/jimmunol.181.6.3933. [DOI] [PubMed] [Google Scholar]
- 66.Berman DM, et al. Mesenchymal Stem Cells Enhance Allogeneic Islet Engraftment in Nonhuman Primates. Diabetes. 2010;59(10):2558–2568. doi: 10.2337/db10-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Solari MG, et al. Marginal mass islet transplantation with autologous mesenchymal stem cells promotes longterm islet allograft survival and sustained normoglycemia. Journal of Autoimmunity. 2009;32(2):116–124. doi: 10.1016/j.jaut.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 68.Ortiz LA, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(14):8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barbash IM, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium - Feasibility, cell migration, and body distribution. Circulation. 2003;108(7):863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 70.Lin P, et al. Dynamic analysis of Bone Marrow Mesenchymal Stem Cells migrating to Pancreatic Islets using coculture Microfluidic Chips: An accelerated migrating rate and better survival of Pancreatic Islets were revealed. Neuroendocrinology Letters. 2009;30(2):204–208. [PubMed] [Google Scholar]
- 71.Lee RH, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(46):17438–17443. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sordi V, et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005;106(2):419–427. doi: 10.1182/blood-2004-09-3507. [DOI] [PubMed] [Google Scholar]
- 73.Bell GI, et al. Transplanted human bone marrow progenitor subtypes stimulate endogenous islet regeneration and revascularization. Stem Cells Dev. 2012;21(1):97–109. doi: 10.1089/scd.2010.0583. [DOI] [PubMed] [Google Scholar]
- 74.Ezquer FE, et al. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type I diabetic mice. Biology of Blood and Marrow Transplantation. 2008;14(6):631–640. doi: 10.1016/j.bbmt.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 75.Lu S, et al. Adipose-derived mesenchymal stem cells protect PC12 cells from glutamate excitotoxicity-induced apoptosis by upregulation of XIAP through PI3-K/Akt activation. Toxicology. 2011;279(1–3):189–95. doi: 10.1016/j.tox.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 76.Caja L, et al. The transforming growth factor-beta (TGF-beta) mediates acquisition of a mesenchymal stem celllike phenotype in human liver cells. J Cell Physiol. 2011;226(5):1214–23. doi: 10.1002/jcp.22439. [DOI] [PubMed] [Google Scholar]
- 77.Park KS, et al. Trophic Molecules Derived From Human Mesenchymal Stem Cells Enhance Survival, Function, and Angiogenesis of Isolated Islets After Transplantation. Transplantation. 2010;89(5):509–517. doi: 10.1097/TP.0b013e3181c7dc99. [DOI] [PubMed] [Google Scholar]
- 78.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. Journal of Cellular Biochemistry. 2006;98(5):1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 79.Park KS, et al. Influence of Human Allogenic Bone Marrow and Cord Blood-Derived Mesenchymal Stem Cell Secreting Trophic Factors on ATP (adenosine-5′-triphosphate)/ADP (adenosine-5′-diphosphate) Ratio and Insulin Secretory Function of Isolated Human Islets From Cadaveric Donor. Transplantation Proceedings. 2009;41(9):3813–3818. doi: 10.1016/j.transproceed.2009.06.193. [DOI] [PubMed] [Google Scholar]
- 80.Lu Y, et al. Mesenchymal stem cells protect islets from hypoxia/reoxygenation-induced injury. Cell Biochem Funct. 2010;28(8):637–43. doi: 10.1002/cbf.1701. [DOI] [PubMed] [Google Scholar]
- 81.Choi SE, et al. IL-6 protects pancreatic islet beta cells from pro-inflammatory cytokines-induced cell death and functional impairment in vitro and in vivo. Transpl Immunol. 2004;13(1):43–53. doi: 10.1016/j.trim.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 82.Ogawa M, et al. Cytokines prevent dexamethasone-induced apoptosis via the activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase pathways in a new multiple myeloma cell line. Cancer Res. 2000;60(15):4262–9. [PubMed] [Google Scholar]
- 83.Emamaullee JA, et al. XIAP overexpression in human islets prevents early posttransplant apoptosis and reduces the islet mass needed to treat diabetes. Diabetes. 2005;54(9):2541–8. doi: 10.2337/diabetes.54.9.2541. [DOI] [PubMed] [Google Scholar]
- 84.Plesner A, et al. The X-linked inhibitor of apoptosis protein enhances survival of murine islet allografts. Diabetes. 2005;54(9):2533–40. doi: 10.2337/diabetes.54.9.2533. [DOI] [PubMed] [Google Scholar]
- 85.Vasavada RC, et al. Growth factors and beta cell replication. International Journal of Biochemistry & Cell Biology. 2006;38(5–6):931–950. doi: 10.1016/j.biocel.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 86.Nakano M, et al. Hepatocyte growth factor is essential for amelioration of hyperglycemia in streptozotocininduced diabetic mice receiving a marginal mass of intrahepatic islet grafts. Transplantation. 2000;69(2):214–221. doi: 10.1097/00007890-200001270-00004. [DOI] [PubMed] [Google Scholar]
- 87.Otonkoski T, et al. Hepatocyte growth-factor scatter factor has insulinotropic activity in human fetal pancreatic cells. Diabetes. 1994;43(7):947–953. doi: 10.2337/diab.43.7.947. [DOI] [PubMed] [Google Scholar]
