Abstract
Background
Endothelin-1 (ET-1) and rho-kinase (ROCK) increase vascular tone in experimental PPHN but whether ET-1 activates ROCK to decrease angiogenesis in the developing lung remains unknown.
Methods
Proximal PAEC were harvested from fetal sheep after partial ligation of the ductus arteriosus in utero (PPHN) and age matched controls. Growth and tube formation were assessed after ET-1 treatment. The effect of ET-1 antagonism on tube formation was studied using ET-1 SiRNA, ET-1 monoclonal antibody (ET-1 mAb), BQ-123 (ETA blocker) and bosentan (ETA/ETB blocker). ET-1 gene and protein and ETA/ETB receptor protein expression were measured in normal and PPHN PAECs. ET-1-ROCK interactions were assessed by measuring ROCK activity after ET-1, ET-1 SiRNA and bosentan treatments and tube formation with ET-1 and Y-27632 (ROCK inhibitor).
Results
ET-1 had no effect on growth, but decreased tube formation in normal and PPHN PAEC. PPHN decreased tube formation in PAEC. ET-1 protein and gene expression were increased and ETB receptor protein decreased in PPHN PAECs. ET-1 SiRNA, ET-1mAb, and bosentan, but not BQ-123, increased tube formation. ROCK activity was increased in PPHN PAECs, and decreased with ET-1 SiRNA and bosentan treatments. Y-27632 prevented the decrease in tube formation with ET-1.
Conclusion
ET-1 impairs angiogenesis of fetal PAECs through ROCK activation. Disruption of ET-1 ROCK interactions may increase vascular growth in PPHN.
Introduction
Persistent pulmonary hypertension of the newborn (PPHN) is a clinical syndrome characterized by elevated pulmonary vascular resistance (PVR) that persists after birth (1). Mechanisms contributing to increased PVR in PPHN include increased vascular tone, hypertensive remodeling and in the most severe cases, impaired angiogenesis (2). Decreased vascular growth often occurs in the setting of PPHN with lung hypoplasia, such as in infants with congenital diaphragmatic hernia (CDH) (2,3). In the presence of lung hypoplasia, decreased arterial number plays a prominent role in maintaining high PVR and contributes to increased mortality. While inhaled NO is effective in treating many newborns with PPHN, PPHN with impaired angiogenesis is often refractory to NO therapy (4). Novel strategies that can stimulate vascular growth and increase arterial number may improve long-term outcomes, however, due to the lack of developmentally relevant models, mechanisms that impair angiogenesis and enhance lung vascular growth in severe PPHN remain poorly understood.
Past studies have shown that partial ligation of the ductus arteriosus (DA) in late gestation fetal sheep provides a useful animal model for studying the pathogenesis and treatment of PPHN (5). In this model, partial DA ligation increases pulmonary artery pressure without causing sustained elevations of pulmonary blood flow or hypoxemia (5). PVR remains elevated at birth, with extra-pulmonary shunting and hypoxemia (5). Along with changes in vascular tone, DA ligation impairs lung vascular growth in vivo and causes sustained abnormalities of PAEC growth and tube formation in vitro (6,7). How hemodynamic stress induced by hypertension disrupts normal endothelial cell signaling and function and impairs vascular growth in PPHN is unknown.
In adult models of experimental pulmonary hypertension, high endothelin-1 (ET-1) activity increases vascular tone and promotes hypertensive structural remodeling with smooth muscle proliferation (8-10). ET-1 is strongly expressed in the developing lung and maintains high PVR in the normal fetus (11,12). ET-1 has been implicated in the pathogenesis of PPHN in experimental models and clinical setting. PPHN in fetal sheep caused by DA ligation is characterized by increased lung ET-1 content and altered ET receptor expression, and is prevented by treatment with a selective ETA receptor antagonist (13,14). In addition, human newborns with severe PPHN have high circulating ET-1 levels (15), and in infants who died with CDH, lung ET-1 levels are also markedly increased (3,16). These studies demonstrate the importance of ET-1 in the pathogenesis of severe PPHN, especially in the setting of lung hypoplasia.
Although most studies have focused on the effects of ET-1 on vascular smooth muscle, past studies of tumor angiogenesis and human umbilical vein endothelial cells have suggested a pro-angiogenic effect of ET-1 on endothelial cells (17,18). ET-1 levels are markedly increased in clinical and experimental PPHN but whether ET-1 regulates angiogenesis in the developing lung and alters angiogenesis in severe PPHN has not been previously studied.
ROCK is a complex signaling pathway responsible for cellular proliferation, migration, barrier function, differentiation and gene expression in diverse vascular beds (19,20). ROCK activity maintains high PVR and contributes to the myogenic response in the normal fetal lung (21,22). In experimental models of neonatal and adult pulmonary hypertension, high ROCK activity causes high vascular tone, increased myogenic reactivity and hypertensive vascular remodeling (20,22-24). ET-1 is an upstream regulator of ROCK activity (25), but whether the ET-1-ROCK interactions cause endothelial cell dysfunction and impaired angiogenesis in PPHN remains unknown.
Therefore, we hypothesized that ET-1 impairs angiogenesis in experimental PPHN and that these effects are mediated through activation of ROCK. In this study, we report increased ET-1 production by PAEC from PPHN fetal sheep and that ET-1 activation of ROCK decreases tube formation in vitro. These findings support the hypothesis that ET-1-ROCK interactions contribute to endothelial dysfunction and decreased vascular growth in PPHN.
Results
ET-1 decreases 3D Tube Formation in Normal PAECs
Growth and tube formation were assessed in normal fetal PAECs with and without ET-1 treatment (100nM). ET-1 treatment had no effect on cell growth, but decreased tube formation in normal PAECs. ET-1 treatment decreased tube length by 15% (p<0.01) and branch points/HPF by 32% (p<0.01) in normal fetal PAECs. (Fig 1).
Figure 1. Decreased 3D Tube Formation by Normal PAECs After ET-1 Treatment.
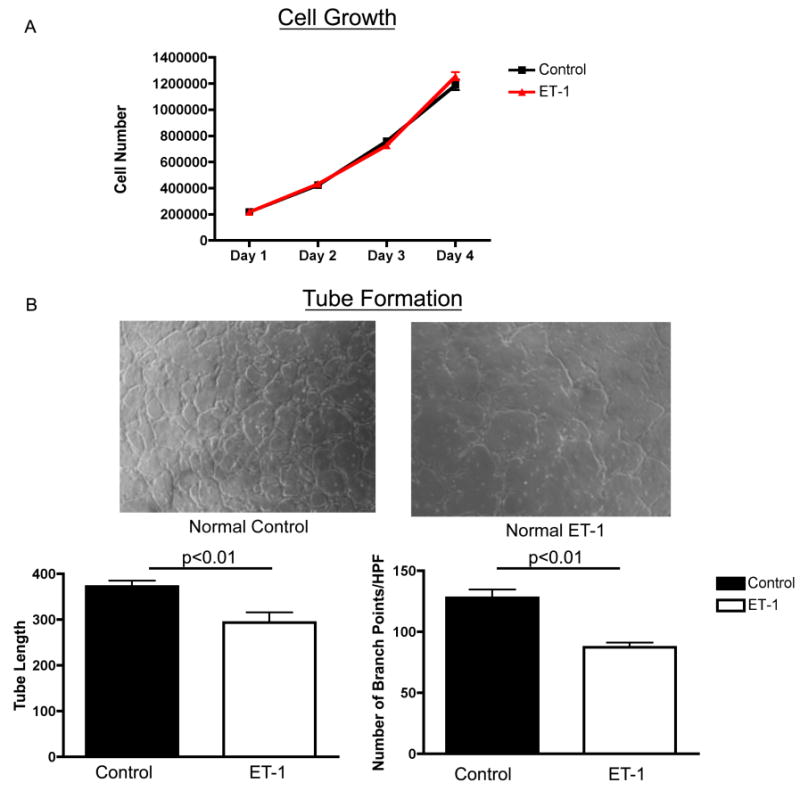
Growth and tube formation were assessed in normal fetal PAECs with and without ET-1 treatment (100nM). ET-1 treatment had no effect on cell growth, but decreased tube formation in normal PAECs. ET-1 treatment decreased tube length by 15% (p<0.01) and branch points/HPF by 32% (p<0.01) in normal fetal PAECs.
