Abstract
Patient: Female, 14
Final Diagnosis: Postobstructive pulmonary edenma
Symptoms: Chest indrawing • bilateral pulmonary crepitations • tachypnea
Medication: —
Clinical Procedure: Controlled ventilatory support • positive end expiratory pressure
Specialty: Intensive care
Objective:
Unusual clinical course
Background:
Postobstructive pulmonary edema (POPE) is a life-threatening complication that occurs after the relief of an upper airway obstruction. POPE occurs rarely in children, primarily after non-lethal hanging.
Case Report:
We report the case of a 14-year-old girl who developed POPE after accidental near hanging. She had chest in-drawing, the SpO2 was 81% on room air, and pulmonary auscultation revealed bilateral crepitations. The chest x-ray showed bilateral diffuse infiltrates consistent with pulmonary edema. The intensive care management consisted of controlled ventilatory support with high-level positive end expiratory pressure. On the third day of hospitalization, the patient was weaned from the ventilator and extubated with a full recovery.
Conclusions:
This case confirms the importance of early recognition of POPE and the value of adapted treatment, which can lead to a favorable outcome and full recovery in cases of near hanging.
Keywords: post-obstructive pulmonary edema, near hanging, child
Bacground
Postobstructive pulmonary edema (POPE), also known as negative-pressure pulmonary edema, is a rare form of no- cardiogenic pulmonary edema. It is a potentially life-threatening complication in which pulmonary edema occurs shortly after relief of an upper airway obstruction. The incidence of POPE has been reported to be as high as 1 in 1000 general anesthetic cases and commonly presents as acute respiratory distress that requires immediate intervention [1], but POPE developing after non-lethal hanging has not been reported widely. We report a case of POPE in an adolescent girl following accidental near-hanging.
Case Report
A 14-year-old girl was admitted to our hospital with a history of accidental hanging. She had been trapped by a rope around the neck while playing in the garden. After this event, she was unconscious. While being transported to the hospital, she started breathing laboriously, breathing difficulty worsened with time, with increased cough and expectoration of bloodstained frothy secretions. At admission, she was unconscious with Glasgow coma scale (GCS) at 7 (E1V1M5), both pupils were equal and reactive to light, she was afebrile, with heart rate of 140/min, respiratory rate of 44/min, and blood pressure of 110/60 mmHg. The SpO2 was 81% on room air. The child had chest indrawing and chest auscultation revealed bilateral crepitations. She had bruises and abrasions on her neck. The other results of physical examination were unremarkable. The child had 2 tonic clonic seizures, treated with 4 mg of Midazolam. In view of severe respiratory distress and neurological worsening, the child was intubated orally using a cuffed 5.5mm endotracheal tube. She received 160 mg of Propofol, 30 mg of Rocuronium and 0.2 mg of Alfentanil. Pink frothy secretions were noted below the glottis. The child was mechanically ventilated with volume-controlled mode and ventilatory settings were: tidal volume 6ml/kg (weight: 40 kg), rate 16/min, I/E: 2.5, FiO2 100%, and PEEP 10 cm H2O. The vital parameters post-ventilation were heart rate of 140/min, blood pressure 120/85 mmHg, and SpO2 88% on FiO2 at 100%. The brain and cervical CT scan and the supra-aortic trunks Doppler ultrasonography results were normal. The electrocardiogram showed a sinus tachycardia. A chest x-ray showed bilateral diffuse infiltrates, primarily on the right, consistent with pulmonary edema, and a normal cardiac silhouette (Figure 1). Arterial blood gases on 100% fraction inspired oxygen revealed pO2 52 mm Hg, pCO2 39 mm Hg, Ph 7.43, and HCO3 25 mEq/L. All hematological and biochemical parameters were normal. During the next 48 hours, the patient was kept intubated and sedated with Midazolam, 0.3 mg/kg/h IV. PEEP was gradually decreased to 5 cm of H2O and FiO2 was simultaneously decreased to 0.5, maintaining SpO2 between 91% and 96%. On the third day of hospitalization, a chest x-ray showed resolution of the pulmonary edema. The patient recovered consciousness and was hemodynamically stable. She was then weaned from the ventilator and extubated. She remained asymptomatic, maintained saturation above 95% in room air, and had normal results on neurological examination. She was discharged home 4 days later.
