Abstract
There is now strong evidence to show that the presence of the cellular prion protein (PrPC) mediates amyloid-β (Aβ) neurotoxicity in Alzheimer's disease (AD). Here, we probe the molecular details of the interaction between PrPC and Aβ and discover that substoichiometric amounts of PrPC, as little as 1/20, relative to Aβ will strongly inhibit amyloid fibril formation. This effect is specific to the unstructured N-terminal domain of PrPC. Electron microscopy indicates PrPC is able to trap Aβ in an oligomeric form. Unlike fibers, this oligomeric Aβ contains antiparallel β sheet and binds to a oligomer specific conformational antibody. Our NMR studies show that a specific region of PrPC, notably residues 95–113, binds to Aβ oligomers, but only once Aβ misfolds. The ability of PrPC to trap and concentrate Aβ in an oligomeric form and disassemble mature fibers suggests a mechanism by which PrPC might confer Aβ toxicity in AD, as oligomers are thought to be the toxic form of Aβ. Identification of a specific recognition site on PrPC that traps Aβ in an oligomeric form is potentially a therapeutic target for the treatment of Alzheimer's disease.—Younan, N. D., Sarell, C. J., Davies, P., Brown, D. R., Viles, J. H. The cellular prion protein traps Alzheimer's Aβ in an oligomeric form and disassembles amyloid fibers.
Keywords: PrP, structure, NMR, electron microscopy, IR, antiparallel β sheet
A key hallmark of alzheimer's disease (AD) is extracellular fibrillar amyloid plaques, composed of a 39- to 43-residue peptide, amyloid-β (Aβ). The amyloid cascade hypothesis indicates that Aβ plays a central role in the disease, as genetic alterations underlying familial AD are associated with mutations in, or increased production of, Aβ (1). Studies on the neurotoxic element of Aβ suggest that small diffusible oligomers, rather than mature amyloid fibers, are the toxic form (2, 3).
A number of studies have indicated a direct high-affinity interaction between Aβ and the benign cellular prion protein (PrPC). Furthermore, studies with mouse models of AD have indicated that the presence of PrPC confers Aβ toxicity. Using an unbiased cDNA expression library screen containing 200,000 proteins, it has been shown that PrPC is the strongest candidate to bind to Aβ (4). The same study showed that the interaction between PrPC and Aβ42 oligomers leads to the inhibition of long-term potentiation (LTP) in the hippocampal slices from normal mice expressing PrPC. Significantly, studies showed that transgenic PrP-knockout mice were immune to Alzheimer's disease (AD) pathology (4). Indeed, PrP-knockout mice can develop Aβ plaques, but not neurotoxicity (5). These findings therefore suggested that PrPC is the main receptor that mediates Aβ toxicity.
The high-affinity interaction between Aβ and the benign PrPC has been reported by a number of groups (4, 6–9). It is believed that PrPC selectively binds to Aβ oligomers (4, 6–9), with an affinity in the nanomolar range (4, 7). Furthermore, the Aβ binding seems to involve the N-terminal half of PrPC (4, 6, 7). However, the initial report to link Aβ toxicity to PrPC has been challenged (8), and certain AD phenotypes have been shown to occur in the absence of PrPC (9–11). These conflicting observations may simply reflect the mouse model used and the nature of the Aβ-oligomer preparation. A number of recent studies support the correlation between the presence of PrPC and Aβ toxic effects, which impair synaptic plasticity and cause special memory defects and axon degeneration (4, 5, 12–15). Hippocampal primary culture and intrahippocampal injection indicate the cytotoxic effects of Aβ oligomers, which are significantly reduced for PrP-null mice (16, 17). A similar effect is observed in cell culture using cell lines that lack PrPC (18). Aβ oligomers also influence PrPC trafficking and inhibit PrPC endocytosis (19). In addition, aggregates from AD brain extracts have been reported to contain Aβ together with PrP (20, 21). PrP has also been shown to influence the processing of the amyloid precursor protein (APP; ref. 22). A genetic association between AD and the PrP has also been suggested (23, 24), with the polymorphism at residue 129 of PrPC representing a small but significant risk factor in sporadic AD, while others have noted no genetic association (25).
Aβ and PrPC are both concentrated at the synaptic terminals (26), and PrPC can also misfold into amyloid fibers. This misfolded PrP is associated with transmissible spongiform encephalopathies (TSEs; ref. 27). Mammalian PrPCs have a high structural and sequence homology (28, 29) and consist of two structurally distinct domains (30). The C-terminal domain (residues 126–231) is predominantly α-helical, while the N-terminal half of PrPC (residues 23–120) is natively disordered (31, 32), contains an octapeptide repeat sequence (residues 58–91), and is notable for its ability to bind Cu2+ ions (33–36). Interestingly, PrPC limits excessive N-methyl-d-aspartate receptor (NMDAR) activity that might otherwise promote neuronal damage (37). Significantly, PrPC only affects the NMDA receptor in a copper-loaded state (14). Aβ has a picomolar affinity for Cu2+ (38), and so may disrupt Cu2+ binding to PrPC, and therefore, in part at least, mediates synaptic injury (14). The mechanism by which PrP mediates Aβ toxicity and NMDA activity is not clear, but may also involve the Fyn receptor (15).
Here, we aim to understand the mechanistic and structural detail of the Aβ:PrP interaction and explain how PrPC might influence Aβ oligomerization and toxicity. We show that PrP profoundly inhibits fiber formation by trapping Aβ in a oligomeric form that is rich in antiparallel β sheet. We map the PrPC recognition site to specific residues in the natively unstructured N-terminal half of PrPC. The ability to trap and concentrate Aβ into toxic oligomers suggests a mechanism by which PrPC might confer Aβ neurotoxicity in AD.
MATERIALS AND METHODS
Recombinant mouse PrP (mPrP) expression and purification
Expression and purification is as described previously (39). The coding region of the full-length mPrP(23–231) was cloned into a pET-23 vector to produce a tag-free protein. In addition, recombinant mPrP fragments containing the unstructured half of PrPC, mPrP(23–126) and the C-terminus structured domain of the mPrP, mPrP(113–231), were expressed. The C-terminal fragment only contains a His tag, to assist in purification. These recombinant proteins were expressed in minimal medium containing 15N ammonium chloride to produce 15N-labeled proteins suitable for nuclear magnetic resonance (NMR) studies. The recombinant PrPC lacks the GPI anchor and glycosylation found in vivo.
Peptide production
All peptides were purchased from Zinsser Analytic (Maidenhead, UK)and Cambridge Research Biochemicals (Cleveland, UK), synthesized using F-moc chemistry, purified as a single peak on HPLC and characterized by mass spectrometry. The purchased peptides included human Aβ peptide, residues 1–40 and 1–42; designated Aβ40 and Aβ42, and shorter PrP peptides, PrP(58–91) and PrP(91–115), human sequence. The N terminus and the C terminus of these two PrP peptides only were acetylated and amidated, respectively, to mimic these residues within the full-length protein.
Aβ solubilization
Aβ40 or Aβ42 was solubilized at 0.7 mg/ml in water at pH 10.5 and gently rocked at 4°C for 72 h, with the pH maintained at 10.5 using NaOH. This process generated essentially monomeric; seed-free, Aβ stocks. After solubilization, the absorbance at 280 nm was used to calculate the concentration of Aβ, with an extinction coefficient of 1280 M−1cm−1. Typically, 10% of the weight was attributed to moisture.