ET-1 decreases 3D Tube Formation in PPHN PAECs
Growth and tube formation were assessed in PPHN fetal PAECs with and without ET-1 treatment. ET-1 treatment had no effect on PPHN PAEC growth. Tube formation by PPHN PAECs was significantly decreased when compared with age-matched controls. Tube length was decreased by 27% (p<0.01) and the number of branch points/HPF was decreased by 32% (p<0.01) in PPHN PAECs. ET-1 treatment (100nM) further decreased tube length by 24% (p<0.05) and number of branch points/HPF by 28% (p<0.05) in PPHN fetal PAECs (Fig 2).
Figure 2. Decreased 3D Tube Formation by PAECs from PPHN fetal Sheep after ET-1 treatment.
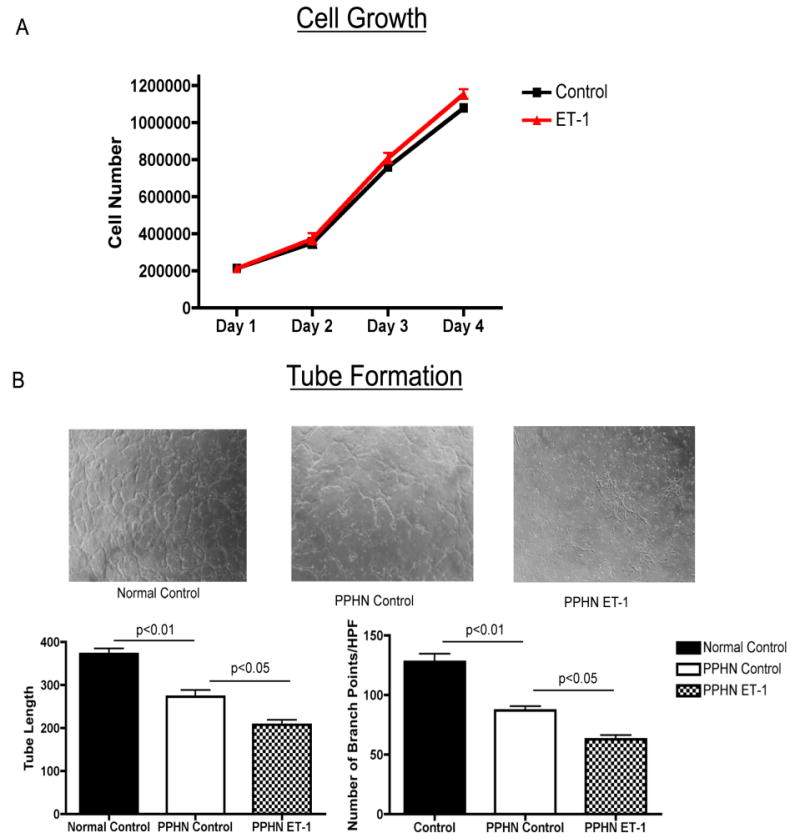
Growth and tube formation were assessed in PPHN fetal PAECs with and without ET-1 treatment. ET-1 treatment had no effect on PPHN PAEC growth. Tube formation by PPHN PAECs was significantly decreased when compared with age-matched controls. Tube length was decreased by 27% (p<0.01) and the number of branch points/HPF was decreased by 32% (p<0.01) in PPHN PAECs. ET-1 treatment (100nM) further decreased tube length 24% (p<0.05) and number of branch points/HPF by 28% (p<0.05) in PPHN fetal PAECs.
Increased ET-1 Production by PPHN PAECS
When compared with controls, ET-1 mRNA expression was increased by 47% and protein expression in the cell supernatant by more than 2 fold in PPHN PAECs (Fig 3).
Figure 3. Increased ET-1 gene and protien expression in PAECs from PPHN Fetal Sheep.
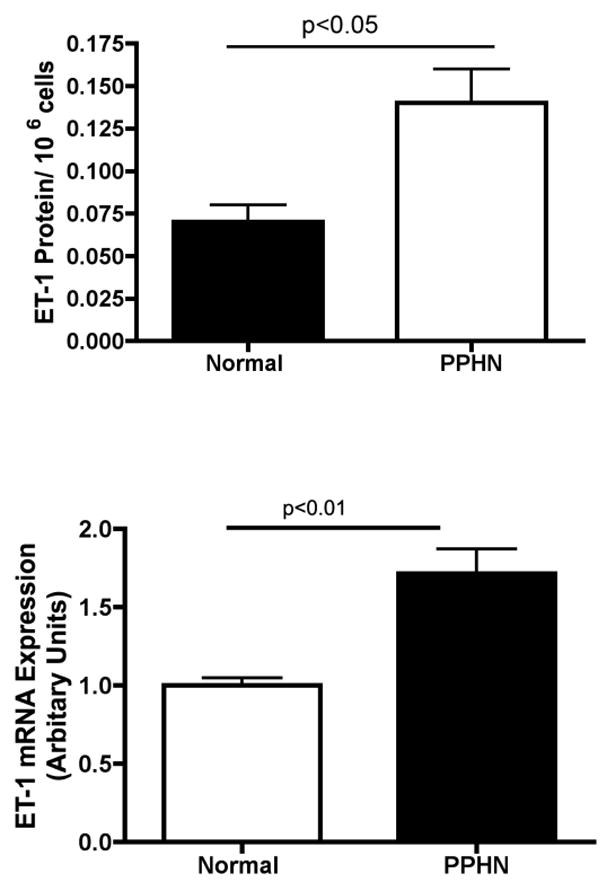
ET-1 mRNA and protein expression were measured in PAECs from normal and PPHN fetal sheep. ET-1 mRNA and protein expression were increased by 47% (p<0.01) and 100% (p<0.05) in PPHN fetal PAECs.
Effect of ET-1 Inhibition on tube formation in normal and PPHN fetal PAECs
Tube formation was assessed in normal and PPHN PAECs after exposure to ET-1mAb and ET-1 SiRNA (4μM). ET-1mAb binds to ET-1 and prevents binding to ETA and ETB receptors. ET-1mAb increased tube length by 36% (p<0.01 for each comparison)(fig not shown) and number of branch points per HPF by 53% and 56% (p<0.01 for each comparison) in normal and PPHN PAECs respectively (Fig 4A). ET-1 SiRNA decreased ET-1 mRNA expression by 50% and 54% in normal and PPHN PAECs (fig 4B). ET-1 SiRNA increaesd tube length by 32% and 46% (p<0.01 respectively)(fig not shown) and number of branch points per HPF by 53% and 61% (p<0.01 respectively) in normal and PPHN (Fig 4b) fetal PAECs. Both ETmAb and ET-1 SiRNA restored tube formation by PPHN fetal PAECs to similar values seen in normal untreated controls.
Figure 4.
