Abstract
Dendritic cell (DC) subsets in the skin and draining lymph nodes (LNs) are likely to elicit distinct immune response types. In skin and skin-draining LNs, a dermal DC subset expressing macrophage galactose-type C-type lectin 2 (MGL2/CD301b) was found distinct from migratory Langerhans cells (LCs) or CD103+ dermal DCs (dDCs). Lower expression levels of Th1-promoting and/or cross-presentation-related molecules were suggested by the transcriptome analysis and verified by the quantitative real-time PCR analysis in MGL2+ dDCs than in CD103+ dDCs. Transfer of MGL2+ dDCs but not CD103+ dDCs from FITC-sensitized mice induced a Th2-type immune response in vivo in a model of contact hypersensitivity. Targeting MGL2+ dDCs with a rat monoclonal antibody against MGL2 efficiently induced a humoral immune response with Th2-type properties, as determined by the antibody subclass. We propose that the properties of MGL2+ dDCs, are complementary to those of CD103+ dDCs and skew the immune response toward a Th2-type response.
Introduction
Dendritic cells (DCs) recognize foreign materials and play a central role in the initiation of a variety of immune responses [1], [2]. However, it is not yet fully understood how DCs determine the type, strength, duration, localization, memory, and other aspects of the immune response. Interestingly, DCs residing in or migrating into various organs seem to be distinct and potentially be classified into subsets according to surface marker molecules and functions [3], [4]. These DC subsets have been suggested to have distinct roles in the initiation of different types of immune responses [3], [5], [6].
At least several DC subsets are known to reside in skin and skin-draining lymph nodes (LNs) [7], [8], [9], [10]. For example, Langerhans cells (LCs) constitute one of the skin DC subsets, and Langerin was thought to be a specific marker of LCs for a period of time [11]. Recently, however, a new DC subset expressing Langerin, the CD103+ dermal dendritic cells (dDCs), was found in the skin immune system, and it was shown to be distinct from migratory LCs based on the expression of distinct surface markers and its unique function [12], [13], [14], [15]. In addition, it was shown that LCs and CD103+ dDCs promote opposite T cell response types, Th17- and Th1- type, respectively, suggesting that skin DC subsets are specialized to induce distinct immune responses [16], [17].
Contact hypersensitivity (CHS) is T cell-mediated immunity with the characteristics of delayed-type hypersensitivity [18], [19]. CHS is experimentally induced by painting haptens diluted in adjuvants onto the skin. Two important phases are involved in CHS reactions: the sensitization phase and the elicitation phase [20]. Classically, LCs were considered to be the main antigen presenting cells (APCs) in the sensitization phase of CHS [18], [21], [22]. However, recent studies using new technologies to deplete LCs have provided confusing information because the depletion of LCs has been shown to promote [23], to have no effect on [12], [24], and to suppress CHS [25], [26], [27], [28]. Furthermore, a new dDC subset, the CD103+ dDCs, was reported to be involved in the initiation of CHS responses [12], [25]. Finally, it was recently shown that antigen presentation by CD103+ dDCs alone did not appear to represent the main pathway involved in sensitization for CHS [29]. Therefore, which skin DC subsets play the dominant role in CHS remains controversial.
We propose in the present report that dDCs expressing macrophage galactose (Gal)-type C-type lectin 2 (MGL2/CD301b) comprise a unique subset. MGL2 is a type II transmembrane lectin containing a single carbohydrate recognition domain that interacts with Gal and N-acethylgalactosamine (GalNAc) as monosaccharides and binds strongly to oligosaccharides with terminal GalNAc residues. Although a single gene encodes human MGL/CD301 [30], mice have two genes: Mgl1 [31] and Mgl2 [32]. We recently examined the distribution of cells expressing MGL2 and concluded that MGL2 was expressed on a portion of conventional DCs [33]. In the skin, MGL2 was expressed on a portion of dDCs that were sufficient to initiate CHS in vivo [34]. However, it was not clear whether MGL2+ cells overlapped with or were distinct from migratory LCs and CD103+ dDCs.
In the present report, MGL2+ dDCs are found to comprise a unique subset that is distinct from migratory LCs, CD103+ dDCs, CD8á+ DCs, or plasmacytoid DCs (pDCs) in the skin immune system. The quantitative real-time PCR analysis demonstrated that the expression levels of Th1-promoting and/or cross-presentation-related molecules were lower in MGL2+ dDCs than in CD103+ dDCs. To assess the role of MGL2+ dDCs in CHS in vivo, MGL2+ dDCs bearing a hapten, fluorescence isothiocyanate (FITC), and CD103+ dDCs bearing the same hapten were sorted and injected into naïve mice; then the subsequent immune responses were examined. Both MGL2+ dDCs and CD103+ dDCs initiated CHS sensitization to a similar extent as revealed by the thickening of the ears. However, MGL2+ dDCs but not CD103+ dDCs were shown to induce a Th2-type immune response in vivo, in the profiles of cytokines and the antibody subclass, in FITC-induced CHS. Furthermore, Th2 skewed humoral immune responses were observed when an antigen was targeted to MGL2+ dDCs in vivo. These results revealed for the first time that MGL2+ dDCs represent a unique dDC subset for the Th2-type immune response.
Materials and Methods
Mice
Five-week-old specific pathogen-free (SPF) BALB/c or C57BL/6 mice of both sexes were purchased from CLEA Japan, Inc. (Tokyo, Japan). Purchased mice, mice transgenic for the OVA323–339-specific and I-Ad-restricted DO11.10 TCR-αβ on a Rag2 –/– (BALB/c) background and Mgl2 –/– mice (BALB/c background) were fed and housed under SPF conditions. All experiments were approved by the Bioscience Committee of the Graduate School of Pharmaceutical Sciences of the University of Tokyo and performed according to the guidelines of the Bioscience Committee of the University of Tokyo.
Sensitization for CHS
BALB/c mice were painted with 200 µl of 0.5 % (w/v) FITC (Dojindo Laboratories, Kumamoto, Japan) in AD, a 1:1 mixture of acetone (Wako Pure Chemical, Osaka, Japan) and dibutylphthalate (Wako), on shaved arms and abdomen.
Preparation of skin cells
Shaved skin was obtained from mice under a naïve state. After removing the adipose tissue, skin samples were minced and digested with 6 mg/ml collagenase (Wako) and 0.1 mg/ml DNaseI (Roche, Basel, Switzerland) in RPMI1640 medium supplemented with 10% fetal calf serum (FCS) for 75 min at 37°C. Digested cells were transferred to new tubes through iron mesh, centrifuged, treated with 10 mM EDTA in PBS for 5 min, washed two times with PBS, and suspended in PBS containing 0.1% BSA and 0.1% sodium azide (FCM buffer).
Preparation of LN cells
Brachial, axillary and inguinal LNs were obtained from BALB/c mice under a naïve state or 1 day after sensitization for CHS. Draining brachial and axillary LNs were obtained from BALB/c mice 1 day after CHS elicitation when mice were sensitized with FITC+ dDCs by footpad injection. LNs were minced and digested with 1 mg/ml collagenase from Clostridium histolyticum (Sigma) in RPMI1640 medium supplemented with 10% FCS for 25 min at 37°C. Digested cells were transferred to a new tube through nylon mesh and washed with PBS containing 0.5% BSA and 2 mM EDTA.
Antibodies
FITC-conjugated anti-mouse IA/IE (monoclonal antibody (mAb) M5/114.15.2), anti-CD11c (mAb N418), anti-CD4 (mAb RM4-5), phycoerythrin (PE)-conjugated anti-mouse CD103 (mAb 2E7), anti-mouse Ep-CAM (mAb G8.8), anti-mouse B220 (mAb RA3-6B2), anti-mouse CD4 (mAb RM4-5), anti-mouse IFN-γ (mAb XMG1.2), anti-mouse IL-17A (mAb TC11-18H10.1), biotin-conjugated anti-mouse CD4 (mAb GK1.5), anti-mouse CD8α (mAb 53-6.7), anti-mouse CD80 (mAb 16-10A1), anti-mouse CD86 (mAb GL-1), anti-mouse B7-H1 (mAb 10F.9G2), anti-mouse B7-H2 (mAb HK5.3), anti-mouse B7-DC (mAb TY25), anti-mouse IL-12/IL-23p40 (mAb C17.8), PE-Cy7-conjugated anti-mouse CD3ε (mAb 145-2C11), Alexa Fluor 488-conjugated anti-mouse Foxp3 (mAb 150D), and allophycocyanin (APC)-conjugated anti-mouse IFN-γ (mAb XMG1.2) were purchased from BioLegend (San Diego, CA). Biotin-conjugated anti-mouse TCRVâ8 (JR2) purchased from BD Biosciences (Oxford, U.K.). PE-conjugated anti-mouse Langerin (mAb eBioL31), anti-mouse IL-4 (mAb 11B11), and biotin-conjugated anti-mouse CD40 (mAb HM40-3) were purchased from eBioscience (San Diego, CA). PE-conjugated anti-mouse mPDCA-1 (mAb JF05-1C2.4.1) was purchased from Miltenyi Biotec (Bergisch Gladbach, Germany). Biotinylated-, APC- or Hilyte647-conjugated anti-mouse MGL2 (mAb URA-1) was prepared using purified antibodies from the hybridoma culture supernatant [33].
Flow cytometry analysis
Cells isolated from mouse tissues and primary cultured cells were incubated with anti-mouse CD16/CD32 mAb (1/100 dilution of ammonium sulfate-precipitated hybridoma culture supernatant; the 2.4G2 hybridoma was purchased from ATCC) to reduce non-specific binding 5 min before the addition of the first antibodies. Cells were then incubated with biotin-, FITC-, PE-, PE-Cy7-, HiLyte647- and/or APC-conjugated antibodies for 30 min. Biotin-conjugated antibodies were visualized with PE-Cy7- or APC-labeled streptavidin (SAv) (BioLegend). To exclude dead cells, except for intracellularly stained cells, all cells were resuspended in FCM buffer containing 7-amino-actinomycin D (7-AAD) (eBioscience). To stain intracellular molecules, cells were fixed and permeabilized with BD Cytofix/Cytoperm (BD Biosciences, Franklin Lakes, NJ) according to the manufacturer's protocol. All procedures were performed on ice. Antibodies and reagents were diluted in FCM buffer. The cells were rinsed once with FCM buffer at the end of each incubation period. Samples were analyzed on a FACS Aria cell sorter (BD). Data were analyzed using FlowJo software (Tree Star, Ashland, OR).
Sorting of MGL2+ dDCs and CD103+ dDCs
After the preparation of LN cells, CD11c+ cells were isolated by an autoMACS Separator (Miltenyi) according to the manufacturer's protocol. After MACS, cells were stained with mAbs and suspended in sterilized 3% FCS-PBS. MHCIIhighMGL2+ dDCs and MHCIIhighCD103+ dDCs were sorted from mice under a naïve state by a FACS Aria cell sorter. FITC+MGL2+ dDCs and FITC+CD103+ dDCs were sorted from BALB/c mice 1 day after sensitization for CHS by a FACS Aria cell sorter. After sorting, the purity of each dDC subset was at least 90% or more.