Fiber growth kinetics
The growth of Aβ fibers was monitored using a 96-well microplate BMG Galaxy and Omega FLUOstar fluorescence reader (BMG Labtech, Ortenberg, Germany), with an excitation filter at 440 nm and an emission filter at 490 nm. Each reading consists of 50–100 flashes, prior to each reading, the plate was agitated for 30 s, orbital (3-mm) shaking, every 30 min. Sterile flat-bottomed plates were used and sealed with Starseal polyolefin sealing film (Starlab, Milton Keynes, UK). The wells hold up to 300 μl; these were used to their full capacity to reduce the volume of air in each plate. The pH of a sample was monitored before and after each experiment; a variation of ±0.05 pH units or less was observed over the course of the experiment. The amyloid binding fluorophore used was thioflavin T (ThT) of fresh stock (2 mM) in water. The fibril growth experiments were typically carried out using 10 μM of essentially monomeric Aβ40 or Aβ42 in the presence of 2 mol eq of ThT. The sample was incubated at 30°C in the presence of 160 mM NaCl and 30 mM 4-(2-hydroxethyl)-1-piperazineethanesulfonic acid (HEPES) buffer (pH 7.4). Fluorescence detection was made by reading from the top of the well, using an orbital averaging sample reading (4 mm diameter). For the disaggregation experiments, small aliquots of PrP(23–231) were added while the 96-well plate was left on the plate holder within the reader.
Fibril growth-curve fitting
Sigmoidal fibril growth curves were fitted to the following equation, as described previously (40, 41):
where y is the ThT fluorescence intensity, x is the time (t) in hours, and xo is the time at which the ThT fluorescence has reached half maximal intensity (t50). The apparent fiber growth rate (kapp) is calculated from the following equation:
The nucleation or lag time (tlag) is taken from the following equation:
NMR
Main-chain assignments for mouse PrPC amide resonances have previously been reported by ourselves (32). NMR samples contain 15N labeled PrP(23–231) (50 μM; pH 6.5) in phosphate buffer with essentially monomeric Aβ40 (50 μM; unlabeled). NMR spectra were acquired at 30°C on a Bruker Avance spectrometer (Bruker Corp., Billerica, MA, USA) operating at 700 MHz for 1H nuclei using a 5-mm inverse detection triple-resonance cryoprobe. Phase-sensitive 2D 1H-15N heteronuclear single quantum coherence (HSQC) spectra were acquired using echo-antiecho gradient selection.
Transmission electron microscopy (TEM)
Carbon-coated 300-mesh grids (SPI Supplies, West Chester, PA, USA) were glow discharged at the start of each experiment. An aliquot (5 μl) of the 10 μM Aβ sample was allowed to absorb onto a grid for 1 min before blotting off. This step was then followed by a 5 μl aliquot 2% (w/v) of phosphotungstic acid (PTA) at pH 7.4, to be absorbed for 1 min, to produce a negatively stained protein loaded grid. Images of the grids were recorded on a Jeol JEM 1230 electron microscope (Jeol Ltd., Akishima, Japan) operated at 80 kV.
Approximate relationship between protein volume (V) and molecular mass (M) is as follows: V (nm3) = M (kDa) × 1.27 nm3/kDa. Oligomers are assumed to be spherical.
Size-exclusion chromatography (SEC)
The Tricorn Superdex 200 10/300 analytical gel-filtration chromatography column (GE Healthcare, Little Chalfont, UK) was used on an automated AKTA FPLC system (GE Healthcare), at a flow rate of 0.5 ml/min. The column is capable of resolving proteins below 600 kDa. The column is equilibrated with 1.5 column volumes of buffer (30 mM HEPES, pH 7.4, and 160 mM NaCl), at 4°C. A 0.5-ml injection volume was used to load the protein onto the column (10 μM Aβ). The approximate molecular weight of the Aβ oligomers was estimated using standard globular proteins.
Conformational Aβ oligomer antibody (A11) dot-blot assay
Aβ, typically 30 μM, was incubated with and without the presence of PrPC (10 μM), then 6 μl of these Aβ preparations was dotted on to a Hybond-ECL nitrocellulose membrane (purchased from Amersham Biosciences, Piscataway, NJ, USA). Once the sample spots were air dried, the membrane was blocked in 10% nonfat dry milk in TBST buffer [containing NaCl, Tris, and Tween 20 as per Invitrogen (Carlsbad, CA, USA) A11 protocol] for 3 h at 4°C. After the incubation, the membrane was washed 3 times with TBST buffer and incubated in 0.5 mg/ml unconjugated rabbit (polyclonal) anti-oligomer primary antibody (A11; cat. no. AHB0052; Invitrogen) in 5% nonfat dry milk in TBST buffer at 25°C for 1 h. After being washed, the membrane was then incubated in the enzyme conjugate (F(ab′)2 fragment of goat anti-rabbit IgG (H+L), alkaline phosphatase conjugate, (F21456; Invitrogen) for 1 h at 25°C. Finally, the membrane was washed once again and then incubated in NBT-BCIP substrate solution (BCBH8024V; Sigma-Aldrich, St. Louis, MO, USA) for 10 min. Stained dots is indicative of the presence of oligomeric species, but not Aβ monomer or fiber (42).
Attenuated total reflectance Fourier transform infrared (ATR-FT-IR) spectroscopy
Spectra were obtained using a Bruker IFS 66/s FT-IR-spectrometer. Spectra represent an average of 32 scans, recorded at 25°C in the spectral range from 3000 to 370 cm−1. All samples were prepared in 30 mM HEPES buffer (pH 7.4) and 160 mM NaCl. Small aliquots (5 μl) of sample were deposited onto the ATR crystal (ZnSe prism) where it was purged with nitrogen gas to generate a protein film.
Ultraviolet circular dichroism (UV-CD)
Far-UV CD spectra were recorded between 210 and 260 nm, with a 0.1-cm pathlength and sampling points every 0.5 nm. Three scans were recorded, and a baseline spectrum was subtracted from each spectrum and zeroed at 255 nm. Data were processed using Applied Photophysics Chirascan Viewer (Applied Photophysics Ltd., Leatherhead, UK), Microsoft Excel (Microsoft Corp., Redmond, WA, USA), and the KaleidaGraph spreadsheet and graph package (Synergy Software, Reading, PA, USA). The direct CD measurements (θ, mdeg) were converted to molar ellipticity, Δε (M−1cm−1), using the equation Δε = θ/(33,000·c·l), where c is the concentration and l is the pathlength.
RESULTS
Substoichiometric amounts of PrPC will completely inhibit Aβ fiber formation
In light of the clear interaction between Aβ and PrPC and its influence on Aβ toxicity (4, 5,12–15) we wondered whether PrPC might influence the process of Aβ oligomerization and fiber formation. First we investigated the influence of varying amounts of full-length PrPC, PrP(23–231), on the kinetics of fibril formation of Aβ40 and Aβ42, using the well-established ThT amyloid binding fluorescence assay. In the absence of PrPC, Aβ40 (10 μM) readily forms fibers, exhibiting a characteristic sigmoidal growth curve with a lag-phase (47 h) followed by rapid elongation of fibers (Fig. 1A). In the presence of equimolar concentration of PrPC (10 μM) no amyloid fibers are detected even after 450 h (Fig. 1B). The same complete inhibition of fiber formation was observed for 0.1 and 0.05 mol eq of PrP(23–231) (Fig. 1C, D). Remarkably, 1:20 concentration of PrPC (500 nM) relative to Aβ40 will strongly inhibit the ability of Aβ40 to form fibers. Only after >400 h of incubation with agitation is there a very weak ThT fluorescence signal observed in the presence of 500 nM PrP. At smaller amounts of PrP(23–231), for example, 250 and 100 nM, inhibition of Aβ40 fiber formation was not apparent (Fig. 1E, F), and similar lag times, elongation rates, and total ThT fluorescence were observed to those of Aβ40 in the absence of PrPC (Supplemental Table S1). The cutoff point at which PrP(23–231) influences Aβ40 fiber growth is very pronounced, with a molar ratio of 1:20 (PrP:Aβ40) almost completely inhibiting fibril growth, while 1:40 has no detectable influence on Aβ40 fibril growth. TEM and IR data confirm a profound change in Aβ structure and lack of Aβ fiber content in the presence of PrPC, described later.
