Abstract
Rationale
Macrophage accumulation in adipose tissue associates with insulin resistance and increased cardiovascular disease risk. We previously have shown that generation of reactive oxygen species (ROS) and monocyte chemotactic factors after exposure of adipocytes to saturated fatty acids (SFAs) such as palmitate occurs via translocation of NADPH oxidase 4 (NOX4) into lipid rafts (LRs). The anti-inflammatory effects of apolipoprotein A-I (apoA-I) and HDL on macrophages and endothelial cells appears to occur via cholesterol depletion of LRs. However, little is known concerning anti-inflammatory effects of HDL and apoA-I on adipocytes.
Objective
To determine whether apoA-I and HDL inhibit inflammation in adipocytes and adipose tissue, and whether this is dependent on LRs.
Methods and Results
In 3T3L-1 adipocytes, apoA-I, HDL and methyl-β-cyclodextrin inhibited chemotactic factor expression. ApoA-I and HDL also disrupted LRs, reduced plasma membrane cholesterol content, inhibited NOX4 translocation into LRs, and reduced palmitate-induced ROS generation and monocyte chemotactic factor expression. Silencing ABCA-1 abrogated the effect of apoA-I, but not HDL, while silencing ABCG-1 or SRB-1 abrogated the effect of HDL but not apoA-I. In vivo, apoA-I transgenic mice fed a high fat, high sucrose, cholesterol-containing diet showed reduced chemotactic factor and pro-inflammatory cytokine expression and reduced macrophage accumulation in adipose tissue.
Conclusion
ApoA-I and HDL have anti-inflammatory effects in adipocytes and adipose tissue similar to their effects in other cell types. These effects are consistent with disruption and removal of cholesterol from LRs, which are regulated by cholesterol transporters such as ABCA-1, ABCG-1 and SRB-1.
Keywords: Adipocytes, ABC transporters, cholesterol, HDL, Apolipoprotein A-I
INTRODUCTION
Obesity, especially visceral obesity, is accompanied by adipose tissue inflammation, which in turn is associated with insulin resistance and an increased risk of cardiovascular disease 1, 2. A hallmark of adipose tissue inflammation is the accumulation of macrophages 3, 4 and other immune cells 5–7, that are recruited to adipose tissue by chemotactic factors such as monocyte chemotactic protein-1 (MCP-1) 8–10 and serum amyloid A3 (SAA3) 9, 10. Reactive oxygen species (ROS) are generated by adipocytes in a NOX4-dependent fashion after exposure to excess glucose and certain saturated fatty acids (SFAs) in vitro, and the generation of ROS is linked to NFκB activation and expression of monocyte chemotactic factor genes 9, 10. Moreover, these chemotactic factors are expressed and macrophages accumulate in adipose tissue when mice are exposed to a diet rich in saturated fat and sucrose 9.
HDL and apolipoprotein A-I (apoA-I) are believed to protect against the development of atherosclerosis, in part due to their role in promoting cholesterol efflux and reverse cholesterol transport (reviewed in 11). However, in addition to these properties, HDL and apoA-I have anti-inflammatory properties [reviewed in 12–14]. The most studied of these is its ability to inhibit adhesion molecule expression by endothelial cells 15, 16, which reduces monocyte adhesion 17. At the level of the artery wall, this property of HDL would reduce recruitment of monocyte-macrophages and inhibit atherogenesis. HDL and apoA-I also have been shown to have anti-inflammatory effects on monocytes 18 and macrophages 19, 20. However, little is known concerning their effect on adipocytes.
HDL and apoA-I can exert an anti-inflammatory effect on macrophages via ABCA-1[ATP-binding cassette (ABC) A-1] and ABCG-1-mediated cholesterol efflux from plasma membrane microdomains known as lipid rafts (LRs), which are enriched in cholesterol, glycosyl-phosphatidylinositol-linked proteins, glycosphingolipids, cholesterol and caveolin 21. Free cholesterol accumulation in LRs is associated with increased signaling via proteins concentrated in these microdomains. NADPH oxidase 4 (NOX4) is the source of ROS after exposure of adipocytes to palmitate in vitro, and its activity is increased by its translocation into LRs following exposure to palmitate. Cholesterol efflux occurs via two main pathways; first, free cholesterol simply diffuses from the plasma membrane through the aqueous medium to HDL, and second, cholesterol efflux is mediated by transporters in the plasma membrane [ABCA-1, ABCG-1 and scavenger receptor (SRB-1)] 22, 23 24. ABCA-1 transports free cholesterol from plasma membrane to lipid-poor apoA-I, and ABCG-1 transports cholesterol to mature HDL particles. These transporters regulate the cholesterol content of LRs by efflux to HDL and apoA-I. In turn, disruption and depletion of cholesterol in LRs blocks signal transduction25. However, whether and how HDL and apoA-I affects inflammation in adipocytes are unknown.
To test the hypothesis that HDL has anti-inflammatory effects 12, 13 on adipocytes analogous to other cell types 18–20, we first exposed fully differentiated 3T3-L1 adipocytes to normal human HDL and apoA-I in vitro to test their effect on palmitate-induced inflammation. We found that HDL and apoA-I inhibited palmitate-induced ROS generation and chemotactic factor expression by decreasing LR formation and translocation of NOX4 into LRs. Moreover, these effects of HDL and apoA-I were dependent on cholesterol transporters in the plasma membrane. To determine whether HDL inhibited adipose tissue inflammation in vivo, we evaluated the effect of increased HDL levels that resulted from overexpression of the human apoA-I transgene on adipose tissue inflammation induced by a high fat, high sucrose, cholesterol-containing diet in mice. We found that adipose tissue inflammation also was ameliorated in human apoA-I transgenic mice. Our finding suggest that HDL and apoA-I have anti-inflammatory properties on adipocytes and adipose similar their effects in other cell types.
METHODS
Reagents and other detailed methods are described in Supplemental Methods.
Preparation of fatty acid–albumin complexes
Free fatty acids (FFAs) were prepared by conjugation with albumin, as described previously 10.
Multiplex real-time quantitative reverse-transcription polymerase chain reaction
(RT-PCR) was performed using the TaqMan Master kit (Applied Biosystems) in the ABI prism 7900HT system 27, 28 (Supplemental Methods).
Quantification of cholesterol levels in cellular membranes of 3T3L-1 adipocytes
Cellular membranes were isolated from 3T3L-1 adipocytes as described previously 29. Membrane preparations were resuspended in 100µl of ethanolic potassium hydroxide (1mol/L) and cholesterol-d7 was added as the internal standard. After saponification, the lipid fractions were extracted from the membrane preparations with hexane and dried under nitrogen gas. Total cholesterol levels were determined after derivatization using liquid chromatography electrospray ionization tandem mass spectrometry (LC-MS/MS) as described previously 30.
In vitro ABCA-1, ABCG-1 and SRB-1 gene silencing
For experiments in which we tested the roles of ABCA-1, ABCG-1 and SRB-1 in mediating ROS generation and the expression of SAA3 and MCP-1, two days after completion of the differentiation protocol 3T3-L1 adipocytes were transiently transfected with small interfering RNA (siRNA) duplexes for ABCA-1, ABCG-1 and SRB-1 or scrambled sequences, which were synthesized and purified by Ambion using the DeliverX system (Panomics), as described previously 10, 29. Briefly, the cells were incubated with 300µl of siRNA/siRNA transfection reagent complex for 3min. After that, 300µl of serum free media was added and incubated for 24h. Four days later mRNA and protein were analyzed for confirm the efficiency of silencing by RT-PCR and Western blotting.
Quantification of reactive oxygen species (ROS)
ROS generation was assessed as CM-H2DCFDA (Molecular Probes) fluorescence, which was monitored by FACS (FACSCanto, Becton-Dickinson) as described previously 10, 29.
Visualization of lipid rafts
To detect lipid rafts in plasma membranes, Alexa Fluor 594 conjugated cholera toxin subunit β (CTB) was used to stain 3T3-L1 adipocytes. Briefly, cultured 3T3-L1 adipocytes were incubated with 1µg/ml of Alexa Fluor 594 conjugated CTB for 15 min at 4 °C. After washing twice with cold PBS, cells were fixed in 4% paraformaldehyde for 20 min at 4 °C. Fixed cells were photographed by fluorescent microscopy (Nikon Eclipse 80i).
Detergent-free lipid raft fractionation
Lipid raft (LR) and non-LR fractions from adipocytes were obtained by Optiprep gradient centrifugation using a detergent-free protocol 31 (Supplemental methods).
Animals, diet, and tissue collection
To investigate the role of HDL and apoA-I in adipose tissue inflammation, adult (ten week-old) male human apoA-I overexpressing transgenic (apoA-Itg/tg ) and control C57BL/6 mice were fed either a high fat, high sucrose, cholesterol-containing (HFHSC diet, 35.5% calories as fat and 36.6% as carbohydrate, 0.15% added cholesterol, BioServ No.F1850) or chow (control) diets for 24 weeks 32. In a parallel experiment, ten-week-old male LDL receptor (LDLR)-deficient and human apoA-I overexpressed transgenic (LDLR−/− apoA-Itg/tg) mice, and control LDLR−/− mice also were fed either HFHS or chow diets for 16 weeks. ApoA-Itg/tg and LDLR-/- mice were bought from the Jackson laboratory, and LDLR−/− apoA-Itg/tg mice were generated from these homozygous mice. At sacrifice, adipose tissues were either fixed in 10% formalin for macrophage staining using a Mac2 antibody33, or snap-frozen at −70°C for isolation of total RNA. Metabolic variables were measured in blood samples obtained from the retro-orbital sinus after a 4h fast. Cholesterol and triglycerides in plasma and fast-phase liquid chromatography (FPLC) fractions were measured using colorimetric assay kits. Lipoproteins were separated from pooled plasma samples by FPLC. Plasma insulin and SAA levels were measured using ELISA as described previously 27. All experimental procedures were undertaken with approval from the Institutional Animal Care and Use Committee of the University of Washington.