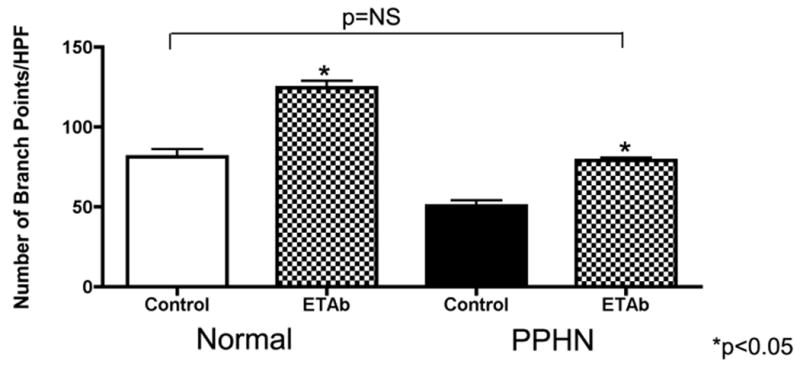
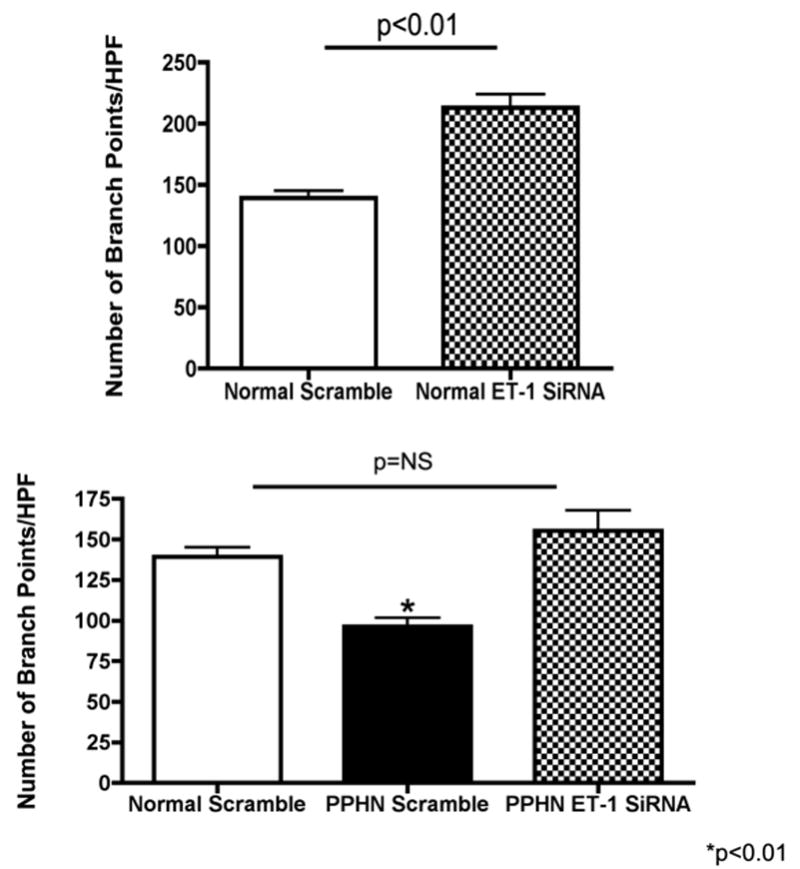
Figure 4a: Anti-ET-1 monoclonal antibody (ET-1mAb) increases tube formation in normal and PPHN fetal PAECs. Tube formation was assessed in normal and PPHN PAECs after exposure to ET-1mAb. ET-1mAb binds to ET-1 and prevents binding to ETa and ETb receptors. Tube length increased by 36% in normal and PPHN PAECs (p<0.01 for each comparison)(fig not shown) and number of branch points per HPF increased by 53% and 56% (p<0.01 for each comparison) in normal and PPHN PAECs respectively. Tube formation by PPHN fetal PAECs was restored to similar values seen in normal untreated controls.
Figure 4b: ET-1 SiRNA increases tube formation in normal and PPHN PAECs. PAECs from normal fetal sheep were treated with ET-1 SiRNA (4μM). ET-1 SiRNA decreased ET-1 mRNA expression by 50% and 54% in normal and PPHN PAECs. ET-1 SiRNA increaesd tube length by 32% and 46% (p<0.01 respectively)(fig not shown) and number of branch points per HPF by 53% and 61% (p<0.01 respectively) in normal and PPHN fetal PAECs. ET-1 SiRNA restored tube formation by PPHN fetal PAECs to similar values seen in normal untreated fetal PAECs.
Decreased ETB Receptor Protein By PAECs from PPHN Fetal Sheep
ETB receptor protein was measured in whole cell lysates from normal and PPHN PAECs (N=4 clones) by western blot analysis. ETB receptor protein expression was decreased by 48% (p<0.01) in PAECs from PPHN fetal sheep (Fig 5a). ETA receptor protein was detected in PASMC but not PAEC whole cell lysates demonstrating absent ETA receptor expression in PAECs (Fig 5b). 20μg of protein was loaded per lane and differences in β-actin protein expression were noted between PASMC and PAEC.
Figure 5.

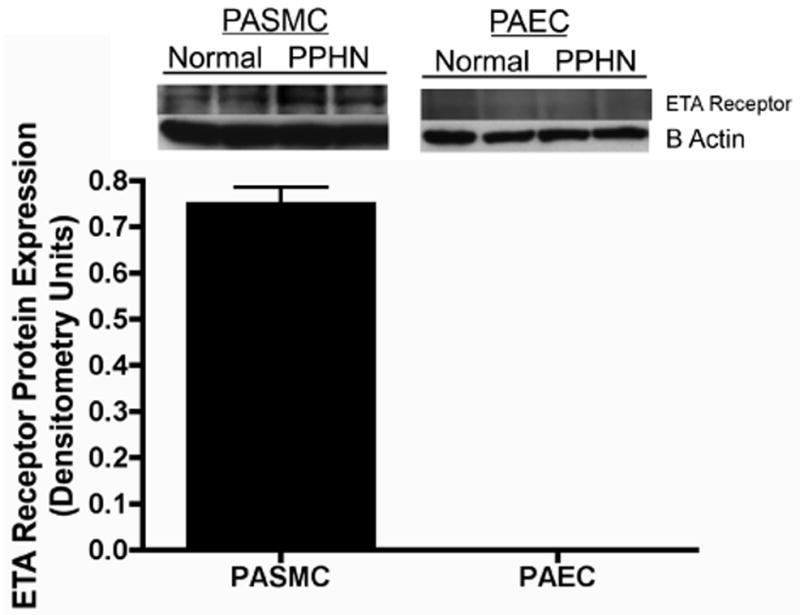
Figure 5a: Decreased ETB Receptor Protein By PAECs from PPHN Fetal Sheep. ETB receptor protein was measured in whole cell lysates from normal and PPHN PAECs (N=4 clones) by western blot analysis. ETB receptor protein expression was decreased by 48% (p<0.01) in PAECs from PPHN fetal sheep.
Figure 5b: Absent ET receptor A expression in normal and PPHN fetal PAECs. Western blot analysis was performed on whole cell lysates from normal and PPHN fetal PAECs (N=4 clones) and normal fetal PASMCs (N=4 clones). ETA receptor protein was detected in PASMC but not PAEC whole cell lysates demonstrating absent ETA receptor expression in PAECs (Fig 6a). 20μg of protein was loaded per lane and differences in β-actin protein expression were noted between PASMC and PAEC.
