Abstract
A large number of perivascular cells expressing both macrophage and melanocyte characteristics (named perivascular-resident macrophage-like melanocytes, PVM/Ms), previously found in the intra-strial fluid–blood barrier, are also found in the blood–labyrinth barrier area of the vestibular system in normal adult cochlea, including in the three ampullae of the semicircular canals (posterior, superior, and horizontal), utricle, and saccule. The cells were identified as PVM/Ms, positive for the macrophage and melanocyte marker proteins F4/80 and GSTα4. Similar to PVM/Ms present in the stria vascularis, the PVM/Ms in the vestibular system are closely associated with microvessels and structurally intertwined with endothelial cells and pericytes, with a density in normal (unstimulated) utricle of 225 ± 43/mm2; saccule 191 ± 25/mm2; horizontal ampullae 212 ± 36/mm2; anterior ampullae 238 ± 36/mm2; and posterior ampullae 223 ± 64/mm2. Injection of bacterial lipopolysaccharide into the middle ear through the tympanic membrane causes the PVM/Ms to activate and arrange in an irregular pattern along capillary walls in all regions within a 48-h period. The inflammatory response significantly increases vascular permeability and leakage. The results underscore the morphological complexity of the blood barrier in the vestibular system, with its surrounding basal lamina, pericytes, as well as second line of defense in PVM/Ms. PVM/Ms may be important to maintain blood barrier integrity and initiating local inflammatory response in the vestibular system.
Keywords: mouse vestibular system, perivascular-resident macrophage-type melanocyte, inflammation, vascular permeability
Introduction
Perivascular-resident macrophages in the brain, retina, and other tissues (Cuadros and Navascues 1998; Kezic and McMenamin 2008; Guth et al. 2009; Brigitte et al. 2010; Yang et al. 2010) play a role in immunological defense and repair (Cui et al. 2009; Ekdahl et al. 2009). The resident macrophages scavenge invading microorganisms and dead cells (Mitrasinovic and Murphy 2002; Mitrasinovic et al. 2005; Nimmerjahn et al. 2005). The resident macrophages are also immune-effector cells that produce superoxide anions, nitric oxide, and inflammatory cytokines during inflammation (Block and Hong 2005; Block et al. 2007; Hanisch and Kettenmann 2007; Cheret et al. 2008).
A large number of perivascular cells which express both macrophage and melanocyte characteristics (perivascular-resident macrophage-like melanocytes, PVM/Ms) are previously found in the intra-strial fluid–blood barrier (Shi, 2010; Zhang et al. 2012). Using double-label fluorescent immunohistochemistry, combined with confocal microscopy, we also found a population of PVM/Ms in the vestibular system, including in horizontal, anterior, and posterior semicircular canal ampullae, utricle, saccule, and semicircular canal. In an earlier study, we demonstrated that PVM/Ms are a hybrid cell type, with morphology and function similar to astrocytes in the blood–brain barrier and glial cells in the blood–retina barrier (Zhang et al. 2012), playing an essential role in regulation of the intra-strial fluid–blood barrier integrity by affecting the expression of tight and adherens junction proteins. In the absence of PVM/Ms, the permeability of the endothelial cell monolayer markedly increases (Zhang et al. 2012). Expression of specific macrophage surface markers and scavenger receptors in strial PVM/Ms is also indicative of their contribution to maintenance and repair of the intra-strial fluid–blood barrier under conditions of tissue injury and inflammation (Shi 2010).
In this study, we characterized PVM/M phenotype, density, and function in the blood–labyrinth barrier of the normal adult vestibular system, particularly under inflammatory conditions. The cells were verified as PVM/Ms by positive immunolabeling for the specific cell surface makers, F4/80 and GSTα4. The vestibular PVM/Ms, like strial PVM/Ms, closely associated with microvessels and intertwined with endothelial cells and pericytes. The functional role of the vestibular PVM/Ms was especially apparent under inflammatory conditions. Injection with bacterial lipopolysaccharide (LPS) through the tympanic membrane in normal C57BL/6J adult mice caused activation and irregularities in the PVM/Ms, asymmetric positioning along capillary walls in all regions, and a significant increase vascular permeability and leakage within a 48-h period.
Materials and methods
Animals
Male C57BL/6J mice (aged 4–6 weeks, stock number 000664) were purchased from Jackson Laboratory (Bar Harbor, ME, USA). The protocol for the care and use of these animals was approved by the Institutional Animal Care and Use Committee at Oregon Health and Science University (IACUC approval number MU7_IS00001157).