Statistical analysis
Statistical significance was determined by Student’s t-tests (two tailed and paired). All data are shown as means ± SD of three independent experiments performed in triplicate. P<0.05 was considered significant.
RESULTS
ApoA-I and HDL disrupt lipid rafts and inhibit palmitate-induced generation of chemotactic factors
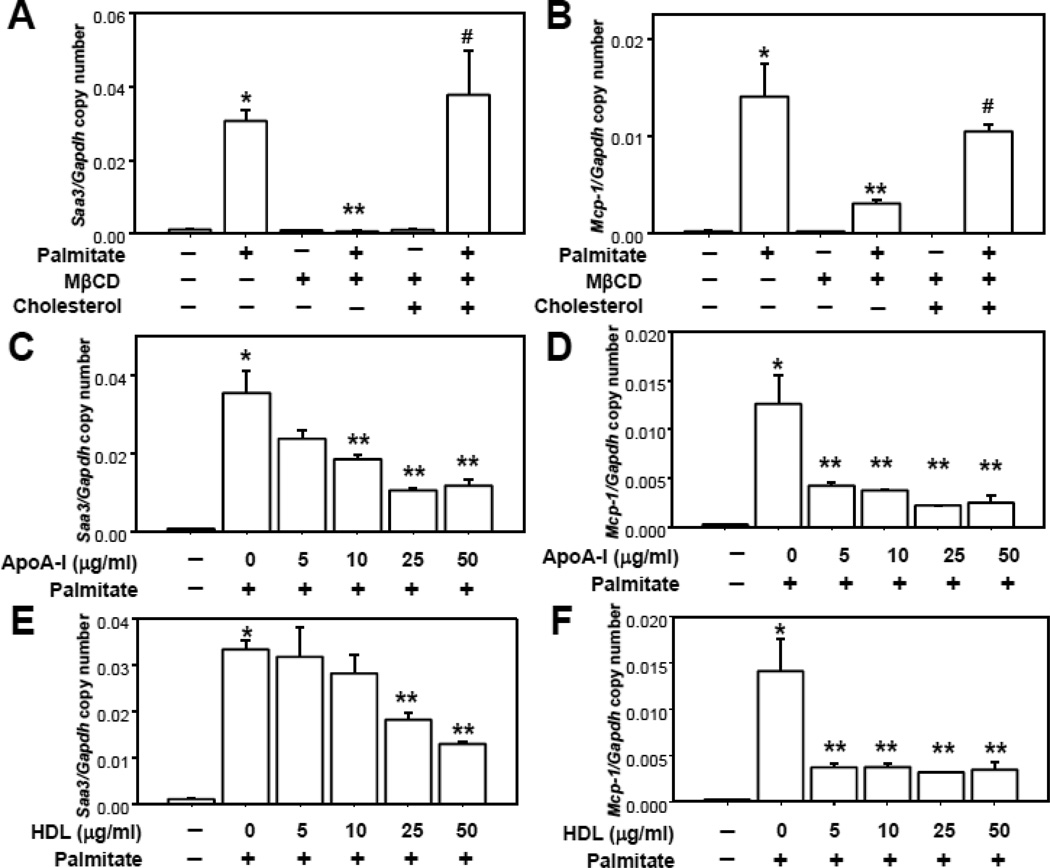
We previously showed that exposure of differentiated 3T3-L1 adipocytes to palmitate (250µmol/L) increased chemotactic factor gene expression10. We also showed that chemotactic factor generation was linked to the translocation of NOX4 into LRs, which led to ROS generation 29. Cholesterol is an essential component of LRs. Depletion of cholesterol in the plasma membrane disrupts LRs and blocks the assembly of proteins, resulting in inhibition of signal transduction 25. Therefore, we investigated whether modulation of membrane cholesterol using methyl-β-cyclodextrin (MβCD), a compound that depletes membrane cholesterol and disrupts LRs 25, 34, affects palmitate-induced chemotactic factor gene expression. MβCD totally blocked palmitate-induced Saa3 and Mcp-1 gene expression (Figure 1A and B). Moreover, adding back cholesterol reversed the effect of MβCD on Saa3 and Mcp-1 gene expression (Figure 1A and B).
Figure 1. MβCD, apoA-I and HDL inhibits chemotactic factor expression.
3T3-L1 adipocytes were pre-exposed to MβCD (10µmol/ml, A and B), cholesterol loaded MβCD (A and B), apoA-I and/or HDL (at the indicated concentrations in µg protein/ml), C–F) for 6h. After that, adipocytes were incubated with or without 250 µmol/L palmitate for 24h. Saa3 and Mcp-1 gene expression was analyzed by multiplex real-time RT-PCR, normalized to GAPDH (A–F). *P < 0.001 vs. control media, **P < 0.001 vs. palmitate, #P < 0.001 vs. palmitate plus MβCD.
When cells were pre-exposed to simvastatin (10µmol/L), an inhibitor of 3-hydroxy-3-methyl-glutaryl (HMG)-CoA reductase, for 24h to reduce cholesterol biosynthesis, we also found that palmitate-induced Saa3 and Mcp-1 gene expression was blocked (data not shown). Since apoA-I and HDL have the ability to remove cholesterol from cell membranes, we also investigated whether apoA-I and HDL could inhibit this effect of palmitate. We first determined whether apoA-I and HDL could decrease the cholesterol level of membranes in 3T3L-1 adipocytes. Indeed, apoA-I and HDL decreased membrane cholesterol levels before as well as after exposure of adipocytes to palmitate (Supplemental figure I). Palmitate exposure alone led to a significant increase in membrane cholesterol (Supplemental figure I). Both apoA-I and HDL blocked the increase of Saa3 and Mcp-1 gene expression induced by palmitate in a dose-dependent manner (Figure 1C–F), although Mcp-1 expression appeared to be more sensitive to the effects HDL and apoA-I possibly because the promoter of the Mcp-1 gene might be more sensitive to the anti-inflammatory effects of HDL and apoA-I than the Saa3 gene.
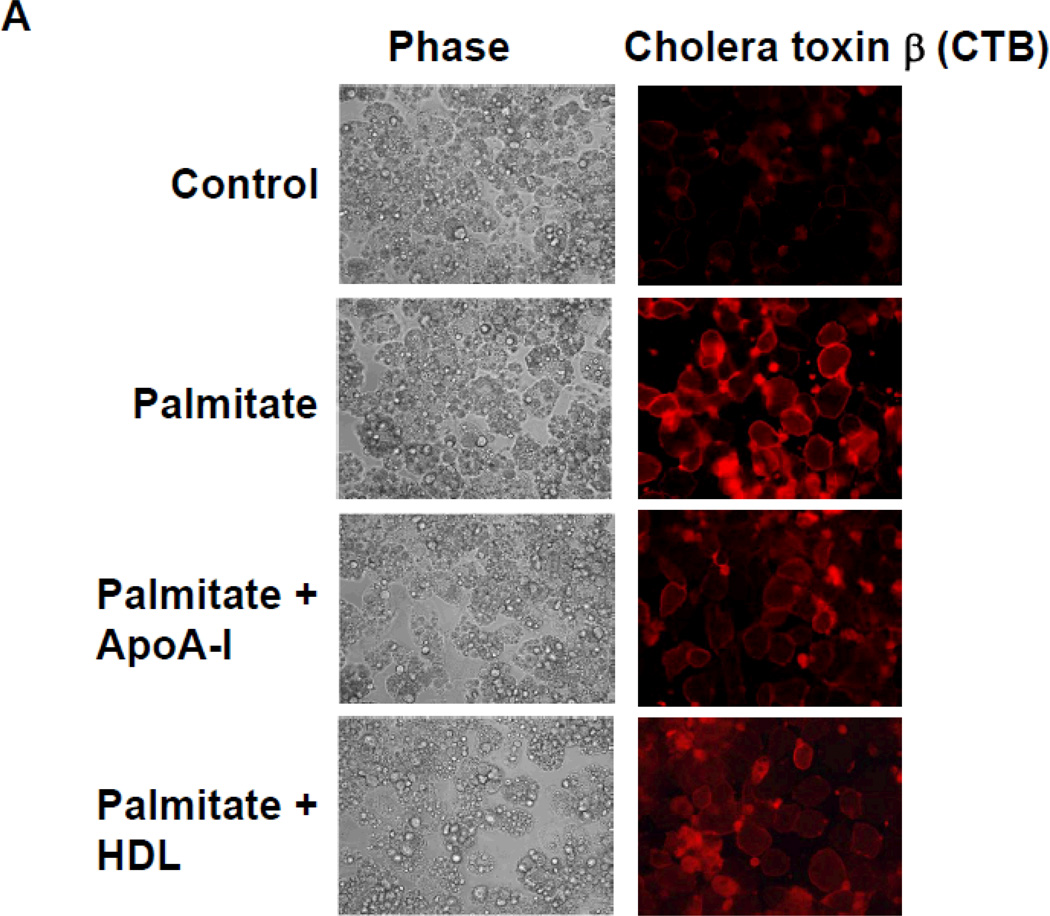
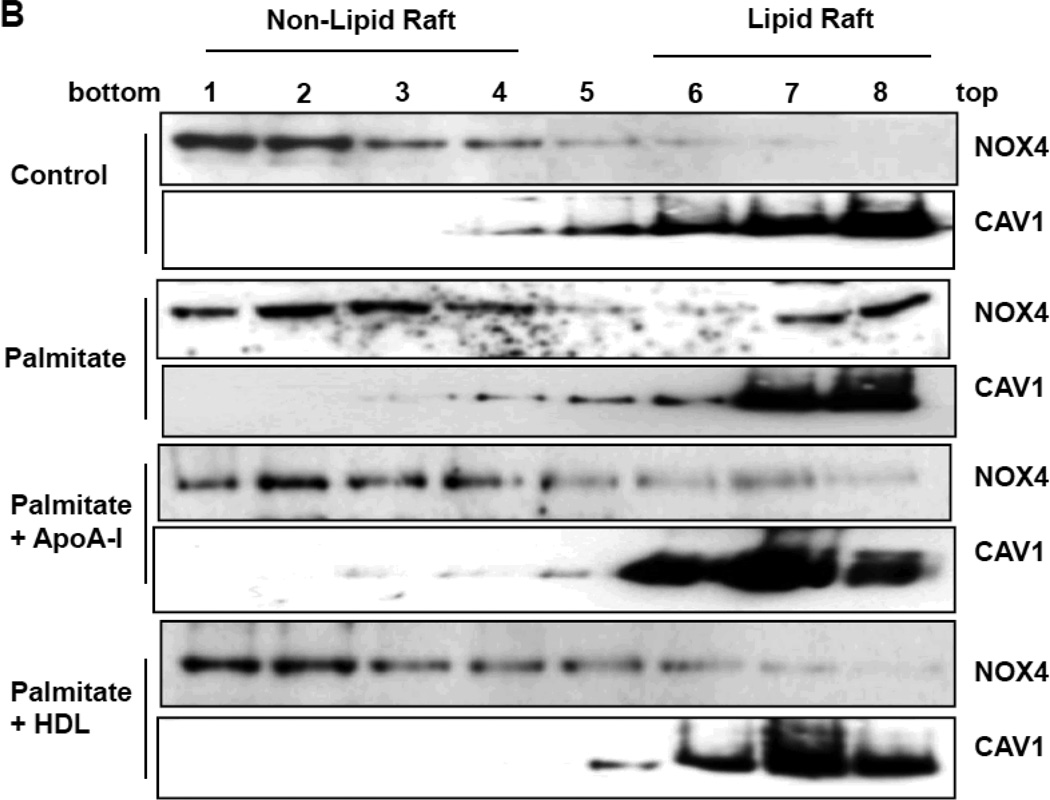
Moreover, co-treatment with apoA-I and HDL synergistically inhibited these chemotactic factor expression induced by palmitate (Supplemental figure II). Since a trace of HDL in the serum used for adipocyte cells culture could potentially affect these results, we also performed experiments using lipoprotein-deficient serum and found no differences between these two conditions (data not shown). To determine whether apoA-I and HDL disrupt LR formation, we visualized the LRs using Alexa Fluor 594 conjugated cholera toxin subunit β (CTB), which selectively binds to LRs in the plasma membrane 35. Exposure