Effect of ETA and combined ETA/ETB receptor blockade on tube formation in normal and PPHN fetal PAECs
Tube formation was assessed in normal and PPHN PAECs in the presence of ETA receptor blockade (BQ-123 1μM) and combined ETA/ETB receptor blockade (bosentan 1μM) with and without ET-1 (100nM) treatment. BQ-123 alone had no effect on tube length (fig not shown) or number of branch points per HPF in normal and PPHN PAECs (Fig 6a). BQ-123 did not prevent the decrease in tube length and number of branch points per HPF with ET-1 treatment. Tube length was decreased by 49% (p<0.01) in normal and 26% (p<0.05) in PPHN PAECs (fig not shown) and branch points per HPF by 27% in normal and 34% in PPHN PAECs (Fig 6a) (p<0.05 for each comparison). Bosentan alone increased tube length by 35% and 42% (p<0.01 for each comparison) in normal and PPHN PAECs respectively (fig not shown). Number of branch points per HPF increased by 45% and 62% (p<0.01 for each comparison) in normal and PPHN PAECs respectively (Fig 6b). Bosentan prevented the decrease in tube length and number of branch points per HPF with ET-1 treatment. With bosentan and ET-1 treatment in combination, tube length was increased by 42% (p<0.01) in normal and 32% (p<0.05) in PPHN PAECs (fig not shown) and branch points per HPF by 48% in normal and 61% in PPHN PAECs (Fig6b)(p<0.05 for each comparison).
Figure 6.
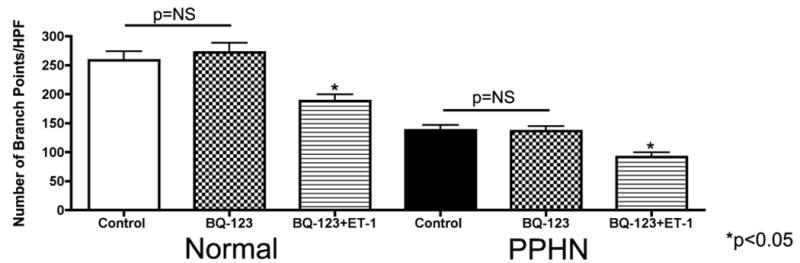
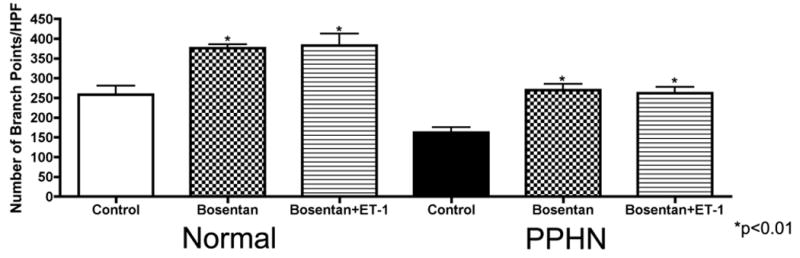
Figure 6a: Effect of ETA receptor blockade on tube formation in normal and PPHN fetal PAECs. Tube formation was assessed in normal and PPHN PAECs in the presence of ETA receptor blockade (BQ-123 1μM) with and without ET-1 (100nM) treatment. BQ-123 alone had no effect on tube length (data not shown) or number of branch points per HPF in normal and PPHN PAECs. BQ-123 did not prevent the decrease in tube length and number of branch points per HPF with ET-1 treatment. Tube length was decreased by 49% (p<0.01) in normal and 26% (p<0.05) in PPHN PAECs (data not shown) and branch points per HPF by 27% in normal and 34% in PPHN PAECs (p<0.05 for each comaprison).
Figure 6b: Effect of bosentan on tube formation in normal and PPHN fetal PAECs. Tube formation was assessed in normal and PPHN PAECs in the presence of non-selective ETA/ETB receptor blockade (bosentan 1μM) with and without ET-1 (100nM) treatment. Bosentan alone increased tube length by 35% and 42% (p<0.01 for each comparison) in normal and PPHN PAECs respectively (data not shown). Number of branch points per HPF increased by 45% and 62% (p<0.01 for each comparison) in normal and PPHN PAECs respectively. Bosentan prevented the decrease in tube length and number of branch points per HPF with ET-1 treatment. With bosentan and ET-1 treatment in combination, tube length was increased by 42% (p<0.01) in normal and 32% (p<0.05) in PPHN PAECs (data not shown) and branch points per HPF by 48% in normal and 61% in PPHN PAECs (p<0.05 for each comparison).
Effect of ET-1 and ET-1 SiRNA on rho-kinase activity in normal and PPHN fetal PAECs
Effect of ET-1 treatment (100nM) and ET-1 SiRNA (4μM) on rho-kinase activity was measured by MYPT-1 and phosphorylated-MYPT-1 (p-MYPT-1) protein by western blot analysis in normal and PPHN PAEC whole cell lysates (N=4 clones). Rho-kinase activity was increased by 65% in PPHN PAECs (p<0.01) and increased by 44% in normal and 55% in PPHN PAECs with ET-1 treatment (Fig 7a)(p<0.01 for each comparison). ET-1 SiRNA treatment decreased p-MYPT-1:MYPT-1 protein ratio by 35% in normal and PPHN PAECs. (Fig 7b)(p<0.01 for each comparison).
Figure 7.
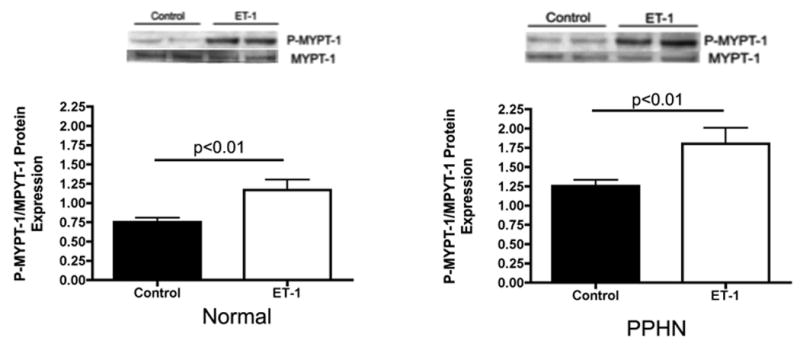

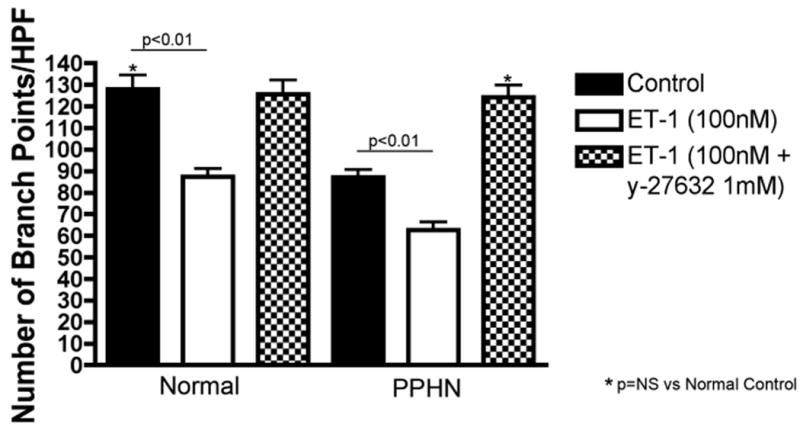
Figure 7a: ET-1 increases rho-kinase activity in normal and PPHN fetal PAECs. Effect of ET-1 treatment (100nM) on rho-kinase activity was measured by MYPT-1 and phosphorylated-MYPT-1 (p-MYPT-1) protein by western blot analysis in normal and PPHN PAEC whole cell lysates (N=4 clones). Rho-kinase activity as measured by p-MYPT-1:MYPT-1 protein ratio increased by 44% in normal and 55% in PPHN PAECs with ET-1 treatment (p<0.01 for each comparison).
