Abstract
Objectives
The aim of this study was to evaluate the discriminant capability of the patient acceptable symptom state (PASS) according to disease activity, in a cohort of Italian patients affected by systemic Lupus erythematosus (SLE).
Methods
Consecutive SLE patients were enrolled. At each visit, the patients underwent a complete physical examination and the clinical/laboratory data were collected in a standardized, computerized, and electronically-filled form. The evaluation of serum complement C3 and C4 levels and determination of autoantibodies was obtained. Disease activity was assessed with the SLEDAI-2K and ECLAM, while chronic damage was measured with the SLICC. Finally, PASS was assessed in all patients by asking to answer yes or no to a single question.
Results
One hundred sixty-five patients were enrolled (M/F 12/153; mean age 40.4±11.8 years, mean disease duration 109.1±96.2 months). No patients refused to answer, suggesting the acceptability of PASS. A total of 80% of patients rated their state as acceptable. The patients with an acceptable status had significantly lower mean SLEDAI-2K and ECLAM scores than the others [1.8±2.7 versus 3.4±2.3(P=0.004); 0.7±0.9 versus 1.4±1.1(P=0.0027)]. No significant differences were observed when considering chronic damage, evaluated with SLICC.
Conclusions
In the clinical practice, SLE patients assessment performed by using complex disease activity indices such as SLEDAI-2K and ECLAM, could be time consuming. In our study, for the first time, we used PASS, a quick and easily comprehensible tool, to evaluate the patients’ status, this single question seems to be able to discriminate patients with different disease activity, especially when this is determined by musculoskeletal involvement.
Introduction
Systemic lupus erythematosus (SLE) is a multifactorial autoimmune disease, involving genetic and environmental factors, characterized by a wide range of autoantibodies and clinical manifestations [1–10]. Monitoring of disease activity is an important aspect in the management of SLE patients, as recently pointed out in a core-set of recommendations proposed by the European League Against Rheumatism (EULAR) [11].
Through the years, many indices have been developed and validated to measure disease activity in SLE patients, such as the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) and the European Consensus Lupus Activity Measurement (ECLAM) [12,13]. Flare is another outcome measure that identifies patients with a worsening of disease activity. Several definitions have been proposed according to the disease activity index adopted, but no consensus has been reached so far [14,15]. More recently, in order to identify patients with a disease course characterized by a persistent status of activity, the concept of persistently active disease (PAD) was proposed [16–18].
Furthermore, in daily clinical practice, evaluation of disease activity is not always feasible due to time consuming and lacking data. Thus, it could be of interest the development of a feasible and time-sparing tool to assess patients’ status.
As recently pointed-out, the Outcome Measures in Rheumatology Clinical Trials (OMERACT) recommended the measurement of patient well-being, identified by a dichotomous conditions: satisfactory versus unsatisfactory status [19,20].
The patient acceptable symptom state (PASS) is a single-question outcome tool to evaluate the level of symptoms at which patients consider themselves well [21]. Data published in the literature report the application of PASS to patients affected by Ankylosing Spondylitis (AS), osteoarthritis (OA), and Rheumatoid Arthritis (RA). All these studies have demonstrated a significant association between PASS and disease activity, evaluated with different indices [22–26].
However, no data are available concerning a possible application of PASS in patients affected by SLE. Thus, the aim of the present study was to evaluate the discriminant capability of PASS according with disease activity in a cohort of Italian SLE patients.
Materials and Methods
Consecutive SLE patients were enrolled between January 2010 and June 2012 at the Lupus Clinic of the Rheumatology Unit, Sapienza University of Rome (“Sapienza Lupus Cohort”).
SLE diagnosis was performed according to the revised 1997 American College of Rheumatology (ACR) criteria [27]. Patients provided written informed consent at the time of the visit. The local ethical committee of “Policlinico Umberto I” of Rome, approved the study.
At each visit, the patients underwent a complete physical examination, the clinical and laboratory data were collected in a standardized, computerized, and electronically-filled form, which includes demographics, education level, past medical history with date of diagnosis, co-morbidities, previous and concomitant treatments.