of adipocytes to palmitate increased CTB-stained microdomains of LRs compared with controls, while pre-exposure to either apoA-I or HDL reduced these microdomains (Figure 2A). Finally we investigated whether apoA-I and HDL blocked NOX4 translocation into LRs isolated by ultracentrifugation. Non-LRs sediment in the lower fractions while LRs float towards the top of the centrifuge tube. The presence of LRs was judged by detection of caveolin-1 (CAV1). In the absence of palmitate exposure, all NOX4 protein was present in non-LR fractions. After exposure to palmitate, NOX4 proteins were translocated into LRs (Figure 2B). However, pre-exposure of cells to either apoA-I or HDL inhibited palmitate-induced translocation of NOX4 into LRs (Figure 2B). These results imply that apoA-I and HDL disrupt the formation of LRs, causing disturbance of palmitate-induced NOX4 translocation, which in turn inhibits chemotactic factor expression.
Figure 2. ApoA-I and HDL disrupt palmitate-induced LR formation and NOX4 translocation into LRs.
3T3-L1 adipocytes were pre-exposed to apoA-I (50µg protein/ml) or HDL (50µg protein/ml) for 6h. After that, adipocytes were incubated with or without 250µmol/L palmitate for 24h. A. LRs were stained by Alexa Fluor 594 conjugated cholera toxin subunit β (CTB) and photographed by fluorescent microscopy (Nikon Eclipse 80i, original magnification ×400). B. LRs were isolated and fractionated by ultracentrifugation using a detergent-free fractionation method. Proteins from OptiPre-gradient fractions were immunoblotted with anti-NOX4 antibody and anti-caveolin-1 (CAV1) antibody (B). Fractions 6 to 8 contain LRs and fractions 1 to 4 are non-LR containing fractions.
The anti-inflammatory effect of apoA-I and HDL on palmitate-induced chemotactic factor production is dependent on ABC transporters and scavenger receptor B1
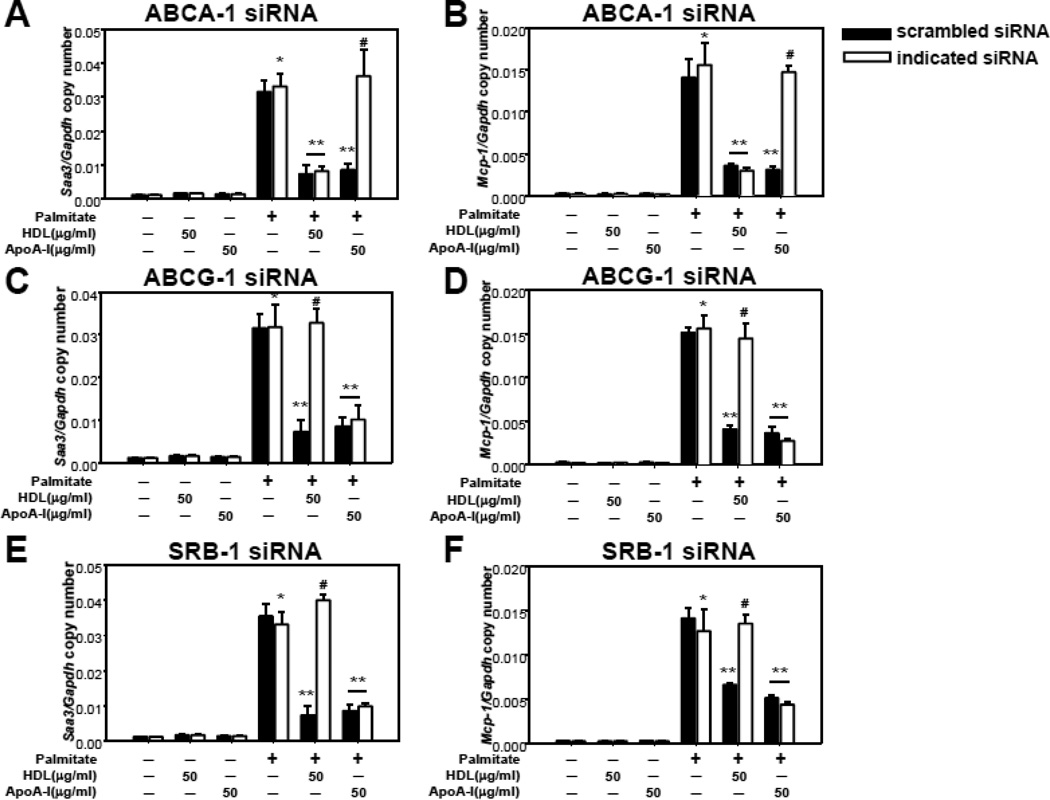
Since reverse cholesterol transport by apoA-I and HDL is mediated by transporters in the plasma membrane (ABCA-1, ABCG-1 and SRB-1), we first tested which of these transporters is important for apoA-I or HDL to exert their effects on palmitate-induced chemotactic factor gene expression. ABCA-1, ABCG-1 and SRB-1 silencing in 3T3-L1 adipocytes by their specific siRNAs was confirmed by demonstrating significant reduction of their respective expression levels by RT-PCR (data not shown) and Western blotting (Supplemental figure III). When ABCA-1 was silenced, the effect of apoA-I on inhibiting Saa3 and Mcp-1 gene expression induced by palmitate was reversed, while HDL still inhibited palmitate-induced expression of these chemotactic factors (Figure 3 A and B). Conversely, the ability of HDL to inhibit palmitate-induced chemotactic factor gene expression was reversed by silencing ABCG-1 or SRB-1, while apoA-I still inhibited these events (Figure 3 C–F).
Figure 3. The effects of apoA-I and HDL on palmitate-induced chemotactic factor gene expression are mediated by ABCA-1, ABCG-1 and SRB-1.
3T3-L1 adipocytes were transfected with a siRNA specific for ABCA-1 (A and B), ABCG-1 (C and D), SRB-1 (E and F) or a scrambled siRNA (negative control) as indicated. 24h later the siRNA was removed and the cells were cultured for a further 3 days. After that, the cells were pre-exposed to apoA-I (50µg/ml) or HDL (50µg protein/ml) for 6h, and then incubated with or without added palmitate (250µmol/L) for 24h. Total RNA was isolated and analyzed by multiplex real-time RT-PCR using Saa3-specific (A, C and E) or Mcp-1-specific (B, D and F) primers and normalized to Gapdh. *P < 0.001 vs. control media, **P < 0.001 vs. palmitate, #P < 0.001 vs. palmitate plus HDL or apoA-I.
