SUMMARY
Sexual dimorphisms in the brain underlie behavioral sex differences, but the function of individual sexually dimorphic neuronal populations is poorly understood. Neuronal sexual dimorphisms typically represent quantitative differences in cell number, gene expression, or other features, and it is unknown if these dimorphisms control sex-typical behavior in one sex exclusively or in both sexes. The progesterone receptor (PR) controls female sexual behavior, and we find many sex differences in number, distribution, or projections of PR-expressing neurons in the adult mouse brain. We have ablated one such PR-expressing neuronal population located in the ventromedial hypothalamus (VMH) using a novel genetic strategy. Ablation of these neurons in females greatly diminishes sexual receptivity. Strikingly, the corresponding ablation in males reduces mating and aggression. Our findings reveal the functions of a molecularly-defined, sexually dimorphic neuronal population in the brain. Moreover we show that sexually dimorphic neurons can control distinct sex-typical behaviors in both sexes.
INTRODUCTION
Males and females show sex differences in many behaviors, including mating and aggression, that result from sexually dimorphic development or activation of the underlying neural circuits. Gonadal sex hormones exert a profound influence on vertebrate sex-typical behaviors by controlling sex differences in the brain (Cooke et al., 1998; Dewing et al., 2003; Gagnidze et al., 2010; Jazin and Cahill, 2010; McCarthy and Arnold, 2011; Morris et al., 2004; Simerly, 2002; De Vries, 1990; Xu et al., 2012; Yang et al., 2006). Most behaviors and neural circuits are shared between the sexes such that sexually dimorphic neuronal clusters represent a small fraction of the neurons within larger brain regions. It has therefore been difficult to discern which dimorphic, hormone-responsive neurons in the brain control each of the various sex differences in physiology and behavior. In addition, neuronal sex differences usually represent quantitative rather than allor-nothing dimorphisms in gene expression or cytological features. It is presently unclear whether such groups of dimorphic neurons regulate gender-typical behaviors in one or both sexes.
Progesterone controls female reproduction, including sexual receptivity, by signaling via its cognate receptor, PR (Levine et al., 2001; Mani et al., 1997). PR is widely distributed in the brain, and the PR+ neurons that regulate sexual receptivity remain to be identified unambiguously (Blaustein and Feder, 1979; Olster and Blaustein, 1990; Quadros et al., 2008). The VMH, which contains a small pool of PR+ neurons in its ventrolateral division (VMHvl), is well characterized for its relevance to female mating in mammals (Blaustein, 2008; Cohen and Pfaff, 1992; Flanagan-Cato, 2011; Rubin and Barfield, 1983). Studies with c-Fos suggest that many VMHvl neurons, including a subset of PR+ neurons, are activated following female mating (Flanagan-Cato et al., 2006). However, lesions or manipulations of neuronal activity of the VMH can lead to no change, a decrease, or an increase in female sexual behavior (Goy and Phoenix, 1963; Kow et al., 1985; Leedy and Hart, 1985; Mathews and Edwards, 1977a, 1977b; Musatov et al., 2006; Pfaff and Sakuma, 1979a, 1979b; Robarts and Baum, 2007; La Vaque and Rodgers, 1975). Some studies also report a concurrent increase in body weight, suggesting a complex role of this region in feeding and mating (King, 2006; Musatov et al., 2007). This phenotypic diversity is likely due to manipulations that variably affect the heterogeneous neuronal subsets within the VMH (Kurrasch et al., 2007), adjacent brain regions, and fibers of passage. Given these challenges, the identity and function of VMHvl neurons that specifically influence female mating remain unclear.
In accord with the notion that the VMHvl influences female sexual behavior, the VMHvl exhibits quantitative cell and molecular sex differences (Dugger et al., 2007; Grgurevic et al., 2012; Matsumoto and Arai, 1983, 1986; Patisaul et al., 2008; Wu et al., 2009; Xu et al., 2012). Intriguingly, lesions or manipulations of neural activity of the VMH or the surrounding neurons have long suggested an important role of this region in controlling aggression (Hess and Akert, 1955; Kruk et al., 1979; Reeves and Plum, 1969; Wheatley, 1944). In fact, this region is activated during male aggression, and correspondingly, electrical activation or inhibition elicits or inhibits fighting, respectively (Kollack-Walker and Newman, 1995; Lin et al., 2011; Veening et al., 2005). However, as with VMH neurons that regulate female receptivity, the identity of VMH neurons that influence aggression is unknown. In principle, these behaviors may be regulated by a single set or by non-overlapping sets of neurons.
We utilized genetic strategies in mice to visualize PR+ neurons and to assess their contributions to mating and aggression. We find many sex differences in PR+ neurons in the adult brain, including in the VMHvl. We have developed a Cre-loxP strategy to ablate any molecularly defined neuronal population via targeted viral delivery of a genetically engineered caspase. Using this approach, we have ablated PR+ VMHvl neurons in adult females and observe a dramatic reduction in sexual receptivity. The corresponding ablation in males reduces mating and territorial aggression. Thus our results define a role of PR+ VMHvl neurons in sex-typical behaviors. Moreover, we establish that a discrete, sexually dimorphic neuronal population influences sexually dimorphic behaviors in both sexes.
RESULTS
Visualizing PR expression in the mouse brain
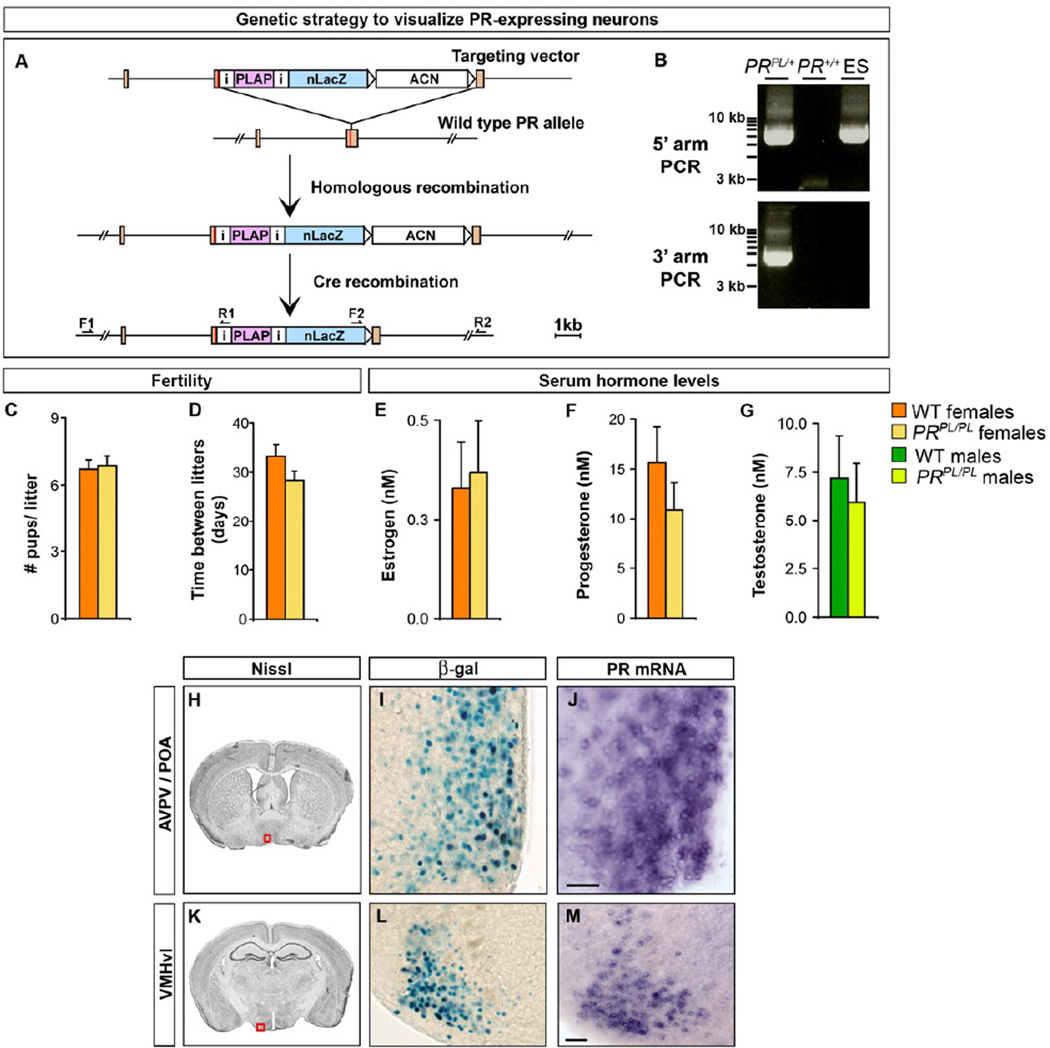
We wished to identify PR+ neurons at high cellular resolution. We inserted an IRES-PLAP-IRES-nuclear LacZ (PL) reporter into the 3’ UTR of PR using gene targeting (Figures 1A, B). As described previously (Shah et al., 2004), this cassette permits expression of placental alkaline phosphatase (PLAP), which labels neuronal processes, and nuclear targeted β-galactosidase (β-gal) in PR+ cells. This strategy maintains the expression and function of PR and permits examination of PR+ neurons in otherwise wildtype (WT) mice. Accordingly, and in contrast to PR−/− mice (Chappell et al., 1997; Lydon et al., 1995), PRPL/PL females were similar to WT females in fecundity and also maintained normal sex hormone titers (Figure 1C–G).
Figure 1. Visualizing PR+ neurons in the mouse brain.
(A) Generating the PRPL allele. ACN is a self-excising neomycin selection cassette (Bunting et al., 1999). Orange rectangles are exons and the red line in the 3’ exon denotes the stop codon.
(B) PCR to detect homologous recombination at the PR locus. Primers used to detect integration of the 5’ (F1, R1) and 3’ (F2, R2) arms of the targeting vector. ACN precludes detection of the 3’ recombination event in ES cells.
(C, D) No difference between WT and PRPL/PL females in litter size and frequency.
(E–G) No difference in titers of sex hormones between WT and PRPL/PL adults.
(H–M) Boxed areas in Nissl-stained coronal sections (Paxinos and Franklin, 2003) through the adult brain depict locations of the regions shown in panels to the right. PR expression in PRPL/+female as labeled by β-gal activity mirrors expression of PR mRNA in adjacent sections.
Scale bars = 50 µm.
Mean ± SEM; n ≥ 12/genotype (C–G); n = 3 (H–M).