Immunohistochemistry
In this study, mice were first deeply sedated with ketamine hydrochloride (100 mg/kg, intramuscularly) and 2 % xylazine hydrochloride (10 mg/kg, Abbott Laboratories, USA). The mice were perfused intravascularly via the left ventricle with phosphate-buffered saline (PBS, pH 7.4), followed by the fixative 4 % paraformaldehyde. The mice were then sacrificed by decapitation, and their cochleae were harvested. The cochleae were then perilymphatically perfused with the same fixative and the vestibular system tissues isolated and placed in PBS with 4 % paraformaldehyde overnight. After being washed in PBS, tissue samples were permeabilized in 0.5 % Triton X-100 (Sigma, USA) for 1 h and immunoblocked with a solution of 10 % goat serum (alternatively rat or rabbit serum) and 1 % bovine albumin in 0.02 mol/L PBS for an additional hour. The specimens were incubated overnight at 4 °C with the primary antibodies (Table 1) diluted in PBS-bovine serum albumin (BSA). After several washes in PBS, the samples were incubated with secondary antibodies (Table 1) for 1 h at room temperature. Capillaries were labeled with lectin Griffonia simplicifolia IB4 (GS-IB4) conjugated to Alexa Fluor 568. The tissues were washed for 30 min, mounted (H-1000, Vector Laboratories, USA), and visualized under an FV1000 Olympus laser-scanning confocal microscope. Controls were prepared by replacing primary antibodies with overnight incubation in PBS–BSA.
TABLE 1.
Primary and second antibodies employed
| Antibodies | Vectors | Identification | Dilution | Source | Specificity |
|---|---|---|---|---|---|
| GSTα4 | Santa Cruz Biotechnology, Santa Cruz, CA | Cat# sc-241483 | 1:50 (dilution with 1 % BSA–PBS) | Goat polyclonal antibody | Reacts with human, mouse, bovine, and porcine |
| F4/80 | San Diego, CA | Cat# 14-4801-85 | 1:50 (dilution with 1 % BSA–PBS) | Rat monoclonal antibody | Reacts with mouse |
| Desmin | Abcam, Cambridge, MA | Cat# Ab32362 | 1:100 (dilution with 1 % BSA–PBS) | Rabbit monoclonal antibody | Reacts with mouse, rat, guinea pig, and human |
| Collagen IV | Abcam, Cambridge, MA | Cat# Ab6586 | 1:100 (dilution with 1 % BSA–PBS) | Rabbit polyclonal antibody | Reacts with mouse, rat, cow, human, and pig |
| Isolectin GS-IB4-conjugated Alexa Fluor 568 conjugate | Invitrogen, Eugene, OR | Cat# 121411 | 1:100 (dilution with 1 % BSA–PBS) | Griffonia simplicifolia, | Reacts with mouse and bovine |
| Alexa Fluor 488-conjugated goat anti-rabbit IgG | Invitrogen, Eugene, OR | Cat# A-11008 | 1:100 (dilution with 1 % BSA–PBS) | Goat | Reacts with rabbit |
| Alexa Fluor 647-conjugated goat anti-rabbit IgG | Invitrogen, Eugene, OR | Cat# A-21245 | 1:100 (dilution with 1 % BSA–PBS) | Goat | Reacts with rabbit |
| Alexa Fluor 488-conjugated goat anti-rat IgG | Invitrogen, Eugene, OR | Cat# A-11006 | 1:100 (dilution with 1 % BSA–PBS) | Goat | Reacts with rat |
| Alexa Fluor 647-conjugated goat anti-rat IgG | Invitrogen, Eugene, OR | Cat# A-21247 | 1:100 (dilution with 1 % BSA–PBS) | Goat | Reacts with rat |
| Alexa Fluor 488-conjugated rabbit anti-rat IgG | Invitrogen, Eugene, OR | Cat# A-21210 | 1:100 (dilution with 1 % BSA–PBS) | Rabbit | Reacts with rat |
| Alexa Fluor 568-conjugated rabbit anti-goat IgG | Invitrogen, Eugene, OR | Cat# A-11079 | 1:100 (dilution with 1 % BSA–PBS) | Rabbit | Reacts with goat |
Assessment of vascular permeability
Vascular permeability in control and LPS-treated cohorts was assessed using a fluorescein isothiocyanate (FITC)-conjugated bovine albumin tracer (FITC-albumin, ∼66 kDa, A-9771, Sigma, USA). The tracer was intravenously administered to the tail vein of anesthetized animals 30 min prior to harvesting. Anesthetized animals were perfused intravascularly through the left ventricle with Hank's balanced salt solution (HBSS). The mice were decapitated and whole mounts of the vestibular system imaged on a fluorescent microscope (Leica DM2500, Germany).
Vascular leakage in each group receiving FITC-albumin was quantitatively analyzed. The mice were anesthetized and perfused intravascularly for 5 min with HBSS. The vestibular system was then removed, homogenized in 1 % Triton X-100 in PBS, and the lysate centrifuged at 16,000 rpm for 20 min. Relative fluorescence of the supernatant was measured on a Tecan GENios Plus Microplate Reader (Tecan Group Ltd, USA), with samples for each group run in quintuplicate. Data were represented as means ± SD.
For in situ detection, Alexa Fluor 568-conjugated goat anti-human IgG (molecular mass, 200 kDa) (A-21090, Invitrogen, USA) was administered by i.v. for 2 h. Anesthetized animals were perfused intravascularly through the left ventricle for 2 min with HBSS followed by 5 min of 4 % paraformaldehyde (PFA) in PBS. The vestibular system from each group was removed and post-fixed overnight with 4 % PFA in PBS, pH 7.2, at 4 °C. Whole-mounted parts of the vestibular system were immunolabeled with anti-collagen IV antibody (Table 1). Tissue samples were imaged with an FV1000 Olympus laser-scanning confocal microscope.