ApoA-I and HDL regulate palmitate-induced formation of LRs, translocation of NOX4 and ROS generation via ABCA-1, ABCG-1 and SRB-1
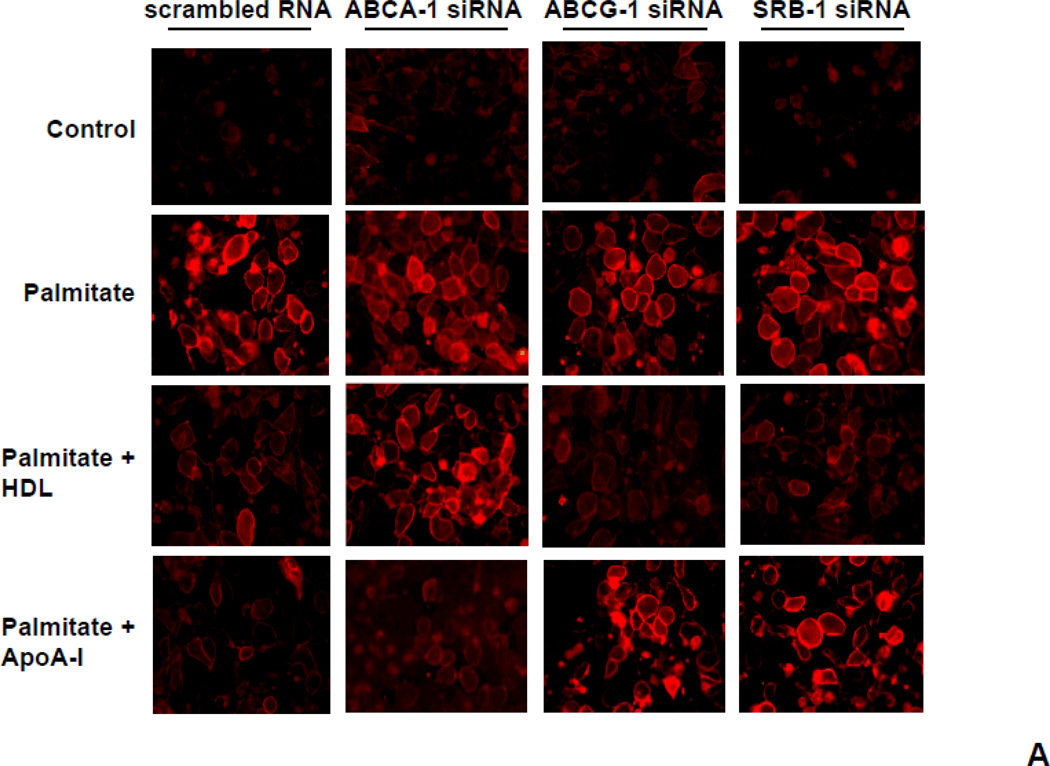
To investigate the roles of ABCA-1, ABCG-1 and SRB-1 in LR formation induced by palmitate, we evaluated CTB-stained microdomains in the plasma membrane of adipocytes in which these transporters had been silenced using siRNA. In control cells, palmitate resulted in increased CTB-stained microdomains. In ABCA-1 silenced cells, apoA-I did not decrease the CTB-stained LRs comparing while HDL did (Figure 4). Conversely, in ABCG-1 or SRB-1 silenced cells, HDL failed to reduce the CTB-stained LRs while apoA-I did (Figure 4).
Figure 4. CTB-stained LRs are modulated by HDL and apoA-I via ABCA-1, ABCG-1 and SRB-1.
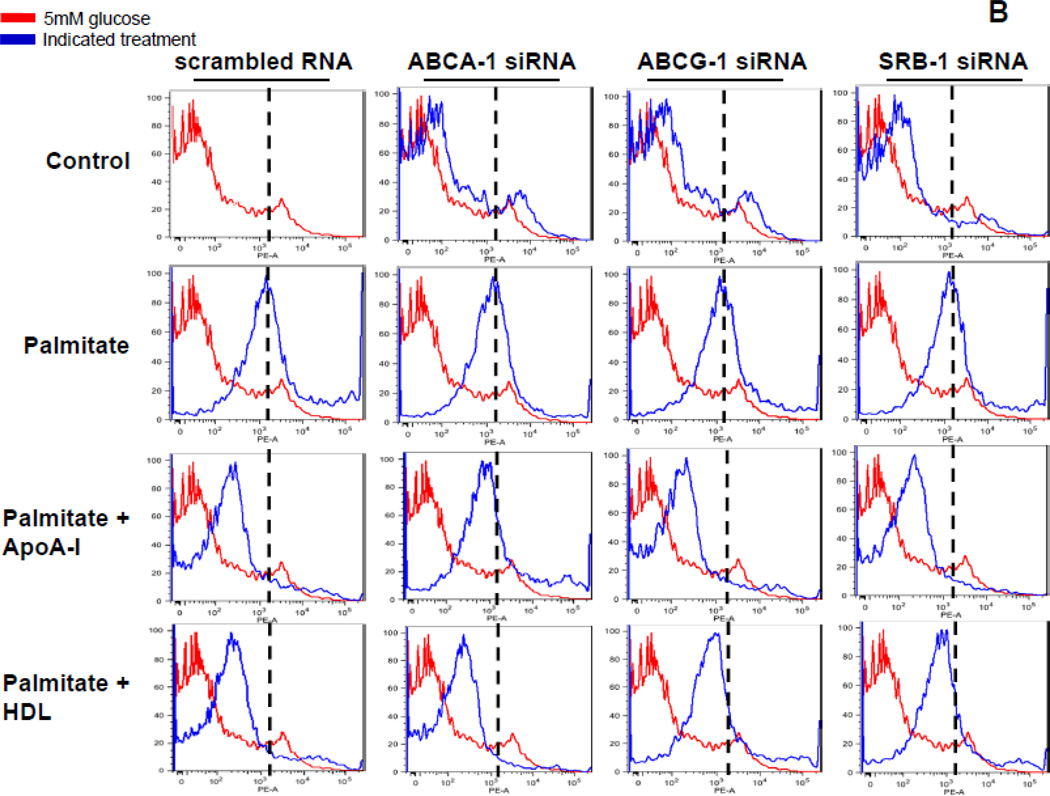
3T3-L1 adipocytes were transfected with a siRNA specific for ABCA-1, ABCG-1, SRB-1 or a scrambled siRNA (negative control) as indicated. 24h later the siRNA was removed and the cells were cultured for a further 3 days. After that, the cells were pre-exposed to apoA-I (50µg/ml) or HDL (50µg protein/ml) for 6h, and then incubated with or without added palmitate (250 µmol/L) for 24h. A. LRs were stained by Alexa Fluor 594 conjugated cholera toxin subunit β (CTB) and photographed by fluorescent microscopy (Nikon Eclipse 80i, original magnification ×400). B. Cells in which LRs were stained by CTB were subjected to FACS analysis to quantify LR formation. Cells exposed to control media are shown in red and the peak value for cells exposed to 250µmol/L palmitate are indicated by the dashed lines. Cells exposed to the indicated treatments are shown in blue.
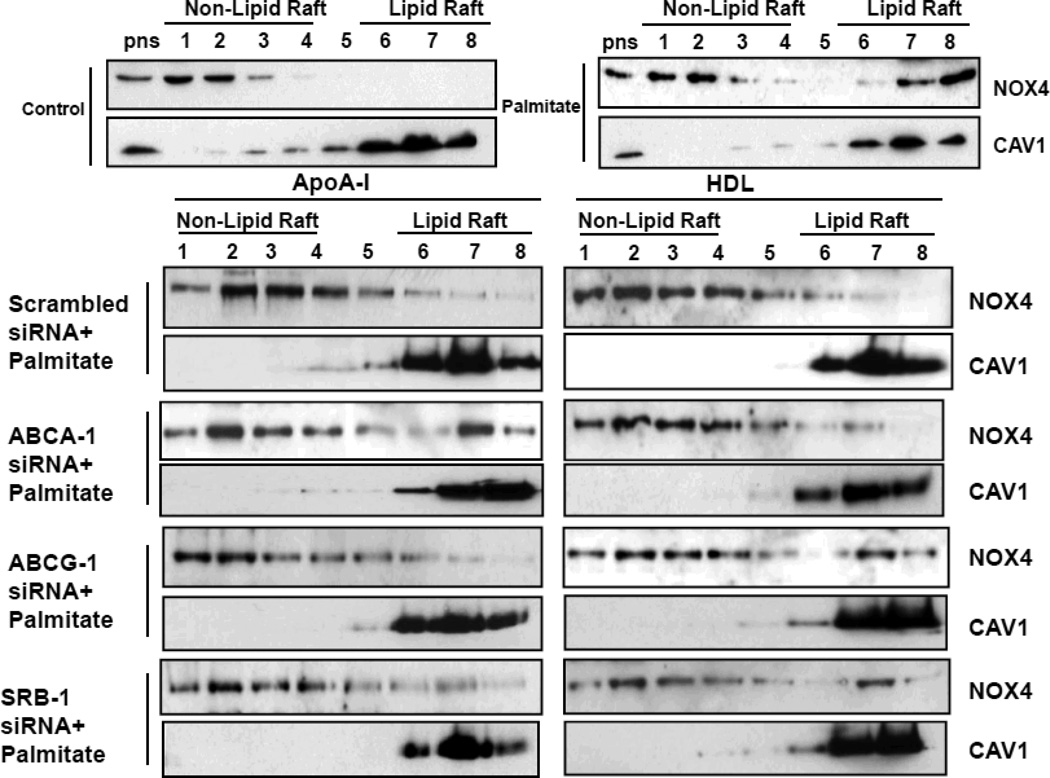
Next, we investigated whether ABCA-1, ABCG-1 and SRB-1 regulate NOX4 translocation into LRs, since we had previously shown that exposure of adipocytes to palmitate lead to translocation of NOX4 to LRs 29. Consistent with the findings with CTB staining, in ABCA-1 silenced cells, palmitate-induced NOX4 translocation into LRs was not blocked by apoA-I while HDL inhibited NOX4 translocation (Figure 5). Conversely, in ABCG-1 or SRB-1 silenced cells, palmitate-induced NOX4 translocation was not blocked by HDL while apoA-I inhibited NOX4 translocation (Figure 5).
