Abstract
Returning to the sea, just like invasion of land, has occurred in many groups of animals and plants. For flowering plants, traits adapted to the terrestrial environments have to change or adopt a new function to allow the plants to survive and prosper in the sea where water motion tends to rotate and move seeds. In this investigation, how seeds of the seagrass Thalassia hemprichii (Hydrocharitaceae), a common monocotyledon in the Indo-Pacific, adapt to the wavy environment was studied. Mature seeds were collected from Dongsha Atoll in South China Sea. The effects of light qualities on seed germination, the seed morphology, the unipolar distribution of starch granules in the endosperms and growth of root hair-like filamentous cells from basal surface of the seeds were all found to differ from those of terrestrial monocotyledons. Physiologically, germination of the seeds was stimulated by blue light rather than red light. Morphologically, the bell-shaped seeds coupled with the unipolar distribution of starch granules in the enlarged bases helped maintain their upright posture on the tidal seafloor. Growth of root hair-like filamentous cells from the basal surface of the seeds prior to primary root growth served to attach onto sediments, providing leverage and attachment required by the primary roots to insert into sediments. These filamentous cells grasped coral sand but not silicate sand, demonstrating a habitat preference of this species.
Introduction
Seagrasses are descendants of terrestrial plants that are able to complete their life cycle in the submerged photic zones in the sea. It was estimated that they entered the sea at three different times between 75 and 17 million years ago based on rbcL DNA sequence analysis [1], [2]. Seagrass meadows are crucial in the marine ecosystems [3], [4], [5]. They serve as nurseries and feeding grounds for diverse marine live forms. These meadows are important sites of carbon sequestration [6], and they stabilize coastline by holding sediments using rhizomes and roots. Four families of seagrasses, Hydrocharitaceae, Posidoniaceae, Cymodoceaceae and Zosteraceae are identified worldwide. These families comprise 12 genera and 58 species, and all of these species are flowering monocotyledons [1], [7]. The diversity of seagrass in different oceanic areas varies, and the Tropical Indo-Pacific bioregion has the highest diversity compared to the other five bioregions [7]. The habitat of some species such as Thalassia hemprichii (Hydrocharitaceae) ranges several thousand kilometers along coastline and extends to remote islands and coral reefs in open oceans [8]. On the other hand, ten species in Phyllopadix, Zostera, Halophila, and Posidonia are listed as endangered or vulnerable species in the IUCN Red List [9].
In order to propagate in the sea, ancestors of seagrasses had to adapt to new stresses imposed by the oceanic environment [10]. The first stress is the high salinity of the seawater which contains about 0.55 M salt. This level of salt is too high for the majority of terrestrial plants to stay alive. The second is the change of light spectrum. Red and far-red lights are absorbed by water easily and thus irradiation within these two wavelength ranges is scarce at the photic seafloor [11]. Red and far-red lights are environmental cues which are perceived by the photoreceptor phytochromes for many developmental programs and physiological responses in flowering plants. These developmental and physiological events include flowering, seed germination, seedling elongation, circadian rhythm and chlorophyll synthesis [12]. How seagrasses initiate these programs in an environment lacking red and far-red light is still not clear. The third significant change is the wavy environment that imposes new constraints on reproduction and dispersal processes employed by flowering plants. Seedling settlement is especially difficult in the constantly moving watery environment. In order to propagate in the sea, seagrasses must have developed mechanisms to cope with these challenges.
Thalassia hemprichii is a successful seagrass species in the Indo-Pacific bioregion [7], [8], [9]. However, this species is diminishing at some locations such as in the southern coast of Taiwan. For successful conservation and restoration, it is important to understand the details of how this species propagates and its habitat requirements. In this study we discovered novel developmental modifications of Thalassia hemprichii seeds and seedlings. These are demonstrations of Thalassia hemprichii’s adaptation to the wavy environment, which contribute to the success of this species in the ocean.
Materials and Methods
Ethics Statement
Collection permits at Dongsha Atoll National Park were granted by Marine National Park Headquarters of Taiwan. No endangered or protected species was used in this study.