The evaluation of serum complement C3 and C4 levels and determination of autoantibodies was obtained. ANA were determined by means of indirect immunofluorescence (IIF) on HEp-2 (titer ≥1:160 or ++ on a scale from + to ++++); anti-dsDNA with ELISA assays (considering levels two folds higher than the cut-off of the reference laboratory) or IIF on Crithidia Luciliae (titer ≥1:10), ENA (including anti-Ro/SSA, anti-La/SSB, anti-Sm, anti-RNP) by ELISA assay considering titers above the cut-off of the reference laboratory, anti-CL of IgG or IgM isotype, by ELISA, in serum or plasma at medium or high titers (e.g., > 40 GPL or MPL or above the 99th percentile), anti-β2Glycoprotein-I (GPI) of IgG or IgM isotype, by ELISA, in serum or plasma (above the 99th percentile), and finally Lupus anticoagulant (LA) was assessed according to the guidelines of the International Society on Thrombosis and Hemostasis (Scientific Subcommittee on lupus anticoagulant/phospholipid-dependent antibodies) [28]. For all the subjects, C3 and C4 concentrations were studied by means of radial immunodiffusion. Urine protein analysis was performed in patients with renal involvement (proteinuria/24 hours).
Disease activity was assessed at each visit with the SLEDAI-2K and ECLAM, while chronic damage was measured by the System Lupus International Collaborating Clinics (SLICC) Damage Index (SDI) [12,13,29].
In patients with more than 2 consecutive visits with a minimum interval of 2 months between them, the assessment of PAD could be performed and PAD was defined as a SLEDAI-2K score > 4, excluding serology alone [16].
In a subgroup of the enrolled patients the evaluation of quality of life by using the Medical Outcomes Study-Short Form 36 (SF-36) was performed [30].
Patient acceptable symptom state evaluation
PASS was assessed in all patients by asking to answer yes or no to a single question [21] that was previously translated into Italian. In the linguistic validation process, two Italian translators, who are native speakers and are experienced in translating health questionnaires, independently translated the question. After this process, the two translators compared their translations and produced a third translation jointly, which was given to a native English-speaking translator for translation back into English. Finally, the question was compared with the original English version and approved.
The original question was "Considering all the different ways your disease is affecting you, if you would stay in this state for the next months, do you consider that your current state is satisfactory?” [21]. For this question patients could give a dichotomized answer (yes or no). The Italian version administered to our SLE cohort was reported in Table S1.
The discriminant ability of PASS for use in SLE patients was assessed by comparing the relationship of PASS responses (yes versus no) with the disease activity indices SLEDAI-2K, ECLAM and PAD, recorded during the same visit. Moreover, the association with the chronic damage, assessed by SDI, was evaluated.
Statistical evaluation
We used version 13.0 of the SPSS statistical package. Normally distributed variables were summarized using the mean ± SD, and non-normally distributed variables by the median and range. Mann-Whitney test was performed accordingly. Univariate comparisons between nominal variables were calculated using chi-square test or Fisher-test where appropriate. All the associations were referred to the last visit at which PASS was evaluated. Two-tailed P values were reported, P values less than 0.05 were considered significant.
Results
One hundred sixty-five patients (M/F 12/153; mean age 40.4±11.8 years, mean disease duration 109.1±96.2 months; mean SLEDAI-2K 2.1±2.8; mean ECLAM 0.8±1.0; mean SDI 0.2±0.6) were evaluated.
No patients refused to answer, suggesting the acceptability of PASS. In our cohort, 136 patients (82.4%) showed a high educational level (more than 8 years of education), while 29 patients showed a low level of education (less than 8 years of education). No patients asked the examiner to repeat the question due to non-comprehension, and there was no difference in the percentage of patients answering yes or no according to the educational level (high level of education: yes 78.7%, no 21.3%; low level of education: yes 86.2%, no 13.8%, P=0.26), suggesting that the question was easy understood.
According with the answer given to PASS, the patients were dichotomized in group 1 (patients answering yes) and group 2 (patients answering no).
In table 1, the main demographic, clinical and laboratory features of SLE patients are reported according with the answer to PASS.