Figure 5. HDL and apoA-I block the palmitate-induced NOX4 translocation via ABCA-1, ABCG-1 and SRB-1.
3T3-L1 adipocytes were transfected with a siRNA specific for ABCA-1, ABCG-1, SRB-1 or a scrambled siRNA (negative control) as indicated. 24h later siRNA was removed and the cells were cultured for a further 3 days. After that, the cells were pre-exposed to apoA-I (50µg/ml) or HDL (50µg protein/ml) for 6h, and then incubated with or without added palmitate (250µmol/L) for 24h. LRs were isolated and fractionated by ultracentrifugation using a detergent-free fractionation method. Proteins from OptiPre-gradient fractions were immunoblotted with anti-NOX4 and anti-caveolin-1 (CAV1) antibodis. PNS: the post-nuclear supernatant fraction.
Finally, we evaluated how these transporters affect NOX4 derived ROS generation. Similar to the findings with CTB staining and NOX4 translocation, silencing ABCA-1 reduced the ability of apoA-I to suppress palmitate-induced ROS generation, while HDL still reduced palmitate-induced ROS (Figure 6). Silencing of ABCG-1 or SRB-1 reduced the ability of HDL to suppress palmitate-induced ROS generation, while apoA-I still reduced palmitate-induced ROS (Figure 6).
Figure 6. ApoA-I and HDL affect palmitate-induced NOX4-derived ROS generation via ABCA-1, ABCG-1 and SRB-1.
3T3-L1 adipocytes were transfected with a siRNA specific for ABCA-1, ABCG-1, SRB-1 or a scrambled siRNA (negative control) as indicated. 24h later the siRNA was removed and the cells were cultured for a further 3 days. After that, the cells were exposed to apoA-I (50µg/ml) or HDL (50µg protein/ml) for 6h, and then incubated with or without added palmitate (250µmol/L) for 24h. Cells were subjected to FACS analysis using CM-H2DCFDA. Results are plotted as counts (number of cells) on the vertical axis, versus DCF fluorescence intensity on the horizontal axis. Cells exposed to control media are shown in red and the peak value for cells exposed to 250µmol/L palmitate are indicated by the dashed lines. Cells exposed to the indicated treatments are shown in blue.
Since silencing these transporters could potentially affect palmitate uptake, thereby changing palmitate concentration in cells, we measured the uptake of 14C-labeled palmitate in adipocytes in which these transporters had been silenced. None of silenced cells, either in the presence or absence of HDL or apoA-I showed alterations in palmitate uptake (data not shown). These findings all indicate that apoA-I exerts its anti-inflammatory effect on adipocytes via ABCA-1, and HDL does so via ABCG-1 and SRB-1.
Adipose tissue inflammation is reduced in human apoA-I transgenic mice
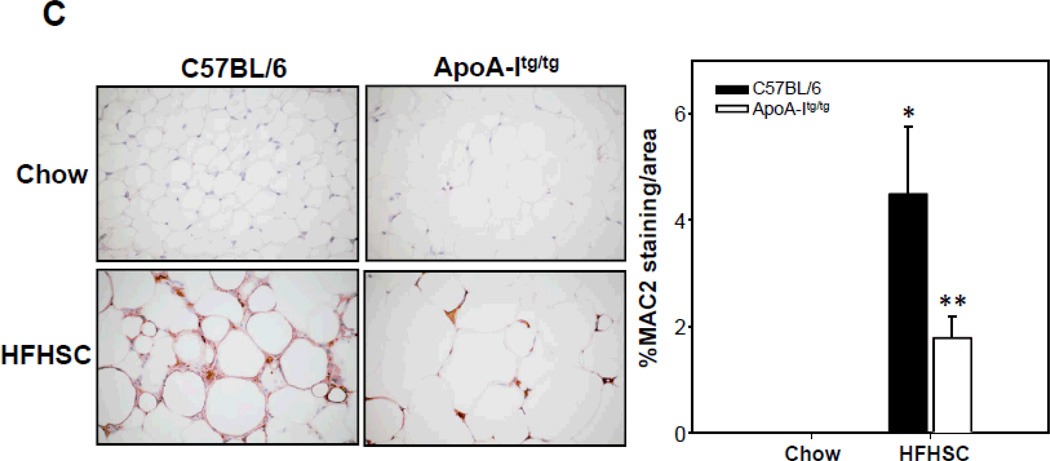
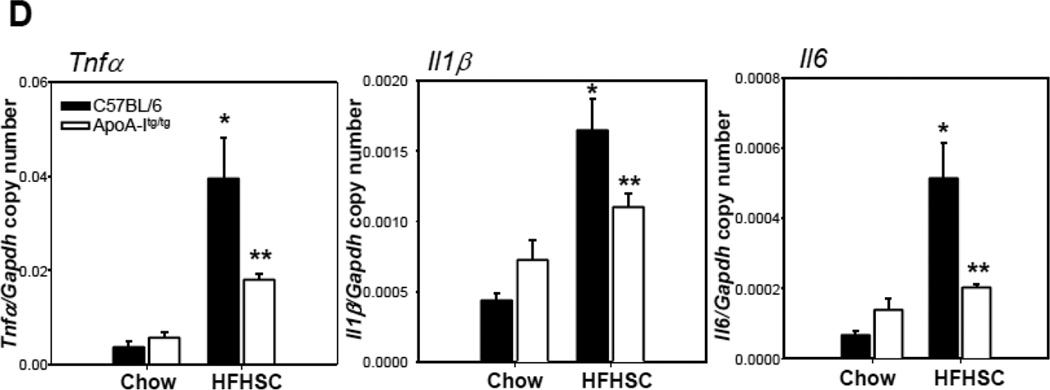
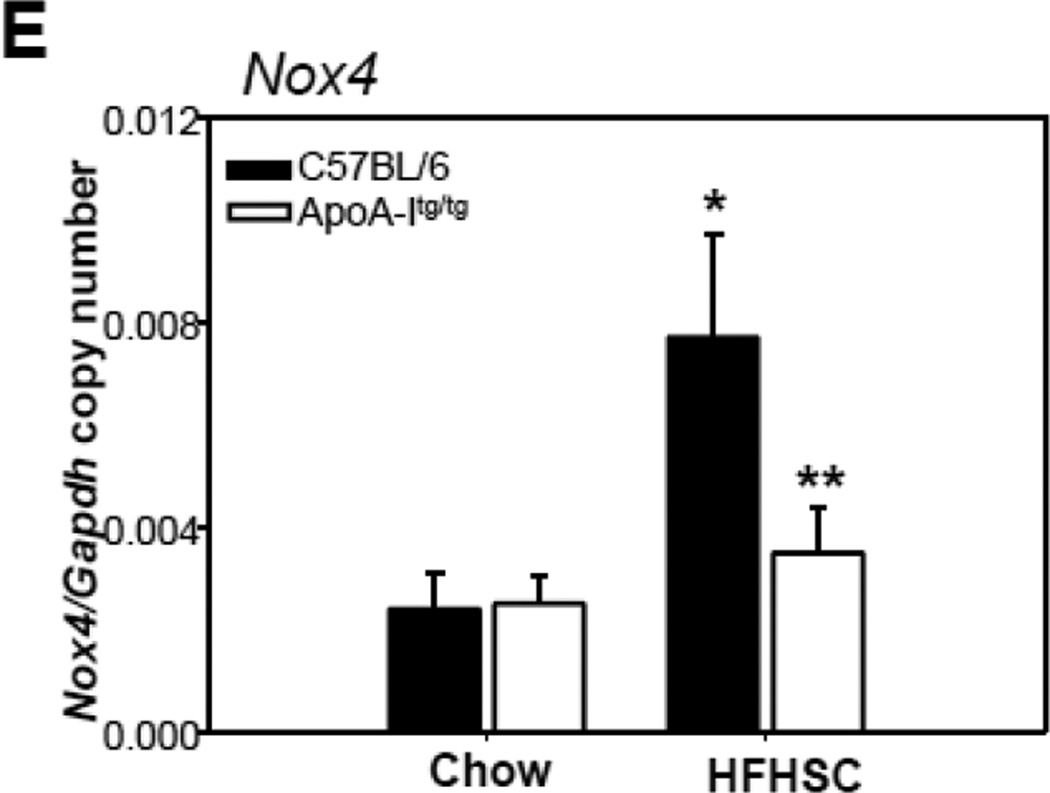
To extend our in vitro findings in 3T3-L1 adipocytes to an in vivo model, we used male apoA-I transgenic mice fed a high-fat, high-sucrose, cholesterol containing diet (HFHSC), which previously has been shown to result in obesity, insulin resistance, and atherosclerosis 32. ApoA-I and HDL cholesterol levels are increased about two fold in mice overexpressing the human apoA-I transgene 36. ApoA-I transgenic mice and their littermates showed the same weight gain with either chow or the HFHSC diet (data not shown). As expected, lipoprotein distribution profiles of apoA-I transgenic mice fed chow or HFHSC diet revealed increased HDL particles (supplemental figure IV A and E). Plasma triglyceride and cholesterol levels were increased in apoA-Itg/tg mice fed the HFHSC diet (supplemental figure IV B and C). However, there were no changes in plasma triglyceride and cholesterol profiles between LDLR−/− and LDLR−/− apoA-Itg/tg mice fed the HFHSC diet (supplemental figure IV F and G). Levels of SAA (a circulating inflammatory marker) showed a trend towards reduction in the apoA-I transgenic mice fed the HFHSC diet, but the changes did not reach statistical significance (supplemental figure IV D and H). Next, we investigated inflammation in adipose tissue. First, in response to consumption of the HFHSC diet expression of chemotactic factor gene, Saa3, was decreased in intra-abdominal adipose tissue of apoA-Itg/tg (Figure 7A) and LDLR−/− apoA-Itg/tg mice (Supplemental figure V) versus controls (C57BL/6 or LDLR−/−), whereas another chemotactic factor gene, Mcp-1, was only decreased in intra-abdominal adipose tissue of LDLR−/− apoA-Itg/tg mice (Figure 7A and Supplemental figure V). Second, mRNA expression of macrophage markers was decreased in intra-abdominal adipose tissue of apoA-Itg/tg (Figure 7B) and LDLR−/− apoA-Itg/tg mice (Supplemental figure V) fed the HFHSC diet. Immunohistochemical staining also showed a decrease of macrophages (detected with Mac2) in intra-abdominal adipose tissue of apoA-Itg/tg mice compared to C57BL/6 mice fed the HFHSC diet (Figure 7C). Third, mRNA expression level of pro-inflammatory cytokines (Tnfα, Il1β and Il6) was decreased in intra-abdominal adipose tissue of apoA-Itg/tg (Figure 7D) and LDLR−/− apoA-Itg/tg mice (Supplemental figure V) fed the HFHSC diet. Interestingly, Nox4 mRNA expression level was decreased in apoA-Itg/tg (Figure 7E) as well as LDLR−/− apoA-Itg/tg mice (Supplemental figure V) fed the HFHSC diet. These findings imply that apoA-I and HDL also exert an anti-inflammatory effect on adipose tissue in vivo.