Figure 7b: ET-1 SiRNA decreases rho-kinase activity in normal and PPHN PAECs. The effect of ET-1 SiRNA (4μM) on rho-kinase activity was measured by MYPT-1 and phosphorylated-MYPT-1 (p-MYPT-1) protein by western blot analysis in normal and PPHN PAEC whole cell lysates (N=4 clones). p-MYPT-1:MYPT-1 protein ratio decreased by 35% in normal and PPHN PAECs with ET-1 SiRNA treatment (p<0.01 for each comparison).
Figure 7c: Rho-kinase inhibition prevents the decreases in 3D tube formation by normal PAECs and rescues the abnormal in vitro PPHN phenotype after ET-1 treatment. 3D tube formation was assessed in normal and PPHN PAECs in the presence and absence of ET-1 treatment (100nM) with and without y-27632 (1μM) (rho-kinase inhibitor). After ET-1 treatment y-27632 increased tube length by 25% (p<0.05)(fig not shown) and branch points/HPF by 32% (p<0.01) in normal PAECs restoring tube formation to normal. In PPHN PAECs, y-27632 increased tube length by 75% (p<0.01)(fig not shown) and branch points/HPF by 95% (p<0.01) restoring tube formation to values seen in normal PAECs.
Effect of rho-kinase inhibition on tube formation after ET-1 treatment in normal and PPHN PAECs
Y-27632 blocked the effect of ET-1 treatment on 3D tube formation in both normal and PPHN PAECs. After ET-1 treatment, Y-27632 increased tube length by 25% (p<0.05) (fig not shown) and branch points/HPF by 32% (p<0.01) in normal PAECs, restoring tube formation to normal (Fig 7c). In PPHN PAECs, Y-27632 increased tube length by 75% (p<0.01) (fig not shown) and branch points/HPF by 95% (p<0.01), achieving similar values as measured in normal PAECs (Fig 7c). In the absence of ET-1, Y-27632 increased tube length by 13% (p<0.05) and 36% (p<0.01) and branch points/HPF by 18% (p<0.05) and 44% (p<0.01) in normal and PPHN PAECs, respectively (data not shown).
Effect of bosentan on rho-kinase activity in normal and PPHN PAECs
The effect of bosentan on rho-kinase activity was measured by MYPT-1 and phosphorylated-MYPT-1 (p-MYPT-1) protein by western blot analysis in normal and PPHN PAEC whole cell lysates (N=4 clones) with and without ET-1 (100nM) treatment. Bosentan decreased p-MYPT-1:MYPT-1 protein ratio by 39% (p<0.01) with ET-1 treatment and 41% (p<0.05) without ET-1 treatment in normal PAECs (Fig 8) and by 36% (p<0.01) with ET-1 treatment and by 32% (p<0.01) without ET-1 treatment in PPHN PAECs (Fig 8).
Figure 8. Effect of bosentan on rho-kinase activity in normal and PPHN PAECs.
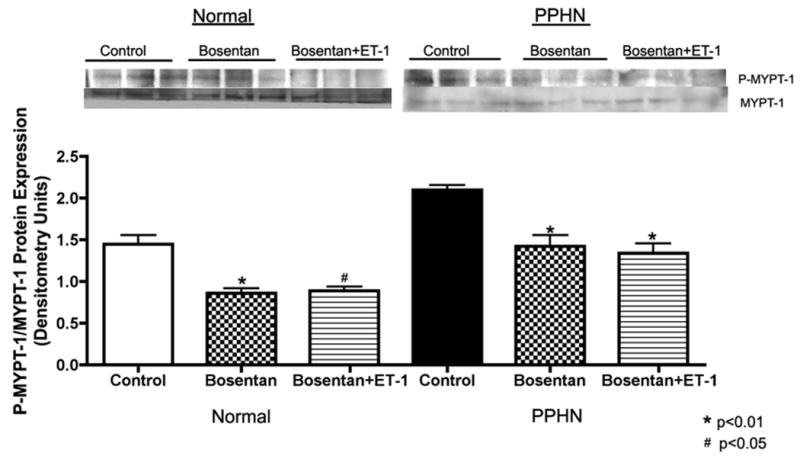
The effect of bosentan on rho-kinase activity was measured by MYPT-1 and phosphorylated-MYPT-1 (p-MYPT-1) protein by western blot analysis in normal and PPHN PAEC whole cell lysates (N=4 clones) with and without ET-1 (100nM) treatment. Bosentan decreased p-MYPT-1:MYPT-1 protein ratio by 39% (p<0.01) with ET-1 treatment and 41% (p<0.05) without ET-1 treatment in normal PAECs and by 36% (p<0.01) with ET-1 treatment and by 32% (p<0.01) without ET-1 treatment in PPHN PAECs.
Discussion
We report that ET-1 had no effect on PAEC growth, but ET-1 decreased tube formation in normal and PPHN fetal PAECs. In comparison with control PAEC, tube formation by PPHN PAECs was decreased. ET-1 protein and gene expression were increased and ETB receptor protein decreased in PPHN PAECs. Treatment of PPHN PAECs with ET-1 SiRNA, ET-1mAb and bosentan, but not BQ-123, increased tube formation. When compared with controls, ROCK activity was increased in PPHN PAECs, and was decreased with ET-1 SiRNA and bosentan treatments. These findings suggest that ET-1 impairs angiogenesis of fetal PAECs through ROCK activation, and that disruption of ET-1 ROCK interactions may improve endothelial dysfunction and increase vascular growth in severe PPHN.
In addition to increased vascular tone and marked hypertensive remodeling of the vascular wall, decreased arterial density also contributes to high PVR in severe PPHN, especially in the setting of lung hypoplasia (2,4,6,7,26). Past studies have shown that pulmonary hypertension during late gestation impairs fetal lung vascular growth in vivo (7), and causes abnormalities in PAECs that persist in vitro (6). The PAEC PPHN phenotype is characterized by decreased growth and tube formation, altered VEGF-NO signaling and increased ROCK activity (6,26). While these findings provide some insights into mechanisms responsible for impaired angiogenesis in severe PPHN, how sustained elevations in pulmonary arterial pressure inhibit lung angiogenesis during development is poorly understood. ET-1 contributes to the development of pulmonary hypertension, (8-10,13,14) but whether ET-1 regulates angiogenesis in the developing lung and contributes to impaired angiogenesis in PPHN has not been previously studied.
ET-1 has been implicated in the pathogenesis of diverse neonatal diseases. Newborn infants with meconium aspiration, respiratory distress syndrome (RDS), birth asphyxia and sepsis all have increased serum ET-1 levels, which correlate with disease severity (27-29). ET-1 also contributes to the development of bronchopulmonary dysplasia (BPD), a chronic lung disease of infancy characterized by decreased alveolar and vascular growth. In newborn infants with RDS, the degree of elevation in plasma ET-1 concentrations predicted higher risk for the subsequent development of BPD (28,29). Past work further suggests that lung fibrosis in BPD may also be ET-1 dependent (30). In newborn lambs with RDS and PPHN, serum ET-1 levels correlated with the degree of elevation in pulmonary artery pressure (31) and human newborns with severe PPHN have high circulating levels of ET-1 (15). In infants with CDH, plasma ET-1 levels predict disease severity and degree of pulmonary hypertension (3) and in infants who died from CDH lung ET-1 levels are also markedly increased (16). Overall, these studies suggest the importance of ET-1 in the pathogenesis of severe neonatal pulmonary hypertension, especially in the setting of lung hypoplasia. Our findings of increased ET-1 mRNA and protein expression in PPHN PAECs and striking improvement in PAEC tube formation with ET-1 inhibition further demonstrate the importance of ET-1 signaling pathways in the pathobiology of PPHN.