Encyclopedic transcriptome analysis
MHCIIhighMGL2+ dDCs, MHCIIhighCD103+ dDCs, FITC+MGL2+ dDCs or FITC+CD103+ dDCs were obtained from forty mice and pooled. Total RNA was obtained using an RNeasy Mini Kit (QIAGEN, Valencia, CA). Individual SAGE libraries from the dDC samples were constructed with 1 µg RNA using the SOLiD SAGE™ kit from Life Technologies (Beverly, MA) according to the manufacturer's protocol. The constructed tags were sequenced by a SOLiD4 sequencer (Life Technologies Japan). The samples were mapped to Refseq using the v1.1 SOLiD™ SAGE™ Analysis software method, which used the 25_1 mapping parameter. After mapping, the number of genes for each dDC subset was normalized by setting the number of total genes to 3000000. After normalization, the number of genes was rounded up to the nearest integral number. Encyclopedic transcriptome analysis was performed, and the fold differences in the relative expression levels of indicated genes (Cxcl2, Cxcl3, Ccl1, Il12b, Xcr1, Naip2, Clec4n, Clec9a and Tlr3) among the dDC subsets (MHCIIhiMGL2+ dDCs, MHCIIhiCD103+ dDCs, FITC+MGL2+ dDCs and FITC+CD103+ dDCs). Differential expressions suggested by the encyclopedic transcriptome analysis were verified by the quantitative real-time PCR more than two times.
Accession number
SAGE tags have been deposited in the NCBI Short Read Archive under the project accession, DRA000852.
Quantitative real-time PCR
Draining brachial and axillary LN cells were obtained 1 day after CHS elicitation from mice sensitized with FITC+MGL2+ dDCs, FITC+CD103+ dDCs or non-sensitized mice. MHCIIhighMGL2+ dDCs, MHCIIhighCD103+ dDCs, FITC+MGL2+ dDCs or FITC+CD103+ dDCs were obtained as shown above. Total RNA was obtained from these cells with an RNeasy mini kit or RNeasy micro kit (QIAGEN). RNA (0.05–0.4 µg) was reverse-transcribed into cDNA using Superscript III (Invitrogen). All procedures were performed according to the manufacturers' instructions. The quantitative real-time PCR was performed on a StepOnePlus Real-time PCR System (Applied Biosystems, Foster City, CA) using Fast SYBR Green master mix (Applied Biosystems) or KAPA SYBR FAST ABI Prism (Kapa Biosystems,Woburn, MA). Primers are listed in Table S3.
Intracellular analysis of IL-12p40 on skin-derived DCs in LNs
LN cells (4.0×106 cells) obtained from BALB/c mice under a naïve state or mice 1 day after sensitization for CHS were cultured for 2 hours in the presence of 10 µg/ml brefeldin A (Sigma) on 24-well plates. After incubation, intracellular IL-12p40 in skin-derived DCs was analyzed by a FACS Aria cell sorter.
Cytokine production from MGL2+ dDCs and CD103+ dDCs
MHCIIhighMGL2+ dDCs, MHCIIhighCD103+ dDCs, FITC+MGL2+ dDCs or FITC+CD103+ dDCs were obtained as shown above. dDCs (2.0×104 cells) were cultured in the presence of 10 µg/ml anti-CD40 agonistic antibody (FGK-45) for 15 hours on 96-well round-bottom plates. Cytokine concentrations in culture supernatant were measured using the Bio-Plex Suspension Array System (Bio-Rad Laboratories, Hercules, CA).
Adoptive transfer and CHS elicitation
Sorted FITC+MGL2+ dDCs and FITC+CD103+ dDCs (2.0×104 cells) were resuspended in 10 µl of Hanks' balanced salt solution (HBSS) and injected into the footpads of BALB/c mice under a naïve state. Six days later, the recipient mice were painted with 20 µl of 0.5% FITC/AD on the skin of the dorsal right ear. The left ear was treated with the vehicle alone as a control. As negative controls for sensitization, BALB/c mice that were injected with HBSS 6 days prior to elicitation (non-sensitized mice) were used. FITC-specific ear swelling was defined as follows: (right ear thickness at 24 h after elicitation – right ear thickness before elicitation) – (left ear thickness at 24 h after elicitation – left ear thickness before elicitation). For intracellular cytokine staining and FITC-specific antibody ELISA, draining brachial and axillary LN cells and sera were obtained 1 day after elicitation from mice sensitized with FITC+MGL2+ dDCs, FITC+CD103+ dDCs or non-sensitized mice.
Intracellular analysis of cytokines in T cells in LNs after CHS elicitation
LN cells (4.0×106 cells) obtained 1 day after CHS elicitation from mice sensitized with FITC+MGL2+ dDCs, FITC+CD103+ dDCs or non-sensitized mice were cultured for 5 hours in the presence of 20 ng/ml phorbol 12-myristate 13-acetate (PMA) (Sigma), 1 µM ionomycin calcium salt from Streptomyces conglobatus (ionomycin) (Calbiochem) and 10 µg/ml brefeldin A in a 24-well plate. After culturing, intracellular cytokines in CD4+ T cells were analyzed by a FACS Aria cell sorter.
FITC-specific antibody ELISA
Ninety-six-well ELISA microplates (Greiner, Monroe, NC) were coated with fluorescein-conjugated BSA (FITC-BSA; 4 µg/ml: Invitrogen) or BSA (4 µg/ml: Calbiochem, Darmstadt, Germany) and incubated at 4°C overnight. The plates were washed with 0.05% Tween-20-PBS and blocked with 10% FCS-PBS for 1 hour at room temperature. Sera from mice were diluted in 10% FCS-PBS and incubated for 2 hours at room temperature. Goat anti-mouse IgG1 and IgG2a and human ads-HRP (Beckman Coulter, Fullerton, CA) were added, and the plates were incubated for 1 hour at room temperature. The substrate, 3,3,5,5-tetramethylbenzidine (Sigma: 0.1 mg/ml) diluted in 0.05 M citrate-phosphate buffer (pH5) containing hydrogen peroxide (Wako), was added, and the reaction continued for 30 minutes. The reaction was terminated by the addition of 2 N sulfuric acid, and the OD 450 nm/570 nm was measured by ARVO X5 (PerkinElmer Japan, Kanagawa, Japan).
OVA-specific CD4+ T cell differentiation assays
CD4+ T cells were purified from the spleens of DO11.10rag2 –/– mice by MACS. FITC+MGL2+ dDCs and FITC+CD103+ dDCs or MHCIIhighMGL2+ dDCs and MHCIIhighCD103+ dDCs were obtained as shown above. dDCs (1.0×104 cells) were cultured in the presence or absence of 2 µg/ml OVA323–339 peptide (Genscript) for 2 hours and added with CD4+ T cells (1.0×105 cells). The co-culture was continued for 3 or 5 days in 96-well round-bottomed plates. After the culture supernatants were obtained, the cells were cultured in the presence of 20 ng/ml PMA, 1 µM ionomycin, and 10 µg/ml Brefeldin A for 5 hours. Cytokine concentrations in culture supernatant were measured using the Bio-Plex Suspension Array System. Intracellular cytokines in T cells were measured by a FACS Aria cell sorter.
Targeting MGL2+ dDCs and analysis of subsequent immune responses
Anti-MGL2 mAbs or isotype control Rat IgG2a (5 µg) together with LPS (1 µg) were injected into the footpad of Mgl2 +/+ or Mgl2 –/– mice (BALB/c background). One day after the injection of biotinylated anti-MGL2 mAbs, cells from draining LNs stained with anti-CD11c, anti-MGL2, and intracellular PE-SAv were analyzed by a FACS Aria cell sorter. One week after injection, sera were obtained. Anti-Rat IgG2a antibodies in sera were determined by ELISA using HRP-goat anti-mouse IgGAM (Zymed), IgM, IgG1, IgG2a, IgG2b, and IgG3 (Beckman Coulter).
Statistical analysis
Data were compared with a two-sided student's t test and presented as the mean ± standard deviation (SD). When p values were less than 0.05, the difference was considered significant.
Results
MGL2+ dDCs are distinct from migratory LCs and CD103+ dDCs in skin under a naïve state
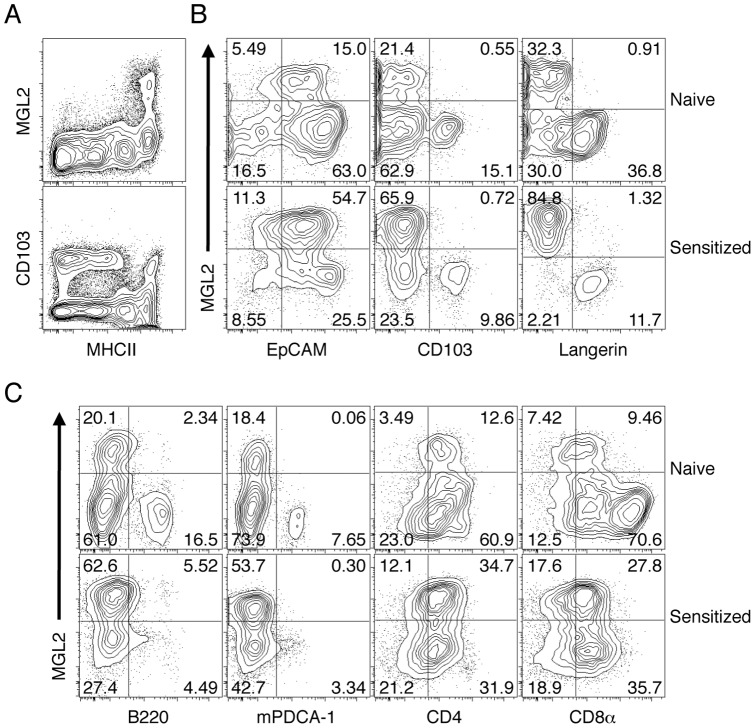
The expression of MGL2 is unique to dermal DCs and is not observed with epidermal cells such as epidermal LCs [34]. However, whether MGL2 is also expressed on migratory LCs, CD103+ dDCs, or other DC subsets distinct from these populations has not been previously studied. We analyzed skin cells from mice under a naïve state by flow cytometry and found that almost all MHCII+ cells were CD11c+ and thus DCs (data not shown). MGL2+ cells belonged to this population and were shown to represent EpCAMlowCD103–Langerin– cells ( Fig. 1 ). Previous reports showed that LCs had characteristics of EpCAMhighCD103–Langerin+ cells [12], [15], [35], and CD103+ dDCs had characteristics of EpCAMlowCD103+Langerin+ cells [12], [15], [35]. Therefore, MGL2+ dDCs should be distinct from migratory LCs and from CD103+ dDCs in skin.