LPS treatment
Animals in the LPS group were trans-tympanically injected with 10 μl emulsion of LPS in 0.9 % sodium chloride (LPS 5 mg/ml, Sigma, USA). The LPS was injected into the middle ear cavity of the left ear for 48 h.
Counts of F4/80- and GSTα4-positive cells
Cells labeled with antibody for F4/80 or cells co-labeled with antibodies for F4/80 and GSTα4 in the vestibular system of control and LPS-treated mice (cohorts of five mice) were counted on a standard epifluorescence microscope with a ×20 objective. Counts were obtained for ten randomly chosen, non-overlapping 150 × 300 μm areas of each group, and the data were represented as means ± SD.
Counts of F4/80- and GS-IB4-positive cells
The percentage of active PVM/Ms was determined for whole mounts of semicircular canal ampullae, utricle, and saccule on control and LPS-treated groups (cohorts of five mice). Cells double labeled for F4/80 (green) and GS-IB4 (red) were counted on a fluorescence confocal microscope with a ×20 objective. The percentage of double-labeled cells was identified by color: green for cells showing green only labeling for F4/80 and yellow for cells positive for both markers F4/80 and GS-IB4. The percentage was calculated as:
Statistics
All statistical analyses were performed using SPSS 17.0. Data, presented as means ± SD, were evaluated using Student’s t test for group comparisons. A 95 % confidence level was considered statistically significant.
Results
Identification of perivascular-resident macrophages in the vestibular system
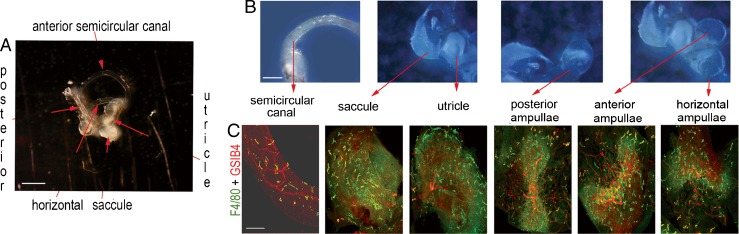
The peripheral vestibular system consists of different end organs including the semicircular canals, utricle, saccule, and three ampullae (Fig. 1A, B). Using fluorescent immunohistochemical labeling for F4/80, combined with confocal microscopy, we found a population of dendrite-shaped resident macrophages with ramified processes (green) distributed in different regions of the vestibular system of normal adult cochlea, but particularly concentrated in capillary regions labeled by GS-IB4 (red) (Fig. 1C).
FIG. 1.
Perivascular-resident macrophage in the vestibular system. A shows a macroview of an isolated mouse vestibular system. The scale bar is 1,000 μm. B Different parts of the normal vestibular system, including the horizontal, anterior, and posterior semicircular canal ampullae, utricle, saccule, and semicircular canal are photographically portrayed. The scale bar is 500 μm. C PVM/Ms in different parts of the normal vestibular system are labeled with antibody for F4/80 (green), with capillaries labeled by GS-IB4 (red). The scale bar is 100 μm.
Perivascular-resident macrophages identified as melanocytes
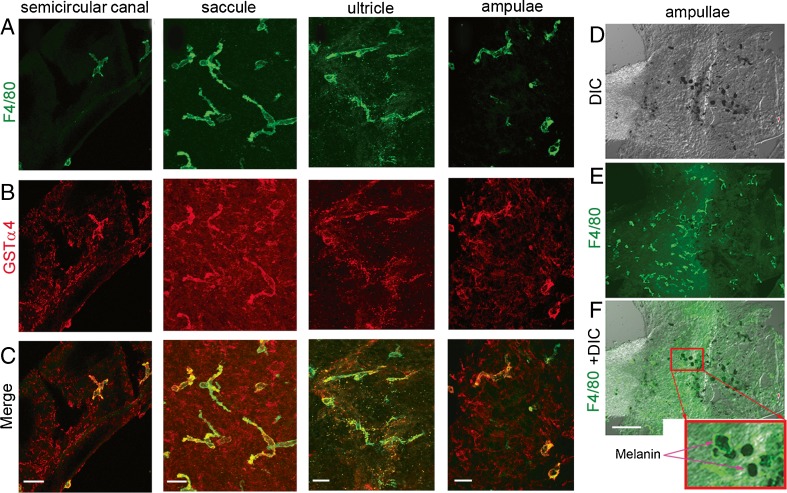
Double-label immunofluorescence showed F4/80-positive cells to express melanocyte marker protein, including cytosolic GSTα4 (Fig. 2A–C). The resident macrophages contain melanin pigment granules in the cytoplasm (Fig. 2D–F). The data indicate that resident macrophages are a hybrid cell type, differing from the classical macrophage, having both macrophage and melanocyte characteristics, thus the moniker, perivascular-resident macrophage-like melanocytes.