Figure 1.
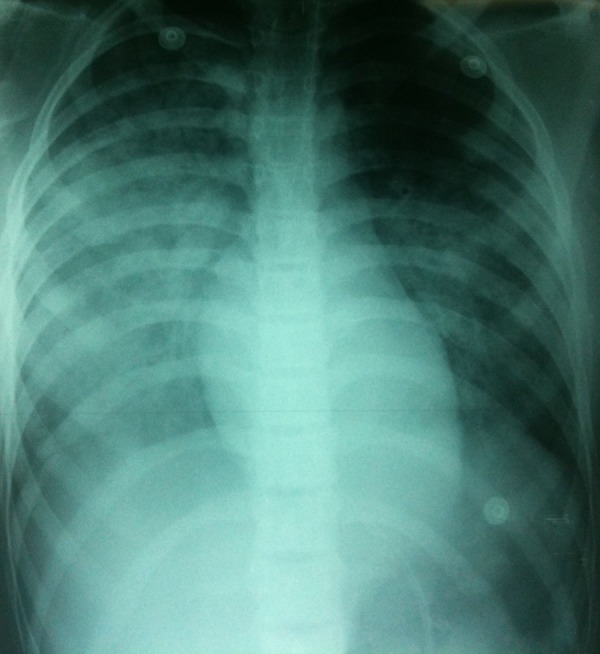
Chest x-ray shows diffuse, bilateral infiltrates consistent with pulmonary edema.
Discussion
The first description of POPE was made in an animal model in 1927 [2] and was first described in humans in 1973 [3]. Two distinct subclasses of POPE have been described: type I is associated with forceful inspiratory effort in the context of an acute airway obstruction, whereas type II occurs after relief of a chronic partial airway obstruction (eg, adenoidectomy, laryngeal mass resection, or reduction of a hypertrophic redundant uvula) [4]. The most common cause of POPE type I remains postextubation laryngospasm. Other causes of obstruction of upper airway leading to POPE include: hanging, mononucleosis, strangulation, near drowning, sleep apnea, biting of the endotracheal tube while intubated, croup and epiglottitis (especially in children), choking, foreign body, postoperative vocal cord paralysis, administration of muscle relaxant at the beginning of an inhalational induction of anesthesia (paralysis of glossal muscles before diaphragm), and following aspiration of pneumothorax or massive pleural effusion [1,5].The incidence of POPE in all anesthetics is 0.05% to 0.1% [6]. POPE may also follow strangulation or near hanging, but such cases are very rare in children. This is probably due to the high mortality rate of suicidal hangings.
The pathophysiology of POPE is multifactorial, involving components of negative pressure pulmonary edema, hypoxia, and a hyperadrenergic state [4]. The initiating event in POPE is the generation of markedly negative transpulmonary pressure during a forceful inspiration against a closed upper airway [7].
This exaggerated negative intrapleural pressure leads to increase in pulmonary blood flow due to an increase in venous return to the right side of the heart; the sudden influx of blood to the thorax also causes a decrease in the flow from the left side as a result of increased afterload. This combination causes increased pulmonary blood volume and elevated pulmonary venous pressures, which lead to an increase in hydrostatic pressures and edema formation [8–10]. In addition, high negative intrapleural pressure is transmitted to the interstitium and causes an increase in the hydrostatic gradient, favoring transudation of fluid from the pulmonary capillary to the pulmonary interstitial space, resulting in pulmonary edema [4]. Healthy young men who generate greater negative intrapleural pressures have an increased incidence of POPE, supporting the importance of vigorous inspiratory efforts in the development of POPE [11]. The hypoxemia due to upper airway obstruction leads to increased pre- and post-capillary pulmonary vascular resistance in a nonuniform manner, thus elevating the pulmonary vascular resistance. The hypoxemia associated with a hyper-adrenergic state precipitates pulmonary edema [12]. Fremont et al concluded that both the edema fluid/plasma protein ratio and the rate of net alveolar fluid clearance in patients with POPE were in the same range as patients with acute hydrostatic pulmonary edema, providing further support for the hypothesis that hydrostatic forces are the primary mechanism behind POPE and that the alveolar epithelium remains functionally intact [13].