Table 1. Main demographic, clinical and laboratory features of 165 SLE patients, sub-grouped according with the response to PASS question.
| PASS-Yes | PASS-No | ||
|---|---|---|---|
| (Group1N=132) | (Group2N=33) | Pvalue | |
| Mean age±SD (years) | 40.2+11.5 | 41.2+13 | 0.09 |
| Sex (M/F) | 11/121 | 1/32 | 0.08 |
| Mean disease duration ± SD (months) | 96+98.8 | 81.9+80.4 | 0.07 |
| Clinical manifestations (N/%) | |||
| Mucocutaneous manifestations (malar/discoid rash, photosensitivity, oral ulcers) | 12/9.2 | 4/12.1 | 0.05 |
| Arthritis | 10/7.7 | 13/39.3 | 0.00003 |
| Serositits (Pleuritis or pericarditis) | 0 | 1/3.0 | 0.16 |
| Renal involvement (proteinuria >0.5gr/day or cellular casts) | 7/5.3 | 2/6 | 1 |
| Hematological manifestations (hemolytic anemia, leukopenia, lymphopenia, thrombocytopenia) | 17/13 | 8/24 | 0.1 |
| Neurological involvement (seizures or psychosis) | 3/2.2 | 1/3 | 0.1 |
| Laboratory assessment (N/%) | |||
| ANA | 93/89 | 24/92 | 0.8 |
| Anti-dsDNA | 36/38.7 | 8/32 | 0.8 |
| anti-Sm | 2/2.3 | 3/21 | 0.05 |
| anti-RNP | 2/2.3 | 2/14 | 0.002 |
| anti-SSA/Ro | 16/18.2 | 6/42 | 0.003 |
| anti-SSB/La | 5/5.6 | 1/7.1 | 1 |
| anti-CL | 19/29 | 4/28 | 1 |
| Anti-β2GPI | 13/27 | 1/10 | 0.003 |
| LA | 11/23 | 1/10 | 0.02 |
| Mean C3 levels ± SD (mg/dl) | 95.6+26.9 | 87.4+23.8 | 0.1 |
| Mean C4 levels ± SD (mg/dl) | 18.1+11.2 | 14.9+6.8 | 0.2 |
| Therapy | |||
| Glucocorticoids (N/%) | 96/72.7 | 26/78.7 | 0.6 |
| Mean glucocorticoids dosage±SD (prednisone equivalents) (mg/weekly) | 48.2+38.5 | 51.8+36.1 | 0.4 |
| Hydroxychloroquine (N/%) | 93/70 | 21/63 | 0.5 |
| Immunosuppressant agents (N/%) | 43/32.5 | 16/48.4 | 0.03 |
| Methotrexate (N/%) | 10/7.6 | 3/9.1 | 0.7 |
| Cyclosporine A (N/%) | 13/9.8 | 7/21.2 | 0.1 |
| Mycophenolate Mofetil (N/%) | 24/18.2 | 6/18.2 | 1 |
| Cyclophosphamide (N/%) | 1/0.7 | 0 | 1 |
| Azathioprine (N/%) | 34/25.7 | 8/24.2 | 1 |
| Rituximab (N/%) | 1/0.7 | 0 | 1 |
Abbreviations. SD: Standard deviation; CL: cardiolipin; LA: Lupus Anticoagulant.
Interestingly, group 1 patients had significantly lower mean SLEDAI-2K and ECLAM scores than group 2 patients [1.8±2.7 versus 3.4±2.3(P=0.004); 0.7±0.9 versus 1.4±1.1(P=0.0027), respectively; Figure 1 and Figure 2]. Although not statistically significant, the percentage of patients with PAD was lower in group 1 than group 2 patients (14.4% versus 21.1%, P=0.2).
Figure 1. SLEDAI values according with answer given to PASS question.