Thalassia hemprichii Plants, Fruits and Seeds
Plants and fruits of T. hemprichii were collected at Dongsha Atoll National Park of Taiwan (20°43′N, 116°42′E) between January and April in both 2011 and 2012. Seeds were gathered from the collected fruits.
Specimen Preparation, Starch Granule Staining and Microscopy
Blocks of sectioned seeds were fixed in modified FPA fixatives (37% formaldehyde: propionic acid: 95% ethanol: glycerol: water = 1∶1∶7∶3∶8, v/v) [13]. Samples were embedded in paraffin and sectioned by a microtome. Sections of 10 µm thickness were dewaxed, rehydrated, and stained by I2-KI solution (1% iodine and 1% potassium iodide in water) until desired intensity was reached [14], [15]. Starch granules were observed and imaged with a light microscope using phase contrast and polarized light.
Treatments for Seed Germination
Reconstituted seawater (32 g crude salt/L H2O) was used for the germination tests. The tests were conducted in a darkroom at room temperature. Seed coats of these seeds were removed and they were placed in 6-well cell culture plates (well size 17×35 mm, depth and diameter, Nunc Inc.), submerged in the reconstituted seawater and illuminated continuously with intensities specified in the results. The seawater was renewed and the seeds were examined each day. Rotten and loss-of-turgor seeds were removed everyday. White light was given by an incandescent bulb. Blue and red lights were given from LEDs (light emitting diode) with spectra peaked at 455 and 645 nm, respectively. Light intensities were measured by a LI-COR photometer. Shoots reaching the height of seed tips were recorded as germinating (Fig. 1C). A total of 277 seeds were used in the germination experiments.
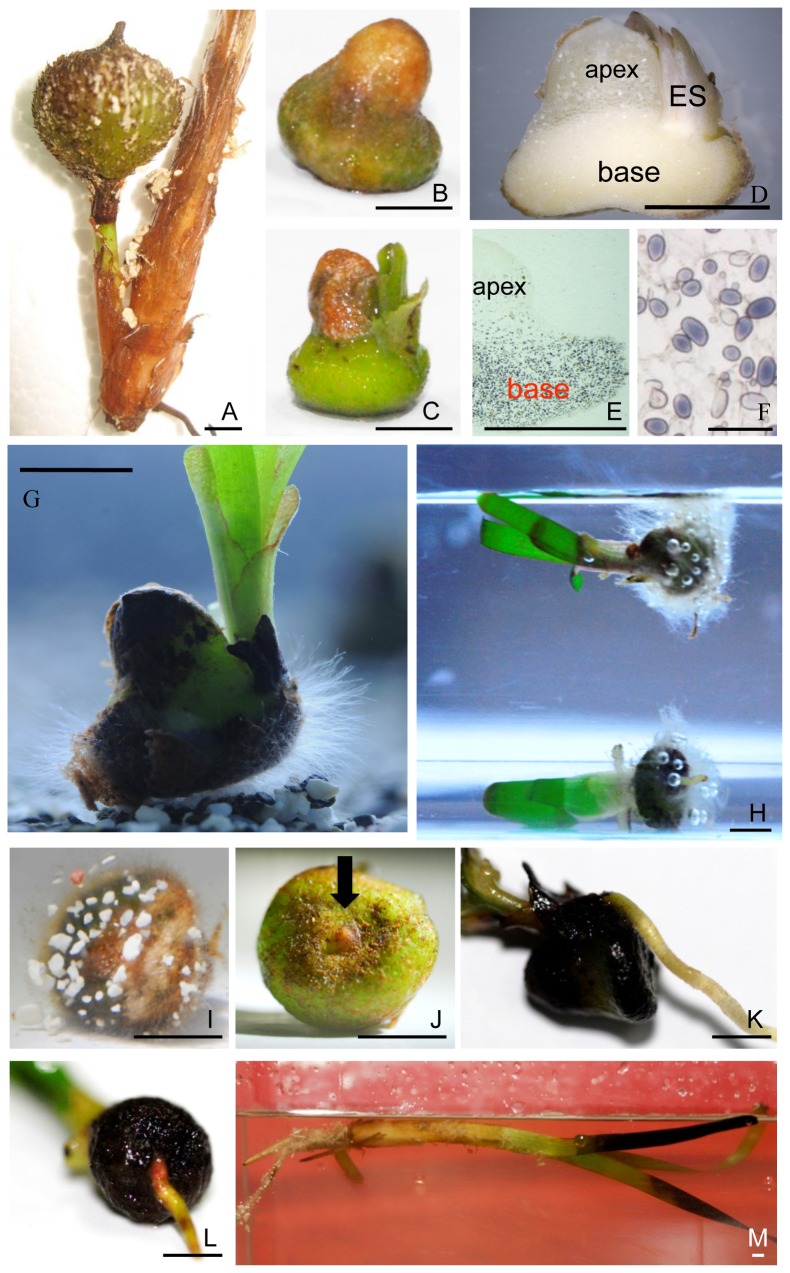
Figure 1. Fruit, seeds, and seedlings of T. hemprichii.