Figure 1.
PrPC inhibits Aβ40 fiber formation. Kinetics of Aβ40 fiber formation was monitored by fluorescence upon ThT binding to amyloid. Aβ40 alone (A), and in the presence of 1 mol eq (B), 0.1 mol eq (C), 0.05 mol eq (D), 0.025 mol eq (E), and 0.01 mol eq (F) of PrP(23–231). Aβ40 monomer (10 μM) was incubated at pH 7.4 in HEPES buffer (30 mM) and NaCl (160 mM) at 30°C with intermittent agitation. As little as 500 nM of PrP(23–231) completely inhibits Aβ40 fiber formation over 250 h.
We also investigated the effect PrP(23–231) has on fiber formation of the more amyloidogenic Aβ42. At two concentrations (5 and 10 μM), a very similar effect is observed; both 1:1 and 1:0.1 Aβ42:PrPC molar ratios inhibit fibril formation (Fig. 2 and Supplemental Fig. S1). When 0.05 mol eq of PrP(23–231) was added, the nucleation period was prolonged, almost doubling the lag time; furthermore, the total maximal fluorescence was also significantly reduced, by more than half. As with Aβ40, the presence of smaller amounts, 0.02 mol eq of PrP(23–231), had little effect on the Aβ42 fibril kinetics.
Figure 2.

PrPC inhibits Aβ42 fiber formation. Kinetics of Aβ42 (5 μM) fiber formation was monitored by fluorescence upon ThT binding to amyloid. Aβ42 alone (A), and in presence of 1 mol eq (B), 0.1 mol eq (C), 0.05 mol eq (D), and 0.02 mol eq (E) of PrP(23–231). Aβ42 monomer (5 μM) was incubated at pH 7.4 in HEPES buffer and 160 mM NaCl at 30°C with intermittent agitation. As little as 1:20 PrP(23–231) inhibits Aβ42 fiber formation over 250 h.
Aβ fiber inhibition is specific, centered at the N-terminal unstructured half of PrPC
Next we investigated how different regions of PrPC influence Aβ fiber growth, whether intact full-length PrPC is required for fibril inhibition, or whether the effect is isolated to a particular region of the protein. The fibril growth kinetics were measured in the presence of a number of recombinant and synthetic peptide fragments (Fig. 3). PrP(113–231), which contains all the structured elements of PrPC, does not inhibit fibril growth even at a 1:1 ratio (Fig. 3B). In particular, there is no increase in the lag time or reduction in total amount of fibers generated (Supplemental Table S2). In contrast, in the presence of only 0.1 mol eq of the unstructured N-terminal domain of PrPC, PrP(23–126), complete inhibition of fibril growth is observed (Fig. 3C). This suggests it is the natively unstructured half of PrPC that is specific to the PrP-Aβ interaction.
Figure 3.

Aβ fiber formation in the presence of PrPC fragments. Kinetics of Aβ40 (10 μM) fiber formation was monitored by fluorescence upon ThT binding to amyloid. Aβ40 alone (A), and in presence of 1 mol eq of PrP(113–231) (B), 0.1 mol eq of PrP(23–126) (C), 0.1 mol eq of PrP(58–91) (D), 0.1 mol eq of PrP(91–115) (E), and 1 mol eq of PrP(91–115) (F). Aβ40 was incubated at pH 7.4 in 30 mM HEPES buffer and 160 mM NaCl at 30°C with intermittent agitation.
These observations prompted us to look at smaller fragments of the natively unstructured domain of PrPC. In particular, the presence of 0.1 mol eq or 1:1 of PrP(58–91), the 4-octarepeat fragment, showed no inhibition of fibril formation (Fig. 3D and Supplemental Table S2). Figure 3E, F also shows fibril growth kinetics measurements for Aβ40 (10 μM) with different concentrations of PrP(91–115); only at relatively high levels of the PrP fragment do we observe an inhibitory effect at a 1:1 ratio, while no significant inhibition effect is observed at a 1:0.1 ratio.
The effects of various fragments of PrPC on Aβ42 were also investigated (Supplemental Fig. S2). As with Aβ40, the N-terminal unstructured domain of PrP(23–126) at just 0.1 mol eq completely inhibits Aβ42 fiber formation over 250 h of incubation, while the 4-octarepeat fragment has no effect on fiber formation, even at 1:1 molar ratio. It is clear that the Aβ40 and Aβ42 fiber inhibition is specific to the natively unstructured N-terminal portion of PrPC, PrP(23–126), which has a similar inhibitory effect as full-length PrPC.
PrPC causes disassembly of preformed Aβ fibrils
Next, the effect of PrP(23–231) on preformed mature Aβ fibrils was investigated. Figure 4 shows the fluorescence of ThT-bound fibers at equilibrium, after 240 h of incubation. At this time point, a small aliquot of PrP(23–231) was added to the Aβ fibers to make a 1:1 stoichiometry. The ThT signal rapidly reduces in intensity; almost half (40±3%) of the total ThT Aβ fiber signal is lost within 90 min of PrPC addition (see also Supplemental Fig. S3). A parallel control experiment involved adding 10 μM of the PrP fragment, PrP(113–231), to mature Aβ40 fibrils (Supplemental Fig. S3). As expected, the structured C-terminal half of PrPC had no effect on the fiber content, as there was no observable decrease in the ThT fluorescence. This suggests that PrP(23–231) not only stops Aβ40 fibril formation, but will also disassemble fully formed Aβ mature fibrils. PrPC does not dissociate all the Aβ fibers over a 100-h period, as only 40–50% of the total ThT fiber signal is lost. It may be that PrPC dissociates Aβ protofibrils rapidly, within an hour, while a subpopulation of more mature fibers is kinetically more resistant to disassembly by PrPC(23–231). TEM images of disassembled fibers, described later, suggest that mature fibers are broken up into much shorter fragments, which causes a reduction in the amount of ThT binding.
Figure 4.
PrPC induced Aβ fiber disassembly. Mature Aβ40 fibers alone (gray) are formed over a 234-h period, after which 1 mol eq of PrP(23–231) is added to 6 of the reaction wells (black). Aβ40 (10 μM) samples were incubated at pH 7.4 in 30 mM HEPES buffer and 160 mM NaCl at 30°C with intermittent agitation. Addition of PrPC caused a 40% reduction in ThT fluorescence within 90 min.
NMR maps the binding site on PrPC
To probe the molecular details of the PrPC:Aβ interaction on a per-residue basis, we used 2D 15N HSQC NMR spectra of full-length PrPC. Essentially monomeric Aβ40 was incubated with PrPC(23–231) using a 1:1 molar stoichiometry, and 15N HSQC spectra were recorded over time, as shown in Fig. 5. Only the amide signals from PrPC are observed in the 15N HSQC spectra, as Aβ is unlabeled. Initial addition of essentially monomeric Aβ40 has little effect on the PrPC 15N HSQC spectra. After 20 h at 30°C, specific PrPC amide resonances start to lose their intensity. In particular, residues from two sections of the natively unstructured N-terminal half of PrPC, between residues T95 and A113, and also residues T33, G35, S43, and T56. While the majority of the signals from PrPC remain unaffected, in particular, none of the C-terminal resonances have their chemical shift or signal intensity affected. Strikingly, the regions of PrPC that are perturbed by Aβ binding correlate very closely with the fragments for the N terminus of PrPC that inhibit fiber formation (Fig. 3). 15N HSQC spectra of PrPC under similar conditions, in the absence of Aβ, remain unchanged for a number of days.