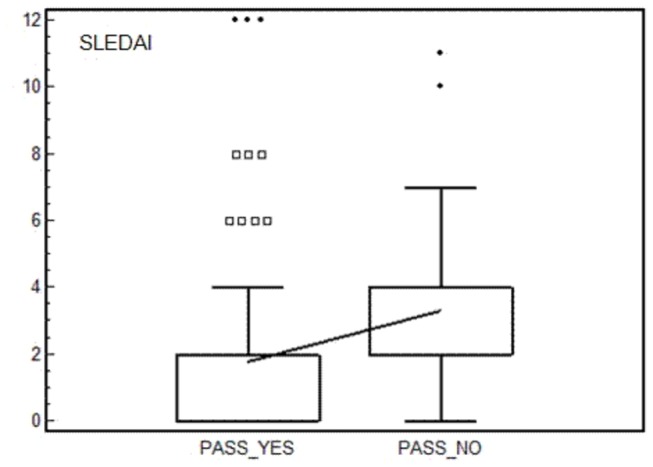
Box and whiskers plot (median, quartiles, range and possible extreme values).
Figure 2. ECLAM values according with answer given to PASS question.
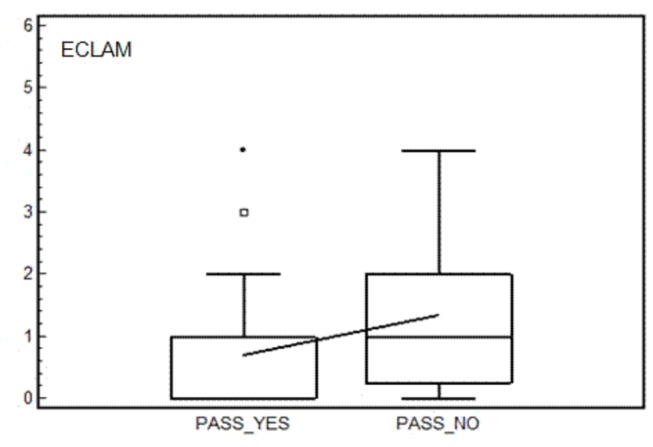
Box and whiskers plot (median, quartiles, range and possible extreme values).
No significant differences were observed when considering chronic damage, evaluated with SDI (group 1: 0.2±0.6, group 2: 0.2±0.5; P=0.09).
Aiming at evaluating the impact of the quality of life on PASS status, 30 patients (M/F 2/28; mean age ± SD 39.8±11.8 years; mean disease duration ± SD 112.4±102.7 months) completed the SF-36 questionnaire. Among these, 21 patients (70%) answered yes to PASS question. In table 2 we reported the mean values of SF-36 items according to PASS status. Patients answering yes showed higher values of all items, except for mental health. A significant difference between the two groups was demonstrated when considering the items “vitality” and “role limitations due to emotional problems” (P=0.01 and P=0.03, respectively).
Table 2. SF-36 items according with answer to PASS question.
| SF-36Domain (mean±SD) | PASS-Yes | PASS-No | P |
|---|---|---|---|
| (N=21) | (N=9) | ||
| Physical functioning | 78.7±24.7 | 63.8±26.3 | 0.05 |
| Role limitations due to physical problems | 66.4±43.4 | 51.2±43.4 | 0.09 |
| Bodily Pain | 69.4±27.4 | 47.3±21.6 | 0.08 |
| General health perceptions | 52.0±24.7 | 67.4±17.1 | 0.07 |
| Vitality | 62.7±25.3 | 42.2±16.2 | 0.01 |
| Social Functioning | 72.1±24.9 | 62.2±19.7 | 0.1 |
| Role limitations due to emotional problems | 64.6±43.3 | 36.2±41.8 | 0.03 |
| Mental health | 70.0±21.4 | 89.8±27.7 | 0.09 |
Abbreviations. SF-36: Short-Form 36; SD: Standard deviation;
Discussion
In the present study, we demonstrated for the first time the ability of the Patient Acceptable Symptom State (PASS) in discriminating patients affected by SLE with different level of the disease activity.
As emphasized during the OMERACT 6 meeting, the patients’ perspective evaluation is a very important outcome to perform a complete assessment, and it could influence clinical decision making [19]. In this context, PASS is a simple, reliable, and valid assessment of well-being and could be easily applied in rheumatology practice. This is a single and quick question, requiring little amount of time to be answered, due to the presence of a Boolean response (yes or no).