FIG. 2.
Perivascular-resident cells identified as melanocyte-like macrophages. A–C PVM/Ms double labeled with antibody for F4/80 and GSTα4 show positive staining for macrophage and melanocyte marker proteins F4/80 (green) and GSTα4 (red). The scale bar is 50 μm. D–F PVM/Ms contain melanin pigment granules. The column-reticular square region in panel F shows melanin granules under a high magnification. The scale bar is 100 μm.
Perivascular-resident macrophage-like melanocytes are in close contact with capillaries
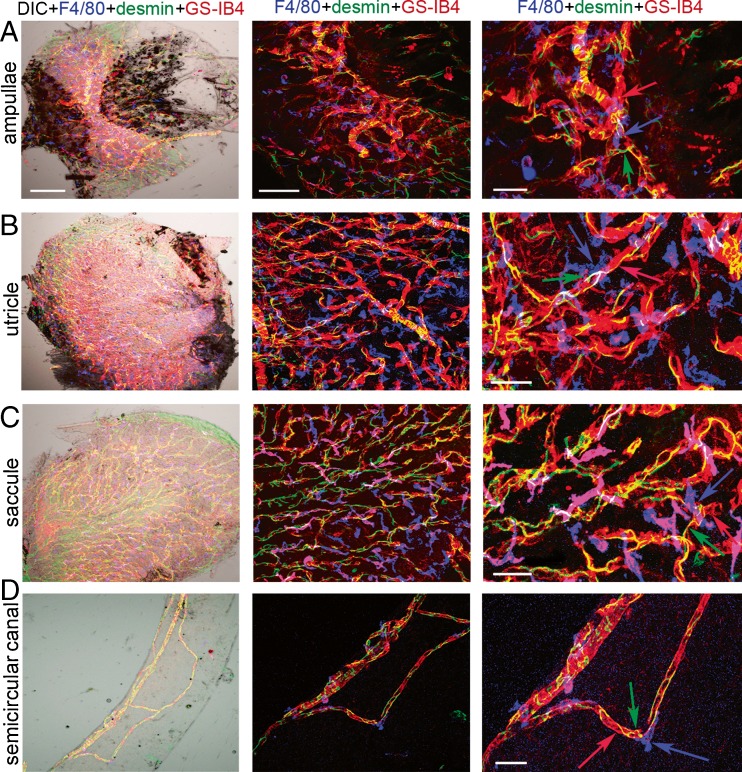
The PVM/Ms, highly invested on the abluminal surface of capillaries and structurally intertwined with endothelial cells, have dendritic processes that interface with the capillary wall. Whole-mounted semicircular canal ampullae, utricle, saccule, and semicircular canal triple labeled for F4/80, desmin, and GS-IB4 to show PVM/Ms (blue) closely associated with pericytes (green) and endothelial cells (red). The three cell types structurally intertwine. The left panels of Figure 3 show the distribution of the three cell types under low-magnification differential interference contrast (DIC) and confocal fluorescence microscopy. The middle and right panels of Figure 3 are low- and high-magnification confocal images which show PVM/Ms adjacently associated with pericytes and endothelial cells.
FIG. 3.
PVM/Ms in the vestibular system are closely associated with microvessels and structurally intertwined with endothelial cells and pericytes. A–D (left panels) Whole-mounted semicircular canal ampullae, utricle, saccule, and semicircular canal triple labeled for F4/80 (blue), desmin (green), and GS-IB4 (red) in low magnification (×20) confocal and DIC images show PVM/Ms, pericytes, and endothelial cells. The scale bar is 100 μm. A–D (middle panels) The confocal image under higher magnification (×40) shows the interaction between the three cell types. The scale bar is 50 μm. A–D (right panels) The zoomed image shows the three cell types to structurally intertwine. The scale bar is 30 μm.