Figure 1. Characterization of MGL2+ dDCs in skin.
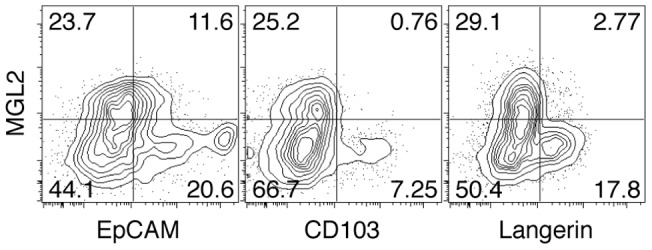
Flow cytometry analysis of skin cell suspensions for DC markers. MHCII+ cells were analyzed for the expression of MGL2, EpCAM, CD103, and Langerin. The experiments were independently performed three times.
MGL2+ dDCs are distinct from LCs or CD103+ dDCs in skin-draining LNs under a naïve state and after sensitization for CHS
In skin-draining LNs under a naïve state, MGL2+ dDCs are CD11c+CD11bhighMHCIIhighCD86+CD40highF4/80+B220−Ly6G−CD8álow and represent a uniform subset [34]. However, MGL2+ dDCs in LNs might have contained cells derived from migratory LCs or CD103+ dDCs. Alternatively, MGL2+ dDCs were distinct from these well-known subsets in skin-draining LNs. To clarify this point, we analyzed LN cells from mice under a naïve state and 1 day after sensitization for CHS with FITC for the distribution of MGL2 and other markers on skin-derived DCs by flow cytometry. All MHCIIhigh cells were confirmed to be CD11c+ under a naïve state and 1 day after percutaneous administration of FITC (data not shown), indicating that MHCIIhigh cells in LNs were DCs derived from the skin [35]. We also confirmed that all cells containing FITC in skin-draining LNs 1 day after sensitization were MHCIIhigh (data not shown). These results indicated that FITC was mainly processed by skin-derived DCs but not by resident DCs in LNs. In addition, MGL2 but not CD103 was specifically expressed on cells in the MHCIIhigh compartment in the flow cytometry charts of cells from skin-draining LNs under a naïve state ( Fig. 2A ). These findings also confirmed that MGL2 was specifically expressed on skin-derived DCs in LNs. The results shown in Fig. 2B revealed that MHCIIhighMGL2+ cells in skin-draining LNs under a naïve state and FITC+MGL2+ cells 1 day after sensitization for CHS with FITC were EpCAMintCD103–Langerin–. Because the LCs that migrate into skin-draining LNs are EpCAMhighCD103–Langerin+ [12], [15], [35], and CD103+ dDCs in LNs are EpCAMintCD103+Langerin+ [12], [15], [35], our results clearly indicate that MGL2+ dDCs are distinct from migratory LCs or CD103+ dDCs in skin-draining LNs both under naïve and sensitized conditions. Based on the notion that skin-draining LNs contain LN-resident CD8á+ DCs [35], [36], [37] and pDCs [37], [38], we assessed whether MGL2 was present on these DC subsets in naïve and sensitized skin-draining LNs. In both cases, MGL2+ cells were CD4lowCD8álowB220–mPDCA-1– ( Fig. 2C ). According to previous reports, the majority of LN-resident DCs highly express CD8á [37], and pDCs express B220 [38] and mPDCA-1 [39], whereas MGL2 was not detected on CD8á+ DCs or on pDCs in skin-draining LNs. Thus, we concluded that there is no overlap between these populations and MGL2+ cells. We also confirmed that CD8á+ DCs or pDCs did not incorporate FITC hapten during experimental CHS as previously shown [24].
Figure 2. Characterization of MGL2+ dDCs in skin-draining LNs.
Flow cytometry analysis of cells from skin-draining LNs for DC markers. (A) LN cells were analyzed for the expression of MGL2 or CD103 and MHCII. (B) MHCIIhigh cells from naïve mice are shown as “Naive” and FITC+ cells from sensitized mice (1 day after FITC painting) are shown as “Sensitized.” MHCIIhigh cells or FITC+ cells were analyzed for the expression of MGL2, EpCAM, CD103, or Langerin. (C) CD11c+ cells from naïve mice, shown as “Naïve,” and FITC+ cells from sensitized mice, shown as “Sensitized,” were analyzed for the expression of MGL2, B220, mPDCA-1, CD4, or CD8á. (A–C) The experiments were independently performed three times.
To explore the differences between MGL2+ dDCs and the other skin-derived DC subsets, we compared the expression of co-stimulatory molecules on MGL2+ dDCs, MGL2–CD103– skin-derived DCs (DN DCs), and CD103+ dDCs obtained from skin-draining LNs under a naïve state and after sensitization for CHS with FITC. The profiles of CD40, CD80, CD86, B7-H1, and B7-H2 were not different among these three skin-derived DC subsets, regardless of the sensitization for CHS (Figs. S1A and S1B). Interestingly, the level of B7-DC on MGL2+ dDCs was higher than for DN DCs or CD103+ dDCs (Figs. S1A and S1B). The levels of CD40, CD80, CD86, B7-H1, and B7-DC on MGL2+ dDCs, DN DCs, and CD103+ dDCs were elevated one day after sensitization for CHS, but B7-H2 was not elevated (Figs. S1A and S1B), indicating that all skin-derived DC subsets underwent maturation to a similar extent after sensitization for CHS.
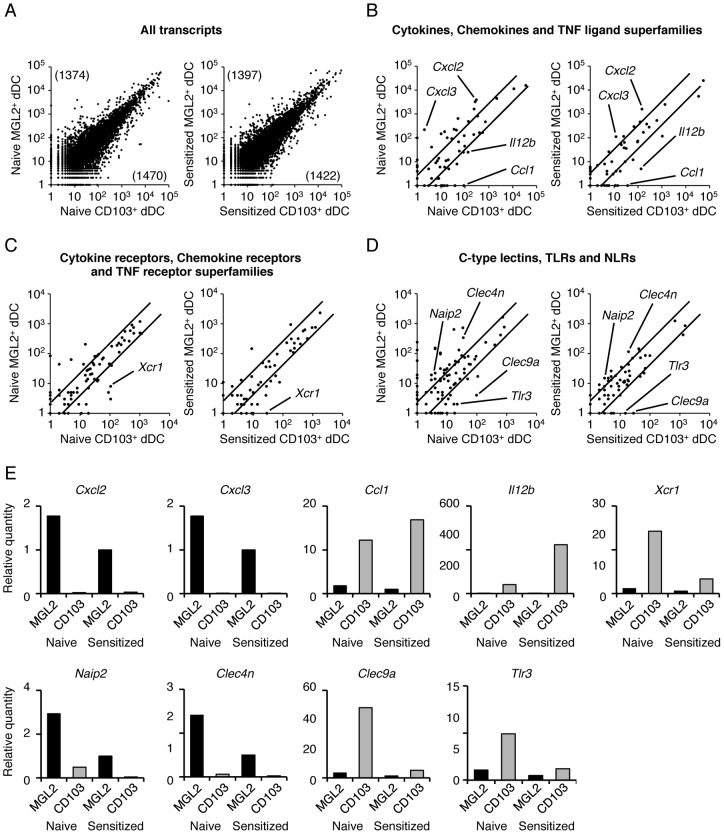
Encyclopedic transcriptome analysis and quantitative real-time PCR for MGL2+ dDCs and CD103+ dDCs
MGL2+ dDCs and CD103+ dDCs before and after sensitization for CHS were subjected to encyclopedic transcriptome analysis. Among the 18521 transcripts that were identified, approximately 1400 transcripts were estimated to be present in numbers that were more than three times greater in MGL2+ dDCs than in CD103+ dDCs under both naïve and sensitized conditions ( Fig. 3A ). Additionally, under both naïve and sensitized conditions, approximately 1400 transcripts were estimated in numbers that were more than three times greater in CD103+ dDCs than in MGL2+ dDCs ( Fig. 3A ). These results suggested that transcript expression profiles were distinct for MGL2+ dDCs and CD103+ dDCs under both naïve and sensitized conditions, despite the fact that both MGL2+ dDCs and CD103+ dDCs are skin-derived dDCs.
Figure 3. Encyclopedic transcriptome analysis of MGL2+ dDCs and CD103+ dDCs and results of quantitative real-time PCR for the transcripts suggested by the transcriptome analysis to have differential levels.
(A–D) Reciprocal two-dimensional plots of the results of transcriptome analysis among MHCIIhighMGL2+ cells, MHCIIhighCD103+ cells, FITC+MGL2+ cells and FITC+CD103+ cells. In these panels, MHCIIhighMGL2+ dDCs are indicated as “Naïve MGL2+ dDC,” MHCIIhighCD103+ dDCs are indicated as “Naïve CD103+ dDC,” FITC+MGL2+ dDCs are indicated as “Sensitized MGL2+ dDC,” and FITC+CD103+ dDCs are indicated as “Sensitized CD103+ dDC.” (A) All transcripts of cells from naïve mice and cells from sensitized mice are shown. The numbers in the parentheses indicate the number of transcripts expressed 3 times greater than the other dDC subset. (B–D) The numbers of transcripts in selected categories are plotted. In the comparisons, the names of the transcripts are indicated when they fit the following criteria: (1) the number is greater than 15 in MGL2+ dDCs or in CD103+ dDCs, and (2) the difference in the number is 5-fold or greater both before and after sensitization. The diagonal lines represent the border for 3-fold differences. (B) Transcripts of cytokines, chemokines and TNF ligand superfamily members are shown. (C) Transcripts of cytokine receptors, chemokine receptors, and TNF receptor superfamily members are shown. (D) Transcripts of C-type lectins, TLRs and NLRs are shown. (E) The quantitative real-time PCR analysis of the expression of indicated genes (Cxcl2, Cxcl3, Ccl1, Il12b, Xcr1, Naip2, Clec4n, Clec9a and Tlr3). They were chosen from the categories indicated above (B), (C), and (D) and the differences between MGL2+ dDCs and CD103+ dDCs based on the transcriptome analysis, appeared to be significant under both untreated and sensitized conditions (Figs. 3B–D). (A–E) Transcriptome analysis was performed once. The quantitative real-time PCR analysis was independently performed more than two times.
We focused on the profiles of cytokines, chemokines, TNF ligand superfamily members. TLRs, NLRs, and C-type lectins, which were thought to be involved in the regulation of DC functions ( Figs. 3B–3D , and Table S1). Fold differences in the relative expression levels of the indicated genes (Cxcl2, Cxcl3, Ccl1, Il12b, Xcr1, Naip2, Clec4n, Clec9a and Tlr3) among the dDC subsets were determined by the quantitative real-time PCR ( Fig. 3E ).