So far, PASS was applied in patients affected by different rheumatic conditions, such as OA, AS, and RA, showing a significant correlation with disease activity [22–26]. At first, PASS was evaluated in patients with knee and hip OA after a 4-weeks period of treatment with NSAIDs. PASS-defined satisfactory status was recorded in 57.7% of patients with knee OA and 50.2% with hip OA [23]. The percentage of patients with knee OA giving a positive response to PASS was lower in a study by Dougados and coll. characterized by a longer follow-up (13 weeks follow-up) in which the response to different Coxib was evaluated [31].
In patients affected by AS, a significant association between the presence of an acceptable symptom state and a reduced disease activity, assessed with BASDAI, BASFI and/or ASDAS, was found, underlining the external validity of PASS [32–34]. Rodriguez-Lozano et al. evaluated the discriminant capacity of PASS showing that AS patients with positive response to PASS assumed significantly lower dosages of NSAIDs and steroids than those patients not achieving an acceptable symptom state [23]. Similarly, a significantly higher frequency of patients with an acceptable status was achieved by AS patients treated with adalimumab compared with patients assuming placebo. Interestingly, patients treated with adalimumab had also lower disease activity [21,22,35].
More recently, PASS was administered to patients affected by RA. It has been shown that a positive response to PASS is associated with a range of moderate disease activity assessed with several composite indices, such as DAS28, CDAI and SDAI [36,37].
Following these previous experiences, we evaluated the discriminant capability of PASS in a cohort of Italian SLE patients. A significant association with disease activity, assessed by SLEDAI-2K and ECLAM was identified. In particular, patients with a positive response to PASS showed significant lower levels of SLEDAI-2K and ECLAM. In this same group, a lower percentage of patients with persistently active disease, defined by PAD, was observed.
When analyzing the clinical manifestations, a significant lower frequency of musculoskeletal involvement was found in patients with an acceptable symptom state. Joint involvement in SLE is very common, affecting up to 90% of patients at any stage of disease. The clinical presentation of joint involvement can widely vary, ranging from arthralgia, without erosions or deformity, to an erosive arthritis with severe functional disability [38]. The results of the present study confirm the impact of the musculoskeletal involvement in the acceptability of the patient’s status. The disability linked to the joint involvement, associated with the inability to perform the daily activities and the possible need of caregivers help, could make unacceptable the status of a SLE patient.
In our cohort, a significant higher percentage of autoantibodies in patients with a negative response to PASS was found. We could hypothesize that the presence of autoantibodies might be associated with a greater disease activity, influencing the acceptability of the patient’s status. Indeed, it’s widely known the association between anti-SSA and anti-RNP antibodies and mucocutaneous involvement in SLE patients [39]. Since body image is a major theme identified by patients, it is not surprising that mucocutaneous involvement and in turn the presence of anti-SSA and anti-RNP antibodies were more frequent in patients not accepting their status [40].
The absence of an association between PASS and SDI in this cohort is not surprising. Indeed, the response to PASS freezes the actual disease status, reflected by the disease activity but not by chronic damage indices. Moreover, in our population the low mean values of SDI probably don’t influence the patient’s status.
Even though SF-36 was administered only to a subgroup of SLE patients, overall a worse quality of life was identified in the patients answering no to PASS question.
Conclusions
PASS is a simple, reliable, and valid patient-reported outcome to assess patients’ well-being. It could provide a highly feasible tool for clinicians to focalize the disease activity status in SLE patients. In daily clinical practice PASS should not be applied as replacement but administered together with the commonly used activity indices. Larger studies are needed to confirm these results and verify its actual application in a disease characterized by a great clinical heterogeneity, such as SLE.
Supporting Information
Translation to Italian language of original question of patient acceptable symptom state (PASS).
(DOC)
Funding Statement
The authors have no support or funding to report.