LPS treatment induces inflammation
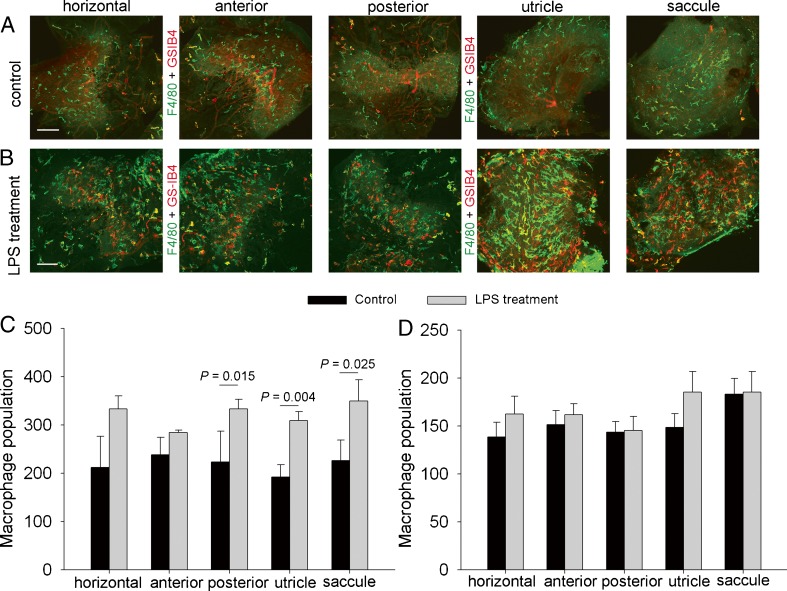
The density of F4/80-positive cells in normal (unstimulated) utricle was 225 ± 43/mm2; saccule 191 ± 25/mm2; horizontal ampullae 212 ± 36/mm2; anterior ampullae 238 ± 36/mm2; and posterior ampullae 223 ± 64 /mm2 (Fig. 4A). Injection of LPS (5 mg/ml) into the middle ear through the tympanic membrane causes a dramatic and statistically significant increase in F4/80-positive cells in all regions within a 48-h period (Fig. 4B). Comparison of F4/80-positive cells in the control and LPS-treated groups is shown in Figure 4C. However, it should also be remarked that approximately 20–40 % of the F4/80-positive cells are not positive for melanocyte marker proteins GSTα4 (Fig. 4D), suggesting macrophage infiltration into the vestibular system due to the inflammation.
FIG. 4.
LPS treatment causes inflammation. A, B Whole-mounted semicircular canal ampullae, utricle, and saccule double labeled for F4/80 (green) and GS-IB4 (red) in the control (A) and LPS-treated groups (B). The scale bar is 100 μm. C, D F4/80-positive macrophage population in whole-mounted semicircular canal ampullae, utricle, and saccule is significantly increased with LPS treatment [C, n = 5, P (control vs LPS treatment/posterior ampullae) = 0.015; P (control vs LPS treatment/utricle) = 0.004; P (control vs LPS treatment/saccule) = 0.025]. The identified PVM/Ms do not show the same level of cell number response to LPS as macrophages (D, n = 5, P (control vs LPS treatment in all regions) > 0.05).
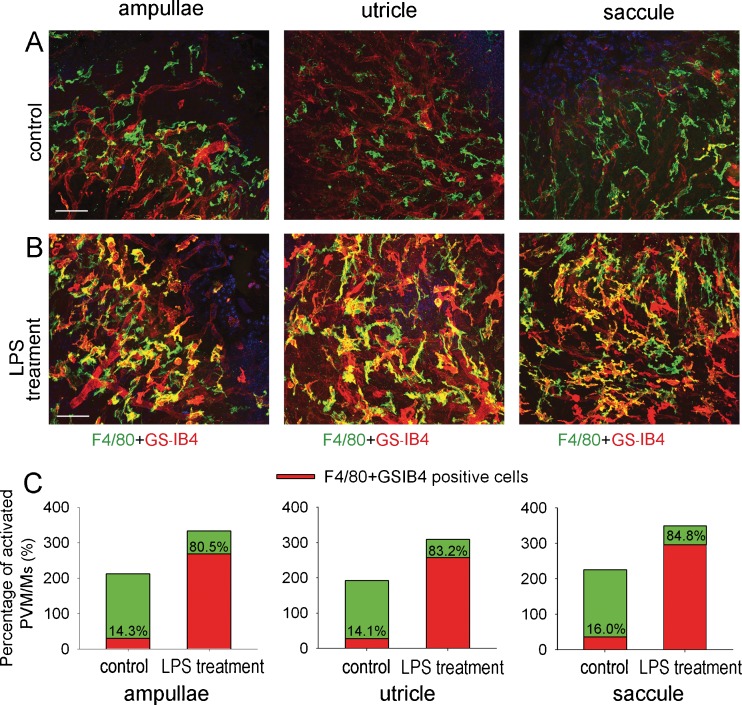
LPS treatment causes PVM/M activation
Activated macrophages display terminal galactopyranosyl group on their membrane surface which binds GS-IB4. Enhanced affinity and binding of lectins to GS-IB4 is well-developed as a marker for macrophage activation (Takacs and Staehli 1987; Tabor et al. 1989). In control tissue, most PVM/Ms do not bind GS-IB4 (Fig. 5A), and only 14–16 % are positively labeled. However, a significantly increased proportion of PVM/Ms are GS-IB4 positive in the LPS-treated animals (Fig. 5B), with 80 to 84 % of PVM/Ms in treated tissues GS-IB4 positive (Fig. 5C).
FIG. 5.
LPS treatment causes activation of PVM/Ms. A, B The PVM/Ms are activated by LPS treatment. Double-labeled whole-mounted ampullae, saccule, and utricle show PVM/Ms labeled with antibody for F4/80 also positive for GS-IB4 in the LPS-treated groups. The scale bar is 50 μm. C, Percentage of activated PVM/Ms in ampullae, utricle and saccule" after 50 um.