From the results, it was found that MGL2+ dDCs expressed Cxcl2 and Cxcl3, both of which were known to attract neutrophils, at higher levels than CD103+ dDCs [40], [41], [42]. MGL2+ dDCs expressed Il12b, which makes up a subunit of IL-12p70 that is critical for Th1 cell differentiation [43], [44] , at lower levels than CD103+ dDCs did. The level of Ccl1, which is known to attract various types of immune cells, in MGL2+ DCs was also lower than in CD103+ dDCs under naïve or sensitized conditions [45], [46], [47] ( Figs. 3B and 3E , and Table S1). Among chemokine receptors and TNF receptor superfamily members, the levels of Xcr1, which is involved in cross-presentation [48], were lower in MGL2+ dDCs than in CD103+ dDCs ( Figs. 3C and 3E , and Table S1). Among the variety of TLRs, NLRs, and C-type lectins that were examined, the levels of Naip2, which is known to form the NAIP-NALC4 inflammasome [49], [50], and the levels of Clec4n, which induces signal transduction and subsequent cytokine production after recognition of α-mannan derived from C. albicans [51], [52], were higher in MGL2+ dDCs than in CD103+ dDCs. Conversely, the levels of Tlr3 and Clec9a, which are involved in cross-presentation [53], [54], [55], [56], [57], [58], [59], [60], [61], were lower in MGL2+ dDCs than in CD103+ dDCs ( Figs. 3D and 3E , and Table S1). The levels of Il18, which is involved in Th2 cell differentiation in the absence of IL-12 [62], were slightly higher in MGL2+ dDCs than in CD103+ dDCs, both under naïve and sensitized conditions (Table S1 and S2). However, the levels of other Th2-promoting molecules such as Il10 [63], Tnfsf4 [64], [65], jag1 [66], [67], jag2 [66] and Crlf2 (TSLP receptor) [68] were very similar between MGL2+ dDCs and CD103+ dDCs both under naïve and sensitized conditions (Table S1 and Table S2). Judging from these results, the levels of molecules promoting a Th1-type immune response and molecules potentially involved in cross-presentation were suggested to be expressed at much lower levels in MGL2+ dDCs than in CD103+ dDCs, whereas the levels of molecules promoting a Th2-type immune response were suggested to be almost similar in MGL2+ dDCs compared to CD103+ dDCs.
The production levels of IL-12 by MGL2+ dDCs are low even after sensitization
As shown above, the levels of Il12b transcript in MGL2+ dDCs were lower than in CD103+ dDCs, both under naïve and sensitized conditions. To quantify the corresponding protein, skin-draining LN cells were obtained from naïve and sensitized mice, and intracellular IL-12p40 on skin-derived DC subsets was measured by flow cytometry after treatment with brefeldin A. The level of intracellular IL-12p40 in MGL2+ dDCs was almost the same as that in DN DCs and was significantly lower than CD103+ dDCs under naïve conditions ( Figs. 4A and 4B ). One day after sensitization for CHS, intracellular IL-12p40 in MGL2+ dDCs was significantly lower than that found in DN DCs or CD103+ dDCs ( Figs. 4 A and 4B). The levels of secreted IL-12p40 and IL-12p70 by MGL2+ dDCs and CD103+ dDCs were also tested after sorting these cells from mice under naïve and sensitized conditions. The cells were cultured for 15 hours in the presence of an agonistic anti-CD40 antibody, and the concentrations of IL-12p40 and IL-12p70 in culture supernatants were examined. Concentrations of IL-12p40 and IL-12p70 in the culture supernatant of MGL2+ dDCs were much lower than those of CD103+ dDCs, both under naïve and sensitized conditions ( Fig. 4 C). These results indicated that MGL2+ dDCs produced a smaller amount of IL-12, a key cytokine for the Th1-type immune response, than CD103+ dDCs [43], [44] under both naïve and sensitized conditions.
Figure 4. Paucity in IL-12 production by MGL2+ dDCs.
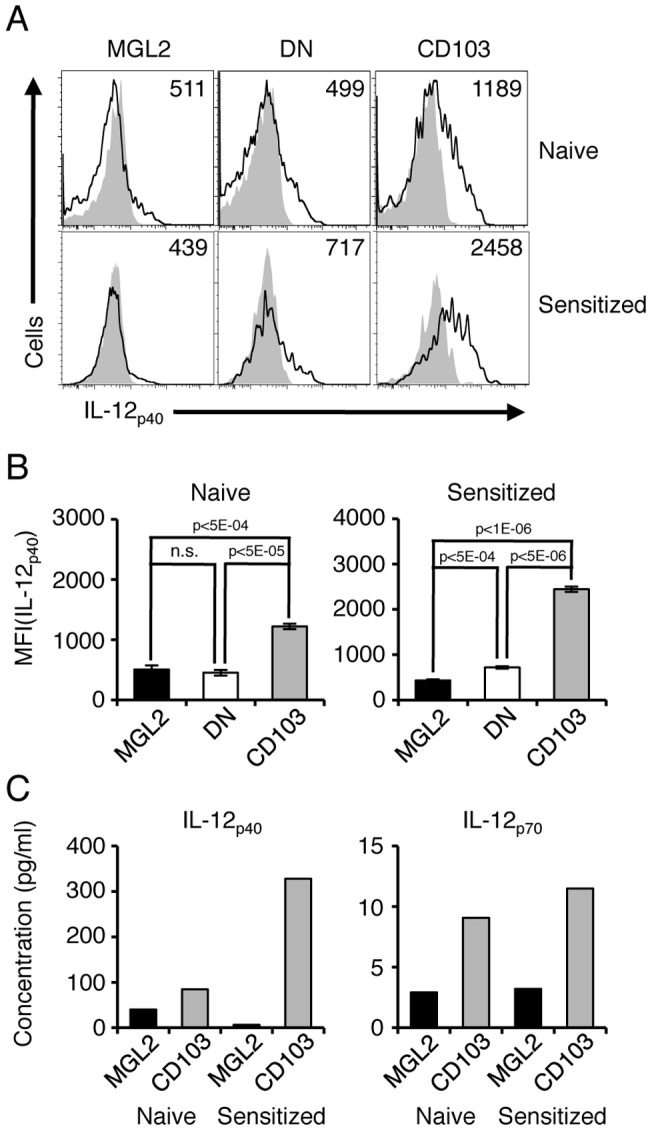
(A) Flow cytometry analysis of intracellular IL-12p40 in MGL2+ dDCs, MGL2–CD103– skin-derived DCs, and CD103+ dDCs in skin-draining LNs under naïve or sensitized conditions. MGL2+ dDCs are shown as “MGL2,” MGL2–CD103– DCs are shown as “DN” and CD103+ dDCs are shown as “CD103.” Cells from naïve mice are shown as “Naïve,” and cells from sensitized mice are shown as “Sensitized.” Areas shaded in gray indicate the staining pattern with an isotype control antibody. The numbers represent the mean fluorescence intensity (MFI) of each skin-derived DC subset. (B) The MFI of IL-12p40 for each skin-derived DC subset is shown in panel A in bar graphs. Data are shown as the mean ± SD of three biological replicates (n = 3); n.s. indicates that the difference is not statistically significant. (A–B) The experiments were independently performed three times. (C) Concentrations of IL-12p40 and IL-12p70 in culture supernatant of MHCIIhighMGL2+ dDCs, MHCIIhighCD103+ dDCs, FITC+MGL2+ dDCs, and FITC+CD103+ dDCs were measured by the Bio-Plex Suspension Array System and are shown in the panels as “Naive MGL2,” “Naïve CD103,” “Sensitized MGL2,” and “Sensitized CD103,” respectively. The experiments were independently performed two times.
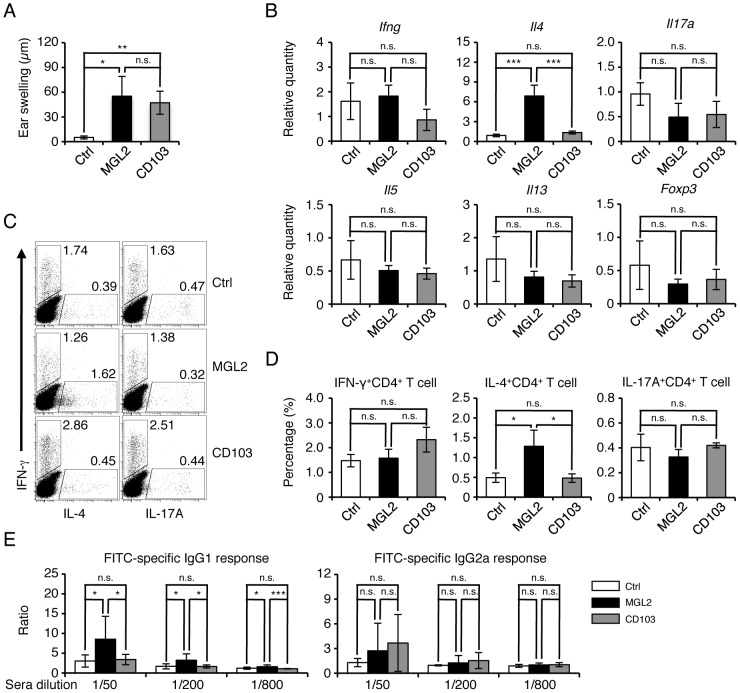
MGL2+ dDCs induce a Th2-type immune response in vivo in a model of contact hypersensitivity
The results shown above demonstrated that the levels of cytokines and other molecules involved in Th1-promotion or cross-presentation were lower in MGL2+ dDCs compared to CD103+ dDCs. MGL2+ dDCs produced very low levels of IL-12, which is known to be a key cytokine for Th1 differentiation [43], [44]. Additionally, we previously demonstrated that MGL2+ dDCs could induce CHS sensitization in vivo [34], indicating that MGL2+ dDCs can induce an immune response in vivo in CHS. Therefore, we assessed the type of immune response induced by the transfer of MGL2+ dDCs in vivo. FITC+MGL2+ dDCs and FITC+CD103+ dDCs were isolated from skin-draining LNs 1 day after sensitization for CHS and subcutaneously injected into naïve mice to compare the role of these dDC subsets in the induction of different types of immune responses. Ear swelling was elicited by FITC to a similar extent in mice that received transfers of these two subsets ( Fig. 5A ). Therefore, both FITC+MGL2+ dDCs and FITC+CD103+ dDCs were capable of CHS sensitization. The expression levels of Il4 in LN cells were much higher in mice that received FITC+MGL2+ dDCs than those in mice that received FITC+CD103+ dDCs or those in untreated mice ( Fig. 5B ). After stimulation with PMA and ionomycin in the presence of brefeldin A, the percentage of IL-4+CD4+ T cells in the total CD4+ T cells of the draining LNs was significantly higher in mice that received FITC+MGL2+ dDCs than in mice that received FITC+CD103+ dDCs or in untreated mice ( Figs. 5C and 5D ). In addition, the FITC-specific IgG1 response was significantly higher in mice that received FITC+MGL2+ dDCs than in mice that received FITC+CD103+ dDCs or in untreated mice ( Fig. 5E ). These results strongly suggest that MGL2+ dDCs, but not CD103+ dDCs, skew the immune response toward a Th2-type in vivo in CHS. Interestingly, the expression levels of Il5 and Il13 in LN cells were not different among these mice ( Fig. 5B ), suggesting that MGL2+ dDCs are specialized to induce Il4 in CD4+ T cells. After transfer of FITC+MGL2+ dDCs or FITC+CD103+ dDCs, the expression levels of Ifng, Il17a and Foxp3 in LN cells in the recipient mice were not significantly different from those in non-sensitized mice ( Fig. 5B ). The percentage of IL-17A+CD4+ T cells in the total CD4+ T cells from the LNs of these mice was not significantly different from that in the total CD4+ T cells of draining LNs from untreated mice ( Figs. 5C and 5D ). The percentage of IFN-γ+CD4+ T cells in the total CD4+ T cells from the draining LNs of mice that received FITC+CD103+ dDCs was slightly higher than that in mice that received FITC+MGL2+ dDCs or untreated mice, but the difference was not statistically significant ( Figs. 5C and 5D ). The FITC-specific IgG2a response was not significantly different but was slightly higher in mice that received FITC+CD103+ dDCs compared to mice that received FITC+MGL2+ dDCs or untreated mice ( Fig. 5E ). From these results, it is likely that CD103+ dDCs comprise a DC subset that has the potential to skew the immune response toward a Th1-type in vivo during the sensitization phase of CHS, as previously suggested [69].