Figure 7. Overexpression of human apoA-I inhibits chemotactic factor expression and macrophage accumulation in adipose tissue of mice.
ApoA-Itg/tg and C57BL/6 control mice were fed chow or a high-fat, high-sucrose, cholesterol-containing (HFHSC) diet for 24 weeks (A–E; n=9). Epididymal fat was isolated and analyzed by real-time RT-PCR using Saa3, Mcp-1 (A), Mac2, F4/80 (B), Tnfα, Il1β, Il6 (D) and Nox4 (E)-specific primers and probes and normalized to Gapdh. Epididymal fat was isolated and analyzed by immunohistochemistry using a Mac-2 antibody (C), which detects murine macrophages. Tissues were photographed using microscopy (original magnification ×60. *P < 0.005 vs. chow, **P < 0.005 vs. C57BL/6 or LDLR-/- in HFHSC.
DISCUSSION
We previously have shown that both excess glucose 9 and saturated fatty acids such as palmitate 10 increase the expression of the monocyte chemotactic factors, SAA3 and MCP-1, which induce monocyte chemotaxis. In this study we show that apoA-I and HDL have anti-inflammatory properties in cultured adipocytes in addition to their well-described effects on vascular cells such as endothelial cells and macrophages 16, 18–20, 37. Addition of either normal human HDL or its major apolipoprotein, apoA-I, both reduced palmitate-induced expression of Saa3 and Mcp-1 in a dose dependent and additive fashion. These effects are likely related to the cholesterol content of adipocyte membranes, since they could be mimicked by removal of cholesterol from cells by exposure to MβCD and restored by adding back cyclodextrin to which cholesterol had been added. Moreover, the anti-inflammatory effect of apoA-I appeared to be ABCA-1 dependent, since it could be blocked by silencing ABCA-1. The effects of intact HDL appeared to depend on ABCG-1 and SRB-1, since they could be reversed by silencing either of these genes. All of these proteins are present in 3T3-L1 adipocytes in vitro. We also were able to show that Saa3 and Mcp-1 expression and macrophage accumulation in adipose tissue were reduced in vivo in mice with high HDL levels as a result of apoA-I over-expression. Finally, we had previously shown that palmitate induction of SAA3 and MCP-1 in adipocytes was dependent on the generation of reactive oxygen species by NOX4 after translocation to lipid rafts 29. In this study we extend those findings by demonstrating that NOX4 translocation to lipid rafts is inhibited by both HDL and apoA-I. Several mechanisms have been proposed by which HDL might be able to exert an anti-inflammatory effect on cells, including induction of scavenger receptor class B 38 and hydrolysis of some HDL components by endothelial lipase 39, both PPAR-α dependent processes. In addition, effects of apoA-I and intact HDL on ABAC1 and ABCG-1-mediated cholesterol efflux from macrophages have been invoked to play a role in the anti-inflammatory properties of HDL in macrophages 19, 20. A similar mechanism appears to be operative in adipocytes, since palmitate increased the cholesterol content of rafts, as assessed by cholera toxin fluorescence, an affect that was reduced by pre-treatment of the cells with either HDL or apoA-I. Additional evidence that the cholesterol content of the cell membrane plays an important role in determining the ability of adipocytes to respond to palmitate is the observation that disruption of cell membrane rafts by the addition of cyclodextrin 40 had the same anti-inflammatory effect as exposure of cells to either HDL or apoA-I, and that the pro-inflammatory effect could be restored by exposing the cells to cyclodextrin that had been pre-loaded with cholesterol.
ABCA-1 transports cholesterol from cell membranes to lipid-poor apoA-I 41, whereas whole HDL accepts cholesterol from both ABCG-1 42, 43 and SRB-1 44. Although ABCG-1 is reported to reside in the endoplasmic reticulum, it could mediate transfer of cholesterol to the plasma membrane from where it could be desorbed to exogenous lipid acceptors such as HDL 45. Our results indicate that silencing ABCA-1 reversed the anti-inflammatory effect of apoA-I but had no influence on the effect of intact HDL. Conversely, silencing ABCG-1 or SRB-1 reversed the inhibitory effect of HDL but not apoA-I on palmitate induction of chemotactic factors. Because of the known functions of these proteins in transferring cholesterol to either apoA-I or HDL, these findings are consistent with the cholesterol content of plasma membranes playing a major role in the ability of adipocytes to respond to the pro-inflammatory effects of palmitate.
Although some controversy exists regarding the definition or even the existence of lipid rafts 46, proteins involved in cell signaling clearly cluster in certain domains of the plasma membrane. Accumulation of free cholesterol of the plasma membrane is associated with increased signaling via TLR4 19, 20, which reside in these domains – the presence of apoA-I or HDL reduces the cholesterol content of these rafts and TLR4 signaling in other cell types 20. The presence of ABCG-1 and HDL also protect against endothelial cell dysfunction in mice fed a western-type diet, consistent with an inhibition of activation of TRL4 by SFA in endothelial cells 47, 48. We recently showed that ROS generation by NOX4, a member of the NADPH family of oxidases, plays a critical role in palmitate-induction of chemotactic factor production in adipocytes, and is the major reason for generation of ROS in these cells29. NOX4 is translocated to the plasma membrane after exposure of adipocytes to palmitate, and disruption of adipocytes membranes by exposure to cyclodextrin blocked both NOX4 translocation and stimulation of chemotactic factor expression induced by palmitate 29. In the present study, we show that pre-exposure of cells to either HDL or apoA-I markedly inhibited the translocation of NOX4 to “lipid raft” fractions of the plasma membrane and ROS generation. Moreover, silencing ABCA-1 reversed the effect of apoA-I but not HDL, whereas silencing ABCG-1 or SRB-1 reversed the effect of HDL but not apoA-I on palmitate-mediated NOX4 translocation to the plasma membrane. In view of the effects of apoA-I and HDL and the silencing of these transporter proteins on chemotactic factor gene expression, these findings are consistent with apoA-I and HDL effects on NOX4 translocation playing an important role in their ability to influence gene expression.
To test whether similar changes occur in vivo, we chose to emulate saturated fatty acid induced adipose tissue inflammation in control mice and in mice expressing the human apoA-I transgene. We previously have shown that a diet rich in saturated fat and sucrose with a moderate amount of added cholesterol, the so-called HFHSC diet, led to profound pro-inflammatory effects on intra-abdominal adipose tissue 27. To test the effect of apoA-I and HDL in vivo, we chose to study apoA-I transgenic mice, which have increased levels of human HDL 36, 49. These mice show a reduction of atherosclerosis when present on an atherosclerosis prone background 49, suggesting that vascular inflammation is reduced in these mice. Our findings indicate that high levels of apoA-I that result from expression of the human apoA-I transgene also inhibit the expression of monocyte chemotactic factors, and the accumulation of macrophages in adipose tissue in mice. Moreover, expression of inflammatory genes such as Tnfα and Il6 were reduced in adipose tissue from these mice, consistent with our in vitro findings. The reason why MCP-1 expression was not reduced in adipose tissue from the apoA-I transgenic mice on the C57BL6 background whereas it was in the transgenic mice on the LDLR deficient background is not clear. However, macrophage and inflammatory genes were reduced in both apoA-I transgenic strains of mice.