In the forebrain, we observed β-gal activity in pools of neurons in specific hypothalamic nuclei, posterodorsal medial amygdala (MeApd), medial division of the posteromedial bed nucleus of the stria terminalis (BNSTmpm), various cortical areas, basal ganglia, and dentate gyrus (Figures 1H–M, 2, S1). This distribution of cells mirrors the expression pattern of PR mRNA in adjacent sections (Figure 1H–M). In regions such as the basal ganglia with low level PR expression that precludes visualization by in situ hybridization, we can detect PR message by RT-qPCR (Figure S1A). The distribution of β-gal+ cells is in accord with histological and pharmacological studies (Becker, 1999; Blaustein and Feder, 1979; Olster and Blaustein, 1990; Quadros et al., 2008). In the case of the basal ganglia, our studies localize PR expression to sparsely distributed neurons across the rostrocaudal axis (Figure S1B–D). In addition, we find unreported PR+ neuronal pools scattered within the basal forebrain (Figure 2), an observation confirmed by RT-qPCR from this region (Figure S1A). The ~1 week t1/2 of β-gal in neurons precludes detection of PR mRNA changes across the 4–6 day estrous cycle (Allen, 1922; Smith et al., 1995). However, the long t1/2 and superb signal:noise of β-gal labeling allows sensitive detection of PR expression. Together, the PRPL reporter mouse confirms and extends previous reports of PR expression in the mouse brain.
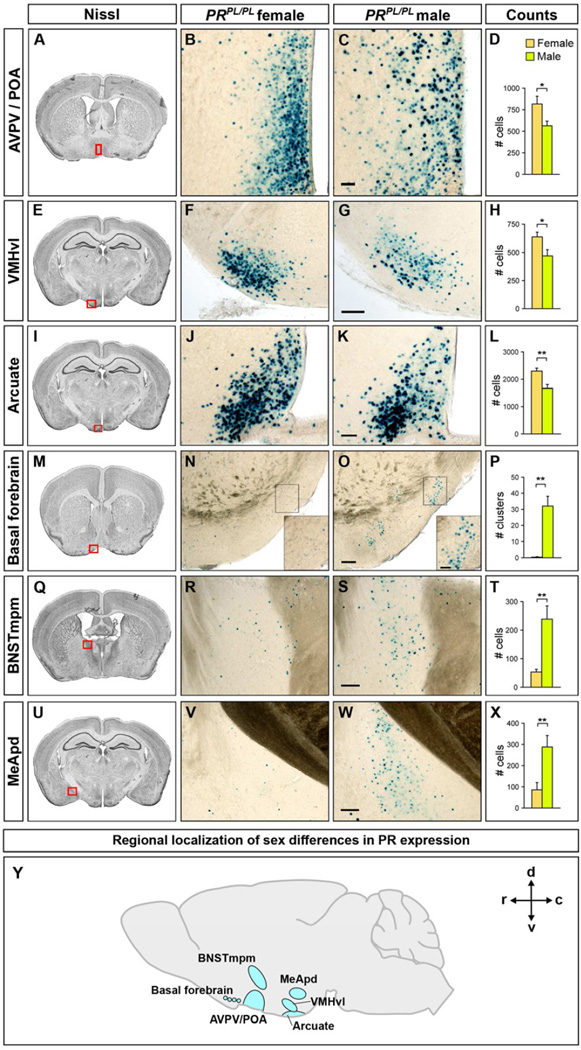
Figure 2. Sexual dimorphism in PR-expression in the adult brain.
Boxed areas in Nissl-stained coronal sections through the adult brain depict regions of PRPL/PLmice labeled for β-gal activity in the panels to the right.
(A–L) More PR+ cells in the female AVPV/POA, VMHvl, and arcuate nucleus.
(M–X) More PR+ cells in the male basal forebrain, BNSTmpm, and MeApd.
(Y) Representation of sexually dimorphic PR expression in different brain regions as projected on to a mid-sagittal section. c, caudal, d, dorsal, r, rostral, v, ventral.
Scale bars = 50 µm (C, K) and 100 µm (G, O, S, W). Inset scale bars = 25 µm.
Mean ± SEM; n ≥ 4/sex; *p < 0.04, **p < 0.01.
Widespread sex differences in the distribution and cell number of PR+ neurons
We observed previously unreported as well as known sex differences in PR+ cells in the adult PRPL brain (Figures 2, S2A, and Table S1). We found more PR+ cells in the female preoptic area (POA), the adjacent anteroventral periventricular hypothalamic nucleus (AVPV), arcuate nucleus, and VMHvl (Figure 2A–L). The VMHvl contains cells expressing the estrogen receptor alpha (ERα or Esr1) (Xu et al., 2012), and we find that >92% PR+ neurons co-label for ERα in both sexes (Figure S2B). We asked whether PR+ VMHvl neurons expressed Cckar, a GPCR required for sexual receptivity and expressed in the female but essentially absent in the male VMHvl (Xu et al., 2012). We observed that 67% ± 3 (Mean ± SEM) of PR+ VMHvl cells co-labeled with Cckar whereas 96% ± 0.2 of Cckar+ VMHvl cells were PR+ (n = 3 PRPL/PL females, ≥500 cells analyzed/brain) (Figure S2C–E). Thus PR+ neurons represent the vast majority of VMHvl neurons that express Cckar, a gene required for female mating.
We observed many clusters of PR+ cells (~15–40 cells/cluster) in the male but not female basal forebrain (Figure 2M–P). Together with a sex difference in androgen receptor expression in this region (Shah et al., 2004), our findings suggest an unappreciated role of the basal forebrain in responding to sex hormones. We also found more PR+ cells in the male BNSTmpm and MeApd (Figure 2Q–X). This increased PR expression is surprising because there is little circulating progesterone in males; our findings are nevertheless consistent with studies indicating a role of PR in male behaviors (Phelps et al., 1998; Schneider et al., 2005, 2009; Witt et al., 1995). As suggested previously (Mani et al., 1994a; Power et al., 1991; Tsutsui, 2012), PR may function in a progesterone- independent manner or locally synthesized progesterone may activate PR in males. Consistent with these sex differences in PR expression, the POA, BNSTmpm, MeApd, arcuate nucleus, and VMHvl have been implicated in sex differences in behavior or physiology (Cooke et al., 1998; Morris et al., 2004; Simerly, 2002), and PR+ neurons in these regions could contribute to such sexually dimorphic output.
We find that the dimorphic PR+ cells co-label with pan-neuronal markers (Figure S2F). However, within any given brain region expressing PR dimorphically, only a subset of neurons is PR+. Even within the VMHvl, only 49% ± 4 of NeuN+ cells co-label with PR (n = 3 brains, ≥103 NeuN+ cells analyzed for PR/brain). There is a sex difference in the soma size of thionin-labeled neurons within the rat VMHvl (Dugger et al., 2007). However, there was no such sex difference in PR+ VMHvl neurons (Figure S2G), suggesting a species difference or that other VMHvl neurons account for this dimorphism. The sex differences in PR expression cannot result solely from sex differences in neuronal numbers. Indeed, no sex difference in neuronal number has been reported in the basal forebrain or VMHvl, and in the POA and arcuate nucleus, which contain more neurons in males (Gorski et al., 1980; Leal et al., 1998), we find more PR+ neurons in females. Finally, the 3–4 fold more PR+ neurons in the male BNSTmpm and MeApd exceeds the <2-fold more neurons in these regions in males (Morris et al., 2008; Shah et al., 2004; Wu et al., 2009). Thus, our studies confirm known sex differences (POA, VMHvl, arcuate nucleus, MeApd) (Blaustein et al., 1980; Brown et al., 1996; Grgurevic et al., 2012; Kudwa et al., 2009; Quadros et al., 2002) and reveal new sexual dimorphisms in PR expression (basal forebrain, BNSTmpm) in the mammalian brain.
Visualizing sex differences in projections of PR+ neurons
We determined whether sexually dimorphic PR+ neurons projected to distinct locations in the two sexes. Consistent with PR expression in interconnected regions such as the POA, BNST, MeA, and VMHvl, we observed a rich distribution of PLAP+ fibers in the PRPL/PL forebrain (data not shown) that precluded identification of dimorphic projection patterns. We devised a genetic strategy to visualize the projections of any subset of PR+ neurons. We first targeted an IRES-Cre recombinase cassette to the 3’ UTR of PR (Figures 3A, S3A, B). As expected, these PR-IRES-Cre (PRCre) mice, like PRPL mice, are viable and fertile, and Cre expression mirrors that of PR in the brain (Figure S3C–F). We also designed a lentiviral vector that expresses PLAP in a Cre-dependent manner (Lenti-lxlplap, Figures 3A, S3G). This lentivirus is replication-incompetent and integrates into the host genome, properties that restrict PLAP expression to Cre+ cells for the life of the cells. This virus infects cells in both WT and PRCre mice, but we only observe PLAP expression in PRCre mice (Figure 3B–E).
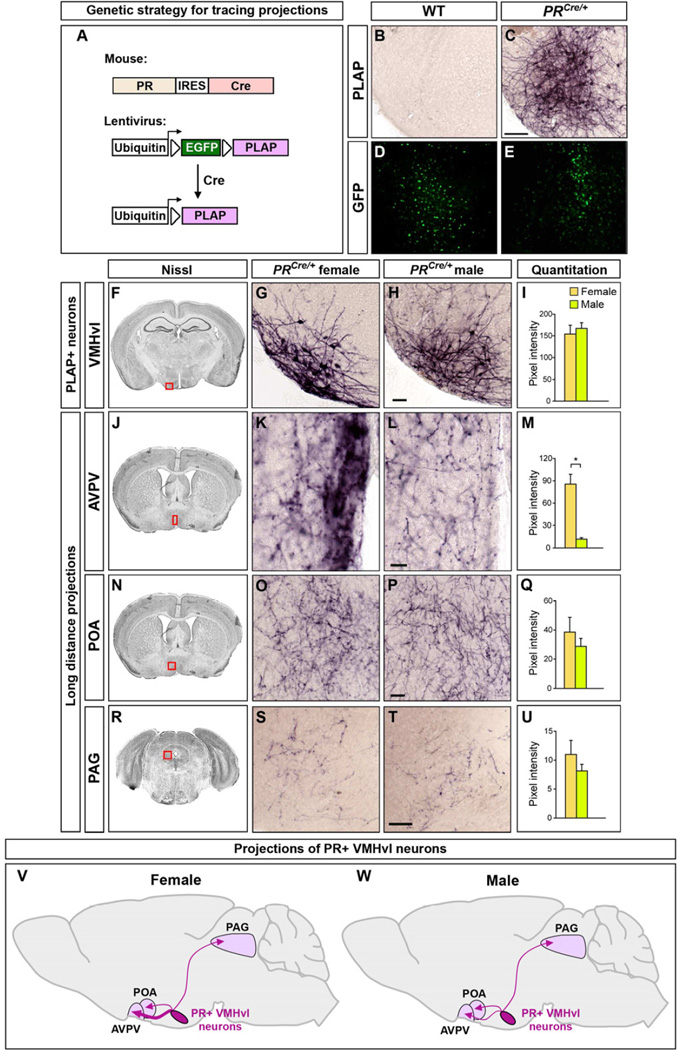
Figure 3. PR+ VMHvl neurons project in a sexually dimorphic manner.