A fruit attached to the base of a shoot (A). A typical seed with seed coat resembling a bell in shape (B). Seed germination at early stage (C). A longitudinal section of a seed illustrating texture difference between the apex and the base; ES, embryonic shoots (D). Uneven distribution of starch granules, stained by iodine in a longitudinal section, between the apex and the base (E). Magnification of the stained starch granules (F). A germinating seed with root hair-like filamentous cells emerging from all over the basal surface before emergence of primary root. Note that the sediment is composed of coral and silicate sands (G). A seedling floating in seawater when its vegetative tissue is dominant (H). A seedling right after germination shown in (G) with root hair-like filamentous cells grasping of small coral sands but not silicate sand at the seed base (I). A basal surface view of a typical seed with a tip of the primary root, indicated by the black arrow, at the base center before germination (J). A primary root emerged beneath the shoots (K). A primary root emerged from the center of seed base (L). A young plant floating in seawater (M). Scale bar in F: 100 µm; in others: 0.5 cm.
Measurement of Seed and Coral Sand Densities
Total weight of a group of randomly selected nine pieces of apical or basal tissues of the seeds was measured by a digital balance. The volume of each group was measured using a 25-mL pipette (internal diameter 1.2 cm). Each group was sunk into the tip-sealed pipette filled with water. The volume of displaced water was measured by the calibrated lines on the pipette. Density was calculated as weight/volume. Density of coral sand was measured using the same principle.
Results and Discussion
Combination of Shape and Starch Granule Distribution in Thalassia hemprichii Seeds Facilitates Seed Orientation in the Wavy Sea
The characteristic morphology of T. hemprichii seeds is a bell shape (Fig. 1B–D, G). In longitudinal sections, we observed that the tissue in the bases was harder and whiter than that of the apices as shown in Fig. 1D which was intriguing. By examining the microscopic structures and components of the apical and the basal cells, large amounts of starch granules were found within the base but few in the apex cells (Fig. 1E and F). The cell wall thickness of the two tissues was equivalent (data not shown). Due to the uneven distribution of starch granules in the seeds, we suspected that the tissue densities of the apex and the base would differ. To verify this assumption, the seeds were cross-sectioned at the waistline to separate the apices and the bases, and their respective densities were measured. The results showed that density of the apex tissue (1.033±0.018 g/cm3, mean ±95% C.I., n = 5) was at the same level as surface seawater (about 1.025 g/cm3). However, density of the base tissue was 12% higher (1.157±0.028 g/cm3, n = 5) than that of the apex tissue (Fig. 2). The bell shape of T. hemprichii seeds coupled with their concentrated starch granules in the enlarged bases help the seeds to adapt to the wavy environment. The function of this adaptation is to ensure at least part of the base remains in contact with seafloor and to allow the root hair-like filamentous cells growing from the basal surface to more readily grasp sediments (see below).
Figure 2. Density difference between the apex and the base of Thalassia hemprichii seeds.