Figure 5. Immune response in mice after transfers of MGL2+ dDCs in vivo.
In the following panels, data obtained from untreated mice, mice that received FITC+MGL2+ cells, and mice that received FITC+CD103+ cells are indicated as “Ctrl,” “MGL2,” and “CD103,” respectively. (A) FITC-specific ear swelling 1 day after elicitation is shown in µm. (B) Relative quantity of mRNA for Ifng, Il4, Il17a, Il5, Il13 and Foxp3 was measured by the quantitative real-time PCR in LN cells collected from recipient mice 1 day after elicitation. The values are shown as the mean ± SD from three independent measurements, and the levels of significance for the differences are: *** for p<0.005 and n.s. for not significant. (C) Results of flow cytometry analysis of intracellular cytokines in CD4+ T cells from LNs collected from recipient mice 1 day after elicitation are shown. The numbers in the panels indicate the percentages of IFN-γ+IL-4–CD4+ T cells, IFN-γ–IL-4+CD4+ T cells, IFN-γ+IL-17A–CD4+ T cells, and IFN-γ–IL-17A+CD4+ T cells in the total CD4+ T cells. (D) The percentages of IFN-γ+IL-4–CD4+ T cells, IFN-γ–IL4+CD4+ cells, and IFN-γ–IL-17A+CD4+ T cells in total CD4+ T cells are illustrated in bar graphs. Values in the Y-axis indicate the mean ± SD from three separate experiments, * indicates that the difference is statistically significant (p<0.05) and n.s. indicates that the difference is not statistically significant. (E) FITC-specific IgG1 and IgG2a in the sera of mice that received a transfer of FITC+MGL2+ cells or FITC+CD103+ cells were determined. The y-axis indicates the relative antibody binding to FITC. Mean ± SD from 9 separate experiments are shown, and *, ***, and n.s. indicate p<0.05, p<0.005, and difference not statistically significant, respectively. (A–E) The experiments were independently performed more than three times.
MGL2+ dDCs alone cannot induce antigen-specific IL-4 production from T cells in vitro
Although a significant contribution of MGL2+ dDCs to the Th2-type immune response is clear, we thought it was important to determine whether MGL2+ dDCs alone induce antigen-specific IL-4 production from T cells. FITC+MGL2+ dDCs and FITC+CD103+ dDCs were isolated from skin-draining LNs 1 day after sensitization for CHS and co-cultured with CD4+ T cells isolated from DO11.10Rag2 –/– mice in the presence of OVA323–339 peptide. The percentage of cytokine-producing T cells 3 or 5 days after co-cultivation and cytokine concentrations in the culture supernatant 3 or 5 days after co-cultivation were measured. In the absence of OVA323–339 peptide or dDCs, T cell proliferation was not observed (data not shown). The percentage of IL-4+CD4+ T cells co-cultured with FITC+MGL2+ dDCs was similar to that co-cultured with FITC+CD103+ dDCs (Fig. S2A). The IL-4 concentration in the supernatant of T cells co-cultured with FITC+MGL2+ dDCs was similar to that of T cells co-cultured with FITC+CD103+ dDCs (Fig. S2B). In addition, the percentage of IFN-γ+CD4+ T cells and the IFN-γ concentration in the supernatant of T cells co-cultured with FITC+CD103+ dDCs were similar to those after co-culture with FITC+MGL2+ dDCs (Figs. S2A and S2B). These results indicated that MGL2+ dDCs and CD103+ dDCs alone could not skew the immune response toward Th2 and Th1, respectively, in vitro.
Th2-type humoral response is induced by targeting MGL2+ dDCs in vivo
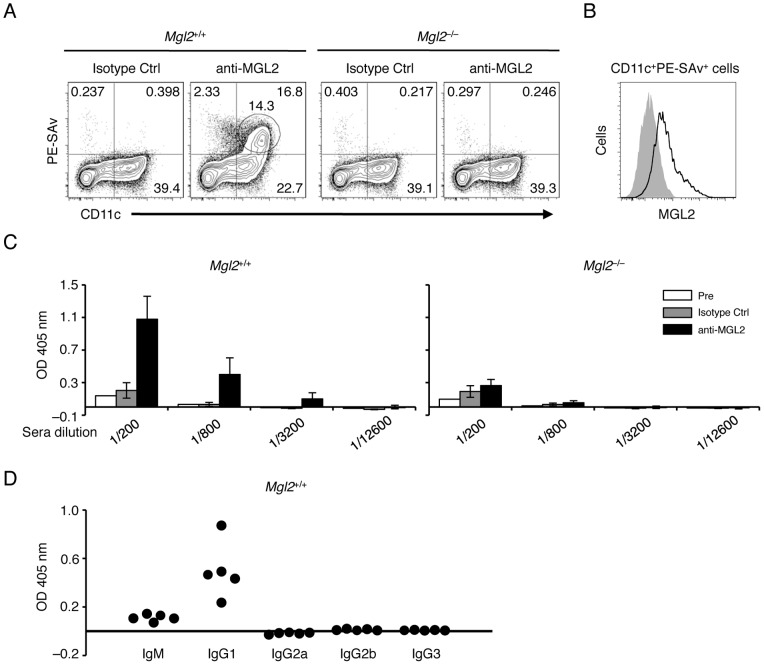
We tested in vivo whether MGL2+ dDCs skewed the immune response toward a Th2-type when an antigen is targeted to MGL2. Molecules reactive to MGL2 were previously shown to be efficiently internalized, processed, and presented to CD4+ T cells, but the type of the immune response was not known [33]. Therefore, we immunized mice with a rat anti-MGL2 mAb together with LPS as an adjuvant, and subsequent immune responses to rat IgG were examined. Anti-MGL2 mAbs, but not isotype controls, were internalized by MGL2+ dDCs in Mgl2 +/+ mice. Similar uptake did not occur in equivalent cells from Mgl2 –/– mice ( Fig. 6A ), indicating that anti-MGL2 mAbs were internalized through MGL2. An antibody response specific for rat IgG was observed in the immunized Mgl2 +/+ mice but not in Mgl2 +/+ mice immunized with isotype controls or in Mgl2 –/– mice immunized with rat anti-MGL2 mAbs or with isotype controls ( Fig. 6B ). IgG1 was the main detected subclass in Mgl2 +/+ mice immunized with anti-MGL2 mAbs ( Fig. 6C ). These results indicate that a Th2-type humoral response took place against an antigen that was taken up by MGL2+ dDCs in vivo. When LPS was not supplemented, the rat IgG-specific antibody response did not occur (data not shown). Therefore, maturation of MGL2+ dDCs, which was induced by LPS but not by cross-linking of cell surface MGL2, seems to be necessary for an efficient Th2-type humoral response.
Figure 6. A Th2-type humoral response is induced by targeting MGL2+ dDCs in vivo.
(A) One day after the injection of biotinylated anti-MGL2 mAbs or rat IgG2a (isotype control) into Mgl2 +/+ or Mgl2 –/– mice, cells from draining LNs were subjected to flow cytometry analysis for biotin residues internalized into CD11c+ DCs using PE-SAv. (B) Flow cytometry analysis of surface MGL2 on the cells, gated for the levels of PE-SAv binding and CD11c circled in the panel A. (C) Antibodies specific for rat IgG2a in sera were detected 1 week after the injection of rat anti-MGL2 mAbs or rat IgG2a (isotype control) into Mgl2 +/+ mice or Mgl2 –/– mice. (D) Sera obtained 1 week after the injection of anti-MGL2 mAbs into Mgl2 +/+ mice were assessed for antibody isotypes that were specific for rat IgG2a. (A–D) The experiments were independently performed three times.
Discussion
The type of an immune response is likely to depend upon the subsets and the degree of maturation of the antigen presenting cells that are present. In the skin immune system, LCs and CD103+ dDCs were previously reported to promote Th17-type and Th1-type immune responses, respectively [16], [17]. However, the types of immune response promoted or suppressed by other dDC subsets in vivo had yet to be elucidated [17]. In the present study, we show that MGL2 serves as a marker for a dDC subset distinct from CD103+ dDCs or LCs both under naïve and sensitized conditions in a model to elicit CHS. Using MGL2 as a marker, we isolated and characterized MGL2+ dDCs from skin and skin-draining lymph nodes.
The increase in the expression levels of co-stimulatory molecules after sensitization was similar among MGL2+ dDCs, DN DCs, and CD103+ dDCs. Thus, the capacities of these skin-derived DC subsets for T cell activation [70], [71] are likely to be comparable to each other in vivo. These results were consistent with previous work in other laboratories showing that both LCs and CD103+ dDCs are involved in CHS sensitization [12], [25], [26], [27], [28] and suggest that MGL2+ dDCs also play important roles for sensitization leading to CHS in vivo [34].