This is the first study to demonstrate that ET-1 decreases fetal lung vascular growth in experimental PPHN. The findings of decreased PAEC tube formation with exogenous ET-1 treatment and that ET-1 SiRNA increased tube formation in normal and PPHN PAECs suggests that ET-1 is responsible in part for impaired angiogenesis in PPHN. In contrast to our findings in the fetal lung, studies of tumor angiogenesis have demonstrated a pro-angiogenic role for ET-1, and that ET-1 increases tumor vascularity in lung cancer through ETA receptor activation (17). In addition, others have shown that ET-1 increases vascular network formation through ETB receptor activation in human umbilical vein endothelial cells (18). These findings differ from our study and may be explained in part by differences in cell type and developmental timing, or perhaps differential receptor activation.
A previous report has suggested that ETA receptor protein may be expressed in PAECs from adults with idiopathic pulmonary hypertension (32), However, although we could readily detect ETA receptor protein expression in fetal PA smooth muscle cells, we could not detect the ETA receptor in PAECs in our study. In addition, BQ-123, a selective ETA receptor blocker, had no affect on tube formation in PAECs and did not prevent the decrease in tube formation during ET-1 treatment. In contrast, the effects of ET-1 on both ROCK activation and tube formation were prevented by bosentan. These interesting findings support our speculation that ETB receptors may mediate ET-1 activation of ROCK and contribute to impaired tube formation in PPHN. While ET-1 activation of ETB receptors increases NO production, other published studies have shown that ET-1 increases reactive oxygen species (33) and thromboxaneA2 (TXA2) release (34) through ETB receptor activation. Both ROS and TXA2 are potent activators of ROCK (35,36) suggesting a putative mechanism linking ETB receptor stimulation with ROCK activation. We speculate that in the setting of neonatal pulmonary hypertension with impaired vascular growth, combined ETA/ETB receptor blockade may be more beneficial than selective ETA receptor blockade alone.
High ET-1 and ROCK activities contribute to high pulmonary vascular tone and myogenic reactivity in the normal fetus (11,12,21,22). With chronic intrauterine pulmonary hypertension, lung ET-1 gene and protein expression and ROCK activity are increased (13,26). However, links between these two potent cell-signaling pathways in neonatal pulmonary hypertension has not been previously studied. This current study reports that ET-1 treatment increases ROCK activity in fetal PAEC and that the adverse effects of ET-1 on tube formation are reversed by ROCK inhibition, suggesting that ET-1 is responsible for increased ROCK activity in PPHN PAEC and that ROCK mediates ET-1 induced reduction of tube formation in vitro. SiRNA knockdown of ET-1 increases tube formation in normal and PPHN PAECs and decreases ROCK activity suggesting that ET-1-ROCK interactions may be responsible for impaired angiogenesis in PPHN. In contrast with our findings in the fetal lung, the role of ROCK in the regulation of angiogenesis in chronic pulmonary hypertension is controversial. In adult rats with pulmonary hypertension due to chronic hypoxia, ROCK inhibition inhibits angiogenesis (37). These findings differ from our study and may be explained in part by differences between species or development timing.
Potential limitations of this study include the use of fetal PAECs harvested from relatively large vessels, and that differences may exist in the behavior of these cells as compared to microvascular PAECs. Since microvascular PAECs may primarily be involved in lung angiogenesis during development in vivo, future studies are needed to compare and contrast ET-1 ROCK interactions in microvascular PAECs to determine if similar cellular mechanisms are responsible for impaired micorvascular endothelial cell function. Another potential limitation is the fact that angiogenesis was only measured in vitro. Whether prolonged ET-1 or ROCK inhibition enhances angiogenesis in vivo remains unknown.
In conclusion, we found that ET-1 impairs angiogenesis in the developing lung through ROCK activation, and contributes to decreased vascular growth in severe PPHN. We speculate that treatment strategies that inhibit ET-1 production, block ETB receptor activity or disrupt ET-1-ROCK interactions in PPHN may enhance lung angiogenesis, which may be especially important in treating pulmonary hypertension in the presence of endothelial dysfunction and lung hypoplasia.
Methods
Isolation and culture of fetal ovine pulmonary arterial endothelial cells
All procedures and protocols were reviewed and approved by the Animal Care and Use Committee at the University of Colorado Health Sciences Center, Aurora, CO. The left and right pulmonary arteries were isolated from late-gestation normal fetal sheep (mixed-breed Columbia-Rambouillet pregnant ewes at 135 days gestation (n=4), term = 147 days) and from fetal sheep that had undergone partial ligation of the ductus arteriosus in utero 7-10 days prior to euthanasia (PPHN)(n=4), as previously described (5). Proximal PAECs were isolated and PAEC phenotype confirmed by cobblestone appearance and positive immunostaining for von Willebrands Factor (vWF), eNOS, vascular endothelial (VE)-cadherin. VEGF-R2 (KDR), positive uptake of ac-LDL and negative staining for desmin as previously described (6). Cells from passage 4 and 5 were used for the study experiments and cells from each animal were kept separate throughout all passages and experiments.
Cell growth
Fetal PAECs from normal and PPHN lambs were plated at 2 × 105 cells/well and allowed to adhere overnight. Cells were grown in DMEM media (Sigma, St. Louis, MO) supplemented with 5% FBS (Sigma, St. Louis, MO) under 3% oxygen conditions with and without ET-1 treatment. Daily cell counts were performed for 4 days using a hemocytometer.
3-D Tube Formation Assay
PAECs were grown to 90% confluence in T150 flasks, then trypsinized and 2.5 × 105 cells per well in 24 well plate resuspended in a collagen mix which is prepared by diluting bovine type 1 collagen (5mg/ml)(R&D Catalog # 3442-100-01, Minneapolis, MN) in sterile water for a 1.3mg/ml final concentration and 10x PBS with phenol red for quantification of pH. After mixing the collagen PAEC suspension, 0.5mls are added per well to a 24 well plate and the plate placed in the incubator for 1 hour to allow for polymerization. Various combinations of ET-1 (100nM)(Sigma, St Louis, MO), Y-27632 (1μM)(Cayman Chemical, Ann Arbor, MI), BQ-123 (1μM) (Sigma, St Louis, MO) and bosentan (1μM)(Actelion Pharmaceuticals, San Francisco, CA) were added, and media changed daily to achieve maximal tube formation. Tube formation was also assessed after exposure to ET-1 SiRNA and control SiRNA. Tube formation was quantified using Fovea 3 software analysis (Microsoft, Redmond, WA) by counting the number of branch points per high power field (HPF) and measuring tube length.
ET-1 Assay
ELISA was performed using the EIA for Endothelin-1 kit (Peninsula Laboratories, LLC # S-1157, San Carlos, CA). PAECs from control and PPHN fetal sheep were grown to 90% confluence in T-75 flasks and cell supernatant collected for solid phase extraction by Sep-Pak C18 columns (Peninsula Laboratories, LLC # Y-1000). Prior to loading on the column 0.25ml 2M HCL was added to each sample to acidify the solution. Solid phase extraction was performed and samples lyophilized overnight using a centrifugal concentrator. Purified lyophilized samples were resuspended in EIA buffer and ELISA was performed in triplicate per manufacturers recommendations. Differences in ET-1 protein between supernatant from normal and PPHN PAECs were measured and quantified.