Figure 5.

15N HSQC NMR of the PrP(23–231) binding to Aβ40 oligomer. A, B) Selected regions of 2D 15N-1H HSQC of PrP(23–231) alone (black) and PrP(23–231) with 1 mol eq of Aβ40 after 40 h incubation (red). Amide resonances that show a marked loss of signal are labeled in blue. C) Peak intensity plotted against time; 6 new peaks are observed after 20 h (various shades of red), as well as reductions in the intensity of A113 and Q98, while S131 and T187 remain unaffected over 40 h. Spectra obtained at 30°C in 50 mM phosphate buffer (pH 6.5) and 50 μM PrPC and Aβ.
Selected regions of the 15N HSQC spectra of PrPC are shown before and after 40 h incubation with Aβ in Fig. 5A, B. The peak intensity of some selected amide resonances are plotted vs. time in Fig. 5C. After 20 h incubation, there is also the appearance of new well-resolved PrP amide signals that increase in intensity with time as Aβ binds to PrPC. Interestingly, after 40 h, these signals have intensities and line widths comparable to unaffected PrPC amide signals. The appearance of new PrP signals in the presence of Aβ indicates slow exchange between free and bound Aβ-PrP complex (on the chemical-shift timescale). Furthermore, the comparable intensity of the new PrP signal implies that most or all of the PrP molecules have Aβ bound, which suggests that at this stage, a simple 1:1 or 1:2 (Aβ dimer) stoichiometric complex has formed.
It is clear from the NMR spectra that PrPC does not interact with disordered monomeric Aβ, as NMR spectra recorded for the first 20 h of incubation are unaffected by Aβ. Aβ must first change its conformation, presumably forming a small oligomeric species (dimeric) or misfolded monomer, before it will bind to PrPC. The PrPC amide line width for the new PrP signals for the PrP:Aβ complex are not appreciably increased, suggesting minimal increase in molecular mass. PrPC must therefore be bound to no more than an Aβ dimer at this stage of the incubation of Aβ with PrPC. We note that the concentrations of PrPC and Aβ required for the NMR experiments described are very different from those that were used to monitor fiber formation in Fig. 1. It is therefore not possible to relate the lag times of Aβ binding to PrPC (Fig. 5C) with nucleation times for fiber formation.
EM indicates PrPC traps Aβ in an oligomeric form and disassembles Aβ fibers
Negative-stain TEM was used to investigate the effect of PrPC on the gross structural morphology of Aβ fibers (Fig. 6 and Supplemental Fig. S4). In the absence of PrPC, incubation of Aβ (10 μM, pH 7.4; under the same conditions used for the ThT-binding fiber-growth experiments) generates the characteristic long unbranched fibers, 10 nm in diameter, while thicker mature fibers are 20 nm in diameter (Fig. 6A). However, in the presence of PrPC, TEM images indicate a complete lack of fiber generation throughout the EM grids. Instead, numerous spherical oligomers are observed (Fig. 6B). The small oligomers are typically between 6 and 10 nm in diameter, suggesting spherical oligomers of 100–500 nm3, which roughly approximates to 20–100 Aβ molecules in size (see Materials and Methods). Smaller oligomers may also be present, observed using SEC. Medium-sized oligomers, 25–30 nm in diameter, equate to Aβ oligomers that might contain a few thousand Aβ molecules. The medium-sized oligomers have halos of very small oligomers, 4–5 nm in diameter (approximately hexamers or dodecamers), around them. There are also a few much larger oligomers, 80–100 nm in diameter, containing 5–10 × 104 Aβ molecules (Fig. 6B–D). There are also donut-shaped oligomers observed, 20 nm in diameter (Fig. 6E), which are postulated to form toxic pore-like structures across cell membranes (43).
Figure 6.

TEM of Aβ in the presence of PrPC. Negative-stain TEM images of Aβ40 fibers alone (A), Aβ incubated with PrP(23–231) (B–E), and PrPC added to mature Aβ40 fibers (F). Aβ40 (10 μM) samples were incubated at pH 7.4 in 30 mM HEPES and 160 mM NaCl at 30°C with intermittent agitation for 300 h. TEM grids were negatively stained using phosphotungstic acid. Only Aβ oligomers are observed where Aβ is incubated with PrPC (0.1 mol eq).
Remarkably, the addition of PrPC to preformed fibers causes marked disassembly of fibers. The resulting assemblies generated are less spherical than those produced with PrPC incubated with Aβ from the start, and have their own distinctive “rice-grain” morphology (Fig. 6F). The oligomers have quite a uniform appearance, typically 60 nm wide and 120 nm long, suggestive of fibers that have been chopped into very short 120-nm rods. Furthermore, under these conditions, some fibers remain after PrPC addition (Supplemental Fig. S4F), as suggested by partial fluorescence on ThT binding (Fig. 4).
SEC indicates dodecameric Aβ oligomers are formed in presence of PrPC
We also used SEC to investigate the size of the Aβ oligomers formed. A series of samples obtained from a fibril growth assay after 250 h of incubation was eluted from a Superdex 200 column (Fig. 7). Fibrils produced from 10 μM of Aβ eluted in the void volume, as shown in the chromatogram in Fig. 7. In addition, the majority of the fibrils may not even enter the column with a precolumn filter. For Aβ fibers incubated in the absence of PrPC, there is no evidence of small oligomeric Aβ species < 200 kDa in size. Aβ was also incubated with 0.2 or 0.05 mol eq of PrP(23–231); ThT fibril growth assay indicated that under these conditions, no fibers were detected. The resulting chromatograms show two bands, eluting at 60–70 kDa (complex I) and 100–120 kDa (complex II). The N-terminal half of PrP, residues 23–126, also inhibits Aβ fiber formation, and very similar chromatograms were observed (Fig. 7B). SEC suggests that the incubation of Aβ with either PrP(23–231) or PrP(23–126) results in the formation of two oligomeric species. Assuming one PrPC molecule per complex, this suggests that complex I has an oligomer size of 8–12 Aβ monomers (Aβ is 4 kDa in size), and complex II is 20–24 monomers in size, the typical size for toxic Aβ-derived diffusible ligands (ADDLs). The smaller (complex I) is the more abundant. The larger oligomers, detected by TEM (Fig. 6B) are too large to enter the column or elute within the void volume.
Figure 7.
SEC of Aβ40 oligomer in the presence of PrPC. Aβ40 was incubated with PrP(23–231) (A) and PrP(23–126) (B). Traces indicate Aβ40 only (top), Aβ40 with 0.5 μM PrP (middle), and Aβ40 with 2 μM PrP (bottom). Labels I and II indicate oligomers I and II, the complexes formed at ∼60 and ∼100 kDa, respectively. Aβ40 (10 μM) samples with and without PrP were incubated at pH 7.4 in 30 mM HEPES and 160 mM NaCl at 30°C with agitation for 250 h. SEC was carried out at 4 °C and pH 7.4, using a Superdex 200 column.
PrPC-generated Aβ oligomers bind the oligomer-specific antibody
To further characterize the Aβ oligomers generated in the presence of PrPC, we used the conformational antibody A11, which is specific to toxic oligomers found in vivo, but does not bind to Aβ fibers or low-molecular-mass Aβ (42). Aβ42 that was incubated with PrP(23–231) to generate oligomers clearly showed positive binding in the dot-blot assay (Fig. 8). In contrast, fibrillar Aβ42, essentially monomeric Aβ42, and PrP(23–231) on its own did not bind the A11 antibody. Furthermore, Aβ42 oligomers generated by the addition of PrP(23–231) to mature Aβ42 fibers, causing them to disassemble, also bound to the A11 antibody. In addition, the N-terminal unstructured fragment PrP(23–126) had a similar effect when incubated with Aβ40, generating A11 antibody binding to the Aβ oligomers.