POPE usually presents with rapid onset of acute respiratory failure, with dyspnea, tachypnea, and respiratory distress. Additional signs are paradoxical ventilation, pink frothy sputum, stridor, and severe agitation [14]. The chest radiograph findings of pulmonary edema support the diagnosis.
In judicial hanging, the death is due to fatal injury to the cervical spine, brain stem, and larynx; but suicide attempts or accidents usually cause compression of the internal jugular veins and the carotid arteries, leading to cerebral hypoxia and airway compression, and finally resulting in global hypoxia [15]. Most hospital deaths following near-fatal hangings are due to severe pulmonary edema or pneumonia [16]; the pulmonary edema may be from a neurogenic origin or secondary to negative intrathoracic pressures (POPE).
The initial phase of neurogenic pulmonary edema results from a centrally mediated, massive, sympathetic discharge. This discharge produces intense, generalized, but transient, vasoconstriction, with a resultant shift of blood from the high-resistance systemic circulation to the low-resistance pulmonary circulation. In addition, the hypoxic pulmonary vasoconstriction also increases permeability by disrupting the permeability barrier. Neurogenic pulmonary edema is often recognized after devastating and usually lethal brain injury [17].
It is likely that the prognosis for patients with POPE after strangulation injury is not as grave as that for patients with neurogenic pulmonary edema. Greater brain injury is caused in the context of neurogenic edema and this would be the major prognostic factor determining the ultimate outcome. Negative-pressure edema may result from transient airway obstruction, and resolution and recovery would be expected. It is difficult, if not impossible, to determine the initial cause of pulmonary edema in survivors of strangulation injury, although good neurological recovery suggests a brief episode of obstruction and negative pressure as the cause [18]. We believe that the pulmonary edema in our case was due to negative pressure secondary to sudden compression of airways. The total neurological recovery after extubation and the normal brain imagery support this diagnosis. Initial unconsciousness in our case may have resulted from transient jugular venous compression aggravated by general hypoxia due to acute pulmonary edema. Other causes of pulmonary edema, including aspiration pneumonia, iatrogenic volume overload, and cardiogenic cause, were excluded.
Treatment strategies vary according to the severity of symptoms of POPE. Supportive measures include maintaining a patent airway and ensuring adequate oxygenation via supplemental oxygen with the addition of positive end-expiratory pressure (PEEP) guided by pulse oximetry and arterial blood gas determinations, but severe cases require intubation and mechanical ventilation with PEEP. The role of diuretics in management of POPE is unclear.
With therapy, almost all patients improve within 24 to 48 hours and have a normal result on chest x-ray [14].
Conclusions
POPE, also known as negative-pressure pulmonary edema, is essentially a complication of laryngospasm. Airway obstruction is the main cause of morbidity and subsequent mortality in the survivors of hangings; pulmonary edema may subsequently develop in such patients. In most instances, POPE is a reversible process once it is recognized and properly treated [19].
