Abstract
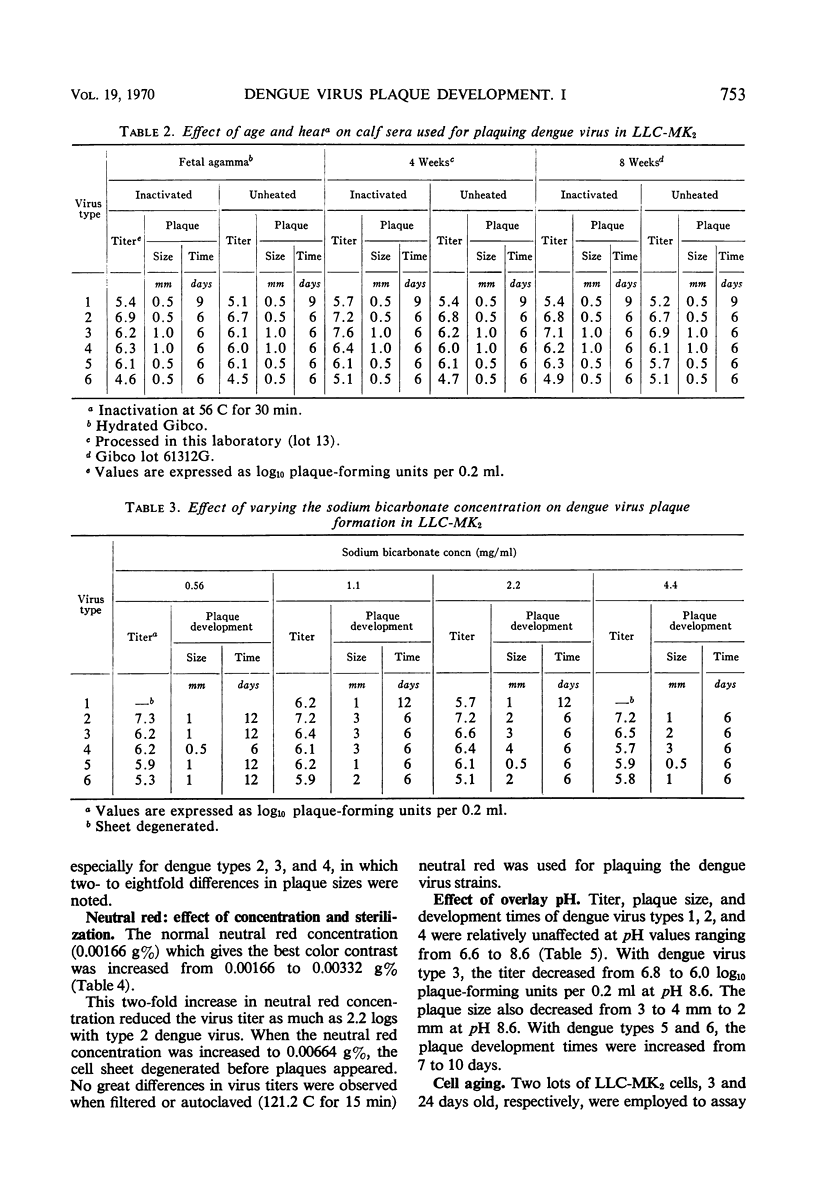
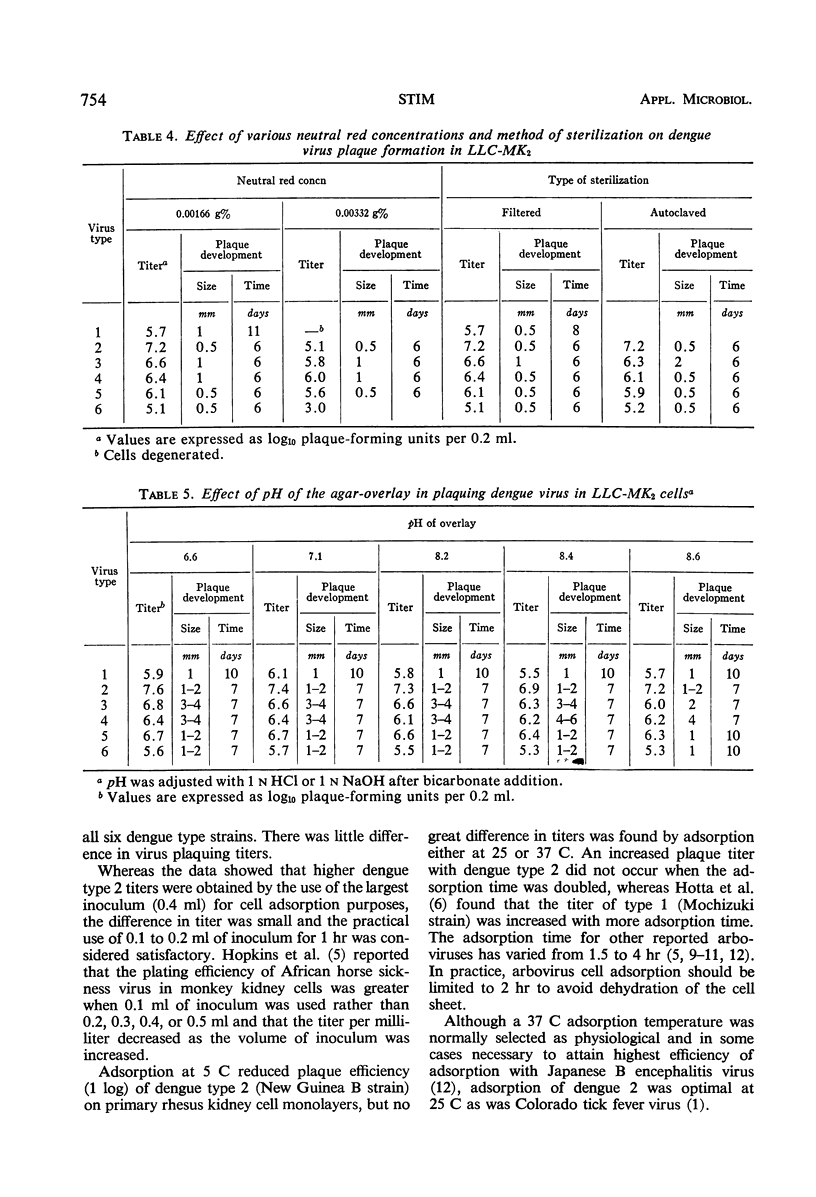
Chemical and physical variables influencing the plaquing of all dengue serotypes in two simian cell systems were studied. Calf serum in the nutrient overlay may be replaced by mouse ascitic fluid or bovine plasma albumin when employing the rhesus monkey kidney LLC-MK2 cell system for plaquing all dengue serotypes. Doubling the serum concentration in the overlay had little effect in modifying dengue types 1, 2, 3, and 4 plaque titers. Newborn agamma, 4-week-old and 8-week-old calf serum gave comparable titers with all dengue virus serotypes. Dengue virus titers, plaque size, and development time were unaffected by sodium bicarbonate concentrations ranging from 1.1 to 4.4 mg/ml of overlay. A twofold increase (0.00332 g%) in the amount of either autoclaved or filtered-sterilized neutral red reduced the dengue 2 virus titer as much as 2.2 logs. An increased Mg++ and decreased Ca++ concentration in the overlay medium increased the efficiency of the plaquing system.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DEIG E. F., WATKINS H. M. PLAQUE ASSAY PROCEDURE FOR COLORADO TICK FEVER VIRUS. J Bacteriol. 1964 Jul;88:42–47. doi: 10.1128/jb.88.1.42-47.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Nutrition needs of mammalian cells in tissue culture. Science. 1955 Sep 16;122(3168):501–514. doi: 10.1126/science.122.3168.501. [DOI] [PubMed] [Google Scholar]
- GEORGIADES J., STIM T. B., MCCOLLUM R. W., HENDERSON J. R. DENGUE VIRUS PLAQUE FORMATION IN RHESUS MONKEY KIDNEY CULTURES. Proc Soc Exp Biol Med. 1965 Feb;118:385–388. doi: 10.3181/00379727-118-29851. [DOI] [PubMed] [Google Scholar]
- HSIUNG G. D., MELNICK J. L. Plaque formation with poliomyelitis, Coxsackie, and orphan (echo) viruses in bottle cultures of monkey epithelial cells. Virology. 1955 Dec;1(5):533–535. doi: 10.1016/0042-6822(55)90041-6. [DOI] [PubMed] [Google Scholar]