(A) Strategy to visualize projections of PR+ neurons.
(B–E) Lenti-lxlplap targeted to the VMH infects cells in PRCre/+ and WT mice as visualized by EGFP+ cells. Only a few cells are PR+ in this region so there is no apparent difference in the number of EGFP+ cells in PRCre and WT mice. PLAP+ soma and local arbors of VMHvl neurons are only observed in PRCre mice.
(F–U) Boxed areas in Nissl-stained coronal sections depict regions shown in panels to the right. Lenti-lxlplap targeted to the VMHvl of adult PRCre/+ mice labels PLAP+ soma and local arbors of VMHvl neurons (F–I). The lentiviral titer limits the number of infected Cre+ neurons and does not highlight the sex difference in the number of these neurons. The variable multiplicity of infection can lead to apparent size differences in PLAP-labeled soma. However, there is no sex difference in the soma size of these neurons (Figure S2G). PR+ VMHvl neurons project to the AVPV, POA, and PAG (J–U). There are more PLAP+ projections to the AVPV in females (J–M).
(V, W) Schematic summarizing projections of PR+ VMHvl neurons. No difference in anatomical extent of projections in different regions, but female AVPV receives more innervation from these neurons.
Scale bars = 100 µm (C), 50 µm (H,P,T), 25 µm (L).
Mean ± SEM; n ≥ 7/sex; *p < 0.001.
See also Figure S3, Tables S2, S3.
The VMH has been implicated in sex-specific behaviors, and we therefore traced the projections of PR+ VMHvl neurons in adults. We initially determined that we could visualize maximal expression of PLAP 7–8 days following delivery of Lenti-lxlplap into the VMH (CFY, unpublished observations). Such injections revealed the soma and local arbors of PR+ VMHvl neurons (Figure 3F–I). In contrast to the wide-ranging projections of the entire VMH (Saper et al., 1976; Krieger et al., 1979), we observed PLAP+ projections of PR+ VMHvl neurons in the AVPV and adjacent periventricular area, POA, and periaqueductal gray (PAG) (Figure 3J–U). Unlike PR+ VMHvl projections in the guinea pig (Ricciardi and Blaustein, 1994), mouse PR+ VMHvl neurons did not appear to project appreciably to the BNST or MeA, suggesting subtle species differences in these cells. Although we observed similar localization of PLAP+ projections of PR+ VMHvl neurons in both sexes (Figures 3J–W, S3H, and Table S2), there was a striking, previously unreported 7-fold increase in PLAP+ fibers in the female AVPV (Figure 3J–M). This sex difference cannot solely result from the dimorphism (~30%) in PR+ VMHvl cell number. In fact, we even observed the dimorphic AVPV projection in PRCre females in whom a few PR+ VMHvl neurons had been infected. Thus, more PR+ female VMHvl neurons project to the AVPV or their axonal termini arborize more extensively. The AVPV is thought to control ovulation, and the PAG can regulate sexual receptivity in females (Sakuma and Pfaff, 1979; Simerly, 2002). In summary, PR+ VMHvl neurons project to a subset of VMH targets, their efferents are sexually dimorphic, and each of their targets can influence sexually dimorphic behaviors or physiology.
A novel genetic approach to ablate adult neurons in vivo
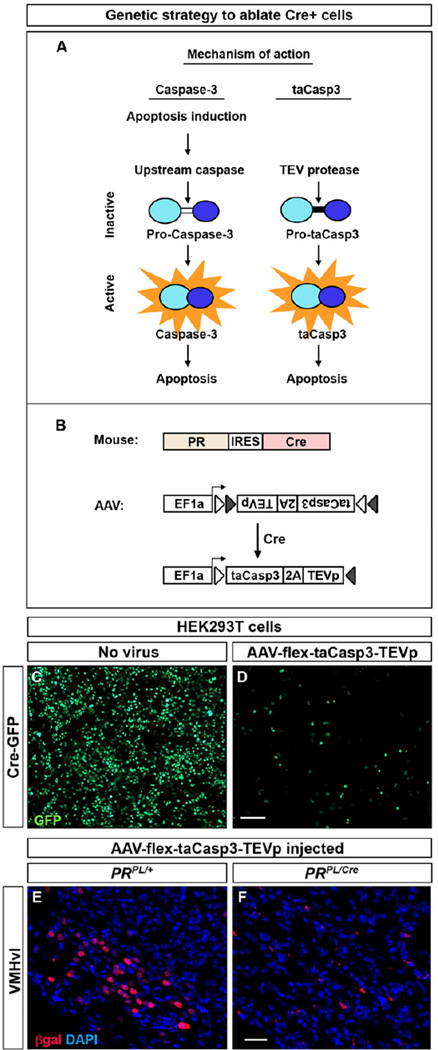
We determined the requirement of PR+ VMHvl neurons in sex-typical behaviors by targeting Cre-dependent, virally encoded toxins to the VMHvl of PRCre mice. Initial studies suggested that virally encoded diphtheria toxin A or tBid (Jiang and Wang, 2004; Maxwell et al., 1986) were partially effective in ablating PR+ neurons in vivo even though they were effective in tissue culture cells (CFY, unpublished observations). We therefore employed a genetically engineered caspase-3, a caspase whose activation commits a cell to apoptosis, to kill adult neurons in vivo (Figure 4A) (Gray et al., 2010). Endogenous caspase-3 normally exists as pro-caspase-3, and apoptotic signals activate upstream caspases that cleave pro-caspase-3 into its active form (Figure 4A). Our designer pro-caspase-3, pro-taCasp3, lacks the cleavage site for upstream caspases and encodes a cleavage site for the heterologous enzyme Tobacco Etch Virus protease (TEVp). Provision of TEVp activates pro-taCasp3 into the apoptosis-inducing taCasp3. We generated an adeno-associated virus (AAV) to drive expression of pro-taCasp3 and TEVp in a Cre-dependent manner (Figures 4B, S4A) (Atasoy et al., 2008). This virus (AAV-flex-taCasp3-TEVp) utilizes the T2A peptide encoding sequence to ensure bi-cistronic expression of pro-taCasp3 and TEVp. Importantly, taCasp3 triggers cell-autonomous apoptosis, thereby minimizing toxicity to adjacent non-Cre+ cells (Gray et al., 2010).
Figure 4. Genetic strategy to ablate neurons in a Cre-dependent manner.
(A) Intramolecular cleavage of endogenous pro-caspase-3 by upstream caspases activates caspase-3, which then induces apoptosis. This intramolecular cleavage site has been replaced by a TEV linker domain (black bar) in inactive taCasp3 (pro-taCasp3) such that only TEV protease activates taCasp3, which then induces apoptosis.
(B) Viral strategy to ablate PR+ neurons conditionally.
(C, D) Cell death 1 week following infection of Cre:EGFP+ HEK293T cells with AAV-flex-taCasp3-TEVp. n = 3 experiments.
(E, F) Ablation of PR+ VMHvl neurons in a PRPL/Cre but not PRPL/+ female injected with AAV-flex-taCasp3-TEVp. n ≥ 10/experimental group.
Scale bar = 100 µm (C, D) and 25 µm (E, F).
See also Figure S4.
Infection of HEK293T cells with this virus leads to rapid Cre-dependent cell death (Figure 4C, D). We next tested whether this virus could ablate adult PR+ neurons by stereotaxically targeting it to the VMHvl of adult PR+/PL or PRCre/PL mice. PR+ VMHvl neurons appeared unaffected in controls but were essentially completely lost in PRCre/PL mice 2–4 weeks following viral delivery (Figures 4E, F, S4B). We tested whether the taCasp3-encoding AAV targeted to the VMHvl diffused to and ablated PR+ cells in distant hypothalamic regions. We therefore enumerated PR+ cells along the rostrocaudal extent of the hypothalamus in a cohort of virally injected control and PRCre mice. This analysis revealed no difference in PR+ cell counts between PRCre and control females (number of PR+ cells: Control, 619 ± 60 and PRCre, 679 ± 150; n = 5/cohort, p = 0.7). Thus taCasp3-mediated ablation appears restricted to the vicinity of the injection site. We observed local spread of the virus to the arcuate and present these findings below. In separate experiments, we found that stereotaxic delivery of the taCasp3-encoding virus ablated Cre+ neurons in different brain regions (CFY, EKU, and MC, unpublished observations), indicating that we have devised a general strategy for targeted ablation of Cre+ cells.
The dimorphic PR+ VMHvl cluster of neurons regulates female sexual behavior
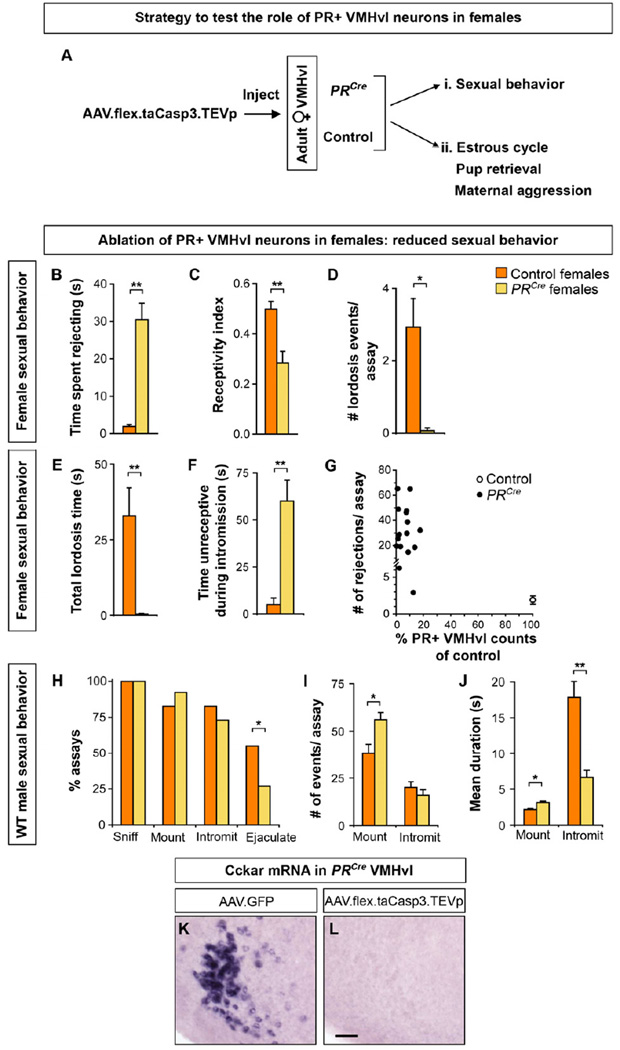
We tested the role of PR+ VMHvl neurons in female mating. We targeted AAV-flex-taCasp3-TEVp bilaterally to the VMHvl of adult PRCre and control females (Figure 5A). To assure optimal sexual receptivity, females were ovariectomized at the time of viral injection and, following recovery, hormonally primed to be in estrus when tested with WT males.