Figure 8.

Aβ oligomer antibody binding dot-blot assay. Samples were dotted on a membrane and examined using antibody A11, which is sensitive to oligomers but not to fibers or monomers. A) Aβ42 essentially monomeric. B) Aβ42 (30 μM) incubated with PrP(23–231) (10 μM). C) Aβ42 fibers. D) Disassembled Aβ42 fibers with 1.5 mol eq of PrP(23–231). E) PrP(23–126) (10 μM) only. F) Aβ40 with 0.1 mol eq of PrP(23–126). G) PrP(23–231) (10 μM) only.
Trapped Aβ oligomers contain antiparallel β sheet
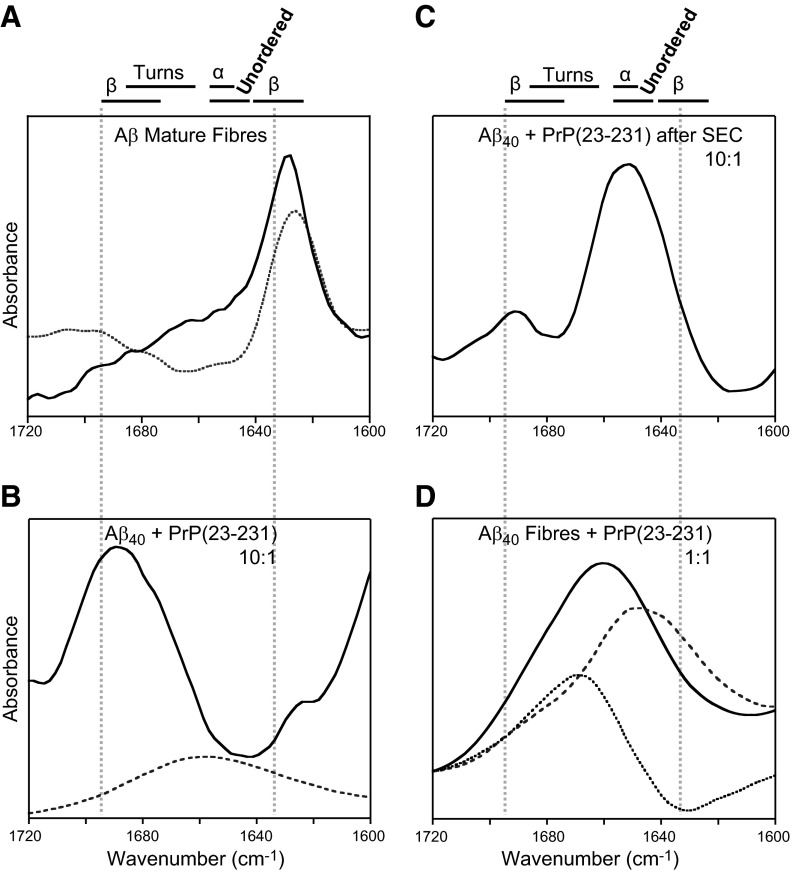
Finally, we wanted to understand the structural nature of the Aβ oligomers formed. IR spectroscopy was used to determine the secondary structure conformation within the Aβ:PrP complex. IR spectra were obtained after incubation of Aβ with full-length PrPC (Fig. 9). As expected, the IR spectra of amyloid fibers of Aβ42 and Aβ40 is dominated by a strong amide-I absorption band centered at 1633 cm−1, characteristic of β sheet (Fig. 9A). Aβ incubated with PrP(23–231) (1:0.1 Aβ:PrP) to generate the oligomeric species indicates a significant reduction in the intensity for the amide-I band at 1633 cm−1; instead, the IR spectrum is dominated by a broad band centered at 1695 cm−1, characteristic of β sheet and turns within the polypeptide chain (Fig. 9B). Interestingly, an amide-I band at 1695 cm−1 can be indicative of β sheet in an antiparallel conformation, and the ratio of the intensity of the band at 1695/1633 can indicate the proportion of antiparallel arrangement of the β strands (44). Indeed, this ratio has been used to distinguish Aβ fibrils from the Aβ-oligomeric structure (45). The mixture of Aβ:PrP has contributions from PrPC as well as Aβ. The IR spectrum for PrPC on its own is also shown (Fig. 9B), although the PrPC contribution at a 1:0.1 ratio is minor.
Figure 9.
IR spectra of Aβ oligomers in the presence of PrPC. A) Structural characterization using IR amide-I band of Aβ40 mature fibers (dashed gray) and Aβ42 mature fibers (solid black). B) Aβ40 monomer incubated with 0.1 mol eq of PrP(23–231) (solid black) or PrP(23–231) alone (dashed gray). C) Aβ40 with PrPC oligomers eluted by SEC. D) Aβ40 mature fibers with 1 mol eq of PrP(23–231) (solid black), PrP(23–231) alone (dashed gray), and difference spectra (dotted). Aβ40 monomer (10 μM) was incubated at pH 7.4 in 30 mM HEPES buffer and 160 mM NaCl at 30°C. Vertical dashed gray lines highlight 1695 and 1633 cm−1. Aβ40 oligomer formed in the presence of PrPC shows an increase in 1695 cm−1 amide-I band.
Figure 9C shows the IR spectra for the 60-kDa Aβ-oligomer complex after it has been passed down the size-exclusion column (Fig. 7). In this spectrum, there is a clear amide I band at 1695 cm−1, typical of antiparallel β sheet, with some absorption at 1633 cm−1. A second stronger amide band is very characteristic of α helix and unstructured conformation centered at 1653 cm−1 and may be largely due to a contribution from the α-helical PrPC (although it is not clear what the precise ratio of Aβ:PrP is within the small 60-kDa oligomers).
The final IR spectrum (Fig. 9D) reveals the effect of PrP(23–231) addition to mature fibers. It is clear that there is a profound change in the appearance of the spectra relative to fibers. There is little signal at 1633 cm−1; instead, there is a broad band (1660–1690) typical of turns and β-sheet conformation. The higher ratios of Aβ:PrP used (1:1) means that the contribution to the spectra from PrP is significant, and the PrPC signal has been subtracted out to create a difference spectrum (Fig. 9D).
Aβ42 was incubated with the smaller unstructured N-terminal PrP fragment (residues 23–126), and the IR spectra were obtained (Supplemental Fig. S5). The incubated sample for the Aβ:PrP (23–126) complex has a relatively weak band at 1633 cm−1 and a stronger broad band between 1660–1690 cm−1, again indicative of turns and β-sheet structure.
Similarly, far-UV-CD spectra of Aβ fibers give a strong CD band at 217 nm, characteristic of β sheet. Spectra of Aβ incubated with the natively unstructured PrP fragment, PrP (23–126), show that the extended β-sheet conformation is largely retained within the oligomers (Supplemental Fig. S6). However, CD is not able to distinguish between parallel and antiparallel β strands.
DISCUSSION
Both PrPC and Aβ are concentrated at the synapse (15, 26) and bind to each other with a nanomolar affinity (4, 7). There is now strong evidence to link Aβ neurotoxicity with the presence of PrPC in mouse models of AD and primary cell culture (4,5,12–15). Here we show that PrPC has a profound influence on Aβ fibril growth kinetics. Remarkably, relatively small amounts of PrP (23–231), 1/20 mol eq (500 nM), will inhibit Aβ40 and Aβ42 fiber formation, confirming a nanomolar affinity for the interaction.