References
- 1. Tsokos GC (2011) Systemic lupus erythematosus. N Engl J Med 365: 2110-2121. doi:10.1056/NEJMra1100359. PubMed: 22129255. [DOI] [PubMed] [Google Scholar]
- 2. Priori R, Medda E, Conti F, Cassara EA, Danieli MG et al. (2003) Familial autoimmunity as a risk factor for systemic lupus erythematosus and vice versa: a case-control study. Lupus 12: 735-740. doi:10.1191/0961203303lu457oa. PubMed: 14596421. [DOI] [PubMed] [Google Scholar]
- 3. Colasanti T, Maselli A, Conti F, Sanchez M, Alessandri C et al. (2012) Autoantibodies to estrogen receptor α interfere with T lymphocyte homeostasis and are associated with disease activity in systemic lupus erythematosus. Arthritis Rheum 64: 778-787. doi:10.1002/art.33400. PubMed: 21968947. [DOI] [PubMed] [Google Scholar]
- 4. Alessandri C, Barbati C, Vacirca D, Piscopo P, Confaloni A et al. (2012) T lymphocytes from patients with systemic lupus erythematosus are resistant to induction of autophagy. FASEB J 26: 4722-4732. doi:10.1096/fj.12-206060. PubMed: 22835828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Valesini G, Alessandri C, Celestino D, Conti F (2006) Anti-endothelial antibodies and neuropsychiatric systemic lupus erythematosus. Ann N Y Acad Sci 1069: 118-128. doi:10.1196/annals.1351.010. PubMed: 16855139. [DOI] [PubMed] [Google Scholar]
- 6. Margutti P, Sorice M, Conti F, Delunardo F, Racaniello M et al. (2005) Screening of an endothelial cDNA library identifies the C-terminal region of Nedd5 as a novel autoantigen in systemic lupus erythematosus with psychiatric manifestations. Arthritis Res Ther 7: R896-9037. doi:10.1186/ar1759. PubMed: 15987492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Conti F, Alessandri C, Bompane D, Bombardieri M, Spinelli FR et al. (2004) Autoantibody profile in systemic lupus erythematosus with psychiatric manifestations: a role for anti-endothelial-cell antibodies. Arthritis Res Ther 6: R366-R372. doi:10.1186/ar1198. PubMed: 15225372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Conti F, Spinelli FR, Alessandri C, Valesini G (2011) Toll-like receptors and lupus nephritis. Clin Rev Allergy Immunol 40: 192-198. doi:10.1007/s12016-010-8208-0. PubMed: 20596796. [DOI] [PubMed] [Google Scholar]
- 9. Conti F, Alessandri C, Perricone C, Scrivo R, Rezai S et al. (2012) Neurocognitive dysfunction in systemic lupus erythematosus: association with antiphospholipid antibodies, disease activity and chronic damage. PLOS ONE 7: e33824. doi:10.1371/journal.pone.0033824. PubMed: 22461897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Govoni M, Bombardieri S, Bortoluzzi A, Caniatti L, Casu C et al. ; Italian Society of Rheumatology; (2012) Factors and comorbidities associated with first neuropsychiatric event in systemic lupus erythematosus: does a risk profile exist? A large multicentre retrospective cross-sectional study on 959 Italian patients. Rheumatology(Oxford) 51: 157-68 [DOI] [PubMed] [Google Scholar]
- 11. Mosca M, Tani C, Aringer M, Bombardieri S, Boumpas D et al. (2010) European League Against Rheumatism recommendations for monitoring patients with systemic lupus erythematosus in clinical practice and in observational studies. Ann Rheum Dis 69: 1269-1274. doi:10.1136/ard.2009.117200. PubMed: 19892750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gladman DD, Ibañez D, Urowitz MB (2002) Systemic lupus erythematosus disease activity index 2000. J Rheumatol 29: 288-291. PubMed: 11838846. [PubMed] [Google Scholar]
- 13. Mosca M, Bencivelli W, Vitali C, Carrai P, Neri R et al. (2000) The validity of the ECLAM index for the retrospective evaluation of disease activity in systemic lupus erythematosus. Lupus 9: 445-450. doi:10.1191/096120300678828640. PubMed: 10981649. [DOI] [PubMed] [Google Scholar]