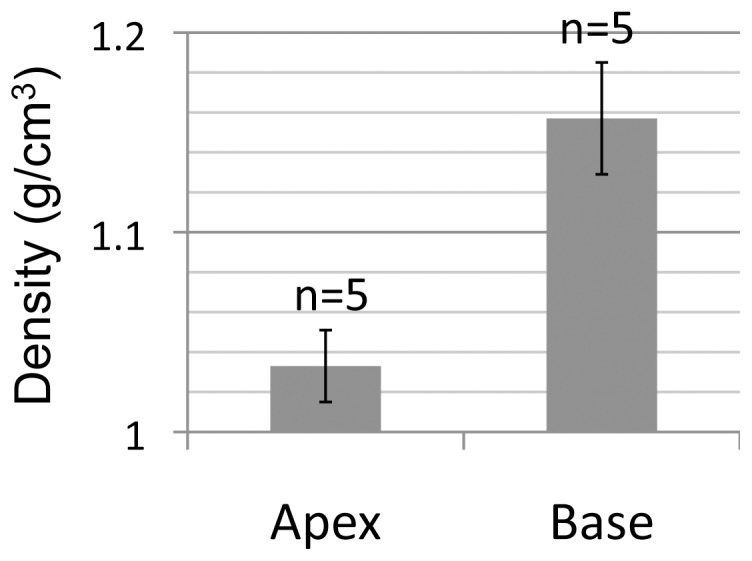
Error bars indicate 95% C.I.
To the best of our knowledge, the bell-shaped seeds and the unipolar distribution of starch granules in the seeds are novel developmental modifications that have not been observed in the evolution of terrestrial plants. Anatomically, the apical and the basal tissues are parts of an endosperm that is used as nutrient reservoir for seedling growth (Fig. 1D). This is confirmed by the observations that the two tissues diminished during seedling growth and eventually disappeared. Each monocotyledon seed contains one embryo and one endosperm, which result from double fertilization. Endosperm cells are triploid, and their genetic activities are permanently switched off when seeds reach maturity. Since the apical and the basal cells are both parts of an endosperm, they are generated from the same triploid progenitor cell. How the differentiation of the two tissues is regulated during the seed developmental process needs to be clarified through further studies.
Growth of Root Hair-like Filamentous Cells from the Basal Surface of the Seeds Prior to Primary Root Emergence Prevents Rolling of the Seeds Caused by Moving Seawater
An additional novel developmental modification of the seeds for the adaptation to the wavy environment is that root hair-like filamentous cells grow before emergence of the primary root during seed germination. As shown in Figure 1G–I, the filamentous cells grew from all over the basal surface of the seeds at an early stage of germination. These filamentous cells were able to grasp coral sand but not silicate sand, presumably by attaching to the small grooves and holes of coral sand grains or due to chemical interactions (Fig. 1I). The density of coral sand was 2.544±0.016 g/cm3 (n = 5) by our measurements, which was much higher than that of the seeds. The growth of the filamentous cells prior to primary roots prevents the seeds from rolling on seafloor in the wavy environment. These cells also provide the gripping power necessary to allow primary roots to insert into compact coral sand beds.
Gravity is detected by a mechanism comprising specialized starch granules called amyloplasts in the root tip cells in flowering plants [16]. In a seed being rolled by currents, the amyloplasts are agitated constantly which prevents the root tips from identifying the gravity vector and thus gravitropic growth is abolished. This would likely lead to death of the plant if its primary root is not able to grow in the right direction to anchor the new seedling.
For terrestrial plants, root hairs are extensions of root epidermal cells, which grow at the maturation zone of a root. The identity of the cells that generate the root hair-like filamentous cells from the basal surface of T. hemprichii seeds has yet to be positively identified. However, evidence suggests they are aleurone cells. Aleurone is a layer of tissue covering the surface of endosperm. These cells and endosperm cells share the same origin. However, endosperm cells are filled with starch and have little biochemical activities. In contrast, aleurone cells contain high level of protein and are biochemically active during seed germination [17]. In grass seeds such as corn, rice, barley and wheat, aleurone cells secrete enzymes into endosperm during seed germination to digest the starchy tissue. The degraded biomaterials are then used as nutrients for seedling growth. Since the endosperm cells are degraded by aleurone cells during germination, it is unlikely this cells could develop structures such as these root hair-like filamentous cells. This suggests that the cells growing the filamentous cells are likely aleurone cells. More studies are required to verify this hypothesis. These filamentous cells perished eventually together with the aleurone and endosperm cells as the seedlings continued their growth.