With the observation that PrPC promotes AD pathology, one might have expected PrPC to accelerate fiber formation of Aβ rather than inhibit it. However, oligomeric forms of Aβ are thought to be the most toxic to neurons (2,3). Here we show that PrPC promotes the formation of oligomeric species over fibers. Indeed, PrPC will disassemble mature fibers; this suggests that PrP drives the equilibrium between the fiber and the oligomer to the oligomeric form; trapping Aβ oligomers. Furthermore, we show that PrPC-trapped Aβ oligomers bind to the conformational antibody A11, which is specific to toxic oligomers found in vivo (42). The ability of PrPC to mediate the form of Aβ present at the synapse suggests a mechanism by which PrPC can confer its toxic effect on neurons.
Our NMR studies show that monomeric unstructured Aβ does not interact with PrPC; Aβ must first change conformation into a misfolded monomer or dimer. Only oligomeric Aβ forms in the presence of PrPC from dodecamers, as indicated by SEC, and larger oligomers, 20–100 mers, as indicated by TEM. The conformational rearrangement of Aβ must trigger recognition by PrPC; PrPC then traps or promotes Aβ misfolding into a conformation that will only progress to assemble into oligomers, but not amyloid fibers. PrPC-bound Aβ oligomers may have increased stability, so that there is a great energy barrier to the formation of fibers (Fig. 10). Interestingly, our IR data indicate antiparallel β-sheet content within the Aβ oligomers generated in the presence of PrPC. A cylindrical barrel structure, a “cylindrin” containing antiparallel β strands, has been suggested as a generic structure of toxic oligomers that will bind to the A11 oligomer-specific antibody (46). Indeed, antiparallel β sheet has been proposed as a signature for Aβ oligomers (45). Whether the transition from antiparallel oligomer to Aβ fibers is on or off pathway, it is clear that the binding of PrPC stabilizes the oligomeric form.
Figure 10.
PrPC stabilizes the oligomeric form of Aβ. Binding of PrPC stops the transition from antiparallel to parallel sheet necessary for a transition from oligomer to fiber. PrPC may stop the rotation of the β-sheet pairing from intra- to intermolecular. The energy barrier to go from antiparallel to parallel arrangement of β sheet is likely to be very large. It is therefore more likely that antiparallel oligomeric arrangements largely disassemble to form the in-register parallel sheets formed in fibers. Whether the transition from antiparallel oligomer to fibers is on or off pathway, it is clear that the binding of PrPC stabilizes the oligomeric form.
The presence of PrPC, even at substoichiometric amounts (1:20 concentration of Aβ), drives Aβ into an oligomeric form, rather than forming amyloid fibers. The ratio of Aβ to PrP to cause complete fiber inhibition implies that a single PrP is sufficient to trap an Aβ oligomer 20 monomers in size, as a 1:1, interaction might leave 95% of Aβ free to form fibers. This is in close agreement with a study by Chen et al. (7), who suggested a binding ratio of 21 Aβ molecules to 1 PrPC. Remarkably, preformed fibers are rapidly broken up by the presence of PrPC, which may enhance their toxicity, as it has been shown that fibers that have been broken up mechanically have increased toxicity relative to one long fiber (47).
The effect of various fragments of PrPC from the N- and C-terminal halves of PrPC indicates that the interaction is specific. The natively unstructured N-terminal half is the key to the Aβ-PrP interaction, while the C-terminal structured domain has no effect on Aβ fibril growth. Furthermore, we have also shown that at a high enough concentration, the short PrP fragment PrP(91–115) is able to strongly inhibit Aβ fibril growth. As the octarepeats alone do not influence Aβ fiber generation, these observations together suggest that the fibril-inhibiting region is centered at residues 91–115 but enhanced by the N-terminal residues 23–58. The Aβ-binding region on PrPC is confirmed by our NMR studies, which show specific amide residues perturbed by the Aβ:PrP interaction, in particular, residues between T95 and A113, but also residues T33, G35, S43, and T56, whereas the C-terminal structured domain is unperturbed by Aβ interactions with PrPC. This agrees very closely with studies mapping the binding region of PrP for Aβ oligomers, which indicate the binding sites of Aβ oligomers to PrPC centered between residues 95–110 (4, 7) and secondary binding enhancement between residues 23–27 (7). In addition to full-length PrPC, which is anchored to the plasma membrane surface, this is also an N-terminal fragment found in vivo, residues 23–113, which is caused by copper catalyzed cleavage (48). This cleaved fragment is able to diffuse within the synaptic space and could also bind to Aβ.
Amyloid fibers and oligomers have common features between proteins, such as exposed hydrophobic residues in an extended β-strand conformation. It has been suggested that the PrPC-Aβ recognition site may be similar to the PrPC-PrPSc interaction that induces template assisted misfolding in prion replication (6). Interestingly, the natively unstructured residues (residues 90–126) that bind to Aβ have been identified as necessary for prion replication in TSEs (49). Furthermore, like PrPC, the scrapie isoform of PrP may also interact with Aβ (50).
In addition to the PrPC, a number of other protein-binding partners have been indicated for Aβ, including serum amyloid P (SAP), transthyretin, apolipoprotein E (APOE), and tau. Furthermore, both the extracellular chaperone, clusterin (51), and serum albumin, the most abundant protein in the cerebrospinal fluid (CSF), inhibit Aβ fiber formation (52), while islet amyloid polypeptide (IAPP) does not (53). PrPC may be unique in its ability to concentrate Aβ into oligomers, while albumin isolates Aβ as a monomer and inhibits larger oligomer formation, as well as fiber generation (52).
The Aβ:PrP interaction represents a major new avenue in understanding Alzheimer's disease. Potential therapeutics could be designed for the treatment of Alzheimer's disease that inhibit the interaction of PrPC with Aβ oligomers (12). Residues in the N-terminal portion of PrPC are identified as target residues. Our studies show that small amounts of PrPC have a profound effect on the equilibrium between Aβ fibrils and oligomers. Thus, PrPC traps Aβ in an oligomeric form, which might explain how PrPC can mediate Aβ's toxic effects.
Supplementary Material
Acknowledgments
This work was supported by a Wellcome Trust project grant (093241/Z/10/Z) and UK Biotechnology and Biological Sciences Research Council Quota studentships.
The authors thank Harold Toms (Queen Mary, University of London) and the UK National Institute for Medical Research for NMR support, and Graham McPhail for assistance with TEM.