Figure 5. PR+ VMHvl neurons regulate female sexual receptivity.
(A) Experimental design to test the role of PR+ VMHvl neurons in female behaviors. Mating was tested with ovariectomized females primed to be in estrus. Other behaviors were tested with gonadally-intact females.
(B–J) PRCre and control females were injected with AAV-flex-taCasp3-TEVp and tested for sexual behavior with WT males.
(B) PRCre females spend more time rejecting male mating attempts, walking away when the male approaches.
(C–E) PRCre females display lower receptivity index (mounts leading to intromission/total mounts) and reduced number and duration of lordosis events.
(F) PRCre females spend more time moving about and being unreceptive during intromission.
(G) Fewer than 20% of PR+ neurons remain in the VMHvl of PRCre females, who reject male mating attempts more than control females.
(H) Males sniff and initiate mating equivalently with PRCre and WT females but ejaculate in fewer assays with PRCre females.
(I) Males mount PRCre females more but without a corresponding increase in intromission.
(J) Males mount PRCre females longer, but intromit for shorter duration.
(K, L) Ablation of PR+ VMHvl neurons in PRCre females results in loss of Cckar expression.
Mean ± SEM; n ≥ 10/experimental group (B–J); n = 3 (K, L); *p < 0.02, ** p < 0.005. Scale bar = 50 µm.
We observed a marked diminution of female sexual behavior in such PRCre females (Figure 5B–G, Movies S1 and S2). As in many vertebrates, female mating in mice is stereotyped and includes permitting the male to approach and mount and dorsiflexing the neck and back (lordosis) upon sensory stimulation to the dorsum (Harvey, 1651; McGill, 1962). This allows the males to intromit (penetrate, as determined by his thrust pattern) and attempt ejaculation. PRCre females rejected mount attempts by kicking or running away (Figure 5B), thereby reducing the fraction of mounts that progressed to intromission (receptivity index, Figure 5C). In sharp contrast to controls, PRCre females walked around during intromission, lordosed rarely, and with a >20-fold reduction in lordosis duration (Figures 5D–F). This reduced sexual behavior of PRCre females affected the WT male partner’s performance (Figure 5H–J). Males were interested in both PRCre and control females, initiating anogenital sniffing, mounting, and intromission equivalently, but were less successful in ejaculating with the former (Figures 5H, S5A, S5B). Accordingly, males intromitted only briefly with PRCre females even though they mounted the females more and for longer duration (Figure 5I, J). Correspondingly the total duration of intromission per assay was also reduced (Control, 279 s ± 41 and PRCre, 121 s ± 19; n ≥ 10, p = 3×10−3). In summary, targeted ablation of adult PR+ VMHvl neurons leads to a significant diminution in female mating.
We next assessed the ablation of PR+ VMHvl cells in these PRCre females. We observed that most (97% ± 1; n = 10 control and 16 PRCre females) PR+ VMHvl neurons were ablated upon injection of the taCasp3-encoding AAV into PRCre females (Figure 5G). Co-injection of this AAV and a constitutively expressed EGFP-encoding AAV revealed spread to the adjacent arcuate nucleus, which contains PR+ neurons (Figure 2I–L) and controls feeding and the estrous cycle (Atasoy et al., 2012; Simerly, 2002). Consistent with the lack of estrous cycle or body weight phenotypes in PRCre mice (see below and Figure S5), our injections spared most PR+ arcuate neurons in PRCre females (74% ± 12 of controls). There was no correlation in the extent of loss of PR+ arcuate neurons and reduced sexual receptivity (R2 = 5×10−3, p = 0.8). Moreover, we found that PRCre females (n = 7) in whom the number of PR+ arcuate neurons was indistinguishable from controls also rejected males and displayed reduced sexual receptivity (Rejections/assay: Controls, 1 ± 1 and PRCre females, 35 ± 7; p ≤ 6 × 10−5, n ≥ 7; Receptivity Index: Controls, 0.5 and PRCre females 0.2 ± 0.1, p ≤ 3 × 10−3, n ≥ 7). Thus PR+ VMHvl neurons are required for normal female sexual behavior.
We tested the specificity of the behavioral deficit in PRCre females following ablation of PR+ VMHvl neurons. Despite their reduced sexual receptivity, these mice sniffed and groomed males normally (Figure S5C, D) (Groom duration: Control, 2 s ± 1 and PRCre, 5 s ±1, n ≥ 10, p ≥ 0.3). There were no overt deficits in tests of anxiety, motivated behavior, motor coordination, and locomotor activity (Figure S5E–H). In contrast to the weight gain subsequent to a VMH lesion (Dhillon et al., 2006; Hetherington and Ranson, 1940; King, 2006; Majdic et al., 2002), PRCre females maintained body weight similar to controls upon ablation of PR+ VMHvl neurons (Figure S5I). Thus, we have partitioned the VMHvl to reveal that PR+ VMHvl neurons are required for normal levels of female sexual receptivity but not for all social or other behaviors and physiology.
In separate studies we ablated PR+ VMHvl neurons but left the ovaries intact to examine whether other female-typical behaviors are regulated by these neurons. This ablation did not disrupt the estrous cycle as assayed by vaginal cytology (Figure S5J). To test for maternal behaviors, we obtained litters from PRCre and control females by co-housing them with WT males. Similar to control females, PRCre females displayed various elements of maternal care toward their litters, including pup retrieval and aggression toward unfamiliar intruders in their cage (Figure S5K–O). Our results therefore show that ablation of PR+ VMHvl neurons reduced female sexual displays without overt disruption of other female-typical behaviors and physiology.
PR regulates female mating (Lydon et al., 1995), and our findings suggest that it functions in the VMHvl to do so, consistent with prior work (Mani et al., 1994a, 1994b; Ogawa et al., 1994; Pollio et al., 1993). Cckar is also required for female mating (Xu et al., 2012). Most Cckar+ VMHvl neurons are PR+ (Figure S2C–E), resulting in a near-complete loss of these cells upon ablation of PR+ VMHvl neurons (Figure 5K, L). It is possible that PR or Cckar act elsewhere to control female mating, and these genes only mark a pool of VMHvl neurons that controls this behavior. We favor a more parsimonious model in which PR and Cckar function in the VMHvl to regulate female mating. In any event, our findings show that PR+, Cckar+ VMHvl neurons are essential for high, WT levels of female sexual behavior.
The dimorphic PR+ VMHvl cluster of neurons regulates mating and aggression in males
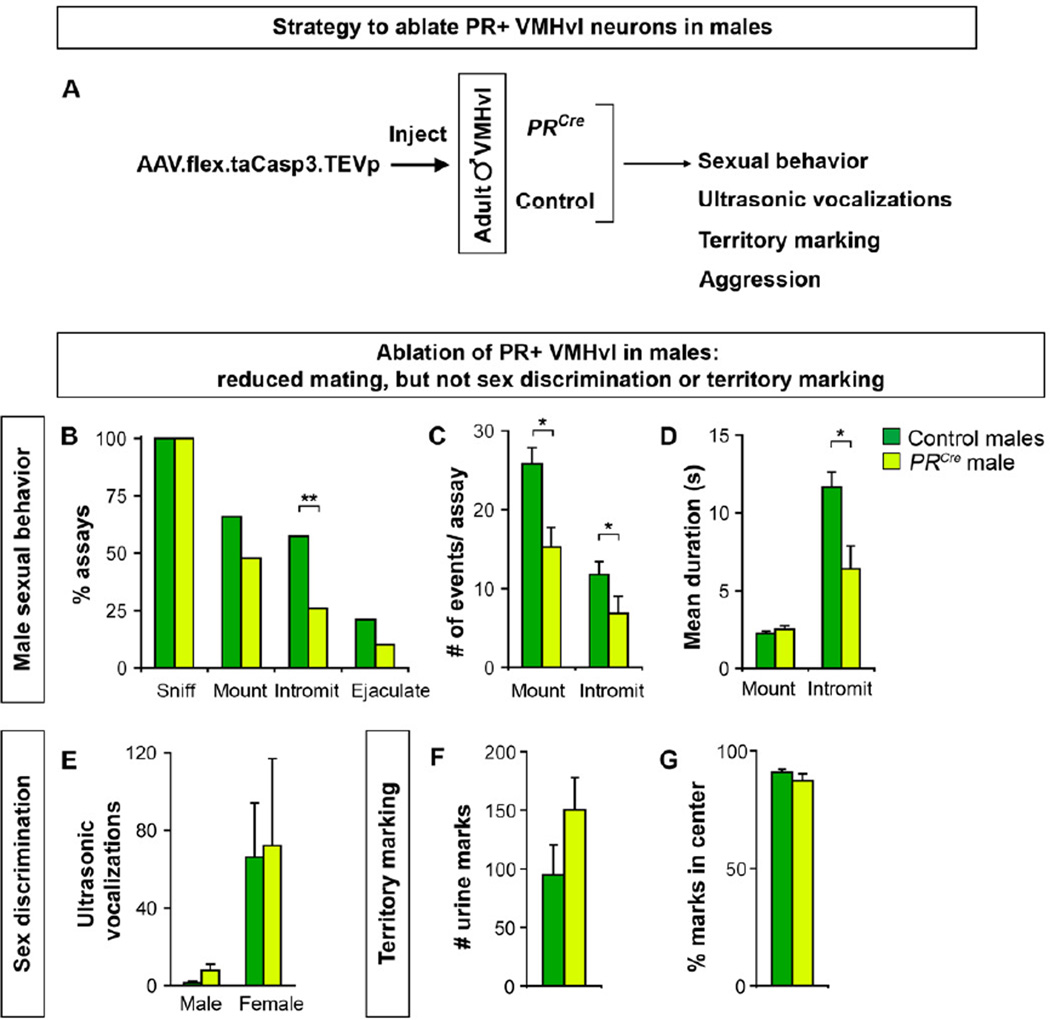
The VMHvl has been implicated in regulating female mating and male fighting. PR+ neurons represent ~50% of VMHvl neurons and these regulate female mating (Figure 5), but fighting could be controlled by PR+ or PR- VMHvl cells. We tested whether PR+ VMHvl neurons regulate male behaviors by ablating them with the taCasp3-encoding AAV (Figure 6A). PRCre and control males were allowed to recover for 4 weeks following viral delivery, singly housed, and tested for mating and fighting.