By encyclopedic transcriptome analyses, expression levels of Th1-promoting cytokines, especially Il12b, and molecules known to be involved in cross-presentation, especially Xcr1, Tlr3 and Clec9a, were lower in MGL2+ dDCs than in CD103+ dDCs and the differences in mRNA levels were verified by the quantitative real-time PCR. In contrast, MGL2+ dDCs contained almost similar levels of transcripts for Th2-promoting molecules compared to CD103+ dDCs. In addition, MGL2+ dDCs have been shown to produce low levels of IL-12, which was previously known to skew the immune response toward a Th1-type [1], [72], even after sensitization for CHS. MGL2+ dDCs were also shown to produce lower levels of IL-6, which skewed the immune response toward a Th17-type [73] after sensitization for CHS (data not shown). These results lead us to hypothesize that MGL2+ dDCs and CD103+ dDCs initiate different types of immune responses.
To prove or disprove this hypothesis, we isolated MGL2+ dDCs and CD103+ dDCs from mice sensitized with FITC and transferred them to naïve mice to test the type of immune response initiated by each subset. MGL2+ dDCs were shown to transfer antigen-specific Th2-type immunity, as revealed by the induction of IL-4+CD4+ T cells and a FITC-specific IgG1 response in vivo. In contrast, CD103+ dDCs were likely to render a Th1-type immune response, based on our finding that these cells expressed Il12b at high levels, produced high amount of IL-12p40 and IL-12p70 in vitro, and induced IFN-ã+CD4+ T cells and FITC-specific IgG2a in vivo. Takeshita and co-workers reported that neutralization of IL-4 during the sensitization phase resulted in a reduction of CHS by more than 60% [74], demonstrating that IL-4 played a significant role in the initiation of CHS. It is likely that MGL2+ dDCs initiate CHS through the regulation of IL-4 production by T cells.
We demonstrated that MGL2+ dDCs and CD103+ dDCs alone could not skew the immune response toward Th2 and Th1, respectively, in vitro. These results suggest the following two possibilities. First, FITC+MGL2+ dDCs alone cannot skew the immune response toward a Th2-type in an antigen-dependent manner unless additional help comes from other cells, such as basophils, previously shown to contribute to the Th2-type immune response [75], [76], and FITC+CD103+ dDCs are in a similar situation for the Th1 response in the context of this model. Secondly, an in vivo niche may play an important additional role in skewing for a Th2 or Th1 response. MGL2+ dDCs did not induce Il5 and Il13 expression in LN cells in vivo after sensitization for CHS, suggesting that MGL2+ dDCs represent a subset specialized to induce IL-4 production from CD4+ T cells.
The numbers of the transcripts that are known to promote a Th2-type immune response, including Il10 [63], Tnfsf4 [64], [65], jag1 [66], [67], jag2 [66], and Crlf2 (TSLP receptor) [68], in MGL2+ dDCs were not largely different from those in CD103+ dDCs, both under naïve and sensitized conditions in our transcriptome analysis. Therefore, in the Th2-type immune response initiated by MGL2+ dDCs in vivo, these molecules might not directly be involved. Very low numbers of transcripts were found for molecules involved in the Th1-type immune response or in cross-presentation, such as Il12b, Xcr1, Tlr3 and Clec9a [43], [44], [48], [53], [54], [55], [56], [57], [58], [59], [60], [61], in MGL2+ dDCs. At the protein level, IL-12p40 and IL-12p70 levels were very low, both under naïve and sensitized conditions, and IL-6 was down-regulated under sensitized conditions in MGL2+ dDCs (data not shown). The expression of co-stimulatory molecules that are important for T cell activation [70], [71] was elevated to a similar extent as other skin-derived DCs after sensitization for CHS. These results suggest that MGL2+ dDCs stimulated naïve T cells via co-stimulatory molecules but failed to induce T cell differentiation toward a Th1-type or Th17-type response. Consequently, the immune response was likely to skew toward a Th2-type in vivo in CHS. MacDonald reported that DCs might induce a Th2-type immune response in the absence of IL-12, and our interpretation is consistent with their findings [77]. Guilliams and co-workers reported that CD103– dDCs, corresponding mostly to MGL2+ dDCs, induced antigen-specific Tregs in vitro under a naïve state [78]. In the present study, however, MGL2+ dDCs did not induce Foxp3 expression in LN cells in vivo in CHS. The discrepancy was most likely due to the increased expression of co-stimulatory molecules after sensitization for CHS.
In the present study, we did not extensively investigate the characteristics of DN DCs, which should correspond mostly to LCs. According to our analyses, DN DCs were shown to produce low levels of IL-12p40 under naïve and sensitized conditions, and the expression levels of co-stimulatory molecules on DN DCs, as shown by flow cytometry analysis, were almost the same as MGL2+ dDCs and CD103+ dDCs. However, we previously demonstrated that MGL2+ dDCs migrate to the outer areas of the T cell cortex in skin-draining LNs 1 day after FITC-painting, whereas the other groups demonstrated that LCs migrate into the T cell cortex 4 days after FITC- or TRITC-painting [24], [34], [79], [80]. Judging from these results, MGL2+ dDCs and LCs might act in distinct areas and at distinct time points despite the fact that IL-12p40 expression is similar between MGL2+ dDCs and LCs. Therefore, MGL2+ dDCs and LCs may play different roles in the course of the immune response in CHS.
A rat monoclonal antibody specific for MGL2 was used to target MGL2+ DCs to induce an immune response in vivo [33]. A Th2-type humoral response was induced, as shown by the antibody subclass, which was consistent with the role of MGL2+ dDCs in CHS. In addition, encyclopedic transcriptome analysis and the quantitative real-time PCR analysis revealed that the expression profiles of C-type lectin receptors in MGL2+ dDCs and CD103+ dDCs were distinct from each other. MGL2+ dDCs expressed Clec4n at high levels, whereas CD103+ dDCs expressed Clec9a at high levels. It was previously shown that cross-presentation and a Th1-type immune response were induced when Langerin, DEC205, or Clec9A were targeted [57]. These results collectively indicate that carbohydrate moieties of proteins direct antigens to defined subsets of DCs, which incorporate, process and present antigens to lead to a subsequent immune response, and suggest that the exogenous antigen taken up into MGL2+ dDCs through MGL2 induces Th2-type immune responses.
In conclusion, we demonstrated that dDCs displaying MGL2 on their surfaces were phenotypically and functionally distinct from LCs or CD103+ dDCs, and MGL2+ dDCs induced a Th2-type immune response. A causal relationship between the surface expression of MGL2 and the Th2-type immune response induced by MGL2+ dDCs remains to be determined.
Supporting Information
The expression of co-stimulatory molecules on dDC subsets in skin-draining LNs. Flow cytometry analysis for the expression of co-stimulatory molecules on MGL2+ dDCs, MGL2–CD103– skin-derived DCs, and CD103+ dDCs from skin-draining LNs under a naïve state or 1 day after FITC painting. MGL2+ dDCs are shown as “MGL2,” MGL2–CD103– skin-derived DCs are shown as “DN,” and CD103+ dDCs are shown as “CD103.” (A) MHCIIhighMGL2+ dDCs (MGL2), MHCIIhigh MGL2–CD103– skin-derived DCs (DN), and MHCIIhighCD103+ dDCs (CD103) in skin-draining LNs from mice under a naïve state were analyzed for the expression of the indicated co-stimulatory molecules. The number indicates the MFI of each co-stimulatory molecule on each skin-derived DC subset. (B) FITC+MGL2+ dDCs (MGL2), FITC+MGL2–CD103– skin-derived DCs (DN), and FITC+CD103+ dDCs (CD103) in skin-draining LNs from mice 1 day after FITC painting were analyzed for the expression of the indicated co-stimulatory molecules. The number indicates the MFI of each co-stimulatory molecule on each skin-derived DC subset. (A–B) The experiments were independently performed three times.
(TIF)
Induction of cytokines in CD4+ T cells by co-culture with MGL2+ dDCs or CD103+ dDCs in vitro . (A) FITC+MGL2+ dDCs or FITC+CD103+ dDCs were co-cultured with CD4+ T cells from DO11.10rag2 –/– mice in vitro. Three days later, these cells were stimulated with PMA/ionomycin in the presence of brefeldin A, and intracellular cytokine levels of T cells were determined by flow cytometry. In these panels, T cells co-cultured with FITC+MGL2+ dDCs are shown as “MGL2,” and T cells co-cultured with FITC+CD103+ dDCs are shown as “CD103.” The numbers indicate the percentages of IFN-γ+IL-4–CD4+ T cells and IFN-γ–IL-4+CD4+ T cells in total CD4+ T cells. (B) Concentrations of IFN-γ and IL-4 in culture supernatants were determined before stimulation with PMA/ionomycin. Culture supernatants in the presence of FITC+MGL2+ dDCs are shown as “MGL2,” and culture supernatants in the presence of FITC+CD103+ dDCs are shown as “CD103.” (A–B) The experiments were independently performed three times.
(TIF)
Encyclopedic transcriptome analysis of MGL2+ dDCs and CD103+ dDCs. The number of transcripts in MHCIIhighMGL2+ cells from naïve mice, MHCIIhighCD103+ cells from naïve mice, FITC+MGL2+ cells from mice 1 day after FITC painting, and FITC+CD103+ cells from mice 1 day after FITC painting are shown. Nine categories – cytokines, chemokines, TNF ligand superfamily members, cytokine receptors, chemokine receptors, TNF receptor superfamily members, C-type lectins, TLRs, and NLRs – were chosen. The lists include items whose expression levels were greater than 15 in MGL2+ dDCs, CD103+ dDCs, or both, either under a naïve state or 1 day after FITC painting. MHCIIhighMGL2+ dDCs are shown as “Naïve MGL2,” MHCIIhighCD103+ dDCs are shown as “Naïve CD103,” FITC+MGL2+ dDCs are shown as “Sensitized MGL2” and FITC+CD103+ dDCs are shown as “Sensitized CD103.”
(TIF)
The expression of Th2-promoting molecules on MGL2+ dDCs and CD103+ dDCs. Comparison of transcripts of Th2-promoting molecules chosen from the encyclopedic transcriptome analysis of MHCIIhighMGL2+ cells from naïve mice, MHCIIhighCD103+ cells from naïve mice, FITC+MGL2+ cells from mice 1 day after FITC painting, and FITC+CD103+ cells from mice 1 day after FITC painting. MHCIIhighMGL2+ dDCs are shown as “Naïve MGL2,” MHCIIhighCD103+ dDCs are shown as “Naïve CD103,” FITC+MGL2+ dDCs are shown as “Sensitized MGL2,” and FITC+CD103+ dDCs are shown as “Sensitized CD103.” The relative numbers of each transcript are indicated.
(TIF)
The list of primers used in the quantitative real-time PCR.
(TIF)
Acknowledgments
We thank Ms. Kyoko Sakai and Ms. Miyuki Ikeda for their assistance in the preparation of this manuscript and Ms. Satomi Yoshinaga for animal care.