Note added in proof: A recent report shows that PrPC will inhibit Ab42 fiber formation (54). However, no Ab oligomers were observed, and the study concluded that the prion protein is an inhibitor of Ab assembly into toxic oligomers. Clearly, there remains much to be resolved regarding the Ab-prion protein interaction.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- Aβ
- amyloid-β
- Aβ40
- amyloid-β residues 1–40
- Aβ42
- amyloid-β residues 1–42
- AD
- Alzheimer's disease
- ATR-FT-IR
- attenuated total reflectance Fourier transform infrared
- HEPES
- 4-(2-hydroxethyl)-1-piperazineethanesulfonic acid
- HSQC
- heteronuclear single quantum coherence
- mPrP
- mouse prion protein
- NMDAR
- N-methyl-d-aspartate receptor
- NMR
- nuclear magnetic resonance
- PrP
- prion protein
- PrPC
- cellular prion protein
- PrPC(23–231)
- cellular prion protein residues 23–231
- SEC
- size-exclusion chromatography
- TBST
- Tris-buffered saline and Tween 20
- TEM
- transmission electron microscopy
- ThT
- thioflavin T
- TSE
- transmissible spongiform encephalopathy
- UV-CD
- ultraviolet circular dichroism
REFERENCES
- 1. Hardy J., Selkoe D. J. (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297, 353–356 [DOI] [PubMed] [Google Scholar]
- 2. Walsh D. M., Klyubin I., Fadeeva J. V., Cullen W. K., Anwyl R., Wolfe M. S., Rowan M. J., Selkoe D. J. (2002) Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535–539 [DOI] [PubMed] [Google Scholar]
- 3. Lambert M. P., Barlow A. K., Chromy B. A., Edwards C., Freed R., Liosatos M., Morgan T. E., Rozovsky I., Trommer B., Viola K. L., Wals P., Zhang C., Finch C. E., Krafft G. A., Klein W. L. (1998) Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. U. S. A. 95, 6448–6453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Laurén J., Gimbel D. A., Nygaard H. B., Gilbert J. W., Strittmatter S. M. (2009) Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature 457, 1128–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gimbel D. A., Nygaard H. B., Coffey E. E., Gunther E. C., Lauren J., Gimbel Z. A., Strittmatter S. M. (2010) Memory impairment in transgenic Alzheimer mice requires cellular prion protein. J. Neurosci. 30, 6367–6374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gunther E. C., Strittmatter S. M. (2010) Beta-amyloid oligomers and cellular prion protein in Alzheimer's disease. J. Mol. Med. (Berl.) 88, 331–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen S., Yadav S. P., Surewicz W. K. (2010) Interaction between human prion protein and amyloid-beta (Abeta) oligomers: role of N-terminal residues. J. Biol. Chem. 285, 26377–26383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Balducci C., Beeg M., Stravalaci M., Bastone A., Sclip A., Biasini E., Tapella L., Colombo L., Manzoni C., Borsello T., Chiesa R., Gobbi M., Salmona M., Forloni G. (2010) Synthetic amyloid-β oligomers impair long-term memory independently of cellular prion protein. Proc. Natl. Acad. Sci. U. S. A. 107, 2295–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Calella A. M., Farinelli M., Nuvolone M., Mirante O., Moos R., Falsig J., Mansuy I. M., Aguzzi A. (2010) Prion protein and Abeta-related synaptic toxicity impairment. EMBO Mol. Med. 2, 306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kessels H. W., Nguyen L. N., Nabavi S., Malinow R. (2010) The prion protein as a receptor for amyloid-beta. Nature 466, E3–E4; discussion E4–E5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cisse M., Sanchez P. E., Kim D. H., Ho K., Yu G. Q., Mucke L. (2011) Ablation of cellular prion protein does not ameliorate abnormal neural network activity or cognitive dysfunction in the J20 line of human amyloid precursor protein transgenic mice. J. Neurosci. 31, 10427–10431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Freir D. B., Nicoll A. J., Klyubin I., Panico S., Mc Donald J. M., Risse E., Asante E. A., Farrow M. A., Sessions R. B., Saibil H. R., Clarke A. R., Rowan M. J., Walsh D. M., Collinge J. (2011) Interaction between prion protein and toxic amyloid beta assemblies can be therapeutically targeted at multiple sites. Nat. Commun. 2, 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barry A. E., Klyubin I., Mc Donald J. M., Mably A. J., Farrell M. A., Scott M., Walsh D. M., Rowan M. J. (2011) Alzheimer's disease brain-derived amyloid-beta-mediated inhibition of LTP in vivo is prevented by immunotargeting cellular prion protein. J. Neurosci. 31, 7259–7263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. You H., Tsutsui S., Hameed S., Kannanayakal T. J., Chen L., Xia P., Engbers J. D., Lipton S. A., Stys P. K., Zamponi G. W. (2012) Abeta neurotoxicity depends on interactions between copper ions, prion protein, and N-methyl-D-aspartate receptors. Proc. Natl. Acad. Sci. U. S. A. 109, 1737–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Um J. W., Nygaard H. B., Heiss J. K., Kostylev M. A., Stagi M., Vortmeyer A., Wisniewski T., Gunther E. C., Strittmatter S. M. (2012) Alzheimer amyloid-beta oligomer bound to postsynaptic prion protein activates Fyn to impair neurons. Nat. Neurosci. 15, 1227–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bate C., Williams A. (2011) Amyloid-beta-induced synapse damage is mediated via cross-linkage of cellular prion proteins. J. Biol. Chem. 286, 37955–37963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kudo W., Lee H. P., Zou W. Q., Wang X., Perry G., Zhu X., Smith M. A., Petersen R. B., Lee H. G. (2012) Cellular prion protein is essential for oligomeric amyloid-beta-induced neuronal cell death. Hum. Mol. Genet. 21, 1138–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hyeon J. W., Kim S. Y., Park J. S., Choi B. Y., Lee S. M., Ju Y. R., An S. S., Kim C. K. (2012) The association between prion proteins and Abeta(1–42) oligomers in cytotoxicity and apoptosis. Biochem. Biophys. Res. Commun. 424, 214–220 [DOI] [PubMed] [Google Scholar]
- 19. Caetano F. A., Beraldo F. H., Hajj G. N., Guimaraes A. L., Jurgensen S., Wasilewska-Sampaio A. P., Hirata P. H., Souza I., Machado C. F., Wong D. Y., De Felice F. G., Ferreira S. T., Prado V. F., Rylett R. J., Martins V. R., Prado M. A. (2011) Amyloid-beta oligomers increase the localization of prion protein at the cell surface. J. Neurochem. 117, 538–553 [DOI] [PubMed] [Google Scholar]
- 20. Zou W. Q., Xiao X., Yuan J., Puoti G., Fujioka H., Wang X., Richardson S., Zhou X., Zou R., Li S. (2011) Amyloid beta interacts mainly with insoluble prion protein in the Alzheimer brain. J. Biol. Chem. 286, 15095–15105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferrer I., Blanco R., Carmona M., Puig B., Ribera R., Rey M., Ribalta T. (2001) Prion protein expression in senile plaques in Alzheimer's disease. Acta Neuropathol. 101, 49–56 [DOI] [PubMed] [Google Scholar]
- 22. Parkin E. T., Watt N. T., Hussain I., Eckman E. A., Eckman C. B., Manson J. C., Baybutt H. N., Turner A. J., Hooper N. M. (2007) Cellular prion protein regulates beta-secretase cleavage of the Alzheimer's amyloid precursor protein. Proc. Natl. Acad. Sci. U. S. A. 104, 11062–11067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gacia M., Safranow K., Styczynska M., Jakubowska K., Peplonska B., Chodakowska-Zebrowska M., Przekop I., Slowik A., Golanska E., Hulas-Bigoszewska K., Chlubek D., Religa D., Zekanowski C., Barcikowska M. (2006) Prion protein gene M129 allele is a risk factor for Alzheimer's disease. J. Neural. Transm. 113, 1747–1751 [DOI] [PubMed] [Google Scholar]
- 24. Bertram L., McQueen M. B., Mullin K., Blacker D., Tanzi R. E. (2007) Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat. Genet. 39, 17–23 [DOI] [PubMed] [Google Scholar]