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Van Kooy MA, Gargiulo RF. Postobstructive pulmonary edema. Am Fam Physician. 2000;62:401–4. [PubMed] [Google Scholar]
- 2.Moore RL, Binger CAL. The response to respiratory resistance: a comparison of the effects produced by partial obstruction in the inspiratory and expiratory phases of respiration. J Exp Med. 1927;45:1065–80. doi: 10.1084/jem.45.6.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capitanio MA, Kirkpatrick JA. Obstructions of the upper airway in children as reflected on the chest radiograph. Pediatr Radiol. 1973;107:159–61. doi: 10.1148/107.1.159. [DOI] [PubMed] [Google Scholar]
- 4.Udeshi A, Cantie SM, Pierre E. Postobstructive pulmonary edema. J Crit Care. 2010;25:508.e1–e5. doi: 10.1016/j.jcrc.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Singh Bajwa SJ, Kulshrestha A. Diagnosis, Prevention and Management of Postoperative Pulmonary Edema. Ann Med Health Sci Res. 2012;2:180–85. doi: 10.4103/2141-9248.105668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mc Conkey PP. Postobstructive pulmonary edema, a case series and review. Anaesth Intensive Care. 2001;29(1):79–80. [PubMed] [Google Scholar]
- 7.Trujillo MH, Fragachan CF, Tortoledo F. Noncardiogenic pulmonary edema following accidental near-hanging. Heart Lung. 2007;36:364–66. doi: 10.1016/j.hrtlng.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Smith-Erichsen NBG. Airway closure and fluid filtration in the lung. Br J Anesth. 1979;51:475–79. doi: 10.1093/bja/51.6.475. [DOI] [PubMed] [Google Scholar]
- 9.Scharf SM, Brown R, Tow DE, Parisi AF. Cardiac effects of increased lung volume and decreased pleural pressure in man. J Appl Physiol Resp Environ Exerc Physiol. 1979;47:257–62. doi: 10.1152/jappl.1979.47.2.257. [DOI] [PubMed] [Google Scholar]
- 10.Lang SA, Duncan PG, Shephard DA, et al. Pulmonary oedema associated with airway obstruction. Can J Anaesth. 1990;37:210–18. doi: 10.1007/BF03005472. [DOI] [PubMed] [Google Scholar]
- 11.Herrick IA, Mahendran B, Penny FJ. Postobstructive pulmonary edema following anesthesia. J Clin Anesth. 1990;2:116–20. doi: 10.1016/0952-8180(90)90064-a. [DOI] [PubMed] [Google Scholar]
- 12.Sarnoff SJ, Burglund E, Sarnoff LC. Neurohemodynamics of pulmonary edema. III. Estimated changes in pulmonary blood volume accompanying systemic vasoconstriction and vasodilation. J Appl Physiol. 1953;5:367–74. doi: 10.1152/jappl.1953.5.7.367. [DOI] [PubMed] [Google Scholar]
- 13.Fremont RD, Kallet RH, Matthay MA, Ware LB. Postobstructive Pulmonary Edema A Case for Hydrostatic Mechanisms. Chest. 2007;131:1742–46. doi: 10.1378/chest.06-2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinha A, Sivanandan S, Ramesh P, et al. Post Obstructive Pulmonary Edema in a Child who attempted Suicidal Hanging. Indian J Pediatr. 2008;75:1075–77. doi: 10.1007/s12098-008-0091-9. [DOI] [PubMed] [Google Scholar]
- 15.Nair S, Jacob J, Aaron S, et al. Pulmonary distress following attempted suicidal hanging. Indian J Med Sci. 2009;63:53–57. [PubMed] [Google Scholar]
- 16.Fischman CM, Goldstein MS, Gardner LB. Suicidal hanging: An association with the adult respiratory distress syndrome. Chest. 1977;71:225–27. doi: 10.1378/chest.71.2.225. [DOI] [PubMed] [Google Scholar]
- 17.Theodore J, Robin ED. Pathogenesis of neurogenic pulmonary edema. Lancet. 1975;2:745–51. doi: 10.1016/s0140-6736(75)90729-1. [DOI] [PubMed] [Google Scholar]
- 18.Kaki A, Crosby ET, Lui A. Airway and respiratory management following non-lethal hanging. Can J Anaesth. 1997;44:445–50. doi: 10.1007/BF03014468. [DOI] [PubMed] [Google Scholar]
- 19.Aggarwal R, Anant S, Vardhan H. Pulmonary Oedema in a survivor of Suicidal Hanging. MJAFI. 2004;60:188–89. doi: 10.1016/S0377-1237(04)80120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