- 88.Kayali AG, et al. The stromal cell-derived factor-1 alpha/CXCR4 ligand-receptor axis is critical for progenitor survival and migration in the pancreas. Journal of Cell Biology. 2003;163(4):859–869. doi: 10.1083/jcb.200304153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kinnaird T, et al. Marrow-Derived Stromal Cells Express Genes Encoding a Broad Spectrum of Arteriogenic Cytokines and Promote In Vitro and In Vivo Arteriogenesis Through Paracrine Mechanisms. Circulation Research. 2004;94(5):678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 90.Cheng Y, et al. Elevated vascular endothelial growth factor production and its effect on revascularization and function of graft islets in diabetic rats. World Journal of Gastroenterology. 2007;13(20):2862–2866. doi: 10.3748/wjg.v13.i20.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Figliuzzi M, et al. Bone Marrow-Derived Mesenchymal Stem Cells Improve Islet Graft Function in Diabetic Rats. Transplantation Proceedings. 2009;41(5):1797–1800. doi: 10.1016/j.transproceed.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 92.Ito T, et al. Mesenchymal stem cell and islet co-transplantation promotes graft revascularization and function. Transplantation. 2010;89(12):1438–45. doi: 10.1097/tp.0b013e3181db09c4. [DOI] [PubMed] [Google Scholar]
- 93.Rackham CL, et al. Co-transplantation of mesenchymal stem cells maintains islet organisation and morphology in mice. Diabetologia. 2011;54(5):1127–35. doi: 10.1007/s00125-011-2053-4. [DOI] [PubMed] [Google Scholar]
- 94.Johansson U, et al. Formation of composite endothelial cell-mesenchymal stem cell islets - A novel approach to promote islet revascularization. Diabetes. 2008;57(9):2393–2401. doi: 10.2337/db07-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Neeman Z, et al. Radiologic aspects of islet cell transplantation. Curr Diab Rep. 2006;6(4):310–5. doi: 10.1007/s11892-006-0066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Juang JH, Hsu BRS, Kuo CH. Islet Transplantation at Subcutaneous and Intramuscular Sites. Transplantation Proceedings. 2005;37(8):3479–3481. doi: 10.1016/j.transproceed.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 97.Kemp CB, et al. Effect of transplantation site on the results of pancreatic islet isografts in diabetic rats. Diabetologia. 1973;9(6):486–91. doi: 10.1007/BF00461694. [DOI] [PubMed] [Google Scholar]
- 98.Juang JH, et al. Outcome of subcutaneous islet transplantation improved by polymer device. Transplantation. 1996;61(11):1557–61. doi: 10.1097/00007890-199606150-00001. [DOI] [PubMed] [Google Scholar]
- 99.Kawakami Y, et al. Modified subcutaneous tissue with neovascularization is useful as the site for pancreatic islet transplantation. Cell Transplant. 2000;9(5):729–32. doi: 10.1177/096368970000900523. [DOI] [PubMed] [Google Scholar]
- 100.Stendahl JC, et al. Growth factor delivery from self-assembling nanofibers to facilitate islet transplantation. Transplantation. 2008;86(3):478–481. doi: 10.1097/TP.0b013e3181806d9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Su J, et al. Anti-inflammatory peptide-functionalized hydrogels for insulin-secreting cell encapsulation. Biomaterials. 2010;31(2):308–314. doi: 10.1016/j.biomaterials.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pileggi A, et al. Reversal of diabetes by pancreatic islet transplantation into a subcutaneous, neovascularized device. Transplantation. 2006;81(9):1318–1324. doi: 10.1097/01.tp.0000203858.41105.88. [DOI] [PubMed] [Google Scholar]
- 103.Ashcroft Frances M, Rorsman P. Diabetes Mellitus and the β Cell: The Last Ten Years. Cell. 2012;148(6):1160–1171. doi: 10.1016/j.cell.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jiang R, et al. Transplantation of placenta-derived mesenchymal stem cells in type 2 diabetes: a pilot study. Front Med. 2011;5(1):94–100. doi: 10.1007/s11684-011-0116-z. [DOI] [PubMed] [Google Scholar]
- 105.Si Y, et al. Infusion of Mesenchymal Stem Cells Ameliorates Hyperglycemia in Type 2 Diabetic Rats. Diabetes. 2012;61(6):1616–1625. doi: 10.2337/db11-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bernardo ME, et al. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007;67(19):9142–9. doi: 10.1158/0008-5472.CAN-06-4690. [DOI] [PubMed] [Google Scholar]
- 107.Rubio D, et al. Molecular characterization of spontaneous mesenchymal stem cell transformation. PLoS One. 2008;3(1):e1398. doi: 10.1371/journal.pone.0001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang M, et al. The immunomodulatory activity of human umbilical cord blood-derived mesenchymal stem cells in vitro. Immunology. 2009;126(2):220–32. doi: 10.1111/j.1365-2567.2008.02891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kang JW, et al. Soluble factors-mediated immunomodulatory effects of canine adipose tissue-derived mesenchymal stem cells. Stem Cells Dev. 2008;17(4):681–93. doi: 10.1089/scd.2007.0153. [DOI] [PubMed] [Google Scholar]
- 110.Veriter S, et al. The impact of hyperglycemia and the presence of encapsulated islets on oxygenation within a bioartificial pancreas in the presence of mesenchymal stem cells in a diabetic Wistar rat model. Biomaterials. 2011;32(26):5945–56. doi: 10.1016/j.biomaterials.2011.02.061. [DOI] [PubMed] [Google Scholar]