Funding Statement
The study was supported in part by a grants from the Japan Chemical Industry Association (JCIA) Long-range Research Initiative (LRI) and Medical Glycomics Project of New Energy Development Organization. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392: 245–252. [DOI] [PubMed] [Google Scholar]
- 2. Steinman RM (2012) Decisions About Dendritic Cells: Past, Present, and Future. Annu Rev Immunol 30: 1–22. [DOI] [PubMed] [Google Scholar]
- 3. Maldonado-López R, Moser M (2001) Dendritic cell subsets and the regulation of Th1/Th2 responses. Semin Immunol 13: 275–282. [DOI] [PubMed] [Google Scholar]
- 4. Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, et al. (2007) Differential antigen processing by dendritic cell subsets in vivo. Science 315: 107–111. [DOI] [PubMed] [Google Scholar]
- 5. Bialecki E, Macho Fernandez E, Ivanov S, Paget C, Fontaine J, et al. (2011) Spleen-Resident CD4+ and CD4-CD8α- Dendritic Cell Subsets Differ in Their Ability to Prime Invariant Natural Killer T Lymphocytes. PLoS One 6: e26919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kasahara S, Clark EA (2012) Dendritic cell-associated lectin 2 (DCAL2) defines a distinct CD8α- dendritic cell subset. J Leukoc Biol 91: 437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Henri S, Guilliams M, Poulin LF, Tamoutounour S, Ardouin L, Dalod M, Malissen B (2010) Disentangling the complexity of the skin dendritic cell network. Immunol Cell Biol 88: 366–375. [DOI] [PubMed] [Google Scholar]
- 8. Guilliams M, Henri S, Tamoutounour S, Ardouin L, Schwartz-Cornil I, et al. (2010) From skin dendritic cells to a simplified classification of human and mouse dendritic cell subsets. Eur J Immunol 40: 2089–2094. [DOI] [PubMed] [Google Scholar]
- 9. Heath WR, Carbone FR (2009) Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat Immunol 10: 1237–1244. [DOI] [PubMed] [Google Scholar]
- 10. van de Ven R, van den Hout MF, Lindenberg JJ, Sluijter BJ, van Leeuwen PA, et al. (2011) Characterization of four conventional dendritic cell subsets in human skin-draining lymph nodes in relation to T-cell activation. Blood 118: 2502–2510. [DOI] [PubMed] [Google Scholar]
- 11. Stoitzner P, Holzmann S, McLellan AD, Ivarsson L, Stössel H, et al. (2003) Visualization and characterization of migratory Langerhans cells in murine skin and lymph nodes by antibodies against Langerin/CD207. J Invest Dermatol 120: 266–274. [DOI] [PubMed] [Google Scholar]
- 12. Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, et al. (2007) Identification of a novel population of Langerin+ dendritic cells. J Exp Med 204: 3147–3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Poulin LF, Henri S, de Bovis B, Devilard E, Kissenpfennig A, et al. (2007) The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J Exp Med 204: 3119–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ginhoux F, Collin MP, Bogunovic M, Abel M, Leboeuf M, et al. (2007) Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J Exp Med 204: 3133–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nagao K, Ginhoux F, Leitner WW, Motegi S, Bennett CL, et al. (2009) Murine epidermal Langerhans cells and langerin-expressing dermal dendritic cells are unrelated and exhibit distinct functions. Proc Natl Acad Sci U S A 106: 3312–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Igyártó BZ, Haley K, Ortner D, Bobr A, Gerami-Nejad M, et al. (2011) Skin-Resident Murine Dendritic Cell Subsets Promote Distinct and Opposing Antigen-Specific T Helper Cell Responses. Immunity 35: 260–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaplan DH, Igyártó BZ, Gaspari AA (2012) Early immune events in the induction of allergic contact dermatitis. Nat Rev Immunol 12: 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Macatonia SE, Knight SC, Edwards AJ, Griffiths S, Fryer P (1987) Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. Functional and morphological studies. J Exp Med 166: 1654–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang B, Fujisawa H, Zhuang L, Freed I, Howell BG, et al. (2000) CD4+ Th1 and CD8+ type 1 cytotoxic T cells both play a crucial role in the full development of contact hypersensitivity. J Immunol 165: 6783–6790. [DOI] [PubMed] [Google Scholar]
- 20. Wang B, Esche C, Mamelak A, Freed I, Watanabe H, et al. (2003) Cytokine knockouts in contact hypersensitivity research. Cytokine Growth Factor Rev 14: 381–389. [DOI] [PubMed] [Google Scholar]
- 21. Toews GB, Bergstresser PR, Streilein JW (1980) Epidermal Langerhans cell density determines whether contact hypersensitivity or unresponsiveness follows skin painting with DNFB. J Immunol 124: 445–453. [PubMed] [Google Scholar]
- 22. Kripke ML, Munn CG, Jeevan A, Tang JM, Bucana C (1990) Evidence that cutaneous antigen-presenting cells migrate to regional lymph nodes during contact sensitization. J Immunol 145: 2833–2838. [PubMed] [Google Scholar]
- 23. Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ (2005) Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity 23: 611–620. [DOI] [PubMed] [Google Scholar]
- 24. Kissenpfennig A, Henri S, Dubois B, Laplace-Builhé C, Perrin P, et al. (2005) Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity 22: 643–654. [DOI] [PubMed] [Google Scholar]
- 25. Bennett CL, van Rijn E, Jung S, Inaba K, Steinman RM, et al. (2005) Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol 169: 569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bennett CL, Noordegraaf M, Martina CA, Clausen BE (2007) Langerhans cells are required for efficient presentation of topically applied hapten to T cells. J Immunol 179: 6830–6835. [DOI] [PubMed] [Google Scholar]
- 27. Noordegraaf M, Flacher V, Stoitzner P, Clausen BE (2010) Functional Redundancy of Langerhans Cells and Langerin(+) Dermal Dendritic Cells in Contact Hypersensitivity. J Invest Dermatol 130: 2752–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zahner SP, Kel JM, Martina CA, Brouwers-Haspels I, van Roon MA, et al. (2011) Conditional Deletion of TGF-{beta}R1 Using Langerin-Cre Mice Results in Langerhans Cell Deficiency and Reduced Contact Hypersensitivity. J Immunol 187: 5069–5076. [DOI] [PubMed] [Google Scholar]
- 29. Honda T, Nakajima S, Egawa G, Ogasawara K, Malissen B, et al. (2010) Compensatory role of Langerhans cells and langerin-positive dermal dendritic cells in the sensitization phase of murine contact hypersensitivity. J Allergy Clin Immunol 125: 1154–1156.e1152. [DOI] [PubMed] [Google Scholar]
- 30. Suzuki N, Yamamoto K, Toyoshima S, Osawa T, Irimura T (1996) Molecular cloning and expression of cDNA encoding human macrophage C-type lectin. Its unique carbohydrate binding specificity for Tn antigen. J Immunol 156: 128–135. [PubMed] [Google Scholar]
- 31. Sato M, Kawakami K, Osawa T, Toyoshima S (1992) Molecular cloning and expression of cDNA encoding a galactose/N-acetylgalactosamine-specific lectin on mouse tumoricidal macrophages. J Biochem 111: 331–336. [DOI] [PubMed] [Google Scholar]
- 32. Tsuiji M, Fujimori M, Ohashi Y, Higashi N, Onami TM, et al. (2002) Molecular cloning and characterization of a novel mouse macrophage C-type lectin, mMGL2, which has a distinct carbohydrate specificity from mMGL1. J Biol Chem 277: 28892–28901. [DOI] [PubMed] [Google Scholar]
- 33. Denda-Nagai K, Aida S, Saba K, Suzuki K, Moriyama S, et al. (2010) Distribution and function of macrophage galactose-type C-type lectin 2 (MGL2/CD301b): Efficient uptake and presentation of glycosylated antigens by dendritic cells. J Biol Chem 285: 19193–19204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kumamoto Y, Denda-Nagai K, Aida S, Higashi N, Irimura T (2009) MGL2 Dermal dendritic cells are sufficient to initiate contact hypersensitivity in vivo. PLoS One 4: e5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Henri S, Poulin LF, Tamoutounour S, Ardouin L, Guilliams M, et al. (2010) CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J Exp Med 207: 189–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, et al. (2008) Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science 322: 1097–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shortman K, Naik SH (2007) Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol 7: 19–30. [DOI] [PubMed] [Google Scholar]
- 38. Asselin-Paturel C, Boonstra A, Dalod M, Durand I, Yessaad N, et al. (2001) Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol 2: 1144–1150. [DOI] [PubMed] [Google Scholar]
- 39. Krug A, French AR, Barchet W, Fischer JA, Dzionek A, et al. (2004) TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity 21: 107–119. [DOI] [PubMed] [Google Scholar]
- 40. Wolpe SD, Sherry B, Juers D, Davatelis G, Yurt RW, et al. (1989) Identification and characterization of macrophage inflammatory protein 2. Proc Natl Acad Sci U S A 86: 612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ahuja SK, Murphy PM (1996) The CXC chemokines growth-regulated oncogene (GRO) alpha, GRObeta, GROgamma, neutrophil-activating peptide-2, and epithelial cell-derived neutrophil-activating peptide-78 are potent agonists for the type B, but not the type A, human interleukin-8 receptor. J Biol Chem 271: 20545–20550. [DOI] [PubMed] [Google Scholar]
- 42. Rainard P, Riollet C, Berthon P, Cunha P, Fromageau A, et al. (2008) The chemokine CXCL3 is responsible for the constitutive chemotactic activity of bovine milk for neutrophils. Mol Immunol 45: 4020–4027. [DOI] [PubMed] [Google Scholar]
- 43. Moser M, Murphy KM (2000) Dendritic cell regulation of TH1-TH2 development. Nat Immunol 1: 199–205. [DOI] [PubMed] [Google Scholar]
- 44. Trinchieri G (1995) Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol 13: 251–276. [DOI] [PubMed] [Google Scholar]
- 45. Gombert M, Dieu-Nosjean MC, Winterberg F, Bünemann E, Kubitza RC, et al. (2005) CCL1-CCR8 interactions: an axis mediating the recruitment of T cells and Langerhans-type dendritic cells to sites of atopic skin inflammation. J Immunol 174: 5082–5091. [DOI] [PubMed] [Google Scholar]
- 46. Hoshino A, Kawamura YI, Yasuhara M, Toyama-Sorimachi N, Yamamoto K, et al. (2007) Inhibition of CCL1-CCR8 interaction prevents aggregation of macrophages and development of peritoneal adhesions. J Immunol 178: 5296–5304. [DOI] [PubMed] [Google Scholar]
- 47. Rot A, von Andrian UH (2004) Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol 22: 891–928. [DOI] [PubMed] [Google Scholar]
- 48. Dorner BG, Dorner MB, Zhou X, Opitz C, Mora A, et al. (2009) Selective expression of the chemokine receptor XCR1 on cross-presenting dendritic cells determines cooperation with CD8+ T cells. Immunity 31: 823–833. [DOI] [PubMed] [Google Scholar]