Potential shortcomings of our study include lack of comparison of gene expression in the adipocyte versus stromal vascular fractions, and of knowledge of the cellular distribution of NOX4 in adipose tissue. These should be determined in future studies of this nature.
Accumulation of macrophages in adipose tissue of both mice and humans is a hallmark of obesity3, 4. Adipose tissue inflammation and obesity are features of the metabolic syndrome in humans 50 and in a mouse model that has many of the features of the metabolic syndrome 27. Moreover, obesity-associated inflammation predisposes to the development of both type 2 diabetes and cardiovascular disease 50. Therefore, strategies to reduce adipose tissue inflammation have potentially important therapeutic implications. Although simply raising HDL cholesterol levels has not been shown to reduce atherosclerosis 51 nor do some genetic polymorphs that raise HDL levels protect against cardiovascular disease, it is clear from human epidemiological studies that HDL has a strong inverse relationship with cardiovascular disease 52. In addition, experiments in mice that either overexpress or are lacking in apoA-I clearly show a very strong relationship with atherosclerosis49, 53. HDL also clearly has anti-inflammatory 12–14 and other 14 potentially atheroprotective properties on cells of the artery wall. The findings presented in this study, which demonstrate anti-inflammatory effects of HDL and apoA-I on adipose tissue inflammation, offer additional possibilities for the prevention of diabetes and atherosclerosis associated with adipose tissue inflammation.
Supplementary Material
Novelty and Significance.
What Is Known?
High density lipoproteins (HDL) and apolipoprotein A-I (apoA-I) have atheroprotective effects, which may be in part due to their anti-inflammatory properties.
The mechanism by which HDL and apoA-I exert their anti-inflammatory effect in macrophages and endothelial cells appears to be via cholesterol depletion of lipid rafts on cell membranes.
What New Information Does This Article Contribute?
Cholesterol depletion of lipid rafts in adipocytes by HDL, apoA-I or methyl-β-cyclodextrin inhibited palmitate-mediated chemotactic factor expression.
HDL and apoA-I have anti-inflammatory effects on adipocytes similar to other cell types such as macrophages and endothelial cells.
Similar findings were observed in adipose tissue in vivo as a result of apoA-I overexpression.
HDL and apoA-I exert anti-inflammatory effects on macrophages and endothelial cells by depleting cholesterol in lipid rafts. However, it unclear whether HDL and apoA-I have similar effects on adipocytes. We found that both HDL and apoA-I inhibit palmitate-mediated induction of monocyte chemotactic factor gene expression in cultured adipocytes by a mechanism consistent with the disruption and the removal of cholesterol from lipid rafts. Moreover, overexpression of apoA-I in vivo reduced chemotactic factor expression and macrophage accumulation in adipose tissue in mice fed a high-fat diet. These findings indicate that HDL and apoA-I have anti-inflammatory effects on adipocytes and adipose tissue, similar to their better-known effects on vascular cells such as macrophages and endothelial cells. Because macrophage accumulation in adipose tissue is an important harbinger of insulin resistance and cardiovascular disease (CVD), these results have important translational implications for the prevention and the management of insulin resistance and obesity-associated CVD by controlling adipose tissue inflammation and its downstream consequences.
Acknowledgments
SOURCES OF FUNDING
NIH grants HL094352, HL092969 and DK-035816
Nonstandard Abbreviations
- MCP-1
monocyte chemoattractant protein-1
- SAA
serum amyloid A
- CAV1
caveolin-1
- FFA
free fatty acids
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IBX
3-isobutyl-1-methylxanthine
- ROS
reactive oxygen species
- LRs
Lipid rafts
- ApoA-I
apolipoprotein A-I
- SFAs
saturated fatty acids
- siRNA
small interfering RNA
- MβCD
Methyl-β-cyclodextrin
- ABCA-1
ATP-binding cassette (ABC) A-1
- ABCG-1
ATP-binding cassette (ABC) G-1
- SRB-1
scavenger receptor B-1 (SRB-1)
- CTB
cholera toxin subunit β
- HFHSC
high fat, high sucrose, cholesterol-containing diet
- NOX4
NADPH oxidase 4
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None
REFERENCES
- 1.Murdolo G, Smith U. The dysregulated adipose tissue: A connecting link between insulin resistance, type 2 diabetes mellitus and atherosclerosis. Nutr Metab Cardiovasc Dis. 2006;16(Suppl 1):S35–S38. doi: 10.1016/j.numecd.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Mathieu P, Lemieux I, Despres JP. Obesity, inflammation, and cardiovascular risk. Clinical pharmacology and therapeutics. 2010;87:407–416. doi: 10.1038/clpt.2009.311. [DOI] [PubMed] [Google Scholar]
- 3.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strissel KJ, DeFuria J, Shaul ME, Bennett G, Greenberg AS, Obin MS. T-cell recruitment and th1 polarization in adipose tissue during diet-induced obesity in c57bl/6 mice. Obesity (Silver Spring) 2010;18:1918–1925. doi: 10.1038/oby.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. Cd8+ effector t cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 7.Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, Wabitsch M, Fischer-Posovszky P, Barth TF, Dragun D, Skurk T, Hauner H, Bluher M, Unger T, Wolf AM, Knippschild U, Hombach V, Marx N. T-lymphocyte infiltration in visceral adipose tissue: A primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1304–1310. doi: 10.1161/ATVBAHA.108.165100. [DOI] [PubMed] [Google Scholar]
- 8.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr Ccr2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han CY, Subramanian S, Chan CK, Omer M, Chiba T, Wight TN, Chait A. Adipocyte-derived serum amyloid a3 and hyaluronan play a role in monocyte recruitment and adhesion. Diabetes. 2007;56:2260–2273. doi: 10.2337/db07-0218. [DOI] [PubMed] [Google Scholar]
- 10.Yeop Han C, Kargi AY, Omer M, Chan CK, Wabitsch M, O'Brien KD, Wight TN, Chait A. Differential effect of saturated and unsaturated free fatty acids on the generation of monocyte adhesion and chemotactic factors by adipocytes: Dissociation of adipocyte hypertrophy from inflammation. Diabetes. 2010;59:386–396. doi: 10.2337/db09-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alwaili K, Awan Z, Alshahrani A, Genest J. High-density lipoproteins and cardiovascular disease: 2010 update. Expert Rev Cardiovasc Ther. 2010;8:413–423. doi: 10.1586/erc.10.4. [DOI] [PubMed] [Google Scholar]
- 12.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of hdl. Circ Res. 2004;95:764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 13.Barter PJ, Puranik R, Rye KA. New insights into the role of hdl as an anti-inflammatory agent in the prevention of cardiovascular disease. Curr Cardiol Rep. 2007;9:493–498. doi: 10.1007/BF02938394. [DOI] [PubMed] [Google Scholar]
- 14.deGoma EM, deGoma RL, Rader DJ. Beyond high-density lipoprotein cholesterol levels evaluating high-density lipoprotein function as influenced by novel therapeutic approaches. J Am Coll Cardiol. 2008;51:2199–2211. doi: 10.1016/j.jacc.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia P, Vadas MA, Rye KA, Barter PJ, Gamble JR. High density lipoproteins (hdl) interrupt the sphingosine kinase signaling pathway. A possible mechanism for protection against atherosclerosis by hdl. J Biol Chem. 1999;274:33143–33147. doi: 10.1074/jbc.274.46.33143. [DOI] [PubMed] [Google Scholar]
- 16.McGrath KC, Li XH, Puranik R, Liong EC, Tan JT, Dy VM, Di Bartolo B, Barter PJ, Rye KA, Heather AK. Role of 3{beta}-hydroxysteroid-{delta}24 reductase in mediating antiinflammatory effects of high-density lipoproteins in endothelial cells. Arterioscler Thromb Vasc Biol. 2009 doi: 10.1161/ATVBAHA.109.184663. [DOI] [PubMed] [Google Scholar]
- 17.Navab M, Berliner JA, Subbanagounder G, Hama S, Lusis AJ, Castellani LW, Reddy S, Shih D, Shi W, Watson AD, Van Lenten BJ, Vora D, Fogelman AM. Hdl and the inflammatory response induced by ldl-derived oxidized phospholipids. Arterioscler Thromb Vasc Biol. 2001;21:481–488. doi: 10.1161/01.atv.21.4.481. [DOI] [PubMed] [Google Scholar]