Figure 6. PR+ VMHvl neurons regulate male sexual behavior.
(A) Experimental design to test the role of PR+ VMHvl neurons in male behaviors.
(B–G) PRCre and control males were injected with AAV-flex-taCasp3-TEVp and tested for mating, ultrasonic vocalizations toward male or female intruders, and territory marking.
(B) PRCre males intromit females in fewer assays.
(C, D) PRCre males mount and intromit females less and have shorter bouts of intromissions.
(E) Both PRCre and control males emit more vocalizations to females.
(F, G) No difference between PRCre and control males in the number and distribution of urine
marks. % marks in center = 100*(# urine marks not abutting cage perimeter/# of all urine marks).
Mean ± SEM; n ≥ 24/experimental group (B–D, F,G), n ≥ 5/experimental group (E); *p < 0.008, **p < 0.001.
See also Figures S6 and S7.
PRCre and control males initiated mounting intruder females equivalently, but PRCre males were less likely to intromit (Figures 6B and S6A). The reduced intromissions likely resulted from the fewer mounts exhibited by PRCre males (Figure 6C). Even when these males intromitted, there was a decrease in the number and duration of intromissions (Figures 6C, D, S6B). The decreased intromission count was significant (n ≥ 16/cohort; p = 5×10−3) even when normalized to the fewer mounts. Thus, ablation of male PR+ VMHvl neurons leads to specific deficits in consummatory elements of mating. This phenotype is not accompanied by deficits in presumptively appetitive behaviors such as sniffing (Figure 6B, S6C–E), sex discrimination, or territory marking. There was no difference between PRCre and control males in sex discrimination as shown by predominantly female-directed ultrasonic vocalization (Figure 6E) (Nyby et al., 1977). Both PRCre and control males also marked their territory equivalently (Figure 6F, G) (Desjardins et al., 1973; Kimura and Hagiwara, 1985). Together, PR+ VMHvl neurons are essential for the normal display of male sexual behavior.
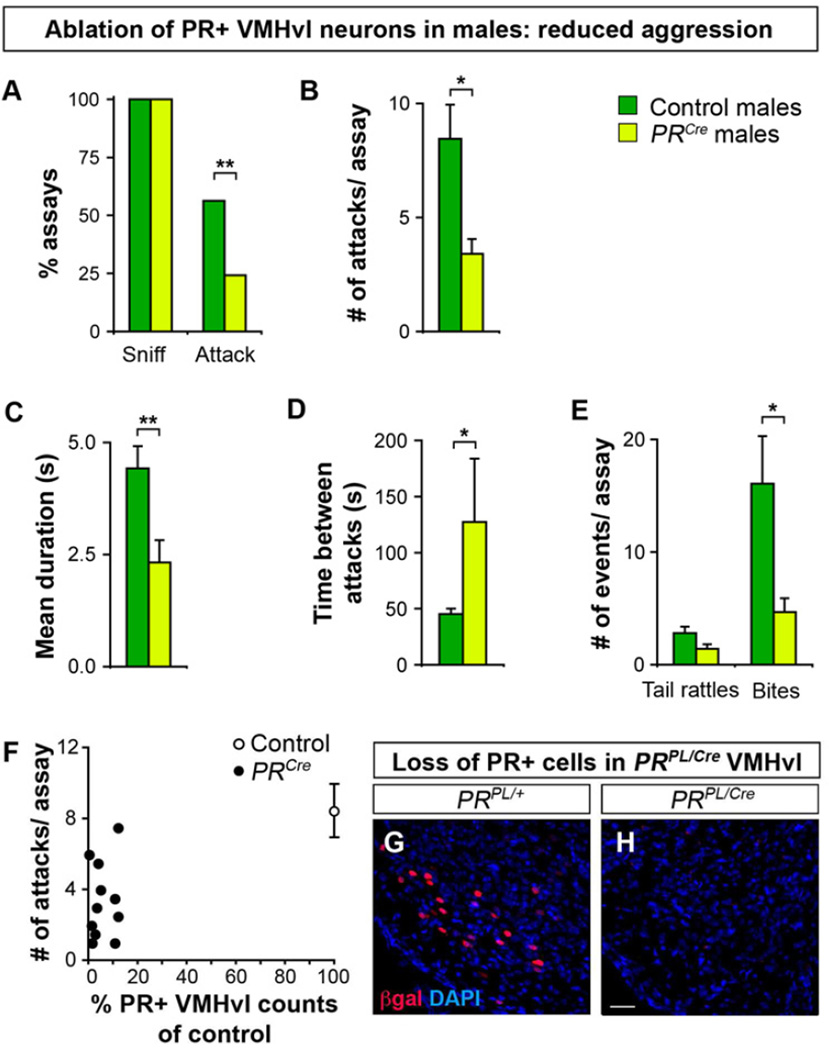
We tested whether ablation of PR+ VMHvl neurons disrupted aggression toward a WT male intruder. PRCre males exhibited a >2-fold reduction in the probability of initiating aggression compared to controls (Figure 7A). Even when PRCre males fought, they attacked less, for a shorter duration, and with a longer inter-attack interval (Figure 7B–D). Male fighting includes tail rattles and overt attacks such as biting. Control and PRCre residents rattled their tails equivalently, but PRCre males bit the intruders over 3-fold less (Figure 7E). Thus ablation of PR+ VMHvl neurons significantly reduces male aggression.
Figure 7. PR+ VMHvl neurons regulate male aggression.
(A–F) PRCre and control resident males were injected with AAV-flex-taCasp3-TEVp targeted to the VMHvl and tested for aggression toward a WT male intruder.
(A) All residents sniff intruders equivalently, but PRCre males attack less.
(B–D) When PRCre males fight, they attack less, for a shorter duration, and with longer intervals between attacks.
(E) PRCre males bite less.
(F) Fewer than 20% of PR+ neurons remain in the VMHvl of PRCre males, who attack intruders less.
Mean ± SEM; n ≥ 24/experimental group; *p < 0.04, **p ≤ 0.009.
(G, H) Ablation of PR+ VMHvl neurons in a PRPL/Cre male injected with AAV-flex-taCasp3-TEVp. Scale bar = 25 µm.
See also Figure S7.
We assessed the ablation of PR+ VMHvl neurons in males tested behaviorally. Most of these neurons (95% ± 1; n = 14 control and 35 PRCre males) were ablated in PRCre males (Figure 7F–H) whereas PR+ arcuate neurons were largely spared (92% ± 12 of controls). There was no correlation in the extent of loss of PR+ neurons in the arcuate and the reduced mating or fighting (mating, R2 = 4 ×10−4, p = 0.9; fighting, R2 = 2 × 10−2, p = 0.7). PRCre males (n = 15) in whom the number of PR+ arcuate neurons was indistinguishable from controls also exhibited deficits in mating and fighting (Percent males intromitting: Controls, 67% and PRCre males, 27%, n ≥ 15, p = 0.02; Percent males attacking: Controls, 75% and PRCre males, 20%, n ≥ 15, p = 1 × 10−3). Taken together, our findings demonstrate that PR+ VMHvl neurons control the normal display of male mating and fighting.
We tested the specificity of the deficits in PRCre males following ablation of PR+ VMHvl neurons. Despite deficits in mating and fighting, these males sniffed and groomed intruders in a WT manner (Figures 6B, 7A, S6C–E, S7A–C). PRCre males performed at WT levels in assays of anxiety, motivated behavior, motor coordination, and locomotor activity (Figure S7D–G). These males maintained normal body weight, and there was no change in the weight of gonads, seminal vesicles, and serum testosterone titers (Figure S7H–J). Thus, PR+ VMHvl neurons are specifically required in males for the high, WT levels of mating and aggression.
DISCUSSION
We have identified a small, sexually dimorphic cluster of ~2,000 PR+ hypothalamic neurons that is essential for the normal display of sexual receptivity in females and sexual and aggressive behaviors in males. Our findings directly demonstrate that sexually dimorphic neurons in the brain influence dimorphic behaviors. Moreover these PR+ neurons are functionally bivalent in that they regulate distinct dimorphic behaviors in the two sexes.
Control of social behaviors by the VMH
Experimental studies and clinical observations have suggested that the VMH or adjacent hypothalamic regions regulate aggression and female mating (Bard, 1928; Blaustein, 2008; Clemente and Chase, 1973; Colpaert and Wiepkema, 1976; Grossman, 1972; Hess and Akert, 1955; Kow et al., 1985; Kruk et al., 1979; Lin et al., 2011; Olivier and Wiepkema, 1974; Pfaff and Sakuma, 1979a, 1979b; Reeves and Plum, 1969; Swaab, 2003; La Vaque and Rodgers, 1975; Wheatley, 1944). Despite intense scrutiny, the neurons that control these behaviors remained unidentified. In fact, whether separate or overlapping neuronal groups control these innate behaviors was also unknown. Our studies reveal the molecular identity of the long sought-after neurons in or around the VMH that influence male fighting and female mating. While other neighboring neurons may also influence these behaviors, we show that PR+ VMHvl neurons are required for the normal display of mating in females and fighting in males. These PR+ neurons also regulate male mating. Non-targeted inhibition of neurons in this region disrupts male fighting but not mating (Lin et al., 2011), suggesting partial inactivation or incomplete targeting of the neurons that regulate male mating. By contrast, our ablation of the PR+ VMHvl population revealed a role for these cells in male mating. Generalized arousal systems may feed into the VMH to enhance social interactions (Schober et al., 2011). We do not observe altered locomotor activity, sensorimotor coordination, or general social interactions in mice lacking PR+ VMHvl neurons, suggesting that these neurons are unlikely to exert a major influence on neural pathways that increase such arousal. In summary, we show that PR+ VMHvl neurons are required for the normal display of mating in both sexes and fighting in males. Given the conservation of genes and neuroanatomy across placental mammals, these VMHvl neurons may regulate mating and aggression in many mammals, including humans.