ET-1 SiRNA
ET-1 siRNA purchased from Santa Cruz Biotechnology (Santa Cruz, CA)(sc-45394) and siRNA knockdown performed per manufacturers recommendations using control SiRNA (sc-3707) as a negative control.
RNA Isolation and Real Time PCR (RT-PCR)
RNA was isolated from 95% confluent PAECs using RLT buffer (Qiagen #79216, Valencia, CA) for lysis of cells and the RNAeasy minikit (Qiagen #74104, Valencia, CA) per manufacturers protocol. Reverse transcriptase reactions were prepared using 2 μg of RNA and Superscript III RT with random hexamers (Invitrogen, Carlsbad, CA). Real-time PCR primers and assays were optimized for ET-1 (Integrated DNA Technologies, Coralville, Iowa) (Forward AGGTTGGAGACCATCAGCAA, and reverse sequence AGCACGGCTGTAGATCACTT) and S15 (Integrated DNA Technologies, Coralville, Iowa). Real-time PCR was performed using 2ng of diluted cDNA and LightCycler 480 SYBR Green I Master (Roch 04707516001, Indianapolis, IN) on the Roche-Light Cycle 480 II real time PCR instrument (Roch, Indianapolis, IN). A relative standard curve of pooled PAEC lysate was generated (six standards prepared at 4-fold serial dilutions) and used for quantification of unknown sample expression. Results were adjusted to S15 expression and expressed relative to the average of the control for each gene.
Western Blot Analysis
PAECs from normal and PPHN fetal sheep were grown on 150mm cloning dishes in DMEM supplemented with 5% serum. At 70% confluence, PAECs were treated with ET-1 (100nM) with or without bosentan (1μM) for 24 hours. 70% confluence was chosen as prior studies have demonstrated downregulation of important cell signaling pathways with confluence (38). At time of harvest PAECs were 95% confluent. For ETA receptor antibody western blot analysis, fetal PASMCs were used as a positive control. Cell lysates were also collected after exposure to ET-1 SiRNA and control SiRNA. Cell lysates from PAECs for all experiments were collected as previously described (6,26). Protein content in the supernatant was determined by the BCA assay (Pierce Biotechnology Inc (catalog # 23225) Rockford, IL), using bovine serum albumin as the standard. 20 μg of protein sample per lane was resolved by SDS polyacrylamide gel electrophoresis, and proteins from the gel were transferred to nitrocellulose membrane. ETB receptor antibody (USA Biologicals # E3110, Boston, MA), ETA receptor antibody (USA Biologicals # E3100), MYPT-1 (#2634 Cell Signaling, Danvers, MA), Phospho-MYPT1 (Thr853) Antibody (#4563 Cell Signaling, Danvers, MA) were detected per manufactures protocol using appropriate controls and molecular weight as identified by the manufacturer for the protein of interest. Densitometry was performed using NIH Image (v1.61) and changes in protein expression were analyzed after normalizing for β-actin expression.
Statistical analysis
Data are presented as means ± SEM. Statistical analysis was performed with the Prism 4 software package (GraphPad Software, San Diego, CA). Statistical comparisons were made using analysis of variance for tube formation assays and western blot analysis using Bonferroni post test analysis. Unpaired t test was used for ELISA and RT PCR analysis. P < 0.05 was considered significant.
Acknowledgments
Statement of Financial Support 1) Entelligence Young Investigator Award, 2) National Institute of Health (NIH) 5K08HL102261. 3) NIH R01 HL068702-05A2
References
- 1.Levin DL, Heymann MA, Kitterman JA, et al. Persistent pulmonary hypertension of the newborn infant. J Pediatr. 1976;89:626–630. doi: 10.1016/s0022-3476(76)80405-2. [DOI] [PubMed] [Google Scholar]
- 2.Hopkins N, McLoughlin P. The structural basis of pulmonary hypertension in chronic lung disease: remodelling, rarefaction or angiogenesis? J Anat. 2002;201:335–348. doi: 10.1046/j.1469-7580.2002.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keller RL, Tacy TA, Hendricks-Munoz K, et al. Congenital diaphragmatic hernia: endothelin-1, pulmonary hypertension, and disease severity. Am J Respir Crit Care Med. 2010;182:555–61. doi: 10.1164/rccm.200907-1126OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldman AP, Tasker RC, Haworth SG, et al. Four patterns of response to inhaled nitric oxide for persistent pulmonary hypertension of the newborn. Pediatrics. 1996;98:706–713. [PubMed] [Google Scholar]
- 5.Abman SH, Shanley PF, Accurso FJ. Failure of postnatal adaptation of the pulmonary circulation after chronic intrauterine pulmonary hypertension in fetal lambs. J Clin Invest. 1989;83:1849–58. doi: 10.1172/JCI114091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gien J, Seedorf G, Balasubramaniam V, et al. Chronic intrauterine pulmonary hypertension impairs angiogenesis and growth of ovine fetal pulmonary artery endothelial cells in vitro. Am J Respir Crit Care Med. 2007;176:1146–1153. doi: 10.1164/rccm.200705-750OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grover TR, Parker TA, Balasubramaniam V, et al. Pulmonary hypertension impairs alveolarization and reduces lung growth in the ovine fetus. Am J Physiol Lung Cell Mol Physiol. 2005;288:L648–L654. doi: 10.1152/ajplung.00288.2004. [DOI] [PubMed] [Google Scholar]
- 8.Chen SJ, Chen YF, Chen QC, et al. Endothelin-receptor antagonist bosentan prevents and reverses hypoxic pulmonary hypertension in rats. J Appl Physiol. 1995;79:2122–2131. doi: 10.1152/jappl.1995.79.6.2122. [DOI] [PubMed] [Google Scholar]
- 9.Davie N, Haleen SJ, Upton PD, et al. ETA and ETB Receptors Modulate the Proliferation of Human Pulmonary Artery Smooth Muscle Cells. Am J Respir Crit Care Med. 2002;165:398–405. doi: 10.1164/ajrccm.165.3.2104059. [DOI] [PubMed] [Google Scholar]
- 10.Miyauchi T, Yorikane R, Sakai S, et al. Contribution of endogenous endothelin-1 to the progression of cardiopulmonary alterations in rats with monocrotaline-induced pulmonary hypertension. Circ Res. 1993;73:887–897. doi: 10.1161/01.res.73.5.887. [DOI] [PubMed] [Google Scholar]
- 11.Ivy DD, le Cras TD, Parker TA, et al. Developmental changes in endothelin expression and activity in the ovine fetal lung. Am J Physiol Lung Cell Mol Physiol. 2000;278:L785–93. doi: 10.1152/ajplung.2000.278.4.L785. [DOI] [PubMed] [Google Scholar]