Thalassia hemprichii Seed Germination was Enhanced by White and Blue Light but not Red Light
As mentioned previously, red and far-red wavelengths are scarce at the seafloor. It is uncertain whether the red/far-red light photoreceptor phytochromes in T. hemprichii have lost their function since no selection pressure was imposed on the photoreceptors over the course of its evolution. Seed germination is a typical test used to examine the function of phytochromes. In most plant species, red light is able to stimulate seed germination even at low levels. On the other hand, mutants with impaired phytochrome function are not able to perceive red light, resulting in significant reduced germination rates compared to wildtype counter parts [18].
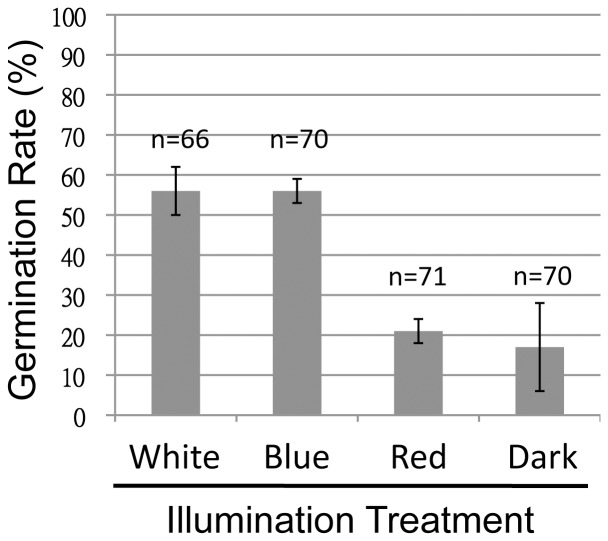
T. hemprichii seeds were germinated under various light conditions. While over 50% of seeds illuminated under white or blue light at 50 µmol/m2s germinated, only 21% of seeds treated under red light of the same intensity germinated. This low percentage was comparable to seeds germinated in darkness which was 17% (Fig. 3). The differences between white/blue and red/dark were all significant (P<0.01, t-tests). To exclude the possibility that the low germination rates under red light was caused by the low light intensity, the germination was re-examined under 50 and 200 µmol/m2s of red light. The results showed that there was no difference between the two treatments over the course of germination (data not shown). Based on these results, it is very likely that the phytochrome or its downstream signaling genes in T. hemprichii have mutated, resulting in a loss of either red light perception or the signaling pathways. Cloning and sequencing of these genes will be necessary to verify these hypotheses.
Figure 3. White and blue light enhances germination of Thalassia hemprichii seeds.
Seeds were immersed in the reconstituted seawater and illuminated under different light treatments. The light intensities were set at 50 µmol/m2s. “n” indicates combined number of seeds in duplicated tests. The experiments were terminated when the germination rate of at least one group exceeded 50%. Error bars indicate 95% C.I.
T. hemprichii seeds are non-dormant, i.e., their germination process starts within a short period of time after seed maturation. Nevertheless, they are still able to fine tune the timing of germination based on the availability of white and blue light wavelengths that are more abundant than red light on the seafloor. However, blue light has rarely been related to seed germination in terrestrial plants. Blue light, as an environmental signal, is perceived by the photoreceptors cryptochromes and phototropins. The canonical physiological roles of blue light receptors include regulation of circadian rhythm, phototropism, and stimulation of stomatal opening [19]. How blue light stimulates T. hemprichii seed germination is currently unclear.