- 18.Murphy AJ, Woollard KJ, Hoang A, Mukhamedova N, Stirzaker RA, McCormick SP, Remaley AT, Sviridov D, Chin-Dusting J. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler Thromb Vasc Biol. 2008;28:2071–2077. doi: 10.1161/ATVBAHA.108.168690. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y, Ishibashi M, Seimon T, Lee M, Sharma SM, Fitzgerald KA, Samokhin AO, Wang Y, Sayers S, Aikawa M, Jerome WG, Ostrowski MC, Bromme D, Libby P, Tabas IA, Welch CL, Tall AR. Free cholesterol accumulation in macrophage membranes activates toll-like receptors and p38 mitogen-activated protein kinase and induces cathepsin k. Circ Res. 2009;104:455–465. doi: 10.1161/CIRCRESAHA.108.182568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in abc transporter-deficient macrophages: Free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–1847. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simons K, Ehehalt R. Cholesterol, lipid rafts, and disease. J Clin Invest. 2002;110:597–603. doi: 10.1172/JCI16390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yancey PG, Bortnick AE, Kellner-Weibel G, de la Llera-Moya M, Phillips MC, Rothblat GH. Importance of different pathways of cellular cholesterol efflux. Arterioscler Thromb Vasc Biol. 2003;23:712–719. doi: 10.1161/01.ATV.0000057572.97137.DD. [DOI] [PubMed] [Google Scholar]
- 23.Oram J, Lawn R. Abca1: The gatekeeper for eliminating excess tissue cholesterol. J Lipid Res. 2001;42:1173–1179. [PubMed] [Google Scholar]
- 24.Wang N, Lan D, Chen W, Matsuura F, Tall AR. Atp-binding cassette transporters g1 and g4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc Natl Acad Sci U S A. 2004;101:9774–9779. doi: 10.1073/pnas.0403506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kabouridis PS, Janzen J, Magee AL, Ley SC. Cholesterol depletion disrupts lipid rafts and modulates the activity of multiple signaling pathways in t lymphocytes. Eur J Immunol. 2000;30:954–963. doi: 10.1002/1521-4141(200003)30:3<954::AID-IMMU954>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 26.Lin Y, Berg AH, Iyengar P, Lam TK, Giacca A, Combs TP, Rajala MW, Du X, Rollman B, Li W, Hawkins M, Barzilai N, Rhodes CJ, Fantus IG, Brownlee M, Scherer PE. The hyperglycemia-induced inflammatory response in adipocytes: The role of reactive oxygen species. J Biol Chem. 2005;280:4617–4626. doi: 10.1074/jbc.M411863200. [DOI] [PubMed] [Google Scholar]
- 27.Subramanian S, Han CY, Chiba T, McMillen TS, Wang SA, Haw A, 3rd, Kirk EA, O'Brien KD, Chait A. Dietary cholesterol worsens adipose tissue macrophage accumulation and atherosclerosis in obese ldl receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:685–691. doi: 10.1161/ATVBAHA.107.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han CY, Chiba T, Campbell JS, Fausto N, Chaisson M, Orasanu G, Plutzky J, Chait A. Reciprocal and coordinate regulation of serum amyloid a versus apolipoprotein a-i and paraoxonase-1 by inflammation in murine hepatocytes. Arterioscler Thromb Vasc Biol. 2006;26:1806–1813. doi: 10.1161/01.ATV.0000227472.70734.ad. [DOI] [PubMed] [Google Scholar]
- 29.Han CY, Umemoto T, Omer M, Den Hartigh LJ, Chiba T, LeBoeuf R, Buller CL, Sweet IR, Pennathur S, Abel ED, Chait A. Nadph oxidase-derived reactive oxygen species increases expression of monocyte chemotactic factor genes in cultured adipocytes. J Biol Chem. 2012;287:10379–10393. doi: 10.1074/jbc.M111.304998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Honda A, Yamashita K, Hara T, Ikegami T, Miyazaki T, Shirai M, Xu G, Numazawa M, Matsuzaki Y. Highly sensitive quantification of key regulatory oxysterols in biological samples by lc-esi-ms/ms. J Lipid Res. 2009;50:350–357. doi: 10.1194/jlr.D800040-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Smart EJ, Ying YS, Mineo C, Anderson RG. A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc Natl Acad Sci U S A. 1995;92:10104–10108. doi: 10.1073/pnas.92.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subramanian S, Goodspeed L, Wang S, Kim J, Zeng L, Ioannou GN, Haigh WG, Yeh MM, Kowdley KV, O'Brien KD, Pennathur S, Chait A. Dietary cholesterol exacerbates hepatic steatosis and inflammation in obese ldl receptor-deficient mice. J Lipid Res. 2011;52:1626–1635. doi: 10.1194/jlr.M016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han CY, Subramanian S, Chan CK, Omer M, Chiba T, Wight TN, Chait A. Adipocyte-derived serum amyloid a3 and hyaluronan play a role in monocyte recruitment and adhesion. Diabetes. 2007 doi: 10.2337/db07-0218. [DOI] [PubMed] [Google Scholar]
- 34.Giocondi MC, Milhiet PE, Dosset P, Le Grimellec C. Use of cyclodextrin for afm monitoring of model raft formation. Biophysical journal. 2004;86:861–869. doi: 10.1016/s0006-3495(04)74161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harder T, Scheiffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol. 1998;141:929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubin EM, Ishida BY, Clift SM, Krauss RM. Expression of human apolipoprotein a-i in transgenic mice results in reduced plasma levels of murine apolipoprotein a-i and the appearance of two new high density lipoprotein size subclasses. Proc Natl Acad Sci U S A. 1991;88:434–438. doi: 10.1073/pnas.88.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barter PJ, Baker PW, Rye KA. Effect of high-density lipoproteins on the expression of adhesion molecules in endothelial cells. Curr Opin Lipidol. 2002;13:285–288. doi: 10.1097/00041433-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Kimura T, Mogi C, Tomura H, Kuwabara A, Im DS, Sato K, Kurose H, Murakami M, Okajima F. Induction of scavenger receptor class b type i is critical for simvastatin enhancement of high-density lipoprotein-induced anti-inflammatory actions in endothelial cells. J Immunol. 2008;181:7332–7340. doi: 10.4049/jimmunol.181.10.7332. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed W, Orasanu G, Nehra V, Asatryan L, Rader DJ, Ziouzenkova O, Plutzky J. High-density lipoprotein hydrolysis by endothelial lipase activates pparalpha: A candidate mechanism for high-density lipoprotein-mediated repression of leukocyte adhesion. Circ Res. 2006;98:490–498. doi: 10.1161/01.RES.0000205846.46812.be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zidovetzki R, Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: Evidence, misconceptions and control strategies. Biochim Biophys Acta. 2007;1768:1311–1324. doi: 10.1016/j.bbamem.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaughan AM, Oram JF. Abca1 and abcg1 or abcg4 act sequentially to remove cellular cholesterol and generate cholesterol-rich hdl. J Lipid Res. 2006;47:2433–2443. doi: 10.1194/jlr.M600218-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Vaughan AM, Oram JF. Abcg1 redistributes cell cholesterol to domains removable by hdl but not by lipid-depleted apolipoproteins. J Biol Chem. 2005 doi: 10.1074/jbc.M505368200. [DOI] [PubMed] [Google Scholar]
- 43.Baldan A, Bojanic DD, Edwards PA. The abcs of sterol transport. J Lipid Res. 2009;50(Suppl):S80–S85. doi: 10.1194/jlr.R800044-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rohrer L, Ohnsorg PM, Lehner M, Landolt F, Rinninger F, von Eckardstein A. High-density lipoprotein transport through aortic endothelial cells involves scavenger receptor bi and atp-binding cassette transporter g1. Circ Res. 2009;104:1142–1150. doi: 10.1161/CIRCRESAHA.108.190587. [DOI] [PubMed] [Google Scholar]
- 45.Tarling EJ, Edwards PA. Atp binding cassette transporter g1 (abcg1) is an intracellular sterol transporter. Proc Natl Acad Sci U S A. 2011;108:19719–19724. doi: 10.1073/pnas.1113021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leslie M. Mysteries of the cell. Do lipid rafts exist? Science. 2011;334:1046–1047. doi: 10.1126/science.334.6059.1046-b. [DOI] [PubMed] [Google Scholar]
- 47.Nicholls SJ, Lundman P, Harmer JA, Cutri B, Griffiths KA, Rye KA, Barter PJ, Celermajer DS. Consumption of saturated fat impairs the anti-inflammatory properties of high-density lipoproteins and endothelial function. J Am Coll Cardiol. 2006;48:715–720. doi: 10.1016/j.jacc.2006.04.080. [DOI] [PubMed] [Google Scholar]
- 48.Terasaka N, Yu S, Yvan-Charvet L, Wang N, Mzhavia N, Langlois R, Pagler T, Li R, Welch CL, Goldberg IJ, Tall AR. Abcg1 and hdl protect against endothelial dysfunction in mice fed a high-cholesterol diet. J Clin Invest. 2008;118:3701–3713. doi: 10.1172/JCI35470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein ai. Nature. 1991;353:265–267. doi: 10.1038/353265a0. [DOI] [PubMed] [Google Scholar]
- 50.Romeo GR, Lee J, Shoelson SE. Metabolic syndrome, insulin resistance, and roles of inflammation--mechanisms and therapeutic targets. Arterioscler Thromb Vasc Biol. 2012;32:1771–1776. doi: 10.1161/ATVBAHA.111.241869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rader DJ, Tall AR. The not-so-simple hdl story: Is it time to revise the hdl cholesterol hypothesis? Nat Med. 2012;18:1344–1346. doi: 10.1038/nm.2937. [DOI] [PubMed] [Google Scholar]
- 52.Emerging Risk Factors C, Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J. Major lipids, apolipoproteins, and risk of vascular disease. Jama. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moore RE, Navab M, Millar JS, Zimetti F, Hama S, Rothblat GH, Rader DJ. Increased atherosclerosis in mice lacking apolipoprotein a-i attributable to both impaired reverse cholesterol transport and increased inflammation. Circ Res. 2005;97:763–771. doi: 10.1161/01.RES.0000185320.82962.F7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.