Distributive neural control of sexually dimorphic behaviors
It is curious that ablation of a highly restricted, molecularly defined set of neurons results in deficits in male mating and fighting. These PR+ neurons may integrate social cues relevant to both behaviors, allowing males to mate or fight appropriately. Such dual control could also reflect further diversity within PR+ VMHvl neurons such that subsets of these neurons regulate one or the other behavior. In fact, in vivo recordings and c-Fos studies (Lin et al., 2011) reveal male VMHvl neurons that are activated during encounters with both sexes as well as neurons that appear responsive to either male or female encounters.
We find that different components of male behaviors require distinct neuronal populations. Males lacking PR+ VMHvl neurons have a male behavioral repertoire: they distinguish between the sexes with vocalizations (Stowers et al., 2002), attack males, and mate with females. Moreover, these males mark territory like WT males, thereby providing an objective indicator that their internal representation of sexual identity is masculine. Nevertheless these males display specific deficits in mating and fighting, indicating that ablation of PR+ VMHvl neurons dissociates the repertoire of masculine behaviors. Such partial behavioral deficits could reflect compensatory mechanisms activated upon the loss of these neurons. However, acute inactivation of the VMH mimics the behavioral deficits we observe (Lin et al., 2011), suggesting a minimal role of compensatory mechanisms. Thus, male mating and fighting are encoded in a distributive or redundant manner in the brain. Similarly, ablation of these neurons reduces female sexual receptivity without overtly disrupting estrous cyclicity or maternal care, indicating that these behaviors and physiology may also be controlled by distinct neuronal groups. Together, our findings show that sex-typical behaviors are represented distributively, and different neuronal populations in the underlying neural circuit control specific components of these behaviors. In fact genes such as Cckar also control these behaviors in a modular manner; for instance, Cckar−/− females show reduced sexual receptivity without alterations in other behaviors or physiology (Xu et al., 2012). Thus modular control of sexually dimorphic behaviors across multiple levels, including genes and neurons, may be a general organizational principle of the underlying neural circuits.
Control of sex-typical behaviors by sexually dimorphic VMHvl neurons
Studies in diverse animals have defined the relevance of particular brain regions to sex-typical behaviors (Brenowitz, 1991; Cooke et al., 1998; Ferveur et al., 1995; Kelley, 1997; Konishi, 1989; Morris et al., 2004). However, within a brain region only specific subsets of neurons are sexually dimorphic (Ng et al., 2009; De Vries and Panzica, 2006; Xu et al., 2012), and with rare exceptions in invertebrates (Kohatsu et al., 2011; von Philipsborn et al., 2011), the function of sexually dimorphic neurons is unknown. Ablation of the ~2000 sexually dimorphic PR+ VMHvl neurons, a fraction of the ~108 neurons in the mouse brain, results in specific deficits in complex social behaviors. Such specificity likely results from manipulation of a molecularly defined subset of neurons. Indeed PR+ neurons represent only ~50% of VMHvl neurons that, in turn, represent a fraction of VMH neurons.
The mechanisms whereby sexually dimorphic neurons control dimorphic behaviors are poorly understood. It is possible that PR+ VMHvl neurons represent unrelated cell types in the two sexes, as evidenced by the sex differences in cell number and distribution, projection targets, and expression of Cckar. This is unlikely because PR+ VMHvl neurons also share many features, including location, projection targets, gene expression (PR, ERα), and developmental lineage (Grgurevic et al., 2012). Thus, it appears that a common pool of PR+ VMHvl neurons is present in both sexes, but their sex differences may allow them to transform synaptic inputs in a sex-specific manner or to relay either male or female-specific input to drive sexually dimorphic behavioral output.
Most behaviors are common to the sexes, suggesting that each sex possesses the motor pathways to display dimorphic behaviors of the opposite sex. Most sex differences in the brain represent quantitative and not all-or-none cellular or molecular sex differences. It is unknown whether these shared but dimorphic neurons regulate sex-typical behaviors in both sexes. Alternately, such neurons may regulate a dimorphic output in one sex, and in the other sex, they may be functionally vestigial, subserve a non-dimorphic function, or suppress a function of the opposite sex (De Vries and Boyle, 1998). We show that PR+ VMHvl neurons are functionally bivalent in the sense that they control sex-typical behaviors in both males and females. This dual function may prove adaptive if such neurons can generate a dimorphic behavior of the opposite sex in the appropriate context; in addition, bivalence may permit facile interchange of sex-typical behaviors between the sexes during speciation. Such flexibility may underlie the rapid evolution of sexually dimorphic traits (Darwin, 1871), including behaviors such as allocation of parental care and social dominance hierarchies. Given such evolutionary considerations, it remains to be seen whether all sexually dimorphic neuronal populations control sex-typical behaviors in both sexes.
EXPERIMENTAL PROCEDURES
Viruses
AAV-flex-taCasp3-TEVp
The plasmid encoding AAV-flex-taCasp3-TEVp (Figure S4A) was generated using routine subcloning. High titer virus of serotype 2/1 (3×1012 IU/mL) was generated from the plasmid at the UNC, Chapel Hill Vector Core.
Lenti-lxlplap
The plasmid encoding this VSVG pseudo-typed lentivirus was generated using standard subcloning (Figure S3G). High titer virus (~108 IU/mL) was generated using standard protocols (Barde et al., 2001).
Stereotaxic surgery
The virus was stereotaxically delivered under anesthesia to the VMHvl (Coordinates: rostrocaudal, −1.48 mm; mediolateral, ±0.78 mm; depth, 5.8 mm; see also Supplemental Procedures) (Paxinos and Franklin, 2003). Injections of taCasp3-encoding AAV were spiked (9:1) with constitutive EGFP-encoding AAV to verify accuracy of the injection placement in control and PRCre mice.
Behavior
Testing was performed as described previously (Juntti et al., 2010; Wu et al., 2009; Xu et al., 2012) (see also Supplemental Procedures). To test for sexual receptivity, females were castrated and, subsequent to estrus induction with estrogen and progesterone, inserted singly into the home cage of a sexually experienced WT male. Lordosis was defined as the female holding still with a dorsiflexed neck while being intromitted. Each experimental cohort included a set of control and PRCre mice.
Supplemental Experimental Procedures
Details regarding animals, histology, data analyses, and the procedures described above can be found in the Supplemental Information. All animal studies were in accordance with IACUC protocols at UCSF.
Supplementary Material
HIGHLIGHTS.
Widespread adult sex differences in progesterone receptor (PR) expressing neurons
Ventrolateral compartment of ventromedial hypothalamus (VMHvl) contains PR+ neurons
PR+ VMHvl neurons exhibit cellular and molecular dimorphisms between the sexes
PR+ VMHvl neurons essential for normal mating in both sexes and fighting in males
ACKNOWLEDGMENTS
We thank C. Saper for sharing reagents; A. Lasek and U. Heberlein for a practical on stereotaxis; R. Axel for discussions; T. Clandinin, H. Ingraham, S. Lomvardas, C. Saper, and Shahlab members for comments on the manuscript; and N. Agarwal, A. Wang, and M. Borius for technical support. This work was supported by a Genentech Graduate Fellowship (CFY); NSF Graduate Fellowship (SAJ); NIH (EKU, F31NS078959); NARSAD, Program in Biomedical Breakthrough Research, Ellison Medical Foundation, and NIH (R01NS049488, R01NS083872, and DP1MH099900) (NMS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Allen E. The oestrous cycle in the mouse. American Journal of Anatomy. 1922;30:297–371. [Google Scholar]
- Atasoy D, Aponte Y, Su HH, Sternson SM. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J. Neurosci. 2008;28:7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard P. A diencephalic mechanism for the expression of rage with special reference to the sympathetic nervous system. American Journal of Physiology. 1928;84:490–515. [Google Scholar]
- Barde I, Salmon P, Trono D. In Current Protocols in Neuroscience. John Wiley & Sons, Inc.; 2001. Production and Titration of Lentiviral Vectors. [DOI] [PubMed] [Google Scholar]
- Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol. Biochem. Behav. 1999;64:803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- Blaustein JD. Neuroendocrine regulation of feminine sexual behavior: lessons from rodent models and thoughts about humans. Annu Rev Psychol. 2008;59:93–118. doi: 10.1146/annurev.psych.59.103006.093556. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Feder HH. Cytoplasmic progestin-receptors in guinea pig brain: Characteristics and relationship to the induction of sexual behavior. Brain Research. 1979;169:481–497. doi: 10.1016/0006-8993(79)90398-6. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Ryer HI, Feder HH. A sex difference in the progestin receptor system of guinea pig brain. Neuroendocrinology. 1980;31:403–409. doi: 10.1159/000123110. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA. Altered perception of species-specific song by female birds after lesions of a forebrain nucleus. Science. 1991;251:303–305. doi: 10.1126/science.1987645. [DOI] [PubMed] [Google Scholar]
- Brown TJ, Yu J, Gagnon M, Sharma M, MacLusky NJ. Sex differences in estrogen receptor and progestin receptor induction in the guinea pig hypothalamus and preoptic area. Brain Res. 1996;725:37–48. doi: 10.1016/0006-8993(96)00241-7. [DOI] [PubMed] [Google Scholar]
- Bunting M, Bernstein KE, Greer JM, Capecchi MR, Thomas KR. Targeting genes for self-excision in the germ line. Genes Dev. 1999;13:1524–1528. doi: 10.1101/gad.13.12.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell PE, Lydon JP, Conneely OM, O’Malley BW, Levine JE. Endocrine defects in mice carrying a null mutation for the progesterone receptor gene. Endocrinology. 1997;138:4147–4152. doi: 10.1210/endo.138.10.5456. [DOI] [PubMed] [Google Scholar]