Thalassia hemprichii Seedlings Anchored to Coral Sand but not Silicate Sand
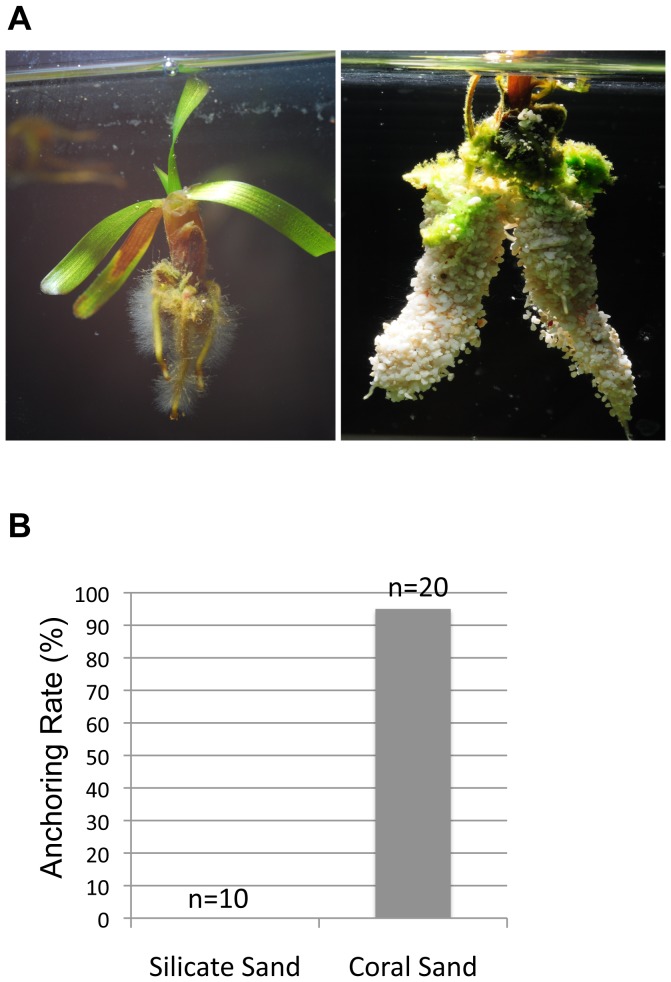
We observed that most T. hemprichii habitats are close to coral reefs or on dead reef platforms rather than on silicate sand beds. In addition, T. hemprichii population is declining in the southern coast of a national park in Taiwan which has become a stretch of silicate sand beach. The two lines of evidence suggest the possibility that the seeds have difficulty in anchoring to a silicate sand bed. To test whether the root hair-like filamentous cells could distinguish sediments, T. hemprichii seeds were germinated on (1) a bed with mixed coral and silicate sand as shown in Figure 1G, and (2) silicate or coral sand bed separately in the same aquarium that were divided by a porous plate and illuminated under 100 µmol/m2s white light 16 hours a day. In the first experiment, the filamentous cells from the basal surface grew first as described previously and they held onto coral sand only as shown in Figure 1I but not silicate sand. In the second experiment, the roots of the coral sand group were able to insert into the bed successfully. On the other hand, the roots in the silicate sand group were unable to insert into the sediment. The seedlings in this latter group eventually floated to the top of the water column when nutrients in the endosperms were depleted and its vegetative tissues became dominant (Fig. 4A, left). Plenty of root hairs were growing from the roots (Fig. 4A, left), which is a typical trait of flowering plants. A seedling of the coral sand group was pulled out of the sediment to demonstrate the holding capacity of the root hairs to coral sand (Fig. 4A, right). The anchoring rates of the seeds to the sediments are dependent on the composition of the sand, as 19 out of 20 seeds in coral sand secured to the sediment but all 10 in the silicate sand group floated (Fig. 4B).
Figure 4. Thalassia hemprichii seedlings anchor to a bed of coral sand but not silicate sand.
Seeds were allowed to germinate on beds of silicate or coral sand in the same aquarium. Seedlings anchored themselves using root and root hairs to grip coral sand (A, right and B) but not silicate sand (A, left and B; Chi-square test, p<0.01). Seedlings not anchored were floating in the water column when the vegetative tissues became dominant (A, left).
We have discovered three novel developmental modifications of the monocotyledon seagrass Thalassia hemprichii, which had not been observed among terrestrial plants. These modifications include the bell-shaped seeds, the unipolar distribution of starch granules in the base of the seeds and the root hair-like filamentous cells growing from the basal surface of the seeds prior to primary roots. The roles of these modifications are to increase the probability of the seedlings to anchor into coral sand beds in the wavy environments. In addition, we also discovered that red light is no longer able to stimulate seed germination of T. hemprichii but white and blue light do. How this species executes the signal of blue light to stimulate seed germination is an interesting question. T. hemprichii seedlings have a preference for coral sand beds but not silicate sand. This helps to explain the difficulty of restoring T. hemprichii population in the black sandy coastal areas.