- 25. Calero O., Bullido M. J., Clarimon J., Frank-Garcia A., Martinez-Martin P., Lleo A., Rey M. J., Rabano A., Blesa R., Gomez-Isla T., Valdivieso F., de Pedro-Cuesta J., Ferrer I., Calero M. (2011) Genetic cross-interaction between APOE and PRNP in sporadic Alzheimer's and Creutzfeldt-Jakob diseases. PLoS One 6, e22090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Herms J., Tings T., Gall S., Madlung A., Giese A., Siebert H., Schurmann P., Windl O., Brose N., Kretzschmar H. (1999) Evidence of presynaptic location and function of the prion protein. J. Neurosci. 19, 8866–8875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prusiner S. B. (1998) Prions. Proc. Natl. Acad. Sci. U. S. A. 95, 13363–13383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Riek R., Hornemann S., Wider G., Billeter M., Glockshuber R., Wuthrich K. (1996) NMR structure of the mouse prion protein domain PrP(121–321). Nature 382, 180–182 [DOI] [PubMed] [Google Scholar]
- 29. Wuthrich K., Riek R. (2001) Three-dimensional structures of prion proteins. Adv. Protein. Chem. 57, 55–82 [DOI] [PubMed] [Google Scholar]
- 30. Donne D. G., Viles J. H., Groth D., Mehlhorn I., James T. L., Cohen F. E., Prusiner S. B., Wright P. E., Dyson H. J. (1997) Structure of the recombinant full-length hamster prion protein PrP(29–231): the N terminus is highly flexible. Proc. Natl. Acad. Sci. U. S. A. 94, 13452–13457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Viles J. H., Donne D., Kroon G., Prusiner S. B., Cohen F. E., Dyson H. J., Wright P. E. (2001) Local structural plasticity of the prion protein. Analysis of NMR relaxation dynamics. Biochemistry 40, 2743–2753 [DOI] [PubMed] [Google Scholar]
- 32. O'Sullivan D. B., Jones C. E., Abdelraheim S. R., Brazier M. W., Toms H., Brown D. R., Viles J. H. (2009) Dynamics of a truncated prion protein, PrP(113–231), from (15)N NMR relaxation: order parameters calculated and slow conformational fluctuations localized to a distinct region. Protein Sci. 18, 410–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Klewpatinond M., Davies P., Bowen S., Brown D. R., Viles J. H. (2008) Deconvoluting the Cu2+ binding modes of full-length prion protein. J. Biol. Chem. 283, 1870–1881 [DOI] [PubMed] [Google Scholar]
- 34. Nadal R. C., Davies P., Brown D. R., Viles J. H. (2009) Evaluation of copper2+ affinities for the prion protein. Biochemistry 48, 8929–8931 [DOI] [PubMed] [Google Scholar]
- 35. Younan N. D., Klewpatinond M., Davies P., Ruban A. V., Brown D. R., Viles J. H. (2011) Copper(II)-induced secondary structure changes and reduced folding stability of the prion protein. J. Mol. Biol. 410, 369–382 [DOI] [PubMed] [Google Scholar]
- 36. Viles J. H. (2012) Metal ions and amyloid fiber formation in neurodegenerative diseases. Copper, zinc and iron in Alzheimer's, Parkinson's and prion diseases. Coord. Chem. Rev. 256, 2271–2284 [Google Scholar]
- 37. Khosravani H., Zhang Y., Tsutsui S., Hameed S., Altier C., Hamid J., Chen L., Villemaire M., Ali Z., Jirik F. R., Zamponi G. W. (2008) Prion protein attenuates excitotoxicity by inhibiting NMDA receptors. J. Cell Biol. 181, 551–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sarell C. J., Syme C. D., Rigby S. E., Viles J. H. (2009) Copper(II) binding to amyloid-beta fibrils of Alzheimer's disease reveals a picomolar affinity: stoichiometry and coordination geometry are independent of Abeta oligomeric form. Biochemistry 48, 4388–4402 [DOI] [PubMed] [Google Scholar]
- 39. O'Sullivan D. B., Jones C. E., Abdelraheim S. R., Thompsett A. R., Brazier M. W., Toms H., Brown D. R., Viles J. H. (2007) NMR characterization of the pH 4 beta-intermediate of the prion protein: the N-terminal half of the protein remains unstructured and retains a high degree of flexibility. Biochem. J. 401, 533–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sarell C. J., Wilkinson S. R., Viles J. H. (2010) Substoichiometric levels of Cu2+ ions accelerate the kinetics of fiber formation and promote cell toxicity of amyloid-β from Alzheimer disease. J. Biol. Chem. 285, 41533–41540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Uversky V. N., Li J., Fink A. L. (2001) Metal-triggered structural transformations, aggregation, and fibrillation of human-synuclein. J. Biol. Chem. 276, 44284. [DOI] [PubMed] [Google Scholar]
- 42. Kayed R., Head E., Thompson J. L., McIntire T. M., Milton S. C., Cotman C. W., Glabe C. G. (2003) Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300, 486–489 [DOI] [PubMed] [Google Scholar]
- 43. Lashuel H. A., Hartley D., Petre B. M., Walz T., Lansbury P. T., Jr. (2002) Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature 418, 291. [DOI] [PubMed] [Google Scholar]
- 44. Miyazawa T., Blout E. R. (1961) The infrared spectra of polypeptides in various conformations: amide I and II bands1. J. Am. Chem. Soc. 83, 719–712 [Google Scholar]
- 45. Cerf E., Sarroukh R., Tamamizu-Kato S., Breydo L., Derclaye S., Dufrene Y. F., Narayanaswami V., Goormaghtigh E., Ruysschaert J. M., Raussens V. (2009) Antiparallel beta-sheet: a signature structure of the oligomeric amyloid beta-peptide. Biochem. J. 421, 415–423 [DOI] [PubMed] [Google Scholar]
- 46. Laganowsky A., Liu C., Sawaya M. R., Whitelegge J. P., Park J., Zhao M., Pensalfini A., Soriaga A. B., Landau M., Teng P. K., Cascio D., Glabe C., Eisenberg D. (2012) Atomic view of a toxic amyloid small oligomer. Science 335, 1228–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xue W. F., Hellewell A. L., Gosal W. S., Homans S. W., Hewitt E. W., Radford S. E. (2009) Fibril fragmentation enhances amyloid cytotoxicity. J. Biol. Chem. 284, 34272–34282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Watt N. T., Taylor D. R., Gillott A., Thomas D. A., Perera W. S., Hooper N. M. (2005) Reactive oxygen species-mediated beta-cleavage of the prion protein in the cellular response to oxidative stress. J. Biol. Chem. 280, 35914–35921 [DOI] [PubMed] [Google Scholar]
- 49. Muramoto T., Scott M., Cohen F. E., Prusiner S. B. (1996) Recombinant scrapie-like prion protein of 106 amino acids is soluble. P. Natl. Acad. Sci. U. S. A. 93, 15457–15462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Morales R., Estrada L. D., Diaz-Espinoza R., Morales-Scheihing D., Jara M. C., Castilla J., Soto C. (2010) Molecular cross talk between misfolded proteins in animal models of Alzheimer's and prion diseases. J. Neurosci. 30, 4528–4535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yerbury J. J., Poon S., Meehan S., Thompson B., Kumita J. R., Dobson C. M., Wilson M. R. (2007) The extracellular chaperone clusterin influences amyloid formation and toxicity by interacting with prefibrillar structures. FASEB J. 21, 2312. [DOI] [PubMed] [Google Scholar]
- 52. Stanyon H. F., Viles J. H. (2012) Human serum albumin can regulate amyloid-beta peptide fiber growth in the brain interstitium: implications for Alzheimer disease. J. Biol. Chem. 287, 28163–28168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Andreetto E., Yan L.-M., Tatarek-Nossol M., Velkova A., Frank R., Kapurniotu A. (2010) Identification of hot regions of the Aβ–IAPP interaction interface as high-affinity binding sites in both cross- and self-association. Angew. Chem. Int. Ed. 49, 3081–3085 [DOI] [PubMed] [Google Scholar]
- 54. Nieznanski K., Choi J. K., Chen S., Surewicz K., Surewicz W. K. (2012) Soluble prion protein inhibits amyloid-β (Aβ) fibrillization and toxicity. J. Biol. Chem. 287, 33104–33108 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.