- Clemente CD, Chase MH. Neurological substrates of aggressive behavior. Annu. Rev. Physiol. 1973;35:329–356. doi: 10.1146/annurev.ph.35.030173.001553. [DOI] [PubMed] [Google Scholar]
- Cohen RS, Pfaff DW. Ventromedial hypothalamic neurons in the mediation of long-lasting effects of estrogen on lordosis behavior. Prog. Neurobiol. 1992;38:423–453. doi: 10.1016/0301-0082(92)90045-g. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Wiepkema PR. Effects of ventromedial hypothalamic lesions on spontaneous intraspecies aggression in male rats. Behav Biol. 1976;16:117–125. doi: 10.1016/s0091-6773(76)91225-6. [DOI] [PubMed] [Google Scholar]
- Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front Neuroendocrinol. 1998;19:323–362. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- Darwin C. The Descent of Man, and Selection in Relation to Sex. Princeton University Press; 1871. [Google Scholar]
- Desjardins C, Maruniak JA, Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science. 1973;182:939–941. doi: 10.1126/science.182.4115.939. [DOI] [PubMed] [Google Scholar]
- Dewing P, Shi T, Horvath S, Vilain E. Sexually dimorphic gene expression in mouse brain precedes gonadal differentiation. Brain Res. Mol. Brain Res. 2003;118:82–90. doi: 10.1016/s0169-328x(03)00339-5. [DOI] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, et al. Leptin Directly Activates SF1 Neurons in the VMH, and This Action by Leptin Is Required for Normal Body-Weight Homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Dugger BN, Morris JA, Jordan CL, Breedlove SM. Androgen receptors are required for full masculinization of the ventromedial hypothalamus (VMH) in rats. Horm Behav. 2007;51:195–201. doi: 10.1016/j.yhbeh.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferveur JF, Störtkuhl KF, Stocker RF, Greenspan RJ. Genetic feminization of brain structures and changed sexual orientation in male Drosophila. Science. 1995;267:902–905. doi: 10.1126/science.7846534. [DOI] [PubMed] [Google Scholar]
- Flanagan-Cato LM. Sex differences in the neural circuit that mediates female sexual receptivity. Front Neuroendocrinol. 2011;32:124–136. doi: 10.1016/j.yfrne.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan-Cato LM, Lee BJ, Calizo LH. Co-localization of midbrain projections, progestin receptors, and mating-induced fos in the hypothalamic ventromedial nucleus of the female rat. Hormones and behavior. 2006;50:52–60. doi: 10.1016/j.yhbeh.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Gagnidze K, Pfaff DW, Mong JA. Gene expression in neuroendocrine cells during the critical period for sexual differentiation of the brain. Prog. Brain Res. 2010;186:97–111. doi: 10.1016/B978-0-444-53630-3.00007-5. [DOI] [PubMed] [Google Scholar]
- Gorski RA, Harlan RE, Jacobson CD, Shryne JE, Southam AM. Evidence for the existence of a sexually dimorphic nucleus in the preoptic area of the rat. J. Comp. Neurol. 1980;193:529–539. doi: 10.1002/cne.901930214. [DOI] [PubMed] [Google Scholar]
- Goy RW, Phoenix CH. Hypothalamic regulation of female sexual behaviour; establishment of behavioural oestrus in spayed guinea-pigs following hypothalamic lesions. J. Reprod. Fertil. 1963;5:23–40. doi: 10.1530/jrf.0.0050023. [DOI] [PubMed] [Google Scholar]
- Gray DC, Mahrus S, Wells JA. Activation of specific apoptotic caspases with an engineered small-molecule-activated protease. Cell. 2010;142:637–646. doi: 10.1016/j.cell.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grgurevic N, Büdefeld T, Spanic T, Tobet SA, Majdic G. Evidence that sex chromosome genes affect sexual differentiation of female sexual behavior. Horm Behav. 2012;61:719–724. doi: 10.1016/j.yhbeh.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman SP. Aggression, avoidance, and reaction to novel environments in female rats with ventromedial hypothalamic lesions. Journal of Comparative and Physiological Psychology. 1972;78:274–283. doi: 10.1037/h0032284. [DOI] [PubMed] [Google Scholar]
- Harvey W. Exercitationes de generatione animalium (London: Typis Du-Gardianis) In: Willis R, translator. The Works of William Harvey. Miami, USA: HardPress Publishing, 2012; 1651. pp. 186–187. [Google Scholar]
- Hess WR, Akert K. Experimental data on role of hypothalamus in mechanism of emotional behavior. AMA Arch Neurol Psychiatry. 1955;73:127–129. doi: 10.1001/archneurpsyc.1955.02330080005003. [DOI] [PubMed] [Google Scholar]
- Hetherington AW, Ranson SW. Hypothalamic lesions and adiposity in the rat. The Anatomical Record. 1940;78:149–172. [Google Scholar]
- Jazin E, Cahill L. Sex differences in molecular neuroscience: from fruit flies to humans. Nat. Rev. Neurosci. 2010;11:9–17. doi: 10.1038/nrn2754. [DOI] [PubMed] [Google Scholar]
- Jiang X, Wang X. Cytochrome C-mediated apoptosis. Annu. Rev. Biochem. 2004;73:87–106. doi: 10.1146/annurev.biochem.73.011303.073706. [DOI] [PubMed] [Google Scholar]
- Juntti SA, Tollkuhn J, Wu MV, Fraser EJ, Soderborg T, Tan S, Honda S-I, Harada N, Shah NM. The androgen receptor governs the execution, but not programming, of male sexual and territorial behaviors. Neuron. 2010;66:260–272. doi: 10.1016/j.neuron.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DB. Generating sexually differentiated songs. Curr. Opin. Neurobiol. 1997;7:839–843. doi: 10.1016/s0959-4388(97)80144-4. [DOI] [PubMed] [Google Scholar]
- Kimura T, Hagiwara Y. Regulation of urine marking in male and female mice: effects of sex steroids. Horm Behav. 1985;19:64–70. doi: 10.1016/0018-506x(85)90006-6. [DOI] [PubMed] [Google Scholar]
- King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol. Behav. 2006;87:221–244. doi: 10.1016/j.physbeh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Kohatsu S, Koganezawa M, Yamamoto D. Female contact activates male-specific interneurons that trigger stereotypic courtship behavior in Drosophila. Neuron. 2011;69:498–508. doi: 10.1016/j.neuron.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Newman SW. Mating and agonistic behavior produce different patterns of Fos immunolabeling in the male Syrian hamster brain. Neuroscience. 1995;66:721–736. doi: 10.1016/0306-4522(94)00563-k. [DOI] [PubMed] [Google Scholar]
- Konishi M. Birdsong for neurobiologists. Neuron. 1989;3:541–549. doi: 10.1016/0896-6273(89)90264-x. [DOI] [PubMed] [Google Scholar]
- Kow LM, Harlan RE, Shivers BD, Pfaff DW. Inhibition of the lordosis reflex in rats by intrahypothalamic infusion of neural excitatory agents: evidence that the hypothalamus contains separate inhibitory and facilitatory elements. Brain Res. 1985;341:26–34. doi: 10.1016/0006-8993(85)91468-4. [DOI] [PubMed] [Google Scholar]
- Krieger MS, Conrad LC, Pfaff DW. An autoradiographic study of the efferent connections of the ventromedial nucleus of the hypothalamus. J. Comp. Neurol. 1979;183:785–815. doi: 10.1002/cne.901830408. [DOI] [PubMed] [Google Scholar]
- Kruk MR, Van der Poel AM, De Vos-Frerichs TP. The induction of aggressive behaviour by electrical stimulation in the hypothalamus of male rats. Behaviour. 1979;70:292–322. doi: 10.1163/156853979x00106. [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Harada N, Honda S-I, Rissman EF. Regulation of progestin receptors in medial amygdala: estradiol, phytoestrogens and sex. Physiol. Behav. 2009;97:146–150. doi: 10.1016/j.physbeh.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurrasch DM, Cheung CC, Lee FY, Tran PV, Hata K, Ingraham HA. The neonatal ventromedial hypothalamus transcriptome reveals novel markers with spatially distinct patterning. J. Neurosci. 2007;27:13624–13634. doi: 10.1523/JNEUROSCI.2858-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal S, Andrade JP, Paula-Barbosa MM, Madeira MD. Arcuate nucleus of the hypothalamus: effects of age and sex. J. Comp. Neurol. 1998;401:65–88. doi: 10.1002/(sici)1096-9861(19981109)401:1<65::aid-cne5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Leedy MG, Hart BL. Female and male sexual responses in female cats with ventromedial hypothalamic lesions. Behavioral Neuroscience. 1985;99:936–941. doi: 10.1037//0735-7044.99.5.936. [DOI] [PubMed] [Google Scholar]
- Levine JE, Chappell PE, Schneider JS, Sleiter NC, Szabo M. Progesterone receptors as neuroendocrine integrators. Front Neuroendocrinol. 2001;22:69–106. doi: 10.1006/frne.2001.0210. [DOI] [PubMed] [Google Scholar]
- Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, Anderson DJ. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470:221–226. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O’Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Majdic G, Young M, Gomez-Sanchez E, Anderson P, Szczepaniak LS, Dobbins RL, McGarry JD, Parker KL. Knockout Mice Lacking Steroidogenic Factor 1 Are a Novel Genetic Model of Hypothalamic Obesity. Endocrinology. 2002;143:607–614. doi: 10.1210/endo.143.2.8652. [DOI] [PubMed] [Google Scholar]
- Mani SK, Allen JM, Clark JH, Blaustein JD, O’Malley BW. Convergent pathways for steroid hormone- and neurotransmitter-induced rat sexual behavior. Science. 1994a;265:1246–1249. doi: 10.1126/science.7915049. [DOI] [PubMed] [Google Scholar]
- Mani SK, Blaustein JD, Allen JM, Law SW, O’Malley BW, Clark JH. Inhibition of rat sexual behavior by antisense oligonucleotides to the progesterone receptor. Endocrinology. 1994b;135:1409–1414. doi: 10.1210/endo.135.4.7925102. [DOI] [PubMed] [Google Scholar]
- Mani SK, Blaustein JD, O’Malley BW. Progesterone receptor function from a behavioral perspective. Horm Behav. 1997;31:244–255. doi: 10.1006/hbeh.1997.1393. [DOI] [PubMed] [Google Scholar]
- Mathews D, Edwards DA. Involvement of the ventromedial and anterior hypothalamic nuclei in the hormonal induction of receptivity in the female rat. Physiol. Behav. 1977a;19:319–326. doi: 10.1016/0031-9384(77)90345-6. [DOI] [PubMed] [Google Scholar]