- 14. Gladman DD, Urowitz MB, Kagal A, Hallett D (2000) Accurately describing changes in disease activity in Systemic Lupus Erythematosus. J Rheumatol 27: 377-379. PubMed: 10685800. [PubMed] [Google Scholar]
- 15. Petri M, Kim MY, Kalunian KC, Grossman J, Hahn BH et al. OC-SELENA Trial (2005) Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med 353: 2550-2558. doi:10.1056/NEJMoa051135. PubMed: 16354891. [DOI] [PubMed] [Google Scholar]
- 16. Nikpour M, Urowitz MB, Ibañez D, Gladman DD (2009) Frequency and determinants of flare and persistently active disease in systemic lupus erythematosus. Arthritis Rheum 61: 1152-1158. doi:10.1002/art.24741. PubMed: 19714602. [DOI] [PubMed] [Google Scholar]
- 17. Conti F, Ceccarelli F, Perricone C, Massaro L, Spinelli FR et al. (2010) Low incidence of flare and persistent active disease in a cohort of Italian patients with systemic lupus erythematosus: comment on the article by Nikpour et al. Arthritis Care Res (Hoboken) 62: 899-900. doi:10.1002/acr.20145. [DOI] [PubMed] [Google Scholar]
- 18. Conti F, Ceccarelli F, Perricone C, Miranda F, Truglia S et al. (2012) Flare, persistently active disease, and serologically active clinically quiescent disease in systemic lupus erythematosus: a 2-year follow-up study. PLOS ONE 7: e45934. doi:10.1371/journal.pone.0045934. PubMed: 23029327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saag KG, OMERACT (2003) 6 brings new perspectives to rheumatology measurement research. J Rheumatol; 30: 639-641 PubMed; : 12672177 [PubMed] [Google Scholar]
- 20. Tubach F, Dougados M, Falissard B, Baron G, Logeart I et al. (2006) Feeling good rather than feeling better matters more to patients. Arth Rheum 55: 526-530. doi:10.1002/art.22110. PubMed: 16874795. [DOI] [PubMed] [Google Scholar]
- 21. Maksymowych WP, Gooch K, Dougados M, Wong RL, Chen N et al. (2010) Thresholds of patient-reported outcomes that define the patient acceptable symptom state in ankylosing spondylitis vary over time and by treatment and patient characteristics. Arth Care Res (Hoboken) 62: 826-834. doi:10.1002/art.27296. [DOI] [PubMed] [Google Scholar]
- 22. Dougados M, Luo MP, Maksymowych WP, Chmiel JJ, Chen N et al. ATLAS STUDY GROUP 2008) Evaluation of the patient acceptable symptom state as an outcome measure in patients with ankylosing spondylitis: data from a randomized controlled trial. Arth Rheum 59 pp. 553-560. [DOI] [PubMed] [Google Scholar]
- 23. Tubach F, Ravaud P, Baron G, Falissard B, Logeart I et al. (2005) Evaluation of clinically relevant states in patient reported outcomes in knee and hip osteoarthritis: the patient acceptable symptom state. Ann Rheum Dis 64: 34-37. doi:10.1136/ard.2004.023028. PubMed: 15130902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heiberg T, Kvien TK, Mowinckel P, Aletaha D, Smolen JS et al. (2008) Identification of disease activity and health status cut-off points for the symptom state acceptable to patients with rheumatoid arthritis. Ann Rheum Dis 67: 967-971. PubMed: 17965118. [DOI] [PubMed] [Google Scholar]
- 25. Kvamme MK, Kristiansen IS, Lie E, Kvien TK (2010) Identification of cutpoints for acceptable health status and important improvement in patient-reported outcomes, in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol 37: 26-31. doi:10.3899/jrheum.090449. PubMed: 19955045. [DOI] [PubMed] [Google Scholar]
- 26. Tubach F, Ravaud P, Martin-Mola E, Awada H, Bellamy N et al. (2012) Minimal clinically important improvement and patient acceptable symptomatic state in pain and function in rheumatoid arthritis, ankylosing spondylitis, chronic back pain, hand osteoarthritis, and hip and knee osteoarthritis: The reflect multinational study. Arth Care Res 64: 1699–1707. doi:10.1002/acr.21747. [DOI] [PubMed] [Google Scholar]
- 27. Hochberg MC (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40: 1725. doi:10.1002/art.1780400929. PubMed: 9324032. [DOI] [PubMed] [Google Scholar]