- Hopkins I. G., Hazrati A., Ozawa Y. Development of plaque techniques for titration and neutralization tests with African horse-sickness virus. Am J Vet Res. 1966 Jan;27(116):96–105. [PubMed] [Google Scholar]
- Hotta S., Fujita N., Maruyama T. Research on dengue in tissue culture. I. Plaque formation in an established monkey kidney cell line culture. Kobe J Med Sci. 1966 Sep;12(3):179–187. [PubMed] [Google Scholar]
- Lee G. C., Grayston J. T., Kenny G. E. Growth of Japanese encephalitis virus in cell structure. J Infect Dis. 1965 Oct;115(4):321–329. doi: 10.1093/infdis/115.4.321. [DOI] [PubMed] [Google Scholar]
- NAGAI K., HAMMON W. M. PLAQUE STUDIES WITH CERTAIN GROUP B ARBOVIRUSES. I. JAPANESE B ENCEPHALITIS VIRUS STRAINS ON HAMSTER KIDNEY AND CHICK EMBRYO TISSUE CULTURE. Proc Soc Exp Biol Med. 1964 Oct;117:154–159. doi: 10.3181/00379727-117-29522. [DOI] [PubMed] [Google Scholar]
- PORTERFIELD J. S. A plaque technique for the titration of yellow fever virus and antisera. Trans R Soc Trop Med Hyg. 1959 Nov;53:458–466. doi: 10.1016/0035-9203(59)90021-5. [DOI] [PubMed] [Google Scholar]
- SCHULZE I. T., SCHLESINGER R. W. Plaque assay of dengue and other group B arthropod-borne viruses under methyl cellulose overlay media. Virology. 1963 Jan;19:40–48. doi: 10.1016/0042-6822(63)90022-9. [DOI] [PubMed] [Google Scholar]
- Sartorelli A. C., Fischer D. S., Downs W. G. Use of sarcoma 180/TG to prepare hyperimmune ascitic fluid in the mouse. J Immunol. 1966 Apr;96(4):676–682. [PubMed] [Google Scholar]
- Stim T. B., Henderson J. R. Further studies on multiplication of dengue viruses in various host systems. Proc Soc Exp Biol Med. 1966 Aug-Sep;122(4):1004–1008. doi: 10.3181/00379727-122-31310. [DOI] [PubMed] [Google Scholar]
- Westaway E. G. Assessment and application of a cell line from pig kidney for plaque assay and neutralization tests with twelve group B arboviruses. Am J Epidemiol. 1966 Nov;84(3):439–456. doi: 10.1093/oxfordjournals.aje.a120657. [DOI] [PubMed] [Google Scholar]