- Mathews D, Edwards DA. The ventromedial nucleus of the hypothalamus and the hormonal arousal of sexual behaviors in the female rat. Hormones and Behavior. 1977b;8:40–51. doi: 10.1016/0018-506x(77)90019-8. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y. Sex difference in volume of the ventromedial nucleus of the hypothalamus in the rat. Endocrinol. Jpn. 1983;30:277–280. doi: 10.1507/endocrj1954.30.277. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y. Male-female difference in synaptic organization of the ventromedial nucleus of the hypothalamus in the rat. Neuroendocrinology. 1986;42:232–236. doi: 10.1159/000124445. [DOI] [PubMed] [Google Scholar]
- Maxwell IH, Maxwell F, Glode LM. Regulated expression of a diphtheria toxin A-chain gene transfected into human cells: possible strategy for inducing cancer cell suicide. Cancer Res. 1986;46:4660–4664. [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat. Neurosci. 2011;14:677–683. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill TE. Sexual behavior in three inbred strains of mice. behavior. 1962;19:341–350. [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat. Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual dimorphism in neuronal number of the posterodorsal medial amygdala is independent of circulating androgens and regional volume in adult rats. J. Comp. Neurol. 2008;506:851–859. doi: 10.1002/cne.21536. [DOI] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. RNAi-mediated silencing of estrogen receptor {alpha} in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proc. Natl. Acad. Sci. U.S.A. 2006;103:10456–10460. doi: 10.1073/pnas.0603045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang X-J, Clegg DJ, Kaplitt MG, Ogawa S. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc. Natl. Acad. Sci. U.S.A. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L, Bernard A, Lau C, Overly CC, Dong H-W, Kuan C, Pathak S, Sunkin SM, Dang C, Bohland JW, et al. An anatomic gene expression atlas of the adult mouse brain. Nat. Neurosci. 2009;12:356–362. doi: 10.1038/nn.2281. [DOI] [PubMed] [Google Scholar]
- Nyby J, Wysocki CJ, Whitney G, Dizinno G. Pheromonal regulation of male mouse ultrasonic courtship (Mus musculus) Anim Behav. 1977;25:333–341. doi: 10.1016/0003-3472(77)90009-4. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Olazábal UE, Parhar IS, Pfaff DW. Effects of intrahypothalamic administration of antisense DNA for progesterone receptor mRNA on reproductive behavior and progesterone receptor immunoreactivity in female rat. J. Neurosci. 1994;14:1766–1774. doi: 10.1523/JNEUROSCI.14-03-01766.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier B, Wiepkema PR. Behaviour changes in mice following electrolytic lesions in the median hypothalamus. Brain Res. 1974;65:521–524. doi: 10.1016/0006-8993(74)90241-8. [DOI] [PubMed] [Google Scholar]
- Olster DH, Blaustein JD. Immunocytochemical colocalization of progestin receptors and beta-endorphin or enkephalin in the hypothalamus of female guinea pigs. J. Neurobiol. 1990;21:768–780. doi: 10.1002/neu.480210510. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Fortino AE, Polston EK. Sex differences in serotonergic but not gamma-aminobutyric acidergic (GABA) projections to the rat ventromedial nucleus of the hypothalamus. Endocrinology. 2008;149:397–408. doi: 10.1210/en.2007-0666. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. Academic Press; 2003. The Mouse Brain in Stereotaxic Coordinates: Compact Second Edition, Second Edition. [Google Scholar]
- Pfaff DW, Sakuma Y. Deficit in the lordosis reflex of female rats caused by lesions in the ventromedial nucleus of the hypothalamus. J. Physiol. (Lond.) 1979a;288:203–210. [PMC free article] [PubMed] [Google Scholar]
- Pfaff DW, Sakuma Y. Facilitation of the lordosis reflex of female rats from the ventromedial nucleus of the hypothalamus. J. Physiol. (Lond.) 1979b;288:189–202. [PMC free article] [PubMed] [Google Scholar]
- Phelps SM, Lydon JP, O’Malley BW, Crews D. Regulation of male sexual behavior by progesterone receptor, sexual experience, and androgen. Horm Behav. 1998;34:294–302. doi: 10.1006/hbeh.1998.1485. [DOI] [PubMed] [Google Scholar]
- Von Philipsborn AC, Liu T, Yu JY, Masser C, Bidaye SS, Dickson BJ. Neuronal control of Drosophila courtship song. Neuron. 2011;69:509–522. doi: 10.1016/j.neuron.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Pollio G, Xue P, Zanisi M, Nicolin A, Maggi A. Antisense oligonucleotide blocks progesterone-induced lordosis behavior in ovariectomized rats. Brain Res. Mol. Brain Res. 1993;19:135–139. doi: 10.1016/0169-328x(93)90158-l. [DOI] [PubMed] [Google Scholar]
- Power RF, Mani SK, Codina J, Conneely OM, O’Malley BW. Dopaminergic and ligand-independent activation of steroid hormone receptors. Science. 1991;254:1636–1639. doi: 10.1126/science.1749936. [DOI] [PubMed] [Google Scholar]
- Quadros PS, Pfau JL, Goldstein AYN, De Vries GJ, Wagner CK. Sex differences in progesterone receptor expression: a potential mechanism for estradiol-mediated sexual differentiation. Endocrinology. 2002;143:3727–3739. doi: 10.1210/en.2002-211438. [DOI] [PubMed] [Google Scholar]
- Quadros PS, Schlueter LJ, Wagner CK. Distribution of progesterone receptor immunoreactivity in the midbrain and hindbrain of postnatal rats. Dev Neurobiol. 2008;68:1378–1390. doi: 10.1002/dneu.20664. [DOI] [PubMed] [Google Scholar]
- Reeves AG, Plum F. Hyperphagia, rage, and dementia accompanying a ventromedial hypothalamic neoplasm. Arch. Neurol. 1969;20:616–624. doi: 10.1001/archneur.1969.00480120062005. [DOI] [PubMed] [Google Scholar]
- Ricciardi KH, Blaustein JD. Projections from ventrolateral hypothalamic neurons containing progestin receptor- and substance P-immunoreactivity to specific forebrain and midbrain areas in female guinea pigs. J. Neuroendocrinol. 1994;6:135–144. doi: 10.1111/j.1365-2826.1994.tb00564.x. [DOI] [PubMed] [Google Scholar]
- Robarts DW, Baum MJ. Ventromedial hypothalamic nucleus lesions disrupt olfactory mate recognition and receptivity in female ferrets. Horm Behav. 2007;51:104–113. doi: 10.1016/j.yhbch.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BS, Barfield RJ. Induction of estrous behavior in ovariectomized rats by sequential replacement of estrogen and progesterone to the ventromedial hypothalamus. Neuroendocrinology. 1983;37:218–224. doi: 10.1159/000123546. [DOI] [PubMed] [Google Scholar]
- Sakuma Y, Pfaff DW. Facilitation of female reproductive behavior from mesensephalic central gray in the rat. Am. J. Physiol. 1979;237:R278–284. doi: 10.1152/ajpregu.1979.237.5.R278. [DOI] [PubMed] [Google Scholar]
- Saper CB, Swanson LW, Cowan WM. The efferent connections of the ventromedial nucleus of the hypothalamus of the rat. J. Comp. Neurol. 1976;169:409–442. doi: 10.1002/cne.901690403. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Burgess C, Sleiter NC, DonCarlos LL, Lydon JP, O’Malley B, Levine JE. Enhanced Sexual Behaviors and Androgen Receptor Immunoreactivity in the Male Progesterone Receptor Knockout Mouse. Endocrinology. 2005;146:4340–4348. doi: 10.1210/en.2005-0490. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Burgess C, Horton TH, Levine JE. Effects of progesterone on male-mediated infant-directed aggression. Behav. Brain Res. 2009;199:340–344. doi: 10.1016/j.bbr.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober J, Weil Z, Pfaff D. How generalized CNS arousal strengthens sexual arousal (and vice versa) Horm Behav. 2011;59:689–695. doi: 10.1016/j.yhbeh.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Shah NM, Pisapia DJ, Maniatis S, Mendelsohn MM, Nemes A, Axel R. Visualizing sexual dimorphism in the brain. Neuron. 2004;43:313–319. doi: 10.1016/j.neuron.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu. Rev. Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Smith RL, Geller AI, Escudero KW, Wilcox CL. Long-term expression in sensory neurons in tissue culture from herpes simplex virus type 1 (HSV-1) promoters in an HSV-1-derived vector. J. Virol. 1995;69:4593–4599. doi: 10.1128/jvi.69.8.4593-4599.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Swaab D. The Human Hypothalamus: Basic and Clinical Aspects Part I: Nuclei of the Human Hypothalamus. Elsevier; 2003. The ventromedial nucleus (VMN; nucleus of Cajal) pp. 239–242. [Google Scholar]
- Tsutsui K. Neurosteroid biosynthesis and action during cerebellar development. Cerebellum. 2012;11:414–415. doi: 10.1007/s12311-011-0341-7. [DOI] [PubMed] [Google Scholar]
- La Vaque TJ, Rodgers CH. Recovery of mating behavior in the female rat following VMH lesions. Physiol. Behav. 1975;14:59–63. doi: 10.1016/0031-9384(75)90142-0. [DOI] [PubMed] [Google Scholar]
- Veening JG, Coolen LM, De Jong TR, Joosten HW, De Boer SF, Koolhaas JM, Olivier B. Do similar neural systems subserve aggressive and sexual behaviour in male rats? Insights from c-Fos and pharmacological studies. Eur. J. Pharmacol. 2005;526:226–239. doi: 10.1016/j.ejphar.2005.09.041. [DOI] [PubMed] [Google Scholar]
- De Vries GJ. Sex Differences in Neurotransmitter Systems. Journal of Neuroendocrinology. 1990;2:1–13. doi: 10.1111/j.1365-2826.1990.tb00385.x. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Boyle PA. Double duty for sex differences in the brain. Behav. Brain Res. 1998;92:205–213. doi: 10.1016/s0166-4328(97)00192-7. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience. 2006;138:947–955. doi: 10.1016/j.neuroscience.2005.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley MD. The hypothalamus and affective behavior in cats: a study of the effects of experimental lesions, with anatomic correlations. Arch NeurPsych. 1944;52:296–316. [Google Scholar]
- Witt DM, Young LJ, Crews D. Progesterone modulation of androgen-dependent sexual behavior in male rats. Physiol. Behav. 1995;57:307–313. doi: 10.1016/0031-9384(94)00247-3. [DOI] [PubMed] [Google Scholar]
- Wu MV, Manoli DS, Fraser EJ, Coats JK, Tollkuhn J, Honda S-I, Harada N, Shah NM. Estrogen masculinizes neural pathways and sex-specific behaviors. Cell. 2009;139:61–72. doi: 10.1016/j.cell.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Coats JK, Yang CF, Wang A, Ahmed OM, Alvarado M, Izumi T, Shah NM. Modular genetic control of sexually dimorphic behaviors. Cell. 2012;148:596–607. doi: 10.1016/j.cell.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Schadt EE, Wang S, Wang H, Arnold AP, Ingram-Drake L, Drake TA, Lusis AJ. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 2006;16:995–1004. doi: 10.1101/gr.5217506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.