- 28. Pengo V, Tripodi A, Reber G, Rand JH, Ortel TL et al. ; Subcommittee on Lupus Anticoagulant /Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis (2009) Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost Yul': 1737-1740. doi:10.1111/j.1538-7836.2009.03555.x. PubMed: 19624461. [DOI] [PubMed] [Google Scholar]
- 29. Gladman DD, Urowitz MB, Goldsmith CH, Fortin P, Ginzler E et al. (1997) The reliability of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index in patients with systemic lupus erythematosus. Arthritis Rheum 40: 809-813. doi:10.1002/art.1780400506. PubMed: 9153540. [DOI] [PubMed] [Google Scholar]
- 30. Ware JE (2000) SF-36 health survey update. Spine 25: 3130–3139. doi:10.1097/00007632-200012150-00008. PubMed: 11124729. [DOI] [PubMed] [Google Scholar]
- 31. Dougados M, Moore A, Yu S, Gitton X (2007) Evaluation of the patient acceptable symptom state in a pooled analysis of two multicentre, randomised, double-blind, placebo-controlled studies evaluating lumiracoxib and celecoxib in patients with osteoarthritis. Arth Res Ther 9: 11. doi:10.1186/ar2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maksymowych WP, Richardson R, Mallon C, van der Heijde D, Boonen A (2007) Evaluation and validation of the patient acceptable symptom state (PASS) in patients with ankylosing spondylitis. Arthritis Rheum 57: 133-139. doi:10.1002/art.22469. PubMed: 17266072. [DOI] [PubMed] [Google Scholar]
- 33. Rodríguez-Lozano C, Gantes MÁ, González B, Hernández-Beriain JA, Naranjo A et al. (2012) Patient-acceptable symptom state as an outcome measure in the daily care of patients with ankylosing spondylitis. J Rheumatol; 39: 1424-1432. doi:10.3899/jrheum.111481. PubMed: 22660807. [DOI] [PubMed] [Google Scholar]
- 34. Wariaghli G, Allali F, Idrissi Z, Berrada K, Hmamouchi I et al. (2012) Evaluation and stability of the Patient Acceptable Symptom State (PASS) over time in patients with ankylosing spondylitis. Clin Exp Rheumatol 30: 106-109. PubMed: 22260741. [PubMed] [Google Scholar]
- 35. Van der Heijde D, Kivitz A, Schiff MH, Sieper J, Dijkmans BA et al. (2006) ATLAS Study group Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arth Rheum 54: 2136-46. [DOI] [PubMed] [Google Scholar]
- 36. Heiberg T, Kvien TK, Mowinckel P, Aletaha D, Smolen JS et al. (2008) Identification of disease activity and health status cut-off points for the symptom state acceptable to patients with rheumatoid arthritis. Ann Rheum Dis 67: 967-971. PubMed: 17965118. [DOI] [PubMed] [Google Scholar]
- 37. Dougados M, Brault Y, Logeart I, van der Heijde D, Gossec L et al. (2012) Defining cut-off values for disease activity states and improvement scores for patient-reported outcomes: the example of the Rheumatoid Arthritis Impact of Disease (RAID). Arth Res Ther 14: 129. doi:10.1186/ar4109. PubMed: 22647431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ball EM, Bell AL (2012) Lupus arthritis-do we have a clinically useful classification? Rheumatology (Oxf) 51: 771-779. doi:10.1093/rheumatology/ker381. [DOI] [PubMed] [Google Scholar]
- 39. Tang X, Huang Y, Deng W, Tang L, Weng W et al. (2010) Clinical and serologic correlations and autoantibody clusters in systemic lupus erythematosus: a retrospective review of 917 patients in South China. Medicine (Baltimore) 89: 62-67. doi:10.1097/MD.0b013e3181cb449c. PubMed: 20075706. [DOI] [PubMed] [Google Scholar]
- 40. McElhone K, Abbott J, Shelmerdine J, Bruce IN, Ahmad Y et al. (2007) Development and validation of a disease-specific health-related quality of life measure, the LupusQol, for adults with systemic lupus erythematosus. Arthritis Rheum 57: 972-979. doi:10.1002/art.22881. PubMed: 17665467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Translation to Italian language of original question of patient acceptable symptom state (PASS).
(DOC)