- 12.Wong J, Fineman JR, Heymann MA. The role of endothelin and endothelin receptor subtypes in regulation of fetal pulmonary vascular tone. Pediatr Res. 1994;35:664–670. doi: 10.1203/00006450-199406000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Ivy DD, Dubus JW, Fox MF, et al. Chronic Intrauterine Pulmonary Hypertension Alters Endothelin Receptor Activity in the Ovine Fetal Lung. Pediatr Res. 1996;39:435–442. doi: 10.1203/00006450-199603000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Ivy DD, Parker TA, Ziegler JW, et al. Prolonged endothelin A receptor blockade attenuates chronic pulmonary hypertension in the ovine fetus. J Clin Invest. 1997;99:1179–86. doi: 10.1172/JCI119274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg AA, Kennaugh J, Koppenhafer SL, et al. Elevated immunoreactive endothelin-1 levels in newborn infants with persistent pulmonary hypertension. J Pediatr. 1993;123:109–14. doi: 10.1016/s0022-3476(05)81552-5. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi H, Puri P. Plasma endothelin levels in congenital diaphragmatic hernia. J Pediatr Surg. 1994;29:1258–1261. doi: 10.1016/0022-3468(94)90818-4. [DOI] [PubMed] [Google Scholar]
- 17.Zaho Y, Spingall D, Hamid P, et al. Localization and characterization of endothelin-1 receptor binding in the blood vessels of human pulmonary tumors. J Cardiovasc Pharmacol. 1995;26:9341–9345. [PubMed] [Google Scholar]
- 18.Salani D, Taraboletti G, Rosano L, et al. Endothelin-1 Induces an Angiogenic Phenotype in Cultured Endothelial Cells and Stimulates Neovascularization In Vivo. Am J Pathol. 2000;157:1703–11. doi: 10.1016/S0002-9440(10)64807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arbajal JM. RhoA inactivation enhances endothelial cell barrier function. RhoA inactivation enhances endothelial cell barrier function. Am J Physiol. 1999;277:C955–C964. doi: 10.1152/ajpcell.1999.277.5.C955. [DOI] [PubMed] [Google Scholar]
- 20.McMurtry IF, Fagan KA, Nagaoka T, et al. Hypoxia and Rho/Rho-kinase Signalling: Lung Development Versus Hypoxic Pulmonary Hypertension. Adv Exp Med Biol. 2003;543:127–137. [PubMed] [Google Scholar]
- 21.Parker TA, Roe G, Grover TR, et al. Rho kinase activation maintains high pulmonary vascular resistance in the ovine fetal lung. Am J Physiol Lung Cell Mol Physiol. 2006 Jun;291:L976–L982. doi: 10.1152/ajplung.00512.2005. 2006. Circ Res. 2007; 101:7-9. [DOI] [PubMed] [Google Scholar]
- 22.Tourneux P, Chester M, Grover T, et al. Fasudil inhibits the myogenic response in the fetal pulmonary circulation. Am J Physiol Heart Circ Physiol. 2008;295:H1505–13. doi: 10.1152/ajpheart.00490.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagaoka T, Casanova N, Bauer N, et al. Rho/Rho kinase signaling mediates increased basal pulmonary vascular tone in chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol. 2004;287:L665–667. doi: 10.1152/ajplung.00050.2003. [DOI] [PubMed] [Google Scholar]
- 24.Oka M, Taraseviciene-Stewart L, Morris KG, et al. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res. 2007;100:923–929. doi: 10.1161/01.RES.0000261658.12024.18. [DOI] [PubMed] [Google Scholar]
- 25.Homma N, Nagaoka T, Morio Y, et al. Endothelin-1 and serotonin are involved in activation of RhoA/Rho kinase signaling in the chronically hypoxic hypertensive rat pulmonary circulation. J Cardiovasc Pharmacol. 2007;50:697–702. doi: 10.1097/FJC.0b013e3181593774. [DOI] [PubMed] [Google Scholar]
- 26.Gien J, Seedorf GJ, Balasubramaniam V, et al. Chronic intrauterine pulmonary hypertension increases endothelial cell Rho kinase activity and impairs angiogenesis in vitro. Am J Physiol Lung Cell Mol Physiol. 2008;295:L680–7. doi: 10.1152/ajplung.00516.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Figueras-Aloy J, Gómez-Lopez L, Rodríguéz-Miguélez JM, et al. Plasma endothelin-1 and clinical manifestations of neonatal sepsis. J Perinat Med. 2004;32:522–6. doi: 10.1515/JPM.2004.126. [DOI] [PubMed] [Google Scholar]
- 28.El Sayed M, Sherif L, Said RN, et al. Endothelin-1 and L-arginine in preterm infants with respiratory distress. Am J Perinatol. 2011;2:129–36. doi: 10.1055/s-0030-1263295. [DOI] [PubMed] [Google Scholar]
- 29.Niu JO, Munshi UK, Siddiq MM, et al. Early increase in endothelin-1 in tracheal aspirates of preterm infants: correlation with bronchopulmonary dysplasia. J Pediatr. 1998;132:965–70. doi: 10.1016/s0022-3476(98)70392-0. [DOI] [PubMed] [Google Scholar]
- 30.Kambas K, Chrysanthopoulou A, Kourtzelis I, et al. Endothelin-1 signaling promotes fibrosis in vitro in a bronchopulmonary dysplasia model by activating the extrinsic coagulation cascade. J Immunol. 2011;186:6568–75. doi: 10.4049/jimmunol.1003756. [DOI] [PubMed] [Google Scholar]
- 31.de Vroomen M, Lopes Cardozo RH, Steendijk P, et al. Endothelin-1 plasma concentration increases in the early phase of pulmonary hypertension development during respiratory distress syndrome: a study in newborn lambs. Early Hum Dev. 2001;63:9–21. doi: 10.1016/s0378-3782(01)00143-8. [DOI] [PubMed] [Google Scholar]
- 32.Hall SM, Davie N, Klein N, et al. Endothelin receptor expression in idiopathic pulmonary arterial hypertension: effect of bosentan and epoprostenol treatment. Eur Respir J. 2011;38:851–860. doi: 10.1183/09031936.00167010. [DOI] [PubMed] [Google Scholar]
- 33.Dong F, Zhang X, Wold LE, et al. Endothelin-1 enhances oxidative stress, cell proliferation and reduces apoptosis in human umbilical vein endothelial cells: role of ETB receptor, NADPH oxidase and caveolin-1. Br J Pharmacol. 2005;145:323–333. doi: 10.1038/sj.bjp.0706193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D'Orleans-Juste P, Claing A, Telemaque S, et al. Block of endothelin-1-induced release of thromboxane A2 from the guinea pig lung and nitric oxide from the rabbit kidney by a selective ETB receptor antagonist, BQ-788. Br J Pharmacol. 1994;113:1257–1262. doi: 10.1111/j.1476-5381.1994.tb17133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jernigan NL, Walker BR, Resta TC. Reactive oxygen species mediate RhoA/Rho kinase-induced Ca2+ sensitization in pulmonary vascular smooth muscle following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2008;295:L515–29. doi: 10.1152/ajplung.00355.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin C, Goggel R, Ressmeyer AR, et al. Pressor responses to platelet-activating factor and thromboxane are mediated by Rho-kinase. Am J Physiol Lung Cell Mol Physiol. 2004;287:L250–L257. doi: 10.1152/ajplung.00420.2003. [DOI] [PubMed] [Google Scholar]
- 37.Hyvelin JM, Howell K, Nichol A, et al. Inhibition of Rho-kinase attenuates hypoxia-induced angiogenesis in the pulmonary circulation. Circ Res. 2005;97:185–91. doi: 10.1161/01.RES.0000174287.17953.83. [DOI] [PubMed] [Google Scholar]
- 38.Zöllner S, Aberle S, Harvey SE, et al. Changes of endothelial nitric oxide synthase level and activity during endothelial cell proliferation. Endothelium. 2000;7:169–84. doi: 10.3109/10623320009165315. [DOI] [PubMed] [Google Scholar]