- 49. Kofoed EM, Vance RE (2011) Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 477: 592–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhao Y, Yang J, Shi J, Gong YN, Lu Q, et al. (2011) The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477: 596–600. [DOI] [PubMed] [Google Scholar]
- 51. Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, et al. (2010) Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 32: 681–691. [DOI] [PubMed] [Google Scholar]
- 52. Saijo S, Iwakura Y (2011) Dectin-1 and Dectin-2 in innate immunity against fungi. Int Immunol 23: 467–472. [DOI] [PubMed] [Google Scholar]
- 53. Schulz O, Diebold SS, Chen M, Näslund TI, Nolte MA, et al. (2005) Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 433: 887–892. [DOI] [PubMed] [Google Scholar]
- 54. Wick DA, Martin SD, Nelson BH, Webb JR (2010) Profound CD8+ T cell immunity elicited by sequential daily immunization with exogenous antigen plus the TLR3 agonist poly(I:C). Vaccine 29: 984–993. [DOI] [PubMed] [Google Scholar]
- 55. Jelinek I, Leonard JN, Price GE, Brown KN, Meyer-Manlapat A, et al. (2011) TLR3-specific double-stranded RNA oligonucleotide adjuvants induce dendritic cell cross-presentation, CTL responses, and antiviral protection. J Immunol 186: 2422–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Desch AN, Randolph GJ, Murphy K, Gautier EL, Kedl RM, et al. (2011) CD103+ pulmonary dendritic cells preferentially acquire and present apoptotic cell-associated antigen. J Exp Med 208: 1789–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Idoyaga J, Lubkin A, Fiorese C, Lahoud MH, Caminschi I, et al. (2011) Comparable T helper 1 (Th1) and CD8 T-cell immunity by targeting HIV gag p24 to CD8 dendritic cells within antibodies to Langerin, DEC205, and Clec9A. Proc Natl Acad Sci U S A 108: 2384–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang JG, Czabotar PE, Policheni AN, Caminschi I, San Wan S, et al. (2012) The Dendritic Cell Receptor Clec9A Binds Damaged Cells via Exposed Actin Filaments. Immunity 36: 646–657. [DOI] [PubMed] [Google Scholar]
- 59. Iborra S, Izquierdo HM, Martinez-Lopez M, Blanco-Menendez N, Reis ESC, et al. (2012) The DC receptor DNGR-1 mediates cross-priming of CTLs during vaccinia virus infection in mice. J Clin Invest 122: 1628–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zelenay S, Keller AM, Whitney PG, Schraml BU, Deddouche S, et al. (2012) The dendritic cell receptor DNGR-1 controls endocytic handling of necrotic cell antigens to favor cross-priming of CTLs in virus-infected mice. J Clin Invest 122: 1615–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schreibelt G, Klinkenberg LJ, Cruz LJ, Tacken PJ, Tel J, et al. (2012) The C-type lectin receptor CLEC9A mediates antigen uptake and (cross-)presentation by human blood BDCA3+ myeloid dendritic cells. Blood 119: 2284–2292. [DOI] [PubMed] [Google Scholar]
- 62. Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H (2001) Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol 19: 423–474. [DOI] [PubMed] [Google Scholar]
- 63. Laouini D, Alenius H, Bryce P, Oettgen H, Tsitsikov E, et al. (2003) IL-10 is critical for Th2 responses in a murine model of allergic dermatitis. J Clin Invest 112: 1058–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Akiba H, Miyahira Y, Atsuta M, Takeda K, Nohara C, et al. (2000) Critical contribution of OX40 ligand to T helper cell type 2 differentiation in experimental leishmaniasis. J Exp Med 191: 375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Flynn S, Toellner KM, Raykundalia C, Goodall M, Lane P (1998) CD4 T cell cytokine differentiation: the B cell activation molecule, OX40 ligand, instructs CD4 T cells to express interleukin 4 and upregulates expression of the chemokine receptor, Blr-1. J Exp Med 188: 297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, et al. (2004) Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell 117: 515–526. [DOI] [PubMed] [Google Scholar]
- 67. Okamoto M, Matsuda H, Joetham A, Lucas JJ, Domenico J, et al. (2009) Jagged1 on dendritic cells and Notch on CD4+ T cells initiate lung allergic responsiveness by inducing IL-4 production. J Immunol 183: 2995–3003. [DOI] [PubMed] [Google Scholar]
- 68. Larson RP, Zimmerli SC, Comeau MR, Itano A, Omori M, et al. (2010) Dibutyl phthalate-induced thymic stromal lymphopoietin is required for Th2 contact hypersensitivity responses. J Immunol 184: 2974–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. King IL, Kroenke MA, Segal BM (2010) GM-CSF-dependent, CD103+ dermal dendritic cells play a critical role in Th effector cell differentiation after subcutaneous immunization. J Exp Med 207: 953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sharpe AH, Freeman GJ (2002) The B7-CD28 superfamily. Nat Rev Immunol 2: 116–126. [DOI] [PubMed] [Google Scholar]
- 71. Lenschow DJ, Walunas TL, Bluestone JA (1996) CD28/B7 system of T cell costimulation. Annu Rev Immunol 14: 233–258. [DOI] [PubMed] [Google Scholar]
- 72. Feili-Hariri M, Falkner DH, Morel PA (2005) Polarization of naive T cells into Th1 or Th2 by distinct cytokine-driven murine dendritic cell populations: implications for immunotherapy. J Leukoc Biol 78: 656–664. [DOI] [PubMed] [Google Scholar]
- 73. Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, et al. (2006) Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441: 235–238. [DOI] [PubMed] [Google Scholar]
- 74. Takeshita K, Yamasaki T, Akira S, Gantner F, Bacon KB (2004) Essential role of MHC II-independent CD4+ T cells, IL-4 and STAT6 in contact hypersensitivity induced by fluorescein isothiocyanate in the mouse. Int Immunol 16: 685–695. [DOI] [PubMed] [Google Scholar]
- 75. Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, et al. (2009) MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol 10: 697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, et al. (2009) Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol 10: 706–712. [DOI] [PubMed] [Google Scholar]
- 77. MacDonald AS, Maizels RM (2008) Alarming dendritic cells for Th2 induction. J Exp Med 205: 13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Guilliams M, Crozat K, Henri S, Tamoutounour S, Grenot P, et al. (2010) Skin-draining lymph nodes contain dermis-derived CD103(-) dendritic cells that constitutively produce retinoic acid and induce Foxp3(+) regulatory T cells. Blood 115: 1958–1968. [DOI] [PubMed] [Google Scholar]
- 79. Kamath AT, Henri S, Battye F, Tough DF, Shortman K (2002) Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood 100: 1734–1741. [PubMed] [Google Scholar]
- 80. Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, et al. (2006) Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity 25: 153–162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The expression of co-stimulatory molecules on dDC subsets in skin-draining LNs. Flow cytometry analysis for the expression of co-stimulatory molecules on MGL2+ dDCs, MGL2–CD103– skin-derived DCs, and CD103+ dDCs from skin-draining LNs under a naïve state or 1 day after FITC painting. MGL2+ dDCs are shown as “MGL2,” MGL2–CD103– skin-derived DCs are shown as “DN,” and CD103+ dDCs are shown as “CD103.” (A) MHCIIhighMGL2+ dDCs (MGL2), MHCIIhigh MGL2–CD103– skin-derived DCs (DN), and MHCIIhighCD103+ dDCs (CD103) in skin-draining LNs from mice under a naïve state were analyzed for the expression of the indicated co-stimulatory molecules. The number indicates the MFI of each co-stimulatory molecule on each skin-derived DC subset. (B) FITC+MGL2+ dDCs (MGL2), FITC+MGL2–CD103– skin-derived DCs (DN), and FITC+CD103+ dDCs (CD103) in skin-draining LNs from mice 1 day after FITC painting were analyzed for the expression of the indicated co-stimulatory molecules. The number indicates the MFI of each co-stimulatory molecule on each skin-derived DC subset. (A–B) The experiments were independently performed three times.
(TIF)
Induction of cytokines in CD4+ T cells by co-culture with MGL2+ dDCs or CD103+ dDCs in vitro . (A) FITC+MGL2+ dDCs or FITC+CD103+ dDCs were co-cultured with CD4+ T cells from DO11.10rag2 –/– mice in vitro. Three days later, these cells were stimulated with PMA/ionomycin in the presence of brefeldin A, and intracellular cytokine levels of T cells were determined by flow cytometry. In these panels, T cells co-cultured with FITC+MGL2+ dDCs are shown as “MGL2,” and T cells co-cultured with FITC+CD103+ dDCs are shown as “CD103.” The numbers indicate the percentages of IFN-γ+IL-4–CD4+ T cells and IFN-γ–IL-4+CD4+ T cells in total CD4+ T cells. (B) Concentrations of IFN-γ and IL-4 in culture supernatants were determined before stimulation with PMA/ionomycin. Culture supernatants in the presence of FITC+MGL2+ dDCs are shown as “MGL2,” and culture supernatants in the presence of FITC+CD103+ dDCs are shown as “CD103.” (A–B) The experiments were independently performed three times.
(TIF)
Encyclopedic transcriptome analysis of MGL2+ dDCs and CD103+ dDCs. The number of transcripts in MHCIIhighMGL2+ cells from naïve mice, MHCIIhighCD103+ cells from naïve mice, FITC+MGL2+ cells from mice 1 day after FITC painting, and FITC+CD103+ cells from mice 1 day after FITC painting are shown. Nine categories – cytokines, chemokines, TNF ligand superfamily members, cytokine receptors, chemokine receptors, TNF receptor superfamily members, C-type lectins, TLRs, and NLRs – were chosen. The lists include items whose expression levels were greater than 15 in MGL2+ dDCs, CD103+ dDCs, or both, either under a naïve state or 1 day after FITC painting. MHCIIhighMGL2+ dDCs are shown as “Naïve MGL2,” MHCIIhighCD103+ dDCs are shown as “Naïve CD103,” FITC+MGL2+ dDCs are shown as “Sensitized MGL2” and FITC+CD103+ dDCs are shown as “Sensitized CD103.”
(TIF)
The expression of Th2-promoting molecules on MGL2+ dDCs and CD103+ dDCs. Comparison of transcripts of Th2-promoting molecules chosen from the encyclopedic transcriptome analysis of MHCIIhighMGL2+ cells from naïve mice, MHCIIhighCD103+ cells from naïve mice, FITC+MGL2+ cells from mice 1 day after FITC painting, and FITC+CD103+ cells from mice 1 day after FITC painting. MHCIIhighMGL2+ dDCs are shown as “Naïve MGL2,” MHCIIhighCD103+ dDCs are shown as “Naïve CD103,” FITC+MGL2+ dDCs are shown as “Sensitized MGL2,” and FITC+CD103+ dDCs are shown as “Sensitized CD103.” The relative numbers of each transcript are indicated.
(TIF)
The list of primers used in the quantitative real-time PCR.
(TIF)