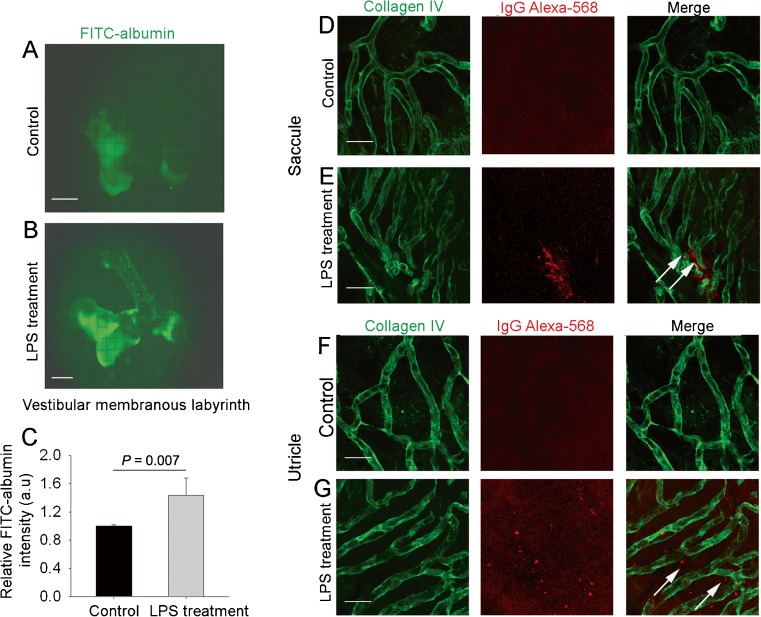
LPS treatment increases vascular leakage
Under normal conditions, the blood–fluid barrier restricts movement of large molecules, and to a lesser extent small molecules, into the ear (Fig. 6A). The barrier becomes highly permeable to large substances such as albumin-FITC and IgG Alexa Fluro-568 with 48 h of LPS treatment. When albumin-FITC is administered through the animal’s tail vein 30 min prior to harvesting, fluorescent tracer accumulation is seen in isolated whole mounts of the vestibular system from leakage of the albumin-FITC (∼66 kDa) (Fig. 6B). LPS-treated animals show significant leakage of albumin-FITC across the fluid–blood barrier (Fig. 6C). Immunohistochemical staining in combination with confocal microscopy shows extravasation of high molecular weight IgG Alexa Fluro-568 tracers in tissues of LPS-treated animals (Fig. 6D–G). The data show inflammation to cause increased vascular permeability.
FIG. 6.
LPS treatment increases vascular leakage. A, B Intravenously injected tracer (FITC-albumin, MW = 66 kD) accumulates throughout the entire vestibular system of LPS-treated animals (B), but not in controls (A). C Capillary leakage of FITC-albumin is significant in LPS-treated mice [n = 6, P (control vs LPS treatment) = 0.007]. D–G Extravasation of intravenously injected IgG Alexa 568 (white arrows) from capillaries, visualized by collagen IV immunostaining (green), was observed in LPS-treated mice, but not in controls. The scale bar is 50 μm.
Discussion
This study shows the vestibular system to contain a large population of PVM/Ms. By confocal imaging, the PVM/Ms are shown to be closely associated with microvessels and structurally intertwined with endothelial cells and pericytes. PVM/Ms in all regions of the vestibular system show marked activation and rearrangement into an irregular pattern with LPS treatment. The concurrent inflammatory response significantly increases vascular permeability and leakage.
In a previous study, we found a large population of perivascular cells in the vicinity of the intra-strial fluid–blood barrier in the stria vascularis of normal adult cochlea. The cells were identified as perivascular-resident macrophages, positive for several macrophage surface molecules including F4/80, CD68, and CD11b (Shi 2010). Further study demonstrated the PVM/Ms to be a hybrid cell type, differing from the classical macrophage. In the present study, we have expanded our study to include the vestibular system and have investigated the morphological complexity of the blood barrier in the vestibular system, including its surrounding basal lamina, pericytes, as well as second line of defense in PVM/Ms.
Resident dendritic cells or macrophages in most tissues have a physiological role which supports homeostasis (Lech et al. 2012). Perivascular-resident macrophages are prevalent in the brain, retina, lung, and intestine (Cuadros and Navascues 1998; Kezic and McMenamin 2008; Guth et al. 2009; Brigitte et al. 2010; Yang et al. 2010) where they have several important homeostatic roles. For example, macrophages are specialized for phagocytic clearance of airborne and ingested pathogens in the lung (Guth et al. 2009; Soroosh et al. 2013) and liver (Yang et al. 2012).
The phenotype and density of mononuclear phagocytes in different tissues vary widely, paralleling the wide range of homeostatic requirements in different tissues. In a normal animal ear vestibular system, PVM/Ms are a hybrid cell type, expressing macrophage and melanocyte marker proteins, including F4/80 and cytosolic GSTα4 (Fig. 2), and containing melanin pigment granules in the cytoplasm. Density of the cells across different regions of the vestibular system ranges between 191 and 238 ± 36/mm2. The PVM/Ms are found invested on the abluminal surface of capillaries and intertwined with endothelial cells and dendritic processes at the interface with the capillary wall.