Unanchored T. hemprichii seedlings and plants are able to float in seawater (Figs. 1H, 1M, and 4A left). This may provide an alternative way of dispersal for this species other than using seeds, if the transported seedlings and plants are able to anchor themselves onto sediments at their destinations. Whether the plants have similar preference over sediment composition for anchoring as germinating seeds needs to be clarified.
Acknowledgments
The authors thank numerous students who assisted this study.
Funding Statement
This work was financed in part by a grant from Asian-Pacific Ocean Study Center of National Sun Yat-sen University and a grant NSC 101-2119-M-110-004-MY5 from National Science Council, Taiwan. This project was also supported by Dongsha Atoll Research Station. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Les DH, Cleland MA (1997) Phylogenetic studies in Alismatidae, II: Evolution of marine angiosperms (seagrasses) and hydrophily. Systematic Botany 22: 443–463. [Google Scholar]
- 2. Janssen T, Bremer K (2004) The age of major monocot groups inferred from 800+ rbcL sequences. Botanical Journal of the Linnean Society 146: 385–398. [Google Scholar]
- 3. Waycott M, Duarte CM, Carruthers TJ, Orth RJ, Dennison WC, et al. (2009) Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proceedings of the National Academy of Sciences USA 106: 12377–12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lucas C, Thangaradjou T, Papenbrock J (2012) Development of a DNA barcoding system for seagrass: Successful but not simple. Plos One 7: e29987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pollard PC, Greenway M (2013) Seagrasses in tropical Australia, productive and abundant for decades decimated overnight. Journal of Bioscience 38: 157–166. [DOI] [PubMed] [Google Scholar]
- 6. Fourqurean JW, Duarte CM, Kennedy H, Marba N, Holmer M, et al. (2012) Seagrass ecosystems as a globally significant carbon stock. Nature Geoscience 5: 505–509. [Google Scholar]
- 7. Short F, Carruthers T, Dennison W, Waycott M (2007) Global seagrass distribution and diversity: A bioregional model. Journal of Experimental Marine Biology and Ecology 350: 3–20. [Google Scholar]
- 8.Short FT, Carruthers TJR, Waycott M, Kendrick GA, Fourqurean JW, et al. (2010) Thalassia hemprichii. In IUCN 2012. IUCN Red List of Threatened Species. Version 2012.2 IUCN.
- 9. Short FT, Polidoro B, Livingstone SR, Carpenter KE, Bandeira S, et al. (2011) Extinction risk assesment of the world’s seagrass species. Biological Conservation 144: 1961–1971. [Google Scholar]
- 10. Wissler L, Codoner FM, Gu J, Reusch TB, Olsen JL, et al. (2011) Back to the sea twice: identifying candidate plant genes for molecular evolution to marine life. BMC Evolutionary Biology 11: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lalli CM, Parsons TR (1993) Biological Oceanography: An Introduction, p. 25: Pergamon Press.
- 12.Taiz L, Zeiger E (2006) Plant Physiology, 4th Ed, 417–441: Sinauer.
- 13.Johansen DA (1940) Plant Microtechnique. New York: McGraw Hill.
- 14.Gahan PB (1984) Plant Histochemistry and Cytochemistry: An Introduction. London: Academic Press.
- 15.Ruzin SE (1999) Plant Microtechnique and Microscopy. Oxford: Oxford University Press.
- 16. Hashiguchi Y, Tasaka M, Morita MT (2013) Mechanism of higher plant gravity sensing. American Journal of Botany 100: 91–100. [DOI] [PubMed] [Google Scholar]
- 17. Becraft PW, Yi G (2011) Regulation of aleurine development in crereal grains. Journal of Experimental Botany 62: 1669–1675. [DOI] [PubMed] [Google Scholar]
- 18. Franklin KA, Quail PH (2010) Phytochrome functions in Arabidopsis development. Journal of Experimental Botany 61: 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Losi A, Gartner W (2011) Old chromophores, new photoactivation paradigms, trendy applications: flavins in blue light-sensing photoreceptors. Photochemistry and Photobiology 87: 491–510. [DOI] [PubMed] [Google Scholar]