Tissue-resident macrophages are mobilized with trauma, as well as by pathogens and toxic insults to tissue homeostasis (Kharraz et al. 2013). In this study, injection of bacterial LPS into the middle ear through the tympanic membrane causes a dramatic increase in infiltration of inflammatory cells (with a significant increase in F4/80-positive cells). The PVM/Ms display marked morphological changes with increased binding to GS-IB4. High fluorescence signals for Alexa Fluor-conjugated GS-IB4 are seen in LPS-treated PVM/Ms (Fig. 5), suggesting the PVM/Ms harbor a terminal galactopyranosyl group on their membrane surface (Maddox et al. 1982). Enhanced affinity for binding this lectin is well recognized as a marker to indicate the degree of macrophage responsiveness with exposure to various stimuli (Adams and Hamilton 1984; Tabor et al. 1989). Consistent with Maddox et al. (1982), we show that activated macrophages express terminal a-linked galactopyranosyl residues on their surface which, conversely, are not found on normal unstimulated macrophages (Fig. 5). Local phagocyte activation augments homeostasis, and the activation of PVM/Ms in the vascular wall of the blood–labyrinth barrier may be the primary contributing factor in local inflammatory response.
PVM/Ms are in close contact with vessels in the intra-strial barrier. Like astrocytes and glial cells in the brain, PVM/Ms in the cochlea have an essential role in regulating barrier integrity and performing other tissue-oriented functions (Prat et al. 2001; Abbott et al. 2006). PVM/Ms in the intra-strial fluid–blood barrier affect membrane integrity by regulating synthesis of tight junction proteins, and loss of PVM/Ms is associated with tissue edema (Zhang et al. 2012). PVM/Ms produce pigment epithelium growth factor, a 50-kDa glycoprotein first identified in retinal pigment epithelium cells (Liu et al. 2012), which plays a role in the stria vascularis as an essential signaling molecule for stabilizing the intra-strial fluid–blood barrier and establishing a normal hearing threshold (Zhang et al. 2012). The role of PVM/Ms in the blood barrier of the vestibular system may be similar to their role in the intra-strial fluid–blood barrier. LPS treatment significantly increases vascular permeability and leakage in all regions and causes irregular arrangement of PVM/Ms along capillary walls within a 48-h period.
Change in vascular architecture is known to be correlated with blood flow and vascular stability (Duvall and Robinson 1987). The rearrangement of PVM/Ms caused by the introduction of LPS toxin into the blood–labyrinth barrier may be related to the increased vascular leakage. Further studies are needed to identify the role of PVM/Ms in blood barrier permeability and for delineating the mechanisms of various inflammatory mediators and bacterial toxins in inducing vascular leakage.
Conclusion
A large number of PVM/Ms are found in the area of the blood–labyrinth barrier of the vestibular system in normal adult cochlea, including in the three ampullae of the semicircular canals (posterior, superior, and horizontal), utricle, and saccule. The cells are identified as PVM/Ms, as they are positive for the macrophage and melanocyte marker proteins, F4/80 and GSTα4. Similar to PVM/Ms present in the stria vascularis, the PVM/Ms in the vestibular system are closely associated with microvessels and structurally intertwined with endothelial cells and pericytes. Tympanic injection of bacterial LPS into the middle ear causes dramatic infiltration of macrophages and irregular rearrangement of resident macrophages (PVM/Ms) along capillary walls within a 48-h period. The inflammatory response significantly increases vascular permeability and leakage. The results indicate PVM/Ms in the vascular wall of the blood–labyrinth barrier may be important factors in maintaining blood barrier integrity and initiating local inflammatory response in the vestibular system.
Acknowledgments
This work was supported by the National Institutes of Health grants NIH NIDCD R01-DC010844 (XS), DC R21DC1239801 (XS), and NIHP30-DC005983.
Contributor Information
Fei Zhang, Email: zhangfe@ohsu.edu.
Jinhui Zhang, Email: zhangjin@ohsu.edu.
Lingling Neng, Email: neng@ohsu.edu.
Xiaorui Shi, Phone: +1-503-4947149, FAX: +1-503-4945656, Email: shix@ohsu.edu.
References
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood–brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Adams DO, Hamilton TA. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Brigitte M, Schilte C, Plonquet A, Baba-Amer Y, Henri A, Charlier C, Tajbakhsh S, Albert M, Gherardi RK, Chretien F. Muscle resident macrophages control the immune cell reaction in a mouse model of notexin-induced myoinjury. Arthritis Rheum. 2010;62:268–279. doi: 10.1002/art.27183. [DOI] [PubMed] [Google Scholar]
- Cheret C, Gervais A, Lelli A, Colin C, Amar L, Ravassard P, Mallet J, Cumano A, Krause KH, Mallat M. Neurotoxic activation of microglia is promoted by a nox1-dependent NADPH oxidase. J Neurosci. 2008;28:12039–12051. doi: 10.1523/JNEUROSCI.3568-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadros MA, Navascues J. The origin and differentiation of microglial cells during development. Prog Neurobiol. 1998;56:173–189. doi: 10.1016/S0301-0082(98)00035-5. [DOI] [PubMed] [Google Scholar]
- Cui Q, Yin Y, Benowitz LI. The role of macrophages in optic nerve regeneration. Neuroscience. 2009;158:1039–1048. doi: 10.1016/j.neuroscience.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvall AJ, 3rd, Robinson KS. Local vs systemic effects of acoustic trauma on cochlear structure and transport. Arch Otolaryngol Head Neck Surg. 1987;113:1066–1071. doi: 10.1001/archotol.1987.01860100044019. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience. 2009;158:1021–1029. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- Guth AM, Janssen WJ, Bosio CM, Crouch EC, Henson PM, Dow SW. Lung environment determines unique phenotype of alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2009;296:L936–L946. doi: 10.1152/ajplung.90625.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Kezic J, McMenamin PG. Differential turnover rates of monocyte-derived cells in varied ocular tissue microenvironments. J Leukoc Biol. 2008;84:721–729. doi: 10.1189/jlb.0308166. [DOI] [PubMed] [Google Scholar]
- Kharraz Y, Guerra J, Mann CJ, Serrano AL, Munoz-Canoves P. Macrophage plasticity and the role of inflammation in skeletal muscle repair. Mediat Inflamm. 2013;2013:491497. doi: 10.1155/2013/491497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lech M, Grobmayr R, Weidenbusch M, Anders HJ. Tissues use resident dendritic cells and macrophages to maintain homeostasis and to regain homeostasis upon tissue injury: the immunoregulatory role of changing tissue environments. Mediat Inflamm. 2012;2012:951390. doi: 10.1155/2012/951390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JT, Chen YL, Chen WC, Chen HY, Lin YW, Wang SH, Man KM, Wan HM, Yin WH, Liu PL, Chen YH. Role of pigment epithelium-derived factor in stem/progenitor cell-associated neovascularization. J Biomed Biotechnol. 2012;2012:871272. doi: 10.1155/2012/871272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox DE, Shibata S, Goldstein IJ. Stimulated macrophages express a new glycoprotein receptor reactive with Griffonia simplicifolia I-B4 isolectin. Proc Natl Acad Sci U S A. 1982;79:166–170. doi: 10.1073/pnas.79.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrasinovic OM, Murphy GM., Jr Accelerated phagocytosis of amyloid-beta by mouse and human microglia overexpressing the macrophage colony-stimulating factor receptor. J Biol Chem. 2002;277:29889–29896. doi: 10.1074/jbc.M200868200. [DOI] [PubMed] [Google Scholar]
- Mitrasinovic OM, Grattan A, Robinson CC, Lapustea NB, Poon C, Ryan H, Phong C, Murphy GM., Jr Microglia overexpressing the macrophage colony-stimulating factor receptor are neuroprotective in a microglial-hippocampal organotypic coculture system. J Neurosci. 2005;25:4442–4451. doi: 10.1523/JNEUROSCI.0514-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Prat A, Biernacki K, Wosik K, Antel JP. Glial cell influence on the human blood–brain barrier. Glia. 2001;36:145–155. doi: 10.1002/glia.1104. [DOI] [PubMed] [Google Scholar]
- Shi X. Resident macrophages in the cochlear blood-labyrinth barrier and their renewal via migration of bone-marrow-derived cells. Cell Tissue Res. 2010;342:21–30. doi: 10.1007/s00441-010-1040-2. [DOI] [PubMed] [Google Scholar]
- Soroosh P, Doherty TA, Duan W, Mehta AK, Choi H, Adams YF, Mikulski Z, Khorram N, Rosenthal P, Broide DH, Croft M. Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. J Exp Med. 2013;210:775–788. doi: 10.1084/jem.20121849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor DR, Larry CH, Jacobs RF. Differential induction of macrophage GSIB4-binding activity. J Leukoc Biol. 1989;45:452–457. doi: 10.1002/jlb.45.5.452. [DOI] [PubMed] [Google Scholar]
- Takacs B, Staehli C. Activated macrophages and antibodies against the plant lectin, GSI-B4, recognize the same tumor-associated structure (TAS) J Immunol. 1987;138:1999–2007. [PubMed] [Google Scholar]
- Yang I, Han SJ, Kaur G, Crane C, Parsa AT. The role of microglia in central nervous system immunity and glioma immunology. J Clin Neurosci. 2010;17:6–10. doi: 10.1016/j.jocn.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Shi Y, He J, Chen Z. The evolving story of macrophages in acute liver failure. Immunol Lett. 2012;147:1–9. doi: 10.1016/j.imlet.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Zhang W, Dai M, Fridberger A, Hassan A, Degagne J, Neng L, Zhang F, He W, Ren T, Trune D, Auer M, Shi X. Perivascular-resident macrophage-like melanocytes in the inner ear are essential for the integrity of the intrastrial fluid-blood barrier. Proc Natl Acad Sci U S A. 2012;109:10388–10393. doi: 10.1073/pnas.1205210109. [DOI] [PMC free article] [PubMed] [Google Scholar]